Abstract
Objective
Nonalcoholic steatohepatitis (NASH) is an unmet need associated with metabolic syndrome. There are no approved therapies for NASH; however, glucagon-like peptide-1 receptor (GLP-1R) and farnesoid-X receptor (FXR) agonists are promising drug targets. We investigated the therapeutic effects of co-administration of a GLP-1R agonist, IP118, with FXR agonist obeticholic acid (OCA) in mice.
Methods
OCA and IP118 alone and in combination were sub-chronically administered to Lepob/Lepob mice with diet-induced NASH or diet-induced obese (DIO) mice. Metabolic (body weight and glucose) and liver (biochemical and histological) endpoints were assessed. NASH severity in Lepob/Lepob mice was graded using a customized integrated scoring system.
Results
OCA reduced liver weight and lipid in NASH mice (both by −17%) but had no effect on plasma ALT or AST levels. In contrast, IP118 significantly reduced liver weight (−21%), liver lipid (−15%), ALT (−29%), and AST (−27%). The combination of OCA + IP118 further reduced liver weight (−29%), liver lipid (−22%), ALT (−39%), and AST (−36%). Combination therapy was superior to monotherapies in reducing hepatic steatosis, inflammation, and fibrosis. Hepatic improvements with IP118 and OCA + IP118 were associated with reduced body weight (−4.3% and −3.5% respectively) and improved glycemic control in OCA + IP118-treated mice. In DIO mice, OCA + IP118 co-administration reduced body weight (−25.3%) to a greater degree than IP118 alone (−12.5%) and further improved glucose tolerance and reduced hepatic lipid.
Conclusion
Our data suggest a complementary or synergistic therapeutic effect of GLP-1R and FXR agonism in mouse models of metabolic disease and NASH.
Keywords: GLP-1, OCA, Combination therapy, NASH, Steatosis, Fibrosis
Abbreviations: NASH, nonalcoholic steatohepatitis; NAFLD, nonalcoholic fatty liver disease; GLP-1R, glucagon-like peptide-1 receptor; FXR, farnesoid-X receptor; OCA, obeticholic acid; ALT, alanine transaminase; AST, aspartate transaminase; BA, bile acid; LFD, low-fat diet; AMLN, Amylin liver NASH diet
Highlights
-
•
Agonists for FXR (e.g., OCA) and GLP-1R are leading clinical candidates for NASH.
-
•
First preclinical evidence for OCA alone or added to GLP-1R agonist IP118 on NASH and metabolism.
-
•
OCA + IP118 exerted greater reductions in key morphologic features of NASH.
-
•
OCA + IP118 synergistically reduced body weight in DIO mice.
-
•
FXR and GLP-1 co-activation may be therapeutically beneficial.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of conditions ranging from simple steatosis to NASH, fibrosis, cirrhosis, and hepatocellular carcinoma, along with substantial liver and whole body metabolic derangements [1], [2]. Currently in the United States, an estimated 30% of the adult population has NAFLD and 3%–5% has NASH [3], which, in turn, is predicted to become the main cause of liver transplantation by 2020 [4]. A growing body of evidence portrays NASH as the hepatic manifestation of the metabolic syndrome since the majority of NASH/NAFLD patients suffer from obesity, diabetes, and insulin resistance [5], [6]. While dysregulated hepatic lipid and insulin-mediated metabolic pathways appear to be major pathophysiological contributors to NASH, the precise mechanisms underlying the development of NAFLD and its progression to NASH have not been fully elucidated, which has affected the development of effective therapeutics [5], [6].
The current standard of care for NAFLD/NASH is limited to ameliorating components of the metabolic syndrome, primarily through weight loss and/or improving insulin sensitivity with lifestyle interventions or surgery [7], [8]. Unfortunately, the long-term effectiveness of these interventions is questionable as most patients are unable to initiate or maintain dietary and lifestyle changes, and surgeries may be unfeasable due to financial or health limitations. Therefore, developing new pharmacological therapies is vital to combating the complex nature of NASH. Considering that insulin resistance is a key factor in the pathogenesis of NASH, several studies have shown promising data using insulin sensitizers. In one major clinical trial (PIVENS Trial), enhancing insulin sensitivity using pioglitazone or vitamin E resulted in significant improvements in NASH pathology including resolution of steatosis, hepatocyte ballooning, and inflammation. Interestingly, these treatments led to a marginal improvement in fibrosis score sufficient to impede, but not reverse, further development in fibrosis [9]. Thus, correcting insulin resistance or obesity is necessary but not sufficient for effectively treating NASH in the majority of patients [10], suggesting the need for broader hepatoprotective actions that also target fibrogenic pathways.
The strong association between obesity, type 2 diabetes, and NASH provides a logical justification for therapies such as the gut-derived incretin hormone GLP-1, that induces weight loss and enhances insulin sensitivity. GLP-1 receptor (GLP-1R) agonists currently approved for the treatment of type 2 diabetes and obesity enhance glycemic control, delay gastric emptying, decrease postprandial glucagon secretion, and reduce food intake/body weight [11]. Studies in rodents have shown that GLP-1R agonism exerts beneficial effects on body weight, liver mass, liver lipid, plasma ALT, and plasma triglycerides [12], [13], [14]. In clinical studies, exenatide therapy lowered plasma ALT levels in patients with type 2 diabetes [15]. Furthermore, intriguing results from the LEAN clinical study with GLP-1R agonist liraglutide demonstrated statistically significant decreases in plasma liver enzymes, histological resolution of steatohepatitis, slowing of fibrosis progression along with metabolic, and diabetic improvements following 44–48 weeks of treatment in patients with biopsy-proven NASH [16]. Together, these data strongly suggest a potential beneficial effect of GLP-1R agonism on NASH.
Another promising treatment approach involves FXR, classically described as the master regulator of bile acid (BA) metabolism [17]. Expression of FXR is mainly confined to liver, intestine, kidney, and, to a lesser extent, adipose tissue, thus allowing FXR to control key metabolic programs [17], [18]. Lipophilic BAs serve as FXR natural ligands and, upon binding to FXR, activate a variety of target genes regulating not only BA synthesis but also lipid and glucose homeostasis as well as genes affecting the regulation of immune responses [17], [18], [19], [20], [21]. Pre-clinical studies in mouse models showed enhanced insulin sensitization and reduced liver steatosis, inflammation, and fibrosis following FXR activation by synthetic agonists [22], [23], [24]. FXR controls glucose metabolism through the regulation of gluconeogenesis and glycogenolysis in the liver and peripheral insulin sensitivity in skeletal muscle and adipose tissue. Promising data were recently published from the FLINT clinical trial where subjects with biopsy-confirmed NASH were treated for 72 weeks with the bile acid obeticholic acid (OCA, or 6-ethylchenodeoxycholic acid), a potent and highly selective FXR agonist synthesized from the natural bile acid chenodeoxycholic acid [9], [20]. OCA demonstrated superiority to placebo in improving several pathological aspects of NASH including steatosis, fibrosis, and inflammation, along with reducing liver enzymes [9]. However, OCA-treated patients showed an unexpected elevation in serum cholesterol and worsening of pruritus [9]. While GLP-1 and FXR agonists are being pursued in clinical trials, no single agent is yet approved for the treatment of NASH.
Therefore, we investigated whether combining long-acting GLP-1R agonist IP118 (a GLP-1R-IgG-Fc-fusion peptide, see Supplementary Materials) with the FXR agonist OCA is more effective than using either compounds separately in slowing or reversing NASH-associated defects including hepatic steatosis and fibrosis.
In Lepob/Lepob mice with diet-induced NASH [14], OCA monotherapy reduced plasma and hepatic lipids, whereas treatment with IP118 alone reduced body weight and improved NASH-associated biochemical indices, hepatic steatosis, and overall NASH score. Interestingly, superior effects were observed with combined administration of IP118 + OCA as this treatment led to improved glycemic control, reduced body weight, liver size, and lipid and liver enzyme levels. Pathologically, combination therapy not only reduced hepatic steatosis and inflammation but also resulted in a marked reduction in fibrosis and overall NASH score.
In diet-induced obese mice (DIO), OCA alone had no effect on body weight or body composition, whereas IP118 elicited the expected reduction in body weight. Intriguingly, co-administration of OCA + IP118 dramatically reduced body weight beyond IP118 alone. Statistically significant reductions in liver lipid were also observed with IP118 + OCA combination treatment but not either treatment alone. Overall, our data represent a significant first step in combining two promising therapeutic agents to successfully reverse NASH-associated steatosis and fibrosis in a mouse model.
2. Material and methods
2.1. Experimental animals and diets
Animal studies were conducted at MedImmune (Gaithersburg, USA) according to protocols reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at MedImmune and in compliance with the applicable national laws and regulations concerning use of laboratory animals and the AstraZeneca Animal Welfare and Bioethics policy. Male C57BL6 (B6) or Lepob/Lepob mice obtained from Jackson Labs (Bar Harbor, ME) and diet-induced obese (DIO) C57BL6J mice obtained from Charles River Laboratories (Frederick, MD) were housed in standard caging at 22 °C in a 12-h light: 12-h dark cycle at standard temperature and humidity conditions with ad libitum access to water and food (except where noted otherwise, e.g. during fasting prior to glucose tolerance tests). Test diets were sourced from Research Diets (New Brunswick, NJ). Lepob/Lepob mice (aged ∼8 weeks) were fed the AMLN diet which is comprised of high fat (40% kcal), high fructose (22% by wt), and high cholesterol (2% by wt), in which the source of fat was trans-fat (Primex partially hydrogenated vegetable oil shortening, Research Diets, D09100301) or a purified low-fat control diet (LFD; 10% kcal/fat) with no fructose or cholesterol (Research Diets, D09100304). Age-matched B6 mice were included as reference mice for terminal biochemical measures and pathology assessment. DIO mice were obtained aged ∼20 weeks, after 16 weeks on purified high fat diet (D12492), which they were maintained on throughout the study, or on high fat diet containing OCA (described below).
2.2. Pharmacology studies
To characterize the development of NASH, we maintained Lepob/Lepob or B6 mice on either a LFD or AMLN diet for 8 weeks and body weight was measured every 2 weeks. At the end of this feeding period body weights and blood samples were collected from non-fasted animals, and main metabolic parameters were assessed (blood glucose, plasma ALT, insulin). Mice were then randomized to appropriate drug treatment groups based on the above parameters (n = 8–10 mice/group). GLP-1R agonist IP118 was dosed at 1 mg/kg subcutaneously, twice-weekly, while vehicle controls received sterile PBS. OCA (Selleckchem, Houston, TX) was dosed via dietary admixture at a concentration of 0.05% (∼30 mg/kg/d). Mice were treated for 4 weeks and maintained on the test diets throughout the duration of the study.
DIO mice were acclimated and randomized to study groups (n = 9/group) by body weight and baseline 6 h fasted blood glucose values (Ascensia Breeze 2, Bayer Healthcare LLC, Mishawaka, IN). Mice received either standard high fat diet (D12492) or the same diet supplemented with 0.05% OCA (OCA sourced from Selleckchem, Houston, TX; diet manufactured by Research Diets, D17051405). Vehicle (PBS) or IP118 was administered subcutaneously twice per week (0.3 mg/kg in 5 mL/kg). Body weight was measured twice weekly, and body composition was assessed via NMR (Bruker MiniSpec LF90II, Bruker BioSpin Corporation, Billerica, MA) at baseline and just prior to necropsy. On day 17, a glucose tolerance test was performed (∼16 h following dose of IP118). Glucose (1.5 g/kg) was administered after a 6 h fast, and blood glucose measured using a handheld glucometer at 0, 15, 30, 60, 120, and 180 min. On day 21, mice were fasted for 6 h then euthanized via CO2 inhalation, and cardiac blood was collected for terminal plasma analyses.
2.3. Measurement of glucose, ALT, AST, plasma and liver lipids
Terminal blood was collected into tubes containing EDTA anticoagulant, centrifuged at 2000 × g for 10 min at ambient temperature, and plasma was immediately frozen prior to measurement of alanine aminotransferase (ALT) and aspartate transaminase (AST), total cholesterol, and triglycerides levels using a biochemistry analyzer (Cobas c-111, Roche Diagnostics USA, Indianapolis, IN). Insulin was measured using a commercial ELISA (MesoScale Diagnostics, Rockville, MD). Total lipids were assessed in frozen liver samples using a Bruker LF-90 minispec system (Bruker BioSpin Corporation, Billerica, MA).
2.4. Histological assessment and quantitative analysis of liver tissue
Tissues were collected in 10% neutral buffered formalin. After 24–48 h, the liver was trimmed to approximately 4 mm and placed into tissue cassettes, processed, and then embedded in molten paraffin (60 °C). Liver paraffin blocks were sectioned at 4 μm, floated on a water bath at 44 °C for 120 s, collected on a positively charged slide, dried in an oven at 75 °C for 45 min, and stained with hematoxylin (50% Gills I and 50% Gills II) and eosin according to standard protocols. Immunohistochemistry (IHC) was performed using Ventana Discovery ULTRA Staining Module (Ventana Medical Systems, Tucson, AZ). For CD68, sections were incubated with rabbit polyclonal antibody (ab125212 Abcam, Inc., Cambridge, MA) for 20 min at 37 °C after antigen unmasking CC1 mild conditioning for 36 min at 95 C. For collagen detection, liver sections were incubated with goat anti-collagen Type I polyclonal antibody (#1310-01, SouthernBiotech, Birmingham, AL) for 20 min at 37 °C after antigen unmasking CC1 standard conditioning for 64 min at 98 °C. All slides were then subjected to the same secondary antibody protocol: discover HRP detection with CM inhibitor for 8 min was then applied, and secondary antibody (Discovery OmniMap anti-rabbit HRP, Ventana Medical Systems) was applied for 16 min at 37 °C. Slides were incubated with ChromoMap DAB for 8 min, counterstained with hematoxylin II for 12 min and bluing reagent for 8 min. All histological assessments were conducted by a pathologist blinded to the treatment condition, and data quantification was conducted utilizing customized algorithms (Definiens, Munich, Germany).
Assessment of steatosis (macrovesicular and microvesicular steatosis and location), hepatocyte ballooning, lobular inflammation, biliary hyperplasia, fibrosis (presence and pattern as demonstrated by IHC), and CD68 immunoreactivity was performed by the pathologist and graded on a 0–2 scale for each parameter. The NASH score was subsequently represented as the sum of the scores for each of these features to reflect the broad pathology present in whole mouse liver. The mean NASH score per group was then calculated. This approach was based on an adapted scoring method by Kleiner/Brunt NAFLD activity score [25].
2.5. Statistical analyses
Single point data was analyzed using one-way ANOVA with Tukey's post hoc test or a Kruskal–Wallis non-parametric ANOVA with Dunn's multiple comparison test when standard deviation between group datasets was evident. Data comparing the effects of diet or drug treatment over time were analyzed using a two-way (e.g., drug*time) repeated measures ANOVA with Tukey's post hoc test to determine statistical differences. Data were graphed and analyzed using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA), with p < 0.05 deemed to be statistically significant. All data are presented as the mean ± SEM.
3. Results
3.1. Combined administration of the FXR agonist OCA and GLP-1 receptor agonist IP118 prevents weight gain and improves glycemic control in mice fed AMLN diet
We initially tested three different concentrations of OCA mixed with the NASH-inducing AMLN diet to identify optimal OCA levels. In this pilot study, OCA at concentrations of 0.01–0.05% did not affect body weight gain or cumulative food intake in Lepob/Lepob mice compared to unaltered AMLN diet (Supplementary Figure S1, A and B). The estimated daily dose ranged from ∼10 to 50 mg/kg/d, and concentrations of 0.025% and 0.05% were associated with reduced hepatic lipid and plasma cholesterol after two weeks (Supplementary Figure S1, C–E). Expression of FXR target genes in the liver and intestine was also reduced with the 0.05% admixture (Supplementary Table S2); thus, we performed future studies utilizing the AMLN diet containing 0.05% OCA.
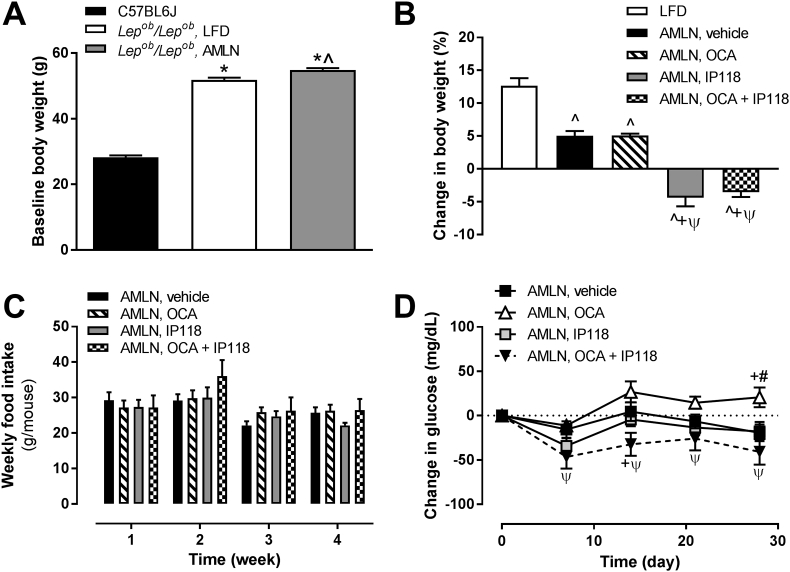
To induce NASH in our animal model, obese and diabetic leptin-deficient (Lepob/Lepob) mice were maintained on the AMLN diet (high in trans-fat, fructose and cholesterol) for a two month pre-treatment period, while control mice were fed a low-fat diet (LFD). At the end of this induction phase a significant increase in body weight was observed in all Lepob/Lepob mice fed either test diets compared to LFD-fed control B6 mice (Figure 1A). Mice remained on AMLN diet and were administered vehicle, the FXR agonist OCA, the GLP-1R agonist IP118, or OCA + IP118 for 4 weeks. During this treatment phase, OCA did not affect body weight, whereas IP118 alone significantly reduced body weight, and the addition of OCA to IP118 did not enhance the effect (Figure 1B). The inability to reduce body weight in OCA-treated groups was not due to failure to induce FXR transcriptional machinery since we observed significantly altered expression of FXR-target genes (e.g. Nr0b2, Cyp7a1, Cyp8b1) in the liver of OCA-treated mice (Supplementary Table S3). Mice from all experimental groups showed similar food intake during the treatment phase (Figure 1C). Mice treated with IP118 alone showed a reduction in non-fasting glucose, which was further reduced in mice receiving dual treatment with OCA + IP118 (Figure 1D). This observed glucose-lowering capability was absent in mice receiving OCA monotherapy. Overall, our data demonstrated that the combined treatment with the FXR agonist OCA and GLP-1R agonist IP118 in Lepob/Lepob mice improved glucose homeostasis and reduced body weight.
Figure 1.
Effect of AMLN diet and activation of GLP-1 receptor, FXR, or both on body weight, food intake, and plasma glucose of obese Lepob/Lepobmice. Changes in body weight during the dietary lead-in period (A) and testing period (B) are demonstrated for lean C57BL6 controls and Lepob/Lepob mice. (C) Weekly food intake during the testing period presented, per treatment group, as amount of food consumed in grams per mouse (g/mouse). (D) Change in plasma glucose during testing period. *p < 0.05 vs. C57BL6 controls; ˆp < 0.05 vs. Lepob/Lepob LFD controls; +p < 0.05 vs. Lepob/Lepob AMLN vehicle-treated; ψp < 0.05 vs. Lepob/Lepob AMLN, OCA; #p < 0.05 vs. Lepob/Lepob AMLN, IP118.
3.2. Improved plasma and biochemical indices of NASH by dual treatment with FXR agonist OCA and GLP-1 receptor agonist IP118
We next assessed the effect of diet and treatments on markers known to be associated with NASH development in humans such as liver mass, liver and plasma lipids, and alanine transaminase (ALT) and aspartate aminotransferase (AST). As demonstrated in Table 1, all parameters assessed (except plasma cholesterol) were significantly increased in Lepob/Lepob mice compared to B6 control mice. Feeding Lepob/Lepob mice AMLN diet also resulted in significant increases in liver mass and plasma triglyceride (22% and 48% respectively, p < 0.05; Table 1) compared to Lepob/Lepob mice on LFD. AMLN-diet fed Lepob/Lepob mice showed a trend towards increased plasma ALT plasma (13%, Table 1) while AST levels showed a significant decrease (−31%, Table 1) compared to Lepob/Lepob mice fed LFD. The levels of these enzymes in all Lepob/Lepob mice were already increased by 9–15-fold over those of B6 mice (Table 1).
Table 1.
Effect of AMLN diet and experimental treatments on liver endpoints and circulating lipids. Liver mass data are presented as percent of body weight; hepatic lipid is shown as percentage of total lipid relative to mass of the sample analyzed. Plasma parameters were determined in non-fasting plasma from terminal samples.
| Strain |
C57BL6J |
Lepob/Lepob |
||||
|---|---|---|---|---|---|---|
| Diet/drug | LFD | LFD | AMLN/Vehicle | AMLN/OCA | AMLN/IP118 | AMLN/OCA + IP118 |
| Liver mass (%) | 4.7 ± 0.2 | 10.2 ± 1.6§ | 12.4 ± 1.3§* | 10.3 ± 0.9§ ˆ | 9.8 ± 0.9§ ˆ | 8.8 ± 0.9§ ˆ # |
| Hepatic lipid (%) | 14.0 ± 4.2 | 32.3 ± 1.9§ | 34.8 ± 2.1§ | 28.7 ± 2.2§* ˆ | 29.6 ± 1.9§ ˆ | 27.2 ± 3.1§* ˆ |
| Plasma triglycerides (mg/dL) | 196 ± 11 | 325 ± 29§ | 479 ± 36§* | 343 ± 52§ ˆ | 436 ± 30§*# | 307 ± 49§ ˆ + |
| Plasma cholesterol (mg/dL) | 96 ± 21 | 93 ± 15 | 101 ± 11 | 81 ± 16 | 105 ± 30# | 69 ± 11ˆ + |
| Plasma ALT (U/L) | 56 ± 20 | 841 ± 212§ | 947 ± 134§ | 907 ± 311§ | 674 ± 195§ ˆ | 577 ± 141§ ˆ # |
| Plasma AST (U/L) | 100 ± 22 | 902 ± 336§ | 622 ± 126§* | 553 ± 165§* | 457 ± 142§* ˆ | 395 ± 90§* ˆ |
§p < 0.05 vs. C57BL6J, *p < 0.05 vs. Lepob/Lepob LFD, ˆp < 0.05 vs. Lepob/Lepob AMLN diet, #p < 0.05 vs. Lepob/Lepob AMLN diet, OCA, +p < 0.05 vs. Lepob/Lepob AMLN diet, IP118. LFD, low-fat diet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AMLN, high trans-fat NASH diet.
Compared to AMLN-fed Lepob/Lepob vehicle-treated control mice, monotherapy with OCA or IP118 significantly reduced liver mass and intrahepatic lipid (−17% and −21% in liver mass, −18% and −15% in intrahepatic lipid, respectively, both p < 0.05; Table 1). However, combined administration of OCA + IP118 was associated with a further decrease in liver weight and lipid content (−29% and −22%, respectively, both p < 0.05; Table 1). In concert with these findings, OCA + IP118 led to the most significant reductions in plasma lipids (−36% and −32% reduction in plasma triglycerides and total cholesterol, respectively, p < 0.05; Table 1) compared to vehicle controls. Treatment with OCA alone exerted its effects primarily on plasma triglyceride levels resulting in a 28% reduction compared to vehicle (p < 0.05; Table 1). In contrast, IP118 alone had little effect on reducing plasma lipids (Table 1). Finally, while plasma levels of liver enzymes ALT and AST were unchanged following treatment with OCA alone, treatment with IP118 alone significantly reduced plasma ALT and AST (−29% and −27% respectively, p < 0.05; Table 1) compared to vehicle group, and the addition of OCA further reduced ALT and AST levels (−39% and −36% respectively, p < 0.05; Table 1).
3.3. Combination therapy with OCA and IP118 reduced steatosis induced by AMLN diet
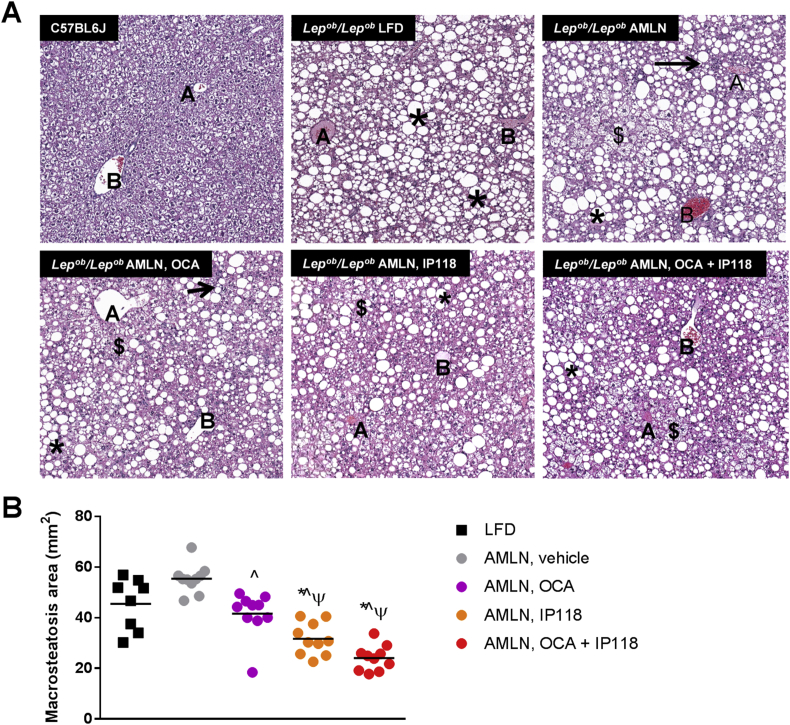
Lack of functional leptin leads to extensive morphologic micro- and macro-vesicular steatosis, and this was evident in all experimental groups of Lepob/Lepob mice compared to B6 controls (Figure 2A). Exposure to AMLN diet for 8 weeks increased hepatic steatosis above that seen in Lepob/Lepob mice maintained on LFD (+22%, p < 0.05, Figure 2). However, this AMLN diet-driven increase in steatosis was lost in mice treated with OCA as they demonstrated a 9% reduction in steatosis compared to vehicle controls (p < 0.05; Figure 2). More dramatic reductions in steatosis were observed in mice treated with IP118 alone and with IP118 + OCA, which exerted the highest reduction in liver lipids (−30% for IP118 and −47% for IP118 + OCA, respectively, p < 0.05, Figure 2).
Figure 2.
Marked reduction in hepatic steatosis following combined treatment with OCA and IP118. (A) Representative hematoxylin and eosin (H&E)-stained liver sections showing lipid accumulation in lean C57BL6 control and Lepob/Lepob mice maintained on control LFD or AMLN diet and treated with vehicle, OCA, IP118, or OCA + IP118. The following symbols within images indicate: central vein (letter A), portal field (letter B), macrosteatosis (*), microsteatosis ($), inflammatory foci (→). Original magnification at 60×. (B) Quantitative analysis of hepatic lipid contents by measuring area covered by macrosteatosis in H&E-stained liver sections showing significant reduction in steatosis in mice receiving OCA + IP118 compared to other groups. *p < 0.05 vs. C57BL6 controls; ˆp < 0.05 vs. Lepob/Lepob LFD controls; +p < 0.05 vs. Lepob/Lepob AMLN vehicle-treated; ψp < 0.05 vs. Lepob/Lepob AMLN, OCA.
3.4. Dual therapy with OCA and IP118 reverses inflammation induced by AMLN diet
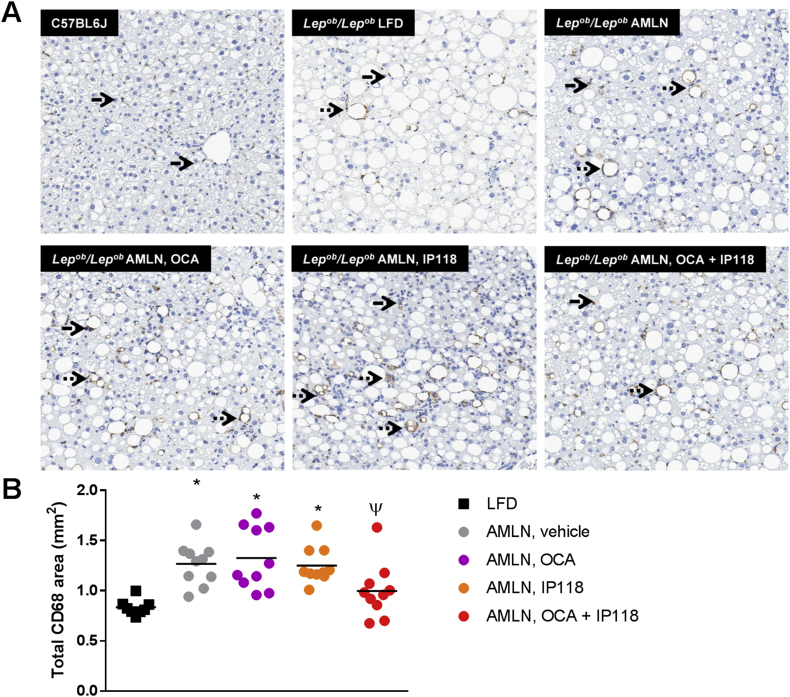
To identify macrophage infiltration (inflammation) in NASH mouse livers we performed immunostaining for the monocyte/macrophage marker CD68. Compared to Lepob/Lepob mice on LFD, AMLN diet resulted in a significant increase in CD68 immunoreactivity (+52%, p < 0.05, Figure 3). Monotherapy with either OCA or IP118 failed to reduce liver inflammation in contrast to mice treated with IP118 + OCA, which exhibited a trend towards reduced CD68 immunostaining compared to vehicle-treated mice (−21%, p = 0.09, Figure 3).
Figure 3.
Reduced hepatic inflammation following combined treatment with OCA and IP118. Representative liver sections (A) immunostained for monocyte/macrophage-specific cell-surface marker CD68 in C57BL6 control and Lepob/Lepob mice maintained on control LFD or AMLN diet and treated with vehicle, OCA, IP118, or OCA + IP118. The following symbols within images indicate: individual phagocytosis (solid arrow), phagocyte cluster (dotted arrow); magnification 100×. (B) Quantitative analysis of inflammatory milieu by measuring area stained for CD68 marker in liver sections where mice receiving OCA + IP118 exhibit significant reduction in inflammation compared to other groups. *p < 0.05 vs. C57BL6 controls; ψp < 0.05 vs. Lepob/Lepob AMLN, OCA.
3.5. Combined administration of OCA and IP118 reverses fibrosis and improves overall NASH score
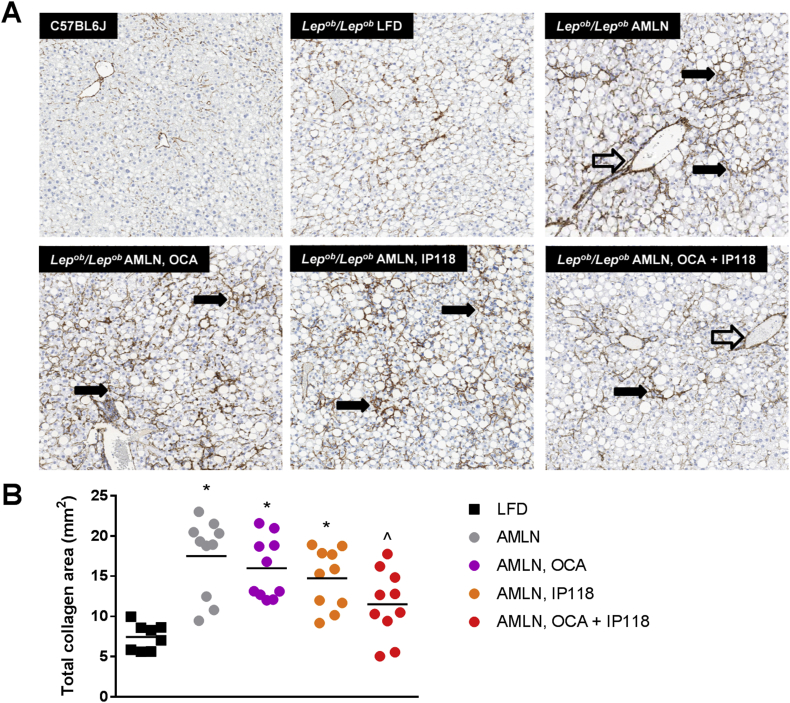
We next assessed the presence of liver fibrosis by immunostaining for collagen-1a1. Similar to humans with NASH, feeding Lepob/Lepob mice AMLN diet resulted in a dramatic collagen staining with perisinusoidal and perivascular patterns, resulting in a 1.4-fold increase in fibrosis compared to Lepob/Lepob mice fed LFD (p < 0.05, Figure 4). Fibrosis was not statistically affected by IP118 or OCA alone; however, administration of IP118 + OCA attenuated the increase in fibrosis induced by AMLN diet. Mice treated with IP118 + OCA exhibited a 34% reduction in fibrosis compared to vehicle controls (p < 0.05, Figure 4).
Figure 4.
Dual treatment with OCA and IP118 reverses fibrosis. Representative liver sections (A) immunostained for the fibrotic marker collagen-1a1 in C57BL6 control and Lepob/Lepob mice maintained on control LFD or AMLN diet and treated with vehicle, OCA, IP118, or OCA + IP118. The following symbols within images indicate: perivascular fibrosis (open arrow), perisinusoidal fibrosis (solid arrow); magnification 60×. (B) Quantitative analysis of collagen-stained fibrotic lesions in liver sections showing mice receiving dual treatment with OCA + IP118 exhibit marked reduction in fibrosis compared to other groups. *p < 0.05 vs. Lepob/Lepob LFD; ˆp < 0.05 vs. Lepob/Lepob AMLN group.
AMLN diet induced a marked increase in total NASH score compared to Lepob/Lepob mice on LFD (score of 16 vs. 9, Table 2). While OCA treatment had a minimal impact on the score (17), IP118 administration was associated with a trend towards a lower NASH score (13.9). The OCA + IP118 combination was associated with a marked reduction in total NASH score (13.5, Table 2).
Table 2.
Summary of histopathology findings. Number of animals within each treatment classified as either none, mild, moderate, or marked for presence of various pathological features of NASH. The average NASH score was calculated as the sum of multiple hepatic pathological features of NASH described in Materials and Methods. LFD, low fat diet.
| Strain |
C57BL6J |
ob/ob |
ob/ob |
ob/ob |
ob/ob |
ob/ob |
|---|---|---|---|---|---|---|
| Diet/drug |
LFD |
LFD |
AMLN/vehicle |
AMLN/OCA |
AMLN/IP118 |
AMLN/OCA + IP118 |
| Animals examined | 6 | 8 | 10 | 10 | 10 | 10 |
| Steatosis | ||||||
| None | 4 | 0 | 0 | 0 | 0 | 0 |
| Mild | 2 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 0 | 4 | 0 | 4 | 7 | 7 |
| Marked | 0 | 4 | 10 | 6 | 3 | 3 |
| Steatosis location | ||||||
| None | 5 | 2 | 0 | 1 | 2 | 1 |
| Mild | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 1 | 0 | 0 | 0 | 0 | 0 |
| Marked | 0 | 6 | 10 | 9 | 8 | 9 |
| Microvesicular steatosis | ||||||
| None | 6 | 7 | 2 | 0 | 6 | 3 |
| Mild | 0 | 1 | 8 | 10 | 4 | 7 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 |
| Marked | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatocyte ballooning | ||||||
| None | 6 | 8 | 6 | 0 | 4 | 5 |
| Mild | 0 | 0 | 4 | 10 | 6 | 5 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 |
| Marked | 0 | 0 | 0 | 0 | 0 | 0 |
| Lobular inflammation | ||||||
| None | 6 | 8 | 4 | 1 | 6 | 3 |
| Mild | 0 | 0 | 4 | 8 | 3 | 7 |
| Moderate | 0 | 0 | 2 | 0 | 1 | 0 |
| Marked | 0 | 0 | 0 | 1 | 0 | 0 |
| CD68 immunoreactivity | ||||||
| None | 6 | 0 | 0 | 0 | 0 | 0 |
| Mild | 0 | 8 | 2 | 4 | 6 | 7 |
| Moderate | 0 | 0 | 8 | 6 | 4 | 3 |
| Marked | 0 | 0 | 0 | 0 | 0 | 0 |
| Biliary hyperplasia | ||||||
| None | 6 | 1 | 0 | 0 | 0 | 0 |
| Mild | 0 | 7 | 0 | 4 | 4 | 6 |
| Moderate | 0 | 0 | 10 | 6 | 6 | 4 |
| Marked | 0 | 0 | 0 | 0 | 0 | 0 |
| Fibrosis | ||||||
| None | 6 | 0 | 0 | 0 | 0 | 0 |
| Mild | 0 | 8 | 2 | 1 | 1 | 4 |
| Moderate | 0 | 0 | 5 | 1 | 6 | 6 |
| Marked | 0 | 0 | 3 | 8 | 3 | 3 |
| Fibrosis pattern | ||||||
| None | 6 | 0 | 0 | 0 | 0 | 0 |
| Mild | 0 | 6 | 1 | 0 | 0 | 0 |
| Moderate | 0 | 2 | 7 | 3 | 5 | 7 |
| Marked | 0 | 0 | 2 | 7 | 5 | 3 |
| Average NASH Score | 0.7 | 9 | 16 | 17 | 13.9 | 13.5 |
3.6. Modest mitochondrial enhancement following dual treatment with GLP-1 receptor and FXR agonists
To better understand the mechanism by which dual activation of GLP-1R and FXR pathways are involved in reversing NASH progression, we assessed hepatic expression of genes involved in mitochondrial β-oxidation of lipids. The expression of Cpt1a, Sirt1, Tfam, and Mfn1 was unaltered following NASH induction. Tfam and Sirt1 expression increased with OCA treatment when compared to Lepob/Lepob mice on LFD, and also following IP118 treatment compared to LFD and vehicle-treated AMLN mice (Supplementary Table S3). Combination treatment did not elicit further increases in mitochondrial gene expression. We further explored the potential for OCA and/or the GLP-1R agonist liraglutide to enhance hepatic mitochondrial activity by measuring oxygen consumption rates in primary hepatocytes isolated from B6 or Lepob/Lepob mice on AMLN diet (NASH hepatocytes). In both systems an acute (4 h) treatment with OCA alone had no effect on oxygen consumption, and the addition of liraglutide also had no effect (Supplementary Figure S2). Drug-induced enhancement in expression of some mitochondrial genes may contribute to the observed improved phenotype in mice but is not likely to be due to a direct effect on enhancing mitochondrial function.
3.7. Co-administration of OCA and IP118 exerts greater cooperative effects on metabolism in DIO mice
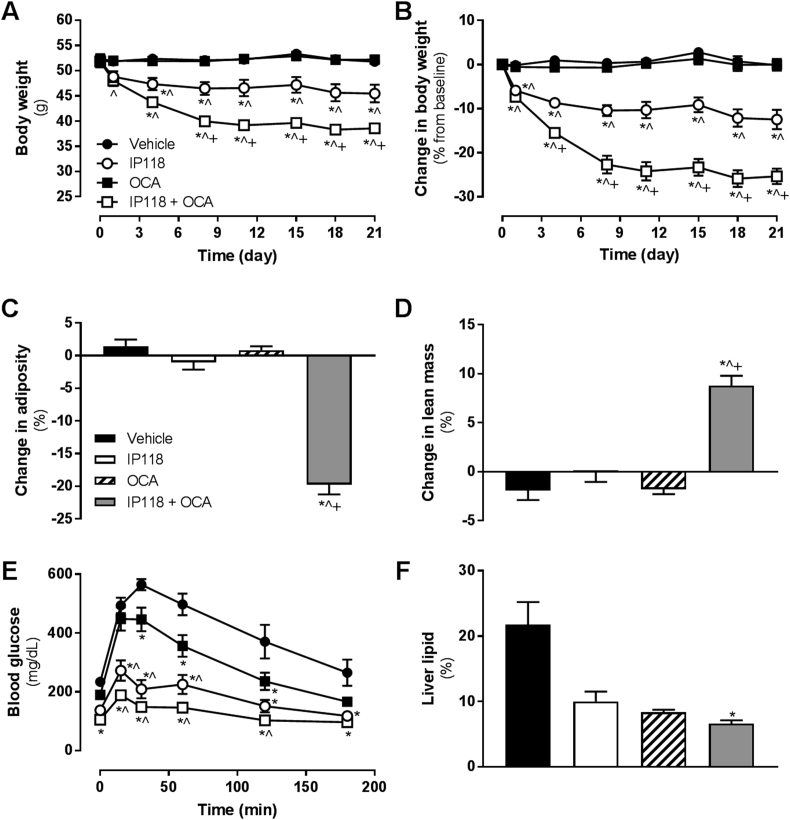
We explored the potential for OCA and IP118 to interact in a different murine model of metabolic disease. In DIO mice, acute (single dose) administration of IP118 improved glucose tolerance, but OCA had no effect alone and did not enhance glucose tolerance when added to IP118 (Supplementary Figure S5). In a subchronic study in DIO mice to assess longer term effects on weight, OCA alone had no effect on body weight or body composition, whereas IP118 elicited the expected reduction in body weight (−12.5%, p < 0.001 vs. vehicle, Figure 5A and B), with no significant change in body composition (Figure 5C and D). Co-administration of OCA + IP118, however, markedly reduced body weight (−25.3%, p < 0.001 vs. all groups, Figure 5A and B), associated with reduced fat mass and increased percent lean mass (Figure 5C and D). On day 17, a glucose tolerance test was performed following a 6 h fast. Despite not altering body weight, OCA treatment was associated with improved glucose tolerance (Figure 5E). IP118 alone also elicited improved glucose tolerance compared to vehicle and OCA groups in the first hour following glucose administration (Figure 5E). OCA + IP118 co-administration tended to further reduce glucose levels, but this did not reach statistical significance compared to IP118 at any timepoint during the test or by assessing area under the curve (Figure 5E, area under the curve data not shown). OCA and IP118 monotherapies tended to reduce hepatic lipid content, whereas the combination OCA + IP118 group showed significantly lower hepatic lipid compared to vehicle controls (Figure 5F).
Figure 5.
Co-administration of OCA and IP118 to diet-induced mice synergistically reduces body weight. Effect of OCA alone or in combination with twice-weekly doses of IP118 on (A) body weight, (B) percent change in body weight from basline, (C) change in adiposity (percent fat mass), and (D) change in percent lean mass. On day 17 of the study, glucose tolerance was explored in an intraperitoneal glucose tolerance test (E). Terminal liver lipid levels are shown in (F). *p < 0.05 vs. vehicle, ˆp < 0.05 vs. OCA, +p < 0.05 vs. IP118.
Terminal plasma analysis revealed significant reductions in total cholesterol in OCA and OCA + IP118-treated mice (Table 3). Plasma ALT levels were slightly elevated in the OCA group (vs. IP118 only), and AST levels were unchanged. Plasma triglycerides were reduced with IP118 and OCA + IP118 treatment relative to OCA alone (Table 3). Insulin levels were lowest in OCA + IP118 group vs. vehicle and OCA alone, corresponding to improved metabolic control (Table 3).
Table 3.
Effect of IP118, OCA or IP118 + OCA administration on plasma metabolic markers of diet-induced obese mice. Summary of terminal plasma levels of ALT, AST, triglycerides, total cholesterol and insulin.
| Metabolite | Vehicle | IP118 | OCA | IP118 + OCA |
|---|---|---|---|---|
| ALT (U/L) | 149 ± 25 | 124 ± 27 | 298 ± 54+ | 131 ± 19 |
| AST (U/L) | 118 ± 13 | 153 ± 19 | 178 ± 21 | 132 ± 12 |
| Cholesterol (mg/dL) | 223 ± 13 | 195 ± 14 | 114 ± 8* | 103 ± 5*+ |
| Triglycerides (mg/dL) | 83 ± 3 | 78 ± 9 | 103 ± 5+ | 73 ± 3ˆ |
| Insulin (ng/mL) | 5.5 ± 0.6 | 2.5 ± 1.2 | 6.2 ± 1.1+ | 1.0 ± 0.2*ˆ |
*p < 0.05 vs. vehicle, ˆp < 0.05 vs. OCA, +p < 0.05 vs. IP118.
The expression of Ucp1 in brown adipose tissue was significantly increased in the OCA + IP118 group compared to all other groups (Supplementary Table S6). Tfam was likewise significantly increased compared to other groups, but Sirt1 and Ppargc1a were not significantly different between groups, and Mfn1 and Cpt1a were slightly reduced (Supplementary Table S6). Liver gene expression analysis revealed significant changes in FXR target genes Nr0b2, Cyp7a1, and Cyp8b1 with either OCA and/or OCA + IP118 treatment and no significant effect on mitochondrial genes (Supplementary Table S6).
4. Discussion
OCA and GLP-1R agonists are being actively pursued as therapeutic options for the treatment of NASH, a currently unmet medical need. Here, we provide preclinical rationale to support exploration of combinatorial approaches to enhance overall efficacy. In this study, the first to report the effects of combined administration of a GLP-1R agonist (IP118) with OCA, we demonstrate superior effects on NASH endpoints in the Lepob/Lepob diet-induced mouse model of NASH with combination therapy and surprisingly robust synergistic effects on body weight in DIO mice.
Several studies have established that pharmacological activation of the GLP-1R in mice and humans reduces hepatic lipid content, improves plasma ALT and lipids, and slows fibrosis progression [12], [14], [16]. Consistent with these findings, administration of IP118 alone to mice maintained on the AMLN diet significantly reduced body weight, liver mass, plasma ALT, and AST and hepatic steatosis, while fibrosis and overall NASH score both showed a trend towards reduction. How GLP-1R activation improves hepatic function is not clearly understood, although improvements in lipogenesis and inflammation have been proposed [26], [27]. Specifically how these effects might be mediated in the liver remains unclear as the presence or absence of GLP-1R on hepatocytes is controversial. There is some evidence for the presence of a functional GLP-1R on human hepatocytes [28], although a comprehensive analysis suggests that full-length GLP-1R mRNA is not present in hepatocytes or macrophages [29]. Notwithstanding the possibility of functional GLP-1R on other cells within the liver (e.g., other immune cell populations), the benefits of GLP-1R therapy on liver endpoints are likely indirectly mediated, possibly via a GLP-1-dependent neural pathway that can alter hepatic glucose production [30].
FXR agonists such as OCA and other non-bile acid analog agonists directly activate a downstream response in hepatocytes and other cells/tissues which promote NASH resolution. While the clinical efficacy of OCA has been reported from the phase 2 FLINT study [9], there is remarkably little literature on the efficacy of OCA, or any other FXR agonist, in a relevant preclinical model of NASH. In our studies, the effects of OCA were limited to improvements in steatosis and circulating lipids, with no effect on metabolic endpoints. These effects are similar to those reported in the FLINT trial, albeit relatively more modest.
In combination with IP118, however, OCA was able to further enhance the more profound benefit of GLP-1R agonist therapy. While weight loss in Lepob/Lepob mice was not enhanced, there was a significant but transient improvement in glucose levels. The potential for improved glucose tolerance and/or insulin action with combination therapy warrants future studies. Notably, OCA has been reported to improve insulin sensitivity in humans via the hyperinsulinemic-euglycemic clamp technique [31]. The mechanism for improved metabolic control remains to be determined. Emerging data link bile acid metabolism to GLP-1 function via activation of FXR and/or the cell surface receptor TGR5, which has been reported to stimulate the release of GLP-1 in vitro [32], [33]. Similar responses to bile acids have been reported in clinical studies in humans [34], [35] and also after bariatric surgery [36]. Thus, OCA via activation of FXR/TGR5 may exert pro-metabolic effects via enhanced GLP-1 action.
To determine whether dual activation of FXR and GLP-1R pathways exert similar beneficial effects on enhancing metabolic phenotype in models other than Lepob/Lepob mice, we co-administered OCA and IP118 to DIO mice. Surprisingly, in contrast to the modest additional body weight and metabolic benefit observed in Lepob/Lepob mice, co-administration resulted in markedly reduced body weight and further improvements in glucose tolerance, hepatic and circulating lipid content, and reduced plasma insulin. The mechanism underlying these benefits remains to be fully explored; however, a significant induction of Ucp1 in brown adipose tissue with IP118 + OCA treatment suggests enhanced energy expenditure. The effect of the combination on other components of energy balance such as food intake and physical activity remains to be explored. Why OCA is able to induce a greater degree of weight loss benefit in combination with IP118 in DIO mice compared to Lepob/Lepob mice on NASH diet is also unknown. Whether the absence of leptin in Lepob/Lepob mice plays a role or whether the presence of NASH perturbs other aspects of bile acid signaling in the context of pharmacological levels of GLP-1R agonists is worth exploring. Our data are reminiscent of that reported in the genetically obese Alms1 mutant mice (foz/foz mice), where OCA was observed to be less efficacious compared to less obese wildtype mice maintained on an atherogenic diet [37].
This is the first report of combined pharmacological activation GLP-1R and FXR with a focus on NASH endpoints. We demonstrated enhanced effects on steatosis and CD68 immunoreactivity and fibrosis, paralleled by the lowest integrated NASH score. The magnitude of the additional benefit was relatively modest compared to IP118 monotherapy effects. The pathologic/histologic benefits, however, should be noted in the context of similar weight loss and food intake. We have previously reported that weight loss per se contributes a significant proportion, but not all, of the benefit of GLP-1 therapy on liver endpoints [14]. A similar level of contribution from overall weight loss is expected here given the lack of effect on food intake and body weight with OCA monotherapy. Coupled with improvement in NASH endpoints, we have shown that OCA and GLP-1R agonism can induce synergistic body weight loss accompanied by reduced hepatic lipid and improved glucose tolerance. Whether these effects are also due to the more extreme weight loss is unknown and requires comparison to calorie restricted mice.
To understand more of the mechanism of action of GLP-1 and OCA administration, we performed gene expression analysis in total liver RNA from both models. Overall, the effects on mitochondrial function were rather modest with subtly increased expression of key mitochondrial function genes. OCA and/or GLP-1 (liraglutide) administration likewise did not directly alter mitochondrial function as assessed by mitochondrial respiratory capacity. Hepatic mitochondrial function declines in the context of NAFLD/NASH [38], [39], which we observed in our model, and can be attenuated in mice with surgical intervention therapy such as roux-en-y gastric bypass [40]. GLP-1 receptor agonists have been shown to improve mitochondrial function in the liver putatively via the master mitochondrial regulator SIRT-1 [41]. FXR agonism has also been reported to enhance mitochondrial function in various cell types [42]. While our gene expression and oxygen consumption data do not support a major benefit of OCA on top of GLP-1 therapy on mitochondrial action, expression of Ucp1 and Tfam was increased with combination treatment in brown adipose tissue. Thus, an improvement in mitochondrial activity in other tissues, including brown and white adipose tissue, may enhance whole body lipid metabolism and energy expenditure, thereby contributing to the observed benefits, as has been reported for OCA in other rodent models [37].
OCA and GLP-1R agonists are being tested in late stage clinical trials for their therapeutic effects in biopsy-proven NASH subjects. Our data provide a rationale to consider exploring a combinatorial approach. Indeed, examples of bile acid moieties conjugated to a stabilized GLP-1R agonist (modified exenatide peptide) have been reported thus illustrating the chemical possibility of such a therapy [43]. Whether the addition of OCA can enhance the metabolic benefits in patients already on a GLP-1 therapy should be tested. Further preclinical studies in other models may be required to understand the impact and nature of the weight-reducing and anti-fibrotic/anti-inflammatory potential of GLP-1/OCA action.
Acknowledgements
We gratefully acknowledge the assistance of Sally Lee, Holly Koelkebeck, and Wanda King (MedImmune, Gaithersburg, MD) for expert technical assistance with tissue processing and immunohistochemistry. We thank Lorenz Rognoni and Farzad Sekhavati (Definiens, Germany) for tissue image analysis. We also thank Donna Goldsteen, Krystal Nacel, and the Laboratory Animal Resource staff (MedImmune, Gaithersburg, MD) for their assistance with animal husbandry and care.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.09.001.
Financial support
These studies were conducted at and funded by MedImmune, LLC. HJ, SW, SG, MB, SO, PR, and JLT are current employees and/or stockholders of MedImmune/AstraZeneca; AC was an employee of MedImmune and stockholder of AstraZeneca at the time this work was performed.
Author contributions
Project conception: AC, JLT; experimental design and interpretation: HJ, AC, MB, JLT; data acquisition and analysis: HJ, SW, SG, MB, SO, PR, JLT; wrote and edited the manuscript: HJ, SG, MB, JLT.
Conflict of interest
All authors are or were employees of MedImmune/AstraZeneca at the time these experiments were designed and conducted.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ascha M.S., Hanouneh I.A., Lopez R., Tamimi T.A., Feldstein A.F., Zein N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 2.Starley B.Q., Calcagno C.J., Harrison S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G., Baranova A., Younossi Z.M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Alimentary Pharmacology and Therapeutics. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M.R., Burns J.M., Pedersen R.A., Watt K.D., Heimbach J.K., Dierkhising R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 8.Lazo M., Solga S.F., Horska A., Bonekamp S., Diehl A.M., Brancati F.L. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratziu V., Pienar L. Pharmacological therapy for non-alcoholic steatohepatitis: how efficient are thiazolidinediones? Hepatology Research. 2011;41:687–695. doi: 10.1111/j.1872-034X.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- 11.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Ding X., Saxena N.K., Lin S., Gupta N.A., Anania F.A. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samson S.L., Gonzalez E.V., Yechoor V., Bajaj M., Oka K., Chan L. Gene therapy for diabetes: metabolic effects of helper-dependent adenoviral exendin 4 expression in a diet-induced obesity mouse model. Molecular Therapy. 2008;16:1805–1812. doi: 10.1038/mt.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevaskis J.L., Griffin P.S., Wittmer C., Neuschwander-Tetri B.A., Brunt E.M., Dolman C.S. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2012;302:G762–G772. doi: 10.1152/ajpgi.00476.2011. [DOI] [PubMed] [Google Scholar]
- 15.Klonoff D.C., Buse J.B., Nielsen L.L., Guan X., Bowlus C.L., Holcombe J.H. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Current Medical Research and Opinion. 2008;24:275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 17.Adorini L., Pruzanski M., Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discovery Today. 2012;17:988–997. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Porez G., Prawitt J., Gross B., Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. The Journal of Lipid Research. 2012;53:1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cave M.C., Clair H.B., Hardesty J.E., Falkner K.C., Feng W., Clark B.J. Nuclear receptors and nonalcoholic fatty liver disease. Biochimica et Biophysica Acta (BBA) 2016;1859:1083–1099. doi: 10.1016/j.bbagrm.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellicciari R., Fiorucci S., Camaioni E., Clerici C., Costantino G., Maloney P.R. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. Journal of Medicinal Chemistry. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 21.Sinal C.J., Tohkin M., Miyata M., Ward J.M., Lambert G., Gonzalez F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 22.Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T.H., Grefhorst A., Abdelkarim M. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. Journal of Biological Chemistry. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S., Wang J., Liu Q., Harnish D.C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. Journal of Hepatology. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Lee F.Y., Barrera G., Lee H., Vales C., Gonzalez F.J. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proceedings of the National Academy of Sciences of United States of America. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Zietek T., Rath E. Inflammation meets metabolic disease: gut feeling mediated by GLP-1. Frontiers in Immunology. 2016;7:154. doi: 10.3389/fimmu.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mells J.E., Anania F.A. The role of gastrointestinal hormones in hepatic lipid metabolism. Seminars in Liver Disease. 2013;33:343–357. doi: 10.1055/s-0033-1358527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta N.A., Mells J., Dunham R.M., Grakoui A., Handy J., Saxena N.K. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–1592. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panjwani N., Mulvihill E.E., Longuet C., Yusta B., Campbell J.E., Brown T.J. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology. 2013;154:127–139. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 30.Yang M., Wang J., Wu S., Yuan L., Zhao X., Liu C. Duodenal GLP-1 signaling regulates hepatic glucose production through a PKC-delta-dependent neurocircuitry. Cell Death & Disease. 2017;8:e2609. doi: 10.1038/cddis.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mudaliar S., Henry R.R., Sanyal A.J., Morrow L., Marschall H.U., Kipnes M. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582 e571. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Brighton C.A., Rievaj J., Kuhre R.E., Glass L.L., Schoonjans K., Holst J.J. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology. 2015;156:3961–3970. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M. A G protein-coupled receptor responsive to bile acids. Journal of Biological Chemistry. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 34.Adrian T.E., Gariballa S., Parekh K.A., Thomas S.A., Saadi H., Al Kaabi J. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia. 2012;55:2343–2347. doi: 10.1007/s00125-012-2593-2. [DOI] [PubMed] [Google Scholar]
- 35.Hansen M., Scheltema M.J., Sonne D.P., Hansen J.S., Sperling M., Rehfeld J.F. Effect of chenodeoxycholic acid and the bile acid sequestrant colesevelam on glucagon-like peptide-1 secretion. Diabetes, Obesity and Metabolism. 2016;18:571–580. doi: 10.1111/dom.12648. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen S., Svane M.S., Kuhre R.E., Clausen T.R., Kristiansen V.B., Rehfeld J.F. Chenodeoxycholic acid stimulates glucagon-like peptide-1 secretion in patients after Roux-en-Y gastric bypass. Physiological Reports. 2017;5 doi: 10.14814/phy2.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haczeyni F., Poekes L., Wang H., Mridha A.R., Barn V., Geoffrey Haigh W. Obeticholic acid improves adipose morphometry and inflammation and reduces steatosis in dietary but not metabolic obesity in mice. Obesity (Silver Spring) 2017;25:155–165. doi: 10.1002/oby.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metabolism. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Petersen K.F., Befroy D.E., Dufour S., Rothman D.L., Shulman G.I. Assessment of hepatic mitochondrial oxidation and pyruvate cycling in NAFLD by (13)C magnetic resonance spectroscopy. Cell Metabolism. 2016;24:167–171. doi: 10.1016/j.cmet.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verbeek J., Lannoo M., Pirinen E., Ryu D., Spincemaille P., Vander Elst I. Roux-en-y gastric bypass attenuates hepatic mitochondrial dysfunction in mice with non-alcoholic steatohepatitis. Gut. 2015;64:673–683. doi: 10.1136/gutjnl-2014-306748. [DOI] [PubMed] [Google Scholar]
- 41.Xu F., Li Z., Zheng X., Liu H., Liang H., Xu H. SIRT1 mediates the effect of GLP-1 receptor agonist exenatide on ameliorating hepatic steatosis. Diabetes. 2014;63:3637–3646. doi: 10.2337/db14-0263. [DOI] [PubMed] [Google Scholar]
- 42.Han C.Y., Kim T.H., Koo J.H., Kim S.G. Farnesoid X receptor as a regulator of fuel consumption and mitochondrial function. Archives of Pharmacal Research (Seoul) 2016;39:1062–1074. doi: 10.1007/s12272-016-0812-y. [DOI] [PubMed] [Google Scholar]
- 43.Son S., Chae S.Y., Kim C.W., Choi Y.G., Jung S.Y., Lee S. Preparation and structural, biochemical, and pharmaceutical characterizations of bile acid-modified long-acting exendin-4 derivatives. Journal of Medicinal Chemistry. 2009;52:6889–6896. doi: 10.1021/jm901153x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.