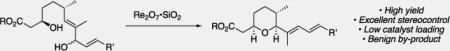
Abstract
Re2O7 catalysis effects efficient and stereoselective dehydrative cyclization reactions from monoallylic diols, with stereocontrol arising from thermodynamic equilibration. This method was applied to a rapid synthesis of the spliceosome inhibitor herboxidiene. The route was also utilized for the synthesis of an analog that highlights the importance of a single methyl group in biasing the conformation in the acyclic region of the molecule.
Keywords: natural products, cyclization, solvolysis, oxygen heterocycles, conformational analysis
TOC Image
Water from a stone: Re2O7 is an extremely effective catalyst for promoting green dehydrative cyclization reactions. Stereochemical control can be achieved through thermodynamic product equilibration. The use of the method in a brief synthesis of the spliceosome inhibitor herboxidiene and an analog illustrated its functional group compatibility and applicability to complex targets. These compounds illustrate the importance of remote steric interactions in promoting biological activity.
Catalytic dehydrative cyclization reactions are extremely attractive, green processes for synthesizing heterocycles because the only waste product is water and because the alcohol that serves as the nucleofuge requires no derivatization. Numerous approaches have recently been reported for achieving dehydrative cyclizations that proceed through either of two basic mechanisms. Soft electrophilic transition metal catalysts based on palladium,[1] gold,[2] or ruthenium[3] coordinate to the alkenes of allylic alcohols and promote cyclization/elimination sequences. Alternatively hard Lewis acids, including FeCl3,[4] BF3•OEt2,[5] arylboronic acids,[6] Bi(OTf)3,[7] and even hot water,[8] react with alcohols to form stablized carbocations that react with appended nucleophiles. Alkene coordination conditions allow for kinetic substrate- or ligand-based stereocontrol while hard Lewis acids allow for thermodynamic control through product equilibration.
We have been exploring[9] the use of Re2O7-catalyzed allylic alcohol transposition reactions[10] in complex molecule synthesis. These processes, in which allylic alcohols react with Re2O7 to form allylic perrhenate esters that rearrange and cleave to form isomeric allylic alcohols, employ appended electrophiles to dictate the regioselectivity of the transposition reaction. However we observed[9c] that allylic alcohols can also serve as precursors to allylic cations (Scheme 1) during an investigation of epoxides as electrophilic traps. This result is consistent with calculations that show substantial charge separation in the transition states of perrhenate ester rearrangements[11] and with the generation of highly stabilized carbocations from allylic alcohols in bimolecular processes.[12] Thus Re2O7 should serve as an effective catalyst for promoting dehydrative cyclizations from allylic alcohols with pendent nucleophiles.[13] This manuscript describes the realization of this objective and defines the structural features that promote high levels of stereocontrol. We apply the protocol to brief total syntheses of the naturally occuring spliceosome inhibitor herboxidiene and an analog that provides useful information regarding the importance of acyclic conformational constraints on biological activity in this compound class.
Scheme 1.
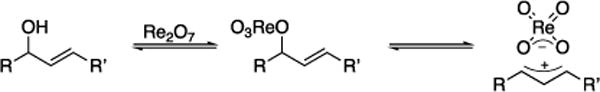
Re2O7-mediated allylic alcohol ionization.
We utilized diol 1 as the initial substrate for developing the method (Scheme 2). Exposing 1 to Re2O7•SiO2[9c] (5 mol%) at rt generated diastereomeric tetrahydropyrans 3 in <10 min through the putative carbocation intermediate 2. While the diastereoselectivity improved at prolonged reaction times the epimerization proved to be too sluggish at ambient temperature for practical purposes. This was remedied by heating the reaction to 40 °C (capped vial). Stirring at this temperature for 20 h provided major product 2 in 82% yield as >30:1 ratio of diastereomers. The stereochemical assignment was based on coupling constants in the 1H NMR spectrum and by analyzing a NOESY experiment.
Scheme 2.
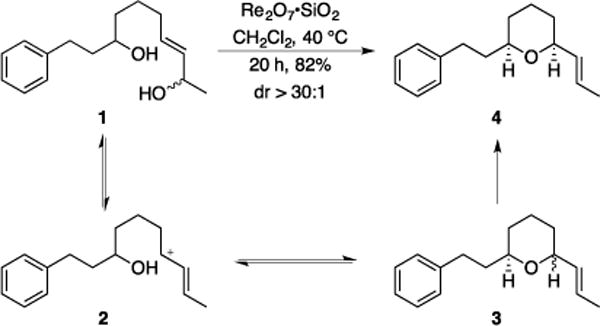
Re2O7-mediated dehydrative cyclization.
Table 1 illustrates the scope of the process. All reactions were conducted at 40 °C for 20 h for consistent comparisons. Secondary allylic alcohol substrates cyclize with good to excellent levels of stereocontrol but primary allylic alcohols, while cyclizing efficiently, do not equilibrate as readily as the secondary alcohols (entries 1 and 2). This is consistent with a mechanism in which allylic cation stability dictates the isomerization rate. Silyl ethers serve as nucleophiles for the reaction (entry 3), albeit with a slight yield reduction (entry 3 relative to entry 4). Trisubstituted alkenes can be generated as single geometrical isomers (entries 3 and 4). The reaction works well when electron-withdrawing groups are proximal to the nucleophilic hydroxyl group (entries 5–9). Notably, these products correspond to common substitution patterns in tetrahydropyran-containing natural products.[14] The cyclization of triol substrate 14 (entry 6) highlights the selectivity of the ionization of allylic alcohols in the presence of non-allylic alcohols. The compatibility of the nitro group provides an entry to structures in which amino acids are incorporated into polyketide biosynthetic pathways.[15] Matching (entry 8) or mismatching influences (entry 9) dictate the degree of stereocontrol when two non-equilibrating stereocenters are present in the substrate.
Table 1.
Dehydrative cyclization scope.[a]
| Entry | Substrate | Product | Yield[b] | dr[c] |
|---|---|---|---|---|
| 1 |
|
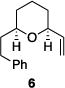
|
81% | 1.7:1 |
| 2 |
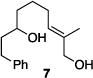
|
|
81% | 2.6:1 |
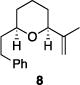
| 3 |
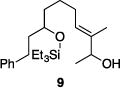
|

|
71% | >30:1 |
| 4 |
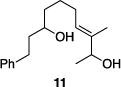
|
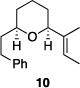
|
86% | >30:1 |
| 5 |
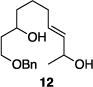
|
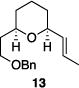
|
86% | 7.6:1 |
| 6 |
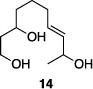
|
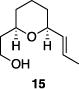
|
85% | >15:1 |
| 7 |
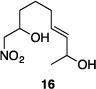
|
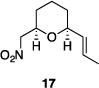
|
84% | 16:1 |
| 8 |
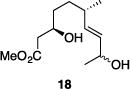
|
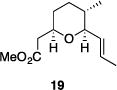
|
77% | >30:1 |
| 9 |
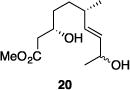
|
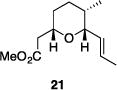
|
56% | 2.9:1 |
See the Supporting Information for experimental procedures and spectral data.
Combined yield of the diastereomeric mixture.
Determined by 1H NMR analysis of the crude product mixture.
Our success in the initial phase of this study led us to explore the applicability of the process to natural product synthesis. Herboxidiene (22), also known as GEX1A, provides an excellent opportunity to validate the capacity of the dehydrative cyclization to access an important synthetic target. Scientists at Monsanto isolated herboxidiene[16] and Oppolzer subsequently established the absolute stereochemistry through degradation and semisynthesis.[17] Yoshida demonstrated that 22 is a potent toxin against several tumor cell lines and enhances survival in murine cancer models.[18] Subsequent studies showed that 22 operates through spliceosome inhibition,[19] which is generating significant interest for selective cancer therapy.[20] Herboxidiene also inhibits angiogenesis,[21] providing further justification for developing a rapid approach to access this compound and its analogs. Several syntheses of 22 have been developed.[22] The dehydrative cyclization protocol, however, provides uniquely rapid access to 22 via diol intermediate 23 (Scheme 3).
Scheme 3.
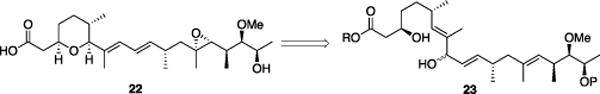
Herboxidiene retrosynthesis.
The synthesis (Scheme 4) of the left-hand fragment commenced with known[23] aldehyde 24, which can be prepared in one step from commercially available citronellene. Asymmetric cycloaddition[24] with AcCl provided β-lactone 25 with good stereocontrol and in high yield. Cross metathesis with methacrolein[25] generated aldehyde 26. While the yield of this transformation is not high, throughput is sufficient since 25 can be recovered and resubjected to the reaction conditions. The synthesis of the right- hand fragment began with a vinylogous Mukaiyama aldol reaction between 27[26] and 28,[27] each available in two steps from commercially available materials, to yield 29 with excellent diastereocontrol in accord with studies by Kobayashi.[28] Conversion to iodide 30 proceeded through facile transformations. Evans alkylation[29] followed by a routine three step sequence generated alkyne 31. Hydrozirconation of 31 followed by transmetalation with Me2Zn[30] and quenching with 26 provided, after in situ β-lactone ethanolysis, cyclization substrate 32 as a mixture of diastereomers. Dehydrative cyclization proceeded extremely efficiently in the presence of Re2O7 (1 mol%). Prolonged exposure to the reaction conditions also effected silyl group cleavage to provide 33 in 82% yield upon increasing the Re2O7 loading to 3 mol%. Conducting this reaction with p-TsOH rather than Re2O7 resulted in a much slower and transformation with extensive by-product formation, demonstrating that Re2O7 is uniquely effective for allylic alcohol ionizations. Vanadium-mediated epoxidation and ester cleavage, in accord with established protocols,[22b,c] provided herboxidiene in 14 steps from commercially available materials for the longest linear sequence. This sequence matches the shortest reported linear sequence[22j] and sets a new standard for the lowest overall step count.
Scheme 4.
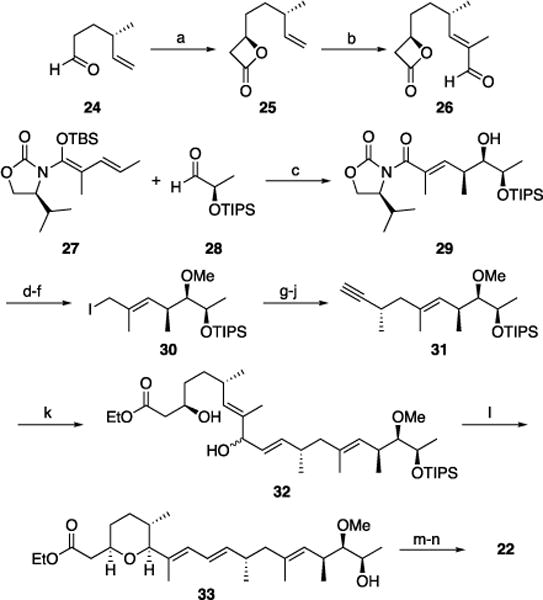
Total synthesis of herboxidiene. Reagents and conditions: a) AcCl, iPr2NEt, trimethylsilylquinidine, LiClO4, CH2Cl2, Et2O, 73%, dr = 9:1; b) Methacrolein, Hoveyda-Grubbs metathesis catalyst, 31%, 59% BRSM; c) TiCl4, CH2Cl2, −78 °C, 71%; d) Me3OBF4, Proton Sponge®, CH2Cl2, 87%; e) LiBH4, MeOH, Et2O, 0 °C, 95%; f) I2, Ph3P, CH3CN, Et2O; g) (R)-4-Benzyl-3-propionyloxazolidin-2-one, NaHMDS, THF, −78 °C, 61% (two steps); h) LiBH4, MeOH, Et2O, 0 °C, 88%; i) Dess-Martin periodinane, NaHCO3, CH2Cl2, 76%; j) Ohira-Bestmann reagent, NaOMe, THF, −78 C to −40 °C, 96%; k) Cp2Zr(H)Cl, CH2Cl2, then Me2Zn, then 26 then NaOEt, 72%; l) Re2O7•SiO2, CH2Cl2, 82%; m) tBuOOH, VO(acac)2, CH2Cl2, −15 °C, 64%; n) K2CO3, H2O, MeOH, reflux, 85%.
The capacity to form the tetrahydropyranyl group rapidly creates ample opportunities for analog synthesis. The structure-activity relationships for 22 have been explored,[22h,31] though the influence of the methyl group at C12 on potency has yet to be established. The considerable effort that is dedicated to introducing this group led us to investigate whether this group is necessary for biological activity. The synthesis of the 12-desmethyl analog (Scheme 5) began with allylic chloride 34, which is available through a sequence that follows the preparation of 30. The addition of lithiated 1-trimethylsilyl-1-propyne[32] followed by in situ silyl cleavage[33] yielded alkyne 35. 12-Desmethyl herboxidiene (36) was accessed by following the route that was developed for the natural product synthesis. This route is three steps shorter than the natural product synthesis and eliminates the need for a chiral auxiliary.
Scheme 5.
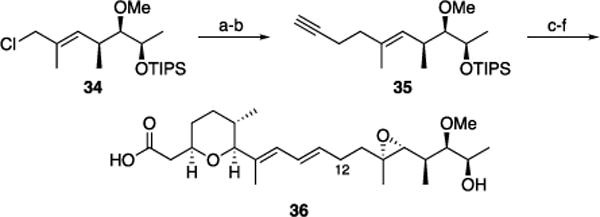
Synthesis of 12-desmethyl herboxidiene: a) 1-Trimethylsilyl-1-propyne, THF, −78 °C, then K2CO3, MeOH, 95%; b) Cp2Zr(H)Cl, CH2Cl2, then Me2Zn, then 26, then NaOMe, 64%; c) Re2O7•SiO2, CH2Cl2, 67%; d) tBuOOH, VO(acac)2, CH2Cl2, −15 °C, 38%, 48% brsm; e) K2CO3, H2O, MeOH, reflux, 85%.
Access to herboxidiene and its desmethyl analog allowed for a comparison of their potencies as cytotoxins. These compounds were evaluated against HeLa (cervical cancer) and 4T-1 (breast cancer) cells using an MTT assay.[34] The IC50 values for 22 were 30 nM and 40 nM, repectively. Minimal cytotoxicity, however, was observed for desmethyl analog 36 at concentrations up to 500 nM, indicating that the absence of the C12 methyl group causes at least a 40-fold drop in potency. This result is remarkable in consideration of the distance of this methyl group from the polar functionality on the molecule.
A conformational analysis of 22 and 36 provided insights on the origin of the potency difference. The methyl group at C12 in 22 promotes the formation of a turn conformation, consistent with the crystal structure[17] and modeling studies. This is illustrated by structure 37 in Figure 1. Rotation of the C12–C13 bond by 120° in either direction generates a highly destabilizing syn-pentane interaction with the methyl group at C14, as illustrated by conformer 38. 1H NMR data support this analysis. The C13 hydrogens of 22 have coupling constants of 4.8 and 10.8 Hz to the C12 hydrogen, indicating a dominant conformation. Moreover the turn conformation enhances the energetic penalty for rotation around the C11–C12 bond since rotation would lead to enhanced steric clashes with the C10 alkenyl hydrogen, as illustrated by 39 (note the perspective change). Removal of the C12 methyl group eliminates the energetic penalty for the syn-pentane interaction, thereby making several additional conformations avaliable through rotation around the C12–13 and C11–C12 bonds.[35] 1H NMR analysis again supports this hypothesis, with the coupling constants of the C12 and C13 hydrogens in 36 being 5.6 and 8.1 Hz, which correlates with a time averaged conformational ensemble. We postulate that 37 represents the binding conformation and removing the methyl group leads to a significant reduction in the population of conformers that interact with the spliceosome.
Figure 1.
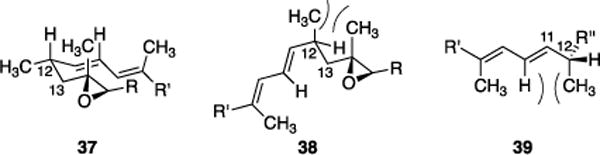
Preference for the turn conformation at C12.
We have demonstrated that Re2O7 is an exceptional catalyst for dehydrative cyclization reactions of monoallylic diols. Stereochemical equilibration leads to high diastereocontrol for transformations in which the products can ionize to form sufficiently stabilized allylic cations. The process shows good functional group tolerance and is experimentally facile, requiring no special precautions to avoid oxygen or water. The method facilitates access to the natural product herboxidiene that validates its applicability to complex molecule synthesis. C12 desmethyl herboxidiene is accessible through a shorter sequence, though the product is less potent as a cytotoxin. The greater potency of the natural product is attributed to the turn conformation that results from the minimization of steric interactions with the C12 methyl group, highlighting the importance of remote steric effects in influencing biological activity.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (R01GM103886) for generous funding of this project. We thank Professor Song Li and Dr. Li Jiang (University of Pittsburgh, Department of Pharmaceutical Sciences) for conducting the MTT assays on compounds 22 and 36.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.a) Hirai Y, Watanabe J, Nozaki T, Yokoyama H, Yamaguchi S. J Org Chem. 1997;62:776. [Google Scholar]; b) Makabe H, Kong LK, Hirota M. Org Lett. 2003;5:27. doi: 10.1021/ol0201916. [DOI] [PubMed] [Google Scholar]; c) Uenishi J, Ohmi M. Angew Chem. 2005;117:2816. doi: 10.1002/anie.200500029. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:2756. [Google Scholar]; d) Hanessian S, Focken T, Oza R. Org Lett. 2010;12:3172. doi: 10.1021/ol101103q. [DOI] [PubMed] [Google Scholar]
- 2.a) Aponick A, Li CY, Biannic B. Org Lett. 2008;10:669. doi: 10.1021/ol703002p. [DOI] [PubMed] [Google Scholar]; b) Unsworth WP, Stevens K, Lamont SG, Robertson J. Chem Commun. 2011;47:7659. doi: 10.1039/c1cc11805f. [DOI] [PubMed] [Google Scholar]; c) Mukherjee P, Widenhoefer RA. Angew Chem. 2012;124:1434. doi: 10.1002/anie.201107877. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:1405. [Google Scholar]; f) Ghebreghiorgis T, Biannic B, Kirk BH, Ess DH, Aponick A. J Am Chem Soc. 2012;134:16307. doi: 10.1021/ja306333a. [DOI] [PubMed] [Google Scholar]
- 3.a) Tanaka S, Seki T, Kitamura M. Angew Chem. 2009;121:9110. doi: 10.1002/anie.200904671. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:8948. [Google Scholar]; b) Miyata K, Kutsuna H, Kawakami S, Kitamura M. Angew Chem. 2011;123:4745. doi: 10.1002/anie.201100772. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:4649. [Google Scholar]
- 4.Guérinot A, Serra-Muns A, Gnamm C, Bensoussan C, Reymond S, Cossy J. Org Lett. 2010;12:1808. doi: 10.1021/ol100422d. [DOI] [PubMed] [Google Scholar]
- 5.Hanessian S, Focken T, Oza R. Tetrahedron. 2011;67:9870. [Google Scholar]
- 6.Zheng H, Ghanbari S, Nakamura S, Hall DG. Angew Chem. 2012;124:6291. doi: 10.1002/anie.201201620. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:6187. [Google Scholar]
- 7.Hoyisha N, Noda K, Mihara Y, Kawai N, Uenishi J. J Org Chem. 2015;80:7790. doi: 10.1021/acs.joc.5b01173. [DOI] [PubMed] [Google Scholar]
- 8.Zhang FZ, Tian Y, Li GX, Qu J. J Org Chem. 2015;80:1107. doi: 10.1021/jo502636d. [DOI] [PubMed] [Google Scholar]
- 9.a) Jung HH, Seiders JR, II, Floreancig PE. Angew Chem. 2007;119:8616. doi: 10.1002/anie.200702999. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:8464. [Google Scholar]; b) Xie Y, Floreancig PE. Chem Sci. 2011;2:2423. [Google Scholar]; c) Xie Y, Floreancig PE. Angew Chem. 2013;125:653. doi: 10.1002/anie.201208132. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:625. [Google Scholar]; d) Xie Y, Floreancig PE. Angew Chem. 2014;126:5026. doi: 10.1002/anie.201402010. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2014;53:4926. [Google Scholar]
- 10.For reviews of Re2O7-mediated allylic alcohol transposition reactions, see:; a) Volchkov I, Lee D. Chem Soc Rev. 2014;43:4381. doi: 10.1039/c4cs00036f. [DOI] [PubMed] [Google Scholar]; b) Bellemin-Laponnaz S. ChemCatChem. 2009;1:357. [Google Scholar]; For relevant examples, see:; c) Narasaka K, Kusama H, Hiyashi Y. Tetrahedron. 1992;48:2059. [Google Scholar]; d) Bellemin-Laponnaz S, Gisie H, Le Ny JP, Osborn JA. Angew Chem. 1997;109:1011. [Google Scholar]; Angew Chem Int Ed Engl. 1997;36:976. [Google Scholar]; e) Morrill C, Grubbs RH. J Am Chem Soc. 2006;128:8142. [Google Scholar]; f) Trost BM, Toste FD. J Am Chem Soc. 2000;122:11262. [Google Scholar]; g) Hutchison JM, Lindsay HA, Dormi SS, Jones GD, Vivic DA, McIntosh MC. Org Lett. 2006;8:3663. doi: 10.1021/ol061072j. [DOI] [PubMed] [Google Scholar]; h) Hansen EC, Lee D. J Am Chem Soc. 2006;128:8142. doi: 10.1021/ja0620639. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Herrmann AT, Saito T, Stivala CE, Tom J, Zakarian A. J Am Chem Soc. 2010;132:5962. doi: 10.1021/ja101673v. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Yun SY, Hansen EC, Volchkov I, Lo WY, Lee D. Angew Chem. 2010;122:4357. doi: 10.1002/anie.201001681. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:4261. [Google Scholar]; k) Hu J, Xu D, Zhang Q, Shang Y, Shi M, Huangfu Y, Liu L, Liang R, Lai Y, He Y, Gao J-m, Xie W. RSC Adv. 2016;6:52583. [Google Scholar]
- 11.Bellemin-Laponnaz S, Le Ny JP, Dedieu A. Chem Eur J. 1999;5:57. [Google Scholar]
- 12.a) Das BG, Nallagonda R, Ghorai P. J Org Chem. 2012;77:5577. doi: 10.1021/jo300706b. [DOI] [PubMed] [Google Scholar]; b) Chavhan SW, McAdam CA, Cook MJ. J Org Chem. 2014;79:11234. doi: 10.1021/jo501992p. [DOI] [PubMed] [Google Scholar]
- 13.A related approach was disclosed during the preparation of this manuscript.; Wan X, Hu J, Xu D, Shang Y, Zhen Y, Hu C, Xiao F, He YP, Lai Y, Xie W. Tetrahedron Lett. 2017;58:1090. [Google Scholar]
- 14.Helfrich EJN, Piel J. Nat Prod Rep. 2016;33:231. doi: 10.1039/c5np00125k. [DOI] [PubMed] [Google Scholar]
- 15.a) Piel J, Butzke D, Fusetani N, Hui D, Platzer M, Wen G, Matsunaga S. J Nat Prod. 2005;68:472. doi: 10.1021/np049612d. [DOI] [PubMed] [Google Scholar]; b) Mosey RA, Floreancig PE. Nat Prod Rep. 2012;29:980. doi: 10.1039/c2np20052j. [DOI] [PubMed] [Google Scholar]
- 16.a) Millerwideman M, Makkar N, Tran M, Isaac B, Biest N, Stonard R. J Antibiotics. 1992;45:914. doi: 10.7164/antibiotics.45.914. [DOI] [PubMed] [Google Scholar]; b) Isaac BG, Ayer SW, Elliot RC, Stonard RJ. J Org Chem. 1992;57:7220. [Google Scholar]
- 17.Edmunds AJF, Trueb W, Oppolzer W, Cowley P. Tetrahedron. 1997;53:2785. [Google Scholar]
- 18.Sakai Y, Yoshida T, Ochiai K, Uosaki Y, Saitoh Y, Tanaka F, Akiyama T, Akinaga S, Mizukami T. J Antibiotics. 2002;55:855. doi: 10.7164/antibiotics.55.855. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa M, Miura T, Kuzuya K, Inoue A, Ki SW, Horinouchi S, Yoshida T, Kunoh T, Koseki K, Mino K, Sasaki R, Yoshida M, Mizukami T. ACS Chem Biol. 2011;6:229. doi: 10.1021/cb100248e. [DOI] [PubMed] [Google Scholar]
- 20.a) Pham D, Koide K. Nat Prod Rep. 2016;33:637. doi: 10.1039/c5np00110b. [DOI] [PubMed] [Google Scholar]; b) Webb TR, Joyner AS, Potter PM. Drug Discovery Today. 2013;18:43. doi: 10.1016/j.drudis.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung HJ, Kim Y, Shin JY, Sohng JK, Kwon HJ. Arch Pharm Res. 2015;38:1728. doi: 10.1007/s12272-015-0625-4. [DOI] [PubMed] [Google Scholar]
- 22.a) Smith ND, Kocienski PJ, Street SDA. Synthesis. 1996:652. [Google Scholar]; b) Blakemore PR, Kocienski PJ, Morley A, Muir K. J Chem Soc Perkin Trans. 1999;1:955. [Google Scholar]; c) Banwell M, McLeod M, Premraj R, Simpson G. Pure Appl Chem. 2000;72:1631. [Google Scholar]; d) Zhang Y, Panek JS. Org Lett. 2007;9:3141. doi: 10.1021/ol701427k. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Murray TJ, Forsyth CJ. Org Lett. 2008;10:3429. doi: 10.1021/ol800902g. [DOI] [PubMed] [Google Scholar]; f) Ghosh AK, Li J. Org Lett. 2011;13:66. doi: 10.1021/ol102549a. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Pellicena M, Krämer K, Romea P, Urpí F. Org Lett. 2011;13:5350. doi: 10.1021/ol202210k. [DOI] [PubMed] [Google Scholar]; h) Lagisetti C, Yermolina MV, Sharma LK, Palacios G, Prigaro BJ, Webb TR. ACS Chem Biol. 2014;9:643. doi: 10.1021/cb400695j. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Yadav JS, Reddy GM, Anjum SR, SubbaReddy BV. Eur J Org Chem. 2014:4389. [Google Scholar]; j) Meng F, McGrath KP, Hoveyda AH. Nature. 2014;513:367. doi: 10.1038/nature13735. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Thirupathi B, Mohapatra DK. Org Biomol Chem. 2016;14:6212. doi: 10.1039/c6ob00321d. [DOI] [PubMed] [Google Scholar]
- 23.Fürstner A, Feyen F, Prinz H, Waldmann H. Angew Chem. 2003;115:5519. doi: 10.1002/anie.200352268. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:5361. [Google Scholar]
- 24.Zhu C, Shen X, Nelson SG. J Am Chem Soc. 2004;126:5352. doi: 10.1021/ja0492900. [DOI] [PubMed] [Google Scholar]
- 25.a) Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J Am Chem Soc. 2000;122:8168. [Google Scholar]; b) Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 26.Shirokawa S-i, Kamiyama M, Nakamura T, Okada M, Nakazaki A, Hosokawa S, Kobayashi S. J Am Chem Soc. 2004;126:13604. doi: 10.1021/ja0465855. [DOI] [PubMed] [Google Scholar]
- 27.McGrath JW, Hammerschmidt F, Preusser W, Quinn JP, Schweifer A. Org Biomol Chem. 2009;7:1944. doi: 10.1039/b821829c. [DOI] [PubMed] [Google Scholar]
- 28.Shinoyama M, Shirokawa S-i, Nakazaki A, Kobayashi S. Org Lett. 2009;11:1277. doi: 10.1021/ol9000312. [DOI] [PubMed] [Google Scholar]
- 29.Evans DA, Ennis M, Mathre DJ. J Am Chem Soc. 1982;104:1737. [Google Scholar]
- 30.Wipf P, Xu W. Tetrahedron Lett. 1994;35:5197. [Google Scholar]
- 31.a) Ghosh AK, Ma N, Effenberger KA, Jurica MS. Org Lett. 2014;16:3154. doi: 10.1021/ol501345d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ghosh AK, Lv K, Ma N, Cárdenas EL, Effenberger KA, Jurica MS. Org Biomol Chem. 2016;14:5263. doi: 10.1039/c6ob00725b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipshutz BH, Bulow G, Fernandez-Lazaro F, Kim SK, Lowe R, Mollard P, Stevens KL. J Am Chem Soc. 1999;121:11664. [Google Scholar]
- 33.Clausen DJ, Wan S, Floreancig PE. Angew Chem. 2011;123:5284. doi: 10.1002/anie.201007757. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:5178. [Google Scholar]
- 34.Mossmann T. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 35.For reviews on the influence of remote steric interactions on biological activity, see:; a) Larsen EM, Wilson MR, Taylor RE. Nat Prod Rep. 2015;32:1183. doi: 10.1039/c5np00014a. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schönherr H, Cernak T. Angew Chem. 2013;125:12480. doi: 10.1002/anie.201303207. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:12256. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


