Abstract
Background
Low bone mineral density (BMD) is a significant co-morbidity in HIV. However, studies evaluating vitamin D supplementation on bone health in this population are limited. This study investigates changes in bone health parameters after 12 months of supplementation in HIV-infected youth with vitamin D insufficiency.
Methods
This is a randomized, active-control, double-blind trial investigating changes in bone parameters with 3 different vitamin D3 doses [18,000 (standard/control dose), 60,000 (moderate dose) and 120,000 IU/monthly (high dose)] in HIV-infected youth 8–25 years old with baseline serum 25-hydroxyvitamin D (25(OH)D) concentrations <30 ng/mL. Bone mineral density and bone turnover markers were measured at baseline and 12 months.
Results
One hundred and two subjects enrolled. Over 12 months, serum 25(OH)D concentrations increased with all doses, but the high dose (i.e. 120,000 IU/monthly) maintained serum 25(OH)D concentrations in an optimal range (≥30 ng/mL or ≥20 ng/mL) throughout the study period for more subjects (85% and 93%, respectively) compared to either the moderate (54% and 88%, respectively) or standard dose (63% and 80%, respectively). All dosing groups showed some improvement in BMD; however, only the high-dose arm showed significant decreases in bone turnover markers for both procollagen type 1 amino-terminal propeptide (−3.7 ng/mL; P=0.001) and B-CrossLaps (−0.13 ng/mL; P=0.0005).
Conclusions
High dose vitamin D supplementation (120,000 IU/month) given over 12 months decreases bone turnover markers in HIV-infected youth with vitamin D insufficiency, which may represent an early, beneficial effect on bone health. High vitamin D doses are needed to maintain optimal serum 25(OH)D concentrations.
Keywords: HIV, vitamin D, randomized-controlled trial, pediatrics and adolescents, bone mineral density, bone turnover markers
INTRODUCTION
People with HIV are living longer than ever before because of combination antiretroviral therapy (cART). In fact, life expectancy nears that of the general population in developed countries1. However, HIV-infected patients are experiencing more medical co-morbidities, such as osteoporosis and fractures, compared to age-matched individuals in the general population2. The cause of bone loss in HIV is multifactorial, including traditional risk factors and alterations in bone metabolism due to a direct effect of antiretrovirals (ARVs) or of HIV viral proteins, and the chronic inflammatory state associated with HIV3.
Low bone mineral density (BMD) and osteoporosis are also much more common among HIV-infected children and young adults than their counterparts in the general population, regardless of whether HIV is acquired perinatally or later in adolescence4–6. Likewise, there are some, albeit sparse, data suggesting that they may be at an increased risk of fractures too7. In fact, bone toxicity is especially concerning in this younger population, as the majority of bone mass acquisition occurs during this period of rapid growth, and the effects of chronic HIV infection and exposure to cART accumulate over many decades. The long-term ramifications of these alterations in bone metabolism and reductions in BMD as this younger population ages are particularly alarming.
Vitamin D is essential for optimal bone health; however, the extent to which vitamin D deficiency contributes to bone disease in HIV is largely unknown. In contrast, in the general population, there are data from randomized-controlled trials (RCTs) showing that vitamin D supplementation improves BMD and modestly decreases the risk of fracture8–10. Even in healthy children, vitamin D supplementation, particularly among those with vitamin D deficiency, shows promise in improving BMD11,12. However, there are only a few published RCTs investigating vitamin D supplementation trials for improved bone health in the adult HIV population13 and HIV-infected youth population14,15, and results have been conflicting.
Exploring vitamin D supplementation may be even more important in HIV as a way of mitigating bone disease, given that some of the bone disease in HIV appears to be related to the associated chronic inflammation and heightened immune response. In addition to its role in bone health, vitamin D has wide-reaching immunomodulatory effects and plays a critical role in immune function including promoting an anti-inflammatory state16–19. Vitamin D insufficiency is widespread in the HIV population20–22, providing even more support that additional RCTs investigating the effect of vitamin D supplementation on bone in this population are urgently needed.
In this current analysis, we sought to explore the effects of vitamin D supplementation on bone health parameters in HIV-infected children and young adults with vitamin D insufficiency in the context of a RCT. Our focus on HIV-infected youth represents an innovative approach to potentially identify efficacious strategies to prevent the development of HIV-related bone disease before the onset of clinical symptoms.
METHODS
Study Design/Population
This is a randomized, active-control, double-blind, 24-month trial designed to measure the effect of vitamin D supplementation in HIV-1-infected youth. Subjects were recruited from the HIV clinics of University Hospitals Cleveland Medical Center, Cleveland, OH and Grady Health System, Atlanta, GA via electronic medical record system queries and case manager/provider referrals. Subjects were eligible if they were between 8–25 years of age with documented HIV-1 infection on a stable cART regimen for ≥12 weeks, with ≥6 months cumulative cART duration, HIV-1 RNA level <1,000 copies/mL, with no intent to change cART regimen, diet, sun exposure or exercise routine during the study period, and a baseline serum 25-hydroxyvitamin D (25(OH)D) concentration <30 ng/mL (Endocrine Society’s current definition of vitamin D sufficiency is ≥30 ng/mL23. Exclusion criteria included routine vitamin D supplementation >400 IU/day, pregnancy or lactation, acute illness or inflammatory condition, malignancy, parathyroid or calcium disorder, diabetes, creatinine clearance <50 mL/min, liver enzymes ≥2.5 times the upper limit of normal, hemoglobin ≤9.0 g/dL, medication use (e.g., chemotherapy agents, systemic steroids) which could affect results, or unwillingness/inability to comply with study procedures.
Intervention consisted of 3 different monthly vitamin D3 (cholecalciferol) doses [18,000 IU/month (standard dose/active control), 60,000 IU/month (moderate dose) or 120,000 IU/month (high dose) (Tischon Corp., Salisbury, MD)]. Doses were chosen to represent an approximate monthly equivalent to 600 IU/daily (standard/control dose), 2,000 IU/daily (moderate dose), and 4,000 IU/daily (high dose), respectively. Six hundred IU/daily is the current Institute of Medicine’s (IOM) recommended dietary allowance (RDA) of vitamin D across our study population. This amount is considered sufficient by the IOM Food and Nutrition Board to meet the requirements of 97.5% of healthy individuals in each life-stage and sex group. The IOM considers a 25(OH)D concentration of ≥20 ng/mL to be sufficient. Likewise, 4,000 IU/daily is the IOM’s current tolerable upper intake level24.
The randomization scheme was computer-generated, stratified by efavirenz (EFV) use at entry (an antiretroviral drug that has been shown to affect 25(OH)D concentrations in some studies25) and provided by investigational pharmacist departments at each site. Each vitamin D3 capsule contained either 9,000 IU, 30,000 IU, or 60,000 IU. Each dose consisted of 2 capsules so that the amount of vitamin D3 totaled the appropriate dose for the subject’s randomized arm (e.g. subjects in the standard dose arm took two 9,000 IU capsules so that the total was 18,000 IU). Regardless of dose, all capsules look identical. All subjects took two capsules at baseline and then monthly after being prompted by a reminder phone call from study staff. Subjects returned for study visits every 3 months. Representative capsules were sent to an independent laboratory (Analytical Research Laboratories, Oklahoma City, OK) at regular intervals during the study period to ensure continued potency of each vitamin D dose.
The study was reviewed and approved by the Institutional Review Boards of University Hospital Case Medical Center, Emory University and Grady Health System. Parents or legal guardians gave informed consent for minors, and patients ≥18 years of age signed their own informed consent. The study was registered on clinicaltrials.gov (NCT01523496).
Here, we present the pre-specified secondary analysis that assessed changes in BMD and bone turnover markers from baseline to 12 months in HIV-infected subjects.
Clinical Assessments
Relevant data were obtained by questionnaire, including demographics, current and past medical history, alcohol intake, tobacco use, and drug habits. Further information was collected from the HIV-infected subjects’ medical records including past and current medical diagnoses, nadir CD4 count, detailed past and current ARV and non-ARV medication use, HIV diagnosis date, and acquisition method (perinatal or horizontal). Targeted physical examination and weight and height measurements were obtained.
Laboratory Assessments
Blood was collected from all participants after at least an 8-hour fast. Serum concentrations of 25(OH)D were measured as the best measure of overall vitamin D status26. Serum 25(OH)D concentrations were measured using either an automated chemiluminescent technique (IDS-iSYS automated machine, Immunodiagnostic Systems, Inc., Fountain Hills, AZ) or a competitive immunoassay (ADVIA Centaur XP System, Siemens Healthcare Diagnostics, Inc., Tarrytown, NY). Parathyroid hormone was measured via ELISA (Immutopics, Inc., Athens, OH).
Plasma bone turnover markers were also measured, including the bone formation markers, osteocalcin (OC) and procollagen type 1 amino-terminal propeptide (P1NP), and the bone resorption marker, B-CrossLaps (B-CTx). Osteocalcin and B-CTx were measured using a sandwich immunoassay on the Roche Elecsys 2010 (Roche Diagnostics, Indianapolis, IN). Median inter-assay coefficients of variance for OC and B-CTx were 5.15–9.40% and 4.13–5.82%, respectively. Procollagen type 1 amino-terminal propeptide was measured using a radioimmunoassay (Immunodiagnostics Systems, Fountain Hills, AZ), and the median inter-assay coefficient of variance was 5.86–15.44%.
Absolute CD4+ T-cell count and plasma HIV-1 RNA level were concomitantly measured as markers of HIV disease activity. Because there were several different assays used in the clinical laboratories of the two study sites to measure HIV-1 RNA levels, there were varying lower limits of detection (<20, <40, <48, <79 and <80 copies/mL) used throughout the study. Less than 80 copies/mL was the highest cut-off that defined an undetectable HIV-1 RNA; thus, we considered any HIV-1 RNA <80 copies/mL as undetectable.
BMD Assessments
Lumbar spine and total hip BMD were assessed via dual-energy x-ray absorptiometry (DXA) at baseline and 12 months. Measurements were obtained for individual subjects on the same scanner at both time points (GE Lunar Prodigy, GE Healthcare). The left hip was utilized in all cases, unless clinically contraindicated, and only the total hip BMD was used as the hip BMD of interest. Lumbar spine BMD was measured at L1-L4.
Statistical Analyses
Analyses were performed using intent-to-treat principles based on randomized treatment assignment which used all available data, and missing data were ignored.
First, bone mineral density data from the moderate- and high-dose arms were compared. It was determined in preliminary analyses that there was no significant difference in the % change for spine or total hip BMD over the study period (see results section). Thus, the subjects randomized to receive the moderate or high doses were considered together (supplementation arm) and compared to subjects randomized to receive the standard dose (active control).
Nominal variables were compared using χ2 analysis or Fisher’s exact test as appropriate. Continuous measures were tested for normality. For between-group comparisons (baseline and changes from baseline to 12 months), normally-distributed variables were compared using the t-test, and non-normally-distributed variables were compared using Wilcoxon rank sum test. For within-group changes from baseline to 12 months, normally-distributed variables were assessed with the paired t-test, and non-normally-distributed variables were assessed with Wilcoxon signed rank test. Spearman correlation coefficients were utilized to investigate associations between changes in bone parameters and variables of interest.
Additional analyses were conducted to further assess changes in serum 25(OH)D concentrations, BMD, and bone turnover markers for the 3 dosing groups separately (standard, moderate, and high). Kruskall-Wallis test was used to compare differences in changes in the bone turnover markers over the 12-month time period among the 3 groups. Linear regression models were then used to compare the changes in bone turnover markers for the 3 groups while adjusting for potential confounders. Confounding variables were chosen based on clinical importance and included race, sex, and the following baseline characteristics: age, body mass index (BMI), Tanner stage (5 vs. <5) and HIV-1 RNA (<80 vs. ≥80 copies/mL).
All statistical tests were two-sided with a 0.05 significance level. Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
Subjects were recruited from January 2012 – July 2014. One hundred and two HIV-infected subjects were enrolled into the study; 81 subjects completed their 12-month visit (Figure 1). Of those 81 subjects, 30, 24, and 27 were in the standard-, moderate-, and high-dose groups, respectively.
Figure 1. Consort diagram of study participants.
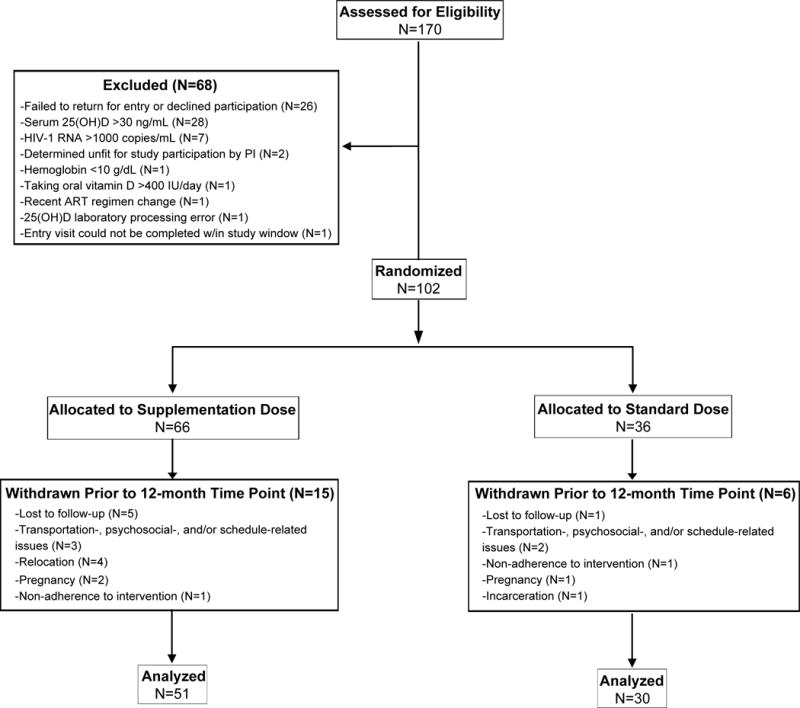
One hundred-seventy patients were screened for enrollment. Sixty-eight subjects screen failed, resulting in 102 subjects enrolled. Twenty-one subjects were withdrawn before their 12-month study visit, leaving 81 subjects. 25(OH)D, 25-hydroxyvitamin D; HIV, human immunodeficiency virus; PI, principal investigator; ART, antiretroviral therapy
As described in the methods, there was no significant difference between % change in spine or hip BMD over the study period between the moderate- and high-dose arms [median (quartile 1, quartile 3) % change in spine and hip BMD, respectively: moderate-dose arm = +3.6 (+0.3, +9.2) and +1.8 (−1.2, +4.3), high-dose arm = +2.1 (−0.1, +5.9) and +0.4 (−0.5, +2.5); P = 0.28 (spine) and P=0.61 (hip)]. Thus, subjects from the moderate- and high-dose arms were combined into one group (supplementation arm) and compared to subjects in the standard-dose arm (active control).
Baseline characteristics are shown in Table 1. Overall, there was a median age of 20 years with 64% male and 89% black. Fifty-six subjects were on a ritonavir-boosted protease inhibitor (PI/r) (darunavir = 29; atazanavir = 20; lopinavir = 7), 39 subjects were on a non-nucleoside reverse transcriptase inhibitor (NNRTI) (EFV = 27; rilpivirine = 9; nevirapine = 2; etravirine = 1), and 9 subjects were on an integrase inhibitor (elvitegravir/cobicistat = 7; dolutegravir = 1; raltegravir = 1). Nucleoside reverse transcriptase inhibitor (NRTI) backbones included emtricitabine/tenofovir (N=91), lamivudine/abacavir (N=7), lamivudine/zidovudine (N=2), stavudine/abacavir (N=1), and lamivudine/zidovudine/abacavir (N=1).
Table 1.
Baseline Characteristics
| Median (Q1, Q3) or no. (%) | Total HIV+ Group (N=102) |
Supplementation Dose* (N=66) |
Standard Dose** (N=36) |
P† |
|---|---|---|---|---|
| Age, years | 20.3 (16.6, 22.8) | 20.9 (17.0, 23.6) | 19.9 (15.9, 21.6) | 0.27 |
| BMI, kg/m2 | 22.5 (19.5, 25.3) | 23 (20, 26) | 21.1 (18.9, 21.6) | 0.07 |
| Male sex | 65 (64%) | 44 (67%) | 21 (58%) | 0.52 |
| Black race | 91 (89%) | 57 (86%) | 34 (94%) | 0.32 |
| Tanner stage | 0.96 | |||
| 1 | 4 (4%) | 2 (3%) | 2 (6%) | |
| 2–4 | 21 (21%) | 13 (20%) | 8 (22%) | |
| 5 | 77 (75%) | 51 (77%) | 26 (72%) | |
| Current smoking | 27 (27%) | 22 (33%) | 5 (14%) | 0.04 |
| Perinatal transmission | 54 (53%) | 35 (47%) | 19 (53%) | 0.68 |
| HIV duration, years | 8.1 (2.3, 15.6) | 8.2 (2.1, 15.8) | 8.0 (2.7, 15.6) | 0.86 |
| ARV duration, years | 3.2 (1.3, 10.0) | 3.2 (1.3, 10.0) | 2.7 (1.3, 10.1) | 1.00 |
| NRTI duration, years | 3.1 (1.3, 9.5) | 3.2 (1.3, 9.7) | 2.6 (1.3, 7.7) | 0.84 |
| Current use of EFV | 27 (25%) | 18 (27%) | 9 (25%) | 1.00 |
| Current use of TDF | 82 (80%) | 55 (83%) | 27 (75%) | 0.31 |
| Current CD4, cells/mm3 | 652 (449, 872) | 652 (451, 879) | 608 (417, 792) | 0.58 |
| Nadir CD4, cells/mm3 | 293 (174, 424) | 317 (190, 472) | 246 (109, 363) | 0.06 |
| HIV RNA, <80 copies/mL | 91 (89%) | 59 (89%) | 32 (89%) | 1.00 |
| HIV RNA, copies/mL (N=11) | 190 (127, 630) | 143 (90, 590) | 654 (382, 805) | 0.10 |
| 25(OH)D, ng/mL | 17 (13, 22) | 18.0 (14.0, 22.0) | 17.3 (11.03, 20.9) | 0.49 |
| PTH, pg/mL | 55.1 (40.7, 74.9) | 53.4 (39.8, 74.9) | 58.1 (44.9, 72.2) | 0.40 |
| Spine BMD, g/cm2 | 1.13 (0.95, 1.24) | 1.17 (1.06, 1.27) | 1.13 (0.89, 1.27) | 0.29 |
| Hip BMD, g/cm2 | 1.05 (0.85, 1.16) | 1.10 (0.90, 1.22) | 0.98 (0.88, 1.13) | 0.05 |
| Spine z-score | −0.5 (−1.4, 0.4) | −0.3 (−1.1, 0.4) | −0.6 (−1.5, 0.2) | 0.31 |
| Hip z-score | −0.5 (−1.4, 0.4) | −0.4 (−0.9, 0.5) | −0.9 (−1.7, −0.2) | 0.04 |
| Osteocalcin, ng/mL | 33.7 (24.6, 49.5) | 33.7 (25.6, 49.5) | 32.8 (22.5, 47.9) | 0.61 |
| B-CrossLaps, ng/mL | 0.82 (0.57, 1.14) | 0.81 (0.59, 1.09) | 0.82 (0.54, 1.25) | 0.85 |
| P1NP, ng/mL‡ | 99.7 (76.8, 150.0) | 99.7 (76.8, 150.0) | 99.8 (73.3, 165.8) | 0.99 |
Supplementation dose = 60,000 IU/month (moderate dose) or 120,000 IU/month (high dose);
Standard dose = 18,000 IU/month (control dose);
P value between supplementation and standard dosing arms;
The upper limit of detection for P1NP was 300 ng/mL. Overall, 21% of subjects had unmeasurable values above the upper limit (21.5% vs. 19.4% in supplementation vs. standard arms, respectively; P=0.80) and were assigned a value of 300 ng/mL for analysis, resulting in a truncated, non-normal distribution. N.B. Bold-faced P values indicate those <0.05.
Q, quartile; BMI, body mass index; HIV, human immunodeficiency virus; ARV, antiretroviral; NRTI, nucleoside reverse transcriptase inhibitor; EFV, efavirenz; TDF, tenofovir; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; BMD, bone mineral density; P1NP, procollagen type 1 amino-terminal propeptide
At baseline, study arms were well-matched for most variables, except that there were statistically more smokers in the supplementation arm than the standard arm, and the hip z-score was statistically lower in the standard arm than the supplementation arm.
Changes in Clinical Characteristics
The time that elapsed from the baseline visit to the 12-month visit were similar for all study arms [mean (standard deviation) number of days between study visits: standard-dose arm = 366.1 (19.5) days vs. supplementation-dose arm = 365.9 (19.0) days; moderate-dose arm = 366.5 (20.7) days vs. high-dose arm = 365.4 (17.8) days].
Thirteen subjects who were still in the study at the 12-month time point changed their ART regimens, reflecting in part updates to the Guidelines for the Use of Antiretroviral Agents in HIV-1-infected Adults and Adolescents27. Seven subjects (3 in supplementation arm; 4 in standard arm) switched a PI/r to rilpivirine (N=1), elvitegravir/cobicistat (N=5), or dolutegravir (N=1). Five subjects (2 in supplementation arm; 3 in standard arm) switched from EFV to rilpivirine (N=1), elvitegravir/cobicistat (N=2), or dolutegravir (N=1). One of these subjects (from the supplementation arm) stopped ARVs completely. One additional subject on a PI/r switched to EFV for a short period and then switched back to a PI/r. Other than the subject who stopped ARVs, no subject changed NRTIs. Overall, 88% of subjects maintained an HIV-1 RNA <1000 copies/mL throughout the 12-month study period (86% in supplementation arm; 90% in standard arm).
There was no difference between the supplementation arm vs. standard arm at 12 months for current CD4 count (712 vs. 665 cells/mm3; P=0.91), % of subjects with HIV-1 RNA <80 copies/mL (78% vs. 90%; P=0.88), or % of subjects at Tanner stage 5 (82% vs. 76%; P=0.57). Body mass index was significantly higher in the supplementation arm when compared to the standard arm (23.8 vs. 21.3 kg/m2, P=0.02).
Changes in Serum 25(OH)D Concentrations
Overall, 25(OH)D concentrations increased significantly over the 12-month study period for all study arms (Table 2), although the increase was greater in the supplementation arm.
Table 2.
Changes in Bone Parameters over Study Period
| Median (Q1, Q3) or no. (%) | HIV+ Combined (N=81) |
P° | Supplementation Dose* (N=51) |
P° | Standard Dose** (N=30) |
P° | P† |
|---|---|---|---|---|---|---|---|
| 25(OH)D, ng/mL | +18.0 (+8.8, +32.0) | <0.001 | +42 (+33, +53) | <0.001 | +31 (+22, +37) | <0.001 | 0.001 |
| PTH, pg/mL | +6.7 (−18.0, +19.9) | 0.30 | +7.2 (−11.5, +15.9) | 0.37 | +1.8 (−23.1, +25.0) | 0.60 | 0.91 |
| % Δ in spine BMD | +2.4 (−0.08, +6.0) | <0.001 | +2.8 (−0.04, +7.2) | <0.001 | +1.4 (−0.16, +3.5) | 0.87 | 0.29 |
| % Δ in hip BMD | +0.87 (−1.6, +2.8) | 0.08 | +0.93 (−0.7, +3.2) | 0.03 | +0.61 (−2.6, +2.1) | 0.002 | 0.37 |
| Spine z-score | +0.1 (−0.1, +0.4) | 0.007 | +0.2 (−0.1, +0.4) | 0.005 | 0.0 (−0.6, +0.3) | 0.51 | 0.15 |
| Hip z-score | 0.0 (−0.2, +0.2) | 0.44 | 0.0 (−0.2, +0.1) | 0.56 | −0.05 (−0.2, +0.2) | 0.50 | 0.68 |
| Osteocalcin, ng/mL | −2.9 (−9.2, +3.8) | 0.03 | −3.3 (−10.1, +3.5) | 0.046 | −2.2 (−8.0, +4.42) | 0.34 | 0.67 |
| B-CrossLaps, ng/mL | −0.09 (−0.24, +0.09) | 0.03 | −0.09 (−0.25, +0.06) | 0.01 | −0.07 (−0.19, +0.15) | 0.74 | 0.23 |
| P1NP, ng/mL‡ | −6.7 (−21.0, +4.6) | 0.003 | −12.8 (−25.4, 0.0) | 0.001 | 0.0 (−13.1, +12.1) | 0.61 | 0.06 |
Supplementation dose = 60,000 IU/month (moderate dose) or 120,000 IU/month (high dose);
Standard dose = 18,000 IU/month (control dose);
P value for changes within group;
P value for differences in changes between the two dosing arms;
The upper limit of detection for P1NP was 300 ng/mL. At the 12-month time point, 11.8% and 23.3% of subjects in the supplementation and standard arms, respectively, had unmeasurable values above the upper limit and were assigned a value of 300 ng/mL for the purpose of calculation of change from baseline. Bold-faced P values indicate those <0.05.
Q, quartile; 25(OH)D, 25-hydroxyvitamin D; BMD, bone mineral density; P1NP, procollagen type 1 amino-terminal propeptide
In the supplementation arm, 71% and 90% of subjects maintained a serum 25(OH)D concentration ≥30 and ≥20 ng/ml, respectively, at every measured time point (3, 6, 9, 12 months) compared to 33% and 67%, respectively, in the standard arm. Similarly, 80% and 90% of subjects had a serum 25(OH)D concentration ≥30 and ≥20 ng/ml, respectively, at the 12-month time point compared to 63% and 80%, respectively, in the standard arm.
Within the supplementation arm, 54% and 88% in the moderate-dose group and 85% and 93% in the high-dose group maintained a serum 25(OH)D concentration ≥30 and ≥20 ng/ml, respectively, at every measured time point. At the 12-month time point, 71% and 88% in the moderate-dose group and 89% and 93% in the high-dose group had a serum 25(OH)D concentration ≥30 and ≥20 ng/ml, respectively.
Through the 12-month time point, there were no study-related adverse events, interruptions in study drug administration, or known non-adherence for any subject in any dosing group. No subject achieved a serum 25(OH)D concentration ≥100 ng/mL. There was one subject who reported mild nausea after taking the cholecalciferol capsules; this subject withdrew from the study prior to the 12-month time point for non-study-related reasons.
Changes in Bone Parameters
Table 2 depicts the changes in bone endpoints. For the HIV-infected subjects combined, % change in spine BMD and spine z-score increased significantly. There was a trend toward a significant increase in % change in hip BMD but not for hip z-score. All three bone turnover markers decreased significantly.
In the supplementation arm, % change in spine and hip BMD and spine z-score increased significantly. In the standard arm, only % change in hip BMD increased significantly. All three bone turnover markers decreased significantly within the supplementation arm, without significant changes in the standard arm.
Changes in bone turnover markers were then further analyzed for the three dosing arms separately (standard, moderate, high). Only the high-dose arm showed significant decreases for both P1NP (P=0.001) and B-CTx (P=0.0005) (Table 3; Figure 2 in supplemental digital content). These changes were statistically significantly different among the three groups for P1NP (P=0.038) and approached significance for B-CTx (P=0.07). Changes in P1NP and B-CTx were then compared among the three groups after adjusting for potential confounders (race, sex, and baseline age, BMI, Tanner stage and HIV-1 RNA). The high-dose arm had statistically significant decreases in P1NP compared to the standard-dose arm (P=0.02), and differences in B-CTx changes approached significance (P=0.07) (Figure 2 in supplemental digital content).
Table 3.
Changes in Bone Turnover Markers over 12 months by Randomized Group
| Median (Q1, Q3) | Standard Dose* (N=30) |
P° | Moderate Dose** (N=24) |
P° | High Dose*** (N=27) |
P° | P† |
|---|---|---|---|---|---|---|---|
| Osteocalcin, ng/mL | −2.2 (−8.0, +4.4) | 0.35 | −2.5 (−8.5, +2.9) | 0.21 | −3.7 (−13.3, +3.5) | 0.14 | 0.84 |
| B-CrossLaps, ng/mL | −0.07 (−0.19, +0.15) | 0.74 | 0.00 (−0.23, +0.14) | 0.98 | −0.13 (−0.31, −0.02) | 0.0005 | 0.07 |
| P1NP, ng/mL‡ | 0.0 (−13.1, +12.1) | 0.61 | −7.4 (−18.5, +5.8) | 0.24 | −15.9 (−32.2, 0.0) | 0.001 | 0.038 |
Standard dose = 18,000 IU/month;
Moderate dose = 60,000 IU/month;
High dose = 120,000 IU/month;
P value for changes within group;
P value for differences in changes among the three dosing groups;
The upper limit of detection for P1NP was 300 ng/mL, and those subjects with an unmeasurable value above the upper limit were assigned a value of 300 ng/mL for the purpose of calculation of change from baseline. Bold-faced P values indicate those <0.05. Q, quartile; 25(OH)D, 25-hydroxyvitamin D; P1NP, procollagen type 1 amino-terminal propeptide
There were no significant differences between % change in spine and hip BMD between the standard arm and moderate-dose arm, nor between the standard arm and high-dose arm (data not shown).
Correlations
There were no significant correlations between % change in spine BMD and either baseline or changes in serum 25(OH)D concentrations. There was a significant negative correlation between % changes in spine BMD and changes in plasma OC (R=−0.36; P=0.0019), but not plasma B-CTx or P1NP. None of the above correlations were significant when % change in hip BMD was considered; however, there was a trend toward an inverse significant relationship with changes in OC (% change: R=−0.19, P=0.0998). Changes in 25(OH)D were not significantly correlated with changes in any of the bone turnover markers.
DISCUSSION
In this current study, we investigated the effects of vitamin D supplementation on BMD and bone markers after 12 months of monthly oral vitamin D3 in HIV-infected youth on cART with vitamin D insufficiency. Serum 25(OH)D concentrations increased significantly after 12 months with all three doses [18,000 IU/month (standard/active control dose), 60,000 IU/month (moderate dose), and 120,000 IU/month (high dose)]; however, serum 25(OH)D concentrations increased significantly more in the supplementation arm (moderate and high doses) compared to the standard arm. Notably, the high dose within the supplementation arm (i.e. 120,000 IU/monthly) maintained serum 25(OH)D concentrations in the optimal range more consistently throughout the study period than either the moderate or standard doses. And, perhaps most importantly, the standard vitamin D dose (600 IU/day; given in this study as 18,000 IU/month) recommended by the IOM to satisfy the vitamin D requirements of 97.5% of healthy individuals (to maintain a serum 25(OH)D concentration ≥20 ng/mL) was insufficient for more than one-third of the subjects randomized to that arm. Likewise, the IOM’s upper tolerable limit of 4,000 IU/day (given in this study as 120,000 IU/month) satisfied the vitamin D requirements of 93% of subjects randomized to that dose. Notably, bone turnover markers decreased significantly more in the supplementation arm (compared to the standard arm), and the decrease was more pronounced with the high dose. However, no significant differences in BMD changes were detected between groups.
Overall, both study arms showed some increases in BMD over the study period, as expected given that some of our population was still growing. And, despite some suggestive data, we were unable to conclusively demonstrate that vitamin D supplementation was related to those improvements. This may merely be a reflection of inadequate sample size and/or insufficient follow-up time to show differences in BMD among the three different doses and/or a lack of a true placebo. In support of this, we observed significant decreases in bone turnover markers within the high-dose arm not seen in the other two dosing arms, with changes in P1NP being the most dramatic. This finding has also been reported in a recent abstract of an open-label study of vitamin D supplementation in HIV-infected youth28, and it may reflect an early, beneficial effect of high-dose vitamin D on bone health in HIV before significant changes are observed in BMD.
Bone undergoes constant remodeling with osteoclasts resorbing older bone and osteoblasts laying down new bone, and the actions of osteoclasts and osteoblasts can be assessed in vivo using markers of bone turnover. This process is normally tightly-coupled, but, in HIV and especially with cART-initiation, accelerated bone resorption and an increased bone formation result in a net loss of bone29,30. It is not clear, however, whether these changes result from alterations in the inflammatory/immune environment, direct antiretroviral effects, or a combination of factors. Indeed, bone metabolism is also mediated in part by inflammatory cytokines that affect the activity of osteoclasts and osteoblasts31. Given the chronic inflammatory state associated with HIV and vitamin D’s known immunomodulatory effects, further studies are needed to investigate the mechanisms and the clinical consequences of the changes that we and others have observed in bone turnover markers with vitamin D supplementation.
It is also important to note that our study design used an active-control arm instead of a true placebo which likely blunted our ability to detect differences in BMD changes among the dosing arms. However, due to ethical concerns about failing to treat subjects with vitamin D insufficiency/deficiency, we chose not to have a true control/placebo arm. Likewise, we utilized a bolus dosing strategy designed to minimize additional pill burden, given the risk of poor adherence to medication among adolescents and young adults. Serum 25(OH)D, the major circulating form of vitamin D and the metabolite most commonly used to assess overall vitamin D status, has a long half-life of ~15 days32, theoretically allowing a longer interval between vitamin D doses. Yet, some data suggest that a daily dosing schedule exerts superior therapeutic effects compared to large bolus doses, which can result in both a steep and rapid increase in circulating 25(OH)D concentrations, followed by a slow decline33. Thus, while bolus dosing strategies are particularly appealing in this population who often struggle with adherence to daily medications, daily dosing may be necessary to achieve clinically-meaningful benefits from vitamin D supplementation.
On the other hand, a recent abstract describing a study using daily vitamin D in adults with HIV also failed to demonstrate any clear benefit on BMD34. One commonality may not be the dosing schedule per se, but instead whether the dose results in a sustained elevation of serum 25(OH)D concentrations in an optimal range. Importantly, in our study, we demonstrated that a higher dose of vitamin D is needed to maintain 25(OH)D at sufficient serum concentrations based on either the IOM’s (≥20 ng/mL) or Endocrine Society’s (≥30 ng/mL) current recommendations and that the current IOM recommended dose (600 IU/daily) is not enough. In fact, these findings alone are important to help inform future trials.
Our study suffered from some limitations worth mentioning. As previously discussed, there was a relatively small sample size and a lack of a true placebo arm which limited the conclusions we could make. Moreover, the correlations we observed among serum 25(OH)D concentrations, BMD, and bone turnover markers, in particular, should be interpreted with caution given the small study population with frequent ART changes. In addition, a 1-year follow-up period may not be long enough to assess changes in BMD. Furthermore, our study lacked adherence measurements to study drug, such as pill counts, although limitations of adherence assessments are well-recognized. Finally, the majority of our study population was African American, and the correlation between serum 25(OH)D concentrations and bone health has been questioned35. As such, in this mostly African American population, serum 25(OH)D concentrations may not be reflective of vitamin D status or free vitamin D concentrations.
In conclusion, we showed that high-dose vitamin D supplementation (120,000 IU/month) decreases bone turnover markers, which may reflect an early, beneficial effect on bone health in HIV that could result in clinically-meaningful changes over time. Likewise, a higher dose of vitamin D supplementation is needed to achieve optimal 25(OH)D concentrations for this population. Our study underscores the complicated nature of bone disease in HIV-infected youth and the potential impact of chronic HIV and cART exposure that will span many decades. Further studies investigating how vitamin D supplementation affects BMD and bone microarchitecture in this vulnerable population are urgently needed.
Supplementary Material
Acknowledgments
Sources of Support: This work was made possible by the National Institute of Child Health and Development at the National Institutes of Health [K23 HD069199 to ARE; R01 HD070490 to GAM; K12 HD072245 to AC], Case Western Reserve University’s Center for AIDS Research (P30 AI36219), Emory University’s Center for AIDS Research (P30 AI050409), Emory+Children’s Pediatric Research Center (Immunology and Flow Cytometry Cores), Clinical and Translational Science Award and the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor and speaker for Gilead. ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor and speaker for Gilead. GAM serves as a consultant for Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Gilead, Pfizer, and ICON, and has received grant funding from Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Merck, AstraZeneca, and Gilead.
Footnotes
Conflicts of Interest: All other declare no conflicts of interest.
ClinicalTrials.gov Identifier: NCT01523496
Previous Publication: Data were presented in part at the Conference on Retroviruses and Opportunistic Infections, 2016. Boston, MA. Abstract 859.
References
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS one. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young B, Dao CN, Buchacz K, Baker R, Brooks JT, Investigators HIVOS Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Apr 15;52(8):1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 3.Hileman CO, Eckard AR, McComsey GA. Bone loss in HIV: a contemporary review. Current opinion in endocrinology, diabetes, and obesity. 2015 Dec;22(6):446–451. doi: 10.1097/MED.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiMeglio LA, Wang J, Siberry GK, et al. Bone mineral density in children and adolescents with perinatal HIV infection. Aids. 2013 Jan 14;27(2):211–220. doi: 10.1097/QAD.0b013e32835a9b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulligan K, Harris DR, Emmanuel P, et al. Low bone mass in behaviorally HIV-infected young men on antiretroviral therapy: Adolescent Trials Network Study 021B. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Aug;55(3):461–468. doi: 10.1093/cid/cis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. Aids. 2014 Jan 28;28(3):345–353. doi: 10.1097/QAD.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirani G, Williams PL, Chernoff M, et al. Changing Trends in Complications and Mortality Rates Among US Youth and Young Adults With HIV Infection in the Era of Combination Antiretroviral Therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Dec 15;61(12):1850–1861. doi: 10.1093/cid/civ687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. Jama. 2005 May 11;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 9.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. The New England journal of medicine. 1997 Sep 04;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 10.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. The New England journal of medicine. 2006 Feb 16;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 11.El-Hajj Fuleihan G, Nabulsi M, Tamim H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. The Journal of clinical endocrinology and metabolism. 2006 Feb;91(2):405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 12.Winzenberg TM, Powell S, Shaw KA, Jones G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi: 10.1002/14651858.CD006944.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Overton ETCE, Brown TT, Tebas P, McComsey GA, Melbourne KM, Napoli A, Hardin R, Ribaudo HJ, Yin MT, ACTG A5280 Study Team High-dose vitamin D and calcium attenuates bone loss with ART initiation: results from ACTG A5280, Abstract 133. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2014; Boston, MA. [Google Scholar]
- 14.Arpadi SM, McMahon DJ, Abrams EJ, et al. Effect of supplementation with cholecalciferol and calcium on 2-y bone mass accrual in HIV-infected children and adolescents: a randomized clinical trial. The American journal of clinical nutrition. 2012 Mar;95(3):678–685. doi: 10.3945/ajcn.111.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havens PL, Mulligan K, Hazra R, et al. Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection. The Journal of clinical endocrinology and metabolism. 2012 Nov;97(11):4004–4013. doi: 10.1210/jc.2012-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008 Apr;10(2):110–117. doi: 10.1007/s11926-008-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutrition reviews. 2008 Oct;66(10 Suppl 2):S125–134. doi: 10.1111/j.1753-4887.2008.00096.x. [DOI] [PubMed] [Google Scholar]
- 18.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. Apr;11(4):344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 19.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. Journal of molecular medicine. 2010 May;88(5):441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross AC, Judd S, Kumari M, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antiviral therapy. 2011;16(4):555–563. doi: 10.3851/IMP1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckard AR, Judd SE, Ziegler TR, et al. Risk factors for vitamin D deficiency and relationship with cardiac biomarkers, inflammation and immune restoration in HIV-infected youth. Antiviral therapy. 2012;17(6):1069–1078. doi: 10.3851/IMP2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutstein R, Downes A, Zemel B, Schall J, Stallings V. Vitamin D status in children and young adults with perinatally acquired HIV infection. Clinical nutrition. 2011 Oct;30(5):624–628. doi: 10.1016/j.clnu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011 Jul;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 24.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): 2011. [PubMed] [Google Scholar]
- 25.Wohl DA, Orkin C, Doroana M, et al. Change in vitamin D levels and risk of severe vitamin D deficiency over 48 weeks among HIV-1-infected, treatment-naive adults receiving rilpivirine or efavirenz in a Phase III trial (ECHO) Antiviral therapy. 2014;19(2):191–200. doi: 10.3851/IMP2721. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007 Jul 19;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 27.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 28.Sudjaritruk T, Aurpibul L, Bunupuradah T, et al. Effects of calcium and vitamin D supplementation on bone health in HIV-infected youth. Abstract 820. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2017; Seattle, WA. [Google Scholar]
- 29.Aukrust P, Haug CJ, Ueland T, et al. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999 Jan;84(1):145–150. doi: 10.1210/jcem.84.1.5417. [DOI] [PubMed] [Google Scholar]
- 30.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2009;51(5):554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 31.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004 Feb;15(1):49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008 Sep;93(9):3430–3435. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 33.Xiao L, Xing C, Yang Z, et al. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. The British journal of nutrition. 2015 Oct 14;114(7):1026–1034. doi: 10.1017/S000711451500207X. [DOI] [PubMed] [Google Scholar]
- 34.Yin MT, RoyChoudhury A, Bucovsky M, et al. A randomized trial of vitamin D3 (3000 IU vs 1000 IU) in HIV+ postmenopausal women. Abstract 682. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2017; Seattle, WA. [Google Scholar]
- 35.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England journal of medicine. 2013 Nov 21;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


