Abstract
Paclitaxel is a powerful antimitotic agent with excellent activity against a range of cancers. Hazel has been described as a paclitaxel-producing species among angiosperms. Fast-growing callus is a prerequisite for the success of callus production and then paclitaxel production. Therefore, optimizing the medium culture for enhancing callus growth is a crucial step for paclitaxel production. In this research, Murashige and Skoog (1962) (MS) medium was optimized for improving callus growth of hazel (Corylus avellana L.). The M10 medium (MS medium with pH 6.0 and supplemented with 1000 mg l−1 spirulina powder, 1000 mg l−1 casein hydrolysate and 3 g l−1 gelrite) significantly improved hazel callus growth. This modified MS medium increased callus fresh weight (55.8%) as compared to the control. M10 medium increased fatty acids yield of callus (66.7%) as compared to the control. Liquid M10 medium maintained growth over a longer period of time and also increased slightly, the paclitaxel production as compared to the control. This novel medium is promising for facilitating the mass production of hazel callus as a source of valuable metabolites including paclitaxel, linoleic and oleic acids.
Introduction
Paclitaxel is a powerful antimitotic agent with excellent activity against a range of cancers1. The major limitation in the extensive use of this valuable secondary metabolite is its low supply, since Taxus spp. contains very low amounts of paclitaxel2. Extraction of paclitaxel from this tree has imposed important ecological effects, resulting in the extinction of Taxus species3. Plant cell suspension culture is considered as the most promising approach to the production of paclitaxel4. The availability of this drug is still restricted and its cost is very high, mainly due to the recalcitrant behavior of Taxus spp. under in vitro conditions2. Therefore, the search for alternative sources of paclitaxel was considered as crucial. In addition to Taxus spp., hazel (Corylus avellana) has also been described as a paclitaxel-producing species through bioprospection among angiosperms5,6. The major advantages of producing paclitaxel through hazel cell cultures are that hazel is widely accessible and its in vitro cultivation is easier than that of yew2,7. It is stated that in vitro cultures of C. avellana can become a promising and cheaper source for paclitaxel production8. Besides the use of the nuts of hazel tree as a source of protein, its leaves are used to relieve the symptoms of hemorrhoidal and varicose veins9. The kernel and green leaf/flower portions of the hazel tree have antioxidant activity10. It is found that the consumption of nuts is protective against cardiac morbidity and mortality11.
In addition to the use of hazel cell cultures for paclitaxel production, hazel plantlets can be regenerated from callus tissues by differentiation induced by exogenous growth regulators. Plant regeneration from calli is possible by somatic embryogenesis or in vitro organogenesis. Meanwhile, infrequent somaclonal variants resulting from genetic diversity in somatic cells, mutations, chromosome aberrations and environmentally induced epigenetic changes can be isolated by plant regeneration of callus12.
Fast-growing callus is a key prerequisite for the success of mass callus production and then paclitaxel production, plant regeneration and transformation. Therefore, optimizing the culture medium for improvement of callus growth is a crucial step in mass callus production. For study of in vitro production of metabolites, in addition to a suitable protocol for callus induction, obtaining large amounts of callus biomass is a prerequisite13. Also, setup of fast-growing in vitro cultures is an important stage for producing secondary metabolites from the plant cell cultures14. One of the key problems in commercial production of secondary metabolites by plant cell cultures is slow growth of plant cells. The large-scale culture of low-growing cells is expensive and also basic laboratory analysis of such cells is tedious and difficult. Additionally, such slow growth rates require extreme precautions against contamination15.
Regulation of medium nutrients significantly improves the callus growth. There are a few reports on the influence of medium composition on embryogenic induction16 and also, the best method for inducing shoot organogenesis17 in hazel, but no sufficient information is available regarding the influence of different nutrient concentrations in the culture medium on callus growth of C. avellana. Recently, a suitable callus was obtained from C. avellana in our laboratory and optimization of hazel callus mass production was tried. For this purpose, effects of different concentrations of some inorganic ingredients of Murashige and Skoog (1962)18 (MS) as well as the effects of spirulina powder (Arthrospira platensis), casein hydrolysate and some amino acids on hazel callus production were investigated.
Results and Discussion
The results of different MS medium amendments for improvement of growth of hazel callus indicated that these amendments enhanced callus growth.
Effects of gelrite, pH 6.0 and medium volume increment on hazel callus growth
The 22 modifications (Table S1) in the first experiment significantly affected all the studied characteristics (Table S2). Accordingly, medium volume increment resulted in the highest amount of relative growth rates (RGR) (0.069 d−1) and relative fresh weight growth (RFWG) (4.65) which was significantly higher than that in the control (0.054 d−1and 2.87, respectively). In the present study, improved RGR and RFWG were obtained by the use of 3 g l−1 gelrite as the gelling agent (Table S2). Bacterial gellan gum (like gelriteTM and phytagelTM) is the superior gelling agent for most plant tissue culture media due to its consistent quality and high purity19. It was reported that gelrite is the best gelling agent for callus growth in Ilex paraguariensis 20. The presence of ionic impurities in both gelrite and agar may affect growth21,22. Agar contains a large amount of sodium and also levels of sulfur and copper are significant. Gelrite has less organic impurities but inorganic ones exist at high concentrations23,24. The addition of 4 g l−1 gelrite as the gelling agent decreased significantly, RGR and RFWG in comparison with 3 g l−1 gelrite (Table S2). Indeed, as a result of the rigidity of the gelling agents, water and nutrient uptake was reduced. Therefore, the growth of callus was reduced. It was reported that gelling agent type and its concentration affect water availability and cytokinin uptake21,22. Furthermore, the chemical and physical characteristics of a culture medium are influenced by both the brand and concentration of the gelling agent22.
Increasing the amount of myoinositol to 3 times as compared to the control resulted in the lowest RGR and RFWG with an average of 0.039 d−1 and 1.64, respectively. Subsequently, omission of plant hormones in the medium caused lowest RGR and RFWG with an average of 0.041 d−1 and 1.80, respectively (Table S2). Improving callus growth with increasing amount of myoinositol up to an optimum concentration and decrease in callus growth with concentration beyond the optimum level in Vitis has been reported25. No significant difference in callus growth was observed between the control and T22 (supplemented with 2 mg l−1 GA3) (Table S2). While auxins26 and cytokinins27 are required for the growth of tissue cultures, the need for gibberellic acid is controversial28.
Since pH of the medium influences the uptake of nutrients by regulating their solubility, the adjustment of the medium pH is necessary29. According to the obtained results, MS medium with pH 6.0 is recommended for the production of hazel callus (Table S2). Effect of the medium pH on callus growth of Aquilaria malaccensisis was reported30. No significant difference in hazel callus growth was obtained by replacement of FeEDTA by FeEDDHA, nor by increasing the amount of KNO3 up to 1.5 times and also using half the amount of NH4NO3 or KNO3, Whereas simultaneous decrease in KNO3 and NH4NO3 by half, reduced significantly, callus growth (Table S2). Nitrogen plays a major role in growth. The cell growth is affected by the form and amount of nitrogen source in culture medium31. Maintaining cultured cells in an undifferentiated state requires an easily attainable supply of nitrogen32. Doubled amount of NH4NO3 as compared to the control reduced callus growth (Table S2). This could be due to the toxicity of the ammonium ion at high concentration.
Effect of casein hydrolysate on growth of hazel callus
Adding six concentrations of casein hydrolysate (0, 500, 1000, 1500, 2000, 2500 and 3000 mgl−1) to MS medium increased the weight and growth rate of hazel callus (Fig. S1). The highest RGR and RFWG of callus with an average of 0.068 d−1and 4.44, respectively were obtained by using 1000 mgl−1 of casein hydrolysate in MS medium which was significantly higher than that of the control. It was reported that media supplemented with casein hydrolysate can improve callus growth in different plants33,34. Casein hydrolysates contain calcium, phosphate, microelements, vitamins and up to 18 amino acids. Among commercially available casein hydrolysates, the supplies provided by enzymatic hydrolysis are favorable. There is a limit to the amount of casein hydrolysate which can be safely used in the culture medium. It is remarked that casein hydrolysate promotes growth in cultures where phosphate deficiency inhibit growth, suggesting that this deficiency is compensated for, by amino acids. It has been inferred that casein hydrolysate is also a source of phosphate35.
Effect of spirulina powder on growth of hazel callus
Arthrospira platensis, also known as spirulina, is a multicellular and filamentous blue-green microalga. It is an edible microbe with a high food value and provides high levels of vitamins, minerals, β-carotene, essential fatty acids and antioxidants36. This investigation on hazel explores the effect of spirulina powder on callus growth rate. Calli were cultured on MS medium supplemented with different concentrations of spirulina powder (0, 100, 500, 1000, 1500 and 2000 mg l−1). The results indicated that spirulina powder in the medium significantly affected hazel callus growth. The highest RGR and RFWG of callus with an average of 0.072 d−1 and 5.03, respectively, were obtained by using 1000 mg l−1 of spirulina powder in MS medium, which was significantly higher than that of the control with a mean of 0.056 d−1 and 3.10, respectively (Fig. S2). The favorable consequence of seaweed extract on growth, yield, quality and environmental stress tolerance of crops has been already shown in in vivo conditions37,38. Acadian marine plant extract powder (AMPEP) is another alga powder that can improve callus growth. This alga powder is obtained from fresh Ascophyllum nodosum and contains the major and minor nutrients, carbohydrates, amino acids and plant growth promoting substances which are required for callus growth39.
Effects of amino acids supplementation on growth of hazel callus
The present investigation showed the effects of some amino acids (glutamine, proline, alanine, phenylalanine, cysteine and methionine) at different concentrations (0, 50, 100, 150 and 200 mgl−1) on the growth of hazel callus. As shown in Fig. S3, the supplementation of these amino acids in culture media resulted in additive effects on RGR and RFWG of callus. Results indicated that using MS medium supplemented with any studied concentration of glutamine, alanine or methionine (Fig. S3) improved callus growth, but the maximum RGR and RFWG of callus were obtained by the use of 50 mg l−1 of glutamine, alanine or methionine. MS medium supplemented with any concentration of proline, phenylalanine or cysteine improved callus growth (Fig. S3). No significant difference was observed between different concentrations of proline, phenylalanine or cysteine (Fig. S3). Therefore, the addition of 50 mg l−1 proline, phenylalanine and cysteine to medium is recommended. Increased rate of callus growth by amino acid supplements have been reported40,41. Amino acids are an accessible nitrogen source for plant cells and can be absorbed much more readily than inorganic in the same medium42. According to the results of some studies43,44, amino acids are not necessary ingredient for many cultural purposes but their addition to the medium can compensate for medium deficiency or provide an accessible source of nitrogen to cultured cells or tissues. With ammonium ion uptake, plant tissues use adenosine triphosphate (ATP) as an energy source to convert it into amino acids45. Therefore, the presence of suitable amino acids in the medium may save some ATPs.
Optimized culture medium for callus growth of hazel
According to the results obtained in the preliminary experiments, some treatments including 3 g l−1 gelrite as the gelling agent, medium pH 6.0, the use of 70 ml of medium instead of 50 ml and the addition of 1000 mgl−1 of casein hydrolysate, 1000 mgl−1 of spirulina powder, 50 mgl−1 of glutamine, proline, alanine, phenylalanine, cysteine and methionine, improved hazel callus growth. Thus, in order to find the optimal culture medium for improvement of hazel callus growth, 14 new modified MS media were prepared and investigated in another study (Table 1). In this final experiment, five grams of callus were cultured in each replication and the data was analyzed after 35 days.
Table 1.
Different modified media (based on MS medium) tested for improving hazel callus growth.
| Treatment | Medium pH = 6.0 | Phytagel (3 g l−1) | Spirulina powder (1000 g l−1) | Glutamine (50 mg l−1) | Proline (50 mg l−1) | Alanine (50 mg l−1) | Phenylalanine (50 mg l−1) | Cysteine (50 mg l−1) | Methionine (50 mg l−1) | casein hydrolysate (1000 g l−1) | Glycine (50 mg l−l) | FeEDDHA (96 mg l−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M0 | — | — | — | — | — | — | — | — | — | — | — | — |
| M1 | * | * | — | — | — | — | — | — | — | — | — | — |
| M2 | * | * | * | — | — | — | — | — | — | — | — | — |
| M3 | * | * | * | * | — | — | — | — | — | — | — | — |
| M4 | * | * | * | * | * | — | — | — | — | — | — | — |
| M5 | * | * | * | * | * | * | — | — | — | — | — | — |
| M6 | * | * | * | * | * | * | * | — | — | — | — | — |
| M7 | * | * | * | * | * | * | * | * | — | — | — | — |
| M8 | * | * | * | * | * | * | * | * | * | — | — | — |
| M9 | * | * | * | * | * | * | * | * | * | * | — | — |
| M10 | * | * | * | — | — | — | — | — | — | * | — | — |
| M11 | * | * | * | — | — | — | — | — | — | * | * | — |
| M12 | * | * | * | — | — | — | — | — | — | * | — | * |
| M13 | * | * | * | * | * | * | * | * | * | * | — | * |
| M14 | * | * | * | — | — | — | — | — | — | * | * | * |
Volume of medium in all treatments was 70 ml.
The results presented in Table 2, clearly show that the new modified MS medium, M10, resulted in the highest amount of fresh weight (63.06 g), dry weight (1.70 g), RGR (0.072 d−1) and RFWG (11.61) which were significantly higher than that in the control (with a mean of 40.48 g, 1.06 g, 0.0597 d−1 and 7.10, respectively) (Fig. 1). The M10 medium contained 70 ml of culture medium per replication with pH 6.0 and supplemented with 1000 mg l−1 spirulina powder, 1000 mg l−1 casein hydrolysate and 3 g l−1 gelrite. It is noteworthy that the difference between M9 (M10 medium supplemented with 50 mg l−1 of six above-mentioned amino acids), M10 and M11 (M10 medium supplemented with 50 mg l−1 of glycine) was not significant (Table 2). Since M10 medium is less costly than M9 and M11, it is economically preferable. Therefore, using the M10 medium to enhance callus growth of C. avellana is advised. M10 medium (M2 medium supplemented with 1000 mg l−1 casein hydrolysate) resulted in a significantly higher RGR and RFWG than the M8 medium (M2 medium supplemented with 50 mg l−1 of six above-mentioned amino acids) (Table 2). Indeed, casein hydrolysate was more effective for hazel callus growth than the addition of the major amino acids. It is thought that casein hydrolysate might contain some unknown growth promoting factors. Data shown in Table 2 indicated that M12 medium (M10 medium supplemented with FeEDDHA) reduced callus growth as compared to M10 medium. As shown in Fig. S4, culture medium was not consumed by the hazel callus. It seems that the absorption and transport of nutrient elements are impaired in this modified MS medium.
Table 2.
Effects of different modified MS media on fresh weight (FW), dry weight (DW), relative growth rate (RGR), relative fresh weight (RFWG) and percentage of callus water content of hazel callus (PCWC).
| Treatment | FW | DW | RGR | RFWG | PCWC |
|---|---|---|---|---|---|
| M0 | 40.48g | 1.06e | 0.0597g | 7.10g | 97.37a |
| M1 | 51.46e | 1.33d | 0.0666e | 9.29e | 97.42a |
| M2 | 56.58d | 1.47c | 0.0693d | 10.32d | 97.41a |
| M3 | 58.48c | 1.53bc | 0.0702cd | 10.70c | 97.38a |
| M4 | 59.06bc | 1.54bc | 0.0705bc | 10.81bc | 97.40a |
| M5 | 59.18bc | 1.53bc | 0.0706bc | 10.84bc | 97.41a |
| M6 | 59.20bc | 1.55b | 0.0706bc | 10.84bc | 97.39a |
| M7 | 59.33bc | 1.55b | 0.0707bc | 10.87bc | 97.38a |
| M8 | 60.73b | 1.58b | 0.0713b | 11.15b | 97.40a |
| M9 | 64.07a | 1.68a | 0.0729a | 11.81a | 97.38a |
| M10 | 63.06a | 1.70a | 0.0724a | 11.61a | 97.30a |
| M11 | 63.77a | 1.67a | 0.0727a | 11.75a | 97.38a |
| M12 | 34.38h | 1.12e | 0.0551h | 5.88h | 96.73b |
| M13 | 41.20fg | 1.32d | 0.0602fg | 7.24fg | 96.79b |
| M14 | 41.20fg | 1.32d | 0.0602fg | 7.24fg | 96.79b |
| LSD (0.05) | 1.78 | 0.07 | 0.0011 | 0.36 | 0.14 |
Means within a column followed by the same letter are not significantly different (p ≤ 0.05).
Figure 1.
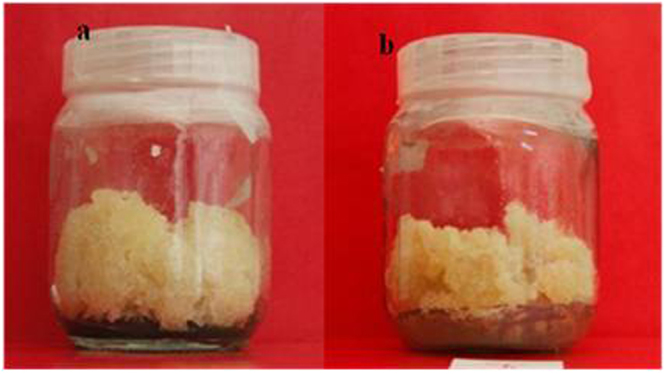
Hazel callus in M10 (a) and control (b) media.
Effect of optimized M10 medium on fatty acids profile of hazel callus
The seed oil of C. avellana and C. americana can be served as occlusive skin-conditioning agents46. Nut oil of C. avellana was reported as a natural cosmetic hazel oil without contaminants, which is used in 85 cosmetic formulations46. Analysis of C. avellana fatty acids profile indicated that linoleic acid (C18:2) in M0 medium and the linoleic acid (C18:2) and oleic acid (C18:1) in M10 medium were the main fatty acids in the fatty acids profile (Fig. 2). The proportion of unsaturated fatty acids was higher than the saturated acids. Palmitic acid (C16:0) and linoleic acid (C18:2) were the major components of saturated and unsaturated fatty acids, respectively. Short (myristic acid, C14:0) and long chain fatty acids (arachidic acid, C20:0) were detected in small amounts. Arachidonic acid (C20:4) was detected only in M10. Unsaturated, saturated and total fatty acids of callus in M10 were higher than in M0 according to Student’s t-test. It is reported that spirulina (Arthrospira platensis) is the source of essential fatty acids47. High fatty acid content of M10 as compared to the control can be explained by the amendments used in M10 medium including spirulina powder as a source of essential fatty acids while the control was spirulina powder-free medium. Linoleic and oleic acids which are used in cosmetic and pharmaceutical products48 represent 91% of the total lipids of callus grown in M10. Previous report49 showed that α-linolenic acid and its ester derivatives have strong antimicrobial activity against various oral pathogens.
Figure 2.
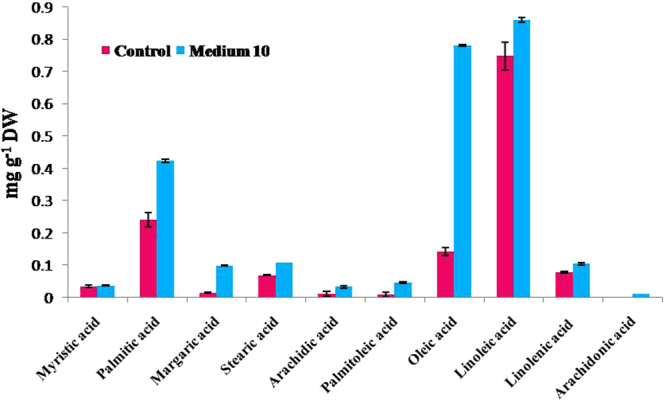
Effects of control and M10 medium on the fatty acids of hazel callus.
Effect of optimized M10 medium on cell growth and paclitaxel production in hazel cell suspension culture
Growth trends of the hazel cell suspensions showed that cell growth in M10 reached the stationary phase 2 days later as compared to the control (Fig. 3). The maximum dry weight in the control and M10 were about 11.39 and 13.64 g l−1, respectively (Fig. 3). The average growth rate over the growth period (AGR = [maximum cell density − initial cell density]/growth period50) was about 0.50 and 0.56 g l−1 day−1 in the control and M10, respectively. The cell growth index (maximum cell density/initial cell density50) in the control and M10 were about 8.78 and 10.49, respectively. The maximum biomass in M10 was 19.8% greater than that in the control. It was considered that this higher biomass was achieved by higher average growth rate and also by maintaining growth over a longer period of time (Fig. 3).
Figure 3.
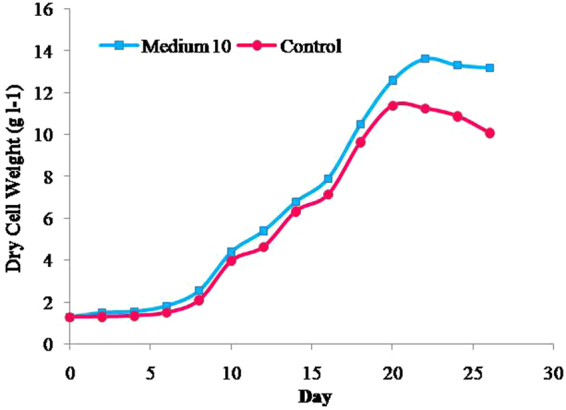
Time courses of hazel cell growth in control and in M10 medium. Average values of three replicates are given.
The average growth rate of M10 was higher than that of the control and the hazel cells grown in M10 medium were able to maintain positive growth during a period of 22 days, whereas the cells grown in the control reached stationary phase within 21 days. These trends may be explained by the amendments used for M10 medium including major and minor nutrients, carbohydrates, amino acids and plant growth promoting substances present in spirulina powder and casein hydrolysate that were absent in the control. The maximum total yield of paclitaxel concentration in the control (77.7 µg l−1) and M10 medium (106.6 µg l−1) was obtained on days 21 and 23, respectively and was produced mainly in the cells (Fig. 4). The dry weight of cells and the intracellular, extracellular and total yield of paclitaxel increased significantly by using M10 medium according to Student’s t-test (Table S3).
Figure 4.
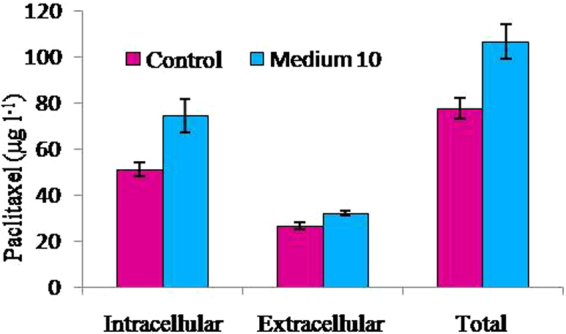
Effects of control and M10 medium on paclitaxel production (a) and dry weight (b).
Conclusion
Paclitaxel production by C. avellana cell culture was found for the first time in 20062 and the use of hazel cell culture for paclitaxel production is promising. Establishment of fast-growing in vitro cultures by using the most suitable medium is a key step towards producing paclitaxel from the hazel cell cultures. However, there are no considerable reports regarding the influences of different nutrient concentrations in the culture medium on callus growth of C. avellana. Therefore, the effects of different concentrations of mineral elements, spirulina powder, casein hydrolysate and some amino acids in MS medium on improvement of the production of hazel callus, was investigated. The modified MS medium (pH 6.0) with 1000 mg l−1 algae powder, 1000 mg l−1 casein hydrolysate and 3 g l−1 gelrite favored the increase of callus biomass of C. avellana. M10 medium as the best treatment increased the fatty acids yield of hazel callus (66.7%) as compared to the control. Also, M10 liquid medium increased the culture period and cell biomass in cell suspension culture. Production of paclitaxel by hazel cell suspension was not as high as that reported for Taxus cell suspension. However, it should be noted that establishment of the fast-growing in vitro culture of hazel may compensate for the lower yield of paclitaxel. It is noteworthy that paclitaxel production in this cell clone was improved by the combined use of phenylalanine and Vanadyl sulfate51. Therefore, it is recommended to improve paclitaxel productivity in two stage cell culture system. The use of M10 liquid medium may favour increase of cell population by improving growth at the first stage of culture (logarithmic phase). Besides, the use of different elicitors and precursors at the second stage (stationary phase) can lead to increased production of paclitaxel via involvement of some related pathways. Also, the lipids of hazel callus may have large clinical and economic applications due to their content of unsaturated fatty acids (linoleic and oleic acids) that are used in cosmetic and pharmaceutical products48. The cell suspension of C. avellana as a promising source of paclitaxel can be effective in reducing the high cost of drug-therapy.
Materials and Methods
Plant materials and tissue culture reagents
The C. avellana callus was obtained from a stable 6-year-old diploid callus. Concisely, callus were derived from seed cotyledons on MS medium supplemented with 0.2 mg l−1 6-benzylaminopurine and 2 mg l−1 dichlorophenoxyacetic acid, and solidified with 8 g l−1 agar agar. The pH of all media was adjusted to 5.8 with either KOH or HCl prior to autoclaving for 20 min at 121 °C. All cultures were incubated in dark at 25 ± 2 °C until the calli emerged. These calli were routinely subcultured every 25 days. To obtain a homogenous callus, several subcultures of calli were carried out on the same medium.
The medium components, plant growth regulators, paclitaxel and fatty acids standards used in the experiments were supplied by Sigma and Merck Chemical Companies.
Culture amendment experiments
Nine independent experiments with five replications were carried out in this research. All experiments except the first one were planned based on Completely Randomized Design (CRD), and the first experiment was set up in a Randomized Complete Block Design (RCBD). In all experiments, each replication consisted of a glass jar with autoclave-resistant plastic caps (5.5 cm in diameter, 8 cm in height and 250 ml in volume) containing 50 ml medium and seven grams of hazel callus. The cultures were incubated in a controlled incubator at 25 °C for 25 days in the dark. The first experiment was set up to test effects of 22 modifications applied in MS medium (Table S1) for improvement of hazel callus growth.
The second experiment was performed to assess the effects of different concentrations of casein hydrolysate (0, 500, 1000, 1500, 2000, 2500 and 3000 mg l−1) on callus growth of C. avellana. The third experiment was designed to study the effect of spirulina (Arthrospira platensis) powder in medium (0, 100, 500, 1000, 1500 and 2000 mg l−1) on the fresh and dry weights of hazel calli. The next six independent experiments were planned to evaluate separately, the effects of different levels (0, 50, 100, 150 and 200 mg l−1) of six amino acids (glutamine, proline, alanine, phenylalanine, cysteine and methionine) on callus growth of C. avellana.
Growth indices and water content of callus
In all the experiments, two growth indices and water content of callus were investigated as follows:
Relative growth rate (day −1). Calli were weighted before culturing on callus production medium and were weighted once again 25 days after culture (W1). Relative growth rate (RGR) was calculated based on fresh weight according to Eq. (1)52.
| 1 |
Relative fresh weight growth (RFWG). Relative fresh weight growth of callus was calculated according to Eq. (2).
| 2 |
Percentage of callus water content (PCWC). All samples of calli were dried to constant weight at 60 °C for 36 h in an oven. This trait was calculated according to Eq. (3).
| 3 |
Lipid analysis
Calli were dried in the oven at 60 °C. Lipid extraction, preparation of the fatty acid methyl esters and GC/MS analysis for C. avellana callus was done according to the procedure described by Bao et al.53. All samples were filtered through 0.22 µm cellulose acetate syringe filters before analysis with GC/MS. The fatty acids in samples were analyzed by Hewlett Packard 5890 gas chromatograph MSD 5972 mass analyzer with a HP-5MS capillary column (Agilent Technologies, Santa Clara, CA).
Measurement of cell growth
The C. avellana cell suspension cultures were obtained by cultivating 5 g callus into 250 ml Erlenmeyer flask containing 100 ml of MS medium supplemented with 0.2 mg l−1 BAP and 2 mg l−1 2,4-D acid and maintained at 25 °C in darkness on gyratory shakers at 110 rpm. Cell suspensions were also subcultured every 15 days until the cells reached homogeneity. Then, 1.5 ± 0.1 g of cells (fresh mass) were cultivated in 100 ml Erlenmeyer flask containing 30 ml MS medium.
The cell growth was determined by measuring the dry cell weight (DCW). Briefly, the biomass in the cell suspension culture was separated from the liquid medium by filtration (Whatman No. 1) and then dried at 60 °C to constant weight to obtain the dry cell weight.
Quantification of paclitaxel
Hazel cells were separated from cell suspension culture through a filter paper (Whatman No. 1). The cell-free medium was subsequently extracted according to the method proposed by Fett-Neto54. Intracellular paclitaxel was extracted from the cells with a procedure described by Luo et al.55. All samples were filtered through 0.22 µm cellulose acetate syringe filters before analysis with HPLC. Paclitaxel in samples was analyzed by HPLC (Waters, USA) with a C18 analysis column (MachereyeNagel EC 250/4.6 Nucleodur). The sample (20 µl) was injected each time and detected at 230 nm using a UV detector. The mobile phase was methanol: water (80:20 v/v) at a flow rate of 1.0 ml/min. The quantification of paclitaxel was based on an external standard of genuine paclitaxel (Sigma).
Statistical analysis
The hypothesis of normality and equal variance were met and conventional parametric statistics was used for the analysis. Analysis of variance and means comparison using least significant difference (LSD) were performed by SAS (SAS 9.1, 2003) and Excel (Excel, 2013) software was used for making graphs.
Availability of data and material
The dataset supporting the conclusions of this article is included in the article.
Electronic supplementary material
Author Contributions
M. Salehi carried out the experiments and the preparation of manuscript under the supervision and advisorship of prof. A. Moeini and prof. N. Safaie, respectively. All authors have read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15703-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.Bestoso F, et al. In vitro cell cultures obtained from different explants of Corylus avellana produce Taxol and taxanes. BMC Biotechnol. 2006;6:45–56. doi: 10.1186/1472-6750-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonsen HT, Drew DP, Lunde C. Perspectives on using physcomitrella patens as an alternative production platform for thapsigargin and other terpenoid drug candidates. Perspect. Medicin. Chem. 2009;3:1–6. doi: 10.4137/PMC.S2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson DM, Ketchum REB, Vance NC, Christen AA. Initiation and growth of cell lines of Taxus brevifolia (Pacific yew) Plant Cell Rep. 1993;12:479–482. doi: 10.1007/BF00236091. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman A, et al. Bioprospecting for Taxol in angiosperm plant extracts-Using high performance liquid chromatography thermospray mass spectrometry to detect the anticancer agent and its related metabolites in Filbert trees. Spectrosc. 1998;13(6):22–32. [Google Scholar]
- 6.Service RF. Hazel trees offer new source of cancer drug. Science. 2000;288:27–28. doi: 10.1126/science.288.5463.27a. [DOI] [PubMed] [Google Scholar]
- 7.Gallego A, et al. Development of a hazel cell culture-based paclitaxel and baccatin III production process on a benchtop scale. J. Biotechnol. 2015;195:93–102. doi: 10.1016/j.jbiotec.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Gallego A, et al. Taxol from Corylus avellana: paving the way for a new source of this anti-cancer drug. Plant Cell Tiss. Organ Cult. 2017;129:1–16. doi: 10.1007/s11240-016-1164-5. [DOI] [Google Scholar]
- 9.Amaral JS, et al. Phenolic profile of hazelnut (Corylus avellana L.) leaves cultivars grown in Portugal. Nat. Prod. Res. 2005;19:157–163. doi: 10.1080/14786410410001704778. [DOI] [PubMed] [Google Scholar]
- 10.Alasalvar C, Karamac M, Amarowicz R, Shahidi F. Antioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy cover. J. Agric. Food Chem. 2006;54:4826–4832. doi: 10.1021/jf0601259. [DOI] [PubMed] [Google Scholar]
- 11.Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch. Intern. Med. 2002;162:1382–1387. doi: 10.1001/archinte.162.12.1382. [DOI] [PubMed] [Google Scholar]
- 12.Larkin PJ, Scowcraft WR. Somaclonal variation: a novel source of variability from cell culture for plant improvement. Theor. Appl. Genet. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- 13.Souza JM, Berkov S, Santos AS. Improvement of friable callus production of Boerhaavia paniculata Rich and the investigation of its lipid profile by GC/MS. An. Acad. Bras. Ciênc. 2014;86(3):1015–1027. doi: 10.1590/0001-3765201420130098. [DOI] [PubMed] [Google Scholar]
- 14.Smetanska I. Production of secondary metabolites using plant cell cultures. Adv. Biochem. Eng. Biotechnol. 2008;111:187–228. doi: 10.1007/10_2008_103. [DOI] [PubMed] [Google Scholar]
- 15.Shuler ML. Production of secondary metabolites from plant tissue culture‐problems and prospects. Ann. N. Y. Acad. Sci. 1981;369(1):65–79. doi: 10.1111/j.1749-6632.1981.tb14178.x. [DOI] [Google Scholar]
- 16.Karasawa MMG, et al. Gametic embryogenesis through isolated microspore culture in Corylus avellana L Plant Cell Tiss. Organ Cult. (PCTOC) 2016;124(3):635–647. doi: 10.1007/s11240-015-0921-1. [DOI] [Google Scholar]
- 17.Silvestri C, et al. Adventitious shoot organogenesis from leaf and petiole explants of European hazelnut. Plant Cell Tiss. Organ Cult. (PCTOC) 2016;126(1):59–65. doi: 10.1007/s11240-016-0976-7. [DOI] [Google Scholar]
- 18.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 19.Huang LC, Kohashi C, Vangundy R, Murashige T. Effects of common components on hardness of culture media prepared with gelrite™. In Vitro Cell Dev. Biol. Plant. 1995;31(2):84–89. doi: 10.1007/BF02632242. [DOI] [Google Scholar]
- 20.Sansberro, P. A., Rey, H. Y., Luna, C. V. & Mroginski, L. A. Influence of gelling agents on Ilex paraguariensis tissue culture. In IV International Symposium on In Vitro Culture and Horticultural Breeding560, 453-456, 10.17660/ActaHortic.2001.560.89 (2000).
- 21.Bornman CH, Vogelmann TC. Effect of rigidity of gel medium on benzyladenine-induced adventitious bud formation and vitrification in vitro in. Picea abies. Physiol. Plant. 1984;61:505–512. doi: 10.1111/j.1399-3054.1984.tb06364.x. [DOI] [Google Scholar]
- 22.Debergh PC. Effects of agar brand and concentration on the tissue culture medium. Physiol. Plant. 1983;50:270–276. doi: 10.1111/j.1399-3054.1983.tb00770.x. [DOI] [Google Scholar]
- 23.Scherer PA, Muller E, Lippert H, Wolff G. Multielement analysis of agar and Gelrite impurities investigated by inductively coupled plasma emission spectrometry as well as physical properties of tissue culture media prepared with agar or gellan gum gelrite. Acta Hortic. 1988;226:655–658. doi: 10.17660/ActaHortic.1988.226.91. [DOI] [Google Scholar]
- 24.Scholten HJ, Pierik RLM. Agar as a gelling agent: Chemical and physical analysis. Plant Cell Rep. 1998;17:230–235. doi: 10.1007/s002990050384. [DOI] [PubMed] [Google Scholar]
- 25.Staudt G. The effect of myo-inositol on the growth of callus tissue in Vitis. J. Plant Physiol. 1984;116(2):161–166. doi: 10.1016/S0176-1617(84)80073-5. [DOI] [PubMed] [Google Scholar]
- 26.Jablonski JR, Skoog F. Cell enlargement and cell division in excised tobacco pith tissue. Physiol. Plant. 1954;7:16–24. doi: 10.1111/j.1399-3054.1954.tb07552.x. [DOI] [Google Scholar]
- 27.Einset JW. Two effects of cytokinin on the auxin requirement of tobacco callus cultures. Plant physiol. 1977;59(1):45–47. doi: 10.1104/pp.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Digby J, Wareing PF. The effect of growth hormones on cell division and expansion in liquid suspension cultures of Acer pseudoplatanus. J. Exp. Bot. 1966;17(4):718–725. doi: 10.1093/jxb/17.4.718. [DOI] [Google Scholar]
- 29.Bhatia P, Ashwath N. Effect of medium pH on shoot regeneration from the cotyledonary explants of tomato. Biotechnol. 2005;4(1):7–10. doi: 10.3923/biotech.2005.7.10. [DOI] [Google Scholar]
- 30.Jayaraman S, Daud NH, Halis R, Mohamed R. Effects of plant growth regulators, carbon sources and pH values on callus induction in Aquilaria malaccensis leaf explants and characteristics of the resultant calli. J. For. Res. 2014;25(3):535–540. doi: 10.1007/s11676-014-0492-8. [DOI] [Google Scholar]
- 31.Kirby, E. G., Leustek, T. & Lee, M. S. Cell and Tissue Culture inForestry (eds Bonga, J. M. & Durzan, D. J.) Ch. 5, 67–88, 10.1007/978-94-017-0994-1_5 (Springer, 1987).
- 32.Hahlbrock K. Further studies on the relationship between the rates of nitrate uptake, growth and conductivity changes in the medium of plant cell suspension cultures. Planta. 1975;124(3):311–318. doi: 10.1007/BF00388694. [DOI] [PubMed] [Google Scholar]
- 33.Cardi J, Monti M. Optimization of callus culture in pea (Pisum sativum) Annali Della Facolta di Scienze. 1993;24:11–22. [Google Scholar]
- 34.Khaleda L, Al-Forkan M. Stimulatory effects of casein hydrolysate and proline in in vitro callus induction and plant regeneration from five deepwater rice (Oryza sativa L.) Biotechnol. 2006;5(3):379–384. doi: 10.3923/biotech.2006.379.384. [DOI] [Google Scholar]
- 35.George, E. F., Hall, M. A. & De Klerk, G. J. Plant Propagation by Tissue Culture (eds George, E. F. et al.) Ch. 3, 65–113 (Springer, 2008).
- 36.Habib, M. A. B., Parvin, M., Huntington, T. C. & Hasan, M. R. A review on culture, production and use of spirulina as food for humans and feeds for domestic animals and fish. FAO (2008).
- 37.Jayaraj J, Wan A, Rahman M, Punja ZK. Seaweed extracts reduces foliar fungal disease on carrot. Crop Prot. 2008;27(10):1360–1366. doi: 10.1016/j.cropro.2008.05.005. [DOI] [Google Scholar]
- 38.Rayorath P. Mundaya et al. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2007;20:423–429. doi: 10.1007/s10811-007-9280-6. [DOI] [Google Scholar]
- 39.Hurtado AQ, Yunque DA, Tibubos K, Critchley AT. Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J. Appl. Phycol. 2009;21(6):633–639. doi: 10.1007/s10811-008-9395-4. [DOI] [Google Scholar]
- 40.El-Sharabasy S, Farag MA, El-Emerym GAE, Safwat G, Diab A. Effect of Amino acids on the growth and production of steroids in date palm using tissue culture technique. Researcher. 2012;4(1):75–84. [Google Scholar]
- 41.Taira T, Haskins FA, Gorz HJ. Callus and suspension cultures of Melilotus alba tissues and cells. Crop Sci. 1977;17:407–411. doi: 10.2135/cropsci1977.0011183X001700030017x. [DOI] [Google Scholar]
- 42.Thom, M., Maretzki, A., Komor, E. & Sakai, W. S. Nutrient uptake and accumulation by sugarcane cell cultures in relation to the growth cycle. Plant Cell Tiss. Organ Cult. (PCTO) 1, 3–14, 10.1007/BF02318898 (1981).
- 43.Armstrong CL, Green CE. Establishment and maintenance of friable embryogenic maize callus and involvement of L-proline. Planta. 1985;164:207–214. doi: 10.1007/BF00396083. [DOI] [PubMed] [Google Scholar]
- 44.Cheng TY. Factors effecting adventitious bud formationof cotyledon culture of douglas fir. Plant Sci. Lett. 1977;9:179–187. doi: 10.1016/0304-4211(77)90096-7. [DOI] [Google Scholar]
- 45.Durzan, D. J. Tissue Culture in Forestry (eds Bonga, J. M. & Durzan, D. J.) Ch. 3, 36–71 (Springer, 1982).
- 46.Andersen FA. Final report on the safety assessment of Corylus avellana (Hazel) seed oil, Corylus americana (Hazel) seed oil, Corylus avellana (Hazel) seed extract, Corylus americana (Hazel) seed extract, Corylus rostrata (Hazel) seed extract, Corylus avellana (Hazel) leaf extract, Corylus americana (Hazel) leaf extract, and Corylus rostrata (Hazel) leaf extract. Int. J. Toxicol. 2001;20:15–20. doi: 10.1080/10915810152902538. [DOI] [PubMed] [Google Scholar]
- 47.Golmakani MT, Rezaei K, Mazidi S, Razavi SH. γ‐Linolenic acid production by Arthrospira platensis using different carbon sources. Eur. J. Lipid Sci. Technol. 2012;114(3):306–314. doi: 10.1002/ejlt.201100264. [DOI] [Google Scholar]
- 48.Rabasco Álvarez AM, González Rodríguez ML. Lipids in pharmaceutical and cosmetic preparations. Grasas y aceites. 2000;51(1):74–96. [Google Scholar]
- 49.Huang CB, George B, Ebersole JL. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch.Oral Biol. 2010;55:555–560. doi: 10.1016/j.archoralbio.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Ho KP. Assessment of various carbon sources and nutrient feeding strategies for Panax ginseng cell culture. Appl. Biochem. Biotechnol. 1999;82:17–26. doi: 10.1385/ABAB:82:1:17. [DOI] [PubMed] [Google Scholar]
- 51.Rahpeyma SA, Moieni A, Jalali Javaran M. Paclitaxel production is enhanced in suspension‐cultured hazel (Corylus avellana L.) cells by using a combination of sugar, precursor, and elicitor. Eng. Life Sci. 2015;15(2):234–242. doi: 10.1002/elsc.201400115. [DOI] [Google Scholar]
- 52.Al-Khayri JM, Al-Bahrany AM. Growth, water content, and proline accumulation in drought-stressed callus of date palm. Biol. Plant. 2004;48(1):105–108. doi: 10.1023/B:BIOP.0000024283.74919.4c. [DOI] [Google Scholar]
- 53.Bao X, Katz S, Pollard M, Ohlrogge J. Carbocyclic fatty acids in plants: biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculia foetida. PNAS. 2002;99(10):7172–7177. doi: 10.1073/pnas.092152999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fett-Neto AG, Zhang WY, DiCosmo F. Kinetics of taxol production, growth and nutrient uptake in cell suspension cultures of Taxus cuspidata. Biotechnol. Bioeng. 1994;44:205–210. doi: 10.1002/bit.260440209. [DOI] [PubMed] [Google Scholar]
- 55.Luo J, Liu L, Wu CD. Enhancement of paclitaxel production by abscisic acid in cell suspension cultures of Taxus chinensis. Biotechnol. Lett. 2001;23(16):1345–1348. doi: 10.1023/A:1010597802741. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


