Abstract
Tetranectin, encoded by the clec3b gene, is a plasminogen kringle-4 binding protein that can be detected in the plasma and the extracellular matrix. In malignancies, tetranectin is thought to enhance proteolytic processes enabling tumor cells to invade and metastasize. Nevertheless, the prognostic value of tetranectin in gastric cancer remains elusive. In this study, we found the expression of tetranectin was decreased in gastric cancer. High intratumoral tetranectin level was positively associated with tumor invasion (P = 0.013), lymph node metastasis (P = 0.005), advanced TNM stage (P = 0.003) and shorter overall survival (OS) (P < 0.001) for patients with gastric cancer. Tetranectin expression was identified as an independent prognostic factor for poor OS, and combining tetranectin expression with other independent prognostic factors generated a predictive nomogram, which showed better prognostic efficiency for OS in patients with gastric cancer. In summary, our study suggests that intratumoral tetranectin is a potential independent unfavorable prognostic biomarker for OS of patients with gastric cancer after gastrectomy.
Keywords: Gastric cancer, Tetranectin, Prognosis, Nomogram.
Introduction
Gastric cancer is an important health problem, being the fifth most common cancer and the third leading cause of cancer-related death worldwide 1. More than 950,000 new diagnoses are made every year 2. In China, although the incidence has decreased over past several decades, gastric cancer still ranks the second most common malignancy with an estimated 679,100 new cases each year3. Due to the lack of specific symptoms at the early stage, over 80% of patients with gastric cancer are diagnosed at an advanced and unsuitable stage for surgical resection, which is the major reason for a poor prognosis 3. Tumor-node-metastasis (TNM) staging system has been widely used to provide reliable prognostic information of gastric cancer, but they still have limited capacity to determine different outcomes for ignoring the heterogeneity of gastric cancer. Considering the increasing level of understanding of the molecular basis of tumor biology, more novel biomarkers are applied in diagnosis, prognosis and treatment 4.
Tetranectin, a homotrimeric C-type lectin originally isolated from plasma, was detected to bind with plasminogen and fibrin 5. It is thought to play an important role in the regulation of proteolytic processes via binding to plasminogen 5. Impaired cutaneous wound healing, delayed fracture healing and features of Parkinson disease were found in mice lacking tetranectin 6-8. In malignancies, preoperative serum tetranectin was supposed to be a significant prognostic marker in advanced ovarian cancer patients 9. The alteration of tetranectin expression in cancer tissue is also considered as a potential biomarker predicting prognosis in breast 10, bladder 11, oral 12 and ovarian cancer 13. Nevertheless, the roles of tetranectin in gastric carcinoma remain poorly understood.
The aim of the present study was to investigate the expression of tetranectin in gastric cancer specimens and explore its associations with clinicopathological factors and prognosis. We also evaluated whether integration of intratumoral tetranectin expression could generate a predictive nomogram to refine the risk stratification system for prognosis of patients with gastric cancer.
Materials and Methods
Patient samples
A total of 328 consecutive gastric cancer patients who received standard gastrectomy with D2 dissection from the same surgical team in Zhongshan Hospital of Fudan University (Shanghai, China) between May 2002 and April 2006 were enrolled in the study. We retrospectively collected the baseline demographic and clinicopathological factors of these patients, including age, gender, tumor location, tumor differentiation, Lauren classification, and tumor stage. Tumor stage and tumor differentiation were reassessed by two independent gastroenterology pathologists according to the 2010 International Union Against Cancer TNM classification system. All patients were followed up until July 2012, with a median follow-up time of 59 months. OS was defined as the time between the dates of surgery and death or last visit. No patients received any preoperative anticancer treatment. All methods were approved by the research medical ethics committee of Fudan University and were carried out in accordance with the approved guidelines. Informed consent on the use of clinical specimens were obtained from all patients.
TCGA and GEO datasets
These data are publically available from the Cancer Genome Atlas and the NCBI Gene Expression Omnibus (accession number: GSE27342 and GSE13911). For the TCGA dataset, all level-3 data were downloaded from the TCGA-STAD portal by using TCGA-Assembler software14. The mRNA expression in TCGA dataset was measured by RNA sequencing V2. The RSEM (RNA-Seq by Expectation-Maximization) counts were further normalized by TMM (trimmed mean of M value) method to estimate the relative RNA production levels using edgeR software15. For the Gene Expression Omnibus dataset reported previously, the mRNA expression was measured by microarray. The probe set intensities were quantified using the GeneChip Operating Software (GCOS) and normalized with GCRMA (GeneChip Robust Multiarray Averaging) using Array Assist Software.
IHC staining and evaluation
Tissue microarray on glass slides was deparaffinized in xylene, dehydrated in graded ethanol and subjected to antigen retrieval in boiling citrate buffer (0.01 M, pH 6.0). Then, the sections were blocked by UltraVision Hydrogen Peroxide Block (Thermo Scientific, CA, USA) and UltraVision Protein Block (Thermo Scientific), followed by primary antibody incubation (1:1000; Abcam, USA). UltraVision Quanto Detection System horseradish peroxidase (HRP) Polymer (Thermo Scientific) and DAB Quanto (Thermo Scientific) were applied staining and hematoxylin was used for counterstaining. We also make an H.E staining of the tissue microarray to discriminate tumor part and other part. The staining in gastric cancer cells, not the stromal part of the cancer tissue, were calculated. The staining intensity was categorized as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. Depending on the staining extent, the area was categorized as follows: 0, <5%; 1, 5-25%; 2, 26-50%; 3, 51-75%; and 4, >75%. The staining score was calculated by combining staining intensity and area, yielding a series of results ranging from 0 to 12 according to our previous report 16. The best cut off point was determined by the ROC analysis.
Statistical analysis
Analyses were performed with SPSS 19.0 (SPSS Inc., Chicago, IL) and R software version 3.0.2 and the “rms” package (R Foundation for Statistical Computing, Vienna, Austria). Chi-square test was used to compare categorical variables. Cox proportional hazards model was used to perform univariate and multivariate analysis. Kaplan-Meier analysis was used to determine the survival and log-rank test was used to compare the patient survival between subgroups. The area under the receiver operating characteristic curves (AUC) at different cut-off values of OS time was calculated to determine the optimal cut-off value of the tetranectin expression in tumors. Nomogram was created by R software using “rms” package. Calibration plots were generated to examine the performance characteristics of the predictive nomogram. The Harrell's concordance index (C-Index) and Akaike information criterion (AIC) were used to measure the prognostic accuracy. All statistical analyses were two-sided and P < 0.05 was regarded as statistically significant.
Results
The expression of tetranectin in gastric cancer
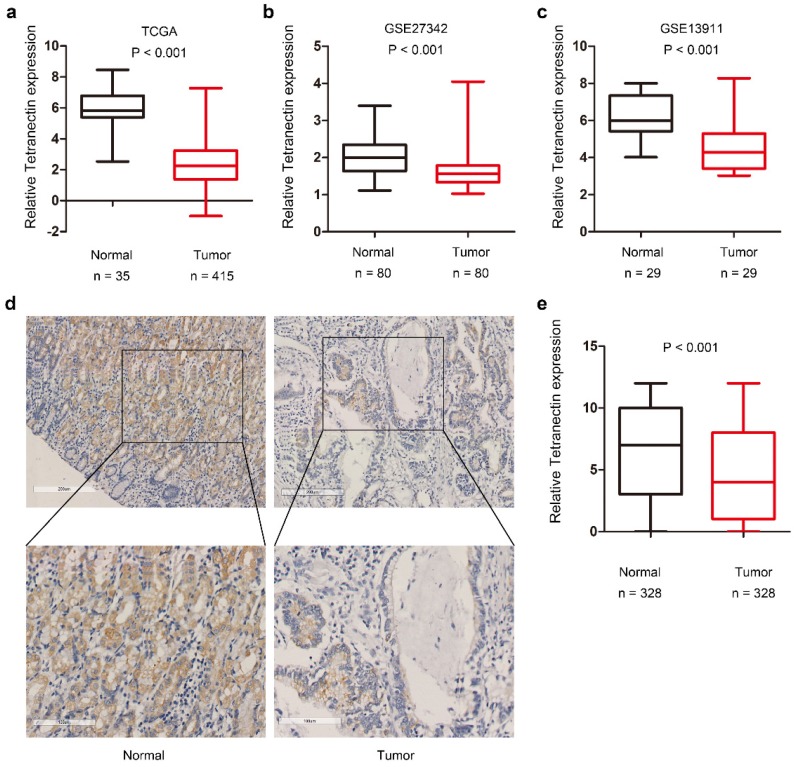
We first screened differentially expressed members of C-type lectin family in the TCGA-STAD dataset. Results demonstrated that among the various members of CLEC family, the mRNA expression of clec3b in gastric cancer tissues displays the most significant fold-change by comparing with that in normal gastric mucosa (Table 1).Statistical analysis also indicated that the tetranectin expression was remarkably down-regulated in gastric cancer tissues in TCGA (P < 0.001), GSE27342 (P < 0.001) and GSE13911 (P < 0.001) datasets (Fig. 1a-c).
Table 1.
Differentially expressed members of CLEC family in TCGA dataset.
| Gene | Log FC | P-value |
|---|---|---|
| clec1a | -0.421 | 0.006 |
| clec2b | -0.603 | 0.000 |
| clec2d | 0.344 | 0.015 |
| clec2l | 0.238 | 0.710 |
| clec3a | -2.617 | 0.000 |
| clec3b | -3.490 | 0.000 |
| clec4a | 0.482 | 0.027 |
| clec4d | 0.482 | 0.202 |
| clec4e | 0.584 | 0.083 |
| clec4f | -1.209 | 0.000 |
| clec4g | -2.273 | 0.000 |
| clec5a | 2.345 | 0.000 |
| clec7a | 0.574 | 0.015 |
| clec9a | -1.324 | 0.000 |
| clec10a | -2.075 | 0.000 |
| clec11a | 0.334 | 0.214 |
| clec12a | 0.477 | 0.113 |
| clec14a | -0.403 | 0.007 |
| clec16a | 0.103 | 0.255 |
| clec17a | -0.118 | 0.902 |
| clec18a | 0.544 | 0.060 |
| clec18b | 0.279 | 0.427 |
| clecl1 | -0.407 | 0.318 |
Abbreviations: FC, fold change.
Figure 1.
The expression of tetranectin in gastric cancer tissues. Relative expression of tetranectin mRNA in gastric cancer and normal gastric epithelium in TCGA (a), GSE27342 (b), GSE13911 (c). (d) Representative IHC staining images of tetranectin and its regional magnification in gastric cancer tissues and normal tissues. (e) IHC score of tetranectin expression in gastric cancer tissues and normal tissues.
Next we investigated the expression of tetranectin in 328 cases of gastric cancer with tissue microarray. The representative staining of tetranectin in tumor tissues and adjacent normal tissues were shown in Figure 1d. In comparison to the H.E staining, it revealed that the expression of tetranectin was mostly in the cytoplasm of gastric epithelial cells, not the stromal part of the cancer tissue (Figure S1). Statistical analysis confirmed that tetranectin staining score in tumor tissue was significantly lower than that in normal gastric epithelium (P < 0.001) (Fig. 1e). These results suggest that the expression of tetranectin is down-regulated in gastric cancer.
Correlation between tetranectin expression and clinicopathological features in gastric cancer
According to the results conducted by receiver operating characteristic (ROC) curve analysis, IHC score of 6 was determined as the cut-off to dichotomize the patients into tetranectin low expression group (score, 0-6; n = 221) and tetranectin high expression group (score, 7-12; n = 107). The correlations between tetranectin expression and clinicopathological features in 328 gastric cancer samples were also explored with the chi-square test (Table 2). Among the variables, higher expression of tetranectin was positively associated with tumor invasion (P = 0.013), lymph node metastasis (P = 0.005), advanced TNM stage (P = 0.003) and younger age (P = 0.024). No other clinicopathological variables showed a significant difference between tetranectin high and low expression group. We also assessed the expression of tetranectin among different tissue components in the same patient. It showed an increasing expression of tetranectin in invasive part than the normal and in situ part (Figure S2).
Table 2.
Correlation between tetranectin expression and clinicopathological characteristics in patients with gastric cancer.
| Clinicopathological Characteristics | No. | Tetranectinlow | % | Tetranectinhigh | % | P -value |
|---|---|---|---|---|---|---|
| 328 | ||||||
| Gender | 0.596 | |||||
| Male | 215 | 147 | 68 | 68 | 32 | |
| Female | 113 | 74 | 65 | 39 | 35 | |
| Age | 0.024 | |||||
| < 60 | 158 | 116 | 73 | 42 | 27 | |
| ≧60 | 170 | 105 | 62 | 65 | 38 | |
| Tumor location | 0.739 | |||||
| Cardiac | 40 | 25 | 63 | 15 | 38 | |
| Body | 82 | 57 | 70 | 25 | 30 | |
| Pylorus | 206 | 139 | 67 | 67 | 33 | |
| Tumor differentiation | 0.703 | |||||
| Well | 13 | 10 | 77 | 3 | 23 | |
| Moderate | 51 | 33 | 65 | 18 | 35 | |
| Poor | 264 | 178 | 67 | 86 | 33 | |
| Lauren classification | 0.266 | |||||
| Diffused Type | 137 | 95 | 69 | 42 | 31 | |
| Intestinal Type | 180 | 121 | 67 | 59 | 33 | |
| Mixed Type | 11 | 5 | 45 | 6 | 55 | |
| T | 0.013 | |||||
| T1 | 71 | 58 | 82 | 13 | 18 | |
| T2 | 30 | 21 | 70 | 9 | 30 | |
| T3 | 84 | 57 | 68 | 27 | 32 | |
| T4 | 143 | 85 | 59 | 58 | 41 | |
| N | 0.005 | |||||
| N0 | 103 | 77 | 75 | 26 | 25 | |
| N1 | 55 | 41 | 75 | 14 | 25 | |
| N2 | 64 | 44 | 69 | 20 | 31 | |
| N3 | 106 | 59 | 56 | 47 | 44 | |
| M | 0.137 | |||||
| M0 | 318 | 217 | 68 | 102 | 32 | |
| M1 | 10 | 4 | 40 | 5 | 50 | |
| TNM stages | 0.003 | |||||
| I | 73 | 61 | 84 | 12 | 16 | |
| II | 76 | 52 | 68 | 24 | 32 | |
| III | 170 | 104 | 61 | 66 | 39 | |
| IV | 9 | 4 | 44 | 5 | 56 |
Prognostic value of tetranectin expression in gastric cancer
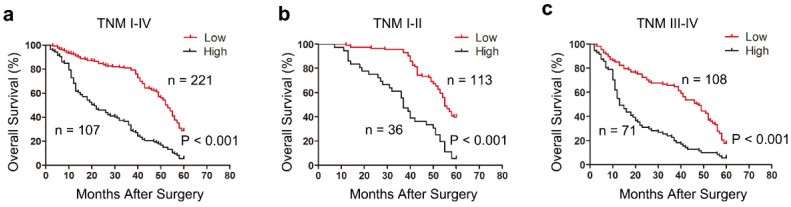
We next explored the relationship between tetranectin expression and overall survival (OS) in patients with gastric cancer after gastrectomy using Kaplan-Meier analysis. The results demonstrated that high tetranectin expression was associated with poor OS (P < 0.001) (Fig. 2a). To further evaluate the efficiency of tetranectin expression in stratifying patients with different TNM stages, we divided the patients into early (I-II) and advanced (III- IV) groups. In both TNM I-II and TNM III-IV groups, tetranectin expression showed statistically significant value in predicting the outcomes of gastric cancer patients (Fig. 2b, c). These data suggest that tetranectin expression is correlated with OS for patients with gastric cancer.
Figure 2.
Kaplan-Meier survival analysis showing the relationship between tetranectin expression and overall survival in all patients (a), patients at TNM I-II stage (b) and patients at TNM III -IV stage (c).
Tetranectin expression is identified as an independent prognosticator in patients with gastric cancer
Univariate Cox regression analysis was conducted to identify the prognostic significance of clinicopathological factors for OS. Lauren classification (P < 0.001), T classification (P < 0.001), N classification (P < 0.001), distant metastasis (P = 0.004), TNM stage (P < 0.001) and tetranectin expression (P < 0.001) were defined as risk factors that may affect the OS of gastric cancer patients (Table 3). After adjustment of covariate factors by using multivariate Cox analysis, we identified Lauren classification (P < 0.001), T classification (P = 0.006), N classification (P = 0.001), distant metastasis (P = 0.001), and tetranectin expression (P < 0.001) as independent prognostic factors for OS (Table 3).
Table 3.
Univariate and multivariate Cox regression analysis of clinicopathological characteristics influencing the overall survival of gastric cancer patients.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | (95 % CI) | P-value | Hazard ratio | (95 % CI) | P-value | |
| Gender: male versus female |
0.954 | 0.737-1.236 | 0.723 | |||
| Age (Year): ≥60 versus < 60 |
1.234 | 0.966-1.576 | 0.093 | |||
| Tumor Location: cardia + body versus antrum | 0.856 | 0.666-1.1 | 0.224 | |||
| Differentiation: poorly +moderately versus well |
1.973 | 0.931-4.182 | 0.076 | |||
| Lauren classification: diffused +mixed versus intestinal | 2.007 | 1.564-2.577 | <0.001 | 1.96 | 1.516-2.534 | <0.001 |
| T classification: T2-4 versus T1 |
2.492 | 1.764-3.521 | <0.001 | 1.754 | 1.173-2.623 | 0.006 |
| N classification: N2+N3 versus N0+N1 |
2.047 | 1.596-2.624 | <0.001 | 1.779 | 1.286-2.461 | 0.001 |
| M classification: M1 versus M0 |
2.708 | 1.385-5.292 | 0.004 | 3.381 | 1.685-6.783 | 0.001 |
| TNM stage: III+IV versus I+II |
2.055 | 1.599-2.641 | <0.001 | 0.952 | 0.665-1.362 | 0.787 |
| Tetranectin expression: high versus low |
3.039 | 2.351-3.927 | <0.001 | 2.848 | 2.192-3.702 | <0.001 |
Abbreviations: P < 0.05 was considered statistically significant.
Combination of tetranectin expression with TNM stage generates a better predictive model for overall survival of gastric cancer patients
To improve the prognostic accuracy for OS of patients with gastric cancer after gastrectomy, we combined tetranectin expression and TNM staging system together to generate a predictive model. ROC curve analysis showed that the combination of tetranectin and TNM stage revealed better prognostic value (AUC [95% CI], 0.749 [0.686-0.813]) than tetranectin expression alone (AUC [95% CI], 0.657 [0.592-0.721]) or TNM stage alone (AUC [95% CI], 0.672 [0.598-0.747]) with statistical significance (Fig. 3a). In addition, the AIC was 2647.21 when estimated according to TNM stage alone, and it decreased to 2595.652 when estimated in combination with tetranectin expression. The C-index was 0.635 when estimated according to TNM stage alone, and it increased to 0.700 when tetranectin expression was added (Table 4).
Figure 3.
ROC curve and nomogram for predicting prognosis in patients with gastric cancer. (a) ROC curve analysis of the sensitivity and specificity for the predictive value of the TNM model, the tetranectin model, and the combined TNM and tetranectin stratification model. (b) Predictive nomogram for 3-year and 5-year overall survival was generated by combining proven independent prognostic factors including Lauren classification, T stage, N stage, M stage and tetranectin expression. (c) Calibration plot for nomogram predicting observed 5-year survival rate. Gray Line: ideal model; vertical bars, 95% confident interval. (d) Kaplan-Meier curves of overall survival based on risk score calculated by nomogram. P-value was assessed by log-rank test.
Table 4.
AIC and Harrell's C-index analysis of the comparison of the predictive accuracies of different models.
| Model | AIC | C-index | 95% CI |
|---|---|---|---|
| TNM | 2647.21 | 0.635 | 0.605-0.666 |
| Tetranectin | 2628.349 | 0.632 | 0.604-0.659 |
| TNM+ Tetranectin | 2595.652 | 0.700 | 0.671-0.730 |
| Nomogram | 2559.376 | 0.741 | 0.715-0.767 |
Abbreviations: AIC, Akaike information criterion; C-index, Harrell's concordance index; CI, confident interval.
We also conducted a predictive nomogram by combing all independent prognostic factors after multivariate Cox regression analysis (Fig. 3b). In the nomogram, a higher total point predicts a worse prognosis. The total point was calculated by adding the score of Lauren classification, T stage, N stage, M stage and tetranectin expression respectively. The calibration curve for predicted 5-year OS performed well with the ideal mode (Fig. 3c). We next stratified the 328 patients with gastric cancer into 3 groups according to the points calculated by the nomogram: low-risk (< 25th percentile), intermediate-risk (25th-75th percentile), and high-risk (> 75th percentile) groups (Fig. 3d). The results showed that the nomogram could effectively discriminate the risk of OS in patients with gastric cancer.
Discussion
It is well recognized that gastric cancer is a highly heterogeneous disease with poor clinical outcome. To provide a predictive model for patients with gastric cancer, traditional TNM staging system and the Lauren classification were commonly used in clinical practice 17, 18. Nevertheless, these predictive models still have a limit in their ability to discriminate a subset of patients when referring to the molecular heterogeneity of gastric cancer 19. Classification of gastric carcinomas based on molecular subtypes are supposed to be used in the near future to determine prognosis and to customise treatment 1. Recently, the Asian Cancer Research Group (ACRG) applied gene expression profiling and defined four distinct gastric cancer molecular subtypes that are associated with distinct genomic alterations, survival outcome and recurrence patterns after surgery 20, which shows prognostic value of gastric cancer by using molecular approaches. Moreover, molecular classification based on human epidermal growth factor receptor 2 (HER2; also known as ERBB2) status has been introduced in gastric cancers because of therapeutic implications 21. In our study, we found a significant association between high tetranectin expression and poor prognosis in gastric cancer patients following surgery, and the molecular mechanism and potential functions of tetranectin in gastric cancer needs further investigation.
Tetranectin belongs to the family of C-type lectins that bind specifically to kringle four of plasminogen 22. C-type lectins are a kind of carbohydrate-binding molecules whose functions span many areas of immunity and homeostasis 23. Recent studies have suggested the potential correlation between C-type lectins and carcinogenesis including gastric cancer 24. Our recent research also indicated that C-type lectin receptor CLEC-2 suppressed AKT signaling and invasive activities of gastric cancer cells by blocking expression of PI3K subunits 25. The expression pattern of tetranectin has also been described in several types of human carcinomas. Serum tetranectin have shown great distinguishing ability between autoimmune pancreatitis and pancreatic cancer 26, as well as benign ovarian lesions and malignant ovarian tumors 27. However, the role of intratumoral tetranectin expression as a tumor prognostic factor remains controversial. High tetranectin expression were found to be associated with poor survival in breast cancer 10 and bladder cancer 11, while positive expression of tetranectin predicted a favorable survival prognosis in women diagnosed with ovarian cancer 13.
The mechanisms of tetranectin in human malignancies are being explored. It has been reported that tetranectin plays an important role in regulating the fibrinolysis and proteolytic procedures by binding to kringle four of circulating plasminogen to enhance activation of plasminogen into plasmin 28. Although the precise biological function of tetranectin has not yet been clarified, it shows co-localization with plasminogen at the invasion front of melanomas, suggesting a role in cancer invasion and metastases 29. Increased diffuse cytoplasmic immunoreactivity of urokinase-type plasminogen activator (u-PA) were also found in neoplastic columnar epithelial cells of colon carcinoma while intense immunoreactivity for tetranectin in the stroma surrounding the tumor cells simultaneously 30. As u-PA is found up-regulated in gastric cancer cells to enhance invasiveness 31, 32, tetranectin were hypothesized to combine with, or get close proximity to u-PA to enhance tumor associated proteolytic activity 30. Since proteolytic activity is essential for tumor growth, invasion, and metastasis 33, it is in accordance with our observations that high expression of tetranectin was associated with increased invasiveness and metastasis in gastric cancer. These data imply that tetranectin may play an important role in proteolytic activity to enhance invasion and metastasis of gastric cancer, while the exact mechanisms still needs further investigation.
Although the clinical significance of tetranectin in gastric cancer has been presented in our study, some limitations of this study should be acknowledged. First, the number of patients enrolled in this study was relatively small. Second, our data lack the serum levels of tetranectin. Third, single cohort seems to be inadequate to reach greater reliability. Thus, a large, multi-center, prospective data is needed to validate these results and more efforts need to be exerted in the future studies.
In summary, our data indicated that high intratumoral expression of tetranectin correlated with tumor invasion and metastasis of gastric cancer, and was identified as an independent inferior prognostic factor of OS for patients after gastrectomy. Incorporation of tetranectin expression with TNM staging system or other predictive models might add some prognostic information for patients' survival.
Supplementary Material
Supplementary figures.
Acknowledgments
This study was funded by grants from National Natural Science Foundation of China (81672925, 31670806, 81572317, 31370808, 81302259, 31500645, 31630088). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. The Lancet; 2016. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarovafoucher E, Lortettieulent J, Rosso S, Coebergh JW, Comber H. et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. European Journal of Cancer. 2015;51:1201–2. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. Ca A Cancer Journal for Clinicians. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Duraes C, Almeida GM, Seruca R, Oliveira C, Carneiro F. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch. 2014;464:367–78. doi: 10.1007/s00428-013-1533-y. [DOI] [PubMed] [Google Scholar]
- 5.Clemmensen I, Petersen LC, Kluft C. Purification and characterization of a novel, oligomeric, plasminogen kringle 4 binding protein from human plasma: tetranectin. European Journal of Biochemistry. 1986;156:327–33. doi: 10.1111/j.1432-1033.1986.tb09586.x. [DOI] [PubMed] [Google Scholar]
- 6.Iba K, Abe Y, Chikenji T. Delayed fracture healing in tetranectin-deficient mice. Journal of Bone and Mineral Metabolism. 2013;31:399–408. doi: 10.1007/s00774-013-0436-y. [DOI] [PubMed] [Google Scholar]
- 7.Iba K, Hatakeyama N, Kojima T, Murata M, Matsumura T, Wewer UM. et al. Impaired cutaneous wound healing in mice lacking tetranectin. Wound Repair & Regeneration. 2009;17:108–12. doi: 10.1111/j.1524-475X.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang ES, Zhang XP, Yao HB, Wang G, Chen SW, Gao WW. et al. Tetranectin knockout mice develop features of Parkinson disease. Cellular Physiology & Biochemistry International Journal of Experimental Cellular Physiology Biochemistry & Pharmacology. 2014;34:277–87. doi: 10.1159/000362998. [DOI] [PubMed] [Google Scholar]
- 9.Begum FD, Høgdall E, Christensen IJ, Kjaer SK, Blaakaer J, Christensen L. et al. Serum tetranectin is a significant prognostic marker in ovarian cancer patients. Acta Obstetricia Et Gynecologica Scandinavica. 2010;89:190–8. doi: 10.3109/00016340903530936. [DOI] [PubMed] [Google Scholar]
- 10.Obrist P, Spizzo G. Aberrant tetranectin expression in human breast carcinomas as a predictor of survival. Journal of Clinical Pathology. 2004;57:417–21. doi: 10.1136/jcp.2003.010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunner A, Ensinger C, Christiansen M, Heiss S, Verdorfer I, Mikuz G. et al. Expression and prognostic significance of Tetranectin in invasive and non-invasive bladder cancer. Virchows Arch. 2007;450:659–64. doi: 10.1007/s00428-007-0409-4. [DOI] [PubMed] [Google Scholar]
- 12.Arellano-Garcia ME, Li R, Liu X, Xie Y, Yan X, Loo JA. et al. Identification of tetranectin as a potential biomarker for metastatic oral cancer. Int J Mol Sci. 2010;11:3106–21. doi: 10.3390/ijms11093106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heeran MC, Rask L, Hogdall CK, Kjaer SK, Christensen L, Jensen A. et al. Tetranectin positive expression in tumour tissue leads to longer survival in Danish women with ovarian cancer. Results from the 'Malova' ovarian cancer study. APMIS. 2015;123:401–9. doi: 10.1111/apm.12368. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Qiu P, Ji Y. TCGA-Assembler: open-source software for retrieving and processing TCGA data. Nature Methods. 2014;11:599–600. doi: 10.1038/nmeth.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biology. 2010;11:1–9. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Min L, Wang X, Zhao J, Chen H, Qin J. et al. Loss of RACK1 Promotes Metastasis of Gastric Cancer by Inducing a miR-302c/IL8 Signaling Loop. Cancer Res. 2015;75:3832–41. doi: 10.1158/0008-5472.CAN-14-3690. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Annals of Surgical Oncology. 2010;17:1471. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Turner ES, Turner JR. Expanding the Lauren classification: a new gastric cancer subtype? Gastroenterology. 2013;145:505–8. doi: 10.1053/j.gastro.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Stock M, Otto F. Gene deregulation in gastric cancer. Gene. 2005;360:1–19. doi: 10.1016/j.gene.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS. et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nature Medicine. 2015;21:449. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 21.Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Gastric Cancer, Version 3.2016; Clinical Practice Guidelines in Oncology. 2016. [DOI] [PubMed]
- 22.Holtet TL, Graversen JH, Thøgersen HC, Etzerodt M, Clemmensen I. Tetranectin, a trimeric plasminogen-binding C-type lectin. Protein Science A Publication of the Protein Society. 1997;6:1511. doi: 10.1002/pro.5560060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutikhin AG, Yuzhalin AE. C-type lectin receptors and RIG-I-like receptors: new points on the oncogenomics map. Cancer Management & Research. 2012;4:39. doi: 10.2147/CMAR.S28983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castaño-Rodríguez N, Kaakoush NO, Mitchell HM. Pattern-recognition receptors and gastric cancer. Frontiers in Immunology. 2014;5:336. doi: 10.3389/fimmu.2014.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Yin J, Wang X, Shao M, Duan F, Wu W. et al. C-type Lectin-like Receptor 2 Suppresses AKT Signaling and Invasive Activities of Gastric Cancer Cells by Blocking Expression of PI3K Subunits. Gastroenterology. 2016;150:1183–95.e16. doi: 10.1053/j.gastro.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Felix K, Hauck O, Fritz S, Hinz U, Schnolzer M, Kempf T. et al. Serum protein signatures differentiating autoimmune pancreatitis versus pancreatic cancer. PLoS One. 2013;8:e82755. doi: 10.1371/journal.pone.0082755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begum FD, Hogdall E, Kjaer SK, Blaakaer J, Christensen IJ, Christensen L. et al. Preoperative serum tetranectin, CA125 and menopausal status used as single markers in screening and in a risk assessment index (RAI) in discriminating between benign and malignant ovarian tumors. Gynecol Oncol. 2009;113:221–7. doi: 10.1016/j.ygyno.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Iba K, Hatakeyama N, Kojima T, Murata M, Matsumura T, Wewer UM. et al. Impaired cutaneous wound healing in mice lacking tetranectin. Wound Repair Regen. 2009;17:108–12. doi: 10.1111/j.1524-475X.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang ES, Sun Y, Guo JG, Gao X, Hu JW, Zhou L. et al. Tetranectin and apolipoprotein A-I in cerebrospinal fluid as potential biomarkers for Parkinson's disease. Acta Neurol Scand. 2010;122:350–9. doi: 10.1111/j.1600-0404.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- 30.Verspaget HW, Clemmensen I, Ganesh S, Christensen L, Sier CF, Griffioen G. et al. Tetranectin expression in human colonic neoplasia. Histopathology. 1994;25:463–7. doi: 10.1111/j.1365-2559.1994.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee DH, Yang Y, Lee SJ, Kim KY, Koo TH, Shin SM. et al. Macrophage inhibitory cytokine-1 induces the invasiveness of gastric cancer cells by up-regulating the urokinase-type plasminogen activator system. Cancer Research. 2003;63:4648–55. [PubMed] [Google Scholar]
- 32.Khoi PN, Xia Y, Lian S, Kim HD, Kim dH, Joo YE. et al. Cadmium induces urokinase-type plasminogen activator receptor expression and the cell invasiveness of human gastric cancer cells via the ERK-1/2, NF-κB, and AP-1 signaling pathways. International Journal of Oncology. 2014;45:1760–8. doi: 10.3892/ijo.2014.2558. [DOI] [PubMed] [Google Scholar]
- 33.Markus G. The Role of Hemostasis and Fibrinolysis in the Metastatic Spread of Cancer. Seminars in Thrombosis and Hemostasis; 1984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.