Abstract
Off target toxicities is one of the hallmarks of conventional chemotherapy as only a tiny percentage of the injected dose actually reaches the tumor(s). Numerous strategies have been employed in attempts to achieve targeted therapeutic delivery to tumors. One strategy that has received immense attention has been the packaging of these chemotherapeutics into nanoparticles and relying on the enhanced permeation and retention (EPR) effect for targeting. However, few, if any, nanoformulations have been used clinically that actually show enhanced drug delivery to tumors. There are a number of biological barriers to successful targeted delivery and nanoparticles large enough to theoretically benefit from the EPR effect predominantly accumulate in the liver and spleen after systemic administration. Nanoparticles that do reach the tumor will experience challenges such as difficulty penetrating deeply into tumors and rapid uptake by macrophages rather than tumor cells. In order to overcome this, researchers are investigating a new drug delivery system by utilizing T-cells, macrophages, or stem cells (Mesenchymal/Neural Stem Cells) and loading them with therapeutic nanoparticles for targeted delivery due to either their organotropic or tumor tropic migratory capabilities. By exploiting the migration and motility of these particular cells, researchers have delivered drug-loaded nanoparticles as well as nanoparticles for use in thermal ablation and magnetic field treatments, with the goals of decreasing off-target toxicities and increasing intratumoral distribution of the therapeutic payload. This is an inherently complex drug delivery system that requires optimization of multiple parameters – including cell type, payload, cell loading, release rate from nanoparticle and more – for success. Here we review recent advances and upcoming challenges for the field.
Keywords: Neural Stem Cells, macrophages, T-cells, Mesenchymal Stem Cells, cell carrier
1. Introduction
The administration of chemotherapy drugs generally results in a small fraction of the administered dose acting at the tumor site, which results in significant off target toxicities. Targeted drug delivery offers the promise of increasing the fraction of drug delivered to the tumor. Such delivery must overcome a number of biological hurdles to arrive at the tumor site and then penetrate the physiological barriers present at the tumor microenvironment [1]. Nanoparticles have long been touted as potential delivery vehicles that could be used for targeted chemotherapy delivery. For systemic administration, this strategy primarily depends on taking advantage of the leaky vasculature seen in tumors due to the enhanced permeability and retention (EPR) effect, however, the clinical utility of this targeting approach appears to be limited. Moreover, even for nanoparticles that reach the tumor, penetration and distribution of the payload throughout the tumor can be challenging [2]. The interstitial fluid pressure at a tumor inhibits nanoparticles from penetrating and extravasating to distal sites within the tumor. The intratumoral pressures are caused by disrupted vasculature and impaired lymphatic drainage [3, 4]. Generally, a small percentage of nanoparticles accumulate in tumor cells closest to the blood vessel, however, the majority are readily internalized by tumor associated macrophages making it extremely difficult to penetrate the tumor [3]. Thus, while nanoparticles have been prepared with a wide variety of drug cargoes and have shown the ability to control the release of these payloads, targeted delivery with just nanoparticles remains challenging.
On the other hand, a number of cell types have been shown to be organotropic or tumor tropic and are being investigated due to their migration abilities, whether they be immune cells that scavenge the body for pathogens or stem cells that home in on inflammatory tissues/tumors.[5, 6] These cells are thought to be beneficial in penetrating the physiological barriers that nanoparticles struggle to pass through. A number of researchers are now trying to leverage these properties by using these cells as carriers for various payloads, which include drug-loaded nanoparticles or relatively inert particles that can be activated for thermal ablation. In order to develop cell carriers to maximize therapeutic potential, a few criteria must first be met. 1) Cell carriers must have tropic abilities that lead to accumulation at tumor sites 2) Cell carriers must be resistant to payload for a certain amount of time in order to allow cells to migrate to tumor sites. 3) Cell carriers must carry an efficacious amount of payload to the tumor site. In this review we will go over various different cell types that have been used as cell carriers for drug-loaded nanoparticles.
2. Nanoparticles used in drug delivery
Nanoparticles are a class of materials in which at least one dimension resides in the nanometer range. Many biological structures, such as proteins, viruses, and bacteria exist with precise shapes, sizes (nanometer size range), and chemistries. Much like these biological structures, nanoparticles can be precisely engineered to be a certain size, geometry, and surface charge in order to mimic their biological counterparts. Some of the unique properties nanoparticles have, which are not typically found in their bulk form, include high surface-to-volume ratio and thermal, electrical, magnetic, and optical characteristics [7, 8]. The specific properties of these biological structures and engineered nanoparticles allow them to interact and function in the body. Due to this, nanoparticles have emerged as a promising technology used in drug delivery.
Some of the most common nanoparticle structures used in drug delivery applications include liposomes, micelles, polymeric nanoparticles and silica nanoparticles.[9, 10] Here, we briefly discuss each of these materials and higlight the most advanced examples representative of each particle type.
Liposomes
Liposomes are nanoparticles that are composed of amphiphilic molecules such as cholesterol or phospholipids. These molecules are non-toxic, non-immunogenic, and biodegradable. Their amphiphilic nature (polar and non-polar moieties) allows the molecules to form lipid layers that self-assemble into closed vesicles (unilamellar or multilamellar), allowing drugs to be encapsulated within.[11] Liposomes can be functionalized with poly(ethylene glycol) (PEG) to prolong circulation in the blood stream, avoiding uptake by macrophages.[12, 13] Doxil and DaunoXome are two commercially available liposomal drug formulations approved by the FDA for cancer therapy. Doxil was the first FDA approved PEGylated biodegradable liposome developed to encapsulate doxorubicin for the treatment of solid tumors. DaunoXome uses a liposomal formula for daunorubicin delivery.[9, 11, 13]
Micelles
Micelles are also composed of amphiphilic molecules, such as diblock and triblock copolymers (PEG-poly(L-aspartate), PEG-poly(L-glutamate), PLGA-PPO-PLGA, PEG-PPO- PEG, etc.). They self-assemble into nanostructures consisting of a hydrophobic core and hydrophilic exterior shell when the amphiphilic polymer has exceeded the critical micelle concentration (CMC).[14] They are able to encapsulate poorly water soluble drugs, due to the hydrophobic core of the micelle. Many of the diblock and triblock copolymers used are biodegradable and biocompatible, allowing for full renal clearance of the micelles over time. The hydrophilic PEG prevents opsonization of serum proteins, prolonging circulation of micelles in the body.[10, 12, 15] Current micellar drug delivery vehicles in phase III clinical trials include Genexol-PM (PEG-poly(D,L-lactide) and NK105 (PEG-poly(aspartate) for the treatment of breast cancer.[16–18]
Polymeric Nanoparticles
Polymeric Nanoparticles can be composed of single polymer chains such as polyesters (PGA, PLA, PLGA), PLGA copolymers (PLGA-PEG), polycaprolactones, chitosan, polyamides, hyaluronic acid, or dextran.[19] PLGA is the most widely used biodegradable polymer in formulating nanoparticles. PLGA is FDA approved for use in humans since it can undergo hydrolysis in the body to form lactic and glycolic acid in the body, both which are non-toxic. PLGA particles have been engineered by different methods, including oil-in-water emulsion methods and nanoprecipitation using an anti-solvent.[20, 21] Eligard is an FDA approved PLGA nanoparticle used for the delivery of leuprolide for the treatment prostate cancer.[22]
Silica Nanoparticles
Silica nanoparticles are being explored in drug delivery due to their biocompatibility (generally regarded as safe by FDA) and scalability. They are synthesized from silica precursors such as tetraethyl orthosilicate (TEOS). The size and porosity of silica nanoparticles can be tuned as well. The Stöber method involves using a combination of water, alcohol, TEOS, and ammonia to produce nonporous silica nanoparticles.[23, 24] Mesoporous silica nanoparticles are can be created by using the Stober method plus added surfactant such as cetyl trimethylammonium bromide (CTAB). CTAB removal by washing of the silica nanoparticles generates pores. Porosity can be tuned depending on the concentration and type of surfactant used. Surface modifications can be easily made as well for PEGylation or drug loading.[25] There are many silica nanoparticles currently in preclinical studies.[26]
Nanoparticles for Delayed Release
Nanoparticles can be used to package hydrophobic drugs without the use of toxic excipients and can be engineered to have a delayed drug release over time [27]. For instance, polymeric nanoparticles made of polyesters, polyamides, or polysaccharides undergo either hydrolytic of enzymatic degradation and are able to release their payload in a controlled manner [27]. Nanoparticles can also be synthesized with pH-sensitive linkers, such as hydrazones, that degrade in the endosomal compartment of cells or acidic tumor environment [27, 28]. Due to the varied composition, ease in tunability, and control over drug release kinetics, drug-loaded nanoparticles are being investigated for use in the treatment of various tumors in a passive manner. However, relying on the passive delivery of nanoparticles is not without its challenges.
3. Challenges in targeting nanoparticles to tumors
The study of nanoparticles as drug delivery vehicles has generally been motivated by targeting tumors with poorly formed and leaky vasculature. In 1986, Matsumura and Maeda described a phenomenon later termed the enhanced permeability and retention effect (EPR effect) in tumors [29]. The EPR effect describes the increased accumulation of particulates in the tumor due to a combination of fenestrations in the vasculature and poor lymphatic drainage from tumors. This EPR effect is considered the primary mechanism for the passive accumulation of nanoparticles in tumors in vivo [30]. The fenestrations in the blood vessels act as a sieve leading to nanoparticles being trapped in the perivascular space. Additionally, the impaired lymphatic drainage prevents the tumor from efficiently clearing the nanoparticles allowing nanoparticles to accumulate at the tumor in greater quantities than in healthy tissues. Many of the systems being developed today in the laboratory for tumor targeting rely exclusively on this passive accumulation of nanoparticles in the tumor. While laboratory development of nanomedicines depends on this EPR effect in rodents, the prevalence of this phenomenon in human tumors is unclear with clinical evidence suggesting that the extent of EPR depends highly on the tumor type [3, 31]. Moreover, this passive accumulation also results in accumulation in the liver, due in part to the similar fenestrations in the blood vessels that are a part of the normal physiology of the liver.
The ease of engineering nanoparticles to be of a certain material, size, shape, charge, and geometry has contributed to the popular notion that nanoparticles can be specifically designed to deliver therapeutics efficiently to the tumor site. However, despite careful control of the physical and chemical properties of nanoparticles and careful control over their surface coating, nanoparticles rarely have higher than 10% injected dose in the tumor with many reports of less than 5% injected dose [32]. This accumulation at the tumor is likely to overestimate the actual amount of nanoparticle that interacts with a cancer cell. In the tumor environment, nanoparticles could accumulate adjacent to the blood vessel or be phagocytosed by macrophages associated with the tumor [33, 34].
While 10% of the injected dose at the tumor may be higher than free drug, the majority of nanoparticles injected, regardless of coating, accumulate in the liver. Using current strategies, it may be possible to delay liver accumulation, but the final biodistribution of nanoparticles is predominantly liver accumulation. In general, liver clearance is highly efficient for many materials in the body and escaping this clearance is restricted to a small minority of materials (e.g. red blood cells) through a tightly regulated process. It is likely that to achieve a dramatic improvement in targeting efficiency to tumors, alternate strategies will have to be developed, such as local administration of nanoparticles, or the cell-based delivery discussed in this review.
4. T-Cells
4.1 Naïve T-cells
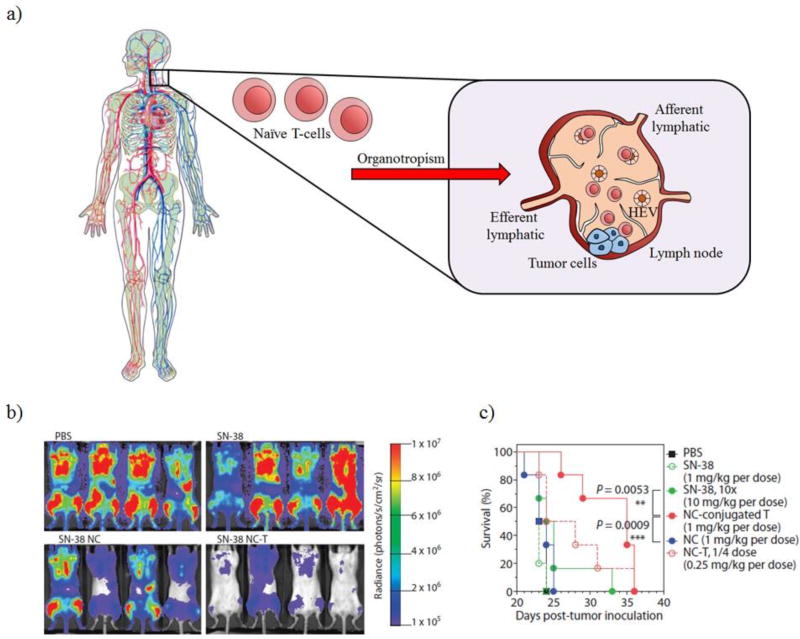
Chemotherapeutics have difficulty reaching certain tissue compartments, such as lymph nodes in lymphoma [35]. After leaving the thymus, naïve T-cells or lymphocytes that have not been activated by antigen-presenting cells (APCs) have the ability to circulate throughout the body migrating and localizing in secondary lymphoid organs, such as the lymph nodes (organotropic) as seen in Figure 1a. Naïve T-cells can enter the lymph nodes within high endothelial venules (HEV) or through the afferent lymphatic vessel. They exit the lymph nodes via the efferent lymphatic vessel [36]. T-cells that have been stimulated by APCs tend to migrate to peripheral tissues that express the antigenic epitope of interest [1, 37]. Due to their organotropic properties, naïve T-cells can be potentially used as cell carriers to treat tumors residing within the lymph nodes. Also, naïve T-cells can be easily isolated in large quantities since they do not recognize any tumor specific antigens. Huang et al. demonstrated that T-cells could be loaded with lipid nanocapsules containing SN-38 (active form of irinotecan) [38]. These lipid nanoparticles were functionalized with maleimides in order to bind to the free thiols present on the surface of the T- cells. Mice treated intravenously with T-cells loaded with SN-38 lipid nanocapsules (1mg/kg) experienced the greatest reduction in tumor burden compared to the free drug alone and the SN- 38 lipid nanoparticles after 16 days as seen in Figure 1b. The survival benefit of this therapy was also evaluated. Mice treated with T-cells loaded with SN-38 lipid nanocapsules experienced a median survival of 35 days, whereas mice treated with SN-38 (1 mg/kg and 10 mg/kg) or SN-38 lipid nanocapsules (1 mg/kg) had a median survival of only 24 days as seen in Figure 1c [38]. This study suggests that the organotropism of T-cells can be used to deliver therapeutic agents, such as SN-38, in order to increase efficacy and slow tumor growth.
Figure 1.
Naïve T-cells can be used as cell carriers due to their organotropism. a) Naïve T-cells circulate throughout the body and are able to home to lymph nodes. b) Bioluminescence imaging of mice treated after 16 days. Naïve T-cells loaded with SN-38 lipid nanocapsules reduced tumor burden. c) Overall survival of mice treated with PBS, SN-38, SN-38 NC, and SN-38 NC conjugated to T-cells.
4.2 Antigen-specific T-cells as carriers
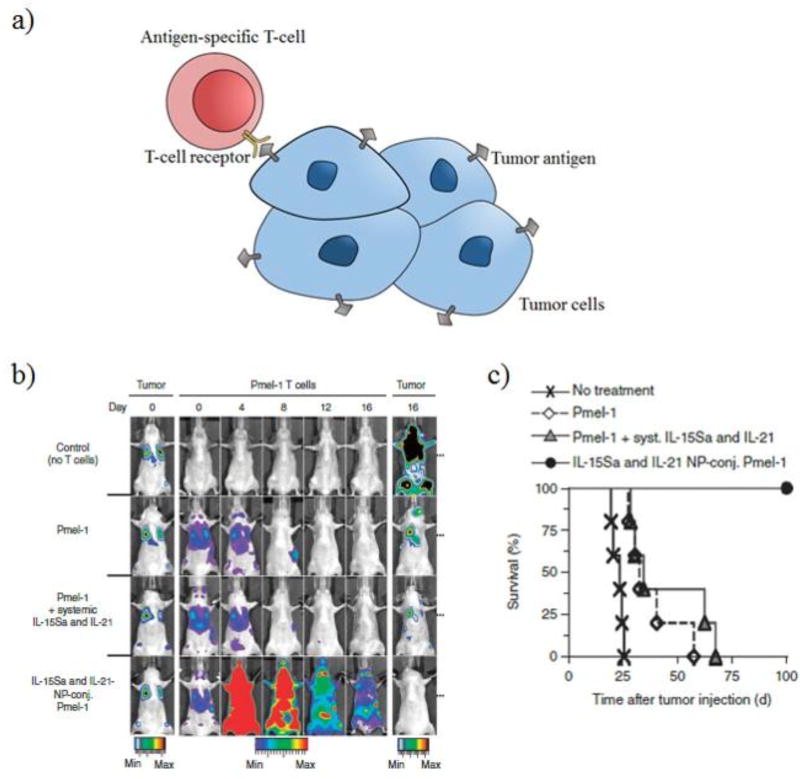
In order to target other cancer types, adoptive T-cell therapy has been explored in which T-cells are primed against a specific tumor antigen. These circulating, activated T-cells have surface receptors that are able to recognize and bind to the antigenic epitope expressed on tumor cells leading to tumor localization as seen in Figure 2a. Stephan et al. demonstrated that liposomal nanoparticles containing adjuvants, such as cytokines (IL-15 and IL-21), could be tethered to the surface of melanoma-specific Pmel-1 effector T-cells using a thiol reactive maleimide group to treat melanoma lung/bone marrow tumors [39]. The release of cytokines was able to stimulate donor T-cells. Irradiation followed by intravenous treatment of Pmel-1 effector T-cells loaded with IL-15 and IL-21 lipid nanoparticles promoted T cell expansion in vivo and was able to irradicate B16F10 melanoma tumors in mice as seen in Figure 2b. A subcutaneous model using B16F10 (flank tumor) was also evaluated. Mice intravenously injected with unmodified Pmel-1 T-cells experienced a median survival of 30 days. Pmel-1 T-cells loaded with cytokine nanoparticles was able to significantly extend survival (100 days) as seen in Figure 2c [39]. Activated T-cells, whether they be tumor infiltrating T- cells or chimeric antigen receptor T-cells, have the potential to be cell carriers for nanoparticle payloads containing either adjuvants that stimulate the donor T-cells to eliminate tumor burden or potentially chemotherapeutics.
Figure 2.
Primed T-cells can be used as cell carriers due to their recognition of tumor antigens. a) T-cells can be primed against antigens present in tumors and expanded ex-vivo. These mature T-cells are able to home to tumor sites and bind to tumor antigens. b) Bioluminescence imaging of mice with B16F10 melanoma over a period of 16 days. c) Overall survival of mice treated with PBS, Pmel-1 T-cells, Pme1-1 Tcells with systemic injection of IL-15Sa and IL-21, and IL-15Sa and IL-21 nanoparticle conjugated to Pmel-1 T-cells.
5. Macrophages and Neutrophils
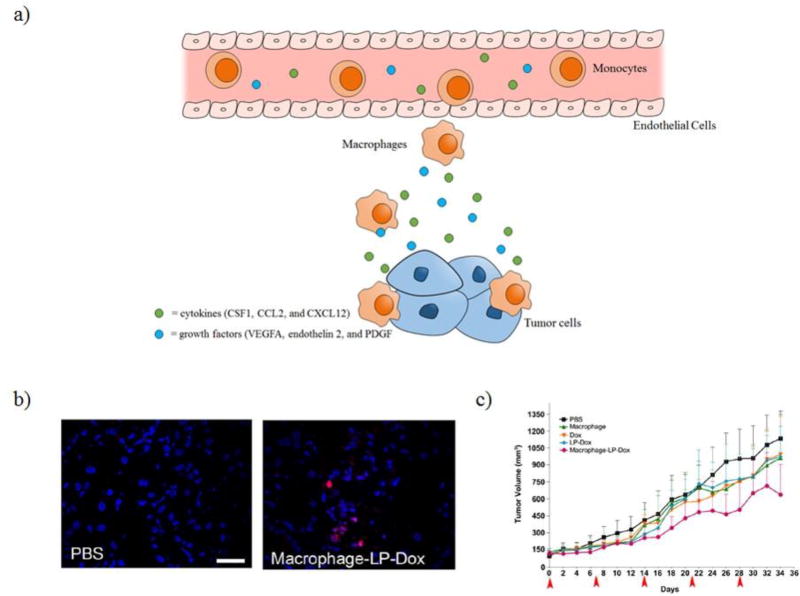
During tumor development, monocytes are recruited to the tumor. This is caused by a chemoattractant gradient consisting of inflammatory cytokines, CSF1, CCL2, CXCL12, and VEGFA, that are secreted by the tumor [40]. Once monocytes have crossed the endothelial basement membrane, they differentiate into macrophages as seen in Figure 3a. After having reached the tumor, these cells are referred to as tumor-associated macrophages (TAMs). Since TAMs maintain their migratory ability after reaching the tumor, they can potentially be used for improving distribution of therapeutics throughout the tumor. Choi et al. demonstrated that both monocytes and macrophages could be loaded with gold nanoshells (silica core surrounded by a thin gold shell) for photothermal ablation after NIR exposure [41]. T47D tumor spheroids were treated with macrophages and gold-silica nanoshells for 3 days and irradiated causing photothermal ablation of the macrophages and the surrounding tumor cells. In this study, however, macrophages were not loaded with gold-silica nanoshells beforehand but rather the two were added simultaneously and uptake by the macrophages was proposed to occur during the experiment. Further studies need to be completed in order to determine the amount of gold nanoparticles these cells can be loaded with while still maintaining viability and migration functionality. Also, the in vitro tumor spheroid experiment is not a representative example of the complex tumor environment and biological barriers and further studies in vivo need to be pursued. In another study, Choi et al. demonstrated that mouse peritoneal macrophages could be loaded with liposomes containing doxorubicin [42]. Macrophages loaded with liposomal doxorubicin (LP-Dox) were intravenously injected in an A549 subcutaneous xenograft mouse model as well as in an A549 lung metastasis model. Doxorubicin was present in tumors after 24 hours in mice treated with macrophages loaded with liposomal doxorubicin, which suggests that the macrophages were able to migrate to both the subcutaneous tumor and the lung metastasis as seen in Figure 3b. In the subcutaneous model, mice that were administered the macrophages loaded with LP-Dox experienced a modest reduction in tumor growth rate as seen in Figure 3c. Neutrophils are another class of phagocytic cells that have been used as nanoparticle transporters. Neutrophils are the most abundant white blood cells in humans and are among the earliest immune cells to migrate towards sites of inflammation. They are also shorter lived than macrophages. For nanoparticle transport by neutrophils, one reported approach is intravenous injection of denatured bovine serum albumin nanoparticles that are specifically taken up by neutrophils. This technique was used to deliver an anti-inflammatory drug in a mouse model of lung inflammation resulting in a reduction in inflammation [43]. In another report, researchers loaded neutrophils ex vivo with paclitaxel encapsulated in liposomes. These cells were injected into mice who had received surgery for a brain tumor and this resulted in an increase in drug delivery to the brain and increased survival relative to treatment with just paclitaxel in liposomes [44]. These studies show the potential of macrophages and neutrophils as tumor tropic cell carriers, however, further loading and efficacy studies still need to be explored. In particular, a control that is frequently absent from cell/NP studies is a comparison with coadministering cells and NPs separately.
Figure 3.
Macrophages can be used as cell carriers due to their migration to tumor sites caused by chemotaxis. a) Macrophages are able to home to the tumor site due to chemoattractant gradients consisting of cytokines and growth factors secreted by tumor cells. b) Fluorescence imaging of A549 lung tumors from mice treated with either PBS or macrophages-LP-Dox. c) Overall survival of mice treated with PBS, macrophages, Dox, LP-Dox, and macrophage-LP-Dox.
6. Mesenchymal Stem Cells
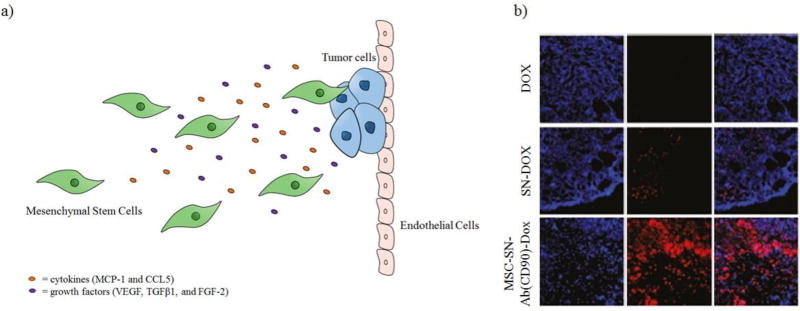
Mesenchymal Stem Cells (MSCs) have the ability to migrate to sites containing tumors and mass inflammation. MSCs can be harvested from the bone marrow or umbilical cord blood. They have the ability to differentiate into osteoblasts, chondrocytes, myocytes, or adipocytes [45]. Migration of MSCs is regulated by chemokines, cytokines, and angiogenesis factors released by tumor cells, such as MCP-1, CCL5, TGFβ1, FGF-2, and VEGF as seen in Figure 4a [46]. In a study done by Li et al., doxorubicin was encapsulated in silica nanoparticles that contain a secondary silica sphere in a central hollow cavity (called silica nanorattle-doxorubicin (SN-Dox)) and were loaded onto MSCs. These nanorattles were functionalized with a monoclonal antibody for CD73 and CD90, which are membrane proteins expressed by MSCs, allowing them to be anchored onto the cell surface of MSCs. However, the loading of nanorattles onto MSCs reduced the ability of these cells by ~40% to migrate towards tumor conditioned media in vitro. Subcutaneous U251 xenograft models were intratumorally injected with MSCs loaded with SN-Dox. Imaging suggested that mice treated with MSCs loaded with SN-Dox experienced a high accumulation and distribution of doxorubicin in the tumor tissue compared to the free doxorubicin seven day post-intratumoral injection as seen in Figure 4b [47]. Efficacy studies were not done. In another study, Sadhukha et al. demonstrated that MSCs loaded with Paclitaxel PLGA nanoparticles could selectively accumulate in lung tumors (A549 lung adenocarcinoma cells) three hours post-intravenous injection [48]. These studies demonstrate the that MSCs are indeed tumor tropic and have potential to be used to deliver nanoformulations of chemotherapeutics, however, long term survival and efficacy need to be addressed. MSCs have also been used as targeted delivery vehicles for viruses, though this lies beyond the scope of this review.[49–52]
Figure 4.
Mesenchymal Stem Cells can be used as cell carriers due to their migration to tumor sites caused by chemotaxis. a) Mesenchymal stem cells are able to home to tumor sites due to cytokines and growth factors secreted by tumor cells. b) Fluorescence imaging of tumor sections 7 days post intratumoral injection.
7. Neural Stem Cells
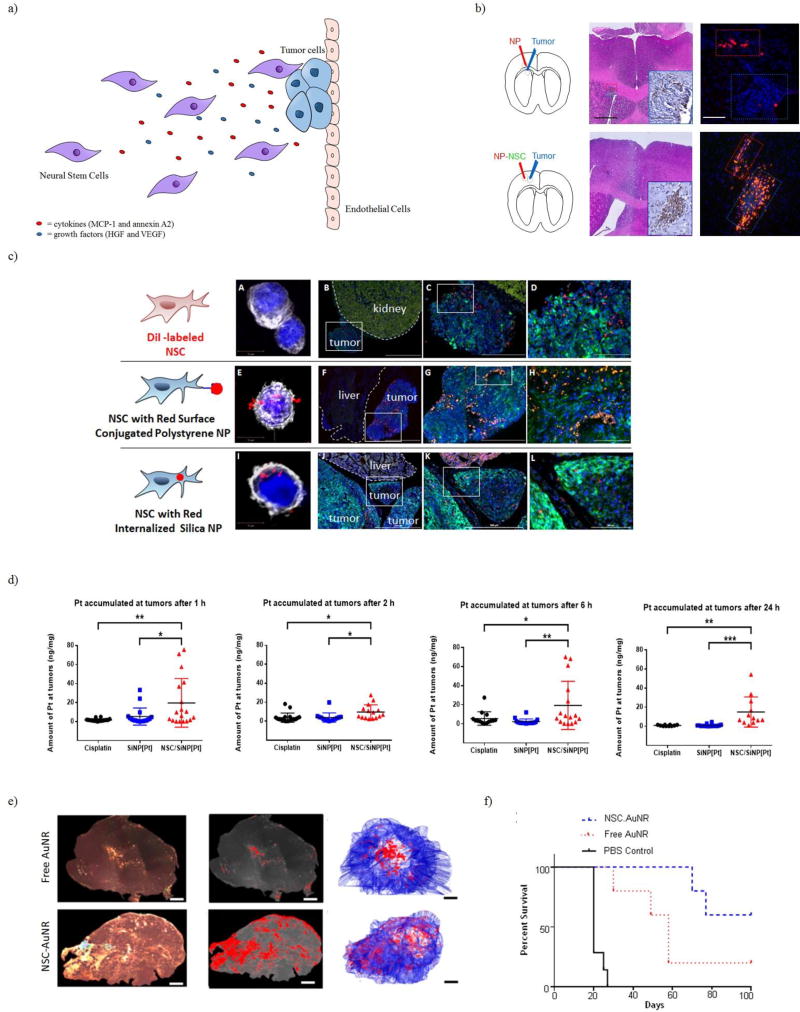
Neural stem cells (NSCs) were the first adult stem cells to be used as cell carriers for therapeutic payloads. These cells are self-renewing and are able to differentiate into neurons, astrocytes, or oligodendrocytes. These cells can be harvested from fetal, neonatal, or postnatal tissues. Similar to MSCs, NSCs can migrate to hypoxic tumor and inflammatory sites secreting chemoattractants (MCP-1/annexin A2) and pro-angiogenic growth factors (HGF/VEGF) as seen in Figure 5a [53]. Since NSCs are able to home to tumor sites via multiple mechanisms, this allows for dynamic targeting. Aboody et al. demonstrated that NSCs injected intracranially and contralateral to the glioblastoma were able to migrate towards the tumor [54]. NSCs transporting polystyrene nanoparticles maintain their tumor tropism in the brain, even when the nanoparticles are relatively large (~800 nm) and conjugated to the surface of the NSCs as seen in Figure 5b [55]. In a study conducted by Cheng et al., NSCs loaded with mesoporous silica nanoparticles containing doxorubicin (MSN-Dox) were able to be detected in intracranial brain tumors 4 hours after contralateral injection in a U87 glioma model though much of the injected dose remained at the initial injection site. Survival was slightly prolonged in mice treated with NSCs loaded with MSN-Dox (both intratumoral and contralateral) compared to mice administered only MSN-Dox [56]. Another study by Mooney et al. demonstrated that pH-responsive (poly(ethylene glycol)- poly((diisopropylamino)ethyl methacrylate) (PEG-PDPAEMA) nanoparticles containing docetaxel could be functionalized onto the surface of NSCs using biotin/avidin conjugation chemistry. Intratumoral injection of NSCs loaded with the docetaxel pH-responsive nanoparticles demonstrated activity for the drug but no efficacy study was performed [57]. Recently, Cao et al. demonstrated tumor tropism through intraperitoneal injection of NSCs with either surface conjugated or internalized silica nanoparticles into a metastatic ovarian cancer model. With these two methods, nanoparticles were present only in the tumor and absent in the adjacent organ as seen in Figure 5c. Cao et al. also demonstrated that cisplatin encapsulated silica nanoparticles (SiNP[Pt]) loaded onto NSCs resulted in selective delivery of the platinum drug to ovarian tumors as seen in Figure 5d [58]. Other therapeutics applications that have been explored include loading NSCs with gold nanorods (AuNRs) for photothermal ablation therapy [59, 60]. NSCs loaded with AuNRs were intratumorally injected into the flank tumor (subcutaneous MDA-MB-231 xenograft model). While free AuNRs injected intratumorally remained primarily localized at the site of injection, AuNRs loaded in NSCs were distributed throughout the tumor as seen in Figure 5e. This resulted in improved photothermal ablation after NIR exposure and overall survival compared to the free AuNR treatment as seen in Figure 5f [60]. Another unique therapy is loading NSCs with magnetic spinning discs (SDs). When a magnetic field is applied to the NSCs loaded with SDs, this causes mechanically induced apoptosis, causing the SDs to be released. The released SDs are able to be internalized by neighboring glioma cells and another treatment using the magnetic field can be applied again, causing membrane disruption and apoptosis [61]. From these various examples provided, NSCs can be loaded with a variety of different payloads, such as chemotherapeutic nanoformulations, gold nanorods, or magnetic spinning disks, to achieve targeted antitumor effects.
Figure 5.
Neural Stem Cells can be used as cell carriers due to their migration to tumor sites caused by chemotaxis. a) Neural Stem Cells are able to home to tumor sites due to cytokines and growth factors secreted by tumor cells. b) Free polystrene nanoparticles and NSCs loaded with polystyrene nanoparticles were injected intracranially near a brain tumor. Dramatically better tumor coverage was achieved with the NSC/NPs c) Fluorescent imaging of tumor and normal organ tissue of metastatic ovarian cancer model intraperitoneally injected with either NSCs, NSCs with surface conjugated polystyrene nanoparticles, and NSCs with internalized silica nanoparticles. In all cases, selective localization to tumor tissue is observed with no signal in the normal tissue d) Mice bearing metastatic ovarian tumors in the IP cavity were intraperitoneally injected with free cisplatin, SiNP[Pt], or NSCs with internalized SiNP[Pt]). Accumulation of platinum was quantified by ICP-MS. e) Intratumoral Distribution of AuNR and NSCs loaded with AuNR. Tumor sections were imaged using dark field microscopy and 3-D projections were generated mapping AuNRs. NSCs loaded with AuNRs resulted in a broader distribution of AuNRs. f) Overall survival of mice with treated with NIR laser that had received and intratumoral injection of either PBS, free AuNRs, or NSCs loaded with AuNRs. For the free AuNRs and NSCs loaded with AuNRs, only mice who had a response to treatment are included in this graph.
8. Conclusions
Nanoparticles are powerful tools for encapsulating drugs and controlling their release. However, nanoparticles alone have shown only modest ability to target tumors when administered systemically. New drug delivery systems are needed in order to overcome biological barriers that inhibit nanoparticle accumulation in tumors. Evidence is extensive that T-cells, macrophages, neutrophils, MSCs, and NSCs have intrinsic homing capabilities either to specific organs affected by tumor metastasis (lymph nodes) or to the tumor itself. These cell carriers are able to selectively deliver a range of therapeutic nanoparticles to tumors and could potentially be used as a new form of therapy. Currently, NSCs are the only cell carriers in clinical trials [62].
Probably the greatest challenge facing the cell/nanoparticle conjugate field is achieving delivery of sufficient dose. For any cell type, there is a realistic maximum for how many cells will arrive at the tumor, and each cell can only carry so many nanoparticles without compromising the cell. Furthermore, encapsulation of drug in a controlled-release nanoparticle means that each nanoparticle contains a significant amount of inert material. Thus, relative to dosing with free drug which can be given in large amount by IV, cell/nanoparticle conjugates are always going to be at a disadvantage for total dose. Indeed, the most advanced cell/nanoparticle conjugates that are being commercialized, are the autocrine T-cell systems where a low dose is acceptable because the payload acts on the carrier cell and only a small amount of cytokine can lead to a large effect [63–65].
For cell/nanoparticle conjugates to have a clinical effect in paracrine applications where the payload affects other target cells like cancer cells, remarkable targeting efficiency will be required and/or extremely potent payloads. A similar situation occurred for antibody-drug conjugates, where benefit is seen only when extremely toxic drugs are delivered [66, 67]. Indeed, for antibody-drug conjugates, which are now having a significant clinical impact, careful optimization of each parameter was required. As cell/nanoparticle conjugates move into the next phase, similar careful studies to optimize cell, nanoparticle and drug payload for each application will be essential.
Acknowledgments
We gratefully acknowledge support from The Rosalinde and Arthur Gilbert Foundation, The Anthony F. & Susan M. Markel Foundation, NIH R01CA197359. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest
References
- 1.Hubbell JA. Prescription for a pharmacyte. Sci Transl Med. 2015;7(291) doi: 10.1126/scitranslmed.aac5665. 291fs23. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura Y, Mochida A, Choyke PL, Kobayashi H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate Chemistry. 2016;27(10):2225–2238. doi: 10.1021/acs.bioconjchem.6b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–7. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura Y, Mochida A, Choyke PL, Kobayashi H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug Chem. 2016;27(10):2225–2238. doi: 10.1021/acs.bioconjchem.6b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su Y, Xie Z, Kim GB, Dong C, Yang J. Design strategies and applications of circulating cell-mediated drug delivery systems. ACS biomaterials science & engineering. 2015;1(4):201–217. doi: 10.1021/ab500179h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephan MT, Irvine DJ. Enhancing cell therapies from the outside in: Cell surface engineering using synthetic nanomaterials. Nano Today. 2011;6(3):309–325. doi: 10.1016/j.nantod.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chemical Reviews. 2016;116(5):2826–2885. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- 8.Murthy SK. Nanoparticles in modern medicine: state of the art and future challenges. International journal of nanomedicine. 2007;2(2):129–41. [PMC free article] [PubMed] [Google Scholar]
- 9.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urologic oncology. 2008;26(1):57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2013;48(3):416–27. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Research Letters. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Advanced drug delivery reviews. 2004;56(11):1649–59. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Lasic DD. Novel applications of liposomes. Trends in biotechnology. 1998;16(7):307–21. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 14.Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine: Nanotechnology, Biology and Medicine. 2010;6(6):714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Liechty WB, Peppas NA. Expert opinion: Responsive polymer nanoparticles in cancer therapy. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2012;80(2):241–6. doi: 10.1016/j.ejpb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharmaceutical Research. 2010;27(12):2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioengineering & Translational Medicine. 2016;1(1):10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, Rha SY, Lee MY, Ro J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast cancer research and treatment. 2008;108(2):241–50. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 19.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chemical reviews. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: An overview of biomedical applications. Journal of Controlled Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 21.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers. 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res. 2016;33(10):2373–87. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 23.Liberman A, Mendez N, Trogler WC, Kummel AC. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surface science reports. 2014;69(2–3):132–158. doi: 10.1016/j.surfrep.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang L, Cheng J. Nonporous Silica Nanoparticles for Nanomedicine Application. Nano today. 2013;8(3):290–312. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang F, Li L, Chen D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Advanced Materials. 2012;24(12):1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 26.Ehlerding EB, Chen F, Cai W. Biodegradable and Renal Clearable Inorganic Nanoparticles. Advanced Science. 2016;3(2) doi: 10.1002/advs.201500223. 1500223-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Yeo Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chemical engineering science. 2015;125:75–84. doi: 10.1016/j.ces.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery. Biomaterials. 2009;30(29):5757–5766. doi: 10.1016/j.biomaterials.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Research. 1986;46(12 Part 1):6387–6392. [PubMed] [Google Scholar]
- 30.Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. Journal of Controlled Release. 2012;164(2):138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 31.Clark AJ, Wiley DT, Zuckerman JE, Webster P, Chao J, Lin J, Yen Y, Davis ME. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(14):3850–3854. doi: 10.1073/pnas.1603018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016;1:16014. [Google Scholar]
- 33.Nichols JW, Bae YH. Odyssey of a cancer nanoparticle: from injection site to site of action. Nano today. 2012;7(6):606–618. doi: 10.1016/j.nantod.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotech. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Wang L, Yao Q, Ling R, Li K, Wang H. Drug concentrations in axillary lymph nodes after lymphatic chemotherapy on patients with breast cancer. Breast Cancer Research. 2004;6(4):R474–R477. doi: 10.1186/bcr819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–73. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 37.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13(5):309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 38.Huang B, Abraham WD, Zheng Y, Bustamante López SC, Luo SS, Irvine DJ. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Science Translational Medicine. 2015;7(291):291ra94–291ra94. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nature medicine. 2010;16(9):1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025. doi: 10.1038/npjbcancer.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi M-R, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. A Cellular Trojan Horse for Delivery of Therapeutic Nanoparticles into Tumors. Nano Letters. 2007;7(12):3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 42.Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, Lee JS, Lee JS, Park HJ, Song SY, Jeong SY, Choi EK. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33(16):4195–203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Chu D, Gao J, Wang Z. Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. ACS nano. 2015;9(12):11800–11. doi: 10.1021/acsnano.5b05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, Wei Z, Wang L, Kong L, Sun H, Ping Q, Mo R, Zhang C. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nano. 2017;12(7):692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 45.Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. International Journal of Clinical and Experimental Medicine. 2010;3(4):248–269. [PMC free article] [PubMed] [Google Scholar]
- 46.Karshieva S, Krasikov LS, Beliavskii AV. Mesenchymal stem cells as an antitumor therapy tool. Molekuliarnaia biologiia. 2013;47(1):50–60. doi: 10.7868/s0026898413010060. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, Fu C, Li Y, Qu Q, Zhang Y, Ji S, Chen L, Chen D, Tang F. Silica Nanorattle–Doxorubicin-Anchored Mesenchymal Stem Cells for Tumor-Tropic Therapy. ACS nano. 2011;5(9):7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 48.Sadhukha T, O'Brien TD, Prabha S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. Journal of Controlled Release. 2014;196:243–251. doi: 10.1016/j.jconrel.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Mader EK, Maeyama Y, Lin Y, Butler GW, Russell HM, Galanis E, Russell SJ, Dietz AB, Peng K-W. Mesenchymal Stem Cell Carriers Protect Oncolytic Measles Viruses from Antibody Neutralization in an Orthotopic Ovarian Cancer Therapy Model. Clinical Cancer Research. 2009;15(23):7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal Stem Cells Effectively Deliver an Oncolytic Adenovirus to Intracranial Glioma. STEM CELLS. 2008;26(3):831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 51.Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A, Rocconi RP, Numnum TM, Everts M, Chow LT, Douglas JT, Siegal GP, Zhu ZB, Bender HG, Dall P, Stoff A, Pereboeva L, Curiel DT. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Research and Treatment. 2007;105(2):157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 52.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Molecular Cancer Therapeutics. 2006;5(3):755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 53.Roger M, Clavreul A, Venier-Julienne M-C, Passirani C, Montero-Menei C, Menei P. The potential of combinations of drug-loaded nanoparticle systems and adult stem cells for glioma therapy. Biomaterials. 2011;32(8):2106–2116. doi: 10.1016/j.biomaterials.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 54.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mooney R, Weng Y, Tirughana-Sambandan R, Valenzuela V, Aramburo S, Garcia E, Li Z, Gutova M, Annala AJ, Berlin JM, Aboody KS. Neural stem cells improve intracranial nanoparticle retention and tumor-selective distribution. Future oncology. 2014;10(3):401–15. doi: 10.2217/fon.13.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Y, Morshed R, Cheng S-H, Tobias A, Auffinger B, Wainwright DA, Zhang L, Yunis C, Han Y, Chen C-T, Lo L-W, Aboody KS, Ahmed AU, Lesniak MS. Nanoparticle-Programmed Self-Destructive Neural Stem Cells for Glioblastoma Targeting and Therapy. Small (Weinheim an der Bergstrasse, Germany) 2013;9(24):4123–4129. doi: 10.1002/smll.201301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mooney R, Weng Y, Garcia E, Bhojane S, Smith-Powell L, Kim SU, Annala AJ, Aboody KS, Berlin JM. Conjugation of pH-Responsive Nanoparticles to Neural Stem Cells. Journal of controlled release : official journal of the Controlled Release Society. 2014;191:82–9. doi: 10.1016/j.jconrel.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao P, Mooney R, Tirughana R, Abidi W, Aramburo S, Flores L, Gilchrist M, Nwokafor U, Haber T, Tiet P, Annala AJ, Han E, Dellinger T, Aboody KS, Berlin JM. Intraperitoneal Administration of Neural Stem Cell-Nanoparticle Conjugates Targets Chemotherapy to Ovarian Tumors. Bioconjugate chemistry. 2017 doi: 10.1021/acs.bioconjchem.7b00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mooney R, Roma L, Zhao D, Van Haute D, Garcia E, Kim SU, Annala AJ, Aboody KS, Berlin JM. Neural stem cell-mediated intratumoral delivery of gold nanorods improves photothermal therapy. ACS nano. 2014;8(12):12450–60. doi: 10.1021/nn505147w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schnarr K, Mooney R, Weng Y, Zhao D, Garcia E, Armstrong B, Annala AJ, Kim SU, Aboody KS, Berlin JM. Gold nanoparticle-loaded neural stem cells for photothermal ablation of cancer. Advanced healthcare materials. 2013;2(7):976–82. doi: 10.1002/adhm.201300003. [DOI] [PubMed] [Google Scholar]
- 61.Muroski ME, Morshed RA, Cheng Y, Vemulkar T, Mansell R, Han Y, Zhang L, Aboody KS, Cowburn RP, Lesniak MS. Controlled Payload Release by Magnetic Field Triggered Neural Stem Cell Destruction for Malignant Glioma Treatment. PloS one. 2016;11(1):e0145129. doi: 10.1371/journal.pone.0145129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aboody KS, Najbauer J, Metz MZ, D’Apuzzo M, Gutova M, Annala AJ, Synold TW, Couture LA, Blanchard S, Moats RA, Garcia E, Aramburo S, Valenzuela VV, Frank RT, Barish ME, Brown CE, Kim SU, Badie B, Portnow J. Neural Stem Cell–Mediated Enzyme/Prodrug Therapy for Glioma: Preclinical Studies. Science Translational Medicine. 2013;5(184):184ra59–184ra59. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christian DA, Hunter CA. Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy. 2012;4(4):425–441. doi: 10.2217/imt.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milling L, Zhang Y, Irvine DJ. Delivering safer immunotherapies for cancer. Advanced Drug Delivery Reviews. 2017 doi: 10.1016/j.addr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Irvine DJ. Materializing the future of vaccines and immunotherapy. 2016;1:15008. [Google Scholar]
- 66.Diamantis N, Banerji U. Antibody-drug conjugates[mdash]an emerging class of cancer treatment. Br J Cancer. 2016;114(4):362–367. doi: 10.1038/bjc.2015.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chari RVJ, Miller ML, Widdison WC. Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy. Angewandte Chemie International Edition. 2014;53(15):3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]