Abstract
Background
Leukaemia remains the most common type of paediatric cancer and its aetiology remains unknown, but considered to be multifactorial. It is suggested that the initiation in utero by relevant exposures and/or inherited genetic variants and, other promotional postnatal exposures are probably required to develop leukaemia. This study aimed to map the incidence and analyse possible clusters in the geographical distribution of childhood acute leukaemia during the critical periods and to evaluate the factors that may be involved in the aetiology by conducting community and individual risk assessments.
Materials and methods
We analysed all incident cases of acute childhood leukaemia (<15 years) diagnosed in a Spanish region during the period 1998-2013. At diagnosis, the addresses during pregnancy, early childhood and diagnosis were collected and codified to analyse the spatial distribution of acute leukaemia. Scan statistical test methodology was used for the identification of high-incidence spatial clusters. Once identified, individual and community risk assessments were conducted using the Paediatric Environmental History.
Results
A total of 158 cases of acute leukaemia were analysed. The crude rate for the period was 42.7 cases per million children. Among subtypes, acute lymphoblastic leukaemia had the highest incidence (31.9 per million children). A spatial cluster of acute lymphoblastic leukaemia was detected using the pregnancy address (p <0.05). The most common environmental risk factors related with the aetiology of acute lymphoblastic leukaemia, identified by the Paediatric Environmental History were: prenatal exposure to tobacco (75%) and alcohol (50%); residential and community exposure to pesticides (62.5%); prenatal or neonatal ionizing radiation (42.8%); and parental workplace exposure (37.5%)
Conclusions
Our study suggests that environmental exposures in utero may be important in the development of childhood leukaemia. Due to the presence of high-incidence clusters using pregnancy address, it is necessary to introduce this address into the childhood cancer registers. The Paediatric Environmental History which includes pregnancy address and a careful and comprehensive evaluation of the environmental exposures will allow us to build the knowledge of the causes of childhood leukaemia.
Keywords: Spatial clustering, childhood leukaemia, environmental health, prenatal exposure, scan statistic
1. Introduction
Leukaemia remains the most common type of paediatric cancer (<15 years old), and represents 30% of all childhood cancers (Hunger and Mullighan, 2015). Acute leukaemia (AL) accounts for more than 95% of all childhood leukaemia cases, including acute lymphoblastic leukaemias (ALL) (78%) and acute myeloblastic leukaemias (AML) (16%) (Puumala et al., 2013; Ries et al., 1999). The peak age of emergence varies, with ages 2-5 for ALL, while AML is more frequent in children younger than age one. In Spain, AL has a crude incidence mean of 46.1 cases per million children, corresponding to 36.0 million cases of ALL and 8.3 million cases of AML (Peris Bonet et al., 2014).
The aetiology of childhood leukaemia remains unknown, but it is believed that both constitutional and environmental factors are involved (Inaba et al., 2013; Knox and Gilman, 1996; Pui et al., 2008). Currently, a well-accepted hypothesis (Inaba et al., 2013) is that leukaemia emerges from precursor mutations initially developed in utero, as well as, from the mutations developed after various exposures to leukaemogenic agents during infancy. Several studies have utilized georeferencing systems to identify and analyse the clustering of childhood leukaemia cases and investigate their possible associations with environmental factors through patient addresses at time of diagnosis (Alexander et al., 1997; Alexander et al., 1998; McNally et al., 2009a). However, in the past decade, only a few studies that analyse the addresses during pregnancy, birth and diagnosis have emerged, showing more conclusive results with pregnancy and birth addresses. These studies support that development of childhood cancers initiates in utero or beginning of infancy, catalysed by a common exposure within a shared geographic area (Kreis et al., 2016; McNally et al., 2009b). Additionally, much progress has been made in the study of the geographical differences in areas with smaller populations, allowing the observation of major differences in low overall prevalence diseases like childhood AL (Ortega-Garcia et al., 2016).
According to the US National Cancer Institute, risk factors (RFs) with conclusive evidence for ALL include: exposure to ionizing radiation in utero, postnatal therapeutic radiation, and genetic conditions, such as Down syndrome (Ries et al., 1999). Additional RFs with inconclusive evidence include: prenatal exposure to tobacco and alcohol, parental occupational exposures, parental and child exposure to pesticides, postnatal infections, advanced maternal age, high birth weight, maternal history of foetal loss, birth order and assisted reproduction technology (Heck et al., 2013; Maule et al., 2009; Reigstad et al., 2016; Ries et al., 1999; Turner et al., 2010; Wigle et al., 2009). On the other hand, breast milk is considered to be a protective factor of childhood leukaemia, with protection increasing as duration of exclusive breastfeeding increases (Amitay and Keinan-Boker, 2015; Ortega-Garcia et al., 2008).
The Paediatric Environmental History (PEH) questionnaire is an integrative tool that allows for the registration of environmental risk factors related to childhood cancer and propose aetiological hypotheses in the study of clusters (Ortega-García et al., 2012). Utilizing a carefully collected PEH could help explore possible environmental exposures involved in the aetiology.
The aim of this paper is to analyse the geographic distribution of childhood AL in a European Region (Murcia, Spain) using the addresses at pregnancy, infancy and time of diagnosis and developing the PEH to conduct individual and community risk assessments.
2. Materials and methods
2.1. Study Population
The Region of Murcia (RM) is a European Region located in southeast Spain, with a total population of 1,470,069 inhabitants in 2011. For the spatial cluster analysis, we consider the administrative division of census districts. In 2011, Region of Murcia was divided into 1220 census districts, with an average of 212 children under the age of 15, and a maximum of 698 and a minimum of 42.
The reference population (risk population) came from the 2001 and 2011 Spanish Census (INE, 2011). The total population (<15 years) was 207,822 in 2001 and 259,083 in 2011. We performed linear interpolation to estimate the population between the censuses. For each census district we used the population at the census times immediately preceding and immediately following. For times before the first census time, the population size is set equal to the population size at that first census time, and for times after the last census time, the population is set equal to the population size at that last census time.
2.2. Patient Registry
The MACAPEMUR (Environment and Paediatric Cancer in the Region of Murcia) database includes all patients under the age of 15 diagnosed with cancer within the RM since 1998. MACAPEMUR is a project that compiles the PEH of newly diagnosed cancer patients (Cárceles-Álvarez et al., 2015; Ferris Tortajada et al., 2004; Ortega-García et al., 2011). The single-province character of the RM and the centralized care reference units of Paediatric Oncohematolgy and the Paediatric Environmental Health Speciality Unit (PEHSU) at the Clinical University Hospital Virgin of Arrixaca facilitated the access to medical records. The hospital registry of the Clinical University Hospital Virgin of Arrixaca registers 100% of the children diagnosed with cancer in the RM. The classification of the cases is done by checking the clinical-pathological diagnosis with the International Classification of Diseases for Oncology (ICD-O-3) (IARC, 2011; Percy et al., 2000) and the International Classification of Childhood Cancer (ICCC-3) (Steliarova-Foucher et al., 2005) within 0-2 months after diagnosis. Over 99% of the cases are morphologically verified. Annually, a medical doctor performs an additional check of all cases to avoid misclassification and/or double registrations.
In all cases, the families are contacted in person or by phone. Once the diagnosis is made, a face-to-face interview is carried out by a doctor trained in paediatric cancer, environmental health and risk communication, who collects information on addresses at pregnancy, early childhood, and diagnosis; as well as another series of environmental data (Cárceles-Álvarez et al., 2015; Ferris Tortajada et al., 2004; Ortega-García et al., 2012). This study was approved by the Ethics Committee and the Institutional Review Board at the Clinical University Hospital Virgin of Arrixaca.
We identified 158 cases diagnosed with leukaemia between 1998-2013, and were grouped according to the criteria of the MACAPEMUR database. All families of patients were contacted to obtain consent and schedule interviews. Information was gathered by a pediatrician through: (1) face-to-face interviews with one or both parents present, (2) complementary phone calls to complete or verify data, (3) compiled data from primary care centres and/or local hospitals with records from the regional databases OMI-AP (Stacks, Consulting e Ingeniería en Software, S.L.U., Barcelona), Selene (UTE Siemens-Indra, Madrid), and Civitas (Steria Ibérica, S.A., Madrid).
The PEH questionnaire comprises a series of concise and basic questions through which the medical doctor identifies environmental exposures of concern and documents human carcinogens characterized by the International Agency for Research on Cancer (IARC) and by the US National Toxicology Program (IARC, 2016; US Department of Health and Human Services, 2014). Moreover, it includes residential address during pregnancy (PA), early childhood (PN) and time of diagnosis (DX) (Table 1) (Ferris Tortajada et al., 2004; Ortega-García et al., 2012). The interview is a semistructured process, where a standardized form is used, and the questions are either open ended or multiple choice. Additionally, the PEH includes visits to patient residences and neighbourhoods to interview local residents in order to identify the geography and RFs within the community. These visits are performed by a multidisciplinary team of doctors, nurses and other professionals with experience in human health risk assessment according to the US Environmental Protection Agency framework (US EPA, 2006). In the visits, a list of environmental hazards that may be associated with each tumour type is checked (IARC, 2016; Ries et al., 1999; US Department of Health and Human Services, 2014).
Table 1.
Main sections of the Paediatric Environmental History.
| Family history and associated constitutional symptoms. |
Pedigree of at least 3 generations for:
|
| Detailed description of sources of exposure during pregnancy. |
| Maternal grandmother's (maternal egg formation) work during pregnancy and drugs in pregnancy. |
| Maternal grandfather's work during pregnancy. |
| Environmental exposures (preconceptional, conceptional, pregnancy, postnatal). |
The data collection is distributed in the following sections:
|
| Type of tumor (tumor data, diagnosis, treatment, and evolution). |
2.3. Statistical Methods
The incidence is described in a statistical summary: Number of cases, Crude Rate (CR) and age-adjusted Standardized Incidence Ratio (SIR) by tumour subtypes was calculated with a corresponding 95% confidence interval. Ages were grouped based on standard categories (<1; 1-4; 5-9;10-14) and used for summary and rate calculations. For the purpose of cluster analysis, leukaemia types were grouped into categories: (1) all leukaemia types, (2) ALL, (3) AML and (4) others. All cases were assigned to the correspondent census district for each address (PA, PN, DX). To identify geographical areas with an excess number of childhood cancer cases (spatial cluster), the scan test methodology (Kulldorff, 1997) was used adjusted by age groups with p-value < 0.05 considered statistically significant (McNally and Eden, 2004). A spatial cluster could arise in a small geographical area or multiple small areas with an increased incidence that is persistent over time (McNally et al., 2009b).
The scan test in its pure spatial version identifies cluster regions with a high incidence, including windows on a map of various shapes and sizes (circular or elliptical), comparing the incidence inside and outside the window. This is a popular test to identify spatial clusters of diseases (e.g. Durand and Wilson, 2006). To understand the operation of the statistic, consider a study area A (in our case the Region of Murcia) that contains a total of C cases distributed in N regions (in our case, 1220 census districts). We assumed that the number of cases of cancer in each census district is distributed according to a Poisson model. Let Z a window of connected census districts. If the window Z includes the centroid of a specific census district, then this census district is included in the window Z. The spatial scan statistic test analyses the null hypothesis of equal risk of childhood cancer incidence inside and outside of any window Z against the alternative hypothesis that there is at least one window (named Z*) with an elevated risk of childhood cancer incidence within of Z*. Formally, the underline hypothesis can then be tested:
H0: The underling risk is equal inside and outside for all windows.
HA: There is at least one window for which the underlying risk is higher inside as compared to outside.
To test this hypothesis, set c(Z) be the observed number of cases in window Z and n(Z) be the expected number of cases in window Z under the null hypothesis. Let LHA(Z) be the likelihood under the alternative hypothesis that there is a cluster in windows Z, and let LH0 be the likelihood under the null hypothesis. Therefore, the likelihood ratio test can be written that:
The scan test is defined as,
Where Θ is the set of all possible circular and/or windows included in study area A. In the previous expression, I(•) is an indicator function. When L is set to scan only for clusters with high rates, I(•) is equal to 1 when the window has more cases than expected under the null hypothesis, and is equal to 0 otherwise. Details, including derivations as a likelihood ratio test, have been given elsewhere (Kulldorff, 1997).
The region Z* which the likelihood ratio is highest is named the Most Likelihood Cluster (MLC). The significance of MLC is obtained by Monte Carlo techniques. We use Monte Carlo simulation generated 999 random replications of the data set. Elliptical shaped windows were chosen to conduct the search. The software used to run the Scan test can be downloaded for free from www.satscan.org.
3. Results
3.1. Descriptive Study
Table 2 shows the incidence of leukaemia in Region of Murcia by sex, age and two subperiods (1998-2005; 2006-2013). The incidence rates are highest under age 5 and slightly higher among males than females. From the first to the second subperiod the incidence crude rate of all leukaemias increased by 9.1 cases per million. SIR for ALL and AML in the second period are greater than 1 showing a significant growth in the second subperiod. Within the subtypes, ALL had the greatest incidence at 31.9 per million children with a statistically significant increase of SIR in the second subperiod (2006-2013). The CR for comparable periods of all leukaemias and subtypes for Spain and Europe is shown in Table 2. Those results show a slightly lower incidence in Region of Murcia than in Spain, but slightly higher than in Europe.
Table 2.
Number of leukaemias, crude rate per millon children (<15) and SIR in the Region of Murcia.
| Time period | Sex | CR (number) by age group | Total | Spain/Europe | SIR1 (SIRL-SIRU) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Girls | Boys | <1 | 1-4 | 5-9 | 10-14 | |||||
| All leukaemias | 1998-2005 | 32 | 32 | 37.0 (4) | 82.6 (36) | 32.4 (18) | 10.1 (6) | 37.8 (64) | 0.91 (0.70-1.16) | |
| 2006-2013 | 45 | 49 | 109.5 (15) | 70.0 (40) | 35.9(24) | 23.9 (15) | 46.9 () | 1.33 (1.11-1.58) | ||
| 1998-2013 | 77 | 81 | 77.5 (19) | 75.5 (76) | 34.3 (42) | 17.2 (21) | 42.7 (158) | 46.1†/41. 7‡ | ||
| ALL | 1998-2005 | 24 | 23 | 27.8 (3) | 55.1 (24) | 27.0 (15) | 8.4 (5) | 27.8 (47) | 0.89 (0.69-1.14) | |
| 2006-2013 | 32 | 38 | 94.9 (12) | 52.5 (30) | 23.9 (16) | 19.1 (12) | 35.4 (70) | 1.35 (1.09-1.64) | ||
| 1998-2013 | 56 | 61 | 65.3 (15) | 53.6 (54) | 25.3 (31) | 13.9 (17) | 31.9 (117) | 36.0†/33. 8‡ | ||
| AML | 1998-2004 | 5 | 8 | 9.3 (1) | 20.7 (9) | 3.6 (2) | 1.7 (1) | 7.7 (13) | 0.92 (0.49-1.57) | |
| 2005-2010 | 10 | 9 | 7.3 (1) | 15.8 (9) | 12.0 (8) | 1.6 (1) | 9.5 (19) | 1.34 (0.88-1.97) | ||
| 1998-2010 | 15 | 17 | 8.2 (2) | 17.9 (18) | 8.2 (10) | 1.6 (2) | 8.7 (32) | 8.3†/6.2‡ | ||
| Other | 1998-2004 | 3 | 1 | 0.0 (0) | 6.9 (3) | 1.8 (1) | 0.0 (0) | 2.4 (4) | 1.11 (0.38-2.54) | |
| 2005-2010 | 3 | 2 | 7.3 (2) | 1.8 (1) | 0.0 (0) | 3.2 (2) | 2.0 (5) | 1.11 (0.38-2.54) | ||
| 1998-2010 | 6 | 3 | 4.1 (2) | 4.0 (4) | 0.8 (1) | 1.6 (2) | 2.2 (9) | 1.9†/--‡ | ||
CR for Spain for period 1988-1997 (Peris-Bonet et al, 2014);
CR for Europe for period 2000-2013 (Steliarova-Foucher et al., 2002)
3.2. Residential addresses during pregnancy, early childhood and diagnosis
Of the 158 reported cases, 12 pregnancy addresses were located outside the Region of Murcia and 6 were unavailable. About 9% of cases moved residence between the time of pregnancy and early childhood, while almost 30% of cases moved between the time of pregnancy and diagnosis. Most residential changes did not involve a change in census district area and those who changed their census district did not change their municipality.
3.3. Spatial clusters of leukaemia in the Region of Murcia
Table 3 shows the results obtained using the spatial scan test for the three address categories. A non-significant cluster (p-value<0.1) was identified for all leukaemia types (Figure 1A) using the pregnancy address. The MLC with statistical significance (p-value<0.05) was identified using the pregnancy addresses for ALL. The cluster included 19 census districts comprised of 9 cases within the municipality of Cieza. The statistical significance of the cluster between pregnancy and other addresses decreased due to cases changing addresses (Figure 1B; Table 3). Note that between pregnancy and diagnostic three cases moved within Cieza (#D, #F, #I) and another (#G) moved outside the municipality (Figure 1C).
Table 3. Spatial clusters of all types of leukaemias and ALL.
| All types of leukaemias | ALL | |||||
|---|---|---|---|---|---|---|
| PA | PN | DX | PA | PN | DX | |
| Total number cases | 147 | 141 | 158 | 107 | 104 | 117 |
| Global annual cases | 3.9 | 3.8 | 4.2 | 2.9 | 2.8 | 3.1 |
| Cluster size in MLC (# census districts) | 18 | 17 | 16 | 19 | 10 | 16 |
| Population inside MLC | 2749 | 2498 | 2321 | 2880 | 193 | 2321 |
| Number cases inside MLC | 10 | 8 | 8 | 9 | 6 | 7 |
| Expected cases inside MLC | 1.7 | 1.50 | 1.57 | 1.32 | 0.71 | 1.16 |
| Annual Cases/100000 | 2 | 20.0 | 21.5 | 19.5 | 23.5 | 18.8 |
| Relative risk | 6.15 | 5.58 | 5.33 | 7.38 | 8.94 | 6.36 |
| Scan test value | 9.16 | 7.03 | 4.32 | 9.91 | 7.37 | 6.61 |
| p-value | 0.09* | 0.39 | 0.62 | 0.03** | 0.31 | 0.52 |
pvalue < 0.1;
p-value<0.05 (in bold);
PA=Pregnancy address; PN= Early childhood address; DX= Diagnosis address; Cluster size=number of census districts that comprise each respective cluster
Figure 1.
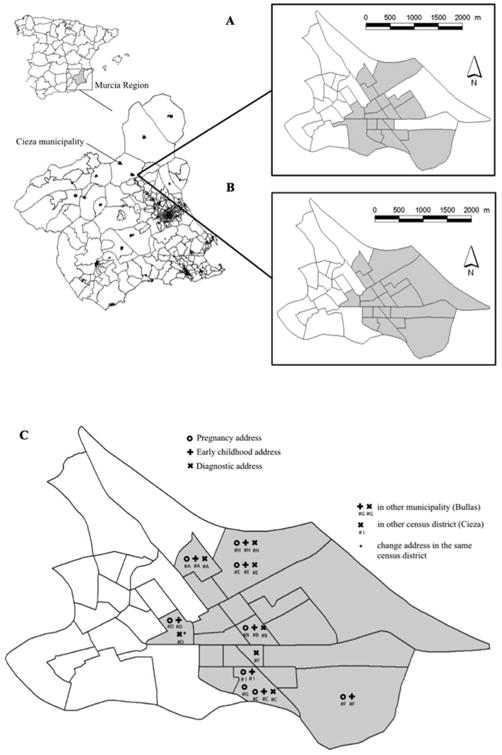
ALL clusters and case movements within the municipality of Cieza. A. Spatial clusters of early childhood addresses by census district. B. Spatial clusters of pregnancy addresses by census district. C. Changes in residential location within the municipality of Cieza.
3.4. Identifying risk and protection factors of a cluster in Cieza
For the 9 cases inside the MLC in Cieza (Table 4), the mean age of diagnosis was 4.8 years, 7 females and 2 males, all diagnosed between 1998 and 2006. Of the 9 cases, 8 were diagnosed with precursor B-cell ALL and 1 T-cell ALL. One patient was diagnosed with Down syndrome (Table 4).
Table 4. Clinical patient information.
| Patient | Sex | Birth Date | DX Date | DX age | Type | Genetics | Exitus |
|---|---|---|---|---|---|---|---|
| 1 | F | 1984 | 1998 | 14 | Precursor B-cell ALL | 46 XX | Yes |
| 2 | F | 1993 | 1999 | 5 | Precursor B-cell ALL | 46 XX | No |
| 3 | F | 1996 | 2000 | 4 | Precursor B-cell ALL | 46 XX, t(12;21), t(9;22) | Yes |
| 4 | F | 1997 | 2002 | 4 | Precursor B-cell ALL | 46 XX | No |
| 5 | F | 1999 | 2003 | 4 | Precursor B-cell ALL | 46 XX, t(12;21) | Yes |
| 6 | M | 1996 | 2004 | 7 | T-cell ALL | 46 XY | No |
| 7 | F | 2003 | 2005 | 2 | Precursor B-cell ALL | 46 XX, t(9;22) | No |
| 8 | M | 2001 | 2005 | 4 | Precursor B-cell ALL | 46 XY | No |
| 9 | F | 2002 | 2006 | 4 | Precursor B-cell ALL | 46 XX, t(12;21), t(9;22),t(11q23), trisomy 21 in majority of the nucleuses analysed | No |
| Summary | 7F-2M | 1984-2003 | 1998-2006 | Mean: 4.8 | 8 Precursor B-cell ALL | 1 cases of trisomy 21 | 3/9 |
DX=Diagnosis address; F=female; M=male.
Only 8 cases involved in the cluster had a full and complete PEH. Table 5 shows the risk factors related to children with ALL obtained by the PEH (Amitay and Keinan-Boker, 2015; Heck et al., 2013; Maule et al., 2009; Reigstad et al., 2016; Ries et al., 1999; Turner et al., 2010; Wigle et al., 2009). The most common modifiable environmental RFs identified and were: prenatal exposure to tobacco (6/8) and alcohol (4/8), residential and community exposure to pesticides (5/8), prenatal or neonatal ionizing radiation (3/7), parental workplace exposure (3/8). Other risk factors included advanced maternal age (3/9) maternal history of foetal loss (2/9). Regarding the protective factor, only 2 were breastfed for over a month and none reached 6 months. All patients were newborns at term, none with high birth weight (>4000 g), and the majority were the first-born (6/9).
Table 5. Summary of Environmental Risk and Protective Factors.
| Cases | IonRx | Mat. Age | Abortions | First born | Occupational exposure (hydrocarbons, and paints) | Pesticides | Atm. Poll | Infs | ELF-EMF Home or school | OH | Tobacco smoke exposure | ART | Any Breastfeeding (weeks) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | - | 24 | No | Yes | - | - | - | - | - | - | - | No | - | |
| 2* | No | 30 | Yes | No | Yes | Yes | 3 | No | Yes | No | Yes++ | No | 4 | |
| 3 | No | 32 | Yes | Yes | - | No | 7 | - | No | Yes | Yes++ | No | 2 | |
| 4 | Yes | 29 | No | Yes | No | No | 8 | Yes | No | No | No | No | 1 | |
| 5 | No | 29 | No | Yes | Yes | No | 7 | - | No | Yes | Yes++ | No | 16 | |
| 6** | Yes | 42 | No | Yes | No | Yes | 0 | Yes | No | Yes | Yes++ | Yes | 1 | |
| 7 | No | 35 | No | No | No | Yes | 8 | Yes | Yes | No | No | No | 0 | |
| 8 | No | 42 | No | Yes | No | Yes | 1 | No | No | No | Yes+ | No | 6 | |
| 9* | Yes | 29 | No | No | Yes | Yes | 0 | No | No | Yes | Yes++ | No | 0 | |
| Exposure reported | 3/7 | 3/9 | 2/9 | 6/9 | 3/8 | 5/8 | 4/8 | 3/6 | 2/8 | 4/8 | 6/8 | 1/10 | ||
| % | 42.8 | 33.3 | 22.2 | 66.6 | 37.5 | 62.5 | 50 | 50 | 25 | 50 | 75 | 10 |
: Regarding addresses at time of diagnosis, three cases moved within Cieza and
: another moved outside the municipality, Ion Rx: Medical Ionizing radiation during pregnancy, Mat. age: Maternal age, Atm. Poll: Atmospheric pollution by Likert-type scale of environmental perception (0-10) in the diagnosis address. Infs: Viral Infections before the diagnosis, ELF-EMF: Extremely Low Frequency Electromagnetic Fields (>/=0.2 uT), OH: alcohol during pregnancy, ART: Assisted reproductive technology.
: both parent smokers during pregnancy
: only the father smoker.
Through the residential and neighbourhood visits, environmental risk exposures for childhood leukaemia (IARC, 2016; Ries et al., 1999; US Department of Health and Human Services, 2014) were evaluated: a) residential exposure to ionizing radiation due to nearby medical facilities; b) atmospheric pollution from heavy-traffic roads, gas stations, industries or other sources; c) exposure to pesticides from field crops; d) exposure to electromagnetic or radiofrequency fields; e) environmental tobacco exposure in the residence; and f) any other exposure that may be considered of risk by the evaluation team. In this study, various risk factors were identified. First, a strong odour originating from exposed sewage waste flowing into the Segura River was identified. Second, the burning of agricultural and rubber waste was a common practice during winter, used to maintain adequate temperature for field crops. Finally, areas adjacent to the river and near the residential areas were frequently aerially fumigated.
4. Discussion
We found a significant ALL cluster using the pregnancy address with a history of exposure to several prenatal and postnatal risk factors in 9 cases of ALL. Our combined study included a georeferentiation analysis and a detailed and careful PEH. These findings support previous research that suggests that ALL is initiated in utero and/or with subsequent post-natal promotion and clonal evolution. There are several studies showing that initial mutations of ALL start in utero, such as Taub et al. (2002), who retrospectively detected leukemogen cells in newborn genetic screening cards of a group of children diagnosed with B-precursor ALL. Moreover, Greaves and Wiemels (2003) found chromosome translocations related to childhood ALL occurring prenatally. Though, these mutations are not sufficient to develop leukaemia, being that other postnatal promotional exposures are necessary. Mori et al. (2002) found that the genomic alterations related to ALL, such as ETV6-RUNX1, are present in the blood of healthy newborns at a higher rate than the cumulative risk of the corresponding leukaemia. This supports the importance of postnatal factors for the development of leukaemia.
We found an incidence rate increase of 9.1 cases per million from 1998-2005 to 2006-2013, consistent with previous studies (Amin et al., 2010; Supriyadi et al., 2011). Additionally, the incidence rate for childhood leukaemia in the Region of Murcia was similar with the national rate (Table 2) (Peris Bonet et al., 2014).
In our study, leukaemias show statistically significant spatial clustering using pregnancy addresses; however, no spatial clusters were detected using diagnosis addresses. Similar results were found in Zhang et al. (2016) showing a decrease in p-values as the temporal lag between the prenatal and diagnostic address increase. However, Chen et al. (2010) explored the extent of ambient air pollutant exposure measurement error due to maternal residential mobility during pregnancy within a New York birth cohort, but no significant impact was found. Compared to adults, the time between the foetal stage until the age of two has a greater sensitivity to mutagenic action of 10-fold risk and a 3-fold adjustment for years 3-15 (Preston, 2004). In recent years there has been an increase in studies linking environmental exposures during pregnancy and the subsequent development of childhood leukaemia (Carlos-Wallace et al., 2016; Heck et al., 2014; Metayer et al., 2016a; Vinson et al., 2011.). This point, associated with the increasingly accepted hypotheses that leukaemia emerges from precursor mutations initially developed in utero, makes it necessary to introduce the address during pregnancy in childhood cancer registries in order to perform more complete studies that help to improve the knowledge of the causes of childhood leukaemia.
Most clusters of childhood cancer have not identified risk factors. We think this is due to the low prevalence, methodological difficulties, and primarily, the absence of an aetiological assessment in each case, causing them to be frequently attributed to chance. The PEH could help improve the descriptive basis for generating consolidated hypotheses (Ortega-García et al., 2012; Ortega-García et al., 2016).
Through the PEH of the 9 cases, we found several RFs associated with ALL which can be grouped into constitutional factors and individual and community exposures in the Cieza municipality.
The PEH allowed us to identify 5 cases with RFs with conclusive evidence, 1 patient with Down syndrome and 3 patients with exposure to medical ionizing radiation during the in utero or neonatal period. Evidence suggests that exposure to ionizing radiation is an associated risk of childhood AL, not only in utero but also in the neonatal and postnatal period (Bartley et al., 2010; Doll and Wakeford, 1997; Infante-Rivard, 2003).
Among the factors with inconclusive evidence, the most common are prenatal exposure to tobacco smoke, alcohol, and solvents and paints by occupational exposures (Metayer et al., 2016a; Wiggle et al., 2009). Some other factors included the short duration of breastfeeding and virus infections before diagnosis (Amitay and Keinan-Boker, 2015; Marcotte et al., 2014). In addition, three community exposures are present in the cluster and maintained over time: air pollution from pesticides, sewage waste, and/or burning of agricultural and rubber waste (Bailey et al., 2015; Gómez-Barroso et al., 2016; Heck et al., 2014; Turner et al., 2010; Vinson et al., 2011).
Tobacco smoke contains carcinogenic compounds, such as benzo (a) pyrene, classified as a human carcinogen (IARC, 2012a). Studies have shown an association between childhood ALL and environmental tobacco smoke exposure during pregnancy (Yan et al., 2015). On the other hand, several studies have found an association between maternal alcohol consumption during pregnancy and increased risk for AML (Latino-Martel et al., 2010; Shu et al., 1996), but this association has not been found for ALL (Yan et al., 2015). Breastfeeding is inversely associated with risk for childhood leukaemia with a dose-response effect (Amitay et al., 2016).
Parental occupational exposure during pregnancy to solvents or paints was found in 3 patients. These exposures have been associated to ALL, with variable evidence (Bailey et al., 2014; Metayer et al., 2016b; Reid et al., 2011).
Cieza has approximately 35,000 inhabitants and is composed of large agricultural areas, frequently exposed to pesticides, intersected by the Segura River. Two meta-analysis published in 2015 (Bailey et al., 2015; Chen et al., 2015) show an association between the pesticide exposure at home and AL. Furthermore, Gómez-Barroso et al (2016) found an excess risk with leukaemia in children living in close proximity to agricultural land. Moreover, residents reported strong odours originating from the open sewers, containing faecal matter and plastic waste, outpouring into the Segura River. Sewer gas is a mix of gases that arise from the decomposition of organic material in sewage waste. Primarily composed of sulfide, methane, ammonia, carbon dioxide and Volatile Organic Compounds (VOC) (Van Langenhove et al., 1985; Vincent, 2001). Pennell et al (2013), found that infiltration of sewer gas into residences can be the source of tetrachloroethylene (PCE) and other VOC, possible leukemogenic agents (Gao et al., 2014; IARC, 2012b). During the winter, burning of agricultural and rubber waste was observed; which produces high concentrations of pollutants, such as polycyclic aromatic hydrocarbons (PAH) like benzene and its derivatives. Heck et al. (2014) observed an increased risk of ALL associated with exposure to PAH. The air and water quality records of the municipality were evaluated during the study period, drawing attention to repetitive contamination peaks of PM10 during the winter months due to the burning of these wastes (“Calidad del aire”, 2016).
A limitation of this study was the limited number of cases used in the analysis due to low prevalence of childhood leukaemia. However, the sample size is representative, since all leukaemia cases diagnosed in the Region of Murcia are included in a long-time period 1998-2013. Second, the increasing migration into the region for diagnosis can falsely increase the case incidence rate. Consequently, we developed an exhaustive search with strict inclusion-exclusion criteria increasing the consistency of our data. Furthermore, the limitation of the border-effect, due to the loss of patients and valuable information because patients receive or continue treatment elsewhere was reduced by revision of hospital registries. The use of observational information for exposure assessment is a limitation of these case studies. Information and memory bias was controlled for by conducting face-to-face interviews and visiting the addresses and neighbourhoods. The PEH is unable to confirm and quantify many exposures. Also, the identification of confounders and understanding of pathophysiology of leukaemia (e.g., gene–environment or infection–environment interaction) is inadequate. However, the PEH seems to be a useful tool to signalize RFs, helping to carry out other more complex studies. While evidence continues to develop, it seems reasonable to create healthier environments for children by avoiding unnecessary ionizing radiation from medical tests during critical periods of development, promoting breastfeeding programs and improving the indoor/outdoor quality air and parental occupational protection.
5. Conclusions
Our study suggests that environmental exposures in utero may be important in the development of childhood leukaemia. Although additional research is clearly needed, our findings have potentially significant implications for current public health and childhood cancer programs. Due to the presence of high-incidence clusters using pregnancy address, it is necessary to introduce this address into the childhood cancer registers. Additionally, a comprehensive and careful analysis of environmental exposures used to detect risk factors could be important to improve healthcare quality, integrity, and for insuring the health and safety of the survivors of childhood cancer.
Highlights.
A spatial cluster of ALL was detected using the pregnancy address.
The spatial cluster is not significant when we use diagnostic address.
Environmental exposures in utero could help to develop a childhood leukaemia.
The Paediatric Environmental History helps to identify environmental risk factors.
Pregnancy address should be included into childhood cancer registries.
Acknowledgments
Funding: The authors wish to express their gratitude to all the participants of the study. This work was supported by Mount Sinai International Exchange Program for Minority Students (grant MD001452) from the National Center on Minority Health and Health Disparities of the U.S. National Institutes of Health; Prof. Fernando A. López-Hernández, grateful for the financial support offered by the projects from Programa de Ayudas a Grupos de Excelencia de la Región de Murcia, Fundación Séneca (#19884-GERM-15) and Ministry of Economy and Competiveness (ECO2015-651758-P).
Footnotes
Human subjects' ethics review: This study was approved by the Ethics Committee and the Institutional Review Board at the Clinical University Hospital of Virgin of Arrixaca.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander FE, Boyle P, Carli PM, Coebergh JW, Draper GJ, Ekbom A, Levi F, McKinney PA, McWhirter W, Michaelis J, Peris-Bonet R, Petridou E, Pompe-Kirn V, Plìsko I, Pukkala E, Rahu M, Storm H, Terracini E, Vatten L, Wray N. Spatial clustering of childhood leukaemia: summary results from the EUROCLUS project. Br J Cancer. 1998;77:818–24. doi: 10.1038/bjc.1998.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander FE, Chan LC, Lam TH, Yuen P, Leung NK, Ha SY, Yuen HL, Li CK, Li CK, Lau YL, Greaves MF. Clustering of childhood leukaemia in Hong Kong: association with the childhood peak and common acute lymphoblastic leukaemia and with population mixing. Br J Cancer. 1997;75:457–63. doi: 10.1038/bjc.1997.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin R, Bohnert A, Holmes L, Rajasekaran A, Assanasen C. Epidemiologic mapping of Florida childhood cancer clusters. Pediatr Blood Cancer. 2010;54:511–8. doi: 10.1002/pbc.22403. [DOI] [PubMed] [Google Scholar]
- Amitay EL, Dubnov Raz G, Keinan-Boker L. Breastfeeding, Other Early Life Exposures and Childhood Leukemia and Lymphoma. Nutr Cancer. 2016;68:968–77. doi: 10.1080/01635581.2016.1190020. [DOI] [PubMed] [Google Scholar]
- Amitay EL, Keinan-Boker L. Breastfeeding and Childhood Leukemia Incidence: A Meta-analysis and Systematic Review. JAMA Pediatr. 2015;169:e151025. doi: 10.1001/jamapediatrics.2015.1025. [DOI] [PubMed] [Google Scholar]
- Bailey HD, Fritschi L, Metayer C, Infante-Rivard C, Magnani C, Petridou E, Roman E, Spector LG, Kaatsch P, Clavel J, Milne E, Dockerty JD, Glass DC, Lightfoot T, Miligi L, Rudant J, Baka M, Rondelli R, Amigou A, Simpson J, Kang AY, Moschovi M, Schüz J. Parental occupational paint exposure and risk of childhood leukemia in the offspring: findings from the Childhood Leukemia International Consortium. Cancer Causes Control. 2014;26:1257–70. doi: 10.1007/s10552-015-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HD, Infante-Rivard C, Metayer C, Clavel J, Lightfoot T, Kaatsch P, Roman E, Magnani C, Spector LG, Th Petridou E, Milne E, Dockerty JD, Miligi L, Armstrong BK, Rudant J, Fritschi L, Simpson J, Zhang L, Rondelli R, Baka M, Orsi L, Moschovi M, Kang AY, Schuz J. Home pesticide exposures and risk of childhood leukemia: Findings from the childhood leukemia international consortium. Int J Cancer. 2015;137:2644–63. doi: 10.1002/ijc.29631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley K, Metayer C, Selvin S, Ducore J, Buffler P. Diagnostic X-rays and risk of childhood leukaemia. Int J Epidemiol. 2010;39:1628–37. doi: 10.1093/ije/dyq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calidad del Aire de la Región de Murcia. [accessed 20 November 2016];2016 Available: http://sinqlair.carm.es/calidadaire/
- Carlos-Wallace FM, Zhang L, Smith MT, Rader G, Steinmaus C. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. Am J Epidemiol. 2016;183:1–14. doi: 10.1093/aje/kwv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;110:162–8. doi: 10.1016/j.envres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Chen M, Chang CH, Tao L, Lu C. Residential Exposure to Pesticide During Childhood and Childhood Cancers: A Meta-Analysis. Pediatrics. 2015;136:719–29. doi: 10.1542/peds.2015-0006. [DOI] [PubMed] [Google Scholar]
- Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–9. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- Durand M, Wilson JG. Spatial analysis of respiratory disease on an urbanized geothermal field. Environ Res. 2006;101:238–245. doi: 10.1016/j.envres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ferris Tortajada J, Ortega García JA, Marco Macián A, García Castell J. Environment and pediatric cancer. An Pediatr (Barc) 2004;61:42–50. doi: 10.1016/s1695-4033(04)78352-6. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Kamijima M, Sakai K, Khalequzzaman M, Nakajima T, Shi R, Wang X, Chen D, Ji X, Han K, Tian Y. Quantitative assessments of indoor air pollution and the risk of childhood acute leukemia in Shanghai. Environ Pollut. 2014;187:81–9. doi: 10.1016/j.envpol.2013.12.029. [DOI] [PubMed] [Google Scholar]
- Gomez-Barroso D, García-Pérez J, López-Abente G, Tamayo-Uria I, Morales-Piga A, Pardo Romaguera E, Ramis R. Agricultural crop exposure and risk of childhood cancer: new findings from a case-control study in Spain. Int J Health Geogr. 2016;15:18. doi: 10.1186/s12942-016-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves MF, Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer. 2003;3:639–49. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- Heck JE, Park AS, Qiu J, Cockburn M, Ritz B. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int J Hyg Environ Health. 2014;217:662–8. doi: 10.1016/j.iheh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Wu J, Lombardi C, Qiu J, Meyers TJ, Wilhelm M, Cockburn M, Ritz B. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect. 2013;121:1385–91. doi: 10.1289/ehp.1306761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373:1541–52. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) ICD-O-3. Lyon, France: 2011. International Classification of Diseases for Oncology. [Google Scholar]
- IARC (International Agency for Research on Cancer) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 100E. World Health Organization; Lyon, France: 2012a. Personal habits and indoor combustions. (online) Available: http://monographs.iarc.fr/ENG/Monographs/vol100E/ [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) ARC Monographs on the Evaluation of Carcinogenic Risks to Humans 106. World Health Organization; Lyon, France: 2012b. Trichloroethylene, Tetrachloroethylene, and Some Other Chlorinated Agents. (online) Available: http://monographs.iarc.fr/ENG/Monographs/vol106/index.php. [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. World Health Organization; Lyon, France: 2016. [accessed 10 October 2016]. (online) Available: http://monographs.iarc.fr/ENG/Monographs/PDFs/index.php. [Google Scholar]
- Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–55. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INE (Instituto Nacional de Estadística) Padrón municipal de habitantes. [accessed 10 October 2016];2011 Available: http://www.ine.es/inebmenu/mnu_cifraspob.htm.
- Infante-Rivard C. Diagnostic x rays, DNA repair genes and childhood acute lymphoblastic leukemia. Health Phys. 2003;85:60–4. doi: 10.1097/00004032-200307000-00012. [DOI] [PubMed] [Google Scholar]
- Knox EG, Gilman EA. Spatial clustering of childhood cancers in Great Britain. J Epidemiol Community Health. 1996;50:313–9. doi: 10.1136/jech.50.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox EG, Gilman EA. Migration patterns of children with cancer in Britain. J Epidemiol Community Health. 1998;52:716–726. doi: 10.1136/jech.52.11.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis C, Grotzer M, Hengartner H, Spycher BD. Space-time clustering of childhood cancers in Switzerland: A nationwide study. Int J Cancer. 2016;138:2127–35. doi: 10.1002/ijc.29955. [DOI] [PubMed] [Google Scholar]
- Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods. 1997;26:1481–1496. doi: 10.1080/03610929708831995. [DOI] [Google Scholar]
- Latino-Martel P, Chan DS, Druesne-Pecollo N, Barrandon E, Hercberg S, Norat T. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1238–60. doi: 10.1158/1055-9965.EPI-09-1110. [DOI] [PubMed] [Google Scholar]
- Marcotte EL, Ritz B, Cockburn M, Yu F, Heck JE. Exposure to infections and risk of leukemia in young children. Cancer Epidemiol Biomarkers Prev. 2014;23:1195–203. doi: 10.1158/1055-9965.EPI-13-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maule MM, Vizzini L, Czene K, Akre O, Richiardi L. How the effect of maternal age on the risk of childhood leukemia changed over time in Sweden, 1960-2004. Environ Health Perspect. 2009;117:299–302. doi: 10.1289/ehp.11938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ, Alexander FE, Vincent TJ, Murphy MF. Spatial clustering of childhood cancer in Great Britain during the period 1969-1993. Int J Cancer. 2009a;124:932–6. doi: 10.1002/ijc.23965. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Bithell JF, Vincent TJ, Murphy MF. Space-time clustering of childhood cancer around the residence at birth. Int J Cancer. 2009b;124:449–55. doi: 10.1002/ijc.23927. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Eden TO. An infectious aetiology for childhood acute leukaemia: a review of the evidence. Br J Haematol. 2004;127:243–63. doi: 10.1111/j.1365-2141.2004.05166.x. [DOI] [PubMed] [Google Scholar]
- Metayer C, Dahl G, Wiemels J, Miller M. Childhood Leukemia: A Preventable Disease. Pediatrics. 2016;138:S45–S55. doi: 10.1542/peds.2015-4268H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metayer C, Scelo G, Kang AY, Gunier RB, Reinier K, Lea S, Chang JS, Selvin S, Kirsch J, Crouse V, Does M, Quinlan P, Hammond SK. A task-based assessment of parental occupational exposure to organic solvents and other compounds and the risk of childhood leukemia in California. Environ Res. 2016;151:174–183. doi: 10.1016/j.envres.2016.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Colman SM, Xiao Z, Ford AM, Healy LE, Donaldson C, Hows JM, Navarrete C, Greaves M. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci USA. 2002;99:8242–7. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Garcia JA, Ferrís-Tortajada J, Torres-Cantero AM, Soldin OP, Torres EP, Fuster-Soler JL, Lopez-Ibor B, Madero-Lopez L. Full breastfeeding and paediatric cancer. J Paediatr Child Health. 2008;44:10–3. doi: 10.1111/j.1440-1754.2007.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Garcia JA, López-Hernández FA, Cárceles-Álvarez A, Santiago-Rodríguez EJ, Sánchez AC, Bermúdez-Cortes M, Fuster-Soler JL. [Analysis of small areas of pediatric cancer in the municipality of Murcia (Spain)] An Pediatr (Barc) 2016;84:154–62. doi: 10.1016/j.anpedi.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Ortega-García JA, López-Hernández FA, Sobrino-Najul E, Febo I, Fuster-Soler JL. Environment and paediatric cancer in the Region of Murcia (Spain): integratingclinical and environmental history in a geographic information system. An Pediatr (Barc) 2011;74:255–60. doi: 10.1016/j.anpedi.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-García JA, Soldin OP, López-Hernández FA, Trasande L, Ferrís-Tortajada J. Congenital fibrosarcoma and history of prenatal exposure to petroleum derivatives. Pediatrics. 2012;130:e1019–25. doi: 10.1542/peds.2011-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell KG, Scammell MK, McClean MD, Ames J, Weldon B, Friguglietti L, Suuberg EM, Shen R, Indeglia PA, Heiger-Bernays WJ. Sewer Gas: An Indoor Air Source of PCE to Consider During Vapor Intrusion Investigations. Ground Water Monit Remediat. 2013;33:119–126. doi: 10.1111/gwmr.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy C, Fritz A, Jack A, Shanmugarathan S, Sobin L, Parkin DM, Whelan S. International Clasification of Diseases for Oncology (ICD-O) 3rd. World Health Organization; Geneva: 2000. [Google Scholar]
- Peris Bonet R, Felipe García S, Martínez Ruiz N, Pardo Romaguera E, Valero Poveda S. Estadísticas 1980-2013. Registro Nacional de Tumores Infantiles; Valencia: 2014. Cáncer infantil en España. [Google Scholar]
- Preston RJ. Children as a sensitive subpopulation for the risk assessment process. Toxicol Appl Pharmacol. 2004;199:132–41. doi: 10.1016/j.taap.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–43. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- Puumala SE, Ross JA, Aplenc R, Spector LG. Epidemiology of childhood acute myeloid leukemia. Pediatr Blood Cancer. 2013;60:728–33. doi: 10.1002/pbc.24464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid A, Glass DC, Bailey HD, Milne E, Armstrong BK, Alvaro F, Fritschi L. Parental occupational exposure to exhausts, solvents, glues and paints, and risk of childhood leukemia. Cancer Causes Control. 2011;22:1575–85. doi: 10.1007/s10552-011-9834-4. [DOI] [PubMed] [Google Scholar]
- Reigstad MM, Larsen IK, Myklebust TA, Robsahm TE, Oldereid NB, Brinton LA, Storeng R. Risk of Cancer in Children Conceived by Assisted Reproductive Technology. Pediatrics. 2016;137:e20152061. doi: 10.1542/peds.2015-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program; Bethesda, MD: 1999. NIH Pub. No. 99-4649. [Google Scholar]
- Shu XO, Ross JA, Pendergrass TW, Reaman GH, Lampkin B, Robison LL. Parental alcohol consumption, cigarette smoking, and risk of infant leukemia: a Childrens Cancer Group study. J Natl Cancer Inst. 1996;88:24–31. doi: 10.1093/jnci/88.1.24. [DOI] [PubMed] [Google Scholar]
- Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103:1457–67. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- Supriyadi E, Widjajanto PH, Purwanto I, Cloos J, Veerman AJ, Sutaryo S. Incidence of childhood leukemia in Yogyakarta, Indonesia, 1998-2009. Pediatr Blood Cancer. 2011;57:588–93. doi: 10.1002/pbc.23109. [DOI] [PubMed] [Google Scholar]
- Taub JW, Konrad MA, Ge Y, Naber JM, Scott JS, Matherly LH, Ravindranath Y. High frequency of leukemic clones in newborn screening blood samples of children with B-precursor acute lymphoblastic leukemia. Blood. 2002;99:2992–6. doi: 10.1182/blood.v99.8.2992. [DOI] [PubMed] [Google Scholar]
- Turner MC, Wigle DT, Krewski D. Residential pesticides and childhood leukemia: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:33–41. doi: 10.1289/ehp.0900966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Public Health Service. Report on Carcinogens. 13th. National Toxicology Program; 2014. [accessed 10 October 2016]. Available: http://ntp.niehs.nih.gov/pubhealth/roc/roc13/index.html. [Google Scholar]
- U.S. EPA. A Framework for Assessing Health Risk of Environmental Exposures to Children (Final) U.S. Environmental Protection Agency; Washington, DC: 2006. EPA/600/R-05/093F. [Google Scholar]
- Van Langenhove H, Roelstraete K, Schamp N, Houtmeyers J. GC-MS identification of odorous volatiles in wastewater. Water Res. 1985;19:597–603. doi: 10.1016/0043-1354(85)90065-X. [DOI] [Google Scholar]
- Vincent AJ. Measurement, Modelling and Control. IWA Publishing; London, United Kingdom: 2001. Sources of odours in wastewater treatment. [Google Scholar]
- Vinson F, Merhi M, Baldi I, Raynal H, Gamet-Payrastre L. Exposure to pesticides and risk of childhood cancer: a meta-analysis of recent epidemiological studies. Occup Environ Med. 2011;68:694–702. doi: 10.1136/oemed-2011-100082. [DOI] [PubMed] [Google Scholar]
- Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117:1505–13. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Xu X, Lui X, Wang X, Hua S, Wang C, Liu X. The associations between maternal factors during pregnancy and the risk of childhood acute lymphoblastic leukemia: A meta-analysis. Pediatr Blood Cancer. 2015;62:1162–70. doi: 10.1002/pbc.25443. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Manjourides J, Cohen T, Hu Y, Jiang Q. Spatial measurement errors in the field of spatial epidemiology. Int J Health Geogr. 2016;15:21. doi: 10.1186/s12942-016-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


