Abstract
Aging and testosterone almost inexorably cause benign prostatic hyperplasia (BPH) in Human males. However, etiology of BPH is largely unknown. Serotonin (5-HT) is produced by neuroendocrine prostatic cells and presents in high concentration in normal prostatic transition zone, but its function in prostate physiology is unknown. Previous evidence demonstrated that neuroendocrine cells and 5-HT are decreased in BPH compared to normal prostate. Here, we show that 5-HT is a strong negative regulator of prostate growth. In vitro, 5-HT inhibits rat prostate branching through down-regulation of androgen receptor (AR). This 5-HT’s inhibitory mechanism is also present in human cells of normal prostate and BPH, namely in cell lines expressing AR when treated with testosterone. In both models, 5-HT’s inhibitory mechanism was replicated by specific agonists of 5-Htr1a and 5-Htr1b. Since peripheral 5-HT production is specifically regulated by tryptophan hydroxylase 1(Tph1), we showed that Tph1 knockout mice present higher prostate mass and up-regulation of AR when compared to wild-type, whereas 5-HT treatment restored the prostate weight and AR levels. As 5-HT is decreased in BPH, we present here evidence that links 5-HT depletion to BPH etiology through modulation of AR. Serotoninergic prostate pathway should be explored as a new therapeutic target for BPH.
Introduction
Benign prostatic hyperplasia (BPH) is one of the main causes of non-neurogenic lower urinary tract symptoms (LUTS) in the aging male1,2. The underlying mechanism responsible for BPH is not understood, and only elucidating the etiology of BPH will increase our ability to treat or even prevent its development.
Currently the most accepted hypothesis for the etiology of BPH is, that proposed by McNeal, in which BPH results from the reawakening of inductive potential in adult prostatic stroma in a specific prostatic region defined as transition zone3–5. This hypothesis claimed that the adult prostatic epithelium retains the ability to respond to inductive stromal signaling with new ductal branching morphogenesis6,7. However this hypothesis does not respond to the critical question of why this reawakening of human adult prostatic stroma occurs.
While there is no BPH without testosterone8, testosterone levels decrease with age9,10 and no direct correlation between testosterone concentration and prostate volume has been established yet11. Moreover, it is widely accepted that physiologic concentrations of testosterone provide an excess of testosterone for optimal prostatic growth suggesting that testosterone is not the etiologic factor responsible for BPH12. On the other hand, several reports have documented an up-regulation of the androgen receptor (AR) in BPH tissue, unveiling a potential role for AR in BPH etiopathogenesis13–15.
The neuroendocrine prostatic cells secrete various neuroendocrine factors with 5-HT being one of the most abundant. The peculiar morphology of some neuroendocrine cells with dendritic processes extending to lumen and projections surrounding the epithelial-stroma interface justify the hypothesis that neuroendocrine products, namely 5-HT, could regulate prostate growth16. Notably, neuroendocrine prostatic cells are mainly located in the transition zone of the normal human prostate17, where BPH originates4. However, comparing BPH tissue with normal transition zone (without BPH) the number of neuroendocrine cells is extraordinarily decreased18–20. Also 5-HT was shown to be significantly depleted in BPH tissue19. Furthermore, a recent study in a large cohort of Scandinavian men revealed that LUTS are associated with benign prostate enlargement and to decreased plasmatic 5-HT concentration21. These findings suggest a potential link between prostatic 5-HT depletion and BPH etiology; however, the function of 5-HT in regulation of benign prostate growth has never been studied.
We hypothesized that 5-HT had an inhibitory function over benign prostate growth and that suppression of prostatic 5-HT production could be responsible for benign prostatic growth. The aim of this study was to define the role of 5-HT in the regulation of benign prostatic growth and to test the pharmacologic modulation of the prostatic serotoninergic system as a new pharmacological target for BPH.
Results
5-HT, 5-Htr1a, and 5-Htr1b specific agonists inhibits rat ventral prostate branching through AR down-regulation
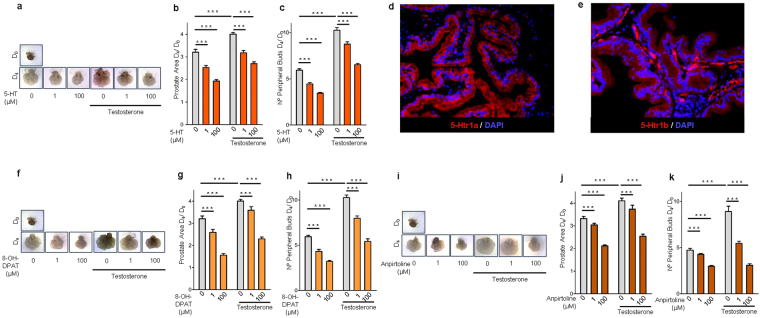
The new epithelial gland formation observed in BPH is normally seen only during prostate branching morphogenesis22. For this reason, we first tested the hypothesis that 5-HT could regulate prostate growth using in vitro cultures of rat ventral prostate explants (VPs) from P1 newborns. During 4 days in culture, 5-HT supplementation induced a significant dose-dependent inhibition of rat VPs growth (Fig. 1a), as expressed by decreased area (Fig. 1b), as well the number of peripheral explant buds (Fig. 1c). In medium conditions without additional testosterone supplementation, inhibitory effect of 5-HT over VPs growth was maximal at 100 µM where a reduction of 40% in prostate area D4/D0 (p < 0.001) and a reduction of 42% in the number of peripheral buds D4/D0 (p < 0.001) was observed in comparison to the control group (0 µM 5-HT). As expected, testosterone supplementation of VPs exerted a strong stimulatory effect on prostate branching morphogenesis, mainly in the number of peripheral buds (Fig. 1c), but again, 5-HT at 100 µM reduced 33% the prostate area D4/D0 (p < 0.001) and 36% the number of peripheral buds D4/D0 (p < 0.001) in comparison to control group (0 µM 5-HT + testosterone).
Figure 1.
5-HT, 5-Htr1a specific agonist and 5-Htr1b specific agonist inhibit prostate branching morphogenesis. (a) Photographs of representative VPs at D0 and at D4 of culture treated with different 5-HT concentrations. (b) Morphometric analysis of the effect of 5-HT on VPs area and (c) number of peripheral buds (n ≥ 12 VPs per group). (d) Immunofluorescence analysis of 5-Htr1a and (e) 5-Htr1b expression in the rat prostate. (f) Photographs of representative VPs at D0 and at D4 of culture treated with different 8-OH-DPAT concentrations. (g) Morphometric analysis of the effect of 8-OH-DPAT on VPs area and (h) number of peripheral buds (n ≥ 12 VPs per group). (i) Photographs of representative VPs at D0 and at D4 of culture treated with different anpirtoline concentrations. (j) Morphometric analysis of the effect of Anpirtoline on VPs area and (k) number of peripheral buds (n ≥ 12 VPs per group). Error bars indicate s.e.m. ***p < 0.001; two-way ANOVA and Bonferroni post hoc test. VPs, ventral prostate explants; D0, day 0; D4, day 4; 5-HT, serotonin.
From all 5-HT receptors, 5-Htr1a and 5-Htr1b were the most extensively studied in the regulation of malignant prostate growth23,24, so we tested if these receptors could contribute to the 5-HT inhibitory function in normal prostate growth. By immunofluorescence, we found that both receptors are strongly expressed in rat prostate but with a slightly different distribution pattern with 5-Htr1a predominantly expressed in prostate epithelium (Fig. 1d), while 5-Htr1b being expressed both in epithelium and stroma (Fig. 1e). To determine the contribution of both receptors in 5-HT inhibition of prostate branching morphogenesis, VPs were treated with drugs that specifically activate 5-Htr1a or 5-Htr1b. The selective 5-Htr1a agonist 8-OH-DPAT, (Fig. 1f,g and h) and the selective 5-Htr1b agonist, anpirtoline, (Fig. 1i, j and k) induced a significant dose-dependent inhibition of VPs growth. The inhibitory effect was maximal in VPs supplemented with testosterone and treated with 100 µM of anpirtoline, where a reduction of 39% in prostate area D4/D0 (p < 0.001) and a reduction of 66% in the number of peripheral buds D4/D0 (p < 0.001) was observed in comparison to the control group (0 µM anpirtoline + testosterone).
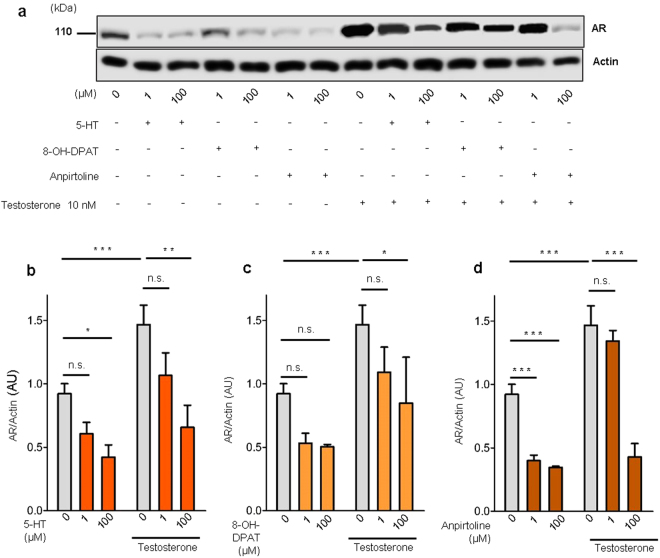
Since, androgens are a major prostatic stimulatory factor, we asked if the 5-HT inhibitory effect was related to the AR stimulatory pathway. By western blot analysis we showed that testosterone supplementation induced AR up-regulation, but 5-HT treatment significantly decreased AR expression either with or without testosterone supplementation (Fig. 2a and b) suggesting that the inhibitory function of 5-HT could be related to inhibition of the AR pathway. Similarly, both the selective 5-Htr1a agonist 8-OH-DPAT, (Fig. 2a and c) and the selective 5-Htr1b agonist, anpirtoline, (Fig. 2a and d) induced a significant AR down-regulation, more evident in anpirtoline treated VPs. Taken together these results indicate that in vitro 5-HT inhibits rat prostate growth through 5-Htr1a and 5-Htr1b, by down-regulating AR.
Figure 2.
5-HT, 5-Htr1a specific agonist and 5-Htr1b specific agonist down-regulates AR expression in rat ventral prostate. (a) Western blot analysis of AR expression in prostate explants treated with increasing doses of 5-HT, specific 5-Htr1a agonist, 8-OH-DPAT, and specific 5-Htr1b agonist, anpirtoline. (b) Quantification of AR protein in VPs treated with different concentrations of 5-HT in medium conditions without or with testosterone supplementation, n ≥ 3 (each sample contained a pool of 4 VPs). (c) Quantification of AR protein in VPs treated with different concentrations of 8-OH-DPAT in medium conditions without or with testosterone supplementation, n ≥ 3 (each sample contained a pool of 4 VPs). (d) Quantification of AR protein in VPs treated with different concentrations of Anpirtoline in medium conditions without or with testosterone supplementation, n ≥ 3 (each sample contained a pool of 4 VPs). Error bars indicate s.e.m. n.s. non-significant; *p < 0.05; **p < 0.01; ***p < 0.001; two-way ANOVA and Bonferroni post hoc test. VPs, ventral prostate explants; AR, androgen receptor; AU, arbitrary units; 5-HT, serotonin.
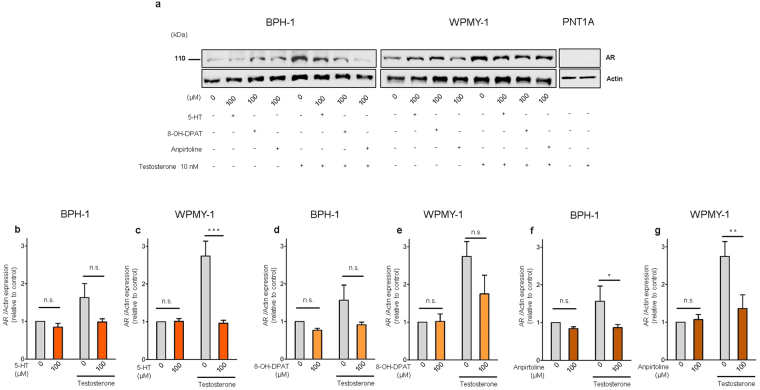
5-HT, 5-Htr1a or 5-Htr1b specific agonists inhibit growth of androgen sensitive human benign prostate cells through AR down-regulation
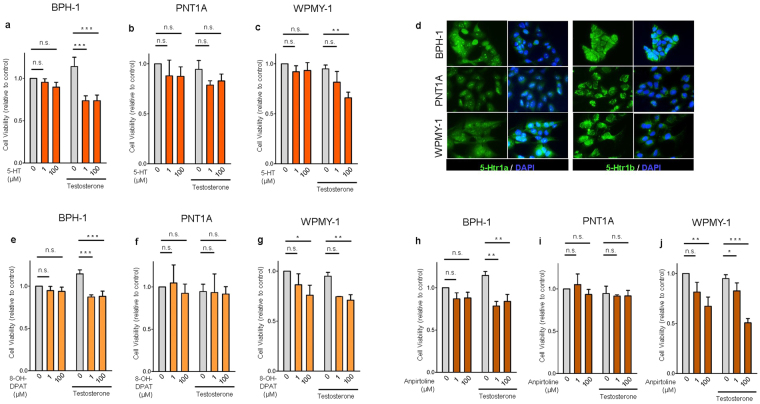
Next, we asked if this mechanism is also present in human prostate. With this purpose we performed in vitro 5-HT treatment of different human cell lines from epithelium of BPH (BPH-1), normal prostate epithelium (PNT1A) and normal prostate stroma (WPMY-1). We found that 5-HT significantly reduced cell viability of BPH-1 and WPMY-1 namely in the presence of testosterone but without changing PNT1A cell viability (Fig. 3a,b,c and Supplementary Fig. 1). The inhibitory effect of 5-HT was maximal in BPH-1 cells supplemented with testosterone. Under these conditions, 100 µM of 5-HT decreased cell viability by 35% compared to control (0 µM 5-HT + testosterone) (p < 0.001).
Figure 3.
5-HT, 5-Htr1a specific agonist and 5-Htr1b specific agonist inhibits cell viability in BPH-1 and WPMY-1 human prostatic cells without any effect in PNT1A cells. (a,b,c) Effect of 5-HT on cell viability analyzed by MTS assay in BPH-1, PNT1A and WPMY-1 cells. (d) Immunofluorescence analysis of 5-Htr1a and 5-Htr1b expression in BPH-1, PNT1A and WPMY-1 cells. (e,f,g) Effect of 5-Htr1a specific agonist, 8-OH-DPAT, and (h,i,j) 5-Htr1b specific agonist, Anpirtoline, on cell viability analyzed by MTS assay in BPH-1, PNT1A and WPMY-1 cells. The data are expressed relative to control condition (0 µM 5-HT without testosterone supplementation) and were reproduced in at least three independent experiments. Error bars indicate s.e.m. n.s. non-significant; 5-HT, serotonin; *p < 0.05; **p < 0.01; ***p < 0.001; two-way ANOVA and Bonferroni post hoc test.
Next, we tested if the growth inhibitory function of 5-HT in androgen human prostate cells BPH-1 and WPMY-1 was mediated by 5-Htr1a and 5-Htr1b. First, we showed that both receptors are expressed in the three human prostate cell lines (Fig. 3d) and that their specific agonists significantly inhibited cell viability (Fig. 3e–j and Supplementary Fig. 1) almost exclusively in the presence of testosterone, but again only in BPH-1 and WPMY-1, without any inhibitory effect in PNT1A cells.
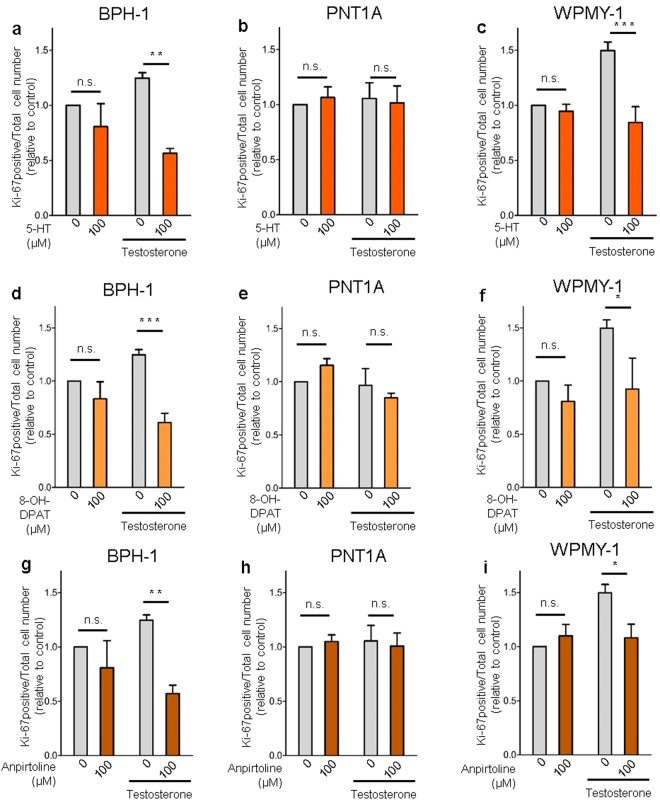
Additionally, Ki-67 staining confirmed that proliferation of BPH-1 and WPMY-1 cells supplemented with testosterone was significantly reduced by 5-HT treatment (Fig. 4a and c), while PNT1A cells proliferation was not affected (Fig. 4b). Similarly, both specific agonists of 5-Htr1a and 5-Htr1b strongly inhibited cell proliferation (Fig. 4d–i) but again only in BPH-1 and WPMY-1 cells.
Figure 4.
5-HT, 5-Htr1a specific agonist and 5-Htr1b specific agonist inhibits cell proliferation in BPH-1 and WPMY-1 human prostatic cells without any effect in PNT1A cells. (a,b,c) Effect of 5-HT, (d,e,f) 5-Htr1a specific agonist, 8-OH-DPAT, and (g,h,i) 5-Htr1b specific agonist, Anpirtoline, on cell proliferation of BPH-1, PNT1A and WPMY-1 cells as quantified by Ki-67 positive cells/total cell number ratio. The data are expressed relative to control condition (0 µM 5-HT without testosterone supplementation) and were reproduced in at least three independent experiments. Error bars indicate s.e.m. n.s. non-significant; 5-HT, serotonin; *p < 0.05; **p < 0.01; ***p < 0.001; two-way ANOVA and Bonferroni post hoc test.
Next, we investigated if this inhibitory function of 5-HT, 5-Htr1a and 5-Htr1b specific agonists was related to changes in the AR pathway. We observed that testosterone induced an up-regulation of AR in both BPH-1 and WPMY-1 cells. 5-HT, 5-Htr1a and 5-Htr1b specific agonists inhibited the AR up-regulation induced by testosterone in both BPH-1 and WPMY-1 cells (Fig. 5b). Regarding 5-HT effect in AR down-regulation this was very significant in WPMY-1 cells after testosterone supplementation (p < 0,001) while in BPH-1 cells a non-significant down-regulation of AR was observed (Fig. 5c and d). Also for both 5-Htr1a and 5-Htr1b specific agonists only Anpirtoline induced a significant down-regulation of AR in both BPH-1 and WPMY-1 cells (Fig. 5d,e,g,h). Additionally, by immunofluorescence analysis we observed that expression of AR after testosterone treatment was decreased in BPH-1 cells treated with 5-HT, 5-Htr1a and 5-Htr1b specific agonists (Supplementary Fig. 2).
Figure 5.
5-HT, 5-Htr1a specific agonist and 5-Htr1b specific agonist down-regulates AR expression in human prostatic cells. (a) Western blot analysis of AR expression in the three cell lines after 5-HT, 8-OH-DPAT and anpirtoline treatment. (b) Quantification of AR in BPH-1 and (c) WPMY-1 cells after 5-HT treatment in medium conditions without or with Testosterone supplementation. (d) Quantification of AR protein levels in BPH-1 and (e) WPMY-1 cells after 8-OH-DPAT treatment in medium conditions without or with Testosterone supplementation. (f) Quantification of AR protein levels in BPH-1 and (g) WPMY-1 cells after Anpirtoline treatment in medium conditions without or with Testosterone supplementation. The data are expressed relative to control condition (0 µM 5-HT without testosterone supplementation) and were reproduced in at least three independent experiments. Full, uncropped gel images are shown. Error bars indicate s.e.m. n.s. non-significant; *P < 0.05; **P < 0.01; ***P < 0.001; two-way ANOVA and Bonferroni post hoc test. AR, androgen receptor; 5-HT, serotonin.
Remarkably, the absence of inhibitory action of 5-HT, 5-Htr1a and 5-Htr1b specific agonists on PNT1A cells viability and proliferation, even in presence of testosterone, co-existed with a complete absence of AR expression in these cells (Fig. 5a). These data strongly argue that 5-HT’s inhibitory function on growth of benign human prostate cells is related with the suppression of the AR pathway.
In vivo ablation of peripheral 5-HT synthesis in mice induces benign prostatic growth
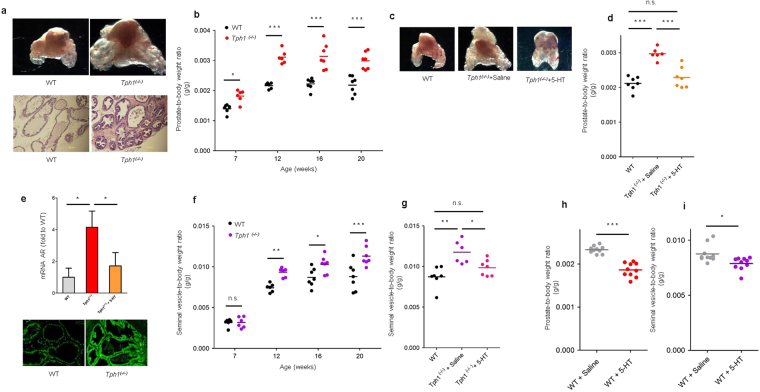
5-HT synthesis is initiated by tryptophan hydroxylase (Tph). Tph type 1 (Tph1) and 2 (Tph2) regulate 5-HT production in non-neuronal and neuronal tissues, respectively25,26. The majority of 5-HT in the body is produced by Tph1. In fact, Tph1 −/− mice exhibit very low levels of circulating 5-HT, while brain serotonin is not affected25. Based on our in vitro findings which suggest that 5-HT has a strong inhibitory action on prostate growth through down-regulation of AR, we used Tph1 −/− mice to evaluate the effect of peripheral 5-HT depletion on mouse prostate gland growth. Remarkably, Tph1 −/− mice exhibited a significantly 37% higher prostate-to-body weight ratio compared to wild-type at 20 weeks (p < 0.001) (Fig. 6a and b) without changes in body weight (Supplementary Fig. 3a). Interestingly, histology of the prostate gland revealed that Tph1 −/− mice exhibit areas of hyperplasia in epithelium and stroma (Fig. 6a, lower panel). To determine if 5-HT treatment could revert higher prostate mass in Tph1 −/− mice, we performed intraperitoneal injections of 5-HT at 10 consecutive days. 5-HT treatment resulted in significant mass reduction in prostate gland compared to levels similar to the wild-type (Fig. 6c and d) again without affecting animal weight (Supplementary Fig. 3b). Next, we asked if the higher prostate mass in Tph1 −/− mice was associated with different expression of AR. We could not demonstrate significant differences of AR expression by western blot analysis in total prostate (data not shown), however by immunofluorescence dorsolateral prostate of Tph1 −/− mice appeared to express more AR (Fig. 6e, lower panel). So, we investigated and demonstrated by qRT-PCR that the dorsolateral prostate of Tph1 −/− mice has increased levels of AR mRNA expression, while 5-HT treatment partially restores it to levels to wild-type mice (Fig. 6e, upper panel), reinforcing our hypothesis.
Figure 6.
Genetic deletion of Tph1 increases prostate gland mass. (a) Representative photographs of prostates from 20 week-old wild-type and Tph1 −/− mice (Top). Images from H&E staining of wild-type and Tph1 −/− prostates (n = 5 per genotype) (Bottom). (b) Prostate-to-body weight ratio of Tph1 −/− mice compared to wild-type at different ages (n = 6–7 for wild-type and Tph1 −/− mice, for each time point). (c) Representative photographs of prostates and (d) prostate-to-body weight ratio from 20 week-old wild-type, Tph1 −/− treated with saline and Tph1 −/− treated with 5-HT (n = 7 WT; n = 6 Tph1 −/− +Saline; n = 7 Tph1 −/− + 5-HT). (e) qRT-PCR for AR expression in dorsolateral lobe of 20 week-old wild-type, Tph1 −/− treated with saline and Tph1 −/− treated with 5-HT (n = 4 wild-type; n = 4 Tph1 −/− +Saline; n = 3 Tph1 −/− + 5-HT) (Top). Immunofluorescence analysis of AR expression in dorsolateral prostate of WT and Tph1 −/− mice (200x) (Bottom). (f) Seminal vesicle-to-body weight ratio of Tph1 −/− mice compared to wild-type mice at different ages (n = 6–7 for wild-type and Tph1 −/−; mice, for each time point). (g) Seminal vesicle-to-body weight ratio from 20 week-old wild-type, Tph1 −/− treated with saline and Tph1 −/− treated with 5-HT (n = 7 WT; n = 6 Tph1 −/− + Saline; n = 7 Tph1 −/− + 5-HT). (h) prostate-to-body weight ratio and (i) seminal vesical-to-body weight ratio in wild-type mice treated with Saline compared to wild-type mice treated with 5-HT during 10 consecutive days (n = 10 for each group). n.s. non-significant; AR, androgen receptor; 5-HT, serotonin; *p < 0.05; **p < 0.01; ***p < 0.001; (B,F) two-way and (D,G) one-way ANOVA and Bonferroni post hoc test. (H,I) Student t test.
It is well established that castration induces a strong reduction in size of the prostate gland as well as in seminal vesicles, while testosterone supplementation makes both organs return to normal size27,28. Interestingly, also seminal vesicles of Tph1 −/− mice were significantly larger than the ones of wild-type (Fig. 6f) suggesting that 5-HT could regulate androgen sensitivity not only in prostate gland but also in seminal vesicles. Again, we observed that the mass of seminal vesicles was partially restored by 5-HT treatment in Tph1 −/− mice (Fig. 6g).
Lastly, we tested if wild-type mice challenged with 5-HT treatment continue responding to 5-HT’s inhibitory action, and we demonstrated that both prostate gland (Fig. 6h) as well as seminal vesicle mass (Fig. 6i) were reduced while animal weight was not affected (Supplementary Fig. 3c).
Discussion
Currently, the etiology of BPH is unknown. However, it is accepted today that BPH is a consequence of aging and the simultaneous presence of testosterone3. BPH almost universally affects human males and a significant part of men will develop bothersome LUTS because of benign prostate enlargement. Although some of these men respond to current medical treatment (mainly α1-adrenoreceptors antagonists and 5 α-reductase inhibitors) a large portion continues to need a surgical procedure to treat resistant LUTS or have even more serious complications of BPH29, creating the emerging necessity for novel therapies.
In this study, we investigated the function of 5-HT in the regulation of non-malignant prostatic growth. Here, we demonstrated for the first time in several in vitro and in vivo models that 5-HT is a powerful negative regulator of prostatic growth through down-regulation of AR. We found that 5-Htr1a and 5-Htr1b are strongly expressed in the rodent prostate gland as well in human benign prostate cells, and that both receptors could mediate the inhibitory action of 5-HT on prostate growth.
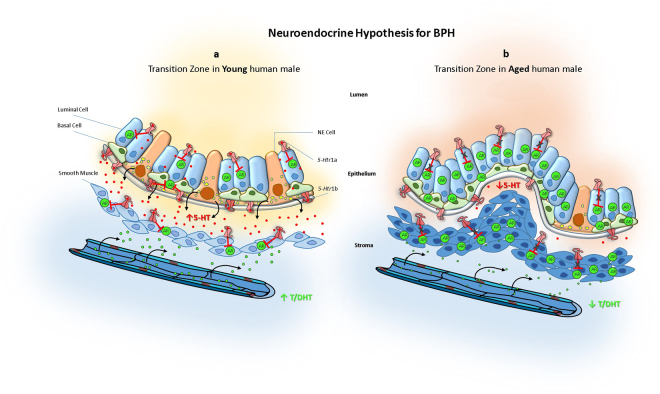
Our in vitro and animal findings lead us to propose a new mechanism to explain the development of BPH in humans (Fig. 7). Our proposed model explains how the depletion of neuroendocrine cells and serotonin observed in prostatic transition zone with aging18–20, could be the etiologic factor responsible for the initiation and progression of BPH. In our model, the depletion of serotonin induces an up-regulation of androgen receptor in the prostatic transition zone leading to the stimulation of benign prostatic growth in this specific prostatic region.
Figure 7.
Neuroendocrine hypothesis for etiopathogenesis of benign prostatic hyperplasia. (a) In young human male, prostate transition zone is enriched with 5-HT producing neuroendocrine cells. Serotonin is secreted to the epithelium-stroma interface and through activation of 5-Htr1a and 5-Htr1b, both in epithelium and stroma, the expression of AR is decreased. Yet, more testosterone is delivery to prostate, down-regulated AR limits benign prostate growth. (b) In aged human male, transition zone loses 5-HT producing neuroendocrine cells causing a depletion in local 5-HT. As a consequence 5-Htr1a and 5-Htr1b release their inhibition over AR expression. Although with aging the delivery of testosterone to the prostate is decreased the up-regulation of AR induces the development of BPH. 5-HT, serotonin; AR, androgen receptor; DHT, Dihydrotestosterone; NE, neuroendocrine; T, testosterone.
Our neuroendocrine model for the etiopathogenesis of BPH would resolve an intriguing question about the crucial participation of androgens in the development of BPH. Previous studies have showed that testosterone does not increase with aging and some studies have even reported that plasmatic testosterone is decreased in the aging human male9–11. In a similar way, intraprostatic testosterone, in particular DHT, are not increased in BPH comparatively to normal prostate30 and even the administration of testosterone in supra-physiologic concentrations to eugonadal men does not induce the development of BPH31,32. This data were the basis for the saturation model of prostate growth proposed by Morgentaler et al. suggesting that prostate gland growth is extraordinarily sensitive to low androgen concentrations (near the castrate range) but insensitive to androgen concentrations above a certain saturation point12. The critical point, in saturation model is that the rate-limiting step for prostatic growth, which is the concentration of AR. Recent studies demonstrated that AR is in fact up-regulated in stroma and epithelium of BPH tissue comparatively to normal prostate, implicating AR in etiophatogenesis of BPH13–15. Our findings seem to provide an explanation for this current view about the participation of androgens in the development of BPH. In this way, the loss of neuroendocrine cells and serotonin in prostatic transition zone up-regulates the AR and then permits the development of BPH, even with a decreased plasmatic concentration of androgens observed in the aging male.
Our first in vitro experimental approach focused on the function of 5-HT in the regulation of rat prostate branching morphogenesis. Because BPH is the result of new branching morphogenesis, this model permits the study the influence of 5-HT exactly in the mechanism by which BPH develops and progresses. Here, we demonstrate that 5-HT strongly inhibit the branching morphogenesis of the prostate gland through down-regulation of AR. In fact, other organs like the prostate which have a development process of branching morphogenesis, such as the mammary gland, 5-HT also have demonstrated a development inhibitory action33.
Although new in the prostate gland, the serotoninergic inhibitory mechanism through down-regulation of AR, is well known in the brain. In fact, the complete masculinization of the brain is dependent of a perinatal surge of testosterone and a simultaneous decrease in hypothalamic 5-HT concentrations34. In the brain, it has been demonstrated that regulation of 5-HT concentration is crucial for normal sexual differentiation, where 5-HT down-regulates AR35,36. In agreement with our findings, Sayed et al. demonstrated that dapoxetine decreased AR expression and prevents testosterone-induced BPH in rats37. However, both in brain and prostate the full mechanism responsible for this down-regulation remains to be elucidated.
The most described 5-HT receptors in prostatic cells, are the 5-Htr1a and 5-Htr1b. Therefore, we characterize its expression in rat prostate for the first time. As we demonstrated both 5-Htr1a and 5-Htr1b are strongly expressed in rat prostate and the activation of these receptors resulted in a significant inhibition of prostate branching morphogenesis, which also occurred through down-regulation of AR. In human prostatic cells the function of 5-HT, 5-Htr1a, and 5-Htr1b have been previously studied but only in malignant cells23,24. These previous reports showed that 5-HT has a proliferative effect in several malignant cell lines through 5-Htr1a and 5-Htr1b. Curiously, this stimulatory effect was mainly evident in androgen-insensitive cells23. Here we studied, for the first time, the function of 5-HT, 5-Htr1a, and 5-Htr1b in human benign prostatic cells. We demonstrated that 5-HT inhibits proliferation of androgen sensitive benign prostatic cells, and this inhibitory function was associated to a down-regulation of AR. This different growth function of 5-HT in benign and malignant cells remains unexplained but the predominant stimulatory effect of 5-HT in androgen-insensitive malignant cells suggests that castration resistance could change the phenotypic response of prostatic cell to neuroendocrine products.
Finally, to test in vivo our mechanistic approach to the etiopathogenesis of BPH, we genetically ablated the peripheral production of 5-HT. Using Tph1−/− mice we demonstrated that prostatic 5-HT depletion induces benign prostatic growth. The inhibition of peripheral 5-HT synthesis in mice, through genetic deletion of Tph1, simulates a decrease in the prostate transition zone 5-HT observed in the aging male. This led us to propose that the decrease in neuroendocrine cells and 5-HT in the human transition zone could contribute to the development of BPH. The increased mass of Tph1 −/− prostates was associated to an up-regulation of AR in dorso-lateral prostate samples suggesting that, at least in part, the excessive prostatic growth in Tph1 −/− could be attributed to AR up-regulation.
In conclusion, our findings suggest that 5-HT is a strong negative regulator of prostate growth through AR down-regulation. As 5-HT is decreased in BPH, we present here evidence that links 5-HT-producing neuroendocrine cell depletion to BPH etiology. Therefore, this new described serotoninergic inhibitory pathway over benign prostatic growth should be explored as a new target for BPH treatment.
Methods
Ethics and animal work
Mice and rats were maintained in accordance with the guidelines of “Guide to the Care and Use of Experimental Animals” National Academy of Science, and the EU Directive 2010/63/EU. This study was approved by the Animal Ethics Committee of the Institution were the study was performed (SECVS 003/2016) and by the National Competent Authority for Animal Protection (DGAV 0421/000/000/2016).
Drugs
5-HT and testosterone were purchased from Sigma-Aldrich (St Louis, Missouri). The 5-Htr1a specific agonist, 8-OH-DPAT and the 5-Htr1b specific agonist, Anpirtoline, were purchased from Tocris-Bioscience (Bristol, UK).
Rat ventral prostate cultures
Newborn male Sprague-Dawley rats were sacrificed 24-hours after birth. Ventral prostate lobes were microdissected using a stereomicroscope (Leica MZ6, Switzerland) and processed for organ culture. Organ culture was performed as previously described38. Briefly, rat ventral prostates (VPs) from P1 newborns were cultured for 4 days at 37 °C in a humidified atmosphere of 5% CO2. Medium and VPs were transferred to porous membranes (Millicell CM filters, Millipore Corp., Bedford, Massachusetts) in 12 well plate for floating explant cultures. Each VPs were dipped into 500 μl of 1:1 mixture of DMEM and Ham’s F-12 nutrient supplemented with 100 μg/mL streptomycin, 100 units/mL penicillin, 10 µg/mL transferrin and 10 µg/mL insulin. Media were replenished at 48 hours of culture. Branching morphogenesis in all groups was monitored daily by a stereomicroscope and photographs were taken at day 0 and day 4. The number of peripheral buds was manually counted and the prostate tissue area was measured in ImageJ using the beProstate plugin (Version 1.0) (developed by Biomedical Engineering Solutions Research Group, Life and Health Sciences Research Institute, University of Minho; available at http://www.besurg.com/sites/default/files/beProstateApp.zip). The differences between day 0 (D0: 0 hours) and day 4 (D4: 96 hours) of culture, were expressed as D4/D0 ratio. A total of 427 VPs were cultured divided in three experimental groups: 5-HT, 8-OH-DPAT and anpirtoline. For each experimental group a dose-effect approach was used. Furthermore, each experimental group was cultured either with or without testosterone supplementation of media ([testosterone] = 10−8 M).
Human prostate cell lines cultures
Three human cell lines were used: PNT1A and WPMY-1 were obtained from American Type Culture Collection (ATCC, Manassas, Virginia) and BPH-1 which was obtained from DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). All the cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM 1x, high glucose; Gibco, Invitrogen, Grand Island, New York) supplemented with 10% FBS (Gibco, Invitrogen, Grand Island, New York) and 1% penicillin/streptomycin solution (DMEM-10), at 37 °C and 5% CO2. For the viability assay, the cells were plated into 96-well plates at a density of 3 × 103 cells per well and allowed to adhere overnight in DMEM medium containing 10% FBS. Subsequently, the cells were treated with increasing concentrations of 5-HT, 8-OH-DPAT and Anpirtoline diluted in 0.5% FBS culture medium, with or without testosterone (10−8M) supplementation. After 72 hours of incubation, cell viability was quantified using CellTiter 96 Aqueous Cell Proliferation Assay (MTS) (Promega, Madison, Wisconsin). The mean percentage of viable cells relative to the vehicle alone (considered as 100% viability) was determined, and the final results were expressed in relation to the control (adjusted to 1). For the proliferation assay, we evaluated the number of Ki-67 positive cells by immunofluorescence. The cells were plated in glass coverslips placed into 12-well plates at a density of 5 × 104 cells per well, and allowed to adhere overnight. Subsequently, the cells were treated with 5-HT (100 µM), 8-OH-DPAT (100 µM) and Anpirtoline (100 µM) diluted in 0.5% FBS culture medium, with or without testosterone (10−8M) supplementation. The total number of cells and the Ki-67 positive cells were manual counted using a fluorescence microscopy (BX16; Olympus). The ratio of Ki-67 positive cells per total number of cells was determined, and the final results were expressed in relation to the control (adjusted to 1).
Tph1−/− Mice and in vivo studies
Only male mice were used for in vivo experiments. They were housed in specific pathogen-free conditions in a room maintained at a constant temperature of 23 °C on a 12-h light-dark cycle. Food and water were provided ad libitum. All treatment groups were age matched and randomized to treatment at the initiation of an experiment. The researchers performing the experiments were blinded to experimental groups during all testing. Animals were excluded from analysis if signs of fight with skin lesions were present. Tph1 −/− mice on a C57BL/6 background were provided by M. Bader (Max Delbrück Center for Molecular Medicine, Berlin, Germany). For assessment of the morphological evolution with age both male wild-type and Tph1 −/− mice were sacrificed at different time points (at least 6 animals for both groups at each time-point): 7, 12, 16 and 20 weeks-old. For pharmacological studies, wild-type and Tph1 −/− mice with 19 week-old were treated daily with intraperitoneal injections of 0.9% saline or 5-HT (100 mg/Kg) during 10 consecutive days (at least 6 animals for each group). Mice were sacrificed and prostate tissue (all lobes combined) and seminal vesicles were micro dissected away from other urogenital and fat tissues. Total prostate was weighted immediately after dissection. The right lobes were separated from the left and processed for histology or western blotting. For histologic analysis hemi-right prostate was fixed in 10% PFA, processed and embedded in paraffin and stained with hematoxylin and eosin (H&E). Hemi-left prostate was separated in three lobes (ventral, dorsolateral and anterior) and processed to qRT-PCR.
Immunofluorescence analysis
Immunofluorescence for AR, Ki-67, 5-Htr1a and 5-Htr1b was performed on formalin-fixed and paraffin-embedded rat ventral prostates or in human prostate cell lines. Briefly, deparaffinized and rehydrated slides were submitted to adequate heat-induced antigen retrieval for 20 min at 98 °C with 10 mM citrate buffer (pH 6.0). Regarding cell lines, all of them were plated in glass coverslips placed into 12-well plates at a density of 5 × 105 cells per well, and allowed to adhere overnight. Then, the cells were fixed in cold methanol by 5 minutes at −20 °C. In both paraffin and cell, for block unspecific ligations the cells/tissues were incubated with a solution of PBS containing 10% FBS for 30 minutes at room temperature followed by incubation with a primary antibody against AR (1:1000 dilution; sc-816: Santa Cruz Biotechnology, Santa Cruz, California), Ki-67 (1:100 dilution, AP10244C; Gennova, Sevilla, Spain), 5-Htr1a (1:100 dilution; sc-10801: Santa Cruz Biotechnology, Santa Cruz, California) and 5-Htr1b (1:100 dilution; sc-28937: Santa Cruz Biotechnology, Santa Cruz, California). The cells/tissues were then washed in a PBS solution with 0.5% FBS and incubated with a goat anti-rabbit antibody conjugated with FITC for cells and with TRITC for tissues (dilution 1:500, Life Technology, Carlsbad, California) for 1 hour at room temperature in the dark. Finally, the cells were counterstained with 40,6-diamidino-2-phenylindole (DAPI). The images were obtained using a fluorescence microscopy (BX16; Olympus).
Western blot analysis
Western blot analysis for androgen receptor (AR) was done in both VPs, mouse prostate tissue and in human cell lines. All the samples were properly processed for western blot analysis and lysed in a buffer containing 50 mM Tris pH 7.6–8, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 10 mM NaPyrophosphate, 1% NP-40 and 1/7 of Protease cocktail inhibitors (Roche). Western blotting was done using standard 10% SDS-PAGE gels, loading 20 µg of protein per lane. For AR detection a specific antibody was used (1:1000 dilution; sc-816: Santa Cruz Biotechnology, Santa Cruz, California). β-Actin was used for loading control (1:500 dilution; sc-1616; Santa Cruz Biotechnology, Santa Cruz, California). After incubation with appropriate secondary antibodies, they were detected by chemiluminescence (Thermo Scientific Pierce ECL Western Blotting) in ChemiDoc™ XRS + System (Bio-Rad). Quantification of western blot results was done using the band densitometry analysis, performed with ImageJ software.
RNA extraction and qRT-PCR
Total RNA was isolated from the dorsolateral prostate of different groups with Trizol (Invitrogen, Carlsbad, California). Then, after quantification using the NanoDrop®, 500 ng of total RNA was reverse transcribed into first strand cDNA using the iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California). Primers used to measure the expression levels of AR was designed using the Primer3 software, on the basis of the respective GenBank sequence. All accession numbers and primer sequences are available on request. The reference gene for hypoxanthine guanine phosphoribosyl transferase (Hprt) (accession number from GenBank: NM_013556) was used as an internal standard for the normalization of the expression of selected transcripts. qRT-PCR was performed on a CFX 96TM real time system instrument (Bio-Rad Laboratories, Hercules, California), with the QuantiTect SYBR Green RT-PCR reagent kit (Qiagen, Hamburg, Germany), using equal amounts of RNA from each one of the samples. Product fluorescence was detected at the end of the elongation cycle. All melting curves exhibited a single sharp peak at the expected temperature.
Statistics
Data are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism by Student’s t test or ANOVA where appropriate. A Bonferroni post hoc test was used to test for significant differences revealed by ANOVA. Statistical significance was confirmed at p < 0.05.
Electronic supplementary material
Acknowledgements
This work has been developed under the scope of the projects NORTE-01-0246-FEDER-000012, NORTE-01-0145-FEDER-000013 and NORTE-01-0145-FEDER-000023, supported by the Northern Portugal Regional Operational Program (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER) and Bolsa de Investigação GSK Inovação em Urologia 2012.
Author Contributions
E.C.D., A.M., O.M., P.M., A.C., C.N.S., R.M., N.A. and M.B. performed the experiments. E.C.D., R.A., and J.C.P. analysed and interpreted the data. E.C.D., E.L. and J.C.P. discussed and planned the experiments. E.C.D. and J.C.P. supervised the project. E.C.D. and J.C.P. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15832-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Egan KB. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol Clin North Am. 2016;43:289–297. doi: 10.1016/j.ucl.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/S0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 3.Aaron L, Franco OE, Hayward SW. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. Urol Clin North Am. 2016;43:279–88. doi: 10.1016/j.ucl.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–345. [PubMed] [Google Scholar]
- 5.McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2:35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- 6.Cunha GR, Ricke WA. A historical perspective on the role of stroma in the pathogenesis of benign prostatic hyperplasia. Differentiation. 2011;82:168–172. doi: 10.1016/j.diff.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha GR. Mesenchymal–epithelial interactions: past, present, and future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 8.Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5 alpha-reductase inhibition in human benign prostatic hyperplasia. Eur Urol. 2000;37:367–80. doi: 10.1159/000020181. [DOI] [PubMed] [Google Scholar]
- 9.Harman SM, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men: Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 10.Morley JE, et al. Longitudinal changes in testosterone, luteinizing hormone and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410–413. doi: 10.1016/S0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 11.Roberts RO, et al. Serum sex hormones and measures of benign prostatic hyperplasia. Prostate. 2004;61:124–31. doi: 10.1002/pros.20080. [DOI] [PubMed] [Google Scholar]
- 12.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–321. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson TM, Sehgal PD, Drew SA, Huang W, Ricke WA. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation. 2013;85:140–149. doi: 10.1016/j.diff.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, et al. Which play a more important role in the development of large-sized prostates (≥80 ml), androgen receptors or oestrogen receptors? A comparative study. Int Urol Nephrol. 2016;48:325–333. doi: 10.1007/s11255-015-1181-z. [DOI] [PubMed] [Google Scholar]
- 15.Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol. 2013;182:1942–1949. doi: 10.1016/j.ajpath.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santamaría L, Ingelmo I, Alonso L, Pozuelo JM, Rodríguez R. Neuroendocrine cells and peptidergic innervation in human and rat prostate. Adv Anat Embryol Cell Biol. 2007;194:1–77. doi: 10.1007/978-3-540-69816-6_1. [DOI] [PubMed] [Google Scholar]
- 17.Santamaría L, Martín R, Martín JJ, Alonso L. Stereologic estimation of the number of neuroendocrine cells in normal human prostate detected by immunohistochemistry. Appl Immunohistochem Mol Morphol. 2002;10:275–281. doi: 10.1097/00129039-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Martín R, et al. Immunohistochemical localization of protein gene product 9.5, ubiquitin, and neuropeptide Y immunoreactivities in epithelial and neuroendocrine cells from normal and hyperplastic human prostate. J Histochem Cytochem. 2000;48:1121–1130. doi: 10.1177/002215540004800809. [DOI] [PubMed] [Google Scholar]
- 19.Cockett AT, di Sant’Agnese PA, Gopinath P, Schoen SR, Abrahamsson PA. Relationship of neuroendocrine cells of prostate and serotonin to benign prostatic hyperplasia. Urology. 1993;42:512–519. doi: 10.1016/0090-4295(93)90260-H. [DOI] [PubMed] [Google Scholar]
- 20.Islam MA, et al. Are neuroendocrine cells responsible for the development of benign prostatic hyperplasia? Eur Urol. 2002;42:79–83. doi: 10.1016/S0302-2838(02)00269-5. [DOI] [PubMed] [Google Scholar]
- 21.Haghsheno MA, et al. Lower urinary tract symptoms are associated with low levels of serum serotonin, high levels of adiponectin and fasting glucose, and benign prostatic enlargement. Scand J Urol. 2015;49:155–161. doi: 10.3109/21681805.2014.936495. [DOI] [PubMed] [Google Scholar]
- 22.Timms BG, Hofkamp LE. Prostate development and growth in benign prostatic hyperplasia. Differentiation. 2011;82:173–83. doi: 10.1016/j.diff.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Dizeyi N, et al. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate. 2004;59:328–336. doi: 10.1002/pros.10374. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui EJ, Shabbir M, Mikhailidis DP, Thompson CS, Mumtaz FH. The role of serotonin (5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell proliferation. J Urol. 2006;176:1648–1653. doi: 10.1016/j.juro.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 25.Walther DJ, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;3:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 27.Berry SJ, Coffey DS, Strandberg JD, Ewing LL. Effect of age, castration, and testosterone replacement on the development and restoration of canine benign prostatic hyperplasia. Prostate. 1986;9:295–302. doi: 10.1002/pros.2990090308. [DOI] [PubMed] [Google Scholar]
- 28.Deanesly R, Parkes AS. Size changes in the seminal vesicles of the mouse during development and after castration. J Physiol. 1933;78:442–450. doi: 10.1113/jphysiol.1933.sp003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarma AV, Wei JT. Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N Engl J Med. 2012;367:248–257. doi: 10.1056/NEJMcp1106637. [DOI] [PubMed] [Google Scholar]
- 30.Krieg M, Nass R, Tunn S. Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab. 1993;77:375–381. doi: 10.1210/jcem.77.2.7688377. [DOI] [PubMed] [Google Scholar]
- 31.Cooper CS, et al. Effect of exogenous testosterone on prostate volume, serum and semen prostate specific antigen levels in healthy young men. J Urol. 1998;159:441–443. doi: 10.1016/S0022-5347(01)63944-2. [DOI] [PubMed] [Google Scholar]
- 32.Pechersky AV, et al. Androgen administration in middle-aged and ageing men: effects of oral testosterone undecanoate on dihydrotestosterone, oestradiol and prostate volume. Int J Androl. 2002;25:119–125. doi: 10.1046/j.1365-2605.2002.00335.x. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda M, et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev. Cell. 2004;6:193–203. doi: 10.1016/S1534-5807(04)00022-X. [DOI] [PubMed] [Google Scholar]
- 34.Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction. 2007;133:331–59. doi: 10.1530/REP-06-0078. [DOI] [PubMed] [Google Scholar]
- 35.Murray JF, et al. Neonatal 5HT activity antagonizes the masculinizing effect of testosterone on the luteinizing hormone release response to gonadal steroids and on brain structures in rats. Eur J Neurosci. 2004;19:387–395. doi: 10.1111/j.0953-816X.2003.03158.x. [DOI] [PubMed] [Google Scholar]
- 36.Dakin CL, Wilson CA, Kalló I, Coen CW, Davies DC. Neonatal stimulation of 5-HT(2) receptors reduces androgen receptor expression in the rat anteroventral periventricular nucleus and sexually dimorphic preoptic area. Eur J Neurosci. 2008;27:2473–2480. doi: 10.1111/j.1460-9568.2008.06216.x. [DOI] [PubMed] [Google Scholar]
- 37.Sayed RH, Saad MA, El-Sahar AE. Dapoxetine attenuates testosterone-induced prostatic hyperplasia in rats by the regulation of inflammatory and apoptotic proteins. Toxicol Appl Pharmacol. 2016;311:52–60. doi: 10.1016/j.taap.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Lipschutz JH, Foster BA, Cunha GR. Differentiation of rat neonatal ventral prostates grown in a serum-free organ culture system. Prostate. 1997;32:35–42. doi: 10.1002/(SICI)1097-0045(19970615)32:1<35::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.