Abstract
Plant roots play a crucial role in regulating key ecosystem processes such as carbon (C) sequestration and nutrient solubilisation. Elevated (e)CO2 is expected to alter the biomass of fine, coarse and total roots to meet increased demand for other resources such as water and nitrogen (N), however, the magnitude and direction of observed changes vary considerably between ecosystems. Here, we assessed how climate and soil properties mediate root responses to eCO2 by comparing 24 field-based CO2 experiments across the globe including a wide range of ecosystem types. We calculated response ratios (i.e. effect size) and used structural equation modelling (SEM) to achieve a system-level understanding of how aridity, mean annual temperature and total soil nitrogen simultaneously drive the response of total, coarse and fine root biomass to eCO2. Models indicated that increasing aridity limits the positive response of fine and total root biomass to eCO2, and that fine (but not coarse or total) root responses to eCO2 are positively related to soil total N. Our results provide evidence that consideration of factors such as aridity and soil N status is crucial for predicting plant and ecosystem-scale responses to future changes in atmospheric CO2 concentrations, and thus feedbacks to climate change.
Introduction
Plant roots play a major role in regulating important ecosystem functions such as nutrient cycling, C sequestration and plant productivity1,2. Coarse roots (>2 mm) can account for up to 40% of total biomass in terrestrial ecosystems and represent a large fraction of the more stable plant C pool3. The proportion of fine roots (<2 mm) varies between ecosystems, from 5–10% of standing total root biomass in forests to more than 50% in grasslands4–6. Even in ecosystems where fine roots represent a small proportion of standing root biomass, they can transfer up to 65% of the C-fixed annually by the canopy to the soil4,7. Increases in atmospheric CO2 concentrations (eCO2) have the potential to alter the standing biomass of fine and coarse roots differently. While fine root responses are generally considered in the context of altered demand for soil resources, the response of coarse/total root biomass to eCO2 can be seen as a proxy for changes in whole plant long-lived biomass8. For example, after eight years of CO2 exposure at the Duke-Free Air CO2 Enrichment (FACE) experiment, plots receiving increased CO2 had 17% greater coarse root biomass compared to ambient plots9, which was similar to the 19% CO2-enhancement of basal area during the same period10.
Field experiments tend to show enhanced belowground biomass under eCO2 9,11,12. However, the direction and magnitude of these responses vary across different study sites. For instance, during 11 years of CO2 treatment in a scrub-oak shrubland in Florida, eCO2 enhanced fine root biomass only after natural disturbances (i.e. fire and hurricane)13, an effect that was attributed to increased resource availability, including nutrients, water and space. Similarly, four years of eCO2 did not result in any enhancement of fine root biomass in a late successional alpine treeline ecosystem in Switzerland14. Interestingly, some field experiments even report negative eCO2 effects on fine root production15,16, suggesting that the response of root biomass to eCO2 may be driven by multiple interactions with other environmental drivers such as climate and soil properties.
Soil resource availability, including soil N and water, may partly explain contrasting root biomass responses to eCO2 17–19. For example, while eCO2 in conjunction with N fertilization has generally been reported to increase coarse and total root biomass11, the magnitude of eCO2 stimulation of fine root biomass can either increase20 or decrease compared to unfertilized plots exposed to eCO2 6,21. Water limitation is generally believed to amplify aboveground plant growth responses to eCO2 17,22, however, much less is known about the importance of water availability in controlling the response of belowground biomass to eCO2, with contrasting results reported in the recent literature19,23,24. For example, there was no interaction between CO2 and drought treatments for root biomass in a temperate grassland ecosystem24, whereas others found a positive effect of eCO2 on total root biomass only under well-watered conditions23. Such contrasting effects under similar treatment combinations may be due to intrinsic differences in climate and soil properties among ecosystems. However, to date, we lack a system-level understanding of the major environmental factors, including climate and nutrient availability, regulating the response of root biomass to eCO2 at the global scale. This information is critical for predicting ecosystem-level responses to global change and to properly integrate biosphere-atmosphere feedbacks into Earth System Models25.
We used structural equation modelling (SEM) to evaluate how environmental factors regulate root biomass responses to eCO2 using data from 24 field-based experiments across multiple climate and vegetation types (Fig. 1). Previous analyses have examined overall eCO2 effects on root pools, and differences between ecosystem types, experimental facilities, plant functional groups and N fertilization11,12,26. In this study, however, we evaluated the role of climate (aridity and temperature) and soil conditions (soil pH, total soil C and N content) in regulating the magnitude of the responses of total, coarse and fine root biomass to eCO2 (as measured using the lnRR-response ratio27). Therefore, we do not focus on main CO2 effects on belowground biomass pools, which have been presented elsewhere11,12.
Figure 1.
Location of the study sites included in the analysis. This map was created using ArcGIS Desktop 10 (Redlands, CA). http://www.esri.com/.
Given that increased plant C uptake under eCO2 may increase the demand for other resources like water and soil nutrients, the magnitude of eCO2 effects on fine root biomass has been predicted to be more pronounced under water and/or N limitation8,28. Herein, we hypothesize that the magnitude of fine root biomass responses to eCO2 (i.e. the response ratio) will be greater in ecosystems with lower levels of soil water (i.e. higher site aridity) or N content as a functional adaptation of plants to meet resource demand. In addition, multiple limitation theory suggests that the magnitude of the CO2 fertilization effect on overall plant growth should be limited by low availability of soil resources such as water and N, and some studies support this hypothesis18,19. Therefore, we hypothesize that low water and soil N content will decrease the magnitude of total root biomass responses to eCO2. We also posit that the response of fine roots to eCO2 will be more sensitive to water and nutrient limitation than that of coarse roots because coarse root biomass represents long-lived, structural tissues that are less responsive to rapid environmental change.
Results
A total of 27 experiments complied with our selection criteria. However, two of these were conducted in peatlands and one in an alpine ecosystem, reporting soil characteristics from a thick organic layer (up to 20–30 cm.). The disproportionate (more than 10-times higher) C and N contents of the organic layer on these ecosystems compared to the remaining sites (mineral soils) included in the database, and the unique characteristics of C, nutrient and decomposition dynamics in these ecosystems29, led to their exclusion from the analysis. Of the remaining 24 experimental sites, 20, 7 and 15 experiments reported total, coarse and fine root biomass respectively (Fig. 1); seven studies reported responses for both coarse and fine root pools. Since some experiments included multiple species or time points, the database comprised 41 case studies for fine root biomass, 20 for coarse root biomass and 37 for fine root biomass. Overall, 18 experiments were conducted in temperate, 3 in arid and 3 in continental climates (Fig. 1). Of these 24 experiments, 11 were conducted in grasslands, 10 in forest/shrubland ecosystems and 3 in agricultural systems.
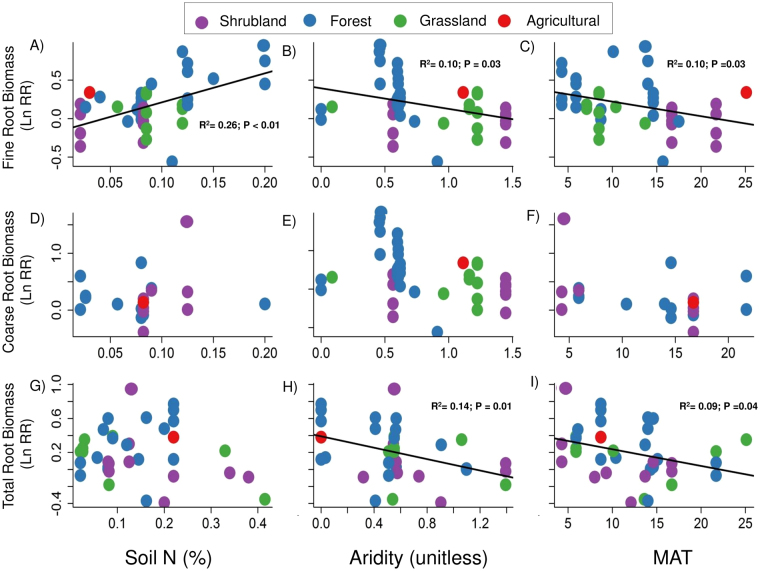
Our a priori model (Fig. 2) incorporated the predictor variables that showed stronger correlations with root responses to eCO2 (Table 1; Fig. 3). Correlation analysis indicated that fine root responses to eCO2 were smaller at low levels of total soil N and high levels of aridity and MAT (Fig. 3A–C). The response of coarse root biomass was not related to aridity, soil N content or MAT (Fig. 3D–F), while the magnitude of eCO2 effects on total root biomass decreased with increasing aridity and MAT (Fig. 3H,I), but was not related to soil N content (Fig. 3G). We found a significant positive correlation between the size of eCO2 effects on fine root biomass and soil total C and a negative correlation between the size of eCO2 effects on fine root biomass and soil C:N ratio, although the correlation coefficients for these variables were weaker than for total soil N (Table 1). Soil pH, C:N ratio and soil C were not correlated with coarse or total root biomass responses to eCO2 (Table 1).
Figure 2.
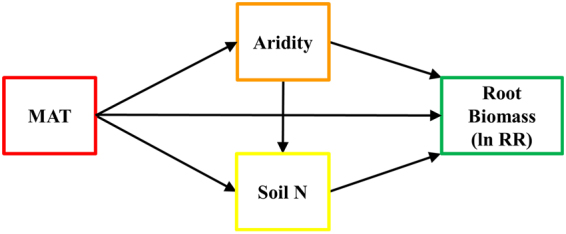
A priori structural equation model depicting the direct and indirect influences of MAT, Aridity and soil N on root biomass responses to eCO2 (lnRR). Boxes indicate measured variables entered in the model.
Table 1.
Correlation coefficients (Pearson r) and p-value (in brackets) between soil pH, soil total C, Soil C:N ratio and mean annual precipitation (MAP) and the response ratios of total root biomass, coarse root biomass and fine root biomass. Bold number indicates significant p-values at 0.05. The sign indicates the direction of the slope. Aridity was calculated as [AI in this dataset – AI in each site] so increases in aridity represent places with low water availability.
| Total Root Biomass (ln RR) | Coarse Root Biomass (ln RR) | Fine Root Biomass (ln RR) | |
|---|---|---|---|
| Soil pH | 0.01 (0.67) | (−) 0.25 (0.11) | (−) 0.27 (0.09) |
| Soil Total C | 0.07 (0.69) | (−) 0.19 (0.44) | 0.334 (0.04) |
| Soil C:N | 0.16 (0.35) | (−) 0.59 (0.65) | (−) 0.35 (0.03) |
| MAP | 0.27 (0.10) | 0.29 (0.33) | (−) 0.32 (0.04) |
Figure 3.
Relationships between cumulative fine, coarse and total root biomass responses to CO2 and site-level explanatory variables used in this study. The solid lines represent the fitted linear correlations. Aridity was calculated as [AI in this dataset – AI in each site] so high values of aridity represent places with low water availability.
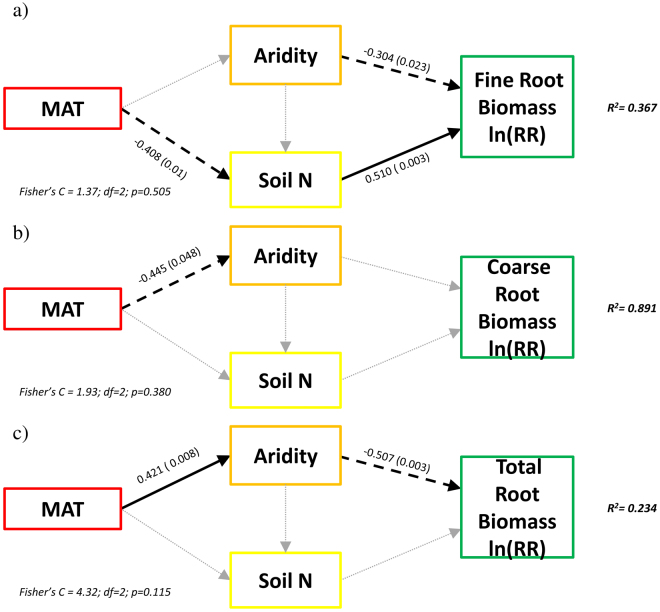
Our SEMs explained 36%, 89% and 23% of the variance found in fine, coarse and total root biomass responses to eCO2, respectively (Fig. 4). Aridity had a direct, negative effect on the responses of fine and total roots to eCO2 (Fig. 4a,c). Moreover, there was a direct, positive relationship between soil total N and fine root responses to eCO2, but not for the total or coarse root pools (Fig. 4a–c). Effects of eCO2 on coarse roots were also unaffected by site aridity (Fig. 4b). Goodness of fit of all SEMs was examined using Fisher’s C tests (Fig. 4).
Figure 4.
Effects of MAT, Aridity and soil N content on: (a) fine root biomass, (b) coarse root biomass and (c) total root biomass response to eCO2. Numbers adjacent to the arrows indicate the effect size of the relationship, while the p-values are shown in brackets. Continuous and dashed black arrows indicate positive and negative relationships, respectively, while grey arrows indicate non-significant relationships. R2 denotes the proportion of the model variance explained. Overall goodness-of-fit test is shown in the bottom of each figure. Aridity was calculated as [AI in this dataset – AI in each site] so increases in aridity represent places with low water availability.
Discussion
Our results provide evidence that enhancement of root biomass by eCO2 is lower at more arid sites, and greater where soil N content is high. Predicted increases in aridity30 may, therefore, limit future biomass responses of both fine and total roots as CO2 concentrations rise. Similarly, expected increases in N deposition31 and associated increases in soil N content may enhance fine root responses to eCO2, although effects on long-lived (coarse) root biomass are less clear. The data presented here summarize the responses of functionally different root compartments to field-based CO2 enrichment in a range of globally dominant ecosystem types (forests, shrublands and grasslands) with contrasting soil conditions and, therefore, have important implications for the understanding of root responses to eCO2 under changing environments.
The more limited stimulation of fine root biomass to eCO2 with increasing aridity may reflect a reduced need to allocate biomass to fine roots where concurrent gains in water use efficiency (WUE) occur. The magnitude of increased WUE under eCO2 has been predicted to be greater in arid-lands, which could explain the trend observed in our analysis32. It has been argued that eCO2 effects on arid-land vegetation tend to reduce the time plants are in water limitation, which prevents over-investment in fine roots33. This, in turn, may promote an increase in aboveground biomass at the expense of fine root biomass32. However, the lack of fine root biomass response to eCO2 under dry conditions does not necessarily imply a lack of eCO2 effect on fine root functional efficiency, since water and nutrient uptake may be more strongly related to other traits such as surface area, length or branching intensity34,35, rather than just biomass. Although there are relatively few data on root morphological responses to eCO2 under field conditions, those studies that have looked at this have reported shifts in these traits towards more acquisitive morphology (e.g. thinner, longer and more branched fine root compartments5,6,12). Our literature search and selection criteria did not, however, provide enough data from independent published studies to analyse this using SEM. Further understanding of the role of eCO2 in driving the responses of plant roots may be improved by including such functional variables.
Previous studies also reported contrasting fine root responses to eCO2 between wet and dry years within the same experiment; those in arid environments have reported a lack of eCO2 effects during dry periods36,37, while studies carried out in moderately or temporarily water-limited ecosystems show a greater relative increase in fine root investment in dry periods38. Taken together, our results and those of earlier studies suggest that eCO2 effects on fine root biomass differ between mesic and arid environments and, therefore, might be particularly influenced by plant life history and the environmental conditions experienced during plant development.
The decreasing (but still positive) response of the total root pool to eCO2 as aridity increased supports our second hypothesis, but challenges the general idea that plant biomass responses are greater (in relative terms) in water-limited ecosystems exposed to eCO2 22,39. There is, however, some empirical support for our observation from field-based CO2 and water manipulation experiments23,24. The general hypothesis that coarse and/or total root growth under eCO2 will be higher under water-limited conditions is usually based on (i) the understanding that WUE typically increases, which may lead to higher soil water content17,40,41 and thus, overall growth; and (ii) the idea that a shift towards relatively greater belowground limitation will increase allocation of new biomass to roots. However, CO2-associated increases in soil water content tend to be relatively small and often restricted to short periods of time in arid ecosystems42 and may, therefore, be insufficient to sustain additional growth of long-lived plant biomass such as coarse/total roots in response to increases in aridity39. Furthermore, productivity responses of stress-tolerant species to eCO2 have been suggested to be lower than for those species associated with more mesic ecosystems43,44, which could also explain the smaller root responses to eCO2 in more water-limited ecosystems. A possible explanation for the lack of larger positive results in arid environments is that a minimum level of soil water is needed for plants to respond positively to eCO2 under water shortage22. Therefore, the previously observed greater positive effects of eCO2 on plant growth in dry periods may be restricted to mesic environments.
As we predicted, effects of eCO2 on fine roots increased as soil N increased. This is in agreement with previous findings that CO2 effects on fine root biomass were larger in N-rich forests compared to N-poor forests21. Given that soil N content mainly represents the organically-bound N pool (i.e. available for plants and microbes in the long-term), the authors argued that a higher biomass of active fine roots – and associated exudates - may be needed to increase N mobilization under higher rates of photosynthetic C assimilation. The mechanism underlying the smaller CO2 response of fine roots under N limitation may also be related to economic trade-offs between C fixation and N acquisition33. The carbon invested in root construction must be fixed from the atmosphere using an N-rich enzyme (i.e. Rubisco). Thus, increased investment of assimilates in fine root material under eCO2 pays for itself in terms of N uptake in N-rich ecosystems, while in N-poor ecosystems, the high C costs of fine root biomass construction and maintenance may restrict plant investment in fine roots.
The response of long-lived plant biomass such as coarse roots to eCO2 has typically been reported to be greater under higher N conditions in both grasslands and forests9,20,45. Surprisingly, we observed no effect of soil N content on coarse and total root biomass responses to eCO2. Several experimental and meta-analytical studies have demonstrated the context-dependent role of mineral N additions on belowground response to eCO2 6,11,45. However, none of these studies have undertaken a quantitative evaluation of the role of soil N content (as opposed to just extractable N) in belowground biomass responses to eCO2. Other studies46 have shown a significant correspondence between increased rates of N mineralization and root biomass responses to eCO2 under contrasting soil N availabilities. Previous meta-analysis21 reported that the direction of eCO2 effects of fine root production differed when comparing N-fertilization experiments and N fertility gradients. For instance, fine root production response to eCO2 was lower in N-fertilized plots compared to unfertilized plots exposed to eCO2, but larger in soils with greater soil N content compared to those with lower N contents21. Under eCO2 conditions, plants may increase N uptake via priming of SOM decomposition47, and from exploration of deeper soil horizons48. Thus, increased C-cost of maintenance of higher fine root biomass and their activity in N-rich ecosystems (Fig. 3C) may preclude C investment to growth of long-lived coarse root tissues18. In addition, potential stoichiometric restrictions of other growth-limiting nutrients such as phosphorus may also limit the response of long-lived biomass to eCO2 despite increased N availability49. Other confounding factors such as the duration of CO2 treatment, plant age and the developmental stage of the study site may also help explain the lack of differences in the relative response of coarse/total root biomass to eCO2 along the N gradient.
Root responses to eCO2 may differ between ecosystem types (e.g., forests vs. grasslands), where fine roots can represent from as little as 10% (in some forests) to more than 70% (in some grasslands) of the standing root biomass4,5. Previous meta-analyses have reported similar (not statistically different) responses of fine and total root biomass to eCO2 (i.e. relative responses) between forest and grasslands11,12, supporting the approach that we adopted in this study. However, it is worth mentioning that the diameter-based classification of root types does not accurately account for functional differences between absorptive and transport/anchorage roots50, and thus, overestimates the percentage of the standing root biomass represented by absorptive roots, particularly in grasslands. An order-based classification could be more appropriate to capture functions (absorption, transport and anchorage) and dynamics (mortality and turnover) of roots under eCO2, particularly in trees, where root branching intensity tends to be higher and is also related to mycorrhizal colonization51. However, an order-based classification has rarely been carried out in field eCO2 experiments, which precluded this type of analysis in our dataset.
By comparing 24 field experiments at a global scale we have demonstrated that root responses to eCO2 appear to be constrained by high aridity and low total soil N. These findings may help inform ecosystem and Earth system model predictions of plant and ecosystem-scale responses to global change, particularly in the context of enhanced N deposition31 and the global expansion of arid ecosystems30. One goal of these models is to simulate feedbacks among ecosystem components and attributes (vegetation, microbes and resources) to predict ecosystem functions for contrasting biomes52. In such efforts, root pools are generally represented as fixed parameters (e.g. CLM4.5 and CABLE), which limits the ability of models to estimate, for example, the land C sink. Our results suggest that, although eCO2 tends to increase belowground biomass, intrinsic ecosystem properties (e.g. soil fertility) and climatic conditions significantly regulate the magnitude of such responses. Carbon, water and nutrient fluxes greatly depend on the biomass of different root components (i.e. fine vs coarse). Therefore, accurate model representation of the variability in responses of the different root fractions to eCO2 may improve model-based predictions of ecosystem functioning in response to changing environmental conditions. Predicted increases in atmospheric CO2 concentrations and N deposition may ultimately lead to ecosystems with higher fine root biomass. In contrast, however, the predicted expansion of arid-lands in a CO2-enriched world may result in lower-than-expected increases in belowground biomass.
Material and Methods
Data collection and extraction
We collected published data from the literature on the responses of root biomass to eCO2 and combined this information with selected climatic (mean annual temperature (MAT), mean annual precipitation (MAP) and aridity index [AI]), geographic (latitude and longitude) and edaphic (pH, organic matter content) data obtained for each study site. We used the meta-analysis published by Nie et al. (2013) as our starting point, but updated the database by searching for relevant studies (Open Top Chamber [OTC] and Free Air CO2 Enrichment [FACE] experiments) published between January 2012 and December 2015 using ISI WEB OF KNOWLEDGE®. We used the keywords “fine root biomass”, “fine root production” and “belowground productivity”, combined with “CO2”, “CO2 fumigation”, “FACE experiment”, “open top chamber” and “global change”. The resulting database led to 315 new articles that, along with the studies included by Nie et al. (2013), were then examined. Studies were only included in our analysis if they:
Reported results of experiments conducted under field conditions. Studies carried out in a greenhouse, growth chamber or using pots were not included.
Evaluated the effect of CO2 treatment on root biomass and production (fine, coarse and/or total). If the experiment was conducted with multiple species, each species was considered as a separate study case. If a published study reported results from multiple soil depths, root biomass results were averaged for the entire soil profile. Although many previous ecological meta-analyses only use results from the last time point of the experiment53,54, it can be argued that, for studies running for long periods (i.e. more than three years), the last point may not be representative of the whole experiment. Therefore, we ran statistical analyses with results averaged from multiple time points across the whole experiment. Given that root biomass responses to eCO2 may decrease from short- to longer periods of treatment13,55 due to more rapid root closure (defined as an equilibrium between production and mortality) in CO2-fumigated plots, when available, we included three time points for those experiments that had been running for more than three years (e.g. Duke FACE, ORNL FACE. In those studies where other treatments (e.g. watering or fertilization) were also applied, we only considered responses to CO2 under ambient conditions for these factors. Given that the main focus of our study is to identify major environmental predictors of the responses of roots to eCO2 in unmanaged ecosystems, the inclusion of other treatments would have obscured the relationships between root responses to eCO2 and climatic or fertility predictors.
Application of these criteria resulted in sufficient data to calculate the CO2 logarithm response ratio (ln RR), obtained as27:
| 1 |
where Xt and Xc are the means of the treatment and control, respectively. When the results were presented graphically, data were extracted using Datathief (www.datathief.org).
For each experiment, the following information was obtained: (1) duration of experimental treatment, (2) soil properties (pH, total C, total N, CN ratio, texture) and (3) ecosystem type (cropland, grassland, shrubland or forest). When such information was not available, authors were contacted to obtain the original data. Mean annual temperature (MAT), mean annual precipitation (MAP) and aridity index (AI; mean annual precipitation/potential evapotranspiration) for each study site were obtained from the WorldClim database56, which provides average climatic values for the period 1950–2000. To improve interpretation, aridity was presented as [maximum value of AI in this dataset – AI in each site] (see57 for a similar approach). Thus, aridity - which is negatively related to AI (r = −1.0; P < 0.001) - represents a metric of water scarcity, instead of a metric of water availability.
Statistical analyses
We used Structural Equation Modelling58 (SEM) to assess the direct and indirect effects of multiple climatic and soil properties on the responses of root biomass to eCO2. Moreover, the use of SEM allowed us to partition the effects that one predictor variable may have on a response variable, and to estimate the strengths of these multiple effects. However, due to the high number of environmental drivers potentially affecting root responses to eCO2, we first used Pearson correlations to explore the association between the effect sizes (ln RR) of fine, coarse and total root biomass and potential explanatory variables (climatic, geographic and edaphic conditions). We only included in our SEM those factors that were shown to be correlated with root responses. Then we established an a priori model (Fig. 2) based on previous knowledge and identified correlations. Since studies along climatic and fertility gradients suggest that temperature, water and N content are the main factors controlling root biomass differences between ecosystems3, our a priori model incorporated these variables59. In accordance with the literature, MAT, aridity and soil total N showed the strongest relationships in the correlation analysis (Table 1; Fig. 3) and so were included in our models as predictor variables. Given the limited number of independent studies that met our selection criteria or provided enough data, we included data from different species within a site and from different publications within the same experiment (for those experiments running for more than three years) in order to meet the data requirements of SEM.
This approach resulted in a hierarchical data structure (i.e. multiple measurements of fine, coarse or total root biomass belonging to different species and time points within experimental sites), which precluded the use of the maximum likelihood method of standard SEM analysis58. We therefore, adopted Shipley’s d-sep method for model evaluation. This approach avoids pseudoreplication by constructing SEM paths as a set of hierarchical linear mixed effects models60,61 and has been suggested to have higher statistical power in studies with small sample size59,62. We fitted linear mixed models for fine, coarse and total root biomass incorporating MAT, aridity, soil total N content as fixed effects, and time and species nested within experimental site as random factors. Linear mixed models were fitted using the “lme” function in the “nlme” package62 and model assumptions were verified by inspecting residuals versus fitted values and quantile–quantile plots. Directional separation analyses were carried out with the “piecewiseSEM” package in R62. Overall goodness-of-fit of the models was tested using Fisher’s C statistic60,61. Non-significant P-values associated with goodness-of fit tests indicate acceptable model fit. Data were transformed when needed to improve linearity in the correlation analysis and SEM models. All statistical analyses were performed using experiment-averaged results.
Given the relatively small number of studies that complied with our selection criteria when compared with other meta-analyses11,12 (see Results), we conducted a standard meta-analysis to elucidate whether our dataset is representative of a larger set of studies. To do so, we calculated cumulative effect sizes for fine, coarse and total root biomass, along with heterogeneity and publication bias assessments (a detailed methodology and results are included in Supplementary Information). Our results are in accordance with previous findings; eCO2 increased fine, coarse and total root biomass by 15, 24 and 23% respectively. In all cases, heterogeneity tests were significant, suggesting that other factors (e.g. climate and soil properties) should be evaluated. In addition, we did not detect publication bias in our dataset, which suggests that our data can be considered as to be a good representation of a larger number of experiments. The finding that only 24 studies complied with our selection criteria (see results) reflects the fact that a large proportion of published studies involving CO2 manipulation had either not been conducted under strictly natural conditions, or did not include an adequate description of the ecosystem’s properties. We call upon the authors of future studies to fully characterize ecosystem properties, so that they can be incorporated into potential meta-analyses.
Electronic supplementary material
Acknowledgements
This research was supported by the Hawkesbury Institute for the Environment Postgraduate Research Scholarship. This study was also funded by the U.S. National Science Foundation (NSF) Long-Term Ecological Research (DEB-9411972, DEB-0080382, DEB-0620652, and DEB-1234162), Biocomplexity Coupled Biogeochemical Cycles (DEB-0322057), Long-Term Research in Environmental Biology (DEB-0716587, DEB-1242531), and Ecosystem Sciences (NSF DEB- 1120064) Programs. We thank John Drake for the constructive comments on earlier versions of this manuscript.
Author Contributions
J.P., R.O.H., M.D.B. and S.A.P. conceived the project. J.P. and S.D. searched and extracted data from published resources. P.B.R. and E.P. provided non-published data. J.P. performed the data analysis (with contributions from R.O.H. and M.D.B.). J.P. wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15728-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hendricks JJ, Nadelhoffer KJ, Aber JD. Assessing the role of fine roots in carbon and nutrient cycling. Trends in Ecology & Evolution. 1993;8:174–178. doi: 10.1016/0169-5347(93)90143-D. [DOI] [PubMed] [Google Scholar]
- 2.Norby RJ, Jackson RB. Root dynamics and global change: seeking an ecosystem perspective. New Phytologist. 2000;147:3–12. doi: 10.1046/j.1469-8137.2000.00676.x. [DOI] [Google Scholar]
- 3.Vogt KA, et al. Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant and Soil. 1995;187:159–219. doi: 10.1007/BF00017088. [DOI] [Google Scholar]
- 4.Jackson RB, Mooney HA, Schulze E-D. A global budget for fine root biomass, surface area, and nutrient contents. Proceedings of the National Academy of Sciences. 1997;94:7362–7366. doi: 10.1073/pnas.94.14.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrillo Y, et al. Disentangling root responses to climate change in a semiarid grassland. Oecologia. 2014;175:699–711. doi: 10.1007/s00442-014-2912-z. [DOI] [PubMed] [Google Scholar]
- 6.Taylor BN, et al. Root length, biomass, tissue chemistry and mycorrhizal colonization following 14 years of CO2 enrichment and 6 years of N fertilization in a warm temperate forest. Tree Physiology. 2014;34:955–965. doi: 10.1093/treephys/tpu058. [DOI] [PubMed] [Google Scholar]
- 7.Lynch DJ, Matamala R, Iversen CM, Norby RJ, Gonzalez-Meler MA. Stored carbon partly fuels fine-root respiration but is not used for production of new fine roots. New Phytologist. 2013;199:420–430. doi: 10.1111/nph.12290. [DOI] [PubMed] [Google Scholar]
- 8.Bazzaz FA. The Response of Natural Ecosystems to the Rising Global CO2 Levels. Annual Review of Ecology and Systematics. 1990;21:167–196. doi: 10.1146/annurev.es.21.110190.001123. [DOI] [Google Scholar]
- 9.Jackson RB, Cook CW, Pippen JS, Palmer SM. Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology. 2009;90:3352–3366. doi: 10.1890/08-1609.1. [DOI] [PubMed] [Google Scholar]
- 10.Moore DJP, et al. Annual basal area increment and growth duration of Pinus taeda in response to eight years of free-air carbon dioxide enrichment. Global Change Biology. 2006;12:1367–1377. doi: 10.1111/j.1365-2486.2006.01189.x. [DOI] [Google Scholar]
- 11.De Graaff M-A, Van Groenigen K-J, Six J, Hungate B, Van Kessel C. Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biology. 2006;12:2077–2091. doi: 10.1111/j.1365-2486.2006.01240.x. [DOI] [Google Scholar]
- 12.Nie M, Lu M, Bell J, Raut S, Pendall E. Altered root traits due to elevated CO2: a meta-analysis. Global Ecology and Biogeography. 2013;22:1095–1105. doi: 10.1111/geb.12062. [DOI] [Google Scholar]
- 13.Day FP, et al. The effects of 11 yr of CO2 enrichment on roots in a Florida scrub-oak ecosystem. New Phytologist. 2013;200:778–787. doi: 10.1111/nph.12246. [DOI] [PubMed] [Google Scholar]
- 14.Handa IT, Hagedorn F, Hättenschwiler S. No stimulation in root production in response to 4 years of in situ CO2 enrichment at the Swiss treeline. Functional Ecology. 2008;22:348–358. doi: 10.1111/j.1365-2435.2007.01372.x. [DOI] [Google Scholar]
- 15.Shaw MR, et al. Grassland responses to global Environmental changes suppressed by Elevated CO2. Science. 2002;298:1987–1990. doi: 10.1126/science.1075312. [DOI] [PubMed] [Google Scholar]
- 16.Bader M, Hiltbrunner E, Körner C. Fine root responses of mature deciduous forest trees to free air carbon dioxide enrichment (FACE) Functional Ecology. 2009;23:913–921. doi: 10.1111/j.1365-2435.2009.01574.x. [DOI] [Google Scholar]
- 17.Morgan JA, et al. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia. 2004;140:11–25. doi: 10.1007/s00442-004-1550-2. [DOI] [PubMed] [Google Scholar]
- 18.Körner, C., Morgan, J. & Norby, R. In Terrestrial Ecosystems in a Changing World (eds Josep G. Canadell, Diane E. Pataki, & Louis F. Pitelka) 9–21 (Springer Berlin Heidelberg, 2007).
- 19.Reich PB, Hobbie SE, Lee TD. Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nature Geosci. 2014;7:920–924. doi: 10.1038/ngeo2284. [DOI] [Google Scholar]
- 20.Pregitzer KS, et al. Interactive Effects of Atmospheric CO2 and Soil-N Availability on Fine Roots of Populus tremuloides. Ecological Applications. 2000;10:18–33. [Google Scholar]
- 21.Dieleman WIJ, et al. Soil [N] modulates soil C cycling in CO2-fumigated tree stands: a meta-analysis. Plant, Cell & Environment. 2010;33:2001–2011. doi: 10.1111/j.1365-3040.2010.02201.x. [DOI] [PubMed] [Google Scholar]
- 22.McMurtrie RE, et al. Why is plant-growth response to elevated CO2 amplified when water is limiting, but reduced when nitrogen is limiting? A growth-optimisation hypothesis. Functional Plant Biology. 2008;35:521–534. doi: 10.1071/FP08128. [DOI] [PubMed] [Google Scholar]
- 23.Derner JD, et al. Above- and below-ground responses of C3–C4 species mixtures to elevated CO2 and soil water availability. Global Change Biology. 2003;9:452–460. doi: 10.1046/j.1365-2486.2003.00579.x. [DOI] [Google Scholar]
- 24.Arndal MF, Schmidt IK, Kongstad J, Beier C, Michelsen A. Root growth and N dynamics in response to multi-year experimental warming, summer drought and elevated CO2 in a mixed heathland-grass ecosystem. Functional Plant Biology. 2013;41:1–10. doi: 10.1071/FP13117. [DOI] [PubMed] [Google Scholar]
- 25.Norby RJ, et al. Model–data synthesis for the next generation of forest free-air CO2 enrichment (FACE) experiments. New Phytologist. 2016;209:17–28. doi: 10.1111/nph.13593. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Manning P, Rist J, Power SA, Marsh C. A global comparison of grassland biomass responses to CO2 and nitrogen enrichment. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:2047–2056. doi: 10.1098/rstb.2010.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. doi: 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2. [DOI] [Google Scholar]
- 28.BassiriRad H, Griffin KL, Reynolds JF, Strain BR. Changes in root NH4+ and NO3− absorption rates of loblolly and ponderosa pine in response to CO2 enrichment. Plant and Soil. 1997;190:1–9. doi: 10.1023/A:1004206624311. [DOI] [Google Scholar]
- 29.Limpens, J., Heijmans, M. M. P. D. & Berendse, F. in Boreal Peatland Ecosystems (eds R. Kelman Wieder & Dale H. Vitt) 195–230 (Springer Berlin Heidelberg, 2006).
- 30.Huang J, Yu H, Guan X, Wang G, Guo R. Accelerated dryland expansion underclimate change. Nature Clim. Change. 2016;6:166–171. [Google Scholar]
- 31.Dentener F, et al. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Global Biogeochemical Cycles. 2006;20:n/a–n/a. doi: 10.1029/2005GB002672. [DOI] [Google Scholar]
- 32.Friedlingstein P, Joel G, Field CB, Fung IY. Toward an allocation scheme for global terrestrial carbon models. Global Change Biology. 1999;5:755–770. doi: 10.1046/j.1365-2486.1999.00269.x. [DOI] [Google Scholar]
- 33.Farrior CE, Rodriguez-Iturbe I, Dybzinski R, Levin SA, Pacala SW. Decreased water limitation under elevated CO2 amplifies potential for forest carbon sinks. Proceedings of the National Academy of Sciences. 2015;112:7213–7218. doi: 10.1073/pnas.1506262112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitter, A. In Plant Roots 15–32 (CRC Press, 2002).
- 35.Coomes DA, Grubb PJ. Impacts of root competition in forest and woodlands: A theoretical framework and review of experiments. Ecological Monographs. 2000;70:171–207. doi: 10.1890/0012-9615(2000)070[0171:IORCIF]2.0.CO;2. [DOI] [Google Scholar]
- 36.Higgins PAT, Jackson RB, Des Rosiers JM, Field CB. Root production and demography in a california annual grassland under elevated atmospheric carbon dioxide. Global Change Biology. 2002;8:841–850. doi: 10.1046/j.1365-2486.2002.00514.x. [DOI] [Google Scholar]
- 37.Ferguson SD, Nowak RS. Transitory effects of elevated atmospheric CO2 on fine root dynamics in an arid ecosystem do not increase long-term soil carbon input from fine root litter. New Phytologist. 2011;190:953–967. doi: 10.1111/j.1469-8137.2011.03654.x. [DOI] [PubMed] [Google Scholar]
- 38.Arnone JA, et al. Dynamics of root systems in native grasslands: effects of elevated atmospheric CO2. New Phytologist. 2000;147:73–85. doi: 10.1046/j.1469-8137.2000.00685.x. [DOI] [Google Scholar]
- 39.Wullschleger SD, Tschaplinski TJ, Norby RJ. Plant water relations at elevated CO2– implications for water-limited environments. Plant, Cell & Environment. 2002;25:319–331. doi: 10.1046/j.1365-3040.2002.00796.x. [DOI] [PubMed] [Google Scholar]
- 40.Hungate BA, et al. Evapotranspiration and soil water content in a scrub-oak woodland under carbon dioxide enrichment. Global Change Biology. 2002;8:289–298. doi: 10.1046/j.1365-2486.2002.00468.x. [DOI] [Google Scholar]
- 41.Lu X, Wang L, McCabe MF. Elevated CO2 as a driver of global dryland greening. Scientific Reports. 2016;6:20716. doi: 10.1038/srep20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newingham BA, et al. No cumulative effect of 10 years of elevated [CO2] on perennial plant biomass components in the Mojave Desert. Global Change Biology. 2013;19:2168–2181. doi: 10.1111/gcb.12177. [DOI] [PubMed] [Google Scholar]
- 43.Rastetter EB, Shaver GR. A Model of Multiple-Element Limitation for Acclimating Vegetation. Ecology. 1992;73:1157–1174. doi: 10.2307/1940666. [DOI] [Google Scholar]
- 44.Inauen N, Körner C, Hiltbrunner E. No growth stimulation by CO2 enrichment in alpine glacier forefield plants. Global Change Biology. 2012;18:985–999. doi: 10.1111/j.1365-2486.2011.02584.x. [DOI] [Google Scholar]
- 45.Sillen WMA, Dieleman WIJ. Effects of elevated CO2 and N fertilization on plant and soil carbon pools of managed grasslands: a meta-analysis. Biogeosciences. 2012;9:2247–2258. doi: 10.5194/bg-9-2247-2012. [DOI] [Google Scholar]
- 46.Reich PB, Hobbie SE. Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nature Clim. Change. 2013;3:278–282. doi: 10.1038/nclimate1694. [DOI] [Google Scholar]
- 47.Drake JE, et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecology Letters. 2011;14:349–357. doi: 10.1111/j.1461-0248.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- 48.Iversen CM, Hooker TD, Classen AT, Norby RJ. Net mineralization of N at deeper soil depths as a potential mechanism for sustained forest production under elevated [CO2] Global Change Biology. 2011;17:1130–1139. doi: 10.1111/j.1365-2486.2010.02240.x. [DOI] [Google Scholar]
- 49.Schleppi P, Bucher-Wallin I, Hagedorn F, Körner C. Increased nitrate availability in the soil of a mixed mature temperate forest subjected to elevated CO2 concentration (canopy FACE) Global Change Biology. 2012;18:757–768. doi: 10.1111/j.1365-2486.2011.02559.x. [DOI] [Google Scholar]
- 50.McCormack ML, et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytologist. 2015;207:505–518. doi: 10.1111/nph.13363. [DOI] [PubMed] [Google Scholar]
- 51.Eissenstat DM, Kucharski JM, Zadworny M, Adams TS, Koide RT. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytologist. 2015;208:114–124. doi: 10.1111/nph.13451. [DOI] [PubMed] [Google Scholar]
- 52.Warren JM, et al. Root structural and functional dynamics in terrestrial biosphere models – evaluation and recommendations. New Phytologist. 2015;205:59–78. doi: 10.1111/nph.13034. [DOI] [PubMed] [Google Scholar]
- 53.Piñeiro J, Maestre FT, Bartolomé L, Valdecantos A. Ecotechnology as a tool for restoring degraded drylands: A meta-analysis of field experiments. Ecological Engineering. 2013;61:133–144. doi: 10.1016/j.ecoleng.2013.09.066. [DOI] [Google Scholar]
- 54.Eldridge DJ, Poore AGB, Ruiz-Colmenero M, Letnic M, Soliveres S. Ecosystem structure, function, and composition in rangelands are negatively affected by livestock grazing. Ecological Applications. 2016;26:1273–1283. doi: 10.1890/15-1234. [DOI] [PubMed] [Google Scholar]
- 55.Stover DB, Day FP, Drake BG, Hinkle CR. The long-term effects of CO2 enrichment on fine root productivity, mortality, and survivorship in a scrub-oak ecosystem at Kennedy Space Center, Florida, USA. Environmental and Experimental Botany. 2010;69:214–222. doi: 10.1016/j.envexpbot.2010.03.003. [DOI] [Google Scholar]
- 56.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 57.Delgado-Baquerizo M, et al. Carbon content and climate variability drive global soil bacterial diversity patterns. Ecological Monographs. 2016;86:373–390. doi: 10.1002/ecm.1216. [DOI] [Google Scholar]
- 58.Grace, J. B. Structural Equation Modeling and Natural Systems. (Cambridge University Press, 2006).
- 59.Shipley, B. Cause and Correlation in Biology: A User’s Guide to Path Analysis, Structural Equations and Causal Inference with R. (Cambridge University Press, 2016).
- 60.Duffy JE, Lefcheck JS, Stuart-Smith RD, Navarrete SA, Edgar GJ. Biodiversity enhances reef fish biomass and resistance to climate change. Proceedings of the National Academy of Sciences. 2016;113:6230–6235. doi: 10.1073/pnas.1524465113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliveira BF, et al. Species and functional diversity accumulate differently in mammals. Global Ecology and Biogeography. 2016;25:1119–1130. doi: 10.1111/geb.12471. [DOI] [Google Scholar]
- 62.Lefcheck J. S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods in Ecology and Evolution. 2016;7:573–579. doi: 10.1111/2041-210X.12512. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.