Abstract
Context:
Little is known about the association between obesity and temporal trends in the incidence of diabetes in children and young adults.
Objective:
We examined the recent incidence of types 1 and 2 diabetes in relation to a high body mass index (BMI) in UK children and young adults.
Design:
Cohort and nested case-control.
Setting:
A total of 375 general practices that contribute to the UK Clinical Practice Research Datalink (CPRD).
Participants:
A total of 369,362 participants aged 2 to 15 years at BMI measurement in CPRD from 1994 to 2013.
Intervention:
None.
Main outcome measures:
Incident type 1 diabetes (T1D) and type 2 diabetes (T2D) diagnoses up to age 25 years.
Results:
A total of 654 incident cases of T2D and 1318 T1D cases were found. The incidence of T2D per 100,000 persons annually increased from 6.4 in 1994 to 1998 to 33.2 in 2009 to 2013; and that for T1D increased from 38.2 to 52.1 per 100,000 persons during the same period. The incidence of T2D increased in both overweight (85th to 95th percentile for age- and sex-specific BMI; P = 0.01) and obese (≥95th percentile; P < 0.01) individuals from 1994 to 2013. Obese individuals, who constituted 47.1% of T2D cases, had a markedly greater risk of incident T2D [odds ratio, 3.75; 95% confidence interval (CI), 3.07 to 4.57], with an incidence rate ratio of 4.33 (95% CI, 3.68 to 5.08) compared with the normal BMI category. No positive linear association was found between obesity (greater BMI) and incident T1D cases.
Conclusions:
Increasing obesity has contributed to the increasing incidence of T2D but not T1D among UK children and young adults, with a fourfold greater risk of developing T2D in obese individuals.
Keywords: body mass index, obesity, diabetes, children, adolescence, epidemiology
Our study results show that increasing obesity has been contributing to the increasing incidence of type 2 diabetes among UK children and young adults, with a fourfold greater risk of developing type 2 diabetes in the obese.
Obesity is a global health epidemic that is shifting toward children and young adults [1-3]. More than one-half of adults, aged ≥18 years, were obese or overweight in 2014 [4], and one of three children leaving primary school were considered obese or overweight in 2015 [5]. The rate of increase in obesity prevalence in children and young adults might have stabilized during the recent decade [6–8]; however, 37% of older children are now overweight or obese in the United Kingdom [6]. Observational and genetic evidence from traditional cohort studies has suggested that obesity is a well-documented risk factor for type 2 diabetes [9–11]. However, recent evidence exploring the association of childhood obesity [defined as the 95th percentile for body mass index (BMI)] with the presence of pediatric diabetes in a real world primary care setting has been less well studied [12–14]. A recent prospective study from Norway and Denmark showed a positive association between the weight increase during the first 12 months of life and the development of type 1 diabetes [15], suggesting that similar to type 2 diabetes, type 1 diabetes might have environmental origins.
Another study showed that the prevalence rates of both type 1 and type 2 diabetes increased among children and adolescents in the United States from 2001 to 2009 [16]. In England and Wales, 26,687 children and young adults aged ≤25 years receive diabetes care, with 95% classified as having type 1 diabetes and 2% having type 2 diabetes [17]. Previous studies of type 2 diabetes in children and young adults had included small number of participants or been conducted in selected cohorts [18, 19]. The present study evaluated the incidence and temporal trends of type 1 and type 2 diabetes and estimated the risk of developing diabetes in association with an elevated BMI in a cohort of children and young adults with BMI recorded from 1994 through 2013.
1. Methods
A. Study Design and Data Source
A cohort study was conducted using the electronic health records from the UK Clinical Practice Research Datalink (CPRD) [20]. The UK CPRD is one of the largest primary care databases of electronic health records worldwide [6] and collates anonymized data on the clinical diagnoses, prescriptions, laboratory test results, referrals to specialists, and hospital admissions [21]. Individuals were sampled from 375 English general practices that participate in the CPRD data linkage scheme, if they were aged 2 to15 years at BMI measurement and had BMI values recorded during the period 1994 to 2013. The Independent Scientific Advisory Committee approved the present protocol (Independent Scientific Advisory Committee no. 13-194).
B. Demographic Information, Measurements, and Outcomes
Demographic information, including sex, date of birth, and dates of the start and end of the record (Supplemental Fig. 1 (65.2KB, docx) ), was analyzed. We searched the database for the BMI, height, and weight records to calculate the BMI, as previously described [6, 22, 23]. Our main outcomes were the diagnosis of type 1 and type 2 diabetes, which were classified according to the medical diagnostic codes, prescriptions for antidiabetic drugs, or a hemoglobin A1c (HbA1c) value. The applicable prescriptions were insulin or an oral glucose-lowering agent, including sulfonylureas, metformin, dipeptidyl-peptidase-4 inhibitors, glitazones, acarbose, and glinides [24]. The first date in the record of a prescription of any of these was assigned as the diabetes start date. Patients attributed with a diabetes diagnosis before 12 months after the start of their CPRD record were excluded because these cases were considered to be prevalent diabetes (Supplemental Figs. 1 and 2 (65.2KB, docx) ) [24].
The patients were considered to have type 1 diabetes if they had a diagnosis of type 1 diabetes or had been prescribed insulin and had never been prescribed oral glucose-lowering medications in the CPRD. Type 2 diabetes was defined as a diagnosis of type 2 diabetes, the prescription of oral glucose-lowering medications only, or a diagnosis of diabetes mellitus or HbA1c ≥6.5% (48 mmol/mol) but no insulin prescription. Those with diabetes that could not be classified were excluded. The excluded cases included diagnoses of both type 1 and type 2 diabetes, prescriptions for both oral glucose-lowering medications and insulin, diagnosis of type 1 diabetes without an insulin prescription, and diagnosis of type 2 diabetes with a prescription for insulin. We also excluded women with a diagnosis of polycystic ovary syndrome or gestational diabetes (Supplemental Fig. 2 (65.2KB, docx) ).
C. Statistical Analysis
The BMI values were converted to Z-scores, adjusting for exact age and sex using the British 1990 growth reference data population [23]. Normal weight was defined as a BMI Z-score <1.04 (<85th percentile on a growth chart). Overweight was defined as 1.04 to 1.64 (85th to 95th percentile) and obese as a Z-score of ≥1.64 (≥95th percentile) of the UK 1990 reference population [23]. We calculated the present age in each calendar year and calculated the person-years of observation from 1994 until the date of diabetes diagnosis for incident cases of diabetes or the end of the record for the remaining individuals. We estimated the incidence rates of diabetes (or age-standardized to the 2013 European Standard Population for reference) [25, 26], with person-years as the exposure variable. Incidence rates (per 100,000 persons per year) were presented for each 5-year period and according to the present age, sex, and BMI categories. We fitted unadjusted or jointly adjusted Poisson regression models to calculate the incidence rate ratios (IRRs) for all variables.
To replicate the Poisson regression models and estimate the relative risks, we also conducted a nested case-control study in which each case with diabetes was matched for sex, year of birth, and general practice with up to four randomly selected controls (without any relevant records for diabetes). We fitted conditional logistic regression models to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for incident diabetes in relation to an elevated BMI. We calculated the ORs per one standard deviation (SD) change in BMI, for overweight and obesity (normal weight as reference) or across BMI quintiles (first quintile as reference). We applied quintile values in controls to categorize the BMI. As a sensitivity analysis, we repeated this analysis using only data for BMI values recorded before the date of the diabetes diagnosis to reduce the likelihood of reverse causality. All data analyses were performed in Stata, version 14 (StataCorp, College Station, TX), and R, version 3.2.3 (R Foundation, Vienna, Austria). Owing to the large sample size of the present study, P < 0.01 was considered statistically significant.
2. Results
A. Population Characteristics
We analyzed the data from 369,362 eligible individuals with the BMI calculated using weight and height values (n = 321,597) or BMI as directly recorded in the CPRD (n = 47,765) from 1994 to 2013. The mean age at the last birthday before the BMI record was 8.8 ± 4.3 years (range, 2 to 15), and 49.5% were female. The median BMI Z-score was 0.36 [interquartile range (IQR), −0.5 to 1.2]. Of the participants, 45,228 were overweight (12.3%) overweight and 61,356 were obese (16.7%). The median BMI Z-score was greater in the obese category in 2009 to 2013 compared with 1994 to 1998 (2.3; IQR, 1.9 to 2.7; vs 2.2; IQR, 1.9 to 2.7; P < 0.01). For the overweight category, no statistically significant increase was observed (P = 0.2).
B. Incidence and Trends for Type 2 and Type 1 Diabetes
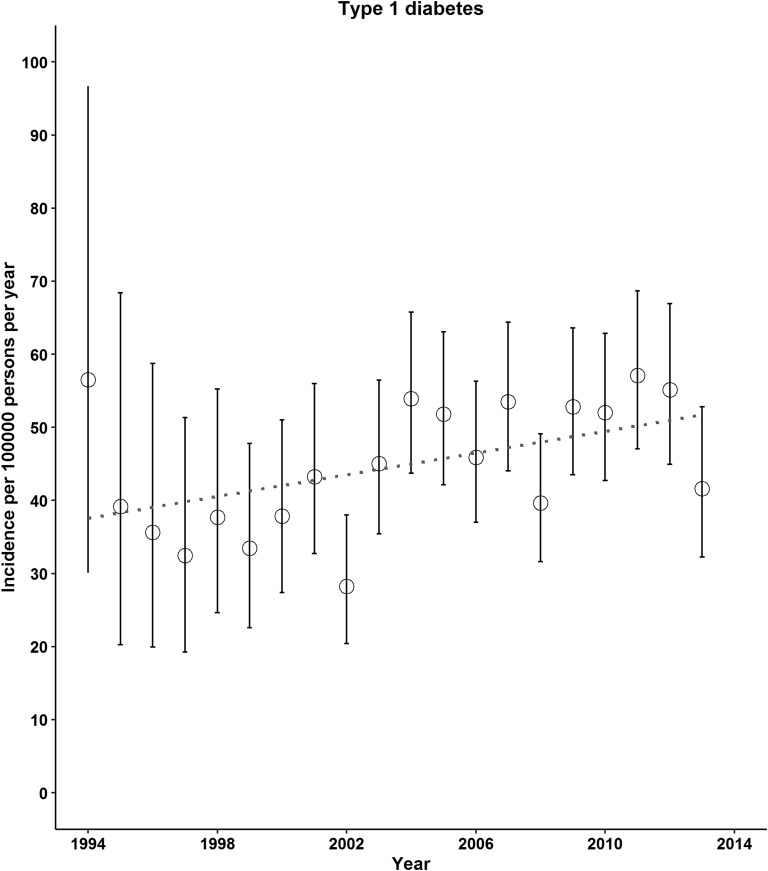
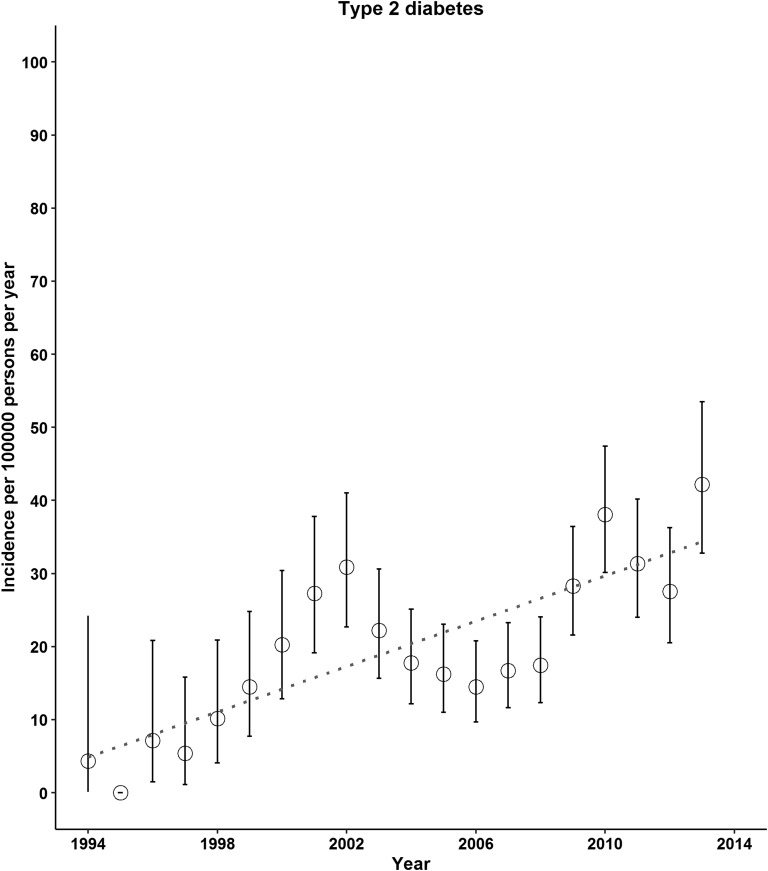
A total of 654 incident cases of type 2 diabetes and 1318 incident cases of type 1 diabetes were found. The incidence of both type 2 and type 1 diabetes increased from 1994 to 2013 (Fig. 1). The mean time lag between the BMI records as exposure and the date of diabetes diagnosis was 0.4 year (IQR, −1.5 to 4.5 years). From 1994 to 1998 through 2009 to 2013, the incidence of type 2 diabetes per 100,000 persons per year increased from 6.4 (95% CI, 3.5 to 10.7) to 33.2 (95% CI, 29.7 to 37.1; P for trend < 0.01; Table 1). We also observed increases in the incidence of type 1 diabetes across 5-year calendar periods: from 38.2 (95% CI, 30.4 to 47.2) in 1994 to 1998 to 52.1 (95% CI, 47.6 to 56.9) in 2009 to 2013 per 100,000 persons per year (P for trend < 0.01; Table 1). Increasing trends in type 2 diabetes incidence were observed for both sexes, for both children and young adults, and for those who were overweight or obese (Table 1). The incidence of type 1 diabetes continued to increase for those aged ≤15 years; however, this trend was evident for females and those with a normal BMI only (Table 1).
Figure 1.
Incidence of type 2 and type 1 diabetes in UK children and young adults, 1994 to 2013. In 1997 and 2010, the diagnostic criteria for diabetes was changed (use of fasting plasma glucose ≥126 mg/dL or HbA1c ≥6.5%, respectively).
Table 1.
Incidence (Rates per 100,000 Persons per Year) of Type 2 and Type 1 Diabetes in UK Children and Young Adults, 1994 to 2013
| Variable | 1994–1998 |
1999–2003 |
2004–2008 |
2009–2013 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | IR (95% CI) | Cases | IR (95% CI) | Cases | IR (95% CI) | Cases | IR (95% CI) | P Value for Trend | |
| Type 2 diabetes | |||||||||
| Total | 14 | 6.4 (3.5–10.7) | 156 | 23.9 (20.3–27.9) | 164 | 16.5 (14.1–19.3) | 320 | 33.2 (29.7–37.1) | < 0.01 |
| Sex | |||||||||
| Female | 7 | 6.3 (2.5–13) | 89 | 27.1 (21.8–33.4) | 90 | 18.3 (14.8–22.5) | 206 | 43.9 (38.1–50.3) | < 0.01 |
| Male | 7 | 6.4 (2.6–13.2) | 67 | 20.6 (15.9–26.1) | 74 | 14.7 (11.6–18.5) | 114 | 23.1 (19–27.7) | < 0.01 |
| Age group, y | |||||||||
| ≤15 | 11 | 5.4 (2.7–9.6) | 89 | 16 (12.8–19.7) | 86 | 11.6 (9.3–14.3) | 149 | 25.6 (21.7–30.1) | < 0.01 |
| 16–25 | 3 | 19.9 (4.1–58.2) | 67 | 69.2 (53.6–87.9) | 78 | 31 (24.5–38.6) | 171 | 44.8 (38.4–52.1) | 0.43 |
| BMI categorya | |||||||||
| Normal | 12 | 7.6 (3.9–13.2) | 99 | 21.3 (17.3–25.9) | 55 | 7.9 (5.9–10.2) | 119 | 17.6 (14.6–21) | 0.48 |
| Overweight | 0 | 0 (0–14) | 12 | 15.3 (7.9–26.7) | 22 | 18.2 (11.4–27.5) | 27 | 22.8 (15–33.2) | 0.01 |
| Obese | 2 | 5.7 (0.7–20.5) | 45 | 40.9 (29.9–54.8) | 87 | 50.7 (40.6–62.6) | 174 | 103.3 (88.5–119.9) | < 0.01 |
| Type 1 diabetes | |||||||||
| Total | 84 | 38.2 (30.4–47.2) | 248 | 37.9 (33.3–42.9) | 484 | 48.8 (44.5–53.3) | 502 | 52.1 (47.6–56.9) | < 0.01 |
| Sex | |||||||||
| Female | 36 | 32.5 (22.8–45) | 110 | 33.5 (27.6–40.4) | 208 | 42.4 (36.8–48.6) | 238 | 50.7 (44.5–57.6) | < 0.01 |
| Male | 48 | 43.8 (32.3–58.1) | 138 | 42.4 (35.6–50) | 276 | 55 (48.7–61.9) | 264 | 53.5 (47.2–60.3) | 0.03 |
| Age group, y | |||||||||
| ≤15 | 81 | 39.5 (31.4–49.1) | 237 | 42.5 (37.3–48.3) | 451 | 60.9 (55.4–66.8) | 434 | 74.6 (67.7–81.9) | < 0.01 |
| 16–25 | 3 | 19.9 (4.1–58.2) | 11 | 11.4 (5.7–20.3) | 33 | 13.1 (9–18.4) | 68 | 17.8 (13.8–22.6) | 0.15 |
| BMI categorya | |||||||||
| Normal | 57 | 36 (27.2–46.6) | 170 | 36.5 (31.2–42.4) | 346 | 49.4 (44.4–54.9) | 365 | 53.9 (48.5–59.8) | < 0.01 |
| Overweight | 18 | 68.4 (40.6–108.2) | 41 | 52.2 (37.4–70.7) | 80 | 66.1 (52.4–82.3) | 62 | 52.4 (40.2–67.2) | 0.58 |
| Obese | 9 | 25.5 (11.7–48.4) | 37 | 33.7 (23.7–46.4) | 58 | 33.8 (25.7–43.7) | 75 | 44.5 (35–55.8) | 0.046 |
Abbreviation: IR, incidence rate.
Normal weight defined as BMI Z-score <1.04 (<85th percentile of growth chart); overweight, BMI Z-score of 1.04–1.64 (85th–95th percentile); obese, BMI Z-score >1.64 (>95th percentile) of 1990 reference population.
C. Incidence Rate and Risk Ratios
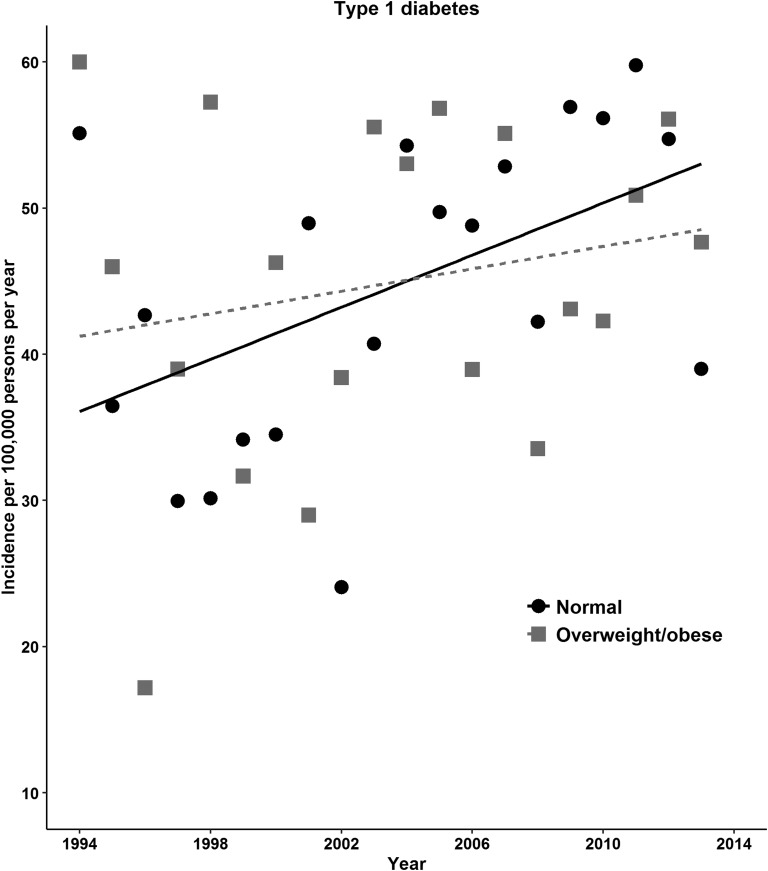
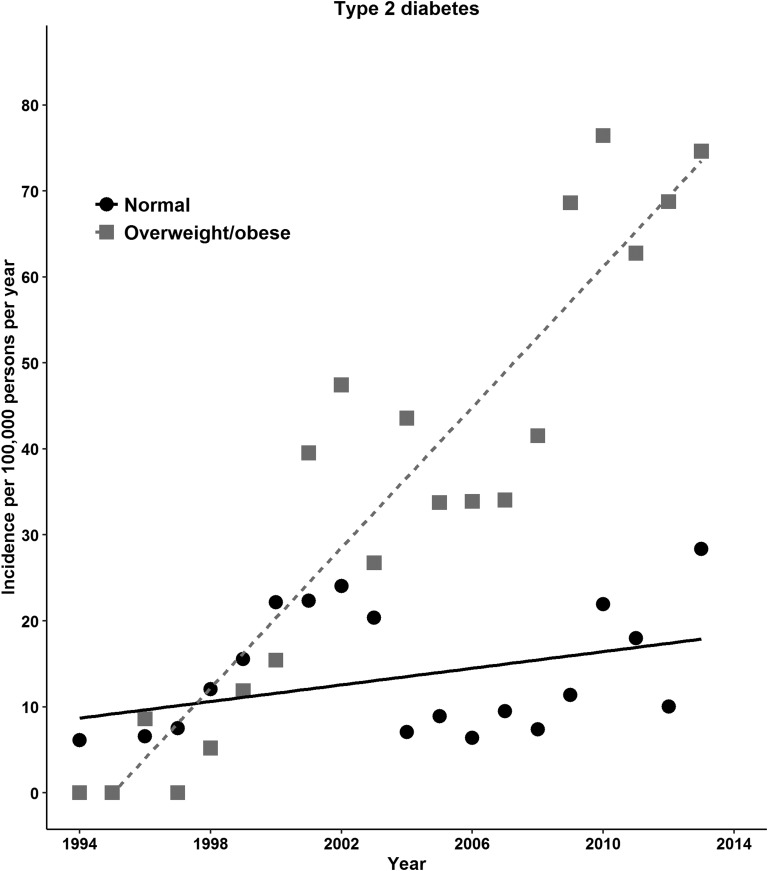
The increase in diabetes risk with time was evaluated using IRRs from Poisson regression models. The adjusted IRRs for type 2 diabetes for 2004 to 2008 or 2009 to 2013 were 1.7 to 3.3 times as high as the rate in 1994 to 1998. For type 1 diabetes, the rates were significantly greater in 2004 to 2008 or 2009 to 2013 compared with the rate in 1994 to 1998 (adjusted IRRs, 1.5 or 2.0; Table 2). The incidence rate of type 2 diabetes was greater in females, but type 1 diabetes was greater in males. The incidence of type 2 diabetes was more evident in those aged 11 to 15 years and in those aged >15 years compared with children aged <5 years. In contrast, the type 1 diabetes incidence decreased with age (adjusted IRR, 0.83 for those aged 11 to 15 years and 0.2 for those aged >15 years compared with those aged <5 years; Table 2). The incidence of type 2 diabetes showed an increase in overweight or obese during 1994 to 2013, and the type 1 diabetes incidence increased gradually in those with normal BMI (Fig. 2). For type 2 diabetes, obese individuals had significantly greater incidence rates than those with a normal BMI (adjusted IRR, 4.3; 95% CI, 3.7 to 5.1; Table 2). We refitted models with two-way interaction terms for BMI categories to assess effect modification by sex, age, and period. Higher rates of type 2 diabetes with obesity were noted in those aged 11 to 15 years and in the last two periods (P < 0.01 for interaction; Supplemental Fig. 3 (65.2KB, docx) ). We observed no similar associations between the BMI categories and type 1 diabetes (Table 2).
Table 2.
Incidence Rate Ratio for Type 2 and Type 1 Diabetes According to Sex, Age, BMI, and Period Categories in UK Children and Young Adults, 1994 to 2013
| Variable | Unadjusted IRR (95% CI) | P Value | Adjusted IRRa (95% CI) | P Value |
|---|---|---|---|---|
| Type 2 diabetes | ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.5 (1.3–1.8) | < 0.01 | 1.5 (1.3–1.8) | < 0.01 |
| Age group, y | ||||
| ≤5 | Reference | Reference | ||
| 6–10 | 1.2 (0.8–1.7) | 0.36 | 1.1 (0.8–1.6) | 0.65 |
| 11–15 | 2.8 (2.0–3.9) | < 0.01 | 2.3 (1.6–3.2) | < 0.01 |
| 16–25 | 4.8 (3.4–6.6) | < 0.01 | 4.7 (3.3–6.6) | < 0.01 |
| BMI categoryb | ||||
| Normal | Reference | Reference | ||
| Overweight | 1.2 (0.9–1.6) | 0.13 | 1.2 (0.9–1.6) | 0.17 |
| Obese | 4.5 (3.8–5.2) | < 0.01 | 4.3 (3.7–5.1) | < 0.01 |
| Period | ||||
| 1994–1998 | Reference | - | Reference | - |
| 1999–2003 | 3.8 (2.2–6.5) | < 0.01 | 3.0 (1.7–5.1) | < 0.01 |
| 2004–2008 | 2.6 (1.5–4.5) | < 0.01 | 1.7 (1.0–3.0) | 0.06 |
| 2009–2013 | 5.2 (3.1–8.9) | < 0.01 | 3.3 (1.9–5.8) | < 0.01 |
| Type 1 diabetes | ||||
| Sex | ||||
| Male | Reference | - | Reference | - |
| Female | 0.8 (0.8–0.9) | < 0.01 | 0.8 (0.8–0.9) | < 0.01 |
| Age group, y | ||||
| ≤5 | Reference | Reference | ||
| 6–10 | 1.1 (0.9–1.2) | 0.46 | 1.0 (0.8–1.1) | 0.70 |
| 11–15 | 0.9 (0.8–1.1) | 0.39 | 0.8 (0.7–1.0) | 0.02 |
| 16–25 | 0.3 (0.2–0.3) | < 0.01 | 0.2 (0.2–0.3) | < 0.01 |
| BMI categoryb | ||||
| Normal | Reference | - | Reference | - |
| Overweight | 1.3 (1.1–1.5) | < 0.01 | 1.3 (1.1–1.5) | < 0.01 |
| Obese | 0.8 (0.7–0.9) | < 0.01 | 0.8 (0.7–0.9) | < 0.01 |
| Period | ||||
| 1994–1998 | Reference | Reference | ||
| 1999–2003 | 1.0 (0.8–1.3) | 0.96 | 1.0 (0.8–1.3) | 0.79 |
| 2004–2008 | 1.3 (1.0–1.6) | 0.03 | 1.5 (1.2–1.9) | < 0.01 |
| 2009–2013 | 1.4 (1.1–1.7) | < 0.01 | 2.0 (1.6–2.5) | < 0.01 |
Model was adjusted for present age, sex, BMI and period.
Normal weight was defined as BMI Z-score <1.04 (<85th percentile of growth chart); overweight, BMI Z-score 1.04–1.64 (85th–95th percentile); and obese, BMI Z-score >1.64 (>95th percentile) of 1990 reference population.
Figure 2.
Incidence of type 2 and type 1 diabetes in UK children and young adults according to BMI category, 1994 to 2013.
The ORs, which approximate the relative risks in the nested case-control analysis, are listed in Table 3 [27]. We observed that obese individuals constituted 47.1% (n = 308 of 654) of type 2 diabetes cases and had approximately four times the risk of incident type 2 diabetes as those with a normal BMI (OR, 3.7; 95% CI, 3.1 to 4.6; Table 3). Overweight and obesity were not similarly associated with type 1 diabetes (Table 2). We observed no association between obesity and incident type 1 diabetes in analyses limited to diabetes cases assigned after BMI records as exposure (Supplemental Table 1 (65.2KB, docx) ). The age-adjusted OR was 4.0 (95% CI, 2.9 to 5.3) for type 2 diabetes when comparing the top quintile and the bottom quintile of the BMI Z-score (Supplemental Table 2 (65.2KB, docx) ). With BMI as a continuous variable, we observed an OR of 1.6 (95% CI, 1.5 to 1.7) per 1-SD increase in BMI Z-score. BMI was positively associated with type 1 diabetes at the middle range of BMI, and the association was nonsignificant for the top quintile vs the bottom quintile (Supplemental Table 2 (65.2KB, docx) ). The corresponding OR per 1-SD increase in BMI was not statistically significant (age-adjusted OR, 1.01; 95% CI, 0.96 to 1.05).
Table 3.
Odds Ratios for Type 2 and Type 1 Diabetes According to BMI Categories in UK Children and Young Adults, 1994 to 2013
| Variable | Unadjusted Model |
Adjusted Modela |
||||
|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Type 2 diabetes | 654 | 8589 | ||||
| Normal BMI | 285 | 5996 | Reference | Reference | ||
| Overweight | 61 | 1028 | 1.3 (0.9–1.7) | 0.13 | 1.3 (0.9–1.8) | 0.12 |
| Obese | 308 | 1565 | 3.9 (3.2–4.8) | < 0.01 | 3.8 (3.1–4.6) | < 0.01 |
| Type 1 diabetes | 1318 | 8589 | ||||
| Normal BMI | 938 | 5996 | Reference | Reference | ||
| Overweight | 201 | 1028 | 1.3 (1.1–1.5) | < 0.01 | 1.3 (1.0–1.5) | 0.02 |
| Obese | 179 | 1565 | 0.8 (0.6–0.9) | < 0.01 | 0.7 (0.6–0.8) | < 0.01 |
Model adjusted for age at BMI and weight or height records in the UK CRPD.
3. Discussion
The present large cohort study was based on primary care electronic health records. Our results showed that the incidence of both type 1 and type 2 diabetes has been increasing among UK children and young adults from 1994 to 2013. A greater incidence (fourfold) of type 2 diabetes was observed in obese (constituting about one-half of cases). Given that no linear association was found between the BMI and the incidence of type 1 diabetes and that a higher BMI was not associated with type 1 diabetes when analyzing only those individuals with a BMI value before the date of diabetes diagnosis, further work is needed to replicate our observational evidence from children and young adults.
Diabetes increasingly imposes a large burden on health and society worldwide, as indicated by estimates that 1 in 11 adults have type 2 diabetes (415 million people) and >0.5 million children (aged ≤14 years) had type 1 diabetes in 2015 [28–30]. Understanding the temporal trends and incidence of diabetes and the modifiable factors is paramount to providing timely prevention or delaying the occurrence of diabetes during the life course [31]. Previous work has mainly reported the burden and incidence of type 1 diabetes in children [32, 33]. Little evidence is available concerning type 2 diabetes and little is known about the potential association of obesity with either type 1 or type 2 diabetes in children and young adults [30, 34]. The SEARCH study for the 2002 to 2003 period has reported that type 2 diabetes in youth is predominantly occurring in higher risk ethnic groups, accounting for 14.9% of all diabetes cases among non-Hispanic white adolescents aged ≥10 years (incidence rates ranged from 5.6 among whites to 49.4 per 100,000 persons per year among Native Americans) [35]. Other studies, which were mainly conducted 10 years earlier in Europe, reported type 2 diabetes as an uncommon condition in white young populations or showed no trend [34, 36]. A prospective surveillance UK study in 2004 to 2005 reported 67 cases of type 2 diabetes of all 363 diabetes diagnoses based on notification by pediatricians in children aged ≤17 years [37]. Using CPRD data, another study analyzing the data from 0.5 million children aged 0 to 18 years showed an eightfold increase in the prevalence of oral antidiabetic drugs, from 0.6 per 100,000 children in 1998 to 5 per 100,000 children in 2005 [38]. A relative risk for incident type 2 diabetes has been estimated as approximately twice as large per 1-SD difference in BMI in European adults. Recently, a longitudinal study showed an association between adolescent BMI and incident diabetes in adults, which was not independent of the adult BMI [30]. Another study in Finland showed a link between a change in childhood BMI and type 2 diabetes in adults [39].
We add to previous observational evidence that childhood or adolescent obesity is associated with an increase in the incidence of type 2 diabetes (but not type 1) in children and young adults. Moreover, increases in type 2 diabetes incidence by obese category were greater among those aged 11 to 15 years and in the last two calendar periods. This pattern is consistent with increased BMI values during the period in the obese category. Although our estimates were greater than those previously reported in white populations, similar data are lacking, in terms of time period and observational setting, to judge recent estimates of type 2 diabetes incidence. We observed that most children and young adults with type 2 diabetes were overweight or obese, in line with the results of the surveillance UK study [37]. Moreover, we estimated that obese children and young adults have approximately four times the risk of incident type 2 diabetes as those with a normal BMI.
Our finding of the increasing incidence of type 1 diabetes in those aged ≤15 years is consistent with previous studies from population-based or primary care data. Although estimates have varied across populations and time, temporal trends have shown an increasing diabetes incidence in children and young adults from the 1990s through the 2000s and beyond [16, 32–37, 40–42]. We, and others, have shown that the incidence of childhood type 1 diabetes has increased during recent decades [32, 38, 40, 42]. Similar to these studies, we observed that the type 1 diabetes incidence continued to increase in female individuals and those aged ≤15 years from 1994 to 2013. However, our study, and other studies in Europe, has shown that the incidence of type 1 diabetes is somewhat greater in male than in female individuals [32]. Although both genetic and environmental factors have been proposed in the etiology of type 1 diabetes, the reason for the increase in type 1 diabetes incidence is unknown. Obviously, the improved survival in children with type 1 diabetes and the potential for successful reproduction are contributors to this increase [32]. No linear association was found between BMI and the incidence of type 1 diabetes in our study. However, we observed a greater incidence among overweight children but a lower incidence among obese children. The results of a few cohort or case-control studies have suggested that weight in the first year of life is positively associated with type 1 diabetes. However, observational evidence for an association between BMI and type 1 diabetes has varied [15]. Given that the autoimmune response encoded by the HLA class genes strongly contributes to the etiology of type 1 diabetes, questions exist regarding whether early life obesity might have a biologically beneficial effect on type 1 diabetes risk later in life [43]. Apart from this, it is plausible that disease-related weight loss or a selection bias might account for an apparently paradoxical association between obesity and type 1 diabetes in our observational study [44].
Taken together, we have updated observational evidence that the rate of type 2 diabetes incidence, although less common than type 1, is increasing among UK children and young adults in parallel with the increasing childhood obesity during the past decades [1, 30, 31, 45]. Our observational findings support the use of a real world practice database to broadly estimate the risks and rates of major global health issues, such as incident diabetes in children. Such evidence can also extend our understanding of disease burden and temporal trends beyond surveillance from the secondary-care pediatric setting and traditional cohorts, which have usually included a limited size and select population [46]. Our results will add value to the research that informs policy makers to identify emerging health care needs and how to prioritize future clinical studies for targeted management of childhood obesity and diabetes. Children with type 2 diabetes potentially have related disorders such as dyslipidemia and hypertension [37, 47]. Given the age of onset, it seems that childhood diabetes is more severe than diabetes in adults. Also, the effects of diabetes-related outcomes on health and society will be greater throughout the life course [2, 48].
The present study had certain limitations. First, data on BMI, height, and weight were not available for all individuals in the CPRD. It is possible that children had their weight and height recorded not as a part of routine practice and that a general practitioner in primary care had decided to measure BMI because of the child’s high weight or health status [21]. In the CPRD, the method of height and weight measurements (e.g., shoes on or off) and the quality of data collected might be varied in each practice; however, such variance can be random, and power can be maintained by the large numbers. Therefore, a potential for selection bias exists that one cannot completely rule out in clinical or observational research, and randomness remains an assumption [21]. Previous studies using the CPRD suggested the consistency of results obtained from primary care data with those from cohort observations and that up-to-date BMI values and obesity prevalence in CPRD were as close as population-based survey data [21, 22]. Furthermore, the magnitude and direction of associations of demographic information and BMI with diabetes types, which were observed in our study, are consistent with those reported in published studies. We also observed similar results when only data on the BMI records before the date of a diabetes diagnosis were used as a sensitivity analysis. As previously reported, most (~77%) children contributed one BMI observation to the CPRD [6]. It also seems plausible that the incidence of diabetes could have been artificially increased, because we used a single measure of BMI to constitute exposure. However, completeness of the BMI data in the CPRD did not fundamentally vary over time among children and young adults from 1994 through 2011 [22], reducing the probability that our results occurred by chance. Although important questions remain regarding how the BMI trajectory measured early in childhood might contribute to the development of type 2 diabetes [31], we did not have enough information about the BMI trend between the time point the BMI was measured and the onset date of diabetes in our study. Second, we used data from clinical cases, prescriptions for diabetes drugs, and HbA1c tests. Nevertheless, we could have missed false-negative cases in the remainder of the CPRD, because diabetes, in particular, type 2 diabetes, can remain undiagnosed for several months to years [29]. Given the large size of the CPRD cohort, combined with the low incidence of type 2 diabetes among children and young adults, we would not expect this to have largely affected our findings [16, 20, 22, 29, 35, 36]. Although we applied some strict criteria for the definition of diabetes types and some misclassification of outcomes or the onset date of diabetes is inevitable, the effect on our estimates was likely small [21]. Other limitations included that the present study could not provide information on autoantibodies for type 1 diabetes, physical activity, puberty effect on changes in the BMI, and the extent of glycemia control. Finally, generalization of these findings is limited, because the data in the UK CPRD are predominantly from those of European ancestry.
In conclusion, the incidence of type 1 and type 2 diabetes among UK children and young adults continued to increase from 1994 to 2013 in both sexes and in those aged ≤15 years. An increasing trend in the incidence of type 2 diabetes was more evident among the overweight and obese. The incidence of type 2 diabetes was four times as high among obese individuals (constituting about one-half of cases) as among those with a normal BMI. No linear association was found between the childhood BMI and the incidence of type 1 diabetes. Further work is needed to explore potential targets for the prevention of childhood obesity and early-onset type 2 diabetes.
Acknowledgments
The present study was based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency; however, the interpretation and conclusions contained in this report are those of the authors alone.
Acknowledgments
M.C.G. was supported by the National Institute for Health Research Biomedical Research Centre at Guy's and St. Thomas' National Health Services Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the UK National Health Service, National Institute for Health Research, or the Department of Health (England). The funders had no role in the design or conduct of the study, collection, management, analysis or interpretation of the data, preparation, review, approval of the manuscript, or the decision to submit the manuscript for publication.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- HbA1c
- hemoglobin A1c
- IRR
- incidence rate ratio
- IQR
- interquartile range
- OR
- odds ratio
- SD
- standard deviation.
References and Notes
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. 2015;373:1307–1317. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health & Chief Medical Officer. Annual report of the Chief Medical Officer 2002. 2003. Available at: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/PublicationsAndStatistics/Publications/AnnualReports/DH_4006432. Accessed 8 April 2016.
- 4.World Health Organization Obesity and overweight. Fact sheet number 311. Geneva: World Health Organization; 2015. [Google Scholar]
- 5.Craig R, Fuller E, Mindell J, eds. Health Survey for England, 2014. Chapter 10: Children's BMI, Overweight and Obesity. NHS Digital (formerly, Health and Social Care Information Centre); publication date 16 December 2015. Available at: http://content.digital.nhs.uk/catalogue/PUB19295. Accessed: 15 April 2016. [Google Scholar]
- 6.van Jaarsveld CH, Gulliford MC. Childhood obesity trends from primary care electronic health records in England between 1994 and 2013: population-based cohort study. Arch Dis Childhood. 2015;100:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lifestyle Statistics Team Health and Social Care Information Centre. National Child Measurement Programme: England, 2012/13 school year; 2013. Available at: http://content.digital.nhs.uk/catalogue/PUB13115/nati-chil-meas-prog-eng-2012-2013-rep.pdf. Accessed 11 April 2016. [Google Scholar]
- 8.Joint Health Surveys Unit. Health Survey for England - 2012, Trend tables. 2013. Available at: https://www.gov.uk/government/statistics/health-survey-for-england-trend-tables-2013. Accessed 10 April 2016.
- 9.Jordan DN, Jordan JL. Pediatric type 2 diabetes mellitus complications: a systematic review of the literature. J Diabet Res Clin Metab 2012;1:24. [Google Scholar]
- 10.Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92:251–265. [DOI] [PubMed] [Google Scholar]
- 11.Or T, Lm T, Mr P. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. 2016;9:235–239. [PMC free article] [PubMed] [Google Scholar]
- 12.Benhalima K, Wilmot E, Khunti K, Gray LJ, Lawrence I, Davies M. Type 2 diabetes in younger adults: clinical characteristics, diabetes-related complications and management of risk factors. Prim Care Diabetes. 2011;5:57–62. [DOI] [PubMed] [Google Scholar]
- 13.Ehtisham S, Hattersley A, Dunger D, Barrett T. First UK survey of paediatric type 2 diabetes and MODY. Arch Dis Child. 2004;89:526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haines L, Wan KC, Lynn R, Barrett TG, Shield JP. Rising incidence of type 2 diabetes in children in the UK. Diabetes Care. 2007;30:1097–1101. [DOI] [PubMed] [Google Scholar]
- 15.Magnus MC, Olsen SF, Granstrom C, Joner G, Skrivarhaug T, Svensson J, Johannesen J, Njolstad P, Magnus P, Stordal K, Stene LC. Infant growth and risk of childhood-onset type 1 diabetes in children from 2 Scandinavian birth cohorts. JAMA Pediatr. 2015;169:e153759. [DOI] [PubMed] [Google Scholar]
- 16.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, Liese AD, Merchant AT, Lawrence JM, Reynolds K, Dolan L, Liu LL, Hamman RF. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royal College of Paediatrics and Child Health. National Paediatric Diabetes Audit Report 2013-14. Available at: http://www.rcpch.ac.uk/system/files/protected/page/2014%20NPDA%20Report%201%202014%20FINAL.pdf. Accessed 11 April 2016.
- 18.Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, Infante AM, Krakoff J, Looker HC. Childhood predictors of young-onset type 2 diabetes. Diabetes. 2007;56:2964–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheelock KM, Sinha M, Knowler WC, Nelson RG, Fufaa GD, Hanson RL. Metabolic risk factors and type 2 diabetes incidence in American Indian children. J Clin Endocrinol Metab. 2016;101:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhaskaran K, Forbes HJ, Douglas I, Leon DA, Smeeth L. Representativeness and optimal use of body mass index (BMI) in the UK Clinical Practice Research Datalink (CPRD). BMJ Open. 2013;3:e003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth H, Khan O, Prevost T, Reddy M, Dregan A, Charlton J, Ashworth M, Rudisill C, Littlejohns P, Gulliford MC. Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diabetes Endocrinol. 2014;2:963–968. [DOI] [PubMed] [Google Scholar]
- 25.Hazra N, Gulliford M. Evaluating pancreatitis in primary care: a population-based cohort study. Br J Gen Pract 2014;64:e295–e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Office for National Statistics Key health statistics from general practice 1998. London: National Statistics; 2000; Series MB6 No. 2. [Google Scholar]
- 27.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 28.International Diabetes Federation IDF Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. [Google Scholar]
- 29.Abbasi A, Peelen LM, Corpeleijn E, van der Schouw YT, Stolk RP, Spijkerman AM, van der A D, Moons KG, Navis G, Bakker SJ, Beulens JW. Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study. BMJ. 2012;345:e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, Derazne E, Tzur D, Shamis A, Vinker S, Rudich A. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuomilehto J. The emerging global epidemic of type 1 diabetes. Curr Diab Rep. 2013;13:795–804. [DOI] [PubMed] [Google Scholar]
- 33.Hamman RF, Bell RA, Dabelea D, D’Agostino RB Jr, Dolan L, Imperatore G, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Pihoker C, Rodriguez BL, Saydah S. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37:3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazeli Farsani S, van der Aa MP, van der Vorst MM, Knibbe CA, de Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia. 2013;56:1471–1488. [DOI] [PubMed] [Google Scholar]
- 35.Dabelea D, Bell RA, D’Agostino RB Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. [DOI] [PubMed] [Google Scholar]
- 36.D’Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care. 2011;34(Suppl 2):S161–S165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haines L, Wan KC, Lynn R, Barrett TG, Shield JP. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007;30:1097–1101. [DOI] [PubMed] [Google Scholar]
- 38.Hsia Y, Neubert AC, Rani F, Viner RM, Hindmarsh PC, Wong IC. An increase in the prevalence of type 1 and 2 diabetes in children and adolescents: results from prescription data from a UK general practice database. Br J Clin Pharmacol. 2009;67:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriksson JG, Kajantie E, Lampl M, Osmond C. Trajectories of body mass index amongst children who develop type 2 diabetes as adults. J Intern Med. 2015;278:219–226. [DOI] [PubMed] [Google Scholar]
- 40.Imkampe AK, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991-2008. Diabet Med 2011;28:811–814. [DOI] [PubMed] [Google Scholar]
- 41.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516–1526. [DOI] [PubMed] [Google Scholar]
- 42.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. [DOI] [PubMed] [Google Scholar]
- 43.Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dixon JB, Egger GJ, Finkelstein EA, Kral JG, Lambert GW. “Obesity paradox” misunderstands the biology of optimal weight throughout the life cycle. Int J Obes. 2005;2015(39):82–84. [DOI] [PubMed] [Google Scholar]
- 45.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A, Flaxman D, Foreman, Gabriel S, Gakidou E, Kassebaum N, Khatibzadeh S, Lim S, Lipshultz SE, London S, Lopez, MacIntyre MF, Mokdad AH, Moran A, Moran AE, Mozaffarian D, Murphy T, Naghavi M, Pope C, Roberts T, Salomon J, Schwebel DC, Shahraz S, Sleet DA, Murray, Abraham J, Ali MK, Atkinson C, Bartels DH, Bhalla K, Birbeck G, Burstein R, Chen H, Criqui MH, Dahodwala, Jarlais, Ding EL, Dorsey ER, Ebel BE, Ezzati M, Fahami, Flaxman S, Flaxman AD, Gonzalez-Medina D, Grant B, Hagan H, Hoffman H, Kassebaum N, Khatibzadeh S, Leasher JL, Lin J, Lipshultz SE, Lozano R, Lu Y, Mallinger L, McDermott MM, Micha R, Miller TR, Mokdad AA, Mokdad AH, Mozaffarian D, Naghavi M, Narayan KM, Omer SB, Pelizzari PM, Phillips D, Ranganathan D, Rivara FP, Roberts T, Sampson U, Sanman E, Sapkota A, Schwebel DC, Sharaz S, Shivakoti R, Singh GM, Singh D, Tavakkoli M, Towbin JA, Wilkinson JD, Zabetian A, Murray, Abraham J, Ali MK, Alvardo M, Atkinson C, Baddour LM, Benjamin EJ, Bhalla K, Birbeck G, Bolliger I, Burstein R, Carnahan E, Chou D, Chugh SS, Cohen A, Colson KE, Cooper LT, Couser W, Criqui MH, Dabhadkar KC, Dellavalle RP, Jarlais, Dicker D, Dorsey ER, Duber H, Ebel BE, Engell RE, Ezzati M, Felson DT, Finucane MM, Flaxman S, Flaxman AD, Fleming T, Foreman, Forouzanfar MH, Freedman G, Freeman MK, Gakidou E, Gillum RF, Gonzalez-Medina D, Gosselin R, Gutierrez HR, Hagan H, Havmoeller R, Hoffman H, Jacobsen KH, James SL, Jasrasaria R, Jayarman S, Johns N, Kassebaum N, Khatibzadeh S, Lan Q, Leasher JL, Lim S, Lipshultz SE, London S, Lopez, Lozano R, Lu Y, Mallinger L, Meltzer M, Mensah GA, Michaud C, Miller TR, Mock C, Moffitt TE, Mokdad AA, Mokdad AH, Moran A, Naghavi M, Narayan KM, Nelson RG, Olives C, Omer SB, Ortblad K, Ostro B, Pelizzari PM, Phillips D, Raju M, Razavi H, Ritz B, Roberts T, Sacco RL, Salomon J, Sampson U, Schwebel DC, Shahraz S, Shibuya K, Silberberg D, Singh JA, Steenland K, Taylor JA, Thurston GD, Vavilala MS, Vos T, Wagner GR, Weinstock MA, Weisskopf MG, Wulf S, Murray; US Burden of Disease Collaborators . The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. [DOI] [PubMed] [Google Scholar]
- 48.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]