SUMMARY
Secretory cells produce diverse cargoes, yet how they regulate concomitant secretory traffic remains insufficiently explored. Rab GTPases control intracellular vesicular transport. To map secretion pathways, we generated a library of lentivirus-expressed dominant-negative Rab mutants and used it in a large-scale screen to identify regulators of hepatic lipoprotein secretion. We identified several candidate pathways, including those mediated by Rab11 and Rab8. Surprisingly, inhibition of Rab1b, the major regulator of transport from the endoplasmic reticulum to the Golgi, differently affected the secretion of the very-low-density lipoprotein components ApoE and ApoB100, despite their final association on mature secreted lipoprotein particles. Since hepatitis C virus (HCV) incorporates ApoE and ApoB100 into its virus particle, we also investigated infectious HCV secretion, and show that its regulation by Rab1b mirrors that of ApoB100. These observations reveal differential regulation of hepatocyte secretion by Rab1b, and advance our understanding of lipoprotein assembly and lipoprotein and HCV secretion.
eTOC blurb
Takacs et al. show Rab1b, a major regulator of transport from the endoplasmic reticulum to the Golgi, differentially controls the secretion of lipoprotein components ApoE and ApoB100 and of infectious hepatitis C virus particles.

INTRODUCTION
Most secretion in eukaryotic cells involves cargo transport from the endoplasmic reticulum (ER) to the plasma membrane (PM). Transported cargoes generally travel through a series of membrane-bound organelles, which include, in order: the ER-Golgi intermediate compartment (ERGIC), the Golgi, the trans-Golgi network (TGN) and, in some cases, the recycling endosomes (Palade, 1975, Goldenring, 2015, Guo et al., 2014, Brandizzi and Barlowe, 2013). This transport picture has emerged from the study of a small number of model cargoes, a typical example being the exogenous expression of the vesicular stomatitis virus glycoprotein (VSV-G). The complexity and diversity of endogenously expressed secretory cargoes, from short polypeptides to ~100-nm wide lipoprotein and virus particles, invite an inquiry into how the concomitant transport of such molecules is regulated. We investigated this question by focusing on the secretion of two lipoprotein components, the apolipoproteins E and B100, and of infectious HCV particles.
ApoB100 is the structural component of very-low-density lipoproteins (VLDL), a class of large (~80 nm) liver-derived lipoproteins which transport triglycerides and cholesterol to distal tissues (Mahley et al., 1984). VLDL production initiates upon the translation and translocation of ApoB100 into the lumen of the hepatocyte ER, and its co-translocational lipidation; the resulting nascent VLDL particle is then transported by the secretory pathway to the PM (Sundaram and Yao, 2010). During secretion, VLDL undergoes maturation events that include its association with ApoE, thought to occur in the Golgi (Gusarova et al., 2007, Sundaram and Yao, 2012). ApoE may also be secreted on its own, as high-density lipoproteins (HDL), a class of smaller lipoproteins involved in cholesterol transport (Zannis et al., 2015). Some progress has been achieved in understanding the molecular regulation of lipoprotein secretion (Tiwari and Siddiqi, 2012). In vitro biochemical experiments have led to the proposal that VLDL is transported from the ER to the Golgi in a specialized transport vesicle. This vesicle does not include ApoE, despite the subsequent, post-Golgi association between ApoE and VLDL, nor does it include albumin, a monomeric secreted protein that is not part of lipoprotein particles (Gusarova et al., 2007, Siddiqi, 2008). How these hepatic cargoes are secreted from living cells remains an open question.
Besides their central role in lipoprotein metabolism, the host factors ApoE and ApoB100 also perform essential functions in the HCV life cycle. This hepatotropic, positive-sense RNA virus affects over 180 million people worldwide, and infections may result in severe liver disease, including liver failure and hepatocellular carcinoma (Mohd Hanafiah et al., 2013, Hoofnagle, 1997). HCV infects human hepatocytes, replicates its genome in a membranous organelle derived from the ER, and assembles new infectious particles that are released into the ER lumen (Scheel and Rice, 2013). Assembled HCV particles exit the cell as cargoes of the vesicular secretory pathway. The Golgi, Rab11a-positive recycling endosomes, and Rab5a/7a/9a-positive endosomes have each been implicated in HCV secretion (Gastaminza et al., 2008, Coller et al., 2012, Wozniak et al., 2016, Lai et al., 2010, Bayer et al., 2016). Secretion of infectious HCV particles requires that the infected cell express apolipoproteins, such as ApoE and ApoB100 (Fukuhara et al., 2014, Hueging et al., 2014), which are associated with the released infectious HCV particles (Catanese et al., 2013). This close functional association of HCV with ApoE and ApoB100 has led to the proposition that the virus may utilize the lipoprotein secretion pathway to exit the host cell (Bartenschlager et al., 2011, Lindenbach, 2013, Lindenbach and Rice, 2013). However, this has not been validated, and recent studies have documented differences between the post-Golgi regulation of lipoprotein and HCV secretion (Benedicto et al., 2015, Mankouri et al., 2016).
To investigate the regulation of lipoprotein and HCV secretion from hepatic cells, we have focused on the Rab family of small GTPases. Well-conserved among eukaryotes, the Rabs control transport carrier behavior between carrier formation at the donor compartment and fusion to the target compartment (Hutagalung and Novick, 2011, Stenmark, 2009). The Rabs cycle between a GDP-bound inactive state and a GTP-bound active state. Rab activation occurs when GTP replaces GDP, a process stimulated by a guanine nucleotide exchange factor (GEF). Upon insertion into membranes, the active GTP-Rabs recruit effectors, including motor proteins and vesicle tethers, thus ensuring proper transport and targeting of the vesicular carrier. Rab function ends upon GTP hydrolysis, usually stimulated by a GTPase activating protein (GAP). While some functional redundancy exists, the Rabs nonetheless quite specifically control discrete segments of vesicular transport and contribute in part to the establishment of membrane identity (Pfeffer, 2013, Barr, 2013). Given that specific Rabs regulate distinct steps of vesicular transport, we hypothesized that inhibition of individual Rabs could help identify the transport pathways utilized during cargo secretion. Here, we inhibited Rab function by expressing a library of dominant-negative (DN) Rab mutants in a hepatic cell line, measured their effects on the secretion of ApoE and ApoB100, and of the control cargo albumin, and explored in greater depth the differential control of hepatic cargo secretion by Rab1b.
RESULTS
A DN Rab screen for regulators of lipoprotein secretion
To identify the transport pathways that mediate lipoprotein secretion, we expressed a panel of 62 DN Rab mutants in a human hepatoma, Huh-7.5-derived cell line (Figure 1A, red). We used a well-characterized mutation in the GTPase’s guanine ring binding site that impairs binding of either GTP or GDP, resulting in potent DN mutants, such as Rab1aN125I (Pind et al., 1994). For Rab15, we used the T22N mutant, which is expected to be locked in the inactive, GDP-bound state (Nuoffer et al., 1994). As controls, we expressed in parallel the WT Rab proteins (Figure 1A, blue). Rab expression was achieved by lentivirus transduction with a vector that co-expressed an mCherry fluorescent protein reporter (Figure S1A). Using this reporter, we quantified the infectious titer of each lentivirus preparation and could monitor transduction efficiencies. To test the effects of varied levels of Rab expression, we used two lentivirus doses, 25 and 100 infectious units (I.U.) per cell. Under these conditions, the overwhelming majority of the target cells became transduced (Figure S1B).
Figure 1. DN Rab screen for regulators of hepatocyte secretion.
A. Screen outline. Hepatic cells were transduced with WT (blue) or DN (red) Rab GTPase-expressing lentiviruses. At 48 h, the cells were washed and allowed to secrete cargo in fresh media over 6 h. Next, secreted cargoes were quantified by ELISA, and cell mass was estimated using a FLuc reporter. The amount of each secreted cargo was normalized to the FLuc activity of the corresponding well. The values obtained were expressed relative to the mean WT value and presented as relative secretion. B–D. Effects of Rab inhibition on hepatic cargo secretion. The secretion of the indicated cargoes: B, albumin; C, ApoB100; and D, ApoE, in the presence of DN Rab expression (red) was compared to the secretion measured in the presence of the WT Rab expression (blue) for each of 62 Rabs. Shown are means ± SD of 3 wells on a Log2 scale. Significant differences between the effects of a WT and its paired DN Rab are highlighted in gray (p<0.0166; unpaired, 2-tailed Student’s t test with Bonferroni correction). Cells were transduced with 100 I.U./cell of Rab-expressing lentiviruses. See also Figures S1 and S2.
After 48 hours (h) of WT or DN Rab expression, we washed the cells, added fresh media, allowed the cells to secrete cargo for 6 h, and then quantified the amounts of ApoB100, ApoE, and albumin released into the media (Figure 1A). In the same wells, we estimated the cellular mass present at the end of the assay using a firefly luciferase (FLuc) reporter constitutively expressed in the Huh-7.5 FLuc cells. Cargo amounts detected by ELISA and cell lysate FLuc activity linearly correlated with the number of cells used in the assay (Figure S1C–D). DN Rab expression did not significantly affect the FLuc activity for ~2/3 of the Rabs tested (Figure S2A). For the other Rabs, the FLuc activities of DN Rab-transduced cells were generally within 2-fold of those of the WT control (Figure S2A), presumably reflecting effects of the expressed Rabs on cell growth over the 2 days of the assay.
To account for variations in the cell mass responsible for secretion, we normalized the amounts of the secreted cargoes by the FLuc activity of the secreting cells (Figure 1A). We compared the normalized secretion value of each DN Rab and each cargo to the corresponding WT value. Significant differences indicated that the Rab might be involved in regulating the secretion of the tested cargo. Both Rab lentivirus doses yielded hits, with the higher dose yielding more hits (Figure 1B–D and S2B). To facilitate the analysis of these results, we defined a Rab effect score. For each DN Rab that caused a significant change (increase or decrease) in the secretion of a cargo, we assigned a score of 1 to that Rab. The maximum score for a given Rab was therefore 6 (3 cargoes tested x 2 doses of Rab-expressing lentivirus, Figure 2A). Rabs previously shown to regulate the exocytic and recycling pathways (Hutagalung and Novick, 2011), such as Rabs 1, 8, 11, or 13, or to maintain the integrity of the Golgi, such as Rabs 2 and 43, yielded the highest scores (Figure 2A). This confirmed that our screen identified putative regulators of constitutive secretion. In contrast, Rab 3 isoforms, involved in regulated secretion in neurons and endocrine cells, and Rabs 5, 7, or 9, involved in endocytic trafficking (Hutagalung and Novick, 2011), yielded smaller scores (Figure 2A). Most of the Rabs that we identified as hits in our screen were also endogenously expressed in the Huh-7.5 cell line (Luna et al., 2015).
Figure 2. Summary of DN Rab screen results.
A. Ranking of Rabs in decreasing order of their DN Rab effect scores. For each cargo for which a DN Rab significantly altered secretion, a score of 1 was assigned to that Rab. Empty bars: scores assigned based on the effects of a Rab virus dose of 25 I.U./cell (Figure S2B); filled bars: scores assigned based on the effects of a dose of 100 I.U./cell (Figure 1B–D). B–D. Selected DN Rabs that significantly affected secretion at a dose of 100 I.U./cell (data synthesized from Figure 1B–D). Secretion in the presence of the DN Rab (red) is shown relative to the secretion in the presence of the WT Rab (blue) on a Log2 scale. The Rabs are listed at the top, and cargoes measured are noted at the bottom of the graphs. Alb, albumin. Significant differences between the effects of a WT and its paired DN Rab are highlighted in gray (p<0.0166; unpaired, 2-tailed Student’s t test with Bonferroni correction). B. DN Rabs that generally inhibited cargo secretion. C. DN Rabs that generally stimulated cargo secretion. D. DN Rabs that had opposing (stimulatory versus inhibitory) effects on at least two of the cargo tested. See also Figure S2.
The effects on cargo secretion, as detected during the original screen, varied among the different DN Rabs. DN Rabs 1c/35, 8a, 13, and 43 primarily impaired cargo secretion, (Figure 2B), while DN Rabs 8b, 11a, 11b, and 12 stimulated it (Figure 2C). DN Rabs 1c/35, 8a, 13, and 12 affected only a subset of the cargoes (Figure 2B–C). Lastly, two DN Rabs promoted the secretion of one cargo and inhibited the secretion of another (Figure 2D). DN Rab1b reduced the secretion of ApoB100 and stimulated that of ApoE, and DN Rab23 inhibited the secretion of albumin and stimulated that of ApoE. Such differential effects of the DN Rabs were the most interesting, as they were consistent with a model in which the tested cargoes were secreted through distinct pathways that were differentially controlled by these Rabs.
mCherry-tagged Rab1b constructs
We analyzed the involvement of Rab1b in the secretion of hepatic cargo in greater detail, because Rab1b regulates cargo transport from the ER to the Golgi (Plutner et al., 1991) and because in vitro experiments have documented differential transport of ApoB100 and either albumin or ApoE from the ER to the Golgi (Gusarova et al., 2007, Siddiqi, 2008). To monitor exogenous Rab1b protein expression, we fused mCherry to the amino terminus of WT Rab1b, and to its GDP-restricted (S22N) and nucleotide-binding (N121I) mutants (Tisdale et al., 1992, Alvarez et al., 2003). Expression of these constructs led to the synthesis of a single mCherry-containing polypeptide of the expected ~49 kDa molecular weight (Figure 3A). Furthermore, the constructs displayed subcellular localizations similar to those described for GFP- and epitope-tagged Rab1: reticulate ER-like, perinuclear Golgi-like, and punctate vesicle-like signals for mCherry-Rab1bWT, ER-like and cytosolic for mCherry-Rab1bS22N; and largely cytosolic and nuclear for mCherry-Rab1bN121I (Figure 3B) (Alvarez et al., 2003, Moyer et al., 2001).
Figure 3. Characterization of mCherry-Rab1b.
A. Western blot of transfected HEK293T cell lysates with an α-DsRed antibody that recognizes mCherry. Transfected constructs are at the top. Molecular weight markers positions are at the left. B. Epifluorescence images of Huh-7.5 cells transduced with lentiviruses expressing the indicated mCherry-Rab1b constructs. C. Effect of mCherry-Rab1b constructs on VSV-G transport. HEK293T cells were co-transfected with VSV-GtsO45-GFP and the constructs listed at the left. The cells were kept at 39.5°C for one day, a set of wells was then shifted to 32°C while a control set was kept at 39.5°C. Lysates were prepared from each well and treated with Endo H, PNGase F, or mock treated. The incubation temperature before lysis and the enzymatic treatment are listed at the top. The lysates were analyzed by Western blotting using an α-GFP antibody. A higher mobility, deglycosylated band (degly) and a lower mobility, glycosylated band (gly) can be seen. D. Effect of mCherry-Rab1b on transferrin uptake. Huh-7.5 cells were transduced with 25 I.U./cell of lentiviruses expressing the indicated constructs. After two days, the cells were exposed to fluorescent transferrin (Tf-AF647), and the amount of endocytosed transferrin was measured by flow cytometry. See also Figure S3.
Previous studies have shown that epitope- or GFP-tagging of Rab1 at its amino terminus does not interfere with Rab1’s localization or its function in secretion, or with the ability of DN Rab1 to impair ER to Golgi transport (Alvarez et al., 2003, Moyer et al., 2001). We nevertheless confirmed that the mCherry-Rab1b constructs affected cargo secretion as previously documented (Tisdale et al., 1992, Alvarez et al., 2003). We utilized VSV-GtsO45-GFP, a tagged version of a temperature sensitive VSV-G mutant that is retained in the ER at the restrictive temperature of 39.5°C, and is synchronously transported through the Golgi to the PM upon shift to 32°C (Presley et al., 1997). Transport of VSV-G out of the ER is accompanied by modification of its N-linked glycan chains by Golgi resident enzymes (Schwaninger et al., 1991). While endoglycosidase H (Endo H) removes the ER-specific immature glycans, resulting in increased mobility of VSV-G in a polyacrylamide gel, it does not remove Golgi-specific modified glycans. Both types of glycans are removed by peptide:N-glycosidase F (PNGase F). We utilized these properties to investigate VSV-G transport in the presence of WT and mutant mCherry-Rab1b. At 39.5°C, VSV-GtsO45-GFP was retained intracellularly (Figure S3) and was digested by both Endo H and PNGase F (Figure 3C), demonstrating retention in the ER. In cells expressing mCherry or mCherry-Rab1bWT, at the permissive temperature of 32°C, VSV-G molecules became insensitive to Endo H digestion and were transported to the PM (Figures 3C and S3). Some VSV-G proteins remained unprocessed in the presence of mCherry-Rab1bWT (Figure 3C), likely the consequence of overexpression of the functional mCherry-Rab1b construct in a subset of cells. In contrast, the S22N and N121I mutants efficiently blocked the maturation of VSV-G to an Endo H-resistant form and its delivery to the PM even at the permissive temperature (Figures 3C and S3), confirming that they are potent inhibitors of ER to Golgi transport. Lastly, Huh-7.5 cells expressing WT or DN mCherry-Rab1b constructs were capable of endocytosing comparable amounts of fluorescent transferrin (Figure 3D). Since DN mCherry-Rab1b expression did not block endocytosis, we argue that pleiotropic cell-wide toxic effects on cell physiology were unlikely a major consequence of mCherry-Rab1bDN expression. These findings further supported using WT and mutant mCherry-Rab1b to investigate the role of Rab1b in hepatic cargo egress.
DN mCherry-Rab1b constructs differentially inhibit the secretion of albumin, ApoE and ApoB100
To better control the expression of the mCherry-Rab1b constructs, we transduced Huh-7.5-TetON cells (Luna et al., 2015) with vectors encoding WT or DN mCherry-Rab1b under the control of a doxycycline (Dox)-inducible promoter. Dox treatment of the resulting cell lines induced mCherry-Rab1b expression in the overwhelming majority of the cells in culture (Figure S4A-C). Furthermore, Huh-7.5-specific cell morphologies were largely retained in the cells expressing the mCherry-Rab1b constructs (Figure S4A). To test the effects of Rab1b inhibition on cargo secretion, we induced mCherry-Rab1b construct expression by treating the cells with Dox for 24 or 48 h. We then washed the cells and allowed them to secrete cargo in fresh media (Figure 4A). At the end of this process, we quantified the amounts of cargo that had been secreted into the medium or that remained cell-associated. Cargo detection by ELISA in both media and cell lysates was linear with respect to the cell number used in the assay (Figure S1C), ensuring our measurements were not skewed by small variations in the cell number present in a well. Furthermore, after 24 h of induction, the protein mass present in the wells was indistinguishable from that found in control, non-induced wells (Figure S4D), and comparable amounts of cargoes were present in each pair of induced-control wells (data not shown).
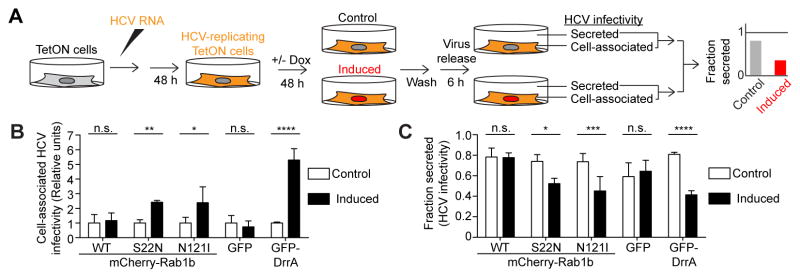
Figure 4. Effects of Rab1b inhibitors on cargo secretion.
A. Experimental outline. Equal numbers of Huh-7.5 TetON cells were induced with Dox for 24 or 48 h, or were left uninduced (Control), then were washed and allowed to secrete cargo in fresh media for 6 h. Secreted and cell-associated cargo amounts were measured by ELISA and a fraction secreted value was calculated (see text). B–F. Fraction secreted values for the indicated cargoes calculated in control uninduced cells (white bars) or cells induced (black bars) to express the following: B, mCherry-Rab1bWT; C, mCherry-Rab1bS22N; D, mCherry-Rab1bN121I; E, GFP; or F, GFP-DrrA. Induction duration (24 or 48 h) is listed under each graph. Shown are means ± SD of 3 wells. G. Effect of Rab1b mutants on cargo secretion from HLCs. Cargo fraction secreted values (mean ± SEM for 7 wells from 2 independent differentiations) were measured in HLCs infected with lentiviruses expressing WT (white bars), S22N (gray bars) or N121I (black bars) mCherry-Rab1b. Significant differences: n.s., not significant, p>0.05; *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; B–F: unpaired, 2-tailed, Student’s t test; G: 2-way ANOVA test. See also Figures S1, S4 and S5.
To estimate cargo secretion efficiencies, we calculated a fraction secreted value, defined as the fraction of the total amount of cargo (secreted + cell-associated) that was secreted. By estimating secretion efficiency in this manner, we inherently accounted for well-to-well differences in cell number and cargo expression level. Indeed, mCherry-Rab1bN121I expression stimulated ApoE and ApoB100 transcript expression and translation rates (Figure S5A–B). However, mCherry-Rab1bN121I expression did not affect total protein translation rates (Figure S5C), indicating that this mutant likely did not cause a metabolic slowdown of the cells.
By comparing fraction secreted values obtained in the presence or absence of mCherry-Rab1b construct expression, we found that mCherry-Rab1bWT did not alter cargo secretion (Figure 4B). In contrast, mCherry-Rab1bS22N impaired the secretion of albumin and ApoB100, but not that of ApoE (Figure 4C), while mCherry-Rab1bN121I impaired the secretion of all three cargoes (Figure 4D). These effects were consistently observed regardless of whether we tested after 24 or 48 h of induction. The decreased ApoE secretion by mCherry-Rab1bN121I seen here does not conflict with the increase of ApoE secretion by Rab1bN121I observed in the DN Rab screen (Figure 2D) since the original screen did not correct for increased ApoE synthesis caused by Rab1bN121I (Figure S5A–B). In the case of mCherry-Rab1bN121I, we also confirmed, using a radioactivity pulse-chase protocol, that it impaired the secretion of newly synthesized albumin, ApoE and ApoB100 (Figure 5). Our results thus indicate that secretion of these cargoes displayed distinct sensitivities to the various modalities of DN-mediated Rab1b inhibition.
Figure 5. mCherry-Rab1bN121I impairs secretion of newly synthesized cargo.
Huh-7.5 TetON mCherry-Rab1bN121I cells were induced with Dox for 24 h (red) or left uninduced (control, blue). A pulse-chase using 35S-Cys/Met was then performed. Shown are fraction secreted values measured for the indicated radiolabeled cargoes at various times during the chase (mean ± SD, n=3 wells).
Inhibition of endogenous Rab1b
Our results demonstrate that DN Rab1b constructs impair the secretion of the tested hepatic cargoes. To test whether inactivation of endogenous Rab1b also affected cargo secretion, we induced expression of DrrA (SidM), a Legionella pneumophila protein. DrrA is injected by the bacterium into the cytosol of infected cells, where it interferes with Rab1 function (Murata et al., 2006, Machner and Isberg, 2006). The DrrA construct we used, GFP-DrrA61–647, (referred to as GFP-DrrA from here on), is targeted to the PM and retains Rab1 GEF activity, but does not exhibit the general cytotoxic effects of full length DrrA (Murata et al., 2006). This construct also preferentially binds and acts on Rab1 GTPases. Its expression interferes with ER to Golgi transport and disrupts Golgi structure (Murata et al., 2006, Machner and Isberg, 2006), as expected of a Rab1 inhibitor. We also engineered a TetON-GFP control cell line. Induction of GFP expression did not affect the secretion of any of the three cargoes tested (Figure 4E). In contrast, GFP-DrrA expression impaired the secretion of albumin and ApoB100, but not that of ApoE (Figure 4F), mirroring the effects of mCherry-Rab1bS22N (Figures 4C). The GFP and the GFP-DrrA proteins were detected in lysates of the respective cell lines only after induction with Dox (Figure S4E), and did not affect total protein mass after 24 h of induction (Figure S4D). Overall, these results establish that inhibition of endogenous Rab1 function differentially impairs hepatic cargo secretion and provide independent confirmation of the results obtained by overexpressing the DN Rab1b constructs.
Differential cargo secretion regulation in human embryonic stem-cell derived hepatocyte-like cells (HLCs)
We tested whether distinct regulation of cargo secretion occurred in a cell system that more closely models primary hepatocytes. We therefore generated HLCs by differentiating human embryonic stem cells along the endoderm-hepatocyte lineage. Importantly, these HLCs displayed many of the physiological characteristics of primary hepatocytes, including the ability to secrete fully lipidated ApoB100 particles that had VLDL-like densities (X.W. and V.L.D.T., unpublished data). We infected these cells with lentivirus particles expressing WT or DN mCherry-Rab1b. Cells were washed after 3 days and allowed to secrete cargo in fresh media. Compared to the WT control, mCherry-Rab1bS22N impaired the secretion of ApoB100 and albumin, but not that of ApoE, while mCherry-Rab1bN121I impaired the secretion of all three cargoes (Figure 4G). These effects mirror those observed in the Huh-7.5 system and establish that HLCs differentially regulate cargo secretion and that the Huh-7.5 cells may be used as an easily tractable system for the study of this process.
Rab1b controls HCV release
To assemble and release infectious HCV particles, infected cells must express apolipoproteins, such as ApoE and ApoB100 (Fukuhara et al., 2014, Hueging et al., 2014). HCV may also employ the same secretion routes as the lipoproteins. We investigated the effect of Rab1b inactivation on the release of infectious HCV. To discern between a proposed Rab1 function in HCV RNA replication (Sklan et al., 2007a, Sklan et al., 2007b) and a putative function in HCV release, we electroporated the TetON cells with in vitro transcribed HCV genomes and allowed viral replication to become established over two days (Figure 6A). Then we induced the expression of the Rab1 inhibitors or control constructs. After two days, we washed the cells and allowed them to release HCV into fresh media and then measured infectious HCV particle titers released or retained intracellularly.
Figure 6. Rab1b controls infectious HCV particle secretion.
A. Experimental outline. Inducible cell lines were electroporated with HCV RNA, then 48 h later Rab1 inhibitor expression (red) was induced with Dox. Control cells were left uninduced. After another 48 h, the cells were washed and allowed to release virus in fresh media, then HCV infectivity titers were measured in paired supernatants and cell lysates. Three electroporations, each with 3 induced and 3 control wells were analyzed. B. Rab1b inhibitors cause accumulation of HCV infectivity intracellularly. Intracellular HCV titers were compared in cells induced to express the indicated constructs (black bars) and in cells left uninduced (control, white bars). C. Rab1b inhibitors impair the release of infectious HCV particles. Fraction secreted values of HCV infectivity are shown in induced conditions (black bars) compared to uninduced controls (white bars). B–C. Shown are means ± SEM. Significant differences (two-way ANOVA): n.s., not significant, p>0.05; *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. See also Figure S6.
Expression of the two DN mCherry-Rab1b constructs and of GFP-DrrA caused intracellular accumulation of infectious HCV (Figure 6B). In the same cells, Rab1b inhibition impaired infectious HCV release (Figure 6C). Intracellular infectious HCV accumulation and decreased viral release were not observed in cells expressing mCherry-Rab1bWT or GFP (Figure 6B, C). These results indicate that infectious HCV particle release requires Rab1 function. Total HCV titers (secreted + cell-associated) were also increased in cells expressing Rab1b inhibitors, but not in control cells (Figure S6A). This is consistent with an accumulation of intracellular infectious titers during the two days of Rab1b inhibitor induction. Lastly, expression of the Rab1b inhibitors did not decrease the levels of HCV RNA found inside the cells (Figure S6B), and similar amounts of the viral protein NS5A could be detected in lysates from induced and non-induced cells (Figure S6C). These findings do not favor a massive inhibition of HCV genome replication by any of the expressed Rab1b inhibitors. Overall, our results are consistent with a model in which mCherry-Rab1bS22N, mCherry-Rab1bN121I, and GFP-DrrA inhibit HCV transport from the ER to the Golgi, and concurrently cause accumulation of infectious HCV particles in an ER-related compartment. Similar results were obtained in cells treated with brefeldin A, a potent blocker of ER to Golgi transport (Gastaminza et al., 2008). Our results therefore establish the involvement of Rab1b in the early stages of exocytic transport of infectious HCV particles.
DISCUSSION
An unbiased DN Rab screen identifies cargo secretion routes
We describe here the construction and use of a comprehensive library of lentivirus-expressed WT and DN human Rab GTPases. We established an experimental platform for the unbiased identification of the Rabs involved in the secretion of a wide variety of cargoes and used it to identify putative regulators of lipoprotein secretion from a hepatic cell line. As lentiviral particles readily infect both primary cells and cell lines and can be easily made and titrated, this DN Rab library can be used to study the secretion of other cargoes and in other cellular contexts.
Rabs putatively involved in lipoprotein secretion
Several DN Rab proteins significantly altered the release of the cargoes studied here when compared to their WT counterparts. Decreased release may be due to inhibition of secretion by the DN Rab, stimulation of secretion by the WT Rab, or a combination of the two effects. Conversely, increased release may be due to stimulation of secretion by the DN Rab, an inhibitory effect of the WT Rab, or a combination of the two effects. Stimulation by a DN Rab could result from blocking a competing vesicular transport pathway, while inhibition by a WT Rab could result from overexpression or competition for a regulator or effector shared between multiple Rabs. As such, further investigation of individual Rab hits will be required to delineate the precise involvement of each Rab in cargo transport. Interesting candidates for analysis include Rabs 1c/35, 8a, 8b, 11a, 11b, 12, 13, and 23, which have previously been implicated in endosomal trafficking, recycling, or secretion (Hutagalung and Novick, 2011). In particular, Rabs 8, 11, and 13 control post-Golgi secretion via the recycling endosome (Ang et al., 2003, Ang et al., 2004, Huber et al., 1993, Chen et al., 1998, Nokes et al., 2008). In a recent study, expression of DN Rabs 1c/35, 8b, 11, 13, and 23, did not affect the secretion of triglycerides, ApoE, or ApoB100 from Huh7 cells (Mankouri et al., 2016). In that study, the authors used a plasmid transfection method of lower efficiency (20%, with a range between 10 and 40% of the cells in culture) and used different Rab-inactivating mutations. The effects of the DN Rabs on lipoprotein secretion may have been missed due to the lower efficiency of expression.
Distinct pathways of pre-Golgi lipoprotein transport
A major finding of our study is that distinct means of inhibiting Rab1b function had different effects on the secretion of ApoE, ApoB100 and albumin. Thus, mCherry-Rab1bN121I inhibited the transport of all the cargoes tested, confirming that their secretion involves Rab1b function. In contrast, mCherry-Rab1bS22N did not impair the secretion of ApoE, but inhibited that of albumin and ApoB100. This discrepancy may be due to the fact that Rab1b functions at several stages of ER-to-Golgi transport (Schwaninger et al., 1991, Plutner et al., 1991). Rab1bS22N was proposed to sequester a Rab1 GEF and preventing it from activating WT Rab1 at the ER (Nuoffer et al., 1994). Indeed, VSV-G retains an ER-like localization in the presence of Rab1bS22N (Tisdale et al., 1992, Alvarez et al., 2003, Nuoffer et al., 1994), and overexpression of a PM-localized Rab1 GEF, DrrA, had the same effects on cargo secretion as mCherry-Rab1bS22N. These results raise the possibility that, while ApoB100 and albumin may exit from the ER in a Rab1b-dependent process, ApoE may not require Rab1b for its initial transport out of the ER. Expression of Rab1bN121I, in contrast, leads to VSV-G accumulation in a punctate pattern, likely at the ERGIC (Pind et al., 1994, Alvarez et al., 2003), implying that this mutant acts at a post-ER exit step of cargo transport. That mCherry-Rab1bN121I blocked secretion of all the cargoes tested in our study suggests that they all utilize a Rab1b-dependent pathway before reaching the Golgi, despite ApoE not requiring Rab1b to exit the ER.
Rab1b functions in infectious HCV particle secretion
Since lipoprotein egress depends on Rab1 function, and since HCV production depends on functional lipoprotein biogenesis (Lindenbach and Rice, 2013), we also investigated the involvement of Rab1 in HCV egress. Our study complements previous findings implicating Rab1, and its cognate GAP, TBC1D20, in HCV genome replication (Sklan et al., 2007a, Sklan et al., 2007b). In these studies, the effects of Rab1b inactivation on the secretion of HCV infectivity were not assessed. Under our experimental conditions, Rab1b inactivation neither decreased the abundance of cell-associated HCV genomes nor inhibited viral protein expression, implying that HCV genome replication was not affected, while particle egress was impaired. Since we allowed the HCV replication complexes to form before we expressed the Rab1 inhibitors, it is likely that, once established, HCV replication may no longer require Rab1 function.
We propose that Rab1b regulates the early, ER to Golgi steps of secretion of infectious HCV particles. Our conclusion is corroborated by the findings that HCV egress is inhibited by relatively short treatments with brefeldin A (Gastaminza et al., 2008), and that the glycan chains on the HCV E2 glycoprotein become partially processed by Golgi enzymes (Vieyres et al., 2010). Our conclusions thus stand despite a recent report proposing that HCV is not secreted through the Golgi system (Bayer et al., 2016), since the interpretation of the assays performed there was hindered by the utilization of a HCV genome strongly impaired in the production and release of infectious HCV particles. We also note that HCV replication was proposed to induce re-organization of the TGN to the proximity of putative viral replication complexes, thus potentially providing a connection between the sites of virus replication and particle assembly, and the TGN compartment involved in particle secretion (Mankouri et al., 2016). Our results nonetheless indicate that virus particle egress utilizes the canonical secretion pathway from the ER to the Golgi, mediated by Rab1b.
HCV secretion displayed a pattern of sensitivity to the Rab1b inactivation methods that mirrored that of ApoB100 and not that of ApoE. Thus, just like ApoB100, HCV secretion was impaired by expression of the N121I and S22N Rab1b mutants, as well as by the Rab1 GEF DrrA, while ApoE secretion was affected only by Rab1bN121I. We propose that HCV and ApoB100-containing nascent VLDL may be transported through early secretory pathways that are similarly regulated. ApoB100 and HCV are indeed similar with respect to their sizes (HCV and VLDL particles, even nascent ones, would have sizes around 50 nm), and N-linked glycosylation status (Catanese et al., 2013, Helle et al., 2010). ApoE, in turn, even if transported as HDL particles, would have smaller dimensions, up to 10 nm, and is O-glycosylated (Wernette-Hammond et al., 1989). Other cargo properties may also be responsible for the observed differences in how secretion is controlled. Regardless, our findings provide further insight into the complex regulation of cargo secretion by hepatocytes, and complement the observations that albumin and the polymeric IgA receptor (Saucan and Palade, 1994), or lipoproteins and HCV are transported through distinct post-Golgi secretion routes (Benedicto et al., 2015, Mankouri et al., 2016).
This study describes a DN Rab expression library that can easily be used to investigate the secretion of various cargoes in a wide range of cellular systems. While focusing on the secretion of hepatic lipoproteins and HCV, we have uncovered similarities and differences in how the secretion of these cargoes is regulated, thus advancing our understanding of the biogenesis of these important bio-molecules.
EXPERIMENTAL PROCEDURES
Detailed methods are in the Supplemental Experimental Procedures.
Cell lines
Cell lines were derived from Huh-7.5 cells (Blight et al., 2002), or its Dox-inducible derivative Huh-7.5 TetON (Luna et al., 2015), by lentiviral or retroviral transduction. Gene expression was induced with 3 μg/mL Dox (Clontech).
DN Rab screen
VSV-G-pseudotyped lentiviral particles expressing WT or DN Rabs were produced in HEK293T cells, concentrated, and titrated on Huh-7.5 cells using a flow cytometry-based assay (Sastry et al., 2002). Lentivirus particles were used at 25 or 100 I.U./cell; 42,000 cells/well were infected in 24-well plates in triplicate. At 48 h, the cells were washed, incubated with fresh media for 6 h, and then media and cell lysates were harvested. FLuc activity was measured in cell lysates using a firefly luciferase assay kit (Promega). Cargo amounts were measured in each media sample using human-specific ELISA kits (Abcam: ApoE, ab108813; ApoB100, ab108807; albumin, ab108788), and were normalized to the luciferase activity of the corresponding cell lysate.
Secretion assays in inducible cell lines
Cells were plated at 2.25×105 cells/well in 6-well plates. The next day, half of the wells were induced with Dox. After 24 or 48 h of induction, the cells were washed and allowed to secrete cargo in fresh media for 6 h, the media and cell lysates were collected and their cargo content was measured by ELISA. Secretion efficiency was calculated as fraction secreted, defined as the ratio between the amount of cargo that was secreted and the total (secreted + cell-associated) amount of cargo present in a well.
Radioactive pulse-chase
Huh-7.5 TetON mCherry-Rab1bN121 cells were induced with Dox for 24 h or left uninduced. The cells were pulse labeled with 35S-Cys/Met (120 μCi/well) for 20 min, then washed and chased in media containing cycloheximide (Millipore, 50 μM) and excess of cold Cys and Met. At indicated time points, media and cell lysates were harvested and total and anti-ApoE, albumin, and ApoB100 immunoprecipitated protein-incorporated radiolabel was quantified.
HCV
In vitro-transcribed genomic RNA of the infectious HCV clone J6/JFH1 was electroporated into Huh-7.5 TetON-derived cell lines (Lindenbach et al., 2005). After 2 days, gene expression was induced using Dox. After two more days, a 6 h secretion assay was performed. Secreted and intracellular HCV infectivity titers were measured in the same well. Cell-associated HCV RNA amounts were measured by qRT-PCR.
Transferrin uptake
A transferrin (Tf)-uptake protocol was adapted from (Fielding et al., 2012). Huh-7.5 cells were serum starved for 1 h, detached, and stained for 5 min with 50 μg/mL Tf-AF647 (Molecular Probes), then either washed, or allowed to uptake Tf for 10 min at 37°C and then washed. Cell-associated Tf-AF647 amounts were measured by flow cytometry.
VSV-G transport
HEK293T or Huh-7.5 cells were co-transfected with pVSV-GtsO45-GFP (Presley et al., 1997) and a plasmid expressing mCherry or mCherry-Rab1b constructs. The plates were incubated for one day at 39.5°C, then one set was shifted to 32°C, while another was kept at 39.5°C. The cells were then fixed and processed for imaging, or were lysed. The lysates were digested with Endo H, PNGase F (New England Biolabs) or left untreated, then analyzed by Western blotting.
Statistics
Two-tailed, unpaired, Student’s t-tests (with or without Bonferroni correction) or 2-way ANOVA tests, as indicated in figure legends, were performed using GraphPad Prism 5 of Microsoft Excel.
Supplementary Material
Highlights.
Generation of a dominant-negative Rab GTPase library
Identification of Rab regulators of lipoprotein secretion
Rab1b differentially controls the secretion of apolipoproteins and of HCV
ApoE, ApoB100 and HCV may be secreted by distinct transport routes
Acknowledgments
We thank P. Bieniasz for providing retrovirus and lentivirus packaging plasmids and the HEK293T cell line, C. Roy (Yale University) for providing the DrrA construct, S. Mazel (Rockefeller University Flow Cytometry Resource Center), K. Thomas and A. North (Rockefeller University Bioimaging Resource Center) for technical advice, to J. Lobby for testing conditions for multiple antibiotic selection, and to the members of the Simon and Rice labs for technical help and critical discussions. This work was supported by the National Institutes of Health (award numbers R01 AI072613 and R01 AI075099 to C.M.R. and R01 GM119585 to S.M.S.), by the Greenberg Medical Research Institute and the Starr Foundation (to C.M.R.), and by The Rockefeller University Center for Basic and Translational Research on Disorders of the Digestive System through the generosity of the Leona M. and Harry B. Helmsley Charitable Trust (to C.N.T. and S.M.S.). C.N.T. was supported in part by a Howard Hughes Medical Institute International Predoctoral Fellowship, V.L.D.T was supported by a postdoctoral fellowship from the German Research Council (Deutsche Forschungsgemeinschaft), and X.W. was supported by a postdoctoral fellowship from Bristol-Myers Squibb at The Rockefeller University. C.M.R. has equity in Apath, LLC, which holds commercial licenses for the Huh-7.5 cell line and certain HCV cell culture systems. The authors are aware of no other conflicts of interest.
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, C.N.T., U.A., V.L.D.T., M.A.S., C.M.R., and S.M.S.; Methodology: C.N.T., V.L.D.T. and X.W.; Formal Analysis: C.N.T. and M.S.I.; Investigation: C.N.T., U.A., V.L.D.T., C.E.G., G.S.S.-B., R.L.B., B.R.F.; Resources: X.W., R.L.B.; Writing: C.N.T. and S.M.S.; Visualization: C.N.T.; Supervision: C.N.T., C.M.R., S.M.S.; Funding Acquisition: C.N.T., C.M.R., S.M.S.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALVAREZ C, GARCIA-MATA R, BRANDON E, SZTUL E. COPI recruitment is modulated by a Rab1b-dependent mechanism. Mol Biol Cell. 2003;14:2116–27. doi: 10.1091/mbc.E02-09-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANG AL, FOLSCH H, KOIVISTO UM, PYPAERT M, MELLMAN I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol. 2003;163:339–50. doi: 10.1083/jcb.200307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANG AL, TAGUCHI T, FRANCIS S, FOLSCH H, MURRELLS LJ, PYPAERT M, WARREN G, MELLMAN I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–43. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARR FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? J Cell Biol. 2013;202:191–9. doi: 10.1083/jcb.201306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTENSCHLAGER R, PENIN F, LOHMANN V, ANDRE P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011;19:95–103. doi: 10.1016/j.tim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- BAYER K, BANNING C, BRUSS V, WILTZER-BACH L, SCHINDLER M. Hepatitis C Virus Is Released via a Noncanonical Secretory Route. J Virol. 2016;90:10558–10573. doi: 10.1128/JVI.01615-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENEDICTO I, GONDAR V, MOLINA-JIMENEZ F, GARCIA-BUEY L, LOPEZ-CABRERA M, GASTAMINZA P, MAJANO PL. Clathrin mediates infectious hepatitis C virus particle egress. J Virol. 2015;89:4180–90. doi: 10.1128/JVI.03620-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGHT KJ, MCKEATING JA, RICE CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–14. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRANDIZZI F, BARLOWE C. Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol. 2013;14:382–92. doi: 10.1038/nrm3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATANESE MT, URYU K, KOPP M, EDWARDS TJ, ANDRUS L, RICE WJ, SILVESTRY M, KUHN RJ, RICE CM. Ultrastructural analysis of hepatitis C virus particles. Proc Natl Acad Sci U S A. 2013;110:9505–10. doi: 10.1073/pnas.1307527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN W, FENG Y, CHEN D, WANDINGER-NESS A. Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell. 1998;9:3241–57. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLER KE, HEATON NS, BERGER KL, COOPER JD, SAUNDERS JL, RANDALL G. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS Pathog. 2012;8:e1002466. doi: 10.1371/journal.ppat.1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELDING AB, WILLOX AK, OKEKE E, ROYLE SJ. Clathrin-mediated endocytosis is inhibited during mitosis. Proc Natl Acad Sci U S A. 2012;109:6572–7. doi: 10.1073/pnas.1117401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUHARA T, WADA M, NAKAMURA S, ONO C, SHIOKAWA M, YAMAMOTO S, MOTOMURA T, OKAMOTO T, OKUZAKI D, YAMAMOTO M, et al. Amphipathic alpha-helices in apolipoproteins are crucial to the formation of infectious hepatitis C virus particles. PLoS Pathog. 2014;10:e1004534. doi: 10.1371/journal.ppat.1004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASTAMINZA P, CHENG G, WIELAND S, ZHONG J, LIAO W, CHISARI FV. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol. 2008;82:2120–9. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDENRING JR. Recycling endosomes. Curr Opin Cell Biol. 2015;35:117–22. doi: 10.1016/j.ceb.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO Y, SIRKIS DW, SCHEKMAN R. Protein sorting at the trans-Golgi network. Annu Rev Cell Dev Biol. 2014;30:169–206. doi: 10.1146/annurev-cellbio-100913-013012. [DOI] [PubMed] [Google Scholar]
- GUSAROVA V, SEO J, SULLIVAN ML, WATKINS SC, BRODSKY JL, FISHER EA. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J Biol Chem. 2007;282:19453–62. doi: 10.1074/jbc.M700475200. [DOI] [PubMed] [Google Scholar]
- HELLE F, VIEYRES G, ELKRIEF L, POPESCU CI, WYCHOWSKI C, DESCAMPS V, CASTELAIN S, ROINGEARD P, DUVERLIE G, DUBUISSON J. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J Virol. 2010;84:11905–15. doi: 10.1128/JVI.01548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOOFNAGLE JH. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26:15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- HUBER LA, PIMPLIKAR S, PARTON RG, VIRTA H, ZERIAL M, SIMONS K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEGING K, DOEPKE M, VIEYRES G, BANKWITZ D, FRENTZEN A, DOERRBECKER J, GUMZ F, HAID S, WOLK B, KADERALI L, et al. Apolipoprotein E codetermines tissue tropism of hepatitis C virus and is crucial for viral cell-to-cell transmission by contributing to a postenvelopment step of assembly. J Virol. 2014;88:1433–46. doi: 10.1128/JVI.01815-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTAGALUNG AH, NOVICK PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–49. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI CK, JENG KS, MACHIDA K, LAI MM. Hepatitis C virus egress and release depend on endosomal trafficking of core protein. J Virol. 2010;84:11590–8. doi: 10.1128/JVI.00587-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDENBACH BD. Virion assembly and release. Curr Top Microbiol Immunol. 2013;369:199–218. doi: 10.1007/978-3-642-27340-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDENBACH BD, EVANS MJ, SYDER AJ, WOLK B, TELLINGHUISEN TL, LIU CC, MARUYAMA T, HYNES RO, BURTON DR, MCKEATING JA, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–6. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- LINDENBACH BD, RICE CM. The ins and outs of hepatitis C virus entry and assembly. Nat Rev Microbiol. 2013;11:688–700. doi: 10.1038/nrmicro3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNA JM, SCHEEL TK, DANINO T, SHAW KS, MELE A, FAK JJ, NISHIUCHI E, TAKACS CN, CATANESE MT, DE JONG YP, et al. Hepatitis C Virus RNA Functionally Sequesters miR-122. Cell. 2015;160:1099–110. doi: 10.1016/j.cell.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHNER MP, ISBERG RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- MAHLEY RW, INNERARITY TL, RALL SC, JR, WEISGRABER KH. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984;25:1277–94. [PubMed] [Google Scholar]
- MANKOURI J, WALTER C, STEWART H, BENTHAM M, PARK WS, HEO WD, FUKUDA M, GRIFFIN S, HARRIS M. Release of Infectious Hepatitis C Virus from Huh7 Cells Occurs via a trans-Golgi Network-to-Endosome Pathway Independent of Very-Low-Density Lipoprotein Secretion. J Virol. 2016;90:7159–70. doi: 10.1128/JVI.00826-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHD HANAFIAH K, GROEGER J, FLAXMAN AD, WIERSMA ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- MOYER BD, MATTESON J, BALCH WE. Expression of wild-type and mutant green fluorescent protein-Rab1 for fluorescence microscopy analysis. Methods Enzymol. 2001;329:6–14. doi: 10.1016/s0076-6879(01)29061-2. [DOI] [PubMed] [Google Scholar]
- MURATA T, DELPRATO A, INGMUNDSON A, TOOMRE DK, LAMBRIGHT DG, ROY CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–7. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- NOKES RL, FIELDS IC, COLLINS RN, FOLSCH H. Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells. J Cell Biol. 2008;182:845–53. doi: 10.1083/jcb.200802176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUOFFER C, DAVIDSON HW, MATTESON J, MEINKOTH J, BALCH WE. A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol. 1994;125:225–37. doi: 10.1083/jcb.125.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–58. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- PFEFFER SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414–9. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIND SN, NUOFFER C, MCCAFFERY JM, PLUTNER H, DAVIDSON HW, FARQUHAR MG, BALCH WE. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994;125:239–52. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUTNER H, COX AD, PIND S, KHOSRAVI-FAR R, BOURNE JR, SCHWANINGER R, DER CJ, BALCH WE. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESLEY JF, COLE NB, SCHROER TA, HIRSCHBERG K, ZAAL KJ, LIPPINCOTT-SCHWARTZ J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–5. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- SASTRY L, JOHNSON T, HOBSON MJ, SMUCKER B, CORNETTA K. Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther. 2002;9:1155–62. doi: 10.1038/sj.gt.3301731. [DOI] [PubMed] [Google Scholar]
- SAUCAN L, PALADE GE. Membrane and secretory proteins are transported from the Golgi complex to the sinusoidal plasmalemma of hepatocytes by distinct vesicular carriers. J Cell Biol. 1994;125:733–41. doi: 10.1083/jcb.125.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEEL TK, RICE CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19:837–49. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWANINGER R, BECKERS CJ, BALCH WE. Sequential transport of protein between the endoplasmic reticulum and successive Golgi compartments in semi-intact cells. J Biol Chem. 1991;266:13055–63. [PubMed] [Google Scholar]
- SIDDIQI SA. VLDL exits from the endoplasmic reticulum in a specialized vesicle, the VLDL transport vesicle, in rat primary hepatocytes. Biochem J. 2008;413:333–42. doi: 10.1042/BJ20071469. [DOI] [PubMed] [Google Scholar]
- SKLAN EH, SERRANO RL, EINAV S, PFEFFER SR, LAMBRIGHT DG, GLENN JS. TBC1D20 is a Rab1 GTPase-activating protein that mediates hepatitis C virus replication. J Biol Chem. 2007a;282:36354–61. doi: 10.1074/jbc.M705221200. [DOI] [PubMed] [Google Scholar]
- SKLAN EH, STASCHKE K, OAKES TM, ELAZAR M, WINTERS M, AROETI B, DANIELI T, GLENN JS. A Rab-GAP TBC domain protein binds hepatitis C virus NS5A and mediates viral replication. J Virol. 2007b;81:11096–105. doi: 10.1128/JVI.01249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENMARK H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- SUNDARAM M, YAO Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr Metab (Lond) 2010;7:35. doi: 10.1186/1743-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNDARAM M, YAO Z. Intrahepatic role of exchangeable apolipoproteins in lipoprotein assembly and secretion. Arterioscler Thromb Vasc Biol. 2012;32:1073–8. doi: 10.1161/ATVBAHA.111.241455. [DOI] [PubMed] [Google Scholar]
- TISDALE EJ, BOURNE JR, KHOSRAVI-FAR R, DER CJ, BALCH WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119:749–61. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIWARI S, SIDDIQI SA. Intracellular trafficking and secretion of VLDL. Arterioscler Thromb Vasc Biol. 2012;32:1079–86. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIEYRES G, THOMAS X, DESCAMPS V, DUVERLIE G, PATEL AH, DUBUISSON J. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J Virol. 2010;84:10159–68. doi: 10.1128/JVI.01180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERNETTE-HAMMOND ME, LAUER SJ, CORSINI A, WALKER D, TAYLOR JM, RALL SC., JR Glycosylation of human apolipoprotein E. The carbohydrate attachment site is threonine 194. J Biol Chem. 1989;264:9094–101. [PubMed] [Google Scholar]
- WOZNIAK AL, LONG A, JONES-JAMTGAARD KN, WEINMAN SA. Hepatitis C virus promotes virion secretion through cleavage of the Rab7 adaptor protein RILP. Proc Natl Acad Sci U S A. 2016;113:12484–12489. doi: 10.1073/pnas.1607277113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZANNIS VI, FOTAKIS P, KOUKOS G, KARDASSIS D, EHNHOLM C, JAUHIAINEN M, CHRONI A. HDL biogenesis, remodeling, and catabolism. Handb Exp Pharmacol. 2015;224:53–111. doi: 10.1007/978-3-319-09665-0_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.