Abstract
The alpha-7 nicotinic acetylcholine receptor (α7 nAChR), consisting of homomeric α7 subunits, is a ligand-gated Ca2+-permeable ion channel implicated in cognition and neuropsychiatric disorders. Enhancement of α7 nAChR function is considered to be a potential therapeutic strategy aiming at ameliorating cognitive deficits of neuropsychiatric disorders such as Alzheimer's disease (AD) and schizophrenia. Currently, a number of α7 nAChR modulators have been reported and several of them have advanced into clinical trials. In this brief review, we outline recent progress made in understanding the role of the α7 nAChR in multiple neuropsychiatric disorders and the pharmacological effects of α7 nAChR modulators used in clinical trials.
Abbreviations: 5-CSRTT, five-choice serial reaction time task; 5-HT, serotonin; ACh, acetylcholine; AD, Alzheimer's disease; ADHD, attention deficit hyperactivity disorder; Aβ, amyloid-β peptide; CNS, central nervous system; DMTS, delayed matching-to-sample; ECD, extracellular domain; GABA, γ-aminobutyric acid; MLA, methyllycaconitine; nAChR, nicotinic acetylcholine receptor; NOR, novel object recognition; PAMs, positive allosteric modulators; PCP, neonatal phencyclidine; PD, Parkinson's disease; PPI, prepulse inhibition; SAR, structure–activity relationship; TMD, transmembrane domains; α-Btx, α-bungarotoxin
KEY WORDS: Alpha7, nAChR, Positive allosteric modulators, Schizophrenia, Alzheimer's disease, Acetylcholine, Ion channel
Graphical abstract
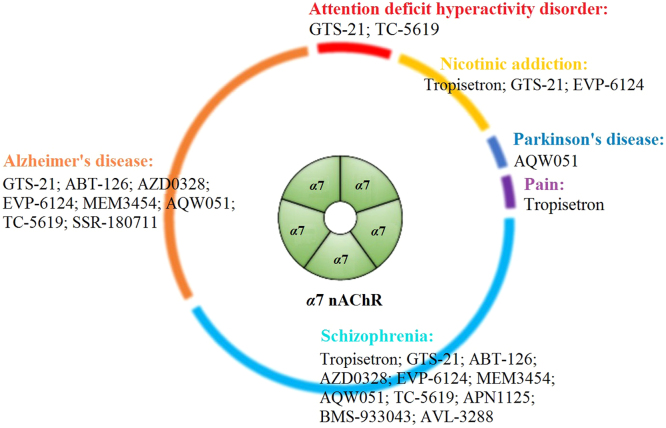
The α7 nAChR is emerging as an attractive target for neuropsychiatric disorders such as schizophrenia and Alzheimer's disease. There has been much effort in recent years toward the development of α7 modulators for many indications. Here, we update the progress made in understanding the neuropharmacological functions of α7 and its modulators in clinical trials.
1. Structure and function of α7 nAChRs in the brain
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels that are activated by the neurotransmitter acetylcholine (ACh) for signaling, and they also respond to drugs including the nicotinic receptor agonist nicotine. The nAChRs can be classified into 5 muscle nAChR subtypes (α1, β1, γ/ε, δ) and 12 neuronal nAChR subtypes (α2–10, β2–4)1., 2.. Among the neuronal nAChR subtypes, the α7 nAChR (also known as α7 receptor) that was first isolated and evaluated in 1990s from avian and rodents are homomeric pentamers widely distributed in the central nervous system (CNS) and periphery organs such as spleen and lymph nodes3., 4., 5., 6., 7.. The five identical α7 nAChR subunits are symmetrically organized around the central pore, and each subunit consists of a large amino-terminal extracellular domain (ECD), four transmembrane domains (TMD, TM1–TM4) and a cytoplasmic domain8. In each homomeric α7 nAChR, there are five ACh binding sites within the ECD, which are located at the interface of every two subunits8., 9..
Compared with other subtypes of nAChRs, the α7 nAChR exhibits unique functional properties including: 1) fast activation and desensitization by agonists (on a millisecond scale); 2) high calcium permeability (PCa/PNa ≈ 10); and 3) selective inhibition by α-bungarotoxin (α-Btx) and methyllycaconitine (MLA)3., 4., 10., 11., 12.. In the brain, α7 nAChRs are abundantly expressed in the regions underlying cognition and memory, such as the hippocampus and frontal cortex8., 13.. In neurons, the presynaptically localized α7 nAChRs are physiologically more important although they are widely localized in the synapses (both pre- and postsynaptically) and extrasynaptically9., 14.. Presynaptic α7 nAChRs play a major role in facilitating glutamate release in the cerebellum, auditory cortex, hippocampus and many other brain areas15., 16., 17., 18., 19., 20.. Together with α4β2 nAChRs, presynaptic α7 nAChRs also stimulate γ-aminobutyric acid (GABA) release in the hippocampus21. Postsynaptic and extrasynaptic α7 nAChRs are also capable of modulating neuronal activity and neurotransmission22. In addition, the α7 nAChRs are also expressed in non-neuronal cells in the brain, including astrocytes, microglia, microvascular endothelial cells, and lymphocytes, playing a role in immunity, inflammation and neuroprotection9., 23., 24., 25., 26., 27., 28..
2. The relevance of α7 nAChR in CNS diseases and therapy
The function of α7 nAChRs is critical for cognition, sensory processing, attention, working memory, and reward. On the contrary, dysfunctional α7 nAChRs are associated with multiple psychiatric and neurologic diseases including schizophrenia, AD, attention deficit hyperactivity disorder (ADHD), addiction, pain and Parkinson's disease (PD). Thus, modulation of α7 nAChR function is an attractive strategy for potential therapy of CNS diseases.
Schizophrenia, with a lifetime prevalence of approximately 1%, chronically and severely afflicts patients all over the world29., 30.. There are at least three distinct symptoms of schizophrenia, including positive symptoms (hallucinations, delusions, thought disorder, and paranoia), negative symptoms (anhedonia, social withdrawal, and thought poverty), and cognitive dysfunction (loss of intellectual abilities such as perception, understanding, working memory, and executive function)29. Almost all the first and second line drugs, including but not limited to chlorpromazine, clozapine, risperidone, olanzapine, and quetiapine, markedly improve positive symptoms for many patients with schizophrenia. However, they show very limited therapeutic effect on negative symptoms and cognitive dysfunction31. Genetic studies show that CHRNA7, the gene encoding α7 nAChR protein, and a partial duplication of CHRNA7, CHRFAM7A, are associated with inhibitory sensory gating deficit in schizophrenia patients32., 33.. It has also been reported that there is diminished mRNA of CHRNA7 and decreased α-Btx binding in post mortem brain tissue samples from patients with schizophrenia34., 35.. It has been reported that exposure to the non-selective nAChR agonist, nicotine, shows the effect of improving or normalizing sensory deficits in schizophrenia36.
Alzheimer's disease (AD) is a chronic neurodegenerative disorder characterized by a slow onset of memory loss and a late development of disorientation, mood swings and behavioral problems37. The cause for AD is still mostly unknown except for less than 10% of cases in which genetic variations have been identified38. One of the most convincing theories is that aberrant extracellular amyloid-β peptide (Aβ) deposits are the fundamental cause of AD39., 40.. Aβ is a peptide of 36–43 amino acids crucially involved in AD as the main component of the amyloid plaques found in the brain neurons of AD patients. Aβ exhibits relatively high binding affinity with α7 nAChRs, and they are co-localized in cortical regions and the hippocampus in the brains of AD patients41., 42.. It is controversial as to whether Aβ and its oligomers, Aβ1–42, are weak agonists or antagonists, but in either role, they are capable of inhibiting endogenous ACh from activating α7 nAChRs by desensitization or non-competitive antagonism43., 44.. The Aβ–α7 nAChR interaction influences neurotransmission, synaptic plasticity, learning and memory45., 46., 47., 48., 49.. Directly or indirectly, the Aβ–α7 nAChR interaction is an important aspect of AD50. From 1993 to 2001, several acetylcholinesterase inhibitors (AChEI) including tacrine (approved in 1993), donepezil (1996), rivastigmine (2000) and galanthamine (2001) which non-selectively enhance nAChR function have been approved for treatment of mild to moderate AD51., 52.. However, there are no AChEIs approved since then. A number of AChEIs such as eptastigmine, phenserine, huperzine A, and dimebon have failed or were discontinued in clinical trials due to adverse effects or insignificant benefits53., 54., 55., 56..
α7 nAChR is also reported to be relevant to other multiple CNS disorders including cigarette addiction, PD and pain57., 58., 59.. The opioid antagonist naltrexone, which inhibits the activity of α7 nAChR, was indicated for potential application in tobacco-use cessation60. Application of the α7 nAChR selective agonist PNU-282987 has been shown to decrease motivation for nicotine use in rats57., 61.. In the temporal cortices of post-mortem PD patients' brains, α7-expressing neurons are significantly less abundant than in the control group62. Accumulating evidence also shows that activation of α7 nAChR can alleviate PD symptoms in animal models58., 63., 64., 65.. Modulation of α7 nAChR function by agonists and positive allosteric modulators (PAMs) exhibits antinociceptive effects in acute and persistent pain66., 67., 68., 69., 70., 71., 72.. Genetic silencing of α7 reveals phenotypes of hyperalgesia and allodynia in mice, whereas α7-hypersensitive mice display decreased pain sensitivity59. Altogether, these studies indicate that α7 nAChR serves as a potential therapeutic target for indications such as schizophrenia, AD, ADHD, addiction, pain, PD and other related CNS disorders.
3. α7 nAChR modulators
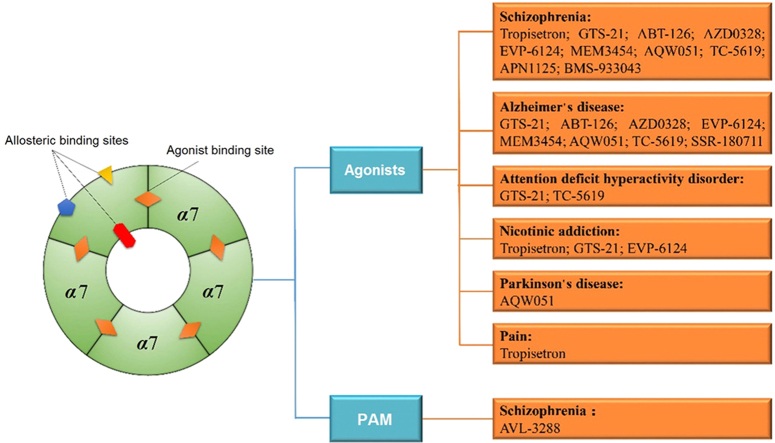
Over the past two decades, medicinal chemists and biologists have carried out extensive studies in identification and evaluation of α7 nAChR modulators. The major focus was in finding potent and selective compounds and bringing them into therapeutic applications. As summarized in Fig. 1 and Table 173., 74., 75., 76., 77., 78., 79., 80., 81., 82., 83., 84., 85., 86., 87., 88., 89., 90., 91., 92., 93., 94., 95., 96., 97., 98., 99., 100., 101., 102., 103., 104., 105., 106., 107., 108., 109., 110., 111., 112., 113., 114., 115., 116., 117., 118., 119., 120., twelve α7 nAChR modulators were tested in clinical trials since 2006.
Figure 1.
Current α7 nAChR agonists and PAMs in clinical trials for different indications. There are 11 drug candidates, of which ten agonists and one PAM are currently being tested for treatment of schizophrenia, nine agonists for AD, three agonists for nicotinic addiction, two agonists for ADHD, and one agonist each for PD and pain.
Table 1.
α7 nAChR agonists and PAM in clinical trials.
| Compound | Classification | Potency & efficacy | Animal model on CNS disorders | Indication | Clinical status (Sponsor) |
|---|---|---|---|---|---|
| Tropisetron | Partial agonist | Binding affinity: | Mice: phencyclidine-induced cognitive deficits75. | Pain | Phase IV (completed in 2009) |
 |
Ki: 6.9 nmol/L (in rα7)73 | ||||
| (University Hospital, Clermont-Ferrand) | |||||
| Electrophysiology activity: | Rats: young and aged rats76; naloxone-induced place aversion77. | ||||
| Hα7 in oocytes: EC50 = 0.6 μmol/L; Emax = 25%74 | |||||
| Smoking cessation; schizophrenia | Phase III (completed in 2011) | ||||
| Mα7 in oocytes: EC50 = 1.3 μmol/L; Emax = 36%73 | |||||
| (Baylor College of Medicine) | |||||
| GTS-21/DMXB-A | Partial agonist | Binding affinity: | Rats: normal or isoflurane-induced cognitive impairment aged rats80., 81., 82.; ibotenic acid-induced dementia83; mecamylamine-caused learning impairment84; auditory gating in isolation-reared rats85; apomorphine/MK-801-elicited PPI deficits86., 87.. | Schizophrenia | Phase II (completed in 2015) |
 | |||||
| Ki: 2000 nmol/L (in hα7)78 | |||||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 11.0 μmol/L; Emax = 9%79 | (University of Colorado) | ||||
| Rα7 in oocytes: EC50 = 5.2 μmol/L; Emax = 32%79 | AD; ADHD | Phase II (completed in 2008) | |||
| (CoMentis) | |||||
| Mice: Aβ-induced cognitive deficits45; deficient sensory inhibition88; aggressive behavior in mouse models89. | |||||
| Tobacco use disorder | Phase II (not yet recruiting) | ||||
| Rabits: aged rabbits90., 91.. | |||||
| (University of Florida) | |||||
| Monkeys: normal monkeys in DMTS task78; Ketamine-induced cognitive deficit92. | |||||
| ABT-126 | Agonist | Binding affinity: | Monkeys: Parkinsonian monkeys95. | AD | Phase II (terminated in 2014) |
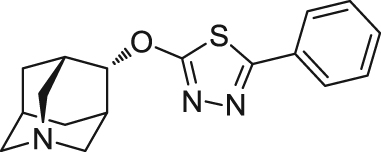 |
Ki: 12–14 nmol/L (in hα7, rα7 and mα7)93., 94. | ||||
| (AbbVie) | |||||
| Schizophrenia | Phase II (terminated in 2014) | ||||
| (AbbVie) | |||||
| AZD0328 | Partial agonist | Binding affinity: | Mice: NOR in normal mice96., 97.. | AD | Phase I (completed in 2008) |
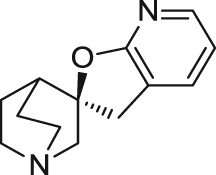 |
Ki: 3.0 and 4.7 nmol/L (in hα7 and rα7)96 | ||||
| Electrophysiology activity: | Monkeys: normal monkeys in delayed response task98. | ||||
| Hα7 in oocytes: EC50 = 338 nmol/L; Emax = 64.7%96 | (AstraZeneca) | ||||
| Schizophrenia | Phase II (terminated in 2008) | ||||
| Rα7 in oocytes: EC50 = 150 nmol/L; Emax = 61.0%96 | |||||
| (AstraZeneca) | |||||
| BMS-933043 | Partial agonist | Binding affinity: | Rats: MK-801-induced cognitive deficits73; S(+)ketamine-induced sensory gating deficits73. | Schizophrenia | Phase I (completed in 2013) |
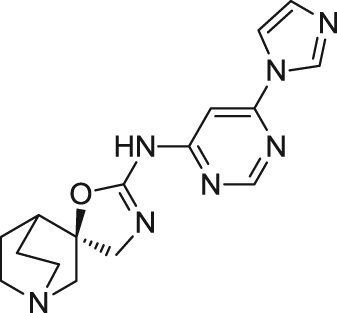 |
Ki: 8.1 and 3.3 nmol/L (in hα7 and rα7)73 | ||||
| Ca2+ flux assays: | |||||
| Hα7 in HEK293 cell line: EC50 = 23.4 nmol/L73 | (Bristol-Myers Squibb) | ||||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 0.29 μmol/L;Emax = 24%73 | |||||
| Rα7 in oocytes: EC50 = 0.14 μmol/L; Emax = 27%73 | |||||
| Mice: MK-801-induced cognitive deficits73. | |||||
| EVP-6124/ Encenicline | Partial agonist | Binding affinity: | Rats: scopolamine-induced deficit99; delay-dependent forgetting in the NOR100; low attentive rats101. | AD; dementia | Phase III (terminated in 2017) |
| Ki: 9.98 nmol/L (in rα7)99 | |||||
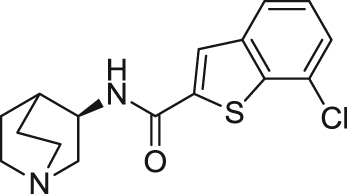 |
(FORUM) | ||||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 0.39 μmol/L; Emax = 42%99 | Schizophrenia; impaired cognition | Phase III (completed in 2016) | |||
| (FORUM) | |||||
| Nicotine dependence; smoking cessation | Phase II (terminated in 2015) | ||||
| (A. Eden Evins) | |||||
| MEM3454/RG3487 | Partial agonist | Binding affinity: | Rats: attentional performance in normal rats103; aged rats102; apomorphine-induced deficits in sensorimotor gating102. | AD | Phase II (completed in 2007) |
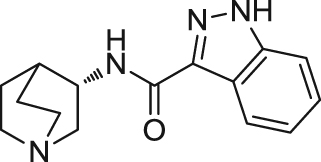 |
Ki: 6 nmol/L (in rα7)102 | ||||
| Electrophysiology activity: | (Memory) | ||||
| Hα7 in oocytes: EC50 = 0.8 μmol/L; Emax = 63%102 | |||||
| Schizophrenia | Phase II (unknown) | ||||
| Hα7 in QM cell line: EC50 = 7.7 μmol/L; Emax = 69%102 | (Memory) | ||||
| AQW051 | Partial agonist | Binding affinity: | Rats: aged rats05105 | Schizophrenia | Phase II (completed in 2013) |
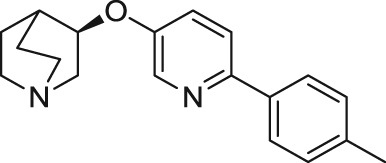 |
Ki: 27 nmol/L104 | ||||
| Ca2+ flux assays: | Mice: NOR in normal mice105 | ||||
| Hα7: EC50 = 7.4 μmol/L; Emax = 73%105 | Monkeys: Parkinsonian monkeys106 | (Novartis) | |||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 7.5 μmol/L; Emax = 75%105 | Levodopa-induced dyskinesia in PD | Phase II (completed in 2013) | |||
| (Novartis) | |||||
| AD | Phase II (terminated in 2009) | ||||
| (Novartis) | |||||
| TC-5619 | Full agonist | Binding affinity: | Mice: th(tk–)/th(tk–) mice108; apomorphine-induced PPI deficits108; NOR in normal mice108. | Schizophrenia | Phase II (completed in 2013) |
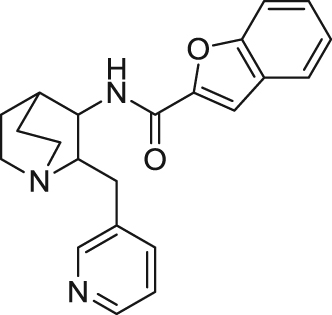 | |||||
| Ki: 1 and 1.4 nmol/L (in hα7 and rα7)107., 108. | |||||
| Electrophysiology activity: | (Targacept) | ||||
| Hα7 in oocytes: EC50 = 33 nmol/L; Emax = 100%108., 109. | AD | Phase I (completed in 2011) | |||
| Rα7 in GH4C1 cell line: EC50 = 17 nmol/L; Emax = 76%107 | (Targacept) | ||||
| ADHD | Phase II (completed in 2012) | ||||
| (Targacept) | |||||
| SSR-180711 | Partial agonist | Binding affinity: | Rats: MK-801/PCP-induced cognitive deficits111; depressive disorders rates111; neurodevelopmental latent inhibition models of schizophrenia112. | AD | Phase II (terminated in 2008) |
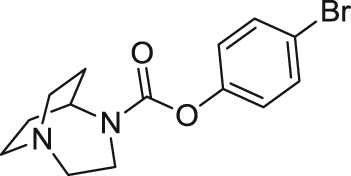 |
Ki: 14 and 22 nmol/L (in hα7 and rα7)110 | ||||
| (Sanofi) | |||||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 4.4 μmol/L; Emax = 51%110 | Mice: chronic mild stress model113; Aβ-induced memory deficits114; phencyclidine-induced cognitive deficits115; forced swim and tail suspension tests116 | ||||
| Hα7 in GH4C1 cell line: EC50 = 0.9 μmol/L; Emax = 36%110 | |||||
| APN1125 | Partial agonist | Electrophysiology activity: | Rats: NOR in normal rats117 | Schizophrenia | Phase I / Phase II (suspended in 2016) |
| (Structure Undisclosed) | Hα7 in oocytes: EC50 = 1.16 μmol/L; Emax = 41%117 | ||||
| (CoMentis) | |||||
| AVL-3288/XY4083/CCMI | Type I PAM | Electrophysiology activity: | Mice: DBA/2 mouse model of sensory-gating deficit118; MK-801-induced hyperlocomotion mode eight-arm radial maze in normal mice118; NOR in normal mice119. | Schizophrenia; schizoaffective disorder | Phase I (recruiting) |
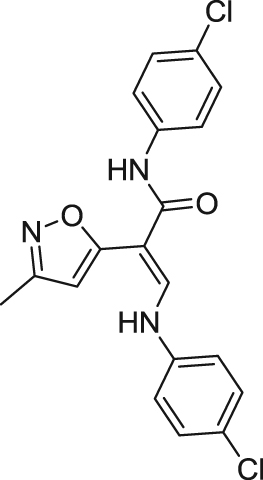 | |||||
| Hα7 in oocytes: EC50 = 0.7 μmol/L; Emax = 9 folds118 | (New York State Psychiatric Institute; University of Colorado) | ||||
| Rats: 5-CSRTT in normal rats119; ketamine-induced cognitive deficits and social withdrawal120. |
DMTS, delayed matching-to-sample; NOR, novel object recognition; PCP, neonatal phencyclidine; 5-CSRTT, five-choice serial reaction time task.
Indications and clinical status of α7 nAChR modulators above are obtained from https://clinicaltrials.gov/.
3.1. α7 nAChR agonists
Currently, most developed α7 nAChR agonists are partial agonists. Unlike full agonists such as endogenous ACh, α7 nAChR partial agonists are orthosteric ligands that can only produce a small maximal current even at concentrations where all receptors occupied121.
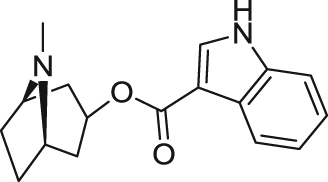
Tropisetron ([(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 1H-indole-3-carboxylate), firstly identified as 5-HT3 receptor antagonist (Ki=5.3 nmol/L), is used clinically in preventing and treating nausea and vomiting after cancer therapy122., 123.. In 2001, Macor et al.123 evaluated activity of several 5-HT3 receptor antagonists on α7 nAChRs and found that tropisetron acted as a selective α7 nAChR partial agonist (Ki=6.9 nmol/L; EC50 =0.6 μmol/L; Emax=25%). Researchers showed that tropisetron could attenuate or improve cognitive deficits in animal models75., 76., 77.. However, tropisetron has not been shown to be effective in improving cognitive deficits in clinical trials. In a phase II clinical trial of tropisetron in patients with schizophrenia, administration of tropisetron significantly improved auditory sensory gating P50 deficits and sustained visual attention, which supports the safety and efficacy of adjunctive tropisetron for treatment of cognitive deficits in schizophrenia124.
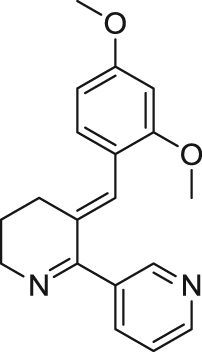
GTS-21 (3-(2,4-dimethoxybenzylidene)-anabaseine), also named DMXB-A, is a derivative of the natural product anabaseine identified as an α7 nAChR agonist and brought into clinical trials125. It has been extensively characterized in vitro and in vivo. This compound acts as a partial agonist in α7 nAChRs and displays better potency and efficacy on rat α7 nAChRs (EC50 =5.2 μmol/L; Emax=32%) than with human nAChRs (EC50 =11 μmol/L; Emax=9%) in Xenopus oocytes79. Selectivity of GTS-21 is not favorable in ion flux studies as it inhibits α4β2 nAChRs (IC50=17 μmol/L) and activates α3β4 nAChRs (EC50=21 μmol/L)78. However, in electrophysiological recordings in Xenopus oocytes, 100 μmol/L GTS-21 barely evoked current from α4β2 and α3β4 nAChRs83. Extensive in vivo studies were carried out to confirm the pharmacological effect of GTS-21 on cognitive deficits and sensory gating models of rodents and primates (Table 1). Scientists from Abbot and the University of South Florida found that intraperitoneally injecting GTS-21 significantly enhanced the learning and memory ability of aged rats in a water maze, 17-arm radical maze, and Lashley III maze tests80., 81.. When the cognition of aged rats was further impaired by isoflurane, GTS-21 still could mitigate such cognitive deficits82. Moreover, acquisition, retention and relearning abilities in eyeblink classical conditioning are much improved in GTS-21-treated aged rabbits than in the vehicle group90., 91.. Cognitive deficits or dementia in rodents and primates as induced by chemical impairment could also be attenuated or normalized by treatment with GTS-21. For instance, Chen et al.45 reported that treatment with GTS-21 (1 mg/kg) perfectly prevented Aβ25–35 induced depression of the α7 nAChR response, which further led to cognitive deficits in mice. These results indicate that GTS-21 may have substantive therapeutic value in the treatment of cognitive deficit in age-associated memory impairment, AD and schizophrenia. Furthermore, sensory gating deficits in rodents could be improved with GTS-21. This compound improved deficient sensory inhibition in DBA/2 mice, and normalized auditory gating in isolation-reared rats, and also ameliorated prepulse inhibition (PPI) deficits induced by apomorphine or MK-80185., 86., 87., 88.. These data show that GTS-21 might have a therapeutic potential for schizophrenia. In 2014, GTS-21 was in phase II clinical studies for treatment of schizophrenia, AD and ADHD. Though GTS-21 failed in improving cognition in schizophrenia patients, high dose of GTS-21 significantly improved negative symptoms in schizophrenia126. However, GTS-21 is not a prototypical α7 nAChR agonist due to its relatively higher affinity for α4β2 nAChRs (Ki=20 nmol/L at human and 19 nmol/L at rat) compared with α7 nAChRs (Ki=2000 nmol/L at human and 650 nmol/L at rat)78. Thus, the clinical benefits of GTS-21 cannot be simply attributed to α7 nAChR pharmacology.
The most explored structure of α7 nAChR agonists to date is quinuclidine derivatives such as spirooxazolidinones and quinuclidine carbamates, amides, and ethers. The first spirooxazolidinone, AR-R17779 ((–)-spiro[1-azabicyclo[2.2.2]octane-3,5ʹ-oxazolidin-2ʹ-one]) was identified and evaluated in vitro and in vivo127., 128.. However, the cross reactivity with 5-HT3 receptors and poor penetration of AR-R17779 into the CNS remains a great challenge for clinical development22. AZD0328 ((29 R)-spiro-[1-azabicyclo[2.2.2]octane-3,29(39H)-furo[2,3-b]pyridine] d-tartrate) is an optimized molecule identified as α7 nAChR agonist by AstraZeneca from the spirooxazolidinone series compounds based on AR-R17779 through structure–activity relationship (SAR) studies96. AZD0328 acts as a partial α7 nAChR agonist exhibiting an EC50 of 338 nmol/L and an efficacy of 65% on Xenopus oocytes expressing human α7 nAChRs96. Compared with the maximal current elicited by serotonin (5-HT) on human 5-HT3A receptors and ACh on human nAChRs, maximal activity of AZD0328 was only about 12% for human 5-HT3A receptors, 4% for α4β2 nAChRs and no activity on α3β4 nAChRs96. Studies showed that AZD0328 is very stable and has favorable pharmacokinetic (PK) properties, which suggests that this compound is acceptable for clinical trials98., 129.. Through activation of α7 nAChRs, AZD0328 is able to enhance cortical dopamine release in rats and improve novel object recognition (NOR) in mice96., 97.. AZD0328 also displays efficacy in improving working memory in a spatial delayed response task in Rhesus macaques98. In 2008, AstraZeneca terminated AZD0328 for a phase II clinical trial for being “unlikely to meet the current target product profile”130.
Reported in 2016, a new spirooxazolidinone named BMS-933043 ((2 R)-N-(6-(1H-imidazol-1-yl)-4-pyrimidinyl)-4ʹH-spiro[4-azabicyclo[2.2.2]octane-2,5ʹ-[1,3]oxazol]-2ʹ-amine) was identified by Bristol-Myers Squibb as a selective partial agonist for the α7 nAChR (Ki=8.1 nmol/L at human α7 nAChRs)73. Preclinical studies showed cognition enhancement and sensory gating improvement in rodents73. This compound was advanced into a phase I clinical trial for schizophrenia in 2012.
Analogs with quinuiclidine, aromatic moieties, and functional linkers such as amides and ethers have been substantially explored. EVP-6124 (((R)-7-chloro-N-quinuclidin-3-yl)benzo[b] thiophene-2-carboxamide) is a representative quinuclidine amide analog developed by FORUM (formerly EnVivo) that acts as a potent partial agonist at α7 nAChR (EC50=0.39 μmol/L, Emax=42%) and an antagonist at 5-HT3 receptors (IC50<10 nmol/L)99. It is also reported that EVP-6124 enhanced dopamine, acetylcholine, and glutamate efflux in the rat cortex and nucleus accumbens131., 132.. In vivo, EVP-6124 reversed scopolamine-induced deficit and improved natural forgetting and low attention in rats99., 101.. Treatment with EVP-6124 in phase I and II trials for mild-to-moderate AD was well tolerated and showed statistically significant improvements compared with placebo on cognitive and functional measures133., 134.. A phase II, a double-blind, randomized, placebo-controlled, parallel-design clinical trial conducted for schizophrenia showed statistical significant cognition improvement in schizophrenia patients135. However, the results of phase III clinical trial from 2012 to 2016 for schizophrenia did not meet the primary clinical end point as high efficacy in placebo group. Consequently, the other two Phase III trials for AD and dementia were suspended in 2017. Like EVP-6124, MEM3454 ((R)-3-(6-p-tolyl-pyridin-3-yloxy)-1-aza-bicyclo[2.2.2]octane)developed by Memory Pharmaceuticals also exhibited antagonism at 5-HT3 receptors and procognitive effects in normal and aged rodents102., 103.. Similarly, MEM3454 enhanced dopamine efflux by nAChR stimulation and ACh efflux primarily mediated via 5-HT3 receptor antagonism136. In a phase II clinical trial, MEM3454 failed to improve cognitive deficits in patients with schizophrenia, but moderate negative symptoms in patients were significantly improved137. Through homology modeling, molecular docking, and pharmacophore elucidation techniques, Targacept designed and synthesized a series of amide quinuclidine compounds, among which TC-5619 (N-[2-(pyridin-3-ylmethyl)-1-azabicyclo[2.2.2]oct-3-yl]-1-benzofuran-2-carboxamide) exhibits excellent activity and selectivity on α7 nAChR (Ki=1 nmol/L at human α7 nAChRs, 2800 nmol/L at human α4β2 nAChRs, IC50> 10 μmol/L at human 5-HT3 receptors)107., 108.. This compound acted as an α7 nAChR full agonist with an EC50 of 33 nmol/L in Xenopus oocytes expressing human α7 nAChRs108., 109.. In vivo studies showed adequate properties of TC-5619, including PK profiles, rapid CNS permeability and procognitive effect in rodents108., 109.. However, after two phase II clinical trials were conducted, it was confirmed that TC-5619 did not improve cognitive deficits and negative symptoms in schizophrenia138., 139., 140..
Recently, Novartis disclosed a quinuclidine ether α7 nAChR partial agonist, AQW051 ((R)-3-(6-p-tolyl-pyridin-3-yloxy)-1-aza-bicyclo[2.2.2]octane). In vitro characterization with human α7 nAChR expressed on Xenopus oocytes yielded an EC50 of 7.5 μmol/L and an efficacy of 75%106. Not only did it show a favorable PK profile and procognitive effects in rodents, this compound also displayed potential in the therapy of PD by reducing l-dopa-induced dyskinesias and extending the duration of l-dopa effects in parkinsonian monkeys104., 105., 106.. AQW051 has been advanced in phase II clinical trials for schizophrenia, AD, and l-dopa-induced PD. It was reported in a phase II randomized, double-blind, placebo-controlled study that evaluated the efficacy and safety of AQW051 in patients with PD and levodopa-induced dyskinesia that AQW051 did not significantly reduce dyskinesia or parkinsonian severity141.
Additionally, structural diversity of α7 nAChR agonists is continuously expanding in the literature from the series of quinuiclidine-based moieties. ABT-126 (2-(((1R,3R,4S,5S,7S)-1-azaadamantan-4-yl)oxy)-5-phenyl-1,3,4-thiadiazole), developed by AbbVie (formerly Abbott), is an azaadamantane derivative that acted as an α7 nAChR agonist (Ki=12–14 nmol/L)93. In a phase II clinical trial in patients with mild-to-moderate AD, ABT-126 demonstrated significant improvement compared with placebo in the primary efficacy endpoint93., 94.. A phase II trial of ABT-126 for treatment of cognitive impairment in schizophrenia was also conducted and revealed that this compound demonstrated a procognitive effect in nonsmoking subjects142. However, in a phase IIb clinical trial, ABT-126 did not demonstrate a consistent effect on cognition in nonsmoking subjects with schizophrenia but a trend toward an effect on negative symptoms143.
Researchers from Sanofi described a diazabicyclononane α7 nAChR partial agonist named SSR180711 (4-bromophenyl (1S,5S)-1,4-diazabicyclo[3.2.2]nonane-4-carboxylate, Ki=14 nmol/L at human α7 nAChRs)110. SSR180711 displayed effects of antidepression, procognition and sensory gating improvement in multiple in vivo studies in rodents112., 113., 114., 116.. However, the phase II clinical trial was terminated in 2008 for insufficient expected benefit and risk. APN1125 developed by Comentis with an undisclosed structure also acted as an α7 nAChR partial agonist (EC50=1.16 μmol/L; Emax=41% at human α7 nAChRs)117. It is currently suspended in a phase I/phase II clinical trial for schizophrenia for business reasons144.
Most of the clinical trials conducted with α7 nAChR agonists showed a paucity of effects. With limited clear reports, we can only assume the lack of sufficient selectivity over 5-HT3 receptors and improper designation of clinical trials might be the cause of the discontinued compounds. However, the crucial function of α7 nAChRs in the brain and the compelling evidence of preclinical studies still suggest that selective agonists activating α7 nAChRs may be an attractive therapeutic strategy for schizophrenia, AD and other CNS diseases.
3.2. α7 nAChR PAMs and ago-PAMs
A large number of compounds modulate α7 nAChR function by binding to allosteric sites instead of the orthosteric site that binds agonists and antagonists. α7 nAChR-positive allosteric modulators (PAMs) are a category of these compounds that can potentiate α7 currents in the presence of an agonist such as acetylcholine. On the basis of their macroscopic effects, α7 nAChR PAMs have been classified and distinguished as type I and type II. Type I PAMs mainly enhance agonist-evoked peak currents without delaying desensitization and do not reactivate desensitized receptors, whereas type II PAMs can delay desensitization and reactivate desensitized receptors22. When compared with agonists, α7 nAChR PAMs are more promising therapeutic tools because of their maintenance of endogenous activation characteristics, better selectivity profile, higher structural diversity and better final effects with an extra neuroprotection effect145. α7 nAChR ago-PAMs can activate receptors from non-orthosteric sites while still retaining the properties of PAMs.
AVL-3288 ((E)-N-(4-chlorophenyl)-3-((4-chlorophenyl)amino)-2-(3-methylisoxazol-5-yl)acrylamide), which also named XY4083 or CCMI, is a representative type I α7 nAChR PAM. Screened from a small library of GABAA receptor PAM analogs, researchers from University of California, Irvine identified a highly selective type I α7 nAChR PAM, AVL-3288118. In rodent models, treatment with AVL-3288 in the presence or absence of agonist both corrected the sensory deficits and improved cognition118., 119., 120., 146.. In 2017, AVL-3288 has advanced into a phase I clinical trial for schizophrenia and schizoaffective disorder, which demonstrated that a type I PAM can be safely administered to humans and that it has potential positive neurocognitive effects in CNS disorders147.
NS1738 (1-(5-chloro-2-hydroxy-phenyl)-3-(2-chloro-5-trifluoromethyl-phenyl)-urea) developed by NeuroSearch and LY2087101 ([2-[(4-fluorophenyl)amino]-4-methyl-5-thiazolyl]-3-thienylmethanone) by Eli Lilly are also type I α7 nAChR PAMs148., 149.. NS1738 was also reported to enhance agonist potency in rescuing scopolamine-induced cognitive deficits148. Both of NS1738 and LY2087101 have not brought into clinical trials yet.
The first selective type II PAM PNU-120596 (1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea) developed by Pfizer was shown to not only potentiate the peak α7 current but also delay desensitization of α7 nAChRs150. Though this compound augmented the procognitive effects of an acetylcholinesterase inhibitor in rodents and non-human primates, it was not able to advance into clinical trial for its potential toxic effects resulting from excessively high calcium influx118., 151.. A-867744 (4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide) is type II PAM with moderate potency and efficacy (EC50=1.12 μmol/L; Emax=733% to ACh-evoked α7 current in Xenopus oocytes) developed by AbbVie152. Other reported type II PAMs such as TQS (4-naphthalene-1-yl-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonic acid amide), JNJ-1930942 (2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol), and RO5126946 ((5-chloro-N-[(1S,3R)-2,2-dimethyl-3-(4-sulfamoyl-phenyl)-cyclopropyl]-2-methoxy-benzamide) also exhibited α7 potentiation effects in vitro and precognition effects in vivo153., 154., 155..
On the basis of the conventional type II α7 nAChR PAM TQS, researchers from Eli Lilly identified a compound named GAT-107 or 4BP-TQS (4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide), which exhibited potent allosteric agonism and allosteric potentiation at α7 nAChRs156. Moreover, with GAT-107 as a tool, it is reported that the direct allosteric activation site is located in the interface of α7 nAChR subunits157., 158..
Exploiting α7 nAChR PAMs and ago-PAMs is still in its early stages, and clinical trials of these compounds are still in their infancy. However, with the property of modulating α7 nAChR activity, α7 nAChR PAMs and ago-PAMs represent an additional therapeutic possibility for CNS diseases.
4. Concluding remarks
Abundant literature has shown us the critical role of α7 nAChRs in cognition, learning, memory, and sensory processing in animal models. Compelling preclinical evidence has shown that α7 nAChR agonists and PAMs could enhance cognition and alleviate sensory gating deficiency.
Most clinical trials of α7 nAChR agonists are terminated or suspended. With the limited data, we are not able to assign the cause of clinical failure. However, almost all of the α7 nAChR agonists show cross-activity with 5-HT3 receptors. Thus, we assume that the lack of selectivity over 5-HT3 receptors might be one reason for the failure of α7 nAChR agonists in clinical trials. In phase II clinical trials for cognitive deficits in schizophrenia, GTS-21 and ABT-126 showed significant improvement in negative symptoms but not in ameliorating cognitive deficits. In addition, EVP-6124 failed to reach the primary clinical endpoint because of the unexpected high effect of the placebo. Therefore, improper design of clinical trials might be another reason for the failure of α7 nAChR agonists in clinical trials.
As for α7 nAChR PAMs and ago-PAMs, the cytotoxic effect of PNU-120596 indicates that a too-potent activity of type II PAM is not favorable in drug discovery. However, the reported procognition and sensory gating improvement effects in animal models demonstrates a promising future for α7 nAChR PAMs. Moreover, positive results of AVL-3288 in a phase I clinical trial indicates that an α7 nAChR PAM is a potential new therapy for cognitive deficit in schizophrenia. Pharmacological studies on α7 nAChR ago-PAMs have not been reported yet. However, based on the activity of GAT-107 in enhancing α7 nAChR function, ago-PAMs remain a positive choice in developing therapeutic solution in CNS disorders.
Taken together, α7 nAChR agonists and PAMs (including ago-PAMs) remain a viable therapeutic strategy for the treatment of AD, schizophrenia, and other neuropsychiatric disorders. While developing α7 nAChR modulators, selectivity and toxicity profiles should be further improved. And before clinical trials, scientific and well-rounded clinical plans should be designed.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Dani J.A., Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 2.Kalamida D., Poulas K., Avramopoulou V., Fostieri E., Lagoumintzis G., Lazaridis K. Muscle and neuronal nicotinic acetylcholine receptors. Struct, Funct Pathog FEBS J. 2007;274:3799–3845. doi: 10.1111/j.1742-4658.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- 3.Seguela P., Wadiche J., Dineley-Miller K., Dani J.A., Patrick J.W. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couturier S., Bertrand D., Matter J.M., Hernandez M.C., Bertrand S., Millar N. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 5.Schoepfer R., Conroy W.G., Whiting P., Gore M., Lindstrom J. Brain α-bungarotoxin binding protein cDNAs and MABs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- 6.Khiroug S.S., Harkness P.C., Lamb P.W., Sudweeks S.N., Khiroug L., Millar N.S. Rat nicotinic ACh receptor α7 and β2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol. 2002;540:425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 8.Taly A., Corringer P.J., Guedin D., Lestage P., Changeux J.P. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- 9.Dineley K.T., Pandya A.A., Yakel J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36:96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertrand D., Galzi J.L., Devillers-Thiéry A., Bertrand S., Changeux J.P. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proc Natl Acad Sci U S A. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng X., Katz M., Gerzanich V., Anand R., Lindstrom J. Human α7 acetylcholine receptor: cloning of the α7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional α7 homomers expressed in Xenopus oocytes. Mol Pharmacol. 1994;45:546–554. [PubMed] [Google Scholar]
- 12.Turek J.W., Kang C.H., Campbell J.E., Arneric S.P., Sullivan J.P. A sensitive technique for the detection of the α7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods. 1995;61:113–118. doi: 10.1016/0165-0270(95)00032-p. [DOI] [PubMed] [Google Scholar]
- 13.Gotti C., Zoli M., Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Wonnacott S. Presynaptic nicotinic ach receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 15.Aramakis V.B., Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barik J., Wonnacott S. Indirect modulation by α7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69:618–628. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- 17.De Filippi G., Baldwinson T., Sher E. Evidence for nicotinic acetylcholine receptor activation in rat cerebellar slices. Pharmacol Biochem Behav. 2001;70:447–455. doi: 10.1016/s0091-3057(01)00653-0. [DOI] [PubMed] [Google Scholar]
- 18.Kawa K. Acute synaptic modulation by nicotinic agonists in developing cerebellar purkinje cells of the rat. J Physiol. 2002;538:87–102. doi: 10.1113/jphysiol.2001.012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radcliffe K.A., Dani J.A. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sher E., Chen Y., Sharples T.J., Broad L.M., Benedetti G., Zwart R. Physiological roles of neuronal nicotinic receptors subtypes: new insights on the nicotinic modulation of neurotransmitter release, synaptic transmission and plasticity. Curr Top Med Chem. 2004;4:283–297. doi: 10.2174/1568026043451393. [DOI] [PubMed] [Google Scholar]
- 21.Maggi L., Sher E., Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand D., Lee C.H., Flood D., Marger F., Donnelly-Roberts D. Therapeutic potential of α7 nicotinic acetylcholine receptors. Pharmacol Rev. 2015;67:1025–1073. doi: 10.1124/pr.113.008581. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins B.T., Egleton R.D., Davis T.P. Modulation of cerebral microvascular permeability by endothelial nicotinic acetylcholine receptors. Am J Physiol Heart Circ Physiol. 2005;289:H212–H219. doi: 10.1152/ajpheart.01210.2004. [DOI] [PubMed] [Google Scholar]
- 24.Sharma G., Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci U S A. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J.X., Yakel J.L. Functional α7 nicotinic ach receptors on astrocytes in rat hippocampal ca1 slices. J Mol Neurosci. 2012;48:14–21. doi: 10.1007/s12031-012-9719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shytle R.D., Mori T., Townsend K., Vendrame M., Sun N., Zeng J. Cholinergic modulation of microglial activation by α7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 27.Vélez-Fort M., Audinat E., Angulo M.C. Functional α7-containing nicotinic receptors of NG2-expressing cells in the hippocampus. Glia. 2009;57:1104–1114. doi: 10.1002/glia.20834. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T., Hide I., Matsubara A., Hama C., Harada K., Miyano K. Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- 29.Tamminga C.A., Holcomb H.H. Phenotype of schizophrenia: a review and formulation. Mol Psychiatry. 2005;10:27–39. doi: 10.1038/sj.mp.4001563. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter Jr, WT, Buchanan R.W. Schizophrenia. N Engl J Med. 1994;330:681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto S., Miyake N., Jarskog L.F., Fleischhacker W.W., Lieberman J.A. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17:1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 32.Freedman R., Coon H., Myles-Worsley M., Orr-Urtreger A., Olincy A., Davis A. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinkus M.L., Lee M.J., Gault J., Logel J., Short M., Freedman R. A 2-base pair deletion polymorphism in the partial duplication of the α7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 2009;1291:1–11. doi: 10.1016/j.brainres.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Court J.A., Lloyd S., Johnson M., Griffiths M., Birdsall N.J., Piggott M.A. Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Dev Brain Res. 1997;101:93–105. doi: 10.1016/s0165-3806(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 35.Guillozet-Bongaarts A.L., Hyde T.M., Dalley R.A., Hawrylycz M.J., Henry A., Hof P.R. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2014;19:478–485. doi: 10.1038/mp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard S., Mexal S., Freedman R. Genetics of smoking and schizophrenia. J Dual Diagn. 2007;3:43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 38.Jarmolowicz A.I., Chen H.Y., Panegyres P.K. The patterns of inheritance in early-onset dementia: Alzheimer's disease and frontotemporal dementia. Am J Alzheimers Dis Other Demen. 2015;30:299–306. doi: 10.1177/1533317514545825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 40.Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S.F., Wu M.N., Wang X.H., Yuan L., Yang D., Qi J.S. Requirement of α7 nicotinic acetylcholine receptors for amyloid β protein-induced depression of hippocampal long-term potentiation in CA1 region of rats in vivo. Synapse. 2011;65:1136–1143. doi: 10.1002/syn.20951. [DOI] [PubMed] [Google Scholar]
- 42.Hellström-Lindahl E., Mousavi M., Zhang X., Ravid R., Nordberg A. Regional distribution of nicotinic receptor subunit mRNAs in human brain: comparison between Alzheimer and normal brain. Mol Brain Res. 1999;66:94–103. doi: 10.1016/s0169-328x(99)00030-3. [DOI] [PubMed] [Google Scholar]
- 43.Dineley K.T., Bell K.A., Bui D., Sweatt J.D. β-Amyloid peptide activates α7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- 44.Pym L., Kemp M., Raymond-Delpech V., Buckingham S., Boyd C.A., Sattelle D. Subtype-specific actions of β-amyloid peptides on recombinant human neuronal nicotinic acetylcholine receptors (α7, α4β2, α3β4) expressed in Xenopus laevis oocytes. Br J Pharmacol. 2005;146:964–971. doi: 10.1038/sj.bjp.0706403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L., Wang H., Zhang Z., Li Z., He D., Sokabe M. DMXB (GTS-21) ameliorates the cognitive deficits in β amyloid25–35-injected mice through preventing the dysfunction of α7 nicotinic receptor. J Neurosci Res. 2010;88:1784–1794. doi: 10.1002/jnr.22345. [DOI] [PubMed] [Google Scholar]
- 46.Chen L., Yamada K., Nabeshima T., Sokabe M. α7 nicotinic acetylcholine receptor as a target to rescue deficit in hippocampal LTP induction in β-amyloid infused rats. Neuropharmacology. 2006;50:254–268. doi: 10.1016/j.neuropharm.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Puzzo D., Privitera L., Leznik E., Fà M., Staniszewski A., Palmeri A. Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu J., Khan G.M., Nichols R.A. Dopamine release in prefrontal cortex in response to β-amyloid activation of α7* nicotinic receptors. Brain Res. 2007;1182:82–89. doi: 10.1016/j.brainres.2007.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wonnacott S. Gates and filters: unveiling the physiological roles of nicotinic acetylcholine receptors in dopaminergic transmission. Br J Pharmacol. 2008;153 Suppl 1:S2–S4. doi: 10.1038/sj.bjp.0707583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parri H.R., Hernandez C.M., Dineley K.T. Research update: α7 nicotinic acetylcholine receptor mechanisms in Alzheimer's disease. Biochem Pharmacol. 2011;82:931–942. doi: 10.1016/j.bcp.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 51.Singh M., Kaur M., Kukreja H., Chugh R., Silakari O., Singh D. Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur J Med Chem. 2013;70:165–188. doi: 10.1016/j.ejmech.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 52.Galimberti D., Scarpini E. Old and new acetylcholinesterase inhibitors for Alzheimer's disease. Expert Opin Investig Drugs. 2016;25:1181–1187. doi: 10.1080/13543784.2016.1216972. [DOI] [PubMed] [Google Scholar]
- 53.Braida D., Sala M. Eptastigmine: ten years of pharmacology, toxicology, pharmacokinetic, and clinical studies. CNS Drug Rev. 2001;7:369–386. doi: 10.1111/j.1527-3458.2001.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein J. Phenserine. Expert Opin Investig Drugs. 2007;16:1087–1097. doi: 10.1517/13543784.16.7.1087. [DOI] [PubMed] [Google Scholar]
- 55.Qian Z., Ke Y. Huperzine A: is it an effective disease-modifying drug for Alzheimer's disease? Front Aging Neurosci. 2014;6:216. doi: 10.3389/fnagi.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chau S., Herrmann N., Ruthirakuhan M.T., Chen J.J., Lanctot K.L. Latrepirdine for Alzheimer's disease. Cochrane Database Syst Rev. 2015;2015:CD009524. doi: 10.1002/14651858.CD009524.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunzell D.H., McIntosh J.M. α7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quik M., Zhang D., McGregor M., Bordia T. α7 nicotinic receptors as therapeutic targets for Parkinson's disease. Biochem Pharmacol. 2015;97:399–407. doi: 10.1016/j.bcp.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alsharari S.D., Freitas K., Damaj M.I. Functional role of α7 nicotinic receptor in chronic neuropathic and inflammatory pain: studies in transgenic mice. Biochem Pharmacol. 2013;86:1201–1207. doi: 10.1016/j.bcp.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Almeida L.E., Pereira E.F., Alkondon M., Fawcett W.P., Randall W.R., Albuquerque E.X. The opioid antagonist naltrexone inhibits activity and alters expression of α7 and α4β2 nicotinic receptors in hippocampal neurons: implications for smoking cessation programs. Neuropharmacology. 2000;39:2740–2755. doi: 10.1016/s0028-3908(00)00157-x. [DOI] [PubMed] [Google Scholar]
- 61.Brunzell D.H., McIntosh J.M., Papke R.L. Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation. Ann N Y Acad Sci. 2014;1327:27–45. doi: 10.1111/nyas.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banerjee C., Nyengaard J.R., Wevers A., de Vos R.A., Jansen Steur E.N., Lindstrom J. Cellular expression of α7 nicotinic acetylcholine receptor protein in the temporal cortex in Alzheimer's and Parkinson's disease—a stereological approach. Neurobiol Dis. 2000;7:666–672. doi: 10.1006/nbdi.2000.0317. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y., Zeng X., Hui Y., Zhu C., Wu J., Taylor D.H. Activation of α7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson's disease. Neuropharmacology. 2015;91:87–96. doi: 10.1016/j.neuropharm.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 64.Sérrière S., Doméné A., Vercouillie J., Mothes C., Bodard S., Rodrigues N. Assessment of the protection of dopaminergic neurons by an α7 nicotinic receptor agonist, PHA 543613 using [18F]LBT-999 in a Parkinson's disease rat model. Front Med (Lausanne) 2015;2:61. doi: 10.3389/fmed.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang D., McGregor M., Bordia T., Perez X.A., McIntosh J.M., Decker M.W. α7 nicotinic receptor agonists reduce levodopa-induced dyskinesias with severe nigrostriatal damage. Mov Disord. 2015;30:1901–1911. doi: 10.1002/mds.26453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Damaj M.I., Meyer E.M., Martin B.R. The antinociceptive effects of α7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 67.Feuerbach D., Lingenhoehl K., Olpe H.R., Vassout A., Gentsch C., Chaperon F. The selective nicotinic acetylcholine receptor α7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56:254–263. doi: 10.1016/j.neuropharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 68.Freitas K., Carroll F.I., Damaj M.I. The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther. 2013;344:264–275. doi: 10.1124/jpet.112.197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freitas K., Ghosh S., Ivy Carroll F., Lichtman A.H., Imad Damaj M. Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology. 2013;65:156–164. doi: 10.1016/j.neuropharm.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freitas K., Negus S.S., Carroll F.I., Damaj M.I. In vivo pharmacological interactions between a type II positive allosteric modulator of α7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br J Pharmacol. 2013;169:567–579. doi: 10.1111/j.1476-5381.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bagdas D., Wilkerson J.L., Kulkarni A., Toma W., AlSharari S., Gul Z. The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br J Pharmacol. 2016;173:2506–2520. doi: 10.1111/bph.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun R., Zhang W., Bo J., Zhang Z., Lei Y., Huo W. Spinal activation of α7-nicotinic acetylcholine receptor attenuates posttraumatic stress disorder-related chronic pain via suppression of glial activation. Neuroscience. 2017;344:243–254. doi: 10.1016/j.neuroscience.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 73.Bristow L.J., Easton A.E., Li Y.W., Sivarao D.V., Lidge R., Jones K.M. The novel, nicotinic α7 receptor partial agonist, BMS-933043, improves cognition and sensory processing in preclinical models of schizophrenia. PLoS One. 2016;11:e0159996. doi: 10.1371/journal.pone.0159996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papke R.L., Schiff H.C., Jack B.A., Horenstein N.A. Molecular dissection of tropisetron, an α7 nicotinic acetylcholine receptor-selective partial agonist. Neurosci Lett. 2005;378:140–144. doi: 10.1016/j.neulet.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 75.Hashimoto K., Fujita Y., Ishima T., Hagiwara H., Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of tropisetron: role of α7 nicotinic receptors. Eur J Pharmacol. 2006;553:191–195. doi: 10.1016/j.ejphar.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 76.Callahan P.M., Bertrand D., Bertrand S., Plagenhoef M.R., Terry A.V., Jr. Tropisetron sensitizes α7 containing nicotinic receptors to low levels of acetylcholine in vitro and improves memory-related task performance in young and aged animals. Neuropharmacology. 2017;117:422–433. doi: 10.1016/j.neuropharm.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 77.Cui R., Suemaru K., Li B., Kohnomi S., Araki H. Tropisetron attenuates naloxone-induced place aversion in single-dose morphine-treated rats: role of α7 nicotinic receptors. Eur J Pharmacol. 2009;609:74–77. doi: 10.1016/j.ejphar.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 78.Briggs C.A., Anderson D.J., Brioni J.D., Buccafusco J.J., Buckley M.J., Campbell J.E. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav. 1997;57:231–241. doi: 10.1016/s0091-3057(96)00354-1. [DOI] [PubMed] [Google Scholar]
- 79.Stokes C., Papke J.K., Horenstein N.A., Kem W.R., McCormack T.J., Papke R.L. The structural basis for GTS-21 selectivity between human and rat nicotinic α7 receptors. Mol Pharmacol. 2004;66:14–24. doi: 10.1124/mol.66.1.14. [DOI] [PubMed] [Google Scholar]
- 80.Arendash G.W., Sengstock G.J., Sanberg P.R., Kem W.R. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res. 1995;674:252–259. doi: 10.1016/0006-8993(94)01449-r. [DOI] [PubMed] [Google Scholar]
- 81.Bjugstad K.B., Mahnir V.M., Kem W.R., Socci D.J., Arendash G.W. Long-term treatment with GTS-21 or nicotine enhances water maze performance in aged rats without affecting the density of nicotinic receptor subtypes in neocortex. Drug Dev Res. 1996;39:19–28. [Google Scholar]
- 82.Kong F.J., Ma L.L., Zhang H.H., Zhou J.Q. α7 nicotinic acetylcholine receptor agonist GTS-21 mitigates isoflurane-induced cognitive impairment in aged rats. J Surg Res. 2015;194:255–261. doi: 10.1016/j.jss.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 83.Meyer E.M., Tay E.T., Papke R.L., Meyers C., Huang G.L., de Fiebre C.M. 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat α7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997;768:49–56. doi: 10.1016/s0006-8993(97)00536-2. [DOI] [PubMed] [Google Scholar]
- 84.Woodruff-Pak D.S. Mecamylamine reversal by nicotine and by a partial α7 nicotinic acetylcholine receptor agonist (GTS-21) in rabbits tested with delay eyeblink classical conditioning. Behav Brain Res. 2003;143:159–167. doi: 10.1016/s0166-4328(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 85.O'Neill H.C., Rieger K., Kem W.R., Stevens K.E. DMXB, an α7 nicotinic agonist, normalizes auditory gating in isolation-reared rats. Psychopharmacology. 2003;169:332–339. doi: 10.1007/s00213-003-1482-2. [DOI] [PubMed] [Google Scholar]
- 86.Callahan P.M., Terry A.V., Jr, Tehim A. The nicotinic α7 receptor partial agonist GTS-21 ameliorates dopaminergic- and glutamatergic-related sensorimotor gating deficits in wistar rats. Biochem Pharmacol. 2011;82:1039. [Google Scholar]
- 87.Callahan P.M., Terry A.V., Jr, Tehim A. Effects of the nicotinic α7 receptor partial agonist GTS-21 on NMDA-glutamatergic receptor related deficits in sensorimotor gating and recognition memory in rats. Psychopharmacology. 2014;231:3695–3706. doi: 10.1007/s00213-014-3509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simosky J.K., Stevens K.E., Kem W.R., Freedman R. Intragastric DMXB-A, an α7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol Psychiatry. 2001;50:493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- 89.Lewis A.S., Garvey K., Mineur Y.S., Picciotto M.R. Reduction of aggressive behavior in mouse models by the selective α7 nicotinic partial agonist GTS-21. Biochem Pharmacol. 2015;97:632–633. [Google Scholar]
- 90.Woodruff-Pak D.S., Green J.T., Coleman-Valencia C., Pak J.T. A nicotinic cholinergic agonist (GTS-21) and eyeblink classical conditioning: acquisition, retention, and relearning in older rabbits. Exp Aging Res. 2000;26:323–336. doi: 10.1080/036107300750015723. [DOI] [PubMed] [Google Scholar]
- 91.Woodruff-Pak D.S., Li Y.T., Kem W.R. A nicotinic agonist (GTS-21), eyeblink classical conditioning and nicotinic receptor binding in rabbit brain. Brain Res. 1994;645:309–317. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- 92.Cannon C.E., Puri V., Vivian J.A., Egbertson M.S., Eddins D., Uslaner J.M. The nicotinic α7 receptor agonist GTS-21 improves cognitive performance in ketamine impaired rhesus monkeys. Neuropharmacology. 2013;64:191–196. doi: 10.1016/j.neuropharm.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 93.Florian H., Meier A., Gauthier S., Lipschitz S., Lin Y., Tang Q. Efficacy and safety of ABT-126 in subjects with mild-to-moderate Alzheimer's disease on stable doses of acetylcholinesterase inhibitors: a randomized, double-blind, placebo-controlled study. J Alzheimers Dis. 2016;51:1237–1247. doi: 10.3233/JAD-150978. [DOI] [PubMed] [Google Scholar]
- 94.Gault L.M., Lenz R.A., Ritchie C.W., Meier A., Othman A.A., Tang Q. ABT-126 monotherapy in mild-to-moderate Alzheimer's dementia: randomized double-blind, placebo and active controlled adaptive trial and open-label extension. Alzheimers Res Ther. 2016;8:44. doi: 10.1186/s13195-016-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McGregor M., Zhang D., Bordia T., Perez X.A., Decker M.W., Quik M. Antidyskinetic effect of the novel α7 nicotinic receptor agonist ABT-126 in parkinsonian monkeys. Biochem Pharmacol. 2015;97:629. [Google Scholar]
- 96.Sydserff S., Sutton E.J., Song D., Quirk M.C., Maciag C., Li C. Selective α7 nicotinic receptor activation by AZD0328 enhances cortical dopamine release and improves learning and attentional processes. Biochem Pharmacol. 2009;78:880–888. doi: 10.1016/j.bcp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Werkheiser J.L., Sydserff S., Hubbs S.J., Ding M., Eisman M.S., Perry D. Ultra-low exposure to α7 nicotinic acetylcholine receptor partial agonists elicits an improvement in cognition that corresponds with an increase in α7 receptor expression in rodents: implications for low dose clinical efficacy. Neuroscience. 2011;186:76–87. doi: 10.1016/j.neuroscience.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 98.Castner S.A., Smagin G.N., Piser T.M., Wang Y., Smith J.S., Christian E.P. Immediate and sustained improvements in working memory after selective stimulation of α7 nicotinic acetylcholine receptors. Biol Psychiatry. 2011;69:12–18. doi: 10.1016/j.biopsych.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Prickaerts J., van Goethem N.P., Chesworth R., Shapiro G., Boess F.G., Methfessel C. EVP-6124, a novel and selective α7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of α7 nicotinic acetylcholine receptors. Neuropharmacology. 2012;62:1099–1110. doi: 10.1016/j.neuropharm.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 100.van Goethem N.P., Prickaerts J., Welty D., Flood D.G., Koenig G. Continuous infusion of the α7 nicotinic acetylcholine receptor agonist EVP-6124 produces no signs of tolerance at memory-enhancing doses in rats: a pharmacokinetic and behavioral study. Behav Pharmacol. 2015;26:403–406. doi: 10.1097/FBP.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 101.Hayward A., Adamson L., Neill J.C. Partial agonism at the α7 nicotinic acetylcholine receptor improves attention, impulsive action and vigilance in low attentive rats. Eur Neuropsychopharmacol. 2017;27:325–335. doi: 10.1016/j.euroneuro.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 102.Wallace T.L., Callahan P.M., Tehim A., Bertrand D., Tombaugh G., Wang S. RG3487, a novel nicotinic α7 receptor partial agonist, improves cognition and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2011;336:242–253. doi: 10.1124/jpet.110.171892. [DOI] [PubMed] [Google Scholar]
- 103.Rezvani A.H., Kholdebarin E., Brucato F.H., Callahan P.M., Lowe D.A., Levin E.D. Effect of R3487/MEM3454, a novel nicotinic α7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:269–275. doi: 10.1016/j.pnpbp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 104.Lopez C.L., Johns D., Weiss M., Feuerbach D. Pharmacological characterisation and phase I evaluation in healthy volunteers of the nAChR agonist, AQW051. Eur Neuropsychopharmacol. 2013;23 Suppl 2:S288–S289. [Google Scholar]
- 105.Feuerbach D., Pezous N., Weiss M., Shakeri-Nejad K., Lingenhoehl K., Hoyer D. AQW051, a novel, potent and selective α7 nicotinic ACh receptor partial agonist: pharmacological characterization and phase I evaluation. Br J Pharmacol. 2015;172:1292–1304. doi: 10.1111/bph.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Paolo T., Gregoire L., Feuerbach D., Elbast W., Weiss M., Gomez-Mancilla B. AQW051, a novel and selective nicotinic acetylcholine receptor α7 partial agonist, reduces l-Dopa-induced dyskinesias and extends the duration of l-Dopa effects in parkinsonian monkeys. Park Relat Disord. 2014;20:1119–1123. doi: 10.1016/j.parkreldis.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Mazurov A.A., Kombo D.C., Hauser T.A., Miao L., Dull G., Genus J.F. Discovery of (2S,3R)-N-[2-(pyridin-3-ylmethyl)-1-azabicyclo[2.2.2]oct-3-yl]benzo[b]furan-2-carboxamide (TC-5619), a selective α7 nicotinic acetylcholine receptor agonist, for the treatment of cognitive disorders. J Med Chem. 2012;55:9793–9809. doi: 10.1021/jm301048a. [DOI] [PubMed] [Google Scholar]
- 108.Hauser T.A., Kucinski A., Jordan K.G., Gatto G.J., Wersinger S.R., Hesse R.A. TC-5619: an α7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia. Biochem Pharmacol. 2009;78:803–812. doi: 10.1016/j.bcp.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hauser T.A., Bencherif M., Lippiello P.M., Jordan K.G., Gatto G.J. TC-5619: an α7 neuronal nicotinic receptor-selective agonist with the potential to treat schizophrenia. Eur Neuropsychopharmacol. 2006;16(Suppl 4):S394. [Google Scholar]
- 110.Biton B., Bergis O.E., Galli F., Nedelec A., Lochead A.W., Jegham S. SSR180711, a novel selective α7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology. 2007;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- 111.Pichat P., Bergis O.E., Terranova J.P., Urani A., Duarte C., Santucci V. SSR180711, a novel selective α7 nicotinic receptor partial agonist: (ii) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007;32:17–34. doi: 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- 112.Barak S., Arad M., De Levie A., Black M.D., Griebel G., Weiner I. Pro-cognitive and antipsychotic efficacy of the α7 nicotinic partial agonist SSR180711 in pharmacological and neurodevelopmental latent inhibition models of schizophrenia. Neuropsychopharmacology. 2009;34:1753–1763. doi: 10.1038/npp.2008.232. [DOI] [PubMed] [Google Scholar]
- 113.Stemmelin J., Cohen C., Bergis O., Griebel G. The α7 nACh receptor agonist, SSR180711, displays antidepressant-like effects in rodents. Behav Pharmacol. 2005;16:S47. [Google Scholar]
- 114.Urani A., Bergis O.E., Griebel G. SSR180711, an α7 nicotinic receptor partial agonist, reverses memory deficits induced by β25–35 amyloid peptide icv administration in mice. Eur Neuropsychopharmacol. 2006;16(Suppl 4):S489. [Google Scholar]
- 115.Hashimoto K., Ishima T., Fujita Y., Matsuo M., Kobashi T., Takahagi M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the novel selective α7 nicotinic receptor agonist SSR180711. Biol Psychiatry. 2008;63:92–97. doi: 10.1016/j.biopsych.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 116.Andreasen J.T., Redrobe J.P., Nielsen E.O. Combined α7 nicotinic acetylcholine receptor agonism and partial serotonin transporter inhibition produce antidepressant-like effects in the mouse forced swim and tail suspension tests: a comparison of SSR180711 and PNU-282987. Pharmacol Biochem Behav. 2012;100:624–629. doi: 10.1016/j.pbb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 117.Koelsch G., Detke M.J., Stevens K.E., Meltzer L.T., Terry A.V., Jr, Callahan P.M. APN1125: a clinical stage α7 nicotinic acetylcholine receptor partial agonist. Biochem Pharmacol. 2015;97:636. [Google Scholar]
- 118.Ng H.J., Whittemore E.R., Tran M.B., Hogenkamp D.J., Broide R.S., Johnstone T.B. Nootropic α7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci U S A. 2007;104:8059–8064. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nikiforuk A., Kos T., Potasiewicz A., Popik P. Positive allosteric modulation of α7 nicotinic acetylcholine receptors enhances recognition memory and cognitive flexibility in rats. Eur Neuropsychopharmacol. 2015;25:1300–1313. doi: 10.1016/j.euroneuro.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 120.Nikiforuk A., Kos T., Hołuj M., Potasiewicz A., Popik P. Positive allosteric modulators of α7 nicotinic acetylcholine receptors reverse ketamine-induced schizophrenia-like deficits in rats. Neuropharmacology. 2016;101:389–400. doi: 10.1016/j.neuropharm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 121.Lape R., Colquhoun D., Sivilotti L.G. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–727. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lacerda J.F., Martins C., Carmo J.A., Lourenço M.F., Pereira A., Rodrigues A. Randomized trial of ondansetron, granisetron, and tropisetron in the prevention of acute nausea and vomiting. Transplant Proc. 2000;32:2680–2681. doi: 10.1016/s0041-1345(00)01841-8. [DOI] [PubMed] [Google Scholar]
- 123.Macor J.E., Gurley D., Lanthorn T., Loch J., Mack R.A., Mullen G. The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective α7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001;11:319–321. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- 124.Shiina A., Shirayama Y., Niitsu T., Hashimoto T., Yoshida T., Hasegawa T. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry. 2010;9:27. doi: 10.1186/1744-859X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Fiebre C.M., Meyer E.M., Henry J.C., Muraskin S.I., Kem W.R., Papke R.L. Characterization of a series of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative is a selective agonist at neuronal nicotinic α7/125I-α-bungarotoxin receptor subtypes. Mol Pharmacol. 1995;47:164–171. [PubMed] [Google Scholar]
- 126.Freedman R., Olincy A., Buchanan R.W., Harris J.G., Gold J.M., Johnson L. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levin E.D., Bettegowda C., Blosser J., Gordon J. AR-R17779, and α7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 128.Mullen G., Napier J., Balestra M., DeCory T., Hale G., Macor J. (−)-spiro[1-azabicyclo[2.2.2]octane-3,5ʹ-oxazolidin-2ʹ-one], a conformationally restricted analogue of acetylcholine, is a highly selective full agonist at the α7 nicotinic acetylcholine receptor. J Med Chem. 2000;43:4045–4050. doi: 10.1021/jm000249r. [DOI] [PubMed] [Google Scholar]
- 129.Zhou D., Zhang M., Ye X., Gu C., Piser T.M., Lanoue B.A. In vitro metabolism of α7 neuronal nicotinic receptor agonist AZD0328 and enzyme identification for its N-oxide metabolite. Xenobiotica. 2011;41:232–242. doi: 10.3109/00498254.2010.536855. [DOI] [PubMed] [Google Scholar]
- 130.ClinicalTrials.gov. Study to asses pharmacodynamics, pharmacokinetics, safety and tolerability of AZD0328 in patients with schizophrenia. 2010.
- 131.Huang M., Felix A.R., Bhuvaneswaran C., Hilt D., König G., Meltzer H.Y. The α7 receptor agonist EVP-6124 increases dopamine and glutamate efflux in rat medial prefrontal cortex and nucleus accumbens. Biochem Pharmacol. 2011;82:1040. [Google Scholar]
- 132.Huang M., Felix A.R., Flood D.G., Bhuvaneswaran C., Hilt D., Koenig G. The novel α7 nicotinic acetylcholine receptor agonist EVP-6124 enhances dopamine, acetylcholine, and glutamate efflux in rat cortex and nucleus accumbens. Psychopharmacology. 2014;231:4541–4551. doi: 10.1007/s00213-014-3596-0. [DOI] [PubMed] [Google Scholar]
- 133.Barbier A.J., Hilhorst M., Van Vliet A., Snyder P., Palfreyman M.G., Gawryl M. Pharmacodynamics, pharmacokinetics, safety, and tolerability of encenicline, a selective α7 nicotinic receptor partial agonist, in single ascending-dose and bioavailability studies. Clin Ther. 2015;37:311–324. doi: 10.1016/j.clinthera.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 134.Deardorff W.J., Shobassy A., Grossberg G.T. Safety and clinical effects of EVP-6124 in subjects with Alzheimer's disease currently or previously receiving an acetylcholinesterase inhibitor medication. Expert Rev Neurother. 2015;15:7–17. doi: 10.1586/14737175.2015.995639. [DOI] [PubMed] [Google Scholar]
- 135.Keefe R.S., Meltzer H.A., Dgetluck N., Gawryl M., Koenig G., Moebius H.J. Randomized, double-blind, placebo-controlled study of encenicline, an α7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40:3053–3060. doi: 10.1038/npp.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang M., Felix A.R., Kwon S., Lowe D., Wallace T., Santarelli L. The α7 nicotinic receptor partial agonist/5-HT3 antagonist RG3487 enhances cortical and hippocampal dopamine and acetylcholine release. Psychopharmacology. 2014;231:2199–2210. doi: 10.1007/s00213-013-3373-5. [DOI] [PubMed] [Google Scholar]
- 137.Umbricht D., Keefe R.S., Murray S., Lowe D.A., Porter R., Garibaldi G. A randomized, placebo-controlled study investigating the nicotinic α7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014;39:1568–1577. doi: 10.1038/npp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lieberman J.A., Dunbar G., Segreti A.C., Girgis R.R., Seoane F., Beaver J.S. A randomized exploratory trial of an α7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38:968–975. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hosford D., Dvergsten C., Beaver J., Segreti A.C., Toler S., Farr M.G. Phase 2 clinical trial of TC-5619, an α7 nicotinic receptor agonist in the treatment of negative and cognitive symptoms in schizophrenia. Eur Neuropsychopharmacol. 2014;24 Suppl 2:S531–S532. [Google Scholar]
- 140.Walling D., Marder S.R., Kane J., Fleischhacker W.W., Keefe R.S., Hosford D.A. Phase 2 trial of an α7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016;42:335–343. doi: 10.1093/schbul/sbv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Trenkwalder C., Berg D., Rascol O., Eggert K., Ceballos-Baumann A., Corvol J.C. A placebo-controlled trial of AQW051 in patients with moderate to severe levodopa-induced dyskinesia. Mov Disord. 2016;31:1049–1054. doi: 10.1002/mds.26569. [DOI] [PubMed] [Google Scholar]
- 142.Haig G.M., Bain E.E., Robieson W.Z., Baker J.D., Othman A.A. A randomized trial to assess the efficacy and safety of ABT-126, a selective α7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016;173:827–835. doi: 10.1176/appi.ajp.2015.15010093. [DOI] [PubMed] [Google Scholar]
- 143.Haig G., Wang D., Othman A.A., Zhao J. The α7 nicotinic agonist ABT-126 in the treatment of cognitive impairment associated with schizophrenia in nonsmokers: results from a randomized controlled phase 2b study. Neuropsychopharmacology. 2016;41:2893–2902. doi: 10.1038/npp.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.ClinicalTrials.gov. Safety, tolerability, and pharmacokinetics of APN1125 in subjects with schizophrenia; 2016.
- 145.Corradi J., Bouzat C. Understanding the bases of function and modulation of α7 nicotinic receptors: implications for drug discovery. Mol Pharmacol. 2016;90:288–299. doi: 10.1124/mol.116.104240. [DOI] [PubMed] [Google Scholar]
- 146.Thomsen M.S., El-Sayed M., Mikkelsen J.D. Differential immediate and sustained memory enhancing effects of α7 nicotinic receptor agonists and allosteric modulators in rats. PLoS One. 2011;6:e27014. doi: 10.1371/journal.pone.0027014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gee K.W., Olincy A., Kanner R., Johnson L., Hogenkamp D., Harris J. First in human trial of a type I positive allosteric modulator of α7-nicotinic acetylcholine receptors: pharmacokinetics, safety, and evidence for neurocognitive effect of AVL-3288. J Psychopharmacol. 2017;31:434–441. doi: 10.1177/0269881117691590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Timmermann D.B., Grønlien J.H., Kohlhaas K.L., Nielsen E.O., Dam E., Jorgensen T.D. An allosteric modulator of the α7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- 149.Broad L.M., Zwart R., Pearson K.H., Lee M., Wallace L., McPhie G.I. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J Pharmacol Exp Ther. 2006;318:1108–1117. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- 150.Hurst R.S., Hajós M., Raggenbass M., Wall T.M., Higdon N.R., Lawson J.A. A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Callahan P.M., Hutchings E.J., Kille N.J., Chapman J.M., Terry A.V., Jr. Positive allosteric modulator of α7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology. 2013;67:201–212. doi: 10.1016/j.neuropharm.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Malysz J., Grønlien J.H., Anderson D.J., Hakerud M., Thorin-Hagene K., Ween H. In vitro pharmacological characterization of a novel allosteric modulator of α7 neuronal acetylcholine receptor, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744), exhibiting unique pharmacological profile. J Pharmacol Exp Ther. 2009;330:257–267. doi: 10.1124/jpet.109.151886. [DOI] [PubMed] [Google Scholar]
- 153.Dinklo T., Shaban H., Thuring J.W., Lavreysen H., Stevens K.E., Zheng L. Characterization of 2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol (JNJ-1930942), a novel positive allosteric modulator of the α7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 2011;336:560–574. doi: 10.1124/jpet.110.173245. [DOI] [PubMed] [Google Scholar]
- 154.Sahdeo S., Wallace T., Hirakawa R., Knoflach F., Bertrand D., Maag H. Characterization of RO5126946, a novel α7 nicotinic acetylcholine receptor-positive allosteric modulator. J Pharmacol Exp Ther. 2014;350:455–468. doi: 10.1124/jpet.113.210963. [DOI] [PubMed] [Google Scholar]
- 155.Grønlien J.H., Håkerud M., Ween H., Thorin-Hagene K., Briggs C.A., Gopalakrishnan M. Distinct profiles of α7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- 156.Gill J.K., Savolainen M., Young G.T., Zwart R., Sher E., Millar N.S. Agonist activation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A. 2011;108:5867–5872. doi: 10.1073/pnas.1017975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Papke R.L., Horenstein N.A., Kulkarni A.R., Stokes C., Corrie L.W., Maeng C.Y. The activity of GAT107, an allosteric activator and positive modulator of α7 nicotinic acetylcholine receptors (nAChR), is regulated by aromatic amino acids that span the subunit interface. J Biol Chem. 2014;289:4515–4531. doi: 10.1074/jbc.M113.524603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Horenstein N.A., Papke R.L., Kulkarni A.R., Chaturbhuj G.U., Stokes C., Manther K. Critical molecular determinants of α7 nicotinic acetylcholine receptor allosteric activation: separation of direct allosteric activation and positive allosteric modulation. J Biol Chem. 2016;291:5049–5067. doi: 10.1074/jbc.M115.692392. [DOI] [PMC free article] [PubMed] [Google Scholar]