Abstract
Objective: The aim of this study was to compare the efficacy of two approaches: multicomponent interventions that focus on working with the carer and dyadic interventions that work with both the carer and the person with dementia. Method: A systematic review involving a search of Medline, EMBASE, and PsycINFO in October 2015 was performed. Randomized controlled trials involving carers of people with dementia and comparing multicomponent interventions with usual care were included. Results: Pooling of all studies demonstrated that multicomponent interventions can reduce depressive symptoms, improve quality of life, reduce carer impact, and reduce behavioral and psychological symptoms of dementia as well as caregiver upset with these symptoms. We were unable to find a significant difference in the effects of dyadic interventions in comparison with carer focused interventions for these outcomes. Discussion: Although effect sizes associated with intervention are small, multicomponent interventions are relatively inexpensive to deliver, acceptable, and widely applicable.
Keywords: dementia, caregivers, nonpharmacological, family
Introduction
Most people with dementia live in their own homes and rely on assistance to manage activities of daily living (ADL) from family and friends (carers; Australian Institute of Health and Welfare, 2012; National Alliance for Caregiving and AARP, 2009). Assistance is commonly required to support personal ADLs (e.g., washing, dressing, grooming, toileting, eating) and instrumental ADLs (e.g., cooking, shopping, and managing finances and health care) as well as providing general surveillance to ensure the safety and well-being of the person with dementia (Alzheimer’s Disease International, 2009). A review of studies that provided information on caregiving reported that carers spent an average of 3.7 hr per day assisting with ADLs and additional time spent providing supervision (Wimo, Winblad, & Jonsson, 2007).
Although caring for someone with dementia can be rewarding, it is also challenging (Thompson, 2013) and associated with high levels of burden (Adelman, Tmanova, Delgado, Dion, & Lachs, 2014), depression (Clare et al., 2002), and reduced quality of life (Argimon, Limon, Vila, & Cabezas, 2004). Carers have reported that they want more education, skills counseling, emotional support, and respite to help them in their caring role (Black et al., 2013; Hughes et al., 2014). There are now many trials demonstrating the efficacy of nonpharmacological interventions where the interventionist predominantly works with the carer with the aim of supporting them to provide care and cope more effectively (Brodaty & Arasaratnam, 2012; Elvish, Lever, Johnstone, Cawley, & Keady, 2013; Gallagher-Thompson et al., 2012; Jones, Edwards, & Hounsome, 2012; Kales, Gitlin, & Lyketsos, 2015; Maslow, 2012; Olazarán et al., 2010; Schoenmakers, Buntinx, & DeLepeleire, 2010; Van’t Leven et al., 2013). Interventions for carers are often multicomponent and tend to involve a combination of education, support, problem solving, and skills training (Kales et al., 2015). Although carer interventions are widely considered to be effective, studies are heterogeneous and involve different participant groups, interventions, comparison conditions, and outcomes. This leads to difficulty in interpreting the evidence and ascertaining which interventions are most effective and should be translated into clinical practice.
One of the distinctions between caregiver intervention programs is whether they work with the person with dementia and carer (dyad) or whether they focus the intervention on the carer alone. A number of the interventions that have been tested are considered dyadic interventions (Van’t Leven et al., 2013). Dyadic interventions may include joint counseling, problem solving, and use of strategies to try and increase independence in ADL and engagement in meaningful activities. Working with the dyad is thought to be more effective because of the synergistic relationship between the person with dementia and the caregiver. Existing systematic reviews have examined the effect of one type of carer intervention or the effect of carer interventions more broadly on a single outcome (Brodaty & Arasaratnam, 2012; Kales et al., 2015; Maslow, 2012).
The aim of this article was to examine the efficacy of carer interventions that involve multiple components (e.g., education, problem solving, skills building, support) and compare effects based on whether or not they were dyadic in nature.
Method
Background to the Review
This review stemmed from work that was completed in the development of clinical practice guidelines for dementia in Australia (Guideline Adaptation Committee, 2016). Clinical practice guidelines should include the most recent literature and be developed in a way that is timely and resource efficient while maintaining methodological quality of the systematic reviews (World Health Organisation, 2012). To manage this, guidelines typically use existing systematic reviews as a source of primary studies and update these reviews with more recent studies where required (Woolf, Schunemann, Eccles, Grimshaw, & Shekelle, 2012). As a component of guideline development, we conducted a systematic review of the efficacy of interventions for carers of people with dementia in comparison with usual care. We identified the most recent, comprehensive, high quality systematic review and used this as a source of primary studies (Olazarán et al., 2010). We then updated this by identifying any newer studies meeting the inclusion criteria and combined all results to present an overall measure of efficacy. This work is available in the Guideline Technical Report (Guideline Adaptation Committee, 2016). Subsequent to development of the guideline, we have examined the effectiveness of multicomponent interventions that were dyadic in nature in comparison with those that were not. We have updated the searches conducted during the guideline development work, included new studies, completed additional data extraction, and conducted novel meta-analyses.
Inclusion Criteria
We included randomized controlled trials published in English. Participants in the included studies were carers of people with any type of dementia. Multicomponent interventions were defined as those that involved a number of different intervention techniques. These intervention techniques included, but were not limited to, education, counseling, information regarding services, enhancing carer skills to provide care, problem solving and strategy development, and increasing resilience and coping skills in the carer. Interventions that were conducted with the carer alone or those that also involved the person with dementia (dyad) were included. Studies were required to compare the intervention with usual care. We also searched for studies that directly compared carer focused with dyadic interventions. The outcomes included were those measuring direct impact for the carer (depression, quality of life, and carer burden and caregiver upset in relation to behavioral and psychological symptoms of dementia) and the person with dementia (ADL function, behavioral and psychological symptoms of dementia).
Search Method
We identified the most recent and comprehensive high quality systematic review. The review was updated by searching for additional randomized controlled trials published after September 2008. Databases searched were Health Technology Assessment, the Cochrane Library (Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects), Medline, EMBASE, PsycINFO, and PubMed using terms including Alzheimer’s, dementia, caregiver, carer, family, assess, treatment, therapy, intervention, support, psychosocial, nonpharmacologic, education (see the appendix for full search strategy). Searches for the guideline were conducted on May 9, 2014, and updated on October 7, 2015.
Data Collection and Analysis
One review author (K.L.) ran the searches and reviewed titles and abstracts to select articles for full text review. Two authors (K.L. and R.M.) conducted full text review to determine which studies met the criteria for inclusion in the review. One author (K.L.) extracted data into the tables and into RevMan Version 5.2 for analysis and a second person checked the data extraction accuracy. Two authors (K.L. and R.M.) independently completed an assessment of risk of bias using the Cochrane Collaboration Risk of Bias tool (Higgins & Green, 2011). This included assessment of (a) random sequence generation, (b) allocation concealment, (c) blinding of participants and personnel, (d) blinding of outcome assessment, (e) incomplete outcome data, and (f) selective reporting. Items were classified as being “low risk,” “high risk,” or “unclear risk of bias.” We did not contact authors to obtain missing information regarding study data or methods.
Study outcomes were pooled in a meta-analysis in RevMan Version 5.2 when means and standard deviations were reported or could be calculated. Where authors did not report means and standard deviations (or these could not be calculated based on the data provided) studies were described narratively. A random effects model was used for pooling all outcomes due to statistical heterogeneity (I2 ≥ 50) and obvious heterogeneity observed on forest plot. Where it was not possible to pool data for an outcome (due to data not presented in an appropriate form for use in meta-analysis) the study results were synthesized in a narrative summary.
Results
The study selection process is presented in Figure 1.
Figure 1.
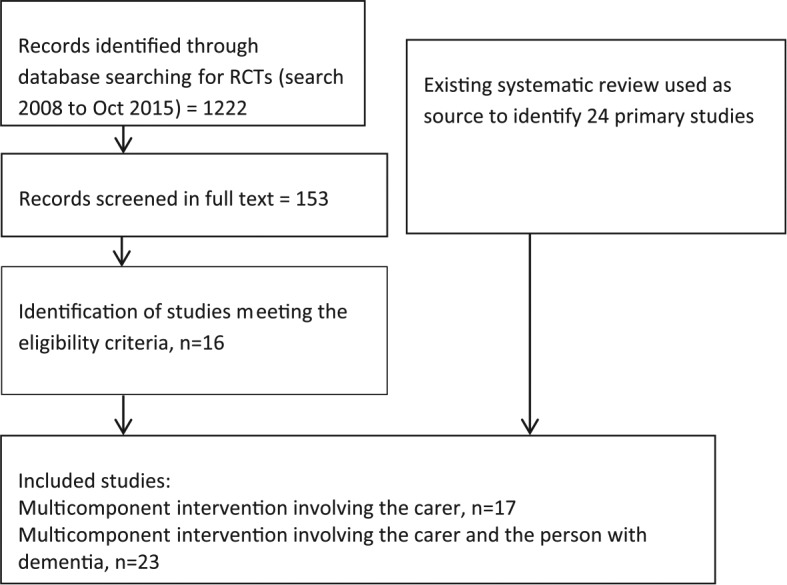
Search for studies.
The most recent high quality systematic review was conducted by Olazarán and colleagues in 2010 (Olazarán et al., 2010). This review included all forms of carer interventions and did not exclude studies based on outcomes reported. The review included six multicomponent interventions involving the carer and 18 multicomponent interventions involving the carer and the person with dementia. The review included studies published up to 2008.
We searched for additional studies published from 2008 to October 2015; 11 studies of multicomponent interventions involving only the carer and five multicomponent dyad interventions were identified. Thus, the total number of studies included in the review was 40. We did not identify any studies that compared carer focused interventions with dyadic interventions. Characteristics of included studies are summarized in Table 1.
Table 1.
Characteristics of Included Studies.
| Reference Country |
N carers | Participants | Intervention | Length of follow-up | Risk of biasa |
|---|---|---|---|---|---|
| Multicomponent carer | |||||
| Lawton 1989 (Lawton, Brody, & Saperstein, 1989) United States |
632 | Carers: Mean age 60 80% female PWD: Mean cognitive symptom severity was 14/30 in the control group and 13/30 in the intervention group (moderate severity). |
Intervention included respite, case management, and counseling. A social worker discussed the carer’s needs, assisted where needed in procuring services, and maintained regular contact (at client’s request at any time, with a 2-month maximum interval, initiated by the social worker if necessary) through the 12 months following the baseline interview. Case management and counseling continued, where needed, throughout the year and the respite offer was made when appropriate. | 12 months | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Mohide 1990 (Mohide et al., 1990) United States |
60 | Carers: Mean age 66 in the intervention group and 69 in the control group Gender 70% in the experimental group and 73% in the control group PWD: Mean MMSE was 13 in the intervention group and 11 in the control group (moderate severity) |
The aim of intervention was to enhance carer competence and achieve a sense of control. Caregiver support nurses scheduled home visits, which were initially weekly. Carers undertook a health assessment, were provided with education, a copy of “The 36-Hour Day,” problem solved strategies to reduce excessive disability, and enhance functional capacity in the PWD. Carers were offered a 4-hr block of in-home respite weekly. Carers were encouraged to attend a monthly 2-hr support group. | 3 and 6 months | 1. Low 2. Unclear 3. High 4. Low 5. High 6. Unclear |
| Eisdorfer 2003 (Eisdorfer et al., 2003) United States (REACH Miami study) |
225 | Carers: Mean age 69 years, 75% female PWD: Across the subgroups, the average MMSE ranged from 10 to 19/30 (moderate severity) |
A structural family therapy intervention plus technology system designed to facilitate linkages of the carers with both their family and supports outside of the home. The system also provided the therapist with enhanced access to the carer and their family members. Intervention took place over 12 months with weekly sessions for the first 4 months, biweekly sessions for the next 2 months, and monthly sessions for the final 6 months. Each session lasted 60 to 90 min. | 6 and 18 months | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. High |
| Mittelman 2004 (Mittelman, Roth, Coon, & Haley, 2004; Mittelman, Roth, Haley, & Zarit, 2004) Gaugler 2008 United States |
406 | Carers: Mean age 71 60% female PWD: The majority of participants had a Global Deterioration Scale score of 4 (mild severity) |
Caregivers in the treatment group received a comprehensive intervention that was designed to maximize the support provided to them. They agreed to participate in individual and family counseling sessions and to join and regularly attend an AD caregiver support group. They and their families could request additional help, advice, or counseling at any time. Education was a key element of the intervention. There was no predefined endpoint to the treatment. Formal counseling was the central structured component of the treatment. There were two sessions with the caregiver alone and four sessions with the caregiver and the family | 4, 8, and 12 months | 1. Low 2. Unclear 3. High 4. High 5. High 6. Unclear |
| Belle 2006 (Belle et al., 2006) Nichols United States |
642 | Carers: Mean age was 57 to 64 in the various treatment and participant groups 81% to 90% female depending on treatment and participant groups PWD: Mean MMSE ranged from 8 to 10/30 across the different subgroups (moderate severity) |
The intervention addressed caregiver depression, burden, self-care, and social support and care recipient problem behaviors through 12 in-home and telephone sessions over 6 months. Key strategies for intervention included education about pleasant events and well-being, stress management techniques, importance of looking after own health, social support, and the symptoms of dementia. Carers were supported to manage BPSD and given prescriptions with step-by-step strategies to manage these. | 6 Months | 1. Low 2. Unclear 3. High 4. Low 5. High 6. Unclear |
| Finkel 2007 (Finkel et al., 2007) United States |
46 | Carers: Mean age 65; 68% female PWD: Severity not reported |
Primary component of intervention delivery was the CTIS. Carers could (a) place and receive calls, (b) send and retrieve messages, (c) access information and services, and (d) conference with several people simultaneously. Intervention was provided over 6 months and included 2 in-home sessions and 12 sessions conducted over the CTIS. Eight individual educational skill-building sessions and six support group sessions were interspersed. | 6 months postintervention | 1. Unclear 2. Unclear 3. High 4. Low 5. High 6. Unclear |
| Tremont 2008 (Tremont, Davis, Bishop, & Fortinsky, 2008) United States |
33 | Carers: Mean age Intervention group 66 Control group 61 Gender not reported PWD: 22 care recipients had mild dementia and 11 had moderate dementia. (mild severity) |
Telephone based psychosocial intervention (called FITT-D). Twenty-three phone calls over 1 year. Involved emotional support, direction to resources, encouraging families, and carers’ health and teaching families and carers’ strategies | 1 year | 1. Unclear 2. Unclear 3. High 4. Low 5. High 6. High |
| Davis 2011 (Davis, Tremont, Bishop, & Fortinsky, 2011) United States |
46 | Carers: Mean age intervention group 57, control group 61 Gender intervention group 83% female, control group 68% female PWD: Severity not reported |
FITT-NH intervention. Delivered via 10 phone calls over 3 months for families and carers whose loved one had moved into a care home. Incorporated emotional adjustment, families– and carers–staff interaction, family functioning, health behaviors, and social support and role change. | Postintervention | 1. Unclear 2. Unclear 3. High 4. Low 5. Low 6. High |
| Kuo 2012 (Kuo et al., 2013) Taiwan |
129 | Carers: Mean age 80, 54% female PWD: 36% had mild dementia, 34% had moderate dementia, 30% had severe dementia (moderate severity) |
Intervention comprised a two-session, in-home training program. Each session was 1 week apart. Sessions lasted 2 to 3 hr. BPSD were identified and a plan formulated to minimize stimuli, and modify daily schedule and environment. The second session involved education and confirming the action plan. One week after the second visit and then once a month for up to 6 months the research nurse made follow-up phone calls. | 2 weeks, 3 months, and 6 months postintervention | 1. Unclear 2. Unclear 3. High 4. Unclear 5. High 6. Unclear |
| Joling 2012 (Joling et al., 2012) Netherlands |
192 | Carers: Mean age intervention group 68, control group 71 Gender 70% female PWD: Mean MMSE was 21/30 (mild severity) |
Six sessions were held over a year. Intervention was tailored to the needs of the families and carers and included psychoeducation, problem solving techniques, and engaging family networks to enhance support. Issues such as management of behavioral problems and coping with feelings of guilt were addressed. Ad hoc telephone counseling was available beyond the scheduled sessions. | Month 12 postintervention | 1. Unclear 2. Unclear 3. High 4. Low 5. Unclear 6. High |
| Gaugler 2013 (Gaugler, Reese, & Mittelman, 2013) United States |
107 | Carer: Mean age 50 Gender: All female PWD: Mean severity on the Global Deterioration Scale was 4.97. (moderate severity) |
Individual and family counseling, support group participation, and ad hoc counseling over a 4-month intervention period. | Quarterly during Year 1 and then every 6 months for a minimum of 2 years | 1. Low 2. Unclear 3. High 4. Low 5. Unclear 6. Unclear |
| Livingston 2013 (Livingston et al., 2013) Knapp 2013 United Kingdom |
260 | Carers: Mean age intervention group 62, control group 56 Gender intervention group 67% female, control group 71% female PWD: Mean severity on the clinical dementia scale was 1.3 indicating mild to moderate severity (mild severity) |
A manual-based coping intervention comprising eight sessions. The program consisted of psychoeducation about dementia, carers’ stress, and where to get emotional support; understanding behaviors of the family member being cared for and behavioral management techniques; changing unhelpful thoughts; promoting acceptance; assertive communication; relaxation; planning for the future; increasing pleasant activities; and maintaining skills learnt. Carers practiced these techniques at home, using the manual and relaxation CDs. | Months 4, 8 | 1. Low 2. Low 3. High 4. Low 5. High 6. Low |
| Martindale-Adams 2013 (Martindale-Adams, Nichols, Burns, Graney, & Zuber, 2013) United States |
154 | Carers: Mean age intervention group 66, control group 65 Gender intervention group 82% female, control group 86% PWD: Mean MMSE 15/30 (moderate severity) |
Telephone support groups involving 5 to 6 families and carers and a group leader. The group met for 14 sessions over 1 year. Families and carers were provided with written materials on managing behaviors of concern and coping with stress. The intervention focused on education, skills building, and support. | Months 6 and 12 | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Low 6. High |
| Gonzalez 2014 (Gonzalez, Polansky, Lippa, Gitlin, & Zauszniewski, 2014) United States |
102 | Carers: Mean age 62 in the intervention group and 58 in the control group Gender: All female PWD: Severity not reported |
Six group sessions on resourcefulness training in groups of 5 to 7 carers who met for 2 hr weekly. The training taught and reinforced cognitive behavioral skills, coping strategies, problem solving, priority setting, and decision making. | Postintervention and 12 weeks after the intervention | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Au 2014 (Au, Wong, Leung, Leung, & Wong, 2014) Hong Kong |
60 | Carers: Mean age 58 in intervention group and 55 in control group. Gender: 78% female PWD: Mean MMSE 15.5 in intervention group and 12.9 in the control group (moderate severity) |
Pleasant event scheduling for the caregivers and discussion and support around coping with caregiving. | Postintervention and 1 month following intervention | 1. Unclear 2. Unclear 3. High 4. Low 5. Unclear 6. Unclear |
| Chen 2015 (Chen, Huang, Yeh, Huang, & Chen, 2015) Taiwan |
46 | Carers: Mean age 55 Gender; 67% female PWD: Approximately 63% had CDR score of 1 and 37% had a CDR score of 2 (mild severity) |
Study nurses provided the intervention of six sessions over 3 months. Sessions included improving knowledge of dementia, providing information regarding support services, techniques to manage BPSD or cognitive difficulties, support in relaxation and coping, and establishing a caregiver self-support system. | Postintervention | 1. Low 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Gaugler 2015 (Gaugler, Reese, & Sauld, 2015) United States |
36 | Carers: Mean age 63, 81% female PWD: NPI-Q severity mean 9.89 out of 30 in the total group (moderate severity) |
Residential Care Transition Module to help families manage their emotional and psychological distress following residential care placement of a cognitively impaired relative. Intervention included psychoeducation, communication, problem solving, behavior management strategies, concrete planning, and counseling. | At 4 months and 8 months | 1. Unclear 2. Unclear 3. High 4. High 5. Unclear 6. Unclear |
| Multicomponent carers plus people with dementia | |||||
| Zarit 1982 (Zarit, Zarit, & Reever, 1982) United States |
35 | Carers: Not reported PWD: Not reported |
Classes with problem solving training involving taking practical steps to manage day-to-day problems caused by the memory loss. Participants, including both the individuals with dementia and the caregivers, were asked to talk about specific instances of “forgetting” that were troublesome. Suggestions for managing these problems were made by group leaders or other participants, taking into account an individual’s severity of memory loss, living arrangements, and personal resources. The classes met twice a week for 1.5 hr a session for 3.5 weeks, or seven sessions | Postintervention | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Chang 1999
(Chang, 1999) United States |
65 | Carers: Mean age 67 All females PWD: Not reported |
The intervention was designed to provide the caregiver with knowledge and skills to improve the PWD’s eating and dressing abilities. It involved (a) videotapes demonstrating assisted modeling behavior (eating and dressing) and (b) a Nurseline support program to reinforce the video information and assist the caregiver to explore coping strategies. | 4, 8, and 12 weeks | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Quayhagen 2000 (Quayhagen et al., 2000) United States |
103 | Carers: Mean age 72 63% female PWD: Severity not reported |
Dyadic counseling, namely, problem (conflict) identification, stress reduction, anger/frustration management, communication enhancement, and conflict resolution. The family (caregiver and PWD) focused on problems and/or conflicts that reduced their ability to interact effectively accompanied by tasks oriented to increasing communication and problem solving skills and enhancing the relationship. The dual participation continued to offer cognitive stimulation to the patient while working on social and emotional issues of concern to the couple. Each of the intervention programs was conducted for 8 weeks and included the participation of both members of the caregiving dyad. Three of the four programs were 1.5 hr in length, while the fourth program, the early-stage day care, was conducted for 4 hr weekly for the patients, but had only two sessions for the caregivers, with respite time an intended aspect of this intervention. | Postintervention | 1. Unclear 2. Unclear 3. High 4. Low 5. Unclear 6. Unclear |
| Buckwalter 1999 (Buckwalter et al., 1999) Gerdner 2002 (Gerdner, Buckwalter, & Reed, 2002) Stolley 2002 (Stolley, Reed, & Buckwalter, 2002) United States |
245 | Carers: Mean age 65 years 75% female PWD: Severity not reported |
Community-based psychoeducational nursing intervention to teach carers to manage BPSD. The individualized plan of care based on the PLST model was presented and practiced utilizing examples with return demonstration during the in-home sessions. The care plan was reviewed, techniques taught, and written materials summarizing the care plan were provided at the second in-home session 1 week later. Referrals for support groups, legal counseling, and case management services were provided as indicated. Experimental subjects received a total of approximately 3 to 4 hr of in-home intervention following baseline assessment and biweekly follow-up phone calls from a research team member for the first 6 months of the study. | 6 months, 12 months | 1. Unclear 2. Unclear 3.High 4. Low 5. Unclear 6. Unclear |
| Chu 2000 (Chu, Edwards, Levin, & Thompson, 2000) Canada |
78 | Carers: 68% of carers older than 75 Equal numbers of males and females PWD: Mean MMSE 22/30 (mild severity) |
The Early Home Care Program provided case management, occupational therapy, physiotherapy, social work, nursing, respiratory therapy, respite, homemaking, personal care assistance, volunteer service, and psychiatric consultation. The objectives were to (a) initiate long-term planning early to issues such as housing, finance, legal matters, and caregiving support; (b) increase the early use of home care and other community services; (c) improve the coping strategies related to psychosocial issues that often hinder long-term planning and service utilization; and (d) improve caregiving strategies related to functional and BPSD. | 3, 6, 10, 14, and 18 months | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Gitlin 2001 (Gitlin, Corcoran, Winter, Boyce, & Hauck, 2001) United States |
171 | Carers: Mean age 61 73% female PWD: Severity of dementia not reported |
Intervention was a targeted, multicomponent program led by an occupational therapist. It involved educating caregivers about the impact of the environment on dementia-related behaviors and helping caregivers simplify objects in the home (e.g., remove clutter), break down tasks (e.g., one- or two-step commands, lay out clothing in the order in which it is to be donned), and involve other members of the family network or formal supports in daily caregiving tasks. For example, occupational therapists provided education about dementia and the relationship between excess stimulation (auditory and visual) and behavioral disturbances such as agitation or resistance to assistance with self-care. Strategies such as removing objects to simplify the home and breaking down tasks provided primary control mechanisms by which caregivers could manage problems areas, such as agitation or the inability to follow directions or initiate tasks by the PWD. The program consisted of five 90-min sessions that were spaced approximately every other week over 3 months. | 3 months | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Garand 2002 (Garand et al., 2002) United States |
39 | Carers: Mean age 65 92% female PWD: Severity of dementia not reported |
Intervention involved two consultations, each lasting approximately 3 hr. During the first home visit, the interventionist focused on developing a therapeutic relationship with the caregiver while teaching underlying principles of the PLST model and instructing caregivers in the use of behavioral logs. At the second home visit, the plan of care was reviewed, specific behavioral techniques were taught, and the therapeutic relationship was reinforced. A plan for home safety was also outlined during this phase of the intervention and supporting literature was left with the caregiver. Referrals for support groups, legal counsel, and case management were provided. Phase 2 of the intervention consisted of telephone contacts with subjects (by the same interventionist), approximately every other week, for 6 months. Throughout both phases of the intervention, caregivers were encouraged to discuss feelings associated with the caregiving experience, as well as general life stressors they encountered, and to be actively involved in the care planning process. | Postintervention; 6 months | 1. Unclear 2. Unclear 3. High 4. High 5. Unclear 6. Unclear |
| Gitlin 2003 (Gitlin, Hauck, Dennis, & Winter, 2005; Gitlin et al., 2003) Gitlin 2005 United States |
255 | Carers: Mean age 61 76% female PWD: Mean MMSE 12/30 (moderate severity) |
“Home Environmental Skill-Building Program” Occupational therapists provided caregivers with education, problem solving, and technical skills (task simplification, communication), and simple home modifications. The goal was to help caregivers modify the environment to support care recipient physical functioning and reduce behavioral occurrences as well as to reduce caregiver burden. Active treatment, consisting of five 90-min home visits and one telephone session, occurred over 6 months. Maintenance, consisting of one home visit and three brief telephone sessions to reinforce strategy use and obtain closure, occurs over the subsequent 6 months. |
6, 12 months | 1. Low 2. Low 3. High 4. Unclear 5. Unclear 6. Unclear |
| Nobili 2004 (Nobili et al., 2004) Italy |
69 | Carers: Mean age 53 in the intervention group and 59 in the control group. Gender: 74% female in the control group and 89% female in the intervention group. PWD: Mean MMSE 12 in the control group and 11 in the intervention group (moderate severity) |
Structured intervention involving one home visit with an OT and one with a psychologist. 1. Analysis of the data collected by researchers during the baseline assessment of patients and principal carer; 2. Home visit by the psychologist to assess and give advice on relationships within the family, care burden of carer and psychological consequences, changes in personality, verbal and nonverbal communication, how problems are dealt by the family and carer, psychological support, and training to carer; 3. Home visit by the occupational therapist to suggest strategies for the control of reactive behaviors and to maintain or improve residual functional ability, modification to home barriers, and adaptation of the environment to meet the patient’s needs to limit dangerous situation. |
6, 12 months | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Bottino 2005 (Bottino et al., 2005) Brazil |
13 | Carer details not reported PWD: Mean MMSE was 23.5 in the intervention group and 21.3 in the control group (mild severity) |
Cognitive rehabilitation consisted of 90-min group sessions, once a week, designed to stimulate patients’ cognitive functions and to enhance ADL and social interaction. Simultaneously, caregivers (either a familiar or a professional caregiver) attended support group sessions. This group aimed to offer support and to prevent early stress caused by the strain and burden of dementia caregiving, sharing information about the disease, and how to take care of the patient. Caregivers were always instructed to repeat some exercises at home in between the group sessions at least 3 times a week. | Postintervention | 1. Unclear 2. Unclear 3. High 4. Low 5. Low 6. Unclear |
| Martin-Cook 2005 (Martin-Cook, Davis, Hynan, & Weiner, 2005) United States |
49 | Carers: Age not reported 70% female PWD: Mean MMSE 19/30 (mild severity) |
Intervention involved four weekly skills-training sessions. In Session 1, the TFLS was administered to the patient while observed by the caregiver. In Session 2, the TFLS was readministered with the addition of breaking tasks into smaller steps, as well as other visual, auditory, tactile, or multimodal cues and prompts to facilitate improved performance. Caregivers were told that the goal was for patients to complete as many of the IADL tasks as independently as possible, but that assistance should be rendered when patients seemed unable to proceed on their own. In Session 3, the caregiver administered the TFLS, using facilitative prompts and cues as appropriate. The study coordinator offered suggestions and input as needed to assist caregivers in cueing specific tasks. Session 4 integrated the experience of the previous three sessions. Individualized suggestions to enhance communication and specific strategies to facilitate cueing on ADL were reviewed. Practical advice regarding home safety and information about community resources, companion service agencies, and other home health services was offered. | Postintervention; 10 weeks postintervention | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Graff 2006 (Graff et al., 2008; Graff et al., 2006; Graff et al., 2007) Graff 2007 Graff 2008 The Netherlands |
135 | Carers: Mean age 66 in intervention group; mean age 61 in control group 70% female Mean MMSE 19/30 (mild severity) |
Treatment consisted of 10 1-hr sessions delivered by OTs and held over 5 weeks and focused on both patients and their primary caregivers. In the first four sessions of diagnostics and goal defining, patients and primary caregivers learnt to choose and prioritize meaningful activities they wanted to improve. The occupational therapist evaluated the possibilities for modifying patients’ homes and environment and observed patients’ ability to perform relevant daily activities and to use compensatory and environmental strategies. Therapists also observed primary caregivers’ supervision skills. In the remaining six sessions, patients were taught to optimize these compensatory and environmental strategies to improve their performance of daily activities. Primary caregivers were trained, by means of cognitive and behavioral interventions, to use effective supervision, problem solving, and coping strategies to sustain the patients’ and their own autonomy and social participation. The total time spent for the intervention, including the time spent for treatment at home (10 hr), narrative analysis, reports, and multidisciplinary briefing, was about 18 hr per patient and caregiver together. |
Postintervention (6 weeks) and 12 weeks | 1. Low 2. Low 3. High 4. Low 5. Low 6. Low |
| Stocking 2007 (Stocking et al., 2007) United States |
149 | Caregiver details not reported PWD: Mean MMSE 20/30 (mild severity) |
Joint completion of the Planning Ahead Together document, a research advance directive. | Postintervention, 2 years | 1. Unclear 2. Unclear 3. High 4. Unclear 5. Unclear 6. Unclear |
| Chien 2008 (Chien & Lee, 2008) Hong Kong |
88 | Carers: Mean age 44 64% female PWD: 80% of the patients presented with low to moderate impairments in ADLs (mild severity) |
Education and support group for family members that lasted for 6 months. A multidisciplinary committee—including a psychiatrist, a social worker, a case manager (nurse) from each center, and the researchers—selected 25 intervention objectives from dementia guidelines and designed an information and psychological support system linking case managers and dementia care services, health professionals, and referrals. Case managers coordinated all levels of family care according to the results of a structured needs assessment. Each family was assigned one case manager who prioritized problem areas and formulated a multidisciplinary education program for each family on effective dementia care—for example, cognitive stimulation. The program consisted of 12 sessions that were held every other week and lasted two hr each. It consisted of five phases—orientation to dementia care (one session), educational workshop about dementia care (three sessions), family role and strength rebuilding (six sessions), community support resources (one session), and review of program and evaluation (one session). The case managers also conducted home visits and brief education about dementia care every other week and family health assessment once per month. | 6, 12 months | 1. Unclear 2. Unclear 3. High 4. Low 5. Low 6. Unclear |
| Gitlin 2008 (Gitlin et al., 2008) United States |
60 | Carers: Mean age 65 88% female PWD: Mean MMSE 12/30 (moderate severity) |
Tailored Activity Program: 6 × 90-min home visits and two 15-min telephone contacts by occupational therapists over 4 months. Three activities per patient were developed based on assessments that identified the person’s capacities. The interventionist developed a brief written activity prescription. Carers were instructed in how to facilitate the activity and also in stress reducing techniques to establish a calm emotional tone. As activities were mastered, interventionists generalized strategies to care problems and instructed them on how to downgrade activities for future declines. | 4 months | 1. Low 2. Unclear 3. High 4. Low 5. Unclear 6. Low |
| Eloniemi-Sulkava 2009 (Eloniemi-Sulkava et al., 2009) Finland |
125 | Carers: Mean age intervention group 78, control group 77 Gender: Approximately 0.75 were female PWD: Mean MMSE 13 in the intervention group and 14 in the control group (moderate severity) |
Family care coordinator, education sessions, geriatrician, support groups for families and carers, and individualized services. Program lasted for up to 24 months | Months 6, 12, and 24 | 1. Low 2. Low 3. High 4. High 5. Low 6. High |
| Logsdon 2010 (Logsdon et al., 2010) United States |
142 | Carers: Mean age intervention group 71, control group 62 Gender 68% PWD: Mean MMSE 23/30 (mild severity) |
Alzheimer’s Association Early Stage Memory Loss Program involves nine sessions for the PWD and families and carers on topics such as information about the condition, relationships, daily living skills, self-esteem, future planning, and legal and financial considerations | Postintervention | 1. High 2. High 3. High 4. Unclear 5. Unclear 6.Unclear |
| Gitlin 2010b
United States (Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010) |
272 | Carers: Mean age 66 Gender 82% female PWD: Mean MMSE 13/30 (moderate severity) |
Intervention occurred over 24 weeks and involved up to nine occupational therapy sessions and two nursing sessions plus three phone calls. Goal setting, home assessment, problem solving and action plans, and strategies to reduce families’ and carers’ stress were used and assistive devices provided. The nurse addressed any potential causes of behavioral symptoms related to medical conditions (e.g., pain, dehydration) | Months 4 and 6 | 1. Low 2. Low 3. High 4. Low 5. Unclear 6. Unclear |
| Gitlin 2010a
(Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010 United States |
237 | Carers: Mean age 82 Gender: 68% female PWD: Mean MMSE 13/30 (moderate severity) |
“COPE” intervention: Assessment (patient capability, medical testing, home environment, family carer communication, concerns), family carer education (patient capabilities, potential effects of medications, pain, constipation, dehydration), and family carer training to address concerns and help reduce stress. Training in problem solving, communication, engaging patients in activities, and simplifying tasks was tailored to the needs of the dyad. Dyads received up to 10 sessions over 4 months with an occupational therapist | Months 4 and 9 | 1. Low 2. Low 3. High 4.Low 5. Unclear 6. Low |
| Chien 2011 (Chien & Lee, 2011) Hong Kong |
92 | Carers: Mean age 45 Gender 66% PWD: Severity ranged from mild (17%), moderate (50%), to severe (33%; moderate severity) |
Program was conducted fortnightly over 5 months. A multidisciplinary group identified intervention goals. The program included case management, education, support and problem solving, information about relationships, community resources, and improvement of home care and finance skills. Peer mentors helped with problem solving. | Week 1, Month 12 and Month 18 | 1. Unclear 2. Unclear 3. High 4. Low 5. Low 6. Unclear |
| Kwok 2012 (Kwok, Lam, & Chung, 2012) Hong Kong |
102 | Carers: Mean age 78 Gender intervention group 59%, control group 56% PWD: Mean MMSE 18/30 (moderate severity) |
Support from case manager via home visits and phone calls, home-based cognitive stimulation activities for the PWD and a telephone hotline to access the case manager. An OT advised on coping strategies, skills training, and behavioral management and linked the person with local services. | Months 4 and 12 | 1. Unclear 2. Unclear 3. High 4. Unclear 5. High 6. Unclear |
| Waldorff 2012 (Waldorff et al., 2012) Phung 2013 Sogaard 2014 Denmark |
330 | Carers: Mean age 66 Gender intervention group 53% female, control group 55% female PWD: Mean MMSE 24/30 (mild severity) |
“DAISY” intervention. Tailored program conducted over 8 to 12 months. Involved up to seven counseling sessions (4-5 with the families and carers present), a group education course about the condition building in peer support, phone call support, written information, and a journal. | 12 months | 1. Low 2. Low 3. High 4. Low 5. High 6. Low |
| Judge 2013 (Judge, Yarry, Looman, & Bass, 2013) United States |
128 | Carers: Mean age 65 Gender 74% female PWD: Mean MMSE 23/30 (mild severity) |
Combines educational skills and cognitive rehabilitation training. Six sessions provided to the dyad covering educational information, effective communication, managing memory, staying active, recognizing emotions, and behaviors | Month 3 | 1. Unclear 2. Unclear 3. High 4. Low 5. High 6. Unclear |
Note. Populations were categorized based on severity. The mean MMSE (or similar) was used where MMSE 19 to 23 was considered mild and MMSE 10 to 18 was considered moderate. REACH = Resources for Enhancing Alzheimer’s Caregiver Health; BPSD = behavioral and psychological symptoms of dementia; PWD = person with dementia; AD = Alzheimer’s disease; CG = caregiver education; NPI-Q = Neuropsychiatric Inventory–Questionnaire; OT = occupational therapist; ADL = activities of daily living; DAISY = Danish Alzheimer Intervention Study; MMSE = Mini-Mental State Examination; CTIS = Computer-Telephone Integration System; FITT-D = Family Intervention: Telephone Tracking–Dementia; FITT-NH = Family Intervention: Telephone Tracking–Nursing Home; CD = compact disc; CDR = Clinical Dementia Rating; PLST = progressively lowered stress threshold; IADL = instrumental activities of daily living; COPE = care of persons with dementia in their environments aRisk of bias: (a) random sequence generation, (b) allocation concealment, (c) blinding of participants and personnel, (d) blinding of outcome assessment, (e) incomplete outcome data, (f) selective reporting.
Summary of Included Studies
The characteristics of carers were similar across all studies. Studies typically recruited more females than males; in most studies, approximately, two thirds of the caregivers were female. Caregivers were typically aged in their 60s and 70s. The severity of symptoms in people with dementia varied widely across studies although most participants had mild to moderate dementia as indicated by Mini-Mental State Examination scores. A summary of the different interventions provided, the follow-up time for the studies, and the outcomes contributing to this review is shown in Table 1. In the dyadic interventions, the person with dementia was involved in various activities including education about dementia, joint problem solving, joint counseling, cognitive stimulation, engagement in pleasant activities, modification of the environment or tasks, peer support, and general case management duties.
Methodological Quality of the Included Studies
Overall, the quality of included studies was considered moderate as presented in Table 1. Due to unclear reporting, it was often difficult to determine methods of randomization and allocation concealment. Few studies had published protocols or were registered on clinical trial registries making it difficult to determine the presence of selective reporting. Four studies appeared to use outcome assessors who were not blinded to allocation, and in a further 16 studies it was unclear as to whether the outcome assessor was aware of allocation.
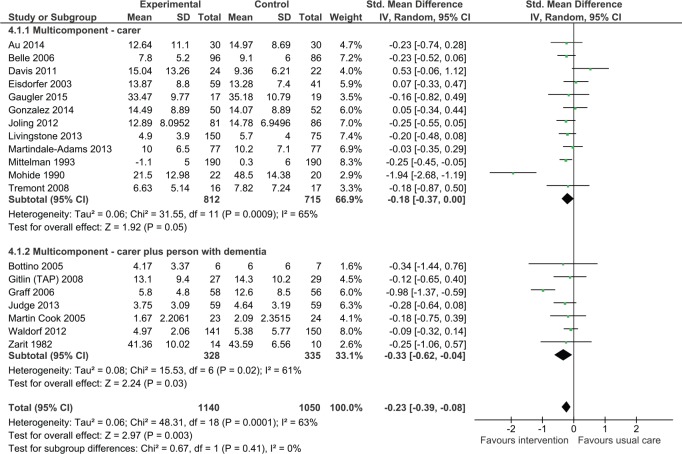
Impact on the carer’s depressive symptoms
Carer only interventions
Multicomponent interventions involving only the carer tended to reduce depressive symptoms although this was of borderline statistical significance (standardized mean difference [SMD] = −0.18, 95% confidence interval [CI] = [−0.37, 0.00], 12 trials, N = 1,527 participants). Two studies could not be included in the meta-analysis; one of these studies reported no effect on depression following treatment (Finkel et al., 2007), whereas the other study reported that carers receiving the intervention had decreased risk of depression (odds ratio = 0.15, confidence interval = [0.04, 0.65], p < .013; Kuo et al., 2013).
Dyadic interventions
Multicomponent interventions involving both the carer and the person with dementia significantly reduced depressive symptoms (Figure 2; SMD = −0.33, 95% CI = [−0.62, −0.04]; seven studies, N = 663). Four studies did not provide data that could be included in the meta-analysis. One of these studies reported no significant effect associated with intervention (Chu, Edwards, Levin, & Thompson, 2000), whereas the other three studies reported a statistically significant improvement in depressive symptoms as a result of the intervention. Buckwalter and colleagues found that those in the intervention group were significantly less depressed than caregivers receiving usual care at 6 months (p = .0007), but outcome at 12 months did not reach statistical significance (Buckwalter et al., 1999). Two other studies reported an improvement in depressive symptoms as a result of the intervention (Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010; Logsdon et al., 2010), with Gitlin and colleagues reporting that 53% of carers in the intervention group reported depressive symptoms versus 68% in the control group (p = .02). There was no statistically significant difference between carer only interventions and dyadic interventions (p = .41).
Figure 2.
Multicomponent intervention versus usual care: Effect on depression postintervention.
Note. CI = confidence interval.
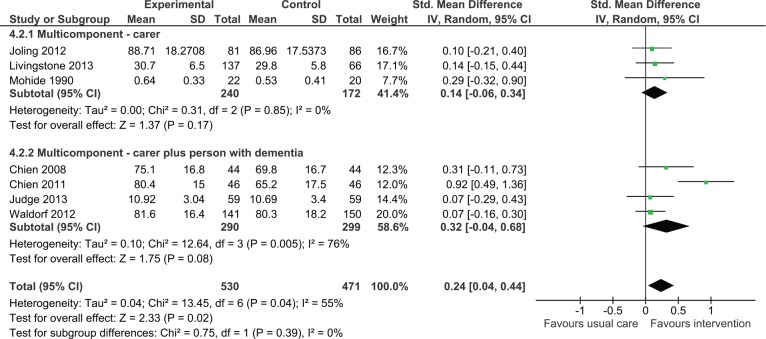
Impact on the carer’s quality of life
Carer only interventions
Although the overall effect on quality of life was not statistically significant, there was a trend in favor of intervention (SMD = 0.14, 95% CI = [−0.06, 0.34], three studies, N = 412). One study that did not report data suitable for contributing to the meta-analysis found no significant improvements in quality of life of carers postintervention (Davis, Tremont, Bishop, & Fortinsky, 2011).
Dyadic interventions
Analysis revealed a trend toward improved quality of life associated with intervention (Figure 3); however, the confidence intervals were wide and the effect was not statistically significant (SMD = 0.32, 95% CI = [−0.04, 0.68], four studies, N = 589). A study conducted by Logsdon and colleagues could not be included in the analysis; this study found no significant differences between treatment and control groups in quality of life (Logsdon et al., 2010).
Figure 3.
Multicomponent interventions versus usual care: Effect on quality of life postintervention.
Note. CI = confidence interval.
When considering all multicomponent interventions (carer only plus dyadic), the analysis demonstrated an overall positive effect (SMD = 0.24, 95% CI = [0.04, 0.44]). There was no statistically significant difference between carer only interventions and dyadic interventions (p = .39).
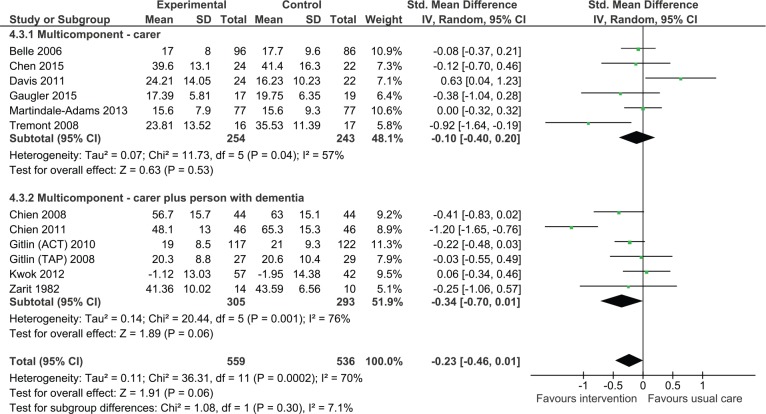
Impact on level of carer burden
Carer only interventions
The analysis found there was no significant effect on carer burden (Figure 4, SMD = −0.10, 95% CI = [−0.40, 0.20], six studies, N = 497). A study conducted by Logsdon and colleagues, which could not be included in the analysis, similarly found no significant reduction in levels of burden (Logsdon et al., 2010).
Figure 4.
Multicomponent interventions versus usual care: Effect on carer burden postintervention.
Note. CI = confidence interval; ACT = Advancing Caregiver Training; TAP = Tailored Activity Program.
Dyadic interventions
Six studies were pooled to examine the efficacy of dyadic training on carer burden. The result was statistically not significant due to wide confidence intervals. However, there was a strong trend suggesting efficacy of intervention (SMD = −0.34, 95% CI = [−0.70, 0.01], six studies, N = 598). One study that could not be included in the analysis found that following intervention there was a significant reduction in carer burden in the intervention group relative to those in the control group. However, at 6 months, there were no differences between groups (Chu et al., 2000). There was no statistically significant difference between carer only interventions and dyadic interventions (p = .30).
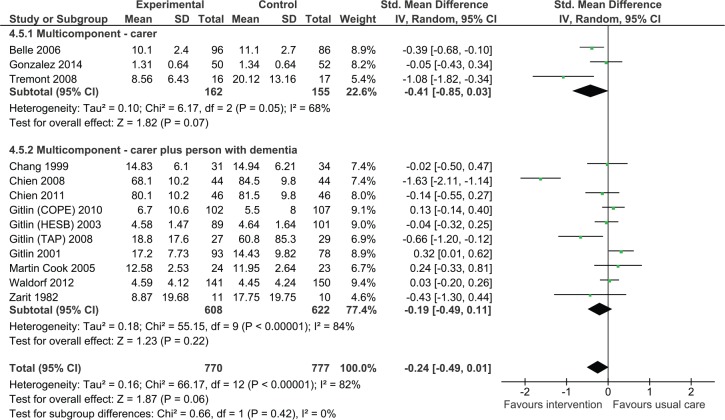
Impact on behavioral and psychological symptoms of dementia
Carer only interventions
We pooled three studies evaluating the effects of intervention on behavioral and psychological symptoms of dementia measured using a tool such as the Neuropsychiatric Inventory or the Revised Memory and Behavior Problem Checklist (Figure 5). Overall, the effect was not significant although the analysis suggested positive effects on intervention (SMD = −0.41, 95% CI = [−0.85, 0.03], three studies, N = 317).
Figure 5.
Multicomponent interventions versus usual care: Effect on behavioral and psychological symptoms of dementia postintervention.
Note. CI = confidence interval; ACT = Advancing Caregiver Training; TAP = Tailored Activity Program; COPE = care of persons with dementia in their environments; HESB = home environmental skill-building program.
Dyadic interventions
We also found that dyadic interventions appeared to be beneficial. However, the results were statistically not significant (SMD = −0.19, 95% CI = [−0.49, 0.11], 10 studies, N = 1,230).
Overall, the meta-analysis suggested a reduction in behavioral and psychological symptoms of dementia (SMD = −0.24, 95% CI = [−0.49, 0.01]). There was no statistically significant difference between carer only interventions and dyadic interventions (p = .42).
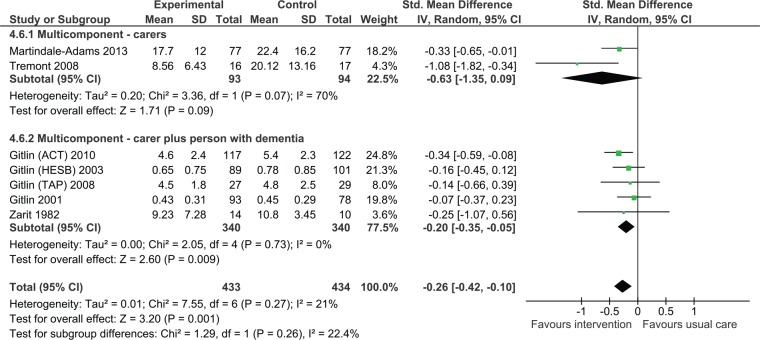
Impact on caregiver upset with behavioral and psychological symptoms of dementia
Fewer studies examined caregiver reaction or upset with symptoms. Overall, we found that multicomponent interventions were effective in reducing caregiver reaction or upset (Figure 6, SMD = −0.26, 95% CI = [−0.42, −0.10], seven studies, 867 participants). The statistical heterogeneity in the analysis was lower than in the other analyses (I2 = 21%).
Figure 6.
Multicomponent interventions versus usual care: Effect on caregiver upset with behavioral and psychological symptoms of dementia postintervention.
Note. CI = confidence interval; ACT = Advancing Caregiver Training; TAP = Tailored Activity Program; COPE = care of persons with dementia in their environments; HESB = home environmental skill-building program.
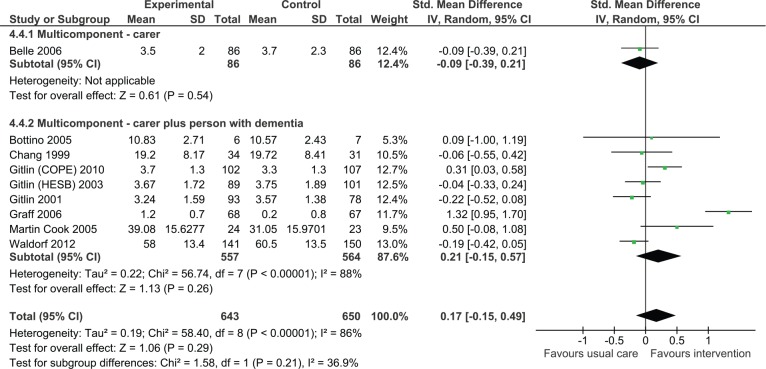
Impact on independence in ADL
Nine studies reported outcomes for ADL in the person with dementia of which eight were dyadic in nature. Overall, the effect was not significant although the direction of effect was positive (Figure 7, SMD = 0.17, 95% CI = [−0.15, 0.57], N = 1,465).
Figure 7.
Multicomponent interventions versus usual care: Effect on activities of daily living postintervention.
Note. COPE = care of persons with dementia in their environments; HESB = home environmental skill-building program.
Effect of interventions based on the severity of symptoms of the person with dementia
There were too few studies to further compare the effect of carer focused and dyadic interventions based on the severity of the symptoms in the person with dementia. However, we did conduct sensitivity analyses to examine whether severity of symptoms was a moderator of effect of caregiver interventions for each outcome. Results are presented in Table 2. It can be seen that studies involving people with milder severity symptoms of dementia appeared to be more effective in reducing levels of depression and burden in carers. Whereas, studies involving people with moderate to severe symptoms of dementia appeared to be more effective in improving the quality of life of the carer.
Table 2.
Effect of Interventions for Carers on Different Outcomes Based on the Severity of Symptoms of the PWD.
| Outcome | Milder severity group | Moderate to severe group |
|---|---|---|
| Carer depression | −0.31 (95% CI = [−0.51, −0.10])*
Eight studies (1,008 participants) |
−0.30 (95% CI = [−0.64, 0.04]) Seven studies (630) participants |
| Carer quality of life | 0.12 (95% CI = [−0.02, 0.25]) Five studies (867 participants) |
0.65 (95% CI = [0.03, 1.26])*
Two studies (134 participants) |
| Carer burden | −0.43, 95% CI = [−0.81, −0.05])*
Three studies (167 participants) |
−0.25 (95% CI = [−0.53, 0.04) Seven studies (858 participants) |
| ADL (person with dementia) | 0.45 (95% CI = [−0.43, 1.33]) Four studies (486 participants) |
0.06 (95% CI = [−0.19, 0.31]) Three studies (571 participants) |
| BPSD global measure | −0.59 (95% CI = [−1.49, 0.30]) Four studies (459 participants) |
−0.18 (95% CI = [−0.42, 0.07]) Five studies (729 participants) |
| BPSD upset | −1.08 (95% CI = [−1.82, −0.34])*
One study (33 participants) |
−0.27 (95% CI = [−0.42, −0.11])*
Four studies (639 participants) |
Note. CI = confidence interval; ADL = activities of daily living.
Significant p<0.05.
Discussion
Family members and friends (informal carers) play an important role in the day-to-day assistance and support of people with dementia. Informal carers typically have poorer outcomes in terms of well-being, depression, quality of life, health status, and use of health care resources (Argimon et al., 2004; Bremer et al., 2015; Spector, Orrell, Charlesworth, & Marston, 2015). This review included 40 studies evaluating multicomponent interventions for carers of people with dementia. Meta-analysis demonstrated that multicomponent interventions can reduce caregiver depressive symptoms, decrease burden, reduce caregiver’s upset with symptoms of dementia and improve the caregiver’s quality of life. Multicomponent interventions can also reduce behavioral and psychological symptoms of dementia, and our analyses suggested beneficial effects in terms of delaying functional decline although some interventions did this more effectively than others.
There were no statistically significant differences between the effects of carer focused interventions and dyadic interventions for any of the outcomes although inspection of the forest plots suggests that dyadic interventions appeared to reduce carer burden to a greater extent. Furthermore, studies evaluating carer focused interventions rarely collected outcome measures relating to the functional independence of the person with dementia, suggesting that this is not one of the aims of treatment. Qualitative research has found that the quality of the relationship between the person with dementia and the carer depends not only on the presence of depression or anxiety in the carer, but also the presence of depression, irritability, behavioral disturbances, and quality of life status of the person with dementia, emphasizing the need for interventions targeting both parties to improve mood and quality of life (Spector et al., 2015). Given the interdependent nature of the health and quality of life of people with dementia and their family members, it is plausible that providing multicomponent interventions that target the dyad, rather than just the caregiver or person with dementia themselves, would be more effective. We did not identify any studies that directly compared carer focused with dyadic interventions. Studies to compare direct differences between carer focused interventions and dyadic interventions are required.
We also examined whether effects varied based on the severity of symptoms of the people with dementia included in the population. Studies were categorized rather arbitrarily based on the average Mini-Mental State Examination score of participants, which provided an overall reflection of the study group. The analyses suggest that there is more potential to reduce depressive symptoms and carer burden when the person has milder severity dementia. Interestingly, there appeared to be more capacity to improve the carer’s quality of life when the person with dementia had moderate to severe symptoms. Quality of life is difficult to change through intervention. It may be that carer quality of life quickly deteriorates when the symptoms of dementia change from mild to moderate/severe and so there is more potential to make a difference at this point. Caution should be applied in interpreting these findings because of the relatively small number of studies involved in the analyses.
This review supports the findings of other reviews of caregiver interventions (Brodaty & Arasaratnam, 2012; Olazarán et al., 2010; Van’t Leven et al., 2013) but adds to the body of literature by examining effect on a number of outcomes, including meta-analysis and comparing different forms of multicomponent interventions. The effect sizes found for all outcomes are considered small (Cohen, 1992). However, living with dementia and providing care for someone with dementia is challenging; hence, even small improvements may be considered clinically significant. This is particularly important in dementia care where there are few effective treatments for the person with dementia.
The total worldwide cost of dementia in 2015 was US$818 billion and this is projected to rise (Prince et al., 2015). This estimate reflects direct medical costs (approx. 20% of global costs), direct social care costs (approx. 40%), and the costs of informal care (valued using an opportunity cost approach and accounting for approximately 40%; Prince et al., 2015). Governments have identified the need to invest money to identify a cure for dementia and to delay onset of dementia. In 2013, the G7 (United States, Japan, Germany, France, United Kingdom, Italy, Canada) launched the “Global Action Against Dementia.” The initiative was designed to increase research funding, promote participation in trials, and enhance collaboration and data sharing. The group declared an ambition to identify a cure, or a disease modifying therapy for dementia by 2025. However, it has been acknowledged that this is an ambitious target and that we cannot and should not delay implementation of treatment and care that has been shown to be effective in improving outcomes for people with dementia (Prince et al., 2015). Although caregiver interventions may not be disease modifying, they have been shown to improve outcomes for both the person with dementia and their carer and there is some evidence of cost-effectiveness (Knapp, Iemmi, & Romeo, 2013).
Nonpharmacological interventions for people with dementia and their carers are heterogeneous in terms of content, dose, and the person delivering the treatment. Studies also involve participants with different severities of dementia and from different cultural backgrounds though the majority of studies were conducted in the United States. Pooling studies in the presence of clinical heterogeneity can be problematic but the reality in clinical practice is that most of these interventions are not replicated “as per protocol.” Clinicians are most likely to provide their own form of multicomponent intervention based on what they have been taught, their organizational culture, and their previous clinical experiences. The benefit of the meta-analyses presented within this review is that they provide an overall indication of the magnitude and types of effect. Indeed, a number of translational studies of caregiver interventions have demonstrated differences between intervention delivery in the context of a highly structured research trial and delivery in “real world” settings (Döpp, Graff, Rikkert, Nijhuis van der Sanden, & Vernooij-Dassen, 2013; Gitlin, Jacobs, & Earland, 2010).
In this review, we did not look at effects on all outcomes (e.g., service use) and we pooled different outcome measures to calculate the SMD, which is more difficult to interpret. We also did not conduct subgroup analyses based on intervention dose, content, and different populations. Subgroup analyses can be insightful but can also be difficult when considering complex interventions as it is hard to determine clinically meaningful cutoff points. For example, how should one compare low and high dose interventions when intervention duration within the studies is consistently spread between 2 and 10 hr?
One of the limitations of this review is that we used an existing systematic review to source studies published until 2008 and then updated searches for more recent studies. Thus, there were two slightly different methods used to source studies. The existing systematic review included all nonpharmacological interventions, whereas we were only interested in caregiver intervention. Our search term was developed based on terms used by the Cochrane Dementia and Cognitive Impairment Group and the BMJ Clinical Evidence terms for study design (British Medical Journal Clinical Evidence, 2016). Furthermore, we relied on the details describing the interventions provided in the publications to categorize studies. This may have led to us categorizing studies as being “education focused” or “support focused” and thus excluded from this review when they actually involved additional content that was poorly described or omitted.
In conclusion, there is a substantial body of evidence that multicomponent interventions can improve a range of important outcomes for both the person with dementia and his or her carer. There was no evidence that the dyadic approach offered an advantage.
Appendix
Search Strategy
Database: Ovid MEDLINE(R)
exp Dementia/
Wernicke Encephalopathy/
Delirium, Dementia, Amnestic, Cognitive Disorders/
dement*.mp.
alzheimer*.mp.
(lewy* adj2 bod*).mp.
(chronic adj2 cerebrovascular).mp.
(“organic brain disease” or “organic brain syndrome”).mp.
(“normal pressure hydrocephalus” and “shunt*”).mp.
“benign senescent forgetfulness”.mp.
(cerebr* adj2 deteriorat*).mp.
(cerebral* adj2 insufficient*).mp.
(pick* adj2 disease).mp.
(creutzfeldt or jcd or cjd).mp.
huntington*.mp.
binswanger*.mp.
korsako*.mp.
or/1-17
(review or review,tutorial or review, academic).pt.
(medline or medlars or embase or pubmed or cochrane).tw,sh.
(scisearch or psychinfo or psycinfo).tw,sh.
(psychlit or psyclit).tw,sh.
cinahl.tw,sh.
((hand adj2 search$) or (manual$ adj2 search$)).tw,sh.
(electronic database$ or bibliographic database$ or computeri?ed database$ or online database$).tw,sh.
(pooling or pooled or mantel haenszel).tw,sh.
(peto or dersimonian or der simonian or fixed effect).tw,sh.
(retraction of publication or retracted publication).pt.
or/20-28
19 and 29
meta-analysis.pt.
meta-analysis.sh.
(meta-analys$ or meta analys$ or metaanalys$).tw,sh.
(systematic$ adj5 review$).tw,sh.
(systematic$ adj5 overview$).tw,sh.
(quantitativ$ adj5 review$).tw,sh.
(quantitativ$ adj5 overview$).tw,sh.
(quantitativ$ adj5 synthesis$).tw,sh.
(methodologic$ adj5 review$).tw,sh.
(methodologic$ adj5 overview$).tw,sh.
(integrative research review$ or research integration).tw.
or/31-41
30 or 42
18 and 43
Caregivers/
(family or caregiver* or carer*).ti,ab.
(assess* or treatment* or therap* or counsel* or intervention* or support or support group* or psychosocial* or nonpharmacologic* or relax* or educat* or psychoeducat* or advice).ti,ab.
46 and 47
45 or 48
44 and 49
limit 50 to (english language and humans and yr = “2005-2014”)
We then conducted a search using a randomized controlled trial filter as follows for studies between January 2008 and 2014.
“randomized controlled trial”.pt.
(random$ or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab.
(retraction of publication or retracted publication).pt.
1 or 2 or 3
(animals not humans).sh.
((comment or editorial or meta-analysis or practice-guideline or review or letter or journal correspondence) not “randomized controlled trial”).pt.
(random sampl$ or random digit$ or random effect$ or random survey or random regression).ti,ab. not “randomized controlled trial”.pt.
4 not (5 or 6 or 7)
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These authors gratefully acknowledge funding provided by the National Health and Medical Research Council (NHMRC) Partnership Centre on Dealing with Cognitive and Related Functional Decline in Older People (Grant No. GNT9100000).
References
- Adelman R., Tmanova L., Delgado D., Dion S., Lachs M. (2014). Caregiver burden: A clinical review. Journal of the American Medical Association, 311, 1052-1059. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease International. (2009). World Alzheimer Report 2009. London, England: Author. [Google Scholar]
- Argimon J., Limon E., Vila J., Cabezas C. (2004). Health-related quality of life in carers of patients with dementia. Family Practice, 21, 454-457. [DOI] [PubMed] [Google Scholar]
- Au A., Wong M. K., Leung L. M., Leung P., Wong A. (2014). Telephone-assisted pleasant-event scheduling to enhance well-being of caregivers of people with dementia: A randomised controlled trial. Hong Kong Medical Journal, 20(3 Suppl. 3), 30-33. [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare. (2012). Dementia in Australia. Canberra: ACT. [Google Scholar]
- Belle S. H., Burgio L., Burns R., Coon D., Czaja S. J., Gallagher-Thompson D., . . . Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II Investigators. (2006). Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: A randomized, controlled trial. Annals of Internal Medicine, 145, 727-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B. S., Johnston D., Rabins P. V., Morrison A., Lyketsos C., Samus Q. M. (2013). Unmet needs of community-residing persons with dementia and their informal caregivers: Findings from the maximizing independence at home study. Journal of the American Geriatrics Society, 61, 2087-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino C. M. C., Carvalho I. A. M., Alvarez A., Avila R., Zukauskas P. R., Bustamante S. E. Z., … Camargo C. H. P. (2005). Cognitive rehabilitation combined with drug treatment in Alzheimer’s disease patients: A pilot study. Clinical Rehabilitation, 19, 861-869. doi: 10.1191/0269215505cr911oa [DOI] [PubMed] [Google Scholar]
- Bremer P., Cabrera E., Leino-Kilpi H., Lethin C., Saks K., Sutcliffe C., … Consortium R. (2015). Informal dementia care: Consequences for caregivers’ health and health care use in 8 European countries. Health Policy, 119, 1459-1471. [DOI] [PubMed] [Google Scholar]
- British Medical Journal Clinical Evidence. (2016). Study design search filters. Retrieved from http://clinicalevidence.bmj.com/x/set/static/ebm/learn/665076.html
- Brodaty H., Arasaratnam C. (2012). Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. The American Journal of Psychiatry, 169, 946-953. [DOI] [PubMed] [Google Scholar]
- Buckwalter K. C., Gerdner L., Kohout F., Hall G. R., Kelly A., Richards B., Sime M. (1999). A nursing intervention to decrease depression in family caregivers of persons with dementia. Archives of Psychiatric Nursing, 13, 80-88. doi: 10.1016/s0883-9417(99)80024-7 [DOI] [PubMed] [Google Scholar]
- Chang B. L. (1999). Cognitive-behavioral intervention for homebound caregivers of persons with dementia. Nursing Research, 48, 173-182. doi: 10.1097/00006199-199905000-00007 [DOI] [PubMed] [Google Scholar]
- Chen H. M., Huang M. F., Yeh Y. C., Huang W. H., Chen C. S. (2015). Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics, 15, 20-25. doi: 10.1111/psyg.12071 [DOI] [PubMed] [Google Scholar]
- Chien W. T., Lee I. Y. (2011). Randomized controlled trial of a dementia care programme for families of home-resided older people with dementia. Journal of Advanced Nursing, 67, 774-787. [DOI] [PubMed] [Google Scholar]
- Chien W. T., Lee Y. M. (2008). A disease management program for families of persons in Hong Kong with dementia. Psychiatric Services, 59, 433-436. doi: 10.1176/appi.ps.59.4.433 [DOI] [PubMed] [Google Scholar]
- Chu P., Edwards J., Levin J., Thompson J. (2000). The use of clinical case management for early stage Alzheimer’s patients and their families. American Journal of Alzheimer’s Disease & Other Dementias, 14, 284-292. [Google Scholar]
- Clare L., Wilson B. A., Carter G., Breen K., Berrios G. E., Hodges J. R. (2002). Depression and anxiety in memory clinic attenders and their carers: Implications for evaluating the effectiveness of cognitive rehabilitation interventions. International Journal of Geriatric Psychiatry, 17, 962-967. doi: 10.1002/gps.735 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1992). A power primer. Psychological Bulletin, 112, 155-159. [DOI] [PubMed] [Google Scholar]
- Davis J. D., Tremont G., Bishop D. S., Fortinsky R. H. (2011). A telephone-delivered psychosocial intervention improves dementia caregiver adjustment following nursing home placement. International Journal of Geriatric Psychiatry, 26, 380-387. [DOI] [PubMed] [Google Scholar]
- Döpp C. M., Graff M. J., Rikkert M., Nijhuis van der Sanden M., Vernooij-Dassen M. (2013). Determinants for the effectiveness of implementing an occupational therapy intervention in routine dementia care. Implementation Science, 8(1), Article 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisdorfer C., Czaja S. J., Loewenstein D. A., Rubert M. P., Arguelles S., Mitrani V. B., Szapocznik J. (2003). The effect of a family therapy and technology-based intervention on caregiver depression. The Gerontologist, 43, 521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloniemi-Sulkava U., Saarenheimo M., Laakkonen M. L., Pietila M., Savikko N., Kautiainen H., … Pitkala K. H. (2009). Family care as collaboration: Effectiveness of a multicomponent support program for elderly couples with dementia. Randomized controlled intervention study. Journal of the American Geriatrics Society, 57, 2200-2208. [DOI] [PubMed] [Google Scholar]
- Elvish R., Lever S.-J., Johnstone J., Cawley R., Keady J. (2013). Psychological interventions for carers of people with dementia: A systematic review of quantitative and qualitative evidence. Counselling & Psychotherapy Research, 13, 106-125. [Google Scholar]
- Finkel S., Czaja S. J., Schulz R., Martinovich Z., Harris C., Pezzuto D. (2007). E-care: A telecommunications technology intervention for family caregivers of dementia patients. American Journal of Geriatric Psychiatry, 15, 443-448. doi: 10.1097/JGP.0b013e3180437d87 [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D., Tzuang Y., Au A., Brodaty H., Charlesworth G., Gupta R., … Shyu Y.-I. (2012). International perspectives on nonpharmacological best practices for dementia family caregivers: A review. Clinical Gerontologist: The Journal of Aging and Mental Health, 35, 316-355. [Google Scholar]
- Garand L., Buckwalter K. C., Lubaroff D., Tripp-Reimer T., Frantz R. A., Ansley T. N. (2002). A pilot study of immune and mood outcomes of a community-based intervention for dementia caregivers: The PLST intervention. Archives of Psychiatric Nursing, 16, 156-167. doi: 10.1053/apnu.2002.34392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler J. E., Reese M., Mittelman M. S. (2013). Effects of the NYU caregiver intervention—Adult child on residential care placement. The Gerontologist, 53, 985-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler J. E., Reese M., Sauld J. (2015). A pilot evaluation of psychosocial support for family caregivers of relatives with dementia in long-term care: The residential care transition module. Research in Gerontological Nursing, 8, 161-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdner L. A., Buckwalter K. C., Reed D. (2002). Impact of a psychoeducational intervention on caregiver response to behavioral problems. Nursing Research, 51, 363-374. doi: 10.1097/00006199-200211000-00004 [DOI] [PubMed] [Google Scholar]
- Gitlin L. N., Corcoran M., Winter L., Boyce A., Hauck W. W. (2001). A randomized, controlled trial of a home environmental intervention: Effect on efficacy and upset in caregivers and on daily function of persons with dementia. The Gerontologist, 41, 4-14. [DOI] [PubMed] [Google Scholar]
- Gitlin L. N., Hauck W. W., Dennis M. P., Winter L. (2005). Maintenance of effects of the home environmental skill-building program for family caregivers and individuals with Alzheimer’s disease and related disorders. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 60, 368-374. [DOI] [PubMed] [Google Scholar]
- Gitlin L. N., Jacobs M., Earland T. V. (2010). Translation of a dementia caregiver intervention for delivery in homecare as a reimbursable Medicare service: Outcomes and lessons learned. The Gerontologist, 50, 847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Burke J., Chernett N., Dennis M. P., Hauck W. W. (2008). Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: A randomized pilot study. American Journal of Geriatric Psychiatry, 16, 229-239. doi: 10.1097/JGP.0b013e318160da72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Corcoran M., Dennis M. P., Schinfeld S., Hauck W. W. (2003). Effects of the home Environmental Skill-Building Program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH initiative. The Gerontologist, 43, 532-546. [DOI] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Dennis M. P., Hodgson N., Hauck W. W. (2010. a). A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: The COPE randomized trial. Journal of the American Medical Association, 304, 983-991. doi: 10.1001/jama.2010.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Dennis M. P., Hodgson N., Hauck W. W. (2010. b). Targeting and managing behavioral symptoms in individuals with dementia: A randomized trial of a nonpharmacological intervention. Journal of the American Geriatrics Society, 58, 1465-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E. W., Polansky M., Lippa C. F., Gitlin L. N., Zauszniewski J. A. (2014). Enhancing resourcefulness to improve outcomes in family caregivers and persons with Alzheimer’s disease: A pilot randomized trial. International Journal of Alzheimer’s Disease, 2014, Article 323478. doi: 10.1155/2014/323478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff M. J. L., Adang E. M. M., Vernooij-Dassen M. J. M., Dekker J., Jonsson L., Thijssen M., … Olderikkert M. G. (2008). Community occupational therapy for older patients with dementia and their care givers: Cost effectiveness study. British Medical Journal, 336, 134-138. doi: 10.1136/bmj.39408.481898.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff M. J. L., Vernooij-Dassen M. J. M., Thijssen M., Dekker J., Hoefnagels W. H. L., Olderikkert M. G. (2006). Community based occupational therapy for patients with dementia and their care givers: Randomised controlled trial. British Medical Journal, 333, 1196-1199. doi: 10.1136/bmj.39001.688843.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff M. J. L., Vernooij-Dassen M. J. M., Thijssen M., Dekker J., Hoefnagels W. H. L., Olderikkert M. G. (2007). Effects of community occupational therapy on quality of life, mood, and health status in dementia patients and their caregivers: A randomized controlled trial. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 62, 1002-1009. [DOI] [PubMed] [Google Scholar]
- Guideline Adaptation Committee, Clinical Practice Guidelines for Dementia in Australia (2016). Clinical practice guidelines and principles of care for people with dementia. Sydney, Australia: NHMRC Cognitive Decline Partnership Centre. [Google Scholar]
- Higgins J., Green S. (2011). Cochrane handbook for systematic reviews of interventions (Version 5.1.0). The Cochrane Collaboration. [Google Scholar]
- Hughes T. B., Black B. S., Albert M., Gitlin L. N., Johnson D. M., Lyketsos C. G., Samus Q. M. (2014). Correlates of objective and subjective measures of caregiver burden among dementia caregivers: Influence of unmet patient and caregiver dementia-related care needs. International Psychogeriatrics, 26, 1875-1883. doi: 10.1017/s1041610214001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joling K. J., van Marwijk H. W., Smit F., van der Horst H. E., Scheltens P., van de Ven P. M., … van Hout H. P. (2012). Does a family meetings intervention prevent depression and anxiety in family caregivers of dementia patients? A randomized trial. PLoS ONE, 7(1), e30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C., Edwards R. T., Hounsome B. (2012). A systematic review of the cost-effectiveness of interventions for supporting informal caregivers of people with dementia residing in the community. International Psychogeriatrics, 24, 6-18. [DOI] [PubMed] [Google Scholar]
- Judge K. S., Yarry S. J., Looman W. J., Bass D. M. (2013). Improved strain and psychosocial outcomes for caregivers of individuals with dementia: Findings from project ANSWERS. The Gerontologist, 53, 280-292. [DOI] [PubMed] [Google Scholar]
- Kales H., Gitlin L. N., Lyketsos C. (2015). Assessment and management of behavioural and psychological symptoms of dementia. British Medical Journal, 350, Article h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M., Iemmi V., Romeo R. (2013). Dementia care costs and outcomes: A systematic review. International Journal of Geriatric Psychiatry, 28, 551-561. [DOI] [PubMed] [Google Scholar]
- Kuo L. M., Huang H. L., Huang H. L., Liang J., Chiu Y. C., Chen S. T., … Shyu Y. I. (2013). A home-based training program improves Taiwanese family caregivers’ quality of life and decreases their risk for depression: A randomized controlled trial. International Journal of Geriatric Psychiatry, 28, 504-513. [DOI] [PubMed] [Google Scholar]
- Kwok T., Lam L., Chung J. (2012). Case management to improve quality of life of older people with early dementia and to reduce caregiver burden. Hong Kong Medical Journal, 18(Suppl. 6), 4-6. [PubMed] [Google Scholar]
- Lawton M. P., Brody E. M., Saperstein A. R. (1989). A controlled-study of respite service for caregivers of Alzheimer’s patients. The Gerontologist, 29, 8-16. [DOI] [PubMed] [Google Scholar]
- Livingston G., Barber J., Rapaport P., Knapp M., Griffin M., King D., … Cooper C. (2013). Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: Pragmatic randomised controlled trial. British Medical Journal, 347, Article f6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon R. G., Pike K. C., McCurry S. M., Hunter P., Maher J., Snyder L., Teri L. (2010). Early-stage memory loss support groups: Outcomes from a randomized controlled clinical trial. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 65, 691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cook K., Davis B. A., Hynan L. S., Weiner M. F. (2005). A randomized, controlled study of an Alzheimer’s caregiver skills training program. American Journal of Alzheimer’s Disease & Other Dementias, 20, 204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale-Adams J., Nichols L. O., Burns R., Graney M. J., Zuber J. (2013). A trial of dementia caregiver telephone support. Canadian Journal of Nursing Research, 45(4), 30-48. [DOI] [PubMed] [Google Scholar]
- Maslow K. (2012). Translating innovation to impact: Evidence based interventions to support people with Alzheimer’s disease and their caregivers at home and in the community. Alliance for Aging Research Administration on Aging MetLife Foundation; Retrieved from https://www.agingresearch.org/publications/view/18#.V4OF07h942w [Google Scholar]
- Mittelman M. S., Roth D. L., Coon D. W., Haley W. E. (2004). Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer’s disease. American Journal of Psychiatry, 161, 850-856. doi: 10.1176/appi.ajp.161.5.850 [DOI] [PubMed] [Google Scholar]
- Mittelman M. S., Roth D. L., Haley W. E., Zarit S. H. (2004). Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer’s disease: Results of a randomized trial. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 59(1), P27-P34. [DOI] [PubMed] [Google Scholar]
- Mohide E. A., Pringle D. M., Streiner D. L., Gilbert J. R., Muir G., Tew M. (1990). A randomized trial of family caregiver support in the home management of dementia. Journal of the American Geriatrics Society, 38, 446-454. [DOI] [PubMed] [Google Scholar]
- National Alliance for Caregiving and AARP. (2009). Caregiving in the U.S. 2009. A Retrieved from http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf
- Nobili A., Riva E., Tettamanti M., Lucca U., Liscio M., Petrucci B., Porro G. S. (2004). The effect of a structured intervention on caregivers of patients with dementia and problem behaviors: A randomized controlled pilot study. Alzheimer Disease & Associated Disorders, 18, 75-82. doi: 10.1097/01.wad.0000126618.98867.fc [DOI] [PubMed] [Google Scholar]
- Olazarán J., Reisberg B., Clare L., Cruz I., Peña-Casanova J., Del Ser T., … Muñiz R. (2010). Nonpharmacological therapies in Alzheimer’s disease: A systematic review of efficacy. Dementia and Geriatric Cognitive Disorders, 30, 161-178. [DOI] [PubMed] [Google Scholar]
- Prince M., Wimo A., Guerchet M., Ali G., Wu Y., Prina M. Alzheimer’s Disease International. (2015). World Alzheimer report 2015: The global impact of dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International. Retrieved from https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf
- Quayhagen M. P., Quayhagen M., Corbeil R. R., Hendrix R. C., Jackson J. E., Snyder L., Bower D. (2000). Coping with dementia: Evaluation of four nonpharmacologic interventions. International Psychogeriatrics, 12, 249-265. [DOI] [PubMed] [Google Scholar]
- Schoenmakers B., Buntinx F., DeLepeleire J. (2010). Supporting the dementia family caregiver: The effect of home care intervention on general well-being. Aging & Mental Health, 14, 44-56. [DOI] [PubMed] [Google Scholar]
- Spector A., Orrell M., Charlesworth G., Marston L. (2015). Factors influencing the person-carer relationship in people with anxiety and dementia. Aging & Mental Health. Advance online publication. doi: 10.1080/13607863.2015.1063104 [DOI] [PubMed] [Google Scholar]
- Stocking C. B., Hougham G. W., Danner D. D., Patterson M. B., Whitehouse P. J., Sachs G. A. (2007). Empirical assessment of a research advance directive for persons with dementia and their proxies. Journal of the American Geriatrics Society, 55, 1609-1612. [DOI] [PubMed] [Google Scholar]
- Stolley J. M., Reed D., Buckwalter K. C. (2002). Caregiving appraisal and interventions based on the progressively lowered stress threshold model. American Journal of Alzheimer’s Disease & Other Dementias, 17, 110-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. (2013). The experience of caring. In Chang E., Johnson A. (Eds.), Living with dementia (pp. 182-197). Camberwell, Australia: Australian Council for Educational Research. [Google Scholar]
- Tremont G., Davis J. D., Bishop D. S., Fortinsky R. H. (2008). Telephone-delivered psychosocial intervention reduces burden in dementia caregivers. Dementia: The International Journal of Social Research and Practice, 7, 503-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Leven N., Prick A.-E. J., Groenewoud J. G., Roelofs P. D., de Lange J., Pot A. M. (2013). Dyadic interventions for community-dwelling people with dementia and their family caregivers: A systematic review. International Psychogeriatrics, 25, 1581-1603. [DOI] [PubMed] [Google Scholar]
- Waldorff F., Buss D., Eckermann A., Rasmussen M., Keiding N., Rishoj S., … Waldemar G. (2012). Efficacy of psychosocial intervention in patients with mild Alzheimer’s disease: The multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). British Medical Journal, 345(7870), e4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A., Winblad B., Jonsson L. (2007). An estimate of the total worldwide societal costs of dementia in 2005. Alzheimer’s Dement, 3, 81-91. doi: 10.1016/j.jalz.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Woolf S., Schunemann H. J., Eccles M. P., Grimshaw J. M., Shekelle P. (2012). Developing clinical practice guidelines: Types of evidence and outcomes; Values and economics, synthesis, grading, and presentation and deriving recommendations. Implementation Science, 7, Article 61. doi: 10.1186/1748-5908-7-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2012). WHO handbook for guideline development. Geneva, Switzerland: Author. [Google Scholar]
- Zarit S. H., Zarit J. M., Reever K. E. (1982). Memory training for severe memory loss: Effects on senile dementia patients and their families. The Gerontologist, 22, 373-377. [DOI] [PubMed] [Google Scholar]