Abstract
Alterations in the composition of the gut microbiota are associated with a number of gastrointestinal (GI) conditions, including diarrhea, inflammatory bowel diseases (IBD) and liver diseases. Probiotics, live microorganisms that may confer a health benefit to the host when consumed, are commonly used as a therapy for treating these GI conditions by means of modifying the composition or activity of the microbiota. The purpose of this overview is to summarize the evidence on probiotics and GI conditions available from Cochrane, a non-profit organization that produces rigorous and high-quality systematic reviews of health interventions. Findings from this overview will help provide more precise guidance for clinical use of probiotics and to identify gaps in probiotic research related to GI conditions.
Keywords: Probiotic, microbiota, gastrointestinal conditions, diarrhea, inflammatory bowel disease, liver disease
Introduction
Probiotics are defined by the World Health Organization as “live microorganisms, which when consumed in adequate amounts confer health and benefit to the host” [1]. Though most commonly consumed worldwide in the form of yogurt or other fermented dairy products, probiotics are found and administered in many different forms including a wide variety of dietary supplements and functional foods. Consumption of probiotics in their various forms is common and increasing rapidly. Within the United States, 3.9 million adults were shown to use probiotic or prebiotic supplements in 2015 —a fourfold increase since 2007 [2]. The increasingly common use of probiotics is also reflected in sales figures that suggest that they are one of the supplement categories most often purchased by consumers. Whereas overall growth in the nutritional supplement industry slowed to 5% in 2014, probiotics grew 14.2% with nearly $1.4 billion in sales [3]. In addition to widespread use among consumers, a recent study revealed that 96% of hospitals used probiotics as part of inpatient clinical care [4]. The increasing use of probiotics in both hospitals and among the public at large demonstrates the increasing public health importance of clinical research on probiotics.
Probiotics, Altered Gut Microbiota and Disease
The increasingly common use of probiotics is supported by a rapidly growing evidence base suggesting a variety of health benefits. More than 25 diseases or health conditions have been associated with the microbiota in the gastrointestinal tract, ranging far beyond gastrointestinal health into the realms of autoimmune disease, emotional health, and other areas [5]. While the relationship between the microbiota and human health is broad ranging and the literature continues to expand, the health conditions that have been most consistently associated with the composition and activity of the microbiota are gastrointestinal in nature. Probiotics are believed to provide an important role in human health by providing a protective effect on the microbiota in the gastrointestinal tract through both colonization and transient activity, depending upon the species. Probiotics have shown therapeutic benefits in adults and children across a broad range of health conditions, including autoimmune diseases [6–9], emotional disorders [10, 11], and even as part of a potential treatment strategy for obesity [12–14]. However, the effects of probiotics have been most studied in gastrointestinal (GI) conditions such as acute infectious diarrhea, inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). Antibiotic therapy is typically prescribed for infectious diarrhea, which has been shown to reduce the diversity of intestinal microbiota. The two main IBD conditions, Crohn’s disease and ulcerative colitis, are also associated with a reduced microbial diversity [5]. Although the exact mechanisms of action are unknown, the use of probiotics is thought to increase microbial diversity by improving the balance of organisms within the intestinal tract and reducing the risk of colonization by pathogenic bacteria [15, 16]. The clinical evidence surrounding probiotics for GI conditions will be the focus of this overview.
Though probiotics have been shown to be efficacious in many randomized controlled trials and systematic reviews for a variety of health conditions, more precise evidence is needed to translate the growing evidence base to appropriate clinical practice. For instance, probiotics are often recommended in clinical practice without the necessary specification of numerous important factors related to the probiotic and the health condition for which they are being recommended. Probiotics are most broadly categorized by their genus (e.g. Lactobacillus), followed by their species (e.g. acidophilus), and most specifically, their strain (e.g. NCFM). The clinical effects of probiotics are dependent on many factors, including the species and strain of the probiotic. Different strains of the same species can yield heterogeneous clinical results [17]. One striking example is the vastly different effects noted among different strains of Escherichia coli (E. coli) species. E. coli 0157:H7 is a food-borne pathogen that can cause hemorrhagic diarrhea, kidney failure, and death. Within the same species, E. coli Nissle 1917 is a probiotic supplement that has been shown to improve inflammatory bowel disease, irritable syndrome, and other GI disorders [18–20]. Despite the heterogeneity of effect of different strains of the same species, strains are rarely specified on most probiotic foods and supplements. This type of heterogeneity in effect often results in discrepancies between clinical outcomes of probiotic interventions. Combinations of different probiotic species and strains within the same capsule also introduce additional uncertainty due to unknown and poorly-studied interactions between probiotic species. Additionally, the effective dosage of probiotics varies by species and strain. Probiotic dosage is most often measured as colony forming units (CFU), and recommendations and clinical effects vary depending on the species and strain used and the pathogen or disease targeted [21]. Thus, identifying species and strains and specifying dosages is critically important to understanding how probiotics may or may not be effective for specific conditions. Lastly, even if the species, strains, and dosages are specified and concordant with the scientific literature, there are currently no product purity and labeling standards for probiotics to ensure that what is listed on the probiotic supplement label is actually in the bottle. The best current process to ensure probiotic purity and bottle-to-bottle consistency is third party laboratory certification, which is flawed due to heterogeneity in testing methods between the various laboratories.
In addition to these confounding factors related to the probiotic intervention, factors related to the design of the clinical trial evaluating the probiotic also have an impact on clinical outcomes. The specific outcomes studied and how they were assessed, the duration of probiotic treatment, and the length of follow-up should be clearly defined in order to more precisely describe the effects of probiotic treatment. The clinical heterogeneity observed in many studies and in clinical practice is a function of the many factors that can influence probiotic efficacy. Thus, the purpose of this overview is to summarize the current evidence of probiotic therapy for GI symptoms to help provide more precise guidance for clinical use and identify future needs for probiotics research. Systematic reviews and meta-analyses of probiotic interventions for GI-related medical conditions performed for the Cochrane organization will be the focus of this overview.
Cochrane and Systematic Reviews of Probiotics
Cochrane, which was founded in 1983 as the Cochrane Collaboration, is one of the first and most highly regarded organizations focused on the production and dissemination of systematic reviews of health care interventions. It is an international non-profit organization that currently includes more than 37,000 contributors, mostly volunteers, from over 130 countries [22]. Cochrane reviews aim to be unbiased; Cochrane does not accept commercial funding and has policies to guard against both commercial and non-commercial conflicts of interest in the production of reviews. Cochrane reviews are also methodologically rigorous and follow structured and transparent methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions [23]. Cochrane reviews have frequently been observed to have higher methodological quality, better reporting, and more precise conclusions than non-Cochrane reviews on the same topics [24–29]. All Cochrane reviews undergo peer review twice; once during the protocol stage and again following completion of the review prior to publication. Both protocols and completed reviews are published in the online Cochrane Library (www.cochranelibrary.com), where there are currently over 2,000 protocols and nearly 7,000 completed reviews. Cochrane reviews are meant to be updated when new evidence becomes available, and many reviews in the Cochrane Library are currently on their fourth or later update. Cochrane reviews may therefore be expected to provide a high-quality, unbiased, and up-to-date assessment of the evidence on health care interventions such as probiotics.
Methods
To identify all reviews in the Cochrane Library whose primary focus was probiotics and the digestive system, two independent authors (EAP, TR) searched the titles and abstracts using the search term “probiotic*”. The authors each read the title and abstract of each retrieved Cochrane review article to verify the inclusion of probiotics and disorders and/or symptoms affecting the digestive system. Cochrane reviews that included any trials comparing oral administration of probiotics to placebo or usual care were included in this overview. For this overview, probiotics were defined as probiotics administered in any form (drink, powder, capsule) as a single species or as a cocktail of multiple species. Cochrane reviews that were withdrawn from publication or Cochrane reviews that contained trials where probiotic treatment was administered through enteral feedings were excluded. Cochrane reviews that did not feature comparisons that isolated the effects of probiotics were also excluded. For example, one Cochrane review compared oral bovine lactoferrin alone, versus oral bovine lactoferrin in combination with a probiotic (Lactobacillus rhamnosus GG), versus placebo [30]. Therefore, this Cochrane review did not include any comparisons isolating the effect of probiotics (e.g., probiotics alone versus placebo).
For each Cochrane review, the two authors extracted data on the number of trials included and the total number of participants. Because there is high variability in microbial composition in the GI system across the lifespan and changes in the microbiota are associated with increasing age [31], we extracted data on the ages of participants included in the trials. We identified the prespecified outcomes of each Cochrane review as well as the Cochrane review authors’ conclusions regarding the prespecified outcomes.
We assigned the conclusions from each Cochrane review into one of the following categories: (A) the Cochrane review indicated good evidence of a benefit from probiotics; (B) the Cochrane review indicated good evidence of no benefit from probiotics; (C) the Cochrane review indicated that there was not sufficient available evidence to allow benefits from probiotics to be determined. The Cochrane review conclusions were based on GRADE (Grading of Recommendations, Assessment, Development and Evaluation), which is Cochrane’s preferred systematic approach for evaluating the quality of evidence for an estimate of effect, as high, moderate, low, or very low [32]. The criterion for good evidence (i.e., a rating of A or B) was at least one statement of “moderate” quality evidence of benefit or lack of benefit when GRADE was used. If the review had only “low” or “very low” quality evidence of benefit or lack of benefit, we assigned a rating of “C”. If GRADE was not used in the Cochrane review, the criterion was a precise effect estimate (e.g. statistical significance) for either benefit or lack of benefit and the authors only mentioned one serious deficiency in the evidence. For example, if there were statistically significant findings of benefit for the primary outcome, and the authors indicated that substantial heterogeneity between trials was the only serious deficiency in the evidence, the conclusions would be rated as “A”. If the review did not have a precise effect estimate for either benefit or lack of benefit, we assigned a rating of “C”. This assignment was carried out by two reviewers making independent assessments of Cochrane review conclusions. In the case of disagreement between the two reviewers, a third reviewer adjudicated.
To determine factors that may influence clinical recommendations of probiotic use, we indicated the number of different probiotic combinations from each Cochrane review, as well as the number of trials that specified strain, dosage, intervention length and follow-up. These variables provide important information regarding potential influences on probiotic efficacy in each trial and were collected from either the main text of the Cochrane review or the “Characteristics of included studies” tables for each trial. We also observed whether subgroup analyses on aspects of the intervention related to species and dosage of probiotics were proposed in the Cochrane review, and if there were sufficient data available to conduct these analyses. Finally, we noted whether adverse events were identified as a prespecified outcome and whether they were discussed within the review.
Results
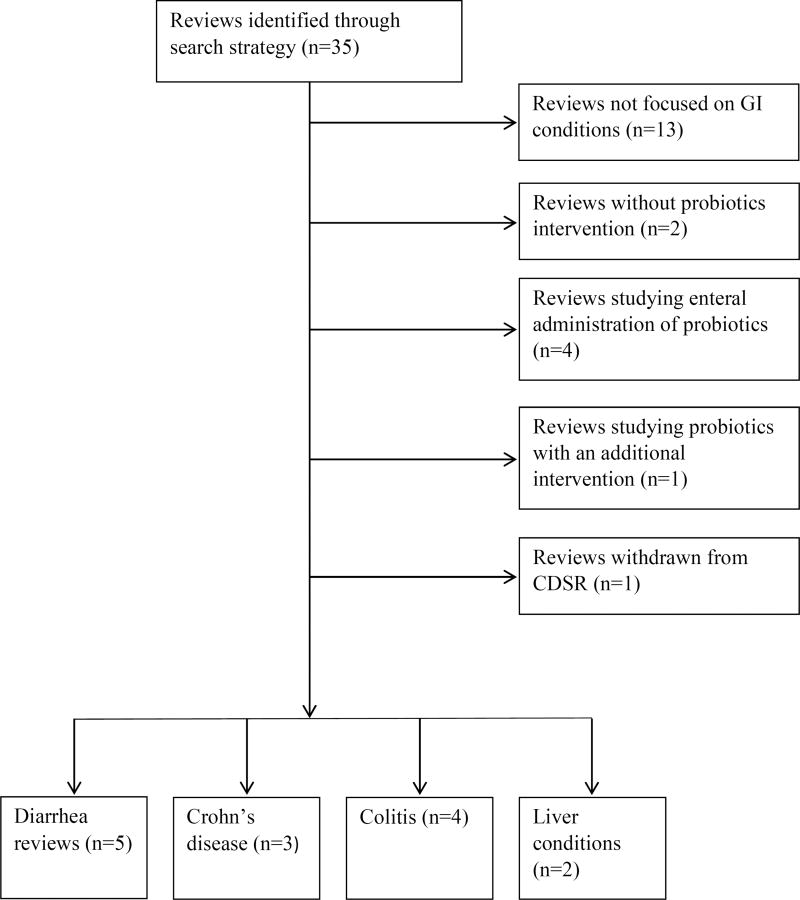
Figure 1 shows the results of our search of the Cochrane Library. We identified 14 Cochrane reviews published between 2006 and 2015 that focused on probiotics and GI-related medical conditions, and grouped them into one of four categories: diarrhea, colitis, Crohn’s disease, and liver conditions.
Figure 1.
Results of searching Cochrane Database of Systematic Reviews (CDSR) for reviews on probiotics and GI conditions.
Table 1 summarizes the total number of trials, the number and age range of participants included in each Cochrane review, the outcomes assessed and the conclusions in each of the Cochrane reviews. Overall, prespecified outcomes were generally similar among reviews within the same condition; however, there was substantial heterogeneity in how these outcomes were operationalized. There were 8 Cochrane reviews that did not explicitly use GRADE to assess the quality of the evidence.
Table 1.
Characteristics of Cochrane Reviews of Probiotics and GI Conditions
| Author Year |
Trials (N) |
Participants (N) |
Age (Years) |
Prespecified outcomes | Conclusion category* |
Review conclusions** |
|---|---|---|---|---|---|---|
| Diarrhea | ||||||
| Goldenberg et al. (2015) [35] *** | 23 | 3938 | 0 to 18 |
|
A | The authors concluded that evidence suggests that Lactobacillus rhamnosus or saccharomyces boulardii at 5 to 40 billion CFU/day may be appropriate in otherwise healthy children. Moderate quality evidence suggesting a protective effect of probiotics in preventing AAD, and very low quality evidence that there is no difference between probiotics and controls in the incidence of adverse effects. |
| Bernaola Aponte et al. (2013) [34]*** | 4 | 464 | 0 to 18 |
|
A | The authors concluded there is insufficient evidence to recommend their use at this time. Moderate quality evidence suggests that probiotics reduce the duration of diarrhea. However, this evidence stems from two studies, one from India and one from Argentina, and authors state that further studies may be necessary before generalizing results to all situations. |
| Allen et al. (2010) [33] | 63 | 8014 | Pediatric: <18 Adults: Not specified |
|
A | Authors conclude that probiotics administered in addition to rehydration therapy resulted in clear reductions in the duration and severity of diarrhea, and were not associated with adverse effects, but that evidence-based treatment guidelines could not be developed due to marked clinical variability between studies. |
| Goldenberg et al. (2013) [36] *** | 31 | 4492 | Pediatric: 0 to 18 Adult: >18 |
|
A | The authors concluded that probiotics are both safe and effective for preventing C. difficile-associated diarrhea. Moderate quality evidence supports a large protective effect of probiotics in preventing C. difficile associated diarrhea, but not in reducing the incidence of infection. Moderate quality evidence supports a reduction in adverse events with probiotics at short term in patients who are not immunocompromised or severely debilitated. |
| Pillai et al. (2010) [37] | 4 | 336 | >18 |
|
C | The authors concluded there was insufficient evidence to recommend probiotic therapy as an adjunct to antibiotic therapy for C. difficile colitis, and no evidence to support the use of probiotics alone. |
| Crohn’s Disease | ||||||
| Rolfe et al. (2006) [40] | 7 | 160 | Pediatric: 5–21 Adults: >18 |
|
C | The authors concluded there was no evidence that probiotic preparations are superior to placebo or aminosalicylates for the maintenance of remission in patients with Crohn’s disease. |
| Butterworth et al. (2008) [38] | 1 | 11 | >18 |
|
C | The authors concluded that there was currently no evidence to support the use of probiotics for the induction of remission in Crohn’s disease. |
| Doherty et al. (2010) [39] | 5 | 363 | Not specified |
|
C | The authors concluded that there was no evidence that probiotics were superior to placebo. |
| Colitis | ||||||
| Naidoo et al. (2011) [43] *** | 4 | 587 | >17 |
|
C | The authors concluded there was no evidence to support the use of probiotics as an alternative to mesalazine for maintenance of remission. |
| Mallon et al. (2010) [42] | 4 | 244 | Not specified |
|
C | The authors concluded there was currently no evidence to suggest that probiotics are superior to placebo or aminosalicylates for the induction of remission in UC, and the use of probiotics as induction therapy for UC could not be recommended at this time. |
| Chande et al. (2009) [41] | 1 | 29 | Not specified |
For studies assessing treatment of active disease:
|
C | The authors concluded that there was no evidence for the effectiveness of probiotics. |
| Singh et al. (2015) [44]*** | 6 | 176 | ≥18 |
|
C | The authors concluded that it is reasonable to treat chronic pouchitis with antibiotics and perhaps probiotics, but additional trials are needed. |
| Liver conditions | ||||||
| Lirussi et al. (2009) [45] | 0 | n/a | n/a |
|
C | No randomized controlled trials fulfilling the inclusion criteria were found and the authors were unable to draw any conclusions about benefit or harm. |
| McGee et al. (2011) [46] *** | 7 | 550 | 30–74 |
|
C | The authors were unable to draw any conclusions about benefit or harm. |
(A) the review indicated good evidence of benefit from probiotics; (B) the review indicated good evidence of no benefit from probiotics; (C) the review indicated that there was not sufficient available evidence to allow benefits from probiotics to be determined
Review conclusions are based on GRADE assessment for the primary outcomes and for adverse events if GRADE was used in the review. For reviews that did not explicitly use GRADE, a summary of the amount and type of evidence from the “Effects of interventions” section was provided.
GRADE criteria used in the review.
Table 2 provides an overview of the intervention components described in each Cochrane review, including probiotics used and the number of trials that specified probiotic strain, dosage, intervention length, and duration of follow-up. Overall, Lactobacillus rhamnosus GG (LGG) was the most commonly studied probiotic, appearing in 10 of the 14 Cochrane reviews, followed by VSL#3 (7/14) and Saccharomyces boulardii (6/14). When taking into account the total number of randomized controlled trials included from all Cochrane reviews, 100 out of 160 trials (63%) specified the strain of the probiotic, 151 of 160 (94%) reported dosage, 126 of 160 (79%) specified intervention length, and 47 of 160 (29%) indicated duration of follow up.
Table 2.
Description of intervention components included in Cochrane reviews of probiotics and GI conditions.
| Author, Year | Exclusive number of probiotics per review N |
Trials that specify strain N (%) |
Trials that indicate dosage N (%) |
Trials that specify intervention length N (%) |
Trials that specify follow-up N (%) |
|---|---|---|---|---|---|
| Goldenberg et al. (2015) [35] | 17 | 11 (48%) | 23 (100%) | 7* (30%) | 17 (74%) |
| Bernaola Aponte et al. (2013) [34] | 4 | 3 (75%) | 4 (100%) | 4 (100%) | 0 (0%) |
| Allen et al. (2010) [33] | 21 | 42 (67%) | 62 (98%) | 45 (71%) | 0 (0%) |
| Goldenberg et al. (2013) [36] | 14 | 21 (68%) | 30 (97%) | 31 (100%) | 24 (77%) |
| Pillai et al. (2010) [37] | 3 | 4 (100%) | 4 (100%) | 4 (100%) | 4 (100%) |
| Rolfe et al. (2006) [40] | 4 | 6 (86%) | 7 (100%) | 7 (100%) | 0 (0%) |
| Butterworth et al. (2008) [38] | 1 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) |
| Doherty et al. (2010) [39] | 4 | 3 (60%) | 1 (20%) | 5 (100%) | 0 (0%) |
| Naidoo et al. (2011) [43] | 3 | 4 (100%) | 4 (100%) | 4 (100%) | 0 (0%) |
| Mallon et al. (2010) [42] | 4 | 1 (25%) | 4 (100%) | 4 (100%) | 0 (0%) |
| Chande et al. (2009) [41] | 1 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) |
| Singh et al. (2015) [44] | 3 | 2 (33%) | 5 (83%) | 6 (100%) | 0 (0%) |
| Lirussi et al. (2009) [45] | n/a | n/a | n/a | (n/a) | n/a |
| McGee et al. (2011) [46] | 7 | 1 (14%) | 5 (71%) | 7 (100%) | 0 (0%) |
Review paper lists the range of intervention length within the results text but it is not listed specifically for each study.
Full details of the probiotic species, strain, dosage, intervention length and follow-up period studied in each trial in each of the Cochrane reviews can be found in the Online Supporting Material Supplemental Table 1. Although each of the fourteen Cochrane reviews discussed adverse events within the text of the review, only 116 of 160 randomized controlled trials (73%) provided specific information regarding adverse events. Three of the Cochrane reviews conducted meta-analyses on adverse events; two Cochrane reviews did not find significant differences between the placebo and probiotic groups, and one Cochrane review reported a statistically significant decrease in the number of adverse events reported in probiotic group vs placebo. Among the 116 randomized individual trials, two trials reported adverse events associated with LGG: 1) mild GI upset with bloating and flatulence and 2) nausea, epigastric pain, constipation, vomiting, and intolerance to meds. One randomized controlled trial reported significantly more adverse events among the S. Boulardii group compared to placebo; symptoms included increase in thirst and constipation. Other reported adverse events were generally mild. When examining the assessments of benefit from probiotics in each Cochrane review, four Cochrane reviews were assigned a rating of “A”, and the remaining ten were assigned a rating of “C”. There were no Cochrane reviews in which it was clear that probiotics were not beneficial (i.e., a rating of “B”). A third reviewer was need to adjudicate in 1/14 (7%) of the cases.
Diarrhea
We identified five Cochrane reviews focused on treatment or prevention of diarrhea-related conditions, including pediatric antibiotic associated diarrhea, acute infectious diarrhea, and Clostridium difficile-associated diarrhea [33–37]. Two of the Cochrane reviews focused on children, one focused on adults, and two studied both populations. Four of the five trials were assigned an “A” review conclusion status. Three of the Cochrane reviews had sufficient data to assess all of their prespecified outcomes, and also performed subgroup analyses on factors related to species and dosage of probiotics. Of the two which did not perform subgroup analyses, one Cochrane review was limited by the small number of included trials and one Cochrane review did not mention whether subgroup analyses were intended. When taking into account the total number of randomized controlled trials included from the Cochrane reviews focusing on diarrhea-related outcomes (125 trials), 65% of trials specified probiotic strain and 98% indicated dosage. The proportion of trials that mentioned intervention length or follow up was 73% and 36%, respectively. The incidence of adverse events was addressed in all five Cochrane reviews, but was a pre-specified outcome in only four Cochrane reviews; 26% of the included trials did not mention assessment of adverse events. Regarding the degree of overlap of clinical trials between different Cochrane reviews, there was very little overlap in clinical trials included in each Cochrane review. Three trials included in the 2013 Cochrane review by Goldenberg et al [36] were also included in the 2015 Cochrane review by Goldenberg et al [35]. There was one trial included in both Cochrane reviews by Bernaola et al [34] and Allen et al [33].
Crohn’s Disease
We identified three Cochrane reviews assessing the efficacy of probiotics for Crohn’s Disease [38–40]. Two of the Cochrane reviews included patients of any age, and one Cochrane review did not specify the age of participants. All of the Cochrane reviews received a “C” rating for the author conclusion statement. One Cochrane review was able to assess one pre-specified outcome; however, additional pre-specified outcomes were not assessed due to lack of available data. Of the two remaining reviews, one assessed four out of five pre-specified outcomes, and the other was able to assess both of two pre-specified outcomes. Two of the Cochrane reviews did not mention whether subgroup analyses were intended and one Cochrane review was unable to conduct subgroup analysis due to insufficient data. However, this review did not list or describe the proposed subgroup analyses in the methods. The three Cochrane reviews included a total of 13 trials. Of these trials, 77% specified strain, 69% indicated dosage, 100% reported the length of the intervention, and 8% reported the duration of follow-up. Adverse events were a pre-specified outcome in all three reviews; however, one Cochrane review containing five trials did not specify which trials examined adverse events and stated within the results text that the risk of withdrawal and serious adverse events were similar to placebo. One trial overlapped in the Cochrane reviews by Rolfe [40] and Doherty [39], and one trial overlapped in the Cochrane reviews by Rolfe [40] and Butterworth [38].
Colitis
We found four Cochrane reviews evaluating the efficacy of probiotic therapy on the treatment of colitis [41–44]. Two of the Cochrane reviews did not specify age of participants included in the trials, one included adults >18years of age, and one included patients of any age. All of the Cochrane reviews were classified as “C” author conclusion statement. Two of the Cochrane reviews were able to assess all of the pre-specified outcomes. One review was able to address two out of six of their pre-specified outcomes, the remaining 4 outcomes were unable to be assessed because of lack of available data. One Cochrane review was unable to complete meta-analyses due to differences in probiotics used, outcomes and trial methodology. Subgroup analyses were not performed in any of the reviews. In one of the reviews, subgroup analyses were stated to have been planned but not performed due to insufficient data; however, this review did not list or describe the proposed subgroup analyses in the methods. None of the remaining Cochrane reviews mentioned whether subgroup analyses were intended. When considering the total number of randomized controlled trials included in these Cochrane reviews (15 trials), 53% of the trials specified strains, 93% indicated dosage, 100% reported the length of the intervention, and only 7% of trials mentioned a follow-up after treatment. The incidence of adverse events was a pre-specified outcome in all four Cochrane reviews; 80% of included trials mentioned assessment of adverse events. None of the clinical trials were repeated among Cochrane reviews.
Liver conditions
There were two Cochrane reviews focused on the impact of probiotics on liver conditions [45, 46]. One review was an empty review [47] because the authors were unable to find any randomized controlled trials applicable to the topic of nonalcoholic steatohepatitis, or fatty liver disease. The second Cochrane review included adults of various ages, depending on the trial criteria. This review was assigned a “C” rating for the author conclusions statement. This review assessed six out of eight of their prespecified outcomes; the two remaining outcomes were not reported in the included trials and therefore could not be assessed. Statistically significant differences were noted in subgroup analyses by genus of probiotic and grade of hepatic encephalopathy. There were seven randomized controlled trials included in this review. Of these trials, 14% specified strain, 71% specified dosage, 100% reported intervention length, and 0% reported follow up. Adverse events were a pre-specified outcome in both reviews and there were no significant differences in adverse events found between probiotic and placebo/no intervention groups and in comparisons of adverse events between probiotics and standard therapy groups.
Discussion
Although probiotics are increasingly commonly used by both the general public and in clinical practice, inference based upon the evidence is currently hampered due to heterogeneity in both the probiotics utilized in clinical trials and in the assessment of outcomes in these studies. This was the first overview of Cochrane reviews of probiotics for GI-related medical conditions. This overview revealed that the heterogeneity in results appears to be related to the use of different probiotic types, doses, and treatment durations within clinical trials studying probiotics. There were a wide variety of probiotic species studied in the trials, of which many did not specify the dose and an even larger proportion did not define the strain. In many cases where strain and dosage was specified, trials using the same probiotic strain utilized different dosages.
This overview revealed that positive outcomes were generally observed with diarrhea-related conditions. All four Cochrane reviews that received an “A” conclusion indicating good evidence of benefit from probiotics focused on the diarrhea-related conditions. This likely reflects that these reviews, which were published between 2010 and 2015, include the most up-to-date and complete picture of currently available evidence. This overview also revealed that there have been a number of clinical trials focused on probiotics in recent years. This is important because most of the ‘C’ reviews were published prior to 2011 and might have had clearer conclusions (either ‘A’ or ‘B’) if they included more recent evidence. The other three categories of GI disorders, Crohn’s disease, colitis and liver conditions, contained Cochrane reviews that received a “C” conclusion indicating that there was not sufficient available evidence to allow benefits from probiotics to be determined. Crohn’s disease and colitis are two conditions associated with changes in the microbiota where primary treatment strategies focus on alleviating symptoms, inactivating the disease and preventing relapses [48]. There is also growing evidence to suggest a connection between alterations in the microbial composition of the gut and chronic liver diseases [49]. Probiotics are more commonly being used as a complementary approach to combat dysbiosis that is associated with these conditions [48, 49]. Searches for newer trials on probiotics for these conditions should be carried out so that the Cochrane reviews may be updated if appropriate, and the review evidence on probiotics may reflect the current underlying evidence base. Updating these older reviews and providing timely evidence should be a priority.
The complexity of the effects of probiotics on GI disorders is due in part to the fact that probiotics are formulated into many different products, including foods, dietary supplements, and functional foods. Furthermore, the term “probiotics” is often used as a catch all term for probiotics, prebiotics (nondigestible food ingredients which can stimulate the growth of gut bacteria) and synbiotics (a combination of a probiotic and prebiotic). The types of probiotics used including the strains, dosages and length of intervention has been highly variable which complicates conclusions drawn from studying probiotics. It is extremely important to recognize that the health benefits attributed to probiotics significantly varies by strain [50]. As noted in Table 2, there was a large variation in the number of trials that specified the strains utilized within the Cochrane reviews. It is critically important that clinical trials specify the strains of the species in the probiotic and do not solely provide the species, given the widely varying health promoting effects by strain. Accordingly, systematic reviews and meta-analyses should report information on the strains of the probiotic species when these data are available.
Additionally, the majority of reviews and included trials did not address the issue of product storage or the quality of probiotics used within the trials. Most studies also did not confirm the viability or microbiological identity of the probiotic species in the product which likely has an influence on subsequent results. We found one review that addressed the viability of the probiotics used within the trials, but this factor was not consistently addressed across reviews, possibly because it was not addressed in the individual trials.
To further complicate the interpretation of the results, there are more than 1000 different species and more than 3 million unique genes that have been discovered within the microbiome [5, 51, 52]. Additionally, diversity of the microbiome significantly varies between healthy individuals; however the pronounced difference is more commonly observed among infants and appears to diminish with age. More importantly, novel bacterial populations such as bifidobacteria and butyrate-producing colon bacteria [53] or Akkermansia muciniphila [54] are currently being studied for potential protective benefits given frequent associations with healthy microbiota in adults, which in the future could eventually be used as a treatment strategy to restore intestinal balance associated with inflammatory gastrointestinal diseases. As the pool of research surrounding the microbiome expands, the interaction of these complex systems and probiotics within the human gut will continue to be explored.
In addition to the aforementioned concerns regarding the variability of the probiotics under study, there was substantial heterogeneity observed among the Cochrane reviews with respect to the outcomes assessed within trials. For example, Goldenberg and colleagues [35] reported that the primary investigators’ definition of diarrhea varied among studies; among the 23 clinical trials included in this Cochrane review, 9 different definitions of diarrhea were used. The use of standardized definitions for outcomes is important because if the outcomes are very different or defined in markedly different ways they may not be appropriate for combining in a meta-analysis. Trials focused on the same health condition and intervention should ideally assess the same clinically-meaningful outcomes and collect them in a similar fashion in order to allow pooling, meta-analysis, and comparison across trials. Core outcome sets can be useful because they ensure that trials collect the same outcomes in standard ways, which increases availability of the most important and relevant information for meta-analyses. One initiative focused on this work is the Core Outcome Measures in Effectiveness Trials (COMET) [55] which recommends including a minimum set of outcomes that should be measured and reported in clinical trials on a specific condition.
Subgroup analyses are often conducted in Cochrane reviews as a means of answering specific questions regarding certain patient or intervention characteristics that may explain some of the heterogeneity within the meta-analysis and reveal differences in intervention effects across subgroup factors. It is important for reviews to pre-specify subgroup analyses to prevent study results from influencing which factors are investigated, therefore possibly leading to misleading results [56]. In our overview of Cochrane systematic reviews, approximately half of the reviews prespecified at least one subgroup analysis in the methods section; although in many instances, there were insufficient data to carry out the proposed subgroup analyses. Five of the fourteen Cochrane reviews planned to examine the type of probiotic used, including dosage, species, or strain, and were unable to do so due to insufficient data. Clinical trials need to report this important information on the probiotics under study in order to apply these results to clinical practice, particularly given the wide range of probiotics and dosages used among the randomized clinical trials. Additionally, none of the reviews that mentioned subgroup analysis planned to examine differences in age. Given the age-related changes in the microbiome, this is an area that needs to be addressed in future studies.
There are a number of strengths of this Cochrane-focused overview of the effect of probiotics in GI disorders. Cochrane reviews are internationally recognized as the gold standard of evidence-based information in healthcare. The methodology utilized through Cochrane are further strengthened by a commitment to transparency and minimizing bias by undergoing rigorous peer review process and avoiding conflicts of interest. The Cochrane collaboration also ensures quality by updating the reviews as new evidence emerges. Another strength of Cochrane reviews is that they specify adverse events as a prespecified outcome. However, a large proportion of the individual RCTs included in the Cochrane reviews did not address adverse events. Often, there was not a consistent record of adverse events within the individual trials, so adverse event data could not be pooled for analysis. This is important when considering the safety of probiotics, and adverse events should be included as a core outcome measure in future clinical trials of probiotics.
A limitation of this study is that information on trials was extracted from the tables and from the body of the text in the Cochrane reviews rather than directly from clinical trial reports. However, this may be expected to be an informative reflection of the evidence that was available to the authors of the Cochrane reviews. An additional limitation of this study is that our overview did not include IBS as one of the GI conditions. IBS is one of the most common reasons that probiotics are consumed in clinical practice and also one of the most commonly studied, with over 80 clinical trials of probiotics for IBS. Although there have been over 150 systematic reviews and/or meta-analyses focused on probiotics and GI conditions, there has yet to be a completed Cochrane review evaluating probiotics for IBS. Cochrane is the gold standard of systematic reviews and future Cochrane reviews should focus on IBS, including both IBS-C and IBS-D, conditions where probiotics are often used due to current lack of pharmacological treatment options.
Conclusion
The results of this overview of Cochrane systematic reviews of probiotics for GI disorders suggests that probiotics can have a beneficial impact on diarrheal conditions and related gastrointestinal symptoms. While encouraging, additional studies are needed to make conclusive inference on the efficacy of probiotics for colitis, Crohn’s disease, and liver disorders. Among the reasons contributing to the inconclusive evidence for these disorders is the heterogeneity in the outcomes assessed across clinical trials, the variable quality of the reporting in the scientific literature on key details of the probiotics that were studied, and the even greater variability in the composition and quality of the probiotics utilized in the studies. Thus, future probiotics clinical trials, systematic reviews, and meta-analyses should specify important and often unreported details including the species, strain, dosage, and manufacturing processes and storage conditions of the probiotics utilized in the study. In addition, future studies would also ideally include core outcome measures that are collected in a standardized manner to allow for more precise assessment of probiotic efficacy. Future systematic reviews should evaluate these aspects of the probiotics intervention as well as whether patient characteristics (e.g. age, dietary intake, antibiotic usage, etc.) and treatment duration are related to treatment effect. Finally, there is a need for updated systematic reviews to reflect the totality of current trial evidence on probiotics interventions.
In the meantime, there are currently a variety of important issues related to the translation of our findings that researchers and clinicians should consider when utilizing probiotics to support gastrointestinal health. For instance, the optimal timing for probiotic intervention in the human lifespan remains an area in need of additional clarity. While some studies have suggested that the microbiota are relatively consistently colonized by early childhood, antibiotic-induced perturbations in the adult microbiota and the many supportive clinical trials and systematic reviews conducted among adult populations suggest that probiotics may offer benefits across the human lifespan. However, referring to the benefits of “probiotics” in a general sense is overly broad and much more specificity is required in this field to reflect the marked differences between various probiotics and more precisely inform the ways in which patients may benefit from probiotic intervention. In light of the varying strain-specific effects of many probiotic species, probiotics that specify the strain of each species in the product are preferable to help ensure known and desirable clinical effects. At present, only a select few commercially-available probiotic supplements specify the strains of the probiotic microorganisms and more products should follow suit. In addition, probiotic manufacturing, shipping, and storage processes can all affect the viability and maintenance of the desired dosage of the probiotic microorganisms by the time they are consumed. Probiotics utilized in research and clinical care should ideally be shipped and stored cold to ensure viability throughout the shipping and storage process. More generally, supportive evidence of the clinical effects and viable potency of the probiotics by the time they arrive to the clinic or consumer is of paramount importance for optimal efficacy. At present, there are no requirements for providing information on the strain, timing of administration, shipping and storage conditions, or evidence of potency on the labels of probiotic products. A consistent labeling standard for probiotics with information on these critical parameters would greatly help researchers, clinicians, and consumer make informed choices in the utilization of probiotics to support gastrointestinal health. While the focus of this overview is on gastrointestinal conditions, consideration of these issues would also be important when utilizing probiotics for other health purposes.
Supplementary Material
Highlights.
The human microbiota is a very complex system influenced by numerous factors.
Positive outcomes were observed with diarrhea-related conditions and probiotic use.
Improved conduct of probiotics studies would enhance inference for all GI outcomes.
Acknowledgments
The authors (EAP, CRD, TR, LSW) were solely responsible for the manuscript preparation, revision and publication decisions. All authors have read and approved the final manuscript.
Funding/Support: This study was supported in part by NIH Grant Number R24 AT001293 from the National Center for Complementary and Integrative Health (NCCIH) and the University of Maryland School of Medicine Summer Program in Obesity, Diabetes, and Nutrition Research Training (NIH grant T35 DK095737).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interests to declare.
References
- 1.Probiotics in food: Health and nutritional properties and guidelines for evaluation. Vol. 85. Rome: World Health Organization and Food and Agricultural Organization of the United Nations; 2006. [Google Scholar]
- 2.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. National health statistics reports. 2015;(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley J, Johnson J, Polito R. 2015 Supplement Business Report. Nutrition Business Journal. 2015 [Google Scholar]
- 4.Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: A descriptive study of 145 U.S. hospitals. American Journal of Infection Control. 2016;44:548–553. doi: 10.1016/j.ajic.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vos WM, de Vos EA. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutrition reviews. 2012;70(Suppl 1):S45–56. doi: 10.1111/j.1753-4887.2012.00505.x. [DOI] [PubMed] [Google Scholar]
- 6.Berggren A, Ahren Lazou I, Larsson N, Onning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. European journal of nutrition. 2011;50:203–210. doi: 10.1007/s00394-010-0127-6. [DOI] [PubMed] [Google Scholar]
- 7.Cao L, Wang L, Yang L, Tao S, Xia R, Fan W. Long-term effect of early-life supplementation with probiotics on preventing atopic dermatitis: A meta-analysis. The Journal of dermatological treatment. 2015;26:537–540. doi: 10.3109/09546634.2015.1027168. [DOI] [PubMed] [Google Scholar]
- 8.Kosiewicz MM, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Frontiers in microbiology. 2011;2:180. doi: 10.3389/fmicb.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makino S, Ikegami S, Kume A, Horiuchi H, Sasaki H, Orii N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. The British journal of nutrition. 2010;104:998–1006. doi: 10.1017/S000711451000173X. [DOI] [PubMed] [Google Scholar]
- 10.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. European journal of clinical nutrition. 2007;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 11.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British journal of nutrition. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 12.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. European journal of clinical nutrition. 2010;64:636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 13.Kadooka Y, Sato M, Ogawa A, Miyoshi M, Uenishi H, Ogawa H, Ikuyama K, Kagoshima M, Tsuchida T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. The British journal of nutrition. 2013;110:1696–1703. doi: 10.1017/S0007114513001037. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, Ngom-Bru C, Berger B, Philippe L, Ammon-Zuffrey C, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. The British journal of nutrition. 2014;111:1507–1519. doi: 10.1017/S0007114513003875. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan A, Nord CE. The place of probiotics in human intestinal infections. International journal of antimicrobial agents. 2002;20:313–319. doi: 10.1016/s0924-8579(02)00199-1. [DOI] [PubMed] [Google Scholar]
- 16.Islam SU. Clinical Uses of Probiotics. Medicine (Baltimore) 2016;95:e2658. doi: 10.1097/MD.0000000000002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders ME. Probiotics: definition, sources, selection, and uses. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(Suppl 2):S58–61. doi: 10.1086/523341. discussion S144–151. [DOI] [PubMed] [Google Scholar]
- 18.Henker J, Laass M, Blokhin BM, Bolbot YK, Maydannik VG, Elze M, Wolff C, Schulze J. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. European journal of pediatrics. 2007;166:311–318. doi: 10.1007/s00431-007-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Alimentary Pharmacology & Therapeutics. 1997;11:853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 20.Malchow HA. Crohn's disease and Escherichia coli A new approach in therapy to maintain remission of colonic Crohn's disease? Journal of clinical gastroenterology. 1997;25:653–658. doi: 10.1097/00004836-199712000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Kligler B, Cohrssen A. Probiotics. American Family Physician. 2008;78:1073–1078. [PubMed] [Google Scholar]
- 22.Cochrane: About Us. 2016. vol. [Google Scholar]
- 23.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. 2011. [Google Scholar]
- 24.Jadad AR, Cook DJ, Jones A, Klassen TP, Tugwell P, Moher M, Moher D. Methodology and reports of systematic reviews and meta-analyses: a comparison of Cochrane reviews with articles published in paper-based journals. Jama. 1998;280:278–280. doi: 10.1001/jama.280.3.278. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen AW, Hilden J, Gotzsche PC. Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: systematic review. BMJ (Clinical research ed) 2006;333:782. doi: 10.1136/bmj.38973.444699.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moseley AM, Elkins MR, Herbert RD, Maher CG, Sherrington C. Cochrane reviews used more rigorous methods than non-Cochrane reviews: survey of systematic reviews in physiotherapy. Journal of clinical epidemiology. 2009;62:1021–1030. doi: 10.1016/j.jclinepi.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Tricco AC, Tetzlaff J, Pham B, Brehaut J, Moher D. Non-Cochrane vs. Cochrane reviews were twice as likely to have positive conclusion statements: cross-sectional study. Journal of clinical epidemiology. 2009;62:380–386.e381. doi: 10.1016/j.jclinepi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Useem J, Brennan A, LaValley M, Vickery M, Ameli O, Reinen N, Gill CJ. Systematic Differences between Cochrane and Non-Cochrane Meta-Analyses on the Same Topic: A Matched Pair Analysis. PloS one. 2015;10:e0144980. doi: 10.1371/journal.pone.0144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Windsor B, Popovich I, Jordan V, Showell M, Shea B, Farquhar C. Methodological quality of systematic reviews in subfertility: a comparison of Cochrane and non-Cochrane systematic reviews in assisted reproductive technologies. Human reproduction (Oxford, England) 2012;27:3460–3466. doi: 10.1093/humrep/des342. [DOI] [PubMed] [Google Scholar]
- 30.Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. The Cochrane database of systematic reviews. 2015;(2):CD007137. doi: 10.1002/14651858.CD007137.pub4. doi:CD007137. [DOI] [PubMed] [Google Scholar]
- 31.Saraswati S, Sitaraman R. Aging and the human gut microbiota-from correlation to causality. Frontiers in microbiology. 2015;5:764. doi: 10.3389/fmicb.2014.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. The Cochrane database of systematic reviews. 2010;(11):CD003048. doi: 10.1002/14651858.CD003048.pub3. doi:CD003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernaola Aponte G, Bada Mancilla CA, Carreazo Pariasca NY, Rojas Galarza RA. Probiotics for treating persistent diarrhoea in children. The Cochrane database of systematic reviews. 2010;(11):CD007401. doi: 10.1002/14651858.CD007401.pub2. doi:CD007401. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg JZ, Lytvyn L, Steurich J, Parkin P, Mahant S, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. The Cochrane database of systematic reviews. 2015;(12):CD004827. doi: 10.1002/14651858.CD004827.pub4. doi:CD004827. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg JZ, Ma SS, Saxton JD, Martzen MR, Vandvik PO, Thorlund K, Guyatt GH, Johnston BC. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. The Cochrane database of systematic reviews. 2013;(5):CD006095. doi: 10.1002/14651858.CD006095.pub3. doi:CD006095. [DOI] [PubMed] [Google Scholar]
- 37.Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. The Cochrane database of systematic reviews. 2008;(1):CD004611. doi: 10.1002/14651858.CD004611.pub2. doi:CD004611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn's disease. The Cochrane database of systematic reviews. 2008;(3):CD006634. doi: 10.1002/14651858.CD006634.pub2. doi:CD006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post-operative recurrence of Crohn's disease. The Cochrane database of systematic reviews. 2009;(4):CD006873. doi: 10.1002/14651858.CD006873.pub2. doi:CD006873. [DOI] [PubMed] [Google Scholar]
- 40.Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall F. Probiotics for maintenance of remission in Crohn's disease. The Cochrane database of systematic reviews. 2006;(4):CD004826. doi: 10.1002/14651858.CD004826.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Chande N, McDonald JW, Macdonald JK. Interventions for treating collagenous colitis. The Cochrane database of systematic reviews. 2008;(2):CD003575. doi: 10.1002/14651858.CD003575.pub5. doi:CD003575. [DOI] [PubMed] [Google Scholar]
- 42.Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. The Cochrane database of systematic reviews. 2007;(4):CD005573. doi: 10.1002/14651858.CD005573.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Naidoo K, Gordon M, Fagbemi AO, Thomas AG, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. The Cochrane database of systematic reviews. 2011;(12):CD007443. doi: 10.1002/14651858.CD007443.pub2. doi:CD007443. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Stroud AM, Holubar SD, Sandborn WJ, Pardi DS. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. The Cochrane database of systematic reviews. 2015;(11):CD001176. doi: 10.1002/14651858.CD001176.pub3. doi:CD001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lirussi F, Mastropasqua E, Orando S, Orlando R. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. The Cochrane database of systematic reviews. 2007;(1):CD005165. doi: 10.1002/14651858.CD005165.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGee RG, Bakens A, Wiley K, Riordan SM, Webster AC. Probiotics for patients with hepatic encephalopathy. The Cochrane database of systematic reviews. 2011;(11):CD008716. doi: 10.1002/14651858.CD008716.pub2. doi:CD008716. [DOI] [PubMed] [Google Scholar]
- 47.Yaffe J, Montgomery P, Hopewell S, Shepard LD. Empty reviews: a description and consideration of Cochrane systematic reviews with no included studies. PloS one. 2012;7:e36626. doi: 10.1371/journal.pone.0036626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheifetz AS, Gianotti R, Luber R, Gibson PR. Complementary and Alternative Medicines Used by Patients With Inflammatory Bowel Diseases. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Elzouki AN. Probiotics and Liver Disease: Where Are We Now and Where Are We Going? J Clin Gastroenterol. 2016;50(Suppl 2):S188–S190. doi: 10.1097/MCG.0000000000000712. Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13–15, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Senok AC, Ismaeel AY, Botta GA. Probiotics: facts and myths. Clin Microbiol Infect. 2005;11:958–966. doi: 10.1111/j.1469-0691.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 51.Brahe LK, Astrup A, Larsen LH. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv Nutr. 2016;7:90–101. doi: 10.3945/an.115.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riviere A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez-Gallego C, Pohl S, Salminen S, De Vos WM, Kneifel W. Akkermansia muciniphila: a novel functional microbe with probiotic properties. Benef Microbes. 2016;7:571–584. doi: 10.3920/BM2016.0009. [DOI] [PubMed] [Google Scholar]
- 55.Williamson P, Clarke M. The COMET (Core Outcome Measures in Effectiveness Trials) Initiative: Its Role in Improving Cochrane Reviews. The Cochrane database of systematic reviews. 2012;(5):ED000041. doi: 10.1002/14651858.ED000041. doi:ED000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryan RCC. Communication Review G: Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Review Group reviews: planning the analysis at protocol stage. 2014;2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.