Abstract
Context:
Exercise-associated muscle cramps are a common clinical problem for athletes.
Objective:
To determine whether acute passive static stretching altered cramp threshold frequency (CTF) of electrically induced muscle cramps.
Design:
Crossover study.
Setting:
Laboratory.
Patients or Other Participants:
Seventeen healthy college-aged individuals.
Intervention(s):
Stretching or no stretching.
Main Outcome Measure(s):
The independent variable was the static stretch versus the no-stretch condition, and the dependent variable was the CTF.
Results:
The CTF increased in both the control (pretest: 18.12 ± 6.46 Hz, posttest: 19.65 ± 7.25 Hz; P = .033) and stretching (pretest: 18.94 ± 5.96 Hz, posttest: 20.47 ± 7.12 Hz; P = .049) groups. No difference between the groups was found (t15 = 0.035, P = .97).
Conclusions:
Acute passive static stretching did not seem to increase the CTF.
Key Words: exercise-associated muscle cramps, electrically induced muscle cramps, Golgi tendon organ reflex response
Key Points
Exercise-associated muscle cramps occur frequently in athletes, affecting as many as 67% of triathletes and 30% to 50% of marathon runners.
Epidemiologic studies have indicated that athletes with more consistent stretching programs experienced cramps less often.
Acute static stretching is the intervention of choice for the immediate relief of exercise-associated muscle cramps; the purported mechanism is the initiation of the Golgi tendon organ reflex response, which inhibits the α-motoneuron.
Exercise-associated muscle cramps (EAMCs) are localized, painful, and involuntary contractions of skeletal muscle that occur during or immediately postexercise and are one of the most common clinical problems reported in athletes.1 They can hinder athletic performance and have been reported to affect 67% of triathletes and between 30% and 50% of marathon runners.2 Investigations of EAMCs are difficult because the spontaneous nature of the muscular phenomenon is unknown and difficult to test in the field. To date, the most frequent method used to provoke EAMCs is electrically induced muscle cramps (EIMCs). The EIMCs are correlated with EAMCs,3 and the method has been previously validated4 with high intrasession and intersession reliability3 while causing little pain and soreness.5
Although the cause of EAMCs is unknown, the traditional mechanism behind them, as reported by Schwellnus et al,1 is associated with dehydration. However, a paucity of empirical evidence links hypohydration to EAMCs. Miller et al6 and Braulick et al7 investigated EIMCs in the nonfatigued limbs of participants at 3% and 5% hypohydration. Neither group demonstrated an effect of hypohydration on the cramp threshold frequency (CTF). Moreover, sodium and carbohydrate drinks are often used to prevent EAMCs before, during, and after athletic events by increasing blood plasma electrolyte concentrations. However, this notion has been challenged because gastric emptying and plasma electrolyte levels immediately after ingestion of sodium and carbohydrate drinks have not resulted in changes in blood plasma concentrations.8,9 (Additional reviews on dehydration and EAMCs can be found elsewhere.10,11)
Given the evidence refuting dehydration as a primary mechanism for EAMCs, focus has been shifted to altered neuromuscular control.12–14 The theory of altered neuromuscular control suggests that hyperexcitability of the motoneuron pool causes sustained α motoneuron firing.1,12 Specifically, the hyperexcitability results from an imbalance between increased muscle-spindle activity and reduced Golgi tendon organ feedback. Because the majority of EAMCs occur at the end of or after activity, fatigue has been implicated.1 Fatigued muscles show increased muscle-spindle activity15 and increased baseline electromyographic (EMG) activity in animals and humans,16 suggesting persistent α-motoneuron activity.
Inconsistent stretching habits and irregular stretching times have been suggested as risk factors for EAMCs.1 Static stretching is a common pre-event modality that has historically been used to improve sport performance or limit performance-related injury or both.17 Static stretching has been reported to alter α-motoneuron pool activity proportionally to the stretching intensity,18 as evidenced by a decreased H-reflex. Palmieri et al19 described the H-reflex as a measure of α-motoneuron pool excitability stemming from spinal afferent stimulation. In brief, low-voltage stimulation of skeletal muscle activates low-threshold spinal afferents originating in the muscle spindles. As the voltage is increased, the H-reflex amplitude (mV) rises to a peak. After the H-reflex peak amplitude is reached, increased mV stimulation results in a second EMG response: the M-response. As the M-response increases, the H-reflex decreases due to antidromic and orthodromic action potentials, producing a recruitment curve and the H : M ratio. The H : M ratio is the ratio of maximal muscle response (M-response) to motoneuron pool excitability (H-reflex peak).19
Furthermore, static stretching may cause a reduction in muscle activation as evidenced by a reduced H : M ratio20 and decreased maximum voluntary contraction. The effects of static stretching on the H-reflex have been seen during, immediately after, and up to 60 minutes poststretching.18,21–23
We accept the proposed mechanism of EAMC as a result of α-motoneuron pool hyperexcitability and elected to investigate static stretching as a perturbation to reduce the excitatory input from the muscle spindles to the α-motoneuron pool. To our knowledge, no authors have researched the acute effect of static stretching on CTF.
Therefore, the purpose of our study was to compare the effects of acute static stretching on CTF using a common stretching duration as recommended by the American College of Sports Medicine.24 We hypothesized that acute static stretching would increase participants' CTF.
METHODS
Study Design
A randomly assigned pretest-posttest crossover design was applied to determine whether acute static stretching (SS) affected the CTF of the flexor hallucis brevis (FHB) and whether that effect was greater than in the control (no-stretching [NS]) group. Each participant acted as his or her own control by completing both conditions. The dependent variable was the CTF, which is the lowest measure of hertz (Hz) required to elicit a cramp.
Participants
A convenience sample of 17 college-aged students was recruited to participate in the study. The participants were all healthy volunteers with no known health conditions. Exclusion criteria were a self-reported injury to the dominant limb within the previous 6 months17; any self-reported metabolic, neuromuscular, or neurologic disease or disorder25; or pregnancy.26 This project was reviewed and approved by the institutional review board and individuals provided written consent before participating.
Procedures
Recruits were asked to avoid participating in strenuous lower body exercise within 24 hours of the scheduled session. They were instructed to report for testing on 4 occasions separated by 7 days. The first 2 sessions (days 1 and 2) were familiarization days, which allowed the participant to become accustomed to the cramping procedure before being assigned to the testing order. At the end of day 2, each participant was randomly assigned to 1 of 2 conditions: SS or NS.
Day 1 (familiarization 1) consisted of signing of consent forms, collection of anthropometric data, and identification of the dominant limb. The dominant limb was determined by asking the participant to kick an imaginary ball.7 Day 1 concluded with an EIMC. The second visit to the lab (familiarization 2) consisted only of 1 EIMC protocol.
Days 3 and 4 were testing days. The conditions were counterbalanced to account for an order effect between days 3 and 4. Participants began their predetermined testing order by receiving either NS on day 3 and SS on day 4 or SS on day 3 and NS on day 4. On each testing day, an EIMC was elicited to establish the pretest CTF, followed by a 10-minute break. After the break, the participant underwent the assigned condition (NS or SS), followed by a second EIMC (posttest CTF).
The SS protocol consisted of 3 bouts of 30-second passive static stretching of the FHB, administered by the tester, separated by 30 seconds of rest. The 3 bouts of stretching totaled 90 seconds, and the entire procedure lasted 3 minutes. The range of the stretch was determined by the participant and his or her perception of mild discomfort,27 also known as the point of discomfort (POD). Posttest CTF was assessed immediately after the intervention ended. The CTF protocol began at 10 Hz below the first CTF: the starting Hz had no effect on CTF, thereby reducing the number of stimulations a participant experienced.28 During the NS condition, the participant remained supine and relaxed for the 3 minutes between the baseline EIMC and the second EIMC. Total time was 13 minutes. Participants received 6 cramp-induction protocols during their 4 laboratory visits as follows:
Day 1: Familiarization CTF
Day 2: Familiarization CTF
Day 3: Pretest CTF, NS or SS, posttest CTF
Day 4: Pretest CTF, NS or SS, posttest CTF
Instruments and EIMC Process
For inducing EIMCs, the FHB is the most commonly used muscle, due to the intersession and intrasession reliability, participant comfort, reproducibility of the cramp, and ease of access to the peripheral nerve.3–5 Participants were instructed to lay supine with the ankle and knee slightly elevated by numbered foam pads. Each participant received the same foam pads during each visit. The foam pads were used to ensure similar positions on each testing day. Participants were instructed to look at the ceiling and were given headphones and access to music to eliminate noise and distracting stimuli.5 To induce the cramp, we placed the 8-mm silver-silver chloride stimulating electrode (model EL 258S; Biopac Systems, Inc, Goleta, CA) slightly inferior and posterior to the medial malleolus, so that the 8-cm dispersive pad was placed over the lateral malleolus. The tibial nerve was then stimulated with an 80-V, 1-millisecond electrical stimulation until full flexion of the FHB was visible. Once the appropriate tibial nerve location was identified, we marked it using a permanent marker. (Participants were asked to reapply the marking if they noticed fading.) The electrode and the dispersive pad were secured using a tight-fitting ankle brace.
To determine the CTF, we set the stimulator (model S88 REV K; Grass Instruments Company, West Warwick, RI) at 2-second trains, 80 V, and 6 pulses per second (pps or Hz). Therefore, at the start of stimulation, there would be a 1-second pause and then a 2-second stimulation period with 6 pulses of 80 V. If no cramp was elicited at 6 Hz, then 2 Hz was added, and stimulation was reapplied after a 1-minute break. The process of adding 2 Hz and giving a 1-minute break between stimuli was continued until a cramp was elicited. The lowest value at which a cramp was elicited was termed the CTF.7 Similar methods are described in detail elsewhere.3,4,29 The stimulus was routed through a stimulation unit (model SIU8T; Grass Instruments Company). Anthropometric data were collected using a medical scale and stadiometer (model 217 stadiometer and model 869 scale; Seca Corp, Chino, CA). All EIMCs were confirmed via EMG analysis (version 4.0.0; BSL Analysis, Biopac Systems, Inc) following the previous literature3,30 and are reported in Figure 1 and the Appendix.
Figure 1. .
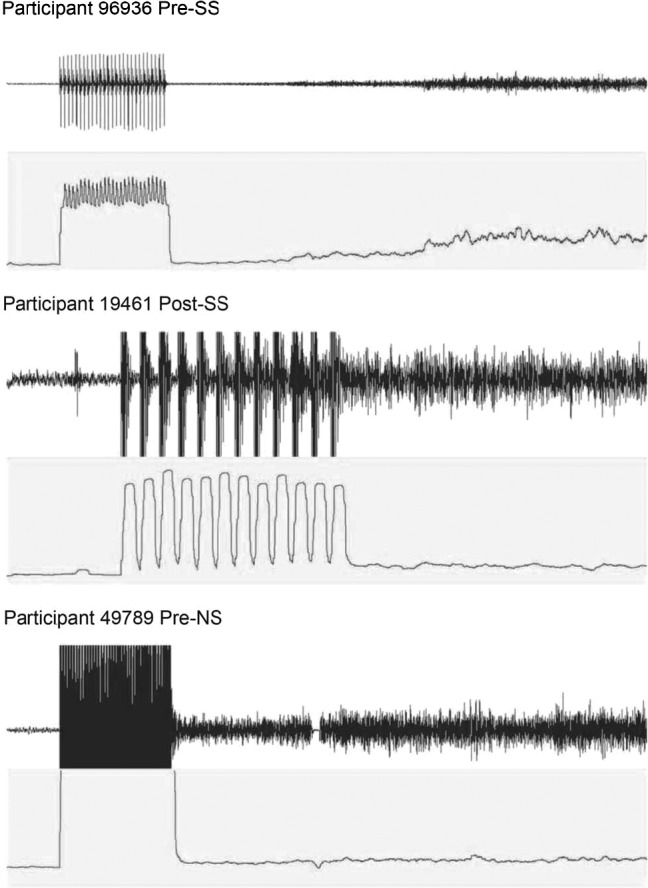
Electromyography and root mean square data for 3 participants. The top graph shows the raw electromyographic data at rest, stimulation, and during the electrically induced muscle cramp. The bottom graph shows the root mean square data during the same time frame.
Statistical Analysis
Descriptive statistics were calculated for study participants' anthropometric values. Before performing the between-conditions statistical analysis, we conducted confirmatory modified t tests31 to identify whether a carryover, period effect, or order effect was present in this crossover design. Before analysis, the data from the 4 identified “crampers” (see definition in next section) were separated from the rest of the data and tested, with and without the modified t test, to verify their inclusion in the group analysis. Participants were then randomized to either NS and then SS or SS and then NS. The single-group crossover design requires 2 within-subject analyses and a between-subjects factor analysis to account for the change in treatment (NS to SS or the converse). The modified t test was also used to assess the change in CTF between the NS and SS conditions. A paired t test was calculated to determine whether statistically significant within-subject differences existed between the pretest and posttest CTF values in each condition. A 1-way analysis of variance (ANOVA) was used to evaluate the differences between the familiarization days and the pretest CTF on each testing day. The paired t test and 1-way ANOVA were determined using SPSS (version 23.0; IBM Corp, Armonk, NY) with significance set at P < .05. All data are presented as mean ± standard deviation.
RESULTS
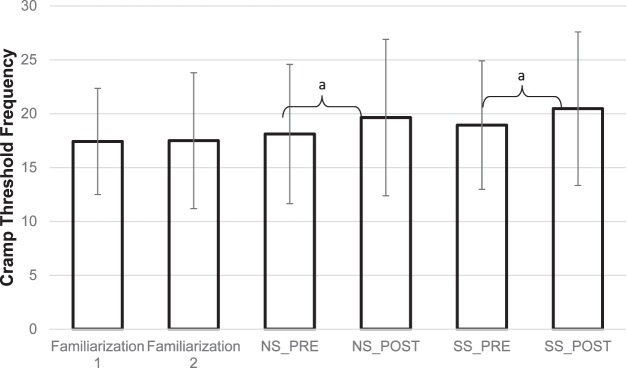
All participants self-reported compliance with the instructions before the experimental days. Participants' anthropometrics were as follows: age = 23 ± 3.3 years, height = 174.10 ± 13.60 m, and weight = 80.67 ± 24.75 kg. Of the 17 participants, 4 were self-reported “crampers.” They did not demonstrate pretest, posttest, or pretest-posttest differences from the self-reported noncrampers in either the standard t test (P ≥ .20) or modified t test for carryover effect (P = .16) or a difference between treatment effects (P = .48). Therefore, the “crampers” were included in the group analysis. The confirmatory analysis did not identify a carryover or period effect (t15 = 1.699, P = .11). No within-subject effects were present between familiarization days and pretest CTFs of each condition (P ≥ .461). Specifically, recorded CTFs were not different between familiarization days 1 and 2 (17.43 ± 4.93 Hz and 17.50 ± 6.30 Hz, respectively; P = .68). No difference occurred between the pretest CTFs of the NS and SS conditions (18.12 ± 6.46 Hz and 18.94 ± 5.96 Hz, respectively; P = .702). However, CTFs increased from pretest to posttest for both the NS and SS conditions (Figure 2). No change in CTF between the SS and NS conditions was found (t15 = 0.035, P = .97).
Figure 2. .
The mean cramp threshold frequency (CTF) for each day and condition. No-stretching (NS) condition pretest CTF = 18.12 ± 6.46 Hz, posttest CTF = 19.65 ± 7.25 Hz. Static stretching (SS) condition pretest CTF = 18.94 ± 5.96 Hz, posttest CTF = 20.47 ± 7.12 Hz. a Denotes differences between pretest and posttest within the NS and SS conditions (P ≤ .05).
DISCUSSION
Our results show that static stretching increased CTF. However, the control condition also demonstrated an increase in CTF. No difference was evident between the conditions (P = .97). To our knowledge, no previous authors have examined the effects of static stretching as a preventive measure to reduce the likelihood of an EIMC as a proxy32 for EAMCs.
These data present static stretching as an ineffective intervention for decreasing the likelihood of a subsequent EIMC, as represented by no difference between the SS and NS conditions. However, static stretching and EAMC have been associated in marathon runners.1 We are the first to directly test the effects of a stretching modality on EIMCs. An increase in CTF is thought to indicate a lower risk of EAMC occurrence because individuals prone to cramping have lower CTFs.3,30 Yet the CTF difference in the control (NS) condition from pretest to posttest is at odds with previously published data3,30 resulting from the same protocol. Miller and Knight3 reported a decrease in CTF from day 1 to day 2 in individuals with a history of cramps, whereas Stone et al30 demonstrated no difference between pretest and posttest control CTFs.30 Miller and Knight3 noted CTFs of 14.9 ± 1.3 Hz for “crampers,” which is consistent with our data (the average CTF for all 4 interventions was 15.275 ± 2.99 Hz). The CTF for the noncrampers was 25.5 ± 1.5 Hz, which is slightly higher than our data (20.50 ± 1.21 Hz). Stone et al30 observed CTFs of 18 ± 4.9 Hz pre-exercise, of 20.0 ± 7.7 Hz prefatigue, and of 32.9 ± 11.7 Hz postfatigue.
Overall, the reason for the difference in the CTF standard deviation is unknown. However, one potential explanation could be due to different methods. Stone et al30 administered the highest tolerable voltage, starting at 80 V, and then started to perturb the frequency. We used the method reported by Miller and Knight,3 who did not alter the stimulus voltage. Yet we report a higher standard deviation than Miller and Knight3 despite using the same protocol; this may be due to the unknown cause of EAMCs or to the static-stretching perturbation. In the latter case, the location of the metatarsophalangeal joint may have limited the range of motion of the stretch, leading to some participants experiencing an insufficient intensity of stretch on the FHB. We designed our study to use a stretch to the POD, with the aim of inhibiting the α motoneuron pool, but the clinician administering the static-stretching condition may not have been able to reach a participant's POD. All participants were asked if they could feel a stretch of the muscles in their feet, which they confirmed. During observations of the participants, we had no obvious indication that the POD was reached, and testers were not comfortable applying additional force to the first digit due to the amount of force already applied. If the static stretch was inadequate, then the inferred mechanism may not have been tested. Moreover, new evidence emerged after the data were collected suggesting that static stretching may not elicit increased inhibition after 3 bouts of 1-minute static stretching of the gastrocnemius.33 Another explanation from our data could be an order effect. The modified t test, which was used to control for the specific order used in the crossover design, provided a value of 1.71 with a P value of .055. It is plausible that an increase in participants could lead to an order effect, which may help explain these results.
Clinically, these data may support the use of other forms of stretching and warm-up protocols such as dynamic stretching and proprioceptive neuromuscular facilitation stretching that aim to improve performance. Static stretching may not reduce the incidence of EAMCs as previously suggested.1
Future researchers should investigate biarticulate muscles, which are known to be more susceptible to cramping,1 and include a measure of the H-reflex poststretch to ensure the stretching perturbation elicits the desired response. In prospective EIMC research studies, investigators should test additional intervention strategies believed to alter neuromuscular function in order to determine their effect on CTF. Testing different muscles may also offer additional insight into skeletal muscle cramping.
In conclusion, our study offers evidence that acute static stretching of the FHB, within the flexibility guidelines of the American College of Sports Medicine, had an acute effect on CTF. However, this effect was not significant when compared with the control condition. Although the same volume of stretching has been reported to cause neural, mechanical, and performance alterations in humans, it failed to result in a significant treatment effect, when compared with controls, in this study. These results are validated by similar same-day EIMC protocol differences34 and day-to-day changes in participants' CTFs5 in studies that did not involve a perturbation. Hence, our day-to-day pattern of CTF changes mimicked those of previous researchers who did not perturb CTF. Thus, we provide evidence that acute static stretching had no effect on CTF in an EIMC model and suggest that static stretching is an ineffective intervention to acutely reduce the likelihood of future EAMCs.
Appendix. .
Root Mean Squares: Average Resting and Electrically Induced Muscle Crampsa Continued on Next Page
| Participant No. |
Before Static Stretching |
After Static Stretching |
||
| Resting | Cramp | Resting | Cramp | |
| RMS ± SD |
RMS ± SD |
RMS ± SD |
RMS ± SD |
|
| Control Condition | ||||
| 19461 | 0.11 ± 0.02 | 0.75 ± 0.20 | 0.12 ± 0.05 | 0.37 ± 0.10 |
| 96936 | 3.69 ± 1.25 | 55.10 ± 29.42 | 3.69 ± 0.80 | 61.98 ± 37.21 |
| 52466 | 9.49 ± 2.13 | 33.98 ± 27.31 | 9.57 ± 2.22 | 46.32 ± 25.28 |
| 53124 | 3.09 ± 1.04 | 37.68 ± 20.44 | 3.87 ± 1.37 | 20.35 ± 52.92 |
| 86112 | 5.24 ± 0.98 | 21.45 ± 13.41 | 5.81 ± 2.00 | 58.31 ± 31.33 |
| 25517 | 2.82 ± 1.10 | 13.91 ± 10.26 | 3.66 ± 1.35 | 33.41 ± 40.57 |
| 34819 | 9.47 ± 2.69 | 20.70 ± 19.69 | 9.83 ± 2.83 | 24.41 ± 35.17 |
| 62981 | 6.16 ± 1.15 | 37.63 ± 12.75 | 3.16 ± 0.84 | 26.16 ± 46.45 |
| 14982 | 4.21 ± 0.97 | 16.01 ± 9.47 | 4.99 ± 1.78 | 26.98 ± 15.32 |
| 49789 | 5.64 ± 1.47 | 38.42 ± 32.77 | 4.19 ± 1.24 | 29.94 ± 30.83 |
| 85418 | 5.82 ± 1.37 | 26.33 ± 7.79 | 4.97 ± 1.34 | 74.41 ± 40.65 |
| 17191 | 12.88 ± 2.82 | 29.72 ± 24.95 | 10.76 ± 2.90 | 46.54 ± 33.07 |
| 38643 | 5.65 ± 1.39 | 23.64 ± 8.61 | 4.47 ± 1.07 | 45.67 ± 23.80 |
| 96613 | 6.61 ± 1.54 | 42.21 ± 28.06 | 9.44 ± 3.09 | 44.10 ± 63.62 |
| 73991 | 9.33 ± 1.56 | 27.04 ± 52.59 | 4.96 ± 1.74 | 31.95 ± 47.57 |
| 12468 | 7.73 ± 1.22 | 123.93 ± 41.68 | 8.44 ± 2.10 | 74.65 ± 34.43 |
| 85357 | 6.71 ± 1.80 | 41.05 ± 25.27 | 12.11 ± 3.83 | 25.05 ± 26.71 |
| Average | 6.16 ± 1.44 | 34.68 ± 21.45 | 6.12 ± 1.80 | 39.45 ± 34.41 |
| SD | 3.05 ± 0.66 | 26.33 ± 13.44 | 3.28 ± 0.96 | 19.88 ± 14.62 |
| Static-Stretching Condition | ||||
| 19461 | 0.11 ± 0.02 | 0.75 ± 0.20 | 0.12 ± 0.05 | 0.37 ± 0.10 |
| 96936 | 3.69 ± 1.25 | 55.10 ± 29.42 | 3.69 ± 0.80 | 61.98 ± 37.21 |
| 52466 | 9.49 ± 2.13 | 33.98 ± 27.31 | 9.57 ± 2.22 | 46.32 ± 25.28 |
| 53124 | 3.09 ± 1.04 | 37.68 ± 20.44 | 3.87 ± 1.37 | 20.35 ± 52.92 |
| 86112 | 5.24 ± 0.98 | 21.45 ± 13.41 | 5.81 ± 2.00 | 58.31 ± 31.33 |
| 25517 | 2.82 ± 1.10 | 13.91 ± 10.26 | 3.66 ± 1.35 | 33.41 ± 40.57 |
| 34819 | 9.47 ± 2.69 | 20.70 ± 19.69 | 9.83 ± 2.83 | 24.41 ± 35.17 |
| 62981 | 6.16 ± 1.15 | 37.63 ± 12.75 | 3.16 ± 0.84 | 26.16 ± 46.45 |
| 14982 | 4.21 ± 0.97 | 16.01 ± 9.47 | 4.99 ± 1.78 | 26.98 ± 15.32 |
| 49789 | 5.64 ± 1.47 | 38.42 ± 32.77 | 4.19 ± 1.24 | 29.94 ± 30.83 |
| 85418 | 5.82 ± 1.37 | 26.33 ± 7.79 | 4.97 ± 1.34 | 74.41 ± 40.65 |
| 17191 | 12.88 ± 2.82 | 29.72 ± 24.95 | 10.76 ± 2.90 | 46.54 ± 33.07 |
| 38643 | 5.65 ± 1.39 | 23.64 ± 8.61 | 4.47 ± 1.07 | 45.67 ± 23.80 |
| 96613 | 6.61 ± 1.54 | 42.21 ± 28.06 | 9.44 ± 3.09 | 44.10 ± 63.62 |
| 73991 | 9.33 ± 1.56 | 27.04 ± 52.59 | 4.96 ± 1.74 | 31.95 ± 47.57 |
| 12468 | 7.73 ± 1.22 | 123.93 ± 41.68 | 8.44 ± 2.10 | 74.65 ± 34.43 |
| 85357 | 6.71 ± 1.80 | 41.05 ± 25.27 | 12.11 ± 3.83 | 25.05 ± 26.71 |
| Average | 6.16 ± 1.44 | 34.68 ± 21.45 | 6.12 ± 1.80 | 39.45 ± 34.41 |
| SD | 3.05 ± 0.66 | 26.33 ± 13.44 | 3.28 ± 0.96 | 19.88 ± 14.62 |
Abbreviations: EIMCs, exercise-induced muscle cramps; RMS, root mean square; SD, standard deviation.
One way to verify EIMCs is by cramp intensity. If the EIMC intensity is above 2 SDs of the resting RMS, then the EIMC is considered valid.
REFERENCES
- 1. Schwellnus MP, Derman EW, Noakes TD. Aetiology of skeletal muscle “cramps” during exercise: a novel hypothesis. J Sports Sci. 1997; 15 3: 277– 285. [DOI] [PubMed] [Google Scholar]
- 2. Kantorowski PG, Hiller WDB, Garrett WE, Jr, Douglas PS, Smith R, O'Toole M. Cramping studies in 2600 endurance athletes [abstract]. Med Sci Sports Exerc. 1990; 22 2: S104. [Google Scholar]
- 3. Miller KC, Knight KL. Electrical stimulation cramp threshold frequency correlates well with the occurrence of skeletal muscle cramps. Muscle Nerve. 2009; 39 3: 364– 368. [DOI] [PubMed] [Google Scholar]
- 4. Stone MB, Edwards JE, Babington JP, Ingersoll CD, Palmieri RM. Reliability of an electrical method to induce muscle cramp. Muscle Nerve. 2003; 27 1: 122– 123. [DOI] [PubMed] [Google Scholar]
- 5. Miller KC, Knight KL. Pain and soreness associated with a percutaneous electrical stimulation muscle cramping protocol. Muscle Nerve. 2007; 36 5: 711– 714. [DOI] [PubMed] [Google Scholar]
- 6. Miller KC, Mack GW, Knight KL., et al. Three percent hypohydration does not affect threshold frequency of electrically induced cramps. Med Sci Sports Exerc. 2010; 42 11: 2056– 2063. [DOI] [PubMed] [Google Scholar]
- 7. Braulick KW, Miller KC, Albrecht JM, Tucker JM, Deal JE. Significant and serious dehydration does not affect skeletal muscle cramp threshold frequency. Br J Sports Med. 2013; 47 11: 710– 714. [DOI] [PubMed] [Google Scholar]
- 8. Miller KC, Mack GW, Knight KL. Gastric emptying after pickle-juice ingestion in rested, euhydrated humans. J Athl Train. 2010; 45 6: 601– 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller KC, Mack G, Knight KL. Electrolyte and plasma changes after ingestion of pickle juice, water, and a common carbohydrate-electrolyte solution. J Athl Train. 2009; 44 5: 454– 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray D, Miller KC, Edwards JE. Does a reduction in serum sodium concentration or serum potassium concentration increase the prevalence of exercise-associated muscle cramps? J Sport Rehabil. 2016; 25 3: 301– 304. [DOI] [PubMed] [Google Scholar]
- 11. Miller KC, Stone MS, Huxel KC, Edwards JE. Exercise-associated muscle cramps: causes, treatment, and prevention. Sports Health. 2010; 2 4: 279– 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwellnus MP. Cause of exercise associated muscle cramps (EAMC): altered neuromuscular control, dehydration or electrolyte depletion? Br J Sports Med. 2009; 43 6: 401– 408. [DOI] [PubMed] [Google Scholar]
- 13. Jansen PH, Joosten EM, Vingerhoets HM. Muscle cramp: main theories as to aetiology. Eur Arch Psychiatry Neurol Sci. 1990; 239 5: 337– 342. [DOI] [PubMed] [Google Scholar]
- 14. Layzer RB. The origin of muscle fasciculations and cramps. Muscle Nerve. 1994; 17 11: 1243– 1249. [DOI] [PubMed] [Google Scholar]
- 15. Hutton RS, Nelson DL. Stretch sensitivity of Golgi tendon organs in fatigued gastrocnemius muscle. Med Sci Sports Exerc. 1986; 18 1: 69– 74. [PubMed] [Google Scholar]
- 16. Sulzer NU, Schwellnus MP, Noakes TD. Serum electrolytes in Ironman triathletes with exercise-associated muscle cramping. Med Sci Sports Exerc. 2005; 37 7: 1081– 1085. [DOI] [PubMed] [Google Scholar]
- 17. Behm DG, Chaouachi A. A review of the acute effects of static and dynamic stretching on performance. Eur J Appl Physiol. 2011; 111 11: 2633– 2651. [DOI] [PubMed] [Google Scholar]
- 18. Hayes BT, Harter RA, Widrick JJ, Williams DP, Hoffman MA, Hicks-Little CA. Lack of neuromuscular origins of adaptation after a long-term stretching program. J Sport Rehabil. 2012; 21 2: 99– 106. [DOI] [PubMed] [Google Scholar]
- 19. Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004; 39 3: 268– 277. [PMC free article] [PubMed] [Google Scholar]
- 20. Weir DE, Tingley J, Elder GCB. Acute passive stretching alters the mechanical properties of human plantar flexors and the optimal angle for maximal voluntary contraction. Eur J Appl Physiol. 2005; 93 5–6: 614– 623. [DOI] [PubMed] [Google Scholar]
- 21. Guissard N, Duchateau J. Effect of static stretch training on neural and mechanical properties of the human plantar-flexor muscles. Muscle Nerve. 2004; 29 2: 248– 255. [DOI] [PubMed] [Google Scholar]
- 22. Avela J, Finni T, Liikavainio T, Niemelä E, Komi PV. Neural and mechanical responses of the triceps surae muscle group after 1 h of repeated fast passive stretches. J Appl Physiol (1985). 2004; 96 6: 2325– 2332. [DOI] [PubMed] [Google Scholar]
- 23. Fowles JR, Sale DG, MacDougall JD. Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol (1985). 2000; 89 3: 1179– 1188. [DOI] [PubMed] [Google Scholar]
- 24. ACSM news releases. American College of Sports Medicine Web site. http://www.acsm.org/about-acsm/media-room/news-releases/2011/08/01/acsm-issues-new-recommendations-on-quantity-and-quality-of-exercise. Accessed July 14, 2017.
- 25. Griffin LY. Neuromuscular training and injury prevention in sports. Clin Orthop Relat Res. 2003; 409: 53– 60. [DOI] [PubMed] [Google Scholar]
- 26. Bertolasi L, De Grandis D, Bongiovanni LG, Zanette GP, Gasperini M. The influence of muscular lengthening on cramps. Ann Neurol. 1993; 33 2: 176– 180. [DOI] [PubMed] [Google Scholar]
- 27. Magnusson SP. Passive properties of human skeletal muscle during stretch maneuvers: a review. Scand J Med Sci Sports. 1998; 8 2: 65– 77. [DOI] [PubMed] [Google Scholar]
- 28. Miller KC, Knight KL. Initial electrical stimulation frequency and cramp threshold frequency and force. J Athl Train. 2012; 47 6: 643– 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller KC, Knight KL, Wilding SR, Stone MB. Duration of electrically induced muscle cramp increased by increasing stimulation frequency. J Sport Rehabil. 2012; 21 2: 182– 185. [DOI] [PubMed] [Google Scholar]
- 30. Stone MB, Edwards JE, Huxel KC, Cordova ML, Ingersoll CD, Babington JP. Threshold frequency of an electrically induced cramp increases following a repeated, localized fatiguing exercise. J Sports Sci. 2010; 28 4: 399– 405. [DOI] [PubMed] [Google Scholar]
- 31. Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Ärztebl Int. 2012; 109 15: 276– 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001; 158 6: 848– 856. [DOI] [PubMed] [Google Scholar]
- 33. Miller KC, Burne JA. Golgi tendon organ reflex inhibition following manually applied acute static stretching. J Sports Sci. 2014; 32 15: 1491– 1497. [DOI] [PubMed] [Google Scholar]
- 34. Minetto MA, Botter A, Ravenni R, Merletti R, De Grandis D. Reliability of a novel neurostimulation method to study involuntary muscle phenomena. Muscle Nerve. 2008; 37 1: 90– 100. [DOI] [PubMed] [Google Scholar]