Abstract
Pyruvate kinase M2 (PKM2), a key protein in glucose and lipid metabolism, has been reported to be related to carcinogenesis in various malignancies. However, its roles in hepatocellular carcinoma with cirrhotic liver (CL) and hepatocellular carcinoma with non-cirrhoticliver (NCL) haves not been investigated. In our study western bloting, qRT-PCR and immunohistochemistry were performed to evaluate the clinical significance of PKM2 protein expression in CL and NCL. The results revealed that PKM2 protein expression was significantly higher in HCC tissues than in their adjacent non-tumour tissues. The high expression rates of PKM2 were more frequently noted in CL (45. 6%) than in NCL (31. 9%) tissues. High PKM2 expression in CL and NCL tissues was significantly associated with vascular invasion (P = 0.002 and P = 0.004, respectively) and intrahepatic metastasis (P < 0.001 and P = 0.019, respectively). Importantly, Kaplan–Meier survival analysis showed that the disease-specific survival (DSS) and recurrence-free survival (RFS) were lower in CL with high PKM2 expression than in NCL with high PKM2 expression (P = 0.003 and P = 0.003, respectively). Overall, high PKM2 expression was more frequently found in CL than in NCL, and PKM2 overexpression was associated with poor survival rates in patients with CL and NCL.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common human cancer and represents the third most frequent cause of cancer deaths worldwide, with an estimated 745 000 deaths occurring annually1–3. Thus far, HCC is one of the few cancers with well-defined major risk factors, such as age and sex (male), hepatitis B and C virus, exposure to toxins (aflatoxin), chronic alcohol abuse and cirrhosis4. The majority of HCC patients often have liver cirrhosis, and the liver cirrhosis is caused by multiple internal and external factors, including alcohol consumption, hepatitis viruses, and fatty liver disease5. In the process of liver cirrhosis, liver cells experience chronic inflammation, fibrosis and scarring, and hepatocellular regeneration, resulting in accumulation of aberrant cells with genetic mutations causally associated with liver malignancy6–8. Chronic liver injury, typically cirrhosis, is the most important and common setting for the development of HCC. However, a portion of HCC cases have been known to occur in patients with a seemingly intact liver9. HCC patients with a non-cirrhotic liver (NCL)represent a relatively small proportion (10–30%) of HCC cases10. Non-cirrhotic HCC is characterized by a large tumour size, has a low risk of liver failure, is more receptive to hepatic and tumor resection and has a generally good prognosis compared to cirrhotic HCC11,12. However, HCC patients with a cirrhotic liver (CL)present with poorer pathological manifestation and worse prognosis, and the 5-year survival rate is only approximately 60% in hepatic resection or liver transplantation patients5. The prognosis of HCC patients with cirrhosis and non-cirrhosis is markedly different. Therefore, there might be different tumourigenic mechanisms between cirrhotic HCC and non-cirrhotic HCC, which highlights the importance of finding effective biomarkers to identify HCC with cirrhosis or non-cirrhosis and to provide personalized detection and therapeutic interventions to improve the survival rate and quality of life.
In the process of tumour growth and progression, changes in substance metabolism accompany uncontrolled cell proliferation13. Moreover, there is growing evidence that cancer is primarily a disease of energy metabolism, especially glucose metabolism14,15. Several recent studies have indicated that aerobic glycolysis (Warburg effect) plays a crucial role in the occurrence and development of tumours16,17. In glycolysis, glucose is enzymatically broken down to pyruvate by pyruvate kinase (PK), thus encouraging oxidative phosphorylation to aerobic glycolysis18. Pyruvate kinase in muscle cells (PKM) exists in two isoforms: PKM1 and PKM2. They are generated due to alternative splicing19. More specifically, pyruvate kinase M2 (PKM2), which converts phosphoenolpyruvate into pyruvate, is a key regulator of aerobic glycolysis because it is an enzyme that catalyses a crucial step20. In recent years, a review of the evidence indicates that high expression of PKM2 has been observed in numerous cancers, such as hilar cholangiocarcinoma, lung cancer, gastric cancer and colorectal cancers21–24. There is increasing evidence that PKM2 is involved in nutritional and metabolic neoplastic disease, but the associations among the protein expression level of PKM2, clinical significance, and the survival rate of HCC patients with cirrhosis or non-cirrhosis is unclear.
In the present study, we investigated the expression level of PKM2 in HCC with cirrhosis or non-cirrhosis and in paracancerous tissues. In addition, we further studied the relationship between PKM2 expression and clinicopathological factors and prognostic significance. We tried to find the influence of PKM2 expression the on the development of HCC with cirrhosis or non-cirrhosis and explored the possibility that PKM2 is a prognostic factors in HCC, and at the same time, we attempted to provide a reliable basis for scientific personalized treatment of HCC.
Results
PKM2 mRNA and protein expression in HCC
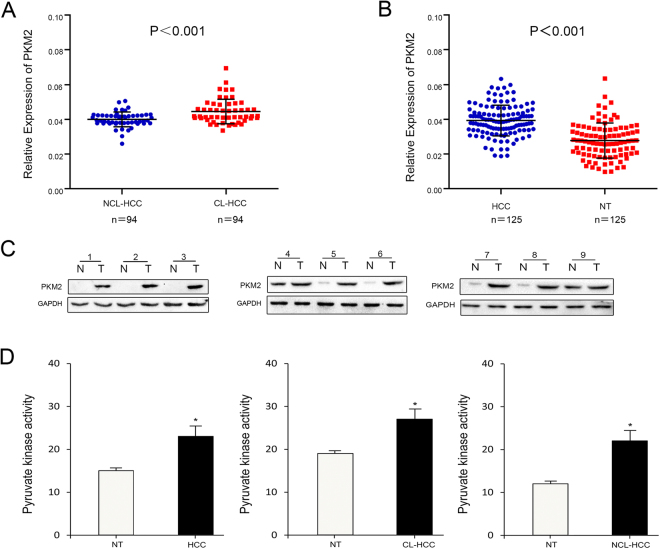
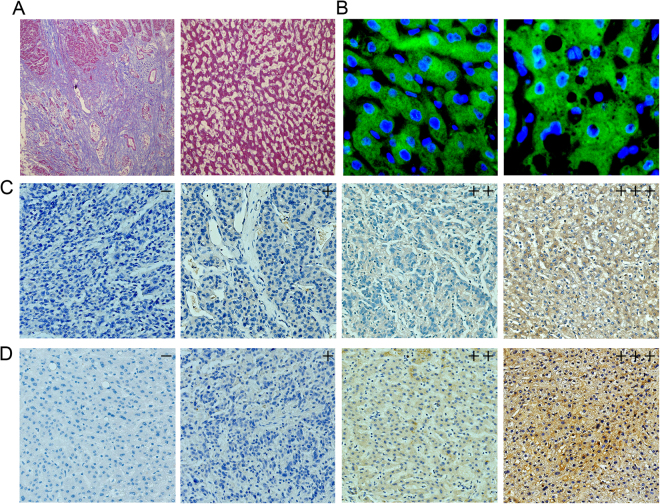
We performed quantitative real-time PCR to examine tissue samples from all patients at the mRNA level. The PCR results showed that tumorous liver tissues exhibited increased PKM2 expression compared with the non-tumorous liver tissues (Fig. 1A,B). We also used western bloting to examine the expressions of PKM2 in each of 9 paired tumorous liver tissues and adjacent non-tumorous liver tissues in cirrhotic HCC and non-cirrhotic HCC. The results showed that tumorous liver tissues exhibited increased PKM2 expression compared with the adjacent non-tumorous liver tissues in cirrhotic HCC and non-cirrhotic HCC (Fig. 1C). We also found that the pyruvate kinase activity was higher in tumorous liver tissues than in non-tumorous liver tissues (P < 0.005) (Fig. 1D). Furthermore, we found a large number of fibre strands between HCC cells in cirrhotic HCC (Fig. 2A), and the expression of PKM2 was mainly concentrated in the cytoplasm (Fig. 2B). We confirmed PKM2 expression in cirrhotic HCC paraffin section and non-cirrhotic HCC paraffin sections. The immunohistochemistry results of indicated that high PKM2 expression was observed in cirrhotic HCC (45. 6%, 57/125) (Fig. 2C) and non-cirrhotic HCC (31. 9%, 30/94) (P < 0.005) (Fig. 2D). However, only high PKM2 expression was observed in the cirrhotic HCC adjacent non-tumour tissues (4%, 5/125) and non-cirrhotic HCC adjacent non-tumour tissues (3.1%, 3/94) (P > 0.005).
Figure 1.
PKM2 mRNA, protein expression and enzyme activity in HCC. (A) The expression levels of PKM2 were measured with quantitative real-time PCR in 94 NCL-HCC and CL-HCC tissues. (B) The expression levels of PKM2 in the 125 HCC tumour and adjacent non-tumour liver tissues (HCC groups consist of randomly selected NCL-HCC and CL-HCC). (C) Protein levels of PKM2 in 9 representative HCC tissues (T) and adjacent non-tumour liver tissues (N) were analysed by western blotting. The uncropped blots details are provided in Supplementary Fig. S1. The relationship between PKM2 and GAPDH is provided in Supplementary Fig. S2. (D) Pyruvate kinase activity in HCC, CL-HCC and NCL-HCC, *P < 0.05.
Figure 2.
Masson’s trichrome staining and PKM2 expression in HCC tissue samples. (A) Masson’s staining in CL-HCC and NCL-HCC tissues (from left to right), ×200. (B) The sub-cellular localization of PKM2 in CL-HCC and NCL-HCC tissues (from left to right), ×200. (C) The expression levels of PKM2 in CL-HCC. (−) Negative expression; (+) Weak expression; (++) Moderate expression; (+++) High expression. Original magnification, ×200. (D) The expression levels of PKM2 in NCL-HCC. (−) Negative expression; (+) Weak expression; (++) Moderate expression; (+++) High expression. Original magnification, ×200.
The relationship between PKM2 and clinicopathological parameters of cirrhotic HCC and non-cirrhotic HCC patients
In our study, 125 cirrhotic HCC cases and 94 non-cirrhotic HCC cases were analysed. There were 32 female (25. 6%) and 93 male (74. 4%) cirrhotic HCC. 30 patients were <45 years old, and 95 patients were ≥45 years old. In the non-cirrhotic HCC group, 27 patients were female (28.7%), 67 patients were male (71.3%), and 27 patients were <45 years old, and 67 patients were ≥45 years old. Our results demonstrated that significant correlations between PKM2 expression in CL and NCL are both closely associated with vascular invasion (P = 0.002 and P = 0.004, respectively), and intrahepatic metastasis (P < 0.001 and P = 0.019, respectively). However, PKM2 status was not significantly associated with gender, age, HBV status, or multiplicity. The details of the basic finding are shown in Table 1.
Table 1.
Patient clinicopathological characteristics and relationship with PKM2 expression.
| Characteristics | N | Cirrhosis HCC | P value | N | Non-cirrhosis HCC | P value | ||
|---|---|---|---|---|---|---|---|---|
| low | high | low | high | |||||
| Gender | ||||||||
| Female | 32 | 18 | 14 | 0.808 | 27 | 19 | 8 | 0.763 |
| Male | 93 | 50 | 43 | 67 | 45 | 22 | ||
| Age | ||||||||
| <45 | 30 | 15 | 15 | 0.579 | 27 | 22 | 5 | 0.077 |
| ≥45 | 95 | 53 | 42 | 67 | 42 | 25 | ||
| Tumor size (cm) | ||||||||
| ≤5 | 48 | 33 | 15 | 0.011 | 40 | 31 | 9 | 0.107 |
| >5 | 77 | 35 | 42 | 54 | 33 | 21 | ||
| AFP (ng/ml) | ||||||||
| ≤20 | 33 | 23 | 10 | 0.04 | 28 | 21 | 7 | 0.349 |
| >20 | 92 | 45 | 47 | 66 | 43 | 23 | ||
| HBsAg | ||||||||
| Positive | 110 | 61 | 49 | 0.521 | 57 | 39 | 18 | 0.622 |
| Negative | 15 | 7 | 8 | 37 | 25 | 12 | ||
| Multiplicity | ||||||||
| Single | 82 | 49 | 33 | 0.097 | 59 | 40 | 19 | 0.938 |
| Multiple (≥2) | 43 | 19 | 24 | 35 | 24 | 11 | ||
| Vascular invasion | ||||||||
| Presence | 67 | 28 | 39 | 0.002 | 52 | 29 | 23 | 0.004 |
| Absence | 58 | 40 | 18 | 42 | 35 | 7 | ||
| Intrahepatic metastasis | ||||||||
| Presence | 59 | 21 | 38 | <0.001 | 37 | 20 | 17 | 0.019 |
| Absence | 66 | 47 | 19 | 57 | 44 | 13 | ||
| TNM stage | ||||||||
| I/II | 82 | 50 | 32 | 0.042 | 58 | 42 | 16 | 0.104 |
| III/IV | 43 | 18 | 25 | 36 | 22 | 14 | ||
| Tumor Differentiation | ||||||||
| poor | 76 | 47 | 29 | 0.037 | 57 | 37 | 20 | 0.413 |
| well | 49 | 21 | 28 | 37 | 27 | 10 | ||
P-values * were calculated using a chi-square (χ2) test. *P < 0.05.
The relationship between high expression of PKM2 and poor disease-specific survival in cirrhotic HCC and non-cirrhotic HCC
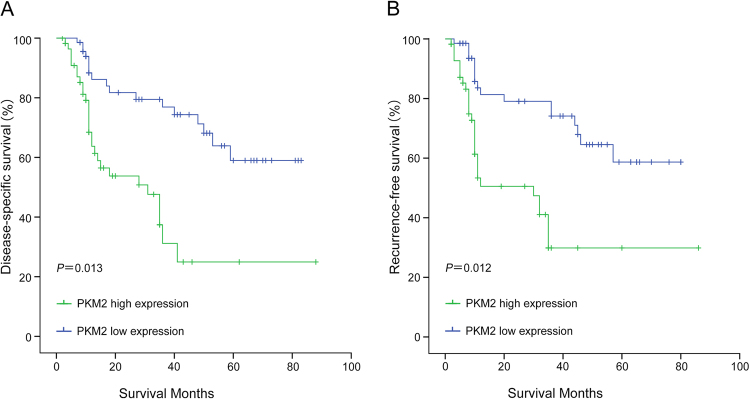
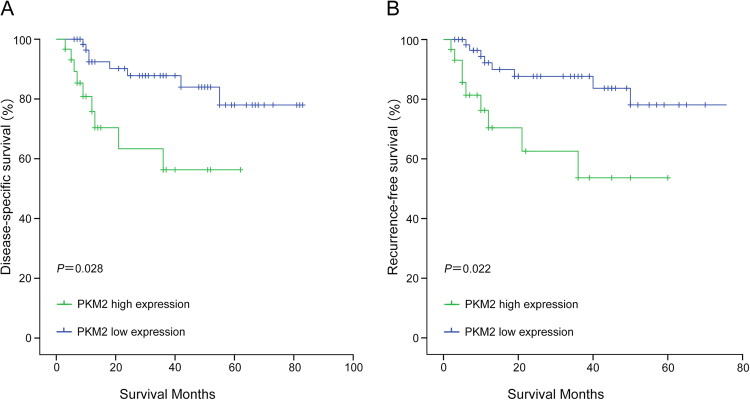
The cumulative survival curves were investigated using the Kaplan-Meier method, and differences in survival times were calculated according to the log rank test. The results show that the 1-year DSS rates in the low PKM2 expression group with cirrhotic HCC and non-cirrhotic HCC were 86. 1% and 86.6%, respectively, and the 3-year DSS rates were 74.8% and 78.3%, respectively. However, the 1-year DSS rates in the high PKM2 expression group with cirrhotic HCC and non-cirrhotic HCC were 73.8% and 75. 8%, respectively. The 3-year DSS rates were 41.0%and 56.3%, respectively. There were significant differences between the two groups in DSS (cirrhotic HCC: P = 0.013, non-cirrhotic HCC: P = 0.028) (Figs 3A and 4A).
Figure 3.
Survival analysis of PKM2 expression using the Kaplan-Meier method. (A) Kaplan–Meier survival curves of DSS in CL-HCC patients according to PKM2 expression. (B) Kaplan–Meier survival curves of RFS in CL-HCC patients according to PKM2 expression.
Figure 4.
Survival analysis of PKM2 expression using the Kaplan-Meier method. (A) Kaplan–Meier survival curves of DSS in NCL-HCC patients according to PKM2 expression. (B) Kaplan–Meier survival curves of RFS in NCL-HCC patients according to PKM2 expression.
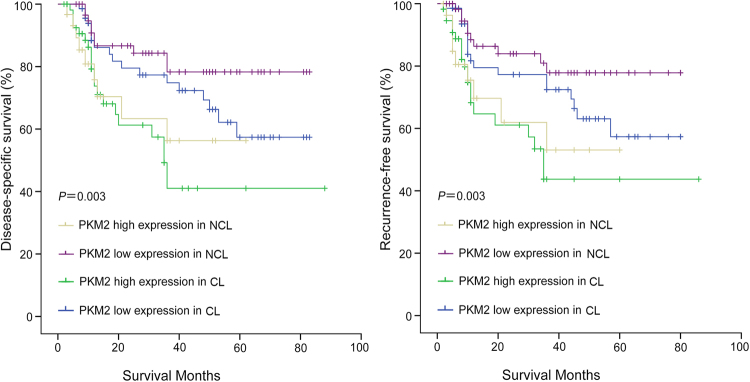
When cirrhotic HCC and non-cirrhotic HCC patients were divided into two groups according to PKM2 expression, through comparative analysis, we found that the disease-specific survival rate was relatively lower in the high PKM2 expression group with cirrhotic HCC than in the high PKM2 expression group with non-cirrhotic HCC (P = 0.003) (Fig. 5A).
Figure 5.
Survival analysis of PKM2 expression using the Kaplan-Meier method. (A) Kaplan–Meier survival curves of DSS between CL-HCC and NCL-HCC patients according to PKM2 expression. (B) Kaplan–Meier survival curves of RFS between CL-HCC and NCL-HCC patients according to PKM2 expression
Univariate analysis revealed that alpha fetoprotein (HR = 1.526, P = 0.047), tumour size (HR = 1.475, P = 0.029), intrahepatic metastasis (HR = 2.191, P = 0.030), TNM stage (HR = 2.857, P = 0.034), vascular invasion(HR = 2.481, P = 0.029), tumour differentiation (HR = 1.753,P = 0.031) and PKM2 expression (HR = 2.240, P = 0.028) were significantly associated with DSS in cirrhotic HCC (Table 2). Tumour size (HR = 3.768, P = 0.036), intrahepatic metastasis (HR = 13.071, P = 0.027), TNM stage (HR = 1.255, P = 0.020), vascular invasion (HR = 1.078, P = 0.017) and PKM2 expression (HR = 5.126, P = 0.014) were adverse prognostic factors affecting DSS in non-cirrhotic HCC after resection (Table 3).
Table 2.
Univariate analysis of different prognostic variables in DSS and RFS of CL-HCC patients using the by Cox proportional hazard model Abbreviations: 95% CI = 95% confidence interval; HR = hazard ratio; TNM stage = tumour node metastasis; P-value < 0.05.
| Variables | DSS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex | ||||||
| male/female | 1.038 | 0.406–2.652 | 0.938 | 0.994 | 0.387–2.557 | 0.990 |
| Age(years) | ||||||
| ≤50/>50 | 0.662 | 0.339–1.292 | 0.227 | 0.689 | 0.358–1.362 | 0.292 |
| AFP(ng/ml) | ||||||
| ≤20/>20 | 1.526 | 0.279–2.990 | 0.047 | 0.528 | 0.279–1.001 | 0.050 |
| HBsAg | ||||||
| yes/no | 0.761 | 0.252–2.297 | 0.628 | 0.737 | 0.243–2.234 | 0.059 |
| Tumor size(cm) | ||||||
| ≤5/>5 | 1.475 | 0.543–1.927 | 0.029 | 3.415 | 1.214–3.805 | 0.009 |
| Multiplicity | ||||||
| Single/multiple | 0.755 | 0.364–1.565 | 0.45 | 0.717 | 0.343–1.499 | 0.377 |
| Intrahepatic Metastasis | ||||||
| yes/no | 2.191 | 0.943–3.854 | 0.03 | 1.212 | 0.748–1.942 | 0.041 |
| TNM stage | ||||||
| (I/II)/(III/VI) | 2.857 | 1.084–7.531 | 0.034 | 3.380 | 1.269–9.003 | 0.015 |
| Vascular Invasion | ||||||
| yes/no | 2.481 | 1.099–5.602 | 0.029 | 2.323 | 1.002–5.383 | 0.049 |
| Tumor Differentiation | ||||||
| Poor/well | 1.753 | 0.910–2.356 | 0.031 | 1.203 | 1.215–1.781 | 0.019 |
| PKM2 expression | ||||||
| low/high | 2.240 | 1.091–4.597 | 0.028 | 2.537 | 1.106–4.965 | 0.028 |
Table 3.
Univariate analysis of different prognostic variables in DSS and RFS of NCL-HCC patients using the Cox proportional hazard model. Abbreviations: 95% CI = 95% confidence interval; HR = hazard ratio; TNM stage = tumour node metastasis; P-value < 0.05.
| Variables | DSS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Sex | ||||||
| male/female | 0.494 | 0.101–2.431 | 0.386 | 0.528 | 0.109–2.548 | 0.426 |
| Age(years) | ||||||
| ≤50/>50 | 0.983 | 0.306–3.163 | 0.977 | 1.145 | 0.356–3.678 | 0.821 |
| AFP(ng/ml) | ||||||
| ≤20/>20 | 0.579 | 0.192–1.744 | 0.331 | 0.815 | 0.272–2.443 | 0.715 |
| HBsAg | ||||||
| yes/no | 0.328 | 0.030–3.560 | 0.359 | 0.409 | 0.038–4.390 | 0.460 |
| Tumor size(cm) | ||||||
| ≤5/>5 | 3.768 | 1.089–13.035 | 0.036 | 2.873 | 0.841–9.818 | 0.092 |
| Multiplicity | ||||||
| Single/multiple | 0.462 | 0.061–3.501 | 0.455 | 0.440 | 0.059–3.275 | 0.423 |
| Intrahepatic Metastasis | ||||||
| yes/no | 13.071 | 1.343–27.203 | 0.027 | 10.985 | 1.206–31.072 | 0.033 |
| TNM stage | ||||||
| (I/II)/(III/VI) | 1.255 | 2.080–3.807 | 0.020 | 1.295 | 0.093–2.937 | 0.039 |
| Vascular Invasion | ||||||
| yes/no | 1.078 | 1.010–2.635 | 0.017 | 1.116 | 0.015–1.866 | 0.036 |
| Tumor Differentiation | ||||||
| Poor/well | 0.854 | 0.210–0.856 | 0.362 | 0.752 | 0.136–0.873 | 0.219 |
| PKM2 expression | ||||||
| low/high | 5.126 | 1.383–9.003 | 0.014 | 4.100 | 4.178–14.268 | 0.027 |
In multivariate analysis, through analysis of the clinical significance of the research data, alpha fetoprotein and hepatitis B virus infection were considered to be closely related with HCC, Therefore, we established three models to carry on the multivariate analysis (Tables 4 and 5). For model 1, the research elements included alpha fetoprotein (AFP) and hepatitis B virus infection status, and the analysis results showed that AFP (HR = 1.412, P = 0.003) was an independent prognostic factor for DSS in patients with cirrhotic HCC; AFP and hepatitis B virus infection status were not prognostic factors for DSS in patients with non-cirrhotic HCC. For model 2, the research elements included AFP, hepatitis B virus infection status and PKM2 expression, and the analysis results showed that AFP (HR = 1.514, P = 0.028) and PKM2 expression (HR = 3.032, P = 0.001) were independent prognostic factors for DSS in patients with cirrhotic HCC; PKM2 expression (HR = 3.960, P = 0.007) was an independent prognostic factor for DSS in patients with non-cirrhotic HCC. Finally, for model 3, the research elements included AFP, hepatitis B virus infection status, tumour size, intrahepatic metastasis, TNM stage, vascular invasion, tumour differentiation and PKM2 expression, and the analysis results showed that tumour size (HR = 1.461, P = 0.019), TNM stage (HR = 3.011, P = 0.018), vascular invasion (HR = 1.895, P = 0.023), tumour differentiation (HR = 1.469, P = 0.043) and PKM2 expression (HR = 2.283, P = 0.034) were independent predictors of DSS in patients with cirrhotic HCC; tumour size (HR = 3.734, P = 0.027), intrahepatic metastasis (HR = 5.832, P = 0.011), TNM stage (HR = 1.260, P = 0.016), vascular invasion (HR = 1.108, P = 0.017), tumour differentiation (HR = 2.387, P = 0.033) and PKM2 expression (HR = 4.857, P = 0.007) were independent predictors of DSS in patients with non-cirrhotic HCC.
Table 4.
Multivariate analysis of different prognostic variables in DSS and RFS of CL-HCC patients using the Cox proportional hazard model.
| Variables | DSS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Model 1 | ||||||
| AFP(ng/ml) | ||||||
| ≤20/>20 | 1.412 | 0.230–1.737 | 0.003 | 1.423 | 0.237–0.756 | 0.004 |
| HBsAg | ||||||
| yes/no | 0.913 | 0.326–2.554 | 0.628 | 0.958 | 0.343–2.678 | 0.925 |
| Model 2 | ||||||
| AFP(ng/ml) | ||||||
| ≤20/>20 | 1.514 | 0.284–1.931 | 0.028 | 1.523 | 0.989–2.947 | 0.032 |
| HBsAg | ||||||
| yes/no | 0.904 | 0.322–2.542 | 0.849 | 0.927 | 0.330–2.604 | 0.885 |
| PKM2 expression | ||||||
| low/high | 3.032 | 1.599–5.749 | 0.001 | 3.020 | 1.602–5.695 | 0.001 |
| Model 3 | ||||||
| AFP(ng/ml) | ||||||
| ≤20/>20 | 0.545 | 0.293–1.013 | 0.055 | 0.551 | 0.298–1.020 | 0.058 |
| HBsAg | ||||||
| yes/no | 0.747 | 0.257–2.175 | 0.593 | 0.753 | 0.258–2.200 | 0.604 |
| Tumor size (cm) | ||||||
| ≤5/>5 | 1.461 | 0.241–1.881 | 0.019 | 1.548 | 0.285–1.053 | 0.041 |
| Intrahepatic Metastasis | ||||||
| yes/no | 0.254 | 0.060–1.073 | 0.062 | 1.236 | 0.0556–1.521 | 0.037 |
| TNM stage | ||||||
| (I/II)/(III/VI) | 3.011 | 1.209–7.502 | 0.018 | 2.519 | 1.019–6.230 | 0.046 |
| Vascular Invasion | ||||||
| yes/no | 1.895 | 0.889–3.993 | 0.023 | 2.051 | 0.988–4.256 | 0.034 |
| Tumor Differentiation | ||||||
| Poor/well | 1.469 | 0.879–1.942 | 0.043 | 1.203 | 0.735–1.547 | 0.039 |
| PKM2 expression | ||||||
| low/high | 2.283 | 1.067–4.888 | 0.034 | 2.181 | 1.054–4.513 | 0.036 |
Abbreviations: 95% CI = 95% confidence interval; HR = hazard ratio; TNM stage = tumour node metastasis; P-value < 0.05.
Table 5.
Multivariate analysis of different prognostic variables in DSS and RFS of NCL-HCC patients using the Cox proportional hazard model.
| Variables | DSS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Model 1 | ||||||
| AFP(ng/ml) | ||||||
| ≤20/>20 | 0.817 | 0.311–2.148 | 0.682 | 0.851 | 0.323–2.236 | 0.743 |
| HBsAg | ||||||
| yes/no | 0.956 | 0.127–7.221 | 0.966 | 0.961 | 0.127–7.529 | 0.970 |
| Model 2 | ||||||
| AFP(ng/ml) | ||||||
| ≤20/>20 | 0.875 | 0.331–2.313 | 0.788 | 0.935 | 0.354–2.471 | 0.892 |
| HBsAg | ||||||
| yes/no | 0.634 | 0.080–4.994 | 0.665 | 0.638 | 0.081–5.025 | 0.669 |
| PKM2 expression | ||||||
| low/high | 3.960 | 1.464–10.712 | 0.007 | 3.812 | 1.417–10.254 | 0.008 |
| Model 3 | ||||||
| AFP(ng/ml) | ||||||
| ≤20/>20 | 0.627 | 0.217–1.815 | 0.390 | 0.862 | 0.298–2.494 | 0.784 |
| HBsAg | ||||||
| yes/no | 0.247 | 0.025–2.422 | 0.230 | 0.306 | 0.032–2.932 | 0.304 |
| Tumor size (cm) | ||||||
| ≤5/>5 | 3.734 | 1.160–12.020 | 0.027 | 2.904 | 0.899–4.381 | 0.045 |
| Intrahepatic Metastasis | ||||||
| yes/no | 5.832 | 1.980–17.095 | 0.011 | 5.354 | 1.810–10.261 | 0.012 |
| TNM stage | ||||||
| (I/II)/(III/VI) | 1.260 | 0.487–1.776 | 0.016 | 2.276 | 1.093–3.818 | 0.020 |
| Vascular Invasion | ||||||
| yes/no | 1.108 | 0.217–1.676 | 0.017 | 1.312 | 1.021–3.819 | 0.030 |
| Tumor Differentiation | ||||||
| Poor/well | 2.387 | 1.302–2.406 | 0.033 | 1.891 | 1.520–3.845 | 0.028 |
| PKM2 expression | ||||||
| low/high | 4.857 | 1.534–15.375 | 0.007 | 3.996 | 1.314–12.154 | 0.015 |
Abbreviations: 95% CI = 95% confidence interval; HR = hazard ratio; TNM stage = tumour node metastasis; P-value < 0.05.
The relationship between high expression of PKM2 and poor recurrence-free survival in cirrhotic HCC and non-cirrhotic HCC
The 1-year RFS rates in the low PKM2 expression group with cirrhotic HCC and non-cirrhotic HCC were 79. 5% and 86.3%, respectively, and the 3-year RFS rates were 72.4% and 77.8%, respectively. However, the 1-year RFS rates in the high PKM2 expression group with cirrhotic HCC and non-cirrhotic HCC were 64.7% and 69.7%, respectively. The 3-year RFS rates were 42.8%and 53.1%, respectively. There was a significant difference between the two groups in RFS (cirrhotic HCC: P = 0.012, non-cirrhotic HCC: P = 0.022) (Figs 3B and 4B).
When cirrhotic HCC and non-cirrhotic HCC were divided into two groups according to PKM2 expression, through comparative analysis, we found that the recurrence-free survival rate was relatively lower in the high PKM2 expression group with cirrhotic HCC than in the high PKM2 expression group with non-cirrhotic HCC (P=0.003) (Fig. 5B).
Univariate analysis revealed that tumour size (HR = 3.415, P = 0.009), intrahepatic metastasis (HR = 1.212, P = 0.041), TNM stage (HR = 3.380,P = 0.015), vascular invasion (HR = 2.323, P = 0.049), tumour differentiation (HR = 1.203, P = 0.019) and PKM2 expression (HR = 2.537, P = 0.028) were significantly associated with RFS in cirrhotic HCC (Table 2). Intrahepatic metastasis (HR = 10.985, P = 0.033), TNM stage (HR = 1.295, P = 0.039), vascular invasion (HR = 1.116, P = 0.036) and PKM2 expression (HR = 4.100, P = 0.027) were adverse prognostic factors affecting RFS in non-cirrhotic HCC after resection (Table 3).
In multivariate analyses, we still used the three different Cox models to analyse the significance of PKM2 for RFS in cirrhotic HCC and non-cirrhotic HCC (Tables 4 and 5). For model 1, the research elements included alpha fetoprotein (AFP) and hepatitis B virus infection status, and the analysis results showed that AFP (HR = 1.423, P = 0.004) was an independent prognostic factor for RFS in patients with cirrhotic HCC; AFP and hepatitis B virus infection status were not prognostic factors for RFS in patients with non-cirrhotic HCC. For model 2, the research elements included AFP, hepatitis B virus infection status and PKM2 expression, and the analysis results showed that AFP (HR = 1.523, P = 0.032) and PKM2 expression (HR = 3.020, P = 0.001) were independent prognostic factors for RFS in patients with cirrhotic HCC; PKM2 expression (HR = 3.812, P = 0.008) was an independent prognostic factor for RFS in patients with non-cirrhotic HCC. Finally, for model 3, the research elements included AFP, hepatitis B virus infection status, tumour size, intrahepatic metastasis, TNM stage, vascular invasion, tumour differentiation and PKM2 expression, and the analysis results showed that tumour size (HR = 1.548, P = 0.041), intrahepatic metastasis (HR = 1.236, P = 0.037), TNM stage (HR = 2.519, P = 0.046), vascular invasion (HR = 2.051, P = 0.034), tumour differentiation (HR = 1.203, P = 0.039) and PKM2 expression (HR = 2.181, P = 0.036) were independent predictors of RFS in patients with cirrhotic HCC; tumour size (HR = 2.904, P = 0.045), intrahepatic metastasis(HR = 5.354, P = 0.012), TNM stage (HR = 2.276, P = 0.020), vascular invasion (HR = 1.312, P = 0.030), tumour differentiation (HR = 1.891, P = 0.028) and PKM2 expression (HR = 3.996, = 0.015) were independent predictors of RFS in patients with non-cirrhotic HCC.
Discussion
Many studies have demonstrated that abnormal glucose metabolism is an important feature of tumour25,26. Regardless of the presence of oxygen, the tumour cells mainly produce energy through glycolysis. The PKM2 gene, located on chromosome 15q22, is a key rate-limiting enzyme in glycolysis and was found to be highly expressed in proliferating cells, especially in tumour cells, and PKM2 upregulation is believed to play a vital role in the Warburg effect and the occurrence a and development of tumors27. However, accurate prediction of the overall survival rates of patients with cirrhosis HCC or non-cirrhosis HCC is still unsatisfactory. Therefore, according to previous studies, we tried to explain the clinicopathological effects and prognostic significance of PKM2 protein expression in cirrhotic HCC and non-cirrhotic HCC. In the present study, we found that PKM2 was more positively expressed in HCC (57.1%) than in adjacent tissues (5.1%) and more frequently expressed in cirrhotic HCC (45.6%) than in non-cirrhosis HCC (31.9%).Meanwhile, PKM2- high expression patients have a poor prognosis. Our results demonstrate that PKM2 is a potentially valuable biomarker to predict the recurrence and survival interval after surgical resection.
In recent years, several studies have indicated that PKM2 is a crucial oncogene in a number of human primary tumours, including lung cancer, cervical cancer, glioblastoma and colorectal cancer28–31. In a study based on 16 months median follow-up of 88 hilar cholangiocarcinoma patients, HK1 and PKM2 levels were found to be higher in human hilar cholangiocarcinoma tumours than in normal tissue samples, and this expression was correlated with lymph node metastasis, tumour differentiation, TNM stage and poor prognosis32. In a previous study that included 266 cases of pancreatic cancer, the results showed that 73% of tumours expressed PKM2 in all cell compartments33. Recent studies have shown that over-expression of PKM2 occours in human HCC34,35. Based on a tissue microarray analysis that included samples from 668 HCC patients with complete clinical records and matched normal samples, Chen al. suggested that PKM2 was an independent prognostic indicator of recurrence-free or overall survival of HCC patients and PKM2 expression was clinicopathologically associated with vascular invasion, tumour number and TNM stage36. In our study, we found that advanced PKM2 expression was a poor independent prognostic indicator and positively related to unfavourable disease-specific survival and recurrence-free survival after surgical dissection. Importantly, we demonstrated that cirrhosis-associated HCC exhibits higher positive PKM2 expression rates than non-cirrhosis-associated HCC. Moreover, the disease-specific survival and recurrence-free survival were lower in cirrhosis HCC than non-cirrhosis HCC with high PKM2 expression. The results of these studies indicate that PKM2 plays an important role in early diagnosis of cancer, evaluation of the effect of tumour and the prognosis of patients.
PKM2 exists in catalytically distinct tetrameric and dimeric states37. Tetrameric PKM2 is mainly in the cytoplasm and participates in synthesis and catabolism of ATP. Dimeric PKM2 is found in cancer cells and is involved in synthesis of nucleic acids and amino acids. PKM2 goes into the nucleus and interacts directly with the HIF-1α subunit and promotes transactivation of HIF-1 target genes by enhancing HIF-1 binding and recruitment of p300, a transcriptional coactivator, which regulates HIF-1 activity. Transcription of the PKM2 gene is also activated by HIF, creating a positive feedback loop that promotes HIF activation and changes glucose metabolism in cancer cells38,39. Dimeric PKM2 can directly phosphorylate signal transducer and activator of transcription 3 (STAT3), which activates inflammatory cytokines, including interleukin-640. PKM2 phosphorylates STAT3 at tyrosine-705 using phosphoenolpyruvate as a phosphate donor, which activates the transcription of mitogen-activated protein kinase 5. The activation of STAT3 in malignant tumour cells may be one of the important molecular markers for tumour progression41. Studies have shown that translocation of PKM2 may activate EGFR42. In PKM2, lysine-433 binds to c-Src-phosphorylated tyrosine-333 on β-catenin, and this results in removal of histone deacetylase 3 (HDAC3) from the promoter, histone H3 acetylation and CCND1 expression43. The transcription of PKM2- dependent β-catenin is required for EGFR promotion of tumour cell proliferation and tumour development44. Recently, a study reported that when the membrane receptor tyrosine kinase (RTKs) -PI3K/AKT-mTOR signalling pathway is over activated, it results in up regulation of PKM2 expression, which leads to an increase in the cellular Warburg effect. The Warburg effect is caused by mTOR using HIF1-mediated PKM gene transcription and c-Myc mediated selective shear to increase the expression of PKM2, which promotes the occurrence and development of tumours45.
A significant feature of malignant tumours cells is that they can switch from oxidative phosphorylation to aerobic glycolysis, known as the Warburg effect, which leads to tumourigenesis and cancer cell proliferation46. Meanwhile, mutation of anti-oncogenes is linked to regulation of proliferation, the cell cycle, and apoptosis and to genetic stability47. PKM2, through dephosphorylation of Cdc25A, promotes PKM2-dependent β-catenin transactivation and c-Myc-mediated upregulation of the expression of the glycolytic genes GLUT1, PKM2 and LDHA. These proteins promote the Warburg effect, cell proliferation and tumourigenesis48.When mitogenic and oncogenic proteins are stimulated by K433 acetylation, FBP binding is inhibited, preventing allosteric activation, and this activates PKM2 protein kinase activity and nuclear localization49. Simultaneously, PKM2 is also regulated by K305 acetylation. Acetylation under high-glucose stimulation targets PKM2 for degradation through chaperone-mediated autophagy and promotes tumour growth50. It was also reported that PKM2 induces EGFR phosphorylation and activates downstream EGFR signalling in triple-negative breast cancer cells51. We found that high PKM2 expression was correlated with vascular invasion and intrahepatic metastasis in our study. More specifically, cirrhosis HCC exhibited higher PKM2 expression and lower survival rates than non-cirrhosis HCC. This demonstrates that high PKM2 expression in tumour tissue can promote tumour cell proliferation and the malignant degree of cancer cells.
In conclusion, in our retrospective study, we are the first to find that PKM2 expression is higher in cirrhosis HCC than in non-cirrhosis HCC. The clinical data analysis indicated that increased PKM2 expression in HCC was correlated with poor prognostic indicators, such as vascular invasion and intrahepatic metastasis. Moreover, cirrhosis HCC, which is more malignant and invasive, has high PKM2 levels were strongly correlated with AFP, multiplicity, TNM stage and tumour differentiation. It is clear that positive PKM2 expression indicates aggressiveness and poor prognosis of HCC. At the same time, the survival analysis results showed that positive PKM2 expression was an independent poor prognostic factor for RFS and DSS. In addition, high PKM2 expression in cirrhosis HCC indicates poorer survival than that in non-cirrhosis HCC. These findings suggest that PKM2 plays an important role in the occurrence and development of tumour and is also an important cause of the tumour malignancy. To the best of our knowledge, this is the first study of PKM2 expression in cirrhosis HCC and non-cirrhosis HCC. Of course, our research is limited, and further studies should investigate the specific mechanism of PKM2 signalling in cirrhosis HCC and non-cirrhosis HCC progression, such as the ways of PKM2 converted to distinct tetrameric and dimeric states, the specific mechanism of dimeric PKM2 entry into the nucleus and the relation of PKM2 and oncogenic signaling pathways. Provide a scientific basis for molecular diagnosis and targeted therapy of cirrhosis HCC and non-cirrhosis HCC.
Materials and Methods
Ethics Statement
The research protocol was reviewed and approved by the Research Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. All experiments were conducted in accordance with approved guidelines of the Second Affiliated Hospital of Chongqing Medical University.
Patients and tissue samples
In our study, all patient tissue samples were collected from May 2004 to May 2010 in the Second Affiliated Hospital of Chongqing Medical University. A total of 219 patients receiveing elective surgery for hepatocellular carcinoma were analysed. These samples included 125 cirrhotic HCC cases and 94 non-cirrhotic HCC cases. All surgical resection specimens were immediately processed after surgical removal, were fixed with 4% paraformaldehyde (pH 7.4) and embedded in paraffin for no longer than 24 h. The histopathological diagnosis of cirrhotic HCC and non-cirrhotic HCC was performed by two experienced independent pathologists in the Department of Pathology Archives of the Second Hospital Affiliated to Chongqing Medical University using haematoxylin and eosin (HE) staining. The diagnosis of cirrhotic HCC and non-cirrhotic HCC was made according to the World Health Organization (WHO) criteria for the study of liver disease.The complete clinical and prognostic data of the study were recorded accurately. The content of the analysis included: age at diagnosis, sex, tumour size, distant metastases, cirrhosis, hepatitis B virus infection, and serum AFP levels (ng/ml), which were obtained from patient medical records. Up to the deadline of May 2016, all respondents with cirrhotic HCC and non-cirrhotic HCC underwent follow-up. The tumour stages were defined according to the seventh edition of the American Joint Committee on Cancer Staging manual. All donors provided written informed consent to donate their samples. The study was performed according to the guidelines of the Declaration of Helsinki and the guidelines of the Ethics Review Committee of the Second Affiliated Hospital of Chongqing Medical University, and each patient signed an informed consent form.
Western blot analysis
The protein in each sample was extracted through tissue homogenation. Similar protein concentrations were loaded into the gel, separated by SDS-PAGE gels electrophoresis (8–12% SDS–PAGE gels), and transferred to a nitrocellulose membrane using the wet transfer method (Bio-Rad, Hercules, CA, USA). The membranes were blocked with TBST (0.05% Tween-20 in TBS) containing 5% skim milk and then incubated overnight with PKM2 (ab150377, Abcam Inc., Cambridge, CA, USA) (1:1000) and GAPDH(ab8245, Abcam Inc., Cambridge, CA, USA) (1:5000) antibodies at 4 °C overnight. Next, the membranes were washed three times in TBST, and then incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibodies(Bio-Rad, Hercules, CA, USA) (1:5000) for 60 minutes at room temperature. Western Blotting Lightning Reagent (203-15291; PerkinElmer) was used to detect the results.
Quantitative real-time PCR
Total RNA was extracted from tissues with Trizol reagent (Takara, Shiga, Japan) according to the manufacturer’s instructions. Primers targeting PKM2 and β-actin (internal control) were synthesized (Shengong Bio, Shanghai, China). Reverse-transcription PCR (RT-PCR) was performed according to instruction provided in a Prime-Script RT Reagent Kit (TaKaRa, Dalian, China). The primer sequences used to amplify PKM2 were 5′-AAGGGTGTGAACCTTCCTGG-3′ and 5′-GCTCGACCCCAAACTTCAGA-3′; β-actin was used as an internal control for normalization.
Immunohistochemistry and evaluation
Briefly, the paraffin-embedded sections (4 μm) were deparaffinized in xylene, and rehydrated in a 100%, 95%, and 85% gradient ethanol series, Antigen retrieval was performed in 10mm citric buffer (pH 6.0) at 100 °C for 15 min. After slides were washed with PBS (pH 7.2), endogenous peroxidase activity was blocked with 3% H2O2 for 15 min. Then, nonspecific protein binding was blocked with goat serum for 15 min at ambient temperature, and the slides were incubated in a moist chamber with antihuman PKM2 rabbit monoclonal antibody at a 1:1000 dilution (rabbit monoclonal antibody, ab38237, 1:1000, Abcam Inc., Cambridge, CA, USA) at 4 °C overnight. Next, after being washed three times in PBS (pH 7.2), slides were incubated with biotinylated secondary antibody at 37 °C for 15 min and visualized with avidin horseradish enzyme at 37 °C for 20 min. The peroxidase reaction was performed using DAB (3,3- diaminobenzidine) for 20 seconds and rinsing in water for 10 min, and then, samples were counterstained with 1% Mayer’s haematoxylin solution, dehydrated in a gradient ethanol series and sealed with neutral gum. To quantify liver fibrosis, the blue pixel content of the images was photographed using the same microscope and magnification times. A semi-quantitative assessment method of scoring was used to judge the expression of PKM2. The evaluation system included staining intensity and the percentage of positive cells, where staining intensity ranged from 0–3 (0, negative; 1, weak; 2, moderate; 3, strong) and the percentage of positive cells ranged from 0–4 (0, negative or <5%1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%). The positive cell percentage scores and the intensity scores were summed to determine the expression of PKM2. Final staining scores <4 were identified as low PKM2 expression, while scores ≥4 were identified as high PKM2 expression.
Measurement of pyruvate kinase activity
Pyruvate kinase activity was measured by Pyruvate Kinase Assay Kit (Abcam, Cambridge, MA, USA) and according to the manufacturer’s instructions.
Co-immunoprecipitation (co-IP) assays
The protein in each sample was extracted through tissue homogenation. The co- immunoprecipitated using the Co-IP Kit (Pierce) according to the manufacturer’s instructions. The immunoprecipitates were washed four times with lysis buffer and stored at −80 °C until needed or were boiled with Laemmli sample buffer, followed by SDS–PAGE and western blotting.
Statistical Analysis
All the experiment data were repeated at least three times. The research data are presented as the mean ± standard deviation for normally distributed continuous variables, and count data are presented as the frequency or percentage for categorical variables. The difference in mRNA expression of PKM2 was determined by wilcoxon matched-pairs signed rank test. Fisher’s exact test or chi square test was used to evaluate the relationships between PKM2 expression and clinicopathological parameters in cirrhotic HCC and non-cirrhotic HCC. The differences in survival between cirrhotic HCC and non-cirrhotic HCC were compared using the Kaplan-Meier method (log-rank test). Univariate and multivariate Cox regression analyses were performed to study the effects of PKM2 expression on different variables in cirrhotic HCC and non-cirrhotic HCC.P < 0.05 was the threshold for statistical significance.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethical committee of the Second Affiliated Hospital of Chongqing Medical University and with the 1964 Helsinki Declaration and its later amendments or comparable.
Ethical standards
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Protein levels of PKM2 in 9 representative HCC tissues
Acknowledgements
This work was supported by Key Foundation of Sichuan Municipal Commission of Health and Family Planning (No. 17ZD008) and the Health Bureau of Chongqing City, projects (No 2015ZDXM012).
Author Contributions
Y.L. and H.W. contributed equally to this manuscript. C.P.L., M.M.D. and J.P.G. designed the research and revised the manuscript. Y.L., H.W., Y.M. and X.L.Y. analyzed the data; Y.L. and H.W. performed the research and wrote the paper. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Yan Liu and Hao Wu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14813-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Changping Li, Email: 506854209@qq.com.
Mingming Deng, Email: 793070544@qq.com.
Jianping Gong, Email: gongjianping11@126.com.
References
- 1.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Media centre. Title of subordinate document. In: Cancer http://www.who.int/mediacentre/factsheets/fs297/en/ (2015)
- 4.Bosetti C, Turati F, Vecchia CL. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevisani F, et al. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Kwon JH, et al. AROS Is a Significant Biomarker for Tumor Aggressiveness in Non-cirrhotic Hepatocellular Carcinoma. J Korean Med Sci. 2015;30:1253–1259. doi: 10.3346/jkms.2015.30.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, et al. Differential reactivation of fetal/neonatal genes in mouse liver tumors induced in cirrhotic and non-cirrhotic conditions. Cancer Sci. 2015;106:972–81. doi: 10.1111/cas.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, et al. Clinicopathologic features and long-term outcomes of Chinese patients with hepatocellular carcinoma in non-cirrhotic liver. Dig Surg. 2008;25:376–382. doi: 10.1159/000170881. [DOI] [PubMed] [Google Scholar]
- 11.Laurent C, et al. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg. 2005;201:656–662. doi: 10.1016/j.jamcollsurg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Chen MF, et al. Prognostic factors after resection for hepatocellular carcinoma in noncirrhotic livers: univariate and multivariate analysis. World J Surg. 2003;27:443–447. doi: 10.1007/s00268-002-6708-7. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutrition & metabolism. 2010;7:7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Lu X, Wang Z. Co-expression of PKM2 and TRIM35 predicts survival and recurrence in hepatocellular carcinoma. Oncotarget. 2015;10:2538–2548. doi: 10.18632/oncotarget.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 17.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Yang W, Lu Z. Nuclear PKM2 regulates the Warburg effect. Cell cycle. 2013;12:3154–3158. doi: 10.4161/cc.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261:13807–13812. [PubMed] [Google Scholar]
- 20.Vander H, Cantley MG, Thompson LC. Science. 2009. C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation; pp. 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu G, et al. PKM2 regulates neural invasion of and predicts poor prognosis for human hilar cholangiocarcinoma. Mol Cancer. 2015;14:193. doi: 10.1186/s12943-015-0462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Z, et al. PKM2 Thr454 phosphorylation increases its nuclear translocation and promotes xenograft tumor growth in A549 human lung cancer cells. Biochem Biophys Res Commun. 2016;13:953–958. doi: 10.1016/j.bbrc.2016.03.160. [DOI] [PubMed] [Google Scholar]
- 23.Tang R, et al. MiR-let-7a inhibits cell proliferation. migration, and invasion by down-regulating PKM2 in gastric cancer. Oncotarget. 2016;7:5972–5984. doi: 10.18632/oncotarget.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi K, Sugito N, Kumazaki M. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363:17–27. doi: 10.1016/j.canlet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation.Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Wang HJ, et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1alpha-mediated glucose metabolism. Proc Natl Acad Sci USA. 2014;111:279–284. doi: 10.1073/pnas.1311249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, et al. PKM2 and ACVR 1C are prognostic markers for poor prognosis of gallbladder cancer. Clin Transl Oncol. 2014;16:200–207. doi: 10.1007/s12094-013-1063-8. [DOI] [PubMed] [Google Scholar]
- 28.Papadaki C, et al. PKM2 as a biomarker for chemosensitivity to front-line platinum-based chemotherapy in patients with metastatic non-small-cell lung cancer. British journal of cancer. 2014;111:1757–64. doi: 10.1038/bjc.2014.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaura B, Bagga R, Patel FD. Evaluation of the Pyruvate Kinase isoenzyme tumor (Tu M2-PK) as a tumor marker for cervical carcinoma. The journal of obstetrics and gynaecology research. 2004;30:193–196. doi: 10.1111/j.1447-0756.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee J, et al. PKM2 uses control of HuR localization to regulate p27 and cell cycle progression in human glioblastoma cells. International Journal of Cancer. 2016;139:99–111. doi: 10.1002/ijc.30041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haug U, Hundt S, Brenner H. Sensitivity and specificity of faecal tumour M2 pyruvate kinase for detection of colorectal adenomas in a large screening study. British journal of cancer. 2008;99:133–135. doi: 10.1038/sj.bjc.6604427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu G, et al. PKM2 regulates neural invasion of and predicts poor prognosis for human hilar cholangiocarcinoma. Mol Cancer. 2015;14:193. doi: 10.1186/s12943-015-0462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammad GH, et al. Pyruvate Kinase M2 and Lactate Dehydrogenase A Are Overexpressed in Pancreatic Cancer and Correlate with Poor Outcome. PLoS One. 2016;18:e0151635. doi: 10.1371/journal.pone.0151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YL, et al. Mechanisms of pyruvate kinase M2 isoform inhibits cell motility in hepatocellular carcinoma cells. World J Gastroenterol. 2015;21:9093–9102. doi: 10.3748/wjg.v21.i30.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, et al. WTRIM35 Interacts with pyruvate kinase isoform M2 to suppress the Warburg effect and tumorigenicity in hepatocellular carcinoma. Oncogene. 2015;23:3946–3956. doi: 10.1038/onc.2014.325. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, et al. Co-expression of PKM2 and TRIM35 predicts survival and recurrence in hepatocellular carcinoma. Oncotarget. 2015;6:2538–2548. doi: 10.18632/oncotarget.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;4:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Luo W, et al. Pandey A and Semenza GL: Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia?inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo W, Semenza GL. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget. 2011;2:551–556. doi: 10.18632/oncotarget.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao X, et al. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45:598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang P, et al. Pyruvate kinase M2 facilitates colon cancer cell migration via the modulation of STAT3 signalling. Cell Signal. 2014;26:1853–1862. doi: 10.1016/j.cellsig.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang W, Lu Z. Nuclear PKM2 regulates the Warburg effect. Cell Cycle. 2013;12:3154–3158. doi: 10.4161/cc.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H, et al. PKM2 depletion induces the compensation of glutaminolysis through β-catenin/c-Myc pathway in tumor cells. Cell Signal. 2014;26:2397–2405. doi: 10.1016/j.cellsig.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Sun Q, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA. 2011;10:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bensinger SJ, Christofk HR. New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol. 2012;23:352–261. doi: 10.1016/j.semcdb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Yang X, Xu T. Molecular mechanism of size control in development and human diseases. Cell Res. 2011;21:715–729. doi: 10.1038/cr.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang J, et al. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat Commun. 2016;7:12431. doi: 10.1038/ncomms12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv L, et al. Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol Cell. 2013;52:340–352. doi: 10.1016/j.molcel.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv L, et al. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol Cell. 2011;42:719–730. doi: 10.1016/j.molcel.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu MC, et al. Extracellular PKM2 induces cancer proliferation by activating the EGFR signaling pathway. Am J Cancer Res. 2016;6:628–638. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein levels of PKM2 in 9 representative HCC tissues