Abstract
Cells within cartilaginous tissues are mechanosensitive and thus require mechanical loading for regulation of tissue homeostasis and metabolism. Mechanical loading plays critical roles in cell differentiation, proliferation, biosynthesis, and homeostasis. Inflammation is an important event occurring during multiple processes, such as aging, injury, and disease. Inflammation has significant effects on biological processes as well as mechanical function of cells and tissues. These effects are highly dependent on cell/tissue type, timing, and magnitude. In this review, we summarize key findings pertaining to effects of inflammation on multiscale mechanical properties at subcellular, cellular, and tissue level in cartilaginous tissues, including alterations in mechanotransduction and mechanosensitivity. The emphasis is on articular cartilage and the intervertebral disc, which are impacted by inflammatory insults during degenerative conditions such as osteoarthritis, joint pain, and back pain. To recapitulate the pro-inflammatory cascades that occur in vivo, different inflammatory stimuli have been used for in vitro and in situ studies, including tumor necrosis factor (TNF), various interleukins (IL), and lipopolysaccharide (LPS). Therefore, this review will focus on the effects of these stimuli because they are the best studied pro-inflammatory cytokines in cartilaginous tissues. Understanding the current state of the field of inflammation and cell/tissue biomechanics may potentially identify future directions for novel and translational therapeutics with multiscale biomechanical considerations.
Keywords: multiscale biomechanics, cytokines, cartilage, intervertebral disc, mechanobiology, mechanotransduction
1. Introduction
Cartilaginous tissues are a type of connective tissue in the musculoskeletal system that functions as a load bearing material and provides joint flexibility and stability during body movement. Cartilaginous tissue is characterized by an extracellular matrix that is rich in proteoglycan and collagen and has high water content. Articular cartilage and the intervertebral disc (IVD) are considered two major cartilaginous tissues in the body. Cartilage and IVD tissue are exposed to loading throughout life. Articular cartilage covers the ends of long bones and functions to bear load and to provide a frictionless sliding during joint movement. Knee cartilage is compressed by ∼3–10% of its overall thickness following various physical activities, such as walking, cycling, running, and knee bending.1,2 Under normal loading, talar cartilage is compressed by 5–35%, with 42% of the contact area having compressive strain higher than 15%.3 The IVD has a heterogeneous structure with distinct regions: the central nucleus pulposus (NP), outer annulus fibrosus (AF), and cartilage endplate (CEP). IVD functions as a cushion to absorb load to protect the vertebral body during body motion, such as bending, twisting, and jumping.4,5 The AF experiences a combination of compressive, tensile and shear stresses during weight-bearing and joint motions.6−10 The NP has also been shown to translate and deform with different loading conditions in experimental studies.11−13
Cells within cartilaginous tissues are “mechanosensitive” and thus respond to mechanical loading. Cells regulate tissue homeostasis and are responsible for synthesis and degradation of matrix proteins. Mechanical loading plays critical roles in cell differentiation, proliferation, biosynthesis, and homeostasis. Overloading or underloading could lead to cell apoptosis, tissue matrix degradation, and increased expression of pro-inflammatory cytokines,14−17 whereas moderate levels of mechanical stimulation maintain normal tissue function and have anti-inflammatory effects.18−20
Inflammation is an important signaling process that occurs in multiple conditions, such as aging, injury, disease, and in response to mechanical loading. These conditions can all serve as primary initiating events of abnormal production of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1α/β (IL-1α/β), IL-6, IL-8, and others. Of these cytokines, TNF-α and IL-1β are the best studied pro-inflammatory cytokines because they play a prominent role in tissue degeneration of IVD (reviewed in ref (21)) and cartilage (reviewed in ref (22 and 23)). In the IVD, these cytokines trigger a range of changes in disc cell phenotypes, including autophagy, senescence, and apoptosis.24−26 It has been shown that TNF-α and IL-1β levels are elevated in degenerate discs and their expression increased with disease severity.27,28 TNF-α and IL-1β induce upregulation of catabolic mediators such as MMP-1, -2, -3, -13, -14, ADAMTS-4/5 and suppress the expression of important matrix genes.27−30 With regard to articular cartilage, levels of TNF-α and IL-1β in joint fluids were elevated acutely following traumatic joint injury compared to uninjured controls,31 and remain elevated in the joints of patients with OA. These cytokines are thought to be involved in the pathophysiology of OA32,33 by stimulating the production of matrix degrading enzymes and other inflammatory cytokines and mediators from chondrocytes and noncartilaginous cells present in the joint (e.g., synovium).34−36
Aging in cartilaginous tissues is characterized by many changes that are similar to those seen in degenerative disease conditions making it challenging to delineate where “healthy” aging and disease interface. Inflammation, which plays a large part in degenerative disease conditions, has also long been associated with aging. The idea of “inflammaging” as first coined by Franceschi et al. in 2000 has shown that systemic levels of inflammatory cytokines and markers increase with age.37,38 On a local tissue level, cartilaginous tissues also exhibit this age dependent increase in inflammatory cytokines and markers (reviewed in ref (39)). For example, aging in the IVD has indeed been shown to be associated with increased levels of TNF and TNF-R expression in NP cells.40 Additional age-associated changes within the IVD may also contribute to an increasingly inflammatory environment in the disc with age. These changes include cell senescence and pro-inflammatory senescent phenotype, increased levels of reactive oxygen species (ROS), accumulation of advanced glycation end products (AGEs), and increased levels of matrix degrading enzymes.39 Furthermore, studies have shown that NF-kB, a key transcription factor in inflammatory signaling pathways, has increased activity with age.41 Articular cartilage presents many of the same age associated changes as seen in the IVD. Cell senesce and pro-inflammatory senescent phenotype, increased presence of matrix degrading enzymes, accumulation of AGEs and ROS which contribute to oxidative stress and damage all promote an inflammatory environment associated with aging in cartilage.42−44
During inflammation and degeneration processes in cartilaginous tissues, there is a shift in extracellular matrix (ECM) homeostasis away from anabolic metabolism toward more catabolic processes. As a result, the biological and biomechanical properties of tissues are altered. The biological changes in articular cartilage and IVD include loss of matrix proteins and altered water content.45−51 The resulting biomechanical alterations in articular cartilage and IVD, however, have different functional manifestation. As cartilage undergoes degeneration in osteoarthritis, articular surfaces become fibrillated and roughened,52 leading to increased surface interaction between articulating cartilage surfaces, decreased shear stiffness, and increased deformation near the surface.53 Compared to normal discs, degenerated discs have a smaller NP area with a lower hydrostatic pressure and a wider posterior annulus with higher stress peaks.4 Structural changes in cartilaginous tissues with degeneration may lead to alterations in load transfer, which may cause pain and lead to further matrix disruption and deterioration.4,11,21,50,53−55
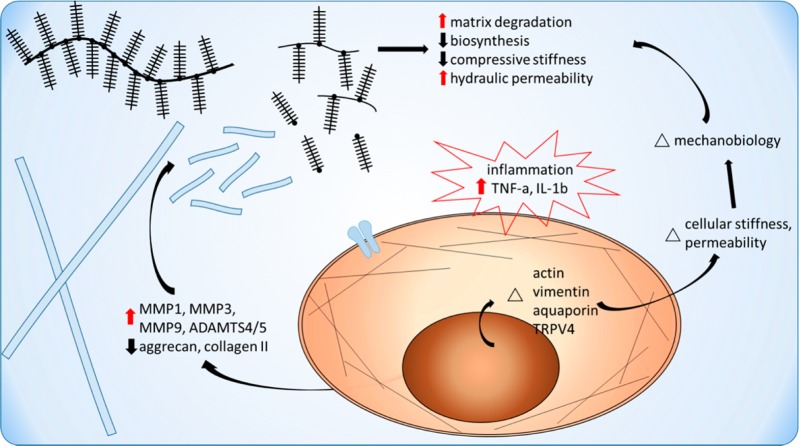
The differential effects of inflammation on cellular processes as well as mechanical function are highly dependent on cell/tissue type, timing, and magnitude. In this review, we summarize key findings pertaining to inflammation, degeneration, and multiscale mechanical properties at the subcellular, cellular, and matrix level in cartilaginous tissues (Figure 1), with emphasis on articular cartilage and NP and AF regions of the IVD. Some inflammatory effects in cartilaginous tissues are similar in articular cartilage and IVD while others are different. This review aims to compare similaries and contrast differences between the two cartilaginous tissue types, to reflect on the functional uniqueness of each tissue/cell type. To recapitulate the pro-inflammatory cascades that occur in vivo, different inflammatory stimuli have been used in vitro and in situ studies, including TNF-α, IL-1α/β, and lipopolysaccharide (LPS). Therefore, this review will focus on the effects of these stimuli because they are the best studied pro-inflammatory cytokines (e.g., TNF-α and IL-1) in cartilaginous tissues. Understanding the current state of the field of inflammation and cell/tissue biomechanics may potentially identify future directions for novel and translational therapeutics with multiscale biomechanical considerations.
Figure 1.
Schematic of integrated effects of pro-inflammatory cytokines on cell and tissue mechanical function and mechanotransduction in cartilaginous tissues.
2. Effects of Inflammation on Tissue Biomechanics
2.1. Inflammation and Matrix Breakdown
Degenerative diseases are characterized by changes in biochemical composition and structure, which alter the tissue biological and biomechanical functions. The biological changes include loss of matrix protein such as proteoglycan and collagen, increase in macroscopic degenerative fibrillation, and altered water content.45−51 Human degenerative IVDs exhibit higher levels of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and others compared to nondegenerative discs.28,40,56,57 The upregulation of pro-inflammatory cytokines not only suppresses the synthesis of ECM proteins (e.g., collagen type II and aggrecan), but also stimulates the release of catabolic proteases (e.g., matrix metallopreoteinase MMPs, aggrecanases ADAMTS4/5).27,58−60 The imbalanced anabolic-catabolic response to inflammation leads to further matrix breakdown and tissue deterioration, which can impair tissue mechanical function. Disc degeneration results in the NP changing from a gelatinous to a more fibrous structure, with the subsequent formation of clefts that eventually extend through the AF.47 Both aging and degree of disc degeneration have been linked to increased levels of pro-inflammatory cytokines40,61 which has itself been shown to up-regulate expression and activity of matrix degrading enzymes and promotes ECM degradation.24,29,62−64
Osteoarthritis (OA) is a disease characterized by degeneration of articular cartilage matrix, bone, synovium, and periarticular tissue.65−67 Similar to degenerative disc diseases, OA involves a complex interplay of biochemical, mechanical, and genetic factors. In the joints of patients with OA, elevated levels of pro-inflammatory cytokines such as TNF-α and IL-1β are present and thought to be involved in the pathophysiology of OA.32,33 These cytokines act on chondrocytes and other cell types in the joint to stimulate the production of matrix degrading enzymes and other inflammatory cytokines and mediators.34−36 The increase in catabolic response in addition to decreased synthesis leads to net catabolic changes in matrix components. Studies investigating the effect of inflammatory stimulation on cartilage explants, with either IL-1α or IL-1β, have shown explants exhibit decreased proteoglycan content as well as increased presence of cleaved and denatured collagen.19,68−71 Inhibitors of matrix degrading enzymes have also been shown to be able to prevent IL-1 induced changes to cartilage explant ECM indicating these inflammatory induced changes are dependent on the production of MMP’s and increased ECM catabolism.70 Following the upregulation of inflammatory mediators and catabolic proteases, there is an increase in collagen type II denaturation and type I procollagen synthesis in degenerated tissue.45 Articular surface of cartilage becomes fibrillated with the presence of matrix cracks and vertical fissures.66
Degenerated cartilaginous tissues often have altered water content compared to healthy normal tissues. Aggrecan, one of the two main components of cartilaginous tissues, is a high-molecular-weight proteoglycan composed of glycosaminoglycan chains attached to a large core protein. Aggrecan exists as large aggregates that form from the interaction of hundreds of aggrecan molecules with hyaluronan.47 Aggrecan aggregates are highly negatively charged molecules, which can absorb and retain water and provide compression-resisting properties of the NP and articular cartilage. Inflammatory factors disturb aggrecan homeostasis by decreasing its synthesis and increasing its catabolism by elevated levels of aggrecanases ADAMTS4/5, which could lead to a net catabolism of aggrecan. The decrease in aggrecan content with tissue degeneration results in differential changes in water content, depending on tissue types. For example, the decrease in aggrecan in the NP ECM directly leads to decrease in fixed charge density and decreased water content in degenerating IVD.46 However, in articular cartilage, water content is increased with tissue degeneration. This is likely because water content in articular cartilage is regulated by a more complex interplay of collagen and aggrecan, with collagen network providing resistance to swelling tendency of aggrecan molecules. In degenerative cartilage, the collagen network is disrupted, resulting in an ECM with higher tendency to swell, and hence having higher water content.50,51,72
2.2. Inflammation and Tissue Mechanical Properties
Changes in ECM composition and structure of articular cartilage and of the IVD due to inflammation considerably change the mechanical properties of the whole tissues. Mechanical damage, aging, and trauma are believed to trigger inflammatory process in many tissues surrounding the joints and the IVD.27,40,60,61,73−76 Mechanical loading, and specifically mechanical injury, regulates inflammatory signaling in articular cartilage (reviewed in refs (77−79)). Effects of inflammation on cartilage mechanical properties have been studied through the context of OA and injury-induced trauma. Through different stages of OA, cartilage stiffness is changed dramatically depending on disease severity. During early stages of OA, cartilage in osteoarthritic joints has decreased shear modulus, tensile stiffness, and compressive strength compared to nonosteoarthritic tissue.80−84 Additionally, differences in cartilage mechanical properties might be dependent on the degree of local inflammatory state, where increased white blood cell count in patients’ synovial fluid is an indicator of reduced cartilage compressive stiffness.81 As the disease progresses, there are increased surface fibrillation, further loss of aggrecan and collagen, and increased water content,55 which result in further deterioration of mechanical integrity.55,85
Inflammation induces ECM catabolism through regulation of matrix degrading enzymes, such as MMPs and aggrecanases, which can have a deleterious effect on the mechanical properties of musculoskeletal tissues (see Table 1). In an organ culture model of intact bovine IVD, TNF-α treatment (100 ng/mL, 6 days) causes tissue stiffening as indicated by a 25% reduction in diurnal displacement and a 40–50% increase in dynamic stiffness from ∼2300 N/mm in unreated samples to 2800 N/mm in TNF-α-treated samples.86 This response was likely dominated by the increased collagen staining rather than the reduced aggrecan content observed on histology. Similarly, AF tissue from degenerate IVD had a 2 fold higher compressive stiffness (∼1000 kPa) (i.e., aggregate modulus) compared to nondegenerate tissue (∼500 kPa),87 which is likely related to condensed matrix associated with water loss during disc degeneration. While few other studies have directly observed the effects of inflammatory stimulation on IVD mechanical properties, treatment with MMPs or other matrix degrading enzymes such as papain has been shown to lead to overall tissue matrix disruption as well as decreased compressive and rotational stiffness of the bovine IVD organ culture.88,89 Similarly, collagenase treatment (0.1 wt %, 120 min) of cartilage explants induced OA-like changes in tissue ECM including a 45% increase in tissue permeability (4.73 to 6.83 m4/(N S) × 10–14) and a 50% decrease in aggregate modulus (0.13 to 0.06 MPa).90 Unlike the IVD, there have been more studies in cartilage observing the direct effect of inflammatory stimulation on cartilage explant properties. Stimulation with IL-1α (5 ng/mL, 7 days) lead to an 80% decrease in compressive equilibrium (0.7 to 0.15 MPa) and a 70% decrease in dynamic moduli (7 to 2 MPa); similar effects are also seen with IL-1β treatement.68,70 In another study, inflammatory stimulation with IL-1α (5 ng/mL, 21 days) was not found to affect cartilage explant tensile modulus but did reduce ultimate tensile strength by 30% (10 to 7.5 MPa) and tensile strain at failure by 33% (0.7 mm/mm to 0.4 mm/mm).69 Similar effects have been observed in engineered cartilage constructs where inflammatory stimulation with IL-1α or IL-1β also shows decreased compressive equilibrium and dynamic modulus as well as decreased proteoglycan content.91−93 Furthermore, studies comparing effect of IL-1α or IL-1β on dense cartilage explants vs less dense engineered cartilage show that engineered cartilage is more susceptible to proteoglycan loss and decreased modulus following IL-1 stimulation.92
Table 1. Changes in Tissue Level Biomechanical Properties in Response to Pro-Inflammatory Stimuli.
| tissue type | inflammatory stimulus/indicator | culture condition | dosage/duration | mechanical property | ECM change | ref |
|---|---|---|---|---|---|---|
| articular cartilage | IL-1α | explant culture | 5 ng/mL | ↓ dyn modulus | ↓ GAG | (68) |
| 7 days | ↓ equil modulus | ↓ collagen | ||||
| articular cartilage | IL-1α | explant culture | 5 ng/mL | ↓ ultimate tensile strength | ↓ GAG | (69) |
| 7–21 days | ↓ tensile failure strain | ↓ Collagen | ||||
| articular cartilage | IL-1α | explant culture | 10 ng/mL | ↓ GAG | (19) | |
| 6 days | ↓ collagen | |||||
| articular cartilage | IL-1α | explant culture | 10 ng/mL | no change in dyn and equil modulus | no change in GAG or Collagen | (92) |
| 14 days | ||||||
| articular cartilage | IL-1α | tissue engineered construct | 10 ng/mL | ↓ equilibrium modulus | ↓ GAG | (93) |
| 7 days | ||||||
| articular cartilage | IL-1α | tissue engineered construct | 10 ng/mL | ↓ dyn modulus | ↓ GAG | (92) |
| 14 days | ↓ equil modulus | ↓ collagen | ||||
| articular cartilage | IL-1α | tissue engineered construct | 10 ng/mL | ↓ dyn modulus | ↓ GAG | (91) |
| 14 days | ↓ equil modulus | |||||
| articular cartilage | IL-1β | explant culture | 10 ng/mL | ↓ GAG | (19) | |
| 6 days | ↓ collagen | |||||
| articular cartilage | IL-1β | explant culture | 10 ng/mL | ↓ GAG | (71) | |
| 28–48 days | ||||||
| articular cartilage | IL-1β | explant culture | 100 ng/mL | ↓ equilibrium modulus | ↓ GAG | (70) |
| 8 days | ||||||
| articular cartilage | IL-1β | tissue engineered construct | 10 ng/mL | ↓ equilibrium modulus | ↓ GAG | (93) |
| 7 days | ||||||
| articular cartilage | IL-1β | tissue engineered construct | 10 ng/mL | ↓ dyn modulus | ↓ GAG | (91) |
| 14 days | ↓ equil modulus | |||||
| articular cartilage | collagenease | explant culture | 0.1 wt % | ↓ aggregate modulus | ↓ GAG | (90) |
| 120 min | ↑ permeability | |||||
| articular cartilage | ↑ white blood cells in synovial fluid | clinical study | ↓ dyn modulus | (81) | ||
| ↓ aggregate modulus | ||||||
| IVD | TNF-α | 3D organ culture | 100 ng/mL | ↑ dyn stiffness | ↓ GAG | (86) |
| 6 days | ↓ creep strain | ↑ collagen | ||||
| IVD | TNF-α | 3D organ culture | 200 ng/mL | ↓ GAG disrupted ECM organization | (24) | |
| 7 days | ||||||
| IVD | TNF-α & IL-1β | 3D organ culture | 100 ng/mL TNF | ↓ GAG disrupted ECM organization | (64) | |
| 10 ng/mL IL-1 | ||||||
| 3–10 days | ||||||
| IVD | Papain | 3D organ culture | 150 U/mL | ↓ compressive stiffness | ↓ GAG | (89) |
| 10 day | ↓rotational stiffness | |||||
| IVD | MMP3 | 3D organ culture | 10 μg/mL | no correlation between disc height and GAG content | (88) | |
| 8 days | ||||||
| IVD | ADAMTS-4 | 3D organ culture | 10 μg/mL | no correlation between disc height and GAG content | (88) | |
| 8 days | ||||||
| IVD | HTRA-1 | 3D organ culture | 10 μg/mL | correlation between disc height and GAG content | (88) | |
| 8 days |
Dyn stands for dynamic and equil stands for equilibrium.
Changes in IVD mechanical properties seen during degeneration are likely affected by matrix fibrillation in addition to ECM degradation and altered hydrostatic pressure (Table 1). Although the role of inflammatory stimulation on hydrostatic pressurization has not been directly investigated, loss of hydrostatic pressure, swelling pressure, and aggregate modulus have all been shown to occur in degenerate human and mouse IVD94,95 and can alter stress and load distribution in the IVD.4 Interaction between tissue mechanical behavior and inflammatory signaling is bidirectional. Mechanical loading indeed has been shown to regulate inflammatory signaling in IVD tissues. Injurious loading such as static loading,96 asymmetrical loading,97 or superphysiological strain73,98 induces increased levels of pro-inflammatory cytokines, which can further perpetuate degenerative changes seen due to injurious loading (reviewed in ref (54)). Abnormal asymmetric loading, consisting of axial compression applied at a 15° angulation to IVD explants where the cartilaginous EP was removed, resulted in spatially dependent changes in inflammatory, ECM, and mechanical effects. Regions exposed to greater stress magnitude (convex side) exhibited increased pro-inflammatory gene expression of IL-1β, IL-6, MMP-1 and ADAMTS4 compared to the control symmetric loading conditions. Additionally, regions under convex loading had lower tissue compressive aggregate modulus compared to regions under concave loading.97 Superphysiological cyclic loading has also been shown to increase pro-inflammatory cytokines in human NP and AF cells, as well as98 in NP explant tissue to facilitate diffusion of inflammatory cytokines and inflammatory induced changes in NP tissue.86
Aging is a risk factor for OA. With advanced aging, loss of ECM hydration and associated PG content decrease the macroscopic compressive properties of cartilage.50,99 At the nanoscale level, increased age results in production of altered aggrecan molecules, with shorter core protein length and shorter side chains.100 Adult aggrecan was also found to be significantly weaker in compression than newborn aggrecan, even at the same total GAG density.100 With age, changes to the collagen network occur, including increased fibril diameter, due to fibril bundling or loss of interfibrillar PGs, and increased collagen cross-linking.101−103 At the macroscopic level, there is a decrease in the tensile-strength properties of cartilage with maturation.104,105 However, at the nanoscale, aging results in a stiffer matrix when measured using nanoindentation.106 These studies provide evidence of the effects of age on the structural and nanomechanical properties of cartilage ECM, with direct implications for alterations in cell–ECM interactions with age.
3. Effects of Inflammation on Cell Biomechanics
3.1. Effects of Inflammation on Cellular Biology
Inflammatory stimuli can alter cytoskeletal components of cells in musculoskeletal tissues. The cytoskeleton of a cell consists of filamentous actin (F-actin), intermediate filaments such as vimentin, and tubulin microtubules, all of which play important roles in maintaining cell biological as well as mechanical functions. Cells from bovine NP of IVD when exposed to pro-inflammatory treatment in vitro exhibited altered F-actin cytoskeleton107 (Table 2). Cells from AF region of IVD when treated with TNF-α in vitro showed an increased content of F-actin and a more diffusely connected α-tubulin network, but no change in vinculin.108 Similarly, chondrocytes cultured in monolayer treated with TNF-α or IL-1 for 24 h increased the expression level of F-actin.109,110 Alterations in cytoskeleton organization due to inflammation is also dependent on culture condition as well as the cellular microenvironment (2D vs 3D, Table 2). In normal NP cells and chondrocytes cultured in 3D, F-actin forms a bright solid ring around the periphery (cortex) of the cells. In situ chondrocytes have less organized F-actin compared to isolated cells and dispersed throughout the cells with focal areas of intense staining.109 With inflammatory treatment, F-actin in isolated NP cells and chondrocytes becomes more punctate and the cortical localization of the filaments is no longer apparent.107,109,111 F-actin organization in isolated cells treated with pro-inflammatory cytokines is similar in structure to F-actin when cells were treated with cytochalasin D, a reagent that disrupts actin polymerization.111 IL-1α did not affect F-actin organization of chondrocytes in situ, but enhanced F-actin expression at the cell periphery.109 When culture condition was changed to 2D, chondrocytes treated with TNF-α or IL-1β exhibited increased stress fiber formation.110 These pro-inflammatory cytokines are also known to cause alteration in tubulin organization. AF cells when treated with TNF-α have a more diffusely connected microtubule network of α-tubulin.108
Table 2. Changes in Cellular Biomechanical Properties in Response to Inflammatory Stimuli.
| cell type | inflammatory stimulus | culture condition | dosage/duration | mechanical property | cytoskeleton change | reference |
|---|---|---|---|---|---|---|
| chondrocyte | IL-1β | 2D | 10 ng/mL | ↑ stiffness | ↑ F-actin | (110) |
| 24 h | ||||||
| chondrocyte | TNF-α | 2D | 40 ng/mL | ↑ stiffness | ↑ F-actin | (110) |
| 24 h | ||||||
| chondrocyte | IL-1α | 3D | 10 ng/mL | ↑ F-actin | (109) | |
| 1 h | altered F-actin distribution | |||||
| chondrocyte | IL-1α | in situ | 10 ng/mL | ↑ F-actin | (109) | |
| 1 h | altered F-actin distribution | |||||
| nucleus pulposus | LPS | 2D | 1 μg/mL | ↑ hydraulic permeability | altered F-actin distribution | (107) |
| 24 h | ||||||
| nucleus pulposus | TNF-α | 2D | 10 ng/mL | ↑ hydraulic permeability | altered F-actin distribution | (107) |
| 24 h | ||||||
| annulus fibrosis | TNF-α | 2D | 10 ng/mL | ↑ F-actin | (108) | |
| 24 h |
3.2. Effects of Inflammation on Cellular Mechanical Properties
Cytoskeletal changes induced by pro-inflammatory cytokines lead to significant changes in cellular biophysical properties (Table 2). Hydraulic permeability and size of NP cells treated with inflammatory factors, such as LPS or TNF-α increased significantly and remained elevated after 1 week post treatment in vitro.107 A linear correlation was observed between hydraulic permeability and cell radius in untreated cells, but not in the inflammatory treated cells. The loss of correlation between cell size and hydraulic permeability suggests that regulation of biophysical properties of NP cells is disrupted irreversibly due to inflammatory stimulation in 2D in vitro culture.107
Alteration in cytoskeleton components also lead to marked changes in cellular biomechanical properties since these elements play major roles in regulating the mechanical properties of cells. F-actin is the main contributor to the cellular stiffness in many cell types while tubulin has a more minor contribution. Disruption of F-actin with cytochalasin D has been shown to result in reduction of chondrocyte stiffness.110−112 The effect of cytochalasin D on chondrocyte stiffness is greater on healthy and normal chondrocytes than those isolated from osteoarthritic cartilage.111 Chondrocytes treated with pro-inflammatory cytokines, such as TNF-α and IL-1β have 50% higher stiffness compared to untreated cells, which might be due to an increase in F-actin.110On the other hand, changes to other cytoskeletal elements such as vimentin under inflammatory conditions are unknown. In general, the contribution of vimentin to cellular physical properties remains debatable. In some studies, chondrocytes with disrupted vimentin using 4–5 mM acrylamide had a reduced stiffness compared to untreated cells,112,113 while other studies showed that chondrocyte stiffness was unaffected by vimentin disruption using same treatment.110,111 The differences in these studies could be due to different testing configuration and methods. In one study, cells were cultured and tested in 2D on tissue culture plastic,110 whereas in other studies cells were cultured in alginate and tested in suspension111 or in alginate.113 The conflicting results of those studies suggest that microenvironment that cells are exposed to may change the phenotype of the cells, and hence, their cytoskeleton structure, cell morphology, and mechanical properties.
The physical interactions between cells and their microenvironment and how those interactions regulate the differentiation state of primary cells have been investigated in several studies using substrates with tunable mechanical stiffness. Studies demonstrate that the differentiation state of chondrocytes and immature NP cells of the IVD can be regulated by mechanical substrate stiffness.114−119 Rat chondrocytes cultured in 2D on polyacrylamide gels with varying stiffness were found to express various degrees of collagen type I and collagen type II.117 On the “stiff” substrate (40 kPa) with stiffness similar to the pericellular matrix surrounding chondrocytes, cells overexpressed Col-1 relative to Col-2, indicative of dedifferentiation.117 When cultured on “soft” substrate (4 kPa) with stiffness on the same order of magnitude as the cell, chondrocytes produced higher level of SOX9, Col-2 and aggrecan.117 NP cells from skeletally immature pigs cultured on “soft” laminin-containing basement membrane extract (BME, 0.3 kPa) produced more proteoglycans than NP cells cultured on rigid plastic surface coated with BME.116 This protective effect of soft substrate on NP cell phenotype is dependent on N-cadherin expression.120 Understanding how cells interact and respond to the in vitro mechanical cues via substrate stiffness may be helpful to further understand cell-matrix interaction in vivo. However, one limitation of such models is that the full repertoire of native cell–matrix interactions is not fully recapitulated, and thus interactions and compensatory mechanisms across varying cell–matrix interactions are difficult to assess.
Various studies have examined the effect of age on chondrocyte mechanical properties from model systems such as rabbit, bovine and from human cartilage.112,121−123 Findings from these studies demonstrate a variety of trends that are dependent on species and/or method of testing. A recent study using a bovine model indicated that chondrocytes exhibit increased stiffness with maturation (between neonatal and adulthood) (from ∼0.5 kpa to 1 kPa), but not with further aging into late adulthood.112 Steklov et al. have reported that the stiffness of chondrocytes obtained from older human individuals (>55 years old) was higher than chondrocytes obtained from younger human individuals (18–35 years old).122 To the contrary, human OA chondrocytes obtained from older patients had an overall lower mechanical stiffness (0.037 N/m) than normal chondrocytes obtained from younger patients (0.096 N/m).121 Thus, it is unclear if the modulation of the biomechanical properties is due to aging or disease. One interpretation could be that increases in elastic properties into adulthood are correlated with healthy aging. However, a peak in biomechanical properties may occur during maturation, followed by decline in cellular biomechanical properties in age-dependent degeneration.
Changes in cell mechanical properties could be interpreted as an adaptation to matrix stiffness changes, in order for cells to survive an altered microenvironment. Cytokines accelerate the degeneration of the extracellular matrix; consequently matrix stiffness is reduced and cell-matrix attachment may be altered. Under inflammatory conditions, cells from articular cartilage become stiffer and more resistant to compressive stress.108,110 A possible explanation is that these cells experience and sense increased stress due to a reduction in surrounding ECM stiffness and compensate by becoming stiffer. One potential effect of the cytokine-induced cellular stiffening is that cells may become less sensitive to small perturbations/alterations in strain/stress. Through cytoskeletal reorganization, cells may alter their mechanical stiffness to adapt to the changes occurring in the ECM.
To address the adaptive hypothesis for multiscale biomechanical alterations in pro-inflammatory conditions, complex nonlinear multiscale computational biomechanical models are needed. Advances in computational biomechanics provide useful tools to study the complex interaction of cells and their microenvironment in many instances where it may be technically challenging (e.g., 3D real time strain analysis of cells under deformation in situ or in vivo) or impossible (e.g., quantification of stress states) to obtain experimental measurements. The finite element (FE) method provides a powerful approach to obtain the response of a complex system from individual contributions of elements. There is an extensive body of work on cellular mechanics of chondrocytes and NP cells using FE modeling.124−131 Various multiscale FE models have been developed to simulate cell-matrix interaction under static127,132 or transient/dynamic compressive loading,133,134 to understand differences in cellular and tissue response to changes in matrix environment due to loading. Using FE modeling in combination with experimental testing, deformation behavior and mechanical properties of cells, as well as cell–matrix interaction could be further investigated, under healthy and degenerate conditions.127,135,136 Development of such models for evaluation of inflammatory effects on multiscale biomechanical properties would further advance the state of understanding in the field.
3.3. Effects of Inflammation on Cellular Mechanotransduction
Alteration in cytoskeleton components and mechanical properties of cells due to inflammation may result in alteration in cell mechanobiology in response to stress or mechanical cues. One way that cells transduce mechanical signal is through deformation of the cytoskeleton.137,138 Pro-inflammatory cytokines that could alter cytoskeleton components and cellular stiffness could also regulate the mechanoresponsiveness of cells to loading. Treatment with TNF-α or IL-1β resulted in a reduced contraction of chondrocytes in response to the contractile agonist histamine.110 Higher stiffness and lower contraction responsiveness indicated that these chondrocytes are in a more contracted state even in the absence of mechanical stimulation. Exposure to IL-1β exacerbated the catabolic effect of pathophysiologically high tensile strain (18%) and prolonged (24 h) tensile strain on AF cells from rabbits.139
The change in cell mechanobiology and mechanosensitivity under pro-inflammatory conditions could be the result of altered mechanotransduction pathways. Cell surface channels, such as water channels and mechanosensitive ion channels are also regulated by pro-inflammatory stimulation. NP cells experience daily fluctuations in water content, with measurements showing 8% water loss upon loading, highlighting the importance of controlling water transport to withstand osmotic and volumetric changes in these cells and for disc function.140 Bovine NP cells exposed to inflammatory stimuli in vitro such as LPS and TNF-α exhibited a decrease in aquaporin-1 expression.107 Inflammatory induced reduction of aquaporin expression is consistent with findings in human IVD, where aquaporin-1 and aquaporin-5 expression decrease with increasing degeneration.141 Transient receptor potential vallinoid-4 (TRPV4) is a calcium channel present in various cell types, including AF and NP cells of the IVD, articular chondrocytes, and cells from peri-articular tissues such as synovium, bone, and muscle.142−147 Inflammation has an indirect effect on TRPV4 expression through modulation of tissue osmolarity. Treatment with TNF-α did not alter TRPV4 expression in isolated NP cells. However, NP and AF cells in whole IVD cultured in media containing TNF-α showed an increase in TRPV4 expression.143 Taken together, these results suggest that the upregulation of TRPV4 in disc cells is likely due to reduction in tissue osmolarity following proteoglycan degradation induced by exposure to pro-inflammatory cytokines such as TNF-α. Degenerated human IVD and cells in synovium of early OA also showed an increased expression of TRPV4 compared to healthy tissue.143,146 TRPV4 is believed to play a central role in the cellular signal transduction in response to mechanical or osmotic stimulations. Inhibition of TRPV4 during dynamic loading prevented up-regulation of pro-anabolic and anticatabolic genes and attenuated the enhancement of matrix accumulation and mechanical properties of chondrocyte-embedded agarose constructs.144 Another family of ion channels implicated in tissue/cell injury is the PIEZO family. PIEZOs are cation-permeable channels that can be activated directly by mechanical signals. PIEZO1 and PIEZO2 are abundantly expressed in normal articular chondrocytes from mice, pigs, and humans as well as in other tissues.148 High strain (≥50% deformation) applied to articular chondrocytes causes increased intracellular Ca2+ influx through PIEZO1 and PIEZO2, which is dependent on actin polymerization.148 Although it has been shown that PIEZO channels involved directly to the mechano-transduction of primary chondrocytes in response to high mechanical strain, how pro-inflammatory cytokines affect PIEZO expression and function remains to be fully investigated.
Integrins are heterodimeric transmembrane glycoproteins, consisting of α and β subunits, and are major mechano-receptors in various tissues within the body.149 Similar types of integrins have been identified in articular chondrocytes and cells from NP and AF regions of the IVD.150−152 Integrin-mediated mechano-transduction involves recognition of the mechanical stimulus by integrins and activation of integrin-mediated signaling pathways leading to biochemical changes. Studies have shown that integrin expression was increased when cells were cultured in inflammatory conditions.153,154 Evidence also exists to support that articular chondrocytes, NP and AF cells from healthy tissues respond to mechanical cues through integrin-mediated pathway, whereas mechanotransduction in similar cell types from degenerate tissues involve different signaling pathways that is not dependent on integrins.155−158 The use of different mechano-transduction pathways by cells isolated from tissues with different health status (heathy vs degenerative) may explain different responses of these cells to similar mechanical stimulation. For example, dynamic compression induced proteoglycan synthesis in normal chondrocytes, whereas similar loading induced a catabolic response in chondrocytes from OA cartilage indicated by upregulation of IL-1β and IL-6.15,158−160 Cyclic tensile strain at physiological frequency (1.0 Hz) induced an anticatabolic response in AF cells isolated from nondegenerated human IVD as indicated by decreased expression of matrix degrading enzymes such as MMP-3 and ADAMTS-4 with no change in expression of matrix genes such as aggrecan and collagen II. Similar loading condition, however, induced a catabolic response in AF cells from degenerated human discs, as indicated by a down-regulation of aggrecan.161,162 Similar differential effects of mechanical stimuli were also observed in AF cells from rabbits.163 Moreover, human NP cells derived from degenerate IVDs exhibited a lack of response to hydrostatic pressure, in contrast to the anabolic response observed in cells derived from nondegenerate IVDs.164 Taken together, these studies suggest that degenerative changes in surrounding microenvironment due to inflammation or disease not only change how the cells respond to mechanical signals (e.g., through different pathways), but also decrease their mechano-sensitivity.
3.4. Disease Modifying Drugs
Several anti-inflammatory, anticatabolic, and pro-anabolic drugs to treat OA and disc degeneration have been recognized as potentially useful therapies to reverse or prevent matrix breakdown and progressive tissue degeneration.21,165−167 Anti TNF-α therapy using etanecerpt, infliximab, and others has been shown to improve pain and behavioral responses in human clinical trials on patients with radicular pain due to lumbar spinal stenosis and patients with sciatica [reviewed in ref (21)]. Anticatabolic glucocorticoids, pro-inflammatory cytokine inhibitors (IL1-ra, anti-TNF-α), MMP inhibitors, and pro-anabolic growth factors (IGF-1, FGF-18, and BMP-7) are disease modifying drugs under investigation for efficacy using cartilage explants.168−171 The use of anti-inflammatory agent Flavopiridol,172,173 an inhibitor of the transcription factor cyclin-dependent kinase 9 (CDK9) has shown efficacy in protecting cartilage from inflammatory induced decrease in PG content and loss of compressive stiffness in vitro.172 Alternatively, the use of the chemical cross-linking agent Genepin has been shown to protect the compressive stiffness of engineered cartilaginous constructs against pro-inflammatory cytokines.174 Due to the avascular nature of articular cartilage and the IVD, systemic administration of these drugs could offer limited effects as a majority of the active molecules may degrade before sufficient diffusion or accumulation into the targeted tissues.
Several approaches using micro- and nanocarriers have been developed for sustained delivery of small drug molecules locally and directly to the target tissue.175−182 Chitosan-based thermosensitive hydrogels have been investigated as drug depots of anti TNF-α therapeutics, which would allow local and sustained release and may increase the efficacy of anti TNF-α treatment.183 Upon intra-articular injection of siRNAs (inhibiting TNF-α) encapsulated in poly(dl-lactide-co-glycolide) (PLGA) microspheres, siRNAs was slowly released and effectively inhibited the expression of TNF-α in murine arthritic joints.180 Similarly, intra-articular injection of IL1-ra encapsulated in PLGA microspheres inhibited joint inflammation in an anterior cruciate ligament transection (ACLT) rat model as indicated by a reduction in lymphocyte proliferation and cartilage degradation. Cartilage and synovial histopathology scores were also reduced. Serum levels of IL1-ra were also significantly lower with injection of PLGA/IL1-ra compared to free IL1-ra.178 Encapsulating dexamethasone, an anti-inflammatory drug, in superparamagnetic iron oxide nanoparticles (SPIONs) demonstrated an increased joint retention and similar anti-inflammatory effect to free dexamethasone in a mouse model.182 When translating the findings of these studies to clinical application, challenges with diffusion and dosage due to animal sizes and clearancepresent potential limitations of these small animal models. Taking advantage of the negative charge density of articular cartilage, Avidin and other polypeptides are also being investigated as positively charged drug carriers into cartilage, where the negatively charged cartilage matrix facilitates Avidin’s retention.175,176 Charge-based intracartilage delivery of a single dose of dexamethasone using Avidin nanocarriers was found to suppress IL-1α induced catabolism long-term.176 These local delivery systems confirm that rapid drug penetration, sustained release, and prolonged joint retention within the target tissue can be efficacious at protecting tissue biomechanics and biochemistry from pro-inflammatory insults.
5. Conclusion
In summary, inflammation following mechanical injury or disease causes up-regulation of pro-inflammatory cytokines in cartilaginous tissue, which not only suppresses the synthesis of ECM proteins, but also stimulates the release of catabolic proteases. At the cellular and subcellular levels, inflammation causes disruption of cytoskeleton structure, altered cellular biomechanical and biophysical properties, and changes in the expression of water and ion channels. These changes could potentially lead to impaired cellular functions in maintaining tissue homeostasis. The imbalanced anabolic-catabolic response to inflammation leads to matrix breakdown and tissue deterioration, which impair tissue mechanical function.
A growing body of research has focused on the effects of inflammation on various cartilaginous cell and tissue properties. However, the relationship between cellular property alterations and macroscopic tissue level alterations has not been delineated. As knowledge of the effect of inflammation on mechanical properties of cartilaginous tissue as well as on the biophysical properties and phenotypes of cells within these tissues continues to grow, we will be able to form a more complete picture of how inflammation-induced changes compare to changes observed within diseased states. Understanding disease mechanism in terms of cell and tissue biomechanics will enrich experimental therapeutic approaches to mitigate and potentially reverse disease progression.
Acknowledgments
We acknowledge our funding sources: National Science Foundation CAREER Award 1151605, National Institute of Health (NIH) R01AR069668, NIH R41 AG050021, and the Feinstein Institute for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
The authors declare no competing financial interest.
References
- Eckstein F.; Lemberger B.; Stammberger T.; Englmeier K. H.; Reiser M. Patellar cartilage deformation in vivo after static versus dynamic loading. J. Biomech 2000, 33 (7), 819–25. 10.1016/S0021-9290(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Kersting U. G.; Stubendorff J. J.; Schmidt M. C.; Brüggemann G. P. Changes in knee cartilage volume and serum COMP concentration after running exercise. Osteoarthritis Cartilage 2005, 13 (10), 925–34. 10.1016/j.joca.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wan L.; de Asla R. J.; Rubash H. E.; Li G. In vivo cartilage contact deformation of human ankle joints under full body weight. J. Orthop. Res. 2008, 26 (8), 1081–9. 10.1002/jor.20593. [DOI] [PubMed] [Google Scholar]
- Adams M. A.; McNally D. S.; Dolan P. ’Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J. Bone Jt. Surg., Br. Vol. 1996, 78 (6), 965–72. 10.1302/0301-620X78B6.1287. [DOI] [PubMed] [Google Scholar]
- Wilke H. J.; Neef P.; Caimi M.; Hoogland T.; Claes L. E. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Philadelphia) 1999, 24 (8), 755–62. 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- Galante J. O. Tensile properties of the human lumbar annulus fibrosus. Acta Orthop. Scand. 1967, 38 (Suppl 100), 1–91. 10.3109/ort.1967.38.suppl-100.01. [DOI] [PubMed] [Google Scholar]
- Nachemson A. Lumbar intradiscal pressure. Experimental studies on post-mortem material. Acta Orthop. Scand. 1960, 31 (suppl 43), 1–104. 10.3109/ort.1960.31.suppl-43.01. [DOI] [PubMed] [Google Scholar]
- Shiraz-Adl A. Strain in fibers of a lumbar disc. Analysis of the role of lifting in producing disc prolapse. Spine (Philadelphia) 1989, 14 (1), 96–103. 10.1097/00007632-198901000-00019. [DOI] [PubMed] [Google Scholar]
- Ebara S.; Iatridis J. C.; Setton L. A.; Foster R. J.; Mow V. C.; Weidenbaum M. Tensile properties of nondegenerate human lumbar anulus fibrosus. Spine (Philadelphia) 1996, 21 (4), 452–61. 10.1097/00007632-199602150-00009. [DOI] [PubMed] [Google Scholar]
- Iatridis J. C.; Kumar S.; Foster R. J.; Weidenbaum M.; Mow V. C. Shear mechanical properties of human lumbar annulus fibrosus. J. Orthop. Res. 1999, 17 (5), 732–7. 10.1002/jor.1100170517. [DOI] [PubMed] [Google Scholar]
- Osti O. L.; Vernon-Roberts B.; Moore R.; Fraser R. D. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J. Bone Joint Surg. Br. 1992, 74 (5), 678–682. [DOI] [PubMed] [Google Scholar]
- Seroussi R. E.; Krag M. H.; Muller D. L.; Pope M. H. Internal deformations of intact and denucleated human lumbar discs subjected to compression, flexion, and extension loads. J. Orthop. Res. 1989, 7 (1), 122–31. 10.1002/jor.1100070117. [DOI] [PubMed] [Google Scholar]
- Shah J. S.; Hampson W. G.; Jayson M. I. The distribution of surface strain in the cadaveric lumbar spine. J. Bone Joint Surg. Br. 1978, 60-B (2), 246–251. [DOI] [PubMed] [Google Scholar]
- Honda K.; Ohno S.; Tanimoto K.; Ijuin C.; Tanaka N.; Doi T.; Kato Y.; Tanne K. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur. J. Cell Biol. 2000, 79 (9), 601–609. 10.1078/0171-9335-00089. [DOI] [PubMed] [Google Scholar]
- Mohtai M.; Gupta M. K.; Donlon B.; Ellison B.; Cooke J.; Gibbons G.; Schurman D. J.; Smith R. L. Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro. J. Orthop. Res. 1996, 14 (1), 67–73. 10.1002/jor.1100140112. [DOI] [PubMed] [Google Scholar]
- Gosset M.; Berenbaum F.; Levy A.; Pigenet A.; Thirion S.; Saffar J. L.; Jacques C. Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis Res. Ther 2006, 8 (4), R135. 10.1186/ar2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando A.; Hagiwara Y.; Tsuchiya M.; Onoda Y.; Suda H.; Chimoto E.; Itoi E. Increased expression of metalloproteinase-8 and −13 on articular cartilage in a rat immobilized knee model. Tohoku J. Exp. Med. 2009, 217 (4), 271–8. 10.1620/tjem.217.271. [DOI] [PubMed] [Google Scholar]
- Gassner R.; Buckley M. J.; Georgescu H.; Studer R.; Stefanovich-Racic M.; Piesco N. P.; Evans C. H.; Agarwal S. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J. Immunol. 1999, 163 (4), 2187–2192. [PMC free article] [PubMed] [Google Scholar]
- Torzilli P. A.; Bhargava M.; Park S.; Chen C. T. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage 2010, 18 (1), 97–105. 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti M.; Gassner R.; Wang Z.; Perera P.; Deschner J.; Sowa G.; Salter R. B.; Agarwal S. Biomechanical signals suppress proinflammatory responses in cartilage: early events in experimental antigen-induced arthritis. J. Immunol. 2006, 177 (12), 8757–66. 10.4049/jimmunol.177.12.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud M. V.; Shapiro I. M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 2014, 10 (1), 44–56. 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M.; Martel-Pelletier J.; Lajeunesse D.; Pelletier J. P.; Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7 (1), 33–42. 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- Lieberthal J.; Sambamurthy N.; Scanzello C. R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage 2015, 23 (11), 1825–34. 10.1016/j.joca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purmessur D.; Walter B. A.; Roughley P. J.; Laudier D. M.; Hecht A. C.; Iatridis J. A role for TNFα in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochem. Biophys. Res. Commun. 2013, 433 (1), 151–6. 10.1016/j.bbrc.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C.; Yan J.; Jiang L. S.; Dai L. Y. Autophagy in rat annulus fibrosus cells: evidence and possible implications. Arthritis Res. Ther 2011, 13 (4), R132. 10.1186/ar3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.; Evans H.; Trivedi J.; Menage J. Histology and pathology of the human intervertebral disc. J. Bone Joint Surg Am. 2006, 88 (Suppl 2), 10–14. 10.2106/00004623-200604002-00003. [DOI] [PubMed] [Google Scholar]
- Le Maitre C. L.; Freemont A. J.; Hoyland J. A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther 2005, 7 (4), R732–45. 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre C. L.; Hoyland J. A.; Freemont A. J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res. Ther 2007, 9 (4), R77. 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguin C. A.; Pilliar R. M.; Roughley P. J.; Kandel R. A. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Philadelphia) 2005, 30 (17), 1940–8. 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- Wang J.; Markova D.; Anderson D. G.; Zheng Z.; Shapiro I. M.; Risbud M. V. TNF and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J. Biol. Chem. 2011, 286 (46), 39738–39749. 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swärd P.; Frobell R.; Englund M.; Roos H.; Struglics A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)--a cross-sectional analysis. Osteoarthritis Cartilage 2012, 20 (11), 1302–8. 10.1016/j.joca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Fukui N.; Purple C. R.; Sandell L. J. Cell biology of osteoarthritis: the chondrocyte’s response to injury. Curr. Rheumatol. Rep. 2001, 3 (6), 496–505. 10.1007/s11926-001-0064-8. [DOI] [PubMed] [Google Scholar]
- Goldring M. B.; Otero M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23 (5), 471–8. 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak J. F.; Pfers I.; Meyer Zum Büschenfelde K. H.; Märker-Hermann E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin. Exp. Rheumatol. 1996, 14 (2), 155–162. [PubMed] [Google Scholar]
- Moos V.; Fickert S.; Müller B.; Weber U.; Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J. Rheumatol. 1999, 26 (4), 870–879. [PubMed] [Google Scholar]
- Murphy G.; Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair?. Nat. Clin. Pract. Rheumatol. 2008, 4 (3), 128–35. 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- Morrisette-Thomas V.; Cohen A. A.; Fülöp T.; Riesco É.; Legault V.; Li Q.; Milot E.; Dusseault-Bélanger F.; Ferrucci L. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 2014, 139, 49–57. 10.1016/j.mad.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C.; Bonafè M.; Valensin S.; Olivieri F.; De Luca M.; Ottaviani E.; De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–54. 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Vo N. V.; Hartman R. A.; Patil P. R.; Risbud M. V.; Kletsas D.; Iatridis J. C.; Hoyland J. A.; Le Maitre C. L.; Sowa G. A.; Kang J. D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 2016, 34 (8), 1289–306. 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier B. E.; Nerlich A. G.; Weiler C.; Paesold G.; Jochum M.; Boos N. Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors, and the activating TNF-alpha-converting enzyme suggests activation of the TNF-alpha system in the aging intervertebral disc. Ann. N. Y. Acad. Sci. 2007, 1096, 44–54. 10.1196/annals.1397.069. [DOI] [PubMed] [Google Scholar]
- Nerlich A. G.; Bachmeier B. E.; Schleicher E.; Rohrbach H.; Paesold G.; Boos N. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann. N. Y. Acad. Sci. 2007, 1096, 239–48. 10.1196/annals.1397.090. [DOI] [PubMed] [Google Scholar]
- Loeser R. F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010, 26 (3), 371–86. 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. A.; Loeser R. F. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 2015, 23 (11), 1966–71. 10.1016/j.joca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. F. Aging processes and the development of osteoarthritis. Curr. Opin. Rheumatol. 2013, 25 (1), 108–13. 10.1097/BOR.0b013e32835a9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou J.; Steffen T.; Nelson F.; Winterbottom N.; Hollander A. P.; Poole R. A.; Aebi M.; Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Invest. 1996, 98 (4), 996–1003. 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce R. H.; Grimmer B. J.; Adams M. E. Degeneration and the chemical composition of the human lumbar intervertebral disc. J. Orthop. Res. 1987, 5 (2), 198–205. 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- Roughley P. J.; Alini M.; Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem. Soc. Trans. 2002, 30 (Pt 6), 869–874. 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- Meachim G. Light microscopy of Indian ink preparations of fibrillated cartilage. Ann. Rheum. Dis. 1972, 31 (6), 457–64. 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meachim G.; Fergie I. A. Morphological patterns of articular cartilage fibrillation. J. Pathol. 1975, 115 (4), 231–40. 10.1002/path.1711150408. [DOI] [PubMed] [Google Scholar]
- Armstrong C. G.; Mow V. C. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J. Bone Joint Surg Am. 1982, 64 (1), 88–94. 10.2106/00004623-198264010-00013. [DOI] [PubMed] [Google Scholar]
- McDevitt C. A.; Muir H. Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J. Bone Joint Surg. Br. 1976, 58 (1), 94–101. [DOI] [PubMed] [Google Scholar]
- Meachim G.; Denham D.; Emery I. H.; Wilkinson P. H. Collagen alignments and artificial splits at the surface of human articular cartilage. J. Anat. 1974, 118 (Pt 1), 101–18. [PMC free article] [PubMed] [Google Scholar]
- Wong B. L.; Bae W. C.; Chun J.; Gratz K. R.; Lotz M.; Sah R. L. Biomechanics of cartilage articulation: effects of lubrication and degeneration on shear deformation. Arthritis Rheum. 2008, 58 (7), 2065–2074. 10.1002/art.23548. [DOI] [PubMed] [Google Scholar]
- Stokes I. A.; Iatridis J. C. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Philadelphia) 2004, 29 (23), 2724–32. 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple-Wong M. M.; Bae W. C.; Chen M. Q.; Bugbee W. D.; Amiel D.; Coutts R. D.; Lotz M.; Sah R. L. Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. Osteoarthritis Cartilage 2009, 17 (11), 1469–76. 10.1016/j.joca.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S. H.; Cho Y. W.; Ahn M. W.; Jang S. H.; Sohn Y. K.; Kim H. S. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine (Philadelphia) 2002, 27 (9), 911–7. 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- Burke J. G.; Watson R. W.; McCormack D.; Dowling F. E.; Walsh M. G.; Fitzpatrick J. M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Jt. Surg., Br. Vol. 2002, 84 (2), 196–201. 10.1302/0301-620X.84B2.12511. [DOI] [PubMed] [Google Scholar]
- Séguin C. A.; Pilliar R. M.; Madri J. A.; Kandel R. A. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Philadelphia) 2008, 33 (4), 356–65. 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- Séguin C. A.; Bojarski M.; Pilliar R. M.; Roughley P. J.; Kandel R. A. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006, 25 (7), 409–18. 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Le Maitre C. L.; Pockert A.; Buttle D. J.; Freemont A. J.; Hoyland J. A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 2007, 35 (4), 652–655. 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- Weiler C.; Nerlich A. G.; Bachmeier B. E.; Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Philadelphia) 2005, 30 (1), 44–53. 10.1097/01.brs.0000149186.63457.20. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler S. J.; Costello P. W.; Freemont A. J.; Hoyland J. A. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther 2009, 11 (3), R65. 10.1186/ar2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan N. E.; Bloom O.; Maidhof R.; Stetson N.; Sherry B.; Levine M.; Chahine N. O. Toll-Like Receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration. Spine (Philadelphia) 2013, 38 (16), 1343–51. 10.1097/BRS.0b013e31826b71f4. [DOI] [PubMed] [Google Scholar]
- Ponnappan R. K.; Markova D. Z.; Antonio P. J.; Murray H. B.; Vaccaro A. R.; Shapiro I. M.; Anderson D. G.; Albert T. J.; Risbud M. V. An organ culture system to model early degenerative changes of the intervertebral disc. Arthritis Res. Ther 2011, 13 (5), R171. 10.1186/ar3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito M. J.; Veale D. J.; FitzGerald O.; van den Berg W. B.; Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64 (9), 1263–1267. 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B.; Goldring S. R. Osteoarthritis. J. Cell. Physiol. 2007, 213 (3), 626–34. 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- Goldring M. B.; Goldring S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–7. 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- Légaré A.; Garon M.; Guardo R.; Savard P.; Poole A. R.; Buschmann M. D. Detection and analysis of cartilage degeneration by spatially resolved streaming potentials. J. Orthop. Res. 2002, 20 (4), 819–26. 10.1016/S0736-0266(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Temple M. M.; Xue Y.; Chen M. Q.; Sah R. L. Interleukin-1alpha induction of tensile weakening associated with collagen degradation in bovine articular cartilage. Arthritis Rheum. 2006, 54 (10), 3267–76. 10.1002/art.22145. [DOI] [PubMed] [Google Scholar]
- Bonassar L. J.; Sandy J. D.; Lark M. W.; Plaas A. H.; Frank E. H.; Grodzinsky A. J. Inhibition of cartilage degradation and changes in physical properties induced by IL-1beta and retinoic acid using matrix metalloproteinase inhibitors. Arch. Biochem. Biophys. 1997, 344 (2), 404–12. 10.1006/abbi.1997.0205. [DOI] [PubMed] [Google Scholar]
- Homandberg G. A.; Ummadi V.; Kang H. High molecular weight hyaluronan promotes repair of IL-1 beta-damaged cartilage explants from both young and old bovines. Osteoarthritis Cartilage 2003, 11 (3), 177–86. 10.1016/S1063-4584(02)00371-0. [DOI] [PubMed] [Google Scholar]
- Buckwalter J. A.; Mankin H. J.; Grodzinsky A. J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [PubMed] [Google Scholar]
- Alkhatib B.; Rosenzweig D. H.; Krock E.; Roughley P. J.; Beckman L.; Steffen T.; Weber M. H.; Ouellet J. A.; Haglund L. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur. Cell Mater. 2014, 28, 98–110. discussion 110–1 10.22203/eCM.v028a08. [DOI] [PubMed] [Google Scholar]
- Scanzello C. R.; McKeon B.; Swaim B. H.; DiCarlo E.; Asomugha E. U.; Kanda V.; Nair A.; Lee D. M.; Richmond J. C.; Katz J. N.; Crow M. K.; Goldring S. R. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011, 63 (2), 391–400. 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M. K.; Kraus V. B. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res. Ther. 2010, 12 (3), 211. 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L.; Heying E.; Nicholson N.; Stroud N. J.; Homandberg G. A.; Buckwalter J. A.; Guo D.; Martin J. A. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage 2010, 18 (11), 1509–17. 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter J. A.; Anderson D. D.; Brown T. D.; Tochigi Y.; Martin J. A. The Roles of Mechanical Stresses in the Pathogenesis of Osteoarthritis: Implications for Treatment of Joint Injuries. Cartilage 2013, 4 (4), 286–294. 10.1177/1947603513495889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res. Clin Rheumatol 2011, 25 (6), 815–23. 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F.; Fermor B.; Keefe F. J.; Kraus V. B.; Olson S. A.; Pisetsky D. S.; Setton L. A.; Weinberg J. B. The role of biomechanics and inflammation in cartilage injury and repair. Clin. Orthop. Relat. Res. 2004, 423, 17–26. 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- Obeid E. M.; Adams M. A.; Newman J. H. Mechanical properties of articular cartilage in knees with unicompartmental osteoarthritis. J. Bone Joint Surg. Br. 1994, 76 (2), 315–319. [PubMed] [Google Scholar]
- Waldstein W.; Perino G.; Jawetz S. T.; Gilbert S. L.; Boettner F. Does intraarticular inflammation predict biomechanical cartilage properties?. Clin. Orthop. Relat. Res. 2014, 472 (7), 2177–84. 10.1007/s11999-014-3583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard R. C.; Burkhardt D.; Ghosh P.; Read R.; Cake M.; Swain M. V.; Murrell G. A. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthritis Cartilage 2003, 11 (1), 65–77. 10.1053/joca.2002.0867. [DOI] [PubMed] [Google Scholar]
- Guilak F.; Ratcliffe A.; Lane N.; Rosenwasser M. P.; Mow V. C. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J. Orthop. Res. 1994, 12 (4), 474–84. 10.1002/jor.1100120404. [DOI] [PubMed] [Google Scholar]
- Xu L.; Flahiff C. M.; Waldman B. A.; Wu D.; Olsen B. R.; Setton L. A.; Li Y. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho). Arthritis Rheum. 2003, 48 (9), 2509–18. 10.1002/art.11233. [DOI] [PubMed] [Google Scholar]
- Bae W. C.; Temple M. M.; Amiel D.; Coutts R. D.; Niederauer G. G.; Sah R. L. Indentation testing of human cartilage: sensitivity to articular surface degeneration. Arthritis Rheum. 2003, 48 (12), 3382–94. 10.1002/art.11347. [DOI] [PubMed] [Google Scholar]
- Walter B. A.; Likhitpanichkul M.; Illien-Junger S.; Roughley P. J.; Hecht A. C.; Iatridis J. C. TNFα transport induced by dynamic loading alters biomechanics of intact intervertebral discs. PLoS One 2015, 10 (3), e0118358. 10.1371/journal.pone.0118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatridis J. C.; Setton L. A.; Foster R. J.; Rawlins B. A.; Weidenbaum M.; Mow V. C. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J. Biomech 1998, 31 (6), 535–44. 10.1016/S0021-9290(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Furtwängler T.; Chan S. C.; Bahrenberg G.; Richards P. J.; Gantenbein-Ritter B. Assessment of the matrix degenerative effects of MMP-3, ADAMTS-4, and HTRA1, injected into a bovine intervertebral disc organ culture model. Spine (Philadelphia) 2013, 38 (22), E1377–87. 10.1097/BRS.0b013e31829ffde8. [DOI] [PubMed] [Google Scholar]
- Chan S. C.; Bürki A.; Bonél H. M.; Benneker L. M.; Gantenbein-Ritter B. Papain-induced in vitro disc degeneration model for the study of injectable nucleus pulposus therapy. Spine J. 2013, 13 (3), 273–83. 10.1016/j.spinee.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Grenier S.; Bhargava M. M.; Torzilli P. A. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J. Biomech 2014, 47 (3), 645–52. 10.1016/j.jbiomech.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima E. G.; Tan A. R.; Tai T.; Bian L.; Ateshian G. A.; Cook J. L.; Hung C. T. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J. Biomech 2008, 41 (15), 3253–9. 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima E. G.; Tan A. R.; Tai T.; Bian L.; Stoker A. M.; Ateshian G. A.; Cook J. L.; Hung C. T. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng., Part A 2008, 14 (10), 1721–30. 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- Tan A. R.; VandenBerg C. D.; Attur M.; Abramson S. B.; Knight M. M.; Bulinski J. C.; Ateshian G. A.; Cook J. L.; Hung C. T. Cytokine preconditioning of engineered cartilage provides protection against interleukin-1 insult. Arthritis Res. Ther 2015, 17, 361. 10.1186/s13075-015-0876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen W.; Elliott D. M. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine (Philadelphia) 2005, 30 (24), E724–9. 10.1097/01.brs.0000192236.92867.15. [DOI] [PubMed] [Google Scholar]
- Martin J. T.; Gorth D. J.; Beattie E. E.; Harfe B. D.; Smith L. J.; Elliott D. M. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J. Orthop. Res. 2013, 31 (8), 1276–82. 10.1002/jor.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. L.; Jiang S. D.; Dai L. Y. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine (Philadelphia) 2007, 32 (23), 2521–8. 10.1097/BRS.0b013e318158cb61. [DOI] [PubMed] [Google Scholar]
- Walter B. A.; Korecki C. L.; Purmessur D.; Roughley P. J.; Michalek A. J.; Iatridis J. C. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage 2011, 19 (8), 1011–8. 10.1016/j.joca.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawri R.; Rosenzweig D. H.; Krock E.; Ouellet J. A.; Stone L. S.; Quinn T. M.; Haglund L. High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain. Arthritis Res. Ther 2014, 16 (1), R21. 10.1186/ar4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X.; Räsänen T.; Messner K. Maturation-related compressive properties of rabbit knee articular cartilage and volume fraction of subchondral tissue. Osteoarthritis Cartilage 1998, 6 (6), 400–9. 10.1053/joca.1998.0143. [DOI] [PubMed] [Google Scholar]
- Lee H. Y.; Han L.; Roughley P. J.; Grodzinsky A. J.; Ortiz C. Age-related nanostructural and nanomechanical changes of individual human cartilage aggrecan monomers and their glycosaminoglycan side chains. J. Struct. Biol. 2013, 181 (3), 264–73. 10.1016/j.jsb.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijl N.; DeGroot J.; Ben Zaken C.; Braun-Benjamin O.; Maroudas A.; Bank R. A.; Mizrahi J.; Schalkwijk C. G.; Thorpe S. R.; Baynes J. W.; Bijlsma J. W.; Lafeber F. P.; TeKoppele J. M. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002, 46 (1), 114–23. . [DOI] [PubMed] [Google Scholar]
- Bank R. A.; Bayliss M. T.; Lafeber F. P.; Maroudas A.; Tekoppele J. M. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem. J. 1998, 330 (1), 345–351. 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R.; Dickson I. R.; Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem. J. 1988, 252 (2), 495–500. 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth V.; Mow V. C. The intrinsic tensile behavior of the matrix of bovine articular cartilage and its variation with age. J. Bone Joint Surg Am. 1980, 62 (7), 1102–17. 10.2106/00004623-198062070-00007. [DOI] [PubMed] [Google Scholar]
- Kempson G. E.; Muir H.; Pollard C.; Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim. Biophys. Acta, Gen. Subj. 1973, 297 (2), 456–72. 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- Stolz M.; Gottardi R.; Raiteri R.; Miot S.; Martin I.; Imer R.; Staufer U.; Raducanu A.; Düggelin M.; Baschong W.; Daniels A. U.; Friederich N. F.; Aszodi A.; Aebi U. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat. Nanotechnol. 2009, 4 (3), 186–92. 10.1038/nnano.2008.410. [DOI] [PubMed] [Google Scholar]
- Maidhof R.; Jacobsen T.; Papatheodorou A.; Chahine N. O. Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. PLoS One 2014, 9 (6), e99621. 10.1371/journal.pone.0099621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhitpanichkul M.; Torre O. M.; Gruen J.; Walter B. A.; Hecht A. C.; Iatridis J. C. Do mechanical strain and TNF-α interact to amplify pro-inflammatory cytokine production in human annulus fibrosus cells?. J. Biomech 2016, 49 (7), 1214–20. 10.1016/j.jbiomech.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard S.; Guilak F. Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis Rheum. 2006, 54 (7), 2164–74. 10.1002/art.21941. [DOI] [PubMed] [Google Scholar]
- Chen C.; Xie J.; Rajappa R.; Deng L.; Fredberg J.; Yang L. Interleukin-1β and tumor necrosis factor-α increase stiffness and impair contractile function of articular chondrocytes. Acta Biochim. Biophys. Sin. 2015, 47 (2), 121–9. 10.1093/abbs/gmu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickey W. R.; Vail T. P.; Guilak F. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J. Orthop. Res. 2004, 22 (1), 131–9. 10.1016/S0736-0266(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Chahine N. O.; Blanchette C.; Thomas C. B.; Lu J.; Haudenschild D.; Loots G. G. Effect of age and cytoskeletal elements on the indentation-dependent mechanical properties of chondrocytes. PLoS One 2013, 8 (4), e61651. 10.1371/journal.pone.0061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild D. R.; Chen J.; Pang N.; Steklov N.; Grogan S. P.; Lotz M. K.; D’Lima D. D. Vimentin contributes to changes in chondrocyte stiffness in osteoarthritis. J. Orthop. Res. 2011, 29 (1), 20–5. 10.1002/jor.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J.; Sen S.; Sweeney H. L.; Discher D. E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126 (4), 677–89. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Chen J.; Irianto J.; Inamdar S.; Pravincumar P.; Lee D. A.; Bader D. L.; Knight M. M. Cell mechanics, structure, and function are regulated by the stiffness of the three-dimensional microenvironment. Biophys. J. 2012, 103 (6), 1188–97. 10.1016/j.bpj.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist C. L.; Darling E. M.; Chen J.; Setton L. A. Extracellular matrix ligand and stiffness modulate immature nucleus pulposus cell-cell interactions. PLoS One 2011, 6 (11), e27170. 10.1371/journal.pone.0027170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W.; Li Y.; Li L.; Zhang W.; Wang S.; Zheng X. YAP-mediated regulation of the chondrogenic phenotype in response to matrix elasticity. J. Mol. Histol. 2013, 44 (5), 587–95. 10.1007/s10735-013-9502-y. [DOI] [PubMed] [Google Scholar]
- Allen J. L.; Cooke M. E.; Alliston T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol. Biol. Cell 2012, 23 (18), 3731–42. 10.1091/mbc.E12-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang P. Y.; Chen J.; Jing L.; Hoffman B. D.; Setton L. A. The role of extracellular matrix elasticity and composition in regulating the nucleus pulposus cell phenotype in the intervertebral disc: a narrative review. J. Biomech. Eng. 2014, 136 (2), 021010. 10.1115/1.4026360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang P. Y.; Jing L.; Chen J.; Lim F. L.; Tang R.; Choi H.; Cheung K. M.; Risbud M. V.; Gersbach C. A.; Guilak F.; Leung V. Y.; Setton L. A. N-cadherin is Key to Expression of the Nucleus Pulposus Cell Phenotype under Selective Substrate Culture Conditions. Sci. Rep. 2016, 6, 28038. 10.1038/srep28038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. H.; Lin Y. H.; Lin S.; Tsai-Wu J. J.; Herbert Wu C. H.; Jiang C. C. Surface ultrastructure and mechanical property of human chondrocyte revealed by atomic force microscopy. Osteoarthritis Cartilage 2008, 16 (4), 480–488. 10.1016/j.joca.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Steklov N.; Srivastava A.; Sung K. L.; Chen P. C.; Lotz M. K.; D’Lima D. D. Aging-related differences in chondrocyte viscoelastic properties. Mol. Cell Biomech. 2009, 6 (2), 113–119. [PubMed] [Google Scholar]
- Duan W.; Wei L.; Zhang J.; Hao Y.; Li C.; Li H.; Li Q.; Zhang Q.; Chen W.; Wei X. Alteration of viscoelastic properties is associated with a change in cytoskeleton components of ageing chondrocytes from rabbit knee articular cartilage. Mol. Cell Biomech. 2011, 8 (4), 253–274. [PubMed] [Google Scholar]
- Alexopoulos L. G.; Setton L. A.; Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005, 1 (3), 317–25. 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Baer A. E.; Laursen T. A.; Guilak F.; Setton L. A. The micromechanical environment of intervertebral disc cells determined by a finite deformation, anisotropic, and biphasic finite element model. J. Biomech. Eng. 2003, 125 (1), 1–11. 10.1115/1.1532790. [DOI] [PubMed] [Google Scholar]
- Baaijens F. P.; Trickey W. R.; Laursen T. A.; Guilak F. Large deformation finite element analysis of micropipette aspiration to determine the mechanical properties of the chondrocyte. Ann. Biomed. Eng. 2005, 33 (4), 494–501. 10.1007/s10439-005-2506-3. [DOI] [PubMed] [Google Scholar]
- Guilak F.; Mow V. C. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J. Biomech 2000, 33 (12), 1663–73. 10.1016/S0021-9290(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Han S. K.; Federico S.; Grillo A.; Giaquinta G.; Herzog W. The mechanical behaviour of chondrocytes predicted with a micro-structural model of articular cartilage. Biomech. Model. Mechanobiol. 2007, 6 (3), 139–50. 10.1007/s10237-006-0016-3. [DOI] [PubMed] [Google Scholar]
- Korhonen R. K.; Herzog W. Depth-dependent analysis of the role of collagen fibrils, fixed charges and fluid in the pericellular matrix of articular cartilage on chondrocyte mechanics. J. Biomech 2008, 41 (2), 480–5. 10.1016/j.jbiomech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Julkunen P.; Wilson W.; Isaksson H.; Jurvelin J. S.; Herzog W.; Korhonen R. K. A review of the combination of experimental measurements and fibril-reinforced modeling for investigation of articular cartilage and chondrocyte response to loading. Comput. Math Methods Med. 2013, 2013, 326150. 10.1155/2013/326150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen R. K.; Julkunen P.; Wilson W.; Herzog W. Importance of collagen orientation and depth-dependent fixed charge densities of cartilage on mechanical behavior of chondrocytes. J. Biomech. Eng. 2008, 130 (2), 021003. 10.1115/1.2898725. [DOI] [PubMed] [Google Scholar]
- Julkunen P.; Wilson W.; Jurvelin J. S.; Korhonen R. K. Composition of the pericellular matrix modulates the deformation behaviour of chondrocytes in articular cartilage under static loading. Med. Biol. Eng. Comput. 2009, 47 (12), 1281–90. 10.1007/s11517-009-0547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.; Guilak F.; Haider M. A. The dynamic mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions under cyclic compressive loading. J. Biomech. Eng. 2008, 130 (6), 061009. 10.1115/1.2978991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine N. O.; Hung C. T.; Ateshian G. A. In-situ measurements of chondrocyte deformation under transient loading. Eur. Cell Mater. 2007, 13, 100–11. 10.22203/eCM.v013a11. [DOI] [PubMed] [Google Scholar]
- Jones W. R.; Ping Ting-Beall H.; Lee G. M.; Kelley S. S.; Hochmuth R. M.; Guilak F. Alterations in the Young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech 1999, 32 (2), 119–27. 10.1016/S0021-9290(98)00166-3. [DOI] [PubMed] [Google Scholar]
- Alexopoulos L. G.; Williams G. M.; Upton M. L.; Setton L. A.; Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J. Biomech 2005, 38 (3), 509–17. 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Ingber D. E. Cellular basis of mechanotransduction. Biol. Bull. 1998, 194 (3), 323–5. 10.2307/1543102. [DOI] [PubMed] [Google Scholar]
- Ingber D. Mechanical signaling. Ann. N. Y. Acad. Sci. 2002, 961, 162–3. 10.1111/j.1749-6632.2002.tb03074.x. [DOI] [PubMed] [Google Scholar]
- Sowa G.; Coelho P.; Vo N.; Bedison R.; Chiao A.; Davies C.; Studer R.; Kang J. Determination of annulus fibrosus cell response to tensile strain as a function of duration, magnitude, and frequency. J. Orthop. Res. 2011, 29 (8), 1275–83. 10.1002/jor.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer J.; Kolditz D.; Gowin R. Water and electrolyte content of human intervertebral discs under variable load. Spine (Philadelphia) 1985, 10 (1), 69–71. 10.1097/00007632-198501000-00011. [DOI] [PubMed] [Google Scholar]
- Johnson Z. I.; Gogate S. S.; Day R.; Binch A.; Markova D. Z.; Chiverton N.; Cole A.; Conner M.; Shapiro I. M.; Le Maitre C. L.; Risbud M. V. Aquaporin 1 and 5 expression decreases during human intervertebral disc degeneration: Novel HIF-1-mediated regulation of aquaporins in NP cells. Oncotarget 2015, 6 (14), 11945–58. 10.18632/oncotarget.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan M. N.; Leddy H. A.; Votta B. J.; Kumar S.; Levy D. S.; Lipshutz D. B.; Lee S. H.; Liedtke W.; Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009, 60 (10), 3028–37. 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B. A.; Purmessur D.; Moon A.; Occhiogrosso J.; Laudier D. M.; Hecht A. C.; Iatridis J. C. Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells. Eur. Cell Mater. 2016, 32, 123–36. 10.22203/eCM.v032a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Conor C. J.; Leddy H. A.; Benefield H. C.; Liedtke W. B.; Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (4), 1316–21. 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusudo T.; Wang Z.; Mizuno A.; Suzuki M.; Yamashita H. TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet-induced obesity. J. Appl. Physiol. 2012, 112 (7), 1223–32. 10.1152/japplphysiol.01070.2011. [DOI] [PubMed] [Google Scholar]
- Bertram K. L.; Banderali U.; Tailor P.; Krawetz R. J. Ion channel expression and function in normal and osteoarthritic human synovial fluid progenitor cells. Channels 2016, 10 (2), 148–57. 10.1080/19336950.2015.1116652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eerden B. C.; Oei L.; Roschger P.; Fratzl-Zelman N.; Hoenderop J. G.; van Schoor N. M.; Pettersson-Kymmer U.; Schreuders-Koedam M.; Uitterlinden A. G.; Hofman A.; Suzuki M.; Klaushofer K.; Ohlsson C.; Lips P. J.; Rivadeneira F.; Bindels R. J.; van Leeuwen J. P. TRPV4 deficiency causes sexual dimorphism in bone metabolism and osteoporotic fracture risk. Bone 2013, 57 (2), 443–54. 10.1016/j.bone.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Lee W.; Leddy H. A.; Chen Y.; Lee S. H.; Zelenski N. A.; McNulty A. L.; Wu J.; Beicker K. N.; Coles J.; Zauscher S.; Grandl J.; Sachs F.; Guilak F.; Liedtke W. B. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (47), E5114–22. 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H.; Thampatty B. P. An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol. 2006, 5 (1), 1–16. 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Loeser R. F. Chondrocyte integrin expression and function. Biorheology 2000, 37 (1–2), 109–116. [PubMed] [Google Scholar]
- Xia M.; Zhu Y. Expression of integrin subunits in the herniated intervertebral disc. Connect. Tissue Res. 2008, 49 (6), 464–9. 10.1080/03008200802325425. [DOI] [PubMed] [Google Scholar]
- Nettles D. L.; Richardson W. J.; Setton L. A. Integrin expression in cells of the intervertebral disc. J. Anat. 2004, 204 (6), 515–20. 10.1111/j.0021-8782.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobanputra P.; Lin H.; Jenkins K.; Bavington C.; Brennan F. R.; Nuki G.; Salter D. M.; Godolphin J. L. Modulation of human chondrocyte integrins by inflammatory synovial fluid. Arthritis Rheum. 1996, 39 (8), 1430–1432. 10.1002/art.1780390826. [DOI] [PubMed] [Google Scholar]
- Barksby H. E.; Hui W.; Wappler I.; Peters H. H.; Milner J. M.; Richards C. D.; Cawston T. E.; Rowan A. D. Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: implications for cartilage destruction and repair. Arthritis Rheum. 2006, 54 (2), 540–50. 10.1002/art.21574. [DOI] [PubMed] [Google Scholar]
- Gilbert H. T.; Nagra N. S.; Freemont A. J.; Millward-Sadler S. J.; Hoyland J. A. Integrin - dependent mechanotransduction in mechanically stimulated human annulus fibrosus cells: evidence for an alternative mechanotransduction pathway operating with degeneration. PLoS One 2013, 8 (9), e72994. 10.1371/journal.pone.0072994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre C. L.; Frain J.; Millward-Sadler J.; Fotheringham A. P.; Freemont A. J.; Hoyland J. A. Altered integrin mechanotransduction in human nucleus pulposus cells derived from degenerated discs. Arthritis Rheum. 2009, 60 (2), 460–9. 10.1002/art.24248. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler S. J.; Wright M. O.; Lee H.; Caldwell H.; Nuki G.; Salter D. M. Altered electrophysiological responses to mechanical stimulation and abnormal signalling through alpha5beta1 integrin in chondrocytes from osteoarthritic cartilage. Osteoarthritis Cartilage 2000, 8 (4), 272–8. 10.1053/joca.1999.0301. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler S. J.; Wright M. O.; Davies L. W.; Nuki G.; Salter D. M. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000, 43 (9), 2091–2099. . [DOI] [PubMed] [Google Scholar]
- Holledge M. M.; Millward-Sadler S. J.; Nuki G.; Salter D. M. Mechanical regulation of proteoglycan synthesis in normal and osteoarthritic human articular chondrocytes--roles for alpha5 and alphaVbeta5 integrins. Biorheology 2008, 45 (3–4), 275–288. [PubMed] [Google Scholar]
- Salter D. M.; Millward-Sadler S. J.; Nuki G.; Wright M. O. Differential responses of chondrocytes from normal and osteoarthritic human articular cartilage to mechanical stimulation. Biorheology 2002, 39 (1–2), 97–108. [PubMed] [Google Scholar]
- Gilbert H. T.; Hoyland J. A.; Millward-Sadler S. J. The response of human anulus fibrosus cells to cyclic tensile strain is frequency-dependent and altered with disc degeneration. Arthritis Rheum. 2010, 62 (11), 3385–94. 10.1002/art.27643. [DOI] [PubMed] [Google Scholar]
- Gilbert H. T.; Hoyland J. A.; Freemont A. J.; Millward-Sadler S. J. The involvement of interleukin-1 and interleukin-4 in the response of human annulus fibrosus cells to cyclic tensile strain: an altered mechanotransduction pathway with degeneration. Arthritis Res. Ther 2011, 13 (1), R8. 10.1186/ar3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa G. A.; Coelho J. P.; Vo N. V.; Pacek C.; Westrick E.; Kang J. D. Cells from degenerative intervertebral discs demonstrate unfavorable responses to mechanical and inflammatory stimuli: a pilot study. Am. J. Phys. Med. Rehabil 2012, 91 (10), 846–55. 10.1097/PHM.0b013e31825f145a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre C. L.; Frain J.; Fotheringham A. P.; Freemont A. J.; Hoyland J. A. Human cells derived from degenerate intervertebral discs respond differently to those derived from non-degenerate intervertebral discs following application of dynamic hydrostatic pressure. Biorheology 2008, 45 (5), 563–575. [PubMed] [Google Scholar]
- Aaltonen K. J.; Virkki L. M.; Malmivaara A.; Konttinen Y. T.; Nordström D. C.; Blom M. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One 2012, 7 (1), e30275. 10.1371/journal.pone.0030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. J. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat. Rev. Rheumatol. 2011, 7 (1), 13–22. 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- Matthews G. L.; Hunter D. J. Emerging drugs for osteoarthritis. Expert Opin. Emerging Drugs 2011, 16 (3), 479–91. 10.1517/14728214.2011.576670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E.; Grodzinsky A. J.; Cummings K.; Plaas A. H.; Cole A. A.; Lee R. T.; Patwari P. Intraarticular injection of heparin-binding insulin-like growth factor 1 sustains delivery of insulin-like growth factor 1 to cartilage through binding to chondroitin sulfate. Arthritis Rheum. 2010, 62 (12), 3686–94. 10.1002/art.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. C.; Evans C. H.; Grodzinsky A. J. Effects of short-term glucocorticoid treatment on changes in cartilage matrix degradation and chondrocyte gene expression induced by mechanical injury and inflammatory cytokines. Arthritis Res. Ther 2011, 13 (5), R142. 10.1186/ar3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon A. J.; Brower-Toland B. D.; Bent S. J.; Saxer R. A.; Wilke M. J.; Robbins P. D.; Evans C. H. Insulinlike growth factor-I gene therapy applications for cartilage repair. Clin. Orthop. Relat. Res. 2000, 379 (379), S201–S213. 10.1097/00003086-200010001-00026. [DOI] [PubMed] [Google Scholar]
- Le Maitre C. L.; Hoyland J. A.; Freemont A. J. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res. Ther 2007, 9 (4), R83. 10.1186/ar2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z.; Yik J. H.; Cissell D. D.; Michelier P. V.; Athanasiou K. A.; Haudenschild D. R. Inhibition of CDK9 prevents mechanical injury-induced inflammation, apoptosis and matrix degradation in cartilage explants. Eur. Cell Mater. 2015, 30, 200–209. 10.22203/eCM.v030a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yik J. H.; Hu Z.; Kumari R.; Christiansen B. A.; Haudenschild D. R. Cyclin-dependent kinase 9 inhibition protects cartilage from the catabolic effects of proinflammatory cytokines. Arthritis Rheumatol. 2014, 66 (6), 1537–46. 10.1002/art.38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima E. G.; Tan A. R.; Tai T.; Marra K. G.; DeFail A.; Ateshian G. A.; Hung C. T. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J. Biomed. Mater. Res., Part A 2009, 91 (3), 692–700. 10.1002/jbm.a.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpayee A. G.; Wong C. R.; Bawendi M. G.; Frank E. H.; Grodzinsky A. J. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials 2014, 35 (1), 538–49. 10.1016/j.biomaterials.2013.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpayee A. G.; Quadir M. A.; Hammond P. T.; Grodzinsky A. J. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthritis Cartilage 2016, 24 (1), 71–81. 10.1016/j.joca.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorth D. J.; Mauck R. L.; Chiaro J. A.; Mohanraj B.; Hebela N. M.; Dodge G. R.; Elliott D. M.; Smith L. J. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1β-mediated degradation of nucleus pulposus in vitro. Arthritis Res. Ther 2012, 14 (4), R179. 10.1186/ar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid K. A.; Ubhe A.; Shaman Z.; D’Souza G. Intra-articular interleukin-1 receptor antagonist (IL1-ra) microspheres for posttraumatic osteoarthritis: in vitro biological activity and in vivo disease modifying effect. J. Exp Orthop 2016, 3 (1), 18. 10.1186/s40634-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki M.; Ishihara T.; Izumo N.; Takatsu M.; Mizushima Y. Treatment of experimental arthritis with poly(D, L-lactic/glycolic acid) nanoparticles encapsulating betamethasone sodium phosphate. Ann. Rheum. Dis. 2005, 64 (8), 1132–1136. 10.1136/ard.2004.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Présumey J.; Salzano G.; Courties G.; Shires M.; Ponchel F.; Jorgensen C.; Apparailly F.; De Rosa G. PLGA microspheres encapsulating siRNA anti-TNFalpha: efficient RNAi-mediated treatment of arthritic joints. Eur. J. Pharm. Biopharm. 2012, 82 (3), 457–64. 10.1016/j.ejpb.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Butoescu N.; Seemayer C. A.; Foti M.; Jordan O.; Doelker E. Dexamethasone-containing PLGA superparamagnetic microparticles as carriers for the local treatment of arthritis. Biomaterials 2009, 30 (9), 1772–80. 10.1016/j.biomaterials.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Butoescu N.; Seemayer C. A.; Palmer G.; Guerne P. A.; Gabay C.; Doelker E.; Jordan O. Magnetically retainable microparticles for drug delivery to the joint: efficacy studies in an antigen-induced arthritis model in mice. Arthritis Res. Ther 2009, 11 (3), R72. 10.1186/ar2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamji M. F.; Hwang P.; Bullock R. W.; Adams S. B.; Nettles D. L.; Setton L. A. Release and activity of anti-TNFalpha therapeutics from injectable chitosan preparations for local drug delivery. J. Biomed. Mater. Res., Part B 2009, 90 (1), 319–326. 10.1002/jbm.b.31289. [DOI] [PMC free article] [PubMed] [Google Scholar]