Abstract
Hodgkin Lymphoma (HL) is a tumor of B-cell origin characterized by Hodgkin and Reed-Stenberg (H/RS) cells embedded in an inflammatory tissue where numerous cytokines/chemokines contribute to shape the microenvironment, leading to the typical clinical symptoms.
We investigated: i) the expression of Interleukin-IL-31 (IL-31) and Thymic Stromal Lymphopoietin (TSLP), two Th2-related cytokines with tumor-promoting and pruritogenic functions, and of the respective receptors in HL invaded lymph nodes by flow cytometry, and ii) the potential association of IL-31/TSLP plasma concentrations with clinical characteristics by ELISA.
H/RS cells and the major immune cell types infiltrating HL lymph nodes expressed intracytoplasmic and surface IL-31/TSLP, and their receptors. A subgroup of patients showing at diagnosis elevated IL-31 and TSLP plasma levels had an International Prognostic Score>2, indicative of high risk of relapse, and a subsequent positive interim PET-scan, indicative of insufficient response to chemotherapy. No correlation was found between IL-31/TSLP plasma levels and overall or event-free survival.
In conclusion, IL-31/TSLP and their receptors are expressed in HL cells and in immune cells infiltrating affected lymph nodes, where both cytokines may contribute to local immune suppression. The clinical impact of IL-31 and TSLP plasma levels has to be further defined in larger patient cohorts.
Keywords: Hodgkin lymphoma, IL-31, TSLP, cytokine receptors, PET
INTRODUCTION
Hodgkin Lymphoma (HL) is a B cell-derived malignancy characterized by low proportions of neoplastic mono-nucleated Hodgkin and multi-nucleated Reed-Stenberg (H/RS) cells in the invaded lymph nodes. HL is subdivided into classical (c) form, occurring in approximately 95% of cases, and nodular lymphocyte predominant (NLP) form (4-5% of cases), considered a different disease [1]. H/RS cells are embedded in a reactive microenvironment including CD4 T cells, B cells, macrophages, dendritic cells, eosinophils, fibroblasts, and basophils/mast cells [2]. This inflammatory microenvironment provides essential signals for H/RS cell survival [2, 3].
Although originating from germinal center (GC) or post-GC B cells [4], H/RS cells are characterized by down-regulation of B-cell markers and expression of CD15 and CD30 [5, 6]. Only a small proportion of HL (1-2%) originates from T cells [7].
Four histological subtypes of cHL have been identified based on HRS morphology and microenvironment composition: nodular sclerosis (80%), mixed cellularity (15%), lymphocyte rich and lymphocyte depleted [1].
The neoplastic tissue in Hodgkin lymphoma produces a wide spectrum of cytokines and chemokines that contribute to shape the microenvironment and lead to the typical clinical symptoms, as fever, night sweats, weight loss or pruritus.
Interleukin-31 (IL-31) is a cytokine related to the IL-6 family secreted by activated Th2 cells, monocytes, macrophages, dendritic cells and mast cells [8–10]. It signals through a heterodimeric receptor complex composed of the IL-31 Receptor Alpha (IL-31RA) and the Oncostatin M Receptor (OSMR) subunits [11–13]. Engagement of the IL-31R with IL-31 results in the activation of JAK1 and, to a minor extent, of JAK2 followed by activation of STAT1/3/5, MAPK, and PI3K signaling pathways [13–15]. It has been shown that IL-31 serum levels are increased in patients with cutaneous T cell lymphoma [16] and, more recently, our group has demonstrated that the IL-31/IL-31R axis promotes tumor growth in Follicular B cell lymphoma [16, 17].
Thymic Stromal Lymphopoietin (TSLP) is expressed by epithelial cells in the thymus, lung, intestine, skin, gut, and tonsil as well as by stromal cells and mast cells [18]. The high affinity TSLP receptor complex is composed of the IL-7R alpha chain/CD127 and the TSLP-specific Receptor component, TSLPR/Crlf2 [19]. The heterodimeric TSLP receptor activates, in addition to STAT5, STAT1/3, STAT4, and STAT6, as well as JAK1 and JAK2 [20]. The TSLP/TSLPR axis has been shown to promote tumor cell survival in both solid tumors and leukemia [21].
The involvement of IL-31 and TSLP as mediators of chronic pruritus in the pathogenesis of various skin diseases is clearly established [22–26]. Pruritus is observed in about 30% of patients with Hodgkin's lymphoma, more often in the nodular sclerosis type with mediastinal mass [27]. No information is available on the relationship among IL-31, TSLP and pruritus in HL patients. This latter issue has been here investigated by testing plasma levels of both cytokines and correlating them to pruritus and other clinical characteristics. In addition, we have investigated the expression of IL-31, TSLP and their receptors in invaded lymph nodes from HL patients in view of the tumor promoting role of these cytokines and the complete lack of information on this latter issue.
RESULTS
Expression of IL-31 and TSLP and the respective receptors in Hodgkin/Reed Sternberg cells and lymphoid cells populating the tumor microenvironment
We have previously demonstrated that IL-31 is expressed on the surface membrane and in the cytoplasm of normal and Follicular Lymphoma B cells [17]. We therefore investigated by flow cytometry the surface and intracellular expression of both IL-31 and TSLP in lymph node biopsies from 10 HL patients. Cell suspensions isolated from invaded lymph nodes were multicolor stained with anti-CD30, -CD15, -CD45 mAbs in combination with the anti-IL-31 or -TSLP mAbs, and analyzed by flow cytometry after gating first on CD45- cells, and then on CD30+, CD15+ H/RS cells (Figure 1A, left panel) [28].
Figure 1. Expression of IL-31, TSLP and their receptors in H/RS cells.
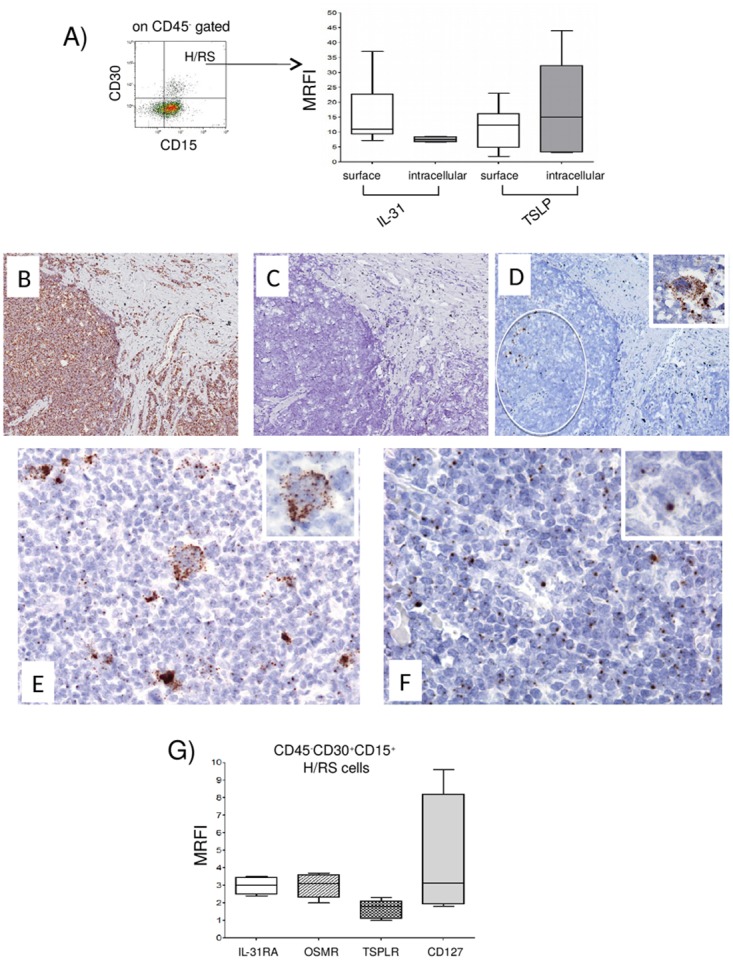
(A) Left panel. A representative gating strategy for H/RS cells identified as CD45, CD30+, CD15+ cells. Right panel. IL-31/TSLP expression was tested by flow cytometry at surface and intracellular levels. Results are expressed in box plot as median MRFI, first and third quartiles, maximum and minimum values, from 10 different HL lymph node cell suspensions. (B-F) In situ hybridization for Ubiquitin (B), dapB (C), CD30 (D), IL-31 (E) and TSLP (F) mRNA in cHL using the RNAscope technology (B, C, D) original magnification x100; E, F x200; insets x400). Ubiquitin mRNA was diffusely expressed (brown dots), whereas the bacterial dapB was completely negative. The CD30 probe hybridized with a proportion of the cells with H/RS morphology (circle and inset). Both IL-31 and TSLP mRNA were detected in the cytoplasm of H/RS cells (inset) and in some of the immune reactive cells present in the background. (G) IL-31RA/OSMR and TSLPR/CD127 chain receptor expression was analyzed by flow cytometry. Results are expressed in box plot as median MRFI, first and third quartiles, maximum and minimum values, from 7 different HL lymph node cell suspensions.
In HL lymph nodes, H/RS cells, that ranged from 1 to 7%, median 3.4%, were found to express IL-31 and TSLP both at the cell surface (median MRFI IL-31= 11, range 7.2-37, n=10; median MRFI TSLP=12, range 2.0-23, n=8) (Figure 1A, right panel, first and third boxes, respectively, from the left) and intracellularly (median MRFI IL-31=7.5, range 6.6-8.6, n=6; median MRFI TSLP=15, range 3.2-44, n=6) (Figure 1A, right panel, second and fourth boxes, respectively, from the left).
To confirm the specificity of IL-31 and TSLP surface staining on H/RS cells, lymph node MNC cell suspensions were incubated in a solution at pH 2.5 for 10 minutes to elute surface-bound cytokines, washed and stained as above. Treatment at acidic pH causes detachment of soluble molecules non specifically adsorbed on the cell surface from the extracellular milieu, whereas it has no effect on endogenous surface molecules [29]. IL-31 and TSLP expression on the surface of H/RS cells was unaffected by treatment at acidic pH (not shown).
In situ hybridization with the RNAscope technology on paraffin sections from three HL lymph nodes using probes for IL-31 and TSLP showed clear punctate staining for both cytokines in cells with the morphology of H/RS cells. (Figure 1B-1F). Ubiquitin mRNA, tested as positive control, was diffusely expressed (brown dots), whereas the bacterial dapB, tested as negative control, was completely negative. The CD30 probe hybridized with a proportion of the cells with H/RS morphology (circle and inset). Both IL-31 and TSLP mRNAs were detected in the cytoplasm of H/RS cells (Figure 1E and 1F, insets) and in some of the immune reactive cells present in the background. In H/RS cells a high number of IL-31-positive dots/cell were evident (Figure 1E).
To investigate the expression of IL-31R and TSLPR in H/RS cells, cell suspensions from seven HL lymph nodes were stained with mAbs to IL-31RA, OSMR, TSLPR and CD127 and analyzed by flow cytometry gating on CD45-, CD30+, CD15+ cells as above. IL-31RA and OSMR, as well as TSLPR and CD127, were detected on H/RS cell surface (median MRFI IL-31RA=3.0, range 2.4-3.5; median MRFI OSMR=3.1, range 2.0-3.7; median MRFI TSLPR =1.8, range 1.0-2.3; median MRFI CD127=3.2, range 1.8-9.6) (Figure 1G, first to fourth boxes from the left, respectively).
Next, we addressed the expression of IL-31/TSLP and their receptors in the major cell types infiltrating the HL microenvironment. To this end, cell suspensions from seven HL lymph nodes and 7 reactive lymph nodes with follicular hyperplasia, tested as controls, were stained with B cell specific CD19 mAb, T helper cell specific CD4 mAb, or macrophage specific CD68 mAb, in combination with anti-IL-31 or -TSLP mAbs. Median values for CD19+ cells, CD4+ cells and CD68+ cells in HL lymph nodes were 39%, 62%, and 10%, respectively, while median values of the same cell populations for reactive lymph nodes were 39%, 47%, and 10%, respectively.
Consistent with our previous report [17], IL-31 was detected on the surface and in the intracellular compartment of CD19+ B cells from both HL and reactive lymph nodes (Figure 2, upper left panel). TSLP was found to be expressed in the same B cell suspensions in the intracellular compartment, whereas it was absent from the cell surface (Figure 2, upper left panel). Expression of IL-31 in CD4+ T cells was detected intracellularly and on the cell surface in both HL and reactive lymph nodes (Figure 2, middle left panel). TSLP was detected in the same cells intracellularly but not at the cell surface (Figure 2, middle left panel). Finally, IL-31 and TSLP were detected both at the cell surface and intracellularly in CD68+ macrophages from HL and reactive lymph nodes (Figure 2, lower left panel). Supplementary Table 1 reports in detail the median MRFI of IL-31 and TSLP detected in immune cell populations from both HL and reactive lymph nodes.
Figure 2. Expression of IL-31, TSLP and their receptors in lymphoid cells populating HL and reactive lymph node microenvironment.
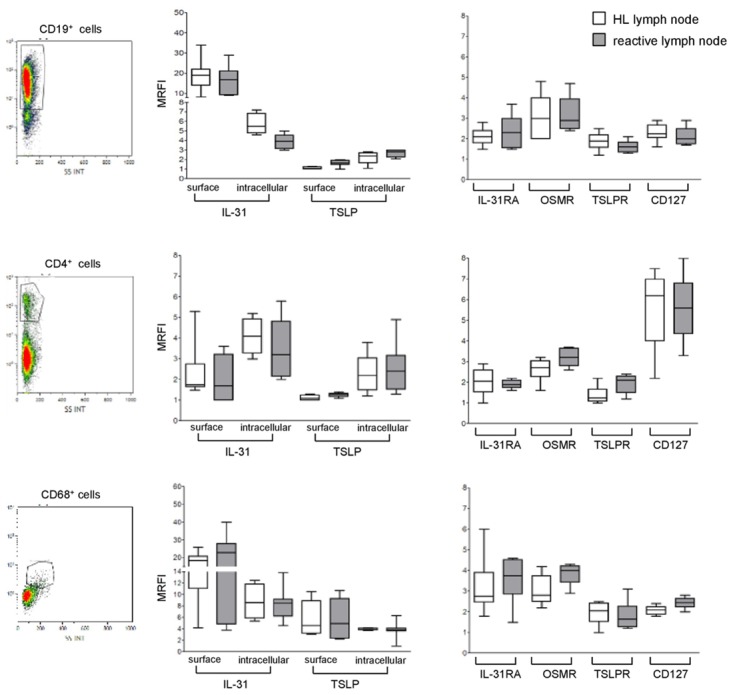
Left panels. A representative gating strategy for B cells (CD19+), T cells (CD4+), and macrophages (CD68+). Middle panels. IL-31/TSLP expression was analyzed by flow cytometry at surface and intracellular levels on CD19+, CD4+, and CD68+ cells for both HL and reactive lymph nodes. Results are shown in box plot as median MRFI, first and third quartiles, maximum and minimum values, from 7 different HL and 7 reactive lymph node cell suspensions. Right panels. IL-31RA/OSMR and TSLPR/CD127 chain receptor expression was analyzed by flow cytometry on CD19+, CD4+ and CD68+ cells for both HL and reactive lymph nodes. Results are shown in box plot as median MRFI, first and third quartiles, maximum and minimum values, from 7 different HL and 7 reactive lymph node cell suspensions.
Surface staining for IL-31 and TSLP was superimposable in the latter cell fractions following pre-incubation at acidic pH (not shown). To confirm the flow cytometric experiments, we performed q-PCR analysis of mRNA in CD19+ B cells, CD4+ T cells and CD68+ macrophages isolated from tonsils, as well as in the L-428, HDLM-2, KM-H2 HL cell lines. The HeLa cell line was tested as positive control. As apparent, all of these cell types but the HL cell lines expressed the TSLP transcript (Supplementary Figure 1).
In additional experiments, we investigated the expression of IL-31R and TSLPR in the same cell suspensions from HL and reactive lymph nodes tested above. Both IL-31R and TSLPR were detected on CD19+ B cells (Figure 2, upper right panel). CD4 T cells expressed CD127, as well as IL-31RA, OSMR and TSLPR (Figure 2, middle right panel). Finally, macrophages expressed IL-31RA and OSMR, as well as TSLPR and CD127 (Figure 2, lower right panel). Median MRFI values for IL-31/TSLP receptors for each immune cell populations in HL and reactive lymph nodes are reported in Supplementary Table 2.
As apparent from both Figure 2 and Supplementary Tables 1 and 2, no differences in the expression of IL-31, TSLP and the respective receptors were found between immune cells present in HL and those present in reactive lymph nodes.
Finally, we investigated the expression by flow cytometry of IL-31, TSLP and the respective receptor chains in HDLM-2, L-428, and KM-H2 HL cell lines. None of the cell lines tested expressed the two cytokines or their receptors (not shown).
IL-31 and TSLP plasma levels in patients with Hodgkin lymphoma
We next analyzed soluble (s)IL-31 and sTSLP levels in plasma samples from HL patients at diagnosis and from healthy controls. sIL-31 was detected in 65/109 (60%) patients (Figure 3) with a wide range from 5 to 7937 pg/ml and a median of 245 pg/ml. sTSLP was detected in 52/75 (69%) patients tested (Figure 3) with a range from 9 to 4209 pg/ml, and a median of 171 pg/ml.
Figure 3. sIL-31/TSLP plasma levels in HL patients.
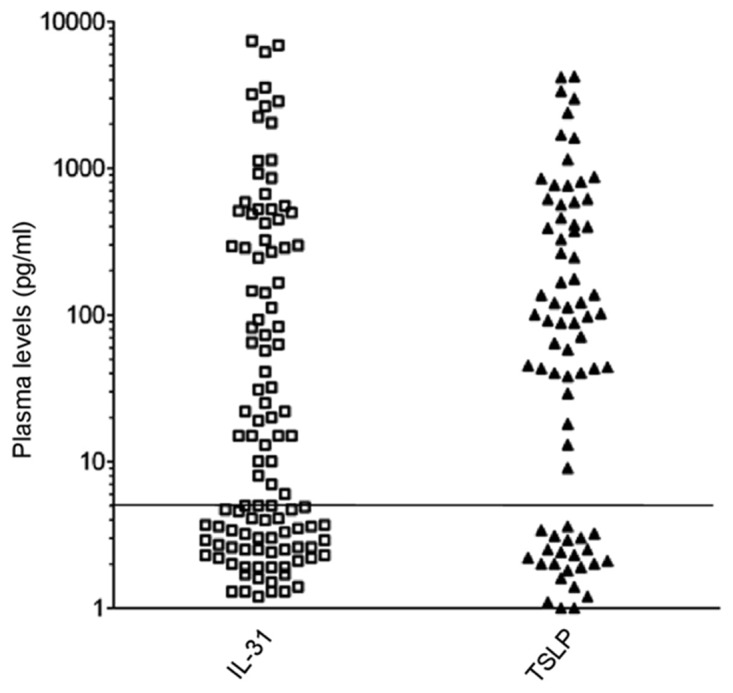
sIL-31/TSLP levels were assayed by ELISA (threshold of detection 5 pg/ml). sIL-31 was detected in 65/109 patients, sTSLP in 52/75 patients. The horizontal line separates positive from negative results.
sIL-31 and sTSLP levels in HL patients did not differ from the levels detected in a group of 84 age-matched controls. Thus, sIL-31 was detected in 42/84 (50%) healthy controls ranging from 8 to 7714 pg/ml (median of 281 pg/ml), while sTSLP was detected in 32/59 (54%) healthy controls ranging from 10 to 2849 pg/ml, with a median of 134 pg/ml (Supplementary Figure 2). A highly significant correlation between sIL-31 and sTSLP concentrations was observed in both HL patients and healthy controls (P<0.0001), suggesting a coordinate production of the two cytokines, possibly operated by the same cells.
We next analyzed for associations between cytokine levels and patient clinical characteristics. HL is often associated with pruritus [27], present in 47% of our patients. Pruritus was defined as: i) intense, if widespread and associated with secondary cutaneous lesions due to scratching and/or need for anti-histamines; and ii) mild, when only localized. sIL-31 or sTSLP were not found to be associated to the presence or degree of itching in the HL patients studied (n=24 no pruritus, n=14 mild, n=21 intense) (Supplementary Figure 3A and 3B, respectively).
The International Prognostic Score (IPS) is the most widely used risk stratification index for HL that incorporates seven clinical parameters independently associated with poor outcome [30]. IPS from 0 to 2 identify low-risk patients, while IPS values>2 are detected in high-risk patients. Significantly higher levels of sIL-31 and sTSLP (P=0.002, n=34/72, median 182 pg/ml for IL-31, P=0.03, n=25/48, median 167 pg/ml for TSLP) were detected in HL patients with an IPS >2 compared to those with IPS 0-2. Among the clinical parameters included in IPS, WBC count >15x103/ml was found to be significantly associated with high sIL-31 and sTSLP levels in HL patients studied (P=0.01, n=19/89 for IL-31; P=0.02, n=15/59 for TSLP). No correlation with other patient characteristics was identified. Tables 1 and 2 show in detail the results on the associations between clinical characteristics and sIL-31 or sTSLP, respectively.
Table 1. Associations between clinical patient characteristics at diagnosis and IL-31 plasma levels in HL patients.
| Variable | Number |
IL-31 level median (pg/ml) |
P | ||
|---|---|---|---|---|---|
| IPS parameters |
Age, years (n=109) |
< 45 >45 |
75 34 |
10 14 |
0.9 |
|
Gender (n=109) |
Female Male |
53 56 |
15 5 |
0.4 | |
|
Stage (n=109) |
I-III IV |
80 29 |
5 83 |
0.1 | |
|
White blood cell count (n=108) |
≤ 15 x 103/ml > 15 x 103/ml |
89 19 |
5 294 |
0.01 | |
|
Lymphocyte count (n=105) |
≥ 600/ml < 600/ml |
93 12 |
7 18 |
0.4 | |
|
Hemoglobin level (n=108) |
≥ 10.5 g/dl < 10.5 g/dl |
89 19 |
7 25 |
0.2 | |
|
Albumin level (n=105) |
≥ 40 g/l < 40 g/l |
55 50 |
5 21 |
0.1 | |
|
IPS (n=106) |
0-2 3-5 |
72 34 |
5 182 |
0.002 | |
|
Histology subtype (n=109) |
cHL Nodular sclerosis cHL Mixed cellularity cHL lymphocyte rich cHL NOS NLPHL |
86 1 4 15 3 |
15 7 9 13 15 |
0.5 | |
|
B-symptoms (n=109) |
Absent Present |
68 41 |
5 22 |
0.2 |
Table 2. Associations between clinical patient characteristics at diagnosis and TSLP plasma levels in HL patients.
| Variable | Number |
TSLP level median (pg/ml) |
P | ||
|---|---|---|---|---|---|
| IPS parameters |
Age, years (n=75) |
< 45 >45 |
52 23 |
80 102 |
0.9 |
|
Gender (n=75) |
Female Male |
39 36 |
58 101 |
0.3 | |
|
Stage (n=75) |
I-III IV |
55 20 |
71 94 |
0.6 | |
|
White blood cell count (n=74) |
≤ 15 x 103/ml > 15 x 103/ml |
59 15 |
45 246 |
0.02 | |
|
Lymphocyte count (n=71) |
≥ 600/ml < 600/ml |
63 8 |
71 112 |
0.7 | |
|
Hemoglobin level (n=74) |
≥ 10.5 g/dl < 10.5 g/dl |
60 14 |
88 80 |
1.0 | |
|
Albumin level (n=73) |
≥ 40 g/l < 40 g/l |
37 36 |
44 117 |
0.3 | |
|
IPS (n=73) |
0-2 3-5 |
48 25 |
44 167 |
0.03 | |
|
Histology subtype (n=75) |
cHL Nodular sclerosis cHL Mixed cellularity cHL NOS NLPHL |
61 1 10 3 |
117 71 63 582 |
0.9 | |
|
B-symptoms (n=75) |
Absent Present |
47 28 |
91 52 |
0.2 |
Early response following 2 cycles of chemotherapy is evaluated using (18F)-fluordeoxyglucose (FDG)-position emission tomography (PET) (interim PET), which represents the strongest predictor to distinguish high-risk from low-risk HL patients [31]. PET images are scored according to the Deauville classification system [31]. FDG uptakes in residual tissue below the uptake in the liver were considered as negative (Deauville score 1-3), while uptake higher than the liver background was considered as positive (Deauville score 4-5) [31, 32]. Interestingly, HL patients with a positive interim PET-scan, indicative of high risk of relapse, had significantly higher levels of sIL-31 (P=0.01, n=13/84, median=298 pg/ml) and sTSLP (P=0.05, n=10/63, median 548 pg/ml) at diagnosis compared to patients with a negative interim PET-scan (Figure 4A and 4B, respectively).
Figure 4. Correlations between sIL-31/sTSLP and Interim Pet in HL patients.
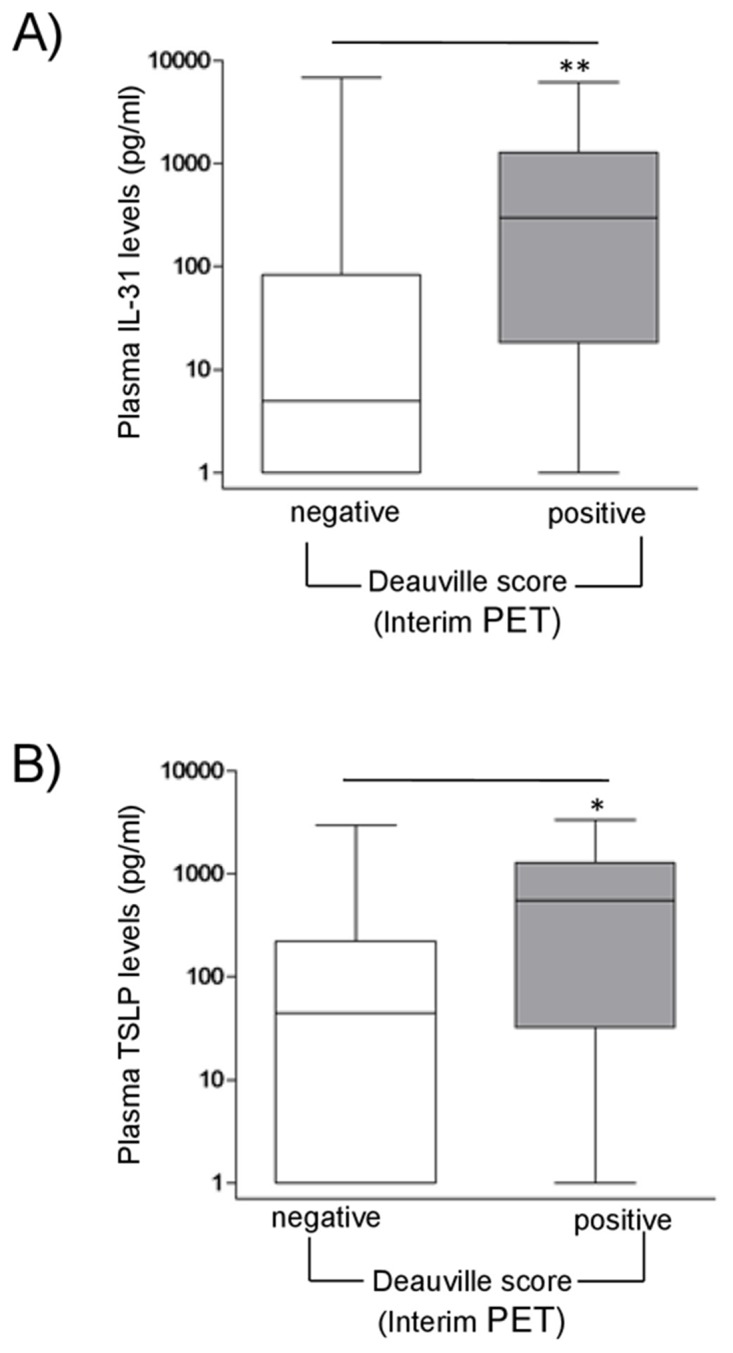
sIL-31 (A) and sTSLP (B) levels (pg/ml) in HL patient plasma were correlated with interim PET scan. PET images were classified according to the Deauville score (1-3 negative; 4-5 positive). HL patients with a positive score had significant higher median levels of sIL-31 and sTSLP than patients with negative score (**P=0.01, n=13/84 and *P=0.05, n=10/63, respectively).
With a median observation of 32 months, 30/109 patients experienced an event. This translated into a 3-year probability of event-free survival (EFS) of 75% (95% C.I., 65-82%). Patients with sIL-31and sTSLP levels higher than the median did not differ in their prognosis from patients with levels below the median. Likewise, HL patients were analyzed for overall survival (OS): the 3-year OS was 90% ( 95% C.I., 82-95%) without any difference between patients with sIL-31/TSLP levels higher or lower than the median (data not shown).
DISCUSSION
In this study, we demonstrate that H/RS cells express intracytoplasmic and surface IL-31 and TSLP, as well as the respective receptors. B cells, macrophages and CD4+ T cells infiltrating HL lymph nodes showed surface and cytoplasmic expression of IL-31, while TSLP was detected on the cell surface of macrophages and in the cytoplasm of B cells, CD4+ T cells and macrophages. IL-31/TSLP and their receptors were expressed with superimposable profiles in the same immune cell fractions from reactive lymph nodes. The finding that both malignant and immune cells in the HL lymph node microenvironment expressed IL-31 and TSLP and the respective receptors suggests that numerous paracrine and/or autocrine interactions may take place in vivo. These cytokines may contribute to cell-to-cell interactions by shedding of soluble forms from the surface membrane or release of the cytoplasmic forms, and ii) direct contact between surface bound cytokine(s) on a cell and the respective receptor(s) on an adjacent cell. In this respect, we have previously demonstrated that IL-31 is not released in soluble form by Follicular Lymphoma B cells, but shed in microvesicles that serve as intercellular messengers. This mechanism, that has not been reported for TSLP and may operate also in H/RS cells [33] could not be investigated due to the paucity of the latter cells in affected lymph nodes.
Plenty cytokines/chemokines released by H/RS cells shape the tumor microenvironment. Thus, for example, IL-5, CCL5, CCL28 attract eosinophils [34–36]; CCL5 attract mast cells, [37] IL-8 neutrophils [34], CCL5, CCL17, CCL22 Th2 cells, and CCL20 T reg cells [38–41]. Although the HL microenvironment is considered as Th2 polarized, this may be an oversimplification, since a recent study demonstrates that T cells infiltrating HL lymph nodes express Th1-type chemokine receptors, cytokines and transcription factors [42].
A question raised by this study is how IL-31 and TSLP can modulate the HL lymph node microenvironment to support tumor growth. In atopic dermatitis, IL-31 induces chemotaxis, Ca2+ mobilization, release of reactive oxygen species, surface expression of adhesion molecules and CCL26 in eosinophils, which in turn release IL-31, contributing to the maintenance of the inflammatory infiltrate [43, 44]. TSLP promotes directly commitment of human bone marrow hematopoietic progenitors to the eosinophil/basophil lineage and elicits mature basophil responses in the periphery [45, 46]. In addition, TSLP can enhance eosinophil survival, up-regulate surface expression of adhesion molecules and induce the release of inflammatory cytokines and chemokines from human eosinophils [47]. Finally, TSLP amplifies M2 macrophage polarization [48, 49]. Since eosinophils and, at a lower extent, basophils/mast cells are important components of the HL lymph node infiltrate, it is conceivable that IL-31 and/or TSLP contribute to the recruitment, survival and activation of these cell types and, more in general, polarize immune responses towards a tumor promoting functional state. A note of caution in the discussion of the potential mechanisms whereby IL-31 and TSLP can shape the HL microenvironment comes from previous studies showing the existence of different IL-31RA isoforms, some of which are devoid of signalling activity [17, 50]. Furthermore, two dominant isoforms of IL-7R alpha chain/CD127 coding for membrane-bound or soluble IL-7R alpha, respectively, have been identified [51], and two isoforms of TSLP with completely different functions have been reported [52].
IL-31 and TSLP are two Th2-related cytokines that promote itch in atopic skin diseases by activating cutaneous somatosensory neurons, either directly or indirectly through stimulation of immune cells [53]. However, no association was detected between concentrations of sIL-31 or sTSLP and presence or degree of itching in a cohort of HL patients at diagnosis. In contrast, we found an association between high plasma levels of both cytokines and increased number of circulating WBC, in particular neutrophils. In this respect, IL-31was found to stimulate in a mouse model the survival of myeloid progenitor cells [54], raising the possibility that in HL patients IL-31 induces neutrophilia, possibly in concert with other cytokines that have myelopoietic activity as CXCL8 [55], Since the WBC count is a prognostic factor included in the IPS score, both IL-31 and TSLP levels were higher in patients with an IPS>2, indicative of high risk disease.
Finally, patients with a positive interim PET-scan, usually indicative of insufficient response to chemotherapy [31], had higher levels of sIL-31 and sTSLP at diagnosis. The association of positive interim PET-scan and high cytokine plasma levels may simply reflect differences in persistence of an inflammatory microenvironment in the scanned lymph nodes at the time of interim PET without truly reflecting disease activity, since we failed to identify any correlation between sIL-31 or sTSLP plasma levels and event-free or overall survival in our patient cohort. In addition, heterogeneity in therapeutic protocols may have influenced the correlations between plasma cytokines, interim PET scan and outcome. Further studies in a larger patient group are needed to better define the clinical impact of IL-31 and TSLP plasma levels.
MATERIALS AND METHODS
Patients and controls
The analysis included 109 patients (56 males and 53 females, 34 of whom >45 years and 75 <45 years), diagnosed with cHL (106 patients) and NLPHL (3 patients) at the Department of Hematology of the Catholic University of Rome, Italy. Six of the patients studied had an age range from 15 to 18 years. Diagnosis of HL was established according to the criteria of the World Health Organization (WHO) classification [1]. cHL histology subtype was the following: 86 Nodular sclerosis, 1 Mixed cellularity, 4 Lymphocyte-rich and 15 that could not be classified into any subtype and are referred to as “not otherwise specified”. Eighty-four healthy donors (45 males and 39 females, 36 of whom >45 years and 48 <45 years,) were recruited from the Division of Immunohematology and Transfusion Centre, Giannina Gaslini Institute, Genoa, Italy. The study was approved by the Institutional Review Board of the Catholic University (P/416/CE/2010) and the Institutional Review Board of the Istituto Giannina Gaslini, Genova, Italy on October 27th, 2005. Informed consent was obtained from both patients and healthy donors according with the Declaration of Helsinki.
Cell isolation
Invaded lymph nodes from 10 HL patients (6 males and 4 females, 3 patients >45 years, 7 patients<45 years) and 7 reactive lymph nodes biopsied for diagnostic purposes, were obtained from the San Martino Hospital-Istituto Scientifico Tumori Biobank (Genova, Italy) and from the Sant'Andrea Hospital (Roma, Italy) (Institutional Review Board n°168/2003). Lymph node mononuclear cells (MNCs) were isolated after a gentle mince and cryopreserved in a freezing solution composed of 50% RPMI 1640 (Sigma Chemical Co., St. Louis, MO), 40% fetal bovine serum (FBS) (Sigma), and 10% DMSO (Sigma). Cells were kept in liquid nitrogen until tested.
The human HDML-2, L-428, KM-H2cell lines, established from HL patients, were provided five months ago by DSMZ (Braunschweig, Germania) that certifies their origin. These cell lines were cultured in RPMI 1640 medium (Sigma Saint Louis, Missouri, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma).
Antibodies for flow cytometry
The monoclonal Antibodies (mAbs) used throughout the study were the following: Phycoerythrin (PE)-conjugated anti-human IL-31RA from R&D System (Minneapolis, MN, USA); phycocyanin (PC)7-CD19; PE-anti-human OSMR, PC7-CD4, Fluorescein Isothiocyanate (FITC)-CD68, PE-CD127, PE-anti-human TSLPR from eBioscience (San Diego, CA). APC-conjugated anti-human IL-31 and unconjugated anti-human TSLP were from Lifespan Biosciences (Seattle, USA) and Abcam, (Cambridge, UK), respectively. Cells were stained with fluorochrome conjugated or unconjugated antibodies followed by secondary reagents. Isotype and fluorochrome matched antibodies were tested as controls. Cells were run on a Gallios instrument (Beckman Coulter, Brea, CA, USA) and data were analyzed using the Kaluza software (Beckman Coulter). On average 30000/40000 events were acquired. Results were expressed as Mean Relative Fluorescence Intensity (MRFI), calculated as follows: fluorescence intensity obtained with specific mAb/fluorescence intensity obtained with irrelevant isotype-matched mAb. For intracellular cytokine staining cells were fixed, permeabilized using cytofix and perm kit (Becton Dickinson, New Jersey, USA) and stained with anti-IL-31and -TSLP or isotype-control mAbs and analyzed as above.
For some experiments, MNCs from three HL and three reactive lymph nodes were suspended in 0.5M NaCl and 0.2M acetic acid (pH 2.5) and held at 4°C for 10 minutes to elute surface-bound cytokines. Cells were then washed twice with PBS and subsequently stained as above.
RNAscope
The RNAscope assay was applied to lymph node paraffin sections from three HL patients using probes to IL-31 and TSLP, as previously described [56]. Briefly, formalin fixed, paraffin embedded (FFPE) tissue sections 2 μm thick were deparaffinized in xylene and then hydrated in an ethanol series. Hybridization was performed with the negative control probe dapB, the positive control probe Probe-Hs-Ubiquitin, and the target probes Probe-Hs-IL-31 and Probe-Hs-TSLP. The preamplifier, amplifier, label probe, and chromogenic detection procedures were performed according to the manufacturer’s instructions (RNAscope® 2.0 HD Reagent Kit, Advanced Cell Diagnostics, Hayward, CA, USA).
RT-PCR
Total RNA was isolated using the RNeasy kit (Qiagen, Milano, Italy) according to the manufacturer’s instructions. RNA was assessed for integrity by gel electrophoresis and quantified by spectrophotometry (Nanodrop Products, Wilmington, DE). One μg of total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Life Technologies, Monza, Italy), according to manufacturer’s instructions. The primer sequences for human TSLP and GAPDH mRNA and the relative PCR conditions were as described [57]. All the primers were purchased from TIB Molbiol (TIB MolBiolS.r.L., Genova, Italy). The amplified products were visualized by electrophoresis on a 2% agarose gels. Images were analyzed by scanning using the VersaDoc instrument (BioRad Laboratories, Segrate, Italy). PCR reactions for each sample were performed at least twice.
ELISA
Plasma samples from HL patients, collected at diagnosis prior to treatment start, and from healthy controls were tested for IL-31 (n=109 and n=84, respectively) and TSLP (n=75 and n=59, respectively) by ELISA (RayBiotech, Inc., Parkway Lane, Norcross, GA, USA). The sensitivity threshold for both the immunoenzymatic assays was lower than 5 pg/ml.
Statistical analysis
Data were reported in box plot in terms of medians, first and third quartiles, minimun and maximum values. The Mann-Whitney U test was used to compare quantitative variables between two groups of observation with 99% confidence interval (GraphPad Prism 3). The Spearman test was used for the correlation between IL-31 and TSLP levels. All statistical tests were two tailed and a P value lower than 0.05 was considered statistically significant. Statistical analyses were performed using Graph Pad Prism 5software.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
The authors acknowledge the excellent secretarial assistance of Mrs. Camilla Valentino.
Abbreviations
- (HL)
Hodgkin lymphoma
- (HRS)
Hodgkin Reed-Stenberg
- (cHL)
classical
- (NLPHL)
nodular lymphocyte predominant HL
- (GC)
germinal center
- (IL)
interleukin
- (IL-31RA)
IL-31 receptor alpha
- (OSMR)
oncostatin M receptor
- (TSLP)
thymic stromal lymphopoietin
- (s)
soluble
- (IPS)
International Prognostic Score
- (FDG)
(18F)-fluordeoxyglucose
- (PET) (interim PET)
position emission tomography
- (EFS)
event-free survival
- (OS)
overall survival
- (WHO)
World Health Organization
- (FBS)
fetal bovine serum
- (mAbs)
monoclonal antibodies
- (PE)
phycoerythrin
- (FITC)
fluorescein isothiocyanate
- (PC)
phycocyanin
- (MRFI)
mean relative fluorescence intensity
- (FFPE)
formalin fixed paraffin embedded
Author contributions
E.F.: designed research, and performed research; S.H: performed research, analyzed data and contribute to write the paper; A.D. N.: performed immunohistochemical studies and contributed to the collection of lymph nodes samples; B.B.: performed immunohistochemical studies; A.C., E.C., E.G., contributed to analyze patient data; V.R.: contributed to analyze PET correlation data; G.T.: contribute to the collection of donor samples; G.F.O.: contribute to the collection of lymph nodes samples; V.P.: contributed to write the discussion; A.C.: designed research, analyzed data, and wrote the paper.
CONFLICTS OF INTEREST
The authors declare that they have no competing financial interests in relation to the work described.
FUNDING
This work was supported by grants from Associazione Italiana Ricerca Cancro (A.I.R.C.), Milano, Italy to V.P. (Project No13003), from Cinque per mille IRPEF- Finanziamento Ricerca Sanitaria and from Ricerca Corrente Ministeriale. E.F. was a recipient of a Fondazione Umberto Veronesi fellowship.
REFERENCES
- 1.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. The classical Hodgkin's lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol. 2010;221:248–263. doi: 10.1002/path.2711. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Sattarzadeh A, Diepstra A, Visser L, van den Berg A. The microenvironment in classical Hodgkin lymphoma: an actively shaped and essential tumor component. Semin Cancer Biol. 2014;24:15–22. doi: 10.1016/j.semcancer.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Marafioti T, Hummel M, Foss HD, Laumen H, Korbjuhn P, Anagnostopoulos I, Lammert H, Demel G, Theil J, Wirth T, Stein H. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95:1443–1450. [PubMed] [Google Scholar]
- 5.Re D, Kuppers R, Diehl V. Molecular pathogenesis of Hodgkin's lymphoma. J Clin Oncol. 2005;23:6379–6386. doi: 10.1200/JCO.2005.55.013. [DOI] [PubMed] [Google Scholar]
- 6.Matsuki E, Younes A. Lymphomagenesis in Hodgkin lymphoma. Semin Cancer Biol. 2015;34:14–21. doi: 10.1016/j.semcancer.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Seitz V, Hummel M, Marafioti T, Anagnostopoulos I, Assaf C, Stein H. Detection of clonal T-cell receptor gamma-chain gene rearrangements in Reed-Sternberg cells of classic Hodgkin disease. Blood. 2000;95:3020–3024. [PubMed] [Google Scholar]
- 8.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen C, Brans R, Czaja K, Skazik C, Marquardt Y, Zwadlo-Klarwasser G, Kim A, Bickers DR, Luscher-Firzlaff J, Luscher B, Baron JM. Ultraviolet B radiation and reactive oxygen species modulate interleukin-31 expression in T lymphocytes, monocytes and dendritic cells. Br J Dermatol. 2011;165:966–975. doi: 10.1111/j.1365-2133.2011.10487.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishii T, Wang J, Zhang W, Mascarenhas J, Hoffman R, Dai Y, Wisch N, Xu M. Pivotal role of mast cells in pruritogenesis in patients with myeloproliferative disorders. Blood. 2009;113:5942–5950. doi: 10.1182/blood-2008-09-179416. [DOI] [PubMed] [Google Scholar]
- 11.Diveu C, Lelievre E, Perret D, Lak-Hal AH, Froger J, Guillet C, Chevalier S, Rousseau F, Wesa A, Preisser L, Chabbert M, Gauchat JF, Galy A, et al. GPL, a novel cytokine receptor related to GP130 and leukemia inhibitory factor receptor. J Biol Chem. 2003;278:49850–49859. doi: 10.1074/jbc.M307286200. [DOI] [PubMed] [Google Scholar]
- 12.Ghilardi N, Li J, Hongo JA, Yi S, Gurney A, de Sauvage FJ. A novel type I cytokine receptor is expressed on monocytes, signals proliferation, and activates STAT-3 and STAT-5. J Biol Chem. 2002;277:16831–16836. doi: 10.1074/jbc.M201140200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347–356. doi: 10.1016/j.cytogfr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dambacher J, Beigel F, Seiderer J, Haller D, Goke B, Auernhammer CJ, Brand S. Interleukin 31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is upregulated in inflammatory bowel disease. Gut. 2007;56:1257–1265. doi: 10.1136/gut.2006.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasraie S, Niebuhr M, Werfel T. Interleukin (IL)-31 activates signal transducer and activator of transcription (STAT)-1, STAT-5 and extracellular signal-regulated kinase 1/2 and down-regulates IL-12p40 production in activated human macrophages. Allergy. 2013;68:739–747. doi: 10.1111/all.12152. [DOI] [PubMed] [Google Scholar]
- 16.Ohmatsu H, Sugaya M, Suga H, Morimura S, Miyagaki T, Kai H, Kagami S, Fujita H, Asano Y, Tada Y, Kadono T, Sato S. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm Venereol. 2012;92:282–283. doi: 10.2340/00015555-1345. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti E, Tripodo C, Pagnan G, Guarnotta C, Marimpietri D, Corrias MV, Ribatti D, Zupo S, Fraternali-Orcioni G, Ravetti JL, Pistoia V, Corcione A. The interleukin (IL)-31/IL-31R axis contributes to tumor growth in human follicular lymphoma. Leukemia. 2015;29:958–967. doi: 10.1038/leu.2014.291. [DOI] [PubMed] [Google Scholar]
- 18.He R, Geha RS. Thymic stromal lymphopoietin. Ann N Y Acad Sci. 2010;1183:13–24. doi: 10.1111/j.1749-6632.2009.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 20.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Kuan E, Ziegler SF. Thymic stromal lymphopoietin and cancer. J Immunol. 2014;193:4283–4288. doi: 10.4049/jimmunol.1400864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Raap U, Weissmantel S, Gehring M, Eisenberg AM, Kapp A, Folster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol. 2012;23:285–288. doi: 10.1111/j.1399-3038.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 24.Singer EM, Shin DB, Nattkemper LA, Benoit BM, Klein RS, Didigu CA, Loren AW, Dentchev T, Wysocka M, Yosipovitch G, Rook AH. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133:2783–2785. doi: 10.1038/jid.2013.227. [DOI] [PubMed] [Google Scholar]
- 25.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jariwala SP, Abrams E, Benson A, Fodeman J, Zheng T. The role of thymic stromal lymphopoietin in the immunopathogenesis of atopic dermatitis. Clin Exp Allergy. 2011;41:1515–1520. doi: 10.1111/j.1365-2222.2011.03797.x. [DOI] [PubMed] [Google Scholar]
- 27.Gobbi PG, Attardo-Parrinello G, Lattanzio G, Rizzo SC, Ascari E. Severe pruritus should be a B-symptom in Hodgkin's disease. Cancer. 1983;51:1934–1936. doi: 10.1002/1097-0142(19830515)51:10<1934::aid-cncr2820511030>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Airoldi I, Gri G, Marshall JD, Corcione A, Facchetti P, Guglielmino R, Trinchieri G, Pistoia V. Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J Immunol. 2000;165:6880–6888. doi: 10.4049/jimmunol.165.12.6880. [DOI] [PubMed] [Google Scholar]
- 29.Pagnan G, Stuart DD, Pastorino F, Raffaghello L, Montaldo PG, Allen TM, Calabretta B, Ponzoni M. Delivery of c-myb antisense oligodeoxynucleotides to human neuroblastoma cells via disialoganglioside GD(2)-targeted immunoliposomes: antitumor effects. J Natl Cancer Inst. 2000;92:253–261. doi: 10.1093/jnci/92.3.253. [DOI] [PubMed] [Google Scholar]
- 30.Moccia AA, Donaldson J, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, Shenkier TN, Slack GW, Skinnider B, Gascoyne RD, Connors JM, Sehn LH. International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30:3383–3388. doi: 10.1200/JCO.2011.41.0910. [DOI] [PubMed] [Google Scholar]
- 31.Zinzani PL, Broccoli A, Gioia DM, Castagnoli A, Ciccone G, Evangelista A, Santoro A, Ricardi U, Bonfichi M, Brusamolino E, Rossi G, Anastasia A, Zaja F, et al. Interim positron emission tomography response-adapted therapy in advanced-stage Hodgkin lymphoma: final results of the phase II Part of the HD0801 study. J Clin Oncol. 2016;34:1376–1385. doi: 10.1200/JCO.2015.63.0699. [DOI] [PubMed] [Google Scholar]
- 32.Gallamini A, Barrington SF, Biggi A, Chauvie S, Kostakoglu L, Gregianin M, Meignan M, Mikhaeel GN, Loft A, Zaucha JM, Seymour JF, Hofman MS, Rigacci L, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica. 2014;99:1107–1113. doi: 10.3324/haematol.2013.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen HP, Engels HM, Dams M, Paes Leme AF, Pauletti BA, Simhadri VL, Durkop H, Reiners KS, Barnert S, Engert A, Schubert R, Quondamatteo F, Hallek M, Pogge von Strandmann E. Protrusion-guided extracellular vesicles mediate CD30 trans-signalling in the microenvironment of Hodgkin's lymphoma. J Pathol. 2014;232:405–414. doi: 10.1002/path.4306. [DOI] [PubMed] [Google Scholar]
- 34.Kuppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 35.Aldinucci D, Lorenzon D, Cattaruzza L, Pinto A, Gloghini A, Carbone A, Colombatti A. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer. 2008;122:769–776. doi: 10.1002/ijc.23119. [DOI] [PubMed] [Google Scholar]
- 36.Hanamoto H, Nakayama T, Miyazato H, Takegawa S, Hieshima K, Tatsumi Y, Kanamaru A, Yoshie O. Expression of CCL28 by Reed-Sternberg cells defines a major subtype of classical Hodgkin's disease with frequent infiltration of eosinophils and/or plasma cells. Am J Pathol. 2004;164:997–1006. doi: 10.1016/S0002-9440(10)63187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer M, Juremalm M, Olsson N, Backlin C, Sundstrom C, Nilsson K, Enblad G, Nilsson G. Expression of CCL5/RANTES by Hodgkin and Reed-Sternberg cells and its possible role in the recruitment of mast cells into lymphomatous tissue. Int J Cancer. 2003;107:197–201. doi: 10.1002/ijc.11370. [DOI] [PubMed] [Google Scholar]
- 38.Cattaruzza L, Gloghini A, Olivo K, Di Francia R, Lorenzon D, De Filippi R, Carbone A, Colombatti A, Pinto A, Aldinucci D. Functional coexpression of Interleukin (IL)-7 and its receptor (IL-7R) on Hodgkin and Reed-Sternberg cells: Involvement of IL-7 in tumor cell growth and microenvironmental interactions of Hodgkin's lymphoma. Int J Cancer. 2009;125:1092–1101. doi: 10.1002/ijc.24389. [DOI] [PubMed] [Google Scholar]
- 39.Baumforth KR, Birgersdotter A, Reynolds GM, Wei W, Kapatai G, Flavell JR, Kalk E, Piper K, Lee S, Machado L, Hadley K, Sundblad A, Sjoberg J, et al. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin's lymphoma cells mediates Up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173:195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma. Am J Pathol. 1999;154:1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamprecht B, Kreher S, Anagnostopoulos I, Johrens K, Monteleone G, Jundt F, Stein H, Janz M, Dorken B, Mathas S. Aberrant expression of the Th2 cytokine IL-21 in Hodgkin lymphoma cells regulates STAT3 signaling and attracts Treg cells via regulation of MIP-3alpha. Blood. 2008;112:3339–3347. doi: 10.1182/blood-2008-01-134783. [DOI] [PubMed] [Google Scholar]
- 42.Greaves P, Clear A, Owen A, Iqbal S, Lee A, Matthews J, Wilson A, Calaminici M, Gribben JG. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood. 2013;122:2856–2863. doi: 10.1182/blood-2013-06-508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung PF, Wong CK, Ho AW, Hu S, Chen DP, Lam CW. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol. 2010;22:453–467. doi: 10.1093/intimm/dxq027. [DOI] [PubMed] [Google Scholar]
- 44.Wong CK, Leung KM, Qiu HN, Chow JY, Choi AO, Lam CW. Activation of eosinophils interacting with dermal fibroblasts by pruritogenic cytokine IL-31 and alarmin IL-33: implications in atopic dermatitis. PLoS One. 2012;7:e29815. doi: 10.1371/journal.pone.0029815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hui CC, Rusta-Sallehy S, Asher I, Heroux D, Denburg JA. The effects of thymic stromal lymphopoietin and IL-3 on human eosinophil-basophil lineage commitment: Relevance to atopic sensitization. Immun Inflamm Dis. 2014;2:44–55. doi: 10.1002/iid3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 48.Han H, Headley MB, Xu W, Comeau MR, Zhou B, Ziegler SF. Thymic stromal lymphopoietin amplifies the differentiation of alternatively activated macrophages. J Immunol. 2013;190:904–912. doi: 10.4049/jimmunol.1201808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirano R, Hasegawa S, Hashimoto K, Haneda Y, Ohsaki A, Ichiyama T. Human thymic stromal lymphopoietin enhances expression of CD80 in human CD14+ monocytes/macrophages. Inflamm Res. 2011;60:605–610. doi: 10.1007/s00011-011-0310-0. [DOI] [PubMed] [Google Scholar]
- 50.Hermanns HM. Oncostatin M and interleukin-31: cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015;26:545–558. doi: 10.1016/j.cytogfr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Hoe E, McKay FC, Schibeci SD, Gandhi K, Heard RN, Stewart GJ, Booth DR. Functionally significant differences in expression of disease-associated IL-7 receptor alpha haplotypes in CD4 T cells and dendritic cells. J Immunol. 2010;184:2512–2517. doi: 10.4049/jimmunol.0902900. [DOI] [PubMed] [Google Scholar]
- 52.Bjerkan L, Schreurs O, Engen SA, Jahnsen FL, Baekkevold ES, Blix IJ, Schenck K. The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. Mucosal Immunol. 2015;8:49–56. doi: 10.1038/mi.2014.41. [DOI] [PubMed] [Google Scholar]
- 53.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol. 2015;51:263–292. doi: 10.1007/s12016-015-8488-5. [DOI] [PubMed] [Google Scholar]
- 54.Broxmeyer HE, Li J, Hangoc G, Cooper S, Tao W, Mantel C, Graham-Evans B, Ghilardi N, de Sauvage FJ. Regulation of myeloid progenitor cell proliferation/survival by IL-31 receptor and IL-31. Exp Hematol. 2007;35:78–86. doi: 10.1016/j.exphem.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baggiolini M. CXCL8 - The first chemokine. Front Immunol. 2015;6:285. doi: 10.3389/fimmu.2015.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo PF, Du MR, Wu HX, Lin Y, Jin LP, Li DJ. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood. 2010;116:2061–2069. doi: 10.1182/blood-2009-11-252940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


