Abstract
Context:
Wnt1-inducible signaling pathway protein 1 (WISP1) is a novel adipokine participating in adipose tissue (AT) dysfunction; so far, no data on WISP1 in diabetes are available.
Objectives:
To evaluate plasma WISP1 in subjects with type 2 diabetes (T2D) and its correlates linked to AT inflammation.
Design and Participants:
For this cross-sectional study, 97 consecutive dysmetabolic patients were recruited at the diabetes outpatient clinics of Sapienza University in Rome; 71 of them had T2D, with (n = 35) or without (n = 36) obesity, and 26 were obese patients without diabetes. Twenty-one normal-weight nondiabetic individuals were enrolled as a control group. Study participants underwent clinical workup and blood sampling for metabolic/inflammatory characterization; magnetic resonance imaging (MRI) data on subcutaneous AT and visceral AT (VAT) area, hepatic fat content, and VAT homogeneity were available for most diabetic patients.
Results:
Plasma WISP1 significantly increased throughout classes of obesity and correlated with greater VAT area, interleukin-8 (IL-8), and lower adiponectin levels, without differing between diabetic and nondiabetic participants. Higher IL-8 was the main determinant of increased WISP1. MRI-assessed VAT inhomogeneity was associated with higher WISP1, IL-8 and C-reactive protein levels, independent of obesity; high WISP1 strongly predicted VAT inhomogeneity (P < 0.001).
Conclusions:
WISP1 levels are increased in obese persons and are directly related to adiposity, independent of glycemic status or insulin resistance; moreover, they are strongly associated with increased plasma IL-8 and signal abnormalities of VAT. The overall data add insights to the mechanisms underlying metabolic alterations and may open a scenario for innovative therapeutic approaches for diabetes prevention and care.
Keywords: adipose tissue, adipokines, type 2 diabetes, inflammation, visceral fat
This study of plasma Wnt1-inducible signaling pathway protein 1 (WISP1) in dysmetabolic patients and controls found that higher WISP1 levels strongly correlated with obesity and adipose tissue dysfunction, independent of diabetes.
Under conditions of excessive caloric intake, adipose tissue (AT) undergoes structural and functional rearrangement characterized by hypertrophy and hyperplasia of adipocytes; insufficient neovascularization; aberrant fibrogenesis; and migration and activation of macrophages, natural killers, and lymphocytes [1, 2]. Thus, the overproduction of proinflammatory adipokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8 [3], along with reduced AT capability of storing free fatty acids and the resultant aberrant efflux of free fatty acids into the circulation, lead to insulin resistance and its related diseases [1, 4–6]. Furthermore, we recently demonstrated that AT dysfunction is a determinant of worse metabolic profile and higher cardiovascular risk in patients with type 2 diabetes [7].
Wnt1-inducible signaling pathway protein 1 (WISP1, or Cyr61/CTGF/NOV) is an extracellular matrix–associated protein belonging to the Cyr61/CTGF/NOV family, which includes matricellular proteins operating at the border between cells and extracellular matrix and exerts regulatory actions on several cellular responses. Thus, WISP1 is involved in a broad spectrum of biological functions and pathological processes [8–16]; is mainly expressed during organ development and under diseased conditions, such as fibrosis [11–15], cancer [9, 10, 13] , and inflammatory diseases [16]; and has recently been proposed as a novel adipokine [17].
WISP1 is widely expressed in visceral (VAT) and subcutaneous (SAT) human AT, is released by fully differentiated human adipocytes, and stimulates cytokine responses in AT-associated macrophages [17]. Circulating WISP1 concentration correlates with its expression in AT, which therefore represents a major source of this adipokine in humans [17]. Among the many cell types on which it exerts proliferative effects, WISP1 has induced proliferation of mesenchymal stem cells and, thus, AT expansion, in experimental models of visceral obesity [18].
Although an association between WISP1 expression in AT inflammation and insulin resistance has been described in persons with normal glucose tolerance [17], no evidence on its role in AT inflammation in conditions of impaired glucose regulation has been produced so far. Increased circulating WISP1 levels were recently reported in a study among women with gestational diabetes; this study identified greater body mass index (BMI) and insulin resistance during pregnancy as determinants of increased WISP1 [19]; however, as far as we know, no study has evaluated circulating WISP1 levels in patients with type 2 diabetes. Therefore, the aim of this study was to investigate the role of plasma WISP1 levels in identifying patterns of AT inflammation in patients with type 2 diabetes.
1. Methods
A. Population
For this study, we recruited 97 consecutive patients referred to our diabetes outpatient clinics for metabolic evaluations. Of these, 71 had a diagnosis of type 2 diabetes, with or without obesity [n = 35, 26 men and nine women, mean age ± standard deviation (SD), 51.9 ± 9.2 years; n = 36, 21 men and 15 women, mean age ± SD, 50.4 ± 9.7 years, respectively], and 26 were obese without diabetes (nine men and 17 women, mean age ± SD, 42.4 ± 9.8 years). Furthermore, a population of 21 normal-weight nondiabetic individuals was recruited as a control group (14 men and seven women, mean age ± SD, 52.3 ± 12.4 years). To be eligible, participants had to fulfil the following inclusion criteria: men or women aged 25–70 years; white ethnicity; no history of excessive alcohol intake (average daily consumption of alcohol >30 g/d in men and >20 g/d in women); and absence of hepatitis B surface antigen and antibodies to hepatitis C virus, cirrhosis or other chronic liver diseases, malignancies, autoimmune diseases, and treatment with corticosteroids and/or other agents affecting the immune system.
The study protocol was reviewed and approved by the Ethics Committee of this hospital, and the study was conducted in conformance with the Helsinki Declaration. All patients provided written consent before the study.
B. Laboratory Determinations
The study population underwent clinical workup, including medical history collection; physical examination with measurement of BMI (kg/m2), waist circumference (cm), and systemic blood pressure [systolic and diastolic (mmHg)]; and fasting blood sampling to assess total cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), triglycerides (mg/dL), aspartate aminotransferase (IU/L), alanine aminotransferase (IU/L), blood urea nitrogen (mg/dL), creatinine (mg/dL), and C-reactive protein (CRP; mg/dL) by standard laboratory methods. The glyco-metabolic profile was evaluated by measuring fasting blood glucose (FBG; mg/dL), insulin (IU/L; PANTEC srl, Italy) and glycosylated hemoglobin (% - mmol/L). The homeostasis model assessments of insulin resistance and insulin secretion were calculated as described elsewhere [20]. Diabetes mellitus was diagnosed according to American Diabetes Association 2009 criteria [21].
Among circulating markers of AT inflammation, we measured the concentrations of IL-8, IL-6, and TNF-α (pg/mL; Multiplex, BioRad Laboratories, Hercules, CA) and adiponectin (μg/mL; enzyme-linked immunosorbent assay; Tema Ricerca srl, Italy) on sera frozen immediately after sampling and stored at −25°C for a few days. WISP1 concentration was detected in plasma samples by enzyme-linked immunosorbent assay commercial kits (ng/mL; RayBiotech Inc., Norcross, GA).
C. MRI
Data on hepatic fat fraction (%) and abdominal SAT and VAT area (cm2), obtained by MRI (1.5-T magnet; Magnetom Avanto, Siemens Medical Systems, Erlangen, Germany), were available for 67 patients with type 2 diabetes enrolled for the Eudract 2011-003010-17 study, as described elsewhere [22]. Briefly, nonalcoholic fatty liver disease (NAFLD) was diagnosed in patients with hepatic fat fraction of ≥5.5% [23]; VAT and SAT were quantified by acquiring a three-dimensional gradient echo T1-weighted volumetric interpolated breath-hold examination sequence on an axial plane modified by Dixon [repetition time, 4.7 msec; echo time, 2.3 msec; flip angle, 10°; matrix, 256 ×192 mm; section thickness, 5 mm (reconstructed 2.5 mm); intersection gap, 0] and analyzing the fat-only data sets with specific software (Slice-O-Matic; Tomovision Inc., Montreal, QC, Canada) [24, 25]. Data were calculated from AT area at L1–L2, L2–L3, L3–L4, and L4–L5 levels; free-form regions of interest and manual threshold were used to select fat tissue within VAT and SAT slides.
A single radiologist, blinded to the clinical characteristics of study participants, conducted an exploratory evaluation of VAT quality by detecting the presence of inhomogeneous areas, likely referable to AT inflammation, in the context of visceral compartment (between L1 and L3). In physiologic conditions, AT signal intensity at MRI is homogenous and markedly brighter than that observed in surrounding tissues and organs on both T2-weighted and T1-weighted sequences. Inflammation is generally characterized by the presence of edema, which represents the main feature of phlogosis and appears as an area with slightly higher signal intensity, in comparison with normal tissue, at T2-weighted sequences with fat saturation. Therefore, VAT nonhomogeneity has been defined as the presence of areas characterized by slightly lower signal intensity on T1-weighted sequences and slightly higher signal intensity on T2-weighted fat-saturated sequences at MRI.
D. Statistical Analysis
All analyses were performed with SPSS statistical package, version 23 (IBM, Armonk, NY). Values are reported as mean ± SD for continuous variables and as a percentage for categorical variables. Non–normally distributed variables underwent log-transformation before the analyses. Comparisons between two groups were performed by Student t test for independent samples and by Pearson χ2 for categorical variables. Analysis of variance analysis with Bonferroni adjustment was performed for comparisons between multiple groups. Comparisons between subgroups of individuals with or without impaired VAT signal intensity, categorized as 0 = absence and 1 = presence of VAT inhomogeneity at MRI, were performed by the nonparametric Mann–Whitney test for continuous variables and Fisher test for categorical variables. Bivariate correlation analyses were calculated by Pearson and Spearman rank correlations or by an age- and sex-adjusted partial correlation test. A two-tailed P value < 0.05 was considered to indicate a statistically significant difference, with a 95% confidence interval.
2. Results
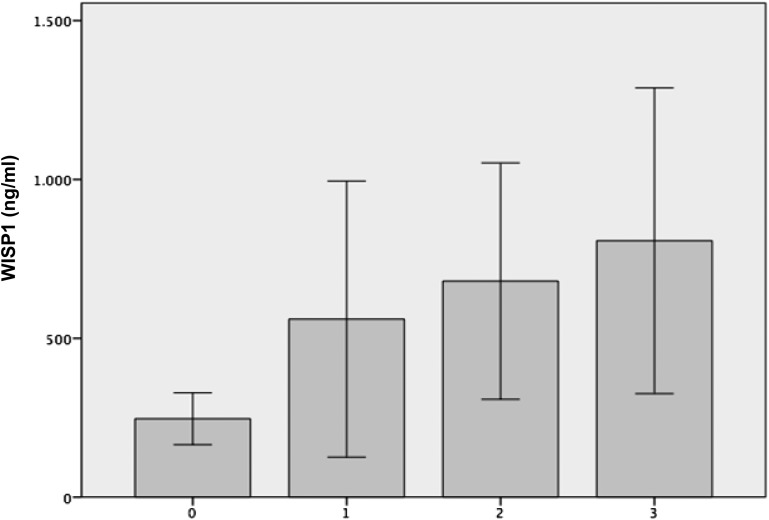
In the total study population (n = 118), the mean plasma WISP1 concentration was 460.5 ± 1540.4 ng/mL; this value linearly increased throughout different classes of obesity (Kruskal–Wallis test; P < 0.001) (Fig. 1). WISP1 levels did not differ significantly between participants with and without type 2 diabetes (432.4 ± 1521 ng/ml vs 535 ± 1639.5 ng/mL; P = 0.29); no specific association between WISP1 levels and diagnosis of diabetes was found even after stratification of the diabetic population for presence of obesity (Table 1).
Figure 1.
Plasma WISP1 levels according to the presence and severity of obesity. 0 = BMI < 29.9 kg/m2; 1 = BMI of 30.0–34.9 kg/m2; 2 = BMI of 35.0–39.9 kg/m2; 3 = BMI ≥ 40 kg/m2. Values are the mean ± standard error of the mean; Kruskall–Wallis test.
Table 1.
Clinical and Biochemical Characteristics of Study Population According to Presence of Type 2 Diabetes and Obesity
| Parameter | NonOb-T2D (n = 36) | Ob-T2D (n = 35) | Ob-NonT2D (n = 26) | NonOb-NonT2D (n = 21) | P Value |
|---|---|---|---|---|---|
| Age (y) | 50.4 ± 9.7 | 51.9 ± 9.2 | 42.4 ± 9.8 | 52.3 ± 12.4 | 0.007a; 0.003b; 0.002c |
| Sex (male/female) | 21/15 | 26/9 | 9/17 | 14/7 | 0.02d |
| BMI (kg/m2) | 26.6 ± 1.8 | 34.2 ± 4.4 | 40.7 ± 6.1 | 24.4 ± 2.9 | <0.001a,b,c,e |
| Waist circumference (cm) | 96 ± 6.9 | 112.8 ± 12.4 | 121.2 ± 14 | 85.8 ± 14.8 | <0.001a,b,c,e |
| T2D duration (y) | 7.6 ± 7.6 | 6.2 ± 5.1 | — | — | NS |
| SBP (mmHg) | 124 ± 13.8 | 135.1 ± 16.9 | 128.1 ± 19.3 | 123.1 ± 9.1 | 0.03e; 0.02f |
| DBP (mmHg) | 79 ± 8 | 86.7 ± 19.6 | 81.2 ± 7.8 | 78.3 ± 5.9 | NS |
| FBG (mg/dL) | 131.7 ± 37.8 | 130 ± 38.2 | 93.6 ± 11.8 | 98.2 ± 16.9 | 0.01e,g; <0.001c,b |
| HbA1c (%-mmol/mol) | 6.3 ± 0.5 | 6.8 ± 1.3 | 5.3 ± 0.5 | — | 0.001c,b |
| Total cholesterol (mg/dL) | 178.1 ± 36.9 | 175.8 ± 38 | 191.4 ± 21.1 | 197.6 ± 26.9 | NS |
| HDL cholesterol (mg/dL) | 49.1 ± 13.3 | 50.3 ± 15.9 | 49.9 ± 19.2 | 51.2 ± 10.9 | NS |
| Triglycerides (mg/dL) | 134.6 ± 70.8 | 138 ± 51.5 | 133.1 ± 37.5 | 114 ± 75.3 | NS |
| AST (IU/L) | 23.5 ± 13.6 | 24.8 ± 10.7 | 23.7 ± 10.5 | 18.1 ± 4.6 | NS |
| ALT (IU/L) | 31.9 ± 24.9 | 33.9 ± 16.7 | 29.8 ± 16.7 | 23.6 ± 12.4 | NS |
| Uric acid (mg/dL) | 5.6 ± 1.3 | 5.8 ± 0.9 | 5.6 ± 1.4 | 5.2 ± 1.04 | NS |
| Blood creatinine (mg/dL) | 0.97 ± 0.3 | 1.06 ± 0.2 | 1 ± 0.1 | 1.05 ± 0.2 | NS |
| FBI | 10.7 ± 5.5 | 13.6 ± 5.1 | 14.6 ± 11.9 | — | NS |
| HOMA-IR | 3.4 ± 1.7 | 4.2 ± 1.8 | 3.5 ± 3.4 | — | NS |
| HOMAβ % | 63.8 ± 64.7 | 105.7 ± 71.2 | 173.2 ± 132 | — | 0.03f; <0.001c |
| IL-8 (pg/mL) | 69.8 ± 109.2 | 79.4 ± 130.4 | 21.1 ± 30.8 | 22.9 ± 29.6 | NS |
| WISP-1 (ng/mL) | 249.4 ± 546.3 | 610.4 ± 2067 | 725 ± 2039.2 | 241 ± 695.7 | NS |
Unless otherwise noted, values are mean ± SD. Bonferroni-adjusted analysis of variance analysis.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HOMAβ%, homeostasis model assessment of insulin secretion; HOMA-IR, homeostasis model assessment of insulin resistance; nonOb-nonT2D, nonobese nondiabetic patients; nonOb-T2D, nonobese diabetic patients; Ob-nonT2D, obese nondiabetic patients; ObT2D, obese diabetic patients; SBP, systolic blood pressure; T2D, type 2 diabetes.
Ob-nonT2D vs nonOb-nonT2D.
Ob-nonT2D vs Ob-T2D.
NonOb-T2D vs Ob-nonT2D.
χ2 Pearson test between groups.
Ob-T2D vs nonOb-nonT2D.
NonOb-T2D vs Ob-T2D.
NonOb-T2D vs nonOb-nonT2D.
Among all clinical and metabolic determinants, bivariate correlation analyses showed that WISP1 levels were strongly associated with higher IL-8 concentration (r = 0.49; P < 0.0001; Supplemental Data 1 (66.2KB, pptx) ) and increased BMI (r = 0.23; P = 0.016); in contrast, no relationship was found with age (r = −0.05; P = 0.6), sex (r = 0.13; P = 0.2), systemic blood pressure [systolic: r = −0.08 (P = 0.4); diastolic: r = −0.13 (P = 0.2)], or metabolic control [FBG: r = −0.06 (P = 0.56); total cholesterol: r = 0.13 (P = 0.17); high-density lipoprotein cholesterol: r = 0.07 (P = 0.44); low-density lipoprotein cholesterol: r = 0.12 (P = 0.2); triglycerides: r = 0.08 (P = 0.4)].
Circulating IL-6 was detectable in 60% of study participants and TNF-α in 45% of study participants [median (25th–75th percentile) IL-6: 1.6 (0–3.9) pg/mL; TNF-α: 0.39 (0–3.93) pg/mL]. No significant differences were found between patients with and without type 2 diabetes [IL-6: patients with diabetes, 1.5 (0–3.36) pg/mL; patients without diabetes: 1.97 (0–4.9) pg/mL; TNF-α: patients with diabetes: 0.68 (0–3.38) pg/mL; patients without diabetes: 0.22 (0–6.8 pg/mL); P = not significant].
Interestingly, patients with increased plasma IL-6 and TNF-α levels (i.e., those in the highest quartile of both circulating cytokines) showed significantly higher WISP1 levels (r = 0.24; P = 0.02) and a trend toward greater IL-8 concentration (r = 0.20; P = 0.054).
In patients with type 2 diabetes, higher WISP1 levels were specifically associated with the presence of VAT—rather than SAT—fat distribution, as expressed by greater VAT area and VAT/SAT ratio, and lower adiponectin levels, whereas no correlation with total body adiposity (i.e., BMI, waist circumference) and NAFLD was observed (Table 2).
Table 2.
Plasma WISP-1 Levels in Patients With Type 2 Diabetes: Bivariate Correlation Analyses
| Parameter | Correlation Coefficienta | P Value |
|---|---|---|
| Sex | −0.15 | 0.23b |
| Age | −0.15 | 0.22 |
| T2D duration | −0.17 | 0.17 |
| BMI | 0.04 | 0.77 |
| Waist circumference | −0.006 | 0.95 |
| SBP | −0.05 | 0.69 |
| DBP | −0.24 | 0.06 |
| FBG | 0.01 | 0.92 |
| HOMA-IR | −0.009 | 0.94 |
| HOMA-β% | −0.11 | 0.41 |
| HbA1c | −0.09 | 0.49 |
| IL-8 | 0.71 | <0.0001 |
| Adiponectin | −0.25 | 0.04 |
| VAT area | 0.56 | 0.005 |
| SAT area | −0.37 | 0.01 |
| VAT/SAT ratio | 0.26 | 0.03 |
| NAFLD (yes/no) | 0.10 | 0.39b |
Abbreviations: DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HOMAβ%, homeostasis model assessment of insulin secretion; HOMA-IR, homeostasis model assessment of insulin resistance; SBP, systolic blood pressure; T2D, type 2 diabetes.
Pearson correlation coefficients are shown.
Spearman correlation coefficient, with WISP-1 as a continuous variable.
The multivariate linear regression analysis performed in the entire study population demonstrated that circulating IL-8 concentration was the main determinant of increased plasma WISP1 levels, independent of sex, age, IL-6, TNF-α, diabetes, and total adiposity (R2 = 0.58; P < 0.001), as shown in Table 3.
Table 3.
Multivariate Linear Regression Analysis
| Parameter |
Unstandardized Coefficients |
Standardized Coefficient: β | t | P Value | |
|---|---|---|---|---|---|
| β | Standard Deviation Error | ||||
| Constant | 722.6 | 884.4 | 0.87 | 0.39 | |
| IL-8 | 9.65 | 1.005 | 0.75 | 9.6 | <0.0001 |
| IL-6 | −3.95 | 18.7 | −0.14 | −0.21 | 0.83 |
| TNF-α | 2.32 | 19.45 | 0.08 | 0.12 | 0.90 |
| BMI | 40.93 | 41.38 | 0.14 | 0.99 | 0.33 |
| Waist circumference | −14.91 | 13.57 | 0.16 | −1.1 | 0.27 |
| Age | −10.34 | 10.25 | −0.07 | −1.00 | 0.32 |
| Sex (M/F) | 36.5 | 248.01 | 0.01 | 0.15 | 0.88 |
| T2D (yes/no) | −189.65 | 254.68 | −0.06 | −0.74 | 0.46 |
R = 0.762; R2 = 0.58; corrected R2 = 0.539; SD error = 931.4877. Plasma WISP1 concentration is the dependent variable.
Abbreviations: T2D, type 2 diabetes.
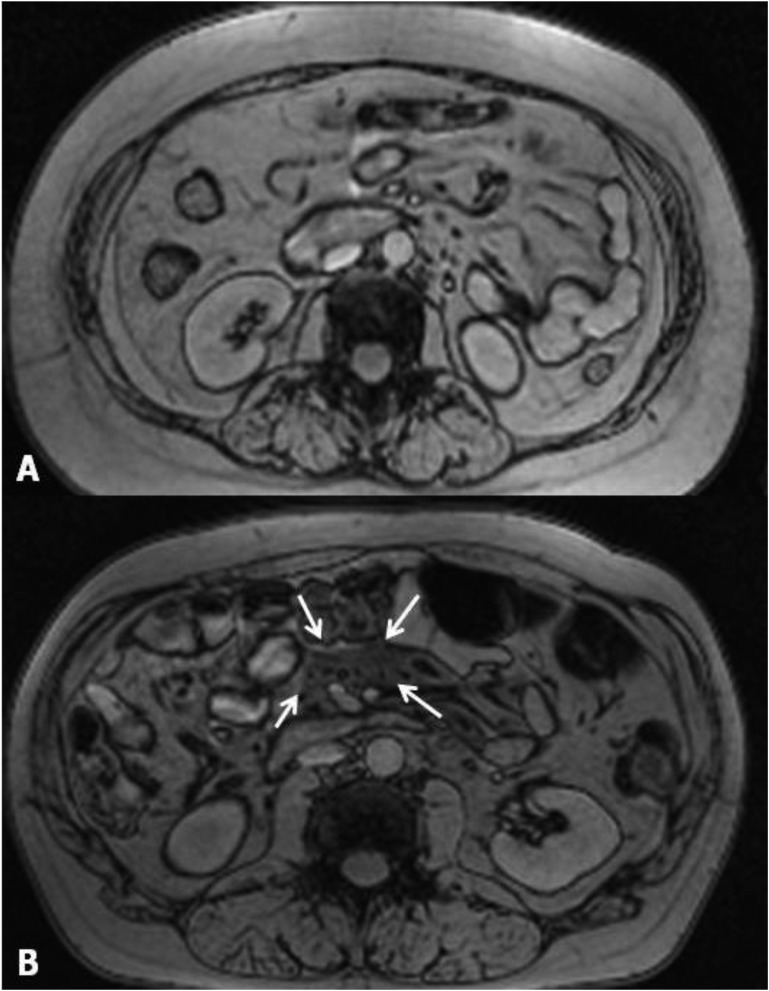
Inhomogeneous areas referable to VAT inflammation were detected in nine out of 67 diabetic patients (13.4%; four without obesity and five with obesity) who underwent MRI (Fig. 2; MRI images from all the patients with VAT inhomogeneity are shown in Supplemental Data 2 (56.7KB, pptx) ). In all these patients, spots with impaired MRI signal, represented by lower intensity on T1-weighted and higher intensity on T2-weighted images in comparison with the surrounding AT (suggestive of edema and inflammation), were localized near the mesenteric root and jejunum vessels. Of note, none of these individuals had a history of abdomen surgery, inflammatory bowel diseases, gastrointestinal infection, or malignancy. Patients with qualitative VAT alterations were all men, had a higher prevalence of insulin therapy, and exhibited peculiar systemic inflammatory profile (characterized by greater WISP1, IL-8, and CRP levels) in comparison with diabetic individuals with homogeneous VAT at MRI (Table 4).
Figure 2.
Abdominal MRI images (1.5-T magnet; Magnetom Avanto; Siemens Medical Systems, Erlangen Germany). (A) Diabetic patient without alterations of VAT homogeneity. (B) Arrows indicate an inhomogeneous area in the context of VAT in another study participant.
Table 4.
Inflammatory Profile and Body Fat Distribution in Patients With Type 2 Diabetes With or Without Impaired VAT Homogeneity
| Parameter | Impaired VAT (n = 9) | Normal VAT (n = 58) | P Value |
|---|---|---|---|
| Age (y) | 55.7 ± 8 | 59.2 ± 9.7 | 0.21 |
| Gender (M/F) | 9/0 | 38/20 | 0.035a |
| BMI (kg/m2) | 32.4 ± 5.6 | 29.5 ± 4 | 0.17 |
| Waist circumference (cm) | 112.9 ± 20.2 | 102 ± 10.5 | 0.20 |
| T2D duration (y) | 6.7 ± 4.9 | 7.1 ± 6.8 | 0.89 |
| WISP-1 (ng/mL) | 1319.9 ± 1480 | 297 ± 1545 | <0.0001 |
| IL-8 (pg/mL) | 159.6 ± 119.7 | 60.1 ± 116.5 | 0.006 |
| CRP (mg/dL) | 7 ± 5.4 | 2.8 ± 3.8 | 0.009 |
| Adiponectin (μg/mL) | 4.6 ± 1.7 | 6.7 ± 3.5 | 0.08 |
| VAT area (cm2) | 236.1 ± 83.8 | 183.1 ± 67.8 | 0.14 |
| SAT area (cm2) | 246.3 ± 163.5 | 238.1 ± 115.2 | 0.9 |
| VAT/SAT | 1.1 ± 0.4 | 0.96 ± 0.6 | 0.19a |
| Prevalence of NAFLD (%) | 55 | 56 | 0.99a |
| Obesity (%) | 55.6 | 50 | 0.75a |
| Insulin therapy (%) | 44.4 | 12 | 0.038a |
Mann–Whitney independent sample U test unless otherwise noted.
Abbreviations: T2D, type 2 diabetes.
Fisher test.
The association between systemic inflammation and impaired VAT homogeneity persisted after adjustment for sex, age, insulin treatment, and abdominal adiposity, as expressed by total VAT and SAT area [WISP1: r = 0.27 (P = 0.03); IL-8: r = 0.34 (P = 0.009); CRP: r = 0.37 (P = 0.004); partial correlation analyses].
Notably, the area under the receiver-operating characteristic curve for WISP1 for identifying the presence of VAT inhomogeneity was 0.87 (P < 0.001; CI: 0.734–1.00); plasma WISP1 levels >130.7 ng/mL were capable of predicting VAT inflammation at MRI with a sensitivity of 78% and a specificity of 83% (Supplemental Data 3 (306.6KB, pdf) ). In contrast, no correlation was found between impaired VAT intensity and indexes of insulin-resistance/secretion, obesity, or glycemic control (data not shown).
3. Discussion
This study demonstrated that circulating WISP1 levels are increased in obese persons and directly related to visceral adiposity, independent of glycemic status or insulin resistance. Furthermore, higher WISP1 is strongly associated with increased IL-8, reduced adiponectin, and impaired VAT homogeneity at MRI.
This study investigated WISP1 in the presence of type 2 diabetes, its potential implication in AT dysfunction, and its related conditions. Recently, Murahovschi et al. [17] conducted an elegant systematic investigation of WISP1 gene expression in human SAT and VAT samples from mostly glucose-tolerant participants with a broad spectrum of body weight. These authors detected high WISP1 expression in AT—mostly in the visceral compartment—and found a negative association with insulin sensitivity and circulating adiponectin levels, whereas WISP1 expression directly correlated with visceral fat content at MRI. These findings suggested a role of this adipokine as a potential marker of visceral fat accumulation and insulin resistance [17]. In agreement with our results obtained in patients with type 2 diabetes, Murahovschi et al. did not find any association between WISP1 expression and the presence of NAFLD.
In our study, WISP1 concentration did not differ significantly between diabetic and nondiabetic participants. Bivariate correlation analyses performed in all study participants showed that plasma levels of this adipokine were not associated with FBG or lipid profile and, in the presence of type 2 diabetes, did not correlate with glycemic control or duration of diabetes.
On the other hand, IL-8 was the main determinant of increased WISP1 concentration in diabetic and nondiabetic patients. Because IL-8 is a well-known mediator of AT inflammation [3], overall our results may suggest that increased WISP1 levels do not portray conditions such as overweight or diabetes per se but rather are a systemic marker of impaired AT homeostasis and function. Studies conducted in other pathological conditions (i.e., lung idiopathic primary fibrosis [11, 12] and cardiac fibrosis [15, 16]) demonstrated that WISP1 displays an active role in determining tissue fibrosis and remodeling. In particular, increased WISP1 levels were detected in fibroblasts from idiopathic primary fibrosis [11]; in these cells, proinflammatory and profibrotic cytokines, such as TNF-α and TGF-β1, induced WISP1 expression through a nuclear factor κB–dependent mechanism; WISP1, in turn, promoted IL-6–mediated fibrosis processes [12]. In addition, WISP1 and WNT pathways took part in processes leading to cardiac fibrosis and myocardial remodeling [14] and mediated angiotensin-induced cardiomyocyte hypertrophy and fibrotic damage [15].
Therefore, it is plausible to speculate that the increase in WISP1, in the presence of AT inflammation, may induce AT remodeling and aberrant fibrogenesis, which is responsible, in turn, for AT loss of function, insulin resistance, and its consequences [26–28]. This hypothesis, ascribing to WISP1 a causal role in AT dysfunction, is strongly corroborated by data from Murahovschi et al. [17] showing that in human macrophages and mesenchymal stem cell–derived adipocytes, 24-hour incubation with WISP1 induces significant and dose-dependent increase of IL-6, TNF-α, IL-1β, and IL-10 messenger RNA expression and protein level in medium [17].
In our study, we explored the possibility of identifying areas of impaired VAT homogeneity at MRI, which could reflect AT inflammation, by qualitative assessment of AT intensity. We found inhomogeneous VAT in a subgroup of patients with type 2 diabetes characterized by a peculiar systemic proinflammatory profile; notably, circulating WISP1 levels showed a great predictive power for VAT inhomogeneity in our population. Previous experimental studies demonstrated that standard routine MRI sequences can depict brown AT, characterized by intense metabolic activity and increased cellularity, in vivo [29, 30].
Similarly, data from the Multi-Ethnic Study of Atherosclerosis showed that the presence of low VAT radiodensity at computed tomography was independently associated with a worse systemic inflammatory profile and higher prevalence of metabolic syndrome in a large US population [31]. The current study investigated the possible role of standard MRI, a radiation-free alternative to computed tomography, in identifying AT inhomogeneity likely referable to local inflammation. This approach could represent a complementary tool for risk stratification in patients at increased cardiovascular risk and warrants further investigation in larger populations and in the context of trials specifically designed for this purpose.
Our study explored WISP1 in the presence of type 2 diabetes and assessed the association between circulating WISP1 levels and markers of AT inflammation. In our study population, WISP1 assessment showed low sensitivity and high SDs, which might represent a limitation for using this molecule as a routine marker for screening AT inflammation. Besides this consideration, increased WISP1 levels have been shown to specifically identify a peculiar phenotype of patients characterized by the presence of visceral fat distribution and worse circulating proinflammatory profile. Furthermore, 93% of patients with plasma WISP1 levels >0 displayed high IL-8 levels, and the association between the circulating concentration of these two molecules persisted after adjustment for possible confounders.
Although we are aware that specific conditions, such as tissue inflammation, may benefit from a biopsy-proven diagnosis, our population did not meet any clinical criteria for performing an invasive procedure, such as AT biopsy. Nevertheless, in our study, AT dysfunction has been estimated by dosing highly accurate circulating biomarkers and exploring qualitative aspects at MRI. Furthermore, our population underwent detailed metabolic phenotyping, allowing us to investigate a broad spectrum of potential correlates of increased WISP1 levels in plasma. Thus, this composite cluster of systemic and imaging data related to AT dysfunction may prove to be useful in identifying dysmetabolic conditions and in the follow-up of targeted treatments.
Finally, we acknowledge that the cross-sectional design of our study does not allow us to establish with certainty a causal nexus between higher WISP1 and IL-8 levels or between VAT inhomogeneity and worse circulating inflammatory profile. Therefore, further studies with longitudinal design are warranted for fully understanding the pathophysiologic processes behind our observations and the possible clinical implications.
In summary, circulating WISP1 levels are increased in obese persons and directly related to adiposity, independent of glycemic status or insulin resistance; moreover, they are strongly associated with increased plasma IL-8 and signal abnormalities of VAT. The overall data add insights to the mechanisms underlying metabolic alterations and may open a scenario for innovative therapeutic approaches to diabetes prevention and care.
Acknowledgments
Acknowledgments
This study has been carried out with the contribution of the Italian Society of Diabetology (SID) Borse di studio Diabete Ricerca-MSD 2014 (I.B.) and was funded by research grants from the Sapienza University Ateneo Scientific Research (M.G.C., I.B.).
Acknowledgments
Disclosure Summary: The author reports no conflicts of interest in this work
Footnotes
- AT
- adipose tissue
- BMI
- body mass index
- CRP
- C-reactive protein
- FBG
- fasting blood glucose
- IL-8
- interleukin-8
- MRI
- magnetic resonance imaging
- NAFLD
- nonalcoholic fatty liver disease
- SAT
- subcutaneous adipose tissue
- SD
- standard deviation
- TNF
- tumor necrosis factor
- VAT
- visceral adipose tissue
- WISP1
- Wnt1-inducible signaling pathway protein-1.
Author contributions: I.B., M.G.B., and M.G.C. designed the study. D.C., F.A.C., L.B., F.L., and M.D.B. coordinated the study, oversaw patient recruitment and data collection, and finalized the dataset. F.A.C., F.M., A.P., and R.D.G. performed laboratory experiments. I.B. and M.G.B. conducted the statistical analyses. M.D.M. performed the MRI and analyzed the data set. I.B. and M.G.C. drafted the paper, which was reviewed by all authors. All authors read and approved the final manuscript.
References and Notes
- 1.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17(4):332–341. [DOI] [PubMed] [Google Scholar]
- 4.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307(5708):373–375. [DOI] [PubMed] [Google Scholar]
- 5.Lin, Chun TH, Kang L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem Pharmacol. 2016;119:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blüher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci (Lond). 2016;130(18):1603–1614. [DOI] [PubMed] [Google Scholar]
- 7.Barchetta I, Angelico F, Del Ben M, Di Martino M, Cimini FA, Bertoccini L, Polimeni L, Catalano C, Fraioli A, Del Vescovo R, Morini S, Baroni MG, Cavallo MG. Phenotypical heterogeneity linked to adipose tissue dysfunction in patients with type 2 diabetes. Clin Sci (Lond). 2016;130(19):1753–1762. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Ye L, Owen S, Weeks HP, Zhang Z, Jiang WG. Emerging role of CCN family proteins in tumorigenesis and cancer metastasis (Review). Int J Mol Med. 2015;36(6):1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiese K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr Neurovasc Res. 2014;11(4):378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berschneider B, Königshoff M. WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease. Int J Biochem Cell Biol. 2011;43(3):306–309. [DOI] [PubMed] [Google Scholar]
- 11.Berschneider B, Ellwanger DC, Baarsma HA, Thiel C, Shimbori C, White ES, Kolb M, Neth P, Königshoff M. miR-92a regulates TGF-β1-induced WISP1 expression in pulmonary fibrosis. Int J Biochem Cell Biol. 2014;53:432–441. [DOI] [PubMed] [Google Scholar]
- 12.Klee S, Lehmann M, Wagner DE, Baarsma HA, Königshoff M. WISP1 mediates IL-6-dependent proliferation in primary human lung fibroblasts. Sci Rep. 2016;6:20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal. 2016;10(2):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao H, Yang JJ, Shi KH, Li J. Wnt signaling pathway in cardiac fibrosis: new insights and directions. Metabolism. 2016;65(2):30–40. [DOI] [PubMed] [Google Scholar]
- 15.Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, Chandrasekar B. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50(6):928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Zhang C, Li X, Yu Y, Liang K, Shan X, Zhao K, Niu Q, Tian Z. WISP1 is increased in intestinal mucosa and contributes to inflammatory cascades in inflammatory bowel disease. Dis Markers. 2016;2016:3547096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Döcke S, Keyhani-Nejad F, Gögebakan Ö, Osterhoff M, Kemper M, Hornemann S, Markova M, Klöting N, Stockmann M, Weickert MO, Lamounier-Zepter V, Neuhaus P, Konradi A, Dooley S, von Loeffelholz C, Blüher M, Pfeiffer AF, Rudovich N. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64(3):856–866. [DOI] [PubMed] [Google Scholar]
- 18.Cernea M, Tang W, Guan H, Yang K. Wisp1 mediates Bmp3-stimulated mesenchymal stem cell proliferation. J Mol Endocrinol. 2016;56(1):39–46. [DOI] [PubMed] [Google Scholar]
- 19.Sahin Ersoy G, Altun Ensari T, Subas S, Giray B, Simsek EE, Cevik O. WISP1 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2017;30(8):942–946. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barchetta I, Del Ben M, Angelico F, Di Martino M, Fraioli A, La Torre G, Saulle R, Perri L, Morini S, Tiberti C, Bertoccini L, Cimini FA, Panimolle F, Catalano C, Baroni MG, Cavallo MG. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. BMC Med. 2016;14:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, Ferrannini E, Defronzo RA. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133(2):496–506. [DOI] [PubMed] [Google Scholar]
- 24.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85(1):115–122. [DOI] [PubMed] [Google Scholar]
- 25.Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab. 2002;87(11):5044–5051. [DOI] [PubMed] [Google Scholar]
- 26.Morrison MC, Kleemann R. Role of macrophage migration inhibitory factor in obesity, insulin resistance, type 2 diabetes, and associated hepatic co-morbidities: a comprehensive review of human and rodent studies. Front Immunol. 2015;6:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guglielmi V, Cardellini M, Cinti F, Corgosinho F, Cardolini I, D’Adamo M, Zingaretti MC, Bellia A, Lauro D, Gentileschi P, Federici M, Cinti S, Sbraccia P. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr Diabetes. 2015;5:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59(6):1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YI, Cypess AM, Sass CA, Brownell AL, Jokivarsi KT, Kahn CR, Kwong KK. Anatomical and functional assessment of brown adipose tissue by magnetic resonance imaging. Obesity (Silver Spring). 2012;20(7):1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holstila M, Virtanen KA, Grönroos TJ, Laine J, Lepomäki V, Saunavaara J, Lisinen I, Komu M, Hannukainen JC, Nuutila P, Parkkola R, Borra RJ. Measurement of brown adipose tissue mass using a novel dual-echo magnetic resonance imaging approach: a validation study. Metabolism. 2013;62(8):1189–1198. [DOI] [PubMed] [Google Scholar]
- 31.Shah RV, Allison MA, Lima JA, Abbasi SA, Eisman A, Lai C, Jerosch-Herold M, Budoff M, Murthy VL. Abdominal fat radiodensity, quantity and cardiometabolic risk: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2016;26(2):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]