1. INTRODUCTION
Increases in smoking, urbanization and the persistence of burning unrefined fuel indoors in low-and middle-income countries (more than 80% of the world’s population) have led to a substantial increase in exposure to environmental pollutants 1–4. Tobacco cigarette smoking contributes to nearly half a million deaths in the U.S. and 5 million deaths worldwide every year 5, 6. Compared to never smokers, tobacco cigarette smokers have an average loss of 13.2 and 14.5 years of life in men and women, respectively 7. Individuals who quit smoking before the age of 44 gain almost a decade of life, compared to those who continue to smoke 8. Even those individuals who quit between the ages of 45–54 gain an average of 6 years of life relative to those who continue to smoke, highlighting the need for counseling patients of any age on smoking cessation and providing resources for the treatment of nicotine addiction 8. Despite declines in tobacco cigarette smoking in high-income societies like the United States (U.S.), a re-emergence of nicotine addiction and related disease is possible due to the manufacture and sale of alternative tobacco products such as electronic cigarettes (e-cigarettes). Further, even in countries that have declines in tobacco cigarette smoking prevalence, certain vulnerable populations (low socioeconomic status, lower education, etc.) continue to have a high smoking prevalence suggesting the need for targeted interventions 9–11.
The most recent World Health Organization (WHO) modelled data shows that 92% of the world’s population live in areas where the air quality levels are not in compliance with air quality standards 4. Climate change is also altering the levels and composition of outdoor (ambient) air pollution and without policies aimed at reducing carbon emissions these changes are likely to continue, further increasing the global burden of environmental disease 12, 13. In 2010, ambient and indoor (household) air pollution were estimated to be responsible for 3 million and 3.9 million deaths that year, respectively 3, 4, 14, making them the 4th and 9th leading risk factors for global disease burden 2.
Mitochondria are highly sensitive to environmental toxicants and the individual components of tobacco smoke and air pollution. Environmental toxicant exposure induces changes in mitochondrial respiration and metabolism 15–25, oxidant generation 26–31, mitochondrial DNA (mtDNA) damage and copy number 25, 29, 30, 32–36, network formation and structure 19, 37–39, clearance of dysfunctional mitochondria through mitophagy 40, apoptosis 24, and reduction-oxidation (redox) signaling 24, 27. Mitochondria contain many iron-rich enzymes making the proteins within the organelle particularly sensitive to oxidant inactivation 41–43. Inactivation of manganese superoxide dismutase (the mitochondrial form of superoxide dismutase) leads to further oxidant generation, and many of the respiratory complexes within the mitochondrion contain heme groups that are subject to inactivation or inhibition by oxidants or carbon monoxide, which directly affects mitochondrial ATP production 43, 44. Several studies have shown that the mtDNA is prone to oxidant/toxicant-induced damage through the formation of strand breaks, thymidine dimer formation, bulky adducts and mtDNA copy number depletion or deletions (reviewed in 45). Thus, environmental toxicant-linked mitochondrial abnormalities likely play a causal or contributory role in disease development and pathogenesis and may serve as biomarkers of toxicant-induced injury.
Mitochondria form a dynamic network within cells undergoing cycles of fission into smaller, rounder mitochondria and fusion re-forming into string-like networks 46–50. The state of the mitochondrial network has been directly linked to the functional status of individual mitochondria 49, 50. Mitochondria with lower membrane potentials are often smaller, more rounded and are thought to be targeted for removal through mitophagy, a specialized form of autophagy 46–50. An inability to remove dysfunctional mitochondria can lead to cellular dysfunction and if sustained, apoptosis 51. Interestingly, cessation of exposure to toxicants often results in restoration of mitochondrial networks, decreased oxidant production, and improved oxidative phosphorylation 17, 52. Hence, the improvements associated with cessation of exposure may be the result of improved clearance of dysfunctional/damaged mitochondria and replacement with a healthy mitochondrial network, through mitochondrial biogenesis.
Our review outlines the contribution of mitochondrial abnormalities to the pathologies resulting from tobacco smoke and air pollution exposure. The chemical composition of tobacco smoke and air pollution are discussed with an emphasis on their similar chemical components that likely explain the similar disease risk profiles attributed to tobacco smoke or air pollution exposure. We also provide an overview on mitochondrial function and genetics. After a brief background on the diseases associated or caused by exposure to tobacco smoke and air pollution, we discuss the potential contribution of mitochondrial abnormalities to the pathogenesis. We provide potential future directions regarding the utility of mitochondrial markers of function and/or damage as biomarkers for monitoring the possible health effects of environmental toxicants and how mitochondrial genetic variation may impact individual susceptibility to disease.
2. CHEMICAL COMPOSITION
Both tobacco smoke and air pollution consist of complex mixtures of gaseous and particulate components that vary in chemical composition and are the result of the combustion of organic compounds. While the complex nature of this mixture makes it difficult to tease out the chemicals responsible for the toxicity of tobacco smoke and air pollution, the toxicity of some of the largest classes of chemicals have been clearly demonstrated, especially particulate matter 53. Particulate matter (PM) may consist of many different chemicals but the size of the particle plays a major role in determining its toxicity, with the smaller particles (≤10 μm in diameter, PM10) capable of reaching the deep lung 54. Fine particulate matter (≤2.5 μm in diameter, PM2.5) is of particular concern due to its potent toxicity especially within the pulmonary and cardiovascular systems 55–57. Additionally, the combination of chemicals present in tobacco smoke and air pollution likely contributes to the overall toxicity of these environmental pollutants 58. The similarities in chemical composition of air pollutants and tobacco smoke likely explain their similar toxicities and overlapping health effects.
2.1 TOBACCO SMOKE
Tobacco smoke is estimated to contain thousands of chemicals, present in particulate matter and gas phases, and is known to be a significant source of oxidants 59, 60. Tobacco smoke is divided into main stream smoke (smoke directly inhaled by the smoker) and side stream smoke, which encompasses the smoke coming off the sides of the burning tobacco product plus smoke exhaled by the smoker (collectively known as second-hand smoke). Both mainstream and side stream smoke consist of carbon monoxide, polycyclic aromatic hydrocarbons, tobacco-specific nitrosamines, nitrogen oxides, aldehydes, volatile organic compounds, nicotine, fine particulate matter, and oxidants 59, 61. The composition of main stream and side stream smoke is similar, but the concentrations of these toxicants and carcinogens differ, largely due to differences in combustion temperatures 59, 60. For a comprehensive review on the chemical composition of tobacco smoke, the review by Stedman, R.L. is an excellent resource 59.
2.2 AIR POLLUTION
Household air pollution is generated from incomplete combustion of solid or unrefined fuels during the burning of wood, coal, and other biomass for cooking, light and warmth 2, 3, 14, 62–65. Household air pollution is a major health risk among impoverished communities in low-and middle-income countries, with women and children having higher health risks due to increased exposure durations relative to men 2, 3, 64, 65. The level and composition of pollutants in household air varies depending upon the ventilation, stove efficiency, source of fuel, duration of exposure, and temperature of combustion 2, 3, 64, 65.
Ambient air pollution is primarily generated by industry and motor vehicle exhaust, although globally, household air pollution contributed an estimated 16% of ambient particulate matter pollution in 2010, with estimates reaching as high as 30% in India in 2012 2, 65. Some studies include both passive and active smoking, burning of biomass both indoors and outdoors, and wind-blown dust as sources of ambient pollution 2. Like household air pollution, ambient air pollution also varies based on the source of fuel exposure duration and combustion temperatures with added variables of industrial activity intensity, level of motor vehicle exhaust, time of day, time of year, weather conditions, local geography and geographical region 66–68.
The primary toxicants or toxicant mixtures associated with air pollution are similar for household and ambient air pollution and include particulate matter, ozone, carbon monoxide, nitrogen dioxide and sulfur dioxide 12, 67, 69. Some fuel sources, such as coal, are highly variable, having very different heating values and containing different levels of impurities such as sulfur, arsenic, silica, lead and mercury which are additional pollutants that can be released into the air during combustion 64.
3. MITOCHONDRIA
Mitochondrial toxicity resulting from pharmaceutical use has been appreciated for many years. Many drugs, including antibiotics, nucleoside reverse transcriptase inhibitors and chemotherapeutics have been identified to have unintended effects on multiple mitochondrial functions or responses (e.g. oxidant generation) reviewed in 45. It is perhaps not surprising that many environmental toxins also “target” the mitochondrion, either through the specific inhibitory action of a mitochondrial enzyme, and/or through the accumulation of a compound due the intrinsic characteristics of the organelle. Lipophilic compounds accumulate in mitochondrial membranes and cationic metals, xenobiotics (including MPP and paraquat) are known to accumulate within the organelle as well 45, likely due the net negative charge and alkaline pH of the organelle’s matrix. These characteristics in addition to the association of the mtDNA with the inner membrane (lipophilic environment), lack of histones, relatively low level of associated proteins for protection (nucleoids), and the presence of cytochrome P450 in mitochondria which can metabolize compounds into active toxins make the organelle a likely target for environmental toxins 45.
Mitochondria originated as separate bacterial organisms that were endocytosed by a primitive prokaryotic “host” cell approximately a billion years ago 70,71, 72. This symbiotic relationship within the proto-eukaryotic cell provided advantages for survival. In addition to the stereotypical characterization of the mitochondrion as the “powerplants of the cell,” mitochondria have evolved to play important roles in the inter-related processes of immune responses, cell signaling, metabolism (bioenergetics and biosynthesis), and nuclear gene expression reviewed in 73. Another consequence of this endosymbiosis is that our genome (as eukaryotes) is a genetic hybrid that consists of the nuclear and mitochondrial genomes (the majority of “mitochondrial” genes encoded by the ancestral mtDNA have been relocated to the nucleus, a process known as endosymbiotic gene transfer). As a result, the two genomes must be coordinately regulated, and the mitochondrion and its “host” are inseparably linked for eukaryotic cell survival and propagation, both genetically and functionally.
3.1. FUNCTIONS
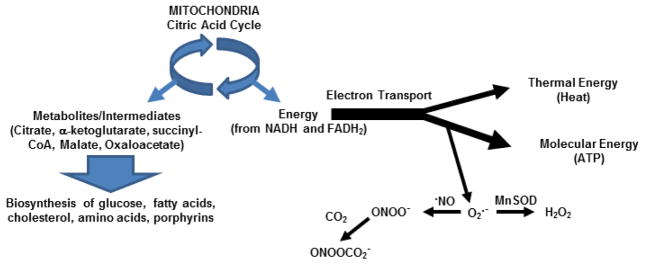
The mitochondrion serves as a source of cellular metabolites (through the citric acid cycle), ATP and oxidants (through electron transport and oxidative phosphorylation), as well as thermal energy (Figure 1). As ATP levels rise and energetic demand decreases, electron carriers remain in a reduced state for longer periods of time, allowing free electrons to readily react with molecular oxygen to form superoxide 74. Superoxide is subsequently converted to hydrogen peroxide, a freely diffusible signaling molecule, by manganese superoxide dismutase. In the presence of nitric oxide, superoxide forms peroxynitrite, a reactive nitrogen species (RNS) that acts as an oxidant, or alternatively, can further react with carbon dioxide to form nitrosoperoxycarbonate, that is capable forming nitrotyrosine adducts 26, 75–79. Contrary to widespread perception, mitochondrial oxidants are not merely “byproducts” of metabolism, but instead, serve as cell signaling mediators for the regulation of metabolism. Mitochondrial oxidants play a role in signaling that alters gene expression of proteins involved in inflammation80, cell death81, and appetite control 82, as well as the MAP kinase and Akt pathways 83 (please see Bonini review herein, “Oxidative signaling in mitochondrial homeostasis” Chapter 4). Consequently, under conditions of positive energy balance (excess reducing equivalents and ATP) or the presence of mitochondrial inhibitors (e.g. components of tobacco smoke such as carbon monoxide and cyanide) mitochondrial oxidant production is altered which may cause damage to mtDNA and impact multiple cell functions by altering the aforementioned cell signaling pathways 84.
Figure 1. Mitochondrial functions.
The citric acid cycle serves as a source of metabolic intermediates (citrate, a-ketoglutarate, etc) for several biosynthetic pathways (glucose, fatty acid, cholesterol synthesis, etc), and electron transport. Energy derived from electrons as they move through the electron transport complexes is utilized to pump protons across the inner mitochondrial membrane to form an electrochemical gradient; this conserved energy is used to generate molecular energy (ATP) at ATP synthase. Energy not conserved for proton pumping is lost in the form of thermal energy (heat). Under conditions of increased ATP/ADP ratios, electron acceptors remain in the reduced state for longer periods of time, and therefore electrons can also react with molecular oxygen to form the oxidant superoxide (O2·−), that can be converted to hydrogen peroxide (H2O2), a cell-signaling molecule by manganese superoxide dismutase (MnSOD) in the mitochondrion, or alternatively, react with nitric oxide (·NO) to form peroxynitrite (ONOO−), an oxidant, which in the presence of carbon dioxide (CO2), can form nitrosoperoxycarbonate (ONOOCO2−), a nitrating agent.
3.2. GENETICS AND MUTATION
Each cell contains hundreds to thousands of mitochondria, and each mitochondrion has 2–10 copies of mtDNA, resulting in thousands of mtDNA copies per cell 85, 86. The mtDNA in mammals encodes 37 genes (13 polypeptides, 22 tRNAs and 2 rRNAs) 87. The mtDNA is especially vulnerable to damage by reactive oxidant species (ROS) as it is tethered to the highly hydrophobic inner mitochondrial membrane within close proximity to sites of ROS generation, and is 93% coding (compared to the nuclear genome which is 2% coding), which means damage to the mtDNA is likely to affect a coding region. Since the 13 polypeptide subunits encoded by the mtDNA are the key catalytic proteins in electron transport complexes (I, III, IV and ATP synthase), mtDNA missense mutations can potentially alter mitochondrial functions. In this respect, mtDNA polymorphisms considered to represent “normal” genetic variation, have been linked with different bioenergetic capacities that may provide an adaptive advantage or altered disease susceptibility 88–90.
4. TOBACCO SMOKE TOXICITY
Active and passive cigarette smoke decrease mitochondrial respiration and membrane potential leading to decreased ATP content and increased oxidant production in a variety of tissues in a dose- and time-dependent manner 15–21, 23, 25, 27–30, 32, 33, 37, 52, 91, 92. Mitochondria are rich in enzymes with active sites easily modified and inactivated by ROS/RNS from the inhalation of tobacco smoke, or from oxidant generation within the mitochondrion (please see Bonini review herein, “Oxidative signaling in mitochondrial homeostasis” Chapter 4). For example, it has been shown that decreased activity of the mitochondrial isoform of superoxide dismutase, manganese SOD or SOD2, increases susceptibility to tobacco smoke exposure 27 and can be inactivated in mice through nitration, a consequence of nitrosoperoxycarbonate formation associated with side-stream tobacco cigarette exposure 32. Further, inactivation of oxidative phosphorylation complexes by ROS/RNS directly impairs proton pumping efficiency leading to decreased mitochondrial membrane potential and ATP production 93. Importantly, studies have shown that cessation of both active and passive smoking improves various measures of mitochondrial function 17, 52.
Other effects of tobacco cigarette smoke include changes in mitochondrial morphology 19, 37, 38 promoting mitophagy in both cell culture and animal models 37, the formation of mtDNA adducts 94, increased mtDNA damage 34 and alterations in mtDNA copy number 35. Acrolein, one of the primary reactive aldehydes within tobacco cigarette smoke, forms bulky DNA adducts which are likely to persist in the mtDNA due to the inefficiency of the mitochondrial DNA polymerase ɣ resulting in transversions 94. However, whether acrolein is one of the primary drivers of mtDNA damage following tobacco smoke exposure has yet to be determined. Components found within tobacco cigarette smoke such as benzene induce alterations in mtDNA copy number 36, however it is important to note that mtDNA copy number is influenced by several factors including age and health status. Differences in mtDNA copy number may be obscured by comorbidities 95 and may be difficult to attribute directly to toxicant exposure. Overall, mtDNA damage may serve as a more reliable indicator of tobacco smoke-induced injury than copy number, based on the conflicting observations concerning copy number 30, 33, 96. However, differences in mtDNA copy number or damage may be difficult to detect following acute exposures or if sufficient time passes between exposure and sample collection, due to DNA repair and/or mitophagy (removal of mitochondria with damaged mtDNA and restoring mitochondrial function) 46–49. Some of the challenges of interpreting alterations in mtDNA copy number and damage to toxicant exposure can be overcome through study design and by determining whether similar alterations are observed across multiple models.
4.1 PULMONARY DISEASES
COPD, emphysema, chronic bronchitis, asthma, obstruction of the small airways, pulmonary hypertension, and acute respiratory illnesses such as pneumonia are all pulmonary diseases causally linked to active smoking in adults 97. In children and adolescents, active smoking causes both impaired lung growth and function as well as asthma- related symptoms and poor asthma control 97. The lung has defense mechanisms against foreign materials that are inhaled which primarily consist of mucociliary clearance, the alveolar-epithelial barrier and the inflammatory immune response 97, 98. However, tobacco smoke and its constituents (especially acrolein, formaldehyde, and oxidants) overwhelm the pulmonary defense systems leading to damage to the cilia and overproduction of mucus thus impairing clearance and disrupting the tight junctions of the epithelial barrier 97, 99. Both the tar and gas phases of tobacco smoke contain high levels of free radicals (as many as 1017 and 1015 spins/gram, respectively) 100. These free radicals react to form ROS and RNS 97, 100, 101 such as the semiquinone radical (tar phase) and the peroxyl radical (gas phase) both of which can contribute to superoxide and hydroxyl radical formation, and the formation of non-radical oxidants, such as hydrogen peroxide 100. Collectively, the radicals and oxidants generated can cause damage to proteins, lipids, and DNA, inactivate antiproteases, deplete antioxidants, and promote a proinflammatory environment by enhancing the phagocytotic respiratory burst and expression of proinflammatory mediator genes in the lung 97, 101.
MtDNA damage, copy number, mitochondrial membrane potential, mitochondrial respiration, ATP content and structural damage have all been observed in tobacco cigarette smoke induced pulmonary disease. MtDNA damage and deletions were shown to be higher in brochoalveolar lavage tissues of smokers compared to cells from non-smokers 34. Nuclear DNA damage was also observed, but the mtDNA damage was significantly higher, while analysis of nuclear DNA to mtDNA ratios showed no significant differences 34. In another study, mtDNA copy number in circulating blood mononuclear cells displayed a dose-dependent increase with the number of cigarettes smoked per day 35. Treatment of primary human bronchial and alveolar epithelial cells with tobacco cigarette smoke-extract (the water-soluble portion of tobacco cigarette smoke) resulted in a dose-dependent decrease in membrane potential, ATP-linked oxygen consumption (and thus ATP content) suggesting impaired oxidative phosphorylation 15, 28. Acrolein exposure induced similar dose-dependent decreases in mitochondrial respiration and resulted in substrate switching to preserve glucose for ATP production in pulmonary alveolar cells, which likely has implications for surfactant production 16. The similar effects of acrolein and cigarette smoke extract on mitochondrial function in pulmonary alveolar cells suggest that acrolein may be one of the key mediators of cigarette smoke-induced pulmonary toxicity.
In addition to the effects on mitochondrial function, treatment of cultured human bronchial epithelial cells with tobacco cigarette smoke extract resulted in mitochondrial structural abnormalities as well including mitochondrial network fragmentation, mitochondrial swelling, and a loss of cristae19, 37, 38. The structural abonormalties in the mitochondria could be attenuated by silencing the mitochondrial fission mediator Fis1 suggestive of up-regulation of mitochondrial fission19, 37, 38. Antioxidant treatment also improved mitochondrial morphology and networks suggesting that oxidative stress likely plays a role in the structural abnormalities induced by tobacco smoke exposure 19, 37, 38. Similar structural abnormalities were also observed in primary human bronchial epithelial cells collected from COPD patients including a loss of mitochondrial cristae and elongated mitochondria that was associated with a down-regulation of genes involved in mitochondrial biogenesis (PGC1α, TFAM) and mitophagy (PINK1) 19. Tobacco cigarette smoke extract treatment in human bronchial epithelial cells increased mitochondrial oxidant generation and activated mitophagy through Parkin and Pink1 which lead to alterations in cellular phenotype consistent with cellular senescence 40. The increase in oxidant production was not observed in cells lacking mitochondria, suggesting that mitochondria were the primary source of oxidants under conditions of tobacco cigarette smoke exposure 28. In mice, eight weeks of tobacco cigarette smoke exposure lead to an up-regulation of proteins involved in oxidative phosphorylation, mitochondrial fusion, and mitochondrial oxidants within the lung tissue suggesting a potential compensation for mitochondrial impairments and dysfunction 17. Hence, acute cigarette smoke exposure appears to induce mitochondrial fission and removal of damaged mitochondria through mitophagy but continued exposure may impair the ability of lung epithelial cells to remove and replace damaged and dysfunctional mitochondria ultimately resulting in diseases such as COPD.
4.2 CARDIOVASCULAR DISEASE
Smoking is a leading risk factor for cardiovascular disease and stroke 9, 102 and together they account for the majority of smoking-related deaths in the U.S. 9, 103, 104. Approximately one-third of coronary heart disease deaths are attributed to tobacco smoke exposure and active smokers have 2–4 times increased risk for stroke 9, 102. Non-smokers exposed routinely to secondhand smoke in the work place or home, have a 25–30% increase in the risk for coronary heart disease – even brief exposures to secondhand smoke have been reported to increase platelet aggregation and damage to the endothelium which is central to vascular homeostasis 97, 102.
Tobacco cigarette smoke exposure alters mtDNA copy number and induces damage to the mitochondrial genome in cardiac and vascular tissues which is associated with cardiovascular disease 32, 33, 105. In a murine model of atherosclerosis, in utero or neonatal exposure to second-hand tobacco cigarette smoke induced mtDNA damage and deletions, and increased mtDNA copy number within the vasculature compared to filter air exposed controls 29, 33. Many of these same associations were also observed in non-human primates following perinatal exposure to side-stream tobacco smoke 30. However, within the non-human primate vasculature, mtDNA copy number was decreased with exposure in contrast to the observations within the vasculature of mice 30, 33. Notably, the effects of second-hand tobacco cigarette smoke exposure during early development persisted into adulthood and was associated with increased atherogenesis 33.
Within the vasculature, damaged mitochondria produce increased oxidants that impact nitric oxide signaling and pro-atherogenic pathways 106–108. Similarly, increased mtDNA damage, oxidant production, and inactivation of mitochondrial proteins (e.g. antioxidants and electron transport complexes) have been observed in the vasculature of mice exposed to cigarette smoke, and associate with increased atherogenesis 27, 33, 91. Concomitantly, aconitase inactivation (a marker for superoxide levels) was elevated in heart and vascular tissues of mice exposed to tobacco smoke 27, 33, 105. Perinatal tobacco cigarette smoke exposure in non-human primates also increased measures of mitochondrial oxidative stress including increased 3-nitrotyrosine and decreased cytochrome c oxidase activity within the abdominal aorta 30. Further, perinatal exposure was associated with increased cellularity in the subintimal space of the aorta, suggestive of increased inflammation consequent of altered mitochondrial redox signaling 30. Importantly, the effects of developmental exposure to tobacco smoke have been shown to have implications for adult vascular disease development in animal models 29, 30, 33.
Tobacco smoke exposure induces cardiac mitochondrial damage and dysfunction. Components of tobacco smoke directly inhibit or inactivate several mitochondrial proteins within the heart 23, 24, 42, 52, 109. Rabbits exposed to tobacco cigarette smoke for a single 30-minute session or 30 minutes twice a day for 2 and 8 weeks (3 cigarettes each session) had decreased myocardial mitochondrial respiration compared to non-exposed rabbits 20, 21. A similar study found that tobacco cigarette smoke exposure of rabbits decreased levels of coenzyme Q and cytochrome c oxidase activity within cardiac mitochondria compared to those of non-exposed rabbits 18. Collectively, these studies are consistent with tobacco smoke exposure mediated dysfunction of electron transport chain components (in this case, cytochrome c oxidase, which would result in decreased respiration) that impact ATP production. More specifically, carbon monoxide exposure in rabbits resulted in small, swollen mitochondria having condensed cristae compared to those observed from unexposed animals 39. Because it is well known that carbon monoxide competes with oxygen for heme binding within cytochrome c oxidase, it is likely that this component of cigarette smoke contributes to inhibiting oxygen consumption and thus inhibits oxidative phosphorylation and decreases ATP generation 24, 42, 109 Consequently, it is not surprising that cytochrome c oxidase activity was found to be lower in peripheral blood lymphocytes from heavy smokers relative to non-smokers 23, 52.
4.3 CANCER
Tobacco smoke contains at least 72 known carcinogens (reviewed in 110, 111) and is a known cause of all histological types of lung cancer (small-cell, large-cell, squamous and adenocarcinoma) with 90% of all lung cancer cases caused by tobacco smoke 97, 111–119. Smoking is also causally linked to cancers of the upper aerodigestive tract (oral, oropharynx, hypopharynx, larynx and oesophagus) as well as pancreas, stomach, bladder, kidney, cervix and acute myeloid leukemia 97, 111, 112, 120–128. It is likely that there are even more, yet undiscovered carcinogens in tobacco smoke and the presence of carcinogens in alternative tobacco products is currently under investigation. Furthermore, some chemical components that are not considered carcinogens, such as nicotine, have been shown to enhance carcinogenicity by promoting tumor growth through stimulation of angiogenesis and inhibition of apoptosis both in vitro and in mouse models of lung cancer 119, 129, 130.
Whereas there is ample evidence that tobacco cigarette smoke exposure can play a role in oncogenesis, the literature examining direct links between tobacco smoke exposure, mitochondria, and cancer is limited. However, it has been observed that changes in mitochondrial function 131–134, oxidant production/signaling 135–137, mtDNA copy number 35, 132, and mtDNA mutagenesis 132, 136, 138, 139 impact tumorgenicity 133, 136, malignancy 134, metastatic capacity 135, 140, 141 and resistance to anti-cancer therapy 142. Recently, differential mitochondrial bioenergetics linked to mitochondrial haplotype background has been shown to effect tumor latency and metastatic progression in a spontaneous mammary tumor model in mice 89. Many of the alterations in mitochondrial biology associated with oncogenesis have also been altered by tobacco smoke exposure suggesting a possible link but further investigation is needed.
4.4 DIABETES
Tobacco smoking is correlated with both the development and exacerbation of type 2 diabetes, the most prevalent form of diabetes accounting for 90–95% of all cases 9, 97, 143–155. Smoking decreases glucose tolerance, insulin sensitivity, and adiponectin levels while promoting hyperinsulinemia, insulin resistance, elevated glycosylated hemoglobin levels, increased fasting blood glucose levels, dyslipidemia and post-prandial lipid intolerance, all of which are associated with the diabetic phenotype 9, 97, 155–160. A meta-analysis of 46 studies published between 1995–2010 revealed that on average, smokers have a 30–40% increased relative risk for developing type 2 diabetes compared to nonsmokers 9. This risk for diabetes increases in a dose-dependent manner with light smokers (less than 20 cigarettes per day) having a 25% increased relative risk while heavy smokers (more than 20 cigarettes per day) have a 50–60% increased risk 9. Smokers also experience poorer control of their diabetes than nonsmokers 9, 97, 154. Cessation of smoking improves insulin sensitivity, despite weight gain, 97, 161, 162 and lowers the relative risk for diabetes to a level similar to that of nonsmokers in both women and men, ≥5 and ≥10 years after quitting, respectively 143, 163. One study, however, reported an 11% improvement in insulin sensitivity after only 8 weeks after smoking cessation suggesting improvements in insulin sensitivity following cessation may occur at even earlier time points 161.
Similar to cancer, the literature linking tobacco smoke, mitochondria and diabetes is not extensive. However, like cancer, there are strong associations between mitochondrial function and diabetes. Maternally Inherited Diabetes and Deafness (MIDD or Ballinger – Wallace Syndrome, OMIM #520000) is characterized by adult onset of sensorineural hearing loss and diabetes (non-insulin dependent). MIDD has been linked with several different mtDNA mutations 164–166 that manifest in altered mitochondrial function. Additionally, numerous reports have associated varied levels of mitochondrial oxidant production with insulin secretion 167–169 or β cell dysfunction 170, 171 (low or high, respectively), with some research showing that they act as necessary signals for glucose induced insulin secretion in rat and mouse islets 167, 168 as well as hypothalamic glucose sensing in rat hypothalamus both in vivo and ex vivo 172. Insulin secretion relies on the closure of ATP-sensitive potassium channels 173, which cause an influx of Ca2+ resulting in exocytosis of insulin vesicles 173–177.
Hence, insulin secretion is highly dependent and sensitive to changes in mitochondrial linked ATP production 175–177. Finally, it has been suggested that diabetes 178–180 and diabetic complications 181 are significantly influenced by mitochondrial genetic background suggesting that mtDNA mutations induced by cigarette smoke exposure may alter diabetes risk. Because tobacco cigarette smoke exposure is also linked with mtDNA damage, altered mitochondrial function, increased oxidant production, it is highly likely that associations between tobacco smoke exposure and diabetic risk are modulated via processes that involve the mitochondrion. Further studies in this area are required.
4.5 REPRODUCTIVE AND DEVELOPMENTAL EFFECTS
Smoking strongly associates with reduced fertility in women and maternal smoking during pregnancy is considered a causal factor of complications including increased incidence of preeclampsia, preterm delivery, and placental abnormalities 182–189. Maternal smoking or exposure to second hand smoke is also a major cause of infant morbidity, mortality, fetal growth restriction, and low birth weight resulting from placental abnormalities 188, 190–193. Even after adjustment for birth weight, infants of mothers who smoke have higher mortality rates than infants of non-smokers with maternal smoking increasing the risk of stillbirth by 40–60% 192.
Tobacco smoke exposure-induced alterations in mitochondrial function may contribute to growth restriction in utero, subsequent low birth weight, and other developmental outcomes 25, 92, 194, 195. Placental abnormalities resulting from tobacco smoke exposure have been attributed to hypoxia and nicotine-mediated vasoconstriction 188, 190–193. Carbon monoxide is a major contributor to the hypoxic in utero environment resulting from maternal tobacco smoking due to its greater affinity for fetal hemoglobin preventing oxygen loading and unloading196–198. Carbon monoxide also binds to complex IV of the electron transport chain inhibiting its activity which likely further contributes to impairments in meeting the high energetic needs of the developing fetus24, 42, 109. The fetal brain is especially sensitive to carbon monoxide toxicity due to its high oxygen consumption and glucose demands which may play a role in the association of cognitive deficits and neurobehavioral effects with maternal smoking 199, 200.
Mitochondrial abnormalities and impairments have been described in placental tissue from mothers who smoked during pregnancy 25. Mitochondria isolated from the placenta of mothers who smoked during pregnancy had a lower respiratory control index and decreased cytochrome c oxidoreductase (complex III) activity compared to placental mitochondria from non-smoking mothers, consistent with impaired mitochondrial respiration 25. Further, complex III activity was inversely related to the number of cigarettes smoked per day and trended towards an association with low birth weights suggesting that mitochondrial dysfunction may contribute to growth restriction in utero 25. According to the Barker hypothesis, developmental exposures to environmental toxicants may result in genetic reprogramming impacting cardiometabolic disease risk later in life and mitochondria may be a key mediator of this reprogramming 201, 202.
Smoking is the leading cause of erectile dysfunction in males between the ages of 20–40, which is not entirely surprising considering the well described effects of smoking on vascular function 203–208. Smokers have impaired endothelial-dependent vasodilation that is the result of oxidant scavenging of a key vasodilator, nitric oxide, which also plays a central role in dilation of the penile vasculature during stimulation of an erection 207, 208. Increased mitochondrial oxidant production contributes to endothelial damage by scavenging nitric oxide and consequently, smoking-induced increases in mitochondrial oxidant production may play a role in the impaired vasodilation and subsequent erectile dysfunction in smokers106.
Tobacco cigarette smoke exposure is also linked to decreased fertility in men 203–206 with several studies reporting decreased sperm motility with mitochondrial dysfunction and/or damage 209, 210. Mitochondria located within the tail of sperm are required to generate the energy to drive the cellular motors in the flagellum and if damaged, may not be able to provide sufficient energy resulting in reduced motility 209, 210. Again, whether the effects of tobacco smoke act through mitochondrial dysfunction is not yet clear. Nevertheless, tobacco smoke exposure decreases mitochondrial function, and therefore it is possible that this could provide a link between the observed association of cigarette smoke and decreased male fertility.
5.0 TOXICITY OF AIR POLLUTION
The six main ambient air pollutants are particulate matter, ground-level ozone, lead, carbon monoxide, nitrogen oxides and sulfur dioxide, termed criteria air pollutants by the United States Environmental Protection Agency. In this review, we have focused on particulate matter and ozone as these are the most well studied pollutants with regards to mitochondrial toxicity.
Particulate Matter
The United States Environmental Protection Agency monitors the levels of particulate matter especially PM10 and PM2.5. Even with PM2.5, sub-fractions have been shown to have distinct effects on pathophysiology with ultrafine particles (≤0.1μM in diameter, PM0.1) being cleared more slowly, retained longer in the lung and able to have significant effects even 6 days after inhalation compared to only 1 day with the larger PM2.5 fraction 211. Particulate matter is a major component of both household and ambient air pollution and has been implicated in mitochondrial toxicity 212–217. Organic PM is formed by gas-to-particle conversion or incomplete combustion of fuels 212. In contrast, engineered PM is generated for use in the production of paint, cosmetics, plastics, paper, and other materials released into the environment during manufacture, distribution and consumer use 213. Ammonium, elemental carbon, organic carbon matter, nitrate, silicon, sodium and sulfate make up about 80% of PM in ambient air; although these components vary regionally and seasonally 218.
PM has been shown to accumulate within the mitochondrion 212 and can disrupt mitochondrial membrane potential 214, damage mitochondrial structure 212, alter the mtDNA (strand breaks and methylation) 214,215–217, and activate the mitochondrial programmed apoptosis in pulmonary tissues. Exposure of murine macrophage and human bronchial epithelial cell lines to diesel exhaust particles suspended in the culture media resulted in a preferential accumulation of PM0.1 within the mitochondrion 212. Further, the accumulation of PM0.1 in mitochondria was further shown to cause structural damage to cristae and induced the formation of myelin figures, indicative of mitochondrial membrane damage 212. In contrast, PM10 or PM2.5 were sequestered in vacuoles rather than mitochondria causing minimal mitochondrial damage 212. Iron-derived free radicals in ambient air particulate matter can also cause DNA damage and decrease mitochondrial membrane potential, ultimately leading to cell death of alveolar epithelial cells via the intrinsic pathway in a dose and time dependent manner 214. Pre-treatment of cells with iron chelators and free radical scavengers protected the alveolar epithelial cells from particulate matter induced-DNA damage, mitochondrial dysfunction, and apoptosis 214. Most larger particles (>0.1μM) are cleared upon entering the respiratory tract; however, PM0.1 have been shown to cross the blood-brain barrier and disseminate through the blood to non-pulmonary organ systems 219, 220. Exposure of rat and human glial cell lines or primary hepatocytes to one of the most widely used engineered PM0.1, titanium dioxide PM0.1, induces oxidative stress 213, causes mitochondrial depolarization 212. Similarly, exposure of primary rat hepatocytes to titanium dioxide PM0.1 suspended in the culture medium for 2.5h induces oxidative stress and causes a loss of mitochondrial membrane potential 221.
Ozone
Ozone has many adverse health effects and is generated through the interaction of volatile organic compounds and nitrogen oxides in the presence of light 69. Ozone is a component of ambient air pollution 68, although the precursors for ozone formation are present in household air pollution 64. As mentioned previously, in many low- and middle-income countries, household air pollution is a significant contributor to ambient air pollution 2, 65. It is well described that exposure to ozone induces oxidative stress 222–224. Proposed mechanisms include the reaction of oxidants with unsaturated fatty acids to form lipid ozonation products and the reaction of secondary products formed in the epithelial lining fluid such as aldehydes, hydrogen peroxide and organic radicals 222–224. Ozone also enhances oxidative stress by depleting antioxidants and surfactants in the epithelial lining fluid 225. Mitochondrial toxicity has been implicated in the pathology of ozone-induced pulmonary and cardiovascular disease including perturbed mitochondrial bioenergetics, decreased mitochondrial membrane potential, increased mitochondrial oxidant production and mtDNA damage in mice, non-human primates and humans 96, 226. In addition, pulmonary ozone exposure decreased endothelial nitric oxide synthase protein levels and indices of nitric oxide production, resulting in vascular dysfunction and accelerated atherogenesis in mice 96. Treatment with the mitochondrial-targeted antioxidant MitoQ, lowered both mitochondrial and cellular oxidant levels, restored membrane potential and attenuated the ozone-induced airway hyper-responsiveness (see Section 5.1) suggesting that ozone mediates its effects, in part, by increasing mitochondrial oxidant production 226. Exposure of rats to ozone levels observed in high pollution cities (0.25ppm) caused chronic oxidative stress in the hippocampus, neurodegeneration, and mitochondrial dysfunction 227. Ozone exposure has also been linked to beta amyloid accumulation in the mitochondria of rat hippocampal cells and may be linked to the development of Alzheimer’s disease due to air pollution 227.
5.1 PULMONARY DISEASES
Particulate matter and ozone along with the rest of the criteria air pollutants cause, trigger, and exacerbate pulmonary diseases in both children and adults while also impairing lung development in children 228–233. The extended duration and heavy breathing associated with manual labor of certain occupations (e.g. transportation, landscaping, construction) as well as the increased exposure to household air pollution among women, children and the elderly in low- and middle-income countries (who spend more time indoors than male adults) results in a greater burden of exposure and subsequent greater pulmonary disease in these sub-populations 14, 63–65, 229, 234. The increasing prevalence of acute respiratory diseases has also been linked to climate change and alterations in ground level ozone pollution distribution 228, 229. Mice exposed to ozone (3 parts per million, 3 hours/day, twice a week for 1 or 6 weeks) exhibit phenotypes similar to human patients with COPD including lung inflammation and airway hyper-responsiveness 226, 235. Mitochondria isolated from the lungs of ozone-exposed mice have increased levels of mitochondrial ROS at both the 1-week and 6-week time points, decreased ATP content, decreased mitochondrial electron transport chain complex I enzyme activity and decreased protein levels of complexes I, III and V compared to control air-exposed mice 226. Treatment of these ozone-exposed mice with MitoQ (5mg/kg intraperitoneally), an antioxidant that targets the mitochondrion, significantly increased mitochondrial membrane potential and decreased the following: ozone-induced airway hyper-responsiveness, bronchoalveolar lavage total cell counts, keratinocyte-derived cytokine levels, mitochondrial ROS levels and cellular ROS levels 226. In contrast, a similar study with a slightly lower ozone exposure of 2.5 parts per million showed that N-acetylcysteine treatment did not attenuate ozone-induced lung injury in the same mouse model of COPD 235.235. The improvement observed in pulmonary function with MitoQ but not N-acetylcysteine suggests that mitochondrially targeted therapies to be more efficacious and that mitochondrial oxidants play a pivotal role in ozone-induced lung injury 235.
5.2 CARDIOVASCULAR DISEASE
Both short and long term exposure to air pollution (especially PM2.5, PM10, ground level ozone) increase the risk of cardiovascular events (hypertensive episodes, myocardial infarction, cardiac arrhythmia, stroke and heart failure) and cardiovascular attributed mortality, especially in individuals with pre-existent cardiovascular disease, the elderly, those that live in urban areas, impoverished communities where unrefined fuels are used indoors and certain occupations 234, 236–244. In adults of more advanced age, increased exposure to ambient air pollution is positively associated with increased mtDNA to nuclear DNA ratio and blood pressure 245. PM2.5 exposure caused structural damage to mitochondria in rat myocardial tissues including mitochondrial swelling, crista disorder and vacuole formation in a dose dependent manner 246. Mice exposed to PM for 3h exhibited impaired cardiac contractility with decreased mitochondrial respiration and ATP production 247. Exposure of healthy rats to diesel exhaust for one month caused decreased mitochondrial aconitase activity in the cardiac tissues 248. Mice exposed to ozone (0.5 ppm, 8h/day for 5 days) had increased heart rate, increased blood pressure and impaired aortic endothelial dependent vasoconstriction while also increasing markers of oxidative/nitrosative stress (increased lipid peroxidation by products, decreased aconitase activity, increased protein nitration) compared to filtered air controls 96. Aortas from mice and infant non-human primates exposed to ozone had increased levels of mtDNA damage compared to filtered air controls 96. Furthermore, atherosclerosis prone apolipoprotein E-deficient (apoE−/−) mice exposed to ozone also exhibited increased atherosclerosis compared to filtered air controls 96. These data taken together suggest that air pollution induced cardiovascular disease at least in part through mitochondrial toxicity.
5.3 CANCER
Ambient air pollution has been causally linked to cancers of the brain, nervous and endocrine systems, skin, cervix, oropharynx, ovary, kidney, liver, bladder, rectum, prostate, breast and blood 249–262 with risk increasing dose- dependently in many cases. Both household and ambient air pollution have been causally linked to lung cancer 64, 249–251, 263–265. Studies of ambient air pollution and cancer have focused on highly exposed cohorts (e.g. occupational exposure, residence proximity) 249, 251, 252, 254–256, 258, 259, 266 and urban areas 250, 253, 257, 260–263, 265. Studies of indoor air pollution and cancer have focused on emissions from incomplete combustion of solid or unrefined fuels (high temperature heating of wood, coal, and other biomass for cooking, light and warmth) in low-and middle-income countries or rural areas where alternative fuel sources are either unavailable or cost prohibitive 64, 65, 264. In Asia (southern, southeastern and eastern), Oceania, and sub-Saharan Africa (eastern, central and western), household air pollution from solid fuels is ranked in the top four risk factors for disease burden, outranking ambient air pollution 2. The World Health Organization estimates that three billion people are exposed to these emissions on a daily basis causing 4.3 million premature deaths a year, 17% of which are from lung cancer 65.
Similar to tobacco smoke, the literature on air pollution effects on mitochondrial toxicity in cancer is limited. It is well established that both ozone and PM mediate carcinogenesis through ROS generation 267–271. Diesel exhaust particles have been shown to induce ROS production, decrease mitochondrial membrane potential, increase mitochondrial structural damage and uncoupling of oxidative phosphorylation 270. Furthermore, studies in human lung carcinoma cell lines as well as primary human and rat lung cell cultures suggest that PM induces apoptosis by increasing mitochondrial ROS production leading to increased p53 expression as well as mitochondria-regulated apoptosis which is thought to cause remodeling and malignant transformation of airway epithelial cells 269.
5.4 DIABETES
Cohort studies suggest that long term exposure to air pollution, specifically PM2.5 and nitrogen dioxide, is associated with increased risk of developing type 2 diabetes and increased diabetes-related mortality 272–279. As with tobacco smoke, research on air pollution induced mitochondrial toxicity in diabetes pathogenesis is limited, however the few performed studies yielded results suggesting that this area of study should be pursued 280, 281. C57BL/6 mice fed a high fat diet (42% fat, for 34 weeks) had increased adipose inflammation and increased insulin resistance when exposed to PM2.5 (6h/day, 5 days/week, for 24 weeks) compared to filtered air exposed controls 280. Exposing this same strain of mouse to PM2.5 for a longer period (40 weeks) but on a standard chow diet still caused insulin resistance and glucose intolerance 282. This prolonged exposure to PM2.5 also decreased mitochondrial number in visceral adipose and decreased mitochondrial size in interscapular brown adipose 282 consistent with a role for mitochondria in air pollution induced type 2 diabetes.
5.5 REPRODUCTIVE AND DEVELOPMENTAL EFFECTS
Ambient air pollution exposure is associated with adverse reproductive health and outcomes in both women and men 283–303. Air pollution has been associated with increased sperm abnormalities including aneuploidy, head morphology and motility decreasing fertility in men 283, 300, 304. The effects of developmental exposure are of great concern especially for pregnant women who live in urban areas with high levels of ambient air pollution or in low-and middle-income countries where women may have extended duration of exposure to household air pollution 14, 62–65. Air pollution has been associated with negative pregnancy outcomes 284, 285, 287–290, 293, 298, 299, including low birth weight 292–295, 302, respiratory illnesses 301 and mortality 301, 303 in the fetus, newborn and infant. Increased in utero exposure to PM2.5, especially in the first trimester of pregnancy, is positively associated with placental mtDNA methylation (in both the displacement loop and the sequence which encodes the mitochondrial ribosomal 12S rRNA) 215. Conversely, increased in utero exposure to PM2.5 or PM10 is negatively associated with mtDNA content with the most significant association in the third trimester 215, 216. MtDNA methylation is controversial because the mtDNA does not have many CpG nucleotides 305–307 and thus, most of the methylations are on non-CpG cytosines 305. Consequently, the techniques used to identify CpG methylation in nuclear DNA may not be appropriate for detecting mtDNA methylation 305. Furthermore, there was little evidence that any of the three catalytically active DNA methyltransferases could access the mitochondrion 215, 305–307 until recently when it was discovered that DNA methyltransferase 1 could target mitochondria 308. Nevertheless, methylation in certain key regions of the mtDNA can have detrimental effects on replication and transcription of mtDNA (displacement loop methylation) 215, 305, or translation of mtDNA encoded RNA (12S rRNA methylation) 215.
6. FUTURE DIRECTIONS
6.1 TOXICITY RELATED TO NEW AND EMERGING TOBACCO PRODUCTS
New and emerging tobacco products such as e-cigarettes have become increasingly popular and are often used in combination with tobacco cigarettes 309, 310. Many of these alternative tobacco products are viewed as a potential means for reducing tobacco-induced disease burden in smokers and as a potential mechanism for aiding smokers in cessation. However, the few studies performed assessing cessation with e-cigarette use have been scarce, small in scale, and had ambiguous results that are often difficult to interpret 311, Moreover, the acute and chronic health effects of e-cigarettes and other alternative products are largely unknown and consequently, studies designed to evaluate risk for cellular injury associated with e-cigarette use are required. Measures of mitochondrial damage and function may provide a means to assess potential injury.
E-cigarettes likely contain fewer toxicants than tobacco cigarettes but they are not toxicant-free. Mass-spectrometry analysis has already demonstrated the presence of aldehydes, free radicals, and metals which are known mitochondrial toxicants and may act through similar disease mechanisms as those triggered by cigarette smoke exposure 312–317. Other tobacco products including hookahs, little cigars, and cigarillos combust tobacco leaves in a fashion very similar to traditional cigarettes and therefore, likely contain the same toxicants and carcinogens found in tobacco cigarette smoke. Consequently, hookah, little cigar, and cigarillo smoke exposure likely induce similar changes to mitochondrial physiology as tobacco cigarette smoke exposure and ultimately, are expected to induce similar morbidities and mortalities but require future investigation. The uncertainty of the chronic effects of e-cigarette and other alternative tobacco product use will likely continue for some time until large scale, longitudinal studies can be performed. However, as we gain a clearer picture regarding the chemical composition of the emitted vapors and smoke given off by these products, we will be able to draw conclusions regarding whether those chemicals at the levels observed have already shown effects on mitochondrial function and disease pathways.
6.2 TOXICITY RELATED TO MITOCHONDRIAL – NUCLEAR GENETIC BACKGROUND
The genetic basis for common disease susceptibility and environmental exposure sensitivity is not well understood. Studies have investigated this question often finding associations of variants with phenotypes in smaller sample cohorts, but these associations are usually lost in more broad-based or larger cohort studies. Our current viewpoint of genetic susceptibility, which typically interrogates only the nuclear genome may, in part, explain differences in disease susceptibility and environmental exposure sensitivity. Mitochondrial genetic variation may be a missing component that modifies penetrance or alters the symptoms associated with a disease. Several reports have shown that certain rare forms of blindness are linked to pathogenic mtDNA mutations and become less penetrant in individuals of African ancestry. Similarly, certain mtDNA polymorphisms alter individual susceptibility to non-syndromic sensorineural deafness, especially in individuals exposed to aminoglycosides 318–320. The evidence showing that mitochondrial genetic background influences disease susceptibility, including cancer, heart disease, and longevity is growing 89, 90, 321, 322. Collectively, these studies have focused on the impact of the mtDNA on disease susceptibility, and while important, a relatively unexplored area of study is the interaction of the two genomes (mitochondrial and nuclear) upon individual response to environmental stimuli (including toxicants). We hypothesize that certain nuclear mutations which convey increased susceptibility to environmental toxicants may have altered penetrance due to the combined influence of mitochondrial genetic background, age, and pre-existent health status. Consequently, a future direction of precision medicine should include an evaluation of both mitochondrial and nuclear genetic backgrounds when considering of the genetic aspects of individual risk.
Acknowledgments
FINANCIAL SUPPORT
This work is supported by NIH grants RO1 103859 (SWB), NIH Predoctoral Training Program in Cardiovascular Pathophysiology T32 HL007918 (MJS), and an American Heart Association Mentored Clinical and Population Research Award 17MCPRP32650002 (JLF). Research reported in this work was also supported by grant #5P50HL120163 from the National Heart, Lung, and Blood Institute (NHLBI) and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King BA, Mirza SA, Babb SD, Group GC. A cross-country comparison of secondhand smoke exposure among adults: findings from the Global Adult Tobacco Survey (GATS) Tob Control. 2013;22:e5. doi: 10.1136/tobaccocontrol-2012-050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin WJ, 2nd, Glass RI, Balbus JM, Collins FS. Public health. A major environmental cause of death. Science. 2011;334:180–1. doi: 10.1126/science.1213088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Ambient Air Pollution: a global assessment of exposure and burden of disease. World Health Organization; 2016. [Google Scholar]
- 5.Antman E, Arnett D, Jessup M, Sherwin C. The 50th anniversary of the US surgeon general’s report on tobacco: what we’ve accomplished and where we go from here. J Am Heart Assoc. 2014;3:e000740. doi: 10.1161/JAHA.113.000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atlanta: Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): 2014. The health consequences of smoking- 50 years of progress: a report of the Surgeon General. [Google Scholar]
- 7.Services USDoHaH. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 8.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 9.Atlanta: Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health. The health consequences of smoking- 50 years of progress: a report of the Surgeon General. 2014. [Google Scholar]
- 10.Lee JG, Griffin GK, Melvin CL. Tobacco use among sexual minorities in the USA, 1987 to May 2007: a systematic review. Tob Control. 2009;18:275–82. doi: 10.1136/tc.2008.028241. [DOI] [PubMed] [Google Scholar]
- 11.Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–40. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- 12.Finlayson-Pitts BJ, Pitts JN., Jr Tropospheric air pollution: ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science. 1997;276:1045–52. doi: 10.1126/science.276.5315.1045. [DOI] [PubMed] [Google Scholar]
- 13.Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, Erwin KN, Levin ED. The toxicology of climate change: environmental contaminants in a warming world. Environ Int. 2009;35:971–86. doi: 10.1016/j.envint.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, Dherani M, Hosgood HD, Mehta S, Pope D, Rehfuess E, Group HCRE. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- 15.van der Toorn M, Slebos DJ, de Bruin HG, Leuvenink HG, Bakker SJ, Gans RO, Koeter GH, van Oosterhout AJ, Kauffman HF. Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1211–8. doi: 10.1152/ajplung.00291.2006. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal AR, Yin F, Cadenas E. Metabolic shift in lung alveolar cell mitochondria following acrolein exposure. Am J Physiol Lung Cell Mol Physiol. 2013;305:L764–73. doi: 10.1152/ajplung.00165.2013. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2012;303:L889–98. doi: 10.1152/ajplung.00219.2012. [DOI] [PubMed] [Google Scholar]
- 18.Gvozdjakova A, Simko F, Kucharska J, Braunova Z, Psenek P, Kyselovic J. Captopril increased mitochondrial coenzyme Q10 level, improved respiratory chain function and energy production in the left ventricle in rabbits with smoke mitochondrial cardiomyopathy. Biofactors. 1999;10:61–5. doi: 10.1002/biof.5520100107. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann RF, Zarrintan S, Brandenburg SM, Kol A, de Bruin HG, Jafari S, Dijk F, Kalicharan D, Kelders M, Gosker HR, Ten Hacken NH, van der Want JJ, van Oosterhout AJ, Heijink IH. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respiratory research. 2013;14:97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gvozdjakova A, Kucharska J, Sany L, Gvozdjak J. The effect of cigarette smoke on cytochrome-oxidase of the heart muscle. Cor Vasa. 1984;26:466–8. [PubMed] [Google Scholar]
- 21.Gvozdjakova A, Bada V, Sany L, Kucharska J, Kruty F, Bozek P, Trstansky L, Gvozdjak J. Smoke cardiomyopathy: disturbance of oxidative processes in myocardial mitochondria. Cardiovasc Res. 1984;18:229–32. doi: 10.1093/cvr/18.4.229. [DOI] [PubMed] [Google Scholar]
- 22.Alonso JR, Cardellach F, Lopez S, Casademont J, Miro O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol Toxicol. 2003;93:142–6. doi: 10.1034/j.1600-0773.2003.930306.x. [DOI] [PubMed] [Google Scholar]
- 23.Miro O, Alonso JR, Jarreta D, Casademont J, Urbano-Marquez A, Cardellach F. Smoking disturbs mitochondrial respiratory chain function and enhances lipid peroxidation on human circulating lymphocytes. Carcinogenesis. 1999;20:1331–6. doi: 10.1093/carcin/20.7.1331. [DOI] [PubMed] [Google Scholar]
- 24.Almeida AS, Figueiredo-Pereira C, Vieira HL. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Front Physiol. 2015;6:33. doi: 10.3389/fphys.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouhours-Nouet N, May-Panloup P, Coutant R, de Casson FB, Descamps P, Douay O, Reynier P, Ritz P, Malthiery Y, Simard G. Maternal smoking is associated with mitochondrial DNA depletion and respiratory chain complex III deficiency in placenta. Am J Physiol Endocrinol Metab. 2005;288:E171–7. doi: 10.1152/ajpendo.00260.2003. [DOI] [PubMed] [Google Scholar]
- 26.Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Harrison CM, Pompilius M, Pinkerton KE, Ballinger SW. Mitochondrial oxidative stress significantly influences atherogenic risk and cytokine-induced oxidant production. Environ Health Perspect. 2011;119:676–81. doi: 10.1289/ehp.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Toorn M, Rezayat D, Kauffman HF, Bakker SJ, Gans RO, Koeter GH, Choi AM, van Oosterhout AJ, Slebos DJ. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L109–14. doi: 10.1152/ajplung.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Knight CA, Mamerow MM, Vickers K, Penn A, Postlethwait EM, Ballinger SW. Prenatal environmental tobacco smoke exposure promotes adult atherogenesis and mitochondrial damage in apolipoprotein E−/− mice fed a chow diet. Circulation. 2004;110:3715–20. doi: 10.1161/01.CIR.0000149747.82157.01. [DOI] [PubMed] [Google Scholar]
- 30.Westbrook DG, Anderson PG, Pinkerton KE, Ballinger SW. Perinatal tobacco smoke exposure increases vascular oxidative stress and mitochondrial damage in non-human primates. Cardiovasc Toxicol. 2010;10:216–26. doi: 10.1007/s12012-010-9085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visalli G, Bertuccio MP, Picerno I, Spataro P, Di Pietro A. Mitochondrial dysfunction by pro-oxidant vanadium: ex vivo assessment of individual susceptibility. Environ Toxicol Pharmacol. 2015;39:93–101. doi: 10.1016/j.etap.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, Ischiropoulos H, Ballinger SW. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation. 2002;105:849–54. doi: 10.1161/hc0702.103977. [DOI] [PubMed] [Google Scholar]
- 33.Fetterman JL, Pompilius M, Westbrook DG, Uyeminami D, Brown J, Pinkerton KE, Ballinger SW. Developmental exposure to second-hand smoke increases adult atherogenesis and alters mitochondrial DNA copy number and deletions in apoE(−/−) mice. PLoS One. 2013;8:e66835. doi: 10.1371/journal.pone.0066835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballinger SW, Bouder TG, Davis GS, Judice SA, Nicklas JA, Albertini RJ. Mitochondrial genome damage associated with cigarette smoking. Cancer research. 1996;56:5692–7. [PubMed] [Google Scholar]
- 35.Hosgood HD, 3rd, Liu CS, Rothman N, Weinstein SJ, Bonner MR, Shen M, Lim U, Virtamo J, Cheng WL, Albanes D, Lan Q. Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis. 2010;31:847–9. doi: 10.1093/carcin/bgq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen M, Zhang L, Bonner MR, Liu CS, Li G, Vermeulen R, Dosemeci M, Yin S, Lan Q. Association between mitochondrial DNA copy number, blood cell counts, and occupational benzene exposure. Environ Mol Mutagen. 2008;49:453–7. doi: 10.1002/em.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aravamudan B, Kiel A, Freeman M, Delmotte P, Thompson M, Vassallo R, Sieck GC, Pabelick CM, Prakash YS. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;306:L840–54. doi: 10.1152/ajplung.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara H, Araya J, Ito S, Kobayashi K, Takasaka N, Yoshii Y, Wakui H, Kojima J, Shimizu K, Numata T, Kawaishi M, Kamiya N, Odaka M, Morikawa T, Kaneko Y, Nakayama K, Kuwano K. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol. 2013;305:L737–46. doi: 10.1152/ajplung.00146.2013. [DOI] [PubMed] [Google Scholar]
- 39.Kjeldsen K, Thomsen HK, Astrup P. Effects of carbon monoxide on myocardium. Ultrastructural changes in rabbits after moderate, chronic exposure. Circ Res. 1974;34:339–48. doi: 10.1161/01.res.34.3.339. [DOI] [PubMed] [Google Scholar]
- 40.Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, Kojima J, Shimizu K, Numata T, Kawaishi M, Odaka M, Morikawa T, Harada T, Nishimura SL, Kaneko Y, Nakayama K, Kuwano K. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy. 2015;11:547–59. doi: 10.1080/15548627.2015.1017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lushchak OV, Piroddi M, Galli F, Lushchak VI. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. 2014;19:8–15. doi: 10.1179/1351000213Y.0000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musatov A, Robinson NC. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res. 2012;46:1313–26. doi: 10.3109/10715762.2012.717273. [DOI] [PubMed] [Google Scholar]
- 43.Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006;41:653–7. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Kakkar P, Singh BK. Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem. 2007;305:235–53. doi: 10.1007/s11010-007-9520-8. [DOI] [PubMed] [Google Scholar]
- 45.Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicological sciences : an official journal of the Society of Toxicology. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–40. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–7. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Chu CT. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Hum Mol Genet. 2010;19:R28–37. doi: 10.1093/hmg/ddq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:C203–10. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–7. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mijaljica D, Prescott M, Devenish RJ. Different fates of mitochondria: alternative ways for degradation? Autophagy. 2007;3:4–9. doi: 10.4161/auto.3011. [DOI] [PubMed] [Google Scholar]
- 52.Cardellach F, Alonso JR, Lopez S, Casademont J, Miro O. Effect of smoking cessation on mitochondrial respiratory chain function. J Toxicol Clin Toxicol. 2003;41:223–8. doi: 10.1081/clt-120021102. [DOI] [PubMed] [Google Scholar]
- 53.Cassee FR, Heroux ME, Gerlofs-Nijland ME, Kelly FJ. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal Toxicol. 2013;25:802–12. doi: 10.3109/08958378.2013.850127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown JS, Gordon T, Price O, Asgharian B. Thoracic and respirable particle definitions for human health risk assessment. Part Fibre Toxicol. 2013;10:12. doi: 10.1186/1743-8977-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295:1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Analitis A, Katsouyanni K, Dimakopoulou K, Samoli E, Nikoloulopoulos AK, Petasakis Y, Touloumi G, Schwartz J, Anderson HR, Cambra K, Forastiere F, Zmirou D, Vonk JM, Clancy L, Kriz B, Bobvos J, Pekkanen J. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology. 2006;17:230–3. doi: 10.1097/01.ede.0000199439.57655.6b. [DOI] [PubMed] [Google Scholar]
- 57.Xing YF, Xu YH, Shi MH, Lian YX. The impact of PM2.5 on the human respiratory system. Journal of thoracic disease. 2016;8:E69–74. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanobetti A, Franklin M, Koutrakis P, Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environmental health : a global access science source. 2009;8:58. doi: 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stedman RL. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968;68:153–207. doi: 10.1021/cr60252a002. [DOI] [PubMed] [Google Scholar]
- 60.Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous Compounds in Tobacco Smoke. Int J Env Res Pub He. 2011;8:613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Apelberg BJ, Hepp LM, Avila-Tang E, Gundel L, Hammond SK, Hovell MF, Hyland A, Klepeis NE, Madsen CC, Navas-Acien A, Repace J, Samet JM, Breysse PN. Environmental monitoring of secondhand smoke exposure. Tob Control. 2013;22:147–55. doi: 10.1136/tobaccocontrol-2011-050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of household solid fuel combustion and of high-temperature frying. The Lancet Oncology. 2006;7:977–8. doi: 10.1016/s1470-2045(06)70969-x. [DOI] [PubMed] [Google Scholar]
- 63.Quansah R, Semple S, Ochieng CA, Juvekar S, Armah FA, Luginaah I, Emina J. Effectiveness of interventions to reduce household air pollution and/or improve health in homes using solid fuel in low-and-middle income countries: A systematic review and meta-analysis. Environ Int. 2017;103:73–90. doi: 10.1016/j.envint.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Cancer WHOIAfRo. Monographs on the Evaluation of Carcinogenic Risks to Humans: Household use of solid fuels and high-temperature frying. Vol. 95. World Health Organization International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans; Lyon, France: 2010. pp. 1–430. [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization. Burning opportunity: clean household energy for health, sustainable development, and wellbeing of women and children. World Health Organization; 2016. pp. 1–113. [Google Scholar]
- 66.LaKind JS, Overpeck J, Breysse PN, Backer L, Richardson SD, Sobus J, Sapkota A, Upperman CR, Jiang C, Beard CB, Brunkard JM, Bell JE, Harris R, Chretien JP, Peltier RE, Chew GL, Blount BC. Exposure science in an age of rapidly changing climate: challenges and opportunities. J Expo Sci Environ Epidemiol. 2016;26:529–538. doi: 10.1038/jes.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brauer M, Amann M, Burnett RT, Cohen A, Dentener F, Ezzati M, Henderson SB, Krzyzanowski M, Martin RV, Van Dingenen R, van Donkelaar A, Thurston GD. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environmental science & technology. 2012;46:652–60. doi: 10.1021/es2025752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.World Health Organization Regional Office for Europe. Global update 2005. World Health Organization, Regional Office for Europe; Copenhagen: 2006. Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. [Google Scholar]
- 69.Seinfeld JH. Urban air pollution: state of the science. Science. 1989;243:745–52. doi: 10.1126/science.243.4892.745. [DOI] [PubMed] [Google Scholar]
- 70.Wallin I. The mitochondria problem. The American Naturalist. 1923;57:255–261. [Google Scholar]
- 71.Margulis L. The origin of mitosing eukaryotic cells. Journal of Theoretical Biology. 1967;14:225–274. [Google Scholar]
- 72.Margulis L. Symbiosis in cell evolution. 1. New York, NY: W.H. Freeman; 1981. [Google Scholar]
- 73.Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion. 2016;30:105–16. doi: 10.1016/j.mito.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annual review of genetics. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alberts B. Molecular Biology of the Cell. 4. New York, NY: Garland Science; 2002. [Google Scholar]
- 76.Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999;31:53–9. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- 77.Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29:345–50. doi: 10.1042/0300-5127:0290345. [DOI] [PubMed] [Google Scholar]
- 78.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Archives of biochemistry and biophysics. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 79.Gow A, Duran D, Thom SR, Ischiropoulos H. Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Archives of biochemistry and biophysics. 1996;333:42–8. doi: 10.1006/abbi.1996.0362. [DOI] [PubMed] [Google Scholar]
- 80.Rogers RJ, Monnier JM, Nick HS. Tumor necrosis factor-alpha selectively induces MnSOD expression via mitochondria-to-nucleus signaling, whereas interleukin-1beta utilizes an alternative pathway. J Biol Chem. 2001;276:20419–27. doi: 10.1074/jbc.M008915200. [DOI] [PubMed] [Google Scholar]
- 81.Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–41. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 82.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 83.Fang Y, Han SI, Mitchell C, Gupta S, Studer E, Grant S, Hylemon PB, Dent P. Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology. 2004;40:961–71. doi: 10.1002/hep.20385. [DOI] [PubMed] [Google Scholar]
- 84.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 85.Wiesner RJ, Ruegg JC, Morano I. Counting target molecules by exponential polymerase chain reaction: copy number of mitochondrial DNA in rat tissues. Biochem Biophys Res Commun. 1992;183:553–9. doi: 10.1016/0006-291x(92)90517-o. [DOI] [PubMed] [Google Scholar]
- 86.Miller FJ, Rosenfeldt FL, Zhang C, Linnane AW, Nagley P. Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: lack of change of copy number with age. Nucleic Acids Res. 2003;31:e61. doi: 10.1093/nar/gng060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–65. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 88.Scott GR, Schulte PM, Egginton S, Scott AL, Richards JG, Milsom WK. Molecular evolution of cytochrome C oxidase underlies high-altitude adaptation in the bar-headed goose. Mol Biol Evol. 2011;28:351–63. doi: 10.1093/molbev/msq205. [DOI] [PubMed] [Google Scholar]
- 89.Feeley KP, Bray AW, Westbrook DG, Johnson LW, Kesterson RA, Ballinger SW, Welch DR. Mitochondrial Genetics Regulate Breast Cancer Tumorigenicity and Metastatic Potential. Cancer research. 2015;75:4429–36. doi: 10.1158/0008-5472.CAN-15-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fetterman JL, Zelickson BR, Johnson LW, Moellering DR, Westbrook DG, Pompilius M, Sammy MJ, Johnson M, Dunham-Snary KJ, Cao X, Bradley WE, Zhang J, Wei CC, Chacko B, Schurr TG, Kesterson RA, Dell’italia LJ, Darley-Usmar VM, Welch DR, Ballinger SW. Mitochondrial genetic background modulates bioenergetics and susceptibility to acute cardiac volume overload. Biochem J. 2013;455:157–67. doi: 10.1042/BJ20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Z, Harrison CM, Chuang GC, Ballinger SW. The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutation research. 2007;621:61–74. doi: 10.1016/j.mrfmmm.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 93.Wallace DC. Bioenergetic origins of complexity and disease. Cold Spring Harb Symp Quant Biol. 2011;76:1–16. doi: 10.1101/sqb.2011.76.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kasiviswanathan R, Minko IG, Lloyd RS, Copeland WC. Translesion synthesis past acrolein-derived DNA adducts by human mitochondrial DNA polymerase gamma. J Biol Chem. 2013;288:14247–55. doi: 10.1074/jbc.M113.458802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fetterman JL, Holbrook M, Westbrook DG, Brown JA, Feeley KP, Breton-Romero R, Linder EA, Berk BD, Weisbrod RM, Widlansky ME, Gokce N, Ballinger SW, Hamburg NM. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc Diabetol. 2016;15:53. doi: 10.1186/s12933-016-0372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chuang GC, Yang Z, Westbrook DG, Pompilius M, Ballinger CA, White CR, Krzywanski DM, Postlethwait EM, Ballinger SW. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Physiol Lung Cell Mol Physiol. 2009;297:L209–16. doi: 10.1152/ajplung.00102.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Services USDoHaH. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed] [Google Scholar]
- 98.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–7. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones JG, Minty BD, Lawler P, Hulands G, Crawley JC, Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1:66–8. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 100.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Annals of the New York Academy of Sciences. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. discussion 27–8. [DOI] [PubMed] [Google Scholar]
- 101.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. American journal of respiratory and critical care medicine. 1997;156:341–57. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 102.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017 doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46:11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 104.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 105.Cakir Y, Yang Z, Knight CA, Pompilius M, Westbrook D, Bailey SM, Pinkerton KE, Ballinger SW. Effect of alcohol and tobacco smoke on mtDNA damage and atherogenesis. Free radical biology & medicine. 2007;43:1279–88. doi: 10.1016/j.freeradbiomed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 106.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxidants & redox signaling. 2011;15:1517–30. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112:1171–88. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vos MH, Liebl U. Time-resolved infrared spectroscopic studies of ligand dynamics in the active site from cytochrome c oxidase. Biochim Biophys Acta. 2015;1847:79–85. doi: 10.1016/j.bbabio.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 110.Hecht SS. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob Res. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cancer WHOIAfRo. Tobacco smoke and involuntary smoking: monographs on the evaluation of carcinogenic risks to humans. World Health Organization International Agency for Research on Cancer. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 112.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature reviews Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 113.Miller GH, Golish JA, Cox CE, Chacko DC. Women and lung cancer: a comparison of active and passive smokers with nonexposed nonsmokers. Cancer detection and prevention. 1994;18:421–30. [PubMed] [Google Scholar]
- 114.Agudo A, Barnadas A, Pallares C, Martinez I, Fabregat X, Rosello J, Estape J, Planas J, Gonzalez CA. Lung cancer and cigarette smoking in women: a case-control study in Barcelona (Spain) Int J Cancer. 1994;59:165–9. doi: 10.1002/ijc.2910590204. [DOI] [PubMed] [Google Scholar]
- 115.Agudo A, Ahrens W, Benhamou E, Benhamou S, Boffetta P, Darby SC, Forastiere F, Fortes C, Gaborieau V, Gonzalez CA, Jockel KH, Kreuzer M, Merletti F, Pohlabeln H, Richiardi L, Whitley E, Wichmann HE, Zambon P, Simonato L. Lung cancer and cigarette smoking in women: a multicenter case-control study in Europe. Int J Cancer. 2000;88:820–7. doi: 10.1002/1097-0215(20001201)88:5<820::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 116.Barbone F, Bovenzi M, Cavallieri F, Stanta G. Cigarette smoking and histologic type of lung cancer in men. Chest. 1997;112:1474–9. doi: 10.1378/chest.112.6.1474. [DOI] [PubMed] [Google Scholar]
- 117.MEDICAL Research Council’s statement on tobacco smoking and cancer of the lung. Lancet. 1957;272:1345–7. [PubMed] [Google Scholar]
- 118.Doll R. The first reports on smoking and lung cancer. Clio medica (Amsterdam, Netherlands) 1998;46:130–40. discussion 141–2. [PubMed] [Google Scholar]
- 119.I A.R.C. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans. 2012;100:1–538. [PMC free article] [PubMed] [Google Scholar]
- 120.Engeland A, Andersen A, Haldorsen T, Tretli S. Smoking habits and risk of cancers other than lung cancer: 28 years’ follow-up of 26,000 Norwegian men and women. Cancer causes & control : CCC. 1996;7:497–506. doi: 10.1007/BF00051881. [DOI] [PubMed] [Google Scholar]
- 121.McLaughlin JK, Hrubec Z, Heineman EF, Blot WJ, Fraumeni JF., Jr Renal cancer and cigarette smoking in a 26-year followup of U.S. veterans. Public health reports (Washington, DC : 1974) 1990;105:535–7. [PMC free article] [PubMed] [Google Scholar]
- 122.McLaughlin JK, Lindblad P, Mellemgaard A, McCredie M, Mandel JS, Schlehofer B, Pommer W, Adami HO. International renal-cell cancer study. I. Tobacco use. Int J Cancer. 1995;60:194–8. doi: 10.1002/ijc.2910600211. [DOI] [PubMed] [Google Scholar]
- 123.Nordlund LA, Carstensen JM, Pershagen G. Cancer incidence in female smokers: a 26-year follow-up. Int J Cancer. 1997;73:625–8. doi: 10.1002/(sici)1097-0215(19971127)73:5<625::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 124.De Stefani E, Correa P, Fierro L, Carzoglio J, Deneo-Pellegrini H, Zavala D. Alcohol drinking and tobacco smoking in gastric cancer. A case-control study. Revue d’epidemiologie et de sante publique. 1990;38:297–307. [PubMed] [Google Scholar]
- 125.De Stefani E, Boffetta P, Carzoglio J, Mendilaharsu S, Deneo-Pellegrini H. Tobacco smoking and alcohol drinking as risk factors for stomach cancer: a case-control study in Uruguay. Cancer causes & control : CCC. 1998;9:321–9. doi: 10.1023/a:1008829321668. [DOI] [PubMed] [Google Scholar]
- 126.You WC, Zhang L, Gail MH, Chang YS, Liu WD, Ma JL, Li JY, Jin ML, Hu YR, Yang CS, Blaser MJ, Correa P, Blot WJ, Fraumeni JF, Jr, Xu GW. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. Journal of the National Cancer Institute. 2000;92:1607–12. doi: 10.1093/jnci/92.19.1607. [DOI] [PubMed] [Google Scholar]
- 127.Friedman GD. Cigarette smoking, leukemia, and multiple myeloma. Annals of epidemiology. 1993;3:425–8. doi: 10.1016/1047-2797(93)90071-b. [DOI] [PubMed] [Google Scholar]
- 128.Garfinkel L, Boffetta P. Association between smoking and leukemia in two American Cancer Society prospective studies. Cancer. 1990;65:2356–60. doi: 10.1002/1097-0142(19900515)65:10<2356::aid-cncr2820651033>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 129.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. The Journal of clinical investigation. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nature medicine. 2001;7:833–9. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 131.Wallace DC. Mitochondria and cancer. Nature reviews Cancer. 2012;12:685–98. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee HC, Huang KH, Yeh TS, Chi CW. Somatic alterations in mitochondrial DNA and mitochondrial dysfunction in gastric cancer progression. World journal of gastroenterology. 2014;20:3950–9. doi: 10.3748/wjg.v20.i14.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 134.Santidrian AF, Matsuno-Yagi A, Ritland M, Seo BB, LeBoeuf SE, Gay LJ, Yagi T, Felding-Habermann B. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. The Journal of clinical investigation. 2013;123:1068–81. doi: 10.1172/JCI64264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–4. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 136.Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, Marshall FF, Wallace DC. mtDNA mutations increase tumorigenicity in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:719–24. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–96. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Copeland WC, Wachsman JT, Johnson FM, Penta JS. Mitochondrial DNA alterations in cancer. Cancer investigation. 2002;20:557–69. doi: 10.1081/cnv-120002155. [DOI] [PubMed] [Google Scholar]
- 139.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–62. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 140.Torroni A, Stepien G, Hodge JA, Wallace DC. Neoplastic transformation is associated with coordinate induction of nuclear and cytoplasmic oxidative phosphorylation genes. J Biol Chem. 1990;265:20589–93. [PubMed] [Google Scholar]
- 141.Malins DC, Polissar NL, Gunselman SJ. Progression of human breast cancers to the metastatic state is linked to hydroxyl radical-induced DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2557–63. doi: 10.1073/pnas.93.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ohta S. Contribution of somatic mutations in the mitochondrial genome to the development of cancer and tolerance against anticancer drugs. Oncogene. 2006;25:4768–76. doi: 10.1038/sj.onc.1209602. [DOI] [PubMed] [Google Scholar]
- 143.Will JC, Galuska DA, Ford ES, Mokdad A, Calle EE. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30:540–6. doi: 10.1093/ije/30.3.540. [DOI] [PubMed] [Google Scholar]
- 144.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2007;298:2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 145.Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. Bmj. 1995;310:555–9. doi: 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wannamethee SG, Shaper AG, Perry IJ. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care. 2001;24:1590–5. doi: 10.2337/diacare.24.9.1590. [DOI] [PubMed] [Google Scholar]
- 147.Uchimoto S, Tsumura K, Hayashi T, Suematsu C, Endo G, Fujii S, Okada K. Impact of cigarette smoking on the incidence of Type 2 diabetes mellitus in middle-aged Japanese men: the Osaka Health Survey. Diabetic medicine : a journal of the British Diabetic Association. 1999;16:951–5. doi: 10.1046/j.1464-5491.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 148.Manson JE, Ajani UA, Liu S, Nathan DM, Hennekens CH. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med. 2000;109:538–42. doi: 10.1016/s0002-9343(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 149.Foy CG, Bell RA, Farmer DF, Goff DC, Jr, Wagenknecht LE. Smoking and incidence of diabetes among U.S. adults: findings from the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2501–7. doi: 10.2337/diacare.28.10.2501. [DOI] [PubMed] [Google Scholar]
- 150.Kawakami N, Takatsuka N, Shimizu H, Ishibashi H. Effects of smoking on the incidence of non-insulin-dependent diabetes mellitus. Replication and extension in a Japanese cohort of male employees. Am J Epidemiol. 1997;145:103–9. doi: 10.1093/oxfordjournals.aje.a009080. [DOI] [PubMed] [Google Scholar]
- 151.Ko GT, Chan JC, Tsang LW, Critchley JA, Cockram CS. Smoking and diabetes in Chinese men. Postgrad Med J. 2001;77:240–3. doi: 10.1136/pmj.77.906.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rimm EB, Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Hennekens CH, Speizer FE. Cigarette smoking and the risk of diabetes in women. Am J Public Health. 1993;83:211–4. doi: 10.2105/ajph.83.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 154.Madsbad S, McNair P, Christensen MS, Christiansen C, Faber OK, Binder C, Transbol I. Influence of smoking on insulin requirement and metbolic status in diabetes mellitus. Diabetes Care. 1980;3:41–3. doi: 10.2337/diacare.3.1.41. [DOI] [PubMed] [Google Scholar]
- 155.Nakanishi N, Nakamura K, Matsuo Y, Suzuki K, Tatara K. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Ann Intern Med. 2000;133:183–91. doi: 10.7326/0003-4819-133-3-200008010-00009. [DOI] [PubMed] [Google Scholar]
- 156.Zavaroni I, Bonini L, Gasparini P, Dall’Aglio E, Passeri M, Reaven GM. Cigarette smokers are relatively glucose intolerant, hyperinsulinemic and dyslipidemic. Am J Cardiol. 1994;73:904–5. doi: 10.1016/0002-9149(94)90823-0. [DOI] [PubMed] [Google Scholar]
- 157.Ronnemaa T, Ronnemaa EM, Puukka P, Pyorala K, Laakso M. Smoking is independently associated with high plasma insulin levels in nondiabetic men. Diabetes Care. 1996;19:1229–32. doi: 10.2337/diacare.19.11.1229. [DOI] [PubMed] [Google Scholar]
- 158.Sargeant LA, Khaw KT, Bingham S, Day NE, Luben RN, Oakes S, Welch A, Wareham NJ. Cigarette smoking and glycaemia: the EPIC-Norfolk Study. European Prospective Investigation into Cancer. Int J Epidemiol. 2001;30:547–54. doi: 10.1093/ije/30.3.547. [DOI] [PubMed] [Google Scholar]
- 159.Eliasson B, Mero N, Taskinen MR, Smith U. The insulin resistance syndrome and postprandial lipid intolerance in smokers. Atherosclerosis. 1997;129:79–88. doi: 10.1016/s0021-9150(96)06028-5. [DOI] [PubMed] [Google Scholar]
- 160.Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992;339:1128–30. doi: 10.1016/0140-6736(92)90730-q. [DOI] [PubMed] [Google Scholar]
- 161.Eliasson B, Attvall S, Taskinen MR, Smith U. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur J Clin Invest. 1997;27:450–6. doi: 10.1046/j.1365-2362.1997.1330680.x. [DOI] [PubMed] [Google Scholar]
- 162.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 163.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10–7. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reardon W, Ross RJ, Sweeney MG, Luxon LM, Pembrey ME, Harding AE, Trembath RC. Diabetes mellitus associated with a pathogenic point mutation in mitochondrial DNA. Lancet. 1992;340:1376–9. doi: 10.1016/0140-6736(92)92560-3. [DOI] [PubMed] [Google Scholar]
- 165.Guillausseau PJ, Massin P, Dubois-LaForgue D, Timsit J, Virally M, Gin H, Bertin E, Blickle JF, Bouhanick B, Cahen J, Caillat-Zucman S, Charpentier G, Chedin P, Derrien C, Ducluzeau PH, Grimaldi A, Guerci B, Kaloustian E, Murat A, Olivier F, Paques M, Paquis-Flucklinger V, Porokhov B, Samuel-Lajeunesse J, Vialettes B. Maternally inherited diabetes and deafness: a multicenter study. Ann Intern Med. 2001;134:721–8. doi: 10.7326/0003-4819-134-9_part_1-200105010-00008. [DOI] [PubMed] [Google Scholar]
- 166.Ballinger SW, Shoffner JM, Hedaya EV, Trounce I, Polak MA, Koontz DA, Wallace DC. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nature genetics. 1992;1:11–5. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- 167.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–81. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–91. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 169.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, Kawashima J, Shirotani T, Ichinose K, Brownlee M, Araki E. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun. 2003;300:216–22. doi: 10.1016/s0006-291x(02)02832-2. [DOI] [PubMed] [Google Scholar]
- 171.Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem. 1999;274:27905–13. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- 172.Leloup C, Magnan C, Benani A, Bonnet E, Alquier T, Offer G, Carriere A, Periquet A, Fernandez Y, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes. 2006;55:2084–90. doi: 10.2337/db06-0086. [DOI] [PubMed] [Google Scholar]
- 173.Sabourin J, Allagnat F. Store-operated Ca2+ entry: a key component of the insulin secretion machinery. Journal of molecular endocrinology. 2016;57:F35–f39. doi: 10.1530/JME-16-0106. [DOI] [PubMed] [Google Scholar]
- 174.Satin LS. Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans. Endocrine. 2000;13:251–62. doi: 10.1385/ENDO:13:3:251. [DOI] [PubMed] [Google Scholar]
- 175.Ashcroft FM, Rorsman P. ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion. Biochem Soc Trans. 1990;18:109–11. doi: 10.1042/bst0180109. [DOI] [PubMed] [Google Scholar]
- 176.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Progress in biophysics and molecular biology. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 177.Wollheim CB, Sharp GW. Regulation of insulin release by calcium. Physiological reviews. 1981;61:914–73. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 178.Poulton J, Luan J, Macaulay V, Hennings S, Mitchell J, Wareham NJ. Type 2 diabetes is associated with a common mitochondrial variant: evidence from a population-based case-control study. Hum Mol Genet. 2002;11:1581–3. doi: 10.1093/hmg/11.13.1581. [DOI] [PubMed] [Google Scholar]
- 179.Fuku N, Park KS, Yamada Y, Nishigaki Y, Cho YM, Matsuo H, Segawa T, Watanabe S, Kato K, Yokoi K, Nozawa Y, Lee HK, Tanaka M. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am J Hum Genet. 2007;80:407–15. doi: 10.1086/512202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pravenec M, Hyakukoku M, Houstek J, Zidek V, Landa V, Mlejnek P, Miksik I, Dudova-Mothejzikova K, Pecina P, Vrbacky M, Drahota Z, Vojtiskova A, Mracek T, Kazdova L, Oliyarnyk O, Wang J, Ho C, Qi N, Sugimoto K, Kurtz T. Direct linkage of mitochondrial genome variation to risk factors for type 2 diabetes in conplastic strains. Genome research. 2007;17:1319–26. doi: 10.1101/gr.6548207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Achilli A, Olivieri A, Pala M, Hooshiar Kashani B, Carossa V, Perego UA, Gandini F, Santoro A, Battaglia V, Grugni V, Lancioni H, Sirolla C, Bonfigli AR, Cormio A, Boemi M, Testa I, Semino O, Ceriello A, Spazzafumo L, Gadaleta MN, Marra M, Testa R, Franceschi C, Torroni A. Mitochondrial DNA backgrounds might modulate diabetes complications rather than T2DM as a whole. PLoS One. 2011;6:e21029. doi: 10.1371/journal.pone.0021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 183.Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181:1026–35. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 184.Chow WH, Daling JR, Weiss NS, Voigt LF. Maternal cigarette smoking and tubal pregnancy. Obstet Gynecol. 1988;71:167–70. [PubMed] [Google Scholar]
- 185.Stergachis A, Scholes D, Daling JR, Weiss NS, Chu J. Maternal cigarette smoking and the risk of tubal pregnancy. Am J Epidemiol. 1991;133:332–7. doi: 10.1093/oxfordjournals.aje.a115885. [DOI] [PubMed] [Google Scholar]
- 186.Saraiya M, Berg CJ, Kendrick JS, Strauss LT, Atrash HK, Ahn YW. Cigarette smoking as a risk factor for ectopic pregnancy. Am J Obstet Gynecol. 1998;178:493–8. doi: 10.1016/s0002-9378(98)70427-2. [DOI] [PubMed] [Google Scholar]
- 187.Bouyer J, Coste J, Shojaei T, Pouly JL, Fernandez H, Gerbaud L, Job-Spira N. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185–94. doi: 10.1093/aje/kwf190. [DOI] [PubMed] [Google Scholar]
- 188.DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995;40:385–94. [PubMed] [Google Scholar]
- 189.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182:465–72. doi: 10.1016/s0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 2010;39:45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 191.Little J, Cardy A, Arslan MT, Gilmour M, Mossey PA United Kingdom-based case-control s. Smoking and orofacial clefts: a United Kingdom-based case-control study. Cleft Palate Craniofac J. 2004;41:381–6. doi: 10.1597/02-142.1. [DOI] [PubMed] [Google Scholar]
- 192.Cnattingius S, Haglund B, Meirik O. Cigarette smoking as risk factor for late fetal and early neonatal death. BMJ. 1988;297:258–61. doi: 10.1136/bmj.297.6643.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–26. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 194.Aagaard-Tillery K, Spong CY, Thom E, Sibai B, Wendel G, Jr, Wenstrom K, Samuels P, Simhan H, Sorokin Y, Miodovnik M, Meis P, O’Sullivan MJ, Conway D, Wapner RJ Eunice Kennedy Shriver National Institute of Child Health HDM-FMUN. Pharmacogenomics of maternal tobacco use: metabolic gene polymorphisms and risk of adverse pregnancy outcomes. Obstet Gynecol. 2010;115:568–77. doi: 10.1097/AOG.0b013e3181d06faf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nukui T, Day RD, Sims CS, Ness RB, Romkes M. Maternal/newborn GSTT1 null genotype contributes to risk of preterm, low birthweight infants. Pharmacogenetics. 2004;14:569–76. doi: 10.1097/00008571-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 196.Hsia CC. Respiratory function of hemoglobin. N Engl J Med. 1998;338:239–47. doi: 10.1056/NEJM199801223380407. [DOI] [PubMed] [Google Scholar]
- 197.Longo LD. Carbon monoxide: effects on oxygenation of the fetus in utero. Science. 1976;194:523–5. doi: 10.1126/science.973133. [DOI] [PubMed] [Google Scholar]
- 198.Longo LD. The biological effects of carbon monoxide on the pregnant woman, fetus, and newborn infant. Am J Obstet Gynecol. 1977;129:69–103. doi: 10.1016/0002-9378(77)90824-9. [DOI] [PubMed] [Google Scholar]
- 199.Storm JE, Fechter LD. Prenatal carbon monoxide exposure differentially affects postnatal weight and monoamine concentration of rat brain regions. Toxicol Appl Pharmacol. 1985;81:139–46. doi: 10.1016/0041-008x(85)90128-0. [DOI] [PubMed] [Google Scholar]
- 200.Storm JE, Fechter LD. Alteration in the postnatal ontogeny of cerebellar norepinephrine content following chronic prenatal carbon monoxide. J Neurochem. 1985;45:965–9. doi: 10.1111/j.1471-4159.1985.tb04088.x. [DOI] [PubMed] [Google Scholar]
- 201.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barker DJ, Martyn CN. The maternal and fetal origins of cardiovascular disease. J Epidemiol Community Health. 1992;46:8–11. doi: 10.1136/jech.46.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hirshkowitz M, Karacan I, Howell JW, Arcasoy MO, Williams RL. Nocturnal penile tumescence in cigarette smokers with erectile dysfunction. Urology. 1992;39:101–7. doi: 10.1016/0090-4295(92)90263-v. [DOI] [PubMed] [Google Scholar]
- 204.Gilbert DG, Hagen RL, D’Agostino JA. The effects of cigarette smoking on human sexual potency. Addict Behav. 1986;11:431–4. doi: 10.1016/0306-4603(86)90022-5. [DOI] [PubMed] [Google Scholar]
- 205.Mannino DM, Klevens RM, Flanders WD. Cigarette smoking: an independent risk factor for impotence? Am J Epidemiol. 1994;140:1003–8. doi: 10.1093/oxfordjournals.aje.a117189. [DOI] [PubMed] [Google Scholar]
- 206.Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, McKinlay JB. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30:328–38. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 207.Powell JT. Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med. 1998;3:21–8. doi: 10.1177/1358836X9800300105. [DOI] [PubMed] [Google Scholar]
- 208.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46:91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 209.Amaral A, Lourenco B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction. 2013;146:R163–74. doi: 10.1530/REP-13-0178. [DOI] [PubMed] [Google Scholar]
- 210.Piomboni P, Focarelli R, Stendardi A, Ferramosca A, Zara V. The role of mitochondria in energy production for human sperm motility. Int J Androl. 2012;35:109–24. doi: 10.1111/j.1365-2605.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- 211.Gong J, Zhu T, Kipen H, Wang G, Hu M, Guo Q, Ohman-Strickland P, Lu SE, Wang Y, Zhu P, Rich DQ, Huang W, Zhang J. Comparisons of ultrafine and fine particles in their associations with biomarkers reflecting physiological pathways. Environmental science & technology. 2014;48:5264–73. doi: 10.1021/es5006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–60. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Huerta-Garcia E, Perez-Arizti JA, Marquez-Ramirez SG, Delgado-Buenrostro NL, Chirino YI, Iglesias GG, Lopez-Marure R. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free radical biology & medicine. 2014;73:84–94. doi: 10.1016/j.freeradbiomed.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 214.Upadhyay D, Panduri V, Ghio A, Kamp DW. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: role of free radicals and the mitochondria. American journal of respiratory cell and molecular biology. 2003;29:180–7. doi: 10.1165/rcmb.2002-0269OC. [DOI] [PubMed] [Google Scholar]
- 215.Janssen BG, Byun HM, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics. 2015;10:536–44. doi: 10.1080/15592294.2015.1048412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, Fierens F, Penders J, Vangronsveld J, Gyselaers W, Nawrot TS. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ Health Perspect. 2012;120:1346–52. doi: 10.1289/ehp.1104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Byun HM, Panni T, Motta V, Hou L, Nordio F, Apostoli P, Bertazzi PA, Baccarelli AA. Effects of airborne pollutants on mitochondrial DNA methylation. Part Fibre Toxicol. 2013;10:18. doi: 10.1186/1743-8977-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bell ML. Assessment of the health impacts of particulate matter characteristics. Research report (Health Effects Institute) 2012:5–38. [PubMed] [Google Scholar]
- 219.Lee JH, Kim YS, Song KS, Ryu HR, Sung JH, Park JD, Park HM, Song NW, Shin BS, Marshak D, Ahn K, Lee JE, Yu IJ. Biopersistence of silver nanoparticles in tissues from Sprague-Dawley rats. Part Fibre Toxicol. 2013;10:36. doi: 10.1186/1743-8977-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. American journal of respiratory and critical care medicine. 2001;164:1665–8. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 221.Natarajan V, Wilson CL, Hayward SL, Kidambi S. Titanium Dioxide Nanoparticles Trigger Loss of Function and Perturbation of Mitochondrial Dynamics in Primary Hepatocytes. PLoS One. 2015;10:e0134541. doi: 10.1371/journal.pone.0134541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pryor WA, Squadrito GL, Friedman M. The cascade mechanism to explain ozone toxicity: the role of lipid ozonation products. Free radical biology & medicine. 1995;19:935–41. doi: 10.1016/0891-5849(95)02033-7. [DOI] [PubMed] [Google Scholar]
- 223.Pryor WA, Church DF. Aldehydes, hydrogen peroxide, and organic radicals as mediators of ozone toxicity. Free radical biology & medicine. 1991;11:41–6. doi: 10.1016/0891-5849(91)90186-7. [DOI] [PubMed] [Google Scholar]
- 224.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Free radical biology and medicine: it’s a gas, man! American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R491–511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 225.Lakey PS, Berkemeier T, Tong H, Arangio AM, Lucas K, Poschl U, Shiraiwa M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Scientific reports. 2016;6:32916. doi: 10.1038/srep32916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J, Bittner A, Rao N, Murphy MP, Kirkham PA, Chung KF, Adcock IM. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. The Journal of allergy and clinical immunology. 2015;136:769–80. doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hernandez-Zimbron LF, Rivas-Arancibia S. Oxidative stress caused by ozone exposure induces beta-amyloid 1–42 overproduction and mitochondrial accumulation by activating the amyloidogenic pathway. Neuroscience. 2015;304:340–8. doi: 10.1016/j.neuroscience.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 228.Schulte PA, Chun H. Climate change and occupational safety and health: establishing a preliminary framework. J Occup Environ Hyg. 2009;6:542–54. doi: 10.1080/15459620903066008. [DOI] [PubMed] [Google Scholar]
- 229.U.S.G.C.R.P. The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment. US Global Change Research Program; Washington, DC: 2016. p. 312. [Google Scholar]
- 230.Islam T, Gauderman WJ, Berhane K, McConnell R, Avol E, Peters JM, Gilliland FD. Relationship between air pollution, lung function and asthma in adolescents. Thorax. 2007;62:957–63. doi: 10.1136/thx.2007.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ Health Perspect. 2006;114:627–33. doi: 10.1289/ehp.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: the AHSMOG Study. Environ Res. 1999;80:110–21. doi: 10.1006/enrs.1998.3894. [DOI] [PubMed] [Google Scholar]
- 233.Kurt OK, Zhang J, Pinkerton KE. Pulmonary health effects of air pollution. Current opinion in pulmonary medicine. 2016;22:138–43. doi: 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Laumbach RJ, Kipen HM, Ko S, Kelly-McNeil K, Cepeda C, Pettit A, Ohman-Strickland P, Zhang L, Zhang J, Gong J, Veleeparambil M, Gow AJ. A controlled trial of acute effects of human exposure to traffic particles on pulmonary oxidative stress and heart rate variability. Part Fibre Toxicol. 2014;11:45. doi: 10.1186/s12989-014-0045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li F, Wiegman C, Seiffert JM, Zhu J, Clarke C, Chang Y, Bhavsar P, Adcock I, Zhang J, Zhou X, Chung KF. Effects of N-acetylcysteine in ozone-induced chronic obstructive pulmonary disease model. PLoS One. 2013;8:e80782. doi: 10.1371/journal.pone.0080782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fiordelisi A, Piscitelli P, Trimarco B, Coscioni E, Iaccarino G, Sorriento D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail Rev. 2017 doi: 10.1007/s10741-017-9606-7. [DOI] [PubMed] [Google Scholar]
- 237.Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Perier MC, Marijon E, Vernerey D, Empana JP, Jouven X. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. Jama. 2012;307:713–21. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 238.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–5. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 239.Pope CA, 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–8. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 240.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 241.Franchini M, Mannucci PM. Air pollution and cardiovascular disease. Thrombosis research. 2012;129:230–4. doi: 10.1016/j.thromres.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 242.Ruidavets JB, Cournot M, Cassadou S, Giroux M, Meybeck M, Ferrieres J. Ozone air pollution is associated with acute myocardial infarction. Circulation. 2005;111:563–9. doi: 10.1161/01.CIR.0000154546.32135.6E. [DOI] [PubMed] [Google Scholar]
- 243.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–9. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 244.Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, Benjamin A, Max P, Bard RL, Brook RD. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension (Dallas, Tex : 1979) 2009;53:853–9. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhong J, Cayir A, Trevisi L, Sanchez-Guerra M, Lin X, Peng C, Bind MA, Prada D, Laue H, Brennan KJ, Dereix A, Sparrow D, Vokonas P, Schwartz J, Baccarelli AA. Traffic-Related Air Pollution, Blood Pressure, and Adaptive Response of Mitochondrial Abundance. Circulation. 2016;133:378–87. doi: 10.1161/CIRCULATIONAHA.115.018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li R, Kou X, Geng H, Xie J, Tian J, Cai Z, Dong C. Mitochondrial damage: an important mechanism of ambient PM2.5 exposure-induced acute heart injury in rats. Journal of hazardous materials. 2015;287:392–401. doi: 10.1016/j.jhazmat.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 247.Marchini T, Magnani N, D’Annunzio V, Tasat D, Gelpi RJ, Alvarez S, Evelson P. Impaired cardiac mitochondrial function and contractile reserve following an acute exposure to environmental particulate matter. Biochim Biophys Acta. 2013;1830:2545–52. doi: 10.1016/j.bbagen.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 248.Gottipolu RR, Wallenborn JG, Karoly ED, Schladweiler MC, Ledbetter AD, Krantz T, Linak WP, Nyska A, Johnson JA, Thomas R, Richards JE, Jaskot RH, Kodavanti UP. One-month diesel exhaust inhalation produces hypertensive gene expression pattern in healthy rats. Environ Health Perspect. 2009;117:38–46. doi: 10.1289/ehp.11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Boffetta P, Dosemeci M, Gridley G, Bath H, Moradi T, Silverman D. Occupational exposure to diesel engine emissions and risk of cancer in Swedish men and women. Cancer causes & control : CCC. 2001;12:365–74. doi: 10.1023/a:1011262105972. [DOI] [PubMed] [Google Scholar]
- 250.Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, Sorensen M, Hansen J, Loft S, Overvad K, Tjonneland A. Air pollution from traffic and cancer incidence: a Danish cohort study. Environmental health : a global access science source. 2011;10:67. doi: 10.1186/1476-069X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Soll-Johanning H, Bach E, Olsen JH, Tuchsen F. Cancer incidence in urban bus drivers and tramway employees: a retrospective cohort study. Occup Environ Med. 1998;55:594–8. doi: 10.1136/oem.55.9.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Boeglin ML, Wessels D, Henshel D. An investigation of the relationship between air emissions of volatile organic compounds and the incidence of cancer in Indiana counties. Environ Res. 2006;100:242–54. doi: 10.1016/j.envres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 253.Castano-Vinyals G, Cantor KP, Malats N, Tardon A, Garcia-Closas R, Serra C, Carrato A, Rothman N, Vermeulen R, Silverman D, Dosemeci M, Kogevinas M. Air pollution and risk of urinary bladder cancer in a case-control study in Spain. Occup Environ Med. 2008;65:56–60. doi: 10.1136/oem.2007.034348. [DOI] [PubMed] [Google Scholar]
- 254.Guo J, Kauppinen T, Kyyronen P, Heikkila P, Lindbohm ML, Pukkala E. Risk of esophageal, ovarian, testicular, kidney and bladder cancers and leukemia among finnish workers exposed to diesel or gasoline engine exhaust. Int J Cancer. 2004;111:286–92. doi: 10.1002/ijc.20263. [DOI] [PubMed] [Google Scholar]
- 255.Ji J, Granstrom C, Hemminki K. Occupational risk factors for kidney cancer: a cohort study in Sweden. World J Urol. 2005;23:271–8. doi: 10.1007/s00345-005-0007-5. [DOI] [PubMed] [Google Scholar]
- 256.Kogevinas M, t Mannetje A, Cordier S, Ranft U, Gonzalez CA, Vineis P, Chang-Claude J, Lynge E, Wahrendorf J, Tzonou A, Jockel KH, Serra C, Porru S, Hours M, Greiser E, Boffetta P. Occupation and bladder cancer among men in Western Europe. Cancer causes & control : CCC. 2003;14:907–14. doi: 10.1023/b:caco.0000007962.19066.9c. [DOI] [PubMed] [Google Scholar]
- 257.Liu CC, Tsai SS, Chiu HF, Wu TN, Chen CC, Yang CY. Ambient exposure to criteria air pollutants and risk of death from bladder cancer in Taiwan. Inhal Toxicol. 2009;21:48–54. doi: 10.1080/08958370802207326. [DOI] [PubMed] [Google Scholar]
- 258.Silverman DT, Hoover RN, Mason TJ, Swanson GM. Motor exhaust-related occupations and bladder cancer. Cancer research. 1986;46:2113–6. [PubMed] [Google Scholar]
- 259.Barregard L, Holmberg E, Sallsten G. Leukaemia incidence in people living close to an oil refinery. Environ Res. 2009;109:985–90. doi: 10.1016/j.envres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 260.Crouse DL, Goldberg MS, Ross NA, Chen H, Labreche F. Postmenopausal breast cancer is associated with exposure to traffic-related air pollution in Montreal, Canada: a case-control study. Environ Health Perspect. 2010;118:1578–83. doi: 10.1289/ehp.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hystad P, Villeneuve PJ, Goldberg MS, Crouse DL, Johnson K. Exposure to traffic-related air pollution and the risk of developing breast cancer among women in eight Canadian provinces: a case-control study. Environ Int. 2015;74:240–8. doi: 10.1016/j.envint.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 262.Parent ME, Goldberg MS, Crouse DL, Ross NA, Chen H, Valois MF, Liautaud A. Traffic-related air pollution and prostate cancer risk: a case-control study in Montreal, Canada. Occup Environ Med. 2013;70:511–8. doi: 10.1136/oemed-2012-101211. [DOI] [PubMed] [Google Scholar]
- 263.Loomis D, Huang W, Chen G. The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: focus on China. Chin J Cancer. 2014;33:189–96. doi: 10.5732/cjc.014.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. The European respiratory journal. 2016;48:889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 265.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B, Xun WW, Katsouyanni K, Dimakopoulou K, Sommar J, Forsberg B, Modig L, Oudin A, Oftedal B, Schwarze PE, Nafstad P, De Faire U, Pedersen NL, Ostenson CG, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Sorensen M, Tjonneland A, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita B, Key TJ, de Hoogh K, Concin H, Nagel G, Vilier A, Grioni S, Krogh V, Tsai MY, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Trichopoulou A, Bamia C, Vineis P, Hoek G. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) The Lancet Oncology. 2013;14:813–22. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 266.DeMarini DM. Genotoxicity biomarkers associated with exposure to traffic and near-road atmospheres: a review. Mutagenesis. 2013;28:485–505. doi: 10.1093/mutage/get042. [DOI] [PubMed] [Google Scholar]
- 267.Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013;10:3886–907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001;14:1371–7. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 269.Soberanes S, Panduri V, Mutlu GM, Ghio A, Bundinger GR, Kamp DW. p53 mediates particulate matter-induced alveolar epithelial cell mitochondria-regulated apoptosis. American journal of respiratory and critical care medicine. 2006;174:1229–38. doi: 10.1164/rccm.200602-203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hiura TS, Li N, Kaplan R, Horwitz M, Seagrave JC, Nel AE. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. Journal of immunology (Baltimore, Md : 1950) 2000;165:2703–11. doi: 10.4049/jimmunol.165.5.2703. [DOI] [PubMed] [Google Scholar]
- 271.Risom L, Moller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutation research. 2005;592:119–37. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 272.Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. European journal of endocrinology. 2014;171:R173–82. doi: 10.1530/EJE-14-0365. [DOI] [PubMed] [Google Scholar]
- 273.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–72. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, Tjonneland A, Overvad K, Sorensen M. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35:92–8. doi: 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Brook RD, Cakmak S, Turner MC, Brook JR, Crouse DL, Peters PA, van Donkelaar A, Villeneuve PJ, Brion O, Jerrett M, Martin RV, Rajagopalan S, Goldberg MS, Pope CA, 3rd, Burnett RT. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36:3313–20. doi: 10.2337/dc12-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR, Copes R. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect. 2013;121:804–10. doi: 10.1289/ehp.1205958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Brook RD, Jerrett M, Brook JR, Bard RL, Finkelstein MM. The relationship between diabetes mellitus and traffic-related air pollution. Journal of occupational and environmental medicine. 2008;50:32–8. doi: 10.1097/JOM.0b013e31815dba70. [DOI] [PubMed] [Google Scholar]
- 278.Kramer U, Herder C, Sugiri D, Strassburger K, Schikowski T, Ranft U, Rathmann W. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118:1273–9. doi: 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Eze IC, Schaffner E, Fischer E, Schikowski T, Adam M, Imboden M, Tsai M, Carballo D, von Eckardstein A, Kunzli N, Schindler C, Probst-Hensch N. Long-term air pollution exposure and diabetes in a population-based Swiss cohort. Environ Int. 2014;70:95–105. doi: 10.1016/j.envint.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 280.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–46. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, Bramble LA, Yang Y, Wang A, Harkema JR, Lippmann M, Rajagopalan S, Chen LC, Sun Q. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol. 2011;8:20. doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicological sciences : an official journal of the Society of Toxicology. 2011;124:88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutation research. 2007;636:95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 284.Jacobs M, Zhang G, Chen S, Mullins B, Bell M, Jin L, Guo Y, Huxley R, Pereira G. The association between ambient air pollution and selected adverse pregnancy outcomes in China: A systematic review. The Science of the total environment. 2017;579:1179–1192. doi: 10.1016/j.scitotenv.2016.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Šrám RJ, Binková B, Dejmek J, Bobak M. Ambient Air Pollution and Pregnancy Outcomes: A Review of the Literature. Environ Health Perspect. 2005;113:375–82. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pedersen M, Stayner L, Slama R, Sorensen M, Figueras F, Nieuwenhuijsen MJ, Raaschou-Nielsen O, Dadvand P. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension (Dallas, Tex : 1979) 2014;64:494–500. doi: 10.1161/HYPERTENSIONAHA.114.03545. [DOI] [PubMed] [Google Scholar]
- 287.Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, Ritz B. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect. 2009;117:1773–9. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Padula AM, Mortimer KM, Tager IB, Hammond SK, Lurmann FW, Yang W, Stevenson DK, Shaw GM. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Annals of epidemiology. 2014;24:888–95. e4. doi: 10.1016/j.annepidem.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pereira G, Belanger K, Ebisu K, Bell ML. Fine Particulate Matter and Risk of Preterm Birth in Connecticut in 2000–2006: A Longitudinal Study. Am J Epidemiol. 2014;179:67–74. doi: 10.1093/aje/kwt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Qian Z, Liang S, Yang S, Trevathan E, Huang Z, Yang R, Wang J, Hu K, Zhang Y, Vaughn M, Shen L, Liu W, Li P, Ward P, Yang L, Zhang W, Chen W, Dong G, Zheng T, Xu S, Zhang B. Ambient air pollution and preterm birth: A prospective birth cohort study in Wuhan, China. International journal of hygiene and environmental health. 2016;219:195–203. doi: 10.1016/j.ijheh.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 291.Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA. Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol. 2002;155:17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- 292.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–24. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108:173–6. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pedersen M, Giorgis-Allemand L, Bernard C, Aguilera I, Andersen AM, Ballester F, Beelen RM, Chatzi L, Cirach M, Danileviciute A, Dedele A, Eijsden M, Estarlich M, Fernandez-Somoano A, Fernandez MF, Forastiere F, Gehring U, Grazuleviciene R, Gruzieva O, Heude B, Hoek G, de Hoogh K, van den Hooven EH, Haberg SE, Jaddoe VW, Klumper C, Korek M, Kramer U, Lerchundi A, Lepeule J, Nafstad P, Nystad W, Patelarou E, Porta D, Postma D, Raaschou-Nielsen O, Rudnai P, Sunyer J, Stephanou E, Sorensen M, Thiering E, Tuffnell D, Varro MJ, Vrijkotte TG, Wijga A, Wilhelm M, Wright J, Nieuwenhuijsen MJ, Pershagen G, Brunekreef B, Kogevinas M, Slama R. Ambient air pollution and low birthweight: a European cohort study (ESCAPE) Lancet Respir Med. 2013;1:695–704. doi: 10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- 295.Ritz B, Yu F. The effect of ambient carbon monoxide on low birth weight among children born in southern California between 1989 and 1993. Environ Health Perspect. 1999;107:17–25. doi: 10.1289/ehp.9910717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wilhelm M, Ritz B. Residential proximity to traffic and adverse birth outcomes in Los Angeles county, California, 1994–1996. Environ Health Perspect. 2003;111:207–16. doi: 10.1289/ehp.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Padula AM, Tager IB, Carmichael SL, Hammond SK, Lurmann F, Shaw GM. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am J Epidemiol. 2013;177:1074–85. doi: 10.1093/aje/kws367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;11:502–11. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 299.Dejmek J, Solansky I, Benes I, Lenicek J, Sram RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108:1159–64. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sram RJ, Binkova B, Rossner P, Rubes J, Topinka J, Dejmek J. Adverse reproductive outcomes from exposure to environmental mutagens. Mutation research. 1999;428:203–15. doi: 10.1016/s1383-5742(99)00048-4. [DOI] [PubMed] [Google Scholar]
- 301.Bobak M, Leon DA. The effect of air pollution on infant mortality appears specific for respiratory causes in the postneonatal period. Epidemiology. 1999;10:666–70. [PubMed] [Google Scholar]
- 302.Pope DP, Mishra V, Thompson L, Siddiqui AR, Rehfuess EA, Weber M, Bruce NG. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiologic reviews. 2010;32:70–81. doi: 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- 303.Woodruff TJ, Grillo J, Schoendorf KC. The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States. Environ Health Perspect. 1997;105:608–12. doi: 10.1289/ehp.97105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Selevan SG, Borkovec L, Slott VL, Zudova Z, Rubes J, Evenson DP, Perreault SD. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ Health Perspect. 2000;108:887–94. doi: 10.1289/ehp.00108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Bellizzi D, D’Aquila P, Scafone T, Giordano M, Riso V, Riccio A, Passarino G. The control region of mitochondrial DNA shows an unusual CpG and non-CpG methylation pattern. DNA research : an international journal for rapid publication of reports on genes and genomes. 2013;20:537–47. doi: 10.1093/dnares/dst029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pollack Y, Kasir J, Shemer R, Metzger S, Szyf M. Methylation pattern of mouse mitochondrial DNA. Nucleic Acids Res. 1984;12:4811–24. doi: 10.1093/nar/12.12.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cardon LR, Burge C, Clayton DA, Karlin S. Pervasive CpG suppression in animal mitochondrial genomes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3799–803. doi: 10.1073/pnas.91.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shock LS, Thakkar PV, Peterson EJ, Moran RG, Taylor SM. DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3630–5. doi: 10.1073/pnas.1012311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Weaver SR, Majeed BA, Pechacek TF, Nyman AL, Gregory KR, Eriksen MP. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2015 doi: 10.1007/s00038-015-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, Cummings KM, Sharma E, Pearson JL, Green VR, Kaufman AR, Bansal-Travers M, Travers MJ, Kwan J, Tworek C, Cheng YC, Yang L, Pharris-Ciurej N, van Bemmel DM, Backinger CL, Compton WM, Hyland AJ. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N Engl J Med. 2017;376:342–353. doi: 10.1056/NEJMsa1607538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4:116–28. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Xu X, Rao X, Wang TY, Jiang SY, Ying Z, Liu C, Wang A, Zhong M, Deiuliis JA, Maiseyeu A, Rajagopalan S, Lippmann M, Chen LC, Sun Q. Effect of co-exposure to nickel and particulate matter on insulin resistance and mitochondrial dysfunction in a mouse model. Part Fibre Toxicol. 2012;9:40. doi: 10.1186/1743-8977-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Wallenborn JG, Schladweiler MJ, Richards JH, Kodavanti UP. Differential pulmonary and cardiac effects of pulmonary exposure to a panel of particulate matter-associated metals. Toxicol Appl Pharmacol. 2009;241:71–80. doi: 10.1016/j.taap.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 314.Visalli G, Bertuccio MP, Picerno I, Spataro P, Di Pietro A. Mitochondria dysfunction by pro-oxidant vanadium: Ex vivo assessment of individual susceptibility. Environ Toxicol Phar. 2015;39:93–101. doi: 10.1016/j.etap.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 315.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372:392–4. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 316.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–86. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, Richie JP., Jr Highly reactive free radicals in electronic cigarette aerosols. Chem Res Toxicol. 2015;28:1675–7. doi: 10.1021/acs.chemrestox.5b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Brown MD, Starikovskaya E, Derbeneva O, Hosseini S, Allen JC, Mikhailovskaya IE, Sukernik RI, Wallace DC. The role of mtDNA background in disease expression: a new primary LHON mutation associated with Western Eurasian haplogroup. J Hum Genet. 2002;110:130–8. doi: 10.1007/s00439-001-0660-8. [DOI] [PubMed] [Google Scholar]
- 319.Torroni A, Petrozzi M, D’Urbano L, Sellitto D, Zeviani M, Carrara F, Carducci C, Leuzzi V, Carelli V, Barboni P, De Negri A, Scozzari R. Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet. 1997;60:1107–21. [PMC free article] [PubMed] [Google Scholar]
- 320.Torroni A, Cruciani F, Rengo C, Sellitto D, Lopez-Bigas N, Rabionet R, Govea N, Lopez De Munain A, Sarduy M, Romero L, Villamar M, del Castillo I, Moreno F, Estivill X, Scozzari R. The A1555G mutation in the 12S rRNA gene of human mtDNA: recurrent origins and founder events in families affected by sensorineural deafness. Am J Hum Genet. 1999;65:1349–58. doi: 10.1086/302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Krzywanski DM, Moellering DR, Westbrook DG, Dunham-Snary KJ, Brown J, Bray AW, Feeley KP, Sammy MJ, Smith MR, Schurr TG, Vita JA, Ambalavanan N, Calhoun D, Dell’Italia L, Ballinger SW. Endothelial Cell Bioenergetics and Mitochondrial DNA Damage Differ in Humans Having African or West Eurasian Maternal Ancestry. Circ Cardiovasc Genet. 2016;9:26–36. doi: 10.1161/CIRCGENETICS.115.001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Latorre-Pellicer A, Moreno-Loshuertos R, Lechuga-Vieco AV, Sanchez-Cabo F, Torroja C, Acin-Perez R, Calvo E, Aix E, Gonzalez-Guerra A, Logan A, Bernad-Miana ML, Romanos E, Cruz R, Cogliati S, Sobrino B, Carracedo A, Perez-Martos A, Fernandez-Silva P, Ruiz-Cabello J, Murphy MP, Flores I, Vazquez J, Enriquez JA. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature. 2016;535:561–5. doi: 10.1038/nature18618. [DOI] [PubMed] [Google Scholar]