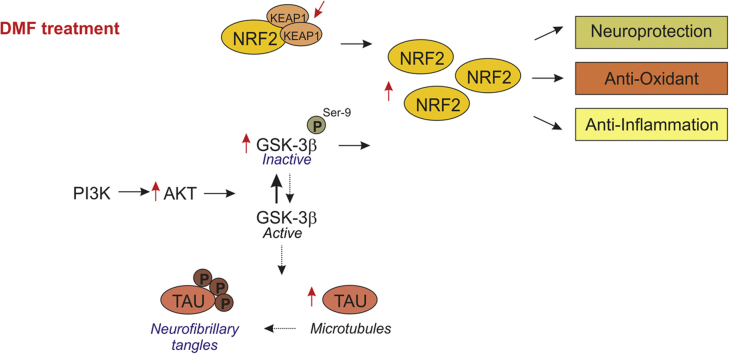
Abstract
Tauopathies are a group of neurodegenerative disorders where TAU protein is presented as aggregates or is abnormally phosphorylated, leading to alterations of axonal transport, neuronal death and neuroinflammation. Currently, there is no treatment to slow progression of these diseases. Here, we have investigated whether dimethyl fumarate (DMF), an inducer of the transcription factor NRF2, could mitigate tauopathy in a mouse model. The signaling pathways modulated by DMF were also studied in mouse embryonic fibroblast (MEFs) from wild type or KEAP1-deficient mice. The effect of DMF on neurodegeneration, astrocyte and microglial activation was examined in Nrf2+/+ and Nrf2−/− mice stereotaxically injected in the right hippocampus with an adeno-associated vector expressing human TAUP301L and treated daily with DMF (100 mg/kg, i.g) during three weeks. DMF induces the NRF2 transcriptional through a mechanism that involves KEAP1 but also PI3K/AKT/GSK-3-dependent pathways. DMF modulates GSK-3β activity in mouse hippocampi. Furthermore, DMF modulates TAU phosphorylation, neuronal impairment measured by calbindin-D28K and BDNF expression, and inflammatory processes involved in astrogliosis, microgliosis and pro-inflammatory cytokines production. This study reveals neuroprotective effects of DMF beyond disruption of the KEAP1/NRF2 axis by inhibiting GSK3 in a mouse model of tauopathy. Our results support repurposing of this drug for treatment of these diseases.
Keywords: DMF, Inflammation, Neurodegeneration, NRF2, Oxidative stress, TAU/ GSK-3
Graphical abstract
Highlights
-
•
DMF mechanisms of action are partially KEAP1-dependent.
-
•
Modulation of GSK-3β phosphorylation by DMF.
-
•
DMF modulates TAU hyperphosphorylation in a tauopathy mouse model.
-
•
DMF attenuates hippocampal neuronal damage, astrogliosis and microgliosis.
1. Introduction
Tauopathies are a group of age-related neurodegenerative diseases that are characterized by the presence of protein aggregates with abnormally phosphorylated TAU [1]. TAU pathology is found in Alzheimer's disease, frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and Pick's disease, among others. The presence of insoluble TAU inclusions and neuronal loss in all of these diseases implies common mechanisms involved in cell injury and death, as well as neuroinflammation. Nowadays, there is no approved pharmacologic treatment for tauopathies that could target the cause of these diseases [2]. For example, to treat cognitive and behavioral symptoms, therapeutic agents such as acetylcholinesterase inhibitors and memantine have been used, but the outcome has not been consistent [2]. Recent research focuses on targeting TAU protein pathology, such as phosphorylation. The best studied TAU kinases are the proline-directed kinases Glycogen Synthase kinase-3β (GSK-3β), CDK5, MAPK (ERK), JNK (SAPK), and p38. GSK-3β is the major kinase to phosphorylate TAU both in vitro and in vivo and has been proposed as a target for therapeutic intervention [3]. Moreover, GSK-3β is a fundamental element in the down-regulation of the antioxidant cell defense elicited by the transcription factor NRF2 [4].
NRF2 was first described as the master regulator of redox homeostasis, but currently it is known to regulate the expression of about 1% of human genes, which contain in their promoter regulatory regions an enhancer sequence termed Antioxidant Response Element [5]. These genes encode a large variety of cytoprotective proteins implicated in biotransformation, antioxidant reactions, and inflammation, by modifying metabolic programs [6]. NRF2 is regulated principally by two different mechanisms. The best established mechanism is the control of protein stability by Kelch-like ECH-associated protein 1 (KEAP1). KEAP1 is an ubiquitin E3 ligase substrate adapter for a Cullin3/Rbx1-dependent E3 ubiquitin ligase complex; henceforth binding of KEAP1 to NRF2 mediates ubiquitination and subsequent proteasomal degradation of NRF2 [7].
The second mechanism is related to GSK-3, which phosphorylates NRF2 creating a recognition site for β-Transducin Repeat Containing E3 Ubiquitin Protein Ligase (β-TrCP). β-TrCP leads to Cullin-1/Rbx1-mediated NRF2 ubiquitination and its subsequent degradation [8]. Since GSK-3β is inhibited by phosphorylation at Ser9 by Ser/Thr protein kinases such as AKT, it has been suggested that NRF2 might be up-regulated through activation of AKT and permanent inactivation of GSK-3 [9], [10].
Many NRF2 activators have been identified. Dimethyl fumarate (DMF) has consistently demonstrated to act in brain and to have neuroprotective effects [11], with the added value of being an already approved drug for relapsing–remitting multiple sclerosis. The oral formulation termed BG-12, has been commercialized with the name of Tecfidera by Biogen [12].
Therefore, in this study we have analyzed the effect of DMF on several signaling pathways and induction of NRF2 signature. We have also used a clinically relevant dose of DMF to analyze its effect on neuronal plasticity markers and neuroinflammation in a preclinical mouse model of tauopathy. The end goal of this study is to validate DMF as a therapeutic drug for tauopathies, modulating NRF2 and GSK-3 signaling.
2. Material and methods
2.1. Cell culture
Keap1−/− and Keap1+/+ MEFs were provided by Dr. Ken Itoh (Center for Advanced Medical Research, Hirosaki University Graduate School of Medicine, Hirosaki, Japan). MEFs from wild-type (PTENwt) and transgenic PTEN (PTENtg) mice were provided by Dr. Manuel Serrano (Tumor Suppression Group, Spanish National Cancer Research Center (CNIO), Madrid, Spain). MEFs were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 2 mM L-glutamine, in 5% CO2 at 37 °C, 50% relative humidity. Medium was changed to serum-free DMEM without antibiotics 16 h before treatments.
2.2. Immunoblotting
Whole brain lysates were prepared as described previously [31]. Immunoblots were performed as described in [4]. The primary antibodies are described in Table 1A.
Table 1a.
List of antibodies used in this study.
| Antibody | Source | Catalog number | Dilution |
|---|---|---|---|
| β-ACTIN | Santa Cruz Biotechnologies | sc-1616 | 1:5000 |
| p-AKT | Cell Signaling | #4058 | 1:1000 |
| AKT total | Santa Cruz Biotechnologies | sc-1618 | 1:1000 |
| CALBINDIN D-28K | Synaptic Systems | 214,002 | 1:500 (IHC) |
| p-CRMP2 | Novus Biologicals | NBP1–03440 | 1:300 (IHC) |
| CRMP2 total | Immuno-Biological Laboratories | 11,096 | 1:20 (IHC) |
| pERK | Cell Signaling | #9106 | 1:1000 |
| ERK total | Cell Signaling | #4695 | 1:1000 |
| GAPDH | Merck-Millipore | CB1001 | 1:15.000 |
| GFAP | DakoCytomation | Z0334 | 1:500 (IHC) |
| p-Ser9-GSK-3β | Cell Signaling | #9336 | 1:1000 |
| GSK-3β total | BD Bioscience | 610,201 | 1:1000 |
| Iba1 | Wako Chemicals | 019–19741 | 1:500 (IHC) |
| LAMIN B | Santa Cruz Biotechnologies | sc-6217 | 1:1000 |
| NRF2 | Dr. Cuadrado's lab (Home-made) | – | 1:2000 |
| p-p38 | Cell Signaling | #9211 | 1:1000 |
| p38 total | Cell Signaling | #9212 | 1:1000 |
| p-TAU (AT8) | ThermoFisher Scientific | #MN1020 | 1:200 (IHC) |
| TAU total | Santa Cruz Biotechnologies | sc-5587 | 1:200 (IHC) |
| ThermoFisher Scientific | #MN1000B | 1:100 (IHC) |
2.3. Preparation of nuclear and cytosolic extracts
Keap1−/− and Keap1+/+ MEFs were seeded in p100 plates (2 × 106 cells/plate). MEFs cells were treated with DMF (20 μM) for different times. Cytosolic and nuclear fractions were prepared as described previously (35). Briefly, cells were washed with cold PBS and harvested by centrifugation at 1100 rpm for 10 min. The cell pellet was resuspended in 3 pellet volumes of cold buffer A (20 mM HEPES, pH 7.0, 0.15 mM EDTA, 0.015 mM EGTA, 10 mM KCl, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 μg/ml leupeptin) and incubated in ice for 30 min. Then, the homogenate was centrifuged at 500g for 5 min. The supernatants were taken as the cytosolic fraction. The nuclear pellet was resuspended in 5 volumes of cold buffer B (10 mM HEPES, pH 8.0, 0.1 mM EDTA, 0.1 mM NaCl, 25% glycerol, 1 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 μg/ml leupeptin). After centrifugation in the same conditions indicated above, the nuclei were resuspended in loading buffer containing 0.5% SDS. The cytosolic and nuclear fractions were resolved in SDS-PAGE and immunoblotted with the indicated antibodies. Since, in general, the commercially available NRF2 antibodies are not specific [13], we had developed our own antibody, that has been previously validated for Western-blot (see [11] and Suppl. Fig. 1).
2.4. Analysis of mRNA levels by quantitative real-time PCR
Total RNA extraction, reverse transcription, and quantitative polymerase chain reaction (PCR) were done as detailed elsewhere (22). Primer sequences are shown in Table 1B. Data analysis was based on the ΔΔCT method with normalization of the raw data to housekeeping genes (Applied Biosystems). All PCRs were performed in triplicates.
Table 1b.
List of primers used in this study.
| Gene product | Forward primer | Reverse primer |
|---|---|---|
| β-actin | 5′ TCCTTCCTGGGCATGGAG 3′ | 5′ AGGAGGAGCAATGATCTTGATCTT 3′ |
| Bdnf | 5′ GATGCCGCAAACATGTCTATGA 3′ | 5′ TAATACTGTCACACACGCTCAGCTC 3′ |
| Gfap | 5′ TCCTGGAACAGCAAAACAAG 3′ | 5′ CAGCCTCAGGTTGGTTTCAT 3′ |
| Hmox1 | 5′ CACAGATGGCGTCACTTCGTC 3′ | 5′ GTGAGGACCCACTGGAGGAG 3′ |
| Il-1β | 5′ CTGGTGTGTGACGTTCCCATTA 3′ | 5′ CCGACAGCACGAGGCTTT 3′ |
| Iba1 | 5′ GTCCTTGAAGCGAATGCTGG 3′ | 5′ CATTCTCAAGATGGCAGATC 3′ |
| iNos | 5′ CCTCCTTTGCCTCTCACTCTTC 3′ | 5′ AGTATTAGAGCGGTGGCATGGT 3′ |
| Nqo1 | 5′ GGTAGCGGCTCCATGTACTC 3′ | 5′ CATCCTTCCAGGATCTGCAT 3′ |
| Nrf2 | 5′ CCCGAAGCACGCTGAAGGCA 3′ | 5′ CCAGGCGGTGGGTCTCCGTA 3′ |
| Osgin-1 | 5′ CGGTGACATCGCCCACTAC 3′ | 5′ GCTCGGACTTAGCCCACTC 3′ |
2.5. Animals and treatments
Colonies of Nrf2-/- mice and Nrf2+/+ littermates were established from funders kindly provided by Prof. Masayuki Yamamoto (Tohoku University Graduate School of Medicine, Sendai, Japan) [14]. Each experimental group comprised 5–8 animals. Recombinant AAV vectors of hybrid serotype 1/2 express mutant hTAUP301L under control of the human synapsin 1 gene promoter and were used as described [15]. Surgical procedures and unilateral intracerebral injection of viral particles into the right hemisphere were performed as described [22]. In brief, 2 μL viral suspension containing 10E8 t.u. was injected at the stereotaxic coordinates −1.94 mm posterior, −1.4 mm lateral, and −1.8 mm ventral relative to bregma. DMF (100 mg/kg) (Sigma-Aldrich) was suspended in 0.8% methocel (Sigma-Aldrich) and given by oral gavage. We did not detect significant weight loss, hair loss or other gross alterations in the DMF-treated mice either in the 3-weeks administration every day.
2.6. Immunofluorescence on mouse tissues
The protocol was previously described [31]. Primary antibodies are described in Table 1A. Secondary antibodies were: Alexa Fluor 546 goat anti-mouse, Alexa 546 goat anti-rabbit and Alexa Fluor 488 goat anti-mouse (1:500, Life technologies, Madrid, Spain). Control sections were treated following identical protocols but omitting the primary antibody.
2.7. Statistical analyses
Data are presented as mean ± SEM. To determine the statistical test to be used, we employed GraphPad Instat 3, which includes the analysis of the data to normal distribution via Kolmogorov-Smirnov test. In addition, statistical assessments of differences between groups were analyzed (GraphPad Prism 5, San Diego, CA) by unpaired Student's t-tests when normal distribution and equal variances were fulfilled, or by the non-parametric Mann–Whitney test. One and two-way ANOVA with post hoc Newman-Keuls test or Bonferroni's test were used, as appropriate.
3. Results
3.1. DMF induces the NRF2 transcriptional signature through KEAP1-dependent and -independent mechanisms
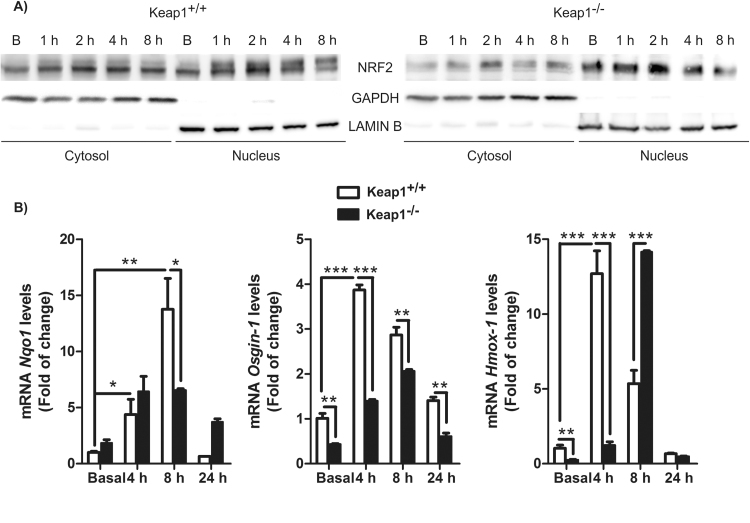
We wanted to corroborate the generally accepted concept that DMF targets KEAP1 by using mouse embryonic fibroblast (MEFs) from wild type (Keap1+/+) or KEAP1-deficient (Keap1-/-) mice. As shown in Fig. 1A, in Keap1+/+ cells, the basal levels of NRF2 were evenly distributed between cytoplasm and nucleus but DMF led to a prolonged nuclear accumulation of NRF2 starting at 1–2 h. In Keap1-/- cells, most of NRF2 was basally located in the nucleus. In these cells, DMF still induced a transient (1–2 h) but significant accumulation of NRF2 both in cytosol and nucleus. Consistent with this, DMF increased the mRNA levels of Nqo1, Osgin-1 and Hmox-1 in both cell types but with a delayed kinetics and lower intensity in Keap1-/- MEFs (Fig. 1B). Our results confirm the regulation of NRF2 in a KEAP1-dependent manner but most importantly, they also provide evidence of KEAP1-independent mechanisms.
Fig. 1.
DMF activates NRF2 signaling through KEAP1-dependent and -independent mechanisms. A) Keap1+/+ and Keap1-/- MEFs were incubated in the presence of DMF (20 μM) for 1, 2, 4 and 8 h, and subcellular fractionations were analyzed by immunoblot: upper panel: NRF2 levels; middle panel: GAPDH levels used as cytosol protein loading control; lower panel: Lamin B level used as nuclear protein loading control. B) Keap1+/+ and Keap1-/- MEFs were treated with DMF and mRNA levels for Nqo1, Osgin1 and Hmox1 were determined by qRT-PCR, normalized to β-actin mRNA levels.
3.2. DMF induces NRF2 through different kinase signaling pathways: key modulation of GSK-3β
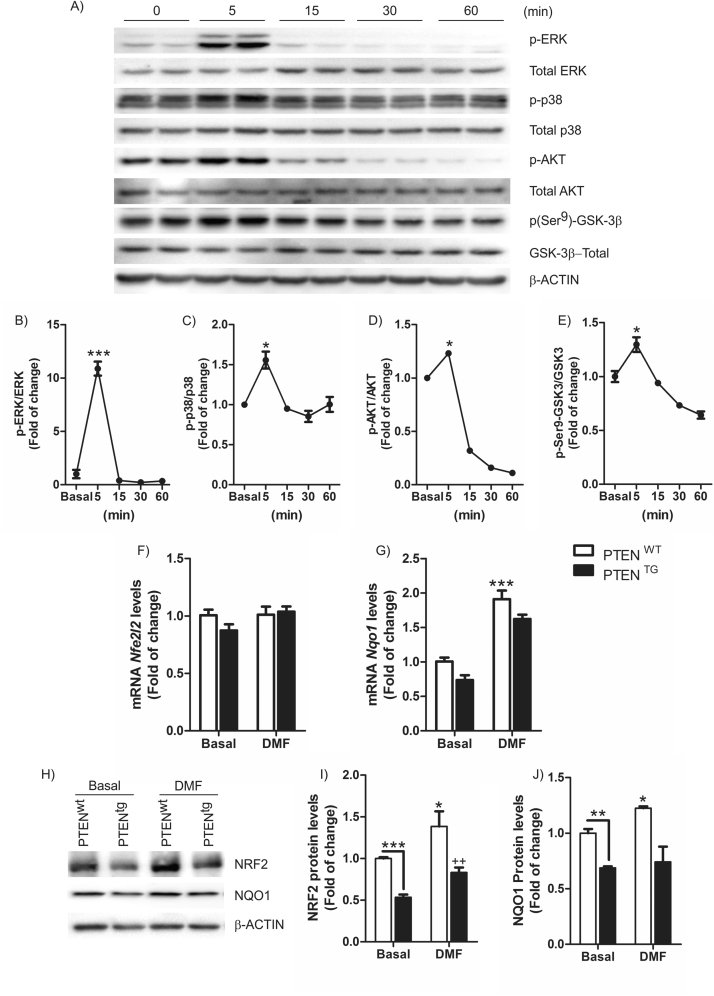
Previous evidence indicate that NRF2 is controlled by mechanisms other than KEAP1. For example, the mitogen-activated protein kinase (MAPK) signaling system responds to oxidative stress, and has been implicated in NRF2 activation [14]. Moreover, our group has described that PI3K/AKT/GSK-3 pathway is essential in NRF2 regulation [8]. Wild-type MEFs treated with DMF (20 μM) showed a time-dependent response effect activating phosphorylation of ERK (Fig. 2A, B) and p38 (Fig. 2A, C), that was maximal within 5 min. The Ser/Thr protein kinase AKT, an upstream regulator of GSK-3β, was also activated after 5 min as determined by increased phosphorylation of S473 (Fig. 2A, D), paralleling similar kinetics of inactivating phosphorylation of GSK-3βSer9 (Fig. 2A, E). Furthermore, MEFs from transgenic mice that overexpress phosphatase and tensin homolog deleted on chromosome 10 (PTEN) (PTENtg) by ∼3-fold relative to control littermates (PTENwt) exhibited impaired NRF2 activity under basal conditions or after treatment with DMF. Thus, DMF (20 μM, 6 h) increased NRF2 and NQO1 protein expression (Fig. 2H-J), while this induction was reduced in PTENtg-MEFs. Interestingly, expression of the NRF2-regulated gene Nqo1 (Fig. 2G), but not Nrf2 (Nfe2l2) was significantly impaired in PTENtg-MEFs, (Fig. 2F). Taken together, these results indicate that activation of the NRF2 pathway by DMF implicates KEAP-1-dependent and independent mechanisms that control its protein stability, including the PI3K/AKT/GSK-3β axis.
Fig. 2.
DMF activates several cell signaling pathways.A) Immunoblot showing phosphorylation levels of ERK, p38, AKT and GSK-3 after treatment with 20 μM DMF of Nrf2+/+-derived MEFs. B-E) Quantification of the corresponding immunoblots. Data are mean ± SEM. The one-way ANOVA test with a Newman-Keuls posterior test was used to evaluate differences in significance between groups: *p < 0.05, ***p < 0.001 compared to basal levels. MEFs from PTENwt and PTENtg transgenic mice were treated with 20 μM DMF for 6 h. F) Determination of Nfe2l2 mRNA levels and E) determination of Nqo1 mRNA levels by qRT-PCR and normalized by β-Actin levels. Data are mean ± SEM (n = 4). H) Immunoblots of PTENwt and PTENtg MEFs treated with 20 μM of tBHQ for 6 h. (I–J) densitometric quantification of NRF2 and NQO1 protein levels of representative blots from (H). Statistical analysis was performed with two-way ANOVA followed by Bonferroni post-hoc test. *p < 0.05; **p < 0.01; and ***p < 0.001 versus PTENwt MEFs and ++p < 0.01 versus PTENtg MEFs.
3.3. Modulation of GSK-3β by DMF and its implication on CRMP2 phosphorylation
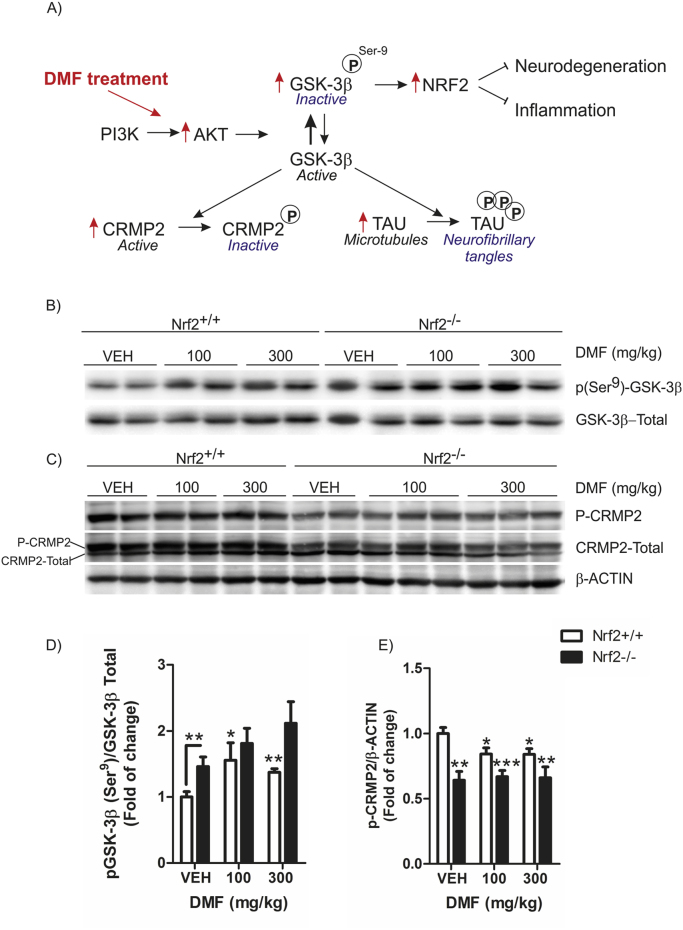
Changes in GSK-3β activity have been associated with several neurodegenerative diseases, through modulation of several substrates which are involved in neuronal polarization by regulating microtubule dynamics, including collapsin response mediator protein 2 (CRMP2) and TAU (Fig. 3A) [15]. To determine if DMF can modulate PI3K/AKT/GSK-3β in vivo as we found in vitro (Fig. 2) and its effects on a substrate like CRMP2, Nrf2+/+ and Nrf2-/- mice were treated with DMF (100 and 300 mg/kg, i.g.) for one hour. Hippocampi of Nrf2-/- animals treated with vehicle showed increased inactivating phosphorylation of GSK-3βSer9 compared to Nrf2+/+ animals (Fig. 3B, D) which correlated with decreased levels of phosphorylated CRMP2 (Fig. 3C, E). DMF increased the phosphorylation levels of GSK-3βSer9 in both genotypes, indicating that this effect is upstream of NRF2 as shown in Fig. 3A. After one hour of DMF treatment, we could not observe a significant change in CRMP2 phosphorylation suggesting that one-hour treatment was sufficient to modulate GSK-3β phosphorylation status, but not to observe downstream effects. These results indicate that DMF modifies GSK-3β activity in mouse hippocampus.
Fig. 3.
DMF modulates GSK-3β activity in mouse hippocampus. Nrf2+/+ and Nrf2−/− mice received 100 or 300 mg/kg intragastric doses of DMF and were sacrificed 4 h later (n = 5 animals per group). A) Diagram of the PI3K/AKT/GSK-3β signaling pathway and its possible modulation by DMF. GSK-3β phosphorylates CRMP2 and TAU, proteins implicated in microtubule dynamics. B) pSer9-GSK-3β protein levels in hippocampus: upper panel, anti-pSer9-GSK-3β antibody; lower panel, anti-GSK-3β total antibody. D) densitometric quantification of protein levels from representative immunoblots of B). C) p-CRMP2 protein levels in hippocampus: upper panel, anti-p-CRMP2 antibody; middle panel, anti- GSK-3β total antibody, lower panel, anti-β-actin. E) densitometric quantification of protein levels from representative immunoblots of C). One-way ANOVA followed by Newman–Keuls post-test was used to assess significant differences among groups. Asterisks denote significant differences with *p < 0.05, **p < 0.01, and ***p < 0.001 comparing the indicated groups.
3.4. DMF modulates TAU hyperphosphorylation in the AAV-TAUP301L mouse model of tauopathy
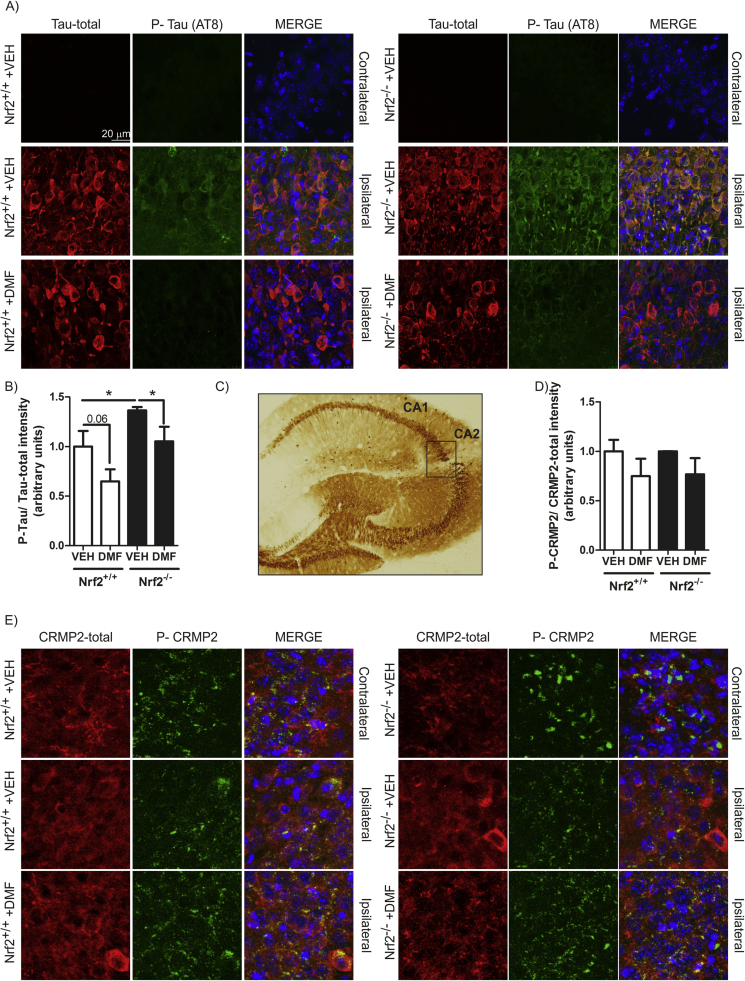
We next examined whether DMF could have beneficial effects on a mouse model of tauopathy based on stereotaxic delivery to the hippocampus of an adeno-associated vector expressing the human mutant TAUP301L protein, under the control of the human synapsin 1 gene promoter (AAV-TAUP301L) [16]. A control adeno-associated virus vector expressing green fluorescence protein did not elicit significant changes in inflammation or gliosis (data not shown). Nrf2+/+ and Nrf2−/− mice were injected with AAV-TAUP301L in the right hippocampus (ipsilateral side) and the left hippocampus was used as control (contralateral side). Animals received a daily administration of DMF (100 mg/kg, i.g.) by oral gavage, which started the same day as the mice were injected with the adeno-associated virus. Three-weeks after, we observed hTAU protein expression in the hippocampi of both genotypes (Fig. 4C), indicating that DMF did not influence AAV-TAUP301L expression. Immunofluorescence analysis showed that in the non-injected side, there was not expression of either total hTAU or phosphorylated-hTAU in both genotypes (Fig. 4A). Interestingly, in hTAUP301L expressing neurons, we observed increased hTAU phosphorylation in Nrf2+/+ hippocampus, which was exacerbated in Nrf2−/− mice (Fig. 4A-B), in agreement with previous work by Jo et al. [17] and our group [18]. Importantly, DMF significantly decreased hTAU phosphorylation in both genotypes (Fig. 4A-B).
Fig. 4.
Phosphorylated TAU levels decrease after DMF treatment in a mouse model of tauopathy.A) Double immunofluorescence staining of 30 µm-thick sections of hippocampus from Nrf2+/+ and Nrf2−/− mice injected with AAV-TAUP301L. Green, anti-phospho-TAU (AT8 antibody). Red, anti-TAU total antibody. B) Quantification of the fluorescence intensity of phospho-TAU related to total TAU total. Values correspond to the mean ± SEM of five samples per group. Differences among groups were assessed by two-way ANOVA followed by Bonferroni's test. *p < 0.05, compared with Nrf2+/+ mice injected with AAV-TAUP301L and vehicle. C) Immunohistochemical staining with anti-human TAU antibody of hippocampi from mice that were injected with AAV-TAUP301L in the right side. D) Quantification of the fluorescence intensity of phospho-CRMP2 related to CRMP2 total staining. Values correspond to the mean ± SEM of five samples per group. E) Double immunofluorescence staining of 30 µm-thick sections of hippocampus from Nrf2+/+ and Nrf2−/− mice injected with AAV-TAUP301L. Green, anti-phospho-CRMP2. Red, anti-CRMP2 total antibody. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
The phosphorylation status of CRMP2 has been associated with microtubule disassemble in Alzheimer's pathology. Therefore, we analyzed CRMP2 phosphorylation induced by TAUP301L (Fig. 4E) and the possible effect of DMF. TAUP301L did not significantly increase the levels of phospho-CRMP2 in agreement with lack of this modification in frontotemporal lobar degeneration associated with mutations in MAPT or with Pick bodies [19]. Moreover, DMF showed a slight tendency to reduce the levels of phosphorylated CRMP2 at the lesioned side (Fig. 4D-E). Taken together, our results show that DMF reduces GSK-3 activity in vivo as determined by a significant and subtle reduction in the phosphorylation levels of its two substrates TAU and CRMP2 respectively.
3.5. DMF protects from hippocampal neuronal damage in the AAV-TAUP301L mouse model
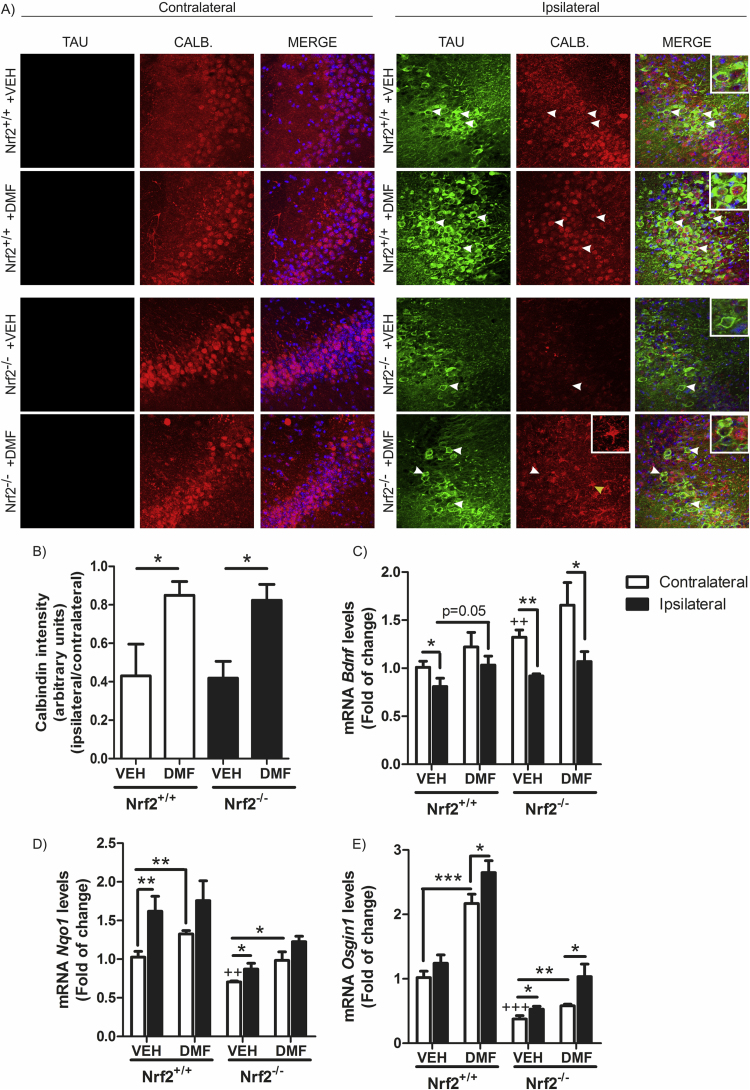
Twenty-one days of TAUP301L-expression did not induce noticeable hippocampal neuronal cell death as measured by Nissl-staining, FluoroJade or Bielschowsky-staining (data not shown). Therefore, we used an alternative and more sensitive method to analyze neuron damage based on calbindin-D28k expression, a major calcium-binding and buffering protein, that has a critical role in preventing neuronal death as well as maintaining calcium homeostasis and synaptic plasticity. Moreover, calbindin-D28K containing neurons do not accumulate neurofibrillary tangles [20]. Immunofluorescence analysis with anti- calbindin-D28K antibody demonstrated that TAUP301L-expressing neurons do not express calbindin-D28K, neither in Nrf2+/+ or Nrf2-/- mice (Fig. 5A-B), indicating that TAUP301L- participates in dysregulation of calcium metabolism. Very relevant, DMF protected against calbindin-D28K loss in TAUP301L-expressing neurons of Nrf2+/+ mice (Fig. 5A). While DMF also increased calbindin-D28K levels in the Nrf2-/- mice, this expression was not at the TAUP301L-expressing neurons but in cells with glial morphology (Fig. 5A-inset), consistent with increased gliosis (see below). These results suggest that TAU-injured neurons in NRF2-deficient mice are sensitized to degeneration or at least to loss functionality and that DMF rescue effect, is NRF2-dependent.
Fig. 5.
TAUP301Lexpression induces hippocampus degeneration which is prevented by DMF.A) Double immunofluorescence staining of 30 µm-thick sections of hippocampus from Nrf2+/+ and Nrf2−/− mice injected with AAV-TAUP301L. Green, anti-TAU. Red, anti-calbindin D-28K antibody. White arrows indicate TAU+/calbindin--neurons in Nrf2+/+ animals treated with vehicle (VEH), while in Nrf2+/+-DMF animals, arrows indicate TAU+/calbindin+-neurons. In Nrf2-/- animals, white arrows indicate TAU+/calbindin--neurons and the yellow arrow indicates a glial cell. B) Quantification of the fluorescence intensity related to calbindin staining. Values correspond to the mean ± SEM of five samples per group. Differences among groups were assessed by two-way ANOVA followed by Bonferroni's test. *p < 0.05 between compared groups. C, D and E) qRT-PCR determination of mRNA levels for Bdnf (C), Nqo1 (D) and Osgin1 (E) in the contralateral and ipsilateral sides of the hippocampus. Differences among groups were assessed by two-way ANOVA followed by Bonferroni's test. Asterisks denote significant differences with *p < 0.05, **p < 0.01, ***p < 0.001, comparing the indicated groups and ++p < 0.01, +++p < 0.001, compared to Nrf2+/+-VEH-contralateral. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
To further characterize the impact of calcium dysregulation, we analyzed several calcium-dependent nuclear effectors including Downstream Regulatory Element Antagonist Modulator (DREAM), Myogenic Transcription Factor-2 (MEF-2), Npas4, Cpg15 and brain-derived neurotrophic factor (BDNF), all of which participate in synaptic connectivity. Messenger RNA gene expression levels of Dream, Mef2c, Npas4 and Cpg15 did not show significant variations in the hippocampus from these mice (data not shown). In connection with tauopathy, it has been described that BDNF levels are reduced in TAUP301L transgenic mouse brains [21]. BDNF has a pivotal role in synaptic plasticity and neuronal survival, playing a key role in neuroprotection. We observed that mRNA levels of Bdnf were significantly reduced by TAUP301L-expression in Nrf2+/+ mice treated with vehicle (Fig. 5C), indicating that TAU induced alterations in calcium homeostasis, according to the results observed above with calbindin-D28K. Interestingly, Nrf2-/- mice showed a compensatory increase in the mRNA levels of Bdnf but TAUP301L-expression decreased significantly this effect. Nrf2+/+ mice treated with DMF recovered Bdnf levels at the ipsilateral side, while Nrf2-/- mice did not, indicating that NRF2 deficiency exacerbates neurodegenerative processes. Consistently with these results, DMF increased Nqo1 and Osgin-1 mRNA expression at the hippocampi of Nrf2+/+ and to a lesser extend in Nrf2−/− mice (Fig. 5D and F, respectively). Nqo1 and Osgin-1 mRNA levels were also increased by TAUP301L expression even in the absence of DMF, suggesting that TAUP301L expression induces antioxidant response. These results suggest that TAUP301L expression induces alterations in two crucial mediators of calcium homeostasis, calbindin-D28K and BDNF, and that DMF prevents these effects mainly through NRF2.
3.6. DMF attenuated TAUP301L -induced astrogliosis and microgliosis
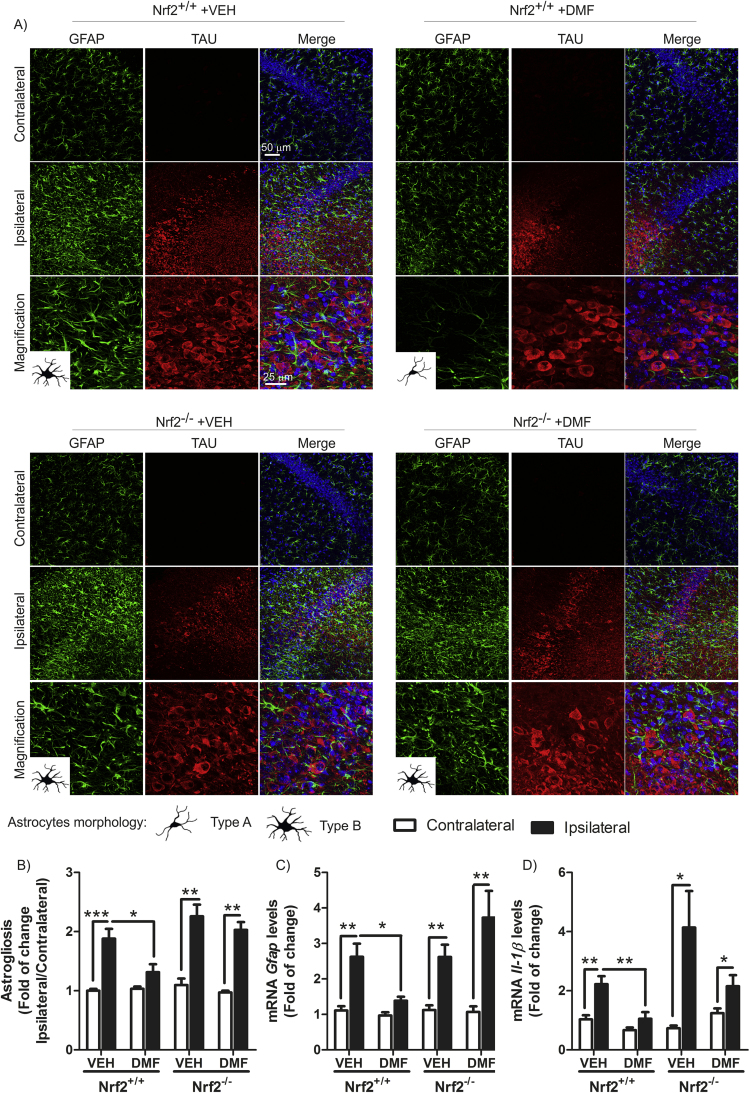
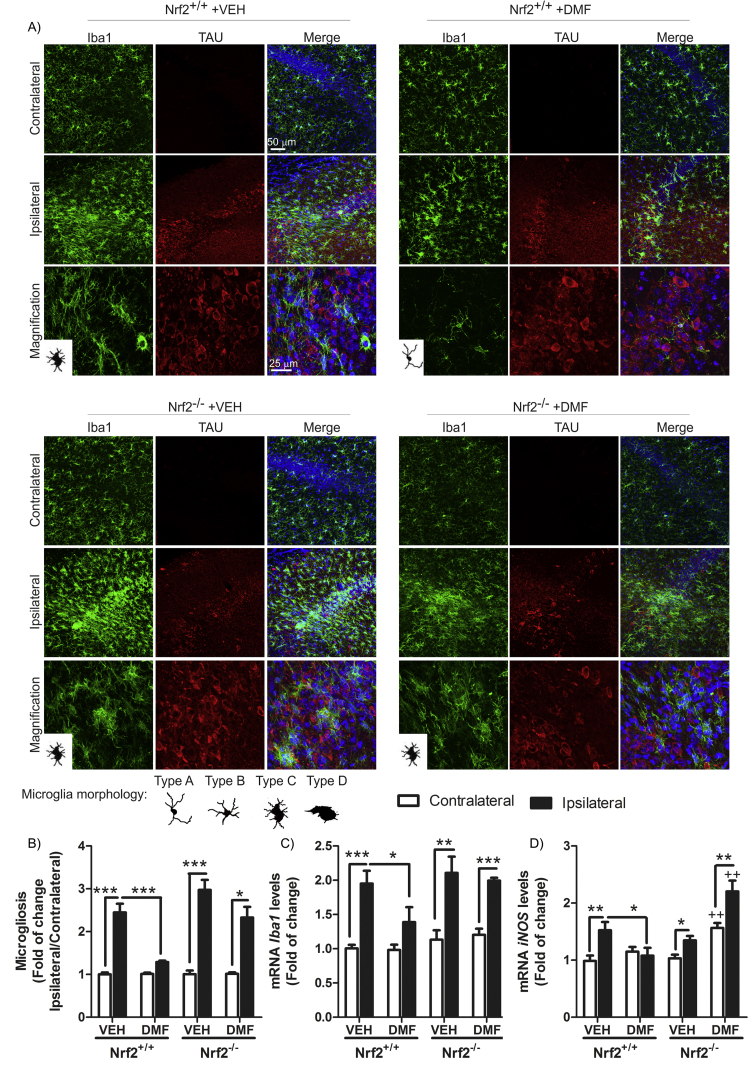
One of the main hallmarks of neurodegeneration is the presence of low-grade chronic inflammation that is characterized by microgliosis and astrogliosis, in this case in the hippocampus. To evaluate whether DMF could modulate the gliosis triggered by TAUP301L expression, we analyzed by immunofluorescence and qRT-PCR the expression levels of GFAP and IBA1, astrocytic and microglial markers, respectively. Regarding astrogliosis, TAUP301L toxicity correlated with a very significant increase in GFAP+ astrocytes at the ipsilateral hippocampal side of Nrf2+/+ and Nrf2−/− mice (Fig. 6A), which was confirmed by stereological quantification (Fig. 6B). This reactive astrogliosis was significantly reduced by the treatment with DMF in Nrf2+/+ but not in Nrf2−/− mice, indicating that the anti-inflammatory effect observed is NRF2-dependent (Fig. 6A, B and C). Astrocytes displayed enlarged bodies and ramifications (Type B morphology), consistent with a reactive state after TAUP301L expression in Nrf2+/+ and Nrf2−/− mice (Fig. 6A left panels). However, only astrocytes from Nrf2+/+ mice treated with DMF were maintained in a resting morphology (Type A) (Fig. 6A right panels). Concerning microglia, TAUP301L expression induced a very significant increase in IBA1+ microglia at the ipsilateral hippocampal side of Nrf2+/+ and Nrf2−/− mice (Fig. 7A), which was confirmed by stereological quantification (Fig. 7B). DMF treatment reduced significantly this microgliosis in Nrf2+/+ but not in Nrf2−/− mice, reinforcing the idea of NRF2-dependent anti-inflammatory effect (Fig. 7A, B and C). Regarding morphology, microglia can switch between a quiescent (type A), initiating microglial activation (type B), activated but non-phagocytic (type C) and a phagocytic state (type D) [22] (Fig. 7 microglia morphology). We observed that microglia morphology was between activated but non-phagocytosis and phagocytic state (Fig. 7A left panels) after TAUP301L expression in both genotypes, and DMF treatment showed a microglial morphology related to a resting state (Fig. 7A right panels) only in Nrf2+/+ mice, corroborating an anti-inflammatory effect of NRF2 activation by DMF. Messenger RNA analysis of two pro-inflammatory markers such as IL-1β and inducible nitric oxide synthase (iNOS) indicate that TAUP301L expression induce Il-1β (Fig. 6D) and iNOS (Fig. 7D) mRNA expression in both genotypes and DMF treatment decreased this expression only in Nrf2+/+ mice. Taken together, our results show that DMF has a beneficial therapeutic effect against tauopathy, modulating neurodegenerative and inflammatory processes.
Fig. 6.
DMF attenuates TAUP301L-induced astrogliosis. A) Photographs show the astrocyte marker GFAP in 30 µm-thick sections of hippocampus. Astrocyte morphology was observed as resting (type A) or reactive (type B). B) Graphs indicate astrocyte number as the ratio of ipsilateral/contralateral sides. qRT-PCR determination of mRNA levels for Gfap (C) and Il-1β (D) in the contralateral and ipsilateral sides of the hippocampus. Differences among groups were assessed by two-way ANOVA followed by Bonferroni's test. Asterisks denote significant differences *p < 0.05, **p < 0.01, ***p < 0.001 comparing the indicated groups.
Fig. 7.
DMF attenuates TAUP301L-induced microgliosis. A) Photographs show immunohistochemistry for the microglial marker IBA1 in 30 µm-thick sections of hippocampus. Microglial morphology was classified as resting (type A), initiating microglial activation (type B), activated but non-phagocytic (type C) and phagocytic (type D) according to Sanchez-Guajardo et al. [22]B) Graphs indicate microglial cell number as the ratio of ipsilateral/contralateral sides. qRT-PCR determination of mRNA levels for Iba1 (C) and iNOS (D) in the contralateral and ipsilateral sides of the hippocampus. Differences among groups were assessed by two-way ANOVA followed by Bonferroni's test. Asterisks denote significant differences *p < 0.05, **p < 0.01, ***p < 0.001 comparing the indicated groups.
4. Discussion
In the last few years, NRF2 has been suggested to be a novel target to slow down the progression of neurodegenerative disorders because it modulates main hallmarks of these diseases like proteinopathy, oxidative stress and chronic inflammation. Indeed, several drugs have been used for proof-of-concept studies indicating that activation of NRF2 may provide a benefit against Parkinson's disease [11], [23] and amyloidopathy of Alzheimer's disease [24], [25], [26], [27], but there is little evidence about the role of NRF2 in tauopathies [16].
NRF2 is ubiquitously expressed in the central nervous system even at low levels, but here we demonstrate that DMF modulates several mechanisms of “brain protection”, essential in tauopathy. Related to the mechanisms that activate NRF2 signature after DMF treatment, our results demonstrate that part of the effects are KEAP1-dependent. This protein contains several cysteine residues that are capable of undergoing redox modifications and adduct formation with electrophilic compounds. Therefore, as expected, NRF2 levels could be modulated by DMF in part through this pathway. But NRF2 signaling can be also activated by other pathways, such as PI3K/AKT/GSK-3β. Our group has reported that GSK-3 phosphorylates critical residues of NRF2 to create a motif that is recognized by the E3 ligase adapter β-TrCP and therefore targets NRF2 for the ubiquiting-proteasome degradation. This proteolytic mechanism is complementary of the KEAP1 system but while KEAP1/NRF2 is a redox sensor, GSK-3/β-TrCP/NRF2 is a cell signaling sensor. The finding that DMF inhibits both proteolytic pathways provides two layers of up-regulation of NRF2 and may make this compound very useful for therapy of neurodegenerative diseases of the elderly, where either KEAP1 of GSK-3 activities are altered.
In this study we demonstrate for the first time that pharmacological treatment with DMF, by disrupting the KEAP1/NRF2 interaction and through the GSK-3β/NRF2 signaling pathway (Fig. 3A), provides a double mechanism of activation of NRF2 that might be used to treat TAU-related neurodegeneration. In our mouse model, where AAV-hTAUP301L is injected in the right hippocampus, TAU became hyperphosphorylated in Nrf2+/+ neurons and this effect was exacerbated in Nrf2−/− mice (Fig. 4) while DMF reduced this phosphorylation through GSK-3β deactivation in both genotypes. GSK-3 is a protein kinase that is abundant in the central nervous system and is one of the main kinases involved in the phosphorylation of TAU, a process that is crucial to the function of the protein [28]. The normal phosphorylation of TAU determines its affinity for microtubule binding, therefore pathological hyperphosphorylation induces the dissociation of TAU from microtubules and following aggregation to form neurofibrillary tangles (NFTs). Modulation of GSK-3β activity has been proposed as a target for therapeutic intervention [3], but therapeutic benefits have not yet been observed. This suggests that the simple modulation of GSK-3 is not sufficient and it is also necessary to influence other aspects of the pathology such as inflammation to obtain a therapeutic benefit. Therefore, the double effect of DMF as an antioxidant modulator of neuroinflammation acting on NRF2 [11], [16], [29], [30], [31] as well as its role in GSK-3 inhibition provides a novel therapeutic approach that should be more efficient than a GSK-3 inhibitor alone.
Although the AAV-hTAUP301L mouse model only resembles early stages of tauopathy without clear evidence of neuronal cell death, we observed changes in the expression levels of calbindin-D28K (Fig. 5A-B), which indicates a disturbance of calcium homeostasis, consistent with incipient neurodegeneration [32]. There is an inverse correlation between hTAUP301L and calbindin-D28K expression in Nrf2+/+ mice, which indicates that in hTAU+ neurons the calcium buffering capacity is impaired and most likely impacts on synaptic Ca2+ dynamics and plasticity [33]. On the other hand, our results show that DMF rescued TAU+ neurons of the Nrf2+/+ mice from calbindin-D28K depletion, therefore potentially recovering synaptic physiology. By contrast, in Nrf2−/− mice, DMF could not restore calbindin-D28K levels. Moreover, we observed an increased expression of calbindin-D28K in glial-shape cells, because DMF could not alleviate astro- and microgliosis. This observation is consistent with the fact that astrocytes also express calbindin-D28K in response to various central nervous system insults [34].
Related to Ca2+ signaling and synaptic activity, increased intracellular calcium induces the expression of BDNF [35]. In fact, BDNF slows down cognitive decline in the elderly, especially in advanced AD [21]. We observed that in Nrf2+/+ mice, TAUP301L expression decreased Bdnf mRNA expression in concordance with impaired Ca2+ signaling and synaptic activity and that DMF restored Bdnf levels. Furthermore, Nrf2−/− mice showed increased basal Bdnf expression in comparison to Nrf2+/+ mice and TAUP301L expression induced a drastic down-regulation which was not prevent by DMF treatment. These results suggest again that calcium homeostasis, which leads to changes in BDNF levels, is deregulated by TAUP301L expression, but can be restored by DMF through targeting of NRF2.
Another hallmark of tauopathies is low grade chronic-inflammation, which is characterized by astrogliosis and microgliosis. In this context, it has been largely reported that NRF2 restrains inflammation in several neurodegenerative models [11], [23], [36] in part by modulating NF-κB signaling [29], [30]. Our results demonstrate that DMF attenuates astrogliosis (Fig. 6) and microgliosis (Fig. 7) and this effect is NRF2-dependent, as DMF had no effect on Nrf2−/− mice. These results were corroborated by stereological quantification and analysis of mRNA levels of GFAP and IBA1. Interestingly, it has been suggested that the anti-inflammatory activity of DMF in patients with multiple sclerosis may occur through alternative pathways independent of NRF2 [37]. Therefore, it is possible that DMF could elicit anti-inflammatory effects through several mechanisms depending on the disease.
Related to neuroinflammation, the pro-inflammatory cytokine IL-1β is a key factor in several tauopathies [38]. Microglial activation and increased expression of IL-1β has been reported in PSP and FTLD-TAU patients, indicating a link between neuroinflammation and pathology. Moreover, exposure of primary neurons to IL-1β exacerbates TAU phosphorylation through aberrant activation of p38–MAPK [39]. In P301S transgenic mouse models, IL-1β or elevated inflammatory responses in the brain increase neuronal TAU phosphorylation and tangle formation [40], [41]. Our results show a correlation between TAUP301L expression, TAU hyperphosphorylation and increased IL-1β expression, and at the same time, we find that DMF reduces TAU phosphorylation and IL-1β expression down to control levels, reinforcing the idea that NRF2 targeting modulates neuroinflammation.
This study is the first to demonstrate the beneficial effects of DMF in a preclinical model of tauopathy, improving the outcome of the main disease-associated hallmarks. The fact that DMF decreased TAU phosphorylation, improved calcium homeostasis and BDNF expression and restrained neuroinflamation, astrogliosis and microgliosis positions DMF as a promising therapeutic strategy for tauopathies.
5. Conclusions
Dimethyl fumarate is able to modulate different signaling pathways to provide protection against tauopathies. Our study validates the repurposing of dimethyl fumarate (Tecfidera) to target GSK-3/NRF2 axis in the brain for treating tauopathies to start clinical trials.
Acknowledgements
The authors thank Rut González and Ángel Juan García-Yagüe for their technical support.
Acknowledgments
Ethics approval
All experiments were performed by certified researchers according to regional, national, and European regulations concerning animal welfare and animal experimentation, and were authorized by the Ethics Committee for Research of the Universidad Autónoma de Madrid and the Comunidad Autónoma de Madrid, Spain, with Ref PROEX 279/14, following institutional, Spanish and European guidelines (Boletín Oficial del Estado (BOE) of 18 March 1988 and 86/609/EEC, 2003/65/EC European Council Directives).
Consent for publication
Not applicable.
Availability of supporting data
Not applicable.
Competing interests
All authors declare no competing interests.
Funding
This work was supported by a Spanish Ministerio de Ciencia e Innovacion Grant SAF2016-76520-R.
Author contributions
ILB and AC contributed to conception and design of the study. SK, generation of AAV6-TAU(P301L) viruses. ILB acquisition and analysis of data. ILB and AC contributed to drafting a significant portion of the manuscript and figures.
Authors' information
Not applicable.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.10.010.
Contributor Information
Antonio Cuadrado, Email: antonio.cuadrado@uam.es.
Sebastian Kügler, Email: sebastian.kuegler@med.uni-goettingen.de.
Isabel Lastres-Becker, Email: ilbecker@iib.uam.
Appendix A. Supplementary material
Fig. S1.
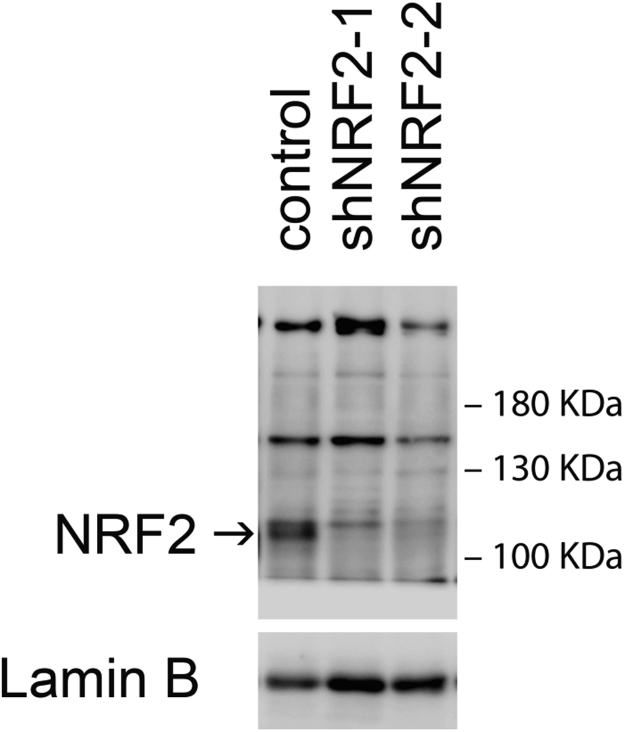
Specificity of NRF2 homemade antibody. U-373 MG human glioblastoma cells interfered with two different shNRF2 lentivirus for three days.
References
- 1.Lee G., Leugers C.J. Tau and tauopathies. Progress. Mol. Biol. Transl. Sci. 2012;107:263–293. doi: 10.1016/B978-0-12-385883-2.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karakaya T., Fusser F., Prvulovic D., Hampel H. Treatment options for tauopathies. Curr. Treat. Options Neurol. 2012;14(2):126–136. doi: 10.1007/s11940-012-0168-7. [DOI] [PubMed] [Google Scholar]
- 3.Medina M., Garrido J.J., Wandosell F.G. Modulation of GSK-3 as a therapeutic strategy on tau pathologies. Front. Mol. Neurosci. 2011;4:24. doi: 10.3389/fnmol.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojo A.I., Sagarra M.R., Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J. Neurochem. 2008;105(1):192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 5.Rushmore T.H., Morton M.R., Pickett C.B. The antioxidant responsive element. activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266(18):11632–11639. [PubMed] [Google Scholar]
- 6.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sanchez-Perez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rada P., Rojo A.I., Chowdhry S., McMahon M., Hayes J.D., Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011;31(6):1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojo A.I., Rada P., Egea J., Rosa A.O., Lopez M.G., Cuadrado A. Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol. Cell. Neurosci. 2008;39(1):125–132. doi: 10.1016/j.mcn.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281(21):14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 11.Lastres-Becker I., Garcia-Yague A.J., Scannevin R.H., Casarejos M.J., Kugler S., Rabano A., Cuadrado A. Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson's disease. Antioxid. Redox Signal. 2016;25(2):61–77. doi: 10.1089/ars.2015.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bomprezzi R. Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther. Adv. Neurol. Disord. 2015;8(1):20–30. doi: 10.1177/1756285614564152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau A., Tian W., Whitman S.A., Zhang D.D. The predicted molecular weight of Nrf2: it is what it is not. Antioxid. Redox Signal. 2013;18(1):91–93. doi: 10.1089/ars.2012.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCubrey J.A., Lahair M.M., Franklin R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006;8(9–10):1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 15.Hur E.M., Zhou F.Q. GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010;11(8):539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lastres-Becker I., Innamorato N.G., Jaworski T., Rabano A., Kugler S., Van Leuven F., Cuadrado A. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain: a J. Neurol. 2014;137(Pt 1):78–91. doi: 10.1093/brain/awt323. [DOI] [PubMed] [Google Scholar]
- 17.Jo C., Gundemir S., Pritchard S., Jin Y.N., Rahman I., Johnson G.V. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014;5:3496. doi: 10.1038/ncomms4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pajares M., Jimenez-Moreno N., Garcia-Yague A.J., Escoll M., de Ceballos M.L., Van Leuven F., Rabano A., Yamamoto M., Rojo A.I., Cuadrado A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy. 2016;12(10):1902–1916. doi: 10.1080/15548627.2016.1208889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson R., van Aalten L., Mann D.M., Platt B., Plattner F., Bedford L., Mayer J., Howlett D., Usardi A., Sutherland C. CRMP2 hyperphosphorylation is characteristic of Alzheimer's disease and not a feature common to other neurodegenerative diseases. J. Alzheimer's Dis. 2011;27:615–625. doi: 10.3233/JAD-2011-110617. [DOI] [PubMed] [Google Scholar]
- 20.Iritani S., Niizato K., Emson P.C. Relationship of calbindin D28K-immunoreactive cells and neuropathological changes in the hippocampal formation of Alzheimer's disease. Neuropathol.: Off. J. Jpn. Soc. Neuropathol. 2001;21(3):162–167. doi: 10.1046/j.1440-1789.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- 21.Jiao S.S., Shen L.L., Zhu C., Bu X.L., Liu Y.H., Liu C.H., Yao X.Q., Zhang L.L., Zhou H.D., Walker D.G. Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer's disease. Transl. Psychiatry. 2016;6(10):e907. doi: 10.1038/tp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Guajardo V., Febbraro F., Kirik D., Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson's disease. PLoS One. 2010;5(1):e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojo A.I., Innamorato N.G., Martin-Moreno A.M., De Ceballos M.L., Yamamoto M., Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58(5):588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- 24.An Y.W., Jhang K.A., Woo S.Y., Kang J.L., Chong Y.H. Sulforaphane exerts its anti-inflammatory effect against amyloid-beta peptide via STAT-1 dephosphorylation and activation of Nrf2/HO-1 cascade in human THP-1 macrophages. Neurobiol. Aging. 2016;38:1–10. doi: 10.1016/j.neurobiolaging.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Kanninen K., Malm T.M., Jyrkkanen H.K., Goldsteins G., Keksa-Goldsteine V., Tanila H., Yamamoto M., Yla-Herttuala S., Levonen A.L., Koistinaho J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol. Cell. Neurosci. 2008;39(3):302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Wruck C.J., Gotz M.E., Herdegen T., Varoga D., Brandenburg L.O., Pufe T. Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Mol. Pharmacol. 2008;73(6):1785–1795. doi: 10.1124/mol.107.042499. [DOI] [PubMed] [Google Scholar]
- 27.von Otter M., Landgren S., Nilsson S., Zetterberg M., Celojevic D., Bergstrom P., Minthon L., Bogdanovic N., Andreasen N., Gustafson D.R. Nrf2-encoding NFE2L2 haplotypes influence disease progression but not risk in Alzheimer's disease and age-related cataract. Mech. Ageing Dev. 2010;131(2):105–110. doi: 10.1016/j.mad.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Lei P., Ayton S., Bush A.I., Adlard P.A. GSK-3 in neurodegenerative diseases. Int. J. Alzheimer's. Dis. 2011;2011:189246. doi: 10.4061/2011/189246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuadrado A., Martin-Moldes Z., Ye J., Lastres-Becker I. Transcription factors NRF2 and NF-kappaB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014;289(22):15244–15258. doi: 10.1074/jbc.M113.540633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Innamorato N.G., Lastres-Becker I., Cuadrado A. Role of microglial redox balance in modulation of neuroinflammation. Curr. Opin. Neurol. 2009;22(3):308–314. doi: 10.1097/WCO.0b013e32832a3225. [DOI] [PubMed] [Google Scholar]
- 31.Lastres-Becker I., Ulusoy A., Innamorato N.G., Sahin G., Rabano A., Kirik D., Cuadrado A. Alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Human. Mol. Genet. 2012;21(14):3173–3192. doi: 10.1093/hmg/dds143. [DOI] [PubMed] [Google Scholar]
- 32.Heizmann C.W., Braun K. Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992;15(7):259–264. doi: 10.1016/0166-2236(92)90067-i. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt H. Three functional facets of calbindin D-28k. Front. Mol. Neurosci. 2012;5:25. doi: 10.3389/fnmol.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yenari M.A., Minami M., Sun G.H., Meier T.J., Kunis D.M., McLaughlin J.R., Ho D.Y., Sapolsky R.M., Steinberg G.K. Calbindin d28k overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke. 2001;32(4):1028–1035. doi: 10.1161/01.str.32.4.1028. [DOI] [PubMed] [Google Scholar]
- 35.Finkbeiner S. Calcium regulation of the brain-derived neurotrophic factor gene. Cell. Mol. Life Sci. 2000;57(3):394–401. doi: 10.1007/PL00000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jazwa A., Rojo A.I., Innamorato N.G., Hesse M., Fernandez-Ruiz J., Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 2011;14(12):2347–2360. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- 37.Schulze-Topphoff U., Varrin-Doyer M., Pekarek K., Spencer C.M., Shetty A., Sagan S.A., Cree B.A., Sobel R.A., Wipke B.T., Steinman L. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc. Natl. Acad. Sci. USA. 2016;113(17):4777–4782. doi: 10.1073/pnas.1603907113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López González I., Garcia-Esparcia P., Llorens F., Ferrer I. Genetic and transcriptomic profiles of inflammation in neurodegenerative diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and tauopathies. Int. J. Mol. Sci. 2016;17(2):206. doi: 10.3390/ijms17020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Liu L., Barger S.W., Griffin W.S.T. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci.: Off. J. Soc. Neurosci. 2003;23(5):1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng J.G., Zhu S.G., Jones R.A., Griffin W.S.T., Mrak R.E. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp. Neurol. 2000;163(2) doi: 10.1006/exnr.2000.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshiyama Y., Higuchi M., Zhang B., Huang S.M., Iwata N., Saido T.C., Maeda J., Suhara T., Trojanowski J.Q., Lee V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]