Abstract
The blood-brain barrier (BBB) plays a vital role in the central nervous system (CNS). A comprehensive understanding of BBB development has been hampered by difficulties in observing the differentiation of brain endothelial cells (BECs) in real-time. Here, we generated two transgenic zebrafish line, Tg(glut1b:mCherry) and Tg(plvap:EGFP), to serve as in vivo reporters of BBB development. We showed that barriergenesis (i.e. the induction of BEC differentiation) occurs immediately as endothelial tips cells migrate into the brain parenchyma. Using the Tg(glut1b:mCherry) transgenic line, we performed a genetic screen and identified a zebrafish mutant with a nonsense mutation in gpr124, a gene known to play a role in CNS angiogenesis and BBB development. We also showed that our transgenic plvap:EGFP line, a reporter of immature brain endothelium, is initially expressed in newly formed brain endothelial cells, but subsides during BBB maturation. Our results demonstrate the ability to visualize the in vivo differentiation of brain endothelial cells into the BBB phenotype and establish that CNS angiogenesis and barriergenesis occur simultaneously.
Keywords: Blood-brain barrier (BBB), Brain endothelial cell (BEC), Barriergenesis, Angiogenesis, Glucose transporter 1 (Glut1), Plasmalemma vesicle-associated protein (Plvap)
INTRODUCTION
While BBB formation and function are known to be dependent upon the brain microenvironment (Alvarez et al., 2011; Armulik et al., 2010; Daneman et al., 2010; Stewart and Wiley, 1981), the precise molecular and cellular mechanisms are only beginning to be elucidated. During BBB development, brain endothelial cells (BECs) acquire both physical (i.e. tight and adherens junctions) and chemical (i.e. efflux and influx transporters) properties that regulate the passage of substances between blood and brain. These barrier properties also prevent the free exchange of many therapeutic agents, presenting a challenging problem for the treatment of many neurological diseases (Abbott et al., 2010; Saunders et al., 2008; Zlokovic, 2008). In fact, it has been estimated that >98% of small molecules cannot penetrate the BBB (Pardridge, 2007). Furthermore, BBB function is often compromised in many CNS diseases including neurodegenerative disorders, brain tumors, stroke, and diabetic retinopathy (Zlokovic, 2008). By unraveling the complex process of BBB development, we may begin to address unresolved issues regarding CNS drug penetration along with BBB damage and repair.
To take an unbiased approach to solving these problems, we turned to the zebrafish, Danio rerio. Zebrafish possess many characteristics suitable for the study of the BBB. Zebrafish are transparent and develop rapidly outside of the mother, making them ideal for observing complex in vivo processes. Zebrafish also produce large numbers of offspring, providing the opportunity for large-scale screens aimed at identifying novel genes and molecular targets for drug discovery. Furthermore, small-molecule screens in zebrafish have identified compounds that inhibit developmental pathways, angiogenesis, and tumor growth, demonstrating their potential usefulness in discovering new drugs for human disease.
Here, we generated transgenic zebrafish to visualize the differentiation and maturation of brain endothelial cells in vivo. As Glut1 is the earliest known marker of BBB formation (Bauer et al., 1995; Dermietzel et al., 1992; Pardridge et al., 1990), we used the zebrafish glut1b promoter to drive expression of mCherry specifically in brain endothelial cells. By imaging live transgenic glut1b:mCherry embryos using time-lapse confocal microscopy, we found that barriergenesis (i.e. the induction of BEC differentiation) occurs simultaneously with CNS angiogenesis. To demonstrate the utility of this transgenic line, we performed a small-scale mutagenesis screen and identified a zebrafish gpr124 mutant. As Gpr124 is essential for CNS angiogenesis and BBB development (Anderson et al., 2011; Cullen et al., 2011; Kuhnert et al., 2010) by promoting canonical Wnt signaling (Posokhova et al., 2015; Zhou and Nathans, 2014; Zhou et al., 2014), the gpr124 mutant validates the utility of our transgenic BBB reporter line. Finally, we generated the plvap:EGFP transgenic line to label immature brain endothelium. In human and mouse, Plvap is initially expressed in brain endothelial cells, subsides during development, and is absent from adult brain vasculature (Hallmann et al., 1995; Leenstra et al., 1993; Niemela et al., 2005; Stan et al., 1999). Here, we found that zebrafish plvap:EGFP behaves as mammalian Plvap, thus demonstrating that our double transgenic glut1b:mCherry;plvap:EGFP zebrafish line serves as a unique model for in vivo imaging of BBB development and maturation.
Materials and Methods
Zebrafish husbandry
AB, TL, and Tg(fli1a:EGFP)y1 strains were acquired from the Zebrafish International Resource Center. Et(cp:EGFP) was previously generated in our lab (Henson et al., 2014). Embryos and larvae were maintained at 28.5°C in egg water (0.03% Instant Ocean in reverse osmosis water). For imaging, 0.003% phenylthiourea (PTU) was used to inhibit melanin production. All experiments were performed in accordance with the St. Jude Children’s Research Hospital and the University of Wisconsin-Madison Institutional Animal Care and Use Committees.
Generation of transgenic zebrafish
To generate the construct for Tg(glut1b:mCherry) and Tg(plvap:EGFP), we used Gateway compatible vectors of the Tol2kit (Kwan et al., 2007). A 2.9 kb fragment of the zebrafish glut1b promoter (accession #: NM_001039808) was PCR amplified from genomic DNA with the following primers: glut1b-promoter (XhoI) F-TATTCTCGAGGGGGCTGATAACATTGACCT; glut1b-promoter (BamHI) R-TCCAGGATCCCAAAAATTGTTCTTTAAAAAAAAC, digested with XhoI and BamHI, and subcloned into the same restriction sites in p5E-MCS. While zebrafish have three glut1 paralogs, we focused our studies on glut1b due to its predominant expression in the zebrafish brain (Tseng et al., 2009). The p5E-glut1b entry clone was combined with middle entry clone pME-mCherry, the 3′ entry clone p3E-polyA, and the pDestTol2pA2 destination vector to create the pDest-glut1b:mCherry construct using the LR Clonase II Plus Enzyme mix (Invitrogen, Carlsbad, CA).
A 2.9 kb fragment of the zebrafish plvap promoter (accession #: NM_001030244; also called vsg1) was PCR amplified from genomic DNA with the following primers: plvap-promoter F-AGGAGCAAATCCATCATTGC; plvap-promoter (SpeI) R-TTGTACTAGTCTGCAGTCCAGTTGTGGGAA, digested with XhoI and SpeI, and subcloned into the same restriction sites in p5E-MCS. The p5E-plvap entry clone was combined with middle entry clone pME-EGFP, the 3′ entry clone p3E-polyA, and the pDestTol2pA2 destination vector to create the pDest-plvap:EGFP construct using the LR Clonase II Plus Enzyme mix (Invitrogen, Carlsbad, CA).
To produce each transgenic line, approximately 30 pg of plasmid DNA and 30 pg of in vitro transcribed Tol2 transposase mRNA were co-injected into single-cell embryos of the AB strain. Embryos with strong transient expression of the transgenes were raised to adults and screened for germline transmission.
Confocal laser scanning microscopy
Zebrafish from 1–6 days postfertilization (dpf) were anesthetized in 0.02% Tricaine and immobilized in 1.2% low melting point agarose (Invitrogen, Carlsbad, CA) in glass bottom culture dishes (MatTek, Ashland, MA). Confocal microscopy was performed using a Nikon Eclipse TE2000E2 microscope equipped with a Nikon C1si or a Nikon Eclipse Ti microscope equipped with a Nikon A1R. For time-lapse imaging, a z stack was acquired every 30 minutes for 30 hours. All images are 2D projections of 3D confocal z stacks or 3D volume rendered images generated using either NIS-Elements MIP or EDF algorithm.
Mutagenesis screen
Twenty three glut1b:mCherry;fli1a:EGFP double transgenic males were mutagenized with N-ethyl-N-nitrosourea (ENU) as previously described (Driever et al., 1996). After one month, F1 pairwise crosses produced 188 F2 families. Pairwise crosses for each F2 family were performed at least six times to identify F3 offspring with homozygous recessive mutations. Using this strategy, 68 F2 families were screened. To identify mutants, F3 larvae at 3–4 dpf were anesthetized and examined for abnormal mCherry and/or GFP expression using a Nikon SMZ1500 epifluorescence stereomicroscope. A mutant line was confirmed if approximately 25% of the total offspring displayed a brain vasculature phenotype.
Whole-exome sequencing
Exome sequencing was performed as described (Ryan et al., 2013). Briefly, genomic DNA was isolated from 40 wild-type sibling and 40 mutant larvae using the Qiagen Mag Attract HMW DNA Kit (Qiagen, Venlo, Netherlands). Three micrograms of genomic DNA were used for SureSelect Target Enrichment System for Illumina Paired-End Multiplexed Sequencing (Agilent, Santa Clara, CA). From the raw sequencing reads, the quality control, mapping and variant calling were performed using CLC Genomic Workbench v6.5 (CLC Bio, Aarhus, Denmark). The reads were trimmed against the sequencing adapters, and only reads with a sequencing quality greater than 20 and a read length greater than 50 bp were retained. The filtered reads were aligned to the zebrafish reference genome sequence (Zv9 assembly), and a list of single-nucleotide variants was generated. We compared mutant variants to wild type to identify a mutant-specific homozygous mutation that impact protein sequence.
Results
Generation of a transgenic brain endothelial cell-reporter line
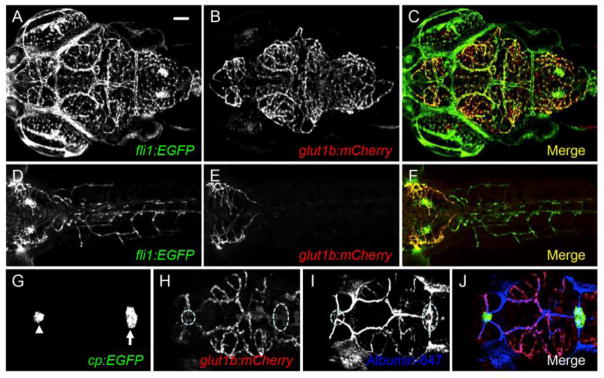
To visualize barriergenesis in vivo, we created a transgenic zebrafish reporter line using the zebrafish glut1b promoter. The fli1a:EGFP transgenic line expresses green fluorescent protein (GFP) in all vascular endothelial cells (Lawson and Weinstein, 2002). We found that mCherry was transiently expressed in the brain vasculature of ~90% of injected embryos (data not shown). Next, we identified several stable transgenic lines and designated one as Tg(glut1b:mCherry) (herein glut1b:mCherry). To characterize the expression pattern of mCherry, we used confocal laser scanning microscopy on live glut1b:mCherry;fli1a:EGFP double transgenic larvae. At 6 dpf, mCherry was expressed specifically in the brain vasculature (Figure 1A–C), but not in the peripheral vasculature (Figure 1D–F). This expression pattern was maintained into adulthood (data not shown). To examine mCherry expression in vasculature of circumventricular organs that lack BBB properties (Saunders et al., 2008), we crossed glut1b:mCherry to cp:EGFP, an enhancer trap line that expresses GFP in the choroid plexus (Henson et al., 2014), and injected albumin Alexa Fluor 647 conjugate to visualize the vasculature. We found that mCherry was not expressed in the vasculature of the choroid plexus (Figure 1G–J). Our results demonstrate that glut1b:mCherry faithfully recapitulates mammalian Glut1 expression in BECs.
Figure 1.
The zebrafish glut1b promoter drives expression in brain endothelial cells. Confocal microscopy of a live glut1b:mCherry; fli1a:EGFP double transgenic larva at 6 dpf. (A–C) mCherry is spatially restricted to the brain vasculature (B) and overlays with endothelial GFP expression from fli1a:EGFP (A, C). Shown here is a dorsal view of the brain vasculature (anterior, left). (D–F) mCherry is not expressed in peripheral endothelial cells. Shown here is the larval trunk (dorsal view) with partial inclusion of the mCherry-expressing hindbrain vasculature (anterior, left). (G–N) mCherry is not expressed in the vasculature of circumventricular organs. (G–J) glut1b:mCherry was crossed to cp:EGFP, an enhancer-trap line that expresses GFP in the myelencephalic (white arrow in G) and diencephalic (white arrowhead in G) choroid plexus. The choroid plexus regions are outlined (white dash) in (H). For G–J, albumin Alexa Fluor 647 (pseudo-colored in blue) was injected as a tracer to visualize blood vessels. Confocal images were captured in the plane of GFP expression. Note the lack of colocalization between mCherry (BBB vessels) and blue (all blood vessels) in the choroid plexus. Scale bar in A is 50 μm for all images.
CNS angiogenesis and barriergenesis occur simultaneously
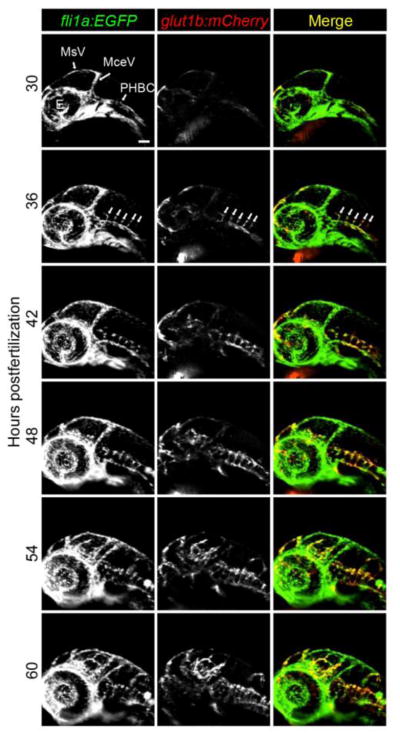
Taking advantage of our in vivo model, we addressed some fundamental questions regarding the development of the BBB. Ongoing arguments contend that BBB formation is either 1) a two-step process where CNS angiogenesis occurs and is followed by barriergenesis, or that 2) both CNS angiogenesis and barriergenesis occur at the same time. To address this issue, we performed time-lapse confocal microscopy to examine the temporal induction of barrier properties. In zebrafish, CNS angiogenesis begins in the hindbrain at approximately 30 hours postfertilization (hpf) when endothelial tip cells originating from the primordial hindbrain channels (PHBCs) migrate into the brain parenchyma (Fujita et al., 2011; Ulrich et al., 2011). These vessels eventually form the central arteries (CtAs) that interconnect the PHBCs with the basilar artery. Using live glut1b:mCherry;fli1a:EGFP double transgenics, we imaged angiogenesis and barriergenesis from 30 to 60 hpf. We observed that endothelial cells express mCherry immediately upon entering the brain (Figure 2; Video 1), indicating that signals within the embryonic brain are sufficient to induce barrier properties. This observation provides the first direct evidence that CNS angiogenesis and barriergenesis occur simultaneously.
Figure 2.
CNS angiogenesis and barriergenesis occur simultaneously. glut1b:mCherry; fli1a:EGFP double transgenic embryos were imaged by time-lapse confocal microscopy. Shown here are snapshots at 6-hour intervals beginning at the onset of CNS angiogenesis (30 hpf). Note that mCherry is immediately expressed as brain endothelial cells migrate into the brain parenchyma from the PHBCs (arrows in 36 hpf panels). See 30 hr time-lapse Videos 1 for more detail. MsV, mesencephalic vein; MCeV, mid-cerebral vein; PHBC, primordial hindbrain channel, E, eye. Scale bar in top left image is 50 μm for all images.
Forward genetic screen identifies gpr124 mutant
To identify genes that regulate BBB development, we performed an ENU mutagenesis screen using glut1b:mCherry;fli1a:EGFP double transgenic fish. Using this strategy, we screened 68 F2 families and identified four independent recessive mutant lines with reduced or ectopic glut1b:mCherry expression and reduced or absent fli1a:EGFP expression within the brain parenchyma. Three of these mutant lines, sj40, sj44, and sj196, showed additional phenotypes, such as brain hemorrhage, brain necrosis, edema, and early lethality. In contrast, one mutant line, sj22, looked phenotypically normal, aside from the lack of CNS angiogenesis, and survived to adulthood (Table 1).
Table 1.
Description of mutant lines
| Mutant allele | Glut1 expression | BEC development | Brain hemorrhage | Brain necrosis | Edema | Survival |
|---|---|---|---|---|---|---|
| sj22 (gpr124) | Reduced | Reduced | No | No | No | ~50% at 1 month |
| sj40 | Ectopic | Reduced | 2 dpf | Yes | Heart | 6–8 dpf |
| sj44 | Reduced | None | 3 dpf | Yes | Heart and eye | 6–8 dpf |
| sj196 | Reduced | Reduced | No | Yes | Heart | 6–8 dpf |
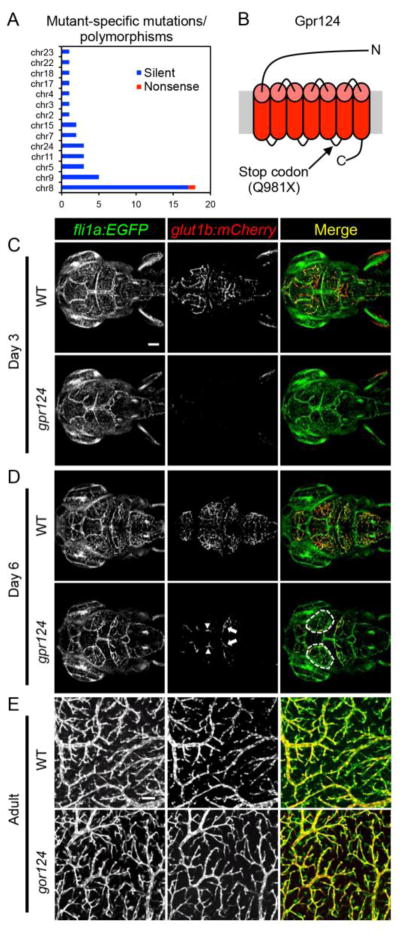
To clone the defective gene in the sj22 mutant, we performed whole-exome sequencing. We identified a total of 44 single-nucleotide differences across 14 chromosomes with 18 of these concentrated to a region on chromosome 8 (Figure 3A). Forty-three of these differences were determined to be silent polymorphisms, while only one mutation on chromosome 8 resulted in a premature stop codon in the gene encoding the orphan G protein-coupled receptor, Gpr124. To confirm the mutation, we sequenced full length gpr124 cDNA from mutants and wild-type siblings (GeneBank Accession #: KT285523) and identified the 2941C>T transition in the open reading frame, resulting in a Q981X mutation located in the intracellular loop between transmembrane-spanning domains 5 and 6 (Figure 3B).
Figure 3.
gpr124 mutants have reduced CNS angiogenesis. (A) Whole-exome sequencing was used to identify the gpr124 mutation. Only one gene on chromosome 8 was found to have a nonsense mutation. (B) A premature stop codon was found in gpr124 resulting in a Q981X mutation located in the intracellular loop between transmembrane domains 5 and 6 (not to scale). (C) At 3 dpf, gpr124 mutants show no CNS angiogenesis and no glut1b:mCherry expression (see Video 2). (D) At 6 dpf, gpr124 mutants form abnormal vessels on the brain surface between the mesencephalic vein and the eye (white dashed outline in Day 6 Merge and Supplemental Figure 1), CNS angiogenesis partially recovered where the mid-cerebral vein sprouts into the parenchyma, and few of these vessels begin to express glut1b:mCherry (arrows), including expression in the mesencephalic vein (arrowheads). The 3D distribution of brain vasculature can be seen more clearly in Video 2. (E) Confocal images of the optic tectum vasculature from wild type (WT) and gpr124 mutant adults at 3 months. Scale bar (C, top left image) is 50 μm for all images in (C) and (D). Scale bar is 100 μm (E, top left image).
Using confocal microscopy, we found that gpr124 mutants at 3 dpf have essentially no CNS angiogenesis within the brain parenchyma and no expression of glut1b:mCherry, while the vessels on the brain surface and the trunk appeared normal (Figure 3C; Video 2; data not shown). By 6 dpf, gpr124 mutants began to recover with some vessels sprouting from the mesencephalic vein into the brain parenchyma and acquiring barrier properties as shown by the induction of glut1b:mCherry (Figure 3D, arrows; Video 2). In addition, abnormal vessels appeared to originate from the mesencephalic vein formed on the brain surface (Figure 3D, dashed lines; Supplemental Figure 1; Video 2). Interestingly, the gpr124 mutants continue to recuperate with ~50% surviving to at least 1 month of age, albeit at a slower growth rate. By 3 months, the adult gpr124 mutants look similar to wild-type siblings and their brains are indistinguishable (data not shown). In fact, the brain vasculature, including expression of glut1b:mCherry, appears normal in adult gpr124 mutants (Figure 3E).
plvap:EGFP visualizes brain endothelial cell maturation
Plasmalemma vesicle-associated protein is expressed in immature brain endothelium and is initially expressed in embryonic brain endothelial cells, but is absent from the adult brain vasculature (Hallmann et al., 1995; Niemela et al., 2005; Stan et al., 1999; Stan et al., 2012). To visualize Plvap expression in zebrafish, we created a transgenic line, Tg(plvap:EGFP) (herein plvap:EGFP), that expresses GFP under control of the zebrafish plvap promoter. We found that the plvap promoter drives expression of GFP in all endothelial cells at 2 dpf both on the brain surface and within the brain parenchyma (Figue 4A, top panels; Video 3). By 6 dpf, GFP expression was considerably reduced within the brain parenchyma (Figue 4A, bottom panels; Video 3). To quantify this change, we measured the ratio of GFP intensity within the brain parenchyma and brain surface at 2 and 6 dpf (n = 18 per group). We found a significant decrease in brain parenchymal expression between 2 and 6 dpf (Figure 4B), but did not find any significant difference in brain surface expression (data not shown). To determine if the decrease in plvap expression correlated with an increase in BBB properties, such as Glut1 expression, we also measured the relative intensity of glut1b:mCherry expression in brain endothelial cells (n = 18 per group). We found that, as GFP decreased, mCherry expression increased significantly from 2 to 6 dpf (Figure 4C).
Figure 4.
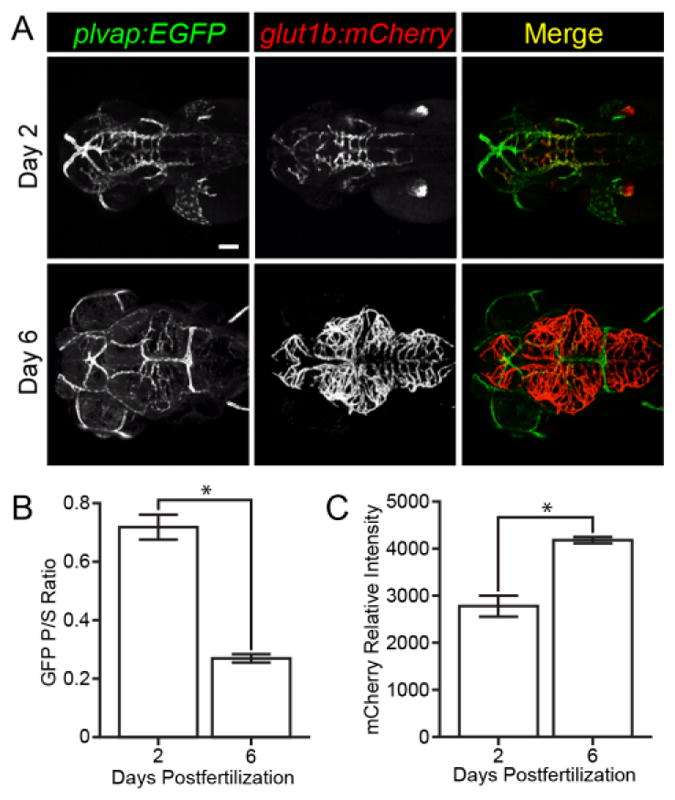
Visualization of blood-brain barrier maturation. (A) Representative images of plvap:EGFP;glut1b:mCherry double transgenic embryos on day 2 and 6 dpf. Green and red channels were captured using the same intensities on day 2 and day 6 to illustrate the decrease in GFP expression and the increase in mCherry expression. Scale bar (A, top left image) is 50 μm for all images. (B) GFP fluorescence intensity was measured in brain parenchymal (P) and brain surface (S) endothelial cells (n = 18 per group) and the ratio of parenchymal/surface intensity (GFP P/S Ratio) was plotted. Note the significant decrease of GFP expression in parenchymal endothelial cells over time. (C) Relative mCherry fluorescence intensity was measured in parenchymal endothelial cells (n = 18 per group) and plotted. Note the significant increase in mCherry expression over time. See volume rendered Video 3 to better visualize surface and parenchymal expression of GFP and mCherry. Error bars represent ± S.E.M.; * p < 0.0001, Unpaired t test.
Discussion
In this study, we report the first direct observation that barriergenesis is initiated at the same time as CNS angiogenesis. To visualize the differentiation of brain endothelial cells, we generated two transgenic reporter lines, glut1b:mCherry and plvap:EGFP. Glut1 is the earliest known marker of barriergenesis (Bauer et al., 1995; Pardridge et al., 1990). It has been reported that BBB tightness plays a pivotal role in the pattern of Glut1 expression during brain differentiation (Dermietzel et al., 1992), suggesting that Glut1 serves as an indicator of BBB function as well as development. In addition, many recent studies rely upon Glut1 expression as an indicator of BBB formation (Armulik et al., 2010; Daneman et al., 2009; Daneman et al., 2010; Stenman et al., 2008; Tam et al., 2012; Vanhollebeke et al., 2015). Other studies have demonstrated that Glut1 is also expressed in zebrafish brain endothelial cells early in development (Tam et al., 2012). For our study, we created a transgenic reporter line, glut1b:mCherry, to visualize Glut1 expression in vivo. We used the zebrafish glut1b promoter sequence, because the mRNA for the glut1b paralog is strongly expressed in the zebrafish brain (Tseng et al., 2009). We chose mCherry for our reporter line because it matures more rapidly than other fluorescent proteins (Shaner et al., 2004), allowing for more accurate observation of temporal expression. We found that the glut1b:mCherry transgenic line expressed mCherry specifically in brain endothelial cells and not in the vasculature of the periphery or circumventricular organs, and found that glut1b:mCherry expression was induced in brain endothelial cells at the onset of CNS angiogenesis.
To validate that our transgenic zebrafish model serves as a reporter for BBB development, we performed a forward genetic screen. We mutagenized our transgenic reporter line and screened for recessive mutations that affected glut1b:mCherry expression. After identifying four independent mutant lines, we cloned the defective gene in one of these mutants by whole-exome sequencing. We found a nonsense mutation in the zebrafish homolog of gpr124. In mice, Gpr124 is also required for CNS angiogenesis and the establishment of the BBB (Anderson et al., 2011; Cullen et al., 2011; Kuhnert et al., 2010). In contrast to embryonic lethality in Gpr124−/− mice, zebrafish gpr124 mutants recover normal brain vasculature and survive to adulthood, indicating that compensatory pathways may overcome the developmental defect. Recent studies indicate that Gpr124 functions ligand-specific coactivator of canonical Wnt/β-catenin signaling (Posokhova et al., 2015; Zhou and Nathans, 2014; Zhou et al., 2014) and that canonical Wnt/β-catenin signaling plays a major role in CNS angiogenesis and the acquisition of BBB properties, such as Glut1 expression (Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008; Zhou et al., 2014). Our gpr124 mutant phenotype is identical to a recently published study that generated two zebrafish gpr124 mutant alleles using TAL effector nucleases (Vanhollebeke et al., 2015). Identification of our gpr124 mutant provides strong validation for our genetic screening strategy in zebrafish.
In contrast to Glut1, Plasmalemma vesicle-associated protein (Plvap) is a widely used marker of immature brain endothelium and fenestrated endothelium (Hallmann et al., 1995; Niemela et al., 2005; Stan et al., 1999; Stan et al., 2012) and its expression is, in part, regulated by VEGF (Strickland et al., 2005). Due to VEGF signaling during CNS angiogenesis, Plvap is initially expressed in brain endothelial cells, but this expression subsides and is absent from the adult brain, except for the vasculature of circumventricular organs (Hallmann et al., 1995). Thus, Plvap is often used as a marker of BBB differentiation (Daneman et al., 2010; Liebner et al., 2008; Zhou and Nathans, 2014; Zhou et al., 2014). Plvap also serves as an indicator of BBB disruption in adult brain and labels brain tumor vasculature (Carson-Walter et al., 2005; Leenstra et al., 1993; Shue et al., 2008). In zebrafish, plvap (also called vessel-specific gene 1, vsg1) is expressed in most blood vessels during early development (Thisse and Thisse, 2008), but later developmental time points had not been examined. To visualize Plvap expression in vivo, we created a transgenic reporter line, plvap:EGFP. We found that this transgenic reporter line drives early expression of GFP in most if not all endothelial cells at 2 dpf, including the brain vasculature. We also found that the brain vascular expression decreases over time and is absent from the adult zebrafish brain. This expression pattern recapitulates the developmental expression of mammalian Plvap (Hallmann et al., 1995; Leenstra et al., 1993). Thus, the plvap:EGFP;glut1b:mCherry double transgenic line allows for live imaging of brain endothelial cell differentiation and maturation and provides a valuable tool for identifying genetic pathways that regulate these processes.
As the BBB matures, BECs become associated with pericytes and astrocytes, ultimately forming the structure referred to as the neurovascular unit (Hawkins and Davis, 2005). During BBB development, neural progenitor cells secret factors, such as VEGF and Wnt ligands, that induce CNS angiogenesis and barriergenesis (Obermeier et al., 2013). This process is followed by BEC interactions with pericytes and astrocytes that help to seal the barrier (Siegenthaler et al., 2013). In zebrafish, pericytes, located in the vicinity of the basilar artery, begin to migrate along the central arteries originating from the PHBCs at approximately 60 hpf, about 30 hours after the start of angiogenesis/barriergenesis (Ando et al., 2016). In contrast, it remains to be determined when radial glial cells (progenitors to astrocytes) differentiate into cells that interact with BECs (Lyons and Talbot, 2014). Future experiments using transgenic zebrafish that label BECs, pericytes, and astrocytes could reveal the real-time, in vivo interactions of these cells during the formation of the neurovascular unit.
Supplementary Material
Highlights.
Zebrafish glut1b:mCherry serves as an in vivo reporter of blood-brain barriergenesis.
Live imaging reveals that CNS angiogenesis and barriergenesis occur simultaneously.
Forward genetic screen using glut1b:mCherry identifies a zebrafish gpr124 mutant.
Zebrafish plvap:EGFP is an in vivo indicator of blood-brain barrier maturation.
Acknowledgments
Funding: This work was supported by the University of Wisconsin-Madison School of Pharmacy, The Hartwell Foundation, St. Jude Children’s Research Hospital, and ALSAC. Funding sources had no involvement in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, Macdonald LE, Thurston G, Daly C, Lin HC, Economides AN, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gale NW. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc Natl Acad Sci U S A. 2011;108:2807–2812. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Fukuhara S, Izumi N, Nakajima H, Fukui H, Kelsh RN, Mochizuki N. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development. 2016;143:1328–1339. doi: 10.1242/dev.132654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Bauer H, Sonnleitner U, Lametschwandtner A, Steiner M, Adam H, Bauer HC. Ontogenic expression of the erythroid-type glucose transporter (Glut 1) in the telencephalon of the mouse: correlation to the tightening of the blood-brain barrier. Brain Res Dev Brain Res. 1995;86:317–325. doi: 10.1016/0165-3806(95)00044-e. [DOI] [PubMed] [Google Scholar]
- Carson-Walter EB, Hampton J, Shue E, Geynisman DM, Pillai PK, Sathanoori R, Madden SL, Hamilton RL, Walter KA. Plasmalemmal vesicle associated protein-1 is a novel marker implicated in brain tumor angiogenesis. Clin Cancer Res. 2005;11:7643–7650. doi: 10.1158/1078-0432.CCR-05-1099. [DOI] [PubMed] [Google Scholar]
- Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, Logsdon D, Hsiao E, Stein EV, Cuttitta F, Haines DC, Nagashima K, Tessarollo L, St Croix B. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci U S A. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Krause D, Kremer M, Wang C, Stevenson B. Pattern of glucose transporter (Glut 1) expression in embryonic brains is related to maturation of blood-brain barrier tightness. Dev Dyn. 1992;193:152–163. doi: 10.1002/aja.1001930207. [DOI] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Fujita M, Cha YR, Pham VN, Sakurai A, Roman BL, Gutkind JS, Weinstein BM. Assembly and patterning of the vascular network of the vertebrate hindbrain. Development. 2011;138:1705–1715. doi: 10.1242/dev.058776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202:325–332. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Henson HE, Parupalli C, Ju B, Taylor MR. Functional and genetic analysis of choroid plexus development in zebrafish. Front Neurosci. 2014;8:364. doi: 10.3389/fnins.2014.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Leenstra S, Troost D, Das PK, Claessen N, Becker AE, Bosch DA. Endothelial cell marker PAL-E reactivity in brain tumor, developing brain, and brain disease. Cancer. 1993;72:3061–3067. doi: 10.1002/1097-0142(19931115)72:10<3061::aid-cncr2820721031>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DA, Talbot WS. Glial cell development and function in zebrafish. Cold Spring Harb Perspect Biol. 2014;7:a020586. doi: 10.1101/cshperspect.a020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela H, Elima K, Henttinen T, Irjala H, Salmi M, Jalkanen S. Molecular identification of PAL-E, a widely used endothelial-cell marker. Blood. 2005;106:3405–3409. doi: 10.1182/blood-2005-01-0254. [DOI] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem. 1990;265:18035–18040. [PubMed] [Google Scholar]
- Posokhova E, Shukla A, Seaman S, Volate S, Hilton MB, Wu B, Morris H, Swing DA, Zhou M, Zudaire E, Rubin JS, St Croix B. GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep. 2015;10:123–130. doi: 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S, Willer J, Marjoram L, Bagwell J, Mankiewicz J, Leshchiner I, Goessling W, Bagnat M, Katsanis N. Rapid identification of kidney cyst mutations by whole exome sequencing in zebrafish. Development. 2013;140:4445–4451. doi: 10.1242/dev.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Ek CJ, Habgood MD, Dziegielewska KM. Barriers in the brain: a renaissance? Trends Neurosci. 2008;31:279–286. doi: 10.1016/j.tins.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotech. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Shue EH, Carson-Walter EB, Liu Y, Winans BN, Ali ZS, Chen J, Walter KA. Plasmalemmal vesicle associated protein-1 (PV-1) is a marker of blood-brain barrier disruption in rodent models. BMC Neurosci. 2008;9:29. doi: 10.1186/1471-2202-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Sohet F, Daneman R. ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Curr Opin Neurobiol. 2013;23:1057–1064. doi: 10.1016/j.conb.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci U S A. 1999;96:13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, McGarry CL, Buitendijk M, Nemani KV, Elgueta R, Kobayashi T, Shipman SL, Moodie KL, Daghlian CP, Ernst PA, Lee HK, Suriawinata AA, Schned AR, Longnecker DS, Fiering SN, Noelle RJ, Gimi B, Shworak NW, Carriere C. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell. 2012;23:1203–1218. doi: 10.1016/j.devcel.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Strickland LA, Jubb AM, Hongo JA, Zhong F, Burwick J, Fu L, Frantz GD, Koeppen H. Plasmalemmal vesicle-associated protein (PLVAP) is expressed by tumour endothelium and is upregulated by vascular endothelial growth factor-A (VEGF) J Pathol. 2005;206:466–475. doi: 10.1002/path.1805. [DOI] [PubMed] [Google Scholar]
- Tam SJ, Richmond DL, Kaminker JS, Modrusan Z, Martin-McNulty B, Cao TC, Weimer RM, Carano RA, van Bruggen N, Watts RJ. Death receptors DR6 and TROY regulate brain vascular development. Developmental cell. 2012;22:403–417. doi: 10.1016/j.devcel.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Tseng YC, Chen RD, Lee JR, Liu ST, Lee SJ, Hwang PP. Specific expression and regulation of glucose transporters in zebrafish ionocytes. American journal of physiology Regulatory, integrative and comparative physiology. 2009;297:R275–290. doi: 10.1152/ajpregu.00180.2009. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Ma LH, Baker RG, Torres-Vazquez J. Neurovascular development in the embryonic zebrafish hindbrain. Dev Biol. 2011;357:134–151. doi: 10.1016/j.ydbio.2011.06.037. [DOI] [PubMed] [Google Scholar]
- Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. Elife. 2015:4. doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest. 2014;124:3825–3846. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.