Abstract
Objective
To determine whether characteristics of glucose dynamics are reflections of β-cell function or rather of inadequate diabetes control.
Materials/methods
We analyzed historical liquid meal tolerance test (LMTT) and continuous glucose monitoring (CGM) data, which had been obtained from 56 non-insulin treated type 2 diabetic outpatients during withdrawal of antidiabetic drugs. Computed CGM parameters included detrended fluctuation analysis (DFA)-based indices, autocorrelation function exponent, mean amplitude of glycemic excursions (MAGE), glucose SD, and measures of glycemic exposure. The LMTT-based disposition index (LMTT-DI) calculated from the ratio of the area-under-the-insulin-curve to the area-under-the-glucose-curve and Matsuda index was used to assess relationships among β-cell function, glucose profile complexity, autocorrelation function, and glycemic variability.
Results
The LMTT-DI was inverse linearly correlated with the short-range α1 and long-range scaling exponent α2 (r = −0.275 and −0.441, respectively, p < 0.01) such that lower glucose complexity was associated with better preserved insulin reserve, but it did not correlate with the autocorrelation decay exponent γ. By contrast, the LMTT-DI was strongly correlated with MAGE and SD (r = 0.625 and 0.646, both p < 0.001), demonstrating a curvilinear relationship between β-cell function and glycemic variability. On stepwise regression analyses, the LMTT-DI emerged as an independent contributor, explaining 20, 38, and 47% (all p < 0.001) of the variance in the long-range DFA scaling exponent, MAGE, and hemoglobin A1C, respectively, whereas insulin sensitivity failed to contribute independently.
Conclusions
Loss of complexity and increased variability in glucose profiles are associated with declining β-cell reserve and worsening glycemic control.
Keywords: Glucose profile dynamics, β-Cell reserve, Disposition index, Type 2 diabetes
Abbreviations: ACF, autocorrelation function; AUC, area under the curve; CGM, continuous glucose monitoring; Cp, C-peptide; DFA, detrended fluctuation analysis; LMTT, liquid meal tolerance test; LMTT-DI, LMTT-based disposition index; MAGE, mean amplitude of glycemic excursions; OHA, oral hypoglycemic agent; SD, standard deviation; TZDs, thiazolidinediones
Introduction
Regulation of glucose concentration is a complex process that involves several hormones among which insulin plays a prominent role. The failing glucoregulation observed in the development of diabetes signals declining β-cell reserve and may be assessed by measurement of glucose dynamics, utilizing indices of glycemic variability and different techniques of time-series analysis. Using detrended fluctuation analysis (DFA), Churruca et al. [1] and Yamamoto et al. [2] described a loss of glucose profile complexity in the progression from normoglycemia to impaired glucose tolerance to overt diabetes, and Ogata et al. [3] have reported that increasing long-range DFA scaling exponents reflect abnormalities in glycemic control. Moreover, Khovanova et al. [4] have recently shown that the dynamics of glucose profiles can be defined by three complementary characteristics: nonstationarity (DFA exponent α), linear predictability (autocorrelation coefficient γ), and amplitude of variation (glucose SD). However, it is not known whether changes in glucose profile dynamics are primarily dependent on exogenous or endogenous factors.
Previous reports 5, 6, Rodbard's recent interpretations [7], and own data 8, 9 support the assumption that β-cell dysfunction might be the underlying pathophysiologic basis of glycemic variability. Furthermore, in a study of patients with early type 2 diabetes Kramer et al. [10] found that improvement of β-cell function was the key determinant of the reduction in glycemic variability in response to short-term intensive insulin therapy. These observations led us to infer that the β-cell reserve may predict changes in various dynamic parameters of glucose profiles. However, whether the inherent structure in glucose profiles mirrors certain aspects of β-cell dysfunction relative to insulin resistance is currently unknown, as is the association among measures of glucose profile dynamics and residual β-cell capacity. Given the increasing use of continuous glucose monitoring (CGM) in glycemic control, it appears essential to reveal such relationships for qualitatively better therapeutic capabilities, e.g. timely adjustment of therapy appropriate to the current β-cell reserve. Achievement of glycemic stability and metabolic flexibility in patients with type 2 diabetes by early therapeutic intervention appears to be a conceptual model to prevent loss of residual β-cell function. We hypothesize that dynamical changes in glucose profiles reflect underlying functional aspects of the failing β-cell during worsening of glycemic control. To address this issue, we used glucose complexity measures, autocorrelation function, and glycemic variability metrics for analysis of glucose dynamics and the validated liquid meal tolerance test-based disposition index (LMTT-DI) for evaluation of β-cell function. Like the oral glucose tolerance test-based measure of insulin secretion and insulin sensitivity [11], the LMTT-DI has been shown to represent an accurate, integrated measure of β-cell function [12]. Thus, this index appeared to be useful in the current study for assessing β-cell function in relation to glucose profile characteristics.
Methods
The present investigation used historical data, which were obtained in an earlier study during withdrawal of diabetes therapy in outpatients with type 2 diabetes mellitus [8]. Retrospective analysis of anonymized ambulatory CGM profiles (MiniMed Solution Software, Medtronic MiniMed) and LMTT data was performed, using a validated β-cell function index and established measures of glucose profile dynamics. Glucose profiles with a minimum of four blood glucose meter calibrations per day and a mean duration of 60-h continuous monitoring had been downloaded and used for calculations of glucose profile parameters. Data not meeting strict validity criteria of the manufacturer were excluded.
Subjects and study procedure
The original study had received ethical approval and, before inclusion, all study participants provided their written informed consent. Thus, no further approval was required for this retrospective data analysis. The characteristics of the patient cohort and the study procedure have been described in detail elsewhere [8]. In brief, before commencement of any of the study procedures antidiabetic medication had been withdrawn and substituted with placebo for 8 days to allow for their pharmacological effects to dissipate. Five patients who had been taking thiazolidinediones (TZDs) were excluded from the study. After a 12-h overnight fast, patients were given a standardized 500 mL liquid meal, containing 75 g carbohydrate, 58 g fat, and 30 g protein to total 1000 kcal.
Calculation of indices of β-cell function and insulin sensitivity
Plasma glucose and insulin concentration during the 150 min of the LMTT were used to calculate indices of β-cell function and insulin sensitivity as described by Maki et al. [12]. The Matsuda insulin sensitivity index was calculated from fasting glucose (G0) and insulin (I0) and the respective mean postmeal glucose (Gm) and insulin (Im) concentrations as 10,000/(G0 × I0 × Gm × Im)0.5. Beta-cell function taking the prevailing insulin sensitivity into account was assessed by the disposition index (LMTT-DI) calculated as total area under the curve for plasma insulin from 0 to 150 min divided by the total area under the curve for plasma glucose from 0 to 150 min multiplied by the Matsuda insulin sensitivity index (LMTT-DI = AUCI/AUCG × Matsuda index) [13].
In comparative analyses, we also evaluated residual β-cell function from AUC of C-peptide (Cp) and AUC of glucose by computation of: ((AUCCp/AUCG) × 1/fasting Cp), as described by Bacha et al. [14]. These two measures of β-cell function, LMTT-DI and the latter index, satisfy the required hyperbolic criteria established by Retnakaran et al. [11].
Calculation of indices of glucose profile dynamics
The following indices were calculated from the CGM datasets:
-
(1)
Glucose complexity and autocorrelation function. The short-term (α1) and long-term (α2) range exponentials were obtained by detrended fluctuation analysis (DFA) according to Yamamoto et al. [2]. Alpha 1 represents the slope of the regression within 1.5 h calculated as n = 5–18 points and α2 the slope of the regression over 1.5 h from n = 18–576 points. The autocorrelation function (ACF) coefficient γ was calculated by a decomposition approach of glucose-time series, as described by Khovanova et al. [3].
-
(2)
Glucose variability. These indices included the mean amplitude of glycemic excursions (MAGE) 15, 16 and overall SD around the sensor-derived mean glucose (SD) [17].
-
(3)
Glucose exposure. The glucose exposure metrics included mean glucose and percentage time in the glucose target range (3.9–10.0 mmol/L).
Statistical analyses
We categorized the patients into thirds on the basis of their disposition index calculated from the liquid meal test. For comparison of continuous variables, we used either one way analysis of variance (ANOVA) or Kruskal–Wallis one way analysis, as appropriate. Control for multiple comparisons was performed using the Holm-Sidak and Dunn's method, respectively. Pearson's correlation analysis, linear and nonlinear regression were used to relate residual β-cell function to measures of glucose complexity, glycemic variability, and glucose exposure. The stepwise multiple regression analysis was used to explore the influence of β-cell function, as measured by the LMTT-DI (independent variable), on glucose profile complexity, glycemic variability, glucose exposure, and various clinical factors (dependent variables). All variables were tested for normality and, where appropriate, were logarithmically transformed as indicated. p < 0.05 was considered statistically significant. To exclude strong interference between independent variables, collinearity statistics were performed. A variance inflation factor (VIF) ≤ 1.40 was found acceptable for inclusion. All statistical analyses were performed using Statistical Package for the Social Sciences software package (version 17.0; SPSS, Chicago, IL).
Results
Table 1 shows the main characteristics of the study participants categorized by thirds of the meal test-based disposition index (LMTT-DI). Duration of diabetes among the LMTT-DI categories was not significantly different in the analysis of variance (p = 0.07). The β-cell functional reserve, including insulin sensitivity, were significantly higher, whereas HbA1c, and fasting plasma glucose values were lower in the highest than in the two lower thirds of LMTT-DI. Baseline antihyperglycemic treatment was different among the three categories. In the top LMTT-DI category, the percentage of patients who had received diet was 72%, whereas the percentage treated with sulfonylurea, either alone or in combination with metformin, was 11%. The opposite was true for the lowest category, where 17% of patients had diet and 72% sulfonylurea alone or in combination with metformin. All other characteristics, including carbohydrate intake, did not differ significantly across the thirds of LMTT-DI.
Table 1.
Main characteristics of patients divided according to thirds of liquid meal tolerance test-based disposition index
| Parameter | Thirds of liquid meal tolerance test-based disposition index |
|||
|---|---|---|---|---|
| 1 (<230) | 2 (230–350) | 3 (>350) | p value | |
| Patients (n) | 18 | 18 | 18 | |
| Sex (male/female) | 9/9 | 8/10 | 11/7 | |
| Age (years) | 65.5 (56.0–69.0) | 65.0 (61.0–71.0) | 64.5 (63.0–69.0) | 0.68 |
| Diabetes duration (years) | 9.0 (4.0–11.0) | 4.5.0 (2.0–9.0) | 2.5 (1.0–10.0) | 0.07 |
| Body mass index (kg/m2) | 29.9 ± 4.3 | 30.6 ± 3.4 | 28.9 ± 3.4 | 0.39 |
| Systolic blood pressure (mm Hg) | 138.1 ± 15.1 | 135.3 ± 12.4 | 134.7 ± 12.7 | 0.73 |
| Diastolic blood pressure (mm Hg) | 82.2 ± 6.7 | 82.8 ± 9.1 | 79.9 ± 5.7 | 0.48 |
| Carbohydrate intake (g/day) | 140 ± 39 | 138 ± 28 | 134 ± 28 | 0.87 |
| Diabetes treatment | ||||
| Diet alone | 3 | 7 | 13 | |
| Sulfonylurea alone | 6 | 5 | 2 | |
| Metformin alone | 2 | 5 | 3 | |
| Sulfonylurea and metformin | 7 | 1 | 0 | |
| Hemoglobin A1C (%) (mmol/mol) | 7.5 (6.5–7.7) 58 (48–61) |
6.1 (5.4–6.5)† 43 (36–48) |
5.9 (5.5–6.1)* 41 (37–43) |
<0.001 |
| Fasting plasma glucose (mmol/L) | 11.8 (9.0–12.0) | 7.7 (7.3–8.2)† | 6.1 (5.8–7.2)* | <0.001 |
| Triglyceride (mmol/L) | 2.6 (1.3–3.3) | 1.6 (1.3–1.9)† | 1.8 (1.5–2.1) | 0.08 |
| HDL-cholesterol (mmol/L) | 1.1 (1.0–1.3) | 1.3 (1.1–1.5) | 1.5 (1.2–1.6)* | 0.025 |
| Fasting plasma insulin (pmol/L) | 114.7 (60.3–166.5) | 111.6 (96.5–138.0) | 90.5 (71.2–100.2) | 0.10 |
| β-cell function | ||||
| LMTT-DI (1/mmol2) | 102.9 (69.2–166.4) | 254.2 (239.6–284.3)† | 467.3 (394.6–547.7)* | <0.001 |
| Insulin sensitivity | ||||
| Matsuda index (1/pmol × mmol) | 3.67 (2.30–5.75) | 4.72 (3.00–6.00) | 6.79 (5.92–9.10)* | 0.003 |
Data are mean ± SD, median (25th–75th percentile) values or n. The disposition index categories are given in 1/mmol2 and were compared using analysis of variance (ANOVA) or the Kruskal–Wallis one way analysis and Holm-Sidak or Dunn's test, where appropriate, for multiple comparisons: p < 0.05, * versus the other two categories and † versus the <230 disposition index category.
LMTT-DI: liquid meal tolerance test-based disposition index.
Comparisons among the categories for glucose profile complexity, autocorrelation function, and various glucose metrics are summarized in Table 2. The data demonstrates that there is an association between the degree of glucose profile complexity and β-cell function, as indicated by the DFA short-range and long-range scaling exponents α, which were found to be smaller in the highest than in the lowest third of LMTT-DI. No statistically significant difference was documented across the categories for α1 and ACF exponent γ. All mean α2-values were less than 1.5.
Table 2.
Comparison of glucose complexity, autocorrelation, glycemic variability, and glucose exposure measures among thirds of residual β-cell function as expressed by the liquid meal tolerance test-based disposition index
| Parameter | Thirds of liquid meal tolerance test-based disposition index |
|||
|---|---|---|---|---|
| 1 (<230) | 2 (230–350) | 3 (>350) | p value | |
| Glucose complexity | ||||
| DFA exponent α1 (<1.5 h) | 1.85 ± 0.13 | 1.84 ± 0.11 | 1.76 ± 0.12 | 0.055 |
| DFA exponent α2 (>1.5 h) | 1.31 ± 0.13 | 1.20 ± 0.17† | 1.15 ± 0.13* | 0.007 |
| Autocorrelation function | ||||
| ACF exponent γ | 0.10 (0.06–0.22) | 0.13 (0.08–0.26) | 0.14 (0.11–0.22) | 0.14 |
| Glycemic variability | ||||
| MAGE (mmol/L) | 6.39 ± 1.81 | 4.48 ± 2.01† | 3.33 ± 1.21* | <0.001 |
| SD (mmol/L) | 2.40 ± 0.68 | 1.97 ± 0.95 | 1.32 ± 0.4* | <0.001 |
| Glucose exposure | ||||
| Mean glucose (mmol/L) | 13.3 (10.5–14.7) | 7.9 (7.2–9.6) | 6.6 (6.0–7.3)* | <0.001 |
| % time in range | 12.0 (3.7–42.3) | 86.0 (65.9–94.3)† | 94.8 (91.7–99.0)* | <0.001 |
Data are mean ± SD or median (25th–75th percentile) values. Disposition index categories are given in 1/mmol2 and were compared using analysis of variance (ANOVA) or the Kruskal–Wallis one way analysis and Holm-Sidak or Dunn's test, where appropriate, for multiple comparisons: p < 0.05, * versus the other two categories and † versus the <230 disposition index category.
DFA: detrended fluctuation analysis; α1: short-range scaling exponent; α2: long-range scaling exponent; ACF: autocorrelation function; MAGE: mean amplitude of glycemic excursion; SD: total SD of all glucose values; % time in range: percentage time of glucose values in range 3.9–10.0 mmol/L.
Levels for MAGE and SD were lowest in the highest third of the LMTT-DI, whereas mean glucose diminished when moving from the lowest to the highest category, indicating that sufficiently preserved β-cell function restrains glucose exposure. Consistently, the percentage of values in the target range of 3.9–10.0 mmol/L glucose increased significantly with increasing disposition index values; the difference between the lowest and highest category amounted to 83%.
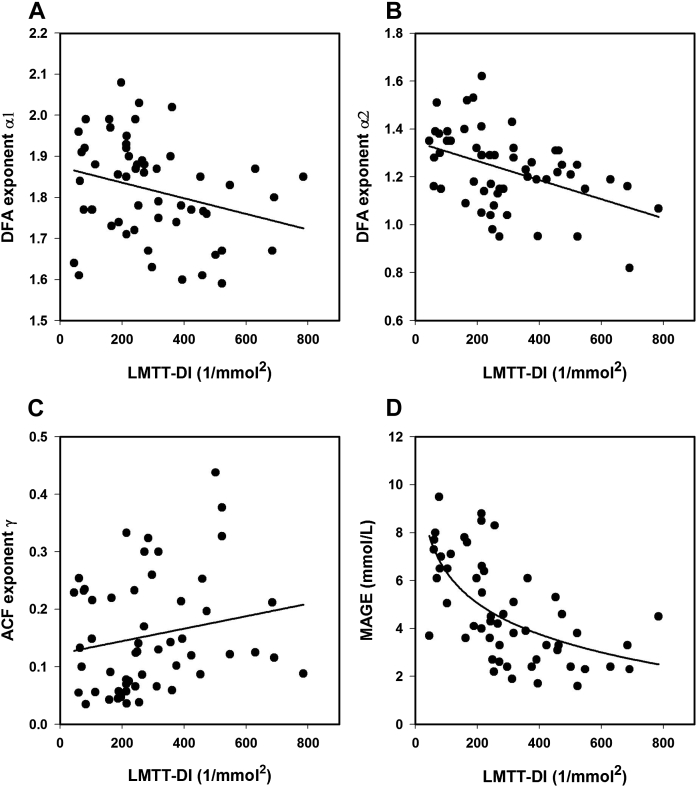
On Pearson's correlation analysis (Supplementary Table 1), the LMTT-DI was significantly associated with indices of glucose complexity and glucose metrics, most strongly with mean glucose (r = −0.779, p < 0.001), but not with the autocorrelation function. Though, a strong correlation was found between the ACF exponent γ and DFA exponent α1. Diabetes duration, considered as another correlate of β-cell function, was included in the correlation analysis. With the exception of mean glucose, there was no significant association between diabetes duration and the different characteristics of glycemic stability ― the correlation with MAGE slightly missed the margin of significance (p = 0.051). When the relationship between the LMTT-DI and these dynamic indices was then assessed by regression analysis, significant correlations, except for ACF γ, were observed. Associations between the LMTT-DI and DFA exponents were found to be inverse linear (Fig. 1) with greater variation at lower disposition index values. Fig. 1A shows the weaker correlation with α1 (r = −0.277, p = 0.040), whereas the stronger correlations with α2 (−0.441, p < 0.001) is shown in Fig. 1B. The correlation between the LMTT-DI and ACF γ (Fig. 1C) failed to reach statistical significance (r = 0.193, p = 0.163). In contrast, the association between the LMTT-DI and MAGE, as presented in Fig. 1D, was curvilinear and stronger (r = −0.625, p < 0.001) than with the two DFA exponents. A similar regression curve as with MAGE was found between the LMTT-DI and SD (r = −0.646, p < 0.001) (data not shown).
Figure 1.
Relationships between the liquid meal tolerance test-based disposition index (LMTT-DI) and (A) DFA exponent α1, (B) DFA exponent α2, (C) AFC exponent γ, and (D) mean amplitude of glycemic excursions (MAGE) in patients with type 2 diabetes. The regression lines were obtained by linear and nonlinear regression equations as indicated for LMTT-DI versus (A) DFA α1 from α1 = −0.0002 (LMTT-DI) + 1.8744 (r = 0.277, p = 0.040), (B) DFA α2 from α2 = − 0.0004 (LMTT-DI) + 1.3465 (r = 0.441, p < 0.001), (C) ACF γ from γ = 0.0001 (LMTT-DI) + 0.1232 (r = 0.193, p = 0.163), and (D) MAGE from MAGE = −1.8713 ln (LMTT-DI) + 14.9775 (r = 0.625, p ≤ 0.001).
The contribution of β-cell function and other clinical factors was assessed by stepwise regression analyses including the LMTT-DI, Matsuda insulin sensitivity index, DFA short-term scaling exponent α1, age, sex, diabetes duration, and carbohydrate consumption as the independent variables and the DFA long-range scaling exponent α2, MAGE, and HbA1c as the dependent variables. The results of these analyses, provided in Table 3, demonstrate that the log-transformed LMTT-DI was the strongest independent contributor to the DFA exponent α2, MAGE, and HbA1c, whereas age, DFA exponent α1, and male sex, respectively, accounted for smaller proportions of the variance in these metrics. The other independent variables above failed to enter the models. When we used ((AUCCp/AUCG) × 1/fasting Cp) as alternative measure of β-cell function in the stepwise regression models, this variable, similar as with the LMTT-DI, independently predicted the variance in DFA exponent α2, MAGE, and HbA1c (β = −0.489, −0.403, and −0.522, respectively, all p < 0.001).
Table 3.
Results of stepwise forward multiple regression analysis
| Dependent variable | Explanatory variable | Regression coefficient (β) | SE | p value | Coefficient of determination (R2) |
|---|---|---|---|---|---|
| DFA exponent α2 | LMTT-DI | −0.463 | 0.061 | <0.001 | 0.200 |
| Age | 0.286 | 0.301 | 0.021 | 0.281 | |
| MAGE | LMTT-DI | −0.525 | 0.066 | <0.001 | 0.375 |
| DFA exponent α1 | 0.324 | 0.165 | 0.002 | 0.483 | |
| Male sex | −0.236 | 0.133 | 0.025 | 0.538 | |
| Hemoglobin A1C | LMTT-DI | −0.687 | 0.021 | <0.001 | 0.472 |
Significance level: p < 0.05.
DFA: detrended fluctuation analysis; LMTT-DI: liquid meal tolerance test-based disposition index; MAGE: mean amplitude of glycemic excursions.
LMTT-DI, MAGE, age, and hemoglobin A1C were log-transformed to assure normality.
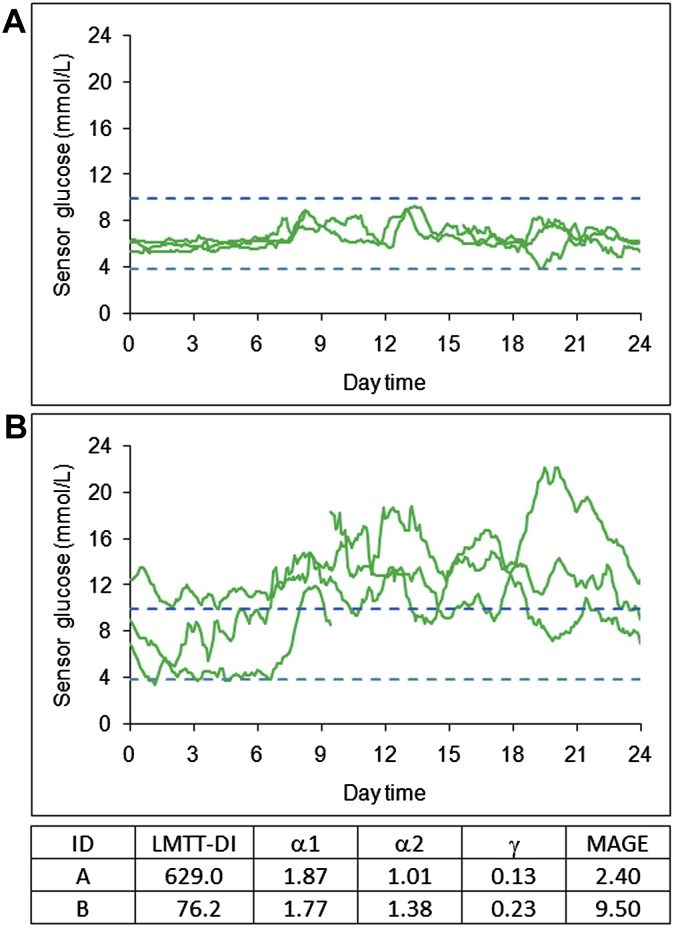
Two characteristic examples of individual CGM tracings given in the Supplementary Fig. 1 (A and B) illustrate the close relationship between residual β-cell function, assessed by the meal-based disposition index, and glucose profile dynamics. Comparison of these patterns shows that the high LMTT-DI value (Supplementary Fig. 1A) was associated with relatively stable and flexible outcomes, i.e. low MAGE, α2, and ACF γ, whereas the low LMTT-DI (Supplementary Fig. 1B) was related to instability, with higher values for α2 (lower glucose complexity), MAGE and ACF γ than for those in example A. Of note, although the HbA1c values were virtually identical in these samples, the pattern dynamics were remarkably different.
Discussion
Our results support the hypothesis that residual β-cell function assessed by the LMTT-based disposition index has a substantial impact on CGM profile dynamics. We observed a significant decline in complexity and levels of glucose metrics concomitant with an increase of glucose readings in the target range across rising categories of the LMTT-DI. While glycemic variability and mean glucose decreased by roughly 50% from the lowest to the highest category, the percentage of glucose values in the target range increased by 81%. We further show that the relationships between β-cell function and glucose complexity factors are inverse linear, whereas those with glucose metrics, exemplified by the regression curve for MAGE and LMTT-DI, are curvilinear. Consistent with our findings, Chen et al. [18] observed hyperbolic relationships between glycemic variability indices and the DI obtained from an oral glucose test across various stages of glucose intolerance. The curvilinear relationship between glycemic variability and the LMTT-DI implies that the better the β-cell function, the better the glycemic stability (lower glycemic variability). It further indicates that a decline of more than 50% β-cell function is required for chronic dysregulation of glycemia to occur. This is compatible with the UKPDS outcomes [19] and our sample CGM tracings (Supplementary Fig. 1). Comparison of these two patient samples shows that, along with the drastic reduction of β-cell function (LMTT-DI), the glucose profiles became remarkably different both in appearance and with regard to the dynamic parameters.
As the multivariate regression analyses clearly demonstrate, the LMTT-DI is an independent predictor of glucose complexity, glycemic variability, and glycemic exposure as well, explaining 20, 38, and 47% of the variance in DFA long-range scaling exponent α2, MAGE, and HbA1c, respectively. The short-range scaling exponent α1, age, and male sex contribute an additional 9 – 6% to the change in α2 and MAGE. Insulin sensitivity, as measured by the Matsuda index, was not associated with variations in the glucose profile dynamics. This may be attributable to a predominant pathogenic role of β-cell function at the stage of overt type 2 diabetes 20, 21. The influence of baseline antidiabetic therapy could be neglected in these models since the relevant variables were determined during withdrawal of oral drugs. Genetic [22] as well as environmental factors [23] or hyperglycemic hormones may be responsible for the residual amount of unexplained variance in the dependent variables. However, their influence is difficult to assess in a retrospective analysis for which the primary goal was to investigate the contribution of β-cell function to CGM measures. It should also be noted that the category with the lowest insulin secretion capacity (LMTT-DI < 230), including the highest proportion of patients who had received treatment (prior to withdrawal) with sulfonylurea either alone or combined with metformin, was characterized by comparatively long diabetes duration, elevated HbA1c and fasting plasma glucose, and diminished HDL-cholesterol levels. The higher fasting glucose levels found in this group compared to the other two groups with higher LMTT-DI values may be partly due to discontinuation of OHA for 8 days. Even short-term moderate hyperglycemia has been shown to be detrimental 5, 6, and exposure to elevated fasting glucose is likely to aggravate the prevailing defects in β-cell function.
Overall, the present paper contributes to the discussion of the natural history of glycemic dysregulation. The fact that insulin is known to prevent β-cell defects [24] by reduction of glucolipotoxicity 25, 26 and inhibitory effects on oxidative stress, as recently shown by Monnier et al. [27] has led to assume that early insulin therapy might be an option to slow down loss of β-cell function. However, the question whether early treatment with insulin 28, 29, 30 can really provide β-cell protection, is not clearly established and requires long-term clinical studies. As pointed out by Del Prato and coauthors [31], any treatment capable of correcting the pathogenetic β-cell abnormalities should ensure glycemic stability. In this context, the proposal by DeFronzo et al. [32] to initiate a triple therapy with antidiabetic agents at the earliest stage of the disease, might preserve β-cell health better than customary approaches.
Apparent limitations of the current investigation are its retrospective nature and the use of a surrogate measure as estimate of pancreatic β-cell function. Nevertheless, among the several indices, the LMTT-DI is a validated, widely accepted measure, taking into account the degree of the patient's insulin sensitivity 12, 13. Previously, we used β-cell function parameters derived from an insulin secretion model [8] that did not account for insulin resistance. In the present work, we have used LMTT-DI and mentioned as alternative a functional parameter ((AUCCp/AUCG) × 1/fasting Cp) that is based on measurement of C-peptide plasma concentrations. It has been claimed that plasma C-peptide more accurately than insulin levels reflects β-cell function [33]. The advantage of measuring C-peptide is that it is cleared in peripheral tissue at a rather constant rate, and it escapes hepatic degradation. Despite the differential kinetics of insulin and C-peptide, our results obtained in the stepwise regression analyses when using either LMTT-DI or (AUCCp/AUCG) × 1/fasting Cp, were comparable. The strength of the investigation is that both glucose profile characteristics and the index of β-cell function were assessed during oral drug withdrawal to largely exclude therapeutic influences. Patients taking TZDs had been excluded, because of long duration of effect after withdrawal.
In summary, we demonstrate the existence of close inverse linear and curvilinear relationships between dynamic glucose profile characteristics and β-cell dysfunction in non-insulin treated patients with type 2 diabetes. Deterioration in glucose dynamics is partly a reflection of the defective β-cell; however, variability metrics such as MAGE are more strongly affected by the residual β-cell function than those of intrinsic dynamics such as long-range negative correlation. The missing correlation between the ACF exponent and the LMTT-DI indicates that linear predictability of glucose is independent of the residual β-cell function. Our results further support the view that maintaining residual β-cell function is most important to prevent derangement of glucose dynamics. Clinical trials are necessary to clarify whether therapies targeted at β-cell status may reduce decomplexification and variability in glycemic profiles and thus improve diabetes control.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Author disclosure statement: All authors declare no potential financial interest or any commercial association that might present a potential conflict of interest. All authors declare that no competing financial interests exist.
Author contributions: K.D.K. contributed to the study conception, data analysis and interpretation as well as to statistical analyses. He wrote the manuscript, contributed to revision of the manuscript for intellectual content and approval of the manuscript. P.H. contributed to the data collection, analysis, and interpretation; revision of the manuscript for intellectual content; and approval of the manuscript. L.V. and A.P. contributed to the study conception and design, data interpretation, revision of the manuscript for intellectual content, and approval of the manuscript. E.S. is the guarantor of this work and, as such, had full access to all the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix.
Supplementary Figure 1.
Sample continuous glucose monitoring tracings obtained from two patients with type 2 diabetes. Gender, age, diabetes duration, body mass index, HbA1c values, mean glucose, and antihyperglycemic therapy were (A) male, 63 years, 10 years, 29.4 kg/m², 6.6%, 6.5 mmol/L, and diet; (B) female, 69 years, 11 years, 23.4 kg/m², 6.5%, 12.4 mmol/L, and sulfonylurea plus metformin. The changes in residual ß-cell function, as measured by the disposition index (LMTT-DI) derived from the liquid meal tolerance test, DFA exponent α1 and α2, ACF γ, and glucose variability (MAGE) corresponding to the patterns of glucose tracings are shown in the table beneath. The dashed horizontal lines (blue) denote the target range (3.9–10.0 mmol/L).
Supplementary Table 1.
Correlation matrix for β-cell function as measured by the liquid meal tolerance test-based disposition index and different characteristics of glycemic stability
| LMTT-DI | Diabetes duration | DFA exponent α1 | DFA exponent α2 | ACF coefficient γ | SD | MAGE | Mean glucose | HbA1c | |
|---|---|---|---|---|---|---|---|---|---|
| LMTT-DI | 1 | ||||||||
| Diabetes duration | −0.23 | 1 | |||||||
| DFA exponent α1 | −0.275* | −0.04 | 1 | ||||||
| DFA exponent α2 | −0.437† | 0.24 | 0.07 | 1 | |||||
| AFC coefficient γ | 0.18 | 0.08 | −0.724† | 0.05 | 1 | ||||
| SD | −0.601† | 0.23 | 0.400* | 0.578† | −0.22 | 1 | |||
| MAGE | −0.597† | 0.269 | 0.416* | 0.583† | −0.24 | 0.923† | 1 | ||
| Mean glucose | −0.779† | 0.480† | 0.18 | 0.470* | −0.16 | 0.638† | 0.638† | 1 | |
| HbA1c | −0.580† | 0.25 | 0.06 | 0.397* | −0.10 | 0.453† | 0.393* | 0.673† | 1 |
The Pearson correlation coefficients (r) are shown. p values (two-sided) are *<0.01 and †<0.001. ACF: autocorrelation function; DFA: detrended fluctuation analysis; LMTT-DI: liquid meal tolerance test-based disposition index; MAGE: mean amplitude of glycemic excursions; SD: standard deviation of mean glucose.
References
- 1.Churruca J., Vigil L., Luna E., Ruiz-Galiana J., Varela M. The route to diabetes: loss of complexity in the glycemic profile from health through the metabolic syndrome to type 2 diabetes. Diabetes Metab Syndr Obes. 2008;1:3–11. doi: 10.2147/dmso.s3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto N., Kubo Y., Ishizawa K., Kim G., Moriya T., Yamanouchi T. Detrended fluctuation analysis is considered to be useful as a new indicator for short-term glucose complexity. Diabetes Technol Ther. 2010;12(10):775–783. doi: 10.1089/dia.2010.0059. [DOI] [PubMed] [Google Scholar]
- 3.Ogata H., Tokuyama K., Nagasaka S., Tsuchita T., Kusaka I., Ishibashi S. The lack of long-range negative correlations in glucose dynamics is associated with worse glucose control in patients with diabetes mellitus. Metabolism. 2012;61(7):1041–1050. doi: 10.1016/j.metabol.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Khovanova N.A., Khovanov I.A., Shabno L., Griffiths F., Holt T.A. Characterisation of linear predictability and non-stationarity of subcutaneous glucose profiles. Comput Methods Programs Biomed. 2013;110(3):260–267. doi: 10.1016/j.cmpb.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Kanat M., Mari A., Norton L., Winnier D., DeFronzo R.A., Jenkinson C. Distinct ß-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes. 2012;61(2):447–453. doi: 10.2337/db11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toschi E., Camastra S., Sironi A.M., Masoni A., Gastadelli A., Mari A. Effect of hyperglycemia on insulin secretion in humans. Diabetes. 2002;51(Suppl. 1):S130–S133. doi: 10.2337/diabetes.51.2007.s130. [DOI] [PubMed] [Google Scholar]
- 7.Rodbard D. Increased glycemic variability at the onset and during progression of type 2 diabetes ― commentary. Diabetes Technol Ther. 2013;15(6):445–447. doi: 10.1089/dia.2013.0146. [DOI] [PubMed] [Google Scholar]
- 8.Kohnert K.D., Augstein P., Zander E., Heinke P., Peterson K., Freyse E.J. Glycemic variability correlates strongly with postprandial ß-cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care. 2009;32(6):1058–1062. doi: 10.2337/dc08-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohnert K.D., Freyse E.J., Salzsieder E. Glycemic variability and ß-cell dysfunction. Curr Diabetes Res. 2012;8(5):345–354. doi: 10.2174/157339912802083513. [DOI] [PubMed] [Google Scholar]
- 10.Kramer C.K., Choi H., Zinman B., Retnakaran R. Glycemic variability in patients with early type 2 diabetes: the impact of improvement of ß-cell function. Diabetes Care. 2014;37(4):1116–1123. doi: 10.2337/dc13-2591. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R., Shen S., Hanley A.J., Vuksan V., Hamilton J.K., Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity. 2008;16(8):1901–1907. doi: 10.1038/oby.2008.307. [DOI] [PubMed] [Google Scholar]
- 12.Maki K.C., McKenney J.M., Farmer M.V., Reeves M.S., Dicklin M.R. Indices of insulin sensitivity and secretion from a standard liquid meal test in subjects with type 2 diabetes, impaired or normal fasting glucose. Nutr J. 2009;8:22. doi: 10.1186/1475-2891-8-22. http://www.nutritionj.com/content/8/1/22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki K.C., Kelley K.M., Lawless A.L., Hubacher R.L., Schild A.L., Dicklin M.R. Validation of insulin sensitivity and secretion indices derived from the liquid meal tolerance test. Diabetes Technol Ther. 2011;13(6):661–666. doi: 10.1089/dia.2010.0240. [DOI] [PubMed] [Google Scholar]
- 14.Bacha F., Gungor N., Lee S., de las Heras J., Arslanian S. Indices of insulin secretion during a liquid mixed-meal test in obese youth with diabetes. J Pediatr. 2013;162(5):924–929. doi: 10.1016/j.jpeds.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Service F.J., Nelson R.L. Characteristics of glycemic stability. Diabetes Care. 1980;3(1):58–62. doi: 10.2337/diacare.3.1.58. [DOI] [PubMed] [Google Scholar]
- 16.Fritzsche G., Kohnert K.D., Heinke P., Vogt L., Salzsieder E. The use of a computer program to calculate the mean amplitude of glycemic excursions. Diabetes Technol Ther. 2011;13(3):319–325. doi: 10.1089/dia.2010.0108. [DOI] [PubMed] [Google Scholar]
- 17.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009;11(9):551–565. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 18.Chen T., Xu F., Su J.B., Wang X.Q., Chen J.L., Wu G. Glycemic variability in relation to oral disposition index in the subjects with different stages of glucose intolerance. Diabetol Metab Syndr. 2013;5:38. doi: 10.1186/1758-5996-5-38. http://www.dmsjournal.com/content/5/1/38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United Kingdom Prospective Study Group: UKPDS 16. Overview of 6 years' therapy of type 2 diabetes: a progressive disease. Diabetes. 1995;44(11):1249–1258. [PubMed] [Google Scholar]
- 20.Jensen C.C., Cnop M., Hull R.L., Fujimoto W.Y., Kahn S.E., American Diabetes Association GENNID Study Group ß-Cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethic groups in the U.S. Diabetes. 2002;51(7):2170–2178. doi: 10.2337/diabetes.51.7.2170. [DOI] [PubMed] [Google Scholar]
- 21.Giannini C., Weiss R., Cali A., Bonadonna R., Santoro N., Pierpout B. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012;61(3):606–614. doi: 10.2337/db11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Permutt M.A., Wasson J., Cox N. Genetic epidemiology of diabetes. J Clin Invest. 2005;115(6):1431–1439. doi: 10.1172/JCI24758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata G.H., Duckworth W.C., Shah J.H., Wendel S.W., Hoffman R.M. Sources of glucose variability in insulin-treated type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES) Clin Endocrinol (Oxf) 2004;60(4):451–456. doi: 10.1111/j.1365-2265.2004.02001.x. [DOI] [PubMed] [Google Scholar]
- 24.Owens D.R. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15(9):776–785. doi: 10.1089/dia.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosetti L. Glucose toxicity: the implication of hyperglycemia in the pathophysiology of diabetes mellitus. Clin Invest Med. 1995;18(4):255–260. [PubMed] [Google Scholar]
- 26.Robertson R.P., Harmon J., Tran P.O., Poitout V. ß-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl. 1):S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 27.Monnier L., Colette C., Mas E., Michel F., Cristol J.P., Boegner C. Regulation of oxidative stress by glycaemic control. Evidence for an independent inhibitory effect of insulin therapy. Diabetologia. 2010;53(3):562–571. doi: 10.1007/s00125-009-1574-6. [DOI] [PubMed] [Google Scholar]
- 28.Weng J., Li Y., Xu W., shi L., Zhang Q., Zhu D. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomized parallel-group trial. Lancet. 2008;371(9626):1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 29.Zeng L., Lu H., Deng H., Mu P., Li X., Wang M. Noninferiority effects on glycemic control and beta-cell function improvement in newly diagnosed type 2 diabetes patients: basal insulin monotherapy versus continuous subcutaneous insulin infusion treatment. Diabetes Technol Ther. 2012;14:35–42. doi: 10.1089/dia.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The ORIGIN Trial Investigators Characteristics associated with maintenance of mean A1C <6.5% in people with dysglycemia in the ORIGIN trial. Diabetes Care. 2013;36:2915–2922. doi: 10.2337/dc12-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Prato S., Bianchi C., Dardano A., Miccoli R. Insulin as an early treatment for type 2 diabetes: origin or end of an old question? Diabetes Care. 2013;36:S198–S204. doi: 10.2337/dcS13-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFronzo R.A. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cersosimo E., Solis-Herrera C., Trautmann M.E., Malley J., Triplitt C.L. Assessment of pancreatic ß-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10:2–42. doi: 10.2174/1573399810666140214093600. [DOI] [PMC free article] [PubMed] [Google Scholar]