Abstract
Progressive liver fibrosis, induced by chronic viral and metabolic disorders, leads to more than one million deaths annually via development of cirrhosis, although no antifibrotic therapy has been approved to date. Transdifferentiation (or “activation”) of hepatic stellate cells is the major cellular source of matrix protein-secreting myofibroblasts, the major driver of liver fibrogenesis. Paracrine signals from injured epithelial cells, fibrotic tissue microenvironment, immune and systemic metabolic dysregulation, enteric dysbiosis, and hepatitis viral products can directly or indirectly induce stellate cell activation. Dysregulated intracellular signaling, epigenetic changes, and cellular stress response represent candidate targets to deactivate stellate cells by inducing reversion to inactivated state, cellular senescence, apoptosis, and/or clearance by immune cells. Cell type- and target-specific pharmacological intervention to therapeutically induce the deactivation will enable more effective and less toxic precision antifibrotic therapies.
Keywords: Myofibroblast, cirrhosis, hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease, nonalcoholic steatohepatitis
Graphical abstract
1. Introduction
Chronic tissue injury leads to a sustained scarring response that gradually disrupts normal cellular functional units and eventually causes failure in multiple epithelial organs such as liver, lung, and kidney, which is estimated to account for one-third of deaths worldwide [1]. Progressive liver fibrosis can be caused by chronic infection of hepatitis B virus (HBV) or hepatitis C virus (HCV), alcohol abuse, non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH), and other relatively rare conditions such as autoimmune hepatitis, hemochromatosis, Wilson’s disease, and primary/secondary biliary cholangitis. Cirrhosis is the terminal stage of progressive liver fibrosis, which is estimated to affect 1% to 2% of global population and results in over 1 million deaths annually worldwide [2, 3].
Lethal complications of cirrhosis include functional liver failure, portal hypertension-induced variceal bleeding, ascites, and hepatic encephalopathy, systemic bacterial infection, and liver cancer, especially hepatocellular carcinoma (HCC) [3]. Annual direct and indirect costs for the care of cirrhosis exceed $12 billion in the U.S. alone [4]. Although approved therapies directly targeting and reversing advanced fibrosis are still lacking, clinical studies have indicated that liver fibrosis and even cirrhosis can be regressed by therapeutic intervention aimed at the primary disease etiology [5]. In this review, we summarize recent findings relevant to direct therapeutic targeting of the major fibrogenic cell type in the liver, the activated hepatic stellate cell (HSC), as an antifibrotic strategy.
2. Cellular sources of matrix-producing myofibroblasts
2.1. Myofibroblasts: driver of tissue fibrogenesis in multiple organs
In chronic fibro-proliferative diseases, affecting multiple organs such as lung, kidney, and liver, presence of myofibroblasts is a key common feature [6]. The myofibroblast is a fibroblast-like cell with contractile properties, which is derived typically from cells of mesenchymal lineage via transdifferentiation, often referred to as “activation”. Proliferating myofibroblasts are the key source of excess extracellular matrix (ECM) molecules such as collagen type I and III as well as other proteins that constitute pathologic fibrous tissues [7]. Paracrine factors such as platelet-derived growth factor (PDGF), transforming growth factor β (TGFβ), and connective tissue growth factor (CTGF), together with other growth factors and cytokines/chemokines, activate cellular signaling pathways of cell proliferation, migration, ECM protein secretion, and contractility that promote transdifferentiation of precursor mesenchymal cells (e.g., pericytes and resident fibroblasts) into fibrogenic myofibroblasts and maintain their activated state [7]. PDGF receptor β (PDGFRβ) and other transmembrane proteins such as integrins are induced on the surface of myofibroblasts and precursors to form positive feedback loops for their perpetuated activation.
2.2. Hepatic stellate cells (HSCs): precursor of myofibroblasts
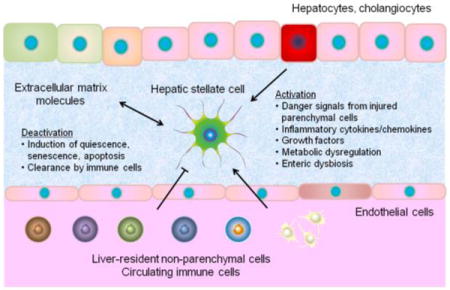
HSCs are resident mesenchymal cells that retain features of resident fibroblasts (embedded in normal stromal matrix) and pericytes (attached to endothelial cells of capillaries), and comprise approximately one-third of nonparenchymal cells and 15% of total resident cells in normal human liver [7]. HSCs reside in a virtual subendothelial space between the basolateral surface of hepatocytes and the anti-luminal side of fenestrated sinusoidal endothelial cell layer (space of Disse) filled with thin permeable connective tissue, where exchange of biomolecules between portal blood flow from gastrointestinal tract and hepatocytes occurs. Intercellular communication via soluble mediators and cytokines/chemokines can occur between neighboring cell types such as hepatocytes, biliary epithelial cells, hepatic progenitor cells (oval cells), Kupffer cells (liver resident macrophages), bone marrow-derived macrophages, liver sinusoidal endothelial cells (LSECs), infiltrating immune cells, and nerve cells [7, 8]. In fibrogenic liver, quiescent HSCs transdifferentiate into proliferative, migratory, and contractile myofibroblasts, manifesting pro-fibrogenic transcriptional and secretory properties (so called “cell activation”), and secrete ECM molecules that accumulate and form scar tissue in the space of Disse that leads to sinusoidal capillarization characterized by loss of endothelial fenestrations [7] (Figure 1). Vitamin A (retinoid) storage in cytoplasmic droplets is a unique characteristic feature of quiescent HSCs, which is gradually lost during the process of transdifferentiation, although its causal relationship with HSC activation is still uncertain [7, 9]. Endothelin-1 (ET-1), a potent vasoconstrictor, is secreted by activated HSCs, and promotes cell proliferation, fibrogenesis, and contraction, which is plausibly linked to portal hypertension in cirrhosis [10].
Figure 1. Extracellular regulation of HSC activation.
HSC activation is promoted (sharp arrow) or inhibited (blocked arrow) by liver resident cells, ECM, and circulating cells via paracrine factors. Red and blue font colors indicate positive and negative regulators of HSC activation, respectively. Pharmacological intervention to each candidate target is shown in parenthesis.
A number HSC-specific marker genes and proteins, including glial fibrillary acidic protein (GFAP), nerve growth factor receptor (p75), desmin, lecithin-retinol acyltransferase (LRAT), integrin αvβ3, mannose 6-phosphate/insulin-like growth factor II receptor (M6P/IGF-IIR), collagen type I, collagen type VI receptor (CVIR), α-smooth muscle actin (αSMA), PDGFRβ, vimentin, and cytoglobin, have been exploited for their histological detection, genetic targeting, cell fate tracing, therapeutic targeting, and imaging [7, 11–14]. In addition, by using genome-wide transcriptome profiling, HSC-specific 122-gene and 194-gene signatures, associated with poorer patient prognoses, have been identified [15, 16].
A widely used experimental culture model is primary human and rodent HSCs, isolated by discontinuous gradient ultracentrifugation with or without cell surface marker-based sorting, that undergo spontaneous fibrogenic activation when grown on a hard plastic dish [17, 18]. Although certain distinct differences in transcriptomic dysregulation have been noted between in vitro and in vivo settings, co-culture with other cell types such as Kupffer cells could partially restore more physiological molecular dysregulation [19]. Immortalized cell lines of human (LX-2 and TWNT-4), mouse (JS-1, GRX, and CoI-GFP), and rat (HSC-T6 and CFSC) HSCs, and rat portal myofibroblasts (RGF and RGF-N2) were established, and have been used for mechanistic analysis of antifibrotic targets and therapies [20–27].
2.3. Resident mesenchymal cells as major source of myofibroblasts
Rodent-based studies have elucidated several potential sources of myofibroblasts. Cell fate tracing, genetically labeling HSCs by using LRAT, involved in the cytoplasmic lipid droplet formation, showed that 82% to 96% of myofibroblasts were derived from HSCs in mice treated with carbon tetrachloride (CCl4), bile duct ligation (BDL), 3,5-diethoxycarbonyl-1,4-dihydrocollidin diet, and Mdr2-knockout mice [28]. Another study, labeling myofibroblasts using Col1a1 promoter in mice, reported that HSCs were the major source of myofibroblasts (>87%) in chemical injury (CCl4), whereas portal fibroblasts were the major source (>70%) in an early stage of cholestatic injury (BDL) [29]. These studies collectively suggest that the liver resident mesenchymal cells, particularly HSCs, are the major source of fibrogenic myofibroblasts, although these rodent-derived, single gene marker-based findings need to be verified in humans. Furthermore, it is still unknown whether cell(s) of origin is/are associated with response to antifibrotic therapies.
2.4. Other potential cellular sources of myofibroblasts
Although relative contribution is plausibly minor or negligible, other cell types in addition to HSCs and portal fibroblasts have been proposed as alternative sources of myofibroblasts. Collagen-producing fibrocytes, distinct from HSCs, are recruited from bone marrow in BDL mice [30, 31]. Mesenchymal stem cells are also suggested as another bone marrow-derived source of myofibroblasts [32]. Wt1-expressing mesothelial cells (approximately 15% of the cell population) may be a source of myofibroblasts near the liver surface via mesothelial–mesenchymal transition (MMT) in CCl4 mice [33]. Epithelial-mesenchymal transition (EMT) of liver parenchymal cells, i.e., hepatocytes and cholangiocytes, is less likely the source of myofibroblasts, although the parenchymal cells could express some of the mesenchymal genes without ECM protein secretion [32, 34–36]. Hedgehog signaling contributes to mesenchymal-to-epithelial transition (MET) of a subpopulation of HSC-derived myofibroblasts into hepatic progenitor cells in the process of liver regeneration in mice [37].
3. Mechanisms of HSC activation
3.1. Extracellular events that promote HSC activation
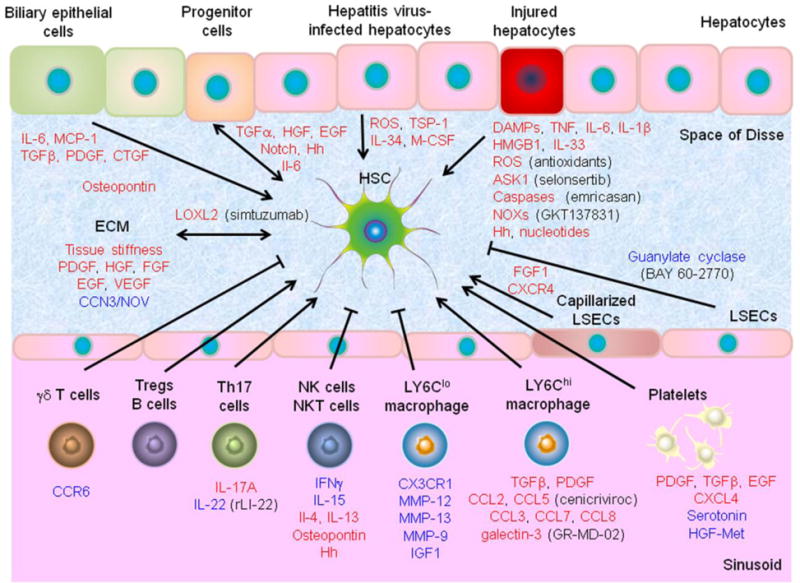
Several cellular events in injured/inflamed hepatic tissue microenvironment as well as extrahepatic factors are known to directly induce HSC activation in etiology-specific or independent manner [38] (Figure 1).
3.1.1. Epithelial cell injury
Progressive fibrotic liver diseases are accompanied with chronic damage to parenchymal epithelial cells, i.e., hepatocytes and cholangiocytes (biliary epithelial cells). According to the liver disease etiology (viral infection, hepatic toxins, metabolic disorders, and autoimmune diseases), various modes of cell death (e.g., apoptosis, necrosis, necroptosis, and autophagic cell death), trigger cell death responses [39]. Immune cell-mediated killing of hepatocytes is a common theme in HBV- and HCV-related liver fibrosis, which involves pro-fibrogenic inflammatory chemokines and their receptors such as C-C motif chemokine ligand 2 (CCL2), CCL21, interleukin-8 (IL-8), IL-17, IL-22, C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, CXCL11, and C-X-C motif chemokine receptor 1 (CXCR1) [8]. Hepatic nuclear factor κB (NF-κB)-inducing kinase (NIK) activates bone marrow-derived macrophages, increases hepatic injury, and promotes HSC activation and fibrogenesis [40]. The dead or dying epithelial cells as well as leukocytes phagocytosing the cells release inflammatory mediators, damage-associated molecular patterns (DAMPs) or danger signals, which initiate and perpetuate a non-infectious “sterile” inflammatory response. Among such mediators, tumor necrosis factor (TNF), IL-6, IL-1β, reactive oxygen species (ROS), hedgehog ligands, and nucleotides can contribute to initiation of HSC activation [8, 41, 42]. Such host biomolecules from injured hepatocytes, “alarmins”, include high mobility group protein B1 (HMGB1) and IL-33 [43, 44]. IL-33 induces IL-13 production in liver resident innate lymphoid cells type II, enhances TGFβ signaling through IL-4Rα and signal transducer and activator of transcription 6 (STAT6) in HSCs, and promotes fibrosis [45]. Loss of the ROS-generating enzymes, NADPH oxidase 1 (NOX1), NOX2, or NOX4, attenuates hepatocyte injury and liver fibrosis in CCl4 and BDL mice [46, 47]. A NOX1/NOX4 inhibitor, GKT137831, reduces hepatic apoptosis and fibrosis in CCl4 and BDL mice [48]. Specific modes of hepatocyte and cholangiocyte death may be monitored by circulating cytokeratins as biomarkers [49]. Apoptosis inhibitors such as a pan-caspase inhibitor, emricasan (IDN-6556), aiming to reduce hepatocyte death, are under evaluation in clinical trials in post-transplant HCV and NASH patients (ClinicalTrials.gov: NCT02138253, NCT02686762, NCT02960204; ENCORE trials). Apoptosis signal-regulating kinase 1 (ASK1) is a mitogen-activated protein kinase (MAP3K) that activates c-Jun N-terminal kinase (JNK) and p38 MAPK in response to various cellular stresses. A phase 2 trial of ASK1 inhibitor, selonsertib (GS-4997), resulted in more than 1 stage of liver fibrosis improvement in 43% of enrolled NASH patients (NCT02466516).
3.1.2. Altered extracellular matrix (ECM)
Progressive deposition of ECM proteins in the space of Disse leads to increased density and stiffness of ECM. In addition, matrix composition shifts from collagen type IV, heparan sulfate proteoglycan, and laminin to fibrillar collagen type I and III. These changes serve as mechanical stimuli to activate HSCs at least partially via integrin signaling pathways, forming positive feedback loops [50, 51]. LX-2 cells cultured on acrylamide gels with 12 kPa pressure, mimicking increased tissue stiffness in fibrotic liver, show higher collagen I expression via RHOA upregulation, which is suppressed by c-Src [52]. Primary HSCs cultured in hydrogels stiffened or softening in situ over time revealed time-course dynamic transcriptional changes during the process of culture activation and regression, respectively [53, 54]. Expanded ECM also serve as reservoir by binding growth factors such as PDGF, hepatocyte growth factor (HGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF), which promote HSC proliferation [6, 55]. Reduced matricellular protein, CCN3/NOV, enhances fibrogenic gene expression in primary HSCs and CFSC cells [56]. Lysyl oxidase-like-2 (LOXL2) is a matrix enzyme expressed by HSC, which catalyzes crosslinking of collagens and elastins, and its inhibition by monoclonal antibody (AB0023) reduces liver and lung fibrosis in experimental models [57]. A humanized monoclonal LOXL2 antibody, simtuzumab (GS-6624), has been tested in phase 2 trials for patients with HCV (NCT01707472), primary sclerosing cholangitis (NCT01672853), and fibrotic (NCT01672866) or cirrhotic (NCT01672879) NASH.
3.1.3. Immune regulation
Macrophages represent a heterogeneous population (10–15% of liver cells), which can have both pro- and anti-fibrogenic effects through paracrine regulation of HSC activation [41, 58]. Macrophage depletion in murine models has revealed their pro-fibrogenic role [59]. Polarized and plastic activation of macrophages is traditionally classified into classic M1 and alternative M2 activation associated with helper T (Th)1 (TNFα, IL-1, IL-12, and inducible nitric oxide synthase [iNOS]) and Th2 (IL-4, IL-10, and IL-13) responses, respectively [60]. p53 depletion in HSCs in mice leads to M2 activation that promotes HCC proliferation by affecting the tumor microenvironment [61]. Lymphocyte antigen 6 complex (LY6C)hi monocyte-derived macrophages are recruited in C-C motif chemokine receptor 2 (CCR2)-dependent manner and secrete pro-fibrogenic mediators such as TGFβ, PDGF, CCL2, CCL3, CCL5, CCL7, and CCL8 [8, 62]. Galectin-3 is a macrophage-derived lectin, which promotes HSC activation and can be pharmacologically inhibited by GR-MD-02, currently in a phase 2 trial in NASH patients (NCT02462967) [63, 64]. In contrast, LY6Clo macrophages represent a fibrolytic subset that expands during fibrosis regression [62]. C-X3-C motif chemokine receptor 1 (CX3CR1) induces differentiation to LY6Clo cells, which promote apoptosis of activated HSCs and secrete matrix metallopeptidase 12 (MMP-12) and MMP-13 to promote ECM degradation [65, 66].
Th17 cells secret IL-17A and IL-22, and are increased in the liver and circulation in chronic liver diseases [67]. IL-17 receptor is expressed on the surface of macrophages and HSCs, and the signaling induces pro-inflammatory cytokines such as TNF, IL-1β, IL-6, and IL-17A, and collagen 1 expression in HSCs by activating STAT3 signaling in mice [68]. IL-22 transgenic mice are protected from CCl4-induced fibrosis through increased HSC senescence by binding to its receptors, IL-10R2 and IL-22R1, up-regulating STAT3 and increasing p53 expression [69]. Recombinant IL-22 also attenuates HSC activation and fibrosis [70]. On the other hand, IL-22 may have adverse effect in HBV infection [71]. Intrahepatic IL-8 producing Foxp3+CD4+ regulatory T cells (Tregs) activate HSCs and promote fibrosis in chronic hepatitis C [72]. γδ T cells restrict liver fibrosis by inducing HSC apoptosis in CCR6-dependent manner [73].
B cells, which account for up to half of lymphocytes in the liver, contribute to fibrogenesis in CCl4 mice [74]. HSC-mediated MyD88-dependent innate B cell activation promotes hepatic fibrosis [75].
Natural killer (NK) cells kill senescent HSCs and produce interferon γ (IFNγ) that induces apoptosis and cell cycle arrest in HSCs [76]. IL-15 increases NK cells and support their killing of HSCs [77]. Regulatory CD4+ T cells can suppress NK cells, and thereby support survival of activated HSCs [78]. Natural killer T (NKT) cells also secrete IFNγ and kill activated HSCs, whereas a subset of NKT cells produce IL-4, IL-13, osteopontin, and hedgehog ligands, and promote HSC activation and liver fibrosis via CXCR6-CXCL16 axis in chronic liver diseases including NASH [79–81].
3.1.4. Other cell types
Liver sinusoidal endothelial cells (LSECs) maintain HSC quiescence, thus inhibiting intrahepatic vasoconstriction and fibrosis development [82]. In pathological conditions, LSECs become capillarized with lost fenestrations, lose the hepatoprotective property, and promote angiogenesis and vasoconstriction. A soluble guanylate cyclase activator, BAY 60-2770, leads to reversal of the capillarization, restores HSC quiescence without directly affecting HSCs, and reduces fibrosis in thioacetamide (TAA)-treated cirrhotic rats [83].
Close proximity between hepatic progenitor cells and HSCs is often observed at the site of ductular reaction, a pathological feature accompanying fibrotic liver diseases, which may have functional implication in HSC activation and/or liver regeneration possibly via contribution of HSCs to progenitor cell pool [84, 85].
Osteopontin, a multifunctional secreted phosphoprotein, induces collagen I expression in HSC by affecting biliary epithelial cells and promoting ductular reaction [86, 87]. Platelets are a source of pro-fibrogenic cytokines and growth factors such as PDGF, TGFβ, and EGF, and they can also suppress collagen expression via HGF-Met signaling pathway [7, 88].
3.1.5. Metabolic dysregulation and enteric dysbiosis
A so-called Western diet can alter the intestinal microbiome (intestinal dysbiosis), and lead to release and increased exposure to pathogen-associated molecular patterns (PAMPs) that can activate HSCs through toll-like receptors (TLRs) [89]. A high-fat diet increases the proportion of intestinal Gram-negative endotoxin-producing bacteria, resulting in higher rates of bacterial translocation, and accelerated fibrogenesis by enhancing HSC activation in CCl4 and BDL mice [90]. Transplantation of the fibrogenic microbiome to control diet-fed mice exacerbates liver damage and fibrosis, suggesting the causal role of microbiome in fibrogenic HSC activation. In contrast, commensal microbiota is hepatoprotective and prevents liver fibrosis by mitigating HSC activation in mice [91]. Lipotoxicity caused by free intracellular cholesterol accumulation sensitizes HSCs to TGFβ-induced activation in NASH mouse model [92].
3.1.6. Chronic infection of hepatitis virus
Chronic infection by hepatotropic viruses, HBV and HCV, has been one of the major risk factors for fibrotic liver diseases worldwide [93]. Given that population-level viral eradication will not be achieved in the next several decades despite the development of highly effective antivirals, directly targeting fibrosis pathways irrespective of viral clearance will remain a viable antifibrotic strategy. Viral genes and proteins can directly or indirectly promote HSC activation. HBV e antigen directly induces activation and proliferation of rat HSCs in vitro via the TGFβ pathway, and core and X proteins similarly activate human LX-2 cells via PDGFβ signaling [94, 95]. Although HCV cannot infect HSCs, viral core and non-structural proteins directly induce inflammatory and pro-fibrogenic pathways in HSCs [96, 97]. HCV core protein may promote EMT of infected hepatocytes through TGFβ signaling [98]. HCV core also increases ECM protein, thrombospondin-1 (TSP-1) and transcription factors, which increase bioavailability and expression of TGFβ [99, 100]. Elevated expression of IL-34 and macrophage colony-stimulating factor (M-CSF) from HCV-infected hepatocytes stimulates maturation of peripheral monocytes into macrophages, which contributes to HSC activation by enhancing TGFβ and PDGFβ signaling, blocking antifibrotic NK cell-derived IFNγ, and inducing MMP-1 [101]. Human immunodeficiency virus (HIV) co-infection is clinically known to accelerate fibrosis progression in HCV-infected individuals, and it can be slowed down by antiretroviral therapies [102, 103]. HIV can directly infect HSCs, and viral envelop protein, gp120, promotes collagen I expression via CXCR4 [104, 105]. Exposure to HIV induces TGFβ, ROS, and NF-κB in a hepatocyte/LX-2 co-culture reporter cell model [106].
3.2. Molecular dysregulation in activated HSCs
Functional studies have elucidated complex dysregulation of molecular pathways in HSCs/myofibroblasts for their perpetuated activation through multiple signaling pathways and other mechanisms as summarized below (Figure 2).
Figure 2. Cellular signaling pathways involved in HSC activation and deactivation.

A variety of cell surface receptor-, nuclear receptor-, and intracellular signal transmitter-mediated mechanisms of HSC activation has been identified. Dysregulation of cellular homeostasis and epigenetic modifications also regulate HSC activation. HSC deactivation can be induced via specific targets. Red and blue font colors indicate positive and negative regulators of HSC activation, respectively. Black font color indicates involvement in both pro- and anti-fibrogenic functions depending on biological context or undetermined role in HSC activation. Pharmacological intervention to each candidate target is shown in parenthesis.
3.2.1. Membrane receptor signaling pathways
3.2.1.1. TGFβ
Activated HSCs secrete latent TGFβ in response to liver injury, which forms an autocrine positive feedback loop driving fibrogenesis via SMAD2/SMAD3, whereas SMAD7 suppresses the activation [7, 107, 108]. Apoptotic body-engulfing macrophages produce TGFβ and activate HSCs [30, 109–111]. Liver-targeted TGFβ1 expression induces fibrosis and chemical carcinogenesis in mice [112, 113]. Therapeutic strategies, e.g., blocking circulating TGFβ1, antagonizing its receptors, or blocking its activation at the cell surface, have been challenging due to adverse effects of systemic TGFβ antagonism, including inflammation and cancer [6, 7, 114]. Knockdown of transient receptor potential melastatin7 (TRPM7) attenuates TGFβ1-induced expression of myofibroblast markers, increases the ratio of MMPs/tissue inhibitor of metalloproteinases (TIMPs), and decrease SMAD2/SMAD3-associated collagen production [115, 116]. Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) mediates TGFβ-induced HSC proliferation, but not collagen production [117]. CUG-binding protein 1 (CUGBP1), induced by TGFβ, binds the 3′-untranslated region (UTR) of antifibrogenic IFNγ mRNA and promotes its decay [118].
3.2.1.2. PDGF
Cell proliferative/inflammatory cytokines, PDGF, FGF, TGFα, p38 MAPK, and JNK, converge on MAPK/extracellular signal-regulated kinase (ERK) pathway activation [6, 119]. PDGF is a potent chemoattractant for HSCs and myofibroblasts [7]. PDGFβ receptor (PDGFRβ) expression is induced during initiation of HSC activation, and enhances inflammatory and fibrogenic responses to chemical injury via ERK, AKT, and NF-κB pathways, without affecting hepatocarcinogenesis in mice [120, 121]. Small molecule tyrosine kinase inhibitors, including imatinib, sorafenib, nilotinib, and sunitinib, suppress proliferative and fibrogenic properties of activated HSCs in organotypic ex vivo culture of fibrotic human liver tissues [122–125]. CD248/endosialin is a PDGF downstream regulator of HSC proliferation during chronic liver injury [126]. A tyrosine phosphatase SHP-1 inhibits proliferation of activated HSCs via PDGF signaling [127]. Knockdown of protein tyrosine phosphatase receptor type O (PTPRO) can suppress PDGFβ-induced HSC proliferation and activation by reducing phosphorylation of ERK and AKT in mice [128]. PDGF-C transgenic mice exhibit HSC activation and develop hepatic steatosis, fibrosis, and cancer despite limited hepatocyte injury and lack of extensive fibrosis [129]. Pigment epithelium-derived factor (PEDF)-derived 34-mer peptide (Asp44-Asn77) down-regulates PDGF receptor expression and blocks HSC activation [130].
3.2.1.3. CTGF
CTGF/CCN2 is a central fibrogenic effector of TGFβ pathway, and its knockout suppresses fibrogenesis in CCl4-treated mice [131]. A human monoclonal antibody to CTGF, FG-3019, was tested in HBV fibrosis patients, although its clinical utility as antifibrotic therapy was not clear due to prominent effect of anti-HBV drug, entecavir (NCT01217632) [132, 133].
3.2.1.4. EGF
EGF receptor is overexpressed and phosphorylated in activated HSCs, and its pharmacological inhibition reduces hepatic fibrosis and HCC nodules in rodent models, although EGF receptor expression is not restricted to HSCs [134]. Genetic knockout of EGF receptor in macrophages reduces HCC development in mice [135].
3.2.1.5. Rho kinases (ROCKs)
ROCKs and family proteins, including Rac1, promote reactive oxygen species (ROS) production and enhance contractility of HSCs, which can be inhibited by kinase inhibitors and other measures such as adenosine [136–139]. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major toxicity [140].
3.2.1.6. Integrins
Integrins are heterodimeric transmembrane proteins composed of α/β subunits that sense cell adhesion, transmit signals from ECM, and promote fibrogenesis by activating latent TGFβ [6, 7]. Genetic deletion of integrin αv as well as its pharmacological blockade by a small molecule, CWHM 12, in activated HSCs reduces CCl4-induced hepatic fibrosis, whereas no such effect is observed for integrins β3, β5, β6, and β8 [51]. Integrin β1 induces PAK proteins and YAP signaling in myofibroblasts and promote liver fibrosis [141]. Integrin α1β6 induces ROS accumulation through Rac1-NADPH oxidase 1 enzyme complex in HSCs [142].
3.2.1.7. Renin-angiotensin system
Angiotensin II, secreted by HSCs, binds to angiotensin II type 1 receptor (AT1R) and promotes liver fibrosis via JAK2, which is associated with portal hypertension [143, 144]. Blocking the renin–angiotensin system by angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) such as losartan may be an effective antifibrotic therapy as suggested by experimental and clinical studies [145, 146]. The AT1R pathway has a synergistic activity with cannabinoid signaling through receptor heterodimerisation during HSC activation, suggesting that combination of ACE/ARB and CB1 receptor antagonist may be an option of antifibrotic therapy [147]. Swertiamarin (Swe), an extract of Gentiana manshurica Kitag, significantly inhibits angiotensin II-induced HSC proliferation and activation via AT1R [148].
3.2.1.8. Wnt/β-catenin signaling
The Wnt pathway regulates a variety of cellular processes such as development, differentiation, and proliferation, and can have both activating and inhibitory effects on HSCs [149]. siRNA-mediated β-catenin knockdown reduces collagen I and III expression, inhibits cell proliferation, and induces apoptosis of HSCs in vitro [150]. Wnt signaling inhibition by Dickkopf-1 (DKK1) abrogates epigenetic repression and restores peroxisome proliferator-activated receptor-γ (PPARγ) activity, and reduces fibrosis [151]. Conversely, HSC-derived delta-like homolog 1 (DLK1) activates HSCs via epigenetic PPARγ repression and promote liver regeneration [152]. A selective inhibitor of the cAMP-response element-binding protein (CBP)/β-catenin interaction, PRI-724, inhibits HSC activation and reduces liver fibrosis in mice [153]. In contrast, β-catenin-dependent canonical Wnt activation is required to maintain quiescent state of HSCs in vitro [154]. Roof plate-specific spondin (RSPO) proteins, potent Wnt pathway agonists, suppressed HSC activation, which is impaired by DKK1 [155]. In activated rat HSCs, non-canonical Wnt pathway activation dominates and confers HSC survival, accompanied with Wnt5a over-expression [156]. A natural Wnt5a inhibitor, secreted frizzled-related protein 5 (Sfrp5), decreases HSC activation and liver fibrosis [157]. These findings collectively suggest that involvement of Wnt pathway in HSC activation is highly complex and biological context-dependent.
3.2.1.9. Hedgehog (Hh) signaling
Hh ligand expression from hepatocytes induces HSC activation and promotes liver fibrosis and hepatocarcinogenesis in a rodent model [158, 159]. Smoothened (Smo), an obligate intermediate in Hh signaling pathway, is required not only for fibrogenic activation of HSCs, but also for epithelial regeneration in mice [37]. Hh signals transduced via activated Smo are transmitted to Gli transcription factors that induce target gene expression [160]. Gli1-positive perivascular mesenchymal cells give rise to 39% of αSMA-positive myofibroblasts after tissue injury [161]. Forskolin, a hedgehog signaling inhibitor, attenuates CCl4-induced liver fibrosis in rats [162]. Pharmacological inhibition of Hh signaling by vismodegib (GDC-0449) attenuates liver fibrosis in BDL and NASH models [163, 164]. Inhibition of Notch or Hh signaling in myofibroblasts leads to mesenchymal-to-epithelial like transdifferentiation with reduced fibrosis in mice [165].
3.2.1.10. Neurochemical receptors
The cannabinoid system is involved in neuro/immune-modulatory function, and has two G protein-coupled receptors, CB1 (pro-fibrogenic) and CB2 (hepatoprotective) [166]. Systemic antagonism of CB1 can induce serious psychiatric adverse effects as seen during trials of rimonabant. Newer generation antagonists with improved target specificity and limited central nervous system penetration such as AM4113 and AM6545 have been developed based on the crystal structure of CB1 [167, 168]. A germline genetic variant in the CNR2 gene (encoding CB2), CB2-63QQ, was found to be associated with more severe necroinflammation in patients with chronic hepatitis C [169]. A selective CB 2 agonist, JWH-133, reduces fibrosis by inducing HSC quiescence/apoptosis and reducing IL-17 production by Th17 cells [166, 170]. Nutritional products such as curcumin (diferuloylmethane), a bioactive polyphenolic ingredient of turmeric, may have potential to inhibit CB1 as well as virtually all cellular pathways involved in HSC activation [171, 172]. Antagonism of a serotonin receptor, 5-hydroxytryptamine 2B receptor (5-HT2B), on HSCs attenuates fibrosis and enhances hepatic regeneration [173]. Inhibition of 5-HT2A similarly suppresses fibrogenic gene expression and induces apoptosis in HSCs [174].
3.2.1.11. Toll like receptors (TLRs)
TLRs are pattern recognition receptors (PRRs) that detect microbial infection and exposure as PAMPs, including bacterial protein products, transcripts, and genomic DNA [8]. Liver is consistently exposed to PAMPs from gastrointestinal and oral microflora, and it is known that plasma and portal level of endotoxin such as lipopolysaccharide (LPS), a Gram-negative bacteria constituent, is elevated in cirrhotic patients due to bacterial dysbiosis and overgrowth, as well as gut leakiness [175]. The composition of the gut microbiome from cirrhosis patients was highly comparable across different populations, suggesting its influence on pathogenesis of fibrotic liver diseases via TLRs [176]. HSCs express TLR4 and TLR9, and TLR2 is induced by TLR4, upon activation by pro-inflammatory and pro-fibrogenic pathways and cytokines including NF-κB, JNK, TGF β, IL-6, IL-8, CCL2 (MCP-1), MIP-2, intercellular cell adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), and E-selectin [177]. LPS-induced TLR4 activation downregulates Bambi (a TGFβ pseudoreceptor) and sensitizes quiescent HSCs to TGFβ activation mediated by MyD88-NF-κB pathway, and enhances liver fibrogenesis in CCl4 and BDL mice [178]. TLR4 activation is required for HCC promotion, but not initiation in mice [179]. TLR9 senses DNA from apoptotic hepatocytes to stop chemotaxis at the site of dying hepatocytes and stimulate collagen synthesis [180]. Bacterial DNA activates TLR9 on resident and recruited macrophages to release IL-1β, which activates HSCs and enhances lipid accumulation, insulin resistance, and injury in hepatocytes, and collectively promotes fibrogenesis and hepatic inflammation [181]. TLR2 stimulates TNFα production from intestinal monocytes that activates the RhoA pathway in intestinal epithelial cells to increase intestinal permeability and bacterial translocation to the liver [182]. TLR2 deficiency leads to attenuated liver fibrosis in choline-deficient amino acid-defined (CDAA) diet-fed NASH mice [183]. Exosome-mediated TLR3 activation in HSCs promotes liver fibrosis by inducing IL-17A production by γδ T cells in mice [184].
3.2.1.12. Lysophospholipid pathway
Lysophosphatidic acid (LPA) is a pleiotropic growth factor-like lysophospholipid, involved in lipid homeostasis. Hepatocyte-derived autotaxin (ATX) catalyzes production of LPA that activates LPA receptor 1 (LPAR1) on HSCs, and increased ATX in various chronic liver diseases has been association with poorer prognosis and cancer risk, [185, 186]. Pharmacological and/or genetic inhibition of ATX and/or LPAR1 attenuates HSC activation and reduces liver fibrosis and cancer in rodent models [185, 186].
3.2.1.13. Adipokines
Adipokines, e.g., leptin and adiponectin, are soluble factors secreted by adipose tissue, which contribute to the pathogenesis of obesity- and metabolic disorder-related complications through their pro- or anti-inflammatory properties [187]. Leptin and adiponectin exert pro- and anti-fibrogenic function, respectively, via their receptors on HSCs [188, 189]. Leptin reduces expression of sterol regulatory element-binding protein-1c (SREBP-1c) and activates HSCs through involvement of β-catenin pathway [190]. Adiponectin attenuates liver fibrosis by inducing nitric oxide (NO) production via adipoR2-AMP-activated protein kinase (AMPK)-JNK/ErK1/2-NF-κB pathway and TIMP-1, and inhibits proliferation and migration of activated HSCs [191, 192]. Adiponectin induces expression of aquaglyceroporin (known to be down-regulated in association with obesity) and inactivates HSCs [193]. Adiponectin-derived synthetic short peptide, ADP355, suppresses focal adhesion kinase (FAK) activity and TGFβ1/SMAD2 signaling and promotes AMPK and STAT3 signaling, and inactivates HSCs in mice [194, 195].
3.2.1.14. Vascular adhesion protein 1 (VAP-1)
VAP-1 (encoded by AOC3) is a membrane sialoglycoprotein expressed by LSECs that promotes lymphocyte recruitment [196]. VAP-1 is also expressed on activated HSCs and associated with hepatic inflammation and fibrosis in NASH patients and mouse models [197]. A selective VAP-1 inhibitor, PXS-4728A, was tested in a phase 1 study.
3.2.1.15. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK)
TWEAK (encoded by TNFSF12) is secreted from macrophages, monocytes, and T lymphocytes upon liver injury, and signals through its receptor, FGF‐inducible 14 (Fn14), to activate liver-resident progenitor cells, while hepatocyte replication is impaired in chronic liver diseases [198]. TWEAK also leads to liver fibrosis progression by directly promoting HSC proliferation. TWEAK, and by enhancing SIRT1 expression and decreasing p53 acetylation, which assumedly inhibits HSC senescence [199, 200].
3.2.1.16. G protein-coupled receptor 91 (GPR91)
Sirtuin 3 (SIRT3), an NAD(+)-dependent protein deacetylase decreased in NAFLD, suppresses αSMA expression via GPR91 on HSCs in methionine- and choline-deficient (MCD) diet-fed NAFLD mice [201].
3.2.1.17. Axl/growth arrest specific 6 (Gas6)
Axl is a receptor tyrosine kinase, activated by its ligand, Gas6. Serum Gas6 level is increased as alcoholic liver disease or chronic hepatitis C progresses [202]. Axl knockout as well as inhibition by BGB324 result in attenuated HSC activation and reduced fibrosis in CCl4 mice.
3.2.2. Nuclear receptor signaling pathways
3.2.2.1. Farnesoid-X-receptor (FXR)
FXR signaling, activated by bile acids, enhances insulin sensitivity, and fatty acid beta-oxidation, and reduces gluconeogenesis and lipogenesis in hepatocytes [89]. A synthetic lipophilic bile acid, obeticholic acid (OCA), has an FXR agonistic activity stronger than the chemically similar ursodeoxycholic acid, and reduced liver fibrosis in non-cirrhotic NASH patients in a phase 2 trial (FLINT trial) [203]. OCA is currently being evaluated in a phase 3 trial in fibrotic NASH patients (REGENERATE trial, NCT02548351). In addition, the following agents targeting FXR signaling are being evaluated in clinical trials: GS-9674, synthetic non-steroidal FXR agonist; INT-767, bile acid analogue agonizing FXR and TGR5 (transmembrane G-protein bile acid receptor expressed on sinusoidal and bile duct epithelial cells) [89, 204]. Although FXR expression and OCA response are limited in HSCs, induction of FXR signaling and resulting suppression of sphingosine-1-phosphate receptor 2 (S1PR2) signaling by dihydroartemisinine (DHA), an artemisinin derivative, restricts HSC contraction and fibrogenesis, and improves portal hypertension in rodent models [205–207].
3.2.2.2. PPARs
PPARs, consisting of α, β/δ, and γ isoforms that share common target DNA sequences but with distinct ligand selectivity and tissue distribution, serve as receptors for a wide range of fatty acids and derivatives; they transcriptionally regulate various metabolic processes to maintain lipid and energy homeostasis [208]. PPARγ has been extensively studied as a potential antifibrogenic target in HSCs [209]. PPARγ suppresses angiogenic PDGFRβ signaling and TGFβ1 via the β-catenin pathway in HSCs [210, 211]. Lipid-induced hepatocyte-derived extracellular vesicles regulate HSCs via miR-128-3p targeting PPARγ [212]. Histone H3K9 demethylase JMJD1A modulates HSCs activation by epigenetic regulation of PPARγ [213]. Leptin inhibits PPARγ expression via GATA binding protein 2 in HSCs [214, 215]. Adiponectin regulates HSC activation via PPARγ-dependent and independent mechanisms [216]. Curcumin regulates PPARγ coactivator-1α expression by AMPK pathway in HSCs in vitro [217]. Activation of PPARγ/p53 signaling is required for curcumin to induce HSC senescence [218]. Thiazolidinediones (TZDs), synthetic insulin sensitizing PPARγ agonists such as pioglitazone and rosiglitazone, improved steatosis and lobular inflammation but not fibrosis in non-cirrhotic NASH patients in a phase 3 trial (PIVENS trial) [219]. Safety concerns about TZDs such as the cardiovascular toxicity of rosiglitazone and weight gain due to redistribution of body fat have led to suboptimal acceptance of these agents as NASH treatments [89]. A dual PPARα/δ agonist, elafibranor (GFT-505), improved histological NASH with no worsening of fibrosis in non-cirrhotic NASH patients in a phase 2 trial, which may suggest indirect suppression of hepatocyte lipotoxicity-driven fibrogenesis (GOLDEN-505 trial) [220]. A follow-up phase 3 trial is currently recruiting patients with fibrotic NASH patients (NCT02704403). A dual PPARα/γ agonist, saroglitazar, is being evaluated in a phase 3 trial for no worsening fibrosis in NASH patients (Indian trial registry, CTRI/2015/10/006236). Novel selective agonists of PPARα (pemafibrate, K-877), PPARγ (INT-131), PPARδ (HPP-593), PPARα/γ (DSP-8658) and a pan-PPAR agonist (IVA337) are under evaluation.
3.2.2.3. Liver X receptors (LXRs)
LXRs are lipid-activated nuclear receptors involved in cholesterol transport, lipogenesis, and anti-inflammatory signaling. LXR activation suppresses HSC activation in mice [221]. Leptin-induced activation of p38 MAPK leads to inhibition of LXR activity and downregulation of SREBP-1c expression, and increases collagen type I expression in HSCs [222]. The therapeutic benefit of LXR agonism may be limited due to induction of de novo lipogenesis by this mechanism [5].
3.2.2.4. Vitamin D receptor (VDR)
In HSCs, TGFβ1 causes a redistribution of genome-wide VDR-binding sites (VDR cistrome) at SMAD3 profibrotic target genes via chromatin remodeling, and VDR ligands reduce TGFβ/SMAD3 target gene expression and inhibit fibrosis [223]. p62/SQSTM1 suppresses fibrogenic and carcinogenic HSC activation by promoting VDR signaling, which is abrogated by VDR knockout in mice [224]. In human primary HSCs, vitamin D treatment similarly suppressed TGFβ-induced fibrogenic transcription, but A1012G single nucleotide polymorphism (G allele) within the VDR gene reduces VDR expression and abolishes the vitamin D effect [225].
3.2.2.5. Retinoid receptors
Retinoic acid, an active metabolite of vitamin A, regulates various physiological processes such as energy metabolism in metabolically active cells through retinoic acid receptors (RARs) and retinoid X receptors (RXRs) that form homo/heterodimers [226]. Retinoid receptor signaling in hepatocytes is implicated in liver carcinogenesis, and a synthetic acyclic retinoid, peretinoin, has been assessed as a potential cancer chemoprevention measure [227, 228]. In HSCs, all-trans retinoic acid (ATRA) (an RAR ligand) moderately inhibits expression of procollagen I, III, IV, fibronectin, laminin, αSMA, TGFβ, and IL-6, but with no effect on cell proliferation, whereas 9-cis (RXR ligand) moderately inhibits proliferation without affecting the fibrogenic gene expression in rodents [229, 230]. These receptors, also together with PPARγ signaling, have additive inhibitory effects on HSC activation [231, 232]. RXR can form heterodimer with VDR to transmit signal from p62 in HSCs to promote fibrogenesis and inflammation, and support carcinogenesis [224].
3.2.2.6. Rev-Erb receptors
Rev-Erbs, consisting of Rev-Erbα and Rev-Erb β, are heme-sensing receptors involved in regulation of metabolism, development, immunity, and circadian rhythm, and are potentially druggable [233]. Rev-Erbα is up-regulated in both nucleus and cytoplasm in activated HSCs in association with increased contractility and fibrogenic gene expression, which can be antagonized by a synthetic ligand, SR6452 [234]. Another synthetic Rev-Erbα ligand, SR9009, similarly reduces fibrogenic gene expression and proliferation in HSCs, accompanied by decreased autophagosome formation [235].
3.2.3. Transcription factors
3.2.3.1. Sex-determining region Y-box 9 (SOX9)
SOX9 has been implicated in control of liver progenitor cells and organ development [236]. A recent study reported that leptin stimulates SOX9 expression, and SOX9 suppresses PPARγ expression by binding to its promoter region to stimulate HSC activation [237]. SOX9 induces osteopontin, and contributes to liver fibrosis in human [238].
3.2.3.2. GATA binding protein 4 (GATA4)
Conditional knockout or haploinsufficiency of a zinc finger transcription factor, GATA4, in hepatic mesenchymal cells leads to fibrogenic HSC activation by directly regulating an antifibrogenic transcription factor, Lhx2, and accelerates fibrosis in mice, and GATA4 expression is reduced in human cirrhotic livers [239, 240].
3.2.3.3. Myocardin-related transcription factor A (MRTF-A)
MRTF-A induces histone H3K4 di/tri-methylation, recruits histone methyltransferase complex (COMPASS), and recruits and interacts with regulatory epigenetic factors, including ASH2, WDR5, and SET1, and activates expression of fibrogenic genes, including TGFβ target genes, which can be suppressed by estradiol [241, 242].
3.2.3.4. Nuclear receptor subfamily 4 group A member 1/2 (NR4A1/2)
NR4A1 and NR4A2 are orphan nuclear receptor family members that act as transcription factors, regulating cell differentiation, proliferation and apoptosis. NR4A1 suppresses TGFβ target genes, and its small molecule agonist, Csn-B, reduces fibrosis in mice [243]. NR4A2 expression is reduced in fibrotic liver, and its genetic knockout in HSCs promoted ECM production and cell proliferation via MAPK pathway [217]. NR4A2 over-expression suppresses ECM production and invasive ability, and induces cell cycle arrest and apoptosis in vitro. Moreover delivery by adenoviral infection in dimethylnitrosamine (DMN)-treated fibrotic rats significantly reduces liver fibrosis [244].
3.2.3.5. Kruppel-like factors (KLFs)
KLF6, a zinc finger transcription factor and tumor suppressor, directly represses collagen I and PDGFRβ expression and increases apoptosis of HSCs, and KLF6 haploinsufficiency increases fibrosis in rats [245]. Statins up-regulates KLF2 expression in sinusoidal endothelial cells, and induces vasoprotective gene expression, and quiescence of HSCs through a KLF2-nitric oxide-guanylate cyclase-mediated paracrine mechanism [246].
3.2.3.6. Aryl hydrocarbon receptor (AhR)
AhR is a ligand-activated transcription factor involved in the regulation of xenobiotic-metabolizing enzymes such as cytochrome P450, and AhR-deficient mice develop liver fibrosis [247]. HSC activation, i.e., TGFβ activation, PPARγ reduction, and increased collagen secretion, induced by AhR deletion requires vitamin A in mice [248]. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibits lipid droplet storage, and enhances cell proliferation and αSMA expression, but not collagen I expression, in the LX-2 human HSC line [249].
3.2.3.7. Gα-interacting vesicle-associated protein (GIV)
GIV/Girdin serves as a cellular hub that transmits signals from multiple receptors such as PDGF and TGFβ, promotes pro-fibrogenic signaling and inhibit anti-fibrogenic pathways [250].
3.2.3.8. Yes associated protein (YAP)
YAP is a transcription co-activator, which is downstream and suppressed by Hippo pathway. YAP also transmits signal from integrin β1 in myofibroblasts, and promotes fibrosis [141]. YAP knockdown inhibits HSC activation, and pharmacological inhibition of YAP suppresses fibrogenesis in mice [251]. YAP triggers accumulation of reactive-appearing ductular cells, increases myofibroblasts, and induces fibrosis in murine NASH model [252].
3.2.3.9. Embryonic stem cell-expressed RAS (ERAS)
ERAS is a RAS family GTPase, which plays an important role in maintaining quiescent HSCs with correlated activation of AKT, STAT3, mTORC2, and Hippo signaling pathways and inactivation of FOXO1 and YAP. [253].
3.2.4. Epigenetic transcriptional dysregulation
Global epigenomic and local epigenetic transcriptional dysregulation in HSC can occur as response or adaptation to environmental and/or etiological stressors, and has been implicated in its fibrogenic activation [254].
3.2.4.1. Genomic DNA methylation, histone modification, and chromatin remodeling
Genome-wide characterization of DNA methylation (5-mC) and hydroxymethylation (5-hmC) of cytosine-phosphoguanine dinucleotides (CpGs) during HSC activation in rat and human has revealed modulation of regulatory enzymes, e.g., induction of DNA methyltransferase (DNMT) 3a/b and suppression of ten-eleven translocation methylcytosine dioxygenase (TET) 2 and 3, accompanied by decreased levels of 5-hmC [255]. HSC activation can be suppressed by inhibitors of DNMT1 (e.g., 5-Aza-2′-deoxycytidine) and enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2), a polycomb group-protein complex catalyzing trimethylation of histone 3 lysine 27 (H3K27me3) (e.g., 3-deazaneplanocin), supporting their regulatory role in the activation [256–258]. DNMT1-mediated DNA hypermethylation of phosphatase and tension homolog (PTEN) leads to HSC activation [259]. Methyl-CpG binding protein 2 (MeCP2), released from translational repression by miR-132, induces HSC activation and liver fibrosis by binding promoter of PPARγ gene and suppresses its expression [256]. In parallel, MeCP2 stimulates EZH2 expression and H3K27 methylation to form a repressive chromatin structure in the 3′ exons of PPARγ, which can be pharmacologically inhibited. ASH1, a transcriptional repressor induced by MeCP2, binds regulatory regions of αSMA, collagen I, TIMP1, and TGFβ1 in activated HSCs, and depletion of ASH1 suppresses fibrogenic gene/protein expression [260]. Histone H3K9 demethylase JMJD1A suppresses HSC activation and liver fibrosis via the H3K9me2 mark in the PPARγ gene promoter [213]. Low-molecular-weight fibroblast growth factor 2 (FGF2(lmw)) attenuates hepatic fibrosis by epigenetic downregulation of Delta-like1 [261]. The bromodomain-containing protein 4 (BRD4), a member of bromodomain and extraterminal (BET) proteins highly enriched at enhancers of pro-fibrogenic genes, can be pharmacologically inhibited by its inhibitor, JQ1 to suppress HSC activation [262]. The PPARγ promoter methylation detected in plasma cell-free circulating DNA stratifies mild and severe fibrosis in patients with NAFLD and alcoholic liver disease, although it was more correlated with methylation in hepatocytes than in myofibroblasts [263].
3.2.4.2. Dietary factors
Ethanol induces an H3K4 methyltransferase, lysine methyltransferase 2A (KMT2A), and transcriptionally activates promoter of multiple ECM genes, including elastin [264]. Ethanol can also induce H3K9 acetylation in HSCs in time- and dose-dependent manner [265]
3.2.4.3. Inheritable epigenetic adaptation
Ancestral history of liver damage is associated with epigenetic suppressive adaptation that leads to higher PPARγ expression, fewer myofibroblasts, and lower expression of profibrogenic factors such as TGFβ in offspring rats [266].
3.2.4.4. Non-coding RNAs
Recent studies have elucidated dysregulation of non-coding RNAs, especially microRNAs (miRs), involved in HSC activation and inactivation by post-transcriptional regulation of mRNA degradation and translation. High-throughput miRNA profiling and other technologies have identified pro-fibrogenic miRNAs over-expressed in activated HSCs, including miR-9a-5p, miR-17-5p, miR-21, miR-27, miR-31, miR-33a, miR-34a/c, miR-125, miR-126, miR-130a/b, miR-181b, miR-214-5p, miR-195, miR-199a/b, miR-221, and miR-222, each of which induces proliferation, collagen secretion, and/or migration via a variety of pathways, and anti-fibrotic miRNAs, including miR-16, miR-19b, miR-29, miR-30, miR-101, miR-122, miR-133a, miR-144, miR-146a, miR-150, miR-155, miR-192, miR-195, miR-335, miR-454, and miR-483, although direction of change could depend on biological and/or experimental contexts [267–269]. miR-378 was recently shown to limit HSC activation by suppressing Gli3 [270]. miR-145 inhibits HSC activation and proliferation by targeting zinc finger E-box-binding homeobox 2 (ZEB2), a key mediator of epithelial-to-mesenchymal transition, via Wnt/β-catenin pathway [271]. Interferon-induced miR-195 down-regulates cyclin E1 and suppresses HSC proliferation [272]. miR-200a over-expression inhibits αSMA expression and suppresses TGFβ-induced proliferation through β-catenin, SIRT1/Notch1, and NRF2/KEAP1 pathways [273–275]. miR-214, delivered to HSCs by exosome, binds 3′-UTR of CTGF gene, and suppresses its expression [130]. The role of long non-coding RNAs (lncRNAs) is less studied compared to miRNAs. lncRNA, H19, which can be silenced by MeCP2, represses expression of insulin-like growth factor 1 receptor (IGF1R) and suppresses HSC proliferation [276]. Circulating cell-free miRNAs, e.g., miR-122, miR-138, miR-143, and miR-185, have been assessed as potential non-invasive biomarkers of HSC activation, liver fibrosis, and/or prognosis in patients mostly affected with HBV or HCV [277–279].
3.2.5. Dysregulation of cellular homeostasis and stress
3.2.5.1. Autophagy, endoplasmic reticulum (ER) stress
Intracellular energy homeostasis is dysregulated during the process of HSC activation to meet the energy demand. Autophagy is a highly regulated stress response pathway induced by chronic liver injury that fuels HSC activation by providing critical ATP substrates [280, 281]. Inhibition of autophagy suppresses HSC activation and proliferation [282]. Autophagy in HSCs can be induced by oxidative stress and ER stress in response to chronic liver injury [283]. The unfolded protein response (UPR) is a highly conserved ER stress effector pathway transmitting signal through either of protein kinase R-like ER kinase (PERK) and inositol-requiring enzyme 1 (IRE1)/X-box transcriptional factor (XBP1) pathways that link ER stress, UPR, and fibrogenic HSC activation in distinct ways: the TGFβ pathway is stimulated in the PERK-mediated, but not IRE1/XBP1-mediated, UPR during HSC activation [284–286]. Advanced glycation end product (AGE)-induced HSC activation requires autophagy in HCV-related fibrosis [287]. Transient receptor potential vanilloid 4 inhibits apoptosis in rat HSC line, HSC-T6, through induction of autophagy [288]. Rev-Erb-activating synthetic ligand, SR9009, and TGFβ similarly affect autophagy, but differentially regulate HSCs [235]. Rev-Erbα and LXR, along with PPARγ, are critical to preserve adipogenic phenotype of HSCs [221, 234]. Deletion of a collagen-specific molecular chaperone, heat shock protein 47 (Hsp47), causes ER stress-mediated apoptosis of HSCs [289]. Vitamin A-coupled liposome nanoparticles loaded with Hsp47-targeting siRNA, ND-L02-s0201, resolves fibrosis in rodent models, and is now under clinical evaluation in a phase 1b/2 trial (NCT02227459) [290]. Reticulon 4B (RTN4B, Nogo-B), associated with ER, is over-expressed in activated HSCs [291, 292]. Hypoxia-inducible factor-1α (HIF1α) regulates autophagy to activate HSCs [293]. Dimethyl α-ketoglutarate reduces CCl4-induced liver fibrosis through inhibition of autophagy in HSCs [294]. Suppression of autophagy in hepatocytes by antagonism of IL-17A inhibits liver fibrosis by restoring the IL-10/STAT3 pathway in BDL and TAA-treated mice [295]. Potentiation of HSC activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation [296].
3.2.5.2. Retinoids/vitamin A
The loss of cytoplasmic retinoids in activated HSCs is linked to retinyl ester hydrolysis that generates fatty acids as part of the autophagic response, although a causal relationship between retinoid metabolism and HSC activation is not established [282]. Retinoids exacerbate rat liver fibrosis by activating latent TGFβ in HSCs [297]. Conversion of retinol to retinoic acid leads to TGFβ and collagen expression via alcohol dehydrogenase 3, and retinol released from HSCs suppresses NK cells and contributes to HSC survival [298]. Vitamin A is required in AhR deficiency-mediated murine HSC activation [248]. Murine HSCs, lacking retinoid droplets due to genetic knockout of LRAT, can be activated, suggesting that retinoid droplets are not indispensable for activation [299]. Vitamin A and insulin are required for the maintenance of HSC quiescence [300]. A retinoic acid receptor β2 agonist reduces HSC activation in NAFLD [301]. Perilipin 2, adipose TG lipase (ATGL), and comparative gene identification-58 (CGI-58) are lipid droplet-associated proteins in rat HSC line, HSC-T6 [302]. LXRs balance lipid stores in HSCs through Rab18, a retinoid responsive lipid droplet protein [303]. Autophagy regulates turnover of lipid droplets via ROS-dependent Rab25 activation in HSC [304]. Alcohol dehydrogenases (ADHs) oxidize retinol to retinaldehyde, which is metabolized to retinoic acid. Loss of ADH3 enhances NK cell activation and IFNγ production, increases HSC apoptosis, and attenuates liver fibrosis in CCl4 and BDL mice [298]. Patatin like phospholipase domain containing 3 (PNPLA3) is highly expressed in HSCs and hepatocytes, and hydrolyzes retinol palmitate to retinol and palmitic acid with influence from retinol availability, and its over-expression is associated with reduced cytoplasmic lipid droplets. A single nucleotide polymorphism (SNP) in the PNPLA3 gene, I148M, is associated with its reduced expression, more advanced NAFLD fibrosis, and HCC, and may have functional implication in HSC activation [305].
3.2.5.3. Protein ubiquitination
Ubiquitination is a post-translational modification, ligating ubiquitin to a substrate protein to regulate its degradation, subcellular localization, and interaction with other proteins. Ubiquitin C-terminal hydrolase 1 (UCHL1), a deubiquitinase, is up-regulated following HSC activation, and promote their proliferation in CCl4 and BDL mice [306]. Small molecule UCHL1 inhibitor, LDN-57444, attenuates PDGF-induced HSC proliferation by modulating phosphorylation of a cell cycle regulator, retinoblastoma protein (Rb).
3.2.5.4. Bone morphogenetic protein 6 (BMP6)
BMP6 is an iron homeostasis regulator. BMP6 decifiency promotes hepatic inflammation and fibrosis in MCD plus high-fat diet-fed mice, but not in other non-NASH models, and recombinant BMP6 protein inhibits HSC activation [307].
4. Deactivation and elimination of fibrogenic HSCs
Termination of chronic liver injury results in regression of liver fibrosis accompanied with resolution of cytokine-rich inflammatory tissue microenvironment as well as reduced or loss of activated HSCs [5]. These observations suggest that deactivating and/or reducing fibrogenic HSCs could be an antifibrotic strategy irrespective of the cause of liver injury.
4.1. Induction of non-fibrogenic state
4.1.1. Reversion, transdifferentiation
Genetic cell lineage tracing studies in mice have showed that activated HSCs can be reverted to a quiescent-like state upon withdrawal of causative agents, although the reverted cells are sensitized to reactivation by exposure to fibrogenic stimuli [308, 309]. More recent study demonstrated that in vivo transcriptional reprogramming by ectopic expression of FOXA3, GATA4, HNF1A, and HNF4A transdifferentiates activated HSCs into hepatocyte-like non-fibrogenic cells, namely induced hepatocytes (iHeps), and reduces liver fibrosis [310].
4.1.2. Senescence
Cellular senescence is a genetically controlled physiological mechanism that restricts cell division within a finite proliferative capacity to prevent accumulation of genetically damaged cells. Senescence mediated by the p53/p21 pathway limits proliferation of activated HSCs, and reduces liver fibrosis in mice [311, 312]. Thus, induction of HSC senescence might serve as an antifibrotic strategy. A CCN family matricellular protein, cysteine-rich protein 61 (CCN1/CYR61), attenuates TGFβ signaling and induces senescence and apoptosis of activated HSCs [142, 313, 314]. Proteomic analysis has revealed that CYR61 and Wnt5a are among several matrix proteins secreted from a human HSC line, LX-2 [315]. A celecoxib derivative, OSU-03012, inhibits the proliferation and activation of HSCs by inducing senescence [316]. Soluble egg antigens (SEA) of schistosoma japonicum induce senescence of activated HSCs by activating FoxO3a/SKP2/p27 pathway [317]. HSC senescence can also be induced by phytochemicals such as curcumin by activating PPARγ/p53 signaling [218]. RAR/RXR and PPARγ may play a role in inducing HSC senescence as antifibrotic therapy [318].
4.2. Induction of HSC death or killing
Activated HSCs are resistant to cell death stimuli by activating survival signals via the NF-κB pathway and inducing anti-apoptotic proteins such as Bcl-2 [5]. Therapeutic induction of susceptibility to cell death signals may reduce the number of ECM-producing transdifferentiated HSCs and serve as an option of antifibrotic therapy. Indeed, liver fibrosis can be reduced in vivo by the NF-κB inhibitor, BAY 11-7082, as well as by proteasome inhibitors, bortezomib and MG132, via NF-κB target gene A1 [319]. Inhibition of neurochemical receptors such as CB1 and 5HT can also induce HSC apoptosis. IFNγ is a hallmark of activated NK and NKT cells that kill senescent HSCs and limits liver inflammation and fibrosis [8, 320]. IFNγ delivery specifically targeting activated HSCs by PDGFRβ-mediated uptake suppresses HSCs and reduces hepatic fibrosis [321]. Nilotinib induces apoptosis and autophagic cell death of activated HSCs via inhibition of histone deacetylases [322]. A multi-kinase inhibitor, sorafenib, similarly induces autophagic cell death and apoptosis in HSCs through the JNK and Akt signaling pathways [323].
5. Summary and future direction
As summarized in this review, comprehensive characterization of molecular dysregulation of HSCs and their functional assessment in engineered animal models have elucidated multiple candidate therapeutic targets and strategies, many of which are currently under evaluation in patients with chronic liver diseases [324]. However, both fibrogenesis and fibrosis regression involve highly heterogeneous and evolving cell populations whose interactions have not yet fully characterized. Single cell/molecule-resolution genome-wide molecular characterization, combined with experimental perturbations and special reconstruction may overcome the limitations of the current strategies that rely upon single gene/protein marker-based cell type determination [325–327]. Newly emerging experimental systems such as multi-cell type and/or 3D culture will enable more physiological modeling of fibrogenic molecular dysregulation [328]. Animal models based on chemical, surgical, and/or genetic interventions have been criticized for their physiological and/or clinical irrelevance [329]. However, unbiased omics-based characterization could enable identification of models that recapitulate specific molecular dysregulation of interest [186]. In addition to these advancements, systematic strategies to translate the experimental model-based findings to clinically meaningful long-term consequences in human fibrotic liver diseases will substantially increase the chance of success in the clinical evaluation of candidate antifibrotic therapies [186].
Acknowledgments
This work is supported by Japan Society for the Promotion of Science, Program for advancing strategic international networks to accelerate the circulation of talented researchers (to T.H.), NIH/NIDDK DK099558, European Union ERC-2014-AdG-671231HEPCIR, Irma T. Hirschl Trust, and US Department of Defense W81XWH-16-1-0363 (to Y.H.). Some cell and organelle images in the figures are from Togo picture gallery, Database Center for Life Science (DBCLS) (licensed under creative commons 4.0).
Abbreviations
- 5-HT(2B)
5-hydroxytryptamine 2B receptor
- ACE
angiotensin-converting enzyme
- AMPK
AMP-activated protein kinase
- ARB
AT1R blocker
- ASK1
apoptosis signal-regulating kinase 1
- AT1R
angiotensin II type 1 receptor
- ATX
autotaxin
- BDL
bile duct ligation
- CB
cannabinoid
- CCL
C-C motif chemokine ligand
- CCl4
carbon tetrachloride
- CCR
C-C motif chemokine receptor
- CpG
cytosine-phosphoguanine dinucleotide
- CVCR
C-X-C motif chemokine receptor
- CXCL
C-X-C motif chemokine ligand
- DAMP
damage-associated molecular pattern
- DKK1
Dickkopf-1
- DNMT
DNA methyltransferase
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- EZH2
enhancer of zeste 2 polycomb repressive complex 2 subunit
- FGF
fibroblast growth factor
- FXR
farnesoid-X-receptor
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HGF
hepatocyte growth factor
- Hh
hedgehog
- HMGB1
high mobility group protein B1
- HSC
hepatic stellate cell
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LPA
lysophosphatidic acid
- LRAT
lecithin-retinol acyltransferase
- LSEC
Liver sinusoidal endothelial cell
- LY6C
lymphocyte antigen 6 complex
- MeCP2
methyl-CpG binding protein 2
- MMP
matrix metallopeptidase
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NF-kB
nucelar factor kBNK, natural killer
- NO
nitric oxide
- OCA
obeticholic acid
- PAMP
pathogen-associated molecular pattern
- PDGF
platelet-derived growth factor
- PPAR
peroxisome proliferator-activated receptor
- PRR
pattern recognition receptor
- RAR
retinoic acid receptor
- ROS
reactive oxygen species
- RXR
retinoid X receptor
- SREBP
sterol regulatory element-binding protein
- STAT
signal transducer and activator of transcription
- TGF
transforming growth facor
- Th
helper T
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TWEAK
tumor necrosis factor-like weak inducer of apoptosis
- TZD
thiazolidinedione
- UPR
unfolded protein response
- UTR
untranslated region
- VAP-1
vascular adhesion protein 1
- VDR
vitamin D receptor
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rockey DC, Bell PD, Hill JA. Fibrosis–a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 2.G.B.D.R.F. Collaborators. Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 4.Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:767–777. doi: 10.1056/NEJMra1504367. [DOI] [PubMed] [Google Scholar]
- 5.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr161. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heymann F, Tacke F. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 9.D’Ambrosio DN, Walewski JL, Clugston RD, Berk PD, Rippe RA, Blaner WS. Distinct populations of hepatic stellate cells in the mouse liver have different capacities for retinoid and lipid storage. PLoS One. 2011;6:e24993. doi: 10.1371/journal.pone.0024993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T, Shi Z, Rockey DC. Preproendothelin-1 expression is negatively regulated by IFNgamma during hepatic stellate cell activation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G948–957. doi: 10.1152/ajpgi.00359.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobie R, Henderson NC. Homing in on the hepatic scar: recent advances in cell-specific targeting of liver fibrosis. F1000Research. 2016;5 doi: 10.12688/f1000research.8822.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhalgh SN, Conroy KP, Henderson NC. Cre-ativity in the liver: transgenic approaches to targeting hepatic nonparenchymal cells. Hepatology. 2015;61:2091–2099. doi: 10.1002/hep.27606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poelstra K, Prakash J, Beljaars L. Drug targeting to the diseased liver. Journal of controlled release: official journal of the Controlled Release Society. 2012;161:188–197. doi: 10.1016/j.jconrel.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Kawada N. Cytoglobin as a Marker of Hepatic Stellate Cell-derived Myofibroblasts. Front Physiol. 2015;6:329. doi: 10.3389/fphys.2015.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang DY, Goossens N, Guo J, Tsai M-C, Chou H-I, Altunkaynak C, et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut. 2016;65:1754–1764. doi: 10.1136/gutjnl-2015-309655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji J, Eggert T, Budhu A, Forgues M, Takai A, Dang H, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology. 2015 doi: 10.1002/hep.27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Analytical biochemistry. 1987;161:207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- 18.Ebrahimkhani MR, Mohar I, Crispe IN. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatology. 2011;54:1379–1387. doi: 10.1002/hep.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Fausther M, Goree JR, Lavoie EG, Graham AL, Sevigny J, Dranoff JA. Establishment and characterization of rat portal myofibroblast cell lines. PLoS One. 2015;10:e0121161. doi: 10.1371/journal.pone.0121161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata N, Watanabe T, Okitsu T, Sakaguchi M, Takesue M, Kunieda T, et al. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12:499–507. doi: 10.3727/000000003108747064. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q, et al. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273:33750–33758. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- 24.Meurer SK, Alsamman M, Sahin H, Wasmuth HE, Kisseleva T, Brenner DA, et al. Overexpression of endoglin modulates TGF-beta1-signalling pathways in a novel immortalized mouse hepatic stellate cell line. PLoS One. 2013;8:e56116. doi: 10.1371/journal.pone.0056116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borojevic R, Monteiro AN, Vinhas SA, Domont GB, Mourao PA, Emonard H, et al. Establishment of a continuous cell line from fibrotic schistosomal granulomas in mice livers. In Vitro Cell Dev Biol. 1985;21:382–390. doi: 10.1007/BF02623469. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65:644–653. [PubMed] [Google Scholar]
- 28.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A. 2014;111:E3297–3305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Kisseleva T, Brenner DA. The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. J Hepatol. 2012;56:965–972. doi: 10.1016/j.jhep.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology. 2017;65:1039–1043. doi: 10.1002/hep.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci U S A. 2013;110:2324–2329. doi: 10.1073/pnas.1214136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholten D, Weiskirchen R. Questioning the challenging role of epithelial-to-mesenchymal transition in liver injury. Hepatology. 2011;53:1048–1051. doi: 10.1002/hep.24191. [DOI] [PubMed] [Google Scholar]
- 36.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123:2380–2394. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace MC, Friedman SL, Mann DA. Emerging and disease-specific mechanisms of hepatic stellate cell activation. Semin Liver Dis. 2015;35:107–118. doi: 10.1055/s-0035-1550060. [DOI] [PubMed] [Google Scholar]
- 39.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783 e764. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen H, Sheng L, Chen Z, Jiang L, Su H, Yin L, et al. Mouse hepatocyte overexpression of NF-kappaB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis. Hepatology. 2014;60:2065–2076. doi: 10.1002/hep.27348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 42.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arshad MI, Piquet-Pellorce C, Samson M. IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int. 2012;32:1200–1210. doi: 10.1111/j.1478-3231.2012.02802.x. [DOI] [PubMed] [Google Scholar]
- 45.McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lan T, Kisseleva T, Brenner DA. Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation. PLoS One. 2015;10:e0129743. doi: 10.1371/journal.pone.0129743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375–1384. doi: 10.1053/j.gastro.2010.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ku NO, Strnad P, Bantel H, Omary MB. Keratins: Biomarkers and modulators of apoptotic and necrotic cell death in the liver. Hepatology. 2016;64:966–976. doi: 10.1002/hep.28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 51.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gortzen J, Schierwagen R, Bierwolf J, Klein S, Uschner FE, van der Ven PF, et al. Interplay of Matrix Stiffness and c-SRC in Hepatic Fibrosis. Front Physiol. 2015;6:359. doi: 10.3389/fphys.2015.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caliari SR, Perepelyuk M, Cosgrove BD, Tsai SJ, Lee GY, Mauck RL, et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci Rep. 2016;6:21387. doi: 10.1038/srep21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caliari SR, Perepelyuk M, Soulas EM, Lee GY, Wells RG, Burdick JA. Gradually softening hydrogels for modeling hepatic stellate cell behavior during fibrosis regression. Integr Biol (Camb) 2016;8:720–728. doi: 10.1039/c6ib00027d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borkham-Kamphorst E, van Roeyen CR, Van de Leur E, Floege J, Weiskirchen R. CCN3/NOV small interfering RNA enhances fibrogenic gene expression in primary hepatic stellate cells and cirrhotic fat storing cell line CFSC. J Cell Commun Signal. 2012;6:11–25. doi: 10.1007/s12079-011-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 58.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 59.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 61.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8:e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrison SA, Marri SR, Chalasani N, Kohli R, Aronstein W, Thompson GA, et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther. 2016;44:1183–1198. doi: 10.1111/apt.13816. [DOI] [PubMed] [Google Scholar]
- 65.Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, et al. The fractalkine receptor CX(3)CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology. 2010;52:1769–1782. doi: 10.1002/hep.23894. [DOI] [PubMed] [Google Scholar]
- 66.Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallowfield JA, Snowdon VK, et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–1975. doi: 10.1002/hep.25567. [DOI] [PubMed] [Google Scholar]
- 67.Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clinical & developmental immunology. 2011;2011:345803. doi: 10.1155/2011/345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776. e761–763. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis. Hepatology. 2012 doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu DH, Guo XY, Qin SY, Luo W, Huang XL, Chen M, et al. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol. 2015;21:1531–1545. doi: 10.3748/wjg.v21.i5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao W, Fan YC, Zhang JY, Zheng MH. Emerging Role of Interleukin 22 in Hepatitis B Virus Infection: a Double-edged Sword. J Clin Transl Hepatol. 2013;1:103–108. doi: 10.14218/JCTH.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langhans B, Kramer B, Louis M, Nischalke HD, Huneburg R, Staratschek-Jox A, et al. Intrahepatic IL-8 producing Foxp3(+)CD4(+) regulatory T cells and fibrogenesis in chronic hepatitis C. J Hepatol. 2013;59:229–235. doi: 10.1016/j.jhep.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Hammerich L, Bangen JM, Govaere O, Zimmermann HW, Gassler N, Huss S, et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology. 2014;59:630–642. doi: 10.1002/hep.26697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thapa M, Chinnadurai R, Velazquez VM, Tedesco D, Elrod E, Han JH, et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology. 2015;61:2067–2079. doi: 10.1002/hep.27761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 77.Jiao J, Ooka K, Fey H, Fiel MI, Rahmman AH, Kojima K, et al. Interleukin-15 receptor alpha on hepatic stellate cells regulates hepatic fibrogenesis in mice. J Hepatol. 2016;65:344–353. doi: 10.1016/j.jhep.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langhans B, Alwan AW, Kramer B, Glassner A, Lutz P, Strassburg CP, et al. Regulatory CD4+ T cells modulate the interaction between NK cells and hepatic stellate cells by acting on either cell type. J Hepatol. 2015;62:398–404. doi: 10.1016/j.jhep.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 79.Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2013;1832:1061–1069. doi: 10.1016/j.bbadis.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J Immunol. 2013;190:5226–5236. doi: 10.4049/jimmunol.1202909. [DOI] [PubMed] [Google Scholar]
- 82.Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol. 2017;66:212–227. doi: 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:918–927 e916. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 85.Kordes C, Sawitza I, Gotze S, Herebian D, Haussinger D. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J Clin Invest. 2014;124:5503–5515. doi: 10.1172/JCI74119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Lopategi A, Ge X, Lu Y, Kitamura N, Urtasun R, et al. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut. 2014;63:1805–1818. doi: 10.1136/gutjnl-2013-306373. [DOI] [PubMed] [Google Scholar]
- 87.Wen Y, Jeong S, Xia Q, Kong X. Role of Osteopontin in Liver Diseases. Int J Biol Sci. 2016;12:1121–1128. doi: 10.7150/ijbs.16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kodama T, Takehara T, Hikita H, Shimizu S, Li W, Miyagi T, et al. Thrombocytopenia exacerbates cholestasis-induced liver fibrosis in mice. Gastroenterology. 2010;138:2487–2498. 2498 e2481–2487. doi: 10.1053/j.gastro.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 89.Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–190. doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]
- 90.De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Candelaresi C, Trozzi L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 91.Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29:1043–1055. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59:154–169. doi: 10.1002/hep.26604. [DOI] [PubMed] [Google Scholar]
- 93.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016 doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zan Y, Zhang Y, Tien P. Hepatitis B virus e antigen induces activation of rat hepatic stellate cells. Biochem Biophys Res Commun. 2013;435:391–396. doi: 10.1016/j.bbrc.2013.04.098. [DOI] [PubMed] [Google Scholar]
- 95.Bai Q, An J, Wu X, You H, Ma H, Liu T, et al. HBV promotes the proliferation of hepatic stellate cells via the PDGF-B/PDGFR-beta signaling pathway in vitro. Int J Mol Med. 2012;30:1443–1450. doi: 10.3892/ijmm.2012.1148. [DOI] [PubMed] [Google Scholar]
- 96.Florimond A, Chouteau P, Bruscella P, Le Seyec J, Merour E, Ahnou N, et al. Human hepatic stellate cells are not permissive for hepatitis C virus entry and replication. Gut. 2015;64:957–965. doi: 10.1136/gutjnl-2013-305634. [DOI] [PubMed] [Google Scholar]
- 97.Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529–540. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 98.Battaglia S, Benzoubir N, Nobilet S, Charneau P, Samuel D, Zignego AL, et al. Liver cancer-derived hepatitis C virus core proteins shift TGF-beta responses from tumor suppression to epithelial-mesenchymal transition. PLoS One. 2009;4:e4355. doi: 10.1371/journal.pone.0004355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benzoubir N, Lejamtel C, Battaglia S, Testoni B, Benassi B, Gondeau C, et al. HCV core-mediated activation of latent TGF-beta via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J Hepatol. 2013;59:1160–1168. doi: 10.1016/j.jhep.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 100.Presser LD, McRae S, Waris G. Activation of TGF-beta1 promoter by hepatitis C virus-induced AP-1 and Sp1: role of TGF-beta1 in hepatic stellate cell activation and invasion. PLoS One. 2013;8:e56367. doi: 10.1371/journal.pone.0056367. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Preisser L, Miot C, Le Guillou-Guillemette H, Beaumont E, Foucher ED, Garo E, et al. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology. 2014;60:1879–1890. doi: 10.1002/hep.27328. [DOI] [PubMed] [Google Scholar]
- 102.Gjaerde LI, Shepherd L, Jablonowska E, Lazzarin A, Rougemont M, Darling K, et al. Trends in Incidences and Risk Factors for Hepatocellular Carcinoma and Other Liver Events in HIV and Hepatitis C Virus-coinfected Individuals From 2001 to 2014: A Multicohort Study. Clin Infect Dis. 2016;63:821–829. doi: 10.1093/cid/ciw380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 104.Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–622. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hong F, Saiman Y, Si C, Mosoian A, Bansal MB. X4 Human immunodeficiency virus type 1 gp120 promotes human hepatic stellate cell activation and collagen I expression through interactions with CXCR4. PLoS One. 2012;7:e33659. doi: 10.1371/journal.pone.0033659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salloum S, Holmes JA, Jindal R, Bale SS, Brisac C, Alatrakchi N, et al. Exposure to human immunodeficiency virus/hepatitis C virus in hepatic and stellate cell lines reveals cooperative profibrotic transcriptional activation between viruses and cell types. Hepatology. 2016;64:1951–1968. doi: 10.1002/hep.28766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, et al. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell and tissue research. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Szondy Z, Sarang Z, Molnar P, Nemeth T, Piacentini M, Mastroberardino PG, et al. Transglutaminase 2−/−mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci U S A. 2003;100:7812–7817. doi: 10.1073/pnas.0832466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174–1183. doi: 10.1016/j.jhep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 111.Meindl-Beinker NM, Matsuzaki K, Dooley S. TGF-beta signaling in onset and progression of hepatocellular carcinoma. Dig Dis. 2012;30:514–523. doi: 10.1159/000341704. [DOI] [PubMed] [Google Scholar]
- 112.Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schnur J, Nagy P, Sebestyen A, Schaff Z, Thorgeirsson SS. Chemical hepatocarcinogenesis in transgenic mice overexpressing mature TGF beta-1 in liver. Eur J Cancer. 1999;35:1842–1845. doi: 10.1016/s0959-8049(99)00224-5. [DOI] [PubMed] [Google Scholar]
- 114.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15–24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 115.Fang L, Huang C, Meng X, Wu B, Ma T, Liu X, et al. TGF-beta1-elevated TRPM7 channel regulates collagen expression in hepatic stellate cells via TGF-beta1/Smad pathway. Toxicology and applied pharmacology. 2014;280:335–344. doi: 10.1016/j.taap.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 116.Fang L, Zhan S, Huang C, Cheng X, Lv X, Si H, et al. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicology and applied pharmacology. 2013;272:713–725. doi: 10.1016/j.taap.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 117.An P, Tian Y, Chen M, Luo H. Ca(2+)/calmodulin-dependent protein kinase II mediates transforming growth factor-beta-induced hepatic stellate cells proliferation but not in collagen alpha1(I) production. Hepatol Res. 2012;42:806–818. doi: 10.1111/j.1872-034X.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- 118.Wu X, Wu X, Ma Y, Shao F, Tan Y, Tan T, et al. CUG-binding protein 1 regulates HSC activation and liver fibrogenesis. Nat Commun. 2016;7:13498. doi: 10.1038/ncomms13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hong F, Chou H, Fiel MI, Friedman SL. Antifibrotic activity of sorafenib in experimental hepatic fibrosis: refinement of inhibitory targets, dosing, and window of efficacy in vivo. Dig Dis Sci. 2013;58:257–264. doi: 10.1007/s10620-012-2325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kocabayoglu P, Lade A, Lee YA, Dragomir AC, Sun X, Fiel MI, et al. beta-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63:141–147. doi: 10.1016/j.jhep.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419–428. doi: 10.1016/j.jhep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 122.Kim Y, Fiel MI, Albanis E, Chou HI, Zhang W, Khitrov G, et al. Anti-fibrotic activity and enhanced interleukin-6 production by hepatic stellate cells in response to imatinib mesylate. Liver Int. 2012;32:1008–1017. doi: 10.1111/j.1478-3231.2012.02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Westra IM, Oosterhuis D, Groothuis GM, Olinga P. Precision-cut liver slices as a model for the early onset of liver fibrosis to test antifibrotic drugs. Toxicology and applied pharmacology. 2014;274:328–338. doi: 10.1016/j.taap.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 124.Liu Y, Wang Z, Kwong SQ, Lui EL, Friedman SL, Li FR, et al. Inhibition of PDGF, TGF-beta, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011;55:612–625. doi: 10.1016/j.jhep.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 125.Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–1926. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 126.Wilhelm A, Aldridge V, Haldar D, Naylor AJ, Weston CJ, Hedegaard D, et al. CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism. Gut. 2016;65:1175–1185. doi: 10.1136/gutjnl-2014-308325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tibaldi E, Zonta F, Bordin L, Magrin E, Gringeri E, Cillo U, et al. The tyrosine phosphatase SHP-1 inhibits proliferation of activated hepatic stellate cells by impairing PDGF receptor signaling. Biochim Biophys Acta. 2014;1843:288–298. doi: 10.1016/j.bbamcr.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 128.Zhang X, Tan Z, Wang Y, Tang J, Jiang R, Hou J, et al. PTPRO-associated hepatic stellate cell activation plays a critical role in liver fibrosis. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;35:885–898. doi: 10.1159/000369746. [DOI] [PubMed] [Google Scholar]
- 129.Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005;102:3389–3394. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsai TH, Shih SC, Ho TC, Ma HI, Liu MY, Chen SL, et al. Pigment epithelium-derived factor 34-mer peptide prevents liver fibrosis and hepatic stellate cell activation through down-regulation of the PDGF receptor. PLoS One. 2014;9:e95443. doi: 10.1371/journal.pone.0095443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hao C, Xie Y, Peng M, Ma L, Zhou Y, Zhang Y, et al. Inhibition of connective tissue growth factor suppresses hepatic stellate cell activation in vitro and prevents liver fibrosis in vivo. Clinical and experimental medicine. 2014;14:141–150. doi: 10.1007/s10238-013-0229-6. [DOI] [PubMed] [Google Scholar]
- 132.Hauff P, Gottwald U, Ocker M. Early to Phase II drugs currently under investigation for the treatment of liver fibrosis. Expert Opin Investig Drugs. 2015;24:309–327. doi: 10.1517/13543784.2015.997874. [DOI] [PubMed] [Google Scholar]
- 133.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis & tissue repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, et al. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nature cell biology. 2014;16:972–981. 971–977. doi: 10.1038/ncb3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, et al. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44:1267–1277. doi: 10.1002/hep.21375. [DOI] [PubMed] [Google Scholar]
- 137.Fukushima M, Nakamuta M, Kohjima M, Kotoh K, Enjoji M, Kobayashi N, et al. Fasudil hydrochloride hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver Int. 2005;25:829–838. doi: 10.1111/j.1478-3231.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 138.Sohail MA, Hashmi AZ, Hakim W, Watanabe A, Zipprich A, Groszmann RJ, et al. Adenosine induces loss of actin stress fibers and inhibits contraction in hepatic stellate cells via Rho inhibition. Hepatology. 2009;49:185–194. doi: 10.1002/hep.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murata T, Arii S, Nakamura T, Mori A, Kaido T, Furuyama H, et al. Inhibitory effect of Y-27632, a ROCK inhibitor, on progression of rat liver fibrosis in association with inactivation of hepatic stellate cells. J Hepatol. 2001;35:474–481. doi: 10.1016/s0168-8278(01)00169-6. [DOI] [PubMed] [Google Scholar]
- 140.Klein S, Van Beuge MM, Granzow M, Beljaars L, Schierwagen R, Kilic S, et al. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220–1227. doi: 10.1016/j.jhep.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 141.Martin K, Pritchett J, Llewellyn J, Mullan AF, Athwal VS, Dobie R, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat Commun. 2016;7:12502. doi: 10.1038/ncomms12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol. 2013;33:2078–2090. doi: 10.1128/MCB.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Granzow M, Schierwagen R, Klein S, Kowallick B, Huss S, Linhart M, et al. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology. 2014;60:334–348. doi: 10.1002/hep.27117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Klein S, Rick J, Lehmann J, Schierwagen R, Schierwagen IG, Verbeke L, et al. Janus-kinase-2 relates directly to portal hypertension and to complications in rodent and human cirrhosis. Gut. 2017;66:145–155. doi: 10.1136/gutjnl-2015-309600. [DOI] [PubMed] [Google Scholar]
- 145.Salama ZA, Sadek A, Abdelhady AM, Darweesh SK, Morsy SA, Esmat G. Losartan may inhibit the progression of liver fibrosis in chronic HCV patients. Hepatobiliary Surg Nutr. 2016;5:249–255. doi: 10.21037/hbsn.2016.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Namisaki T, Noguchi R, Moriya K, Kitade M, Aihara Y, Douhara A, et al. Beneficial effects of combined ursodeoxycholic acid and angiotensin-II type 1 receptor blocker on hepatic fibrogenesis in a rat model of nonalcoholic steatohepatitis. J Gastroenterol. 2016;51:162–172. doi: 10.1007/s00535-015-1104-x. [DOI] [PubMed] [Google Scholar]
- 147.Rozenfeld R, Gupta A, Gagnidze K, Lim MP, Gomes I, Lee-Ramos D, et al. AT1R-CB(1)R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. The EMBO journal. 2011;30:2350–2363. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li S, Wang Q, Tao Y, Liu C. Swertiamarin Attenuates Experimental Rat Hepatic Fibrosis by Suppressing Angiotensin II-Angiotensin Type 1 Receptor-Extracellular Signal-Regulated Kinase Signaling. J Pharmacol Exp Ther. 2016;359:247–255. doi: 10.1124/jpet.116.234179. [DOI] [PubMed] [Google Scholar]
- 149.Miao CG, Yang YY, He X, Huang C, Huang Y, Zhang L, et al. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie. 2013;95:2326–2335. doi: 10.1016/j.biochi.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 150.Ge WS, Wang YJ, Wu JX, Fan JG, Chen YW, Zhu L. beta-catenin is overexpressed in hepatic fibrosis and blockage of Wnt/beta-catenin signaling inhibits hepatic stellate cell activation. Mol Med Rep. 2014;9:2145–2151. doi: 10.3892/mmr.2014.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G39–49. doi: 10.1152/ajpgi.00263.2007. [DOI] [PubMed] [Google Scholar]
- 152.Zhu NL, Asahina K, Wang J, Ueno A, Lazaro R, Miyaoka Y, et al. Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration. J Biol Chem. 2012;287:10355–10367. doi: 10.1074/jbc.M111.312751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Osawa Y, Oboki K, Imamura J, Kojika E, Hayashi Y, Hishima T, et al. Inhibition of Cyclic Adenosine Monophosphate (cAMP)-response Element-binding Protein (CREB)-binding Protein (CBP)/beta-Catenin Reduces Liver Fibrosis in Mice. EBioMedicine. 2015;2:1751–1758. doi: 10.1016/j.ebiom.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kordes C, Sawitza I, Haussinger D. Canonical Wnt signaling maintains the quiescent stage of hepatic stellate cells. Biochem Biophys Res Commun. 2008;367:116–123. doi: 10.1016/j.bbrc.2007.12.085. [DOI] [PubMed] [Google Scholar]
- 155.Xinguang Y, Huixing Y, Linlin W, Wanxin W, Xiaojun W, Linghua Y. RSPOs facilitated HSC activation and promoted hepatic fibrogenesis. Oncotarget. 2016;7:63767–63778. doi: 10.18632/oncotarget.11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Corbett L, Mann J, Mann DA. Non-Canonical Wnt Predominates in Activated Rat Hepatic Stellate Cells, Influencing HSC Survival and Paracrine Stimulation of Kupffer Cells. PLoS One. 2015;10:e0142794. doi: 10.1371/journal.pone.0142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chatani N, Kamada Y, Kizu T, Ogura S, Furuta K, Egawa M, et al. Secreted frizzled-related protein 5 (Sfrp5) decreases hepatic stellate cell activation and liver fibrosis. Liver Int. 2015;35:2017–2026. doi: 10.1111/liv.12757. [DOI] [PubMed] [Google Scholar]
- 158.Chung SI, Moon H, Ju HL, Cho KJ, Kim do Y, Han KH, et al. Hepatic expression of Sonic Hedgehog induces liver fibrosis and promotes hepatocarcinogenesis in a transgenic mouse model. J Hepatol. 2016;64:618–627. doi: 10.1016/j.jhep.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 159.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329. e1311–1311. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gorojankina T. Hedgehog signaling pathway: a novel model and molecular mechanisms of signal transduction. Cell Mol Life Sci. 2016;73:1317–1332. doi: 10.1007/s00018-015-2127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.El-Agroudy NN, El-Naga RN, El-Razeq RA, El-Demerdash E. Forskolin, a hedgehog signalling inhibitor, attenuates carbon tetrachloride-induced liver fibrosis in rats. Br J Pharmacol. 2016;173:3248–3260. doi: 10.1111/bph.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pratap A, Singh S, Mundra V, Yang N, Panakanti R, Eason JD, et al. Attenuation of early liver fibrosis by pharmacological inhibition of smoothened receptor signaling. Journal of drug targeting. 2012;20:770–782. doi: 10.3109/1061186X.2012.719900. [DOI] [PubMed] [Google Scholar]
- 164.Hirsova P, Ibrahim SH, Bronk SF, Yagita H, Gores GJ. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 2013;8:e70599. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xie G, Karaca G, Swiderska-Syn M, Michelotti GA, Kruger L, Chen Y, et al. Crosstalk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology. 2013;58:1801–1813. doi: 10.1002/hep.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mallat A, Teixeira-Clerc F, Lotersztajn S. Cannabinoid signaling and liver therapeutics. J Hepatol. 2013;59:891–896. doi: 10.1016/j.jhep.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 167.Gueye AB, Pryslawsky Y, Trigo JM, Poulia N, Delis F, Antoniou K, et al. The CB1 Neutral Antagonist AM4113 Retains the Therapeutic Efficacy of the Inverse Agonist Rimonabant for Nicotine Dependence and Weight Loss with Better Psychiatric Tolerability. Int J Neuropsychopharmacol. 2016;19 doi: 10.1093/ijnp/pyw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y, et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell. 2016;167:750–762 e714. doi: 10.1016/j.cell.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Coppola N, Zampino R, Bellini G, Macera M, Marrone A, Pisaturo M, et al. Association between a polymorphism in cannabinoid receptor 2 and severe necroinflammation in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2014;12:334–340. doi: 10.1016/j.cgh.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 170.Guillot A, Hamdaoui N, Bizy A, Zoltani K, Souktani R, Zafrani ES, et al. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology. 2014;59:296–306. doi: 10.1002/hep.26598. [DOI] [PubMed] [Google Scholar]
- 171.El Swefy S, Hasan RA, Ibrahim A, Mahmoud MF. Curcumin and hemopressin treatment attenuates cholestasis-induced liver fibrosis in rats: role of CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:103–116. doi: 10.1007/s00210-015-1181-7. [DOI] [PubMed] [Google Scholar]
- 172.Tang Y. Curcumin targets multiple pathways to halt hepatic stellate cell activation: updated mechanisms in vitro and in vivo. Dig Dis Sci. 2015;60:1554–1564. doi: 10.1007/s10620-014-3487-6. [DOI] [PubMed] [Google Scholar]
- 173.Ebrahimkhani MR, Oakley F, Murphy LB, Mann J, Moles A, Perugorria MJ, et al. Stimulating healthy tissue regeneration by targeting the 5-HT(2)B receptor in chronic liver disease. Nat Med. 2011;17:1668–1673. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim DC, Jun DW, Kwon YI, Lee KN, Lee HL, Lee OY, et al. 5-HT2A receptor antagonists inhibit hepatic stellate cell activation and facilitate apoptosis. Liver Int. 2013;33:535–543. doi: 10.1111/liv.12110. [DOI] [PubMed] [Google Scholar]
- 175.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016 doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 176.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 177.Kiziltas S. Toll-like receptors in pathophysiology of liver diseases. World journal of hepatology. 2016;8:1354–1369. doi: 10.4254/wjh.v8.i32.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 179.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 181.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–334 e327. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330–1340 e1331. doi: 10.1053/j.gastro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577–589. doi: 10.1002/hep.26081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Seo W, Eun HS, Kim SY, Yi HS, Lee YS, Park SH, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by gammadelta T cells in liver fibrosis. Hepatology. 2016;64:616–631. doi: 10.1002/hep.28644. [DOI] [PubMed] [Google Scholar]
- 185.Kaffe E, Katsifa A, Xylourgidis N, Ninou I, Zannikou M, Harokopos V, et al. Hepatocyte Autotaxin expression promotes liver fibrosis and cancer. Hepatology. 2016 doi: 10.1002/hep.28973. [DOI] [PubMed] [Google Scholar]
- 186.Nakagawa S, Wei L, Song WM, Higashi T, Ghoshal S, Kim RS, et al. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell. 2016;30:879–890. doi: 10.1016/j.ccell.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655–1669. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saxena NK, Anania FA. Adipocytokines and hepatic fibrosis. Trends in Endocrinology & Metabolism. 2015;26:153–161. doi: 10.1016/j.tem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhai X, Yan K, Fan J, Niu M, Zhou Q, Zhou Y, et al. The beta-catenin pathway contributes to the effects of leptin on SREBP-1c expression in rat hepatic stellate cells and liver fibrosis. Br J Pharmacol. 2013;169:197–212. doi: 10.1111/bph.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dong Z, Su L, Esmaili S, Iseli TJ, Ramezani-Moghadam M, Hu L, et al. Adiponectin attenuates liver fibrosis by inducing nitric oxide production of hepatic stellate cells. J Mol Med (Berl) 2015;93:1327–1339. doi: 10.1007/s00109-015-1313-z. [DOI] [PubMed] [Google Scholar]
- 192.Ramezani-Moghadam M, Wang J, Ho V, Iseli TJ, Alzahrani B, Xu A, et al. Adiponectin reduces hepatic stellate cell migration by promoting tissue inhibitor of metalloproteinase-1 (TIMP-1) secretion. J Biol Chem. 2015;290:5533–5542. doi: 10.1074/jbc.M114.598011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tardelli M, Moreno-Viedma V, Zeyda M, Itariu BK, Langer FB, Prager G, et al. Adiponectin regulates aquaglyceroporin expression in hepatic stellate cells altering their functional state. J Gastroenterol Hepatol. 2016 doi: 10.1111/jgh.13415. [DOI] [PubMed] [Google Scholar]
- 194.Kumar P, Smith T, Rahman K, Mells JE, Thorn NE, Saxena NK, et al. Adiponectin modulates focal adhesion disassembly in activated hepatic stellate cells: implication for reversing hepatic fibrosis. FASEB J. 2014;28:5172–5183. doi: 10.1096/fj.14-253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang H, Zhang H, Zhang Z, Huang B, Cheng X, Wang D, et al. Adiponectin-derived active peptide ADP355 exerts anti-inflammatory and anti-fibrotic activities in thioacetamide-induced liver injury. Sci Rep. 2016;6:19445. doi: 10.1038/srep19445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169:983–992. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- 197.Weston CJ, Shepherd EL, Claridge LC, Rantakari P, Curbishley SM, Tomlinson JW, et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest. 2015;125:501–520. doi: 10.1172/JCI73722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dwyer BJ, Olynyk JK, Ramm GA, Tirnitz-Parker JE. TWEAK and LTbeta Signaling during Chronic Liver Disease. Frontiers in immunology. 2014;5:39. doi: 10.3389/fimmu.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wilhelm A, Shepherd EL, Amatucci A, Munir M, Reynolds G, Humphreys E, et al. Interaction of TWEAK with Fn14 leads to the progression of fibrotic liver disease by directly modulating hepatic stellate cell proliferation. J Pathol. 2016;239:109–121. doi: 10.1002/path.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang F, Zhang M, Wang A, Xu M, Wang C, Xu G, et al. TWEAK increases SIRT1 expression and promotes p53 deacetylation affecting human hepatic stellate cell senescence. Cell Biol Int. 2016 doi: 10.1002/cbin.10706. [DOI] [PubMed] [Google Scholar]
- 201.Li YH, Choi DH, Lee EH, Seo SR, Lee S, Cho EH. Sirtuin 3 (SIRT3) Regulates alpha-Smooth Muscle Actin (alpha-SMA) Production through the Succinate Dehydrogenase-G Protein-coupled Receptor 91 (GPR91) Pathway in Hepatic Stellate Cells. J Biol Chem. 2016;291:10277–10292. doi: 10.1074/jbc.M115.692244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barcena C, Stefanovic M, Tutusaus A, Joannas L, Menendez A, Garcia-Ruiz C, et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J Hepatol. 2015;63:670–678. doi: 10.1016/j.jhep.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology. 2017;65:350–362. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 206.Xu W, Lu C, Zhang F, Shao J, Zheng S. Dihydroartemisinin restricts hepatic stellate cell contraction via an FXR-S1PR2-dependent mechanism. IUBMB Life. 2016;68:376–387. doi: 10.1002/iub.1492. [DOI] [PubMed] [Google Scholar]
- 207.Xu W, Lu C, Zhang F, Shao J, Yao S, Zheng S. Dihydroartemisinin counteracts fibrotic portal hypertension via farnesoid X receptor-dependent inhibition of hepatic stellate cell contraction. FEBS J. 2016 doi: 10.1111/febs.13956. [DOI] [PubMed] [Google Scholar]
- 208.Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13:36–49. doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- 209.Tsukamoto H, She H, Hazra S, Cheng J, Miyahara T. Anti-adipogenic regulation underlies hepatic stellate cell transdifferentiation. J Gastroenterol Hepatol. 2006;21(Suppl 3):S102–105. doi: 10.1111/j.1440-1746.2006.04573.x. [DOI] [PubMed] [Google Scholar]
- 210.Zhang F, Kong D, Chen L, Zhang X, Lian N, Zhu X, et al. Peroxisome proliferator-activated receptor-gamma interrupts angiogenic signal transduction by transrepression of platelet-derived growth factor-beta receptor in hepatic stellate cells. J Cell Sci. 2014;127:305–314. doi: 10.1242/jcs.128306. [DOI] [PubMed] [Google Scholar]
- 211.Qian J, Niu M, Zhai X, Zhou Q, Zhou Y. beta-Catenin pathway is required for TGF-beta1 inhibition of PPARgamma expression in cultured hepatic stellate cells. Pharmacol Res. 2012;66:219–225. doi: 10.1016/j.phrs.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 212.Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell Mol Gastroenterol Hepatol. 2015;1:646–663 e644. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jiang Y, Wang S, Zhao Y, Lin C, Zhong F, Jin L, et al. Histone H3K9 demethylase JMJD1A modulates hepatic stellate cells activation and liver fibrosis by epigenetically regulating peroxisome proliferator-activated receptor gamma. FASEB J. 2015;29:1830–1841. doi: 10.1096/fj.14-251751. [DOI] [PubMed] [Google Scholar]
- 214.Zhou Y, Jia X, Qin J, Lu C, Zhu H, Li X, et al. Leptin inhibits PPARgamma gene expression in hepatic stellate cells in the mouse model of liver damage. Mol Cell Endocrinol. 2010;323:193–200. doi: 10.1016/j.mce.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 215.Zhou Q, Guan W, Qiao H, Cheng Y, Li Z, Zhai X, et al. GATA binding protein 2 mediates leptin inhibition of PPARgamma1 expression in hepatic stellate cells and contributes to hepatic stellate cell activation. Biochim Biophys Acta. 2014;1842:2367–2377. doi: 10.1016/j.bbadis.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 216.Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARgamma-dependent and -independent mechanisms. Am J Pathol. 2011;178:2690–2699. doi: 10.1016/j.ajpath.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhai X, Qiao H, Guan W, Li Z, Cheng Y, Jia X, et al. Curcumin regulates peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression by AMPK pathway in hepatic stellate cells in vitro. Eur J Pharmacol. 2015;746:56–62. doi: 10.1016/j.ejphar.2014.10.055. [DOI] [PubMed] [Google Scholar]
- 218.Jin H, Lian N, Zhang F, Chen L, Chen Q, Lu C, et al. Activation of PPARgamma/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell death & disease. 2016;7:e2189. doi: 10.1038/cddis.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147–1159 e1145. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 221.Beaven SW, Wroblewski K, Wang J, Hong C, Bensinger S, Tsukamoto H, et al. Liver X receptor signaling is a determinant of stellate cell activation and susceptibility to fibrotic liver disease. Gastroenterology. 2011;140:1052–1062. doi: 10.1053/j.gastro.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yan K, Deng X, Zhai X, Zhou M, Jia X, Luo L, et al. p38 mitogen-activated protein kinase and liver X receptor-alpha mediate the leptin effect on sterol regulatory element binding protein-1c expression in hepatic stellate cells. Mol Med. 2012;18:10–18. doi: 10.2119/molmed.2011.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Duran A, Hernandez ED, Reina-Campos M, Castilla EA, Subramaniam S, Raghunandan S, et al. p62/SQSTM1 by Binding to Vitamin D Receptor Inhibits Hepatic Stellate Cell Activity, Fibrosis, and Liver Cancer. Cancer Cell. 2016;30:595–609. doi: 10.1016/j.ccell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Beilfuss A, Sowa JP, Sydor S, Beste M, Bechmann LP, Schlattjan M, et al. Vitamin D counteracts fibrogenic TGF-beta signalling in human hepatic stellate cells both receptor-dependently and independently. Gut. 2015;64:791–799. doi: 10.1136/gutjnl-2014-307024. [DOI] [PubMed] [Google Scholar]
- 226.Zhang R, Wang Y, Li R, Chen G. Transcriptional Factors Mediating Retinoic Acid Signals in the Control of Energy Metabolism. Int J Mol Sci. 2015;16:14210–14244. doi: 10.3390/ijms160614210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shimizu M, Shirakami Y, Hanai T, Imai K, Suetsugu A, Takai K, et al. Pharmaceutical and nutraceutical approaches for preventing liver carcinogenesis: chemoprevention of hepatocellular carcinoma using acyclic retinoid and branched-chain amino acids. Mol Nutr Food Res. 2014;58:124–135. doi: 10.1002/mnfr.201300538. [DOI] [PubMed] [Google Scholar]
- 228.Okita K, Izumi N, Matsui O, Tanaka K, Kaneko S, Moriwaki H, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2015;50:191–202. doi: 10.1007/s00535-014-0956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hellemans K, Grinko I, Rombouts K, Schuppan D, Geerts A. All-trans and 9-cis retinoic acid alter rat hepatic stellate cell phenotype differentially. Gut. 1999;45:134–142. doi: 10.1136/gut.45.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hisamori S, Tabata C, Kadokawa Y, Okoshi K, Tabata R, Mori A, et al. All-transretinoic acid ameliorates carbon tetrachloride-induced liver fibrosis in mice through modulating cytokine production. Liver Int. 2008;28:1217–1225. doi: 10.1111/j.1478-3231.2008.01745.x. [DOI] [PubMed] [Google Scholar]
- 231.Sharvit E, Abramovitch S, Reif S, Bruck R. Amplified inhibition of stellate cell activation pathways by PPAR-gamma, RAR and RXR agonists. PLoS One. 2013;8:e76541. doi: 10.1371/journal.pone.0076541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bruck R, Weiss S, Aeed H, Pines M, Halpern Z, Zvibel I. Additive inhibitory effect of experimentally induced hepatic cirrhosis by agonists of peroxisome proliferator activator receptor gamma and retinoic acid receptor. Dig Dis Sci. 2009;54:292–299. doi: 10.1007/s10620-008-0336-5. [DOI] [PubMed] [Google Scholar]
- 233.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li T, Eheim AL, Klein S, Uschner FE, Smith AC, Brandon-Warner E, et al. Novel role of nuclear receptor Rev-erbalpha in hepatic stellate cell activation: potential therapeutic target for liver injury. Hepatology. 2014;59:2383–2396. doi: 10.1002/hep.27049. [DOI] [PubMed] [Google Scholar]
- 235.Thomes PG, Brandon-Warner E, Li T, Donohue TM, Jr, Schrum LW. Rev-erb agonist and TGF-beta similarly affect autophagy but differentially regulate hepatic stellate cell fibrogenic phenotype. Int J Biochem Cell Biol. 2016;81:137–147. doi: 10.1016/j.biocel.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 236.Yin C. Molecular mechanisms of Sox transcription factors during the development of liver, bile duct, and pancreas. Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Qiao H, Cao Q, Fu Y, Guan W, Cheng F, Wu J, et al. Sex-determining region Y-box 9 acts downstream of NADPH oxidase to influence the effect of leptin on PPARgamma1 expression in hepatic stellate cells. Biochim Biophys Acta. 2016;1862:2186–2196. doi: 10.1016/j.bbadis.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 238.Pritchett J, Harvey E, Athwal V, Berry A, Rowe C, Oakley F, et al. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology. 2012;56:1108–1116. doi: 10.1002/hep.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Delgado I, Carrasco M, Cano E, Carmona R, Garcia-Carbonero R, Marin-Gomez LM, et al. GATA4 loss in the septum transversum mesenchyme promotes liver fibrosis in mice. Hepatology. 2014;59:2358–2370. doi: 10.1002/hep.27005. [DOI] [PubMed] [Google Scholar]
- 240.Wandzioch E, Kolterud A, Jacobsson M, Friedman SL, Carlsson L. Lhx2−/− mice develop liver fibrosis. Proc Natl Acad Sci U S A. 2004;101:16549–16554. doi: 10.1073/pnas.0404678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tian W, Hao C, Fan Z, Weng X, Qin H, Wu X, et al. Myocardin related transcription factor A programs epigenetic activation of hepatic stellate cells. J Hepatol. 2015;62:165–174. doi: 10.1016/j.jhep.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 242.Tian W, Fan Z, Li J, Hao C, Li M, Xu H, et al. Myocardin-related transcription factor A (MRTF-A) plays an essential role in hepatic stellate cell activation by epigenetically modulating TGF-beta signaling. Int J Biochem Cell Biol. 2016;71:35–43. doi: 10.1016/j.biocel.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 243.Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med. 2015;21:150–158. doi: 10.1038/nm.3777. [DOI] [PubMed] [Google Scholar]
- 244.Chen P, Li J, Huo Y, Lu J, Wan L, Yang Q, et al. Adenovirus-mediated expression of orphan nuclear receptor NR4A2 targeting hepatic stellate cell attenuates liver fibrosis in rats. Sci Rep. 2016;6:33593. doi: 10.1038/srep33593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ghiassi-Nejad Z, Hernandez-Gea V, Woodrell C, Lang UE, Dumic K, Kwong A, et al. Reduced hepatic stellate cell expression of Kruppel-like factor 6 tumor suppressor isoforms amplifies fibrosis during acute and chronic rodent liver injury. Hepatology. 2013;57:786–796. doi: 10.1002/hep.26056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Marrone G, Russo L, Rosado E, Hide D, Garcia-Cardena G, Garcia-Pagan JC, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J Hepatol. 2013;58:98–103. doi: 10.1016/j.jhep.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 247.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 248.Andreola F, Calvisi DF, Elizondo G, Jakowlew SB, Mariano J, Gonzalez FJ, et al. Reversal of liver fibrosis in aryl hydrocarbon receptor null mice by dietary vitamin A depletion. Hepatology. 2004;39:157–166. doi: 10.1002/hep.20004. [DOI] [PubMed] [Google Scholar]
- 249.Harvey WA, Jurgensen K, Pu X, Lamb CL, Cornell KA, Clark RJ, et al. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) increases human hepatic stellate cell activation. Toxicology. 2016;344–346:26–33. doi: 10.1016/j.tox.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lopez-Sanchez I, Dunkel Y, Roh YS, Mittal Y, De Minicis S, Muranyi A, et al. GIV/Girdin is a central hub for profibrogenic signalling networks during liver fibrosis. Nat Commun. 2014;5:4451. doi: 10.1038/ncomms5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 252.Machado MV, Michelotti GA, Pereira TA, Xie G, Premont R, Cortez-Pinto H, et al. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J Hepatol. 2015;63:962–970. doi: 10.1016/j.jhep.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nakhaei-Rad S, Nakhaeizadeh H, Gotze S, Kordes C, Sawitza I, Hoffmann MJ, et al. The Role of Embryonic Stem Cell-expressed RAS (ERAS) in the Maintenance of Quiescent Hepatic Stellate Cells. J Biol Chem. 2016;291:8399–8413. doi: 10.1074/jbc.M115.700088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hardy T, Mann DA. Epigenetics in liver disease: from biology to therapeutics. Gut. 2016 doi: 10.1136/gutjnl-2015-311292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Page A, Paoli P, Moran Salvador E, White S, French J, Mann J. Hepatic stellate cell transdifferentiation involves genome-wide remodeling of the DNA methylation landscape. J Hepatol. 2016;64:661–673. doi: 10.1016/j.jhep.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138:705–714. 714 e701–704. doi: 10.1053/j.gastro.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mann J, Oakley F, Akiboye F, Elsharkawy A, Thorne AW, Mann DA. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell death and differentiation. 2007;14:275–285. doi: 10.1038/sj.cdd.4401979. [DOI] [PubMed] [Google Scholar]
- 258.El Taghdouini A, Sorensen AL, Reiner AH, Coll M, Verhulst S, Mannaerts I, et al. Genome-wide analysis of DNA methylation and gene expression patterns in purified, uncultured human liver cells and activated hepatic stellate cells. Oncotarget. 2015;6:26729–26745. doi: 10.18632/oncotarget.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bian EB, Huang C, Ma TT, Tao H, Zhang H, Cheng C, et al. DNMT1-mediated PTEN hypermethylation confers hepatic stellate cell activation and liver fibrogenesis in rats. Toxicology and applied pharmacology. 2012;264:13–22. doi: 10.1016/j.taap.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 260.Perugorria MJ, Wilson CL, Zeybel M, Walsh M, Amin S, Robinson S, et al. Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast transdifferentiation. Hepatology. 2012;56:1129–1139. doi: 10.1002/hep.25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pan RL, Xiang LX, Wang P, Liu XY, Nie L, Huang W, et al. Low-molecular-weight fibroblast growth factor 2 attenuates hepatic fibrosis by epigenetic down-regulation of Delta-like1. Hepatology. 2015;61:1708–1720. doi: 10.1002/hep.27649. [DOI] [PubMed] [Google Scholar]
- 262.Ding N, Hah N, Yu RT, Sherman MH, Benner C, Leblanc M, et al. BRD4 is a novel therapeutic target for liver fibrosis. Proc Natl Acad Sci U S A. 2015;112:15713–15718. doi: 10.1073/pnas.1522163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hardy T, Zeybel M, Day CP, Dipper C, Masson S, McPherson S, et al. Plasma DNA methylation: a potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut. 2016 doi: 10.1136/gutjnl-2016-311526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Page A, Paoli PP, Hill SJ, Howarth R, Wu R, Kweon SM, et al. Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J Hepatol. 2015;62:388–397. doi: 10.1016/j.jhep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kim JS, Shukla SD. Histone h3 modifications in rat hepatic stellate cells by ethanol. Alcohol Alcohol. 2005;40:367–372. doi: 10.1093/alcalc/agh170. [DOI] [PubMed] [Google Scholar]
- 266.Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med. 2012;18:1369–1377. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kitano M, Bloomston PM. Hepatic Stellate Cells and microRNAs in Pathogenesis of Liver Fibrosis. J Clin Med. 2016;5 doi: 10.3390/jcm5030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yu F, Lu Z, Huang K, Wang X, Xu Z, Chen B, et al. MicroRNA-17-5p-activated Wnt/beta-catenin pathway contributes to the progression of liver fibrosis. Oncotarget. 2016;7:81–93. doi: 10.18632/oncotarget.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Coll M, El Taghdouini A, Perea L, Mannaerts I, Vila-Casadesus M, Blaya D, et al. Integrative miRNA and Gene Expression Profiling Analysis of Human Quiescent Hepatic Stellate Cells. Sci Rep. 2015;5:11549. doi: 10.1038/srep11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hyun J, Wang S, Kim J, Rao KM, Park SY, Chung I, et al. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat Commun. 2016;7:10993. doi: 10.1038/ncomms10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhou DD, Wang X, Wang Y, Xiang XJ, Liang ZC, Zhou Y, et al. MicroRNA-145 inhibits hepatic stellate cell activation and proliferation by targeting ZEB2 through Wnt/beta-catenin pathway. Mol Immunol. 2016;75:151–160. doi: 10.1016/j.molimm.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 272.Sekiya Y, Ogawa T, Iizuka M, Yoshizato K, Ikeda K, Kawada N. Down-regulation of cyclin E1 expression by microRNA-195 accounts for interferon-beta-induced inhibition of hepatic stellate cell proliferation. Journal of cellular physiology. 2011;226:2535–2542. doi: 10.1002/jcp.22598. [DOI] [PubMed] [Google Scholar]
- 273.Sun X, He Y, Ma TT, Huang C, Zhang L, Li J. Participation of miR-200a in TGF-beta1-mediated hepatic stellate cell activation. Mol Cell Biochem. 2014;388:11–23. doi: 10.1007/s11010-013-1895-0. [DOI] [PubMed] [Google Scholar]
- 274.Yang JJ, Tao H, Liu LP, Hu W, Deng ZY, Li J. miR-200a controls hepatic stellate cell activation and fibrosis via SIRT1/Notch1 signal pathway. Inflamm Res. 2016 doi: 10.1007/s00011-016-1020-4. [DOI] [PubMed] [Google Scholar]
- 275.Yang JJ, Tao H, Hu W, Liu LP, Shi KH, Deng ZY, et al. MicroRNA-200a controls Nrf2 activation by target Keap1 in hepatic stellate cell proliferation and fibrosis. Cell Signal. 2014;26:2381–2389. doi: 10.1016/j.cellsig.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 276.Yang JJ, Liu LP, Tao H, Hu W, Shi P, Deng ZY, et al. MeCP2 silencing of LncRNA H19 controls hepatic stellate cell proliferation by targeting IGF1R. Toxicology. 2016;359–360:39–46. doi: 10.1016/j.tox.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 277.El-Ahwany E, Nagy F, Zoheiry M, Shemis M, Nosseir M, Taleb HA, et al. Circulating miRNAs as Predictor Markers for Activation of Hepatic Stellate Cells and Progression of HCV-Induced Liver Fibrosis. Electronic physician. 2016;8:1804–1810. doi: 10.19082/1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Li BB, Li DL, Chen C, Liu BH, Xia CY, Wu HJ, et al. Potentials of the elevated circulating miR-185 level as a biomarker for early diagnosis of HBV-related liver fibrosis. Sci Rep. 2016;6:34157. doi: 10.1038/srep34157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wang WJ, Lai RT, Lu J, Xiang XG, Zhao GD, Tang WL, et al. Correlation between circulating miR-122 and prognosis of chronic HBV-related liver failure. Journal of digestive diseases. 2016;17:334–339. doi: 10.1111/1751-2980.12348. [DOI] [PubMed] [Google Scholar]
- 280.Mallat A, Lodder J, Teixeira-Clerc F, Moreau R, Codogno P, Lotersztajn S. Autophagy: a multifaceted partner in liver fibrosis. Biomed Res Int. 2014;2014:869390. doi: 10.1155/2014/869390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Thoen LF, Guimaraes EL, Dolle L, Mannaerts I, Najimi M, Sokal E, et al. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55:1353–1360. doi: 10.1016/j.jhep.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 282.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hernandez-Gea V, Hilscher M, Rozenfeld R, Lim MP, Nieto N, Werner S, et al. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol. 2013;59:98–104. doi: 10.1016/j.jhep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Koo JH, Lee HJ, Kim W, Kim SG. Endoplasmic Reticulum Stress in Hepatic Stellate Cells Promotes Liver Fibrosis via PERK-Mediated Degradation of HNRNPA1 and Up-regulation of SMAD2. Gastroenterology. 2016;150:181–193 e188. doi: 10.1053/j.gastro.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 285.Kim RS, Hasegawa D, Goossens N, Tsuchida T, Athwal V, Sun X, et al. The XBP1 Arm of the Unfolded Protein Response Induces Fibrogenic Activity in Hepatic Stellate Cells Through Autophagy. Sci Rep. 2016;6:39342. doi: 10.1038/srep39342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Heindryckx F, Binet F, Ponticos M, Rombouts K, Lau J, Kreuger J, et al. Endoplasmic reticulum stress enhances fibrosis through IRE1alpha-mediated degradation of miR-150 and XBP-1 splicing. EMBO molecular medicine. 2016;8:729–744. doi: 10.15252/emmm.201505925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.He Y, Zhu J, Huang Y, Gao H, Zhao Y. Advanced glycation end product (AGE)-induced hepatic stellate cell activation via autophagy contributes to hepatitis C-related fibrosis. Acta Diabetol. 2015;52:959–969. doi: 10.1007/s00592-015-0763-7. [DOI] [PubMed] [Google Scholar]
- 288.Zhan L, Yang Y, Ma TT, Huang C, Meng XM, Zhang L, et al. Transient receptor potential vanilloid 4 inhibits rat HSC-T6 apoptosis through induction of autophagy. Mol Cell Biochem. 2015;402:9–22. doi: 10.1007/s11010-014-2298-6. [DOI] [PubMed] [Google Scholar]
- 289.Kawasaki K, Ushioda R, Ito S, Ikeda K, Masago Y, Nagata K. Deletion of the collagen-specific molecular chaperone Hsp47 causes endoplasmic reticulum stress-mediated apoptosis of hepatic stellate cells. J Biol Chem. 2015;290:3639–3646. doi: 10.1074/jbc.M114.592139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 291.Zhang D, Utsumi T, Huang HC, Gao L, Sangwung P, Chung C, et al. Reticulon 4B (Nogo-B) is a novel regulator of hepatic fibrosis. Hepatology. 2011;53:1306–1315. doi: 10.1002/hep.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Men R, Wen M, Dan X, Zhu Y, Wang W, Li J, et al. Nogo-B: A potential indicator for hepatic cirrhosis and regulator in hepatic stellate cell activation. Hepatol Res. 2015;45:113–122. doi: 10.1111/hepr.12324. [DOI] [PubMed] [Google Scholar]
- 293.Deng J, Huang Q, Wang Y, Shen P, Guan F, Li J, et al. Hypoxia-inducible factor-1alpha regulates autophagy to activate hepatic stellate cells. Biochem Biophys Res Commun. 2014;454:328–334. doi: 10.1016/j.bbrc.2014.10.076. [DOI] [PubMed] [Google Scholar]
- 294.Zhao J, Peng L, Cui R, Guo X, Yan M. Dimethyl alpha-ketoglutarate reduces CCl4-induced liver fibrosis through inhibition of autophagy in hepatic stellate cells. Biochem Biophys Res Commun. 2016;481:90–96. doi: 10.1016/j.bbrc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 295.Zhang XW, Mi S, Li Z, Zhou JC, Xie J, Hua F, et al. Antagonism of Interleukin-17A ameliorates experimental hepatic fibrosis by restoring the IL-10/STAT3-suppressed autophagy in hepatocytes. Oncotarget. 2016 doi: 10.18632/oncotarget.14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Jiang S, Zhang Y, Zheng JH, Li X, Yao YL, Wu YL, et al. Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation. Pharmacol Res. 2016;117:82–93. doi: 10.1016/j.phrs.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 297.Okuno M, Moriwaki H, Imai S, Muto Y, Kawada N, Suzuki Y, et al. Retinoids exacerbate rat liver fibrosis by inducing the activation of latent TGF-beta in liver stellate cells. Hepatology. 1997;26:913–921. doi: 10.1053/jhep.1997.v26.pm0009328313. [DOI] [PubMed] [Google Scholar]
- 298.Yi HS, Lee YS, Byun JS, Seo W, Jeong JM, Park O, et al. Alcohol dehydrogenase III exacerbates liver fibrosis by enhancing stellate cell activation and suppressing natural killer cells in mice. Hepatology. 2014;60:1044–1053. doi: 10.1002/hep.27137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kluwe J, Wongsiriroj N, Troeger JS, Gwak GY, Dapito DH, Pradere JP, et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60:1260–1268. doi: 10.1136/gut.2010.209551. [DOI] [PubMed] [Google Scholar]
- 300.Yoneda A, Sakai-Sawada K, Niitsu Y, Tamura Y. Vitamin A and insulin are required for the maintenance of hepatic stellate cell quiescence. Experimental cell research. 2016;341:8–17. doi: 10.1016/j.yexcr.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 301.Trasino SE, Tang XH, Jessurun J, Gudas LJ. A retinoic acid receptor beta2 agonist reduces hepatic stellate cell activation in nonalcoholic fatty liver disease. J Mol Med (Berl) 2016;94:1143–1151. doi: 10.1007/s00109-016-1434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Eichmann TO, Grumet L, Taschler U, Hartler J, Heier C, Woblistin A, et al. ATGL and CGI-58 are lipid droplet proteins of the hepatic stellate cell line HSC-T6. J Lipid Res. 2015;56:1972–1984. doi: 10.1194/jlr.M062372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.O’Mahony F, Wroblewski K, O’Byrne SM, Jiang H, Clerkin K, Benhammou J, et al. Liver X receptors balance lipid stores in hepatic stellate cells through Rab18, a retinoid responsive lipid droplet protein. Hepatology. 2015;62:615–626. doi: 10.1002/hep.27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhang Z, Zhao S, Yao Z, Wang L, Shao J, Chen A, et al. Autophagy regulates turnover of lipid droplets via ROS-dependent Rab25 activation in hepatic stellate cell. Redox Biol. 2016;11:322–334. doi: 10.1016/j.redox.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Bruschi FV, Claudel T, Tardelli M, Caligiuri A, Stulnig TM, Marra F, et al. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology. 2017 doi: 10.1002/hep.29041. [DOI] [PubMed] [Google Scholar]
- 306.Wilson CL, Murphy LB, Leslie J, Kendrick S, French J, Fox CR, et al. Ubiquitin C-terminal hydrolase 1: A novel functional marker for liver myofibroblasts and a therapeutic target in chronic liver disease. J Hepatol. 2015;63:1421–1428. doi: 10.1016/j.jhep.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Arndt S, Wacker E, Dorn C, Koch A, Saugspier M, Thasler WE, et al. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut. 2015;64:973–981. doi: 10.1136/gutjnl-2014-306968. [DOI] [PubMed] [Google Scholar]
- 308.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083 e1022. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, et al. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell. 2016;18:797–808. doi: 10.1016/j.stem.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 311.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 312.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Borkham-Kamphorst E, Schaffrath C, Van de Leur E, Haas U, Tihaa L, Meurer SK, et al. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-beta signaling. Biochim Biophys Acta. 2014;1843:902–914. doi: 10.1016/j.bbamcr.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 314.Klein S, Klosel J, Schierwagen R, Korner C, Granzow M, Huss S, et al. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab Invest. 2012;92:1440–1450. doi: 10.1038/labinvest.2012.106. [DOI] [PubMed] [Google Scholar]
- 315.Rashid ST, Humphries JD, Byron A, Dhar A, Askari JA, Selley JN, et al. Proteomic analysis of extracellular matrix from the hepatic stellate cell line LX-2 identifies CYR61 and Wnt-5a as novel constituents of fibrotic liver. J Proteome Res. 2012;11:4052–4064. doi: 10.1021/pr3000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhang J, Wang M, Zhang Z, Luo Z, Liu F, Liu J. Celecoxib derivative OSU-03012 inhibits the proliferation and activation of hepatic stellate cells by inducing cell senescence. Mol Med Rep. 2015;11:3021–3026. doi: 10.3892/mmr.2014.3048. [DOI] [PubMed] [Google Scholar]
- 317.Duan Y, Pan J, Chen J, Zhu D, Wang J, Sun X, et al. Soluble Egg Antigens of Schistosoma japonicum Induce Senescence of Activated Hepatic Stellate Cells by Activation of the FoxO3a/SKP2/P27 Pathway. PLoS Negl Trop Dis. 2016;10:e0005268. doi: 10.1371/journal.pntd.0005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Panebianco C, Oben JA, Vinciguerra M, Pazienza V. Senescence in hepatic stellate cells as a mechanism of liver fibrosis reversal: a putative synergy between retinoic acid and PPAR-gamma signalings. Clinical and experimental medicine. 2016 doi: 10.1007/s10238-016-0438-x. [DOI] [PubMed] [Google Scholar]
- 319.Anan A, Baskin-Bey ES, Bronk SF, Werneburg NW, Shah VH, Gores GJ. Proteasome inhibition induces hepatic stellate cell apoptosis. Hepatology. 2006;43:335–344. doi: 10.1002/hep.21036. [DOI] [PubMed] [Google Scholar]
- 320.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. Journal of leukocyte biology. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bansal R, Prakash J, Post E, Beljaars L, Schuppan D, Poelstra K. Novel engineered targeted interferon-gamma blocks hepatic fibrogenesis in mice. Hepatology. 2011;54:586–596. doi: 10.1002/hep.24395. [DOI] [PubMed] [Google Scholar]
- 322.Shaker ME, Ghani A, Shiha GE, Ibrahim TM, Mehal WZ. Nilotinib induces apoptosis and autophagic cell death of activated hepatic stellate cells via inhibition of histone deacetylases. Biochim Biophys Acta. 2013;1833:1992–2003. doi: 10.1016/j.bbamcr.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hao H, Zhang D, Shi J, Wang Y, Chen L, Guo Y, et al. Sorafenib induces autophagic cell death and apoptosis in hepatic stellate cell through the JNK and Akt signaling pathways. Anticancer Drugs. 2016;27:192–203. doi: 10.1097/CAD.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 324.Weiskirchen R, Tacke F. Liver Fibrosis: From Pathogenesis to Novel Therapies. Dig Dis. 2016;34:410–422. doi: 10.1159/000444556. [DOI] [PubMed] [Google Scholar]
- 325.Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, et al. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Dixit A, Parnas O, Li B, Chen J, Fulco CP, Jerby-Arnon L, et al. Perturb-Seq: Dissecting Molecular Circuits with Scalable Single-Cell RNA Profiling of Pooled Genetic Screens. Cell. 2016;167:1853–1866 e1817. doi: 10.1016/j.cell.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.van Grunsven LA. 3D in vitro models of liver fibrosis. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 329.Crespo Yanguas S, Cogliati B, Willebrords J, Maes M, Colle I, van den Bossche B, et al. Experimental models of liver fibrosis. Arch Toxicol. 2016;90:1025–1048. doi: 10.1007/s00204-015-1543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


