Abstract
Large animal models play an essential role in the study of tissue engineering and regenerative medicine (TERM), as well as biomechanics. The porcine model has been increasingly used to study the musculoskeletal system, including specific joints, such as the knee and temporomandibular joints, and tissues, such as bone, cartilage, and ligaments. In particular, pigs have been utilized to evaluate the role of skeletal growth on the biomechanics and engineered replacements of these joints and tissues. In this review, we explore the publication history of the use of pig models in biomechanics and TERM discuss interspecies comparative studies, highlight studies on the effect of skeletal growth and other biological considerations in the porcine model, and present challenges and emerging opportunities for using this model to study functional TERM.
Keywords: : animal models, ligament and tendon, cartilage, bone, skeletal muscle
Introduction
Large animal models play an important role in translating tissue engineering and regenerative medicine (TERM) innovations from the laboratory to clinical use (See references for several reviews on this topic1–4). Such models have been widely used to study the musculoskeletal system that functions as a structural support system, while facilitating a wide range of movement during sports and activities of daily living. However, these tissues are often injured during trauma, such as tendon rupture, or the progression of disease, such as osteoarthritis. Within the context of injury, these large animal models are necessary to gain an understanding of injury mechanisms and implications, assess surgical interventions similar to those used in humans, and test potential TERM treatments in a mechanically demanding environment. Appropriate models also feature homology relative to human structure and function and the ability to evaluate the complex biomechanical properties of joints and tissues at multiple scales. In addition to its utility in studying the pulmonary, nervous, and cardiovascular systems,5–13 the pig model has a long history of use for studying the biomechanics of specific joints like the knee or the temporomandibular joint (TMJ), and specific tissues, including bone, cartilage, and ligaments.14–18 Expanding on previous translational studies in adult animals, recent increases in pediatric orthopedic injuries in humans have created a demand for TERM studies in animal models undergoing skeletal growth.19,20 These populations are unique due to the ever-changing size and shape of tissues and joints, as well as altered biological and biomechanical environments during normal growth or in response to injury.
In this review, we first describe the increased use of the pig model in the fields of biomechanics and TERM in general, and for musculoskeletal tissues, specifically, over the past few decades. We will then summarize studies comparing the porcine model to other large animal models. The remaining sections of this review will focus on the use of the porcine model to study the effects of skeletal growth, while also considering other biological factors. We will examine these topics within the context of selected individual tissues and joints and describe relevant TERM applications. Finally, we conclude with a discussion of emerging directions within the porcine model and challenges and opportunities for its use in the TERM field.
Review of the Use of the Porcine Model in TERM
To assess the recent use of specific large animal models for biomechanics and TERM, we conducted several literature searches on April 6, 2017, through Web of Science (Thomson Reuters, Web of Science Core Collection). Given the dates of origin for the terms “tissue engineering” and “regenerative medicine,” we restricted our search to 1990 through 2016.21,22 The following species-specific key words were used: “pig OR porcine,” “cow OR bovine,” “dog OR canine,” “sheep OR ovine,” “horse OR equine,” or “goat OR caprine.” These were individually combined with the key words “biomechanic* OR mechanic*” and/or “tissue engineer* OR regenerative medicine.” This allowed us to search for the use of each species in biomechanics and TERM separately. It also allowed us to isolate studies that utilized biomechanics within a TERM study.
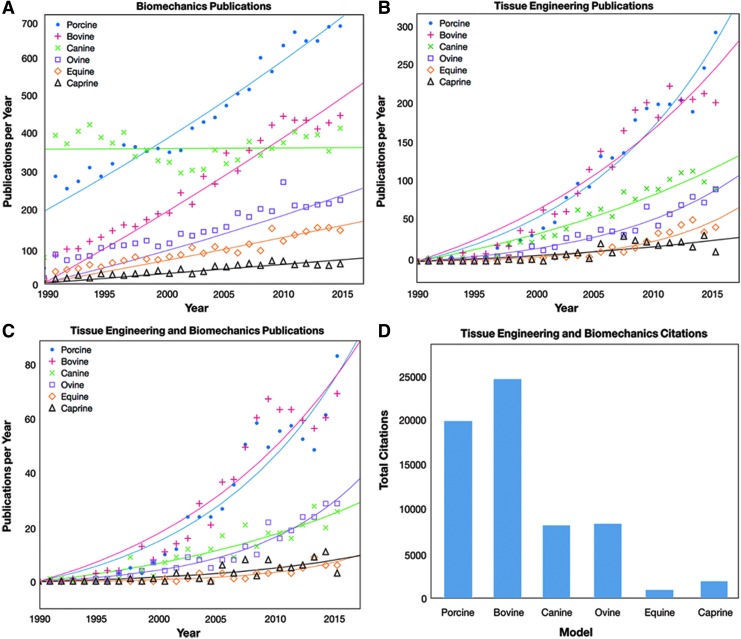
Data detailing the number of publications referring to biomechanics and TERM in the porcine, bovine, canine, ovine, equine, and caprine models are plotted relative to year in Figure 1A–C. In terms of biomechanics (Fig. 1A), the porcine, bovine, and canine models had the highest number of publications per year among all six species. Interestingly, the number of publications per year remained relatively steady over time for the canine model (∼400 publications per year), while the porcine model showed a large increase relative to other species (278 publications per year in 1991 vs. 671 publications per year in 2016). Similarly, the bovine model experienced major increases in publications (12 publications per year in 1990 vs. 437 publications per year in 2016). Gradual increases over time were found for the ovine, caprine, and equine models, but the number of publications per year for these three models remained lower than the porcine model at all years.
FIG. 1.
A comparison of the use of six common large animal models (porcine, bovine, canine, ovine, equine, and caprine) in peer-reviewed publications over the past three decades. Publications with the terms “biomechanics” or “mechanics” (A) experienced major increases in the porcine model, although interestingly these terms were used in combination with the canine model consistently since 1990. Publications involving the terms “tissue engineering” or “regenerative medicine” (B) experienced significant increases in publication rate since the early 1990 s, with the greatest increases found in the porcine and bovine models. When these search terms were combined (C), similar trends were found to the tissue engineering search at a lower scale. Data in (A–C) were fit with either an exponential or linear regression, depending on the shape of the data. The impact of these tissue engineering studies can be seen through the number of times these publications have been cited in studies involving both TERM and biomechanics (D) with nearly 20,000 citations for articles involving the porcine model since 1990. Color images available online at www.liebertpub.com/tec
The number of TERM studies rose for all six animal models from 1990 to 2016, as shown in Figure 1B. Publication rates per year were low for all of the models during the early 1990 s (maximum of five articles per year within a single species between 1990 and 1995). TERM publications using the porcine model experienced the greatest increases with time (277 publications per year in 2016), while publications using the bovine model (192 publications per year in 2016), the canine model (88 publications per year in 2016), and the ovine model (87 publications per year in 2016) also experienced substantial increases in publication rate.
Following individual searches for TERM and biomechanics publications, a refined search was performed for the subset of TERM publications utilizing biomechanics. As expected, this search yielded similar trends to the TERM search with lower numbers of publications across all species (Fig. 1C). For example, publications using the porcine model still exhibited the greatest increase from 1990 to 2016, but the percentage of TERM studies utilizing biomechanics was only 28% of the number of TERM publications alone for the porcine model. This percentage varied between 14% and 32% across species. Similarly, the percentage of biomechanical TERM studies ranged from 2% to 11% across species relative to the number of studies utilizing biomechanics in the same species.
The impact of these studies is also apparent. Articles involving both TERM and biomechanics in the porcine model have been cited almost 20,000 times (19,899 times to be exact, Fig. 1D). This number of citations approximately equals those of canine, ovine, equine, and caprine models combined (19,451 citations total) and speaks to the broad impact of the porcine model for TERM and biomechanics. The impact of studies involving both TERM and biomechanics in the bovine model has also been extremely high, with 24,575 citations (Fig. 1D). Of note, the vast majority of studies in the bovine model are related to cartilage or bone (68% of TERM studies and 80% of TERM/biomechanics studies within our search parameters). Given the size and availability of the joints from abattoirs, the bovine model is commonly used in vitro as a source of cells and tissues, but its translational value is limited. In fact, only 16% of TERM articles in the bovine model involved in vivo studies, and very few, if any, involved implantation within cows themselves.
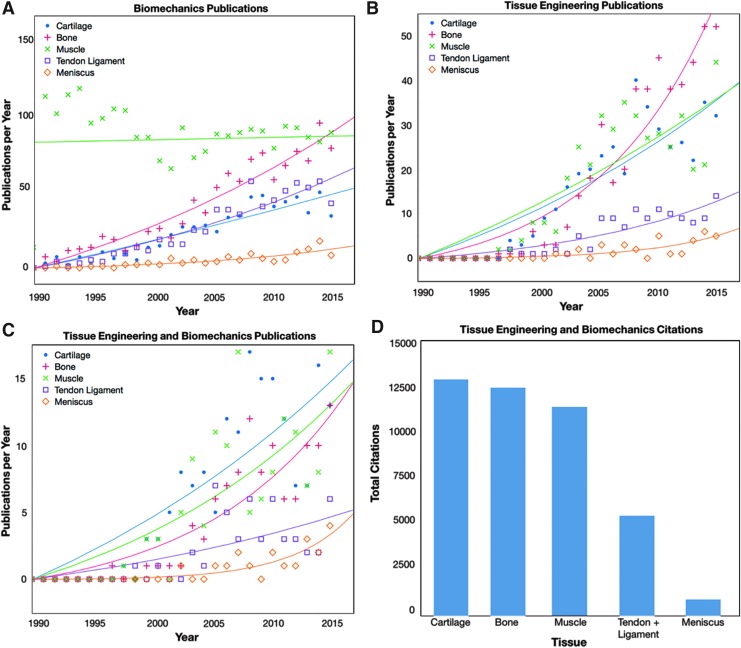
A more specific search of the publication history related to the porcine model and individual musculoskeletal tissues was also performed. For this literature review (Web of Science, Thomson Reuters, Web of Science Core Collection, April 13, 2017), the pig-specific, TERM-specific, and biomechanics-specific search terms listed above were combined with a tissue-specific search term: Specific “cartilag*,” “bone*,” “muscle* OR muscul*,” “ligament* OR tendon*,” and “menisc*.”
Within a search for biomechanics publications (Fig. 2A), studies involving muscle maintained a high annual publication rate from the early 1990 s, whereas studies involving the remaining tissues started at relatively low publication rates and increased exponentially in the later years. For TERM studies (Fig. 2B), annual publication rates increased over time for all tissues, with bone, muscle, and cartilage studies reaching peak rates of 40–50 publications per year. The data sets are much noisier than those in Figure 1 due to the lower number of publications. A combined search of TERM and biomechanics studies found trends similar to studies on TERM alone (Fig. 2C), with the greatest publication levels in cartilage, bone, and muscle models. These publications greatly impacted the TERM/biomechanics field (Fig. 2D), with total citations of combined TERM and biomechanics studies reaching 8000–13,000 (or an average of 31–54 citations per publication) each for bone, cartilage, muscle, and tendon/ligament.
FIG. 2.
Within the porcine model, several musculoskeletal tissues have been the focus of tissue engineering and regenerative medicine studies. For biomechanics (A), studies on bone, cartilage, and muscle tissues had the highest increases in publications per year. Similarly, for tissue engineering and regenerative medicine, studies on cartilage, bone, and muscle have been most frequently published (B). When these search terms were combined, similar trends were found (C) with the highest annual rates in cartilage, bone, and muscle. Data in Figure 1A–C were fit with either an exponential or linear regression, depending on the shape of the data. The impact of studies regarding individual musculoskeletal tissues can be seen through the total citations in each tissue (D), with highest impacts in cartilage, bone, and muscle, followed by tendon/ligament research. Color images available online at www.liebertpub.com/tec
Interspecies Comparisons
Several studies have attempted to compare the anatomy and biomechanics of tissues and joints between species as well as the applicability to TERM studies. Published data on interspecies anatomical and biomechanical differences are provided in Supplementary Tables S1 and S2 (Supplementary Data are available online at www.liebertpub.com/tec), respectively. Summaries of these findings for bone, cartilage, and soft tissues of the knee joint, muscle, and the TMJ disc are described in the following sections.
Bone
To assess interspecies differences in bone, Liebschner reviewed biomechanical parameters from the bones of humans, cows, dogs, sheep, pigs, horses, donkeys, rabbits, rats, mice, monkeys, turkeys, and chickens.1 A lack of industry standards for biomechanical testing of bones created difficulty in directly comparing the models across several studies, but the literature suggested that larger animal models are more beneficial in general for biomechanical evaluation of bone treatments, while small animal models may be preferred for studying biological implications of bone treatments. In addition, the author highlighted two major requirements for preclinical models in tissue engineering: the ability to recapitulate classical surgical techniques in the animal model and the ability to replicate standard human outcomes in the model.
Articular cartilage
Similarly, comparative physiology studies and reviews have been performed in cartilage research to determine appropriate animal models for research in cartilage growth, degeneration, and repair. Comparing the relative biochemical and biomechanical properties of the articular cartilage of the facet joints15 across species (humans, Yucatan miniature swine (often termed “minipigs”), New Zealand White rabbits, and Rhesus Macaque monkeys), the minipig had slightly thicker cartilage with a higher shear modulus compared to the rabbit. In another study, the biomechanical properties of articular cartilage of the stifle joint were not only species-specific but also site dependent with higher permeability and lower aggregate modulus in the patellar groove cartilage relative to the high weight-bearing regions of the femoral condyles across five species (human, cow, dog, monkey, and rabbit).23
In addition to interspecies studies of healthy cartilage, some groups have investigated the use of animal models in the context of injury. Ahern et al. reviewed data on single site cartilage defects in murine, laprine, ovine, canine, porcine, caprine, equine, and human subjects,24 specifically using cartilage thickness values from an interspecies comparison study25 and a study focusing on porcine cartilage.26 This study reported that the most accurate reflection of cartilage defects in humans (percentage of cartilage relative to bone) was found in larger models and only the equine model matched the thickness of human cartilage. As other interspecies reviews for cartilage tissue engineering have suggested,27 small animal models are beneficial for early-phase testing, while larger models such as pigs, sheep, dogs, and horses are necessary to understand the effect of a biomechanical and biochemical environment similar to that seen in humans.
Anterior cruciate ligament of the knee and other soft tissues
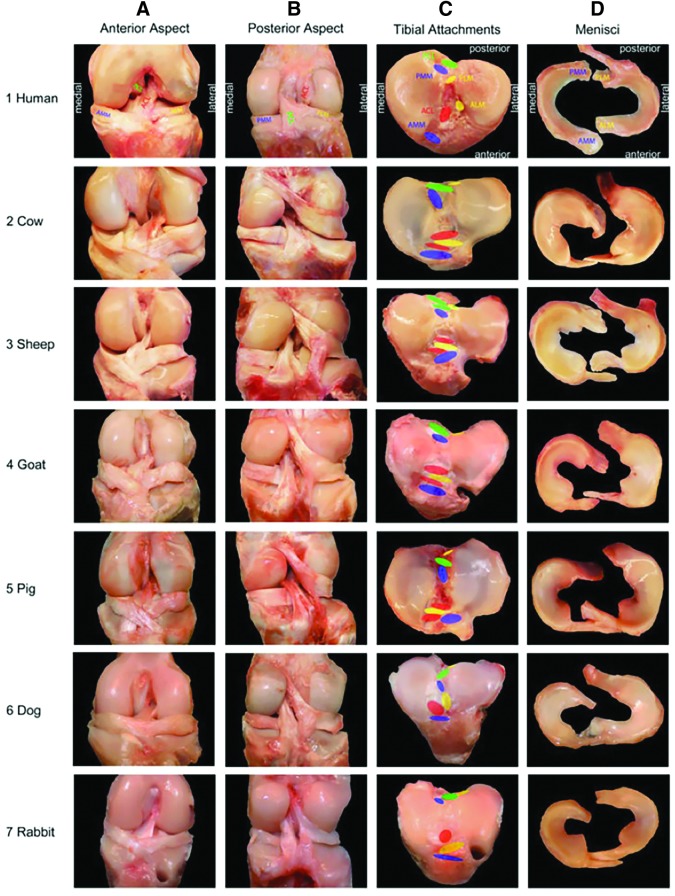
In terms of fibrous soft tissues of the knee, Proffen et al. performed a comparative anatomy study of the knee joint (or equivalent stifle joint) between cow, sheep, goat, dog, pig, rabbit, and human joints.16 All of the major soft tissue structures (ligaments, meniscus, etc.) were identified in all models (Fig. 3). Unsurprisingly, differences in the limits of full extension were evident between the human knee and the animal stifle joints (0° of flexion in humans vs. 22–45° of flexion in the animals). Finer anatomical differences also existed. For example, the bundles of the anterior cruciate ligament (ACL) in the cow, sheep, and pig joints were separated by the anterior insertion of the lateral meniscus on the tibial plateau. Based on gross anatomy alone, the authors concluded that sheep, cows, and goats had the most similar ACL to humans. In terms of dimensions, the absolute width and length measurement of the ACL were significantly similar between humans and cows, sheep, and pigs. Importantly, all species exhibited an ACL featuring multiple bundles, consistent with other work including more exotic species such as deer, bears, lions, and antelope.28,29 Interestingly, this study found less similarity between all the animal models and humans for the posterior cruciate ligament (PCL). The authors proposed that this may be related to the altered biomechanical demands of the knee in bipeds and quadrupeds.
FIG. 3.
Anatomical images of the knee (stifle) joints from human, cow, sheep, goat, pig, dog, and rabbit models reflect many gross similarities in the musculoskeletal tissues. Specific features of interest include the tissues and insertion sites of the anterior and posterior medial meniscus, anterior and posterior lateral meniscus, anterior cruciate ligament, and the posterior cruciate ligament (Adapted from Proffen et al.,16 with permission from Elsevier). Color images available online at www.liebertpub.com/tec
Biomechanical comparisons of the ACL in several species have also been performed.18 Specifically, the in situ forces in the anteromedial (AM) and posterolateral (PL) bundles under an applied anterior drawer load were compared across humans, pigs, goats, and sheep using a modified materials testing system. Relative to humans, the magnitude of force in the ACL and both bundles and the direction of force in the ACL and AM bundle were significantly different in goats and sheep. The only statistically significant difference between the pig and human was in the magnitude and direction of force in the PL bundle. The authors concluded that the pig model was a better analog for human knees based on in situ biomechanics, although all three remain common preclinical models.30–35
Additional studies using the porcine model within a robotic testing system illustrated how restricting degrees of freedom (DOF) within the knee joint under applied loads can greatly impact the direction of force in the ACL and the relative distribution of force across the AM and PL bundles.36 Furthermore, recent studies in the human ACL have highlighted regional heterogeneity in the mechanical properties within each ACL bundle.37 It is unclear how well pigs or other large animal models match this subbundle biomechanical heterogeneity.
With respect to the meniscus, Proffen et al. found that pigs, sheep, and goats were most comparable to humans in regard to size, while the goat model also had similar insertion site locations to humans.16 Further studies by Takroni et al. compared human, sheep, and pig menisci and found that sheep menisci were more similar than pig menisci to human tissues in terms of tissue volume and weight, although the human menisci had significantly greater circumference than both animal models.38 In addition to studies on the body of the meniscus, interspecies comparisons have been made regarding the meniscofemoral ligaments (MFLs). The posterior MFL was present in humans, dogs, sheep, and shire horses, whereas there were no comparable tissues found for the human anterior MFL in other species.39
Additional studies have compared the biomechanical properties of the human meniscus to those found in baboons, cows, dogs, pigs, and rabbits.17 Across all models, the posterior region of the medial meniscus featured a lower shear modulus and aggregate modulus relative to the anterior and central regions. The authors noted that no single animal model is ideal across the board for meniscus studies, but the bovine model was most similar to humans for aggregate and shear modulus, and the canine and baboon models were the most similar to humans in terms of permeability. Additional studies comparing human menisci to those from pigs, sheep, dogs, monkeys, and cows found that sheep exhibited the most similar aggregate modulus and permeability values to human data.40 Other reviews of animal models for meniscus repair have confirmed that no one model is the current gold standard and the specific hypothesis should be carefully considered when selecting an appropriate large animal model.41
Muscle
The architecture of four rotator cuff muscles has been compared across eleven species: mouse, rat, rabbit, dog, goat, sheep, pig, cow, capuchin, chimpanzee, and human.42 Given the wide range of body size across these models, general muscle architecture parameters, such as physiological cross sectional area (PCSA) relative to body size and fiber length to muscle length ratios, were quantified. The study found a roughly linear scaling relationship between body size and muscle architecture. However, there were some distinct differences in relative architectural parameters across the groups. Specifically, based on the relative PCSA values, the ungulate models (goat, pig, sheep, and cow models) were primarily “infraspinatus dominant,” while the remaining models were “subscapularis dominant.” While the nonhuman primates had more similarities to humans than the remaining models, the authors noted an inverse relationship between relative supraspinatus mass and increasing evolutionary similarity to humans, suggesting that there are differences in the structural and functional properties of the rotator cuff muscles even between humans and the most appropriate animal models.
TMJ disc
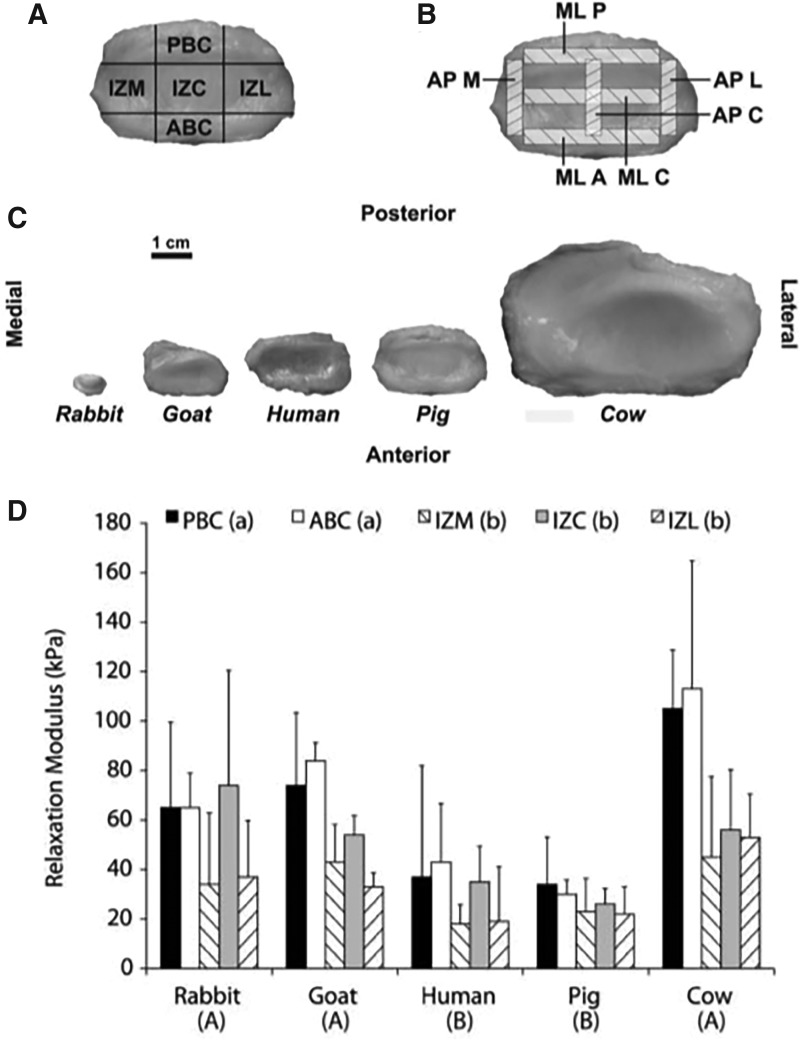
The TMJ is a small joint located in the jaw that facilitates articulation of the temporal fossa and the mandibular condyle with a TMJ disc and several discal attachments designed to maintain stability and distribute loads during multiplanar motions. In a comparative anatomical study of the TMJ disc, Kalpakci et al. investigated the gross anatomy, biochemical composition, and functional properties of the TMJ discs in humans, pigs, cows, rabbits, and goats43 (Fig. 4). Key findings included differences in biomechanical properties between herbivore (cow, goat, and rabbit) and omnivore models (human and pig), as the functional demands of the TMJ discs vary based on the diet of the animal. For example, discs from the herbivore models are designed for translatory jaw motion and had higher glycosaminoglycan (GAG) content and compressive properties compared to discs from omnivores, which function in both rotary and translatory motion. Overall, this study determined that the porcine model was most appropriate as a human analog due to the significant similarities in anteroposterior and mediolateral dimensions, collagen and GAG content, and mechanical behavior under compressive loading.
FIG. 4.
Regions of the TMJ disc. Regions of the TMJ disc for compression testing (A) are labeled as PBC, IZM, IZC, and IZL, and ABC. Regions selected for tensile testing are shown (B) labeled as MLP, MLC, MLA, APM, APC, and APL. These regions were identified and tested across five species, with differences in gross anatomy represented (C). Compressive relaxation moduli at equilibrium across the animal models and disc regions are shown below (D), statistically significant differences exist between groups that do not share a letter (p < 0.05) (Adapted from Kalpakci et al.43 with permission from SAGE Journals). ABC, anterior band central; APM, anteroposterior medial; APC, AP central; APL, AP lateral; MLP, mediolateral posterior; MLC, ML central; MLA, ML anterior; IZM, intermediate zone medial; IZC, IZ central; IZL, IZ lateral; PBC, posterior band central; TMJ, temporomandibular joint.
The large size of the porcine model also allows for the examination of tissues traditionally difficult to assess. For example, Murphy et al. were able to assess the response of the six discal attachments of the TMJ separately under anterior–posterior and medial–lateral tension,14 with the highest modulus values found for the posterior superior attachment under medial–lateral tension. Analysis of the collagen alignment revealed anisotropy in all of the attachments, except for the anterior inferior attachment. Native porcine TMJ discs also exhibit region-specific compressive properties, with increased stiffness in the posterior and medial aspects of the tissue.44 These regionally specific and directionally dependent properties of the TMJ disc provide important design criteria for TERM studies.
Effect of Skeletal Growth in the Porcine Model
One challenge in the use of animal models for translational research is the definition of age equivalency. Age can be defined using several scales, including chronological age, sexual age, and skeletal age.45 Within the pig model, chronological growth occurs on a significantly accelerated timeline compared to human growth. Many pig strains reach sexual maturity between 4.5 and 6 months of age,46 and this age range is often used to describe “early adolescence.” Skeletal age in humans is classically measured from a left-hand radiograph, and the lack of similar bony anatomy in the porcine forelimb makes this a difficult comparison.47 Depending on the particular bone, physes in hind limbs of Yorkshire pigs close between 12 and 20 months of age.46 As such, this age range (and sometimes a little older) is often used to define the end of “adolescence.” We will use the following terms to describe age: young (0–4.5 months), early adolescent (4.5–6 months), adolescent (6–18 months), late adolescent (18–24 months), and adult (>24 months).
A second consideration is that dozens of porcine strains exist. Some, such as Yorkshire pigs and cross-breeds, are bred to gain weight rapidly for use in the food industry. Universities with agricultural programs often have closed herds of these animals. Often, the closely controlled genetics of these herds is useful from a scientific genetic standpoint (control groups can be siblings). Several strains are dubbed “minipigs,” including Yucatan, Hanford, Lee-Sung, Gottingen, and Changfeng minipigs, among others.48–52 Many of these minipig lines exist in closed herds and have been bred specifically to limit rapid growth and increase docility, easing their use in biomedical research.
Several studies have examined biological differences between pig strains in terms of complications due to anesthesia53 or the response of the skin to light due to differences in skin pigmentation.54 In terms of biomechanics, Germscheid et al. studied the medial collateral ligament (MCL) and the biomechanical differences between Yorkshire and red Duroc strains.55 While MCL dimensions of both breeds were comparable to human MCLs, MCLs from the Duroc strain were larger in cross-sectional area and had lower tensile failure stress compared to MCLs from the Yorkshire strain. These findings suggest that interstrain differences exist for the porcine model, although direct comparisons between strains have been limited to date and require further investigation.
A third consideration is the importance of sex of the animals being studied. Unfortunately, research investigating the importance of sex in the porcine model is limited (as summarized in Supplementary Table S3). In Gottingen minipigs ranging in age from 11 to 55 (mean 24) months, males had significantly higher mean cartilage thickness than females in the lateral facet of the trochlear groove.56 Separate work showed that sex, but not birth weight, had an effect on glucose intolerance in Yucatan minipigs.57 Females had higher visceral and subcutaneous adiposity and subsequent glucose intolerance than males after 10 months of the same feeding regimen, which is consistent with human data. More recently, Kiapour et al. studied an ACL transection model in adolescent (15 months on average) Yucatan minipigs repaired either with a conventional reconstruction using a soft tissue graft or combining with a collagen-platelet-rich plasma (PRP) treatment and found several differences between sexes.58 For example, females had 19% lower yield load and 12% lower stiffness than males after 15 weeks of healing. Furthermore, in animals treated with conventional reconstruction, females had larger areas of articular cartilage damage. These data suggest that tissue engineering strategies may need to be tailored to the sex of the patient. Given that the influence of porcine strain and sex on the biomechanical properties of musculoskeletal tissues or the results of TERM strategies are not fully elucidated, we will state the strain and sex of the animals used, when specified. The role of age on biomechanics and TERM has been studied in the porcine model for several musculoskeletal tissues, which we will discuss in the following subsections. A summary of the skeletal growth-related biomechanical changes in the porcine model can be found in Table 1.
Table 1.
Skeletal Growth-Related Biomechanical Differences in the Porcine Model
| Tissue property | Value | Age group | n | Breed | Sex | Reference |
|---|---|---|---|---|---|---|
| Elastic modulus (GPa) | ||||||
| Bone, cancellous | ∼10 | 9.4 ± 0.4 weeks | 5 | — | Female | Willems et al.59 |
| ∼14 | 102.6 ± 5.0 weeks | 5 | — | Female | ||
| Bone, cortical | ∼11 | 9.4 ± 0.4 weeks | 5 | — | Female | |
| ∼14 | 102.6 ± 5.0 weeks | 5 | — | Female | ||
| Bone, cortical, thin lamellae | ∼13 | 6 months | 3 | — | — | Feng et al.61 |
| ∼16 | 12 months | 3 | — | — | ||
| ∼19 | 42 months | 3 | — | — | ||
| Bone, cortical, thick lamellae | ∼16 | 6 months | 3 | — | — | |
| ∼17.5 | 12 months | 3 | — | — | ||
| ∼22.5 | 42 months | 3 | — | — | ||
| Young's modulus* (MPa) | ||||||
| Cartilage, superficial zone | ∼0.3 | 4 weeks | 2 | — | — | Gannon et al.66 |
| ∼0.5 | 8 weeks | 2 | — | — | ||
| ∼0.5 | 1 year | 2 | — | — | ||
| ∼1.0 | 3 years | 2 | — | — | ||
| Cartilage, middle zone | ∼0.3 | 4 weeks | 2 | — | — | |
| ∼0.5 | 8 weeks | 2 | — | — | ||
| ∼1.5 | 1 year | 2 | — | — | ||
| ∼1.0 | 3 years | 2 | — | — | ||
| Cartilage, deep zone | ∼0.3 | 4 weeks | 2 | — | — | |
| ∼0.5 | 8 weeks | 2 | — | — | ||
| ∼4.0 | 1 year | 2 | — | — | ||
| ∼2.0 | 3 years | 2 | — | — | ||
| Compressive modulus** (kPa) | ||||||
| Cartilage, normal | 652 ± 354 | 3–4 months | 3 | — | Male | Shimomura et al.73 |
| 457 ± 212 | 12 months | 3 | — | Male | ||
| Cartilage, untreated defect | 174 ± 225 | 3–4 months | 3 | — | Male | |
| ∼250 | 12 months | 3 | — | Male | ||
| Cartilage, treated defect | 875 ± 493 | 3–4 months | 3 | — | Male | |
| 293 ± 268 | 12 months | 3 | — | Male | ||
| Tensile linear stiffness (N/mm) | ||||||
| ACL, normal | 136 ± 3.0 | 6–9 months | 8 | YM | — | Murray et al.85 |
| 150 ± 13.8 | 12–13 months | 8 | YM | — | ||
| 154 ± 27.3 | 36–60 months | 5 | YM | — | ||
| ACL, untreated injury | 51 ± 8.0 | 6–9 months | 8 | YM | — | |
| 43 ± 10.0 | 12–13 months | 8 | YM | — | ||
| 31 ± 8.6 | 36–60 months | 5 | YM | — | ||
| ACL, treated injury | 71 ± 5.9 | 6–9 months | 8 | YM | — | |
| 79 ± 7.5 | 12–13 months | 8 | YM | — | ||
| 47 ± 6.4 | 36–60 months | 5 | YM | — | ||
| Tensile yield load (N) | ||||||
| ACL, normal | 952 ± 36.2 | 6–9 months | 8 | YM | — | |
| 1414 ± 107.9 | 12–13 months | 8 | YM | — | ||
| 1721 ± 103.6 | 36–60 months | 5 | YM | — | ||
| ACL, untreated injury | 237 ± 45.7 | 6–9 months | 8 | YM | — | |
| 185 ± 43.8 | 12–13 months | 8 | YM | — | ||
| 121 ± 35.6 | 36–60 months | 5 | YM | — | ||
| ACL, treated injury | 300 ± 27.4 | 6–9 months | 8 | YM | — | |
| 369 ± 39.1 | 12–13 months | 8 | YM | — | ||
| 220 ± 29.3 | 36–60 months | 5 | YM | — | ||
For tissue property, ACL indicates anterior cruciate ligament; for breed, YM indicates Yucatan minipig; a dash indicates the parameter was not applicable or unspecified; values preceded by “∼” indicate estimates from presented graphs; *at 15% unconfined compressive strain; **at 100 μm/sec strain rate.
Bone
The effect of skeletal growth on bone properties has been studied in the porcine model. Specifically, cancellous and cortical bone samples from mandibular condyles have been compared between young (9 weeks on average) and adult pigs (103 weeks on average).59 Cortical bone stiffness as measured by nanoindentation was ∼40% higher in the adult specimens, although cancellous bone was not significantly different between age groups. Interestingly, the number of lysylpyridinoline collagen crosslinks, which was found to be negatively correlated with stiffness, was not significantly different between age groups for either cancellous or cortical bone. In an effort to develop a novel procedure for imaging collagen fibers in bone, Ambekar et al. used second harmonic generation (SHG) microscopy to image femoral cortical specimens from pigs ranging from youth to adulthood (1, 3.5, 6, or 30 months old) and found that the number of bony structural osteons increases with skeletal growth, as does the relative organization of collagen fibers.60 These changes were also found to be accompanied by an ∼15% decrease in bone tissue porosity from 1- to 30-month-old specimens.
Nanoindentation testing has also been performed on different microstructural regions, namely laminar bone, interstitial bone, and osteons, in hydrated cortical specimens from porcine femurs aged 6, 12, or 42 months.61 Generally, elastic modulus and hardness were found to increase from adolescence to adulthood, although this effect varied by the region tested. For example, in thin lamellae of laminar bone, modulus increased about 20% from 6 to 12 months and about 33% from 12 to 42 months, while in thin lamellae in osteons, modulus only increased ∼13% from 12 to 42 months. Newer microindentation techniques have found growth-related changes in bone biomechanics similar to those found using nanoindentation.62 Additional studies have examined how the failure properties of bone change during growth in pigs aged 1, 3.5, 6, 14.5, 24, or 48 months and suggest an increased risk of fracture with skeletal growth.63 With the body of data rapidly increasing, it is clear that skeletal growth plays a key role in determining the functional properties of porcine bone, although these time-dependent relationships can be complex.
Articular cartilage
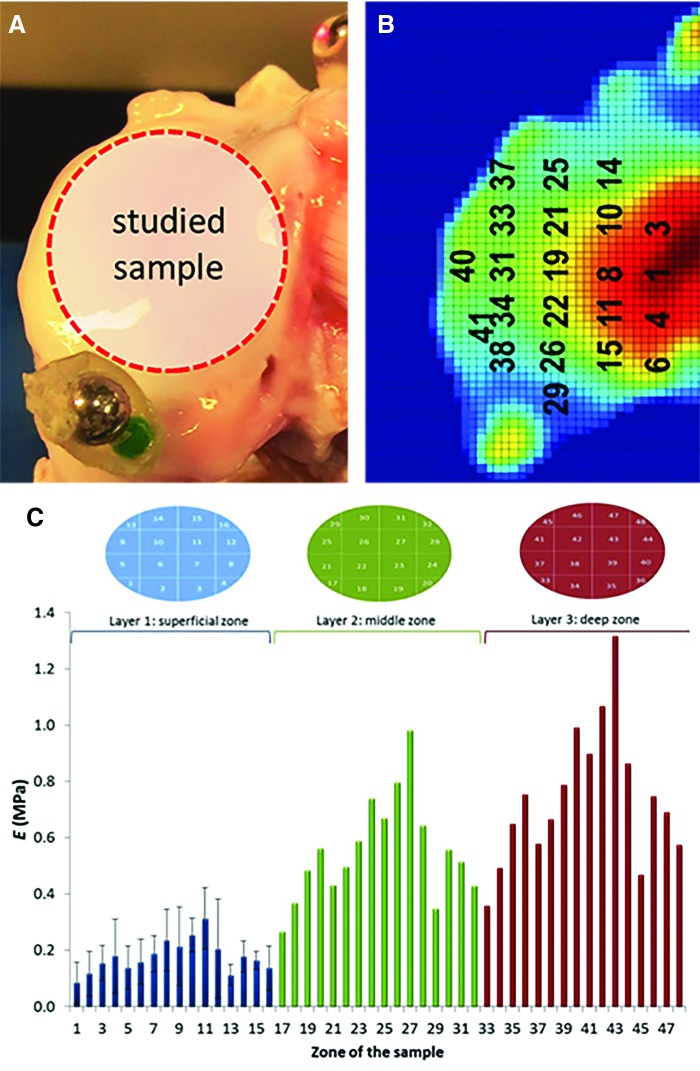
Skeletal growth also influences the biomechanical properties of articular cartilage. Cartilage consists of superficial, middle, and deep layers that vary in their matrix composition and organization.64 Gannon et al. isolated osteochondral cores from pigs aged 4 weeks, 1 year, and 3 years and performed compression testing with or without the superficial region. Confined compressive dynamic modulus was reduced in superficial region-deficient adolescent and adult pigs (1 and 3 years old) by ∼31% and 27% on average, respectively, but was not significantly changed in the young pigs (4 weeks old), which is consistent with the idea that immature cartilage is largely isotropic, with an underdeveloped superficial region.65 Additional work has identified that the shift from a homogeneous to heterogeneous mechanical response throughout the depth of cartilage is likely related to structural changes.66 Relative to young (4 weeks old) pigs, cartilage in adult pigs had a fivefold increase in collagen fiber diameter and an order-of-magnitude increase in the compressive modulus of the deep zone of cartilage. Building on this topic, indentation testing and biochemical analysis of a 16-point grid were used to develop computational models of porcine articular cartilage to show the impact of tissue heterogeneity on tissue swelling behavior67 (Fig. 5). Importantly, swelling was lower in computational models of heterogeneous cartilage. These studies show the ability of the porcine model to provide location-specific data to study heterogeneity within a single tissue.
FIG. 5.
Image of a representative sample (A) and the mathematical model of free cartilage swelling in a region of interest (B). Variations in the mechanical properties (C) of the cartilage were found between the superficial, middle, and deep zones of the tissue (Adapted from Manzano et al.67 with permission under Creative Commons Attribution license). Color images available online at www.liebertpub.com/tec
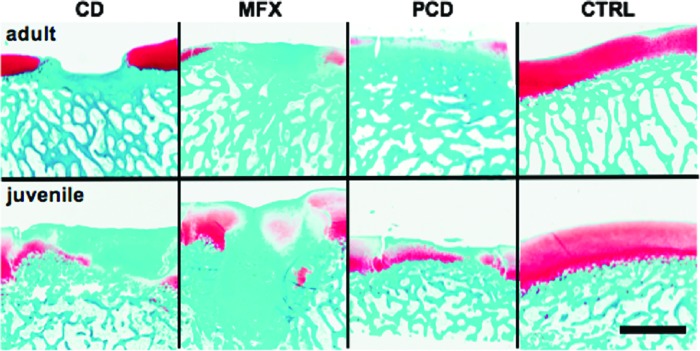
Considering cartilage tissue engineering and the creation of cartilage defects to study injury, one issue is that the cartilage continues to thin during adolescence from 6 to 18 months in the minipig model,68 while the subchondral bone becomes much stiffer and more difficult to penetrate. Given that the vast majority of focal cartilage defects do not involve violation of the subchondral bone in humans, it is useful to create cartilage-only defects in large animal models. Yet in response to the creation of such a defect, skeletally immature pigs show a robust subchondral bone remodeling response,68–71 while this response is less apparent in skeletally mature animals68 (Fig. 6). This remodeling response can also be limited in skeletally immature animals by not violating the calcified cartilage.70 Nevertheless, bone remodeling creates a challenge for tissue engineering studies as the combination of bony remodeling and in vivo loading can cause engineered cartilage constructs to “sink” into the subchondral bone, making an incongruent cartilage surface. The appropriate type of defect (cartilage only or with bone) is also dependent on the type of injury one intends to model. Unlike focal cartilage defects, treatment of osteochondritis dissecans lesions that typically occur in skeletally immature humans involves both the bone and cartilage.72
FIG. 6.
Articular cartilage healing and bony remodeling in late adolescent/adult (18 months old) and early adolescent (6 months old, noted as “juvenile” in images) pigs. Safranin-O/Fast Green staining of representative samples of articular cartilage (red) and bone 6 weeks after injury for CD, MFX, PCD, and CTRL groups (scale bars = 2 mm) (Adapted from Pfeifer et al.,68 with permission from Mary Ann Liebert, Inc.). CD, chondral defects; MFX, microfracture; PCD, partial chondral defect; CTRL, control. Color images available online at www.liebertpub.com/tec
The influence of skeletal growth on the outcomes of engineered tissues has been studied in the porcine model. In one study, Shimomura et al. implanted a tissue-engineered construct derived from synovial mesenchymal stem cells into chondral defects within the femoral condyles of young (3–4 months) and adolescent (12 months) male pigs. Despite the differences in age, there were no significant differences in compressive modulus of repaired tissue between age groups 6 months postoperatively.73 Further work should aim to more clearly elucidate how skeletal growth affects biomechanical properties of cartilage. Challenges for TERM in both skeletally immature and mature animals include integration of constructs with the native cartilage74,75 and the replication of zonal heterogeneity in a single tissue as well as multitissue complexity, such as in osteochondral units.76
Anterior cruciate ligament
The changes in the knee joint during growth have been characterized from birth through skeletal maturity in female Yorkshire pigs using high-field magnetic resonance imaging77 (Fig. 7). General anatomic changes with skeletal growth include a drastic increase in size, a relative reduction in the amount of cartilage, and the closing of the growth plates alongside specific changes in the fibrous soft tissues. For the ACL, there is a marked increase in both the sagittal and coronal angle of the ACL relative to the tibial plateau throughout growth. Interestingly, the sagittal angle continued to increase throughout adolescence, while the orientation changes in the coronal plane plateaued at early adolescence. Previous studies in human growth78 have made similar findings, suggesting that the porcine model may be an appropriate translational model.
FIG. 7.
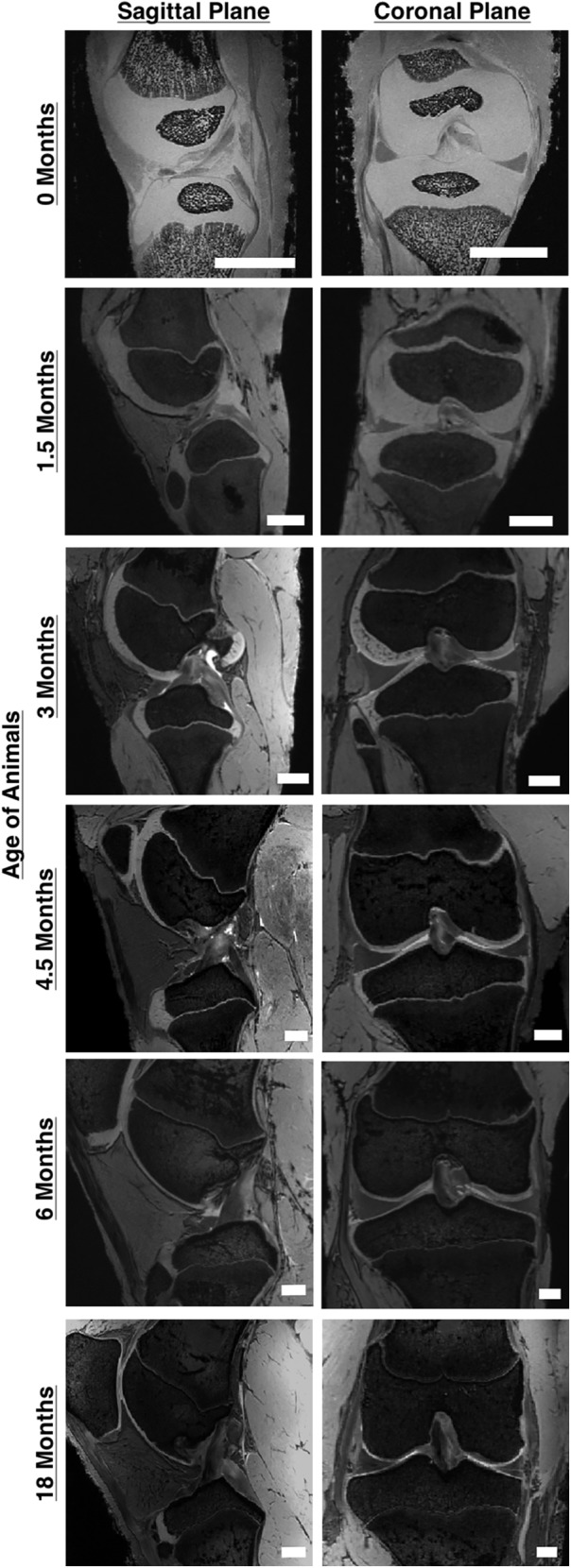
Significant gross anatomical changes occur in the porcine knee between birth and skeletal maturity. Major changes include significant size increases, ossification of the articular cartilage in the epiphyseal region, fusion of the growth plates, and changes in the anatomic orientation of the cruciate ligaments (scale bars = 10 mm) (Adapted from Cone et al.,77 electronically published with permission from John Wiley and Sons, Inc.).
The pig model has been extensively utilized in the development of tissue engineering treatments for the ACL and to evaluate the role of skeletal growth.79–81 One of these approaches, the use of PRP and a collagen scaffold in ACL reconstruction and repair, has been the focus of Murray et al.82–84 A collagen-PRP composite was used to augment a soft tissue graft to reconstruct transected ACLs in early adolescent (6–9 months), adolescent (12–13 months), and adult (36–60 months) Yucatan minipigs. Interestingly, the yield load of the femur-graft-tibia complex was 67% higher in adolescent pigs after 15 weeks of healing compared to adults, while stiffness was 68% and 51% higher in adolescent pigs and juveniles, respectively, compared to adults,85 which may be related to an increased density of cells populating the graft site in younger pigs.86
Other studies have examined the use of the collagen-PRP composite to stimulate healing as an alternative to replacement with a soft tissue graft, with positive results in terms of biomechanical outcomes relative to ACL reconstruction, as well as prevention of cartilage degeneration.83 In vitro studies suggested that the use of PRP increased cell activity and collagen expression, while lowering the rate of apoptosis in cells from immature and adolescent pigs, but had less of an effect on adult pigs.87 This may be due to the decreasing expression level of growth factor receptors in porcine ACL fibroblasts with increasing age.88 As this approach moves toward clinical trials,89 it will be interesting to see if the chondroprotective effects of this regenerative medicine approach hold in human patients. Nevertheless, the influence of skeletal growth on the effectiveness of tissue-engineered strategies is an exciting area for exploration.
Muscle
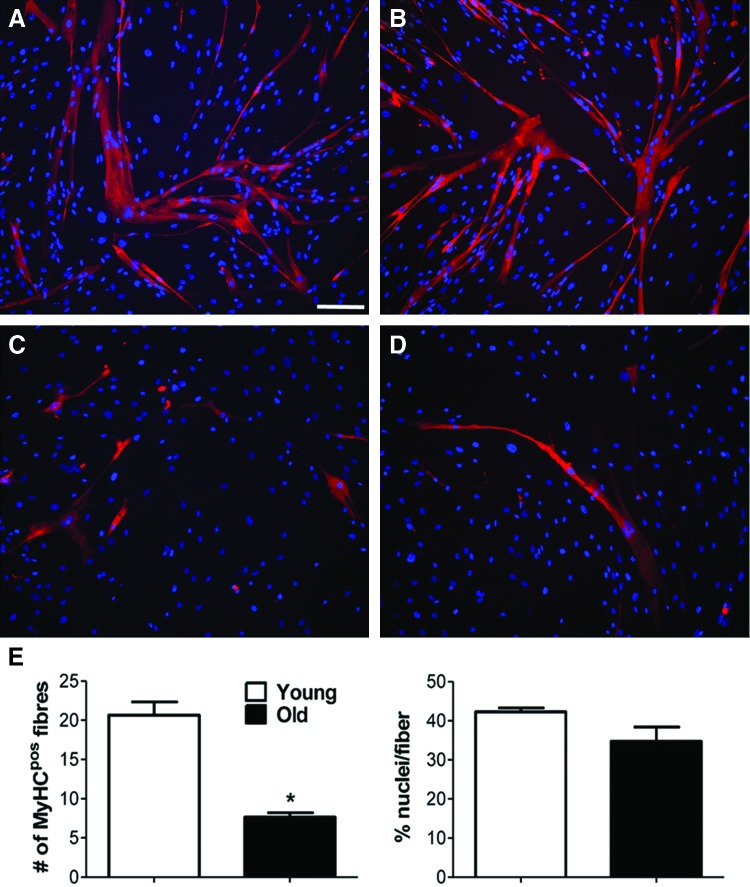
Skeletal muscle has also garnered interest as a target for TERM strategies. Of these approaches, decellularized extracellular matrix scaffolds, such as small intestinal submucosa (SIS), are often obtained from porcine sources. In a rat abdominal wall defect model, histological and immunohistochemical evaluation revealed approximately double the number of slow myosin fibers formed in animals treated with 3-week-old SIS compared to animals treated with 3-month-old SIS, and about five times as many slow myosin fibers formed in animals treated with 3-month-old SIS compared to those treated with 6-month-old SIS.90 Furthermore, collagenous connective tissue formation in both the 3-week- and 3-month-old groups was ∼30% less than in the untreated group and the 6-month-old group.90 Fuoco et al. studied the use of a poly(ethylene glycol) (PEG)-based hydrogel and its ability to enhance muscle tissue regeneration. Muscle pericytes (MP) were isolated from the quadriceps of “piglet” and “boar” specimens (ages not specified) and cultured in vitro (Fig. 8). When combined with human umbilical vein endothelial cells, the ability to form myotubes and capillary-like structures, as determined by myosin heavy chain expression level, in young MP was ∼125% higher compared with adult cells. However, when a cell suspension of adult MP was combined with a UV crosslinkable PEG hydrogel, the difference in myosin heavy chain expression was only ∼9%. It was concluded that the PEG hydrogel promoted skeletal muscle regeneration and vascularization and may mitigate age-related effects of cells.91 Perruchot et al. identified additional differences in cell surface markers expressed by stromal vascular cells isolated from porcine adipose and muscle tissue aged 1, 23, or 57 weeks.92 However, the role of the age of cells on the interaction with biomaterials requires further study.
FIG. 8.
Representative images of immunofluorescent staining using anti-myosin heavy chain antibody with DAPI (nuclei) counterstain for young (A, B) and old (C, D) muscle-derived pericytes; mean number of myosin-positive fibers extracted from images and mean number of myofibers normalized to nuclei (E); * indicates p < 0.05 for comparison between young and old groups; scale bar = 50 μm; (Adapted from Fuoco et al.91 with permission under Creative Commons Attribution license). Color images available online at www.liebertpub.com/tec
TMJ disc
Studies focusing on TMJ discs have also highlighted the importance of skeletal maturity for tissue engineering. Allen et al. isolated fibrochondrocytes from female adolescent porcine TMJ discs (∼8–12 months old) and cultured them for 35 days. It was found that gene expression levels of collagen types I and II decrease 23% and 73% per passage, respectively, which has implications for tissue engineering strategies involving terminally differentiated fibrochondrocytes.93 Expression of TGF-β isoforms in mandibular condylar cartilage also varies with skeletal growth. For example, TGF-β1 level expressed as a percentage of total TGF-β immunoreactivity was two orders of magnitude higher in 24- and 36-month-old animals compared to newborns, while TGF-β3 levels were not significantly different for any age group.94
With regard to TERM strategies for craniomaxillofacial bone,95 polycaprolactone scaffolds were created using selective laser sintering for the filling of defects created in mandibles of young minipigs. As assessed by computed tomography, the young pigs were able to regenerate bone tissue on and in the scaffolds upon observation at both 1 and 3 months, suggesting that this pilot study may have potential as a long-term surgical option. Given that the minipigs were 6–8 months old (early adolescence) at the time of surgery and still growing, this study highlights the ability of engineered tissues to grow with the animal and has particular promise for the treatment of pediatric age groups.
Discussion
In this review, we describe the increased use of the porcine model to study biomechanics and TERM both in vitro and in vivo. A review of the publication history strongly indicates the increased use of the porcine model to study bone, muscle, cartilage, and ligaments, among other tissues. Skeletal growth-related effects have been extensively explored within the pig model, but other biological variables, such as strain differences, have been underexplored. Within these studies, the pig model has allowed advances in the biomechanical understanding of these complex tissues at multiple scales, while providing a platform to test new and innovative TERM approaches.
Every animal model has advantages and disadvantages, and much information can be gained by comparing across models. For specific joints and tissues, the porcine model exhibits homology with humans in terms of anatomy and/or biomechanics, as several interspecies studies have noted. In the case where the porcine model or another animal model could be utilized, increased use of the porcine model may be due to wide availability of live animals and cadaveric specimens through university-controlled herds, commercial farms, and abattoirs. Large litter sizes relative to other large animal models also allow for the steady and, depending on the strain of pig, economical supply of animals. Importantly, the suitability of the porcine model for one tissue/joint cannot be used to justify its use for another tissue/joint without appropriate anatomical and biomechanical comparative studies. Along with the tissues and joints mentioned in this review, many groups have investigated the use of translational animal models for additional tissues and joints, including the shoulder.96 Moreover, if controlled rehabilitation or exercise is important to the research question at hand, the canine or equine models can be utilized. These two species, in particular, are currently treated clinically with regenerative medicine approaches, and thus, provide an additional avenue for translation.
Skeletal growth can have a significant impact on TERM studies and clinical interventions. Any engineered tissue must be capable of changing in size and shape while adapting to an ever-changing biological and biomechanical environment. The skeletally immature pig model provides a unique opportunity to investigate the magnitude and timing of normal changes to the tissues and joints during growth, while testing TERM solutions within a rapidly growing and remodeling environment similar to those found in the pediatric human population. A limitation is the difficulty of assigning age within the porcine model. We have assigned the terms “youth,” “early adolescence,” “late adolescence,” and “adulthood” based on a combination of sexual and skeletal maturity. However, in the literature, a pig could be termed “adolescent” and have an age of anywhere from 6 to 18 months. We have attempted to resolve this discrepancy by using well-defined terms and including the age of the animals when known, and we hope that the rigorous reporting of age will increase as the field moves forward.97
Of note, not all biological variables have been well studied for all musculoskeletal tissues. The limited number of studies examining certain topics, such as comparisons between strains, clearly shows the need for more research in this area. Indeed, several highly specialized strains have been developed for certain functions, such as to gain weight quickly (in the case of Yorkshire pigs) or slowly (in the case of minipigs). Many of these strains exist in closed herds. This poses advantages and disadvantages for translational research. On one hand, the decreased variability in animals within a single herd may allow lower sample sizes with stronger confidence when comparing treatment groups. On the other hand, results may limit reproducibility in other strains or generalization to other species. Thus, it is imperative that basic information on the animals used, including the source and strain of the animals, should be explicitly stated.97 Moreover, there likely exist interactions between variables (age and sex, for example), and thus, multivariate study designs may be required to definitely isolate the effects of each variable alone and in combination.
The porcine model does provide unique potential for the evaluation of tissue engineering in the future. Both a variety of strains and a range of genetic haplotypes within strains are available through commercial vendors. In addition, several closed herds of specialized strains and crossbreeds are maintained through academic research laboratories. These strains have been used to study graft remodeling following ACL reconstruction,98 macular degeneration,99 and intestinal epithelial stem cell-driven regeneration in the porcine intestinal epithelium.100 Of particular note is the ability to create genetically modified pigs. Such animals can be used as genetic tools similar to those in rodent models, but in a large animal model, allowing more relevant physiology and surgical procedures similar to humans. As an example, custom pigs exist that can express green fluorescent protein.101 Immunocompromised and “humanized” animals could be created to tackle issues surrounding allotransplantation or xenotransplantation, and recent work has suggested that genetically modified “designer” pigs could be raised to produce organs with reduced potential for immune rejection for human transplantation.102,103 Xenografts are already utilized for rotator cuff repair and some are advocating their use as an alternative to human autografts and allografts, although acceptable results using these grafts are still unclear.104–108
A limitation of this review is that the literature search for the analysis of publication history was restricted to the year 1990 onward. We acknowledge that seminal work in biomechanics was conducted in large animal models for decades before 1990 (obvious from the number of biomechanics publications already present in 1990). However, our goal was to link biomechanics studies with tissue engineering, a term coined in the late 1980 s by Prof. Y.C. Fung109 and one that did not gain in popularity until the 1990 s.
In summary, the porcine model is becoming increasingly popular as a translational model for musculoskeletal TERM. This valuable model offers some unique opportunities to explore research questions in biomechanics and biology. Furthermore, as the TERM field moves forward, it provides an essential, and in many cases unique, preclinical platform to evaluate new approaches.
Supplementary Material
Acknowledgments
The authors acknowledge funding from the National Institutes of Health (R03 AR068112) and the National Science Foundation (DGE-1252376, CBET-1133427).
Disclosure Statement
No competing financial interests exist.
References
- 1.Liebschner M.A.K. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 25, 1697, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Johnstone B., Alini M., Cucchiarini M., Dodge G.R., Eglin D., Guilak F., Madry H., Mata A., Mauck R.L., Semino C.E., and Stoddart M.J. Tissue engineering for articular cartilage repair—the state of the art. Eur Cell Mater 25, 248, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Reichert J.C., Saifzadeh S., Wullschleger M.E., Epari D.R., Schutz M.A., Duda G.N., Schell H., van Griensven M., Redl H., and Hutmacher D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30, 2149, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Muschler G.F., Raut V.P., Patterson T.E., Wenke J.C., and Hollinger J.O. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev 16, 123, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Weymann A., Patil N.P., Sabashnikov A., Korkmaz S., Li S.L., Soos P., Ishtok R., Chaimow N., Patzold I., Czerny N., Schmack B., Popov A.F., Simon A.R., Karck M., and Szabo G. Perfusion-decellularization of porcine lung and trachea for respiratory bioengineering. Artif Organs 39, 1024, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Schenke-Layland K., Vasilevski O., Opitz F., Konig K., Riemann I., Halbhuber K.J., Wahlers T., and Stock U.A. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol 143, 201, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Angouras D.C., Dosios T.J., Dimitriou C.A., Chamogeorgakis T.P., Rokkas C.K., Manos T.A., and Sokolis D.P. Surgical thoracic sympathectomy induces structural and biomechanical remodeling of the thoracic aorta in a porcine model. J Surg Res 172, 68, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Roberts N., Morticelli L., Jin Z., Ingham E., and Korossis S. Regional biomechanical and histological characterization of the mitral valve apparatus: implications for mitral repair strategies. J Biomech 49, 2491, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Ritchie J., Jimenez J., He Z., Sacks M.S., and Yoganathan A.P. The material properties of the native porcine mitral valve chordae tendineae: an in vitro investigation. J Biomech 39, 1129, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Elkin B.S., Ilankova A., and Morrison B. Dynamic, regional mechanical properties of the porcine brain: indentation in the coronal plane. J Biomech Eng 133, 071009, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Thibault K.L., and Margulies S.S. Age-dependent material properties of the porcine cerebrum: effect on pediatric inertial head injury criteria. J Biomech 31, 1119, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Hasan A., Ragaert K., Swieszkowski W., Selimovic S., Paul A., Camci-Unal G., Mofrad M.R.K., and Khademhosseini A. Biomechanical properties of native and tissue engineered heart valve constructs. J Biomech 47, 1949, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Mavrilas D., and Missirlis Y. An approach to the optimization of preparation of bioprosthetic heart-valves. J Biomech 24, 331, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Murphy M.K., Arzi B., Hu J.C., and Athanasiou K.A. Tensile characterization of porcine temporomandibular joint disc attachments. J Dent Res 92, 753, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Leary S.A., Link J.M., Klineberg E.O., Hu J.C., and Athanasiou K.A. Characterization of facet joint cartilage properties in the human and interspecies comparisons. Acta Biomater 2017. doi.org/10.1016/j.actbio.2017.03.017 [DOI] [PubMed]
- 16.Proffen B.L., McElfresh M., Fleming B.C., and Murray M.M. A comparative anatomical study of the human knee and six animal species. Knee 19, 493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweigart M.A., Zhu C.F., Burt D.M., DeHoll P.D., Agrawal C.M., Clanton T.O., and Athanasiou K.A. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann Biomed Eng 32, 1569, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Xerogeanes J.W., Fox R.J., Takeda Y., Kim H.S., Ishibashi Y., Carlin G.J., and Woo S.L-Y. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Eng 26, 345, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Collins S.L., Layde P., Guse C.E., Schlotthauer A.E., and Van Valin S.E. The incidence and etiology of anterior cruciate ligament injuries in patients under the age of 18 in the state of wisconsin. Pediatr Ther 4, 196, 2014 [Google Scholar]
- 20.Werner B.C., Yang S., Looney A.M., and Gwathmey F.W., Jr Trends in pediatric and adolescent anterior cruciate ligament injury and reconstruction. J Pediatr Orthop 36, 447, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Fisher M.B., and Mauck R.L. Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue Eng Part B Rev 19, 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lysaght M.J., and Crager J. Origins. Tissue Eng Part A 15, 1449, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Athanasiou K.A., Rosenwasser M.P., Buckwalter J.A., Malinin T.I., and Mow V.C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res 9, 330, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Ahern B.J., Parvizi J., Boston R., and Schaer T.P. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage 17, 705, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Frisbie D.D., Cross M.W., and McIlwraith C.W. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthopaed 19, 142, 2006 [PubMed] [Google Scholar]
- 26.Hembry R.M., Dyce J., Driesang I., Hunziker E.B., Fosang A.J., Tyler J.A., and Murphy G. Immunolocalization of matrix metalloproteinases in partial-thickness defects in pig articular cartilage—a preliminary report. J Bone Joint Surg Am Vol 83a, 826, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Chu C.R., Szczodry M., and Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev 16, 105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tantisricharoenkul G., Linde-Rosen M., Araujo P., Zhou J., Smolinski P., and Fu F.H. Anterior cruciate ligament: an anatomical exploration in humans and in a selection of animal species. Knee Surg Sports Traumatol Arthrosc 22, 961, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Zaino N.L., Hedgeland M.J., Ciani M.J., Clark A.M., Kuxhaus L., and Michalek A.J. White-tailed deer as an ex vivo knee model: joint morphometry and acl rupture strength. Ann Biomed Eng 45, 1093, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Proffen B.L., Perrone G.S., Fleming B.C., Sieker J.T., Kramer J., Hawes M.L., and Murray M.M. Effect of low-temperature ethylene oxide and electron beam sterilization on the in vitro and in vivo function of reconstituted extracellular matrix-derived scaffolds. J Biomater Appl 30, 435, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teuschl A., Heimel P., Nurnberger S., van Griensven M., Redl H., and Nau T. A novel silk fiber-based scaffold for regeneration of the anterior cruciate ligament: histological results from a study in sheep. Am J Sports Med 44, 1547, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Mahalingam V.D., Behbahani-Nejad N., Horine S.V., Olsen T.J., Smietana M.J., Wojtys E.M., Wellik D.M., Arruda E.M., and Larkin L.M. Allogeneic versus autologous derived cell sources for use in engineered bone-ligament-bone grafts in sheep anterior cruciate ligament repair. Tissue Eng Part A 21, 1047, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher M.B., Liang R., Jung H.J., Kim K.E., Zamarra G., Almarza A.J., McMahon P.J., and Woo S.L-Y. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc 20, 1357, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boguszewski D.V., Shearn J.T., Wagner C.T., and Butler D.L. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: is the porcine knee acl dependent? J Orthop Res 29, 641, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Atarod M., Frank C.B., and Shrive N.G. Increased meniscal loading after anterior cruciate ligament transection in vivo: a longitudinal study in sheep. Knee 22, 11, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Livesay G.A., Rudy T.W., Woo S.L-Y., Runco T.J., Sakane M., Li G., and Fu F.H. Evaluation of the effect of joint constraints on the in situ force distribution in the anterior cruciate ligament. J Orthop Res 15, 278, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Skelley N.W., Castile R.M., Cannon P.C., Weber C.I., Brophy R.H., and Lake S.P. Regional variation in the mechanical and microstructural properties of the human anterior cruciate ligament. Am J Sports Med 44, 2892, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Takroni T., Laouar L., Adesida A., Elliott J.A., and Jomha N.M. Anatomical study: comparing the human, sheep and pig knee meniscus. J Exp Orthop 3, 35, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupte C.M., Bull A.M., Murray R., and Amis A.A. Comparative anatomy of the meniscofemoral ligament in humans and some domestic mammals. Anat Histol Embryol 36, 47, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Joshi M.D., Suh J.K., Marui T., and Woo S.L-Y. Interspecies variation of compressive biomechanical properties of the meniscus. J Biomed Mater Res 29, 823, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Arnoczky S.P., Cook J.L., Carter T., and Turner A.S. Translational models for studying meniscal repair and replacement: what they can and cannot tell us. Tissue Eng Part B Rev 16, 31, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Mathewson M.A., Kwan A., Eng C.M., Lieber R.L., and Ward S.R. Comparison of rotator cuff muscle architecture between humans and other selected vertebrate species. J Exp Biol 217, 261, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalpakci K.N., Willard V.P., Wong M.E., and Athanasiou K.A. An interspecies comparison of the temporomandibular joint disc. J Dent Res 90, 193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazaeli S., Ghazanfari S., Everts V., Smit T.H., and Koolstra J.H. The contribution of collagen fibers to the mechanical compressive properties of the temporomandibular joint disc. Osteoarthritis Cartilage 24, 1292, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Baxter-Jones A.D., Eisenmann J.C., and Sherar L.B. Controlling for maturation in pediatric exercise science. Pediatr Exerc Sci 17, 18, 2005 [Google Scholar]
- 46.Reiland S. Growth and skeletal development of the pig. Acta Radiol Suppl 358, 15, 1978 [PubMed] [Google Scholar]
- 47.Greulich W.W., and Pyle S.I. Radiographic atlas of skeletal development of the hand and wrist. Am J Med Sci 238, 393, 1959 [Google Scholar]
- 48.Goetz J.E., Fredericks D., Petersen E., Rudert M.J., Baer T., Swanson E., Roberts N., Martin J., and Tochigi Y. A clinically realistic large animal model of intra-articular fracture that progresses to post-traumatic osteoarthritis. Osteoarthritis Cartilage 23, 1797, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Radfar L., and Sirois D.A. Structural and functional injury in minipig salivary glands following fractionated exposure to 70 gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 96, 267, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Jiang C.C., Chiang H., Liao C.J., Lin Y.J., Kuo T.F., Shieh C.S., Huang Y.Y., and Tuan R.S. Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J Orthop Res 25, 1277, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Reisig G., Kreinest M., Richter W., Wagner-Ecker M., Dinter D., Attenberger U., Schneider-Wald B., Fickert S., and Schwarz M.L. Osteoarthritis in the knee joints of gottingen minipigs after resection of the anterior cruciate ligament? Missing correlation of mri, gene and protein expression with histological scoring. PLoS One 11, e0165897, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia W., Liu W., Cui L., Liu Y., Zhong W., Liu D., Wu J., Chua K., and Cao Y. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater 71, 373, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Linkenhoker J.R., Burkholder T.H., Linton C.G., Walden A., Abusakran-Monday K.A., Rosero A.P., and Foltz C.J. Effective and safe anesthesia for yorkshire and yucatan swine with and without cardiovascular injury and intervention. J Am Assoc Lab Anim Sci 49, 344, 2010 [PMC free article] [PubMed] [Google Scholar]
- 54.Eggleston T.A., Roach W.P., Mitchell M.A., Smith K., Oler D., and Johnson T.E. Comparison of two porcine (sus scrofa domestica) skin models for in vivo near-infrared laser exposure. Comp Med 50, 391, 2000 [PubMed] [Google Scholar]
- 55.Germscheid N.M., Thornton G.M., Hart D.A., and Hildebrand K.A. A biomechanical assessment to evaluate breed differences in normal porcine medial collateral ligaments. J Biomech 44, 725, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Gotterbarm T., Breusch S.J., Schneider U., and Jung M. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim 42, 71, 2008 [DOI] [PubMed] [Google Scholar]
- 57.McKnight L.L., Myrie S.B., Mackay D.S., Brunton J.A., and Bertolo R.F. Glucose tolerance is affected by visceral adiposity and sex, but not birth weight, in yucatan miniature pigs. Appl Physiol Nutr Metab 37, 106, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Kiapour A.M., Fleming B.C., Proffen B.L., and Murray M.M. Sex influences the biomechanical outcomes of anterior cruciate ligament reconstruction in a preclinical large animal model. Am J Sports Med 43, 1623, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willems N.M., Mulder L., Bank R.A., Grunheid T., den Toonder J.M., Zentner A., and Langenbach G.E. Determination of the relationship between collagen cross-links and the bone-tissue stiffness in the porcine mandibular condyle. J Biomech 44, 1132, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Ambekar R., Chittenden M., Jasiuk I., and Toussaint K.C., Jr Quantitative second-harmonic generation microscopy for imaging porcine cortical bone: comparison to sem and its potential to investigate age-related changes. Bone 50, 643, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Feng L., Chittenden M., Schirer J., Dickinson M., and Jasiuk I. Mechanical properties of porcine femoral cortical bone measured by nanoindentation. J Biomech 45, 1775, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Rasoulian R., Raeisi Najafi A., Chittenden M., and Jasiuk I. Reference point indentation study of age-related changes in porcine femoral cortical bone. J Biomech 46, 1689, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Baro J., Shyu P., Pang S., Jasiuk I.M., Vives E., Salje E.K., and Planes A. Avalanche criticality during compression of porcine cortical bone of different ages. Phys Rev E 93, 053001, 2016 [DOI] [PubMed] [Google Scholar]
- 64.Mow V.C., andHuiskes R., eds. Basic Orthopaedic Biomechanics and Mechano-Biology. Philadelphia: Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 65.Gannon A.R., Nagel T., Bell A.P., Avery N.C., and Kelly D.J. The changing role of the superficial region in determining the dynamic compressive properties of articular cartilage during postnatal development. Osteoarthritis Cartilage 23, 975, 2015 [DOI] [PubMed] [Google Scholar]
- 66.Gannon A.R., Nagel T., Bell A.P., Avery N.C., and Kelly D.J. Postnatal changes to the mechanical properties of articular cartilage are driven by the evolution of its collagen network. Eur Cell Mater 29, 105; discussion 121–103, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Manzano S., Armengol M., Price A.J., Hulley P.A., Gill H.S., Doblare M., and Hamdy Doweidar M. Inhomogeneous response of articular cartilage: a three-dimensional multiphasic heterogeneous study. PLoS One 11, e0157967, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfeifer C., Fisher M.B., Saxena V., Kim M., Henning E.A., Steinberg D.R., Dodge G.R., and Mauck R.L. Age-dependent subchondral bone remodeling and cartilage repair in a minipig defect model. Tissue Eng Part C 21, 850, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher M.B., Belkin N.S., Milby A.H., Henning E.A., Soegaard N., Kim M., Pfeifer C., Saxena V., Dodge G.R., Burdick J.A., Schaer T.P., Steinberg D.R., and Mauck R.L. Effects of mesenchymal stem cell and growth factor delivery on cartilage repair in a mini-pig model. Cartilage 7, 174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fisher M.B., Belkin N.S., Milby A.H., Henning E.A., Bostrom M., Kim M., Pfeifer C., Meloni G., Dodge G.R., Burdick J.A., Schaer T.P., Steinberg D.R., and Mauck R.L. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: implications for tissue engineering. Tissue Eng Part A 21, 850, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim I.L., Pfeifer C.G., Fisher M.B., Saxena V., Meloni G.R., Kwon M.Y., Kim M., Steinberg D.R., Mauck R.L., and Burdick J.A. Fibrous scaffolds with varied fiber chemistry and growth factor delivery promote repair in a porcine cartilage defect model. Tissue Eng Part A 21, 2680, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pfeifer C.G., Kinsella S.D., Milby A.H., Fisher M.B., Belkin N.S., Mauck R.L., and Carey J.L. Development of a large animal model of osteochondritis dissecans of the knee: a pilot study. Orthop J Sports Med 3, 2325967115570019, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimomura K., Ando W., Tateishi K., Nansai R., Fujie H., Hart D.A., Kohda H., Kita K., Kanamoto T., Mae T., Nakata K., Shino K., Yoshikawa H., and Nakamura N. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials 31, 8004, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Fisher M.B., Henning E.A., Soegaard N.B., Dodge G.R., Steinberg D.R., and Mauck R.L. Maximizing cartilage formation and integration via a trajectory-based tissue engineering approach. Biomaterials 35, 2140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujie H., Nansai R., Ando W., Shimomura K., Moriguchi Y., Hart D.A., and Nakamura N. Zone-specific integrated cartilage repair using a scaffold-free tissue engineered construct derived from allogenic synovial mesenchymal stem cells: biomechanical and histological assessments. J Biomech 48, 4101, 2015 [DOI] [PubMed] [Google Scholar]
- 76.Mellor L., Huebner P., Cai S., Mohiti-Asli M., Taylor M., Spang J.T., Shirwaiker R.A., and Loboa E.G. Fabrication and evaluation of electrospun, 3d bioplotted, and combination electrospun/3d bioplotted scaffolds for tissue engineering applications. BioMed Res Int 2017, 6956794, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cone S.G., Simpson S.G., Piedrahita J.A., Fordham L.A., Spang J.T., and Fisher M.B. Orientation changes in the cruciate ligamnets of the knee during skeletal growth: a porcine model. J Orthop Res 2017. [Epub ahead of print]; DOI: 10.1002/jor.23594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim H.K., Laor T., Shire N.J., Bean J.A., and Dardzinski B.J. Anterior and posterior cruciate ligaments at different patient ages: MR imaging findings. Radiology 247, 826, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Fan H.B., Liu H.F., Toh S.L., and Goh J.C.H. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials 30, 4967, 2009 [DOI] [PubMed] [Google Scholar]
- 80.Magarian E.M., Fleming B.C., Harrison S.L., Mastrangelo A.N., Badger G.J., and Murray M.M. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sport Med 38, 2528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X., He J.K., Bian W.G., Li Z., Li D.C., and Snedeker J.G. A novel silk-tcp-peek construct for anterior cruciate ligament reconstruction: an off-the shelf alternative to a bone-tendon-bone autograft. Biofabrication 6, 015010, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Fleming B.C., Spindler K.P., Palmer M.P., Magarian E.M., and Murray M.M. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med 37, 1554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray M.M., and Fleming B.C. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med 41, 1762, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Proffen B.L., Sieker J.T., and Murray M.M. Bio-enhanced repair of the anterior cruciate ligament. Arthroscopy 31, 990, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murray M.M., Magarian E.M., Harrison S.L., Mastrangelo A.N., Zurakowski D., and Fleming B.C. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am 92, 2039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mastrangelo A.N., Haus B.M., Vavken P., Palmer M.P., Machan J.T., and Murray M.M. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res 28, 1100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng M., Johnson V.M., and Murray M.M. Effects of age and platelet-rich plasma on acl cell viability and collagen gene expression. J Orthop Res 30, 79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vavken P., Saad F.A., and Murray M.M. Age dependence of expression of growth factor receptors in porcine acl fibroblasts. J Orthop Res 28, 1107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murray M.M., Flutie B.M., Kalish L.A., Ecklund K., Fleming B.C., Proffen B.L., and Micheli L.J. The bridge-enhanced anterior cruciate ligament repair (bear) procedure: an early feasibility cohort study. Orthop J Sports Med 4, 2325967116672176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sicari B.M., Johnson S.A., Siu B.F., Crapo P.M., Daly K.A., Jiang H., Medberry C.J., Tottey S., Turner N.J., and Badylak S.F. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 33, 5524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuoco C., Sangalli E., Vono R., Testa S., Sacchetti B., Latronico M.V., Bernardini S., Madeddu P., Cesareni G., Seliktar D., Rizzi R., Bearzi C., Cannata S.M., Spinetti G., and Gargioli C. 3d hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering. Front Physiol 5, 203, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perruchot M.H., Lefaucheur L., Barreau C., Casteilla L., and Louveau I. Age-related changes in the features of porcine adult stem cells isolated from adipose tissue and skeletal muscle. Am J Physiol Cell Physiol 305, C728, 2013 [DOI] [PubMed] [Google Scholar]
- 93.Allen K.D., and Athanasiou K.A. Effect of passage and topography on gene expression of temporomandibular joint disc cells. Tissue Eng 13, 101, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Moroco J.R., Hinton R., Buschang P., Milam S.B., and Iacopino A.M. Type ii collagen and tgf-betas in developing and aging porcine mandibular condylar cartilage: immunohistochemical studies. Cell Tissue Res 289, 119, 1997 [DOI] [PubMed] [Google Scholar]
- 95.Smith M.H., Flanagan C.L., Kemppainen J.M., Sack J.A., Chung H., Das S., Hollister S.J., and Feinberg S.E. Computed tomography-based tissue-engineered scaffolds in craniomaxillofacial surgery. Int J Med Robot 3, 207, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Soslowsky L.J., Carpenter J.E., DeBano C.M., Banerji I., and Moalli M.R. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 5, 383, 1996 [DOI] [PubMed] [Google Scholar]
- 97.Pfeifer C.G., Fisher M.B., Carey J.L., and Mauck R.L. Impact of guidance documents on translational large animal studies of cartilage repair. Sci Transl Med 7, 310re319, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeuchi H., Niki Y., Matsunari H., Umeyama K., Nagashima H., Enomoto H., Toyama Y., Matsumoto M., and Nakamura M. Temporal changes in cellular repopulation and collagen fibril remodeling and regeneration after allograft anterior cruciate ligament reconstruction: an experimental study using kusabira-orange transgenic pigs. Am J Sports Med 44, 2375, 2016 [DOI] [PubMed] [Google Scholar]
- 99.Sommer J.R., Estrada J.L., Collins E.B., Bedell M., Alexander C.A., Yang Z., Hughes G., Mir B., Gilger B.C., Grob S., Wei X., Piedrahita J.A., Shaw P.X., Petters R.M., and Zhang K. Production of elovl4 transgenic pigs: a large animal model for stargardt-like macular degeneration. Br J Ophthalmol 95, 1749, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Gonzalez L.M., Williamson I., Piedrahita J.A., Blikslager A.T., and Magness S.T. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One 8, e66465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sper R.B., Koh S., Zhang X., Simpson S., Collins B., Sommer J., Petters R.M., Caballero I., Platt J.L., and Piedrahita J.A. Generation of a stable transgenic swine model expressing a porcine histone 2b-egfp fusion protein for cell tracking and chromosome dynamics studies. Plos One 12, e0169242, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang L., Guell M., Niu D., George H., Lesha E., Grishin D., Aach J., Shrock E., Xu W., Poci J., Cortazio R., Wilkinson R.A., Fishman J.A., and Church G. Genome-wide inactivation of porcine endogenous retroviruses (pervs). Science 350, 1101, 2015 [DOI] [PubMed] [Google Scholar]
- 103.Phelps C.J., Koike C., Vaught T.D., Boone J., Wells K.D., Chen S.H., Ball S., Specht S.M., Polejaeva I.A., Monahan J.A., Jobst P.M., Sharma S.B., Lamborn A.E., Garst A.S., Moore M., Demetris A.J., Rudert W.A., Bottino R., Bertera S., Trucco M., Starzl T.E., Dai Y., and Ayares D.L. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 299, 411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ono Y., Dávalos Herrera D.A., Woodmass J.M., Boorman R.S., Thornton G.M., and Lo I.K.Y. Healing rate and clinical outcomes of xenografts appear to be inferior when compared to other graft material in rotator cuff repair: a meta-analysis. J ISAKOS Joint Disord Orthopa Sports Med 1, 321, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stone K.R., Abdel-Motal U.M., Walgenbach A.W., Turek T.J., and Galili U. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation 83, 211, 2007 [DOI] [PubMed] [Google Scholar]
- 106.Gupta A.K., Hug K., Boggess B., Gavigan M., and Toth A.P. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sport Med 41, 872, 2013 [DOI] [PubMed] [Google Scholar]
- 107.Shea K.P., Obopilwe E., Sperling J.W., and Iannotti J.P. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elb Surg 21, 1072, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Sclamberg S.G., Tibone J.E., Itamura J.M., and Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elb Surg 13, 538, 2004 [DOI] [PubMed] [Google Scholar]
- 109.Skalak R., and Fox C.F., Eds. Tissue Engineering: Proceedings of a Workshop Held at Granlibakken. Lake Tahoe, CA: Alan R. Liss, 1988 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.