According to Kantor's findings, the majority of Americans support human–animal chimera research, including the creation of human–animal chimeric embryos (HACEs) for disease studies and human–animal chimeras to grow organs for transplant to humans, whereas only about 20% oppose it 1. His survey included questions on ethical issues concerning HACE research, that is, humanized animals in which human pluripotent stem cells transplanted to create human–animal chimeras contribute to brain and germline. The results revealed that among people who expressed support for such research, 77.4% supported studies in which brain contains human cells, and among those who opposed such research, 87.5% did not support the contribution of human cells to brain. These data have important implications for the formulation of future regulations.
Between February and April 2016, we conducted a survey on HACE research (Supporting Information) 2. Regarding the production of HACEs, 81.0% of the public and 92.4% of researchers indicated support. Regarding the production of human–animal chimeras, 64.5% of the public and 83.8% of researchers indicated support. Compared to Kantor's findings, the results of this survey showed a high level of support for the production of HACEs and human–animal chimeras. The acceptance rates were higher than those of a survey conducted by Inoue and his colleagues (referred in Kantor) for the general public and researchers in Japan with respect to the production of HACEs with the purpose of producing organs for transplant 2, 3. One possible reason for this difference is that the subjects were asked about their level of acceptance for various steps: HACE research was classified into three steps and the research objectives of each step were explained in detail (Supporting Information).
Conventionally, ethical problems in HACE research, including concerns about whether human–animal chimeras will have human‐like cognitive abilities, and, more recently, whether human–animal hybrids or humans will be produced, have been debated 4, 5, 6, 7, 8. Therefore, in addition to the brain, which was included in Kantor's survey, our survey also included six items related to liver, sperm/egg, skin, blood, and heart to gauge the level of acceptance for the inclusion of human cells in animal organs, tissues, and cells (Supporting Information).
Similar to conventional ethical concerns, the results of our survey suggest that both the general public and researchers are more concerned about human cells in brain and sperm/egg than in other organs or tissues (see Figure 1). Moreover, subjects were asked about acceptance for various degrees of human cells included (0%–100%) in each organ (Supporting Information). The results revealed that 48.5% of the general public and 45.7% of researchers never accept human cells contributing to the brain (0%), and 52.1% of the public and 74.3% of researchers never accept human cells contributing to the germline (0%).
Figure 1.
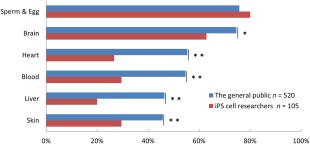
Level of unacceptance regarding the inclusion of human cells in organs/tissues/cells of human–animal chimeras. *, p < .05, χ2 test; **, p < .01, χ2 test. In our survey, subjects were presented with four choices for each organ (item): “acceptable,” “somewhat acceptable,” “somewhat unacceptable,” and “unacceptable.” The above figure summarizes the results for subjects who responded “somewhat unacceptable” or “unacceptable.”
The survey results suggest the necessity of exploring measures to avoid issues regarding humanized brain and gametes in HACE research. HACE research is likely to be more widely accepted in the future by overcoming technological challenges along with the appropriate dissemination of relevant information.
Acknowledgments
We acknowledge the support of a Grant‐in‐Aid for Scientific Research (no. 26253032) from the Ministry of Education Culture, Sports Science, and Technology (MEXT), and the Uehiro Foundation on Ethics and Education. We are also grateful to thank Dr. Peter Karagiannis (Center for iPS Cell Research and Application, Kyoto University, Japan) for his comments and suggestions on earlier drafts of this article. Writing assistance was utilized in the production of this manuscript. The authors asked Editage (www.editage.jp/translation/) for English translating.
Author Contributions
T.S., T.H., and M.F.: conception and design, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Tsutomu Sawai, Taichi Hatta, Misao Fujita
Uehiro Research Division for iPS Cell Ethics, Center for iPS Cell Research and Application, Kyoto University, Kyoto, Japan
Supporting information
Supporting Information.
References
- 1. Kantor J. Public support in the U.S. for human‐animal chimera research: Results of a representative cross‐sectional survey of 1,058 adults [letter]. Stem Cells Transl Med 2017;6:1442–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sawai T, Hatta T, Fujita M. Public attitudes in Japan towards human‐animal chimeric embryo research using human induced pluripotent stem cells. Regen Med 2017;12:233‐248. [DOI] [PubMed] [Google Scholar]
- 3. Inoue Y, Shineha R, Yashiro Y. Current public support for human‐animal chimera research in Japan is limited, despite high levels of scientific approval. Cell Stem Cell 2016;19:152–153. [DOI] [PubMed] [Google Scholar]
- 4. Greely HT. Human/nonhuman chimeras: Assessing the issues. In: Beauchamp TL, Frey RG, eds. Oxford Handbook of Animal Ethics. Oxford: Oxford University Press, 2011:671–698. [Google Scholar]
- 5. Palacios‐González C. Human dignity and the creation of human‐nonhuman chimeras. Med Health Care Philos 2015;18:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palacios‐Gonzalez C. Ethical aspects of creating human‐nonhuman chimeras capable of human gamete production and human pregnancy. Monash Bioeth Rev 2015;33:181–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shaw D, Dondorp W, Geijsen N et al. Creating human organs in chimera pigs: An ethical source of immunocompatible organs? J Med Ethics 2015;41:970–974. [DOI] [PubMed] [Google Scholar]
- 8. Bourret R, Martinez E, Vialla F et al. Human‐animal chimeras: Ethical issues about farming chimeric animals bearing human organs. Stem Cell Res Ther 2016;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


