Summary
There are two basic pathways for formation of calcium based kidney stones. Most idiopathic calcium oxalate (CaOx) stones are formed in association with sub-epithelial plaques of calcium phosphate (CaP), known as Randall’s plaques, on renal papillary surfaces. Crystal formation and retention within the terminal collecting ducts, the ducts of Bellini, leading to the formation of Randall’s plugs, is the other pathway. Both pathways require supersaturation leading to crystallization, regulated by various crystallization modulators produced in response to changing urinary conditions.
High supersaturation, as a result of a variety of genetic and environmental factors, leads to crystallization in the terminal collecting ducts, eventually plugging their openings into the renal pelvis. Stasis behind the plugs may lead to the formation of attached or unattached stones in the tubular lumen. Deposition of crystals on the plug surface facing the pelvic or tubular urine may result in stone formation on the Randall’s plugs.
Kidneys of idiopathic stone formers may be subjected to oxidative stress as a result of increased urinary excretion of calcium/oxalate/phosphate and/or decrease in the production of functional crystallization inhibitors or in relation to co-morbidities such as hypertension, atherosclerosis, or acute kidney injury. We have proposed that production of reactive oxygen species (ROS) causes dedifferentiation of epithelial/endothelial cells into osteoblast type cells and deposition of CaP in the basement membrane of renal tubules or vessels. Growth, aggregation and melding of CaP crystals leads to the formation of plaque which grows by further calcification of interstitial collagen and membranous vesicles. Plaque becomes exposed to pelvic urine once the covering papillary epithelium is breached. Surface layers of CaP are replaced by CaOx through direct transformation or demineralization of CaP and mineralization of CaOx. Alternatively, or in addition, CaOx crystals nucleate directly on the plaque surface. Stone growth may also depend upon supersaturation in the pelvic urine, triggering further nucleation, growth and aggregation.
Keywords: Calcium Oxalate, Nephrolithiasis, Randall’s Plugs, Randall’s Plaques, Kidney Stones
INTRODUCTION
The first urinary stones were found in Egyptian mummies from 4000–5000 BC. Treatments for stones are mentioned in ancient Egyptian medical writings from 1500 B.C. Between the 5th and the 3rd century B.C., physicians swore to refrain from participating in stone surgery, as the Hippocratic Oath states “I will not use the knife, not even on sufferers from stone, but will withdraw in favor of such men as are engaged in this work.” In medieval times, surgery for stones, was performed by barbers who were called lithotomists. For 500 years, lithotomists travelled around Europe with their “lithotomy tables” cutting for stones. In 1561 the first suprapubic lithotomy was performed by Pierre Franco, to remove a bladder stone.
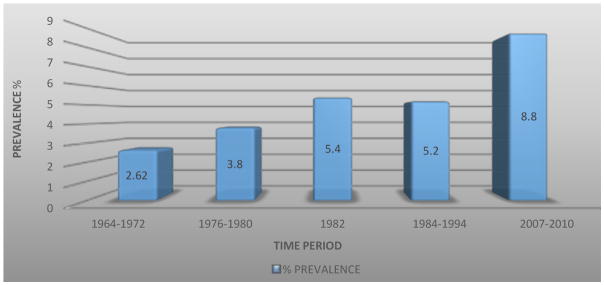
The National Health and Nutrition Examination Surveys (NHANES) which data has been helpful in the analysis of trends in kidney stone prevalence in the United States, demonstrated an increase in prevalence from 5.2% between 1988–1994 (1) to 8.8% between 2007–2010 (2). The Urologic Diseases of America project, funded by the NIDDK, estimated an annual cost of urinary stone disease to be $10 billion, in 2012, making this the most costly urologic condition (Figure 1).
Figure 1.
The prevalence of kidney stones has been steadily increasing in the last five decades. According to the last NHANES data analysis the unadjusted overall prevalence of kidney stones has increased to 8.8%, with 10.6% and 7.1% in men and women respectively (C. Scales et al, 2012).
Aims to comply with the Hippocratic Oath have driven modern day urologists to find minimally invasive ways to carry out such surgeries. In 1929, Young was the first to report the performance of ureteroscopy. Since then, techniques have been developed, to avoid open surgical procedures and need to “cut for stone”. An important goal for urologists and scientists is to elucidate the etiology of stone formation in an effort to prevent stone episodes and recurrence.
Kidney stones are found either free in the urine in the renal collecting system, terminal collecting ducts, or attached to the renal papillary surface or epithelial surface of the terminal collecting ducts (3–5). In 1937, Randall proposed that kidney stone formation requires “an initiating lesion that precedes the formation of a renal calculus” (6). He reported that 17% of the 429 pairs of kidneys examined at autopsy showed “deposition of calcium in the walls and intertubular spaces of the renal papilla.” He described these plaques as a consequence of “a natural reparative process to some form of tubular damage”. After dissection of 1154 pairs of kidneys, Randall found that 19.6% had evidence of a calcified lesion in at least one renal papilla and 65 kidneys had a primary renal stone attached to the papillary plaque (7, 8). He suggested that interstitial deposits of calcium phosphate (CaP) and calcium carbonate erupt on the renal papillary surface forming pre-calculus lesion type I (8), currently known as Randall’s plaques (9). He also suggested that excessive urinary supersaturation or “hypersecretion” results in crystallization of stone forming salts and plugging of the collecting duct, forming pre-calculus lesion type II (8), now called Randall’s plugs (10). Thus Randall has suggested two pathways for the formation of kidneys stones. One through changes in the renal parenchyma and formation of Randall ‘s plaques (RPL) and another requiring alteration in the urinary composition or supersaturation and development of Randall’s plugs (RPG).
Randall’s Plaque
Morphological Characteristics of Randall’s Plaques
Anatomical evidence for RPL has been demonstrated by various investigators including Stoller (9), Haggitt and Pitcock (11), Cooke (12,13) Evan (9,14) and Khan (4,10,15,16). Even though renal papillae of both the stone formers and non-stone formers have plaques, they are more common in kidneys from stone-forming patients (7). Haggitt and Pitcock (17), found alizarin red positive laminated spherules in the renal interstitium which on closer examination by TEM were found associated with collagen fibers not only in the interstitium, but also in the basement membrane of the collecting ducts. Interestingly, all RPLs do not support stone formation, as kidneys of non-stone formers also show the presence of calcified deposits in the renal interstitium (17). Cooke and associates (12,13), examined 62 normal kidneys. Four of them had interstitial calcifications located mostly in the basement membrane of the loops of Henle extending into the medullary interstitium as well as the surrounding collecting ducts and blood vessels. Stoller et al performed high resolution radiography of cadaveric kidneys (9) and found that 57% of the kidneys had sub-epithelial RPL which extended deep within the papillae associated with collecting ducts and vasa recta. Evan and associates analyzed renal papillae from a variety of stone patients (18). They concluded that idiopathic calcium stones develop attached to the sub-epithelial RPL (19), without any signs of cell injury, inflammation, interstitial fibrosis, and intratubular crystal deposition. They hypothesized that interstitial crystal deposits of idiopathic stone formers migrate from the basement membrane of the loops of Henle into the surrounding interstitium and become associated with type 1 collagen, fusing into a “syncytium in which islands of mineral appear to float in an organic sea” (18,20). In addition to CaP crystals, plaques also contain organic matrix positive for osteopontin (OPN) (21,22), heavy chain of inter-α-inhibitor (23,24) collagen (13,17,25) and zinc (26).
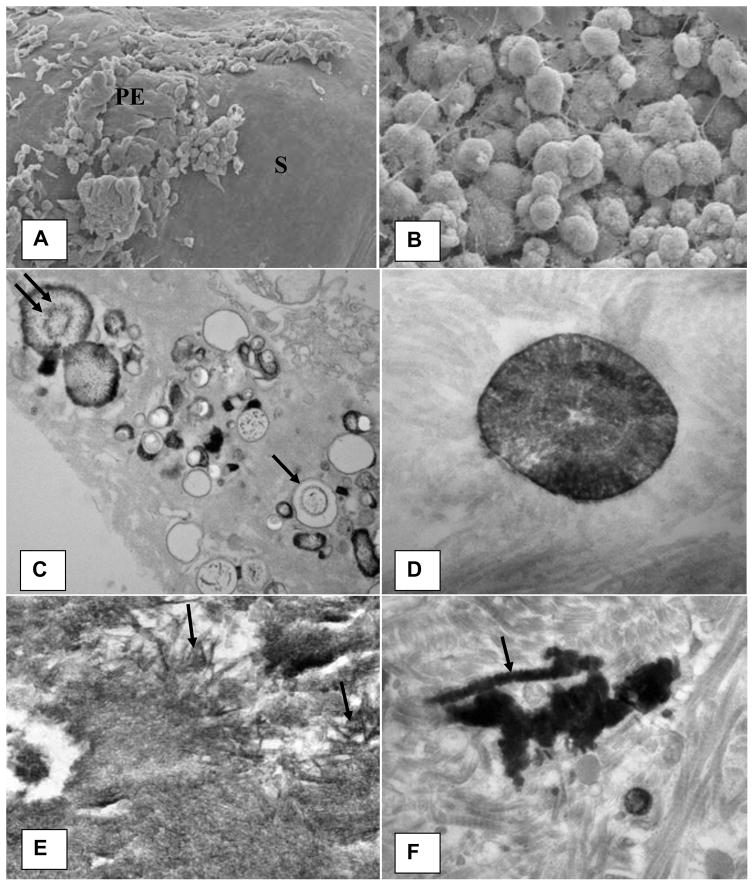
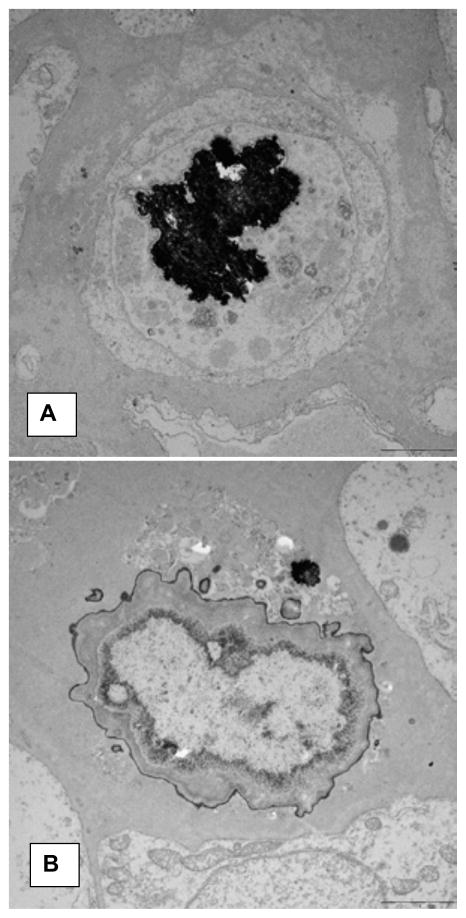
Khan et al. performed detailed scanning (SEM) and transmission electron microscopic (TEM) analysis of the RPL and renal papillary tissue at the stone attachment site (16). The plaque appeared as a small protuberance (Figure 2A). SEM analyses showed plaques to be aggregates of spherical CaP crystals mixed with fibers of varying thickness and other cellular products (Figure 2B).
Figure 2.
Microscopic appearance of the Randall’s plaque. A). Plaque appears as a bulge on the papillary surface. Epithelium (PE) is eroding exposing sub-epithelial amorphous layer (S). B). Spherical deposits of CaP mixed with fibrous material are present under the papillary surface epithelium. C). Calcification consisting of large and small vesicles (arrows), some with crystalline material inside (double arrow) are present embedded in an amorphous to granular to fibrous substrate. D). Concentrically laminated spherical unit surrounded by fibrous substrate. E). Calcification consisting of large dark deposits. Center of the deposit showing plate-like individual crystals (arrows). F). Calcification surrounded by what appear to be collagen fibers with distinct banding pattern (arrow). Arrow points to a calcified collagen fiber showing beaded exterior. Adapted from Khan and Canales, 2015, Urolithiasis 43 Supplement 1:109–123 (10).
There were two distinct types of interstitial CaP deposits which in addition to crystals also contained membrane bound vesicles, collagen and some unidentified fibrillar material (16). Needle shaped electron dense entities, most probably nucleating CaP crystals, were found in some of the vesicles (Figure 2C). Basement membrane of the renal tubules as well as surrounding interstitium was inhabited by 0.5 μm to 2 μm in diameter concentrically laminated spherical deposits (Figure 2D). The center of the calcification contained amorphous to needle or thin plate shaped crystals (Figure 2E). Other types of calcification, generally located in the interstitium and under the papillary epithelium, included dark and dense deposits of elongated strands mixed with aggregating spherulitic crystals. Some of the collagen fibers, particularly on the periphery, appeared calcified (Figure 2F). Energy dispersive x-ray microanalyses of the spherical crystals showed them to contain calcium and phosphorus, suggestive of their CaP nature. Electron diffraction analyses identified the crystals as hydroxyapatite and also revealed that the center of the calcification was more crystalline than the periphery (16).
Pathogenesis of Randall’s Plaques: Hypoxia, Reactive Oxygen Species and cell Injury
Randall’s plaque formation and its transformation into a stone nidus occurs in at least four distinct phases. First, there is the initial crystal deposition in the papillary interstitium in and around renal tubules and blood vessels, which is followed by the 2nd phase-growth and expansion. In the 3rd phase, epithelium covering the plaque is breached, exposing it to the pelvic urine. The 4th phase involves deposition of CaOx on the plaque, in which the outer layer of hydroxyapatite is replaced by CaOx or CaOx nucleated on the crystal associated organic matrix.
Most of the understanding of pathogenesis is based on the urinary characteristics of stone formers and morphological studies of human renal tissues. Even though results of such observational studies can only provide a snapshot at the end of a long process, they are useful in development of theories about stone pathogenesis and interpreting results of experimental studies performed to determine the mechanistic details. Randall’s plaque formation is associated with low urinary volume, low urinary pH and high urinary excretion of calcium (18,20,27). Calcium phosphate crystals either form directly in the basement membrane of the loops of Henle (28,29) or initially form in the supersaturated urine of the renal tubules and then transported into the basement membrane or interstitium (30).
Randall proposed that plaque formation involved damage to the epithelial lining of the collecting ducts and interstitial connective tissue. Calcium deposition appeared to primarily occur in the basement membrane of the damaged epithelium forming ring like structures around the tubules. He suggested that interstitial subepithelial deposits of calcium phosphate and calcium carbonate, arising from pathologic conditions of the renal papilla, erode through to the papillary surface forming the plaque (8). Vermooten also suggested that deposition of calcium in the papillary interstitium and basement membrane of the collecting ducts was associated with senescence (31).
Evan et al. however found no signs of cell injury, inflammation, or interstitial fibrosis (19,25,32). Khan et al however, showed calcification in association with damaged renal tubules and suggested that crystal deposition started within the membranous vesicles formed as a result of cellular injury (16). They further proposed that injury and inflammation play a significant role in the formation of RPL (33–35). Recent studies (36) of the plaque by Taguchi et al demonstrated upregulation (>2-fold) of LCN2 (lipocalin 2), IL11 (interleukin 11), PTGS1 (prostaglandin-endoperoxide synthase 1), GPX3 (glutathione peroxidase 3), and MMD (monocyte to macrophage differentiation associated) and downregulation (0.5-fold) of SLC12A1(solute carrier family 12 member 1) and NALCN (sodium leak channel, non-selective) genes in the plaque tissue. These genes are associated with activated mitogen-activated protein kinase, the Akt/phosphatidylinositol 3-kinase pathway, and proinflammatory cytokines involved in the development of oxidative stress and causing renal injury.
The scenario presented above is based upon renal tubular epithelium playing a critical role in the development of Randall’s plaques. Recent epidemiological studies, however, suggest that renal vascular system may also play a significant role in plaque development (33,37–39). Stoller et al showed that plaque is not limited to the basement membrane of the loops of Henle but reaches deep into the interstitium and basement membrane of the vasa recta (37). Thus plaque formation is somewhat similar to calcified plaques in the renal arteries and capillaries. There is evidence for the association between stone formation and aging and cardiovascular diseases, including hypertension, myocardial infarction, diabetes, chronic kidney disease, metabolic syndrome (38–40).
Idiopathic CaOx Stone formation is a risk factor for development of hypertension. Uric acid stone formers have higher prevalence of diabetes mellitus. Hypertensive and diabetic patients are at a greater risk for the formation of kidney stones. In addition, tissue hypoxia, production of reactive oxygen species and development of oxidative stress are characteristic features of vascular diseases with renal association (34,35,39). Results of a number of clinical and experimental investigations suggest that reactive oxygen species (ROS) may be involved in the pathogenesis of idiopathic stone disease (35,41,42). CaOx stone patients excrete significantly higher amounts of N-acetyl-β-glucoseaminidase (NAG), β-galactosidase, α-glutathione S-transferase (α-GST), malondialdehyde (MDA) and thiobarbituric acid-reactive substances (TBARS) (41), which are biomarkers of injury and production of ROS. Urinary 8-hydroxydeoxyguanosine(8-OHdG), a marker of oxidative damage of DNA, which is increased in stone patients and positively correlated with tubular damage as assessed by urinary excretion of NAG (43). Antioxidant deficit is common in recurrent idiopathic stone formers and was unrelated to the presence or absence of stones (44). It has been suggested that “renal disorders which lead to OS appear to be a continuum. Stress produced by one disorder may trigger the other under the right circumstances” (39).
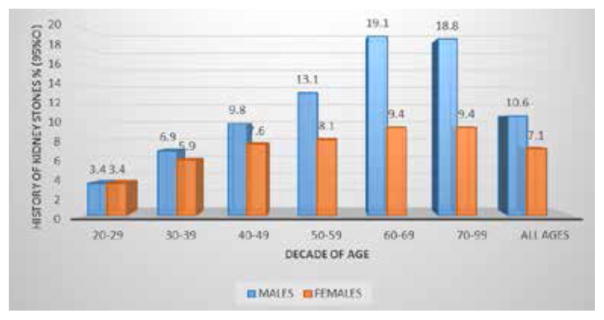
The association between aging and kidney stone risk and prevalence was observed by Scales et al, 2012 (Figure 3) (2). Prevalence, history of kidney stones and history of passing stones was more frequent at the sixth decade of age (95% CI). In addition, a multivariate regression model predicting history of kidney stones demonstrated an odds ratio of 2.18 for age over 60 y/o (95% CI). A possible proposed mechanism may be through glycation end products (AGEs).
Figure 3.
The history of having kidney stones increases with age according to NHANES data analysis on unadjusted percent prevalence of kidney stones by age and gender. These findings were more pronounced in males compared to females, 19.1% vs 9.4% respectively, in their sixth decade of age. (C. Scales et al. 2012)
AGE’s, are the product of non-enzymatic glycation and oxidation of nucleic acids, proteins and lipids. This process has been shown to be associated with aging and pathologic conditions such as hyperglycemia among others (45) (Figure 4).
Figure 4.
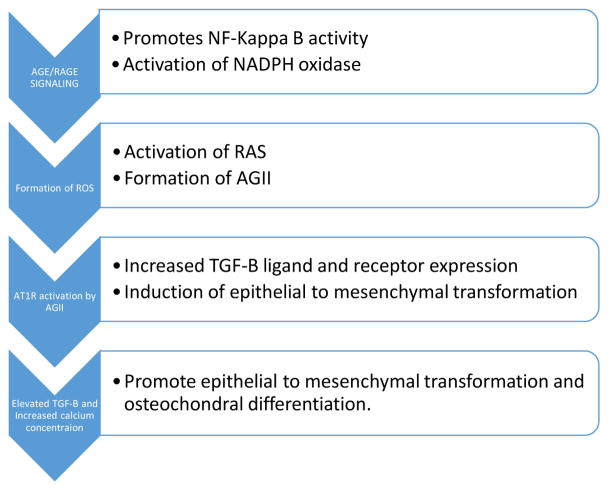
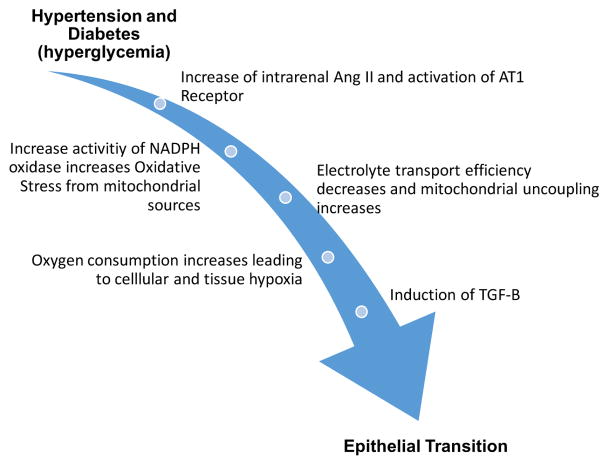
AGE/RAGE signaling pathway for the formation of NADPH oxidase mediated reactive oxygen species (ROS), angiotensin receptor activation and induction of TGF-B expression. TGF-B have been proposed to be a major regulator of the induction of epithelial to mesenchymal transformation (EMT) in the kidney (45). TGF-B and increased calcium concentration promote mesenchymal to osteochondral cell differentiation (59).
Recent findings demonstrate that accumulation of AGE products might be involved in vascular calcifications associated with diabetes (46). Diabetic cellular mechanisms of disease include glucose-induced excessive formation of reactive oxygen species, increased glucose flux through the polyol pathway, formation of advanced glycation end-products and oxidative stress (47), which result in decreased renal oxygenation and subsequent activation of hypoxia-inducible factors. Ultimately this chain of events leads to EMT. These pathways have been demonstrated as a chain reaction involving HIF1, TGF-B and increased expression of AQP1 channels (48). This hypoxic pathway has been demonstrated to lead to chronic kidney disease (49, 50).
The relationship between diabetes and vascular calcification are thought to involve reactive oxygen species which induce vascular smooth muscle cell (VSMC) transformation to osteogenic phenotype by regulating RUNX-2 transcription factor (51,52). Advanced glycation end-products in diabetic patients and older individuals can promote vascular calcification mediated by NADPH oxidase induced reactive oxygen species (53). Cytokines such as IL (interleukin)-1β, IL-6, IL-8, (TNF) tumor necrosis factor-α, TGF (transforming growth factor)-β produced by macrophages also induce transformation of VSMCs (54). Inflammatory cells also produce proteolytic enzymes such as metalloproteinases (MMP)-2 and -9 which degrade matrix and promote calcification (55–58). In the diabetic state, hypoxia mediated induction of profibrotic and proinflammatory molecules, such as TGF-B and TNF-alpha, induce proteinuria, tubulointerstitital fibrosis, infiltration of immune cells and EMT progressing to chronic kidney disease (48).
Renal cells which undergo EMT and specifically undergo osteoblastic differentiation, start the process of tissue calcification via specific cellular functional changes, such as loss of epithelial features and gaining osteogenic features similar to the changes seen in VSMC (59). Explanations of this specific phenomenon have included high extracellular calcium as a pivotal element in EMT to osteoblastic differentiation. This osteoblastic differentiation is believed to be the common precursor of vascular and kidney interstitial calcifications (Figure 5) (48). In addition, association of diabetes with higher incidence of uric acid stones is due to impaired kidney ammoniagenesis, alkalinization defects, and abnormal low pH in urine (60).
Figure 5.
Relationship between Diabetes (hyperglycemia) and Hypertension with Hypoxia. Hypertension mediated increased angiotensin receptor activity and oxidative stress and diabetic mediated mitochondrial dysfunction from excess tubular oxygen consumption lead to a common pathway of tissue hypoxia. The results will include induction of TGF-B among other factors. TGF-B among other factors. TGF-B will be responsible of epithelial to mesenchymal transformation (48).
In the hypertensive state, superoxide anion (O2-) levels are increased 10 fold, leading to IMI supersaturation by significantly enhanced NaCl reabsorption in the ATLH in animal models. In addition, increased levels of superoxide lead to increased production of hydroxyl radicals, which in turn leads to oxidative damage to DNA, proteins, and lipids (61). In a similar study in hypertensive rat kidneys, it was demonstrated that precapillary oxygen shunting reduced the pO2 of cortical nephrons. The pO2 was reduced further in spontaneously hypertensive rats because of less efficient oxygen usage for Na+ transport (62). HTN induced hypoxia is associated with solute supersaturation via the pathways described above. RAS have been implicated as the hypoxic mediated renal insult in hypertension. Explanation of RAS involvement is related to the increased angiotensin receptor, oxidative stress, and precapillary oxygen shunting leading to renal hypoxia. The high renal oxygen demand is due to tubular oxygen consumption necessary for solute reabsorption. The combination of hypertension, angiotensin II, and oxidative stress initiates events leading to renal damage and reduced function. There is a greater than a 10 fold increase in osmolality and decrease in oxygen-carrying capacity between renal cortex and papillary tip, which is believed to be the consequence of the laminar microvascular blood flow changes to turbulent flow at the tip of the inner papilla (51). Tissue hypoxia is now recognized as a unifying pathway to chronic kidney disease.
Obesity has been linked to intracellular hypoxia and production of reactive oxygen species via fat metabolism. It has been demonstrated that hypoxia is involved with dysregulated production of adipocytokines. Adipocytokines have been identified to be partly responsible for obesity-linked metabolic disorders. Studies demonstrate that adiponectin mRNA is downregulated during hypoxia (63). Adiponectin is an adipocytokine. It is associated with prevention of inflammation, atherosclerosis, and improved lipid and glucose metabolism. The connection between vascular inflammation and atherosclerotic plaque formation and adiponectin is via increased expression of tissue inhibitors of metalloproteinases (TIMPs) in human macrophages through IL-10 induction. Adiponectin then prevents tissue degradation in vasculature by matrix metalloproteinases (MMP’s) thus preventing rupture of atherosclerotic plaque and possible acute thrombotic episodes (64). It may be that in nephrolithiasis, inner medullary interstitial hypoxia, in the setting of obesity, may contribute to tissue disruption and Randall’s plaque ulceration via this mechanism (Figures 4, 5).
Deposition of CaP in the renal medullary interstitium i.e. the formation of Randall’s plaques has also been reported in a variety of animal models (65). However, none have shown a CaOx stone growing on a subepithelial plaque, even after experimental induction of hyperoxaluria (66). Nonetheless, such models provide interesting information on the role of various elements involved in the initial stages of plaque formation. NHERF-1(sodium-hydrogen exchanger regulatory factor-1) and THP (Tamm Horsfall Protein) null mice produce interstitial apatite plaques in the renal papillae, similar to that of human plaque (67). THP null mice also produce both intratubular and interstitial CaP deposits (Figure 6).
Figure 6.
Transmission electron microscopic analyses of the renal papilla of THP null mice. A). Calcified deposit inside a loop of Henl3. B). Calcification in the renal interstitium. Copious amount of organic material is mixed with dark staining CaP crystals.
NHERF null mice are hypercalciuric and hyperphosphaturic and produce small interstitial CaP deposits associated with both the loops of Henle and inner collecting ducts. A few smaller deposits are also seen in the basement membrane of their loops of Henle. 88% of the 15 month old THP-null mice produce numerous large deposits in the renal papillary interstitium. The deposits consisting of HA, surround both the thin loops of Henle as well as inner medullary collecting ducts without involving their basement membrane. The mice are hypercalciuric and hypocitraturic. Their urine is supersaturated with respect to brushite (68). OPN null mice also produce interstitial CaP deposits in the renal papillae without an increase urinary excretion of calcium, phosphorus (69). Urine of the OPN null mice is also not supersaturated with respect to brushite. Interestingly both THP and OPN are inhibitors of mineralization (70) are anti-inflammatory and have been identified in the renal interstitium (22,71). Targeted ablation of ABCC6 gene, responsible for generalized arterial calcification and ENPP1 gene which encodes for ectonucleotide pyro-phosphatase/phosphodiesterase 1 in mice leads to late onset ectopic mineralization mainly manifested in skin, eyes and cardiovascular tissues. When placed on an accelerated diet of high phosphorus and low magnesium these mice develop extensive mineralization in the renal interstitium mainly restricted to renal medullary tubules and arcuate and renal arteries (72). Magnesium is a well-known inhibitor of mineralization (73). Mineral deposits consisted of CaP and stained positive for both OPN and fetuin. The production of both OPN, THP, fetuin and other macromolecular mineralization inhibitors is regulated by reactive oxygen species (74).
Pathogenesis of plaque formation is considered mostly a physicochemical process (75). We (76) as well as some others (77) have proposed that Randall’s plaque formation is similar to vascular calcification in the kidneys in which vascular smooth cells (VSMC) acquire osteogenic phenotype (78–80), when exposed to higher concentrations of calcium and phosphate (81–84). Expression of osteoblast specific genes is increased and smooth muscle cell specific genes are decreased (85,86).
Bone morphogenetic proteins, BMP 2 and BMP 4, and Wnt signaling pathways are activated through up-regulation of transcription factor, Runt-related transcription factor 2 (RUNX2). Reactive oxygen species are likely involved in the VSMC transformation to osteogenic phenotype by regulating RUNX-2 transcription factor (51,52). It is our hypothesis that oxidative stress caused by abnormal urinary environment such as hyperoxaluria, hypercalciuria and hypocitraturia and formation of crystals, produce specific changes in the renal epithelial cells (4).
Tubular cells lose their epithelial character and become osteogenic. We tested our hypothesis in a rat model of hyperoxaluria by performing genome wide analysis of differentially expressed genes in the kidneys and determined changes consistent with the dedifferentiation of epithelial cells into bone producing cells. Genes for runt related transcription factors (RUNX1 and 2), zinc finger protein Osterix, bone morphogenetic proteins (BMP2 and 7), bone morphogenetic protein receptor (BMPR2), collagen, osteocalcin, osteonectin, osteopontin (OPN), matrix-gla-protein (MGP), osteoprotegrin (OPG), cadherins, fibronectin (FN) and vimentin (VIM) were up regulated while those for alkaline phosphatase (ALP) and cytokeratins 10 and 18 were down regulated (87). In an animal model of CaP nephrolithiasis, the genetic hypercalciuric rat, basal levels of BMP2, RUNX2, and Osterix are increased in the kidneys with intratubular calcium phosphate deposits (88). Knockdown of the vitamin D receptor in the rats reduced the expression levels of BMP2, RUNX2, and Osterix as well as CaP crystal deposition in the kidneys.
Support for the transformation of renal epithelial cells into osteogenic phenotype comes from many cell culture studies. Exposure to CaOx crystals increased vimantin expression in normal rat kidney epithelial cells in (89). The inner renal medullary collecting duct epithelial cells of rats grown in calcifying media produced calcifying nodules positive for bone morphogenetic proteins (90). When MDCK cells were grown in monolayers on a collagen gel (91,92), small blisters/domes/nodules developed after 30 days. The nodules contained aggregated spherulites of needle shaped CaP crystals. MDCK cells grown in agr produced spherical colonies enclosing CaP crystals (92,93).
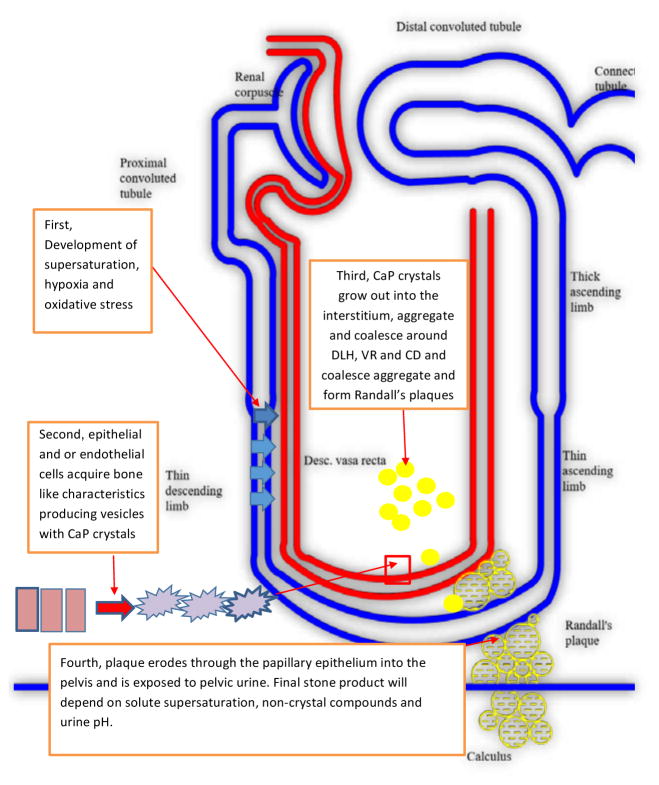
Thus plaque and associated idiopathic CaOx stone formation involves many steps (Figure 7). It starts mainly with the development of supersaturation due to hyperoxaluria, hypercalciuria and hypocitraturia. There may or may not be the formation of and retention of intratubular CaOx crystals. There is a transformation of epithelium/endothelium to osteogenic phenotype. CaP phosphate crystals are deposited on the basal side. Any CaP formed in the tubular lumen is transported into the basement membrane/interstitium through phagocytosis or through a loss of tubular epithelium. Interstitial CaP crystals aggregate, coalesce forming a calcification front. Collagen becomes involved and is calcified. The front keeps growing eventually reaching the papillary epithelium which is breached through the actions of metalloproteinases. Once epithelium is eroded, CaP is exposed to the pelvic/calyceal urine. Pelvic urine is metastable with respect to CaOx, comparatively slow moving and at places stagnant, conditions promoting the nucleation of CaOx on the exposed plaque surface. By addition of more crystals a stone is formed attached to the Randall’s plaque.
Figure 7.
Schematic presentation of various steps involved in the formation of Randall’s plaque and development of kidney stones. Supersaturation of intratubular solute including hypercalciuria promote oxidative stress in tubular cells and epithelial to mesenchymal differentiation; cells acquire osteogenic like features and produce CaP crystals. Crystals extrude out of cells, coalesce and aggregate to form Randall’s plaques at the inner medullary interstitium. Growth of plaque induces erosion through papillary epithelium into urinary lumen. Solute supersaturation, non-crystal compounds and pH of urine in will determine final stone composition.
Randall’s Plugs
Morphological Characteristics of Randall’s Plugs
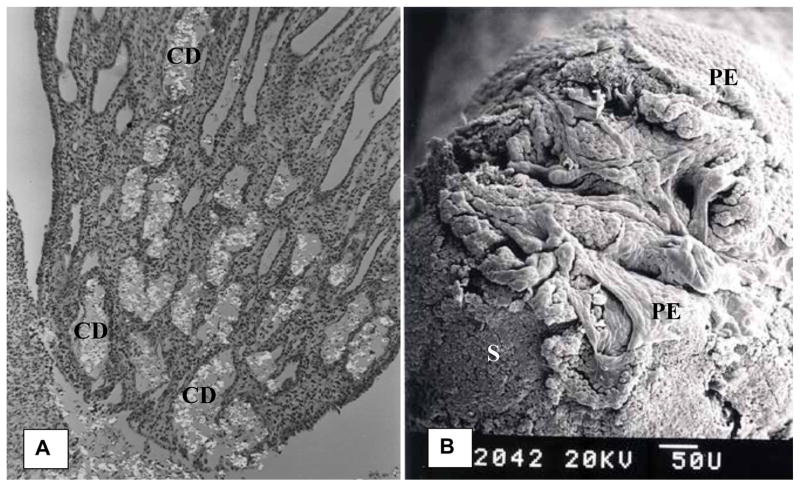
Randall’s plugs are crystalline deposits clogging the terminal collecting ducts, dusts of Bellini and their openings into the renal pelvis (Figure 8). Cystine, brushite, CaP stones in primary hyperparathyroidism, and CaOx stones of primary hyperoxaluria and post-bariatric surgery have all been found attached to Randall’s plugs (15,18,94).
Figure 8.
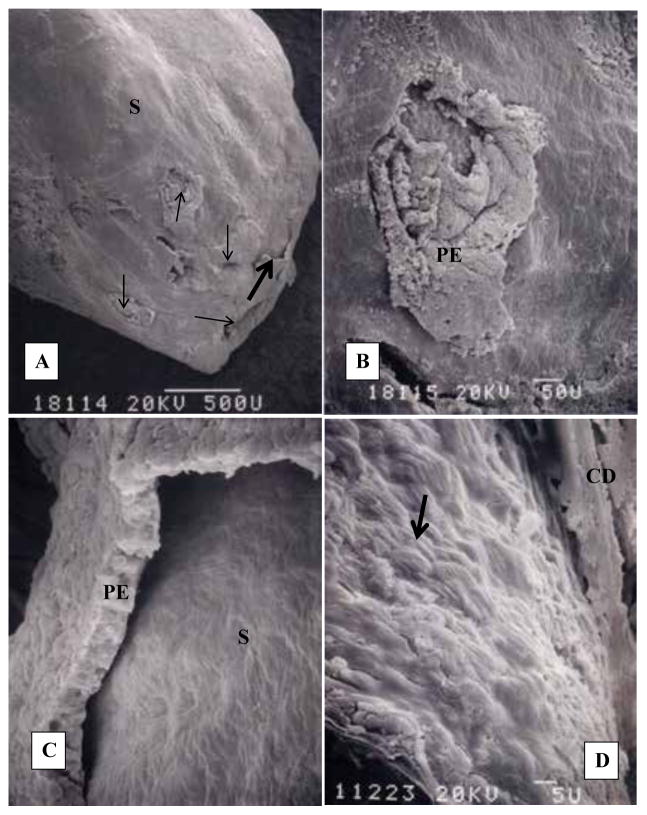
Human renal papilla with Randall’s plug. A). CaOx stone (S) surface is exposed with the loss of epithelium. Thin arrows point out the openings of the ducts of Bellini. B). Highly magnified area showing one of the ductal openings. Part of the surface epithelium (PE) is still intact. C). Highly magnified area of A. pointed out by a single thick arrow. Surface epithelium (PE) is pulling away from the underlying stone (S). D). Stone surface showing tips of monoclinic plate like crystals of CaOx monohydrate. Adapted from Khan and Canales, 2015, Urolithiasis 43 Supplement 1:109–123 (10).
A recent study (94) has provided newer evidence that even some idiopathic CaOx stones may develop on Randall’s plugs formed as a result of intratubular deposits of CaP. It should however be noted that no one has yet reported an idiopathic stone growing over a Randall’s plug. The plugs are generally exposed to two different urinary environments, one is bathed in the pelvic urine and another in tubular. As a result, the two sides of the plugs may have different crystalline composition.
We performed morphological examination using light and scanning and transmission electron microscopy of Randall’s plugs, consisting of CaOx crystals, removed from the terminal collecting ducts of a patient with primary hyperoxaluria with bilateral renal calculi (15). Stone composition was determined by x-ray diffraction and energy dispersive x-ray microanalysis. X-rays revealed bilateral staghorn calculi. All stones consisted of CaOx mixed with small amount of apatite. A large stone measuring 1.7×1.5×0.8 cm, was found completely lodged inside the renal papilla. X-ray diffraction analyses showed that stone comprised of 29% CaOx monohydrate, 62% CaOx dihydrate, 4% hydroxyapatite and 5% organic material.
Papillary tissue showed dilated inner medullary collecting ducts and ducts of Bellini, interstitial fibrosis, focal tubular epithelial hyperplasia, interstitial calcification and intratubular deposits of birefringent CaOx crystals/small stones. Transmission electron microscopy showed an enlarged interstitium, with myofibroblasts, abundant collagen, fibrillar material as well as electron dense deposits of CaP. Cellular degradation products including membrane bound vesicles concentrically laminated spherulites with needle shaped apatite crystals were associated with the large interstitial CaP calcification. Basement membrane of the tubular epithelium showed thickening and layering. Some tubular epithelial cells appeared necrotic with swollen mitochondria and vesiculating endoplasmic reticulum. At places tubular epithelium lifted from the basement membrane while at other sites epithelial cells ruptured releasing their contents into the tubular lumen. Thus the kidneys of this patient with primary hyperoxaluria has Randall’s plug of CaOx within the terminal collecting duct and Randalls’ plaque in the papillary interstitium.
Randall’s plugs of the brushite stone formers which are mostly brushite, also cause serious damage to the surrounding papillary tissue. There are dilated tubules, with localized loss of epithelium, interstitial fibrosis and scattered interstitial Cap deposition or plaque formation (95).
Pathogenesis of Randall’s Plugs
Randall proposed that plugs or as he called them, Type II lesions, are formed as a result of excessive urinary supersaturation and necrosis of the tubular epithelium. The presence of plugs in patients who excrete highly supersaturated urine supports Randall’s position. Cystinurics develop cystine stones over cystine plugs (96). Patients with primary hyperoxaluria develop CaOx stones in addition to CaOx plugs (15,18). In cases where urine is supersaturated with respect to more than one salt, plugs as well as stones contain more than one type of mineral (18). The plugs form, in part, as a result of higher supersaturation with respect to the precipitating salt (6,7,17) and are generally associated with renal tubular injury and focal inflammation as indicated by Randall (7).
Use of excessive amounts of medications such as magnesium trisilicate, ciprofloxacin, sulfa medications, triamterene, indinavir etc. can lead to the formation of kidney stones (97), most likely through plugging of the terminal collecting ducts. Poorly soluble dietary components can also crystallize in the renal tubules and produce kidney stones. Melamine in the baby formulas led to the formation of kidney stones in thousands of infants (98).
Results of animal model studies also support the contention that supersaturation is the driving force for the formation of stones associated with plug formation. Experimental induction of mild hyperoxaluria with increased urinary CaOx supersaturation leads to the deposition of CaOx crystals in the collecting ducts of rat kidneys (Figure 9) (99). Similarly urolithiasis induced by the administration of oxamide in diet leads to urinary excretion of oxamide crystals and their deposition in the terminal collecting ducts of the rat kidneys (100–102).
Figure 9.
Papillary tip of a rat with hyperoxaluria. A). Light microscopic appearance of a longitudinal section showing birefringent CaOx monohydrate crystals plugging the collecting ducts (CD). B). Scanning microscopy of the papillary tip. Surface epithelium (PE) is eroding exposing CaOx deposit (S) plugging the ducts of Bellini. Openings of the ducts appear impaired.
Plugging involves not only crystal formation and growth, which can be explained through urinary supersaturation, but also crystal retention within a tubule with flowing urine. It should therefore depend not only on supersaturation but also tubular dimension, crystal growth rates, diurnal variations in urinary flow, as well crystal aggregation. The inner diameter of the inner medullary collecting ducts ranges between 35–60μm and of the ducts of Bellini 60 to 100μm (3). Single crystals are too small to be able to occlude the tubules (5) and may require attachment to the tubular wall (103,104) followed by growth and/or aggregation with other crystals (3). Alternatively, crystals formed in the glomerular filtrate can become large enough to be trapped in the collecting ducts, particularly those at the end of the long loops of Henle. Aggregation can increase the size in a short span of time (105) and stone formers have been reported to excrete large crystal aggregates (106).
It has been suggested that in the collecting ducts urine flows in discreet boluses propelled by the peristaltic waves (107). Such a movement would result in significant turbulence increasing the possibility of crystal contact and aggregation (108). Plug formation may also be assisted by the fact that luminal diameter the ducts is much larger than the opening of the ducts of Bellini, which are narrow and slit-like (104). Urine may flow at different velocities through the tubule (109), slower near the at the epithelial surface. As a result, crystals would travel at different speeds, faster in the middle of the urinary stream and relatively slower at the tubular epithelium. The crystal moving near the epithelium, would not only have a chance to grow bigger compared to the fast moving ones but also have increased contact time with the epithelial cells. Renal tubules also drain upward, which would affect the crystal movement. Taking into account these hydrostatic factors, Robertson concluded that crystals forming at the end of the descending limb of the loops of Henle and travelling close to the tubular epithelium can become large enough to be trapped in the tubular lumen (110).
Ductal plugging is often associated with small concretions in the inner medullary collecting ducts as well as renal pelvis and calyx (15,27). Such stones have been seen in patient with hyperoxaluria (15) as well as patients with uric acid and cystine stones (27). Urine behind the ductal plugs should be stagnant and may promote crystal formation and aggregation because of high supersaturation which normally exists in these patients. Similarly, pelvic urine around the plugged papillae may also be stagnant and promote stone formation through crystallization in a supersaturated urine.
Thus plugs as well as other free stones associated with plugs may from freely in the urine through crystal formation, growth and aggregation. Animal model and tissue culture studies have shown that crystals are injurious to the epithelial cells. Injured cells are prone to support crystal attachment. Once crystals are retained, ensuing injury would accelerate further crystallization and retention leading to more stone formation.
CONCLUSIONS
A Unified Theory on Pathogenesis of Kidney Stones
Stone formation is a complex multistep process. A variety of theories have been presented to explain stone pathogenesis based upon urinary supersaturation, crystallization inhibitors, and vascular insult. Kidney stones being mostly crystalline, all of the theories agree that stone formation requires urinary supersaturation with respect to the crystalline component (4,30,111–116). Since crystallization may lead to tubular blockage and injury, affecting renal function, kidneys normally produce mineralization inhibitors (70,74,117,118). Degree of supersaturation, which is dependent on increased urinary excretion of ions involved in crystallization (116, 119–123) would however, determine whether stones start in the tubular lumen or the renal interstitium. As discussed above a variety of stones start as a result of high supersaturation and crystallization in the tubular lumens. But the most common kidney stones, the idiopathic calcium oxalate stones, are formed attached to subepithelial plaques of calcium phosphate. Mechanism involved in the formation of plaques are however uncertain. It is proposed that plaques start in the basement membrane of the loops of Henle (27) mostly because of high urinary calcium (124). But there is no experimental proof. High urinary calcium in rodents does not lead to the formation of interstitial plaques (125–127).
Randall’s plaques are common even in non-stone forming individuals (113,128) as well as in stone patients with plug based kidney stones (15). Perhaps plaque formation, at least in some stone patients, is a result of papillary injury caused by plugging and secondary to the tubular blockage. Another theory proposes that plaques are formed secondary to vascular insult near tip of the renal papilla (37). The hypothesis is supported by the fact that majority of stones are associated with chronic kidneys diseases such as hypertension, atherosclerosis, hyperlipidemia etc. We have previously shown that production of reactive oxygen species and development of oxidative stress are common in both the idiopathic stones formers and chronic kidney disease patients (34, 39). It is remarkable that patients with hypertension can eventually develop idiopathic kidney stones and kidney stones may lead to hypertension. We have proposed a hypothesis combining the current theories (4,10).
Renal epithelial cells of stone formers become stressed when challenged by increased urinary excretion of calcium/oxalate/phosphate and/or decrease in the production of functional crystallization inhibitors or perhaps with renal insults, and the abnormal process of aging. There is production of reactive oxygen species causing de-differentiation of renal epithelial/endothelial cells into osteoblast like cells. CaP crystals are deposited in the renal tubular epithelial/vascular endothelial basement membrane. Small spherical units of CaP grow from less than a micron to a few microns in diameter and spread out beyond the basement membrane. CaP spherulites aggregate and form larger deposits or plaques which increase in size by further addition of crystals on the periphery. The growing plaque comes into contact with collagen fibers and membranous degradation products which also undergo calcification (16).
Interstitial CaP deposition produces localized inflammation and fibrosis, providing substrate for further calcification until the front reaches the papillary surface. Urothelium is most likely breached through the activity of matrix metalloproteinases, loss of cellular polarity, architecture, and cell to cell junctions. Once the surface epithelium loses its integrity, the plaque is exposed to the metastable pelvic urine, and becomes a nidus for stone formation. Macromolecules present in the pelvic urine and are produced by renal epithelial cells in response to reactive oxygen species (74), deposit on the plaque surface. Surface layers of HA are replaced by CaOx through direct transformation or demineralization of CaP and mineralization of CaOx. Alternatively, or in addition, CaOx crystals directly nucleate on the macromolecules deposited on plaque surface. Further growth is again dependent upon the urinary supersaturation and is accomplished by crystal nucleation, growth and aggregation on the periphery.
REFERENCES AND RECOMMENDED READINGS
(*of special interest, **of outstanding interest)
- *1.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Kok DJ, Khan SR. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int. 1994;46:847. doi: 10.1038/ki.1994.341. [DOI] [PubMed] [Google Scholar]
- **4.Khan SR, Pearle MS, Robertson WG, et al. Kidney stones. Nat Rev Dis Primers. 2016;2:16008. doi: 10.1038/nrdp.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlayson B, Reid F. The expectation of free and fixed particles in urinary stone disease. Invest Urol. 1978;15:442. [PubMed] [Google Scholar]
- 6.Randall A. The Origin and Growth of Renal Calculi. Ann Surg. 1937;105:1009. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Randall A. Papillary pathology as a precursor of primary renal calculus. Journal of Urology. 1940;44:580. [Google Scholar]
- 8.Randall A. The etiology of primary renal calculus. International Abstract of Surgery. 1940;71:209. [Google Scholar]
- 9.Stoller ML, Low RK, Shami GS, et al. High resolution radiography of cadaveric kidneys: unraveling the mystery of Randall’s plaque formation. J Urol. 1996;156:1263. doi: 10.1016/s0022-5347(01)65565-4. [DOI] [PubMed] [Google Scholar]
- **10.Khan SR, Canales BK. Unified theory on the pathogenesis of Randall’s plaques and plugs. Urolithiasis. 2015;43(Suppl 1):109. doi: 10.1007/s00240-014-0705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shilov IV, Seymour SL, Patel AA, et al. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Weller RO, Nester B, Cooke SAR. Calcification in the human renal papilla: an electron microscope study. Journal of Pathology. 1971;107:211. doi: 10.1002/path.1711070308. [DOI] [PubMed] [Google Scholar]
- *13.Cooke SAR. The site of calcification in the human renal papilla. British Journal of Surgery. 1970;57:890. doi: 10.1002/bjs.1800571205. [DOI] [PubMed] [Google Scholar]
- 14.Evan AP, Unwin RJ, Williams JC., Jr Renal stone disease: a commentary on the nature and significance of Randall’s plaque. Nephron Physiol. 2011;119:p49. doi: 10.1159/000330255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Khan SR, Finlayson B, Hackett R. Renal papillary changes in patient with calcium oxalate lithiasis. Urology. 1984;23:194. doi: 10.1016/0090-4295(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 16.Khan SR, Rodriguez DE, Gower LB, et al. Association of Randall plaque with collagen fibers and membrane vesicles. J Urol. 2012;187:1094. doi: 10.1016/j.juro.2011.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haggit RC, Pitcock JA. Renal medullary calcification: a light and electron microscopic study. The Journal of Urology. 1971;106:342. doi: 10.1016/s0022-5347(17)61284-9. [DOI] [PubMed] [Google Scholar]
- *18.Coe FL, Evan AP, Lingeman JE, et al. Plaque and deposits in nine human stone diseases. Urol Res. 2010;38:239. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller NL, Gillen DL, Williams JC, Jr, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU Int. 2009;103:966. doi: 10.1111/j.1464-410X.2008.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evan AP, Lingeman JE, Coe FL, et al. Role of interstitial apatite plaque in the pathogenesis of the common calcium oxalate stone. Semin Nephrol. 2008;28:111. doi: 10.1016/j.semnephrol.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 2009 doi: 10.1007/s00467-009-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evan AP, Coe FL, Rittling SR, et al. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localization. Kidney Int. 2005;68:145. doi: 10.1111/j.1523-1755.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 23.Evan AP, Bledsoe S, Worcester EM, et al. Renal inter-alpha-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Int. 2007;72:1503. doi: 10.1038/sj.ki.5002569. [DOI] [PubMed] [Google Scholar]
- 24.Evan A, Lingeman J, Coe FL, et al. Randall’s plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006;69:1313. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 25.Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpentier X, Bazin D, Combes C, et al. High Zn content of Randall’s plaque: A mu-X-ray fluorescence investigation. J Trace Elem Med Biol. 2011;2011 doi: 10.1016/j.jtemb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- *27.Coe FL, Evan AP, Worcester EM, et al. Three pathways for human kidney stone formation. Urol Res. 2010;38:147. doi: 10.1007/s00240-010-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sepe V, Adamo G, La Fianza A, et al. Henle loop basement membrane as initial site for Randall plaque formation. Am J Kidney Dis. 2006;48:706. doi: 10.1053/j.ajkd.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Bushinsky DA. Nephrolithiasis: site of the initial solid phase. J Clin Invest. 2003;111:602. doi: 10.1172/JCI18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiselius HG. A hypothesis of calcium stone formation: an interpretation of stone research during the past decades. Urol Res. 2011;39:231. doi: 10.1007/s00240-010-0349-3. [DOI] [PubMed] [Google Scholar]
- 31.Vermooten V. Renal calculi; etiology and differential diagnosis. Tex State J Med. 1951;47:96. [PubMed] [Google Scholar]
- 32.Evan AP, Coe FL, Lingeman JE, et al. Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat Rec (Hoboken) 2007;290:1315. doi: 10.1002/ar.20580. [DOI] [PubMed] [Google Scholar]
- 33.Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol. 2014;3:256. doi: 10.3978/j.issn.2223-4683.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan SR. Stress oxidative: nephrolithiasis and chronic kidney diseases. Minerva Med. 2013;104:23. [PubMed] [Google Scholar]
- *35.Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol. 2013;189:803. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taguchi K, Hamamoto S, Okada A, et al. Genome-Wide Gene Expression Profiling of Randall’s Plaques in Calcium Oxalate Stone Formers. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Stoller ML, Meng MV, Abrahams HM, et al. The primary stone event: a new hypothesis involving a vascular etiology. J Urol. 2004;171:1920. doi: 10.1097/01.ju.0000120291.90839.49. [DOI] [PubMed] [Google Scholar]
- 38.Bagga HS, Chi T, Miller J, et al. New insights into the pathogenesis of renal calculi. Urol Clin North Am. 2013;40:1. doi: 10.1016/j.ucl.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012;40:95. doi: 10.1007/s00240-011-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *40.Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens. 2008;21:257. doi: 10.1038/ajh.2007.62. [DOI] [PubMed] [Google Scholar]
- *41.Huang HS, Ma MC, Chen CF, et al. Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology. 2003;62:1123. doi: 10.1016/s0090-4295(03)00764-7. [DOI] [PubMed] [Google Scholar]
- *42.Tungsanga K, Sriboonlue P, Futrakul P, et al. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res. 2005;33:65. doi: 10.1007/s00240-004-0444-4. [DOI] [PubMed] [Google Scholar]
- *43.Boonla C, Wunsuwan R, Tungsanga K, et al. Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res. 2007;35:185. doi: 10.1007/s00240-007-0098-0. [DOI] [PubMed] [Google Scholar]
- 44.Schwille PO, Manoharan M, Schmiedl A. Is idiopathic recurrent calcium urolithiasis in males a cellular disease? Laboratory findings in plasma, urine and erythrocytes, emphasizing the absence and presence of stones, oxidative and mineral metabolism: an observational study. Clin Chem Lab Med. 2005;43:590. doi: 10.1515/CCLM.2005.103. [DOI] [PubMed] [Google Scholar]
- 45.Sutariya B, Jhonsa D, Saraf MN. TGF-beta: the connecting link between nephropathy and fibrosis. Immunopharmacol Immunotoxicol. 2016;38:39. doi: 10.3109/08923973.2015.1127382. [DOI] [PubMed] [Google Scholar]
- 46.Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46:217. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Palm F. Intrarenal oxygen in diabetes and a possible link to diabetic nephropathy. Clin Exp Pharmacol Physiol. 2006;33:997. doi: 10.1111/j.1440-1681.2006.04473.x. [DOI] [PubMed] [Google Scholar]
- 48.Hansell P, Welch WJ, Blantz RC, et al. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol. 2013;40:123. doi: 10.1111/1440-1681.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palm F, Carlsson PO, Fasching A, et al. Diabetes-induced decrease in renal oxygen tension: effects of an altered metabolism. Adv Exp Med Biol. 2006;578:161. doi: 10.1007/0-387-29540-2_26. [DOI] [PubMed] [Google Scholar]
- 50.Zou AP, Cowley AW., Jr Reactive oxygen species and molecular regulation of renal oxygenation. Acta Physiol Scand. 2003;179:233. doi: 10.1046/j.0001-6772.2003.01206.x. [DOI] [PubMed] [Google Scholar]
- *51.Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Byon CH, Yuan K, et al. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tada Y, Yano S, Yamaguchi T, et al. Advanced glycation end products-induced vascular calcification is mediated by oxidative stress: functional roles of NAD(P) H-oxidase. Horm Metab Res. 2013;45:267. doi: 10.1055/s-0032-1329965. [DOI] [PubMed] [Google Scholar]
- 54.Watson KE, Bostrom K, Ravindranath R, et al. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irwin CL, Guzman RJ. Matrix metalloproteinases in medial arterial calcification: potential mechanisms and actions. Vascular. 2009;17(Suppl 1):S40. doi: 10.2310/6670.2008.00086. [DOI] [PubMed] [Google Scholar]
- 56.Pai A, Leaf EM, El-Abbadi M, et al. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764. doi: 10.1016/j.ajpath.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basalyga DM, Simionescu DT, Xiong W, et al. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110:3480. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vyavahare N, Jones PL, Tallapragada S, et al. Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol. 2000;157:885. doi: 10.1016/S0002-9440(10)64602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He D, Lu Y, Hu H, et al. The Wnt11 Signaling Pathway in Potential Cellular EMT and Osteochondral Differentiation Progression in Nephrolithiasis Formation. Int J Mol Sci. 2015;16:16313. doi: 10.3390/ijms160716313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lieske JC, de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis. 2006;48:897. doi: 10.1053/j.ajkd.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Edwards A, Layton AT. Modulation of outer medullary NaCl transport and oxygenation by nitric oxide and superoxide. Am J Physiol Renal Physiol. 2011;301:F979. doi: 10.1152/ajprenal.00096.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welch WJ, Baumgartl H, Lubbers D, et al. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 2001;59:230. doi: 10.1046/j.1523-1755.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 63.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 64.Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- *65.Wu XR. Interstitial calcinosis in renal papillae of genetically engineered mouse models: relation to Randall’s plaques. Urolithiasis. 2015;43(Suppl 1):65. doi: 10.1007/s00240-014-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mo L, Huang HY, Zhu XH, et al. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004;66:1159. doi: 10.1111/j.1523-1755.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 67.Evan AP, Weinman EJ, Wu XR, et al. Comparison of the pathology of interstitial plaque in human ICSF stone patients to NHERF-1 and THP-null mice. Urol Res. 2010;38:439. doi: 10.1007/s00240-010-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *68.Liu Y, Mo L, Goldfarb DS, et al. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. Am J Physiol Renal Physiol. 2010;299:F469. doi: 10.1152/ajprenal.00243.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **69.Mo L, Liaw L, Evan AP, et al. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Hors-fall protein, or both. Am J Physiol Renal Physiol. 2007;293:F1935. doi: 10.1152/ajprenal.00383.2007. [DOI] [PubMed] [Google Scholar]
- *70.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci. 2004;9:1450. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 71.El-Achkar TM, McCracken R, Rauchman M, et al. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol. 2011;300:F999. doi: 10.1152/ajprenal.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Q, Chou DW, Price TP, et al. Genetic modulation of nephrocalcinosis in mouse models of ectopic mineralization: the Abcc6(tm1Jfk) and Enpp1(asj) mutant mice. Lab Invest. 2014;94:623. doi: 10.1038/labinvest.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan SR, Shevock PN, Hackett RL. Magnesium oxide administration and prevention of calcium oxalate nephrolithiasis. J Urol. 1993;149:412. doi: 10.1016/s0022-5347(17)36106-2. [DOI] [PubMed] [Google Scholar]
- **74.Khan SR, Joshi S, Wang W, et al. Regulation of macromolecular modulators of urinary stone formation by reactive oxygen species: transcriptional study in an animal model of hyperoxaluria. Am J Physiol Renal Physiol. 2014;306:F1285. doi: 10.1152/ajprenal.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halperin ML, Cheema Dhadli S, Kamel KS. Physiology of acid-base balance: links with kidney stone prevention. Semin Nephrol. 2006;26:441. doi: 10.1016/j.semnephrol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Khan SR, Glenton PA, Backov R, et al. Presence of lipids in urine, crystals and stones: implications for the formation of kidney stones. Kidney Int. 2002;62:2062. doi: 10.1046/j.1523-1755.2002.00676.x. [DOI] [PubMed] [Google Scholar]
- 77.Gambaro G, D’Angelo A, Fabris A, et al. Crystals, Randall’s plaques and renal stones: do bone and atherosclerosis teach us something? J Nephrol. 2004;17:774. [PubMed] [Google Scholar]
- *78.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 79.Shanahan CM. Vascular calcification. Curr Opin Nephrol Hypertens. 2005;14:361. doi: 10.1097/01.mnh.0000172723.52499.38. [DOI] [PubMed] [Google Scholar]
- 80.Briet M, Burns KD. Chronic kidney disease and vascular remodelling: molecular mechanisms and clinical implications. Clin Sci (Lond) 2012;123:399. doi: 10.1042/CS20120074. [DOI] [PubMed] [Google Scholar]
- 81.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 82.Kapustin AN, Davies JD, Reynolds JL, et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109:e1. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 83.Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell-derived matrix vesicles. Trends Cardiovasc Med. 2012;22:133. doi: 10.1016/j.tcm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- *84.Shanahan CM, Crouthamel MH, Kapustin A, et al. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109:697. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shroff RC, Shanahan CM. The vascular biology of calcification. Semin Dial. 2007;20:103. doi: 10.1111/j.1525-139X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 86.Jono S, Shioi A, Ikari Y, et al. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176. doi: 10.1007/s00774-005-0668-6. [DOI] [PubMed] [Google Scholar]
- **87.Joshi S, Clapp WL, Wang W, et al. Osteogenic changes in kidneys of hyperoxaluric rats. Biochim Biophys Acta. 2015;1852:2000. doi: 10.1016/j.bbadis.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jia Z, Wang S, Tang J, et al. Does crystal deposition in genetic hypercalciuric rat kidney tissue share similarities with bone formation? Urology. 2014;83:509 e7. doi: 10.1016/j.urology.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Miyazawa K, Aihara K, Ikeda R, et al. cDNA macroarray analysis of genes in renal epithelial cells exposed to calcium oxalate crystals. Urol Res. 2009;37:27. doi: 10.1007/s00240-008-0164-2. [DOI] [PubMed] [Google Scholar]
- *90.Kumar V, Farell G, Yu S, et al. Cell biology of pathologic renal calcification: contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J Investig Med. 2006;54:412. doi: 10.2310/6650.2006.06021. [DOI] [PubMed] [Google Scholar]
- *91.Kageyama S, Ohtawara Y, Fujita K, et al. Microlith formation in vitro by Madin Darby canine kidney (MDCK) cells. Int J Urol. 1996;3:23. doi: 10.1111/j.1442-2042.1996.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 92.Naito Y, Ohtawara Y, Kageyama S, et al. Morphological analysis of renal cell culture models of calcium phosphate stone formation. Urol Res. 1997;25:59. doi: 10.1007/BF00941907. [DOI] [PubMed] [Google Scholar]
- 93.Senzaki H, Yasui T, Okada A, et al. Alendronate inhibits urinary calcium microlith formation in a three-dimensional culture model. Urol Res. 2004;32:223. doi: 10.1007/s00240-004-0409-7. [DOI] [PubMed] [Google Scholar]
- 94.Linnes MP, Krambeck AE, Cornell L, et al. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int. 2013 doi: 10.1038/ki.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *95.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 96.Evan AP, Coe FL, Lingeman JE, et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69:2227. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 97.Matlaga BR, Shah OD, Assimos DG. Drug-induced urinary calculi. Rev Urol. 2003;5:227. [PMC free article] [PubMed] [Google Scholar]
- 98.Gabriels G, Lambert M, Smith P, et al. Melamine contamination in nutritional supplements--Is it an alarm bell for the general consumer, athletes, and ‘Weekend Warriors’? Nutr J. 2015;14:69. doi: 10.1186/s12937-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan SR, Glenton PA, Byer KJ. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 2006;70:914. doi: 10.1038/sj.ki.5001699. [DOI] [PubMed] [Google Scholar]
- 100.Khan SR, Hackett RL, Finlayson B, et al. Light and scanning electron microscopic studies of oxamide urolithiasis in rats. Scan Electron Microsc. 1981;155 [PubMed] [Google Scholar]
- 101.Gill WB, Vermeulen CW. Oxamide Crystalluria and Urolithiasis, Rat and in Vitro Observations. Invest Urol. 1964;1:339. [PubMed] [Google Scholar]
- 102.Borden TA, Vermeulen CW. The renal papilla in calculogenesis of oxamide stones. Invest Urol. 1966;4:125. [PubMed] [Google Scholar]
- 103.Khan SR. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res. 1995;23:71. doi: 10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- 104.Khan SR. Experimental calcium oxalate nephrolithiasis and the formation of human urinary stones. Scanning Microsc. 1995;9:89. [PubMed] [Google Scholar]
- 105.Finlayson B. The concept of a continuous crystallizer. Its theory and application to in vivo and in vitro urinary tract models. Invest Urol. 1972;9:258. [PubMed] [Google Scholar]
- 106.Robertson WG, Peacock M, Nordin BE. Calcium oxalate crystalluria and urine saturation in recurrent renal stone-formers. Clin Sci. 1971;40:365. doi: 10.1042/cs0400365. [DOI] [PubMed] [Google Scholar]
- 107.Moe SM. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur J Clin Invest. 2006;36(Suppl 2):51. doi: 10.1111/j.1365-2362.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 108.Khan SR, Hackett RL. Retention of calcium oxalate crystals in renal tubules. Scanning Microsc. 1991;5:707. [PubMed] [Google Scholar]
- *109.Schulz E, Hengst E, Brundig P, et al. Disturbed urinary transport in the pelvicalyceal system in calcium-oxalate stone patients. Urol Res. 1987;15:109. doi: 10.1007/BF00260943. [DOI] [PubMed] [Google Scholar]
- *110.Robertson WG. Kidney models of calcium oxalate stone formation. Nephron Physiol. 2004;98:p21. doi: 10.1159/000080260. [DOI] [PubMed] [Google Scholar]
- 111.Finlayson B. Physicochemical aspects of urolithiasis. Kidney Int. 1978;13:344. doi: 10.1038/ki.1978.53. [DOI] [PubMed] [Google Scholar]
- 112.Robertson WG. A risk factor model of stone-formation. Front Biosci. 2003;8:s1330. doi: 10.2741/1181. [DOI] [PubMed] [Google Scholar]
- 113.Evan AP, Worcester EM, Coe FL, et al. Mechanisms of human kidney stone formation. Urolithiasis. 2015;43(Suppl 1):19. doi: 10.1007/s00240-014-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kok DJ. Clinical implications of physicochemistry of stone formation. Endocrinol Metab Clin North Am. 2002;31:855. doi: 10.1016/s0889-8529(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 115.Siener R, Hesse A. Fluid intake and epidemiology of urolithiasis. Eur J Clin Nutr. 2003;57(Suppl 2):S47. doi: 10.1038/sj.ejcn.1601901. [DOI] [PubMed] [Google Scholar]
- 116.Hess B, Ryall RL, Kavanagh JP, et al. Methods for measuring crystallization in urolithiasis research: why, how and when? Eur Urol. 2001;40:220. doi: 10.1159/000049776. [DOI] [PubMed] [Google Scholar]
- 117.Ryall RL. Urinary inhibitors of calcium oxalate crystallization and their potential role in stone formation. World J Urol. 1997;15:155. doi: 10.1007/BF02201852. [DOI] [PubMed] [Google Scholar]
- *118.Ryall RL. Macromolecules and urolithiasis: parallels and paradoxes. Nephron Physiol. 2004;98:p37. doi: 10.1159/000080262. [DOI] [PubMed] [Google Scholar]
- 119.Werness PG, Brown CM, Smith LH, et al. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol. 1985;134:1242. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
- 120.Robertson WG, Peacock M, Nordin BE. Activity products in stone-forming and non-stone-forming urine. Clin Sci. 1968;34:579. [PubMed] [Google Scholar]
- 121.Tiselius HG. Risk formulas in calcium oxalate urolithiasis. World J Urol. 1997;15:176. doi: 10.1007/BF02201855. [DOI] [PubMed] [Google Scholar]
- 122.Rodgers AL, Allie-Hamdulay S, Jackson G, et al. Simulating calcium salt precipitation in the nephron using chemical speciation. Urol Res. 2011;39:245. doi: 10.1007/s00240-010-0359-1. [DOI] [PubMed] [Google Scholar]
- 123.Wesson JA, Worcester EM, Wiessner JH, et al. Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int. 1998;53:952. doi: 10.1111/j.1523-1755.1998.00839.x. [DOI] [PubMed] [Google Scholar]
- 124.Kuo RL, Lingeman JE, Evan AP, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int. 2003;64:2150. doi: 10.1046/j.1523-1755.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 125.Bushinsky DA, Frick KK, Nehrke K. Genetic hypercalciuric stone-forming rats. Curr Opin Nephrol Hypertens. 2006;15:403. doi: 10.1097/01.mnh.0000232881.35469.a9. [DOI] [PubMed] [Google Scholar]
- 126.Khan SR. Nephrocalcinosis in animal models with and without stones. Urol Res. 2010;38:429. doi: 10.1007/s00240-010-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khan SR, Glenton PA. Calcium oxalate crystal deposition in kidneys of hypercalciuric mice with disrupted type IIa sodium-phosphate cotransporter. Am J Physiol Renal Physiol. 2008;294:F1109. doi: 10.1152/ajprenal.00620.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **128.Haggitt RC, Pitcock JA. Renal medullary calcifications: a light and electron microscopic study. J Urol. 1971;106:342. doi: 10.1016/s0022-5347(17)61284-9. [DOI] [PubMed] [Google Scholar]