Abstract
Associations between yogurt intake and risk of diet-related cardiometabolic diseases (CMDs) have been the subject of recent research in epidemiologic nutrition. A healthy dietary pattern has been identified as a pillar for the prevention of weight gain and CMDs. Epidemiologic studies suggest that yogurt consumption is linked to healthy dietary patterns, lifestyles, and reduced risk of CMDs, particularly type 2 diabetes. However, to our knowledge, few to no randomized controlled trials have investigated yogurt intake in relation to cardiometabolic clinical outcomes. Furthermore, there has been little attempt to clarify the mechanisms that underlie the potential beneficial effects of yogurt consumption on CMDs. Yogurt is a nutrient-dense dairy food and has been suggested to reduce weight gain and prevent CMDs by contributing to intakes of protein, calcium, bioactive lipids, and several other micronutrients. In addition, fermentation with bacterial strains generates bioactive peptides, resulting in a potentially greater beneficial effect of yogurt on metabolic health than nonfermented dairy products such as milk. To date, there is little concrete evidence that the mechanisms proposed in observational studies to explain positive results of yogurt on CMDs or parameters are valid. Many proposed mechanisms are based on assumptions that commercial yogurts contain strain-specific probiotics, that viable yogurt cultures are present in adequate quantities, and that yogurt provides a minimum threshold dose of nutrients or bioactive components capable of exerting a physiologic effect. Therefore, the primary objective of this review is to investigate the plausibility of potential mechanisms commonly cited in the literature in order to shed light on the inverse associations reported between yogurt intake and various cardiometabolic health parameters that are related to its nutrient profile, bacterial constituents, and food matrix. This article reviews current gaps and challenges in identifying such mechanisms and provides a perspective on the research agenda to validate the proposed role of yogurt in protecting against CMDs.
Keywords: bioactive peptides, cardiometabolic diseases, dairy, fermented dairy, food matrix, hypertension, microbiota, obesity, type 2 diabetes, yogurt
Introduction
Obesity, type 2 diabetes (T2D), dyslipidemia, and hypertension are important risk factors for cardiometabolic diseases (CMDs). Dairy foods, intrinsic to the Western diet and food-based dietary guidelines, may have beneficial effects on CMD risk factors (1). The potential health benefits of yogurt consumption have received much attention over the past few years, with countless reviews published on the topic. To date, the benefits of yogurt intake on cardiometabolic health are primarily sustained by observational evidence. The positive impact of yogurt intake on T2D risk is strongly supported by the consistent findings of 3 separate meta-analyses of prospective cohort studies (2–4). In these studies, the magnitude of risk reduction of T2D for yogurt intake was 22% (95% CI: 60–102%) for every 200 g serving/d (4), 18% (95% CI: 70–96%) for every 244 g serving/d (3), and 14% (95% CI: 83–90%) for 80 g/d compared with no intake (2). A closer look at individual prospective cohort studies shows a neutral relation between yogurt and T2D risk in 6 cohorts (3, 5–8) and an inverse relation in 6 other cohorts (3, 9–12). In other prospective cohort studies, yogurt (total, high-fat, and low-fat) consumption was inversely associated with fasting plasma glucose (13) and had a neutral relation to abnormal glucose homeostasis in other studies (14–16). Drouin-Chartier et al. (17) performed a systematic review of meta-analyses and concluded that high-quality evidence supports a favorable relation between yogurt intake and T2D risk, and this relation is not likely to be modified by the addition of new observational studies. However, to our knowledge, no randomized controlled trials (RCTs) have yet been conducted to determine the effects of yogurt intake on metabolic parameters of T2D.
A favorable association between yogurt and adiposity indicators, such as BMI (in kg/m2), weight, and waist circumference, is also supported by observational studies (13, 16, 18) and an RCT (19). To our knowledge, although no meta-analyses have yet been conducted, findings from 2 systematic reviews suggest that there is accumulating evidence supporting the role of yogurt consumption in weight management from both clinical and observational studies (20, 21).
To date, moderate-quality evidence (17) from prospective cohort studies (22–27) and one clinical study on metabolic parameters (28) has emerged to support a neutral relation between yogurt and cardiovascular disease (CVD) or coronary artery disease (29). Two meta-analyses have examined yogurt and fermented milks in relation to CVDs and reported slightly different conclusions, likely owing to different study inclusion criteria and a very small number of studies (30, 31). Wu and Sun (31) reported no significant association between yogurt consumption and incident CVD based on 9 studies; however, several of the studies included other fermented dairy products. The authors also concluded that a daily dose of 200 g yogurt may be associated with a lower risk for CVD based on 3 studies with a borderline significant inverse association (RR: 0.92; 95% CI: 0.85, 1.00) (31). Guo et al. (30) reported a marginal 2% lower risk for CVD (95% CI: 97–99%) with each 20 g serving of fermented milk/d (sour milk, cheese, and yogurt) but this was attenuated during sensitivity analyses. The relationship was not significant for yogurt (30). To date, observational evidence indicates a neutral association between yogurt consumption and CVD risk.
The overwhelming weight of evidence from observational studies has indicated either beneficial or neutral effects of yogurt consumption on various CMDs. Despite the seemingly positive benefits of yogurt consumption on CMDs, it is extremely difficult to rule out confounding factors in observational studies, given yogurt’s positive association with healthier lifestyles and dietary patterns, which have been discussed elsewhere (32, 33). Yogurt contains a multitude of nutrients that have the potential to act in a functional manner on CMDs. However, these confounders make it difficult to ascertain whether it is the nutritional properties of yogurt that are indeed responsible for the beneficial associations observed in epidemiologic studies, rather than its association with a healthier lifestyle. Nevertheless, high-quality prospective cohort studies with large populations have carefully accounted for various known lifestyle confounders such as physical activity, diet, and smoking and still indicated favorable relations between yogurt intake and CMD (3, 13, 18, 34, 35). These consistent results suggest that the nutrient composition of yogurt has an independent role in promoting cardiometabolic health, although causal evidence will require RCTs and mechanistic studies. Furthermore, the conflicting effects of different dairy products that contain a similar nutrient composition (e.g., milk compared with yogurt) on health suggest that the yogurt matrix and ferments play an important role in potentiating the health benefits of its nutrients (36).
To our knowledge, few RCTs have been conducted to validate the causal relation between yogurt consumption and CMD risk factors. Past studies have generally been conducted in the context of a low-fat or energy-restricted diet (19, 37), have not necessarily isolated yogurt from other dairy products (38, 39), or have used probiotics (40, 41). Moreover, few studies have been conducted in animal models of obesity, T2D, and CVD to demonstrate the independent effect of yogurt intake on CMDs and the underlying mechanisms that could explain the positive relation between yogurt consumption and cardiometabolic risk factors reported in observational human studies. Although many reviews have been published on yogurt and CMDs, the lack of mechanistic studies in both clinical and animal models means that authors are left to speculate on potential mechanisms and cite epidemiologic studies or narrative reviews to support hypotheses (Table 1). This review addresses speculated mechanisms of action that have been reported in the literature to support the beneficial relation between yogurt consumption and reduced risk of CMDs. We explore research gaps that must be addressed to provide evidence that explains why yogurt is not only a “nutritional” marker of a healthy diet but is also a nutrient-rich food that exerts beneficial physiologic actions to reduce the risk of CMDs.
TABLE 1.
Examples of mechanisms proposed by authors of key observational studies and reviews on beneficial effects of yogurt and cardiometabolic diseases1
| Reference | Design | Health parameters | Proposed mechanism(s) | Source(s) |
| Guo et al. (30) | Dose-response meta-analysis of prospective cohort studies | CVD and CAD | The food matrix reduces absorption of lipids and SCFAs by bacteria produced in the large intestine | RCT using probiotic commercial yogurt (40) |
| Chen et al. (3) | Prospective cohort study and meta-analysis | T2D | Probiotic bacteria improves lipid and cholesterol profiles | Clinical study with probiotic yogurt (41); narrative review of health benefits of probiotic and fermented products (43) |
| Gijsbers et al. (2) | Dose-response meta-analysis of prospective cohort studies | T2D | Probiotic bacteria improves blood cholesterol, which is associated with lower T2D risk | Narrative review of health benefits of probiotic and fermented products (43) |
| Yogurt contains vitamin K-2, which is associated with lower T2D risk | Authors’ own hypothesis | |||
| Wu and Sun (31) | Meta-analysis | CVD | Yogurt contains calcium, vitamin D, sphingolipids, and probiotics | Narrative review of mechanisms of dairy products on colorectal cancer (44) |
| Probiotic microorganisms support a healthy microbiota reducing risk for CVD by having an effect on weight reduction | Narrative review of role of the microbiota on metabolism (45) | |||
| Yogurt interferes with cholesterol synthesis | Narrative review of fermented dairy and CVD risk (46) | |||
| Mozaffarian (42) | Narrative review | T2D and CAD | Probiotics in yogurt act on the microbiota to reduce weight gain | Prospective cohort studies (18, 47, 48); clinical and animal studies with probiotic yogurt (49, 50); narrative review on the microbiota and health (51) |
| Ferments synthesize vitamin K-2, which can improve insulin sensitivity | Narrative review of fermented foods and vitamin K (52); placebo-controlled trial of vitamin K-2 supplementation on insulin sensitivity (53) | |||
| Sayon-Orea et al. (21) | Systematic review | Obesity and metabolic syndrome | Calcium intake reduces lipogenesis, increases lipolysis, increases fat oxidation, and produces calcium soaps that increase fat excretion | Clinical studies with dairy and adiposity (19) and calcium and fat oxidation (54); narrative reviews on calcium and metabolic health (55, 56) |
| Probiotic yogurt bacteria influence gut health, reduce inflammation, regulate appetite, and improve immune responses, intestinal barrier function, and lipid profiles | Narrative reviews about probiotics, gut health, and cardiometabolic diseases (57–60) | |||
| Jacques and Wang (36) | Systematic review | Weight change | Yogurt has higher concentrations of nutrients per serving size than milk and some nutrients may be more bioavailable | USDA National Nutrient Database; narrative review on milk and the metabolic syndrome (61) |
| Calcium, whey, casein, bioactive peptides, and FAs facilitate fat mass and weight loss | Narrative review on dairy and weight (62); clinical study on calcium and dairy on weight loss (19, 63) | |||
| Calcium promotes lipolysis and lipid oxidation and reduces lipogenesis | Narrative review on dairy and weight (62) | |||
| Yogurt is a source of probiotic bacteria, which promotes gut microbiota health and weight control | Narrative review on the microbiota (64); clinical and animal study with probiotic yogurt (49); animal study on factors that affect the microbiota (65) | |||
| Yogurt is more satiating than other foods | Clinical studies with yogurt and other foods (66–68) | |||
| Salas-Salvadó et al. (69) | Narrative review | T2D | Yogurt affects satiety, decreasing adiposity (formation of calcium soaps, high calcium intake and satiety from high protein) and mediating insulin resistance and diabetes risk | Authors’ own view supported by the following: observational studies on yogurt and adiposity (16, 18, 35, 48, 49, 70–74); meta-analysis of RCTs of the effects of dairy on weight loss (75); clinical study on effects of dairy intake on fat loss (19); narrative review on high-fat dairy and cardiometabolic diseases (76) |
| Dairy products have an effect on insulin sensitivity or insulin secretion from the pancreas | Authors’ own view supported by the following: observational studies between yogurt, dairy, and T2D risk (3, 11, 74); clinical study on effects of dairy intake on fat loss (63); narrative reviews of protein-induced satiety (77), effects of yogurt on cardiometabolic disease (78, 79), and role of vitamins and minerals in T2D prevention (80) | |||
| Influence of fermentation | Observational studies on yogurt, dairy, and T2D (81–83) | |||
| Alterations to the gut microbiota affecting insulinemia and glucose regulation | Narrative review on the microbiota and insulin resistance (84); observational study on dairy intake and insulinemia (85); meta-analysis of RCTs on effects of probiotics on glycemic control (86) | |||
| Probiotics prevent weight gain or promote weight loss and reduce gut inflammation | Narrative reviews on probiotics, the microbiota, and health (58, 60); observational studies on yogurt intake and weight (36); meta-analysis of RCTs on effects of probiotics on lowering lipids and CVD risk factors (87) |
CAD, coronary artery disease; CVD, cardiovascular disease; RCT, randomized controlled trial; T2D, type 2 diabetes.
Role of the Yogurt Matrix
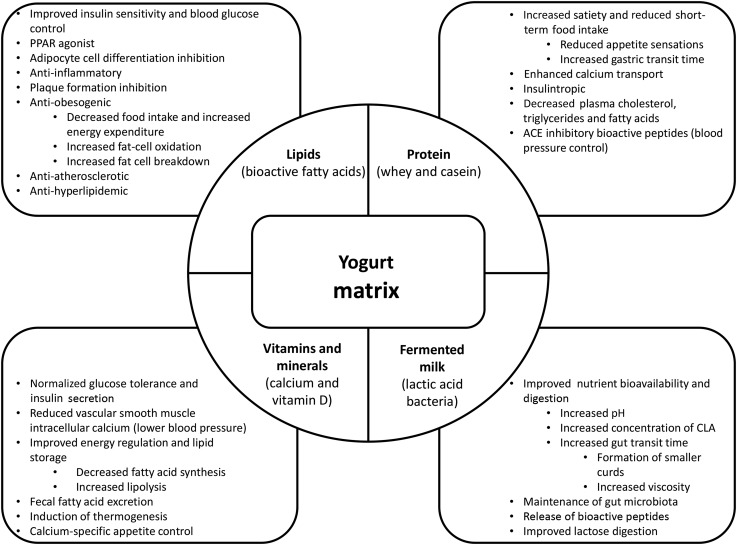
A group of 18 experts published a consensus statement in May 2017, highlighting the importance of the dairy matrix and examining the effects of whole dairy along with single nutrients. The experts agreed that dairy products do not have a positive association with CVD or diabetes, but fermented dairy products generally have an inverse association and different types of dairy products may have distinct effects on disease risk. The effects of whole dairy on CMDs may be different from the effects of individual dairy nutrients, and processing techniques may improve nutrient-matrix interactions potentially modifying metabolic effects (88). An example of potential nutrient-matrix interactions in yogurt is illustrated in Figure 1.
FIGURE 1.
Suspected mechanisms of action responsible for yogurt's protective cardiometabolic properties. ACE, angiotensin-converting enzyme.
The matrix effect of a food goes beyond the individual nutrients, suggesting that the physical structure, created by a combination of nutritive components, can act independently of its individual components during digestion and metabolism (88–90). Compared with an isolated nutrient, the yogurt matrix protects its nutrients and bioactive compounds against degradation (e.g., enhanced viability of live cultures) and allows for nutrient-nutrient interactions (e.g., enhanced calcium absorption during fermentation) (91). The buffering capacity of milk is known to promote higher viability of bacteria in the gastrointestinal tract because of maintenance of higher pH values in the stomach (92). The gel structure of yogurt supports the viability of live cultures by providing a physical barrier against degradation and protecting them against hydrogen ions and organic acids. Furthermore, exopolysaccharides produced by lactic acid bacteria (LAB) play a structural role in yogurt by increasing its viscosity, further enhancing the viability of live bacteria by protecting it during its passage through the gastrointestinal tract (92). In an in vitro digestion simulation, green tea extract was given with a dairy matrix (yogurt, milk, or cheese) or a control (water). Dairy matrices exhibited better capacity to maintain antioxidant activity than water alone (93). A matrix can also be altered during food processing (mechanical, chemical, or biochemical), which can impact the bioavailability of nutrients (94). The physical structure of the matrix and the processing methods (heat treatment and acidification) may considerably impact milk protein digestion and amino acid availability (95). Digestion kinetics of amino acids may be regulated by gastric emptying, which is closely linked to the physical properties of the food matrix (96). It has been speculated that the yogurt matrix may explain the reduced incidence of T2D in yogurt consumers compared with milk and other dairy products (3, 88). Further studies comparing yogurt with other fermented dairy products are needed to confirm the unique protective nature of the yogurt matrix against CMD. It would also be of interest to identify processing techniques that could enhance the physical structure of the yogurt matrix to optimize its nutritional properties (94).
Role of Fermentation
Bioactive peptides, exopolysaccharides, and CLA are among the beneficial compounds released during yogurt fermentation (97). Peptides can be released from protein through 3 known mechanisms: 1) hydrolysis with digestive enzymes (e.g., pepsin, trypsin, and chymotrypsin), 2) fermentation with proteolytic starter cultures, and 3) proteolysis with enzymes derived from microorganisms (98). Yogurt provides all 3 opportunities for bioactive peptide release from milk proteins as follows: 1) release occurs during fermentation of milk with yogurt starter cultures, 2) then the bacteria themselves release enzymes that act on proteins in yogurt, and 3) enzymes in the digestive tract further hydrolyze bioactive peptides from proteins. Cell envelope–associated proteinases contained in LAB are able to break down milk protein into oligo-, di-, and tripeptides, which are then hydrolyzed into peptides and amino acids by endopeptidases and aminopeptidases. These small peptides have alternative transportation routes, allowing them to be absorbed quickly and partially bypass gastrointestinal digestion (99). Once absorbed, these bioactive peptides can directly exert physiologic effects on cardiometabolic parameters. A recent study using an in vitro gastric simulation model concluded that milk fermented with lactic acid acted in a complementary manner to produce bioactive peptides with functional health properties (100). Therefore, yogurt may have greater potential for functional health properties, given the synergistic relation between yogurt microorganisms and human digestion. In one study, milk fermentation with commercial yogurt LAB starters resulted in increased angiotensin-converting enzyme (ACE)–inhibitory activity. Gly-Thr-Trp and Gly-Val-Trp were specific ACE-inhibitory tripeptides identified. The antihypertensive effects of these peptides were then validated with an animal model by feeding diluted whey protein (peptide concentration 5 mg/mL) to spontaneously hypertensive rats for 8 wk. There were significant reductions in systolic and diastolic blood pressure in the treatment group compared with the control group (101). Sequences of bioactive peptides released from fermented milk made with traditional yogurt cultures, Lactobacillus delbrueckii subsp. bulgaricus, and Streptococcus thermophilus, have been characterized with antioxidant (102), mucin-stimulating, ACE-inhibitory, opioid (103, 104), and immunomodulatory properties (105). Similar properties have been characterized in peptide fractions released from probiotic cultures (e.g., L. casei and L. helveticus) in fermented milks (99).
The fermentation process increases the concentration of CLA, potentially making yogurt a better source than milk; however, the extent of this conversion depends on the milk source (dairy cattle breed and animal diet), bacterial strain, and supplemental ingredients (106, 107). For example, the highest concentration of CLA was found by cofermenting milk with S. thermophilus and L. acidophilus supplemented with maltodextrin, resulting in a 38% greater concentration of CLA than a control (108). CLA is potentially a valuable health-promoting FA and may be more concentrated in certain types of yogurt. The CLA content in yogurt can be optimized during processing by selected LAB strains for fermentation (109). Fermentation of milk into yogurt also improves nutrient availability, increases the quantity of nutrients, and enhances digestion and absorption of nutrients by the host. Gut transit time is increased via activity of LAB species that promote the formation of smaller curds and increased viscosity (60). The decrease in the pH of yogurt through fermentation creates an acidic environment that is ideal for mineral absorption (110). Thus, there is a high degree of absorbability of calcium in yogurt, which is of particular interest for lactose-intolerant individuals (60).
In addition to beneficial bacteria, milk contains oligosaccharides, which have a prebiotic role and favorably manipulate the gut microbiota by enhancing the growth of beneficial species (bifidobacteria and lactobacilli) at the expense of potentially pathogenic species (clostridia, enterocci, eubacteria, and enterobacteria). Although oligosaccharides are only found in trace amounts in bovine milk (111), fermentation with different cultures can produce a yogurt with higher concentrations of fermentable oligosaccharides that have the potential to provide prebiotic benefits (112). There has been little characterization or quantification of bioactive metabolites produced by different bacterial strains during fermentation, and results are extrapolated to human health rather than rigorously tested (99). Furthermore, little is known about the doses of bioactive peptides or other fermentation metabolites needed to induce a physiologic effect, but they likely act synergistically with the dairy matrix (99, 113).
Effects on the Gut Microbiota
The trillions of bacteria colonizing the gut live in symbiosis to assist digestion; however, the benefits of this relation extend well beyond intestinal function and have a profound impact on energy homeostasis. Disruption to gut-microbial balance, also known as dysbiosis, can promote obesity, inflammation, and T2D (114). Obesity-linked dysbiosis involves a shift in bacterial communities in the gut, characterized by a decrease in Bacteroides relative to that of Firmicutes, which is known to promote low-grade inflammation (115). Furthermore, this dysbiosis has been linked to greater energy harvest from the diet and increased fat storage (65). Diet is a key factor in obesity-associated gut dysbiosis and T2D (116). It has been hypothesized that yogurt has positive effects on the microbiota (21), which may contribute to its cardiometabolic health benefits (117). However, the mechanisms linking yogurt to gut health are largely based on the assumption that all yogurts contain probiotic bacteria and that commercial yogurts contain sufficient probiotics to exert a physiologic effect. According to a consensus statement from the International Scientific Association for Probiotics and Prebiotics, probiotics should be defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (118). With that definition in mind, the European Food Safety Authority supports that yogurt made from cultures (S. thermophilus and L. bulgaricus) have demonstrated effects for assisting lactose digestion (119) and may be considered a probiotic for individuals with lactose intolerance (118). However, the health benefits of these classic yogurt cultures on CMDs or metabolic parameters are yet to be established.
Yogurts made with alternate cultures and specific strains such as Bifidobacterium lactis BB12 and L. acidophilus are considered probiotic in some countries and are authorized to carry nonspecific health claims indicating that the product may help maintain the gut flora by providing organisms that are naturally found in the gut (120). Although it is unclear what percentage of yogurts on the market are made with probiotic cultures, the use of alternative or complementary cultures is common practice in commercial yogurts regardless of whether they are accompanied by health claims. Yogurt provides an ideal vehicle for probiotics in food products. Probiotic effects of these yogurts, however, are strain specific (121, 122), and over the years, there has been speculation about the broadly defined benefits of commercially available probiotic yogurts (123). Furthermore, not all probiotic products disclose strain information and some need as many as 20 servings to be effective (124). Nevertheless, there are commercial yogurts that can improve glycemic control and cholesterol in individuals with diabetes when consumed on a regular basis in doses as little as 0.5–2 servings/d (41, 125, 126). Epidemiologic studies have yet to distinguish between yogurts made with classic, probiotic, or alternative cultures, further limiting speculation about associations between yogurt consumed (probiotic or not) and benefits to the microbiota. Further characterization of both classic and alternative yogurt cultures is needed to establish potential probiotic effects, particularly on the gut microbiota.
Aside from probiotics, consuming yogurts with live cultures may have important beneficial effects on maintaining a healthy gut microbiota. There is a growing body of literature about the beneficial effects of live cultures, used in both traditional and commercial foods. Fermenting foods with live bacteria releases bioactive peptides and bacteriocins from its food matrix (113). β-Casein–derived peptides released from fermenting milk with classic yogurt cultures (L. bulgaricus and S. thermophilus) can promote goblet cells to secrete mucin in vitro and in vivo (104). Enhancing the intestinal mucosal layer contributes to protecting against the colonization of pathogenic bacteria, acidic pH, luminal proteases, and mechanical damage and reduces intestinal inflammation (127). Ingesting live bacteria can quickly metabolize simple carbohydrates producing lactate, acetate, or propionate. Therefore, these bacteria can influence dietary carbohydrate breakdown, potentially altering metabolic outputs, and can provide substrates (i.e., lactate, acetate and propionate) for resident bacteria growth. They may also have direct effects on pathogenic bacteria abundance by decreasing the pH, competing for niches, or producing exopolysaccharides and bacteriocins (128).
Ingesting milk fermented with B. lactis, S. thermophilus, L. bulgaricus, and Lactococcus lactis stimulated beneficial metabolite production (butyrate) and decreased pathobionts (40) that cause inflammation and compromise the intestinal barrier function. Microbiota dysregulation and impaired gut barrier function can contribute to low-grade inflammation and altered metabolic function (glucose and lipid homeostasis), leading to obesity and T2D (117). The benefits of yogurt (made with cultures) on digestive health through modulating enteric pathogens and regulating toxemia were proposed more than a century ago by Nobel Prize winner Elie Metchnikoff (129). Mechanisms explaining Metchnikoff’s hypothesis are only now starting to be elucidated, but research in this area is nascent. Recently, Putt et al. (130) determined that a commercial low-fat yogurt increased tight junctions of an inflammation-disrupted intestinal barrier in a Caco-2 cell model, with greater bioactivity in the gastric phase than the intestinal phase. Yogurt’s ability to restore inflammation-induced intestinal barrier dysfunction in Caco-2 cells can contribute to improving the pathology of diseases like obesity (130).
The contribution of commercial yogurt consumption to gut-microbial balance has not yet been adequately examined in human models, but like other probiotics, yogurt would need to be consumed on a regular basis in sufficient amounts to confer any benefits. The shelf life of commercial yogurts reduces the viability; however, bacteria may not need to be viable throughout the entire digestive tract to provide health benefits (131). This is evidenced by beneficial effects of yogurt bacteria (S. thermophilus and L. bulgaricus) on lactose digestion despite its poor viability (low abundance in fecal matter) (60). Further research is needed to test the capacity of commercially available yogurts, and not just probiotic specific strains, to contribute to gut-microbial balance. Given the increasingly important role of gut microbiota in health and disease (132, 133), particularly weight management (60, 134), and the popularity, international accessibility, and affordability of commercial yogurt and the potential implications of yogurt in preventing gut dysbiosis and improving gut health, this topic warrants immediate research attention.
Functional Yogurt Nutrients
Protein, whey, and bioactive peptides
Bovine milk protein contains whey and casein in a 20:80 ratio, providing an excellent concentrated source of high-quality protein and essential amino acids. Unlike milk, however, the protein content of yogurt may be further enhanced during the manufacturing process converting milk into a standardized commercially available yogurt. This process may involve the addition (or substitution) of other milk-based products such as milk powders, condensed milk, buttermilk powder, milk protein concentrates, whey products, and caseinates (135). The positive effects of yogurt on CMDs (e.g., obesity, diabetes, and metabolic syndrome) have been frequently attributed to the potential for yogurt to contribute to satiety and subsequently reduce energy intake (69, 136).
Although few clinical studies have been conducted on the impact of yogurt intake on CMD endpoints, some have shown reduced energy intake after yogurt consumption compared with fruit or dairy drinks (66) and chocolate (67). It has also been reported that yogurt has a greater effect on subjective appetite ratings than milk, cheese, or an isovolumetric quantity of water (137). The role of individual yogurt components, particularly protein, on metabolic regulatory mechanisms has received much attention. The protein content and composition of yogurt has been suggested to contribute to appetite control by inducing gastrointestinal hormone secretion, resulting in increased satiety, suppressed short-term food intake, and diet-induced thermogenesis (138–140). Whey protein, known as a “fast protein,” is quickly digested, leading to a rapid rise in plasma amino acid concentrations that peaks 40 min to 2 h after its consumption and returns to basal levels after 3–4 h. In contrast, the casein fraction, known as a “slow protein,” clots in the stomach under acidification and results in lower amino acid concentrations that increase at a slower rate but maintain a plateau over a period of ≥7 h after its consumption (141).
Yogurt can contribute to satiety and reduced food intake in part through its whey protein content. The satiating effects of isoenergetic whey protein isolate (WPI), glycomacropeptide, and maltodextrin carbohydrate were previously compared in preload drinks given 120 min before a test meal. Food intake was significantly lower in the WPI and glycomacropeptide groups than the carbohydrate group, and the WPI was more satiating than the carbohydrate control (142). Other studies have also demonstrated short-term satiety-inducing effects of protein-rich yogurt (143) and intact whey protein (138). The satiety-inducing effects of whole whey proteins are thought to be driven by postprandial increases in circulating amino acids and plasma cholecystokinin and glucagon-like polypeptide-1 (144). Of whey proteins, tryptophan-rich α-lactalbumin is specifically thought to increase satiety and reduce appetite and food intake (145). An α-lactalbumin–supplemented breakfast was associated with significantly lower energy and food intakes in an ad libitum lunch 3 h later than breakfasts supplemented with casein, soy, or whey (without glycomacropeptide) proteins. It has been proposed that α-lactalbumin, which is rich in tryptophan and low in large neutral amino acids, can promote secretion of serotonin, influencing food intake (146).
Whey protein in yogurt could be responsible in part for the protective effects on T2D risk. Consuming small amounts of whey protein before a meal has been suggested to help control plasma glucose through both insulin-dependent and insulin-independent mechanisms. Independent mechanisms are based on whey protein’s effect on the gastrointestinal hormones regulating stomach emptying, incretins that potentiate insulin efficacy, and the gastric-emptying rate before a meal (139). Indeed, whey proteins have been shown to increase insulin secretion in T2D (147). Studies have reported that administering whey protein 170–300 min before a meal can reduce glucose responses and increase insulin and incretin responses (138, 139, 148). These findings demonstrate that even small amounts of whey can help control glycemia independently from changes in insulin secretion or clearance. The potential mechanism underlying this effect appears to involve the release of glucagon-like polypeptide-1 and peptide tyrosine-tyrosine, which is associated with a lower premeal gastric-emptying rate (139). These mechanisms are plausible but have yet to be substantiated, because nutrients that may be responsible for satiety (e.g., whey proteins) have not been consistently demonstrated clinically or have only been demonstrated in animal models. It is uncertain whether a single standard portion of commercial yogurt contains adequate whey to have a physiologic effect on satiety or blood glucose regulation. Furthermore, yogurt may simply act as a replacement for energy-dense foods (136).
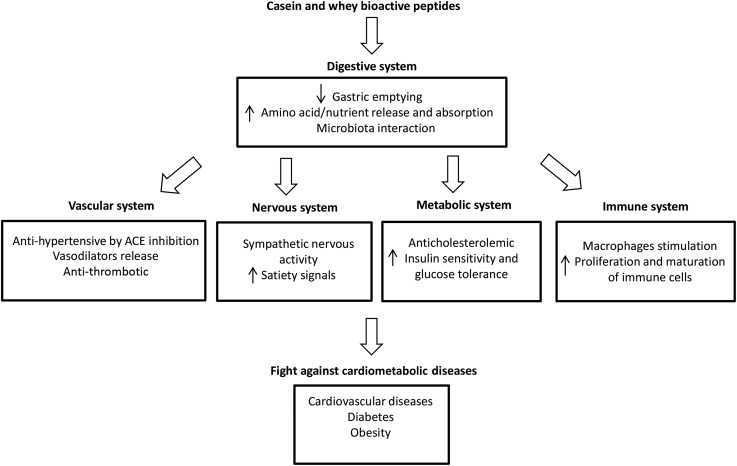
Bioactive peptides that affect cardiac health are released from milk proteins during digestion and are also present in yogurt (145). These peptides contain between 2 and 20 amino acids and, depending on their sequence, may possess hormone-like activity as regulatory compounds exerting antihypertensive, antimicrobial, antithrombic, immunomodulatory, and hypocholesterolemic effects (149, 150). Known sequences of peptides derived from whey (e.g., β-lactoglobulin, α-lactalbumin, and lactoferrin) and casein (e.g., β-casein, κ-casein, and α-casein) proteins have specific health effects (Figure 2) (145).
FIGURE 2.
Potential physiologic effects of bioactive yogurt peptides. ACE, angiotensin-converting enzyme.
ACE-inhibitory peptides are the most widely studied bioactive peptides in milk and fermented milk (98, 150). ACE-inhibitory activity has been identified in >50 sequences of bioactive peptides derived from casein, which are thought to be responsible for the hypotensive properties of dairy, particularly the tripeptides iso(leucine)-proline-proline and valine-proline-proline (129, 149). A meta-analysis of 30 randomized clinical trials investigating the effects of lactotripeptide ingestion on blood pressure suggested that they may be a positive treatment for blood pressure, particularly for Japanese individuals; however, inconsistencies between studies generated through a strong publication bias should be considered (151). Although an overall inverse association between dairy intake and hypertension has been observed (152), this effect has not been consistently found in the yogurt dairy subgroup (153). It should be noted that although the ACE-inhibitory mechanisms of bioactive peptides have been widely investigated and provide a case for yogurt’s antihypertensive effects, the doses used in clinical trials are likely much higher than could be consumed from yogurt, leaving us to question its clinical relevance. Nevertheless, ACE-inhibitory bioactive peptides have been characterized in many fermented dairy (yogurts and cheeses), some of which have been described as having moderate to high activity, but antihypertensive effects of these peptides have yet to be confirmed in relevant animal models, and eventually in human studies (99).
Bioactive peptides from dairy proteins may also play a role in metabolic regulation and contribute to improve insulin sensitivity. Indeed, glycomacropeptide generated from κ-casein has been reported to prevent high-glucose–induced insulin resistance in HepG2 liver cells (154), to reduce lipid accumulation and enhance antioxidant properties in obese rats (155), and to improve CVD risk markers in humans (156). Moreover, glycomacropeptide is a bioactive peptide with demonstrated prebiotic effects that has the potential to modulate proteobacteria in the gut, promoting growth of beneficial bacteria in mice (157), which may contribute to gut health and reduced metabolic endotoxemia, resulting in lower risk for obesity-linked CMDs. To our knowledge, the characterization of bioactive peptides from different strains of yogurt cultures has not been thoroughly studied and the physiologic doses of peptides in humans remain unknown. The ability of bioactive peptides derived from milk proteins to reduce cardiometabolic risk is plausible, but without further research, we can only speculate that yogurt consumption contributes to CMD protection through mechanisms that involve bioactive peptides.
FAs
The nature of lipids in yogurt is almost identical to its milk source but may vary slightly in both quality and composition depending on various factors related to the origin of the milk. The dairy cow breed, its feed, lactation stage, and nutritional status affect the lipid composition (158). Milk contains ~3% PUFAs, 25% MUFAs, and 72% SFAs. Dairy products are a natural source of medium-chain fatty acids (MCFAs), which account for 7–8% of total FAs in butter, milk, yogurt, and cheese (159). MCFAs (4:0 to 12:0) are preferentially hydrolyzed by lipase into diacylglycerides, absorbed, and transported via the portal vein to the liver, where they undergo β-oxidation. Long-chain SFAs, on the other hand, are packaged into chylomicron TGs and enter the systemic circulation through the lymphatic system and are likely stored (160, 161). These differences in metabolism make MCFAs a source of rapid energy that is not likely to be deposited as fat in the body (162, 163), and they likely explain its beneficial effects on weight control and body composition (164, 165).
Long-chain SFAs (lauric acid, myristic acid, palmitic acid, and stearic acid) found in milk have unique profiles and can have contrasting effects on cholesterol (166). As a group, long-chain SFAs are known to increase total cholesterol; however, the magnitude and increase differs in action on either LDL or HDL cholesterol. According to a meta-analysis of 60 controlled trials, when carbohydrates are replaced with SFAs, lauric acid is a potent cholesterol increaser, but this is largely attributable to its ability to increase HDL cholesterol and decrease the total:HDL cholesterol ratio. Other long-chain SFAs have similar, albeit not as potent, effects on HDL cholesterol when replacing carbohydrates, which may be related to their chain length (167). Replacing carbohydrates with long-chain SFAs (lauric acid, myristic acid, palmitic acid, and stearic acid) also increases serum TG concentrations (167). The mitigating effects of different individual SFAs on cholesterol may suggest that the SFAs typically found in yogurt have neutral effects on certain cardiometabolic risk factors such as total:HDL cholesterol ratios, although this remains to be confirmed by RCTs in humans or preclinical studies in relevant animal models.
A growing number of studies suggest that dairy MCFAs may exert protective effects against CMDs. Diets containing MCFAs have been reported to exert antidiabetic effects in both animals and humans. Researchers have observed improved glucose tolerance and protection against insulin resistance (168, 169) and preserved insulin action in skeletal muscle and adipose tissue (170) in rats and humans (171, 172). Indeed, increased insulin-mediated glucose metabolism was found in both diabetic and nondiabetic subjects consuming medium-chain compared to long-chain SFAs (171). Although the molecular mechanisms have not yet been delineated, it was suggested that bioactive lipids, including MCFAs, could increase adiponectin and lipolysis and enhance insulin sensitivity, thus alleviating insulin resistance (173). Improved glucose tolerance in rats fed a medium-chain TG diet has also been attributed to higher serum adiponectin through transcriptional activation of the adiponectin gene (169). Given the increased availability of whole-milk yogurts, understanding the relation between dairy fat and weight regulation is important for health professionals to make appropriate dietary recommendations.
Mechanisms explaining the inverse association between high-fat yogurt and CMD risk may also implicate the bioactive properties of specific dairy fats such as CLA (174). Indeed, several animal studies have consistently demonstrated antiobesogenic effects of CLA (175–178). CLA has been reported to improve glucose tolerance (178), to decrease diabetes onset in rats (179), and to improve metabolic parameters in human subjects with T2D (180). Other bioactive lipids present in dairy can exert metabolic properties. For example, vaccenic acid was reported to improve blood lipids via activation of PPARα and PPARγ expression in rats (181). Phytanic acid, another bioactive fat produced by ruminant animals, has been reported to act as an agonist of PPARα, PPARγ, and also retinoid X receptor pathways, triggering uncoupling protein 1 expression in brown adipose tissue, which was associated with an improved metabolic profile and increased insulin sensitivity in mice (174, 182–184). Butyric acid is a 4-carbon SCFA found in milk fat but is best known for its presence in the colon as a by-product of microbial fermentation. Studies in animal models have shown that butyric acid can improve insulin sensitivity and increase thermogenesis, protecting against diet-induced obesity and insulin resistance (163, 185). Although the current intake of yogurt in a typical diet likely does not provide sufficient quantities of any specific bioactive lipid capable of inducing a physiologic response, bioactive lipid species may act in concert to improve energy balance and glucose and lipid homeostasis (namely through activation of PPAR pathways) (174). There is a conceptual basis for the health benefits of MCFAs and CLA found in yogurt, but the mechanisms involved have largely been investigated in animal models and remain to be validated in humans. Furthermore, these mechanisms may not be relevant to individuals who consume low-fat yogurt and are not relevant for those who consume nonfat yogurt.
Calcium
Calcium concentrations in the blood are not directly affected by dietary calcium but are modulated by hormonal responses and vitamin D that help maintain a constant narrow range in the circulation. Yogurt provides a good source of dietary calcium and vitamin D (in fortified yogurt). Its intake influences vitamin D and hormonal responses, thereby contributing to the regulation of intracellular calcium concentrations. Groups with high dairy consumption have a lower risk of developing T2D than those who do not consume dairy (186). It remains plausible that the contribution to dietary calcium intake from yogurt consumption (~20% of DRIs) plays a role in glucose and insulin regulation. It is also possible that calcium plays an indirect role in preventing T2D through mechanisms related to reducing nutrient deficiencies, controlling hypertension (187), managing adiposity (188), and reducing systemic inflammation (189).
Pittas et al. (189) outlined several mechanisms whereby calcium influences glucose tolerance through 1) improved release of insulin from pancreatic β cells, 2) improved insulin action, and 3) modulation of cytokines to reduce systemic inflammation (189). Regarding the first mechanism, insulin secretion is a calcium-dependent process (189) and hypocalcemia has been associated with impaired insulin release (190, 191). In vitamin D–depleted rats, calcium repletion was able to normalize glucose tolerance and insulin secretion (192), whereas patients who were resistant to 1,25-dihydroxyvitamin D (1,25-OH2D) were prone to abnormal insulin secretion if they were also hypocalcemic (193). Pittas et al. (189) speculated that inadequate calcium or vitamin D intake modifies the intracellular or extracellular calcium equilibrium, potentially interfering with insulin release in response to a glucose load. Calcium concentrations can also affect insulin action through various pathways, including the regulation of intracellular calcium ion concentrations (189); an optimal range of the calcium ion concentration is important to maximize insulin-mediated glucose transport in adipocytes (187). Both vitamin D and calcium influence characteristic mechanisms modulating the development of insulin resistance and T2D (189) such as intracellular insulin signaling and AMP-activated protein kinase pathways, both of which are involved in the regulation of muscle glucose metabolism (194–196). Although yogurt is an excellent source of calcium, only fortified yogurts contain vitamin D.
A calcium-deficient diet induces adaptive mechanisms to maintain extracellular calcium concentrations (197). The impairment of intracellular calcium flux is a fundamental underlying factor for both hypertension and obesity (187). Low dietary calcium results in less calcium absorption in the gut and decreased extracellular calcium concentrations, which activates an increase in parathyroid hormone and 1,25-OH2D (197).
Observational studies have provided conflicting results between the relation between yogurt consumption and hypertension (13, 49, 198, 199), leading to the suggestion that yogurt has a neutral effect on blood pressure (17). However, consuming 3–4 portions of dairy, in combination with adequate fruits and vegetables, has antihypertensive effects (200), which are thought to be a result of adequate intakes of calcium (201). The DASH (Dietary Approaches to Stop Hypertension) Trial diet promotes adequate dairy consumption within the context of a diet rich in fruit and vegetables as a nonpharmacologic approach to reducing hypertension (202, 203). The beneficial effect of calcium intake on hypertension is thought to be superior when calcium is derived from dairy sources than from calcium supplements (204).
The exact mechanism by which dietary calcium is inversely related to hypertension is poorly understood and has yet to be substantiated (205). Nevertheless, it has been postulated that inadequate calcium intake activates parathyroid hormone and 1,25-OH2D. Promotes an increase in cytosolic calcium concentrations that increase contractile tone in vascular smooth muscle, causing vasoconstriction and promoting hypertension (197, 206, 207). Another potential mechanism is that calcium may indirectly promote endothelial NO synthase activity and NO-mediated vasodilation, increasing insulin secretion or action. Indeed, endothelial NO synthase expression and activation is defective in hypertensive individuals, and this is believed to contribute to elevated blood pressure (208).
The potential role of calcium in obesity prevention and weight maintenance is thought to be crucial (209) and to involve pathways related to energy regulation and lipid storage (204). Low dietary calcium, as mentioned previously, is presumed to modulate the presence of 1,25-OH2D, which in turn influences intracellular calcium concentrations in various cells (including in adipocytes). Increases in intracellular calcium promote TG storage in adipocytes by stimulating lipogenesis and inhibiting lipolysis (204, 210). Low calcium intakes may decrease fat mobilization and utilization (54), particularly during caloric restriction (211). A diet rich in calcium would, however, have an antiobesogenic effect by decreasing FA synthesis and increasing lipolysis (204). Furthermore, calcium has been found to promote fecal FA excretion via the formation of insoluble calcium soaps with FAs. Although this effect has been demonstrated clinically, it is very modest (204). Although it contributes to a net reduction in energy balance, fecal fat excretion remains very small (7.4% compared with 6.8%), and it is unclear whether this is sufficient to promote clinically relevant weight loss (204). However, a meta-analysis of 13 calcium supplementation (dairy and supplements) RCTs measuring fecal fat loss concluded that increasing dairy calcium in the diet to 1241 mg/d resulted in a 5.2-g increase in fecal fat loss compared with a diet low in dairy calcium (<700 mg/d) (212). In diets with high dairy intake (∼1200 kcal/d), fecal fat loss would represent a reduced intake of ~50 kcal/d, which may promote weight stability and prevent weight regain (212).
Perspectives on the Research Agenda and Future Challenges
Yogurt is a nutrient-rich fermented dairy product that is part of a healthy diet and lifestyle. Yogurt should be promoted, along with nuts, fish, fruits, and vegetables, within healthy dietary patterns, food-based dietary guidelines, and therapeutic diets such as the Dietary Approaches to Stop Hypertension Trial diet to prevent CMDs (42). However, current evidence stemming from either animal models or clinical studies is not yet sufficient or strong enough to support yogurt-specific recommendations for the prevention of CMDs such as obesity and diabetes. Further experimental and clinical studies are clearly needed to demonstrate the impact of yogurt intake on relevant health parameters and key CMD risk factors. To establish yogurt-specific recommendations, an ambitious research agenda is needed. Research priorities to validate the health benefits of yogurt, identify the mechanisms, and determine an appropriate dose could be summarized as follows:
1) Conduct clinical studies that support the overwhelming observational evidence on yogurt and reduced incidence of T2D. These studies should include investigating the short-term effects of yogurt consumption on blood glucose control and long-term effects of regular yogurt intake on prediabetes and T2D development. Ideal studies would use appropriate controls to compare yogurt supplementation to a nutritionally equivalent food and usual dietary intake. Similar studies are warranted for other cardiometabolic risk factors that contribute to the metabolic syndrome (e.g., adiposity, blood pressure, or blood lipid profiles).
2) Investigate the effects of yogurt cultures on gut-microbial balance, metabolic endotoxemia reduction, inflammation, and metabolism regulation. Testing whether live yogurt cultures are capable of influencing positive changes to the gut-microbial balance or whether the effects are strain specific and limited to probiotic cultures is important. Animal studies may be best suited to testing numerous types of yogurt cultures within relatively short periods before conducting clinical studies that have high participant burden and are costly. Given the importance of the gut microbiota in the development of obesity and T2D, a better understanding of how an everyday product like yogurt can contribute to gut microbiota and intestinal health warrants attention.
3) An in-depth examination of the yogurt matrix is needed to distinguish it from other foods with similar nutrient compositions. Animal studies (and when possible, clinical studies) should use appropriate nutrient equivalent controls to yogurt (e.g., milk or acidified milk) to test that beneficial effects are not limited to the nutrient composition of yogurt.
4) Strain-specific effects of yogurt cultures on the production of bioactive nutrients are important for identifying the best cultures to produce quality commercial products. Metabolomics will be useful to characterize and quantify bioactive nutrients in yogurt produced by specific strains, followed by the establishment of physiologic doses.
It is important to emphasize that further research is not needed to establish yogurt as a nutrient-rich dairy product that can be consumed on a regular basis for good nutritional health. However, the research priorities mentioned above would help to establish whether yogurt-specific recommendations for particular health parameters and diseases are warranted. Some of these priorities are feasible and can be conducted with relative ease (e.g., clinical trials for insulin resistance regulation). However, other priorities, such as determining the physiologic doses of bioactive nutrients in yogurt or examining effects of yogurt on the gut microbiota, are complex and would require a series of multiple complementary experiments or may depend on field-specific technological advances.
Challenges and recommendations for preclinical studies in animal models
The advantage of testing and validating mechanisms on animal models is the ability to have a reasonable degree of control over environmental and genetic factors, which are not possible to fully control in clinical studies (213). Animal models further allow matching for key dietary factors (percentage of lipids, proteins, and carbohydrates), better control of dosage of the yogurt matrix (e.g., through gavage), and examination of the underlying mechanisms of action of yogurt in more detail than a clinical investigation. The impact of yogurt intake on insulin resistance in a high-fat feeding or genetic model of obesity and T2D can be ascertained using tracer-coupled hyperinsulinemic-euglycemic clamp studies. Both glucose uptake and production by insulin target tissues can be used to define molecular mechanisms through determination of insulin signaling intermediates in such tissues (214). This is key for several reasons, particularly to ensure that the gut microbiota is stable and consistent between experimental animals, which is normally achieved after 1 wk of adaptation to a new animal facility. However, it is also known that animals kept in different animal facilities carry different microbiota, and this limitation must be considered when preclinical studies investigating the impact of diets or dietary factors in different locations are compared. Developing diets for laboratory animals that closely resemble human dietary patterns should also be considered to emulate the day-to-day human diet rather than use traditional nutrient-focused diets currently and widely used for animal studies. To better illustrate one obvious limitation of most animal studies investigating the impact of any food components on CMDs, casein is by far the predominant (if not the only) source of dietary protein used in such studies. Yogurt studies in animal models are scarce and, unfortunately, are limited by the fact that a dairy protein, casein, is the common protein source used in control diets (215). In order to be closer to the human reality, it is recommended that investigators include more human-relevant protein sources in animal diets, especially meats, poultry, and eggs.
Challenges and recommendations for clinical intervention studies in humans
RCTs that consider specific criteria are needed to establish the effects of a potential yogurt intervention on various health parameters and in various populations, including children, healthy individuals, and vulnerable individuals such as those with obesity or T2D. These studies would need to include a sufficient sample size, various types and quantities of yogurt, a suitable control, and important biomarker measurements to assess potential hormonal mechanisms in humans. To our knowledge, most clinical studies have examined the effects of yogurts with added probiotics (216, 217) or supplemented yogurts (218) compared with plain (traditional) yogurt and have not used an appropriate control to assess the effects of conventional yogurt. These studies investigate the potential of yogurt as a vector for functional ingredients (e.g., antioxidants, probiotics, or vitamin D) rather than a nutrient-rich food with a unique matrix containing live cultures. The few RCTs that have investigated the impact of yogurt are difficult to compare because they tend to use different routes of administration, study populations, experimental procedures, indicators of gut microbiota health, strains of LAB, and controls (219). Furthermore, given the short duration of most RCTs and the limitations to sample size, the dose of yogurt administered would likely need to be greater than the amounts reported to be associated with lower body weight and T2D incidence in prospective cohort studies.
Conclusions
Given the limitations presented above, the ideal experimental design to validate yogurt’s role and mechanisms of action on CMD prevention would consist of a multistep proof-of-concept study based on strong epidemiologic studies, well-standardized laboratory experiments in relevant animal models using humanized diets, and proof-of-concept human studies and clinical trials. An appropriate placebo for yogurt would be similar in texture, viscosity, and nutrient composition but would be absent of viable yogurt bacteria and fermentation metabolite products resulting from their activity. Together, the results of such studies would represent the best opportunity to provide a global validation of yogurt’s role in preventing CMD and would lead to the identification of the specific mechanism(s) responsible for the purported beneficial health effects of yogurt.
Of the individual yogurt components, calcium, protein, bioactive nutrients, and live cultures are likely among the primary factors responsible for the beneficial effects of yogurt on CMD risk factors, but the individual and synergistic roles of these nutrients within the food matrix also must be examined. Animal data would provide the most relevant and robust outcome to design quality RCTs with appropriate control groups. Overall, this multistep research strategy should eventually reveal the direct role of yogurt on cardiometabolic health, above its already demonstrated role as a dietary marker of a healthier lifestyle.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ACE, angiotensin-converting enzyme; CMD, cardiometabolic disease; CVD, cardiovascular disease; LAB, lactic acid bacteria; MCFA, medium-chain fatty acid; RCT, randomized controlled trial; T2D, type 2 diabetes; WPI, whey protein isolate; 1,25-OH2D, 1,25-dihydroxyvitamin D.
References
- 1.Bohl M, Bjornshave A, Larsen MK, Gregersen S, Hermansen K. The effects of proteins and medium-chain fatty acids from milk on body composition, insulin sensitivity and blood pressure in abdominally obese adults. Eur J Clin Nutr 2017;71:76–82. [DOI] [PubMed] [Google Scholar]
- 2.Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016;103:1111–24. [DOI] [PubMed] [Google Scholar]
- 3.Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med 2014;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 5.Kirii K, Mizoue T, Iso H, Takahashi Y, Kato M, Inoue M, Noda M, Tsugane S. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia 2009;52:2542–50. [DOI] [PubMed] [Google Scholar]
- 6.Soedamah-Muthu SS, Masset G, Verberne L, Geleijnse JM, Brunner EJ. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br J Nutr 2013;109:718–26. [DOI] [PubMed] [Google Scholar]
- 7.Grantham NM, Magliano DJ, Hodge A, Jowett J, Meikle P, Shaw JE. The association between dairy food intake and the incidence of diabetes in Australia: the Australian Diabetes Obesity and Lifestyle Study (AusDiab). Public Health Nutr 2013;16:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouwer-Brolsma EM, Dhonukshe-Rutten RA, van Wijngaarden JP, Zwaluw NL, Velde N, de Groot LC. Dietary sources of vitamin B-12 and their association with vitamin B-12 status markers in healthy older adults in the B-PROOF study. Nutrients 2015;7:7781–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis KL, Wei F, de Boer IH, Howard BV, Liu S, Manson JE, Mossavar-Rahmani Y, Phillips LS, Shikany JM, Tinker LF. A diet high in low-fat dairy products lowers diabetes risk in postmenopausal women. J Nutr 2011;141:1969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Choi HK, Ford E, Song Y, Klevak A, Buring JE, Manson JE. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care 2006;29:1579–84. [DOI] [PubMed] [Google Scholar]
- 11.Díaz-López A, Bulló M, Martínez-González MA, Corella D, Estruch R, Fitó M, Gómez-Gracia E, Fiol M, García de la Corte FJ, Ros E, et al. Dairy product consumption and risk of type 2 diabetes in an elderly Spanish Mediterranean population at high cardiovascular risk. Eur J Nutr 2016;55:349–60. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor LM, Lentjes MA, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Dietary dairy product intake and incident type 2 diabetes: a prospective study using dietary data from a 7-day food diary. Diabetologia 2014;57:909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babio N, Becerra-Tomas N, Martinez-Gonzalez MA, Corella D, Estruch R, Ros E, Sayon-Orea C, Fito M, Serra-Majem L, Aros F, et al. Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly Mediterranean population. J Nutr 2015;145:2308–16. [DOI] [PubMed] [Google Scholar]
- 14.Pereira MA, Jacobs DR Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA 2002;287:2081–9. [DOI] [PubMed] [Google Scholar]
- 15.Snijder MB, van Dam RM, Stehouwer CD, Hiddink GJ, Heine RJ, Dekker JM. A prospective study of dairy consumption in relation to changes in metabolic risk factors: the Hoorn Study. Obesity (Silver Spring) 2008;16:706–9. [DOI] [PubMed] [Google Scholar]
- 16.Sayón-Orea C, Bes-Rastrollo M, Marti A, Pimenta AM, Martin-Calvo N, Martinez-Gonzalez MA. Association between yogurt consumption and the risk of metabolic syndrome over 6 years in the SUN study. BMC Public Health 2015;15:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drouin-Chartier J-P, Brassard D, Tessier-Grenier M, Côté JA, Labonté M-È, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr 2016;7:1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zemel MB, Richards J, Mathis S, Milstead A, Gebhardt L, Silva E. Dairy augmentation of total and central fat loss in obese subjects. Int J Obes (Lond) 2005;29:391–7. [DOI] [PubMed] [Google Scholar]
- 20.Eales J, Lenoir-Wijnkoop I, King S, Wood H, Kok FJ, Shamir R, Prentice A, Edwards M, Glanville J, Atkinson RL. Is consuming yoghurt associated with weight management outcomes? Results from a systematic review. Int J Obes (Lond) 2016;40:731–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayon-Orea C, Martinez-Gonzalez MA, Ruiz-Canela M, Bes-Rastrollo M. Associations between yogurt consumption and weight gain and risk of obesity and metabolic syndrome: a systematic review. Adv Nutr 2017;8:146S–54S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avalos EE, Barrett-Connor E, Kritz-Silverstein D, Wingard DL, Bergstrom JN, Al-Delaimy WK. Is dairy product consumption associated with the incidence of CHD? Public Health Nutr 2013;16:2055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Praagman J, Franco OH, Ikram MA, Soedamah-Muthu SS, Engberink MF, van Rooij FJ, Hofman A, Geleijnse JM. Dairy products and the risk of stroke and coronary heart disease: the Rotterdam Study. Eur J Nutr 2015;54:981–90. [DOI] [PubMed] [Google Scholar]
- 24.Bonthuis M, Hughes MC, Ibiebele TI, Green AC, van der Pols JC. Dairy consumption and patterns of mortality of Australian adults. Eur J Clin Nutr 2010;64:569–77. [DOI] [PubMed] [Google Scholar]
- 25.Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Dairy foods and risk of stroke. Epidemiology 2009;20:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson E, Larsson SC, Wolk A, Akesson A. Association between dairy food consumption and risk of myocardial infarction in women differs by type of dairy food. J Nutr 2013;143:74–9. [DOI] [PubMed] [Google Scholar]
- 27.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr 2011;93:158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadrzadeh-Yeganeh H, Elmadfa I, Djazayery A, Jalali M, Heshmat R, Chamary M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br J Nutr 2010;103:1778–83. [DOI] [PubMed] [Google Scholar]
- 29.Alexander DD, Bylsma LC, Vargas AJ, Cohen SS, Doucette A, Mohamed M, Irvin SR, Miller PE, Watson H, Fryzek JP. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr 2016;115:737–50. [DOI] [PubMed] [Google Scholar]
- 30.Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 2017;32:269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L, Sun D. Consumption of yogurt and the incident risk of cardiovascular disease: a meta-analysis of nine cohort studies. Nutrients 2017;9:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panahi S, Fernandez MA, Marette A, Tremblay A. Yogurt, diet quality and lifestyle factors. Eur J Clin Nutr 2017;71:573–9. [DOI] [PubMed] [Google Scholar]
- 33.Tremblay A, Panahi S. Yogurt consumption as a signature of a healthy diet and lifestyle. J Nutr 2017;147:1476S–80S. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y, Wang H, Hollis JH, Jacques PF. The associations between yogurt consumption, diet quality, and metabolic profiles in children in the USA. Eur J Nutr 2015;54:543–50. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Gonzalez MA, Sayon-Orea C, Ruiz-Canela M, de la Fuente C, Gea A, Bes-Rastrollo M. Yogurt consumption, weight change and risk of overweight/obesity: the SUN cohort study. Nutr Metab Cardiovasc Dis 2014;24:1189–96. [DOI] [PubMed] [Google Scholar]
- 36.Jacques PF, Wang H. Yogurt and weight management. Am J Clin Nutr 2014;99:1229S–34S. [DOI] [PubMed] [Google Scholar]
- 37.van Meijl LE, Mensink RP. Low-fat dairy consumption reduces systolic blood pressure, but does not improve other metabolic risk parameters in overweight and obese subjects. Nutr Metab Cardiovasc Dis 2011;21:355–61. [DOI] [PubMed] [Google Scholar]
- 38.Azadbakht L, Haghighatdoost F, Karimi G, Esmaillzadeh A. Effect of consuming salad and yogurt as preload on body weight management and cardiovascular risk factors: a randomized clinical trial. Int J Food Sci Nutr 2013;64:392–9. [DOI] [PubMed] [Google Scholar]
- 39.Shlisky JD, Durward CM, Zack MK, Gugger CK, Campbell JK, Nickols-Richardson SM. An energy-reduced dietary pattern, including moderate protein and increased nonfat dairy intake combined with walking promotes beneficial body composition and metabolic changes in women with excess adiposity: a randomized comparative trial. Food Sci Nutr 2015;3:376–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veiga P, Pons N, Agrawal A, Oozeer R, Guyonnet D, Brazeilles R, Faurie JM, van Hylckama Vlieg JE, Houghton LA, Whorwell PJ, et al. Changes of the human gut microbiome induced by a fermented milk product. Sci Rep 2014;4:6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V, Akbarian-Moghari A. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci 2011;94:3288–94. [DOI] [PubMed] [Google Scholar]
- 42.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 2016;133:187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 2006;100:1171–85. [DOI] [PubMed] [Google Scholar]
- 44.Norat T, Riboli E. Dairy products and colorectal cancer. A review of possible mechanisms and epidemiological evidence. Eur J Clin Nutr 2003;57:1–17. [DOI] [PubMed] [Google Scholar]
- 45.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 2009;15:1546–58. [DOI] [PubMed] [Google Scholar]
- 46.Tapsell LC. Fermented dairy food and CVD risk. Br J Nutr 2015;113(Suppl 2):S131–5. [DOI] [PubMed] [Google Scholar]
- 47.Smith JD, Hou T, Ludwig DS, Rimm EB, Willett W, Hu FB, Mozaffarian D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am J Clin Nutr 2015;101:1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Troy L, Rogers G, Fox C, McKeown N, Meigs J, Jacques P. Longitudinal association between dairy consumption and changes of body weight and waist circumference: the Framingham Heart Study. Int J Obes (Lond) 2014;38:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, et al. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One 2013;8:e68596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park D-Y, Ahn Y-T, Park S-H, Huh C-S, Yoo S-R, Yu R, Sung M-K, McGregor RA, Choi M-S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One 2013;8:e59470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Power SE, O’Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr 2014;111:387–402. [DOI] [PubMed] [Google Scholar]
- 52.Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr 2013;4:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi HJ, Yu J, Choi H, An JH, Kim SW, Park KS, Jang HC, Kim SY, Shin CS. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care 2011;34:e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melanson EL, Sharp TA, Schneider J, Donahoo WT, Grunwald GK, Hill JO. Relation between calcium intake and fat oxidation in adult humans. Int J Obes Relat Metab Disord 2003;27:196–203. [DOI] [PubMed] [Google Scholar]
- 55.Zemel MB, Teegarden D, Van Loan M, Schoeller DA, Matkovic V, Lyle RM, Craig BA. Dairy-rich diets augment fat loss on an energy-restricted diet: a multicenter trial. Nutrients 2009;1:83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zemel MB. Role of dietary calcium and dairy products in modulating adiposity. Lipids 2003;38:139–46. [DOI] [PubMed] [Google Scholar]
- 57.Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr 2014;54:175–89. [DOI] [PubMed] [Google Scholar]
- 58.Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect 2014;44:1–8. [DOI] [PubMed] [Google Scholar]
- 59.DiBaise JK, Frank DN, Mathur R. Impact of the gut microbiota on the development of obesity: current concepts. Am J Gastroenterol Suppl 2012;1:22–7. [Google Scholar]
- 60.Pei R, Martin DA, DiMarco DM, Bolling BW. Evidence for the effects of yogurt on gut health and obesity. Crit Rev Food Sci Nutr 2017;57:1569–83. [DOI] [PubMed] [Google Scholar]
- 61.Pfeuffer M, Schrezenmeir J. Milk and the metabolic syndrome. Obes Rev 2007;8:109–18. [DOI] [PubMed] [Google Scholar]
- 62.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr 2005; 24(Suppl):537S–46S. [DOI] [PubMed] [Google Scholar]
- 63.Zemel MB, Thompson W, Milstead A, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res 2004;12:582–90. [DOI] [PubMed] [Google Scholar]
- 64.Kallus SJ, Brandt LJ. The intestinal microbiota and obesity. J Clin Gastroenterol 2012;46:16–24. [DOI] [PubMed] [Google Scholar]
- 65.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 66.Tsuchiya A, Almiron-Roig E, Lluch A, Guyonnet D, Drewnowski A. Higher satiety ratings following yogurt consumption relative to fruit drink or dairy fruit drink. J Am Diet Assoc 2006;106:550–7. [DOI] [PubMed] [Google Scholar]
- 67.Chapelot D, Payen F. Comparison of the effects of a liquid yogurt and chocolate bars on satiety: a multidimensional approach. Br J Nutr 2010;103:760–7. [DOI] [PubMed] [Google Scholar]
- 68.Almiron-Roig E, Grathwohl D, Green H, Erkner A. Impact of some isoenergetic snacks on satiety and next meal intake in healthy adults. J Hum Nutr Diet 2009;22:469–74. [DOI] [PubMed] [Google Scholar]
- 69.Salas-Salvadó J, Guasch-Ferré M, Diaz-López A, Babio N. Yogurt and diabetes: overview of recent observational studies. J Nutr 2017;147:1452S–61S. [DOI] [PubMed] [Google Scholar]
- 70.Rautiainen S, Wang L, Lee IM, Manson JE, Buring JE, Sesso HD. Dairy consumption in association with weight change and risk of becoming overweight or obese in middle-aged and older women: a prospective cohort study. Am J Clin Nutr 2016;103:979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crichton GE, Alkerwi A. Whole-fat dairy food intake is inversely associated with obesity prevalence: findings from the Observation of Cardiovascular Risk Factors in Luxembourg study. Nutr Res 2014;34:936–43. [DOI] [PubMed] [Google Scholar]
- 72.Vergnaud AC, Peneau S, Chat-Yung S, Kesse E, Czernichow S, Galan P, Hercberg S, Bertrais S. Dairy consumption and 6-y changes in body weight and waist circumference in middle-aged French adults. Am J Clin Nutr 2008;88:1248–55. [DOI] [PubMed] [Google Scholar]
- 73.Samara A, Herbeth B, Ndiaye NC, Fumeron F, Billod S, Siest G, Visvikis-Siest S. Dairy product consumption, calcium intakes, and metabolic syndrome-related factors over 5 years in the STANISLAS study. Nutrition 2013;29:519–24. [DOI] [PubMed] [Google Scholar]
- 74.Murphy N, Norat T, Ferrari P, Jenab M, Bueno-de-Mesquita B, Skeie G, Olsen A, Tjonneland A, Dahm CC, Overvad K, et al. Consumption of dairy products and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS One 2013;8:e72715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;96:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr 2013;52:1–24. [DOI] [PubMed] [Google Scholar]
- 77.Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, Lejeune M, Luscombe-Marsh N, Westerterp-Plantenga M. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav 2008;94:300–7. [DOI] [PubMed] [Google Scholar]
- 78.Astrup A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: epidemiologic and experimental studies. Am J Clin Nutr 2014; 99(Suppl):1235S–42S. [DOI] [PubMed] [Google Scholar]
- 79.El-Abbadi NH, Dao MC, Meydani SN. Yogurt: role in healthy and active aging. Am J Clin Nutr 2014; 99(Suppl):1263S–70S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martini LA, Catania AS, Ferreira SR. Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr Rev 2010;68:341–54. [DOI] [PubMed] [Google Scholar]
- 81.Sluijs I, Forouhi NG, Beulens JW, van der Schouw YT, Agnoli C, Arriola L, Balkau B, Barricarte A, Boeing H, Bueno-de-Mesquita HB, et al. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct study. Am J Clin Nutr 2012;96:382–90. [DOI] [PubMed] [Google Scholar]
- 82.Ericson U, Hellstrand S, Brunkwall L, Schulz CA, Sonestedt E, Wallstrom P, Gullberg B, Wirfalt E, Orho-Melander M. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr 2015;101:1065–80. [DOI] [PubMed] [Google Scholar]
- 83.Nestel PJ, Mellett N, Pally S, Wong G, Barlow CK, Croft K, Mori TA, Meikle PJ. Effects of low-fat or full-fat fermented and non-fermented dairy foods on selected cardiovascular biomarkers in overweight adults. Br J Nutr 2013;110:2242–9. [DOI] [PubMed] [Google Scholar]
- 84.Caricilli AM, Saad MJA. The role of gut microbiota on insulin resistance. Nutrients 2013;5:829–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drehmer M, Pereira MA, Schmidt MI, Molina MDB, Alvim S, Lotufo PA, Duncan BB. Associations of dairy intake with glycemia and insulinemia, independent of obesity, in Brazilian adults: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Am J Clin Nutr 2015;101:775–82. [DOI] [PubMed] [Google Scholar]
- 86.Ruan Y, Sun J, He J, Chen F, Chen R, Chen H. Effect of probiotics on glycemic control: a systematic review and meta-analysis of randomized, controlled trials. PLoS One 2015;10:e0132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann Med 2015;47:430–40. [DOI] [PubMed] [Google Scholar]
- 88.Thorning TK, Bertram HC, Bonjour JP, de Groot L, Dupont D, Feeney E, Ipsen R, Lecerf JM, Mackie A, McKinley MC, et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr 2017;105:1033–45. [DOI] [PubMed] [Google Scholar]
- 89.Marette A, Picard-Deland E. Yogurt consumption and impact on health: focus on children and cardiometabolic risk. Am J Clin Nutr 2014;99:1243S–7S. [DOI] [PubMed] [Google Scholar]
- 90.Lecerf J-M, Legrand P. Les effets des nutriments dépendent-ilsdes aliments qui les portent ? L’effet matrice. Cahiers de nutrition et de diététique 2015;50:158–64. [Google Scholar]
- 91.Jacobs DR Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr 2009;89:1543S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mohammadi R, Sohrabvandi S, Mortazavian AM. The starter culture characteristics of probiotic microorganisms in fermented milks. Eng Life Sci 2012;12:399–409. [Google Scholar]
- 93.Lamothe S, Azimy N, Bazinet L, Couillard C, Britten M. Interaction of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment. Food Funct 2014;5:2621–31. [DOI] [PubMed] [Google Scholar]
- 94.Turgeon SL, Rioux L-E. Food matrix impact on macronutrients nutritional properties. Food Hydrocoll 2011;25:1915–24. [Google Scholar]
- 95.Barbé F, Ménard O, Le Gouar Y, Buffière C, Famelart M-H, Laroche B, Le Feunteun S, Dupont D, Rémond D. The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chem 2013;136:1203–12. [DOI] [PubMed] [Google Scholar]
- 96.Le Feunteun S, Barbe F, Remond D, Menard O, Le Gouar Y, Dupont D, Laroche B. Impact of the dairy matrix structure on milk protein digestion kinetics: mechanistic modelling based on mini-pig in vivo data. Food Bioprocess Technol 2014;7:1099–113. [Google Scholar]
- 97.Fernández M, Hudson JA, Korpela R, de los Reyes-Gavilán CG. Impact on human health of microorganisms present in fermented dairy products: an overview. Biomed Res Int 2015;2015:412714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Korhonen H. Milk-derived bioactive peptides: from science to applications. J Funct Foods 2009;1:177–87. [Google Scholar]
- 99.Frias J, Martinez-Villaluenga C, Penas E, editors. Fermented foods in health and disease prevention. London: Elsevier; 2016. [Google Scholar]
- 100.Jin Y, Yu Y, Qi Y, Wang F, Yan J, Zou H. Peptide profiling and the bioactivity character of yogurt in the simulated gastrointestinal digestion. J Proteomics 2016;141:24–46. [DOI] [PubMed] [Google Scholar]
- 101.Chen G-W, Tsai J-S, Sun Pan B. Purification of angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. Int Dairy J 2007;17:641–7. [Google Scholar]
- 102.Sabeena Farvin KH, Baron CP, Nielsen NS, Otte J, Jacobsen C. Antioxidant activity of yoghurt peptides: part 2 – characterisation of peptide fractions. Food Chem 2010;123:1090–7. [Google Scholar]
- 103.Plaisancié P, Boutrou R, Estienne M, Henry G, Jardin J, Paquet A, Léonil J. Beta-Casein(94-123)-derived peptides differently modulate production of mucins in intestinal goblet cells. J Dairy Res 2015;82:36–46. [DOI] [PubMed] [Google Scholar]
- 104.Plaisancié P, Claustre J, Estienne M, Henry G, Boutrou R, Paquet A, Léonil J. A novel bioactive peptide from yoghurts modulates expression of the gel-forming MUC2 mucin as well as population of goblet cells and Paneth cells along the small intestine. J Nutr Biochem 2013;24:213–21. [DOI] [PubMed] [Google Scholar]
- 105.Zingone F, Bucci C, Iovino P, Ciacci C. Consumption of milk and dairy products: facts and figures. Nutrition 2017;33:322–5. [DOI] [PubMed] [Google Scholar]
- 106.Shantha NC, Ram LN, O’Leary J, Hicks CL, Decker EA. Conjugated linoleic acid concentrations in dairy products as affected by processing and storage. J Food Sci 1995;60:695–8. [Google Scholar]
- 107.Aneja RP, Murthi TN. Conjugated linoleic acid contents of Indian curd and ghee. Indian J Dairy Sci 1990;43:231–8. [Google Scholar]
- 108.Oliveira RPS, Florence ACR, Silva RC, Perego P, Converti A, Gioielli LA, Oliveira MN. Effect of different prebiotics on the fermentation kinetics, probiotic survival and fatty acids profiles in nonfat symbiotic fermented milk. Int J Food Microbiol 2009;128:467–72. [DOI] [PubMed] [Google Scholar]
- 109.Sosa-Castañeda J, Hernández-Mendoza A, Astiazarán-García H, Garcia HS, Estrada-Montoya MC, Gonzalez-Córdova AF, Vallejo-Cordoba B. Screening of Lactobacillus strains for their ability to produce conjugated linoleic acid in milk and to adhere to the intestinal tract. J Dairy Sci 2015;98:6651–9. [DOI] [PubMed] [Google Scholar]
- 110.Allen LH. Calcium bioavailability and absorption: a review. Am J Clin Nutr 1982;35:783–808. [DOI] [PubMed] [Google Scholar]
- 111.Zivkovic AM, Barile D. Bovine milk as a source of functional oligosaccharides for improving human health. Adv Nutr 2011;2:284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lamoureux L, Roy D, Gauthier SF. Production of oligosaccharides in yogurt containing bifidobacteria and yogurt cultures. J Dairy Sci 2002;85:1058–69. [DOI] [PubMed] [Google Scholar]
- 113.Hill D, Sugrue I, Arendt E, Hill C, Stanton C, Ross RP. Recent advances in microbial fermentation for dairy and health. F1000Res 2017;6:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anhê FF, Varin TV, Le Barz M, Desjardins Y, Levy E, Roy D, Marette A. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr Obes Rep 2015;4:389–400. [DOI] [PubMed] [Google Scholar]
- 115.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell 2012;148:1258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shen J, Obin MS, Zhao LP. The gut microbiota, obesity and insulin resistance. Mol Aspects Med 2013;34:39–58. [DOI] [PubMed] [Google Scholar]
- 117.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol 2013;27:73–83. [DOI] [PubMed] [Google Scholar]
- 118.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 119.EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the substantiation of health claims related to live yogurt cultures and improved lactose digestion. EFSA J 2010;8:1763. [Google Scholar]
- 120.Canadian Food Inspection Agency. Probiotic claims [Internet]. 2016 [cited 2016 Aug 15]. Available from: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/health-claims/eng/1392834838383/1392834887794?chap=9#s21c9.
- 121.Rijkers GT, de Vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P. Health benefits and health claims of probiotics: bridging science and marketing. Br J Nutr 2011;106:1291–6. [DOI] [PubMed] [Google Scholar]
- 122.Azaïs-Braesco V, Bresson JL, Guarner F, Corthier G. Not all lactic acid bacteria are probiotics, ...but some are. Br J Nutr 2010;103:1079–81. [DOI] [PubMed] [Google Scholar]
- 123.Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 2002;109:678–84. [DOI] [PubMed] [Google Scholar]
- 124.Scourboutakos M, Franco-Arellano B, Murphy S, Norsen S, Comelli E, L’Abbé M. Mismatch between probiotic benefits in trials versus food products. Nutrients 2017;9:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012;28:539–43. [DOI] [PubMed] [Google Scholar]
- 126.Mohamadshahi M, Veissi M, Haidari F, Javid AZ, Mohammadi F, Shirbeigi E. Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: a randomized controlled clinical trial. J Res Med Sci 2014;19:531–6. [PMC free article] [PubMed] [Google Scholar]
- 127.Gibson PR, Muir JG. Reinforcing the mucus: a new therapeutic approach for ulcerative colitis? Gut 2005;54:900–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Derrien M, van Hylckama Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol 2015;23:354–66. [DOI] [PubMed] [Google Scholar]
- 129.Shah NP, editor. Yogurt in health and disease prevention. London: Elsevier; 2017. [Google Scholar]
- 130.Putt KK, Pei R, White HM, Bolling BW. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct 2017;8:406–14. [DOI] [PubMed] [Google Scholar]
- 131.Martini MC, Lerebours EC, Lin WJ, Harlander SK, Berrada NM, Antoine JM, Savaiano DA. Strains and species of lactic acid bacteria in fermented milks (yogurts): effect on in vivo lactose digestion. Am J Clin Nutr 1991;54:1041–6. [DOI] [PubMed] [Google Scholar]
- 132.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010;90:859–904. [DOI] [PubMed] [Google Scholar]
- 133.Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, Ngom-Bru C, Berger B, Philippe L, Ammon-Zuffrey C, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr 2014;111:1507–19. [DOI] [PubMed] [Google Scholar]
- 134.Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol 2012;7:91–109. [DOI] [PubMed] [Google Scholar]
- 135.Chandan RC, Kilara A, editors. Manufacturing yogurt and fermented milks. Hoboken (NJ): Wiley-Blackwell; 2013. [Google Scholar]
- 136.Panahi S, Tremblay A. The potential role of yogurt in weight management and prevention of type 2 diabetes. J Am Coll Nutr 2016;35:717–31. [DOI] [PubMed] [Google Scholar]
- 137.Dougkas A, Minihane AM, Givens DI, Reynolds CK, Yaqoob P. Differential effects of dairy snacks on appetite, but not overall energy intake. Br J Nutr 2012;108:2274–85. [DOI] [PubMed] [Google Scholar]
- 138.Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr 2010;91:966–75. [DOI] [PubMed] [Google Scholar]
- 139.Akhavan T, Luhovyy BL, Panahi S, Kubant R, Brown PH, Anderson GH. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J Nutr Biochem 2014;25:36–43. [DOI] [PubMed] [Google Scholar]
- 140.Anderson GH, Tecimer SN, Shah D, Zafar TA. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J Nutr 2004;134:3011–5. [DOI] [PubMed] [Google Scholar]
- 141.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 1997;94:14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chungchunlam SM, Henare SJ, Ganesh S, Moughan PJ. Dietary whey protein influences plasma satiety-related hormones and plasma amino acids in normal-weight adult women. Eur J Clin Nutr 2015;69:179–86. [DOI] [PubMed] [Google Scholar]
- 143.El Khoury D, Brown P, Smith G, Berengut S, Panahi S, Kubant R, Anderson GH. Increasing the protein to carbohydrate ratio in yogurts consumed as a snack reduces post-consumption glycemia independent of insulin. Clin Nutr 2014;33:29–38. [DOI] [PubMed] [Google Scholar]
- 144.Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr 2003;89:239–48. [DOI] [PubMed] [Google Scholar]
- 145.Nongonierma AB, FitzGerald RJ. Bioactive properties of milk proteins in humans: a review. Peptides 2015;73:20–34. [DOI] [PubMed] [Google Scholar]
- 146.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. A breakfast with alpha-lactalbumin, gelatin, or gelatin + TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clin Nutr 2009;28:147–55. [DOI] [PubMed] [Google Scholar]
- 147.Mortensen LS, Holmer-Jensen J, Hartvigsen ML, Jensen VK, Astrup A, de Vrese M, Holst JJ, Thomsen C, Hermansen K. Effects of different fractions of whey protein on postprandial lipid and hormone responses in type 2 diabetes. Eur J Clin Nutr 2012;66:799–805. [DOI] [PubMed] [Google Scholar]
- 148.Ma J, Jesudason DR, Stevens JE, Keogh JB, Jones KL, Clifton PM, Horowitz M, Rayner CK. Sustained effects of a protein ‘preload’ on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract 2015;108:e31–4. [DOI] [PubMed] [Google Scholar]
- 149.Nagpal R, Behare P, Rana R, Kumar A, Kumar M, Arora S, Morotta F, Jain S, Yadav H. Bioactive peptides derived from milk proteins and their health beneficial potentials: an update. Food Funct 2011;2:18–27. [DOI] [PubMed] [Google Scholar]
- 150.Rai AK, Sanjukta S, Jeyaram K. Production of angiotensin i converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit Rev Food Sci Nutr 2017;57:2789–800. [DOI] [PubMed] [Google Scholar]
- 151.Fekete ÁA, Givens DI, Lovegrove JA. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients 2015;7:659–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, Gross MD, Jacobs DR Jr. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 2005;82:1169–77; quiz 1363–4. [DOI] [PubMed] [Google Scholar]
- 153.Beydoun MA, Gary TL, Caballero BH, Lawrence RS, Cheskin LJ, Wang Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am J Clin Nutr 2008;87:1914–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Song JJ, Wang Q, Du M, Li TG, Chen B, Mao XY. Casein glycomacropeptide-derived peptide IPPKKNQDKTE ameliorates high glucose-induced insulin resistance in HepG2 cells via activation of AMPK signaling. Mol Nutr Food Res 2017;61: 1600301. [DOI] [PubMed] [Google Scholar]
- 155.Xu SP, Mao XY, Cheng X, Chen B. Ameliorating effects of casein glycomacropeptide on obesity induced by high-fat diet in male Sprague-Dawley rats. Food Chem Toxicol 2013;56:1–7. [DOI] [PubMed] [Google Scholar]
- 156.Keogh JB, Clifton P. The effect of meal replacements high in glycomacropeptide on weight loss and markers of cardiovascular disease risk. Am J Clin Nutr 2008;87:1602–5. [DOI] [PubMed] [Google Scholar]
- 157.Sawin EA, De Wolfe TJ, Aktas B, Stroup BM, Murali SG, Steele JL, Ney DM. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am J Physiol Gastrointest Liver Physiol 2015;309:G590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boylston TD, Beitz DC. Conjugated linoleic acid and fatty acid composition of yogurt produced from milk of cows fed soy oil and conjugated linoleic acid. J Food Sci 2002;67:1973–8. [Google Scholar]
- 159.Nagao K, Yanagita T. Medium-chain fatty acids: functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol Res 2010;61:208–12. [DOI] [PubMed] [Google Scholar]
- 160.Marten B, Pfeuffer M, Schrezenmeir J. Medium-chain triglycerides. Int Dairy J 2006;16:1374–82. [Google Scholar]
- 161.Lemarié F, Beauchamp E, Legrand P, Rioux V. Revisiting the metabolism and physiological functions of caprylic acid (C8:0) with special focus on ghrelin octanoylation. Biochimie 2016;120:40–8. [DOI] [PubMed] [Google Scholar]
- 162.Babayan VK. Medium chain triglycerides and structured lipids. Lipids 1987;22:417–20. [DOI] [PubMed] [Google Scholar]
- 163.Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 2016;57:943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bueno NB, de Melo IV, Florencio TT, Sawaya AL. Dietary medium-chain triacylglycerols versus long-chain triacylglycerols for body composition in adults: systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr 2015;34:175–83. [DOI] [PubMed] [Google Scholar]
- 165.Mumme K, Stonehouse W. Effects of medium-chain triglycerides on weight loss and body composition: a meta-analysis of randomized controlled trials. J Acad Nutr Diet 2015;115:249–63. [DOI] [PubMed] [Google Scholar]
- 166.Haug A, Hostmark AT, Harstad OM. Bovine milk in human nutrition–a review. Lipids Health Dis 2007;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- 168.Wein S, Wolffram S, Schrezenmeir J, Gasperikova D, Klimes I, Sebokova E. Medium-chain fatty acids ameliorate insulin resistance caused by high-fat diets in rats. Diabetes Metab Res Rev 2009;25:185–94. [DOI] [PubMed] [Google Scholar]
- 169.Takeuchi H, Noguchi O, Sekine S, Kobayashi A, Aoyama T. Lower weight gain and higher expression and blood levels of adiponectin in rats fed medium-chain tag compared with long-chain tag. Lipids 2006;41:207–12. [DOI] [PubMed] [Google Scholar]
- 170.Turner N, Hariharan K, TidAng J, Frangioudakis G, Beale SM, Wright LE, Zeng XY, Leslie SJ, Li JY, Kraegen EW, et al. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: potent tissue-specific effects of medium-chain fatty acids. Diabetes 2009;58:2547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Eckel RH, Hanson AS, Chen AY, Berman JN, Yost TJ, Brass EP. Dietary substitution of medium-chain triglycerides improves insulin-mediated glucose metabolism in NIDDM subjects. Diabetes 1992;41:641–7. [PubMed] [Google Scholar]
- 172.Han JR, Deng B, Sun J, Chen CG, Corkey BE, Kirkland JL, Ma J, Guo W. Effects of dietary medium-chain triglyceride on weight loss and insulin sensitivity in a group of moderately overweight free-living type 2 diabetic Chinese subjects. Metabolism 2007;56:985–91. [DOI] [PubMed] [Google Scholar]
- 173.Nagao K, Yanagita T. Bioactive lipids in metabolic syndrome. Prog Lipid Res 2008;47:127–46. [DOI] [PubMed] [Google Scholar]
- 174.Parodi PW. Cooperative action of bioactive components in milk fat with PPARs may explain its anti-diabetogenic properties. Med Hypotheses 2016;89:1–7. [DOI] [PubMed] [Google Scholar]
- 175.Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Lipids 1997;32:853–8. [DOI] [PubMed] [Google Scholar]
- 176.Sisk MB, Hausman DB, Martin RJ, Azain MJ. Dietary conjugated linoleic acid reduces adiposity in lean but not obese Zucker rats. J Nutr 2001;131:1668–74. [DOI] [PubMed] [Google Scholar]
- 177.Takahashi Y, Kushiro M, Shinohara K, Ide T. Dietary conjugated linoleic acid reduces body fat mass and affects gene expression of proteins regulating energy metabolism in mice. Comp Biochem Physiol B Biochem Mol Biol 2002;133:395–404. [DOI] [PubMed] [Google Scholar]
- 178.Ryder JW, Portocarrero CP, Song XM, Cui L, Yu M, Combatsiaris T, Galuska D, Bauman DE, Barbano DM, Charron MJ, et al. Isomer-specific antidiabetic properties of conjugated linoleic acid. Improved glucose tolerance, skeletal muscle insulin action, and UCP-2 gene expression. Diabetes 2001;50:1149–57. [DOI] [PubMed] [Google Scholar]
- 179.Houseknecht KL, Heuvel JPV, Moya-Camarena SY, Portocarrero CP, Peck LW, Nickel KP, Belury MA. Dietary conjugated linoleic acid normalizes impaired glucose tolerance in the Zucker diabetic fatty fa/fa rat. Biochem Biophys Res Commun 1998;244:678–82. [DOI] [PubMed] [Google Scholar]
- 180.Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr 2002;22:505–31. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y, Jacome-Sosa MM, Ruth MR, Lu Y, Shen J, Reaney MJ, Scott SL, Dugan ME, Anderson HD, Field CJ, et al. The intestinal bioavailability of vaccenic acid and activation of peroxisome proliferator-activated receptor-alpha and -gamma in a rodent model of dyslipidemia and the metabolic syndrome. Mol Nutr Food Res 2012;56:1234–46. [DOI] [PubMed] [Google Scholar]
- 182.Barberá MJ, Schlüter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor α activates transcription of the brown fat uncoupling protein-1 gene: a link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem 2001;276:1486–93. [DOI] [PubMed] [Google Scholar]
- 183.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng Y-H, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013;123:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim SH, Plutzky J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab J 2016;40:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009;58:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tremblay A, Gilbert JA. Milk products, insulin resistance syndrome and type 2 diabetes. J Am Coll Nutr 2009;28(Suppl 1):91S–102S. [DOI] [PubMed] [Google Scholar]
- 187.Zemel MB. Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertension. Mol Cell Biochem 1998;188:129–36. [PubMed] [Google Scholar]
- 188.Davies KM, Heaney RP, Recker RR, Lappe JM, Barger-Lux MJ, Rafferty K, Hinders S. Calcium intake and body weight. J Clin Endocrinol Metab 2000;85:4635–8. [DOI] [PubMed] [Google Scholar]
- 189.Pittas AG, Lau J, Hu F, Dawson-Hughes B. Review: the role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gedik O, Zileli MS. Effects of hypocalcemia and theophylline on glucose tolerance and insulin release in human beings. Diabetes 1977;26:813–9. [DOI] [PubMed] [Google Scholar]
- 191.Yasuda K, Hurukawa Y, Okuyama M, Kikuchi M, Yoshinaga K. Glucose tolerance and insulin secretion in patients with parathyroid disorders. Effect of serum calcium on insulin release. N Engl J Med 1975;292:501–4. [DOI] [PubMed] [Google Scholar]
- 192.Beaulieu C, Kestekian R, Havrankova J, Gascon-Barre M. Calcium is essential in normalizing intolerance to glucose that accompanies vitamin d depletion in vivo. Diabetes 1993;42:35–43. [DOI] [PubMed] [Google Scholar]
- 193.Hochberg Z, Borochowitz Z, Benderli A, Vardi P, Oren S, Spirer Z, Heyman I, Weisman Y. Does 1,25-dihydroxyvitamin D participate in the regulation of hormone release from endocrine glands? J Clin Endocrinol Metab 1985;60:57–61. [DOI] [PubMed] [Google Scholar]
- 194.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 2004;53:330–5. [DOI] [PubMed] [Google Scholar]
- 195.Draznin B, Sussman K, Kao M, Lewis D, Sherman N. The existence of an optimal range of cytosolic free calcium for insulin-stimulated glucose transport in rat adipocytes. J Biol Chem 1987;262:14385–8. [PubMed] [Google Scholar]
- 196.Ohno Y, Suzuki H, Yamakawa H, Nakamura M, Otsuka K, Saruta T. Impaired insulin sensitivity in young, lean normotensive offspring of essential hypertensives: possible role of disturbed calcium metabolism. J Hypertens 1993;11:421–6. [DOI] [PubMed] [Google Scholar]
- 197.Weaver C, Heaney RP, editors. Calcium in human health. Totowa (NJ): Humana Press; 2006. [Google Scholar]
- 198.Gopinath B, Flood VM, Burlutsky G, Louie JC, Baur LA, Mitchell P. Dairy food consumption, blood pressure and retinal microcirculation in adolescents. Nutr Metab Cardiovasc Dis 2014;24:1221–7. [DOI] [PubMed] [Google Scholar]
- 199.Wang H, Fox CS, Troy LM, McKeown NM, Jacques PF. Longitudinal association of dairy consumption with the changes in blood pressure and the risk of incident hypertension: the Framingham Heart Study. Br J Nutr 2015;114:1887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zemel MB. Calcium modulation of hypertension and obesity: mechanisms and implications. J Am Coll Nutr 2001;20(Suppl):428S–35S; discussion 40S–2S. [DOI] [PubMed] [Google Scholar]
- 201.Wang L, Manson JE, Sesso HD. Calcium intake and risk of cardiovascular disease a review of prospective studies and randomized clinical trials. Am J Cardiovasc Drugs 2012;12:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 203.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin P-H, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med 2010;170:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr 2002;21:146S–51S. [DOI] [PubMed] [Google Scholar]
- 205.Cormick G, Ciapponi A, Cafferata ML, Belizan JM. Calcium supplementation for prevention of primary hypertension. Cochrane Database Syst Rev 2015;(6):CD010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ 2003;27:201–6. [DOI] [PubMed] [Google Scholar]
- 207.Belizán JM, Villar J, Repke J. The relationship between calcium intake and pregnancy-induced hypertension: up-to-date evidence. Am J Obstet Gynecol 1988;158:898–902. [DOI] [PubMed] [Google Scholar]
- 208.Zhang YH. Neuronal nitric oxide synthase in hypertension - an update. Clin Hypertens 2016;22:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tremblay A, Gilbert JA. Human obesity: is insufficient calcium/dairy intake part of the problem? J Am Coll Nutr 2011; 30(Suppl 1):449S–53S. [DOI] [PubMed] [Google Scholar]
- 210.Major GC, Chaput JP, Ledoux M, St-Pierre S, Anderson GH, Zemel MB, Tremblay A. Recent developments in calcium-related obesity research. Obes Rev 2008;9:428–45. [DOI] [PubMed] [Google Scholar]
- 211.Melanson EL, Donahoo WT, Dong F, Ida T, Zemel MB. Effect of low- and high-calcium dairy-based diets on macronutrient oxidation in humans. Obes Res 2005;13:2102–12. [DOI] [PubMed] [Google Scholar]
- 212.Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, Tremblay A, Astrup A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev 2009;10:475–86. [DOI] [PubMed] [Google Scholar]
- 213.Hackam DG. Translating animal research into clinical benefit. BMJ 2007;334:163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tremblay F, Lavigne C, Jacques H, Marette A. Dietary cod protein restores insulin-induced activation of phosphatidylinositol 3-kinase/Akt and GLUT4 translocation to the T-tubules in skeletal muscle of high-fat-fed obese rats. Diabetes 2003;52:29–37. [DOI] [PubMed] [Google Scholar]
- 215.Johnson MS, Jumbo-Lucioni P, Watts AJ, Allison DB, Nagy TR. Effect of dairy supplementation on body composition and insulin resistance in mice. Nutrition 2007;23:836–43. [DOI] [PubMed] [Google Scholar]
- 216.Chang BJ, Park SU, Jang YS, Ko SH, Joo NM, Kim SI, Kim CH, Chang DK. Effect of functional yogurt NY-YP901 in improving the trait of metabolic syndrome. Eur J Clin Nutr 2011;65:1250–5. [DOI] [PubMed] [Google Scholar]
- 217.Agerholm-Larsen L, Bell ML, Grunwald GK, Astrup A. The effect of a probiotic milk product on plasma cholesterol: a meta-analysis of short-term intervention studies. Eur J Clin Nutr 2000;54:856–60. [DOI] [PubMed] [Google Scholar]
- 218.Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, Shariatzadeh N, Gharavi A, Heravifard S, Tayebinejad N, et al. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 2011;93:764–71. [DOI] [PubMed] [Google Scholar]
- 219.Adolfsson O, Meydani SN, Russell RM. Yogurt and gut function. Am J Clin Nutr 2004;80:245–56. [DOI] [PubMed] [Google Scholar]