Abstract
This article reviews and summarizes 200 years of Parkinson’s disease. It comprises a relevant history of Dr. James Parkinson’s himself and what he described accurately and what he missed from today’s perspective. Parkinson’s disease today is understood as a multietiological condition with uncertain etiopathogenesis. Many advances have occurred regarding pathophysiology and symptomatic treatments, but critically important issues are still pending resolution. Among the latter, the need to modify disease progression is undoubtedly a priority. In sum, this multiple-author article, prepared to commemorate the bicentenary of the shaking palsy, provides a historical state-of-the-art account of what has been achieved, the current situation, and how to progress toward resolving Parkinson’s disease.
Keywords: Shaking Palsy, Parkinson’s disease, 200 years anniversary
Introduction (J.A. Obeso, M. Stamelou, and A.J. Stoessl)
With this article, the journal Movement Disorders commemorates the second centenary of the publication of the shaking palsy and joins several events organized by the International Parkinson’s Disease and Movement Disorders Society for this year. For the present article, a large number of esteemed colleagues dedicated to the study and advancement of movement disorders research summarize the hallmark advances that have taken place during the past 2 centuries in defining, understanding, and treating Parkinson’s disease (PD). For obvious reasons, the article reflects differences in styles and diverse viewpoints. Nevertheless, we believe this article represents a state-of-the-art account of PD and will serve to remind us of how much has been accomplished and how much more remains to be done. It is also our deepest hope that this article will inspire the next generation of movement disorders clinicians and researchers to continue on this journey until we have reached our ultimate goal of defining the cause and finding the cure for PD.
I. The Past (C. Goetz, W. Poewe, and C. Marras)
This section provides a summary of Dr. Parkinson and his principal life’s circumstances and essential medical achievements with special emphasis on his description of the “shaking palsy.”
a. Dr. James Parkinson—The Man and the Publication in the Context of His Time
James Parkinson (1775–1824) was a general medical practitioner who lived and worked in Shoreditch, a village outside of London during the 18th century and a neighborhood in London today.1 A modest plaque (Fig. 1A) marks No. 1 Hoxton Square, where he lived and practiced (Fig. 1B). His father, Dr. John Parkinson, was an apothecary and surgeon, and James served as his young apprentice on medical rounds. James later studied at London Hospital Medical College, received his diploma in 1784, and was elected as a fellow to the Medical Society of London in 1878. Specific early interests in neurological topics are undocumented, but Parkinson’s student lecture notes from attendance at Sir John Hunter’s lectures on tremor and paralysis (1785) were later transcribed and published as the Hunterian Reminiscences by Parkinson’s son, John W. K. Parkinson.2
FIG. 1.
Current picture of the house where James Parkinson lived and worked in East London and the commemorative plaque.
Parkinson was a prolific author, and the topics of his publications were highly varied. He was a political activist of his era and wrote several pamphlets on social and governmental reform efforts under the pseudonym of Old Hubert. Using his own name, he authored a number of very successful and respected books for the public on health and safety precautions, among them The Villager’s Friend and Physician 3 and the fully illustrated children’s book on safety titled Dangerous Sports.4 More focused medical texts included an early essay on the effects of lightning5 and later treatises on gout6 and typhoid fever.7 Outside of the realms of medicine and public health, James Parkinson was celebrated during his life for his geological and paleontological expertise. He was a founding member of the Geological Society and wrote several treatises on fossils, including the 3-volume Organic Remains of a Former World.8 His name is remembered in the classification of fossils, including the Nautilus parkinsoni and the Nipa parkinsoni.
Regarding the topic of key interest to this article, Parkinson published An Essay on the Shaking Palsy in 1817.9 In this 5-chapter, 66-page monograph, he considered the historical background of the condition he was describing, its signs and symptoms, individual case observations on 6 subjects, differential diagnosis, etiology, and contemporary treatment. Admitting the paucity of information, both past and present, Parkinson aimed to present his “opinions to the examination of others, even in their present state of immaturity and imperfection” (p. 3).9 As a highly astute observer, Parkinson described a disease of insidious onset and a progressive, disabling course. He described rest tremor, flexed posture, and festination. He did not specifically account for bradykinesia or rigidity, and in line with the term palsy, he considered the patients to be weak, although he acknowledged that the impairment “depends not on general weakness, but merely on the interruption of the flow of the nervous influence to the affected parts” (p. 63; see next section for further details).9
The Essay was acknowledged in the medical community, and multiple reviews praised the work,10 including a compliment admiring Parkinson’s “characteristic modesty and the acuteness of his observation” (p. 60).11 Modern historians have reported on the wide reference to the work in England during the first decades after Parkinson’s publication,12 documenting that some cases mimicked the disorder that Parkinson described, but others were more likely mistaken examples of other conditions.
Outside of England, the primary person to bring attention to James Parkinson’s contribution was Jean-Martin Charcot, the premier 19th-century clinical neurologist. In his formal lectures and informal case presentations, Charcot attracted a large international audience of physicians and trainees, and therefore his classroom became a pivotal venue for neurological communication.13 In his lecture on June 12, 1888, Charcot presented a case of parkinsonism to his colleagues and he told his audience about Parkinson’s:
remarkable article on paralysis agitans. . . It is a small pamphlet almost impossible to find. . .As short as the work is, it contains a number of superb ideas. . . Read the entire book and it will provide you with the satisfaction and knowledge that one always gleans from a direct clinical description made by an honest and careful observer. (p. 528)14
Charcot added extensive details to Parkinson’s observations and identified bradykinesia and rigidity as key features of the disease. He acknowledged that tremor was typical, but not an essential diagnostic feature, and contested using “palsy” and “paralysis” as descriptors because patients were not distinctly weak. As such, and in deference to Parkinson, he suggested that the correct nosographic designation should be Parkinson’s disease.14 Charcot’s international endorsement and wide studies of PD, parkinsonian variants, and other tremor conditions were pivotal to the global establishment of PD in the neurological nosology.
b. What Dr. Parkinson Described Accurately and What He Missed
Even after 200 years and the breathtaking acceleration of PD research during the past 50 years, James Parkinson’s original account still excels in its succinctness and careful attention to observational detail. A multitude of clinical facets of PD, as we know it today, was captured in the 1817 seminal essay with amazing clarity—including key elements of the natural history of PD, several of the salient motor features, and some of its nonmotor elements. Finally, Dr. Parkinson fully realized the devastating progression of disability in this disorder, and his report provides instructive insights into the disease course of what we must consider today as untreated PD.
i. Resting Tremor
First and perhaps most of all, James Parkinson made a remarkable description of tremors. He described separately those that are “produced by attempts at voluntary motion versus those which occur whilst the body is at rest” (p. 20),9 giving credit to Sylvius de la Boe,15 and clearly classified the tremors seen in his cases as rest tremor “occurring whilst the affected part is supported and unemployed, and being even checked by the adoption of voluntary motion” (p. 23).9 Parkinson also drew attention to the fact that rest tremor per se would not preclude the performance of fine motor acts: “Thus an artist, afflicted with the malady here treated of, whilst his hand and arm is palpitating strongly, will seize his pencil, and the motions will be suspended, allowing him to use it for a short period” (pp. 23–24).9 Moreover, he recognized the unilateral onset of rest tremor—a phenomenon we still use today as an essential element of clinical diagnostic criteria for PD16,17—and he correctly pointed out that tremor would usually begin in the hands or arms before spreading to the legs.
ii. Gait
James Parkinson noted a specific gait disorder including shuffling (“the legs are not raised to that height which the will directs”; p. 5), reduced step length (“the patient being . . .irresistibly impelled to take much quicker and shorter steps”; p. 7), and festination (“. . .. . ..adopt unwillingly a running pace”; p. 7).9 He accurately described the balance problems and danger of falling in advanced disease and specifically pointed out the relationship between a forward-flexed posture, festination, and risk of falling (“in some cases it is found necessary to substitute running for walking, since otherwise the patient, on proceeding only a very few paces, would inevitably fall”; p. 7).9 Parkinson not only pointed out the characteristic flexed posture peculiar to patients with PD but also the severe degrees of this trunk flexion that we now call camptocormia: “the propensity to lean forward becomes invincible. . . the upper part of the body is thrown so far forward as to render it difficult to avoid falling on the face” (p. 6).9
iii. Bradykinesia and Rigidity
Of the 3 cardinal motor features on which we rest a clinical diagnosis of PD today, Parkinson only described rest tremor with unequivocal clarity, whereas descriptions of rigidity or any reference to the stiffness of muscles are not found in his essay. In fact, Charcot later attributed the recognition of rigidity as a characteristic sign of PD to himself, stating that this phenomenon had been overlooked by Parkinson.14 More important, Parkinson misinterpreted the progressive loss of motor function, which he was able to observe in his cases, as a form of weakness—a state of “lessened muscular power”—and hence his choice of the term paralysis agitans. Nevertheless, it appears that he correctly observed features of bradykinesia when he stated: “one of the legs is discovered slightly to tremble, and is also found to suffer fatigue sooner than the leg on the other side”; “the hand failing to answer with exactness to the dictates of the will” (p. 4) or “The legs are not raised to that height, or with that promptitude which the will directs” (p. 5).9
It seems that the first poignant description of the peculiarity of parkinsonian bradykinesia as something quite distinct from weakness was a description by a patient, the German scholar Wilhelm von Humboldt. In a letter written to a lady friend in 1830, when he was in his early 60s, he responded to her remarks about his deteriorating handwriting by stating the following:
You are completely right as to my hand’s difficulty in writing. . .there occurs either trembling or a situation I prefer calling clumsiness rather than weakness. Writing, if it is to be firm and clear, requires a lot of sometimes very minute and hardly noticeable finger movements that need to be made in rapid sequence but with clear distinction from each other. In aging [the condition he considered the origin of his problems] suppleness is missing in this respect. The same applies also to other acts such as buttoning up during dressing, etc, while the hand maintains its strength for grabbing, carrying, holding, etc.18
iv. Nonmotor Symptoms
Today a large variety of nonmotor symptoms are considered an integral part of the disease, and there is strong evidence that some of these, such as hyposmia, constipation, or rapid eye movement (REM) sleep behavioral disorder, may even be the earliest disease manifestations, occurring years before any of the defining motor features are present.19 Clearly, Parkinson did not have the opportunity to carefully question or even examine his patients for these given that 5 of his 6 cases he could observe only casually on the street. Although he prematurely declared “the senses and intellect being uninjured” (p. 1), he explicitly commented on several other typical nonmotor facets occurring in the most advanced disease stages: “the sleep becomes much disturbed. . .the bowels. . .now, in most cases, demand stimulating medicines of considerable power” (p. 7), and toward the end “the urine and faces are passed involuntarily” (p. 9).9 Although many contemporary reviews of PD list salivation among the nonmotor symptoms of PD, Parkinson took care to point out that this phenomenon is really the result of failing motor control for deglutition: “the saliva fails of being directed to the back part of the fauces, and hence it is continually draining from the mouth” (p. 8).9
v. Natural History and Progression
Even today, neurologists continue to be struck by the fact that many PD patients seem to be curiously unaware of their symptoms early in the disease—even at a time when those close to them clearly begin to notice changes in movement and behavior. James Parkinson accurately captured this by stating “so slight and nearly imperceptible are the first inroads of this malady and so extremely slow is its progress, that it rarely happens, that the patient can form any recollection of the precise period of its commencement” (p. 3).9 Not only did he stress the slowness of progression of PD but also made precise observations on the temporal evolution of certain milestones, particularly in his description of case VI (pp. 14–18).9 Here Parkinson noted that it took about 3 years from the onset of first perceived symptoms in the left hand and arm before the right arm also became affected and that only after another 3 years tremor also appeared in the legs. According to Parkinson’s observations, at least 11 or 12 years elapsed after onset of disease before this man was severely disabled with difficulties walking unaided and marked loss of dexterity of his hands as a result of severe tremor impacting on activities of daily living such as writing or feeding (pp. 14–18). Overall, this would translate into a course of disease of 12 years or more from onset to what we now classify as Hoehn and Yahr stage IV—a time period amazingly close to that described by Hoehn and Yahr in their seminal paper exactly 150 years later.20 Dividing the course of PD into distinct stages, by the way, was an idea already expressed in Parkinson’s essay: “It seldom happens that the agitation extends beyond the arms within the first two years; which period, therefore, if we were disposed to divide the disease into stages, might be said to comprise the first stage” (p. 57).9
vi. Underlying Pathology
James Parkinson felt obliged to remind his audience that he had no solid information or evidence on which to base any conclusions about the “proximate or remote causes” of this disease and designated his ideas on this as “conjecture founded on analogy” (p. 33).9 From today’s perspective, it is nevertheless intriguing to read his introductory sentence on the “supposed proximate cause” of PD: “A diseased state of the medulla spinalis . . . and extending, as the disease proceeds, to the medulla oblongata” (pp. 33–34)—wording that seems to contain the recent concept of spread of pathology along interconnected neural pathways.9 Also, his conjecture of early pathology involving the medulla oblongata today does not sound at all unfamiliar. Parkinson was certainly right in stressing the need for pathological study to gain further insight into the causes for this illness and in expressing the hope for his essay to contribute to “the leading of attention of those who humanely employ anatomical examination in detecting the nature and causes of diseases particularly to this malady” (pp. 65–66).9
vii. The Etiology
Parkinson’s idea about etiopathogenesis of the disease was centered in the medulla and noted in his Essay on the Shaking Palsy that “The great degree of mobility in that portion of the spine which is formed by the superior cervical vertebrae, must render it, and the contained parts, liable to injury from sudden distortions.” 9 However, he further noted that “In no case which has been noticed, has the patient recollected receiving any injury of this kind.” Instead he hypothesized that “taking all circumstances into due consideration, particularly the very gradual manner in which the disease commences, and proceeds in its attacks; as well as the inability to ascribe its origin to any more obvious cause, we are led to seek for it in some slow morbid change in the structure of the medulla, or its investing membranes, or theca, occasioned by simple inflammation, or rheumatic or scrophulous affection.”9 Thus James Parkinson suggested in his Essay that an inflammatory condition, possibly instigated by a chronic infection, might play a key role in the disease. It is interesting that 200 years later the possible role of infectious agents is still being debated.
Through the 1800s, stress and other environmental precipitants were considered as causes of PD by opinion leaders such as Charcot and Gowers.14,21 The great pandemic of encephalitis lethargic in the early 1900s and subsequent cases of postencephalitic parkinsonism fueled a view that parkinsonism was largely a sequel of infectious disease or other similar viral illnesses.22
viii. Treatment
Unsurprisingly, the recommendations Parkinson made with respect to treatment of this disease in 1817 appear obscure to us today. There are, however, 2 statements in chapter 5 (“Considerations Respecting the Means of Cure”) that can be nothing but endorsed 2 centuries later. One reads like an early plea for target validation before proceeding with drug development: “Until we are better informed respecting the nature of this disease, the employment of internal medicines is scarcely warrantable” (p. 62). The other, with hindsight, was clearly overoptimistic but seems to have come closer to reality 200 years later: “there appears to be sufficient reason for hoping that some remedial process may ere long be discovered, by which, at least, the progress of the disease may be stopped” (pp. 56–57).9 In anticipation of modern concepts of disease-modifying interventions, Parkinson also felt that “the earlier the remedies are resorted to, the greater will be the probability of success” (p. 60).9 So early diagnosis and treatment with the goal of preventing disease progression was the vision J. Parkinson had for the treatment of the disease named after him and it still is the holy grail in current therapeutic research.
II. The Present: Facts and Features Dr. Parkinson Couldn’t Envisage
This section summarizes several aspects of PD that are now evident because of greater clinical insight, longer follow-up, pathological studies of the central and peripheral nervous system, and technological advances. Yet, and remarkably, the essential clinical features of the paralysis agitans not only remain as initially described but also prevailed as essential components of assessment, diagnosis, and interpretations.
a. Clinical Heterogeneity and Differential Diagnosis of PD (A.E. Lang and M. Stamelou)
PD is an extremely heterogeneous disorder.23 Age of onset ranges from as early as the third decade of life to extreme old age. The disorder is still defined by the presence of classical motor features, including the hallmark presence of bradykinesia in all patients, rest tremor in the majority, and rigidity. Postural reflex disturbances include flexed postures of the trunk and limbs as well as postural instability, generally occur later in the evolution and are no longer considered essential diagnostic features. These motor signs are often preceded by nonmotor manifestations such as olfactory dysfunction in approximately 90%, constipation, REM behavior disorder, and depression/anxiety. 24 As the disease progresses, the clinical picture becomes a composite of levodopa-related motor complications, nondopaminergic motor features such as speech and swallowing problems, freezing of gait and falls, and increasingly disabling nonmotor features such as autonomic failure, psychiatric disturbances, and dementia. The spectrum of clinical features and disease course manifested by individual patients varies greatly; some have an apparently benign disorder with a sustained response to levodopa and minimal nondopaminergic symptoms, whereas others demonstrate a more malignant course with an early predominance of nondopaminergic motor and nonmotor features.23 The reasons for these clinical differences are poorly understood. Age and age of onset are the best recognized influencing factors. Thus, the younger the onset, the longer that levodopa-responsive features predominate, albeit complicated by motor fluctuations. Independent of age of onset, older patients experience more levodopa-resistant motor signs, autonomic impairment, and cognitive decline.25
Distinct clinical presentations, varying combinations of symptoms and signs, rates of progression, and time to development of more treatment-resistant symptoms suggests the presence of biologically distinct subtypes (ie, “PDs”). Various methods have been used to define different PD subtypes, including selected motor signs, nonmotor features (eg, cognitive dysfunction), ages of onset, and rates of progression. Subtyping has been based on presenting clinical features, rates of evolution of the disease, and/or the occurrence of specific features at a point in the disease course (e.g., the development of dementia). Two main approaches to deriving subtype classifications have been an empirical approach based on clinical observations and data-driven analytic classifications where there are no a priori hypotheses as to how variables should be grouped together to establish specific subtypes in advance of the analysis.
The most common empirical clinical approach to subtypes has been to divide patients on the basis of dominant motor features. This approach has distinguished patients presenting with a tremor-dominant form from a postural instability gait disorder or akinetic/rigid dominant form, with some patients falling into an indeterminate category. It has often been claimed that tremor-dominant patients have a more benign or slowly progressive course of the disease. However, review of the relevant literature has been variably interpreted. Rather than representing distinct biological subtypes, the clinical heterogeneity demonstrated by these subtypes may simply represent different stages of the disease.26
An important advancement in our understanding of PD that has occurred since the early attempts to subdivide patients on the basis of presenting motor symptoms has been the recognition of the prevalence and broad spectrum of early and later nonmotor features. Early studies evaluating nonmotor features in PD subtypes assessed their occurrence in the tremor-dominant versus postural instability gait disorder or akinetic/rigid dominant groups with evidence linking early autonomic dysfunction and later cognitive decline to the latter category.27 Once again, these associations may be largely an artifact of stage of disease rather than a result of distinct pathogenic subtypes.
A large number of different subtypes have been proposed on the basis of data-driven studies. Until relatively recently, these studies incorporated predominantly motor clinical information (including speed of progression), as well as age of onset, motor complications of levodopa, and a limited number of nonmotor features such as cognitive impairment, depression, and anxiety. These approaches have defined highly variable subtypes, and there has been little attempt to apply the results to subsequent studies of etiology, disease progression, or treatment responses. Recently, as our knowledge of the spectrum of nonmotor and nondopaminergic features has evolved, recent data-driven cluster analyses have included more comprehensive evaluations of the role of these along with the more traditional motor features. Early urinary dysfunction characterized a “nonmotor dominant” subgroup and suggested a more malignant course in one study.28 More rapid progression of all clinical features was predicted by the presence of REM behavioral disorder, mild cognitive impairment, and orthostatic hypotension in another.29
To date, PD subtypes have been characterized on the basis of readily apparent and evaluable clinical features. The use of biomarkers to characterize or enhance patient subtyping is in its infancy and application of multiple approaches promises to revolutionize this field (see later). The first and most widely applied of these has been genomics. The discovery of a mutation in the alpha-synuclein gene introduced the possibility of subtyping by etiology (ie, monogenetic vs sporadic). As discussed later, there are considerable phenotypic and prognostic differences in various forms of monogenetic PD. Recent studies have begun to demonstrate an important influence of genetic factors on the clinical aspects of the more common sporadic disease. For example, a higher genetic risk score, calculated from the status of 28 loci shown to increase PD risk in genomewide association studies, was found to predict an earlier age of onset,30 whereas variability in the alpha-synuclein gene (single nucleotide polymorphisms and a specific haplotype) have been found to be associated with dementia.31 As more reliable biomarkers are established, it is expected that we will have a much better understanding of the clinical heterogeneity of the disorder. An important active research goal is to define methods of distinguishing subtypes at the earliest stages of the disease with future expectation that advances in precision medicine will allow the application of patient subtype-specific disease modification strategies.32
The differential diagnosis of PD is relevant for prognosis, treatment, and research, and despite major advances in the field, it still remains largely clinical. In fact, the accuracy of clinical diagnosis of PD has remained the same the past 25 years, as shown by a recent meta-analysis of 28 studies33 (13 with pathology confirmation). The UK Brain Bank diagnostic clinical criteria16 were more sensitive (90.8% vs 81.3%), but less specific (34%) compared to the expert clinical diagnosis (83.5%),33 and the most common misdiagnoses included other tremor disorders, atypical parkinsonian conditions, secondary parkinsonisms, and other dementias.33 Recently, new clinical criteria for PD diagnosis have been published on behalf of the International PD and Movement Disorders Society that do not include dementia any longer as an exclusion criterion.17 Dementia with Lewy bodies has been invariably described in the literature as an atypical parkinsonian condition, as a PD phenotype, or as one end of the spectrum of Lewy-body diseases. Indeed, dementia with Lewy bodies, PD dementia (PDD), and PD share common pathological and clinical features and rather represent a spectrum reflecting the distribution of Lewy-body pathology, which is now acknowledged and taken into account in the recently proposed PD clinical criteria.17 The new criteria accept the diagnosis of PD independent of when dementia arises (before or within the first year as well as after that) as long as the clinical criteria for PD are fulfilled. However, this proposal has triggered considerable debate in the field and is presently open to further evaluation and discussion.34,35
Patients diagnosed as having PD but who have a normal DaTSCAN are often referred to as having SWEDDs (Scans Without Evidence of Dopaminergic Deficit). Patients with asymmetric rest tremor and a normal DaTSCAN represent a relatively common situation that can be misdiagnosed as PD. It has been shown that SWEDDs represent quite a heterogeneous group of disorders; some of these patients have dystonic tremor,36 whereas others develop an abnormal DaTSCAN at a longer follow-up, raising the possibility of either benign tremulous PD or false-negative initial DaTSCANs.36,37
Another relevant aspect is the differential diagnosis between essential tremor and PD and PD with atypical parkinsonism (eg, multiple system atrophy, progressive supranuclear palsy [PSP], and corticobasal degeneration) that can be quite challenging in particular early in the disease course. When these disorders present with their classic phenotypes,38–41 clinical signs or evolution that are inconsistent with or atypical for PD facilitates the differential diagnosis.
A number of red flags have been described and incorporated in the recently published PD criteria17 that may help identifying atypical signs earlier. For example, postural instability is no longer a clinical criterion for the definition of parkinsonism; in contrast, it is suggested as a red flag for an atypical condition when present the first 3 years. However, the major problem in the differential diagnosis of atypical parkinsonism with PD remains the well-recognized fact that a large number of patients with atypical parkinsonism will not present with these hallmark features early or ever during the course of the disease.17 This phenotypic variability, the increasing probability of copathology with advanced age, and the lack of reliable biomarkers for these disorders make the early differential diagnosis sometimes impossible. Imaging may be helpful, in particular if specific MRI changes precede satisfaction of clinical criteria,42 but accuracy of diagnosis based on MRI findings and PET/single-photon emission computerized tomography (SPECT) imaging is not higher than clinical expertise in clinicopathological studies.33
Drug-induced parkinsonism can be generally diagnosed when a history of intake of dopamine-depleting drugs and a normal DaTSCAN are present.43 Vascular parkinsonism or diffuse cerebral small vessel disease44 as well as normal pressure hydrocephalus have usually typical clinical signs such as lower body parkinsonism, freezing, urinary and cognitive dysfunction, and characteristic imaging findings. However, the recent association of normal pressure hydrocephalus phenotype to PSP pathology45 and the identification of late-onset genetic leucoencephalopathies presenting with parkinsonism may complicate the correct diagnosis.46 Last, there is a constantly increasing list of disorders that may present with parkinsonism and may be misdiagnosed as PD at early stages, such as spinocerebellar ataxias, Fragile X tremor–ataxia syndrome, and others. These rarely constitute a differential diagnostic problem later in the disease course; however, at the initial stages, the syndrome’s definition and a detailed family history, when appropriate, are important for their early identification.
b. Psychiatric and Cognitive Manifestations (D. Weintraub and D.J. Burn)
Regarding mental symptoms and cognition, it is often cited that Parkinson did describe severe depression in 1 case history (“A more melancholy object I never beheld. The patient, naturally a handsome, middle-sized, sanguine man, of a cheerful disposition, sanguin and an active mind, appeared much emaciated, stooping, and dejected”). However, this was not 1 of the 6 illustrative cases but, instead, “an interesting case of Palsy occasioned by a fall, attended with uncommon symptoms.” This patient appeared to have developed neurological symptoms after experiencing a traumatic brain injury, and thus may not have met current clinical criteria for PD. Indeed, there is scant mention of cognitive or thinking abilities in the essay. In the definition of “shaking palsy” Parkinson stated, “the senses and intellects being uninjured.” Later, when describing an illustrative case, he stated, “the powers of his mind, unimpaired.” This implied that he did not observe cognitive impairment in his patients, but the words “intellects” and “powers of his mind” were not defined.
There are many possible reasons why Parkinson would not have observed or written more about the mental impairments that we now know are common in PD. First, modern descriptions of and diagnostic criteria for mental illness were not even introduced until the early 19th century. Second, the duration of disease and age at death were not provided for the patients he followed, and it is possible that they may not have lived long enough, or long enough with PD, to have widespread cortical Lewy bodies or comorbid neurodegenerative disease pathology, which are associated with cognitive impairment. Third, the untreated parkinsonism he observed throughout the disease course may have been severe enough to mask the presentation of psychiatric symptoms. Finally, some psychiatric disorders are associated with the introduction of dopamine replacement therapy or other PD therapies that were not available 200 years ago.
Current State of the Field
The high prevalence of cognitive impairment and protean psychiatric complications has changed how we conceptualize PD.47,48 This has manifested itself in the recently proposed revised clinical diagnostic criteria, 17,49 which allows for dementia to be a comorbid condition at the time of diagnosis. In addition, the recognition that some nonmotor symptoms can predate the onset of motor symptoms has led to proposed criteria and risk stratification for prodromal PD.50 The most significant nonmotor symptom in PD is progressive cognitive impairment. Once thought to primarily affect executive abilities in a minority of patients, it is now known that a range of cognitive domains can be affected49 and that dementia (PDD) may affect 80% of patients long term.51 Approximately 25% to 30% of nondemented patients have mild cognitive impairment (PD-MCI),52 and cognitive deficits have been reported in newly diagnosed and even prodromal PD. Diffuse cortical Lewy-body disease pathology is the major contributing pathology to PDD, but about one third of PDD patients also meet criteria for comorbid Alzheimer’s disease. A range of neurotransmitter deficits (acetylcholine, dopamine, and norepinephrine) and genetic mutations (APOE E4, BDNF Val53 Met, COMT Val54 Met, MAPT, and glucocebrosidase (GBA) polymorphisms) have been implicated. Unfortunately, this recognition and knowledge has not translated into significant treatment advances, with only 1 large positive therapeutic study for PDD.53
Prevalence rates for all depression subtypes in PD combined range from 15% to 50%, with such disparity reflecting in part somatic symptom overlap between depression and PD. Depression in PD likely results from a complex interaction of psychological and neurobiological factors, the latter related to impairments in the striatal-thalamic-prefrontal cortex and basotemporal limbic circuitry and in a range of brain stem monoamines (ie, dopamine, serotonin, and norepinephrine). Antidepressant use is common in PD, with positive efficacy data recently for tricyclic antidepressant, 54 selective serotonin reuptake inhibitor,55 mixed serotonin-norepinephrine reuptake inhibitor, and dopamine agonist medications. In addition, cognitive behavior therapy has been shown to be efficacious, 5 but its role in the management of cognitive impairment in PD is not yet cleart.56
Among the disorders of affect, both anxiety and apathy in PD have received less attention than depression despite their frequent occurrence (30%–40% for each disorder). Anxiety can present as generalized anxiety disorder, panic attacks (often in the context of non-motor manifestations), and social phobia.
Psychosis was reported uncommonly prior to the introduction of levodopa, but now the cumulative prevalence of PD psychosis is 60% if one includes minor hallucinations.57 A recent study reported that the latter are common even in newly diagnosed, untreated patients.58 Hallucinations were once thought to be almost exclusively visual, but auditory, tactile, and olfactory hallucinations are also relatively common. Proposed biological mechanisms include the hypersensitivity of mesocorticolimbic D2/D3 receptors as a result of chronic dopaminergic therapy, cholinergic deficits, and a serotonergic/dopaminergic imbalance. The management of comorbid medical conditions and decreasing dosages of nonessential medications may offer temporary relief. Among antipsychotics, quetiapine is commonly used, although proper evidence from clinical trials is lacking, whereas clozapine is being shown efficacious but rarely used, particularly because of the limitations associated with the possibility of provoking leukopenia. A new antipsychotic, pimavanserin (a selective 5HT2A inverse agonist) was recently approved in the United States specifically for PD psychosis.59 All antipsychotics, including pimavanserin, carry a black box warning for increased mortality, a finding first reported in general dementia patients and more recently in PD.
The recent recognition that impulse control disorders (ICDs; eg, compulsive gambling, buying, sexual behavior, and eating) are relatively common in PD coincided with the introduction of D2/3-selective dopamine agonists (DA). Untreated PD patients are not at increased risk for ICD behaviors, but the cross-sectional prevalence is 17% or more in DA-treated patients,60 and both higher dose levodopa and amantadine are also associated with ICDs. Dopamine dysregulation syndrome (ie, compulsive PD medication use) and other impulsive-compulsive disorders (eg, punding) may occur, but are not as well studied. ICD and dopamine dysregulation syndrome patients have sensitized D2/D3 receptors and decreased dopamine transporter availability, and genetic risk factors for incident ICD behaviors were recently identified. ICD behaviors typically resolve after discontinuing DA treatment; however, some patients develop a DA withdrawal syndrome. 61 The relationship between DBS and ICDs is complex, with both improvement and worsening reported post-DBS surgery. Indeed, DBS is increasingly used as a treatment to address the problem of reducing dopaminergic drugs without inducing motor deterioration in patients with ICD.62 Cognitive impairment (particularly impaired verbal fluency) post-DBS surgery has consistently been reported, with some evidence that these effects are preventable or modifiable. Psychiatric findings from controlled studies show an overall improvement in depression and anxiety symptoms, with no clear evidence that DBS itself leads to suicide behaviors.63 Another psychiatric disorder associated with PD treatment is nonmotor fluctuations that can occur with chronic levodopa treatment, with bothersome anxiety, slowness of thinking, fatigue, and dysphoria reported primarily during “off” periods.
In summary, the cumulative prevalence of psychiatric and cognitive complications is far higher than previously thought. These complications are associated with excess disability, worse quality of life, poorer outcomes, and increased caregiver burden. Their etiology and neurobiology is complex, involving a mix of PD and other neurodegenerative disease pathology, PD treatments, and genetic influences. There have been significant advances in the assessment of these disorders (eg, screening instruments, rating scales, and diagnostic criteria). However, despite these advances, current treatment options for nonmotor symptoms in PD remain limited, leaving large areas of unmet therapeutic need.
c. Pathological Basis (G.M. Halliday)
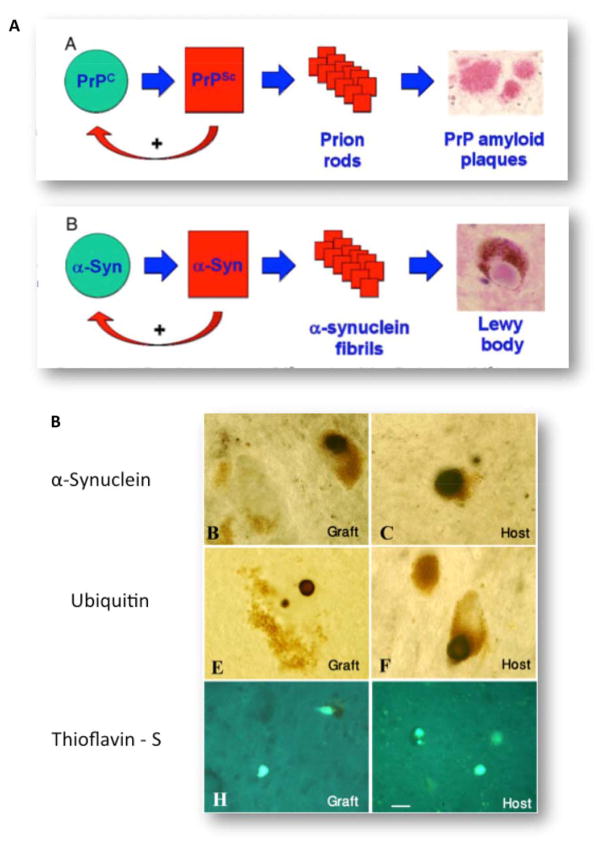
Dr. Parkinson did not know what was the underlying pathology of the shaking palsy. During the next century, many pathological theories were espoused,61 with Bloq and Marinesco first suggesting that the substantia nigra (SN) was involved in 1893, a theory supported by others. In 1912, Friedrich Heinrich Lewy identified the cellular inclusion bodies in patients with paralysis agitans, but it was Constantin Trétiakoff who put these 2 separate pathologies together in 1919, suggesting that both were found in most patients with PD.63
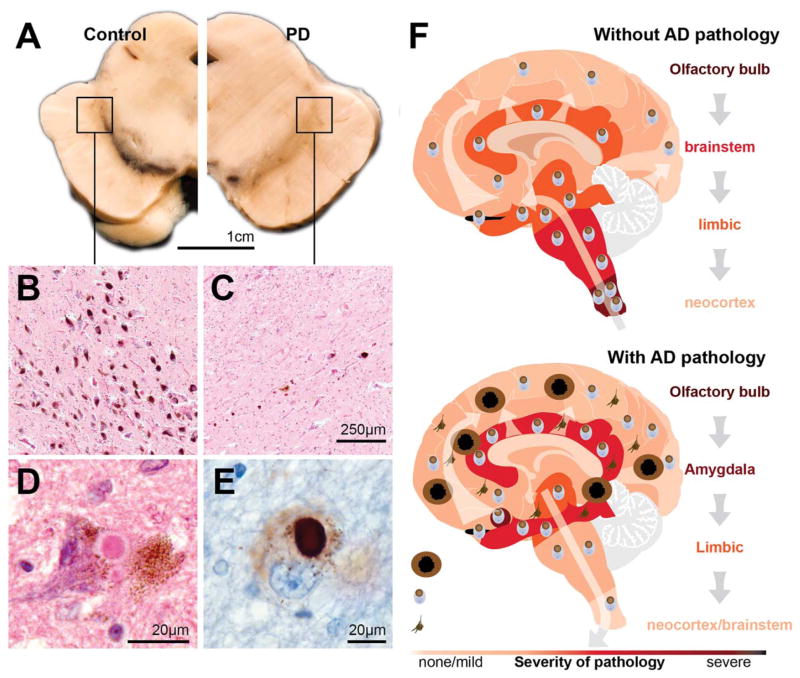
The concept that degeneration of the SN was central to the syndrome was cemented by 2 additional discoveries, the first by Arvid Carlsson on the role of dopamine in the brain and the second by Oleh Hornykiewicz, who demonstrated that the largest group of dopaminergic neurons are found in the SN with their terminals in the caudate nucleus.64 The degeneration of the dopaminergic pigmented neurons in the SN is the most consistent neuropathological feature found in all patients with clinical PD, but also occurs in many other clinical parkinsonian neurodegenerative conditions— a sensitive and necessary neuropathology, but not specific for PD. However, the pattern of dopamine cell loss in the SN is distinctive for PD, with the most severe loss found in the ventrolateral region of the SN, whereas dopaminergic neurons in the nearby ventral tegmental area are nearly entirely spared.65 Moderate to severe loss of the pigmented dopamine neurons in the SN is found in all patients with clinical PD and forms 1 of 2 pathological lesions required for a definitive diagnosis66 (Fig. 2A–C). The cell loss is marked in all those with clinical disease, suggesting that most of the degeneration occurs very early, a concept that has been validated in careful studies of patients with short disease durations53 and those considered to harbor preclinical disease.67
FIG. 2.
The main pathologies in patients with clinical Parkinson’s disease and the pathological progression. (A) Transverse hemisection of the midbrain of a control at left and a patient with clinical Parkinson’s disease (PD) at right showing the marked reduction in the black pigment within the substantia nigra region. (B–C) Haematoxylin and eosin stained section of the ventrolateral region identified by the box in A showing at higher magnification the pigmented neurons of the substantia nigra in a control without PD (B) and a person with clinical PD (C). (D–E) Intracytoplasmic Lewy bodies in remaining pigmented neuron of the substantia nigra of a patient with PD showing the eosinophilic core and paler halo in haematoxylin and eosin stain (D) and the dark aggregation of α-synuclein using immunoperoxidase with cresyl violet counterstaining (E). (F) Cartoon representation (based on data from Toledo et al73) of the two major patterns of Lewy-body pathology in patients with (below) and without (above) Alzheimer’s disease (AD) pathology. In those with clinical PD and little AD pathology, Lewy bodies begin in the olfactory bulb and medulla oblongata then infiltrate higher brain stem regions, then limbic brain regions, and finally the neocortex. In those with AD pathology, this pattern is different. Lewy bodies concentrate in limbic regions of the brain prior to infiltrating to other regions.
The rapid advance in research techniques during the past 20 years has determined that there is not a single cause but several causes all leading to the common preferential early loss of dopaminergic neurons in the SN in patients with clinical PD.68 The 2 most obvious culprits are the many different genes involved (autosomal dominant, autosomal recessive, and risk genes) and some environmental factors (hydrocarbon exposure, less coffee intake and cigarette smoking, constipation, reduced physical activity69). These varied etiologies impact on different cellular pathways that merge to cause dysfunction and then the death of these specific dopaminergic neurons—these include protein misfolding and aggregation, disruption of autophagic catabolism, endoplasmic reticulum stress, mitochondrial dysfunction, and/or the loss of calcium homeostasis—and for an individual the balance between these pathways may vary greatly. This suggests that future treatment strategies will need to be more personalized, with an integrated knowledge of these factors in individuals for effectively preventing or slowing this aspect of the disease.
The second diagnostic marker of idiopathic PD (but not many genetic forms70,71) is the presence of misfolded protein in the form of Lewy bodies in at least the brain stem66 (Fig. 2D,E). The formation and composition of Lewy bodies have been well studied. The major component protein of Lewy bodies is alpha-synuclein, but more than 90 other molecules are now recognized in these abnormal cellular structures. Importantly, the precursor structures of Lewy bodies have also been described with many studies showing that different manipulations that change the solubility and binding affinities of the alpha-synuclein protein cause its intracellular precipitation. Similar to the neuronal loss in patients with PD, Lewy pathologies (LPs) are now known to occur in many elderly people, with such inclusions also a sensitive and necessary neuropathology, but not specific for PD.66 In fact, the greatest numbers of people with LPs in their brains are patients with the pathology of Alzheimer’s disease, where up to 60% have these inclusions.72
Distinctive patterns of LPs are now known to occur in different types of patients, with the most recent study showing that coexisting Alzheimer pathology has a marked influence on the distribution and progression of LP in the elderly (Fig. 2F). In those without Alzheimer pathology (usually <70 at onset), LPs concentrate in the olfactory bulb and brain stem, moving to limbic and neocortices overtime, as originally described by Kosaka and colleagues73 and subsequently by Braak and colleagues.74 The time course of pathological progression is usually very slow, with 50% of patients having limbic LPs on average 13 years following onset, and all having such pathology by 18 years.75 In patients with Alzheimer pathology (often older at onset), LPs dominate the limbic system and only later may become more widespread. These varied patterns of vulnerability to Lewy-body formation depending on other disease processes occurring in the brain at the same time also suggest that future treatment strategies will need to be more personalized for these aspects.
In sum, the following 2 cellular pathologies are consistently found in patients with idiopathic PD: loss of dopaminergic neurons in the ventrolateral region of the SN and LP in the brain stem.66 The marked dopaminergic cell loss at the time of diagnosis is the mainstay, whereas LP is highly variable in location and quantity (dependent on a number of less well-defined factors). The relationship between these 2 cellular pathologies and the role of LPs in the neurodegeneration observed in PD awaits further studies.
d. Experimental Models (E. Bezard and S. Przedborski)
In retrospect, one can only be amazed by the pace of development and validation of experimental models of PD occurring in the past few decades. Experimental models are now available in organisms such as yeast, worms, flies, rodents, and even nonhuman primates. This impressive list does not come without any drawback, however, because all of these models are merely approximations and not phenocopies of PD, hence raising the following legitimate question: which among all of these models of PD is the best? Although this challenging question is of critical importance, it may deserve a whole discussion in its own right, and here we reflect on a few models, which during the PD 200-year journey, have profoundly impacted the field of research.
A first such model emerged from the landmark study of Carlsson and colleagues76 who in 1957 showed that the administration of the monoamine depleter reserpine to mice and rabbits rendered these animals “markedly tranquilized,” which meant that they showed reduced motor activity. Furthermore, these authors found that on administration of the dopamine precursor L-3,4-dihydroxyphenylalanine (L-dopa), these animals regained near normal motor behavior. This striking set of observations provided the first evidence of a crucial role for dopamine in motor control and paved the way to a slew of clinical trials that ultimately led to the use of L-dopa substitution therapy in PD, one of the most effective symptomatic treatments for this disease.
The second and almost as important breakthrough in PD modeling took place in 1968, when Ungerstedt reported that lesioning the nigrostriatal pathway via a stereotaxic injection of the 6-hydroxydopamine (6-OHDA) in the rat SN was an effective means of removing dopamine unilaterally.77 Since then, the unilateral injection of 6-OHDA has been tested in discrete sites along the nigrostriatal pathway other than the SN and remains the model by excellence of right/left unbalance in dopaminergic input to the basal ganglia in rodents, resulting in a quantifiable circling behavior. Over the years, this circling behavior in rodents has become one of the gold-standard motor activities assessed to predict the antiparkinsonian properties of experimental drugs and the success of transplantation and gene therapies in repairing the lesioned pathways.
The third impactful development took place in 1982, when Langston and colleagues78 discovered that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was the cause of a profound and irreversible neurological condition almost indistinguishable from PD. MPTP was then used in a host of animal species and showed that this neurotoxin was able to reproduce most of the clinical and neuropathological hallmarks of PD in monkeys and in mice, at least regarding the degeneration of the nigrostriatal pathway. Not only have the MPTP models advanced our understanding of the pathophysiology of PD thanks to a host of molecular and cellular biology experiments but also they have allowed the development of the latest symptomatic breakthrough in the management of PD that is the surgical ablation and deep brain stimulation of the subthalamic nucleus and globus pallidus pars interna (see below section on surgery).
With the discovery of the first gene mutation that causes PD,79 a new area in modeling began that instead of using toxins to produce a PD-like phenotype relied on engineering the animal genome to express known PD mutations. Thus, in parallel to the race for gene mutations, new animal models of PD, in both invertebrates80 and mammalians,81 emerged at a rapid pace. Remarkably, most genetic models of PD in rodents show either no or quite subtle phenotypes, such as functional abnormalities of the nigrostriatal pathways. Ironically, 1 engineered mouse line that exhibits an overt PD-like degeneration of the nigrostriatal pathway is the MitoPark mouse82 that harbors a dopaminergic neuron-specific gene deletion for the mitochondrial transcription factor-A, a gene linked to migraines but not PD.
Despite the lack of conclusive PD phenotypes in most of these genetic models, many of these have unquestionably opened research avenues that can be considered as real paradigm shifts. Two such instances are worth mentioning here briefly. First, the alpha-synuclein-based animal models, which consistently have provided hints that misfolded alpha-synuclein assemblies, on the form of oligomers or fibrils, are the likely toxic species. From this initial view on alpha-synuclein biology, the field has then progressively moved toward the following popular pathogenic hypothesis: once misfolded, alpha-synuclein becomes a pathological seed that promotes the misfolding of other alpha-synuclein molecules, whereby propagating and enhancing the degenerative process of PD. Illustrating this idea is the work of Luk and colleagues83 in which an injection of recombinant alpha-synuclein preformed fibrils in the striatum of wild-type mice is shown to induce the formation of endogenous alpha-synuclein aggregates as well as signs of nigrostriatal dopaminergic pathway degeneration. Even more striking is the demonstration by Recasens and colleagues84 that intranigral or intrastriatal inoculation of extracts from Lewy bodies—alpha-synuclein-rich proteinaceous inclusions typical of PD—in both mice and monkeys also resulted in a progressive nigrostriatal neurodegeneration. Second is the case of PINK1 and Parkin mutations that in humans cause recessive forms of familial PD and in flies major defects of mitochondria.85 These observations reinvigorated the discussion of mitochondria defect in PD pathogenesis, but this time no longer from the angle of a deficit in bioenergics that have been at the forefront since the early 1990s, but now from the new angle of impaired mitophagy,8,85 a key component of the cellular mechanisms of mitochondrial quality control.
It is no doubt that some experimental models have played seminal roles in driving PD research. It should be recognized that none of the currently available models of PD are perfect. However, it is fair to conclude that when these models, including most classic models, are used carefully, their contributions to our understanding of the neurobiology of PD86 and their role in promoting new therapies87 are phenomenal and clearly outweigh the shortcomings.
e. Neuroimaging—Visualizing Brain Changes (S. Lehericy, A.J. Stoessl, and D. Brooks)
This is undoubtedly one of the most highly developing areas for the diagnosis and assessment of PD and one that James Parkinson could not possibly foresee at his time. This section addresses the role of functional neuroimaging techniques in PD.
i. MRI
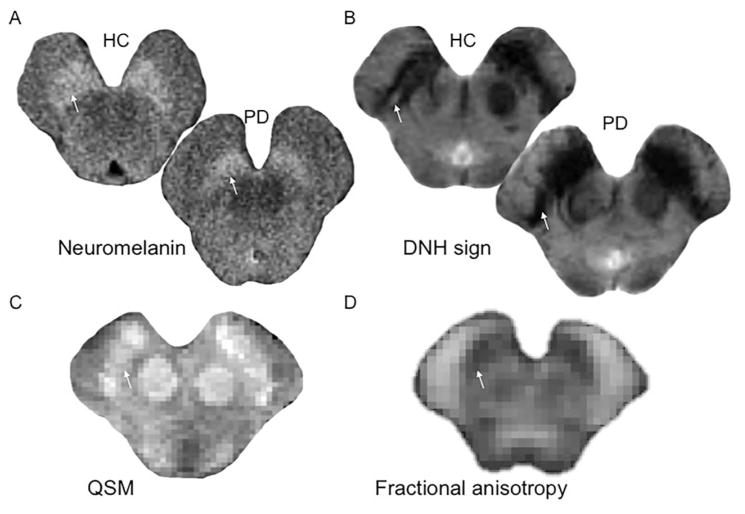
For decades, clinical MRI in PD was considered normal. Over the years, progress in magnetic resonance techniques has allowed the detection of structural, functional, and connectivity changes in the SN as well as other regions affected in PD. In the SN, increased iron content was among the first changes that were evidenced. Initially detected visually on T2*-weighted images in the mid-1980s, increased iron load was then quantified using T2* mapping, followed more recently by quantitative phase and susceptibility mapping. All techniques provide measures that are proportional to iron content in the tissue. Increased iron content may predominate in the lateral segments of the SN and in some studies correlated with the UPDRS motor score.88 A number of studies have reported altered diffusion properties in the SN, characterized by reduced fractional anisotropy, but the large variability of the results question the reliability of this measure as a biomarker. 89 The improved modeling of water diffusion has been proposed to overcome these limitations. For instance, new metrics allowing the characterization of neurite orientation dispersion and density or free water may be more sensitive to PD pathology in the nigra.90 Reduced connectivity of the SN with the basal ganglia and thalamus were also evidenced in PD patients using tractography-based methods and resting state functional connectivity.91
In contrast to quantitative mapping, measurements of the morphological changes of the SN seemed unreliable to detect changes related to PD, but 2 recent techniques appear promising particularly for clinical use. Using high-resolution T2*-weighted MRI at 7T or more recently susceptibility-weighted imaging at 3T MRI, a pocket of relatively high-signal intensity in the normal lateral SN was lost in PD.92 This area corresponded to the histologically defined nigrosome-1 that is affected early and more severely in PD and is loaded with iron in PD, which explains the loss of T2* hyperintensity. This sign (called the dorsal nigral hyperintensity or DNH sign) has a diagnostic accuracy of more than 90%.92 The second technique relies on the paramagnetic properties of neuromelanin, a pigment that is contained in the SN pars compacta (SNc). High-resolution spin echo T1-weighted images are sensitive to neuromelanin and show the SNpc as an area of high signal intensity.93 Reduced size and signal intensity of the SN were reported in PD patients using neuromelanin-sensitive imaging with a high diagnostic accuracy. Both techniques may be used in clinical practice as these changes can be detected by simple radiological reading. A combination of measures, for example, increased iron content and reduced fractional anisotropy, changes in nigrosome-1 containing area or neuromelanin imaging, may result in better separation of PD patients from control subjects as compared with each technique separately, as shown for iron load and fractional anisotropy with 95% global accuracy,94 but this remains to be determined. Last, functional connectivity methods using functional MRI at rest in PD showed that dopamine depletion leads to a remapping of cerebral connectivity characterized by decreased coupling in the cortico-striatal sensorimotor network and between the striatum and the brain stem95 and increased coupling, probably compensatory, in the associative network. Changes varied in relation to predominant motor manifestations and were modulated by levodopa.96 Recent results using functional MRI at rest has also shown that average connectivity in the basal ganglia may distinguish patients with PD from healthy controls.97
MRI has also helped determine the brain correlates of motor and nonmotor features of PD using various techniques such as voxel-based morphometry, cortical thickness measurements, microstructural changes using diffusion imaging, and functional MRI at rest or during task performance (Fig. 3). For motor features, functional and structural98 connectivity studies have suggested that freezing of gait was related to connectivity deficit between the pedunculopontine area, the basal ganglia, and the frontal cortex. Akineto-rigid and tremor-dominant forms of PD were associated with structural and functional changes predominating in the basal ganglia—cortical and cerebello-thalamocortical networks, respectively,99 with tremor-related activity first arising in the internal part of the globus pallidus and propagating to the cerebello-thalamocortical circuit.100 Dyskinesias following the administration of soluble levodopa were associated with abnormal modulation of striato-cortical networks in PD patients. Reduced neuromelanin signal in the locus coeruleus/subcoeruleus area was observed in PD patients with rapid eye movement sleep behavior disorders (RBD)101 as well as in patients with idiopathic RBD. Cognitive decline in PD was associated with greater atrophy in many brain regions, including the frontal, parietal, medial, and lateral temporal areas and substantia innominata, which is more extensive in PD with dementia when compared with PD-MCI and accelerates with disease progression.102,103 Changes in functional connectivity in anterior brain regions seemed to be related to executive dysfunction, whereas changes in more posterior regions may be related to the evolution to dementia.104 Cognitive heterogeneity in PD may be mediated through common genetic variation of several genes including catechol-o-methlytransferase, supporting a frontally based dysexecutive syndrome reflecting dysfunction in dopaminergic networks, and microtubule-associated protein tau and Apoliprotein E (APOE), reflecting a more posterior cortically based cognitive syndrome dependent on age and tau genotype. Atrophy was also reported in limbic regions in association with depression105 and in brain regions responsible for processing visuoperceptual information in association with visual hallucinations.106
FIG. 3.
(A) Spin echo T1-weighted 3T images sensitive to neuromelanin showing a reduction of the area of hyperintensity of the substantia nigra (arrow) in the PD patient as compared with the healthy control (HC). (B) T2*-weighted 7T images showing the normal dorsal nigral hyperintensity (DNH) in the substantia nigra of the HC (arrow) that is not visible in the PD patient. (C) Quantitative susceptibility map of the SN in a control subject showing the substantia nigra as an area of high signal intensity indicating high susceptibility as result of iron deposition (arrow). (D) Fractional anisotropy map of the Substantia Nigra (SN) in a control subject. The arrow indicates the substantia nigra.
MRI techniques are also helpful for differentiating between PD and atypical parkinsonism. Whereas structural changes are mild in PD, changes in PSP and parkinsonian-type multiple system atrophy are largely more prominent including atrophy, increased iron load, increased diffusivity and signal changes in specific brain regions. In PSP, changes predominate in the midbrain, the superior cerebellar peduncles and less so in the basal ganglia. In parkinsonian-type multiple system atrophy, changes predominate in the basal ganglia, pons, and cerebellum. Quantification of these changes, in isolation or in combination, using various techniques has shown sensitivity and specificity in distinguishing PD from other parkinsonian syndromes, their use in clinical practice remains limited because of the lack of normative databases and availability of these techniques in clinical centers.
ii. Positron Emission Tomography and Single Photon Emission Computed Tomography
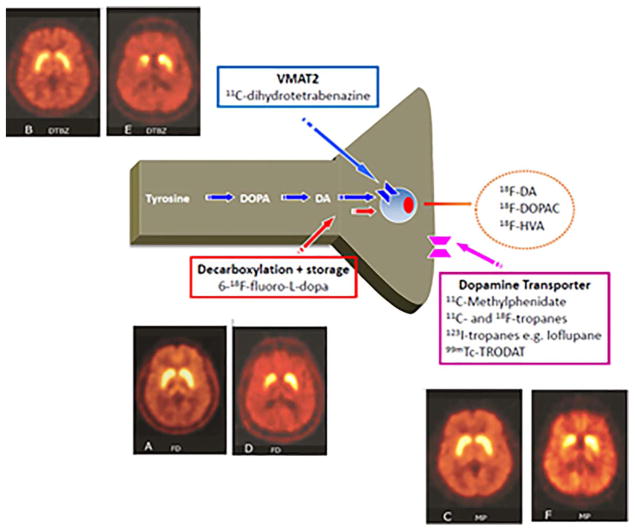
A variety of approaches (Fig. 4) can be used to study the membrane dopamine transporter (DAT; single photon emission computed tomography [SPECT] or positron emission tomography [PET] with a number of 99mTc, 123I, 11C, or 18F tracers, the majority of which are cocaine analogs), the vesicular monoamine transporter type 2 (11C- or 18F-dihydrotetrabenazine PET), or decarboxylation of levodopa to dopamine and the subsequent trapping of dopamine in synaptic vesicles (F-DOPA PET). Radionuclide imaging of presynaptic dopaminergic function using any of these approaches shows a characteristic pattern of asymmetric involvement, with a rostral-caudal gradient in which the posterior putamen is maximally affected (Fig. 4, right). However, although the preferential involvement of putamen over caudate is typical of PD, presynaptic dopaminergic imaging will not reliably differentiate between PD and atypical forms of parkinsonism such as MSA and PSP. This may be possible using metabolic imaging with 18F-fluorodeoxyglucose, where relatively specific covariance patterns (the socalled PD-related pattern [PDRP]) have been described. 107 DAT SPECT using 123I-ioflupane has been approved by the U.S. Food and Drug Administration for the purpose of differentiating between essential tremor and PD.
FIG. 4.
Tracers for presynaptic dopaminergic function. The vesicular monoamine transporter 2 (VMAT2) is responsible for packaging monoamine transmitters into synaptic vesicles. 6-18F-fluoro-L-dopa is a radioactive analog of levodopa that is decarboxylated into 6-18F-fluoro-L-dopamine, which is subsequently stored in synaptic vesicles but then undergoes slow egress and enzymatic degradation. Once dopamine is release from the synapse, it is taken up by the dopamine transporter (DaT), which can be labeled using a variety of 11C and 18F (for PET) and 131I or 99mTc (for SPECT) tracers. For each tracer, the left panel shows a healthy control subject, whereas the right shows a patient with mild Parkinson’s disease. In the latter, there is asymmetric reduction of tracer uptake, maximally affecting the posterior striatum. From Chandran & Stoessl, in Jankovic & Tolosa, Parkinson’s Disease and Movement Disorders, Wolters Kluwer, 2015.
1. Early and Preclinical Detection, Disease Progression
Although the use of dopaminergic imaging may play a relatively limited role in routine clinical diagnostic use, it is sometimes difficult to be certain of diagnosis, particularly in early disease. These approaches may therefore be extremely useful for selection of patients to participate in trials of disease modifying therapies, where a reliance on clinical assessment may result in the inclusion of approximately 15% of patients who do not have dopamine deficiency. The cardinal features of PD do not present until one has lost 30% to 50% of nigral dopamine neurons and close to 80% of striatal dopamine; imaging can detect preclinical dopamine dysfunction several years prior to disease manifestation in individuals at high risk, including those with RBD108 or with a pathogenic dominantly inherited mutation.109 Although the diagnostic utility of preclinical detection may be argued, this approach can be useful as an endophenotype to assist in the identification of new mutations and will ultimately help identify those most likely to benefit from disease-modifying therapies.
Both DAT110 and F-DOPA111 imaging correlate reasonably well with nigral dopamine cell counts; functional imaging has therefore been used to study the progression of PD (and the effects of disease-modifying strategies). Such studies demonstrate that dopaminergic markers decline according to an exponential function, with change occurring most rapidly in early (or presumably presymptomatic) phases of disease.112 Reverse extrapolation of the exponential defining this pattern of decline suggests that vesicular monoamine transporter type 2 binding declines first (more than 15 years prior to disease onset), followed by a decline in DAT binding (some 10–15 years prior), and finally by F-DOPA uptake.113 Although all of the markers correlate somewhat with disease severity, the relationship between change in tracer uptake and change in clinical function is unfortunately limited. There are accordingly several examples where the apparent benefits of a pharmacological or cell-based therapy on imaging have failed to translate into convincing clinical impact. Although this has led to understandable frustration, even those most skeptical of these imaging approaches recognize that they are necessary for the assessment of disease modifying treatments. However, the results must be interpreted with caution and within the broader context of clinical status.
2. Functional Imaging: Motor Complications
Fluctuations in motor response to levodopa are associated with reduced F-DOPA uptake, in keeping with reduced capacity to store dopamine in synaptic vesicles. By prolonging scanning times, F-DOPA imaging can be used to estimate dopamine turnover, which is increased with disease progression.114 11C-raclopride binds to D2/D3 receptors with relatively low affinity and its binding is hence subject to competition from endogenous dopamine. By performing raclopride PET scans before and after an intervention, one can estimate the impact of the intervention on dopamine release. Levodopa itself induces dopamine release, which increases with disease duration. In patients with dyskinesias, the release is of higher magnitude 1 hour after medication, but returns to baseline sooner when compared with patients with a stable response, in keeping with the increase in dopamine turnover as assessed by F-DOPA.115 Indeed, an aberrant pattern of dopamine release is seen in PD patients who are stable at the time of scanning but who later go on to develop fluctuations. Levodopa-induced dyskinesias thus likely reflect an aberrant pattern of dopamine release; this may arise from conversion of levodopa to dopamine in surviving serotonergic neurons, as suggested in animal models and supported by imaging studies.116 Dyskinesias have also been linked to reduced opioid receptor,117 increased adenosine A2A 118, and reduced phosphodiesterase 10A119 binding.
3. Visualizing Nonmotor Complications
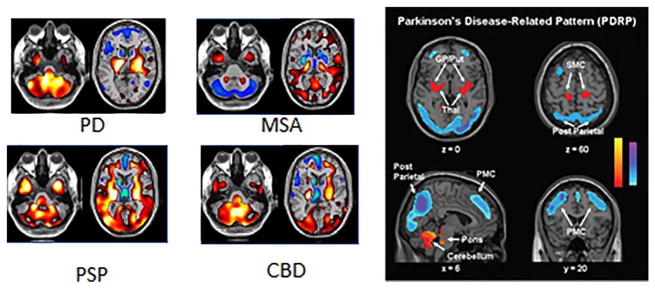
It is now recognized that nonmotor complications affect nearly all cases of PD during the course of their illness and can present ahead of motor disability. Such complications include cognitive dysfunction and frank dementia; depression, anxiety, and psychosis; sleep disorders; altered cardiac reflexes, gastric stasis, constipation, and impotence; and a reduced threshold to pain. Of PD patients, 80% will develop dementia if they survive for 20 years with their illness, and this complication can be more disabling than their locomotor problems.120 Dementia may arise as a consequence of cortical Lewy-body disease, coexistent Alzheimer or small vessel pathology, and the degeneration of monoaminergic and cholinergic projections to cortical areas. Levels of 18F-2-fluoro-2-deoxyglucose (FDG) uptake reflect hexokinase activity which in turn reflects neuronal synaptic activity. In nondemented PD patients, absolute levels of cortical FDG uptake generally fall within normal limits, but covariance analysis reveals an abnormal profile of relatively increased lentiform nucleus and reduced fronto-parietal metabolism.107 This has been labeled the PD-related pattern (PDRP; Fig. 5), and its degree of expression correlates with the degree of motor disability.107 The PDRP normalizes after successful treatment with both dopaminergic drugs or DBS. A distinct profile, the PD-related cognitive pattern has been shown to correlate with cognitive dysfunction.107 This profile is characterized by relatively reduced medial frontal and posterior parietal metabolism with increased cerebellar cortex and dentate nucleus FDG signal. In frankly demented PD patients, FDG PET shows reduced absolute levels of glucose utilization that targets the posterior cingulate, parietal, and temporal association areas.121 This pattern is present to a lesser extent in PD-MCI.122 Although the pattern of glucose hypometabolism in demented PD patients resembles that seen in Alzheimer’s disease, PDD cases later pathologically proven to have cortical Lewy-body disease show significantly greater occipital hypometabolism than that seen in Alzheimer’s patients.123
FIG. 5.
Glucose metabolism in parkinsonian disorders. PD is associated with increased metabolism in the basal ganglia, thalamus, pons, and cerebellum, with concomitant reductions of metabolism in premotor and parietal cortex (the so-called PD related pattern or PDRP, right panel), whereas multiple system atrophy (MSA) is associated with reduced metabolism in basal ganglia and cerebellum, progressive supranuclear palsy (PSP) with reduced metabolism in medial frontal cortex and thalamus, and corticobasal degeneration (CBD) with asymmetrically reduced metabolism in cortex and basal ganglia. Taken from (left) Eckert T, et al. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage 2005;26:912–921 and (right) Asanuma K, et al. Network modulation in the treatment of Parkinson’s disease. Brain 2006;129:2667–2678.
The PET ligand 11C-PIB, a neutral thioflavin-T analogue developed to image β-amyloid plaques in dementia, has been employed to assess the prevalence of amyloid in PD patients.124 Amyloid plaques can be detected with 11C-PIB PET in a majority of dementia with Lewy-body cases, but only a minority of PDD or PD-MCI.125 This suggests that amyloid pathology is not a major contributor to the cognitive problems of PD. However, the presence of amyloid at baseline in PD has been shown to predict more rapid cognitive deterioration over 4 years. It may be that amyloid acts as a trigger for cognitive decline, whereas Lewy-body pathology determines the nature of the behavioral deficits. Tau aggregates are also found in PD cases with cognitive decline. The role that they play in causing cognitive decline is currently under investigation using markers such as 18F-THK5351 PET and 18F-AV1451. The latter appears highly sensitive to tau aggregation processes in patients with PSP.126
Postmortem studies suggest that the loss of cholinergic neurons from the nucleus basalis occurs early in PD. Cholinergic terminal function in PD has been assessed with 11C-NMP4A and 11C-PMP PET, markers of acetylcholine esterase activity.123,127 Nondemented PD patients showed posteriorly reduced cholinergic function in parietal and occipital cortex with 11C-NMP4A PET, and this spreads to involve frontal cortex when dementia is present. Levels of cortical acetylcholinesterase activity in PD correlate with MMSE scores and performance on executive tests such as card sorting and trail making.
A majority of PD patients experience depressive symptoms. It has been suggested that serotonergic loss might contribute to depression in PD; however, the findings from neuroimaging studies have been inconsistent. 123I-β-CIT SPECT is a marker of brain stem serotonin transporter availability. 123I-β-CIT uptake was similar in PD patients with and without depression and did not correlate with Hamilton Depression Rating Scale scores.128 In contrast, an 11C-DASB PET study on drug-naïve PD cases found increased serotonin transporter availability in the raphe and limbic cortex in depressed patients.129 The authors argued that this finding supported the presence of a synaptic serotonin deficiency state in depressed PD. Remy and colleagues130 used 11C-RTI-32 PET, a marker of dopamine and noradrenaline transporters, to assess PD patients with and without depression. The depressed PD patients had lower 11C-RTI-32 binding in locus coeruleus and areas of the limbic system than nondepressed PD patients. This finding suggests that the loss of limbic dopamine and noradrenaline are also relevant to the pathogenesis of depression in patients with PD.
Sleep disorders are a characteristic of PD. The most prevalent problem is excessive daytime somnolence, and this is most often seen when patients are receiving dopamine agonists, but it can also be seen where only levodopa is being taken and occasionally in drug-naïve cases. Using 11C-DASB PET, it has been reported that raphe and thalamic serotonin transporter availability are reduced in excessive daytime somnolence cases, the reductions correlating with severity of somnolence.131 RBD is also a problem in up to 50% of PD cases and can be a prodromal feature of the disorder. Here, there is a failure to lose muscle tone during dreaming and affected patients can shout, kick, or punch, injuring themselves and partners. 18F-dopa PET is a marker of monoamine storage capacity. It has been reported that midbrain uptake of 18F-dopa correlates with decreased duration of REM sleep in PD.132 123I-MIBG SPECT is a marker of adrenergic terminal function and can be used to study functional integrity of cardiac sympathetic innervation in PD. Myocardial:mediastinal 123I-MIBG signal ratios are reduced in more than 80% of PD cases, even when cardiac autonomic reflexes remain intact.133 11C-donepezil PET is a marker of acetylcholine esterase activity in systemic organs. In PD cases, the intestine and pancreas show a significant reduction in 11C-donepezil uptake, and the myocardial signal is also mildly reduced. Reduced 11C-donepezil uptake could reflect reduced vagal cholinergic systemic innervation as the dorsal nucleus of the vagus is targeted by Lewy-body pathology.
In summary, the use of radioimaging has helped to determine or confirm the mechanisms underlying cognitive, mood, sleep, and autonomic problems associated with PD. PET imaging has highlighted the multisystem nature of PD and emphasizes the need for tailoring symptomatic therapies to individual patients. It also provides potential biomarkers for objectively monitoring the efficacy of putative neuroprotective agents directed at nonmotor complications.
f. Pathophysiology: Circuits and Mechanisms (J.R. Rothwell, M. Hallett, and D. DeLong)
James Parkinson did not speculate on pathophysiology. How do the signs and symptoms of PD come about? How can it combine excess muscle activity in the form of tremor and rigidity with poverty of action as in bradykinesia?
i. Overall Summary of Basal Ganglia Operation
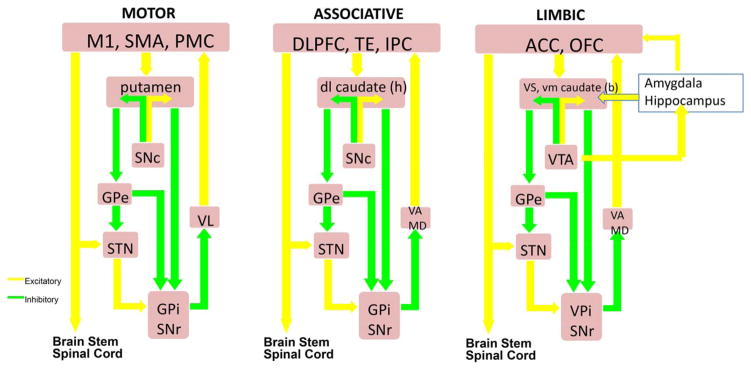
Figure 6 highlights the main connections between basal ganglia nuclei and the concept that within these connections, information flow remains to a certain degree segregated via parallel processing of input from broad regions of the cerebral cortex, termed motor, associative, and reward/reinforcement (limbic).134 Each of the loops deals with different regions of the cortex and therefore different functions, although presumably performing similar operations on the inputs. Output is sent from cortex and thalamus to the input nuclei of the basal ganglia (striatum = caudate + putamen in humans). This is transformed and transmitted through 2 separate pathways, known as the direct and indirect pathways, to the much smaller output nuclei (GPi and SNpr). These send segregated information back to respective cortical areas in the frontal cortex via the thalamus and also to the brain stem. The motor loop involving the precentral motor fields and their projections through the basal ganglia has been studied far more than the other loops because it plays a significant role in the motor signs and symptoms of PD and other movement disorders.
FIG. 6.
Classic scheme of cortico-basal ganglia connectivity highlighting the main motor, associative-cognitive, and emotional-limbic domains. M1, primary motor cortex; SMA, supplementary motor area; PMC, pre-motor cortex; SNc, substantia nigra pars compacta; GPe, globus pallidus pars externa; STN, subthalamic nucleus; GPi, globus pallidus pars interna; SNr, substantia nigra pars reticulate; VL, ventralis lateralis; VA, ventralis anterior; MD, medio-dorsal.
A fundamental starting point for virtually all models of basal ganglia function and dysfunction is that output from the GPi and SNr are characterized by high-frequency GABAergic (inhibitory) neurons that provide a continuous level of inhibition on thalamic and brain stem targets.135 The concept of opponent pathways, within each of the striato-pallidal projections, states that the striatal-GPi/SNr direct pathway has a facilitatory influence on movement by disinhibition of the thalamus, whereas the indirect striatal-GPe-GPi/SNr pathway suppresses movement by increasing inhibition. PD has been viewed as a member of a “family” of movement disorders involving the basal ganglia motor loop in which there is a disorder of the balance between excitation and inhibition, resulting in hypokinetic disorders, such as PD, and hyperkinetic disorders, such as chorea-ballismus or dystonia.
Although PD is a complex neurodegenerative disorder, the cardinal motor features of PD result from dopamine deficiency within the motor loop. The basal ganglia are widely believed to play a role in reinforcement learning. Unexpected rewards produce a phasic burst of dopamine. By altering the effectiveness of connections between cortical inputs and the direct and indirect pathways, dopamine can reinforce patterns of activity and play a role in reinforcement learning.
If asked at this point, Dr Parkinson might have demurred that “his disease is not a disorder of learning.” Present concepts allow for this by positing that dopamine operates in at least 2 different ways. In addition to the phasic bursts that have attracted so much attention, dopamine neurones fire regularly at a slow rate of about 5 Hz to sustain a background tonic level of dopamine within the striatum. Loss of tonic, rather than phasic dopamine, appears to be a crucial factor responsible for major parkinsonian motor manifestations. This is probably why dopamine replacement therapy works: it replaces to some extent the missing tonic dopamine release.
Exactly how tonic levels of dopamine sustain normal basal ganglia function is not completely clear at present. One possibility is that it regulates the moment-to-moment effectiveness of synaptic connections in the striatum. If dopamine is deficient, this may shift the balance of activity in direct and indirect pathways toward inhibition, causing a poverty of movement.134 In addition, it may change the way populations of neurones interact in the overall cortexbasal ganglia-thalamus-cortex loops. This increases the probability that they will oscillate together and reduce their ability to shift patterns of basal ganglia output, resulting in bradykinesia.136
ii. Neuronal Activity Changes in PD
The cardinal features of PD (bradykinesia and muscular rigidity) are a result of the progressive loss of dopaminergic neurons in the dorsolateral SN pars compacta (SNc), which innervate the motor portions of the basal ganglia, the putamen in particular, and also the STN, GPi/SNr, thalamus, and cortex. Although parkinsonism is readily reversed by the administration of levodopa, the frequent development of levodopa-induced dyskinesias and motor fluctuations137 and the later development of dopamine-unresponsive gait and balance difficulties and freezing of gait in advanced disease are major factors in the progressive loss of quality of life in many patients.
Studies of the effects of dopamine loss on basal ganglia networks in animal models administered DA toxins such as 6-OHDA and MPTP as well as more recent optogenetic models have strengthened the view that in parkinsonism, firing rate changes in the direct and indirect pathways are characterized by a reduction of neuronal discharge in GPe, and increased activity in the STN and the output nuclei, GPi and SNr. The resulting increased inhibitory basal ganglia output was, at first, believed to lead to bradykinesia by excessively inhibiting thalamocortical neurons, (the so-called “rate model” of parkinsonism). Further studies have shown, however, that changes in discharge patterns are of equal or even greater importance in the pathophysiology of parkinsonism than rate changes.136 One of the clearest changes is oscillatory activity of the basal ganglia of animal models and patients with PD. Oscillations can also be identified in local field potentials recorded from macroelectrode recordings, which reflect synchronous membrane potential fluctuations in neuronal populations. Recordings in unmedicated patients reveal the predominance of oscillations in the beta range (10–25 Hz) in the STN,138 GPi, and cortex, whereas oscillations in the 60 Hz to 80 Hz (gamma) range are enhanced following the administration of levodopa. In PD patients who develop levodopa–induced dyskynesias (LIDs) and/or pathological impulsivity, a peak in the theta band is recorded in the STN.139 Although still controversial, it is widely believed that abnormal beta band synchrony and STN motor cortex coupling play a critical and important role in the production of bradykinesia138,140
iii. Circuits
The importance of the STN and the indirect pathway in the pathophysiology of parkinsonism is evident from findings in nonhuman primates and the many patients surgically treated. The STN also receives input from the cortex, the “hyperdirect” pathway, which is postulated to play a role in the cancellation of an inappropriate movement.141 It is likely that other structures and feedback loops, such as those involving the pedunculopontine nucleus (PPN) and the Centromedianum (CM) also contribute to parkinsonism. In addition, the CM-striatal projection has been found to degenerate in PD patients.142 In parkinsonian animals, lesioning of the PPN, which may include the brainstem locomotor region, has been associated with akinesia, whereas increased PPN activity is associated with an amelioration of parkinsonism. The PPN is a complex structure54 that has close connections with the basal ganglia and brain stem nuclei that project to the spinal cord, and cholinergic neurons of the PPN degenerate in PD. However, the actual role of the PPN in the pathophysiology of PD and gait in particular remain controversial, and also, therefore, the possibility of using PPN as a surgical target.143
Changes in cerebellar circuits and in interactions between the cerebellum and the basal ganglia are now also recognized to be important in the pathophysiology of PD, especially in the case of parkinsonian tremor.100 Parkinsonian tremor appears to be strongly related to abnormal oscillatory activity in the cerebellar receiving territory of the thalamus (nucleus ventralis intermedius [Vim]). DBS or lesions of this area are among the most effective treatments of (isolated) parkinsonian tremor. Tremor clearly also responds in many patients to dopaminergic therapy, but it is not clear how dopamine loss is linked to tremor (see “The Origin and Mechanisms of Shaking” section later). A role of the basal ganglia in tremor is strongly supported by the beneficial effects of STN and GPi ablation and DBS therapies.
Although this discussion has focused on the role of disturbances in the motor circuit resulting from DA loss, it is clear that disturbances in other neurotransmitters, in particular cholinergic and glutamatergic, are also strongly implicated in PD. It is also important to recognize that DA loss in the associative and limbic circuits may produce disturbances of nonmotor function and that the dopamine unresponsive signs and symptoms of advanced PD result from widespread cortical and subcortical pathology.
It should also be pointed out that the signs and symptoms of PD and other movement disorders do not necessarily reflect the functions of the basal ganglia. The dramatic and immediate beneficial effects of ablation and DBS of GPi in both primate models and patients with PD and dystonia, with little or no impairment of movement or behavior, argues against an essential role of the basal ganglia in the on-line control of movement and favors the view, now strongly supported by animal and human studies, that the basal ganglia motor circuit serves higher order functions such as reinforcement learning and the regulation of general movement “vigor” and motivation.144
g. Etiopathogenesis: Environmental Factors (C. Marras, C. Tanner, W. Ross, and W. Langston)
The role of environmental risk factors and way of living is probably fundamental to many insidiously evolving diseases. Neurodegenerative diseases in general, and PD in particular, are likely influenced by environment and daily activities. This has been recognized for a long time, but the interest and importance has waxed and waned for decades.
A landmark was the discovery in 1983 that MPTP caused a selective injury to dopaminergic neurons and a PD-like illness.78 Owing to structural and mechanistic similarities between MPTP and commonly used pesticides, this finding led to a major shift toward environmental neurotoxicants as a cause of PD. In the several decades that followed, numerous environmental risk factors were identified, such as rural living and well water consumption, but unitary environmental explanations were elusive. At the same time, the evidence for genetic contributions was accumulating in the form of families with multiple affected members, including some families with pathologically proven Lewy-body PD that could be a familial disorder.145 During the past 2 decades, genetic risk factors and causative genes have continued to be discovered, increasing the weight of evidence that genetic factors are important (although not exclusive) contributors to etiology. The observation that early pathological change is seen in the gut and the olfactory systems has recently led to the hypothesis that an infectious agent may be the triggering event.
Although parkinsonism may rarely be caused by a single gene mutation or environmental exposure, most cases of PD likely have a multifactorial etiology, the net result of harmful and protective environmental and genetic factors. Pesticides, solvents, polychlorinated biphenyls, and head injury have been associated with greater risk of PD. Behaviors and lifestyle factors (such as smoking and caffeine intake) have most often been associated with lower risk of PD.
Rural living, agricultural occupation, and well water consumption, presumably surrogates for agricultural chemicals and pesticide use, have been associated with PD in more than 80 populations worldwide.146,147 Specific pesticides have been linked to PD, and most of these have also been found to cause parkinsonism in animal models.147,148 In some cases, the adverse effect of a pesticide is increased in persons with genetically determined altered function of toxicant handling. 148 Conversely, other behaviors appear to protect against the adverse effects of pesticide exposure. 149 Table 1 summarizes these results, showing the complex interplay between environmental exposures, genetics, and behaviors. Analogous relationships are also being investigated for PD-related environmental exposures other than pesticides.
TABLE 1.
Pesticides, effect modification, and risk of Parkinson’s disease
| Toxicant | Exposure type | Effect size | Genetic modifier | Modified effect size | Environmental modifier | Modified effect size |
|---|---|---|---|---|---|---|
| Paraquat | Occupational; residential | ~ 2 – 3 fold ↑ risk | GST T1 null | ~ 11 fold ↑ risk |
|
|
| Rotenone | Occupational | ~ 2 – 3 fold ↑ risk | Unknown | — |
|
|
| Organochlorines : dieldrin, DDT, hexachlorocyclohexanes | Occupational; gardening; residential; | ~ 2 fold ↑ risk |
|
|
Unknown | — |
| Organophosphates: malathion, parathion, chlorpyrifos, diazinon | Occupational, residential | ~ 2 – 5 fold ↑ risk | PON1 variant | ~ 2 – 3 fold ↑ risk | Unknown | — |
| Permethrin | Occupational; Impregnated clothing; home use | Not significant | — | — | No PPE | ~ 4 fold ↑risk |
| Benomyl | Ambient occcupational or residential | ~ 0.5 fold ↑ risk | SKP 1) variant | ~ 7 fold ↑ risk | Unknown | — |
DA, dopamine; PUFAs, polyunsaturated fatty acids; GST T1, Glutathione S Tranferase T1; ABC B1, ATP binding cassette B1; CYP, cytochrome P 450; PM, poor metabolizer; PON1, Paraoxonase 1; PPE, personal protective equipment; UPS, ubiquitin protease system; SKP 1, S phase kinase associated protein 1 variant.
Other toxicants associated with increased PD risk in humans and parkinsonism-associated toxicity in animal models include the chlorinated solvents (trichloroethylene, perchloroethylene, carbon tetrachloride), formerly used in dry cleaning, degreasing, and viscose rayon manufacturing,148 and polychlorinated biphenyls, formerly used as coolants and lubricants.149,150 Importantly, these toxicants are environmentally persistent. Although many have been banned, they remain in soil and water.
Mild to moderate head injury is associated with a higher risk of PD in most but not all studies,147 an inconsistency that may be a result of genetic heterogeneity. For example, persons with head injury with variants in the promotor region of the gene encoding alpha-synuclein that increase alpha-synuclein levels had 2- to 5-fold increased risk of PD, whereas the risk was not increased in persons with a head injury but without the variants.147 Multiple head injuries have been associated with greater risk in some studies.
Cigarette smoking has consistently been associated with a reduced risk of PD, although the underlying mechanism of this relationship remains elusive.151 Current smokers have less than half the risk of developing PD when compared with nonsmokers. Many studies report a dose effect: a longer duration of smoking or greater number of cigarettes smoked are associated with an increasingly lower PD risk. Although there are hundreds of chemicals in cigarette smoke, nicotine is commonly implicated as the causative agent. Smokeless tobacco users are also at lower risk of PD, indirectly supporting an effect of nicotine.151 Variants in several genes have been reported to modify the effect of smoking on PD risk, suggesting a gene–environment interaction, but the effects appear to differ by gender and replication is needed.152
Coffee and tea drinking as well as caffeine consumption are also associated with a lower risk of PD.153 There is a nearly 60% reduction in risk in male high-coffee drinkers when compared with nondrinkers, with an evident dose effect. Interestingly, the relationship is less clear in women, possibly as a result of a weakening effect of hormone replacement therapy.153 Thus far, the CYP1A2 gene involved in caffeine metabolism and the ADORA2A gene that encodes the adenosine A2A receptor that is blocked by caffeine have been the most studied for gene environment interactions, but so far the results have been inconsistent.154 Other factors associated with a reduced risk of PD include physical activity, certain dietary patterns, and the use of certain drugs including nonsteroidal antiinflammatory agents and calcium channel blockers.147,148
The prevailing views on the cause of PD have swung like a pendulum between environmental influences and heritable factors since James Parkinson’s seminal description. At present, the evidence supports a combined etiological model of environmental influences and genetic factors, culminating in a common pathophysiologic process. Unraveling the common pathogenetic threads underlying these gene– environment interactions presents a significant challenge, but with the reward of better understanding, more effective treatments, and potentially interventions to prevent PD.
h. Etiopathogenesis: Genetics (B.V. Klein)
This is another area where information, knowledge, and concepts have expanded and changed substantially during the past few decades. Indeed, not only Dr. Parkinson himself but most (if not all) engaged in PD research until recently could not envisage the developments that have taken place regarding genetics and PD.
The year 2017 also marks the 20th anniversary of the discovery of the first gene causing a monogenic form of PD, that is, alpha-synuclein (SNCA).79,155 However, only a minority (ie, ~5%) of cases is currently attributed to well-defined genetic causes. Indeed, postencephalitic and MPTP-induced parkinsonism, lack of convincing concordance rates among twins, and the identification of environmental risk factors had long supported the hypothesis of an exogenous cause of PD until the identification of monogenic forms of PD in 1997 dismantled the previously held dogma of an exclusively nongenetic etiology.
i. The Shaking Palsy Can Be Caused by Mutations in Single Genes
James Parkinson did not mention any possible contribution of hereditary factors to the shaking palsy in his chapter on possible causes of PD.9 Admittedly, from his observations, no clues toward a genetic etiology could have been drawn, and it remains true that the vast majority of PD patients indeed appear sporadic. Furthermore, with his description of inheritance patterns, Mendel started drawing attention to familial traits in a systematic fashion only in 1865, 40 years after James Parkinson’s report of PD.
According to the recommendations of the Movement Disorder Society Task Force156 for the Nomenclature of Genetic Movement Disorders, confirmed forms of monogenic PD with a PARK designation can be grouped on clinical grounds into those presenting with (i) classical PD, (ii) early-onset PD but clinically still very similar to nongenetic PD, and (iii) atypical parkinsonism (Table 2). As James Parkinson focused on the description of typical late-onset PD cases, only the 3 monogenic forms frequently presenting with classical, late-onset PD will be detailed below.
TABLE 2.
List of monogenic PD and parkinsonism (adapted from ref. 210)
| Designation and reference | GeneReviews and OMIM Reference | Clinical clues | Inheritance | Previous locus symbol |
|---|---|---|---|---|
| 1. Classical PD | ||||
| PARK-SNCA | GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1223/ OMIM 168601 |
Missense mutations cause classical parkinsonism. Duplication or triplication mutations in this gene cause early onset parkinsonism with prominent dementia. | AD | PARK1 |
| PARK-LRRK2 | GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1208/ OMIM 607060 |
Clinically typical PD | AD | PARK8 |
| PARK-VPS35 | GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1223/ OMIM 614203 |
Clinically typical PD | AD | PARK17 |
| 2. Early-onset PD | ||||
| PARK-Parkin | GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1155/ OMIM 600116 |
Often presents with dystonia, typically in a leg | AR | PARK2 |
| PARK-PINK1 | GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1223/ OMIM 605909 |
Often presents with psychiatric features | AR | PARK6 |
| PARK-DJ1 | GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1223/ OMIM 606324 |
AR | PARK7 | |
| 3. Parkinsonism | ||||
| PARK-ATP13A2 | GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1223/ OMIM 606693 |
Kufor-Rakeb syndrome with parkinsonism and dystonia; Additional features: Supranuclear gaze palsy, spasticity/pyramidal signs, dementia, facial-faucial-finger mini-myoclonus, dysphagia, dysarthria, olfactory dysfunction |
AR | PARK9 |
| PARK-FBXO7 | GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1223/ OMIM: 260300 |
Early onset parkinsonism with pyramidal signs | AR | PARK15 |
| PARK-DNAJC6 | GeneReviews: n/a OMIM 615528 | May present with mental retardation and seizures | AR | PARK19 |
| PARK-SYNJ1 | GeneReviews: n/a OMIM 615530 | May have seizures, cognitive decline, abnormal eye movements, and dystonia | AR | PARK20 |
AD, autosomal dominant; AR, autosomal recessive; n/a, not available.
PARK-SNCA
Although mutations in SNCA155 are an extremely rare cause of PD, SNCA is likely the most intensely investigated PD gene not only with respect to causative mutations but also to risk variants as well as the function of the gene and the encoded protein. The observation of mutation (type)-specific clinical expression appears to be unique to SNCA when compared with other PD-causing genes. SNCA triplication carriers have about a 10-year earlier onset than duplication carriers. In accordance with a dosage effect, SNCA triplication carriers also have a more severe phenotype and faster disease progression than duplication carriers. Intriguingly, other PD-related genes, such as leucine-rich repeat kinase 2 (LRRK2) and GBA (acid beta-glucosidase) have also been linked to alterations of SNCA levels. Although cell-to-cell transmission of alpha-synuclein has been demonstrated in both cell culture and animal models, the exact sequence and molecular mechanisms of propagation of PD’s neuropathology throughout the human brain remain elusive.157
PARK-LRRK2
Mutations in LRRK2 are the most common pathogenic changes linked to autosomal dominant PD.158,159 They account for 3% to 41% of familial cases and are also found at a lower rate in apparently sporadic cases.160 The phenotype of LRRK2 p.G2019S mutations is indistinguishable from that of Idiopathic Parkinson’s disease (iPD), although tremor is more common and leg tremor may be a useful diagnostic clue.161 The p.G2019S mutation is by far the most prevalent because of a founder effect. LRRK2 has a guanosine-5-triphosphate–regulated serine/threonine kinase activity with pathogenic LRRK2 variants increasing autophosphorylation or kinase activity, raising the potential not only for a mechanistic understanding of the effect of LRRK2 mutations but also for the development of biomarkers and of LRRK2 kinase inhibitors as a causal therapeutic target.
PARK-VPS35
Two independent studies used whole-exome sequencing to identify the same p.D620N (c.1858G>A) mutation in the vacuolar protein sorting 35 homolog (VPS35) gene as the cause of autosomal-dominant PD.162,163 The p.D620N mutation cose-gregates with a phenotype similar to iPD and has incomplete, age-associated penetrance. VPS35 is a component of the retromer complex and is involved in retrograde transport from the endosomes to the trans-Golgi network. It localizes to dendritic spines and is involved in fundamental neuronal processes, including excitatory synaptic transmission and synaptic recycling.
During the past 2 decades, detailed multimodal analyses of individuals with these and other monogenic forms of PD have collectively provided unique opportunities to pursue the mechanisms of neuronal degeneration in PD highlighting the Bermuda triangle of PD pathogenetic mechanisms with (i) impaired protein turnover, (ii) mitochondrial dysfunction, and (iii) disrupted synaptic and endosomal vesicle and protein trafficking and recycling (Fig. 7).
FIG. 7.
Bermuda triangle of disease mechanisms implicated in monogenic (and idiopathic) Parkinson’s disease, highlighting the role of confirmed genes for monogenic PD and parkinsonism, as well as for the GBA gene in the context of protein degradation, mitochondrial function, and synaptic and endosomal vesicle and protein recycling.
ii. There Are Multiple (Genetic) Forms of PD
The identification of different monogenic forms of PD (Table 2) has clearly established the existence of several distinct entities of PD. With the aim to develop personalized, causal treatment approaches, it may indeed be helpful to split PD into different PDs. However, before being able to subclassify different forms of PD in a useful fashion, the multiple hypo- and hyperkinetic movement disorders observed in PD had to be recognized as comprising one clinical syndrome with variable expressivity and combinations of its cardinal signs. James Parkinson is credited with this key observation and recognition: “it [PD] has not yet obtained a place in the classification of nosologists; some have regarded its characteristic symptoms as distinct and different diseases, and others have given its name to diseases differing essentially from it.”1
Even with today’s insights from genetics and pathology, defining PD remains a challenge.164 Moreover, variants in PD-causing and other genes can confer risk to PD, which may eventually lead to further substratification of PD patients. The most recent and largest genomewide association study conducted on about 19,000 PD patients and about 100,000 controls of Caucasian ancestry, established consistent association of 26 independent genetic loci with PD risk (Table 3).165
TABLE 3.
Overview of the 26 genetic risk variants showing consistent association with Parkinson’s disease in genome-wide association studies
| SNP | Location (hg38) | Nearest gene | Alleles | Risk allele freq | OR | P value |
|---|---|---|---|---|---|---|
| rs35749011 | 1:155,162,810 | SLC50A1 (GBA) | A/G | 0.017 | 1.824 | 1.37 × 10−29 |
| rs114138760* | 1:154,925,709 | PMVK (GBA) | C/G | 0.012 | 1.574 | 3.80 × 10−7 |
| rs823118 | 1:205,754,444 | NUCKS1 | T/C | 0.559 | 1.122 | 1.66 × 10−16 |
| rs10797576 | 1:232,528,865 | SIPA1L2 | T/C | 0.140 | 1.131 | 4.87 × 10−10 |
| rs6430538 | 2:134,782,397 | ACMSD | C/T | 0.570 | 1.143 | 9.13 × 10−20 |
| rs1474055 | 2:168,253,884 | STK39 | T/C | 0.128 | 1.214 | 1.15 × 10−20 |
| rs12637471 | 3:183,044,649 | MCCC1 | G/A | 0.807 | 1.188 | 2.14 × 10−21 |
| rs34311866 | 4:958,159 | TMEM175 (GAK) | G/A | 0.191 | 1.272 | 1.02 × 10−43 |
| rs34884217* | 4:950,422 | TMEM175 (GAK) | A/C | 0.913 | 1.247 | 1.10 × 10−6 |
| rs11724635 | 4:15,735,728 | BST1 | A/C | 0.553 | 1.126 | 9.44 × 10−18 |
| rs6812193 | 4:76,277,833 | FAM47E | C/T | 0.636 | 1.103 | 2.95 × 10−11 |
| rs356182 | 4:89,704,960 | SNCA | C/T | 0.367 | 1.316 | 4.16 × 10−73 |
| rs7681154* | 4: 89,842,802 | SNCA | C/A | 0.498 | 1.189 | 7.09 × 10−19 |
| rs9275326 | 6:32,698,883 | HLA-DQB1 | C/T | 0.906 | 1.211 | 1.19 × 10−12 |
| rs13201101* | 6:32,375,827 | C6orf10 | T/C | 0.053 | 1.192 | 3.84 × 10−6 |
| rs199347 | 7:23,254,127 | GPNMB | A/G | 0.590 | 1.110 | 1.18 × 10−12 |
| rs117896735 | 7:119,777,065 | INPP5F | A/G | 0.014 | 1.624 | 4.34 × 10−13 |
| rs329648 | 11:133,895,472 | MIR4697HG | T/C | 0.354 | 1.105 | 9.83 × 10−12 |
| rs76904798 | 12: 40,220,882 | LRRK2 | T/C | 0.143 | 1.155 | 5.24 × 10−14 |
| rs11060180 | 12:122,819,039 | CCDC62 | A/G | 0.558 | 1.105 | 6.02 × 10−12 |
| rs11158026 | 14:54,882,151 | GCH1 | C/T | 0.665 | 1.106 | 5.85 × 10−11 |
| rs2414739 | 15:61,701,935 | VPS13C | A/G | 0.734 | 1.113 | 1.23 × 10−11 |
| rs14235 | 16:31,110,472 | BCKDK | A/G | 0.381 | 1.103 | 2.43 × 10−12 |
| rs17649553 | 17:45,917,282 | MAPT | G/A | 0.774 | 1.300 | 2.37 × 10−48 |
| rs12456492 | 18:43,093,415 | RIT2 | G/A | 0.307 | 1.106 | 7.74 × 10−12 |
| rs8118008 | 20:3,187,770 | DDRGK1 | A/G | 0.657 | 1.111 | 3.04 × 10−11 |
iii. Translational Genetics May Result in Personalized (Genotype-Specific) Treatments
An improved understanding of monogenic PD and of the genetic contribution to sporadic PD is highly desirable because it is conceivable that at least a subset of PD may be causally treatable. In this context, neurogenetics provides a unique opportunity to identify and study individuals with this neurodegenerative disorder in its earliest stages, which most likely are the ones most amenable to treatment. Interestingly, James Parkinson already pointed to this very aspect in his article by stating the following: “By these repeated observations, he [James Parkinson] hoped that he had been led to a probable conjecture as to the nature of the malady, and that analogy had suggested such means as might be productive of relief, and perhaps even of cure, if employed before the disease had been too long established.”9
This table was adapted from Table 1 and Supplementary Table 3 of the original study.13 It lists the 22 most significant SNPs per locus (defined in 1 Mb boundaries) that showed genome-wide significant (p <5 × 10−8) association with Parkinson’s disease (PD) status.7 Furthermore, it displays 4 SNPs (labeled with a star [*]) that showed significant association (ie, p <1 × 10−5 following Bonferroni correction) with PD risk upon conditioning on the most significant SNP in the same genetic region (ie, corresponding to the SNP listed in this table in the preceding line). Note that the nearest gene assigned to each SNP here (as determined according to RefGene as available on the UCSC genome browser (https://genome.ucsc.edu/)) does not necessarily represent the functional element underlying the genetic association. The genes in parentheses refer to the more commonly used gene names for the respective locus. Full names of all official gene names listed here can be found in the EntrezGene database (http://www.ncbi.nlm.nih.gov/gene/). Alleles = the first allele represents the risk allele. hg38, human genome build 38; Freq, frequency; MAPT, microtubule-associated protein tau; OR, odds ratio; SNP, single nucleotide polymorphism.
Several animal models have been employed to study genetic forms of PD. As an example, in Drosophila, phenotypes caused by mutations in the PD gene PINK1 are particularly well characterized and show defects of flying ability and mitochondrial abnormalities, including reduced ATP levels, impaired enzymatic activity of Complex I, disrupted membrane potential, and abnormal mitochondrial morphology.166 A dominant modifier screen of PINK1 performed in Drosophila identified vitamin K2 as a suppressor of the PINK1 mutant phenotype, which functions as an electron carrier molecule in mitochondria leading to improved ATP production.167 It has thus been hypothesized that stratifying PD patients according to different degrees of mitochondrial dysfunction based on their genetic predisposition may result a differential response to mitochondrial enhancers. This hypothesis is being explored further in the first therapeutic clinical trial in the field of PD with genotype-specific patient selection while this manuscript was in preparation.
iv. Future Perspectives
The advent of whole-exome and whole-genome sequencing technologies promises a new wave of genetic discoveries for PD, particularly in the area of rare variants of intermediate penetrance (similar to the LRRK2-G2019S or the GBA variants), which escaped detection in the previous genomewide approaches, but might represent a relevant missing piece in the complex etiological landscape of PD. The role of epigenetic modifications, chromatine modifications, and genomic regulation of gene expression in the pathogenesis of PD represent other exciting areas of future explorations, enabled by the novel generations of sequencing technologies.168
Detailed and systematic reporting of phenotypic data of patients with monogenic PD is currently lagging behind the advances in PD genetics and poses an important unmet need for successful translational efforts. In this context, it is imperative that as yet unconfirmed and newly detected PD genes undergo careful independent validation before being added to diagnostic PD gene panels. An improved pathogenetic understanding of the known PD genes and proteins will be key for the development of causative treatment strategies, as are the elucidation and possible exploitation of mechanisms of endogenous disease protection as suggested by reduced penetrance of many PD gene mutations.
i. Symptomatic Treatments: The Significance of Levodopa Therapy (J. Jankovic)
In 1967, 150 years after James Parkinson’s publication Essay on the Shaking Palsy, George C. Cotzias and his colleagues169 published the seminal paper that launched the levodopa era of PD. The initial beneficial effects were confirmed 2 years later by Yahr and colleagues 170 in a double-blind, placebo-controlled trial of levodopa in 60 patients with parkinsonism, 81% of whom achieved “significant overall improvement.” Although other drugs have been introduced since that time, levodopa has remained the most effective treatment of PD-related motor symptoms. Despite its clear benefits, there are many limitations associated with initial levodopa treatment (eg, nausea, drowsiness, orthostatic lightheadedness) and with chronic levodopa therapy (eg, motor fluctuations and dyskinesia, hallucinations, and other psychiatric side effects). With a better understanding of its clinical and pharmacological effects, the response to levodopa can be optimized by skillfully adjusting its dosing and tailoring it to the needs of individual patients. Furthermore, new formulations of levodopa are being developed to improve the treatment response.171,172
Levodopa has been used clinically for half a century, but its mechanism of action in PD is still not fully understood. The traditional notion that the amino acid acts primarily as a dopamine precursor (by being decarboxylated into dopamine and then replenishing the deficient neurotransmitter in the striatum) has been challenged because this presumed mechanism does not explain the loss of the long duration response, the development of dyskinesias, and other clinical-pharmacologic effects. Furthermore, studies have found that levodopa itself serves as a neurotransmitter and it is converted nonenzymatically to several biologically active compounds, such as 2,4,5-trihydroxyphenylalanine, an excitotoxin.173 Besides its potential excitotoxic effect, there has been a concern that levodopa might be neurotoxic as a consequence of reactive oxygen species generated by the drug’s oxidative metabolism. Although there is some support for levodopa-induced neurotoxicity from in vitro studies, numerous in vivo, clinical, and pathological studies have failed to provide compelling evidence that levodopa causes cell death.174
The possibility that levodopa may accelerate neuronal degeneration and clinical progression was partly addressed by the ELLDOPA (Early vs Late Levodopa) study,175 spearheaded by Stan Fahn and the Parkinson Study Group.176 The ELLDOPA study was a prospective, double-blind trial in which 361 patients with early (duration of symptoms was less than 1 year) and untreated PD were randomly assigned to receive levodopa in doses of 150, 300, or 600 mg/day or placebo. The endpoint was the change in the Unified PD Rating Scale (UPDRS) score between baseline and final visit performed after 9 months of drug treatment and 2 weeks of drug washout. As expected, patients on any dosages of levodopa showed significant reductions in UPDRS score when compared with placebo, even 2 weeks after levodopa discontinuation.175 This finding has been interpreted as evidence against levodopa neurotoxicity because if levodopa were toxic the UPDRS score in the levodopa groups should deteriorate to a greater degree than in the placebo group after drug washout. Thus the ELLDOPA study strongly suggested that levodopa does not worsen the progression of PD. Furthermore, it was the first controlled study that conclusively demonstrated a dose-dependent motor benefit and levodopa-induced dyskinesias.
There were some limitations to the study. For example, the 2-week washout may not have been sufficient because of potential long-duration (weeks or months) pharmacodynamic effect of levodopa. Furthermore, contrary to the clinical outcome, DAT SPECT scans showed significantly greater loss of the DAT density in the levodopa groups compared to placebo. This, however, may reflect the effect of levodopa on regulation of the DAT rather than an actual reduction in dopaminergic terminals.
Motor complications (ie, fluctuations and dyskinesias) associated with chronic levodopa therapy represent a major challenge in the treatment of patients with PD.177,178 In a large, prospective study, 29% of 352 patients originally enrolled in the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP) study who received levodopa therapy for a mean of 12 months were found to have levodopa-induced dyskinesia and after 20.5 months of levodopa therapy half experienced a wearing-off phenomenon. 179 In the ELLDOPA study, 16.5% of patients in the 600-mg group developed dyskinesia after only 40 weeks of treatment.175
Levodopa-induced dyskinesia can be classified into “peak-dose dyskinesia,” “diphasic dyskinesia,” and “off-period dystonia,” but some patients have a combination of these and other forms of dyskinesias.177,180 Peak-dose dyskinesia is typically manifested by stereotypic movements of the neck and head (“head bobbing”), swaying (choreiform) movements of the trunk, or ballistic movements of the limbs. Some patients also experience “respiratory dyskinesia.” Diphasic dyskinesia is characterized by a pattern of parkinsonism-dyskinesia-improvement-dyskinesia-parkinsonism. It is typically manifested by the onset of unilateral leg stereotypy followed by other abnormal involuntary movements involving the ipsilateral and then contralateral body within 10 to 15 minutes after ingesting levodopa, lasting about 15 minutes, after which time there is improvement of parkinsonism for several hours, followed by recurrence of transient dyskinesia as the levodopa levels decline.180
Dystonia, such as flexion of the toes or inversion of the foot when walking, can be the presenting symptom of untreated PD, especially common in patients with young-onset PD. Patients with PD, however, also frequently develop levodopa-related dystonia, typically in a form of wearing-off dystonia that usually occurs as nocturnal or early-morning dystonia or during interdose wearing-off periods manifested by toe curling, foot flexion, and inversion and painful muscle spasms.
Although being “on” without dyskinesia is clearly the most desirable state, in many patients the dyskinesia is relatively mild and they prefer being “on” with dyskinesia rather than “off.” Some patients, however, have troublesome and occasionally even disabling dyskinesia. Although dyskinesia usually occurs when patients are “on” and their parkinsonian symptoms are relatively well controlled, some, particularly those with MSA, may experience dyskinesia (especially involving the oro-facial-mandibular area) without clinical relief in their motor symptoms. Levodopa-related paroxysmal dyskinesias are sudden, unpredictable abnormal involuntary movements that occur irrespective of the motor “on” or “off” states, referred to as the “yo-yo” response. This complication tends to occur particularly in young-onset patients. The broad spectrum of nonmotor manifestations of the “off” state, including wearing-off anxiety, depression, and sweating, is being increasingly recognized.
The various motor and nonmotor complications of levodopa therapy are largely related to the abnormal pharmacokinetics and pharmacodynamics of levodopa. Although the mechanism of increasingly shorter half-life of levodopa and the apparently dopamine receptor supersensitivity are not well understood, there has been an emergence of new medical and surgical therapeutic strategies designed to smooth out the motor fluctuations and reduce dyskinesia. These include the development of new formulations of levodopa and novel delivery systems (Table 4).
TABLE 4.
Novel formulations and deliveries of levodopa
|
j. Symptomatic Treatments: The Role of Neurosurgery (A. Lozano)
Along with medical and pharmacological developments in the 19th and 20th centuries, neurosurgery has also made substantial contributions and developed a surprisingly large number of surgical strategies; some with questionable rationale, others better conceived.
After a brief period of surgery in the periphery with dorsal root and sympathetic nervous system surgery in the early part of the 20th century, the first serious forays into surgical treatments were directed at alleviating tremor by the interruption of transmission along the corticospinal tracts. Neurosurgeons tackled all stations from the cortex to the cerebral peduncles and pyramids with varying degrees of success and unwanted effects. Although these lesions alleviated tremor, the accompanying motor weakness, although disabling, was apparently preferable.
A breakthrough in surgery came in the 1940s when Russel Meyers resected the head of the caudate and showed that it was possible to intervene in the basal ganglia to alleviate parkinsonian symptoms.181 This lay the foundation for the notion that the so-called extrapy-ramidal system could be reached and manipulated for clinical benefit without the weakness that accompanied lesioning the pyramidal system. During the course of time, various basal ganglia targets were examined, sometimes deliberately with a solid neuroanatomical rationale but often unintentionally or through surgical misadventure. One of Irving Cooper’s serendipitous observations occurred when he was forced to ligate the anterior choroidal artery to control bleeding during a pedunculotomy for PD. Surgery was aborted and the patient awoke with no deficits and with clear clinical benefit on the basis of the resulting ischemic lesion of the subthalamic nucleus, the globus pallidus, and their projections.182 This led to a flurry of arterial ligation surgeries, but the variability and unpredictability of outcomes and the not infrequently accompanying hemiplegia and hemianopia made this procedure fall into disfavor and contributed to redirecting attention on the pallidum and its outflow tracts as favorite surgical targets. Further serendipitous findings of excellent results associated the discovery that misplaced intended pallidal lesions that in reality, at autopsy, were instead found in the motor thalamus183 led to a shift toward thalamotomy as the preferred surgical treatment of PD.
The surgical ablation of basal ganglia targets, predominantly the pallidum and later the thalamus, was at its peak in the 1950s and 1960s and continued at a good pace until the introduction of levodopa and the realization of its striking benefits. We then entered a period of near extinction of surgical procedures until the 1990s when 2 factors led to the reexamination of surgery: first, improvements in stereotactic techniques with the advent of CT scans and MRIs, which greatly increased surgical accuracy, and second, the realization of the limitations of pharmacological treatments and the appearance of drug-induced dyskinesias with disease progression and the chronic use of levodopa.
In 1992, Laitinen and colleagues184 published a seminal paper on the reexamination of pallidotomy in the treatment of PD, which he had learned from Leksell 3 decades earlier.185 The rediscovery and reintroduction of pallidotomy rekindled interest in surgery for PD fostered by a few studies in the MPTP monkey model (ie, Bergman, Wichman, and DeLong in Baltimore, United States; Aziz and Crossman in Manchester, United Kingdom; Guridi and Obeso, Spain; and Benazzouzz and Gross, France), showing that lesion or high-frequency stimulation of the STN could reverse parkinsonism.186 This coincided with the development and application of DBS in the Vim of the thalamus to treat tremor, which allowed Grenoble’s group in France to pioneer the STN for DBS in the early 1990s.187
DBS had been used since the 1960s for a number of psychiatric and pain disorders.188 It had been used sporadically to treat movement disorders in the 1970s and 1980s, but it was the introduction of STN DBS by Benabid and Pollak and their team that caused a major impact, only comparable to the one seen when levodopa was started for PD in the 1960s. DBS for movement disorders targeting the STN, GPi, and Vim has now been performed in more than 150,000 patients with a rate of accrual of more than 10,000 per year. DBS surgery for Parkinson’s is practiced across more than 700 centers in the world. Despite its widespread use, the mechanism of action of how DBS works is still incompletely understood189 and hotly debated.
Recent developments in the field of DBS surgery include high-field MRI for target identification, intraoperative MR localization to help electrode placement, and the performance of DBS procedures under general anesthesia. Emerging advances in DBS include the introduction of directional leads to be able to sculpt the volume of tissue activated at stimulating sites and the initial experience with chronic continuous recording and closed-loop stimulation for PD. There is also a reemergence of the use of ultrasound in movement disorders. Meyers and colleagues190 applied ultrasound through a craniotomy to make pallidal outflow lesions decades ago. Today, multiarray focused ultrasound is used with MRI guidance without the need to open the skull, the procedure (targeting the Vim) has been shown safe and efficacious in open and blind trials 191,192 and approved to treat essential tremor in the United States and Europe. Conceivably, this approach will soon be extended to treat tremor in PD and beyond by moving to target classic antiparkinsonian nuclei.193
There are a number of additional surgical therapies at various stages of investigation, including cellular transplants that are being reexamined with not only embryonic cells but also engineered cells, including various stem cells, various forms of gene therapy involving both modulation of dopamine processing and metabolism and neurotrophic factors, and also the infusion of various neurotrophic proteins. These strategies have had some false starts but are being revisited in many centers, hopefully with the benefits of having learned from previous therapies.
Despite 100 years of surgical therapies, there are still no means of slowing down or stopping PD—perhaps the greatest remaining challenge. Other challenges include making surgical procedures simpler and available to the many patients who could benefit but because of socioeconomic factors are unable to access this life-changing therapy.
III. The Future: Unresolved Challenges and Developments
a. The Origin and Mechanisms of Shaking (G. Deuschl and H. Bergman)
This topic deals with a core aspect of the whole article and celebration since the “shaking” was 1 of the 2 fundamental observations made by James Parkinson and has remained distinctive of the disorder. Incredibly enough, 200 years later the exact mechanisms underlying tremor in PD and how this differs from those mediating the “palsy” (ie, akinesia) and rigidity are not at all well understood
i. Clinical Features and Variants of Tremor in PD
The tremor is often one of the first manifestations as J. Parkinson himself outlined in the following: “The first symptoms perceived are, a slight sense of weakness, with a proneness to trembling in some particular part; sometimes in the head, but most commonly in one of the hands and arms.” (p. 2)9 Today we know that there are different variants of tremor in PD as suggested by clinical observation and quantitative measurements.194 The most typical and common one is the classic parkinsonian tremor at rest and in the focus of this account as it relates most likely to the basal ganglia. The other types are clinically characterized by a postural and action tremor, which is mainly associated with tremor at rest, but the action tremor has often a higher frequency and may be disabling for daily activities. A further high-frequency action tremor is clinically associated with rigidity.194
Classic PD tremor is characterized as a tremor at rest, which is usually suppressed in amplitude or completely stopped during movement onset. It can reoccur when the arms are held outstretched, and in severe cases the suppression during movement is only mild leading to a severe handicap also during action.
Parkinsonian tremor is the most specific sign of PD and occurs in probably more than 90% of the patients at some time during the disease. However, it shows no correlation with the progression of rigidity and akinesia scores. PD tremor is not as reliably responsive to dopamine replacement therapy as other typical motor signs. Although in many cases there is a good and reproducible response to levodopa, there is also a significant percentage of patients who do not respond well enough. The solution of this apparent paradox is most likely that tremor suppression is, on the one hand, a threshold phenomenon with respect to the dose of levodopa required. On the other hand, patients treated with dopaminergic drugs or who have received DBS may not immediately respond but need some days or weeks to perfectly respond, which may be a feature of adaptation of the tremor circuit. The latter, however, may be in part related to electrode location because tremor may also be stopped within less than a second within the operating room.
ii. Anatomical Basis and Degeneration Pattern of Tremor
The patter of nigro-striatal degeneration has been suggested to differ for tremor-dominant versus akinesia-dominant patients with PD. Tremor severity is generally not related with putaminal dopamine depletion measured with Fluorodopa-PET195 or with SPECT DaTSCAN, nor to the reduction in the number of SN cells.196 However, a relation with serotonergic transmission has been suggested. Previous PET studies have already revealed reduction of raphe serotoninergic receptors8,195 related to parkinsonian tremor. This has now been confirmed with (123)I-FP-CIT SPECT in a large group of de novo patients.197 Interestingly, the raphe serotonin transporter availability was inversely correlated with rest tremor severity, and tremulous PD had less raphe serotonin transporter density but less severe striatal dopaminergic deficits than PD patients without tremor. Based on these findings, a specific combination of degenerative changes of the dopaminergic cells and serotoninergic cells is probably needed for the expression of PD tremor.
The anatomy of PD tremor is only poorly understood. One of the main claims of earlier papers was that the cells of the Area 8 close to the SN and caudal to the red nucleus are preferentially degenerating.198,199 This is in keeping with the suggested differential pattern of degeneration between tremor-dominant patients and akinesia-dominant patients. One study200 has shown DaTSCAN patterns of striatal deficit suggestive of a predominant loss in the lateral SN and the retrorubral field (coinciding with Area 8).200 Another study found a similar abnormality with higher pallidal dopamine depletion in PD patients with tremor than in PD without tremor, which was also interpreted to reflect a more severe degeneration of Area 8.99 However, this finding was not reproduced in another study.201 Accordingly, the pathological basis of parkinsonian tremor remains undefined and a putative cell loss pattern of mesencephalic subregions has not been convincingly demonstrated.
iii. Network Patterns of Tremor
In the past, theories of tremor genesis were focused on the idea that there are specific cell groups that have the tendency to oscillate and a mechanism that is related to the neurodegeneration synchronizes their oscillations.202 However, it cannot be excluded that the oscillations are generated within brain networks as a result of changes in the effective connectivity between the network elements. 203 There is much evidence that there is a network engaging tremor of any type, consisting of the cerebellum, the thalamus, and the prefrontal cortex/primary motor cortex. This has been shown with functional techniques such as high-density electro-encephalography203 and magneto-encephalography, which were used to measure the cortical coherence with the trembling muscle. Similarly, metabolic coherence patterns with 18F-deoxyglucose-PET showed a similar network.204
Further activation studies using tremor intensity as a measure to correlate the blood-oxygen-level dependent effect on MRI have shown that tremor onset is best correlated with a network within the basal ganglia (switching on tremor), whereas the tremor severity (dimmer of tremor) is related to the cerebello-cortical network.99 This has been labeled as the switch/dimmer hypothesis of parkinsonian tremor, and it nicely demonstrates that new technologies can show entirely new aspects of tremor. The development of such oscillating loops and the mechanisms by which they are starting to oscillate need to be studied in more detail.
In sum, the available studies so far are suggestive but not conclusive regarding a specific regional pattern of degeneration underlying parkinsonian tremor with a more severe degeneration of specific parts of the midbrain dopaminergic neurons. In addition, the amount of degeneration of additional cell groups may contribute to the specific phenotype of tremor-dominant PD. The convincing evidence to date is in favor of a stronger degeneration of serotoninergic raphe cells. How these anatomical and transmitter changes lead to oscillations is so far unknown.
iv. Tremor Generating Oscillators for Parkinsonian Tremor
Despite all of the important observations on the networks active in tremor disorders, we do not know the mechanisms by which these structures/networks oscillate. Several hypotheses have been put forward.205,206
The first suggestion is the thalamic oscillator hypothesis assuming that thalamic cells oscillate and drive PD tremor, probably through the motor cortex. Thalamic cells are known to possess the property to oscillate based on their intrinsic conductance properties207 (low threshold calcium spikes).206,207 It was assumed that these spontaneous oscillations are synchronized between large cell groups by so far unknown mechanisms of degeneration. As the low threshold calcium mechanism could not be confirmed during single-unit recordings in parkinsonian patients,208 this hypothesis is no longer strongly supported. The modern understanding of the complex architecture of the basal ganglia-thalamo-cortical closed-loop networks further suggests a network rather than a single cell oscillator as the main mechanism underlying parkinsonian tremor.
The second proposal is the basal ganglia pacemaker hypothesis models, which share the common feature that mechanisms within the basal ganglia lead to the synchronization of cells and circuits. The finding that in vitro a preparation of globus-pallidum externum and the subthalamic nucleus are able to oscillate209 stimulated the idea that micro-circuits within the basal ganglia can produce such oscillations. The mechanism by which this loop starts to oscillate following striatal dopamine depletion is unknown, and so far this model has not been further supported by new data.
The loss-of-segregation hypothesis is based on recordings in monkeys with MPTP parkinsonism. Although neurons within the basal ganglia loops are usually segregated, 210 they lose their separation in the parkinsonian state,200 and while the different channels are getting connected, they synchronize and ultimately lead to a rhythmic output of the final common path. This model can explain many of the clinical observations such as the fact that the muscles are usually correlated in 1 extremity, rarely between the arm and leg of 1 side and never between the different sides.
v. The Future and Tremor in PD
Although the first 200 years following the description of the “shaking palsy” by James Parkinson have resulted in major advances of our understanding of PD tremor, there is still a lot to be achieved. First, improved functional imaging and physiological methods should help us to better clarify the cellular/network mechanisms of PD tremor. Hopefully, such better understanding of tremor pathophysiology would enable better methods of treatment. We hope that in the near future, we will be able to offer our patients personalized medicine with optimal adjustment of dopaminergic/serotonergic manipulation.
b. Parkinson Disease: One or Many Diseases? (E. Tolosa, M. Rodriguez-Volante, and S. Fahn)
i. Parkinson Disease: A Clinical Concept
Patients with PD commonly present with the classical triad of rest tremor, rigidity, and bradykinesia, and over time with other motor signs such as flexed posture, freezing of gait, and postural instability. Although not necessary for the diagnosis, nonmotor symptoms of autonomic dysfunction as well as changes in olfaction, sleep, mood, behavior, and cognition frequently accompany the classic motor manifestations.
The great majority of patients clinically diagnosed as PD have intraneuronal inclusions (Lewy bodies and Lewy neurites) in the brain stem on neuropathogical examination, and many clinicians currently support the use of the term Parkinson’s disease to refer to a specific condition characterized by slowly progressive parkinsonism associated with brain stem Lewy-body pathology in the absence of signs suggestive of another disease.
The presence of Lewy-body pathology, therefore, would be a necessary requirement for the diagnosis of PD, but Lewy bodies can occur in the central nervous system in conditions other than PD. The role that these intraneuronal synuclein aggregates play in causing neurodegeneration is inconclusive (see the “Pathological Basis” section earlier in this article and the later “Neurodegeneration in PD” section211). As mentioned previously (see the “Clinical Heterogeneity and Differential Diagnosis” section), the clinical diagnosis of Lewy-body PD might be difficult in some circumstances, 212 and the diagnostic certainty is not optimal.211 It is now well recognized and accepted that diagnostic errors for PD occur in a high proportion of cases, although this is highly influenced by the degree of expertise as is the case in any area of clinical medicine. As more reliable biomarkers become established, the problems with diagnosis and clinical heterogeneity of PD will attenuate as has occurred with many other neurological disorders (ie, spino-cerebellar ataxias, inherited neuropathies, etc.). An important current research goal is to advance recognizing and separating clinical subtypes at the earliest stages of the disease, with future expectations that therapies will become more discriminative and closely related to the underlying mechanism.
ii. Lewy-Body PD
Is Lewy-body PD 1 disease or many diseases? The clinical and pathological features of Lewy-body PD can occur in different disorders, that is, Lewy bodies are not pathognomonic of PD, and Lewy bodies can even occur in asymptomatic subjects (so called incidental Lewy-body disease). Other reasons frequently advanced to suggest that PD is not a single distinct entity are variability in clinical manifestations (clinical heterogeneity) and multiple genetic etiologies.
A number of clinical subtypes have been defined on the bases of prospective studies and on data-driven methods using various forms of cluster analyses. Examples of such subtypes include tremor-predominant PD, postural instability gait disorder (postural instability and gait disorder) PD, and young-onset PD.26 A PD patient from a given subtype, for example, a patient with predominantly tremor, not only presents differently but behaves differently in terms of the clinical course and even response to therapy and could have a different cause than those who, for example, never develop tremor. Are these subtypes biologically distinct subtypes of PD, with different genetic and environmental etiologies and with distinct pathologies? Will they require different disease-modifying and symptomatic treatments? The evidence that such phenotypes represent different biological entities, though, is minimal.26 No evidence yet supports this contention. We do not know what underlies clinical heterogeneity in PD, a phenomenon also common in other neurodegenerative disorders (eg, Huntington disease). Moreover, PD associated with a known genetic cause is clinically just as heterogeneous as is sporadic PD. Tremor-dominant, akinetic, or young-onset cases, for example, are all associated with autosomal-dominant LRRK2. SNCA mutations can cause in 1 single family cases of PD with variable degrees of cognitive deterioration at onset.213
Studies on the etiology of Lewy-body PD suggest that the disorder could actually have many causes and, thus, by definition, represent more than 1 disease. The cause of Lewy-body PD is unknown in more than 95% of cases. In these cases, the great majority are sporadic (ie, nonfamilial), and several etiologies have been considered. Of course, there are many other causes of dopaminergic cell damage but most of these do not really mimic PD as defined here. Viral and toxic etiologies have been particularly pursued because the pandemics of encephalitis lethargic caused progressive parkinsonism in the 1920s,214 the MPTP story in California discussed previously, and neuroleptic exposure 215 has been considered as another potential cause. These cases can sometimes resemble classical PD in the clinic, but in none of these environment-triggered cases have Lewy bodies been observed in the brain.
Although the cause of sporadic cases remains a mystery, advances in molecular biology have clearly shown that Lewy-body PD can have a genetic cause. Mutations in genes located in different chromosomes and encoding different proteins have now been identified as causing the typical clinical picture of PD, responsive to levodopa, and associated with nigral cell loss and have shown abundant intraneuronal alpha-synuclein aggregates in the brain stem.216 Of all PD, 5% is estimated to be caused by such genetic mutations (Table 2). These pathogenic mutations cause (i) a clinical picture generally indistinguishable from sporadic PD and (ii) nigral cell loss frequently associated with Lewy-type pathology in the central nervous system.
Should these various genetic types of PD associated with different causal mutation be considered different disorders? Is LRRK2-associated PD the same disease as SNCA- or parkin-associated PD? It remains possible that in these various forms of parkinsonism, the final common pathway leading to cell death is similar, related to deposition of abnormal synuclein intraneuronally or via some other mechanism.217 If this were the case, the final pathogenesis would be the same for the different genetic parkinsonisms, an argument for PD as “1 disease.” All of the evidence, though, indicates that these different mutations cause cell death through different pathogenic mechanisms. In addition, some of these genetic cases can have a pathological substrate different from Lewy-body disease, strongly suggesting that synuclein-related cell death is not occurring in such cases. A striking example of this is LRRK2-associated PD that can present with tau pathology, as in PSP, or with nonspecific nigral degeneration akin to autosomal recessive parkin PD, without identifiable protein aggregates in the central nervous system.
James Parkinson wrote in his Essay on The Shaking Palsy that
The disease, respecting which the present inquiry is made, is of a nature highly afflictive. Notwithstanding which, it has not yet obtained a place in the classification of nosologists; some have regarded its characteristic symptoms as distinct and different diseases, and others have given its name to diseases differing essentially from it; whilst the unhappy sufferer has considered it as an evil, from the domination of which he had no prospect of escape (p. 1).
Now, 200 years later, we are still lacking proper and irrefutable evidence to define PD as a single disease or several diseases.
Whether PD is 1 or many diseases will in many ways depend on semantics and definitions about terms as elusive and controversial as disease, morbid entity, or disorder. If PD is defined as a clinical syndrome associated with a distinctive pathology (and most experts would agree on this gold standard definition), then the answer about 1 or many disease is not straightforward. The different etiologies being identified for Lewy-body PD result in a uniform clinical picture including response to medical and surgical therapy that supports the idea that Lewy-body PD is a “single, distinct, nosological entity with many causes” (p. 218). Advances in molecular biology and in the characterization of the pathophysiology of the different genetic subtypes of PD may lead us to consider that PD is not 1 but many diseases, each in need for perhaps distinct, different therapies.
c. Preclinical Markers and an Approach to Early Diagnosis (R. Postuma, D. Berg, and K. Marek)
By definition, neurodegenerative disorders develop over time. This implies that there is a stage of disease during which neurodegeneration has commenced, but full clinical manifestations have not developed. These early stages provide critical windows of opportunity; neuroprotective therapy that if applied early enough, could impact substantially on disease evolution and may even prevent clinical PD from occurring. This has led to a broad concerted effort to define the earliest stages of PD.
A few years ago, a task force of the Movement Disorders Society, building on previous systems,218,219 divided early PD into the following 3 stages17:
Preclinical: neurodegeneration is present but without measurable symptoms or signs—this would require biomarker diagnosis. By definition, patients are not just in an at-risk state (eg, young persons with highly penetrant gene mutations); some neurodegeneration is required to have preclinical PD.
Prodromal: symptoms/signs are present, but they are insufficient to diagnose clinical PD.
Clinical: this implies the presence of parkinsonism (bradykinesia with fatiguing/decrement plus 1 of rest tremor or rigidity).
i. Why Do Preclinical and Prodromal PD Exist?
The pathogenesis and possible spread of synuclein are summarized elsewhere in this paper. The central hypothesis of a slowly progressive/spreading neurodegenerative process allows the existence of prodromal and preclinical PD. There are 2 key features of PD pathology’s topography that allow a diagnosis of early PD.
1. Redundancy
In all neurological systems, but perhaps especially in the basal ganglia, there is considerable redundancy. This implies that substantial neurodegeneration needs to take place before symptoms and signs are detectible. Moreover, once motor signs develop, it may take several years before they are severe enough to diagnose clinical PD. It has been suggested that by the time a person is diagnosed with PD, 35% to 70% of SNpc neurons have died, with additional neurons that are hypofunctional.220 Dopaminergic functional neuroimaging suggests that denervation ranges from 35% to 70% at diagnosis. Estimates of the duration of the clinically measurable motor prodromal are scant, but studies suggest an average duration ranging from 4 to 10 years on motor examination, with similar duration estimates using conventional dopaminergic imaging.
2. Nonmotor PD
Whereas redundancy is common in many neurodegenerative disorders, a unique advantage of PD is that it has a nonmotor prodrome.221 Arguably one of the most important new insights during the past 20 years is that PD can start outside of the SNpc. Staging systems for PD have been proposed74,222; all share the recognition that olfactory and lower brain stem structures often are affected first. Moreover, peripheral nervous structures such as Meissner’s plexus in the gut and cardiac autonomic fibers can develop early degeneration; it remains controversial whether this peripheral neurodegeneration can occur before CNS degeneration.
There is now a rapidly growing list of proven biomarkers and clinical markers of prodromal PD. These are summarized in Table 5.
TABLE 5.
Markers of prodromal PD
| Marker | Level of evidence | Approximate relative risk | Lead time | Testing cost/burden |
|---|---|---|---|---|
| Olfaction | High | 5 | ?? | Low/Moderate |
| REM Sleep Behavior Disorder | High | 50 | 13 years | Low (screens) to High (PSG) |
| Somnolence | Moderate | 1.8 | ?? | Low |
| Restless legs (late onset) | Low | 1.5 | Short | Low |
| Constipation | High | 2.5 | > 15 years | Low |
| Orthostatic hypotension | Moderate | ? 2–10? | 2–5 years? | Low |
| Urinary dysfunction | Low-Moderate | 2.1 | ?? >5 years | Low |
| Erectile dysfunction | Low-Moderate | 1.2 mild 3.8 severe |
5–10 years | Low |
| Depression/anxiety | High | 1.8 | Uncertain ?Biphasic | Low, but follow-up higher |
| Color vision | Low | 2.5 | >3 years? | Moderate |
| Subtle parkinsonism | Moderate | 10 | 4–5 years | Moderate - High (Expert) |
| Quantitative motor testing | Moderate | 3–4 | 5 years | Moderate |
| SN ultrasound | Moderate | 15 | Uncertain ? risk marker? | Moderate-High |
| Dopaminergic PET/SPECT | Low (but high plausibility) | 20 | 5 years | High |
| PD-related pattern on SPECT/PET | Low | ? | ? | High |
| Hippocampal hyperperfusion | Low | ? | ? | High |
| GI synuclein pathology | Low | 2? | ? | High |
For this table, only markers with prospective evidence of predictive value are included. For level of evidence, low implies a single study, moderate implies >1 high-quality study, high implies >4 high-quality studies. Lead time refers to the approximate time that the marker deviates from normal values (the time at which testing is reliably abnormal cannot be estimated for most markers). For testing cost, low indicates can be screened by questionnaire (does not require visit), moderate implies in-person assessment required but low cost (eg, research assistant), high implies extensive or expensive evaluation (>$300).
ii. Clinical Markers
Most markers of prodromal PD directly reflect symptoms and signs; some can be detected clinically, whereas others require biomarker confirmation (eg, polysomnography). In general, clinical markers are the most-established means to diagnose prodromal PD.
1. Motor Changes
Although there is little question that subtle motor findings should be measurable before clinical PD, studies defining the actual predictive value are limited. Two studies have assessed UPDRS examination in non-PD patients. In a general population study, Berg and colleagues223 found a score-dependent increase in PD risk with UPDRS, ranging from relative risk (RR) = 5.7 for any score above 0 to 16.5 for scores ≥4. In a cohort of patients with idiopathic RBD, elevated UPDRS scores clearly increased parkinsonism risk in a score-dependent manner. A cutoff of >3 points excluding action tremor could predict parkinsonism 2 years later with 94% sensitivity and 97% specificity. There are ongoing studies looking at quantitative measures of prediction. Again in idiopathic RBD, Purdue Pegboard and the alternate tap test could predict PD with 70% to 80% sensitivity and 75% to 82% specificity even 3 years before PD diagnosis. There is considerable promise for the future with potential markers such as automated gait and balance analysis224 and smartphone-based applications to noninvasively monitor mobility and motor speed on a large scale.225
2. Nonmotor Changes
RBD
RBD is a parasomnia that is characterized by the loss of the normal atonia of REM sleep, allowing the apparent enactment of dreams during REM sleep. Screening questionnaires can detect RBD with specificities as high as 90%,226 but prevalence is low (1%–1.5%), meaning that positive predictive value of screening procedures will be low. So polysomnography is required for a definitive diagnosis. RBD is unique among all markers for having by far the highest specificity/predictive value. In both single-center and multicenter studies, the large majority of patients with idiopathic RBD will develop a neurodegenerative synucleinopathy, including PD, dementia with Lewy bodies, and MSA.227–229 Moreover, the majority of idiopathic RBD patients meet criteria for prodromal PD.50 The pronounced risk and prolonged latency makes idiopathic RBD patients a compelling target population for neuroprotective trials and eventual therapy. The lead time for RBD is unusually long: in the largest studies, the median interval between RBD symptom onset and defined neurodegenerative disease is 13 years. It should be noted that only about one third of early PD patients have RBD, and it is likely that many have a subtype of disease characterized particularly by dementia and worse autonomic function.29
Olfaction
There have now been at least 7 prospective studies demonstrating that reduced olfactory ability can predict PD. In general, olfaction has better predictive value than most other nonmotor markers, with relative risks in published studies ranging from 3.5 to 7 (5.2 and 3.9 in 2 population-based studies). 222 It may also have high sensitivity, because even in early PD, >80% of patients have some measurable hyposmia. Even with relative risks around 5, it should be noted that the majority of those with hyposmia will never develop PD. For example, in the Honolulu Asia Aging study, 549 persons in the lowest quartile all had severe hyposmia, but only 10 (2%) developed PD 4 years later.230 It is unclear when olfactory loss occurs in prodromal stages; some studies show no predictive value of olfactory loss over longer than 2 to 4 years,231,232 although others document measurable abnormalities up to 8 years before clinical onset.232
Autonomic dysfunction
There are now at least 6 prospective studies documenting that constipation increases the risk of future PD.50 These all find similar RR, of approximately 2. This is similar to many other nonmotor markers (below); having a RR this low means that a large majority of the affected will never develop PD. A total of 2 studies have found that orthostatic hypotension can predict PD. One followed patients referred to a specialty autonomic center and found that more than 20% eventually developed PD (with additional patients developing dementia with Lewy bodies and MSA).63,232 A second from a primary care database found that those presenting with orthostatic hypotension had an increased risk of developing PD up to 10 years later. Studies have suggested that reduced heart-rate variability (a marker of cardiac autonomic denervation) may increase risk of PD (with relative risk approximating 2–3).66,232 Symptoms of bladder dysfunction were associated with an approximately doubled risk of PD in primary care studies. Finally, erectile dysfunction was associated increased risk; 1 study found a 3.8-fold increase among those with severe erectile dysfunction66,232 (only 3% of the population had this severe dysfunction), whereas a second, which included all grades of erectile dysfunction, found a much lower RR of 1.2.64,232
Mood dysfunction
Depression and anxiety are commonly comorbid in PD. Many studies document that both mood disorders are associated with higher PD risk, but the relative risk is low, ranging from 1.5 to 2.5, with resulting low specificity.50 The lead time is unclear; it may be biphasic with an early tendency toward anxiety (eg, the putative Parkinson personality) combined with a second depression emerging soon before PD onset.53,233
Somnolence and other sleep disorders
Other sleep disorders may predict PD, but with less evidence and lower predictive value. Two population-based studies found that excessive napping or somnolence doubled the risk of PD.230,234 Lead time is uncertain, but it is easily screened.
3. Blood and CSF Biomarkers
In striking contrast to clinical markers, evidence for blood/CSF variables as prodromal markers is extremely limited, although this may change in the near future for specific types of PD.235
4. Neuroimaging–Dopaminergic Imaging
Dopaminergic PET/SPECT
Dopaminergic PET/SPECT shows a 35% to 65% loss of innervation at diagnosis, implying that milder loss should be evident earlier. So far, however, there has been no published prospective study demonstrating predictive value. Several studies document abnormalities in about 40% of patients with idiopathic RBD, which appear to progress over time. Moreover, in the Parkinson At-Risk Study, patients with dopaminergic denervation had more hyposmia and constipation.236 Follow-up, published in abstract form only, has found that those who had innervation below 65% of expected values had a 20-fold increased risk of developing PD.237 If confirmed, this would imply that dopamine transporter scanning is second only to idiopathic RBD in positive predictive value for PD.
SN Ultrasound
The SN in PD has an increased echogenicity, which can be assessed with ultrasound. In a population-based study, increased echogenic signal was associated with a 20-fold increased risk of PD (although RBD studies have been more uncertain).223 Hyperechogenicity is present early in PD and in general more pronounced contralaterally to the clinically more affected side. It is also found in normal young people, suggesting long latency and the potential to be a risk marker.
5. Tissue Diagnosis of Early-Stage PD
The ability to diagnose PD by documenting tissue synuclein deposition during life would be a major advance. Studies have assessed skin and gastrointestinal biopsy with varying results. Concerns about the correct antibodies, preparation protocols, and interpretation of nonspecific staining are not fully resolved. It is clear that many normal controls have some synuclein deposition in peripheral tissues. Although it is theoretically possible that all of these have some form of early PD, it also suggests poor specificity. A recent Danish study examined gastrointestinal biopsy samples from a national database in patients who eventually developed PD.238 They documented increased phosphorylated synuclein staining in those destined to develop PD; 45% of prodromal PD samples were positive (median interval = 7 years before PD diagnosis), compared to 26% of controls and 48% those who already had clinical PD. A recent study in idiopathic RBD found synuclein deposition in 8/9 submandibular gland samples (compared to none in controls).239 If specificity issues can be resolved, there is considerable future promise for tissue biopsy in early detection of PD.
6. Combining Predictive Markers: The Movement Disorders Society Prodromal Criteria
As is now clear, there are a wide variety of proven markers of prodromal PD. Each has vastly different predictive abilities with different lead times. How can they be combined to estimate the chance that a given individual has prodromal PD? In 2015, a task force of the International Parkinson Disease and Movement Disorders Society published diagnostic criteria for prodromal PD.50 This uses a Bayesian naive classifier to estimate risk in individuals. The baseline likelihood of prodromal PD is estimated based on age. Then sensitivities and specificities of risk markers (eg, genetics, lifestyle risk factors) and diagnostic variables are converted to likelihood ratios; positive diagnostic tests increase disease probability (ie, LR+), and negative tests decrease probability (LR-). These likelihood ratios are multiplied by each other and then added to a baseline risk to provide an individual’s probability of having prodromal PD. This approach is unique among diagnostic criteria for neurologic disease and, most critically, can be continuously adapted as new diagnostic information becomes available.
In sum, the field of prodromal PD is rapidly expanding, with new diagnostic markers discovered each year. It is now possible to define with reasonable certainty the probability that a specific person has prodromal PD. Once neuroprotective therapy has been developed, systematic screening for prodromal PD and resultant prompt treatment could even prevent clinical PD from ever becoming clinically relevant.
d. Neurodegeneration in PD (D.G. Standaert and D.J. Surmeier)
The cardinal motor features of PD are attributable to the degeneration of pigmented dopaminergic neurons in the SNc and downstream loss of dopaminergic inputs to the striatum. However, although the degeneration of SNc dopaminergic neurons is central to the motor symptomatology of PD, this is not the only site of pathology in the disease. In the early part of the 20th century, Lewy discovered that there were abnormal intracellular inclusions in neurons at many places in the brains of PD patients. The discovery that mutations or overexpression of alpha-synuclein was a cause of PD240,241 and that alpha-synuclein was a primary component of Lewy bodies242 opened the door to a much wider exploration of PD pathology. Using alpha-synuclein immunocytochemisty, Braak and colleagues identified a broad range of PD-associated LP in human brains.17 Since the Braak hypothesis was advanced nearly 2 decades ago, there has been a concerted effort to connect LP, neuronal dysfunction, and neuronal death in PD. Efforts to establish a correlation between the severity of LP, clinical features, and cell death in postmortem PD brains have so far been unsuccessful. Although neuronal loss is correlated with LP in some regions, in others it is not.243 Moreover, the temporal sequencing of LP posited by Braak and colleagues has been difficult to confirm because only about half of clinically diagnosed PD patients have a pattern of LP that conforms to the model.244 A major limitation in this effort is the inability to monitor LP in living patients, particularly in the early stages of the disease.
i. Beyond LP
What other factors contribute to neurodegeneration in PD? Two leading themes are the following: (1) proteasomal and lysosomal dysfunction leading to abnormal protein handling and aggregation and (2) mitochondrial impairment.
Misfolded or damaged proteins and dysfunctional organelles need to be degraded or released for neurons to remain functional and healthy. This job is handled by the proteasome and by autophagy. Genetic mutations affecting both have been implicated in PD.245,246 Moreover, it is easy to connect this type of proteostatic dysfunction and LP, at least in principle. Indeed, lysosomal dysfunction might promote alpha-synuclein accumulation, which in turn might worsen lysosomal dysfunction.
Mitochondria and oxidative phosphorylation are essential for neuronal survival. Because of the bioenergetic demands associated with regenerative activity, neuronal mitochondria appear to be operating near their capacity much of the time, leaving little room for error or dysfunction. Several observations suggest that mitochondrial impairment through exposure to environmental toxins, genetic mutations, or age push neurons over a bioenergetic cliff that leads to death. First, Langston and colleagues78 discovered cases of parkinsonism in young people that were induced by MPTP.78 MPTP is converted by monoamine oxidases to MPP+, a potent mitochondrial toxin. Rotenone, another environmental toxin associated with PD, also is a mitochondrial poison.247,248 Second, in PD brains, the activity of Complex I (a key part of the mitochondrial electron transport chain) is diminished in the SN, suggesting mitochondrial damage.247 Third, in PD brains there is an accumulation of mitochondrial DNA deletions in the SNc.249 Fourth, several of the genes linked to familial forms of PD control mitochondrial function either by regulating oxidant defenses, mitophagy, or biogenesis.250 Together, these observations support the thesis that impaired mitochondrial function is a core component of the PD neurodegenerative process.
ii. Selective Neuronal Vulnerability
Although LP, impaired protein turnover, and mitochondrial dysfunction may all play a role in PD pathogenesis, none of these mechanisms explains the most characteristic feature of the disease: selective neuronal vulnerability. Only a small subset of neurons in the brain ever degenerates in PD or shows other signs of pathology. Yet all neurons express alpha-synuclein, rely on mitochondria, and depend on efficient protein turnover.
There are 2 leading theories of what dictates the pattern of pathology in PD. One is that the pattern of pathology is dictated by the spread of a prion-like strain of alpha-synuclein and that this spread is determined by the brain connectome. An alternative theory is that neurons at risk in PD have a preexisting phenotype that renders them vulnerable to the PD triumvirate—alpha-synuclein aggregates, mitochondrial dysfunction, and defective protein turnover.
The prion hypothesis is a derivative of the original work of Braak and colleagues,74 which proposed that PD pathology originated in brain areas that innervate the periphery (the olfactory epithelium and dorsal motor nucleus of the vagus) and spread centrally. This is a compelling idea, but it is important to remember that this is a hypothesis based on study of a large number of brains with differing pathology. Moreover, there is as yet still no direct evidence for spread in a living human.75 Nevertheless, 2 more recent observations have buttressed this view. First, in patients receiving neural transplants for PD, a small percentage (<5%–10%) of the neurons grafted manifest Lewy-like pathology only a decade or so after transplant. 7,251 Second, inoculation of rodent and primate brain with either fragments of fibrillar alpha-synuclein or proteins derived from human LP can spread and induce neurodegeneration.7,83,84
Although there is no doubt that proteins can spread from one neuron to the next in vitro or in models inoculated with large amounts of fibrillar alpha-synuclein, the question is whether this is the primary factor governing selective vulnerability in PD. There are reasons to doubt this. First, although all PD brains manifest LP in a stereotyped collection of nuclei, there are many deviations from the original staging model of Braak and colleagues, arguing against a stereotyped spread of LP through the brain.252,253 Second, the distribution of LP in PD does not conform to a simple connectome rule.254 Thus, the number or strength of synaptic connections with a seeding site does not dictate the probability of manifesting LP. For example, the most robust (and reciprocal) innervation of the SNc is onto striatal spiny projection neurons, which do not manifest LP in PD.72,88 The appearance of Lewy-like pathology in striatal grafts255 is also at odds with a simple synaptic spreading model because there is no obvious seeding LP in the striatum (where the axons of grafted neurons are) of late-stage PD patients, and graft pathology is always limited to a small percentage of dopaminergic neurons, leaving neighboring grafted GABAergic neurons unaffected.
An alternative hypothesis is that the pathology in PD can be explained by intrinsic phenotypes of vulnerable neurons. The following 3 uncommon traits are shared by the small collection of neurons at risk in PD: long, highly branched axons with many alpha-synuclein enriched vesicular release sites; autonomous pacemaking activity that triggers a pronounced elevation in cytosolic Ca2+ concentration; and high basal mitochondrial oxidant stress.87,256 Each of these traits plays to the PD triumvirate. Neurons with a large number of vesicular release sites should have elevated cellular expression of alpha-synuclein, a protein component of vesicles, making pathology more likely. In these neurons, autonomous pacemaking increases intracellular Ca2+, a known precipitant of alpha-synuclein aggregation and fibril formation, acting both directly on alpha-synuclein and by activation of the Ca2+-dependent protease, calpain. Mitochondrially generated reactive oxygen species and reactive nitrogen species also promote alpha-synuclein aggregation and produce mitochondrial DNA deletions, such as those seen in PD patients. The big axonal arbor, elevated cytosolic Ca2+, and sustained mitochondrial oxidant stress should increase the burden on autophagic and proteasomal function by increasing the flux of damaged proteins and organelles (ie, mitochondria), making these neurons more susceptible to aging and mutations compromising the function of key components, such as parkin or beta-glucocerebrosidase (GBA).
SNc dopaminergic neurons also have the added liability of using dopamine as a neurotransmitter.257 For example, the conjunction of dopamine, Ca2+ influx through plasma membrane Cav1 channels, and alpha-synuclein disrupt chaperone mediated autophagy, promoting neurodegeneration.258 The convergence of dopamine and the other 3 traits of at-risk neurons provide a ready explanation for the decided vulnerability of SNc dopaminergic neurons to aging, environmental toxins, and genetic mutations.
Are the 2 theories of selective vulnerability incompatible? No. It could be that there is spread of misfolded alpha-synuclein in the brain, but that most neurons are capable of successfully dealing with this burden and only a subset—defined by their intrinsic vulnerability—succumb to the challenge spreading poses. Having imaging tools to monitor the evolution of LP in the brain of presymptomatic and symptomatic patients would allow this hypothesis to be tested. However, if this is true it has implications for therapeutic strategies in the early stages of the disease. Although spreading could be attenuated with strategies such as immunotherapy, injury to the brain could also be reduced by diminishing intrinsic determinants of vulnerability. The most obvious of those is Ca2+ entry through Cav1 channels. There is growing epidemiological evidence consistent with the notion that the use of Cav1 channel inhibitors is associated with a reduced risk of PD; these findings and preclinical mechanistic studies motivated an ongoing, National Institutes of Health–sponsored phase III disease modification trial in early-stage PD with 1 of these inhibitors (isradipine). Another National Institutes of Health–sponsored phase III trial in early-stage PD is examining the potential neuroprotective value of inosine, which should boost antioxidant defenses.
iii. Inflammation and Neuronal Death in PD
In established PD, there is clear evidence for activation of both the innate and adaptive arms of the immune system. In the brain, the cells primarily responsible for innate immunity are the resident microglia, involved in constant surveillance of the brain microenvironment. Postmortem studies of the PD brain and PD model systems consistently demonstrate striking microglial activation.259 This activation can also drive adaptive immune responses, including T cell infiltration, production of immunoglobins, and modifications of circulating myeloid cell populations, all of which have also been observed in studies of PD patients and animal models of the disease.
The nature of the upstream events responsible for triggering neuroinflammation in PD is still uncertain. Misfolded and oxidized forms of alpha-synuclein are potent inflammogens in many assays, and even neuromelanin, which is presumably released on the death of dopaminergic neurons, can stimulate the activation of brain microglia.260 However, clear proof that any of these are responsible is still lacking. Once the response is triggered, however, the downstream pathways lead to activation of many processes that are likely to cause neuron death, including production of cytokines, activation of phagocytes, and production of complement. Indeed, neuroinflammation may be the final common pathway for cell death arising from a variety of different upstream triggers in PD.
Neuroinflammation may offer an important opportunity to intervene in PD. Unlike processes that lie far upstream, such as protein misfolding, Ca++ flux and mitochondrial stress, neuroinflammation is likely to be active in patients with clinically manifest PD. Recent studies in animal models have demonstrated that antiinflammatory strategies can prevent neuron death even in the presence of strong upstream stressors. This idea is supported by epidemiological observations that suggest that some nonsteroidal antiinflammatory treatments may reduce the incidence of clinically manifest PD.234
In sum, neuronal death is fundamental to PD and its main clinical manifestations. It is highly patterned, being restricted to specific neuronal populations and structures. A patterned distribution of LP is also a fundamental feature of PD, but the relationship between LP and neuronal death is uncertain. Although the proposition that LP is attributable to the prion-like spread of pathological species of alpha-synuclein has gained wide acceptance, this process alone cannot explain the pattern of LP in PD. If there is spread, this spread is likely to be gated by cell autonomous factors that render neurons vulnerable to alpha-synuclein pathology. These cell autonomous factors could also promote neuronal degeneration. The damage induced by these processes could trigger neuroinflammation, which may be a final common pathway of cell death in PD.
iv. PD: A Prion-Like Disorder? (C.W. Olanow)
Much recent attention has focused on the possibility that PD is a prion disorder. Prions are infections agents that do not contain nucleic acids and are comprised solely of misfolded proteins. The key event in prion biology is the conversion of a protein from its native state (eg, PrPC) to a misfolded isoform (eg, PrPSC), which can polymerize to form toxic oligomers/rods/aggregates and amyloid plaques that cause neurodegeneration (Fig. 8A). The infectious process is sustained by the prion conformer reaction in which the misfolded protein acts as a template to promote misfolding of the wild-type protein in a chain reaction–like manner. This neurodegenerative process can spread to unaffected brain regions and can be transferred to independent individuals. The precise molecular basis of the prion conformer reaction and the mechanism responsible for neurodegeneration are presently not well understood. Prion disorders have been described in animals (eg, cattle [mad cow disease], sheep [scrapie], deer, mink) and in humans (eg, Kuru, Cruetzfeld-Jakob disease, Gerstmann-Schenkler-Strauss disease, fatal hyperinsomnia). It has also now been noted that mites, plants, and fungi can be vectors for prions.
FIG. 8.
(A) Schematic illustration of the similarity between the PrP and alpha-synuclein proteins in terms of their potential to misfold to form betarich sheets, and polymerize to form oligomers/rod, and amyloid plaques/Lewy Bodies (adapted from ref. 1). (B) Comparison of Lewy pathology in grafted embryonic dopamine neurons (graft) and in the host substantia nigra (host). Note the similarity in staining for alpha-synuclein, ubiquin, and thioflavin-S (indicative of beta sheet formation). These observations raise the possibility that misfolded alpha-synuclein has spread from affected neurons in the PD brain to unaffected implanted dopamine neurons in a prion-like manner (adapted from ref. 266).
The idea that PD might be a prion disorder is a reasonable consideration because the disease is characterized pathologically by the accumulation of protein aggregates in neuronal cell bodies (Lewy bodies) and processes (Lewy neurites). Following the discovery that alpha-synuclein mutations cause a rare familial form of PD,79 it became apparent that alpha-synuclein is a principal component of LP and that LP is widespread, affecting multiple regions of the central and peripheral nervous system.
It is now appreciated that, as with the prion-protein PrPC, alpha-synuclein exists in an alpha configuration when bound to membrane, but has the potential to misfold to form beta-rich sheets, and potentially toxic oligomers/aggregates that polymerize to form amyloid structures (Lewy bodies; Fig. 8B). Furthermore, pathologic studies by Braak and colleagues suggest that in at least some cases, LP in PD is first seen in the dorsal motor nucleus of the vagus nerve and the olfactory region, with subsequent spread to involve the SNc and the cerebral cortex in a sequential and predictable manner. These findings are consistent with the possibility that alpha-synuclein spreads in a prion-like manner.
The prion hypothesis received additional support from the dramatic finding of LP in healthy embryonic dopamine neurons that had been transplanted into the striatum of PD patients approximately 10 to 14 years earlier251,261 already commented in the previous section. These inclusions are identical to the LP seen in SNc neurons262 and stain with Thioflavin-S indicating beta sheet formation. These observations raise the possibility that misfolded alpha-synuclein in the PD brain has been transferred to the healthy implanted dopamine neurons in a prion-like manner.
There followed a fascinating set of experiments demonstrating that alpha-synuclein can be taken up by previously unaffected neurons in both in vitro and in vivo models. Desplats and colleagues263 showed that alpha-synuclein inclusions develop in fluorescent-labeled embryonic neurons that had been transplanted into the brain of transgenic mice that overexpressed alpha-synuclein. Luk and colleagues264 and Volpicelli-Daley and colleagues265 injected preformed alpha-synuclein fibrils into the striatum of transgenic mice overexpressing alpha-synuclein and demonstrated the formation of alpha-synuclein aggregates in host SNc neurons, neurodegeneration, and behavioral changes, which spread to affect multiple brain regions. These observations have now been reproduced in wild-type mice,83 but importantly, not in alpha-synuclein null mice. This indicates that host alpha-synuclein is essential for the process to occur, consistent with the concept of prion-like templating.
Finally, there is now evidence that alpha-synuclein pathology can be transferred between animals. Innoculates derived from the brains of the elderly and clinically affected alpha-synuclein transgenic mice accelerate mortality when injected into the brains of younger and clinically unaffected transgenic mice.266 Of particular interest are the findings of Recasens and colleagues,84 who injected rodents with extracts derived from Lewy bodies of PD patients and reported widespread LP with nigral degeneration and behavioral change.84 This same group also reported LP and dopamine neuronal degeneration after inoculation into nonhuman primates, but only a small number of animals were studied.84 Collectively, these studies support the concept that alpha-synuclein pathology can spread, although it has not been established if this occurs via a cell-autonomous or nonautonomous process.
Thus, a body of evidence has accumulated suggesting that alpha-synuclein might be a prion and PD a prion disorder.266 It should be noted, however, that some studies have seen transfer of LP in rodents following injection of inoculates derived from Lewy bodies of patients with MSA, but not PD patients. Furthermore, the pathology of PD differs from that of classic prion disorders, and direct evidence of transfer has not yet been demonstrated in human beings. These findings could relate, however, to differences in the metabolism, clearance, and cell vulnerability associated with different prion species, and epidemiologic evidence may be difficult to establish because PD is a common disorder, and there could be a long latency from the time of transmission. If PD is a prion disorder, it is reasonable to consider how and where the process might initiate. Mutant alpha-synuclein is prone to misfold, but mutations are rare and account for only a small number of familial cases. Impairment in the proteasomal and lysosomal clearance mechanisms could cause alpha-synuclein to accumulate and misfold, and indeed alterations in both proteasomal and lysosomal systems have been reported in PD, but it is not clear if these changes are primary or secondary. There has also been interest in the potential of toxic or infectious processes to initiate the prion process. In this regard, it should be appreciated that olfactory nerve filaments are the only nerve terminals that extend to the exterior of the body, and terminals of the dorsal motor nucleus of the vagus innervate the upper gastrointestinal tract where they reside within the mucosa just micrometers from the lumen. These regions are proposed to be the earliest CNS regions affected in PD, raising the possibility of peripheral exposure to a toxic or inflammatory processes with subsequent spread to the CNS. Indeed, alpha-synuclein accumulation in the periphery has been well described,5 with some studies suggesting the presence of LP in the colon prior to the onset of the classic motor features of PD.267 Misfolded proteins could also form stochastically in a random fashion and lead to the accumulation of aggregated proteins and prions in vulnerable individuals. Each of these processes could contribute in varying degrees to the initiation of a prion process and the development of PD in different individuals.
Importantly, the prion hypothesis identifies a series of novel targets and candidate therapeutic interventions that might prevent the formation or facilitate the clearance of misfolded alpha-synuclein and thereby have disease-modifying effects.268,269
e. Neuroprotection and Disease Modification (J. Kordower, P. Paolo, and A.E. Schapira)
i. Drug Trials
Neuroprotection and disease modification are often used interchangeably, although subtle differences between them exist. Neuroprotection literally implies the protection of neurons from death or dysfunction from ongoing pathological processes. Disease modification reflects an intervention that modifies the natural clinical course of an untreated disease. A third term, neurorescue, may be used when an intervention targets the adverse pathogenetic pathways directly and is capable not only of protecting neurons but also restoring their diminished function.270 Medicine as practiced in the time of James Parkinson had no access to cures, although reference was often made to treatments, the effects of which likely relied as much on the benefits of placebo as on any biological therapeutic action. The dawning of the period of scientific, and specifically, medical enlightenment, was responsible for changing our focus to understanding the causes of a disease and the processes that were responsible for it.
More than 170 years were to pass before a clinical trial sought to test the efficacy of a drug to slow PD, based on its ability to intervene in a specific pathogenetic pathway. On the basis that nigrostriatal degeneration was caused in part by the production of free radicals, along with the discovery that MPTP, a protoxin that causes PD in humans and experimental animals requires monoamine oxidase for its conversion to the toxic molecule MPP+, a hypothesis was derived that blockade of monoamine oxidase might slow the progression of PD. Toward this end, a large, multicenter study called DATATOP tested this hypothesis.271 More than 900 early untreated patients were randomized to deprenyl (selegiline), tocophenol (vitamin E), both treatments, or placebo, and the primary endpoint was the time it took for patients to require levodopa. The trial was terminated prematurely when an interim analysis revealed a significant delay in the time to levodopa in the deprenyl and the deprenyl-plustocophrenol groups, suggesting disease modification. However, it was subsequently demonstrated that deprenyl has prodopaminergic effects, and thus it was impossible to separate symptomatic benefit from any disease-modifying effects. Similar findings were found with the SINDEPAR (Sinemet-Deprenyl-Parlodel) study that used the change in motor score between initial visit and final visit after washout of all study medications as the primary endpoint. However, here too there were concerns about confounding symptomatic effects because antiparkinsonian medications have now been shown to have a long duration response that can persist for weeks and perhaps even months after withdrawal. Another monoamine oxidase inhibitor have been tested. The ADAGIO trial showed positive effects at a 1 mg, but not 2 mg, dose and the effect size was quite small.272 Thus the U.S. Food and Drug Administration failed to label this drug as disease modifying.
Based on another pathogenetic pathway considered relevant to the pathogenesis of PD, coenzyme Q10 was used to enhance mitochondrial function. An initial trial demonstrated some disease-modifying benefit, but this treatment failed in phase III clinical trials,273 and similar negative trials employing creatine have also been reported. lnflammation is a major component of PD pathogenesis, and a clinical trial using Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist that reduces proinflammatory cytokines and modulates mitochondrial biogenesis, was performed.70,273 However, this trial failed to meet its primary endpoint as well. Other trials70 used imaging techniques to assess neuroprotection as the primary endpoint as opposed to clinical disease modification, and although initial studies suggested that pramipexole (CALM-PD) and ropinirole (REAL-PD) studies slowed the loss of dopamine imaging markers relative to levodopa, these changes are better interpreted in terms of drug-related dopamine regulation than structural neuroprotection.274 Indeed, a follow-up trial (PROUD-PD) using a primary clinical endpoint failed to establish disease modification with pramipexol.
Ongoing trials have kept the interest of neuroprotection at the forefront of PD research. Recently, Surmeier and colleagues256 demonstrated the prevalence and subsequent toxicity of calcium influx on nigral neurons that could be reversed in animal models by calcium channel blockers. Based on these studies, isradipine is being evaluated in phase 1 and II clinical trials.275 As reviewed by Kalia and colleagues,70 urate possesses antioxidant properties in vitro, and the elevation of urate levels in rodent models can protect SNpc dopaminergic neurons from 6-OHDA toxicity. Epidemiological studies showed that higher serum urate levels are associated with a reduced risk of developing PD.273 Inosine is a urate precursor that, when taken orally, can elevate serum urate, and initial dose-ranging and safety studies have found a dose range than appears successful. Future phase II studies are underway.
ii. Trophic Factors
Dopaminergic trophic factors have been of great interest for many years.276 A variety of trophic factors support the viability of dopaminergic midbrain neurons in vitro and in vivo.275 Of these, none have received as much attention as members of the glial family of ligands, glial derived neurotrophic factor (GDNF) and neurturin.275 GDNF was first shown to support the viability of midbrain dopaminergic neurons in culture. Then in a series of elegant studies, gene delivery of GDNF prevented the structural and functional consequences of nigrostriatal degeneration induced by a number of dopaminergic toxins, including MPTP and 6-OHDA. As this database was building, AMGEN performed a clinical trial testing the safety and efficacy of GDNF following intraventricular delivery in patients with PD. The preclinical data supporting this delivery approach was limited,277 and this trial reported no efficacy or significant side effects.278 A patient who came to autopsy failed to demonstrate any dopamine regeneration, likely a result of poor penetration of the trophic factor from the ventricular space.279 Enthusiasm for this approach then waned until the demonstration that intrastriatal gene delivery of GDNF could augment nigrostriatal function in aged monkeys and prevent the structural and functional consequences of MPTP-induced parkinsonism in nonhuman primates.280 This led to open-label and double-blind trials testing the efficacy of intrastriatal GDNF protein in PD patients.281 Although some open-label trials reported benefit,282 a double-blind trial did not meet its primary endpoint.283 Ceregene Inc. attempted similar studies with AAV-2 neurturin, a sister molecule to GDNF. Again, preclinical studies demonstrated strong neuroprotection in multiple animal models. However, multiple clinical trials, including 2 phase II trials, failed to meet the primary endpoint.284
Uncertainty remains about the potential efficacy of trophic factors in PD.53 None of these studies delivered the trophic factor in an optimal way, and it is likely that the hypothesis that these trophic factors could be successful was never fully tested. Another problem hindering their success is the fact that comprehensive nigrostriatal degeneration of the putamen occurs within about 5 years of diagnosis, and even if the dose was correct and the delivery method adequate, there may not have been enough substrate left in the PD brain for the trophic factor to work. Still, new trials are underway with far superior delivery methods and we are anxious to see if they will be more effective.
With the discovery that (i) genetic missense mutations as well as (ii) gene duplications and triplications in alpha-synuclein cause PD, coupled with the finding (iii) that alpha-synuclein is a major component of Lewy bodies and (iiii) the potential that alpha-synuclein is a prion-like disease that propagates misfolded proteins, alpha-synuclein has become the major target for PD therapeutics.285 Initial preclinical efforts concentrated on synuclein-lowering treatments such as SiRNAs directed against alpha-synuclein.286 However, recent studies using viral delivery of alpha-synuclein SiRNAs found this approach to be toxic in both rodents and monkeys,285,287 and thus it is unclear if this means of providing neuroprotection has a future. More exciting are attempts to disaggregate aggregated synuclein, facilitate its clearance by augmenting autophagy pathways, or using antibodies to prevent its propagation from the periphery to the brain and once in the brain across the neuraxis.288 Farthest along are vaccines against alpha-synuclein as both active and passive immunization approaches have been attempted. Active immunotherapy, championed by Affiris,289 attempts to stimulate the immune system by presenting antigens in a way that triggers an immune response. Passive immunotherapy, championed by Biogen,290 directly targets the disease at hand by using, for the most part, monoclonal antibodies directed at different parts of the alpha-synuclein molecule. Initial phase 1 safety trials are currently underway, and press releases have indicated that these approaches have excellent safety and tolerability profiles.
The hypothesis of cell-to-cell propagation of alpha-synuclein seems to support the use of monoclonal antibodies mainly binding the aggregated forms of the protein.291 However, it should be considered that this hypothesis does not exclude that alpha-synuclein, especially in the early phase of the disease, might exert its clinical deficits by causing synaptic and phenotypic dysfunction via its oligomeric forms.292 Thus, in the future it will be important to assess the ability of distinct monoclonal antibodies to recognize not only aggregated forms of the protein but also oligomeric forms of alpha-synuclein that potentially might be responsible for the synaptic-related behavioral and motor deficits observed in experimental models of PD and possibly occurring in the premotor stages of the human disease.
The recognition that glucocerebrosidase (GBA) mutations are the most important genetic risk factor for PD and that GBA activity is deficient in PD patients with or without mutations293 have provided important insights into a novel pathogenetic pathway. The importance of GBA is its reciprocal relationship to alpha-synuclein and the potential to reduce alpha-synuclein levels by enhancing GBA activity. GBA small molecule chaperones have been shown to both enhance GBA activity and reduce alpha-synuclein levels in cell culture,294 and a clinical trial of a GBA chaperone in PD is now underway.
In summary, disease modification resulting from neuroprotection remains at the forefront of basic, translational, and clinical research in PD. However, there remain many obstacles both to identifying therapies capable of modifying the natural course of PD and to testing their efficacy in clinical trials.295 Using a combination of therapies targeting multiple pathogenetic pathways simultaneously offers some attraction. 296 There has been a focus of attention on influencing the motor progression of PD, although it is increasingly recognized that nonmotor aspects have a greater impact on patient quality of life as the disease progresses. In this respect, those putative agents such as the GBA chaperones and alpha-synuclein immunomodulation that will target both CNS and peripheral synuclein are particularly attractive.297
Clearly, although great progress has taken place in PD, a therapy that interferes with the fundamental disease process and slows or stops disease progression is long overdue.
Conclusions (J.A. Obeso, M. Stamelou, and A.J. Stoessl)
This article has attempted to provide a comprehensive essay on the evolution of PD during the past 200 years. No doubt, if the author of the Shaking Palsy could read it, he would be most surprised and probably highly satisfied by the major impact of his monograph. Nevertheless, it is crystal clear today that so many advances had been insufficient and so much remains to be better understood. Long-term evolution has been achieved thanks to enormous progress in symptomatic treatments, but the evolution of PD is still associated with cumulative and intolerable disability. The association of aging with neurodegeneration as parallel processes is a new phenomenon for neurodegenerative diseases best, if not only, represented in PD. Unless a strikingly effective neuroprotective therapy is developed and applied very early, the interaction between aging and neurodegeneration will oblige us to reconsider mechanisms of disease and newer therapeutic approaches. PD is only found in humans (vide infra), and this by itself represents an important challenge when considering the animal models we use to test putative therapeutic agents, and none fully replicate the clinical disorder. Let us hope that the promising and expected advances in neuro-imaging and molecular genetics will permit more refined and target-specific studies in patients and subjects at risk, allowing for a better understanding of the how PD begins and progresses. Ultimately, all has to be aimed to stop the neurodegenerative process as a first definitive step toward finding a cure. We are afraid this will still take much effort and time. We envisage progress to occur not so much in the form of a single “headline breaking news” but, rather, gradually, as has been the case in oncology, by learning to use multiple therapeutic agents and measures that will make neurodegeneration milder and less disabling over the years. Indeed, PD has already been modified during the past 30 years or so, as the predominant phenotype of patients then (ie, severe motor and psychiatric complications) has dramatically changed to other domains and concerns, particularly associated with longer disease evolution and aging. On the other hand, growing interest is now taking place to redefine PD in many clinical subtypes, a putative genetic correlation of such subcategories and the strong influence of nonmotor manifestations.298 Whether this new approach will help to fast forward a better understanding and resolution of PD is uncertain. We prognosticate a period of confusion. On the other hand, technological advances applied to PD, the use of potent modeling systems, and big data acquisition from global sources (ie, Google, cell phones, etc.) will likely unravel a wealth of precious data.299 Overall, we remain highly optimistic and expectant for major advances that will emerge out of the difficulties and uncertainties associated with too much information during a short period of time. This has occurred before in the area of PD and movement disorders but always has had a positive outcome. Thus, we look forward with the greatest hope to the future and with anticipation that there will be no need for a 300-year anniversary of PD!
Footnotes
Relevant conflicts of interests/financial disclosures: Nothing to report.
References
- 1.Goldman JG, Goetz CG. History of Parkinson’s disease. Handb Clin Neurol. 2007;83:107–128. doi: 10.1016/S0072-9752(07)83005-3. [DOI] [PubMed] [Google Scholar]
- 2.Parkinson J, Hunter J. Hunterian Reminiscences; Being the Substance of a Course of Lectures on the Principles and Practice of Surgery, Delivered by J. Hunter, in the Year 1785. London: Sherwood, Gilbert, and Piper; 1833. [Google Scholar]
- 3.Parkinson J. The Villager’s Friend and Physician. London: C Whittingham; 1804. [Google Scholar]
- 4.Parkinson J. Dangerous Sports: A Tale. London: HD Symonds; 1807. [Google Scholar]
- 5.Parkinson J. Some account of the effects of lightning. Mem M Soc Lon. 1789;2:490–500. [Google Scholar]
- 6.Parkinson J. Observations on the Nature and Cure of Gout. London: HD Symonds; 1805. [Google Scholar]
- 7.Parkinson J, Parkinson J. On the treatment of infectious or typhoid fever. Lond Med Repository. 1824;1:197. [Google Scholar]
- 8.Parkinson J. An Examination of the Mineralized Remains of the Vegetables and Animals of the Antediluian World; Generally Termed Extraneous Fossils. London: 1833. Organic Remains of a Former World. [Google Scholar]
- 9.Parkinson J. An Essay on the Shaking Palsy. London: Whittingham and Rowland Sherwood, Neely and Jones; 1817. [Google Scholar]
- 10.Herzberg L. An essay on the shaking palsy: reviews and notes on the journals in which they appeared. Mov Disord. 1990;5(2):162–166. doi: 10.1002/mds.870050213. [DOI] [PubMed] [Google Scholar]
- 11.Burrows G, Thomson A. Editor’s note. Lond Med Repository. 1817;8:59–61. [Google Scholar]
- 12.Louis ED. The shaking palsy, the first forty-five years: a journey through the British literature. Mov Disord. 1997;12(6):1068–1072. doi: 10.1002/mds.870120638. [DOI] [PubMed] [Google Scholar]
- 13.Fahn S, Goetz CG. Charcot, the clinician: the Tuesday lessons: excerpts from nine case presentations on general neurology delivered at the Salpêtrière Hospital in 1887–88 by Jean-Martin Charcot. Mov Disord. 1987;4(1):123–140. [PubMed] [Google Scholar]
- 14.Charcot JM. Lectures on the Diseases of the Nervous System, Delivered at La Salpêtrière. London: The New Sydenham Society; 1877. [Google Scholar]
- 15.Stern G. Did parkinsonism occur before 1817? J Neurol Neurosurg Psychiatry. 1989;52(suppl):11–12. doi: 10.1136/jnnp.52.suppl.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 18.Horowski R, Horowski L, Phil C, Vogel S, Poewe W, Kielhorn FW. An essay on Wilhelm von Humboldt and the shaking palsy: first comprehensive description of Parkinson’s disease by a patient. Neurology. 1995;45(3):565–568. doi: 10.1212/wnl.45.3.565. [DOI] [PubMed] [Google Scholar]
- 19.Pont-Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PDStudy) Mov Disord. 2015;30(2):229–237. doi: 10.1002/mds.26077. [DOI] [PubMed] [Google Scholar]
- 20.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17(5):427–427. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 21.Gowers WR. A Manual of Diseases of the Nervous System. London: J. & A. Churchill; 1886. [Google Scholar]
- 22.Schwab RS, Doshay LJ, Garland H, Bradshaw P, Garvey E, Crawford B. Shift to older age distribution in parkinsonism: a report on 1,000 patients covering the past decade from three centers. Neurology. 1956;6(11):783–790. doi: 10.1212/wnl.6.11.783. [DOI] [PubMed] [Google Scholar]
- 23.Lang AE, Obeso JA. Time to move beyond nigrostriatal dopamine deficiency in Parkinson’s disease. Ann Neurol. 2004;55(6):761–765. doi: 10.1002/ana.20102. [DOI] [PubMed] [Google Scholar]
- 24.Lang AE. A critical appraisal of the premotor symptoms of Parkinson’s disease: Potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord. 2011;26(5):775–783. doi: 10.1002/mds.23609. [DOI] [PubMed] [Google Scholar]
- 25.Kempster PA, Williams DR, Selikhova M, Holton J, Revesz T, Lees AJ. Patterns of levodopa response in Parkinson’s disease: a clinico-pathological study. Brain. 2007;130(8):2123–2128. doi: 10.1093/brain/awm142. [DOI] [PubMed] [Google Scholar]
- 26.Nutt JG. Motor subtype in Parkinson’s disease: different disorders or different stages of disease? Mov Disord. 2016;31(7):957–961. doi: 10.1002/mds.26657. [DOI] [PubMed] [Google Scholar]
- 27.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord. 2016;31(8):1095–1102. doi: 10.1002/mds.26510. [DOI] [PubMed] [Google Scholar]
- 28.Erro R, Vitale C, Amboni M, et al. The heterogeneity of early Parkinson’s disease: a cluster analysis on newly diagnosed untreated patients. PLoS ONE. 2013;8(8):e70244. doi: 10.1371/journal.pone.0070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fereshtehnejad S-M, Romenets SR, Anang JBM, Latreille V, Gagnon J-F, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression. JAMA Neurol. 2015;72(8):863–873. doi: 10.1001/jamaneurol.2015.0703. [DOI] [PubMed] [Google Scholar]
- 30.Nalls MA, Escott-Price V, Williams NM, et al. Genetic risk and age in Parkinson’s disease: continuum not stratum. Mov Disord. 2015;30(6):850–854. doi: 10.1002/mds.26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guella I, Evans DM, Szu-Tu C, et al. α-synuclein genetic variability: a biomarker for dementia in Parkinson disease. Ann Neurol. 2016;79(6):991–999. doi: 10.1002/ana.24664. [DOI] [PubMed] [Google Scholar]
- 32.Espay AJ, Brundin P, Lang AE. Precision medicine for disease modification in Parkinson disease. Nat Rev Neurol. 2017;13(2):119–126. doi: 10.1038/nrneurol.2016.196. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86(6):566–576. doi: 10.1212/WNL.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 34.Boeve BF, Dickson DW, Duda JE, et al. Arguing against the proposed definition changes of PD. Mov Disord. 2016;31(11):1619–1622. doi: 10.1002/mds.26721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postuma RB, Berg D, Stern M, et al. Abolishing the 1-year rule: how much evidence will be enough? Mov Disord. 2016;31(11):1623–1627. doi: 10.1002/mds.26796. [DOI] [PubMed] [Google Scholar]
- 36.Batla A, Erro R, Stamelou M, et al. Patients with scans without evidence of dopaminergic deficit: a long-term follow-up study. Mov Disord. 2014;29(14):1820–1825. doi: 10.1002/mds.26018. [DOI] [PubMed] [Google Scholar]
- 37.Wile DJ, Dinelle K, Vafai N, et al. A scan without evidence is not evidence of absence: scans without evidence of dopaminergic deficit in a symptomatic leucine-rich repeat kinase 2 mutation carrier. Mov Disord. 2016;31(3):405–409. doi: 10.1002/mds.26450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Respondek G, Stamelou M, Kurz C, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord. 2014;29(14):1758–1766. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 42.Mestre TA, Gupta A, Lang AE. MRI signs of multiple system atrophy preceding the clinical diagnosis: the case for an imaging-supported probable MSA diagnostic category. J Neurol Neurosurg Psychiatry. 2016;87(4):443–444. doi: 10.1136/jnnp-2014-309645. [DOI] [PubMed] [Google Scholar]
- 43.Brigo F, Erro R, Marangi A, Bhatia K, Tinazzi M. Differentiating drug-induced parkinsonism from Parkinson’s disease: an update on non-motor symptoms and investigations. Parkinsonism Relat Disord. 2014;20(8):808–814. doi: 10.1016/j.parkreldis.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Vizcarra JA, Lang AE, Sethi KD, Espay AJ. Vascular parkinsonism: deconstructing a syndrome. Mov Disord. 2015;30(7):886–894. doi: 10.1002/mds.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magdalinou NK, Ling H, Smith JDS, Schott JM, Watkins LD, Lees AJ. Normal pressure hydrocephalus or progressive supranuclear palsy? A clinicopathological case series. J Neurol. 2013;260(4):1009–1013. doi: 10.1007/s00415-012-6745-6. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed RM, Murphy E, Davagnanam I, et al. A practical approach to diagnosing adult onset leukodystrophies. J Neurol Neurosurg Psychiatry. 2014;85(7):770–881. doi: 10.1136/jnnp-2013-305888. [DOI] [PubMed] [Google Scholar]
- 47.Weintraub D, Burn DJ. Parkinson’s disease: the quintessential neuropsychiatric disorder. Mov Disord. 2011;26(6):1022–1031. doi: 10.1002/mds.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease: the ICICLE-PD study. Neurology. 2013;80(3):276–281. doi: 10.1212/WNL.0b013e31827deb74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoogland J, Boel JA, de Bie RMA. Mild cognitive impairment as a risk factor for Parkinson’s disease dementia [published online ahead of print 2017] Mov Disord. doi: 10.1002/mds.27002. [DOI] [PubMed] [Google Scholar]
- 50.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 51.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology. 2001;56(6):730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 52.Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351(24):2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 54.Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology. 2008;72(10):886–892. doi: 10.1212/01.wnl.0000336340.89821.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richard I, McDermott M, Kurlan R, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78:1229–1236. doi: 10.1212/WNL.0b013e3182516244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson’s disease: a randomized, controlled trial. Am J Psychiatry. 2011;168(10):1066–1074. doi: 10.1176/appi.ajp.2011.10111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fénelon G, Soulas T, Zenasni F, de Langavant LC. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord. 2010;25(6):763–766. doi: 10.1002/mds.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pagonabarraga J, Martinez-Horta S, Fernández de Bobadilla R, et al. Minor hallucinations occur in drug-naive Parkinson’s disease patients, even from the premotor phase. Mov Disord. 2016;31:45–52. doi: 10.1002/mds.26432. [DOI] [PubMed] [Google Scholar]
- 59.Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 60.Weintraub D, Koester J, Potenza M, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 61.Krack P, Martinez-Fernandez R, del Alamo M, Obeso JA. Current applications and limitations of surgical treatments for movement disorders. Mov Disord. 2017;32(1):36–52. doi: 10.1002/mds.26890. [DOI] [PubMed] [Google Scholar]
- 62.Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson Disease. Arch Neurol. 2010;67(1):58–63. doi: 10.1001/archneurol.2009.294. [DOI] [PubMed] [Google Scholar]
- 63.Parent M, Parent A. Substantia nigra and Parkinson’s disease: a brief history of their long and intimate relationship. Can J Neurol Sci. 2010;37(03):313–319. doi: 10.1017/s0317167100010209. [DOI] [PubMed] [Google Scholar]
- 64.Fahn S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord. 2014;30(1):4–18. doi: 10.1002/mds.26102. [DOI] [PubMed] [Google Scholar]
- 65.Hall H, Reyes S, Landeck N, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain. 2014;137(9):2493–2508. doi: 10.1093/brain/awu193. [DOI] [PubMed] [Google Scholar]
- 66.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 67.Iacono D, Geraci-Erck M, Rabin ML, et al. Parkinson disease and incidental Lewy body disease: just a question of time? Neurology. 2015;85(19):1670–1679. doi: 10.1212/WNL.0000000000002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michel PP, Hirsch EC, Hunot S. Understanding dopaminergic cell death pathways in Parkinson disease. Neuron. 2016;90(4):675–691. doi: 10.1016/j.neuron.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 69.Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JPA. Environmental risk factors and Parkinson’s disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9. doi: 10.1016/j.parkreldis.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Kalia LV, Kalia SK, Lang AE. Disease-modifying strategies for Parkinson’s disease. Mov Disord. 2015;30(11):1442–1450. doi: 10.1002/mds.26354. [DOI] [PubMed] [Google Scholar]
- 71.Doherty KM, Silveira-Moriyama L, Parkkinen L, et al. Parkin disease: a clinicopathologic entity? JAMA Neurology. 2013;70(5):571–579. doi: 10.1001/jamaneurol.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122(2):187–204. doi: 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- 73.Kosaka K, Tsuchiya K, Yoshimura M. Lewy body disease with and without dementia: a clinicopathological study of 35 cases. Clin Neuropathol. 1988;7(6):299–305. [PubMed] [Google Scholar]
- 74.Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 75.McCann H, Cartwright H, Halliday GM. Neuropathology of α-synuclein propagation and braak hypothesis. Mov Disord. 2015;31(2):152–160. doi: 10.1002/mds.26421. [DOI] [PubMed] [Google Scholar]
- 76.Carlsson A, Lindqvist M, Magnusson TOR. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180(4596):1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 77.Ungerstedt U. 6-hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 78.Langston J, Ballard P, Tetrud J, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 79.Polymeropoulos MH. Mutation in the a-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 80.Feany MB, Bender WW. A drosophila model of Parkinson’s disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 81.Masliah E. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 82.Ekstrand MI, Terzioglu M, Galter D, et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104(4):1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luk KC, Kehm V, Carroll J, et al. Pathological alpha-synuclein transmission initiates parkinson-like neurodegeneration in non-transgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Recasens A, Dehay B, Bové J, et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neuro-degeneration in mice and monkeys. Ann Neurol. 2014;75(3):351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 85.Schon EA, Przedborski S. Mitochondria: the next (neurode) generation. Neuron. 2011;70(6):1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Visanji NP, Brotchie JM, Kalia LV, et al. α-synuclein-based animal models of Parkinson’s disease: challenges and opportunities in a new era. Trends Neurosci. 2016;39(11):750–762. doi: 10.1016/j.tins.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 87.Brundin P, Atkin G, Lamberts JT. Basic science breaks through: New therapeutic advances in Parkinson’s disease. Mov Disord. 2015;30(11):1521–1527. doi: 10.1002/mds.26332. [DOI] [PubMed] [Google Scholar]
- 88.Martin WRW, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: A potential biomarker of disease status. Neurology. 2008;70(Pt 2):1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- 89.Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: a region-of-interest and voxel-based study at 3T and systematic review with meta-analysis. NeuroImage Clin. 2013;3:481–488. doi: 10.1016/j.nicl.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ofori E, Pasternak O, Planetta PJ, et al. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain. 2015;138(8):2322–2331. doi: 10.1093/brain/awv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharman M, Valabregue R, Perlbarg V, et al. Parkinson’s disease patients show reduced cortical-subcortical sensorimotor connectivity. Mov Disord. 2012;28(4):447–454. doi: 10.1002/mds.25255. [DOI] [PubMed] [Google Scholar]
- 92.Kwon D-H, Kim J-M, Oh S-H, et al. Seven-tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol. 2012;71(2):267–277. doi: 10.1002/ana.22592. [DOI] [PubMed] [Google Scholar]
- 93.Castellanos G, Fernández-Seara MA, Lorenzo-Betancor O, et al. Automated neuromelanin imaging as a diagnostic biomarker for Parkinson’s disease. Mov Disord. 2015;30(7):945–952. doi: 10.1002/mds.26201. [DOI] [PubMed] [Google Scholar]
- 94.Peran P, Cherubini A, Assogna F, et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010;133(11):3423–3433. doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- 95.Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 2012;135(12):3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esposito F, Tessitore A, Giordano A, et al. Rhythm-specific modulation of the sensorimotor network in drug-naïve patients with Parkinson’s disease by levodopa. Brain. 2013;136(3):710–725. doi: 10.1093/brain/awt007. [DOI] [PubMed] [Google Scholar]
- 97.Szewczyk-Krolikowski K, Menke RAL, Rolinski M, et al. Functional connectivity in the basal ganglia network differentiates PD patients from controls. Neurology. 2014;83(3):208–214. doi: 10.1212/WNL.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136(8):2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Helmich RC, Janssen MJR, Oyen WJG, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 2011;69(2):269–281. doi: 10.1002/ana.22361. [DOI] [PubMed] [Google Scholar]
- 100.Caligiore D, Pezzulo G, Baldassarre G, et al. Consensus paper: towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum. 2016;16(1):203–229. doi: 10.1007/s12311-016-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garcia-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain. 2013;136(7):2120–2129. doi: 10.1093/brain/awt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mak E, Su L, Williams GB, et al. Baseline and longitudinal grey matter changes in newly diagnosed Parkinson’s disease: ICICLEPD study. Brain. 2015;138(10):2974–2986. doi: 10.1093/brain/awv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Delgado-Alvarado M, Gago B, Navalpotro-Gomez I, Jiménez-Urbieta H, Rodriguez-Oroz MC. Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Mov Disord. 2016;31(6):861–881. doi: 10.1002/mds.26662. [DOI] [PubMed] [Google Scholar]
- 104.Olde Dubbelink KTE, Schoonheim MM, Deijen JB, Twisk JWR, Barkhof F, Berendse HW. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology. 2014;83(22):2046–2053. doi: 10.1212/WNL.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 105.Kostic VS, Agosta F, Petrovic I, et al. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology. 2010;75(10):857–863. doi: 10.1212/WNL.0b013e3181f11c1d. [DOI] [PubMed] [Google Scholar]
- 106.Goldman JG, Stebbins GT, Dinh V, et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain. 2014;137(3):849–859. doi: 10.1093/brain/awt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32(10):548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797–805. doi: 10.1016/S1474-4422(11)70152-1. [DOI] [PubMed] [Google Scholar]
- 109.Adams JR. PET in LRRK2 mutations: comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain. 2005;128(12):2777–2785. doi: 10.1093/brain/awh607. [DOI] [PubMed] [Google Scholar]
- 110.Kraemmer J, Kovacs GG, Perju-Dumbrava L, Pirker S, Traub-Weidinger T, Pirker W. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov Disord. 2014;29(14):1767–1773. doi: 10.1002/mds.25975. [DOI] [PubMed] [Google Scholar]
- 111.Snow BJ, Tooyama I, McGeer EG, et al. Human positron emission tomographic [18F]Fluorodopa studies correlate with dopamine cell counts and levels. Ann Neurol. 1993;34(3):324–330. doi: 10.1002/ana.410340304. [DOI] [PubMed] [Google Scholar]
- 112.Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal progression of sporadic Parkinson’s disease: a multi-tracer positron emission tomography study. Brain. 2009;132(11):2970–2979. doi: 10.1093/brain/awp209. [DOI] [PubMed] [Google Scholar]
- 113.de la Fuente-Fernández R, Schulzer M, Kuramoto L, et al. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol. 2011;69(5):803–810. doi: 10.1002/ana.22284. [DOI] [PubMed] [Google Scholar]
- 114.Sossi V, de la Fuente-Fernandez Rl, Holden JE, Schulzer M, Ruth TJ, Stoessl J. Changes of dopamine turnover in the progression of Parkinson’s disease as measured by positron emission tomography: their relation to disease-compensatory mechanisms. J Cereb Blood Flow Metab. 2004;24(8):869–876. doi: 10.1097/01.WCB.0000126563.85360.75. [DOI] [PubMed] [Google Scholar]
- 115.de la Fuente-Fernandez R. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain. 2004;127(12):2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- 116.Politis M, Wu K, Loane C, et al. Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Invest. 2014;124(3):1340–1349. doi: 10.1172/JCI71640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Piccini P, Weeks RA, Brooks DJ. Alterations in opioid receptor binding in Parkinson’s disease patients with levodopa-induced dyskinesias. Ann Neurol. 1997;42(5):720–726. doi: 10.1002/ana.410420508. [DOI] [PubMed] [Google Scholar]
- 118.Ramlackhansingh AF, Bose SK, Ahmed I, Turkheimer FE, Pavese N, Brooks DJ. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology. 2011;76(21):1811–1816. doi: 10.1212/WNL.0b013e31821ccce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niccolini F, Foltynie T, Reis Marques T, et al. Loss of phosphodiesterase 10A expression is associated with progression and severity in Parkinson’s disease. Brain. 2015;138(10):3003–3015. doi: 10.1093/brain/awv219. [DOI] [PubMed] [Google Scholar]
- 120.Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 121.Metter EJ, Riege WH, Kameyama M, Kuhl DE, Phelps ME. Cerebral metabolic relationships for selected brain regions in Alzheimer’s, Huntington’s, and Parkinson’s diseases. J Cereb Blood Flow Metab. 1984;4:500–506. doi: 10.1038/jcbfm.1984.74. [DOI] [PubMed] [Google Scholar]
- 122.Pappata S, Santangelo G, Aarsland D, et al. Mild cognitive impairment in drug-naive patients with PD is associated with cerebral hypometabolism. Neurology. 2011;77(14):1357–1362. doi: 10.1212/WNL.0b013e3182315259. [DOI] [PubMed] [Google Scholar]
- 123.Bohnen NI, Koeppe RA, Minoshima S, et al. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. J Nuc Med. 2011;52(6):848–855. doi: 10.2967/jnumed.111.089946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79(12):1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 126.Sanchez-Contreras M, Heckman MG, Tacik P, et al. Study of LRRK2 variation in tauopathy: Progressive supranuclear palsy and corticobasal degeneration. Mov Disord. 2016;32(1):115–123. doi: 10.1002/mds.26815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65(11):1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- 128.Kim SE, Choi JY, Choe YS, Choi Y, Lee WY. Serotonin transporters in the midbrain of Parkinson’s disease patients: a study with 123I-beta-CIT SPECT. J Nucl Med. 2003;44(6):870–876. [PubMed] [Google Scholar]
- 129.Politis M, Wu K, Loane C, et al. Depressive symptoms in PD correlate with higher 5-HTT binding in raphe and limbic structures. Neurology. 2010;75(21):1920–1927. doi: 10.1212/WNL.0b013e3181feb2ab. [DOI] [PubMed] [Google Scholar]
- 130.Remy P. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128(6):1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 131.Pavese N. Imaging the aetiology of sleep disorders in dementia and Parkinson’s disease. Curr Neurol Neurosci Rep. 2014;14(12):501. doi: 10.1007/s11910-014-0501-5. [DOI] [PubMed] [Google Scholar]
- 132.Hilker R, Razai N, Ghaemi M, et al. [18F]fluorodopa uptake in the upper brainstem measured with positron emission tomography correlates with decreased REM sleep duration in early Parkinson’s disease. Clin Neurol Neurosurg. 2003;105(4):262–269. doi: 10.1016/s0303-8467(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 133.Matsui H, Nishinaka K, Oda M, Komatsu K, Kubori T, Udaka F. Does cardiac metaiodobenzylguanidine (MIBG) uptake in Parkinson’s disease correlate with major autonomic symptoms? Parkinsonism Relat Disord. 2006;12(5):284–288. doi: 10.1016/j.parkreldis.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 134.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 135.Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13(7):277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- 136.Guridi J, Alegre M. Oscillatory activity in the basal ganglia and deep brain stimulation. Mov Disord. 2016;32(1):64–69. doi: 10.1002/mds.26714. [DOI] [PubMed] [Google Scholar]
- 137.Piconi B, Hernandez L, Obeso JA, Calabresi P. Motor complications in Parkinson’s disease: molecular and electrophysiological mechanisms of dyskinesias. Mov Disord. 2017:32. doi: 10.1002/mds.27261. [DOI] [PubMed] [Google Scholar]
- 138.Neumann W-J, Degen K, Schneider G-H, et al. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov Disord. 2016;31(11):1748–1751. doi: 10.1002/mds.26759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rodriguez-Oroz MC, Lopez-Azcarate J, Garcia-Garcia D, et al. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain. 2010;134(1):36–49. doi: 10.1093/brain/awq301. [DOI] [PubMed] [Google Scholar]
- 140.Cagnan H, Duff EP, Brown P. The relative phases of basal ganglia activities dynamically shape effective connectivity in Parkinson’s disease. Brain. 2015;138(6):1667–1678. doi: 10.1093/brain/awv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- 142.Smith Y, Galvan A, Ellender TJ, et al. The thalamostriatal system in normal and diseased states. Front Syst Neurosci. 2014;8:5. doi: 10.3389/fnsys.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hamani C, Lozano AM, Mazzone PAM, et al. Pedunculopontine nucleus region deep brain stimulation in Parkinson disease: surgical techniques, side effects, and postoperative imaging. Stereotact Funct Neurosurg. 2016;94(5):307–319. doi: 10.1159/000449011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Panigrahi B, Martin KA, Li Y, et al. Dopamine is required for the neural representation and control of movement vigor. Cell. 2015;162(6):1418–1430. doi: 10.1016/j.cell.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 145.Golbe LI, Di Iorio G, Bonavita V, Miller DC, Duvoisin RC. A large kindred with autosomal dominant Parkinson’s disease. Ann Neurol. 1990;27(3):276–282. doi: 10.1002/ana.410270309. [DOI] [PubMed] [Google Scholar]
- 146.Pezzoli G, Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology. 2013;80(22):2035–2041. doi: 10.1212/WNL.0b013e318294b3c8. [DOI] [PubMed] [Google Scholar]
- 147.Tanner CM, Goldman SM, Ross GW, Grate SJ. The disease intersection of susceptibility and exposure: Chemical exposures and neurodegenerative disease risk. Alzheimers Dement. 2014;10(3):S213–S225. doi: 10.1016/j.jalz.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 148.Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol. 2014;54(1):141–164. doi: 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- 149.Furlong M, Tanner CM, Goldman SM, et al. Protective glove use and hygiene habits modify the associations of specific pesticides with Parkinson’s disease. Environ Int. 2015;75:144–150. doi: 10.1016/j.envint.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weisskopf MG, Knekt P, O’Reilly EJ, et al. Polychlorinated biphenyls in prospectively collected serum and Parkinson’s disease risk. Mov Disord. 2012;27(13):1659–1665. doi: 10.1002/mds.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64(7):990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- 152.Hancock DB, Martin ER, Fujiwara K, et al. NOS2A and the modulating effect of cigarette smoking in Parkinson’s disease. Ann Neurol. 2006;60(3):366–373. doi: 10.1002/ana.20915. [DOI] [PubMed] [Google Scholar]
- 153.Palacios N, Gao X, McCullough ML, et al. Caffeine and risk of Parkinson’s disease in a large cohort of men and women. Mov Disord. 2012;27(10):1276–1282. doi: 10.1002/mds.25076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yamada-Fowler N, Söderkvist P. Coffee, genetic variants, and Parkinson’s disease: gene–environment interactions. J Caffeine Res. 2015;5(1):3–10. doi: 10.1089/jcr.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nussbaum RL. Genetics of synucleinopathies. Cold Spring Harb Perspect Med. 2017:a024109. doi: 10.1101/cshperspect.a024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marras C, Lang A, van de Warrenburg BP, et al. Nomenclature of genetic movement disorders: recommendations of the International Parkinson and Movement Disorder Society Task Force. Mov Disord. 2016;31(4):436–457. doi: 10.1002/mds.26527. [DOI] [PubMed] [Google Scholar]
- 157.Walsh DM, Selkoe DJ. A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat Rev Neurosci. 2016;17(4):251–260. doi: 10.1038/nrn.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paisán-Ruíz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44(4):595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 159.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 160.Brice A. Genetics of Parkinson’s disease: LRRK2 on the rise. Brain. 2005;128(12):2760–2762. doi: 10.1093/brain/awh676. [DOI] [PubMed] [Google Scholar]
- 161.Healy DG, Falchi M, O’Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vilariño-Güell C, Wider C, Ross OA, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89(1):162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zimprich A, Benet-Pagès A, Struhal W, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Berg D, Postuma RB, Bloem B, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord. 2014;29(4):454–462. doi: 10.1002/mds.25844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46(9):989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vanhauwaert R, Verstreken P. Flies with Parkinson’s disease. Exp Neurol. 2015;274:42–51. doi: 10.1016/j.expneurol.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 167.Vos M, Esposito G, Edirisinghe JN, et al. Vitamin K2 is a mitochondrial electron carrier that rescues Pink1 deficiency. Science. 2012;336(6086):1306–1310. doi: 10.1126/science.1218632. [DOI] [PubMed] [Google Scholar]
- 168.Olgiati S, Quadri M, Bonifati V. Genetics of movement disorders in the next-generation sequencing era. Mov Disord. 2016;31(4):458–470. doi: 10.1002/mds.26521. [DOI] [PubMed] [Google Scholar]
- 169.Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967;276(7):374–379. doi: 10.1056/NEJM196702162760703. [DOI] [PubMed] [Google Scholar]
- 170.Yahr MD, Duvoisin RC, Schear MJ, Barrett RE, Hoehn MM. Treatment of parkinsonism with levodopa. Arch Neurol. 1969;21(4):343–354. doi: 10.1001/archneur.1969.00480160015001. [DOI] [PubMed] [Google Scholar]
- 171.Lotia M, Jankovic J. New and emerging medical therapies in Parkinson’s disease. Expert Opin Pharmacother. 2016;17(7):895–909. doi: 10.1517/14656566.2016.1149163. [DOI] [PubMed] [Google Scholar]
- 172.Freitas ME, Ruiz-Lopez M, Fox SH. Novel levodopa formulations for Parkinson’s disease. CNS Drug. 2016;30(11):1079–1095. doi: 10.1007/s40263-016-0386-8. [DOI] [PubMed] [Google Scholar]
- 173.LeWitt PA, Fahn S. Levodopa therapy for Parkinson disease: a look backward and forward. Neurology. 2016;86(14 suppl 1):S3–S12. doi: 10.1212/WNL.0000000000002509. [DOI] [PubMed] [Google Scholar]
- 174.Olanow CW. Levodopa: effect on cell death and the natural history of Parkinson’s disease. Mov Disord. 2014;30(1):37–44. doi: 10.1002/mds.26119. [DOI] [PubMed] [Google Scholar]
- 175.Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 176.Jankovic J, Bressman S, Dauer W, Kang U. Clinical and scientific perspectives on movement disorders: Stanley Fahn’s contributions. Mov Disord. 2015;30(1):1862–1869. doi: 10.1002/mds.26445. [DOI] [PubMed] [Google Scholar]
- 177.Vijayakumar D, Jankovic J. Drug-induced dyskinesia, part 1: treatment of levodopa-induced dyskinesia. Drugs. 2016;76(7):759–777. doi: 10.1007/s40265-016-0566-3. [DOI] [PubMed] [Google Scholar]
- 178.Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord. 2014;30(1):80–89. doi: 10.1002/mds.26125. [DOI] [PubMed] [Google Scholar]
- 179.Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP patients requiring levodopa. Ann Neurol. 1996;39(1):37–45. doi: 10.1002/ana.410390107. [DOI] [PubMed] [Google Scholar]
- 180.Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA. Levodopa-induced dyskinesias in Parkinson’s disease: clinical and pharmacological classification. Mov Disord. 1992;7(2):117–124. doi: 10.1002/mds.870070204. [DOI] [PubMed] [Google Scholar]
- 181.Myers RH. Surgical procedure for postencephalitic tremor, with notes on the physiology of the premotor fibers. Arch Neurol Psychiatry. 1940;44:445–459. [Google Scholar]
- 182.Cooper IS. Ligation of the anterior choroidal artery for involuntary movements; parkinsonism. Psychiatr Q. 1953;27(2):317–319. doi: 10.1007/BF01562492. [DOI] [PubMed] [Google Scholar]
- 183.Cooper IS, Bravo G. Chemopallidectomy and chemothalamectomy. J Neurosurg. 1958;15(3):244–250. doi: 10.3171/jns.1958.15.3.0244. [DOI] [PubMed] [Google Scholar]
- 184.Laitinen LV, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg. 1992;76(1):53–61. doi: 10.3171/jns.1992.76.1.0053. [DOI] [PubMed] [Google Scholar]
- 185.Svennilson E, Torvik A, Lowe R, Leksell L. Treatment of parkinsonism by stereotactic thermolesions in the pallidal region. A clinical evaluation of 81 cases. Acta Psychiatrica Scandinavica. 1960;35(3):358–377. doi: 10.1111/j.1600-0447.1960.tb07606.x. [DOI] [PubMed] [Google Scholar]
- 186.Rowland NC, Sammartino F, Lozano AM. Advances in surgery for movement disorders. Mov Disord. 2016;32(1):5–10. doi: 10.1002/mds.26636. [DOI] [PubMed] [Google Scholar]
- 187.Pollak P, Benabid AL, Gross C, et al. Effects of the stimulation of the subthalamic nucleus in Parkinson disease. Rev Neurol (Paris) 1993;149(3):175–176. [PubMed] [Google Scholar]
- 188.Bishop MP, Elder ST, Heath RG. Intracranial self-stimulation in man. Science. 1963;140(3565):394–396. doi: 10.1126/science.140.3565.394. [DOI] [PubMed] [Google Scholar]
- 189.Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77(3):406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 190.Meyers R, Fry WJ, Fry FJ, Dreyer LL, Schultz DF, Noyes RF. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J Neurosurg. 1959;16(1):32–54. doi: 10.3171/jns.1959.16.1.0032. [DOI] [PubMed] [Google Scholar]
- 191.Lipsman N, Schwartz ML, Huang Y, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12(5):462–468. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 192.Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- 193.Weintraub D, Elias WJ. Focus ultrasound therapy for movement disorders: current data and expected developments. Mov Disord. 2017;32:20–27. doi: 10.1002/mds.26599. [DOI] [PubMed] [Google Scholar]
- 194.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Mov Disord. 2008;13(S3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 195.Pavese N, Evans AH, Tai YF, et al. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology. 2006;67(9):1612–1617. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- 196.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Qamhawi Z, Towey D, Shah B, et al. Clinical correlates of raphe serotonergic dysfunction in early Parkinson’s disease. Brain. 2015;138(10):2964–2973. doi: 10.1093/brain/awv215. [DOI] [PubMed] [Google Scholar]
- 198.Hirsch E. Dopamine, tremor, and Parkinson’s disease. Lancet. 1992;340(8811):125–126. doi: 10.1016/0140-6736(92)90457-e. [DOI] [PubMed] [Google Scholar]
- 199.Jellinger KA. Post mortem studies in Parkinson’s disease—is it possible to detect brain areas for specific symptoms? J Neural Transm. 1999;56:1–29. doi: 10.1007/978-3-7091-6360-3_1. [DOI] [PubMed] [Google Scholar]
- 200.Eggers C, Kahraman D, Fink GR, Schmidt M, Timmermann L. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov Disord. 2011;26:416–423. doi: 10.1002/mds.23468. [DOI] [PubMed] [Google Scholar]
- 201.Isaias IU, Marzegan A, Pezzoli G, et al. A role for locus coeruleus in Parkinson tremor. Front Hum Neurosci. 2012;5:179. doi: 10.3389/fnhum.2011.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20(22):8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Muthuraman M, Heute U, Arning K, et al. Oscillating central motor networks in pathological tremors and voluntary movements. What makes the difference? NeuroImage. 2012;60(2):1331–1339. doi: 10.1016/j.neuroimage.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 204.Mure H, Hirano S, Tang CC, et al. Parkinson’s disease tremor-related metabolic network: Characterization, progression, and treatment effects. NeuroImage. 2011;54(2):1244–1253. doi: 10.1016/j.neuroimage.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bergman H, Deuschl GN. Pathophysiology of Parkinson’s disease: from clinical neurology to basic neuroscience and back. Mov Disord. 2002;17(S3):S28–S40. doi: 10.1002/mds.10140. [DOI] [PubMed] [Google Scholar]
- 206.Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A, Bergman H. Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol. 2006;16(6):629–637. doi: 10.1016/j.conb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 207.Llinas R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 208.Zirh TA, Lenz FA, Reich SG, Dougherty PM. Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neuroscience. 1998;83(1):107–121. doi: 10.1016/s0306-4522(97)00295-9. [DOI] [PubMed] [Google Scholar]
- 209.Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and dexternal globus pallidus. Nature. 1999;400(6745):677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- 210.Bar-Gad I, Heimer G, Ritov Y, Bergman H. Functional correlations between neighboring neurons in the primate globus pallidus are weak or nonexistent. J Neurosci. 2003;23(10):4012–4016. doi: 10.1523/JNEUROSCI.23-10-04012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dickson DW. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012;2(8):a009258–a009258. doi: 10.1101/cshperspect.a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rajput AH, Rajput A. Accuracy of Parkinson disease diagnosis unchanged in 2 decades. Neurology. 2014;83(5):386–387. doi: 10.1212/WNL.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 213.Papadimitriou D, Antonelou R, Miligkos M, et al. Motor and nonmotor features of carriers of the p. A53T alpha-synuclein mutation: a longitudinal study. Mov Disord. 2016;31(8):1226–1230. doi: 10.1002/mds.26615. [DOI] [PubMed] [Google Scholar]
- 214.Vilensky JA, Gilman S, McCall S. A historical analysis of the relationship between encephalitis lethargica and postencephalitic parkinsonism: a complex rather than a direct relationship. Mov Disord. 2010;25(9):1116–1123. doi: 10.1002/mds.22908. [DOI] [PubMed] [Google Scholar]
- 215.Erro R, Bhatia KP, Tinazzi M. Parkinsonism following neuroleptic exposure: a double-hit hypothesis? Mov Disord. 2015;30(6):780–785. doi: 10.1002/mds.26209. [DOI] [PubMed] [Google Scholar]
- 216.Kasten M, Klein C. Genetic risk loci for Parkinson’s disease: moving from state to trait? Mov Disord. 2015;30(6):747–749. doi: 10.1002/mds.26246. [DOI] [PubMed] [Google Scholar]
- 217.Kumaran R, Cookson MR. Pathways to parkinsonism redux: convergent pathobiological mechanisms in genetics of Parkinson’s disease. Hum Mol Genet. 2015;24(R1):R32–R44. doi: 10.1093/hmg/ddv236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson’s disease. Mov Disord. 2012;27(1):54–60. doi: 10.1002/mds.24051. [DOI] [PubMed] [Google Scholar]
- 219.Berg D, Lang AE, Postuma RB, et al. Changing the research criteria for the diagnosis of Parkinson’s disease: obstacles and opportunities. Lancet Neurol. 2013;12(5):514–524. doi: 10.1016/S1474-4422(13)70047-4. [DOI] [PubMed] [Google Scholar]
- 220.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 221.Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain. 2012;135(6):1860–1870. doi: 10.1093/brain/aws093. [DOI] [PubMed] [Google Scholar]
- 222.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Berg D, Marek K, Ross GW, Poewe W. Defining at-risk populations for Parkinson’s disease: lessons from ongoing studies. Mov Disord. 2012;27(5):656–665. doi: 10.1002/mds.24985. [DOI] [PubMed] [Google Scholar]
- 224.Hasmann SE, Berg D, Hobert MA, et al. Instrumented Functional Reach Test Differentiates Individuals at High Risk for Parkinsons™ disease from controls. Front Aging Neurosci. 2014;6:286. doi: 10.3389/fnagi.2014.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sánchez-Ferro Á, Elshehabi M, Godinho C, et al. New methods for the assessment of Parkinson’s disease (2005 to 2015): a systematic review. Mov Disord. 2016;31(9):1283–1292. doi: 10.1002/mds.26723. [DOI] [PubMed] [Google Scholar]
- 226.Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913–916. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Postuma RB, Iranzo A, Hogl B, et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol. 2015;77(5):830–839. doi: 10.1002/ana.24385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14(8):744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 229.Iranzo A, Fernández-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS ONE. 2014;9(2):e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65(9):1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- 231.Postuma RB, Gagnon J-F, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol. 2011;69(5):811–818. doi: 10.1002/ana.22282. [DOI] [PubMed] [Google Scholar]
- 232.Gibbons CH, Freeman R. Clinical implications of delayed orthostatic hypotension. Neurology. 2015;85(16):1362–1367. doi: 10.1212/WNL.0000000000002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gustafsson H, Nordstrom A, Nordstrom P. Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology. 2015;84(24):2422–2429. doi: 10.1212/WNL.0000000000001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76(10):863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vilas D, Shaw LM, Taylor P, et al. Cerebrospinal fluid biomarkers and clinical features in leucine-rich repeat kinase 2 (LRRK2) mutation carriers. Mov Disord. 2016;31(6):906–914. doi: 10.1002/mds.26591. [DOI] [PubMed] [Google Scholar]
- 236.Jennings D, Siderowf A, Stern M, et al. Imaging prodromal Parkinson disease: the Parkinson Associated Risk Syndrome Study. Neurology. 2014;83(19):1739–1746. doi: 10.1212/WNL.0000000000000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jennings D, Stern M, Siderowf A, Eberly S, Oakes D, Marek K. Longitudinal imaging and phenoconversion in the PARS Prodromal Cohort [abstract] 2015;30(suppl 1):998. [Google Scholar]
- 238.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79(6):940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 239.Vilas D, Iranzo A, Tolosa E, et al. Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2016;15(7):708–718. doi: 10.1016/S1474-4422(16)00080-6. [DOI] [PubMed] [Google Scholar]
- 240.Gruschus JM. Did α-synuclein and glucocerebrosidase coevolve? Implications for Parkinson’s disease. PLoS ONE. 2015;10(7):e0133863. doi: 10.1371/journal.pone.0133863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Singleton AB. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 242.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 243.Milber JM, Noorigian JV, Morley JF, et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology. 2012;79(24):2307–2314. doi: 10.1212/WNL.0b013e318278fe32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG. Patterns and stages of alpha-synucleinopathy: relevance in a population-based cohort. Neurology. 2008;70(13):1042–1048. doi: 10.1212/01.wnl.0000306697.48738.b6. [DOI] [PubMed] [Google Scholar]
- 245.Moors T, Paciotti S, Chiasserini D, et al. Lysosomal dysfunction and α-synuclein aggregation in Parkinson’s disease: diagnostic links. Mov Disord. 2016;31(6):791–801. doi: 10.1002/mds.26562. [DOI] [PubMed] [Google Scholar]
- 246.Xilouri M, Brekk OR, Stefanis L. Autophagy and alpha-synuclein: relevance to Parkinson’s disease and related synucleopathies. Mov Disord. 2016;31(2):178–192. doi: 10.1002/mds.26477. [DOI] [PubMed] [Google Scholar]
- 247.Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson’s disease. IUBMB Life. 2001;52(3–5):135–141. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- 248.Greenamyre JT, Cannon JR, Drolet R, Mastroberardino P-G. Lessons from the rotenone model of Parkinson’s disease. Trends Pharmacol Sci. 2010;31(4):141–142. doi: 10.1016/j.tips.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cantuti-Castelvetri I, Lin MT, Zheng K, et al. Somatic mitochondrial DNA mutations in single neurons and glia. Neurobiol Aging. 2005;26(10):1343–1355. doi: 10.1016/j.neurobiolaging.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 250.Giannoccaro MP, La Morgia C, Rizzo G, Carelli V. Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson’s disease. Mov Disord. 2017;32(3):346–363. doi: 10.1002/mds.26966. [DOI] [PubMed] [Google Scholar]
- 251.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 252.Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: a critical analysis of α-synuclein staging. Neuropathol Appl Neurobiol. 2008;34:284–295. doi: 10.1111/j.1365-2990.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 253.Halliday G, McCann H, Shepherd C. Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson’s disease? Expert Rev Neurother. 2012;12:673–686. doi: 10.1586/ern.12.47. [DOI] [PubMed] [Google Scholar]
- 254.Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci. 2017;18(2):101–113. doi: 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kordower JH, Brundin P. Lewy body pathology in long-term fetal nigral transplants: is Parkinson’s disease transmitted from one neural system to another? Neuropsychopharmacology. 2009;34(1):254. doi: 10.1038/npp.2008.161. [DOI] [PubMed] [Google Scholar]
- 256.Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem. 2012;288(15):10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 258.Mosharov EV, Larsen KE, Kanter E, et al. Interplay between cytosolic dopamine, calcium, and α-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 260.Zucca FA, Segura-Aguilar J, Ferrari E, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol. 2017;155:96–119. doi: 10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Li J-Y, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 262.Jellinger KA. Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord. 2011;27(1):8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- 263.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Luk KC, Song C, O’Brien P, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Olanow CW. Do prions cause Parkinson disease? The evidence accumulates. Ann Neurol. 2014;75(3):331–333. doi: 10.1002/ana.24098. [DOI] [PubMed] [Google Scholar]
- 267.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27(6):716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 268.Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28:31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]
- 269.Olanow CW, Kordower JH. Targeting α-synuclein as a therapy for Parkinson’s disease: the battle begins. Mov Disord. 2017;32(2):203–207. doi: 10.1002/mds.26935. [DOI] [PubMed] [Google Scholar]
- 270.Schapira AHV. Science, medicine, and the future: Parkinson’s disease. BMJ. 1999;318(7179):311–314. doi: 10.1136/bmj.318.7179.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med. 1989;321(20):1364–1371. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- 272.Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361(13):1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 273.Parkinson Study Group SURE-PD Investigators. Schwarzschild MA, Ascherio A, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71(2):141–150. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schapira AHV, McDermott MP, Barone P, et al. Pramipexole in patients with early Parkinson’s disease (PROUD): a randomised delayed-start trial. Lancet Neurol. 2013;12(8):747–755. doi: 10.1016/S1474-4422(13)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kordower JH, Bjorklund A. Trophic factor gene therapy for Parkinson’s disease. Mov Disord. 2013;28(1):96–109. doi: 10.1002/mds.25344. [DOI] [PubMed] [Google Scholar]
- 276.Collier TJ. Rebuilding the nigrostriatal dopamine pathway: 30 years and counting. Exp Neurol. 2014;256:21–24. doi: 10.1016/j.expneurol.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 277.Lapchak PA, Araujo DM, Hilt DC, et al. Topographical distribution of [125I]-glial cell line-derived neurotrophic factor in unlesioned and MPTP-lesioned rhesus monkey brain following a bolus intraventricular injection. Brain Res. 1998;789(1):9–22. doi: 10.1016/s0006-8993(97)01495-9. [DOI] [PubMed] [Google Scholar]
- 278.Nutt JG, Burchiel KJ, Comella CL, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60(1):69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 279.Kordower JH, Palfi S, Chen E-Y, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann Neurol. 1999;46(3):419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 280.Kordower JH. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290(5492):767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 281.Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2007;59(3):459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 282.Bartus RT, Baumann TL, Brown L, Kruegel BR, Ostrove JM, Herzog CD. Advancing neurotrophic factors as treatments for age-related neurodegenerative diseases: developing and demonstrating “clinical proof-of-concept” for AAV-neurturin (CERE-120) in Parkinson’s disease. Neurobiol Aging. 2013;34(1):35–61. doi: 10.1016/j.neurobiolaging.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 283.Marks WJ, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2–neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7(5):400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 284.Warren OC, Bartus RT, Baumann TL, et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: a double-blind, randomized, controlled trial. Ann Neurol. 2015;78(2):248–257. doi: 10.1002/ana.24436. [DOI] [PubMed] [Google Scholar]
- 285.Dehay B, Vila M, Bezard E, Brundin P, Kordower JH. Alpha-synuclein propagation: new insights from animal models. Mov Disord. 2015;31(2):161–168. doi: 10.1002/mds.26370. [DOI] [PubMed] [Google Scholar]
- 286.Collier TJ, Redmond DE, Steece-Collier K, Lipton JW, Manfredsson FP. Is alpha-synuclein loss-of-function a contributor to parkinsonian pathology? Evidence from non-human primates. Front Neurosci. 2016;10:12. doi: 10.3389/fnins.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Bergström A-L, Kallunki P, Fog K. Development of passive Immunotherapies for synucleinopathies. Mov Disord. 2015;31(2):203–213. doi: 10.1002/mds.26481. [DOI] [PubMed] [Google Scholar]
- 288.McCormack AL, Mak SK, Henderson JM, Bumcrot D, Farrer MJ, Di Monte DA. α-synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PLoS ONE. 2010;5(8):e12122. doi: 10.1371/journal.pone.0012122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Games D, Valera E, Spencer B, et al. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J Neurosci. 2014;34(28):9441–9454. doi: 10.1523/JNEUROSCI.5314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Diogenes MJ, Dias RB, Rombo DM, et al. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci. 2012;32(34):11750–11762. doi: 10.1523/JNEUROSCI.0234-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tozzi A, de Iure A, Bagetta V, et al. Alpha-synuclein produces early behavioral alterations via striatal cholinergic synaptic dysfunction by interacting with GluN2D N-methyl-D-aspartate receptor subunit. Biol Psychiatry. 2016;79(5):402–414. doi: 10.1016/j.biopsych.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 292.Gegg ME, Burke D, Heales SJR, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72(3):455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Schapira AHV, Gegg ME. Glucocerebrosidase in the pathogenesis and treatment of Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(9):3214–3215. doi: 10.1073/pnas.1300822110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.McNeill A, Magalhaes J, Shen C, et al. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137(5):1481–1495. doi: 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Olanow CW, Kieburtz K, Schapira AHV. Why have we failed to achieve neuroprotection in Parkinson’s disease? Ann Neurol. 2009;64(S2):S101–S110. doi: 10.1002/ana.21461. [DOI] [PubMed] [Google Scholar]
- 296.Calabresi P, Di Filippo M. Multitarget disease-modifying therapy in Parkinson’s disease? Lancet Neurol. 2015;14(10):975–976. doi: 10.1016/S1474-4422(15)00227-6. [DOI] [PubMed] [Google Scholar]
- 297.Schapira AHV, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet. 2014;384(9942):545–555. doi: 10.1016/S0140-6736(14)61010-2. [DOI] [PubMed] [Google Scholar]
- 298.Chaudhuri KR, Jenner P. 200 years since James Parkinson’s essay on “The Shaking Palsy” Have we made progress? Mov Disord. 2017:32. doi: 10.1002/mds.27104. [DOI] [PubMed] [Google Scholar]
- 299.Payami H. The emerging science of precision medicine and pharmacogenomics for Parkinson’s disease. Mov Disord. 2017:32. doi: 10.1002/mds.27099. [DOI] [PMC free article] [PubMed] [Google Scholar]