Abstract
Background
The link between PM2.5 exposure and adverse health outcomes is well documented from studies across the world. However, the reported effect estimates vary across studies, locations and constituents. We aimed to conduct a meta-analysis on associations between short-term exposure to PM2.5 constituents and mortality using city-specific estimates, and explore factors that may explain some of the observed heterogeneity.
Methods
We systematically reviewed epidemiological studies on particle constituents and mortality using PubMed and Web of Science databases up to July 2015. We included studies that examined the association between short-term exposure to PM2.5 constituents and all-cause, cardiovascular, and respiratory mortality, in the general adult population. Each study was summarized based on pre-specified study key parameters (e.g., location, time period, population, diagnostic classification standard), and we evaluated the risk of bias using the Office of Health Assessment and Translation (OHAT) Method for each included study. We extracted city-specific mortality risk estimates for each constituent and cause of mortality. For multi-city studies, we requested the city-specific risk estimates from the authors unless reported in the article. We performed random effects meta-analyses using city-specific estimates, and examined whether the effects vary across regions and city characteristics (PM2.5 concentration levels, air temperature, elevation, vegetation, size of elderly population, population density, and baseline mortality) can explain the observed heterogeneity.
Results
We found a 0.89% (95% CI: 0.68, 1.10%) increase in all-cause, a 0.80% (95% CI: 0.41, 1.20%) increase in cardiovascular, and a 1.10% (95% CI: 0.59, 1.62%) increase in respiratory mortality per 10 µg/m3 increase in PM2.5. Accounting for the downward bias induced by studies of single days, the all-cause mortality estimate increased to 1.01% (95% CI: 0.81, 1.20%). We found significant associations between mortality and several PM2.5 constituents. The most consistent and stronger associations were observed for elemental carbon (EC) and potassium (K). For most of the constituents, we observed high variability of effect estimates across cities.
Conclusions
Our meta-analysis suggests that (a) combustion elements such as EC and K have a stronger association with mortality, (b) single lag studies underestimate effects, and (c) estimates of PM2.5 and constituents differ across regions. Accounting for PM mass in constituent’s health models may lead to more stable and comparable effect estimates across different studies.
Systematic review registration
PROSPERO: CRD42017055765
Keywords: Particulate matter constituents, fine particulate matter (PM2.5), mortality, time series, acute effects, meta-analysis
1 Introduction
Ambient air pollution, one of the leading causes of mortality and disability worldwide, was associated with approximately 3.7 million premature deaths (6.7% of all deaths) in 2012 (Lim et al., 2012, WHO, 2014). Air pollution is usually described in terms of the criteria air pollutants: particulate matter (PM), ozone (O3), sulfur dioxide (SO2), nitrogen oxides (NOx), carbon monoxide (CO), benzene, and lead (Pb). Of these, PM affects more people than any other pollutant (Brook et al., 2010).
Air quality standards and regulatory guidelines for inhalable PM (PM10, PM with aerodynamic diameter ≤ 10 µm) and fine PM (PM2.5, PM with aerodynamic diameter ≤ 2.5 µm) have been established by health and regulatory authorities across the world. Air quality standards are usually set mostly based on epidemiological studies, and to a lesser extent on toxicological studies, examining the effects of PM mass on human health (McClellan, 2002). PM2.5 can reach deep into the lungs, and the associations between PM2.5 and cardiovascular and respiratory mortality and morbidity are well documented (WHO, 2013).
However, PM2.5 is a complex mixture of several constituents with different physicochemical properties and toxicity, the proportion of which over the total particle mass varies by source and season (Son et al. 2012; Valdés et al., 2012; Dai et al., 2014; Basagaña et al., 2015). For example, Elemental (or Black) and Organic Carbon (EC/BC, OC), are emitted from traffic (EC) and combustion sources (EC,OC), vegetation (OC), and atmospheric photochemical reactions (OC); and have been previously associated with short-term cardiovascular (CVD) and respiratory diseases (Delfino et al., 2010; Janssen et al., 2012; Kim et al., 2012). Other combustion sources such as biomass burning (potassium, K, as the main trace element) have been associated with CVD and respiratory admissions, as well as CVD mortality (Mar et al., 2006; Andersen et al., 2007; Sarnat et al., 2008). Oil combustion particles, particularly vanadium (V) and nickel (Ni), have been associated with CVD and respiratory hospital admissions (Andersen et al., 2007; Zanobetti et al., 2009; Kioumourtzoglou et al., 2014a). Nitrate (NO3−) and sulfate (SO42−) are secondary ions formed from the oxidation of nitrogen oxides and sulfur gases emitted during fossil and coal combustion and biogenic activities. Epidemiological evidence has also implicated exposure to NO3− and SO42− in increased CVD (Zanobetti et al., 2009; Ito et al., 2011; Kioumourtzoglou et al., 2014a) and respiratory (Atkinson et al., 2010; Kim et al., 2012; Son et al., 2012) hospital admissions.
The underlying biological mechanism by which PM2.5 constituents and sources are associated with cardiorespiratory health effects has been proposed by several studies. For example, transition metals (e.g., V) enhance inflammation and oxidative stress (Brook et al. 2010) and can be mobilized by SO42− (Ghio et al., 1999); BC and SO42− have been associated with changes in vascular (O’ Neill et al., 2005) and lung function (Lepeule et al., 2014); BC has also been associated with decreased DNA methylation which leads to oxidative stress and CVD (Baccarelli et al., 2009); and wood smoke with systemic oxidative stress, coagulation, inflammation and lipid peroxidation (Barregard et al., 2006).
Identifying the PM2.5 constituents that are the most harmful to human health can help regulatory authorities, researchers, and physicians to reduce or prevent exposure to those constituents and sources. Yet, there is substantial inconsistency in the observed health effect estimates between epidemiological studies, and it is still not clear which constituent(s) are associated with the highest risks to human health (Cassee et al., 2013; Wyzga and Rohr, 2015). Atkinson et al. (2015) performed a meta-analysis on the adverse health effects of PM2.5 constituents based on epidemiological time-series studies conducted up to 2013. The strongest association was found for EC but the number of existing studies was insufficient to perform a meta-analysis for metals.
Between 2013 and 2015, a large number of studies on the health effects of short-term exposure to PM2.5 constituents, covering a broad spectrum of elements and geographic locations, have been published. We performed an extended meta-analysis of studies on short-term exposure to PM2.5 constituents and mortality using city-specific estimates, and explored factors that may explain some of the potentially observed heterogeneity. We systemically reviewed observational epidemiological studies regarding PM composition and mortality, and used the city-specific effect estimates to explore the variability of the effect estimates across locations.
2 Methods
Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017055765. A complete PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (Moher et al., 2009) can be found in the supplementary material.
2.1 Studies selection
We conducted a systematic search for studies on particle constituents and mortality using PubMed and Web of Science databases up to July 31, 2015. We also searched for additional studies using the ‘similar articles’ tool in PubMed, and the reference lists of the eligible studies. Since BC has been described by several terms in past studies, we conducted a separate search for BC to include all possible terms. For this reason, we used two separate keyword sets: (“particulate” OR “particles” OR “PM”) AND (“metals” OR “sulfates” OR “sulfate” OR “nitrate” OR “nitrates” OR “ammonium” OR “carbon” OR “elements” OR “constituents” OR “species”) AND “mortality”; and (“black carbon” OR “black smoke” OR “light reflectance” OR “blackness” OR “light absorption” OR “soot”) AND “mortality.” Synonyms of PM, constituents, and mortality were included using Medical Subject Headings (MeSH) terms. Following the PRISMA guidelines, article titles and abstracts were first reviewed independently by two of the authors (SA, SIP) to include epidemiological studies on particle constituents and mortality. The final inclusion of studies was based on full text evaluation. In case of disagreement, a third researcher (JS) resolved any discrepancies. Studies were considered eligible, if: i) they examined and reported a risk estimate for the association between exposure to PM2.5 constituent and mortality in the general adult population, and ii) they were published in a peer-reviewed journal.
2.2 Data extraction
For each study, the two independent reviewers (SA, SIP) extracted information on location, time period, sample size, population, diagnosis standard (mortality International Classification of Diseases, ICD, code), study design (e.g., time-series), study characteristics, particle constituents examined, lag pattern used, and health model covariates into Microsoft Word. We then entered into a Microsoft Excel sheet the city-specific regression coefficients and their standard errors (reported in the study, or calculated from reported relative risk or percent change in mortality and their 95% confidence intervals) for each constituent and cause of mortality for the meta-analysis. For multi-city studies, we requested the city-specific regression coefficients and standard errors from the authors unless they were reported in the article. The extracted data was independently reviewed by a third investigator (MAK) for quality assurance/quality control.
Based on previous studies, we used the lag with the strongest association for each mortality cause: the previous day (lag 1) for all-cause and respiratory mortality and same day (lag 0) for cardiovascular mortality (Peng et al., 2005; Son et al., 2012; Krall et al., 2013). While higher associations of air pollution and respiratory mortality had been found for longer exposure windows than one day before death (Zanobetti et al., 2003; Grass and Cane, 2008), most of the studies examined lag 1 day. Therefore, in the meta-analysis, we included the studies with lag 1 or 0–1 average for all-cause and respiratory mortality, and lag 0 or 0–1 average for cardiovascular mortality. Studies with distributed lag models 0–3, 0–5, and 0–6 were also included.
2.3 Risk of bias assessment
To our knowledge, there is no established tool for risk of bias assessment for time series and case-crossover studies. Therefore, we assessed the risk of bias within each study based on the Office of Health Assessment and Translation (OHAT) tool by the National Institutes of Environmental Health Sciences-National Toxicology Program (NIEHS-NTP), and the Navigation Guide by the University of California, San Francisco (OHAT, 2015; Lam et al., 2016). Both of these tools assess the risk of bias of individual studies based on several risk of bias domains (e.g., selection bias, confounding, measurement, missing data, reporting) in a similar way. Each domain is evaluated as “low”, “probably low”, “probably high”, “high”, or “not applicable” risk according to specific criteria. We assessed our studies for selection bias, confounding, exposure assessment, outcome assessment, incomplete outcome data, selective reporting, and conflict of interest based on pre-specified criteria (Table A.1).
Based on OHAT guidelines, it is recommended to remove studies for which the key elements (for observational human studies: exposure assessment, outcome assessment, and confounding) and most of the other criteria are characterized as ‘high’ or ‘probably high’ risk.
2.4 Data analysis
Our analysis focused on PM2.5 mass and its constituents: SO42−, NO3−, ammonium (NH4+), EC, black smoke (BS), OC, sodium (Na), magnesium (Mg), aluminum (Al), silicon (Si), chlorine (Cl), K, calcium (Ca), titanium (Ti), V, manganese (Mn), iron (Fe), Ni, Cu, and Zn, and their association with all-cause non-accidental, cardiovascular, and respiratory mortality for the entire population (all ages). For black carbon, we used all three measurement methods, referred to as EC (thermal optical transmittance, and reflectance) and BS (optical method). We did not convert BS to EC because their relation depends on geographic location and season (Janssen et al., 2011). In addition, we used both PM2.5 and PM10 SO42− since most SO42− is present mostly in the fine fraction (Masri et al., 2015). Also, since most sulfur (S) is in the SO42− form in fine particles (Masri et al., 2015; Achilleos et al., 2016), we converted S effect estimates to SO42− estimates by dividing the regression coefficients and SEs by their molar mass ratio (SO42−/S). Similarly, organic carbon matter (OCM) coefficients were converted to OC coefficients (OC = OCM/1.4) for comparability with the rest of the studies (Krall et al., 2013).
We first estimated the pooled effect estimates for total PM2.5 mass. We applied a random-effects meta-analysis, using the inverse of the effect estimates variance (within plus the between-studies/cities variance) as weights, to estimate the association between PM2.5 and cause-specific mortality (Berkey et al., 1998). The same approach was used for constituents of PM2.5, for which we had at least three coefficient estimates. We performed a separate analysis for the population ≥65 years of age. It should be noted that the PM2.5 meta-analysis includes only studies that also reported constituent-specific effect estimates and we did not conduct an exhaustive literature review on papers only assessing the association between PM and mortality.
One of the main objectives of the study was to estimate pooled constituent-specific effect estimates from city-specific estimates. Most studies we included did not account for potential confounding by the total PM mass in any way. However, since recent studies have shown that the association between a PM2.5 constituent and a health outcome could indeed be confounded by total mass, some more recent studies accounted for this in the health models (Mostofsky et al., 2012). The studies we included in the meta-analyses did so by including an interaction term between the proportion of the constituent concentration to total PM2.5 mass and PM2.5 mass in the health model. In doing so, they estimated whether the increased contribution of that specific constituent to the total mass modified the association between the average PM and mortality. Since these are two separate ways to assess constituent-specific associations, we ran separate meta-analyses for these two types of effect estimates. For studies that did not account for total PM2.5 mass, we simply pooled the constituent specific effect estimates. For the studies that used an interaction between the proportion of the component to total PM2.5 mass concentrations and PM2.5 mass, we pooled the interaction coefficients. These correspond to the additive effect estimate to the average PM2.5 effect estimate (i.e. the main effect of PM2.5 in the model) if all of the PM2.5 mass were the studied constituent.
We also tested for inter-city heterogeneity in the reported effect estimates, and we provided the p-values of the I2-based Cochran Q test and the I2 metric of inconsistency (Der Simonian and Laird, 2015). We considered I2 >50% to represent substantial heterogeneity (Higgins et al., 2003). We screened for publication bias using funnel plot analysis with standard error as the measure of study size and Egger’s regression test of asymmetry (Egger et al., 1997; Sterne and Egger, 2001), and adjusted for publication bias following the “trim and fill” method if needed (Duval and Tweedie, 2000). These statistical methods were applied for the PM constituents with more than 10 estimates as suggested by Sterne et al. (2011).
To explore factors that explain the potentially observed heterogeneity across the city-specific estimates, and whether they modify the association between constituents and mortality, we ran meta-regression models including (a) an indicator variable for lag pattern to examine the difference between single and average lag days, and (b) an indicator variable for region to examine regional differences, including only the regions with data from more than one city. US cities were further classified according to NOAA US climate regions (http://www.ncdc.noaa.gov/monitoring-references/maps/us-climate-regions.php): Northwest, West, Southwest, West North Central, East North Central, Central, South, Northeast, and Southeast. We combined West North Central and East North Central US regions because data were available for only one city in the West North Central region (Omaha, NE); we referred to the two combined regions as the North Central US.
We also examined city characteristics (PM2.5 concentration levels, air temperature, elevation, vegetation, the size of the elderly population, population density, and baseline mortality) that were found to influence mortality in previous epidemiological studies (Zanobetti et al., 2012; Burtscher, 2014; Shi et al., 2015), to explain some of the observed heterogeneity by including each city-specific variable separately in the meta-regression model (Table B.1). Since some of these variables are correlated, we also conducted a factor analysis using a non-orthogonal rotation method and regressed the city-specific effect estimates on the identified factors in the meta-regression.
Meta-regression analyses were performed only when a significant association and substantial heterogeneity (I2>50%) were found and when more than five city effect estimates were available.
Statistical significance was assessed at the α = 0.05 level, unless otherwise reported. For our statistical analyses, we used the “meta”, “rmeta” and “mvmeta” packages in R Statistical Software, version 3.2.1 (The R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Studies and cities included
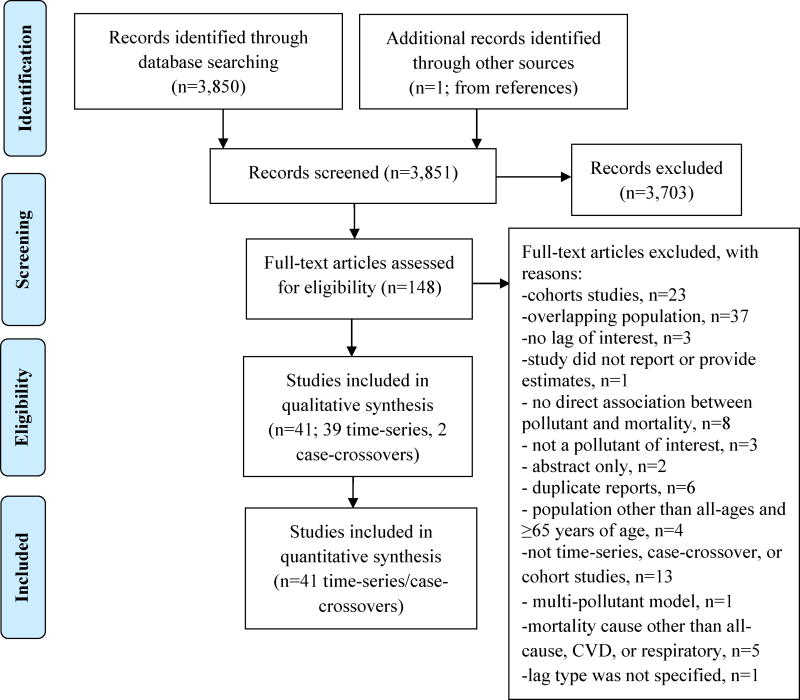
A total of 3,850 peer-reviewed articles were identified from our search, and one additional study through references screening. Of the 3,851 articles, 837 studies were identified from BC/BS search. The number of included studies was reduced to 148 after title and abstract screening (Fig. 1). The association between chronic exposure to PM constituents and mortality is not extensively studied, and therefore we did not identify many original cohort studies since most of them were reanalyzing previous published data. Hence, we combined the time-series and case-crossover design studies to examine the association between short-term exposure to PM2.5 constituent and all-cause, cardiovascular, and respiratory mortality. Studies were screened for overlapping population and final inclusion was based on the most recent publication date and largest number of deaths; 37 studies were excluded for overlapping population. We identified 41 studies (142 cities) that met inclusion criteria and were included in the meta-analysis; 37 studies were used for all-ages analysis and nine for the subgroup analysis of the population ≥65 years of age (Table 1, Table C.1).
Figure 1.
PRISMA flow diagram (modified from Moher et al. 2009).
Table 1.
Studies included in the meta-analysis.
| # | Study | Cities | Additional comments | |
|---|---|---|---|---|
| All ages | ≥65 years old | |||
| 1 | Basagaña et al. 2015 | 4EU | ||
| 2 | Kim et al. 2015 | 1 USA | ||
| 3 | Li et al. 2015 | 1 WP | ||
| 4 | Ostro et al. 2015 | 2 EU | 2 EU | |
| 5 | Wilson et al. 2015 | 1 USA | ||
| 6 | Dai et al. 2014 | 75 USA | ||
| 7 | Heo et al. 2014 | 1 WP | ||
| 8 | Geng et al. 2013 | 1 WP | 1 WP | |
| 9 | Krall et al. 2013 | 72 USA | ||
| 10 | Huang et al. 2012 | 1 WP | ||
| 11 | Sacks et al. 2012 | 1 USA | ||
| 12 | Son et al. 2012 | 1 WP | ||
| 13 | Valdés et al. 2012 | 1 SA | ||
| 14 | Fischer et al. 2011 | 1 EU | Country of Netherlands | |
| 15 | Klemm et al. 2011 | 1 USA | ||
| 16 | Atkinson et al. 2010 | 1 EU | ||
| 17 | Cakmak et al. 2009 | 1 SA | ||
| 18 | Fischer et al. 2009 | 1 EU | Country of Netherlands | |
| 19 | Carder et al. 2008 | 3 EU | ||
| 20 | Brook et al. 2007 | 10 CA | City-specific estimates were not provided | |
| 21 | Stankovic et al. 2007 | 1 EU | 1 EU | |
| 22 | Analitis et al. 2006 | 11 EU | ||
| 23 | Burnett et al. 2004 | 12 CA | City-specific estimates were not provided | |
| 24 | Filleul et al. 2004 | 1 EU | 1 EU | |
| 25 | Aga et al. 2003 | 14 EU | ||
| 26 | Fairley et al. 2003 | 1 USA | ||
| 27 | Villeneuve et al. 2003 | 1 CA | ||
| 28 | Ballester et al. 2002 | 6 EU | ||
| 29 | Le Tertre et al. 2002 | 3 EU | ||
| 30 | Anderson et al. 2001 | 1 EU | ||
| 31 | Goldberg et al. 2001a | 1 CA | Not included in the meta-analysis with the studies specific estimates because of duplicate population with Burnett et al. 2004 | |
| 32 | Goldberg et al. 2001b | 1 CA | 1 CA | |
| 33 | Katsouyanni et al. 2001 | 11 EU | ||
| 34 | Samoli et al. 2001 | 2 EU | ||
| 35 | Burnett et al. 2000 | 8 CA | City-specific estimates were not provided | |
| 36 | Gwynn et al. 2000 | 1USA | ||
| 37 | Hoek et al. 2000 | 1 EU | Country of Netherlands | |
| 38 | Klemm et al. 2000 | 6 USA | ||
| 39 | Lippmann et al. 2000 | 1 USA | ||
| 40 | Anderson et al. 1996 | 1 EU | ||
| 41 | Ballester et al. 1996 | 1 EU | ||
EU, European Union region; CA, Canada; SA, South America; USA, United States of America; WP, West Pacific
Eighteen studies were conducted in Europe, ten in the USA, five in West Pacific, six in Canada, and two in South America. However, we had more cities from USA than any other region. From the 41 selected studies (14 multi-city and 27 single-city studies), we were able to obtain the city-specific estimates from 11 multi-city studies. Therefore, the main analysis included 129 city-specific estimates from 38 studies. We repeated the analysis using study-specific estimates (single city estimates and the pooled estimate from multicity studies) in order to include the remaining three multicity studies. These three studies examined the association between PM2.5 constituents and all-cause mortality and they refer to the same population.
Two of our all-ages studies (76 cities) examined the association between PM2.5 constituents and mortality by including an interaction between the proportion of constituent to PM2.5 mass concentrations and total PM2.5 mass (Valdés et al., 2012; Dai et al., 2014). One study (4 cities) reported the association of non-adjusted and PM-adjusted effect estimates using the constituent residual method (Basagaña et al., 2015). For the latter, we used only the un-adjusted estimates because it was the only study applying the residual method.
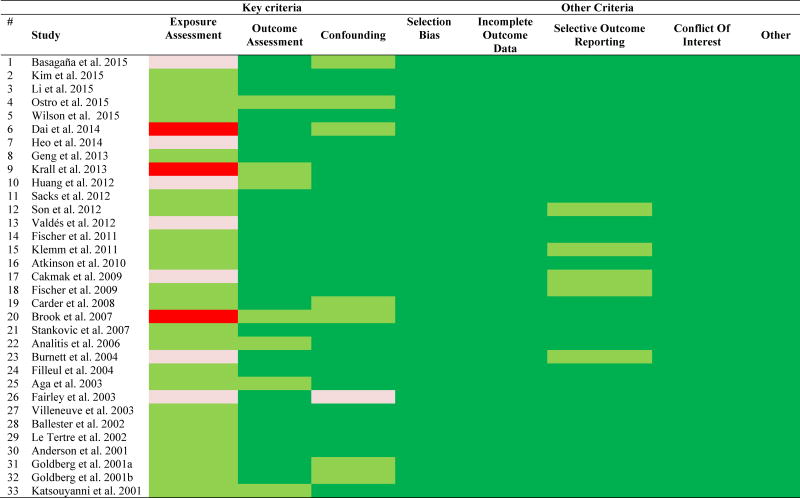
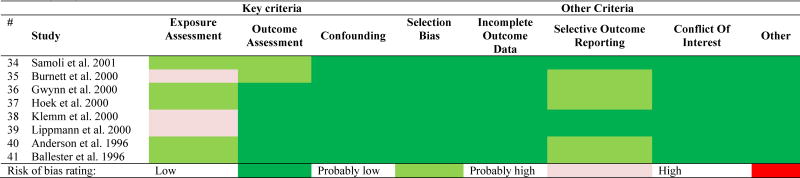
3.2 Risk of bias assessment
The risk of bias ratings for the individual studies are shown in Table 2 and more analytically in Appendix D. Most of the studies were rated with ‘low risk’ in most domains, except for exposure assessment which was rated mostly as ‘probably low’ risk and in some cases it reached to ‘high’ risk. In these types of studies design, we always have some risk of exposure misclassification because it is very difficult to assess the true average population exposure that gives attenuated effect estimates (Dominici et al., 2000; Zeger et al., 2000). The risk is higher for more spatially heterogeneous pollutants, e.g. BC which is a traffic tracer with local sources, versus SO42− that is more homogeneous in space (Sarnat et al., 2010).
Table 2.
Risk of bias rating for each study.
None of our studies had a ‘high’ or ‘probably high’ risk rating in all of the key elements (exposure assessment, outcome assessment, and confounding) and therefore no studies were excluded from the analyses.
3.3 Pooled effect estimates
For the meta-analysis, we used mortality city-specific effect estimates for PM2.5 mass and constituents, derived from time-series and case-crossover studies.
3.3.1 All-cause mortality
We found a 0.89% (95% CI: 0.68, 1.10%; number of cities, ncities=114) increase in all-cause mortality per 10 µg/m3 increase in PM2.5, and a significant heterogeneity in the PM2.5 effect estimates across the cities (Table E.1).
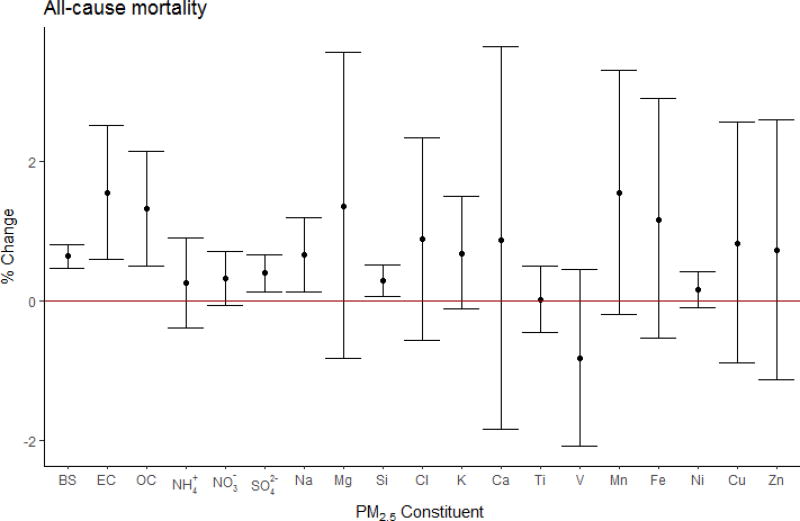
The pooled mortality effect estimates of each constituent, expressed as percent change per inter-quantile range (IQR; the average of IQRs across studies) of each constituent, are presented in Figure 2.
Figure 2.
All-cause mortality pooled effect estimates using city-specific estimates, expressed in percent change in mortality per IQR* increase in PM2.5 constituent with 95 % confidence intervals. (*BS:10, EC:2.6, OC:6.1, NH4+:4.7, NO3−:5.0, SO42−:5.1, Na:0.48, Mg:0.17, Si:0.21, Cl: 1.1, K:0.53, Ca:0.17, Ti:0.017, V:0.007, Mn:0.009, Fe:0.15, Ni:0.005, Cu:0.014, Zn:0.054) µg/m3).
We observed significantly positive associations between all-cause mortality and BS (pooled effect estimate, βpooled : 6.00×10−4; 95% CI: 4.04×10−4, 7.96×10−4), EC (βpooled : 6.00×10−3; 95% CI: 2.28 ×10−3, 9.72×10−3), OC (βpooled : 2.10×10−3; 95% CI: 0.73×10−3, 3.47×10−3), SO42− (βpooled : 8.00×10−4; 95% CI: 4.08×10−4, 1.19×10−3), Na (βpooled : 1.36×10−2; 95% CI: 0.28×10−2, 2.44×10−2), and Si (βpooled : 1.42×10−2; 95% CI: 0.32×10−2, 2.52×10−2). The results also suggested an association between all-cause mortality and NO3− (βpooled : 7.00×10−4; 95% CI: −8.40×10−5, 1.48×10−3), K (βpooled : 1.31×10−2; 95% CI: −2.19×10−3, 2.84×10−2), and Mn (βpooled : 1.70; 95% CI: −0.21, 3.62). We observed significant heterogeneity (I2 >50%) for BS (I2=60%), OC (I2=60%), Ca (I2=86%), Mn (I2=91%), Fe (I2=90%), Cu (I2=90%), and Zn (I2=93%) effect estimates across cities. In addition, we observed positive associations with EC (βpooled : 1.03×10−2; 95% CI: 1.08×10−4, 2.05×10−2), K (βpooled : 7.22×10−2; 95% CI: 2.07×10−2, 1.24×10−1), and Cu (βpooled : 0.77; 95% CI: 0.19, 1.35) in the meta-analysis from the adjusted models (number of studies, nstudies=1, ncities=75).
In addition, we performed the meta-analysis for EC, BS, and SO42− mortality effect estimates among the elderly population (≥65 years of age). EC (2.35%; 95% CI: 1.05, 3.66 % increase per 2.6 µg/m3; nstudies =3, ncities=4) and BS (0.74%; 95% CI: 0.46, 1.02% increase per 10 µg/m3; nstudies=2, ncities=15) were statistically significantly associated with all-cause mortality. None of the studies examining associations among the elderly adjusted for PM2.5 mass.
3.3.2 Cardiovascular mortality
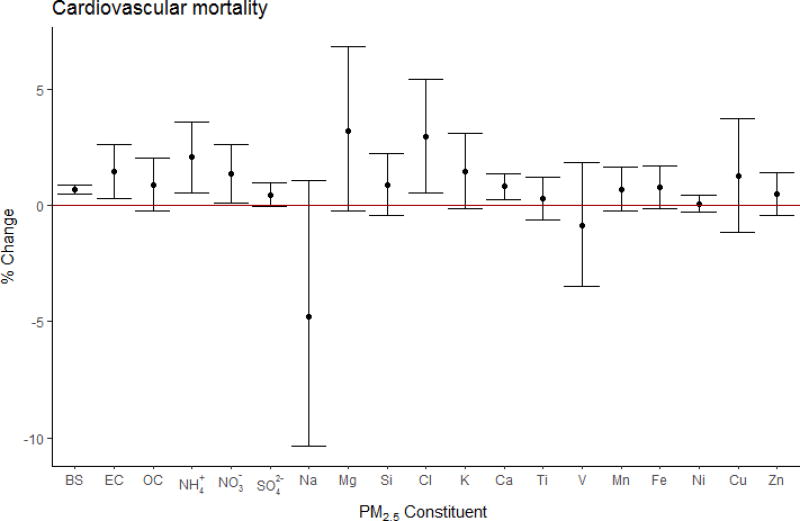
We found a 0.80% (95% CI: 0.41, 1.20%; ncities=89) increase in cardiovascular mortality per 10 µg/m3 increase in PM2.5, and a significant heterogeneity in the PM2.5 effect estimates across the cities (Table E.2).
The observed pooled associations between PM constituents and cardiovascular mortality were not as consistent as all-cause mortality. Positive associations were observed with BS (βpooled : 7.00×10−4; 95% CI: 5.04×10−4, 8.96×10−4), EC (βpooled : 5.70×10−3; 95% CI: 1.19×10−3, 1.02×10−2), NH4+ (βpooled : 4.40×10−3; 95% CI: 1.26×10−3, 7.54×10−3), NO3− (βpooled : 1.50×10−3; 95% CI: 0.32×10−3, 2.68×10−3), Cl (βpooled : 2.64×10−2; 95% CI: 0.48×10−3, 4.80×10−2), and Ca (βpooled : 4.77×10−2; 95% CI: 1.48×10−2, 8.06×10−2); with some evidence for SO42− (βpooled : 9.00×10−4; 95% CI: −8.00×10−5, 1.88×10−3), Fe (βpooled : 5.17×10−2; 95% CI: −8.47×10−3, 1.12×10−1), K (βpooled : 2.77×10−2; 95% CI: −3.07×10−3, 5.85×10−2), and Mg (βpooled : 0.19; 95% CI: −0.02, 0.40) (Fig. 3). We did not observe any significant heterogeneity of the estimates across cities (Table D.2). No significant associations were found for the PM2.5 adjusted effect estimates, except for V (βpooled: 2.60; 95% CI: 0.27, 4.93; nstudies=1, ncities=75).
Figure 3.
Cardiovascular mortality pooled effect estimates using city-specific estimates, expressed in percent change in mortality per IQR* increase in PM2.5 constituent with 95 % confidence intervals. (*BS:10, EC:2.6, OC:6.1, NH4+:4.7, NO3−:5.0, SO42−:5.1, Na:0.48, Mg:0.17, Si:0.21, Cl: 1.1, K:0.53, Ca:0.17, Ti:0.017, V:0.007, Mn:0.009, Fe:0.15, Ni:0.005, Cu:0.014, Zn:0.054) µg/m3).
3.3.3 Respiratory mortality
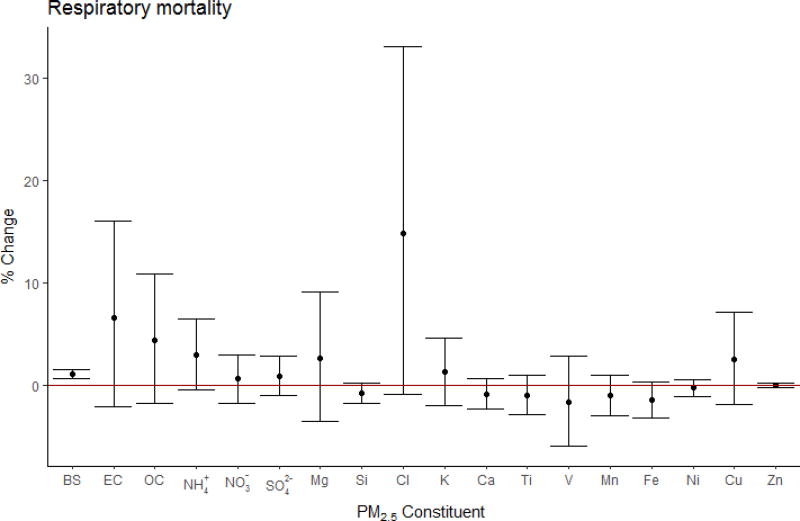
We found a 1.10% (95% CI: 0.59, 1.62%; ncities=86) increase in respiratory mortality per 10 µg/m3 increase in PM2.5, and no significant heterogeneity in the PM2.5 effect estimates across the cities (Table E.3). Among PM components, we found positive associations between respiratory mortality and BS only (βpooled : 1.10×10−3; 95% CI: 0.60×10−3, 1.50×10−3) (Fig. 4). High heterogeneity was detected for EC, OC, and Cl across cities (I2 >50%). We observed positive associations with the PM2.5-adjusted V (βpooled : 5.15; 95% CI: 0.42, 9.89; nstudies=1, ncities=75) and Zn (βpooled : 1.00×10−3; 95% CI: 2.00×10−5, 1.98×10−3; nstudies=2, ncities=76), with some evidence for SO42− (βpooled : 6.10×10−3; 95% CI: −4.48×10−3, 1.67×10−2; nstudies=2, ncities=76), as well.
Figure 4.
Respiratory mortality pooled effect estimates using city-specific estimates, expressed in percent change in mortality per IQR* increase in PM2.5 constituent with 95 % confidence intervals. (* BS: 10, EC: 2.6, OC: 6.1, NH4+: 4.7, NO3−: 5.0, SO42−: 5.1, Na: 0.48, Mg: 0.17, Si: 0.21, Cl: 1.1, K: 0.53, Ca: 0.17, Ti: 0.017, V: 0.007, Mn: 0.009, Fe: 0.15, Ni: 0.005, Cu: 0.014, Zn: 0.054 µg/m3).
3.3.4 Additional analyses
The meta-analysis was repeated using study-specific estimates to include the three multi-city studies for which we did not obtain the city-specific estimates, and results are included in the supplementary material (Tables E.1–E.3, Fig. F.1–F.4). We were able to assess for publication bias for PM2.5 (all-cause, cardiovascular, respiratory) and SO42− (all-cause, cardiovascular) effect estimates. Even if there was some asymmetry in the funnel plots (e.g., PM2.5 and all-cause), Egger’s test showed no statistically significant asymmetry in the plots and therefore no further adjustments for publication bias were made (Fig. G.1–G.5).
The results of the study-specific analysis were in good agreement with the city-specific analysis.
3.4 Meta-regression analyses
We found significant heterogeneity in PM2.5 effect estimates and in many unadjusted constituent estimates. In contrast, the heterogeneity among the adjusted effect estimates was low (I2<25%) for all constituents and mortality causes (see Tables E.1–E.3). Meta-regression analyses were performed only when a significant association and substantial heterogeneity (I2>50%) were found and when more than five city effect estimates were available.
3.4.1 Exposure window
First, we explored whether the effect estimates for the all-age population varied by the exposure window examined, namely, a single-day exposure versus a two-day average exposure. However, we were not able to assess the impact of exposure duration in the associations between constituents and mortality, since most studies used a single day exposure window and thus, no constituent satisfied our criteria for meta-regression except PM2.5 mass. The all-cause mortality effect estimate of PM2.5 for a single day exposure (0.50%; 95% CI: 0.06, 0.94%; ncities=29; lag1) was statistically significantly lower than the two-day average exposure (1.01%; 95% CI: 0.77, 1.26%; nc =85; lag0–1). Similar effects, but not statistically significant, were observed for cardiovascular and respiratory mortality.
3.4.2 Regional differences
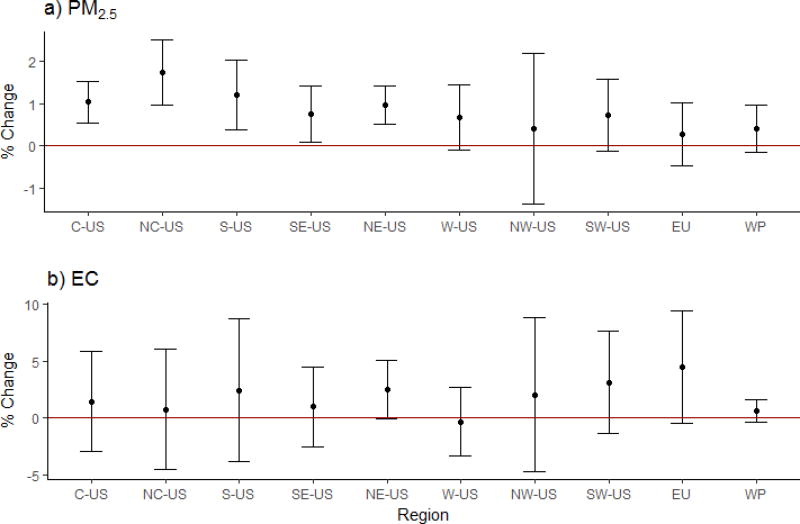
We also explored whether the associations between short-term exposure to PM2.5 and EC, and all-cause mortality vary across the regions (Fig. 5). Among the constituents, only EC satisfied the criteria for inclusion in the meta-regression. Our analysis included PM2.5 and PM-unadjusted EC city-specific effect estimates from the US (ncities-PM2.5=101, ncities-EC=72), Europe (ncities-PM2.5=6, ncities-EC=4), and West Pacific (ncities-PM2.5=5, ncities-EC=4). Table H.1 presents the cities we included in each region. The highest effect estimates on all-cause mortality were observed in North Central US (PM2.5: 1.74%; 95% CI: 0.97, 2.50% per 10 µg/m3), and Europe (EC: 4.45%; 95% CI: −0.44, 9.42% per 2.6 µg/m3). Regional differences explained most of the variability in the PM2.5 (difference in the I2 before and after adding the regions, ΔI2=28%), and EC (ΔI2=84%) effect estimates. The remaining heterogeneity in PM2.5 (I2=23%) was still statistically significant (Q=130.7, p=0.03) after inclusion of the regions.
Figure 5.
All-cause mortality combined effect estimates, expressed in percent change in mortality per 10 µg/m3 increase in PM2.5 (a), and 2.6 µg/m3 increase in EC (b). C-US indicates Central US; NC-US, North Central US; S-US, Southern US; SE-US, Southeastern US; NE-US, Northeastern US; W-US, Western US; NW-US, Northwestern US; SW-US, Southwestern US; EU, Europe; WP, West Pacific.
3.4.3 City characteristics
We also observed modification of the association between PM2.5 mass, and its constituents, and mortality by several variables. In the case of PM2.5 and all-cause mortality, we controlled for the exposure window (single day versus two-day average exposure) in the meta-regression models to avoid bias caused by the lag pattern. We found higher PM2.5 effect estimates in cities with lower summer temperatures (ΔI2=16%), and with some evidence for higher elevation (ΔI2=1%, p-value of elevation = 0.065). No effect modification was observed for cardiovascular mortality. BS showed stronger association with all-cause mortality in cities with lower elevation (ΔI2=9%), vegetation (ΔI2=3%), and temperature difference (ΔI2=9%). Stronger associations were also found in cities with low vegetation (ΔI2=70%) for EC, and higher altitudes (ΔI2=91%) for EC and OC (ΔI2=60%).
We identified five factors that explained 82% of the variance of the city-specific variables: PM2.5 concentration, winter temperature, temperature difference, vegetation (annual and summer), elevation, size of elderly population, population density and baseline mortality. Factor 1 was described by high vegetation and low elevation; factor 2 by high elevation and temperature difference, and low winter temperature; factor 3 by high mortality rate and elderly population; factor 4 by population density; and factor 5 by high vegetation and low PM2.5 levels. However, these factors did not explain the observed variability across the cities.
We repeated the meta-regression analyses using only the US cities effect estimates on all-cause mortality. The city-specific variables and their factors did not explain the observed variability.
4 Discussion
Our systematic review identified studies that examined the association between short term exposure to PM2.5 constituents and mortality in the Americas, Europe, and Western Pacific. SO42− has been the most studied PM constituent, followed by EC/BC. BS has been studied only in Europe since 1990s.
In this meta-analysis, we derived the pooled effect estimates for PM2.5 and for each of the constituents on mortality using city specific effect estimates. PM2.5 had a stronger association with respiratory mortality, than with all-cause and cardiovascular mortality. We also found that studies using a single day of PM2.5 as the exposure variable under-estimated the effect of PM mass on mortality compared to studies using a two-day average, which could be useful for future studies and risk assessments.
We found significantly positive associations for several PM2.5 constituents: EC, BS, OC, SO42−, Na, and Si with all-cause mortality with some evidence for NO3−, Mn, and K; BS, EC, NH4+, NO3−, Cl, and Ca, with cardiovascular mortality, with some evidence for K; and BS with respiratory mortality. The association between mortality and EC and BS was stronger among the elderly. Restricting to studies that controlled for PM mass, we found associations with EC, K, and Cu (for all-cause deaths); V (for cardiovascular and respiratory deaths); and Zn (for respiratory deaths).
Our analysis based on PM2.5 unadjusted estimates suggests that Na and Si have the highest effect (coefficient) on all-cause mortality, and Cl on cardiovascular mortality. However, we are not convinced that these elements have the highest toxicity among all PM2.5 constituents for several reasons. First, we found high heterogeneity in the effect sizes in the unadjusted meta-analyses, but much less heterogeneity in the meta-analysis of the PM2.5 adjusted city coefficients. This indicates that failure to control for PM2.5 mass is contributing to substantial variability in the estimates, which may obscure detecting which components truly have the highest toxicity. Moreover, the effects from the PM2.5-unadjusted models may be confounded by total PM2.5 and other constituents that co-vary, since some constituents are present in high proportion or highly correlated with the total mass. In this case, the health effects of the constituent(s) may be due to the total PM2.5 mass or other constituents that are emitted from the same sources (Mostofsky et al., 2012). Controlling for PM2.5 mass, EC, K, and Cu were associated with all-cause mortality, and V with cardiovascular and respiratory mortality. The consistency of the EC and K associations in the two analyses are more convincing of a true effect.
Second, the exposure measurement error of each constituent is different and potentially larger than that of the total PM2.5. In time-series studies, larger exposure measurement error is expected to result in attenuated effect estimates (Zeger et al., 2000). Measurement error can be caused by measurement/analytical errors, indoor-outdoor relationships which are affected by weather, and source spatial heterogeneity (Koutrakis et al., 2005; Sarnat et al., 2010; Bell et al., 2011). For example, traffic emissions exhibit large spatial variability, whereas this is not the case for regional pollutants like SO42− (Sarnat et al., 2010). Moreover, larger contribution of locally-generated particles has been shown to result in significantly increased measurement error due to spatial heterogeneity in the PM2.5 mass concentrations (Kioumourtzoglou et al., 2014b). BC, even though it is a heterogeneous pollutant, it appears to be an important predictor of health effects suggesting that it is either directly toxic or acts as a surrogate of harmful traffic emissions.
The included studies varied in several study design characteristics, which could bias and modify the estimates, such as: i) method of chemical analysis (e.g., some constituents can be measured with ion chromatography, inductively coupled plasma mass spectrometry, or X-ray fluorescence; EC with reflectance or thermal optical method) since the form of the constituent studied (elemental or ion) can give different results (Cao et al., 2012), ii) number and type of constituents examined, iii) sampling frequency; most of the studies used daily PM2.5 mass data but PM2.5 constituent’s measurements were available in a more reduced frequency (e.g., every third or sixth day) which could introduce error in the estimated effects (Klemm et al., 2011), iv) number and type (e.g., background, urban) of sites per city, v) specifications of the health regression model (e.g., degrees of freedom (df) used per year for time variable, adjusting or not for influenza and holidays, lag and df used to control for weather variables), and vi) mortality cause; most of the studies examined the association with all-cause mortality but very few studies examined other causes of death (e.g., Chronic Obstructive Pulmonary Disease, ischemic heart disease).
We observed large variability among mortality effect estimates across cities and studies. The coefficients of PM2.5 constituents were too few to explore the impact of study-specific characteristics and whether these modify the association between constituent and mortality, except in the case of the exposure window (single versus average lag exposure). Regional differences explained most of the observed variability. The regional differences in the effect estimates are probably due to spatial variation in PM2.5 composition and sources, and individual or community characteristics such as income, education, smoking, prevalence of air conditioning use, and other potential effect modifiers that vary across locations (Bell et al., 2011; Dai et al., 2014; Kioumourtzoglou et al., 2016). However, we were not able to explain much of the variability in the effect estimates with city-specific variables (PM2.5 concentration, temperature, elevation, vegetation, population density, baseline mortality, elderly population size). Even though some city characteristics explained some of the observed variability, their effect did not remain statistically significant when non-US cities were removed from the analysis. These include summer temperature that showed a negative effect, elevation a positive effect, and vegetation that resulted in a negative and positive effect. Among these, elevation had the most consistent positive effect and agrees with Burtscher (2014) findings.
To our knowledge, this is the first meta-analysis on PM2.5 constituents (ions, metals, elements) and mortality. One other strength of this analysis is the use of city-specific estimates that gave the ability to: i) exclude duplicate populations without excluding studies, since some multi-city studies were overlapping; and ii) explore whether city characteristics can modify the effect estimates. Our studies were selected based on a priori protocol and the analysis included studies that examined the same lag exposure to ensure comparability. However, the exposure window was selected based on the strongest association between mortality and PM2.5, but not with PM2.5 constituents since there is no sufficient evidence per constituent about this.
5 Conclusions
In our meta-analysis, we found high variability among the individual time-series studies and their conclusions on which constituent(s) has (have) the highest association with mortality. This makes it difficult to conclude which component per se has the highest toxicity, but from both estimates (PM2.5 - adjusted and unadjusted), EC and K, traffic and wood combustion elements, had a stronger association with mortality than other constituents.
The observed variability across constituent’s effect estimates remains a key question. For this reason, further research is needed to improve air pollution health models. For example, accounting for PM mass in constituent’s health models may lead to more stable and comparable effect estimates across different studies. Future studies can also examine the effect of other PM constituents and properties (e.g., oxidative stress) and whether these may explain some of the observed variability across the effect estimates.
Supplementary Material
Highlights.
A meta-analysis of acute effects of PM2.5 constituents on mortality was conducted.
EC and K had the strongest and most consistent association with mortality.
Single lag studies underestimate effects.
Mortality effects of PM2.5 and constituents differ across regions.
Acknowledgments
This study was supported by the Harvard Cyprus Program and the Cyprus International Initiative for Environmental and Public Health in association with Harvard T.H. Chan School of Public Health. In addition, this publication was made possible by U.S. EPA grant numbers RD83479801 and RD83587201, and NIH T32 grant ES007069. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA. Further, U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication. The authors will also like to thank Dr. X. Basagaña, Dr. J.R. Krall, Ms. L. Dai, Prof. K. Katsouyanni, Dr. E. Samoli, Dr. F. Ballester, Prof. R. Agius, Dr. M. Carder, Dr. H. Kan, Dr. A. Zanobetti, and Dr. A. Valdes for providing us the city-specific mortality effect estimates of their studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achilleos S, Wolfson JM, Ferguson ST, Kang CM, Hadjimitsis DG, Hadjicharalambous M, et al. Spatial variability of fine and coarse particle composition and sources in Cyprus. Atmos Res. 2016;169:255–270. [Google Scholar]
- Aga E, Samoli E, Touloumi G, Anderson HR, Cadum E, Forsberg B, et al. Short-term effects of ambient particles on mortality in the elderly: results from 28 cities in the APHEA2 project. Eur Respir J Suppl. 2003;40:28s–33s. doi: 10.1183/09031936.03.00402803. [DOI] [PubMed] [Google Scholar]
- Analitis A, Katsouyanni K, Dimakopoulou K, Samoli E, Nikoloulopoulos AK, Petasakis Y, et al. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology. 2006;17:230–233. doi: 10.1097/01.ede.0000199439.57655.6b. [DOI] [PubMed] [Google Scholar]
- Andersen ZJ, Wahlin P, Raaschou-Nielsen O, Scheike T, Lof S. Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J Expo Sci Environ Epidemiol. 2007;17:625–636. doi: 10.1038/sj.jes.7500546. [DOI] [PubMed] [Google Scholar]
- Anderson HR, Ponce de Leon A, Bland JM, Bower JS, Strachan DP. Air pollution and daily mortality in London: 1987–92. BMJ. 1996;312:665–669. doi: 10.1136/bmj.312.7032.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HR, Bremner SA, Atkinson RW, Harrison RM, Walters S. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom: associations with fine and coarse particles, black smoke and sulphate. Occup Environ Med. 2001;58:504–510. doi: 10.1136/oem.58.8.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RW, Fuller GW, Anderson HR, Harrison RM, Armstrong B. Urban ambient particle metrics and health: a time-series analysis. Epidemiology. 2010;21:501–511. doi: 10.1097/EDE.0b013e3181debc88. [DOI] [PubMed] [Google Scholar]
- Atkinson RW, Mills IC, Walton HA. Fine particle constituents and health - A systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J Expos Sci Environ Epidemiol. 2015;25:208–214. doi: 10.1038/jes.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179:572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester F, Corella D, Pérez-Hoyos S, Hervás A. Air pollution and mortality in Valencia, Spain: a study using the APHEA methodology. J Epidemiol Community Health. 1996;50:527–533. doi: 10.1136/jech.50.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester F, Sáez M, Pérez-Hoyos S, Iñíguez C, Gandarillas A, Tobías A, et al. The EMECAM project: a multicentre study on air pollution and mortality in Spain: combined results for particulates and for sulfur dioxide. Occup Environ Med. 2002;59:300–308. doi: 10.1136/oem.59.5.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3.Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Barregard L, Sällsten G, Gustafson P, Andersson L, Johansson L, Basu S, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol. 2006;18:845–853. doi: 10.1080/08958370600685798. [DOI] [PubMed] [Google Scholar]
- Basagaña X, Jacquemin B, Karanasiou A, Ostro B, Querol X, Agis D, et al. MED-PARTICLES Study group. Short-term effects of particulate matter constituents on daily hospitalizations and mortality in five South-European cities: results from the MED-PARTICLES project. Environ Int. 2015;75:151–158. doi: 10.1016/j.envint.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. J Expo Sci Environ Epidemiol. 2011;21:372–384. doi: 10.1038/jes.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey CS, Hoaglin DC, Antczak-Bouckoms A, Mosteller F, Colditz GA. Meta-analysis of multiple outcomes by regression with random effects. Stat Med. 1998;17:2537–2550. doi: 10.1002/(sici)1097-0258(19981130)17:22<2537::aid-sim953>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bilotta GS, Milner AM, Boyd IL. Quality assessment tools for evidence from environmental science. Environmental Evidence. 2014;3(14):1–14. [Google Scholar]
- Brook JR, Burnett RT, Dann TF, Cakmak S, Goldberg MS, Fan X, Wheeler AJ. Further interpretation of the acute effect of nitrogen dioxide observed in Canadian time-series studies. J Expo Sci Environ Epidemiol. 2007;17(Suppl 2):S36–44. doi: 10.1038/sj.jes.7500626. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Brook J, Dann T, Delocla C, Philips O, Cakmak S, et al. Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal Toxicol. 2000;12(Suppl 4):15–39. doi: 10.1080/08958370050164851. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Stieb D, Brook JR, Cakmak S, Dales R, Raizenne M, et al. Associations between short-term changes in nitrogen dioxide and mortality in Canadian cities. Arch Environ Health. 2004;59(5):228–36. doi: 10.3200/AEOH.59.5.228-236. [DOI] [PubMed] [Google Scholar]
- Burtscher M. Effects of Living at Higher Altitudes on Mortality: A Narrative Review. Aging Dis. 2014;54:274–280. doi: 10.14336/AD.2014.0500274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakemak S, Dales RE, Vida CB. Components of particulate air pollution an mortality in Chile. Int J Occup Environ Health. 2009;15:152–158. doi: 10.1179/oeh.2009.15.2.152. [DOI] [PubMed] [Google Scholar]
- Cao J, Xu H, Xu Q, Chen B, Kan H. Fine particulate matter constituents and cardiopulmonary mortality in a heavily polluted Chinese city. Environ Health Perspect. 2012;120:373–378. doi: 10.1289/ehp.1103671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carder M, McNamee R, Beverland I, Elton R, Van Tongeren M, Cohen GR, et al. Interacting effects of particulate pollution and cold temperature on cardiorespiratory mortality in Scotland. Occup Environ Med. 2008;65:197–204. doi: 10.1136/oem.2007.032896. [DOI] [PubMed] [Google Scholar]
- Carroll ML, DiMiceli CM, Sohlberg RA, Townshend JRG. 250m MODIS Normalized Difference Vegetation Index. Collection. Vol. 4. University of Maryland; College Park, Maryland: 2004. [Google Scholar]
- Cassee FR, Héroux ME, Gerlofs-Nijland ME, Kelly FJ. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhalation Toxicology. 2013;25:802–812. doi: 10.3109/08958378.2013.850127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Tjoa T, Gillen DL, Staimer N, Polidori A, Arhami M, et al. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21:396–404. doi: 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Zanobetti A, Koutrakis P, Schwartz JD. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environ Health Perspect. 2014;122:837–842. doi: 10.1289/ehp.1307568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Simonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Zeger SL, Samet JM. A measurement error model for time-series studies of air pollution and mortality. Biostatistics. 2000;1:157–175. doi: 10.1093/biostatistics/1.2.157. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley D. Mortality and Air Pollution for Santa Clara County, California, 1989–1996. Revised Analyses of Time-Series Studies of Air Pollution and Health. Health Effects Institute. Special Report. 2003:97–106. [Google Scholar]
- Filleul L, Le Tertre A, Baldi I, Tessier JF. Difference in the relation between daily mortality and air pollution among elderly and all-ages populations in southwestern France. Environ Res. 2004;94:249–253. doi: 10.1016/S0013-9351(03)00080-X. [DOI] [PubMed] [Google Scholar]
- Fischer P, Ameling C, Marra M, Cassee FR. Absence of trends in relative risk estimates for the association between Black Smoke and daily mortality over a 34 years period in The Netherlands. Atmos Environ. 2009;43:481–485. [Google Scholar]
- Fischer PH, Marra M, Ameling CB, Janssen N, Cassee FR. Trends in relative risk estimates for the association between air pollution and mortality in The Netherlands, 1992–2006. Environ Res. 2011;111:94–100. doi: 10.1016/j.envres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Geng F, Hua J, Mu Z, Peng L, Xu X, Chen R, et al. Differentiating the associations of black carbon and fine particle with daily mortality in a Chinese city. Environ Res. 2013;120:27–32. doi: 10.1016/j.envres.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Gent JF, Koutrakis P, Belanger K, Triche E, Holford TR, Bracken MB, et al. Symptoms and medication use in children with asthma and traffic related sources of fine particle pollution. Environ Health Perspect. 2009;117:1168–1174. doi: 10.1289/ehp.0800335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Stoneheurner J, McGee JK, Kinsey JS. Sulfate content correlates with iron concentrations in ambient air pollution particles. Inhal Toxicol. 1999;11:293–307. doi: 10.1080/089583799197104. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Burnett RT, Bailar JC, 3rd, Brook J, Bonvalot Y, Tamblyn R, et al. The association between daily mortality and ambient air particle pollution in Montreal, Quebec. 1. Nonaccidental mortality. Environ Res. 2001a;86:12–25. doi: 10.1006/enrs.2001.4242. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Burnett RT, Bailar JC, 3rd, Brook J, Bonvalot Y, Tamblyn R, et al. The association between daily mortality and ambient air particle pollution in Montreal, Quebec. 2. Cause-specific mortality. Environ Res. 2001b;86:26–36. doi: 10.1006/enrs.2001.4243. [DOI] [PubMed] [Google Scholar]
- Goldman GT, Mulholland JA, Russell AG, Strickland MJ, Klein M, Waller LA, Tolbert PE. Impact of exposure measurement error in air pollution epidemiology: effect of error type in time-series studies. Environ Health. 2011;10:61. doi: 10.1186/1476-069X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass D, Cane M. The effects of weather and air pollution on cardiovascular and respiratory mortality in Santiago, Chile during the winters of 1988–1996. Int J Climatol. 2008;28:1113–1126. [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynn RC, Burnett RT, Thurston GD. A time-series analysis of acidic particulate matter and daily mortality and morbidity in the Buffalo, New York, region. Environ Health Perspect. 2000;108:125–133. doi: 10.1289/ehp.00108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J, Schauer JJ, Yi O, Paek D, Kim H, Yi SM. Fine particle air pollution and mortality: importance of specific sources and chemical species. Epidemiology. 2014;25:379–388. doi: 10.1097/EDE.0000000000000044. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Verhoeff A, van Wijnen J, Fischer P. Daily mortality and air pollution in The Netherlands. J Air Waste Manag Assoc. 2000;50:1380–1389. doi: 10.1080/10473289.2000.10464182. [DOI] [PubMed] [Google Scholar]
- Huang W, Cao J, Tao Y, Dai L, Lu SE, Hou B, Wang Z, Zhu T. Seasonal variation of chemical species associated with short-term mortality effects of PM2.5 in Xi'an, a Central City in China. Am J Epidemiol. 2012;175(6):556–566. doi: 10.1093/aje/kwr342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Mathes R, Ross Z, Nádas A, Thurston G, Matte T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect. 2011;119:467–473. doi: 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen NAH, Hoek G, Simic-Lawson M, Fischer P, van Bree L, ten Brink H, et al. Black carbon as an additional Indicator of the adverse health effects of airborne particle compared with PM10 and PM2.5. Environ Health Perspect. 2011;119:1691–1699. doi: 10.1289/ehp.1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen NAH, Gerlofs-Nijland ME, Lanki T, Salonen RO, Cassee F, Hoek G, et al. Health Effects of Black Carbon. WHO Regional Office for Europe; Copenhagen: 2012. [Google Scholar]
- Katsouyanni K, Schwartz J, Spix C, Touloumi G, Zmirou D, Zanobetti A, et al. Short term effects of air pollution on health: a European approach using epidemiologic time series data: the APHEA protocol. J Epidemiol Community Health. 1996;50:S12–S18. doi: 10.1136/jech.50.suppl_1.s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouyanni K, Touloumi G, Samoli E, Gryparis A, Le Tertre A, Monopolis Y, et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12:521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Kim SY, Peel JL, Hannigan MP, Dutton SJ, Sheppard L, Clark ML, et al. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect. 2012;120:1094–1099. doi: 10.1289/ehp.1104721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Dutton SJ, Sheppard L, Hannigan MP, Miller SL, Milford JB, et al. The short-term association of selected components of fine particulate matter and mortality in the Denver Aerosol Sources and Health (DASH) study. Environ Health. 2015;14:49. doi: 10.1186/s12940-015-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Coull BA, Dominici F, Koutrakis P, Schwartz J, Suh H. The impact of source contribution uncertainty on the effects of source-specific PM2.5 on hospital admissions: a case study in Boston, MA. J Expo Sci Environ Epidemiol. 2014a;24:365–371. doi: 10.1038/jes.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Spiegelman D, Szpiro AA, Sheppard L, Kaufman JD, Yanosky JD, et al. Exposure measurement error in PM2.5 health effects studies: a pooled analysis of eight personal exposure validation studies. Environmental Health. 2014b;13(1):2. doi: 10.1186/1476-069X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Schwartz J, James P, Dominici F, Zanobetti A. PM2.5 and mortality in 207 US cities: Modification by temperature and city characteristics. Epidemiology. 2016;27:221–227. doi: 10.1097/EDE.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RJ, Mason RM, Jr, Heilig CM, Neas LM, Dockery DW. Is Daily Mortality Associated Specifically with Fine Particles? Data Reconstruction and Replication of Analyses. J Air Waste Manag Assoc. 2000;50:1215–1222. doi: 10.1080/10473289.2000.10464149. [DOI] [PubMed] [Google Scholar]
- Klemm RJ, Thomas EL, Wyzga RE. The impact of frequency and duration of air quality monitoring: Atlanta, GA, data modeling of air pollution and mortality. J Air Waste Manag Assoc. 2011;61:1281–1291. doi: 10.1080/10473289.2011.617648. [DOI] [PubMed] [Google Scholar]
- Koutrakis P, Suh HH, Sarnat JA, Brown KW, Coull BA, Schwartz J. Characterization of particulate and gas exposures of sensitive subpopulations living in Baltimore and Boston. Res Rep Health Eff Inst. 2005;131:1–65. [PubMed] [Google Scholar]
- Krall JR, Anderson GB, Dominici F, Bell ML, Peng RD. Short-term exposure to particulate matter constituents and mortality in a national study of U.S. urban communities. Environ Health Perspect. 2013;121:1148–1153. doi: 10.1289/ehp.1206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall R, Ito K, Thurston GD. Distributed lag analyses of daily hospital admissions and source-apportioned fine particle air pollution. Environ Health Perspect. 2011;119:455–460. doi: 10.1289/ehp.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, et al. A Systematic Review and Meta-Analysis of Multiple Airborne Pollutants and Autism Spectrum Disorder. PLoS ONE. 2016;11:e0161851. doi: 10.1371/journal.pone.0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepeule J, Litonjua AA, Coull B, Koutrakis P, Sparrow D, Vokonas PS, et al. Long-term effects of traffic particles on lung function decline in the elderly. Am J Respir Crit Care Med. 2014;190:542–548. doi: 10.1164/rccm.201402-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tertre A, Quénel P, Eilstein D, Medina S, Prouvost H, Pascal L, et al. Short-term effects of air pollution on mortality in nine French cities: a quantitative summary. Arch Environ Health. 2002;57:311–319. doi: 10.1080/00039890209601414. [DOI] [PubMed] [Google Scholar]
- Li P, Xin J, Wang Y, Li G, Pan X, Wang S, et al. Association between particulate matter and its chemical constituents of urban air pollution and daily mortality or morbidity in Beijing City. Environ Sci Pollut Res Int. 2015;22:358–368. doi: 10.1007/s11356-014-3301-1. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Ito K, Nádas A, Burnett RT. Association of particulate matter components with daily mortality and morbidity in urban populations. Res Rep Health Eff Inst. 2000;95:5–72. [PubMed] [Google Scholar]
- Mar TF, Ito K, Koenig JQ, Larson TV, Eatough DJ, Henry RC, et al. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. J Expo Sci Environ Epidemiol. 2006;16:311–320. doi: 10.1038/sj.jea.7500465. [DOI] [PubMed] [Google Scholar]
- Masri S, Kang CM, Koutrakis P. Composition and sources of fine and coarse particles collected during 2002–2010 in Boston, MA. J. Air Waste Manage Assoc. 2015;65:287–297. doi: 10.1080/10962247.2014.982307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan RO. Setting ambient air quality standards for particulate matter. Toxicology. 2002;181–182:329–347. doi: 10.1016/s0300-483x(02)00459-6. [DOI] [PubMed] [Google Scholar]
- Mittleman MA. Optimal referent selection strategies in case-crossover studies: a settled issue. Epidemiology. 2005;16:715–716. doi: 10.1097/01.ede.0000183170.92955.25. 1. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med. 2009;6(6):e1000097. doi: 10.1371/journal.pmed.1000097. Available: www.prisma-statement.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky E, Schwartz J, Coull BA, Koutrakis P, Wellenius GA, Suh HH, et al. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol. 2012;176:317–326. doi: 10.1093/aje/kws018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHAT (Office of Health Assessment and Translation) Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. Division of the National Toxicology Program. National Institute of Environmental Health Sciences. 2015 Available: https://ntp.niehs.nih.gov/pubhealth/hat/noms/index-2.html#Ongoing-Methods-Development-Activities.
- O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Ostro B, Rauch S, Green S. Quantifying the health impacts of future changes in temperature in California. Environ Res. 2011;111:1258–1264. doi: 10.1016/j.envres.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Ostro B, Tobias A, Karanasiou A, Samoli E, Querol X, Rodopoulou S, et al. MEDPARTICLES Study Group. The risks of acute exposure to black carbon in Southern Europe: results from the MED-PARTICLES project. Occup Environ Med. 2015;72:123–129. doi: 10.1136/oemed-2014-102184. [DOI] [PubMed] [Google Scholar]
- Peng RD, Dominici F, Pastor-Barriuso R, Zeger SL, Samet JM. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161:585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- Peng RD, Dominici F, Louis TA. Model choice in time series studies of air pollution and mortality. J R Statistic Soc A. 2006;169:179–203. [Google Scholar]
- Sacks JD, Ito K, Wilson WE, Neas LM. Impact of covariate models on the assessment of the air pollution-mortality association in a single- and multipollutant context. Am J Epidemiol. 2012;176:622–634. doi: 10.1093/aje/kws135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoli E, Schwartz J, Wojtyniak B, Touloumi G, Spix C, Balducci F, et al. Investigating regional differences in short-term effects of air pollution on daily mortality in the APHEA project: a sensitivity analysis for controlling long-term trends and seasonality. Environ Health Perspect. 2001;109:349–353. doi: 10.1289/ehp.01109349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DM, et al. Research Report. Vol. 94. Health Effects Institute; Cambridge MA: 2000. The National Morbidity, Mortality and Air Pollution Study, Part, II: Morbidity and Mortality from Air Pollution in the United States. [PubMed] [Google Scholar]
- Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, et al. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect. 2008;116:459–466. doi: 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat SE, Klein M, Sarnat JA, Flanders WD, Waller LA, Mulholland JA, et al. An examination of exposure measurement error from air pollutant spatial variability in time-series studies. J Expo Sci Environ Epidemiol. 2010;20:135–146. doi: 10.1038/jes.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Kloog I, Zanobetti A, Liu P, Schwartz JD. Impacts of temperature and its variability on mortality in New England. Nat Clim Chang. 2015;5:988–991. doi: 10.1038/nclimate2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JY, Lee JT, Kim H, Yi O, Bell ML. Susceptibility to air pollution effects on mortality in Seoul, Korea: a case-crossover analysis of individual-level effect modifiers. J Expo Sci Environ Epidemiol. 2012;22:227–234. doi: 10.1038/jes.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanković A, Nikić D, Nikolić M, Bogdanović D. Short-term effects of air pollution on cardiovascular mortality in elderly in Nis, Serbia. Cent Eur J Public Health. 2007;15:95–98. doi: 10.21101/cejph.a3425. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- Valdés A, Zanobetti A, Halonen JI, Cifuentes L, Morata D, Schwartz J. Elemental concentrations of ambient particles and cause specific mortality in Santiago, Chile: a time series study. Environ Health. 2012;11:82. doi: 10.1186/1476-069X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve PJ, Burnett RT, Shi Y, Krewski D, Goldberg MS, Hertzman C, et al. A time-series study of air pollution, socioeconomic status, and mortality in Vancouver, Canada. J Expo Anal Environ Epidemiol. 2003;13:427–435. doi: 10.1038/sj.jea.7500292. [DOI] [PubMed] [Google Scholar]
- Wells G, Shea B, O’Connell D, Robertson J, Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis 2011 [Google Scholar]
- WHO (World Health Organization) Europe. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2013. Review of evidence on health aspects of air pollution—REVIHAAP project. Available: http://www.euro.who.int/ [Google Scholar]
- WHO (World Health Organization) Geneva, Switzerland: Public Health, Social and Environmental Determinants of Health Department, World Health Organization; 2014. Burden of disease from Ambient Air Pollution for 2012. Available: http://www.who.int/phe/ [Google Scholar]
- Wilson EW. The relationship between daily cardiovascular mortality and daily ambient concentrations of particulate pollutants (sulfur, arsenic, selenium, and mercury) and daily source contributions from coal power plants and smelters (individually, combined, and with interaction) in Phoenix, AZ, 1995–1998: A multipollutant approach to acute, time-series air pollution epidemiology: I. J Air Waste Manag Assoc. 2015;65:599–610. doi: 10.1080/10962247.2015.1033067. [DOI] [PubMed] [Google Scholar]
- Wyzga RE, Rohr AC. Long-term particulate matter exposure: Attributing health effects to individual PM components. J Air Waste Manag Assoc. 2015;65:523–543. doi: 10.1080/10962247.2015.1020396. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Franklin M, Koutrakis P, Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;8:1–12. doi: 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, O’Neill MS, Gronlund CJ, Schwartz JD. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc Natl Acad Sci. 2012;109:6608–6613. doi: 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J, Samoli E, Gryparis A, Touloumi G, Peacock J, et al. The temporal pattern of respiratory and heart disease mortality in response to air pollution. Environ Health Perspect. 2003;111:1188–1193. doi: 10.1289/ehp.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.