Supplemental Digital Content is available in the text
Keywords: breast cancer, meta-analysis, neutrophil to lymphocyte ratio, prognosis, survival
Abstract
Background:
Inflammation and cancer are closely related to each other. As a parameter that can reflect inflammation and host immune reaction, elevated blood neutrophil to lymphocyte ratio (NLR) has been confirmed to be correlated with poor prognosis in a variety of cancers. However, this remains controversial in breast cancer. Thus, we performed this updated meta-analysis to further clarify whether high NLR could be a predictor of survival in breast cancer patients.
Methods:
We searched on PubMed Database and Cochrane Library. Overall survival (OS), disease-free survival (DFS), and cancer-specific survival were used as outcome events, and hazard ratio (HR) was chosen as the parameter to evaluate the correlation.
Result:
Eighteen eligible studies were involved in this meta-analysis. The synthesized analysis demonstrated that elevated NLR was associated with poor DFS [HR = 1.72, 95% confidence interval (95% CI) = 1.30–2.27], OS (HR = 1.87, 95% CI = 1.41–2.48), and cancer-specific survival (HR = 2.09, 95% CI = 1.04–4.21). The correlation was stronger in triple-negative breast cancer (TNBC) (OS: HR = 2.58, 95% CI = 1.63–4.06; DFS: HR = 3.51, 95% CI = 1.97–6.24).
Conclusion:
Higher NLR was correlated to poor prognosis of breast cancer patients. As a clinical parameter that we can easily obtain, NLR might be a potential predictor in patients’ survival to assist with physicians’ treatment decisions.
1. Introduction
Breast cancer has been the most common cancer in women all over the world, and 1.8 million women died of it in 2013.[1–3] Five major subtypes of breast cancer (luminal A and B, Her2, basal, and normal-like) are very different in clinical characters and prognosis.[4] Inflammation plays an important role in cancer. More and more studies have found that cancer and inflammation are closely related to each other. Not only inflammation results in cancer but also cancer causes inflammation. Serum interleukin (IL)-6, IL-8 that are linked to inflammation and macrophages in the tumor microenvironment (TME) have been identified as unfavorable factors for cancer patients’ survival.[5,6] Furthermore, anti-inflammation agents such as aspirin have been put in a new height in cancer prevention and therapy.
As a parameter that can reflect inflammation and host immune reaction, blood neutrophil to lymphocyte ratio (NLR) has received much attention in predicting the prognosis of cancer. Large numbers of studies have demonstrated that high NLR is related to poor prognosis of malignant tumor, including breast cancer, lung cancer, gastric cancer, colorectal cancer, and prostate cancer.[7–11] Most studies showed that elevated NLR could be a predictor of poor prognosis in breast cancer patients. However, some studies could not support this conclusion very well.[12,13]
In the meta-analysis by Chen et al,[14] only 8 earlier studies were enrolled. Therefore, we performed this meta-analysis in which we included the newest studies to further clarify the relationship between NLR and prognosis of breast cancer patients.
2. Materials and method
2.1. Search strategy and selection criteria
A selective literature search was performed by 2 reviewers (Liu and Qu) using PubMed Database and the Cochrane Library with following terms: “neutrophil-to-lymphocyte ratio” or “neutrophil to lymphocyte ratio” or “NLR” and “breast cancer” or “tumor” or “carcinoma” and “prognosis” or “outcome” or “survival”. This meta-analysis was conducted in accordance with the guidelines provided by the PRISMA statement.
We included publications of clinical research aimed to study the association between NLR and breast cancer prognosis. NLR of peripheral blood is tested before any treatment including surgery or neoadjuvant treatment. Meanwhile, those studies should choose disease-free survival (DFS) or overall survival (OS) or disease-specific mortality (DSS) as the outcome events. Each included study was approved by an ethics committee or institutional review board. Exclusion criteria were as follows: publications that we could not extract sufficient data that we were interested in; nonclinical research, abstracts, reviews, letters, case reports, meta-analyses, and proceedings; duplicate publications; and subgroup of the included articles.
2.2. Data extraction and quality assessment
The first author, publishing year, county of origin, stage of studying population, treatment, the number of patients included in the analysis, molecular subtype of the patients, end-point of follow-up including DFS, OS, DSS, and parameters to analyze the correlation between prognosis and NLR were extracted from each eligible study by 2 independent investigators. Disagreements were resolved by discussion or consensus with a third reviewer.
2.3. Data synthesis and analysis
The hazard ratio (HR), its 95% confidence interval (95% CI), or P value were extracted, respectively, and HR of high NLR group was finally synthesized into pooled analysis to estimate the correlation between NLR and survival. All analyses were performed by using STATA Statistical Software, version 12.0 (Stata Corp, College Station, TX). Cochran Q test was chosen to evaluate the heterogeneity and I2 was carried out to estimate the degree of heterogeneity among the included studies. Fixed-effect model was used to calculate HR if I2 ≤ 50% and P > 10%, and significant heterogeneities (I2 > 50) among the studies were resolved with the random-effects model. Subgroup analysis was conducted to determine the source of heterogeneity. Sensitivity analyses were performed to determine the stability of the result. Publication bias was analyzed using Begg and Egger funnel plot. P>|t| ≤1 0% is considered to exist publication bias. Trim and fill method was used to assess the influence of publication bias on pooled results.
3. Results
3.1. Search result
According to our search strategy, a total of 107 publications were identified, and among them, 35 duplicated publications were excluded. Then, 42 unrelated publications were excluded by reviewing titles and abstracts. Further, we review the left 30 publications carefully, and, find that among them, 6 studies did not provide enough data for our analysis, or did not measure NLR at initial treatment, studied chemotherapy response instead of survival, or publications were meta-analysis and review. The left 23 studies were eligible.[9,12,13,15–34] However, when we perform a review of the 23 publications again, we find that patients in the study by Chen et al[33] is a subgroup of Jia et al.[20] As a result, the study by Chen et al[33] was excluded finally. Regrettably, 2 publications by Azab et al[25,34] reported a dose–response HR, as patients’ NLR was divided into quartiles. To avoid heterogeneity in study method, these 2 were excluded. Although 2 publications by Forget et al[24,32] had different research aims, they all reported HR for DFS or OS in different neutrophil-to-lymphocyte ratios. Taking the number of patients and quality of the 2 studies into consideration, we chose one of them in our analysis. In addition, only 1 literature[12] chose OR as a parameter to assess the correlation between NLR and breast cancer survival. Finally, 18 studies were conducted in our meta-analysis. Figure 1 is the flowchart that describes our literature search progress. Among these 18 studies, 12 publications reported HR for DFS and 11 for OS. Four publications provided the HR for cancer-specific survival (CSS). Table 1 presents some details of the 18 included studies.
Figure 1.
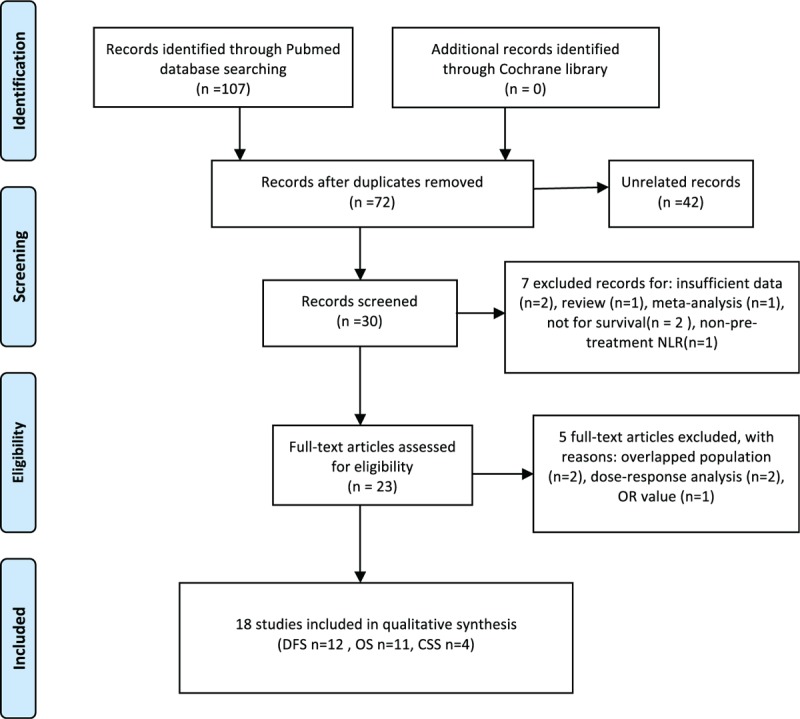
Flow graph of searching process.
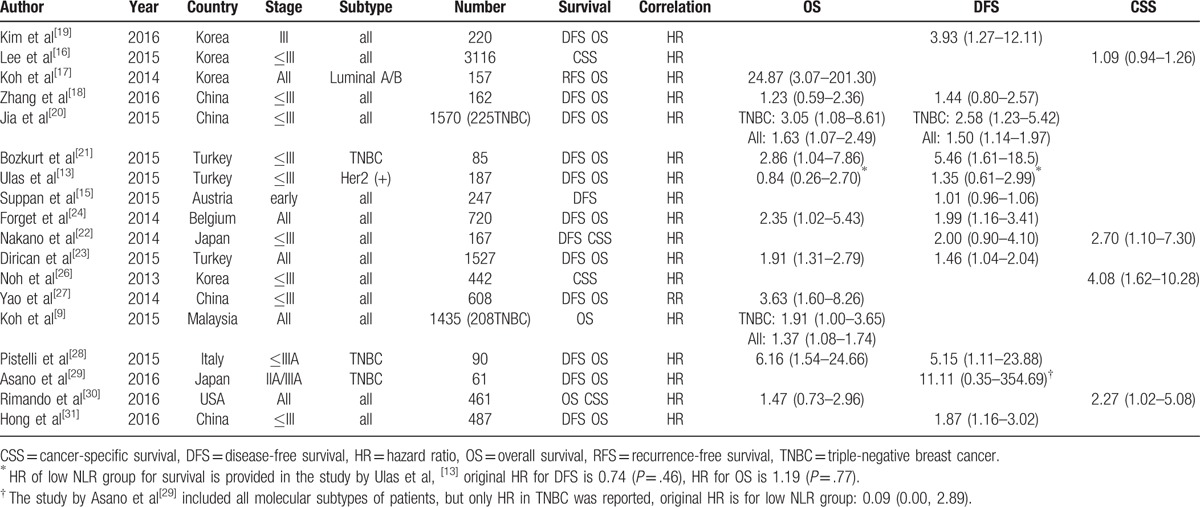
Table 1.
Main characteristics of 18 eligible studies.
3.2. NLR and DFS
Twelve studies with 5523 patients provided HR for OS. Among them, 6 studies came from East or Southeast Asia, and the others were located in Turkey, East Europe, or the USA, mainly recruiting Caucasian patients. We defined those with less than 200 patients as small number studies. Therefore, half of the 12 studies were categorized into small number group. Pooled results showed that higher pretreatment NLR patients are prone to get a poorer outcome in DFS (HR = 1.72, 95% CI = 1.30–2.27, I2 = 76%, Pheterogeneity < .001, Fig. 2). Deletion of any of these studies did not significantly alter our pooled result, but the heterogeneity changed obviously after exclusion of study by Suppan et al[15] when sensitivity analysis was performed (Table 2, Supplementary Figure 3). We predicted that the study by Suppan et al[15] might be an origin of heterogeneity. Then subgroup analysis was conducted, and the result showed that high heterogeneity still existed in Caucasian and large number study group when the study by Suppan et al[15] was present (Table 3). As Suppan et al[15] depicted in their article, early breast cancer patients were chosen in his study, very different from others. Thus, another synthesized analysis was performed when this study was absent. As expected, all the subgroups manifested association between NLR and DFS without statistical heterogeneity (Supplementary Figure 2). These indicated that this study played a critical role in heterogeneity of our pooled analysis.
Figure 2.
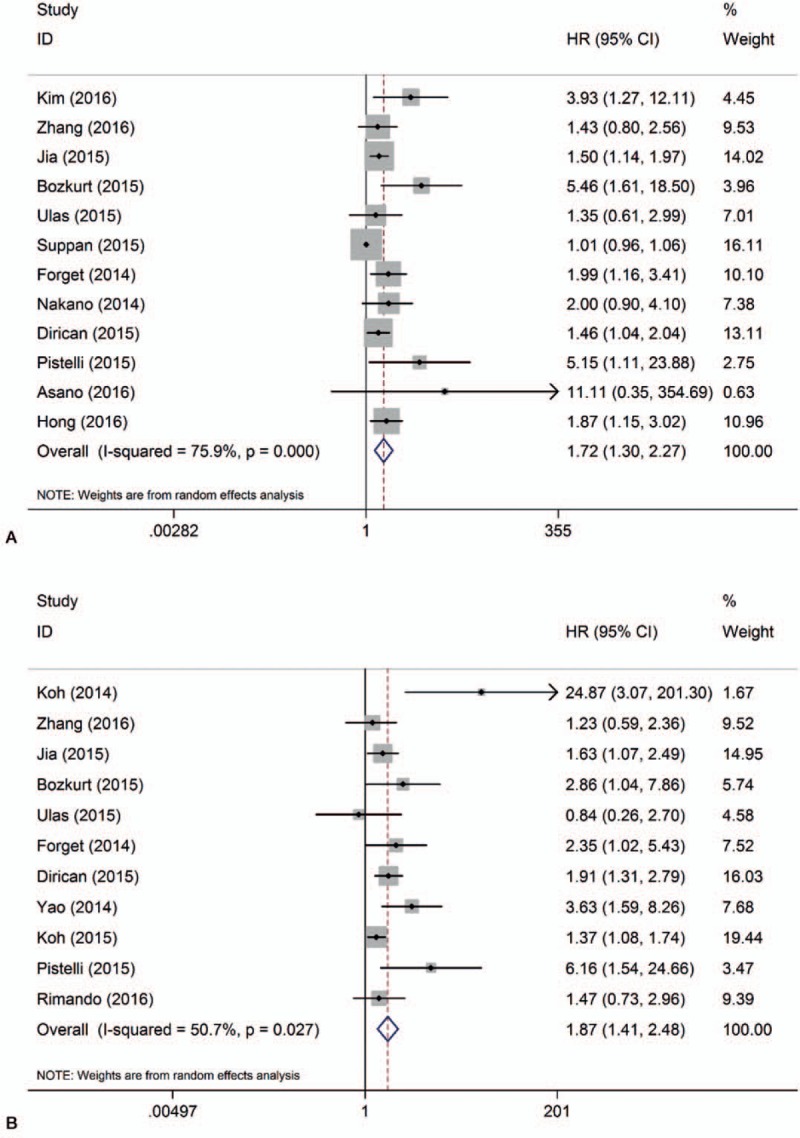
Forrest plots of survival: (A) HR for disease-free survival; (B) HR for overall survival.
Table 2.
Result for sensitivity analysis of DFS and OS with random effect model.
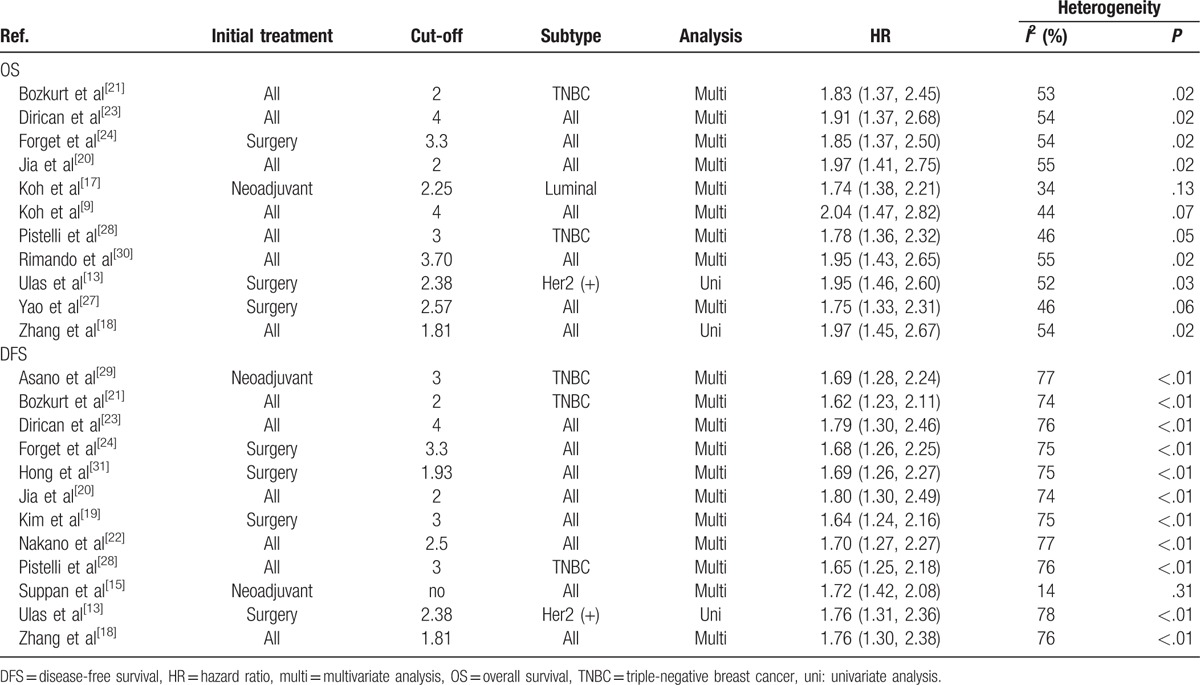
Table 3.
Result for subgroup analysis.
3.3. NLR and OS
Eleven studies comprising 7002 patients reported HR for OS. In the study by Ulas et al,[13] HR of low NLR group for survival was reported rather than that of high NLR group. Therefore, we had to convert HR into data of high NLR group to keep consistent with others’ reports. Pooled analysis demonstrated that high NLR significantly decreased OS (HR = 1.87, 95% CI = 1.41–2.48, I2 = 51%, Pheterogeneity = .027). In order to find out the origin of heterogeneity, we performed a subgroup analysis taking ethnicity, tumor stage, and the number of patients into consideration, respectively. Although adverse effect of higher NLR still existed in each subgroup, significant heterogeneity was also observed in Asian, small number, or all tumor stage subgroup) as summarized in Table 3. The study by Koh et al[17] might be a common factor, as it was categorized to those subgroups with a high heterogeneity. However, sensitivity analysis indicated that the study by Koh et al[17] did not significantly affect the stability of our result. Four of the eleven studies included single molecular subtype patients (1 luminal, 1 Her2 positive, 2 TNBCs) instead of all the subtype compared with the other 7. Thus, another pooled analysis excluding all these 4 studies presented accordant correlation between NLR and OS without significant heterogeneity suggesting that these 4 studies potentially affected heterogeneity of total analysis (Supplementary Figure 1).
3.4. NLR and triple-negative breast cancer
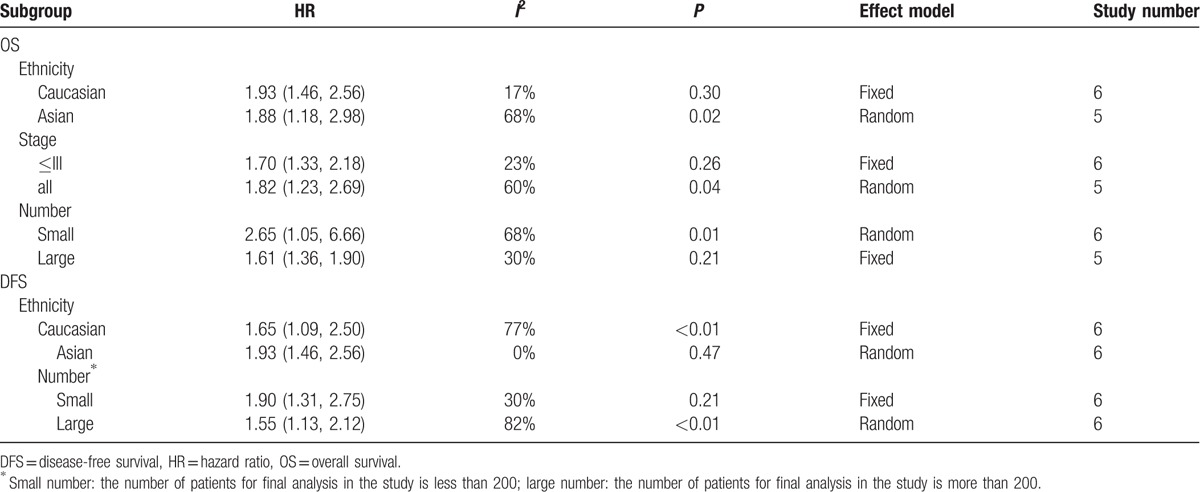
Triple-negative breast cancer (TNBC) is a heterogeneous and clinically aggressive disease with poorer prognosis compared with Luminal or Her2 (+) subtype.[35,36] We wanted to identify whether high NLR had adverse effects on the survival of TNBC. Fortunately, 3[21,28,29] out of 18 studies were aimed to study NLR and survival in TNBC patients. Although all subtypes of breast cancer patients were included in the studies by Jia et al[20] and Koh et al,[9] HR for survival in TNBC was provided additionally in their report. Pooled analysis in which the above 5 studies were included demonstrated that NLR had a stronger association with both OS (HR = 2.58, 95% CI = 1.63–4.06, I2 < 0.1%, Pheterogeneity = .48) and DFS (HR = 3.51, 95% CI = 1.97–6.24, I2 < 0.1%, Pheterogeneity = .61) in TNBC (Fig. 3).
Figure 3.
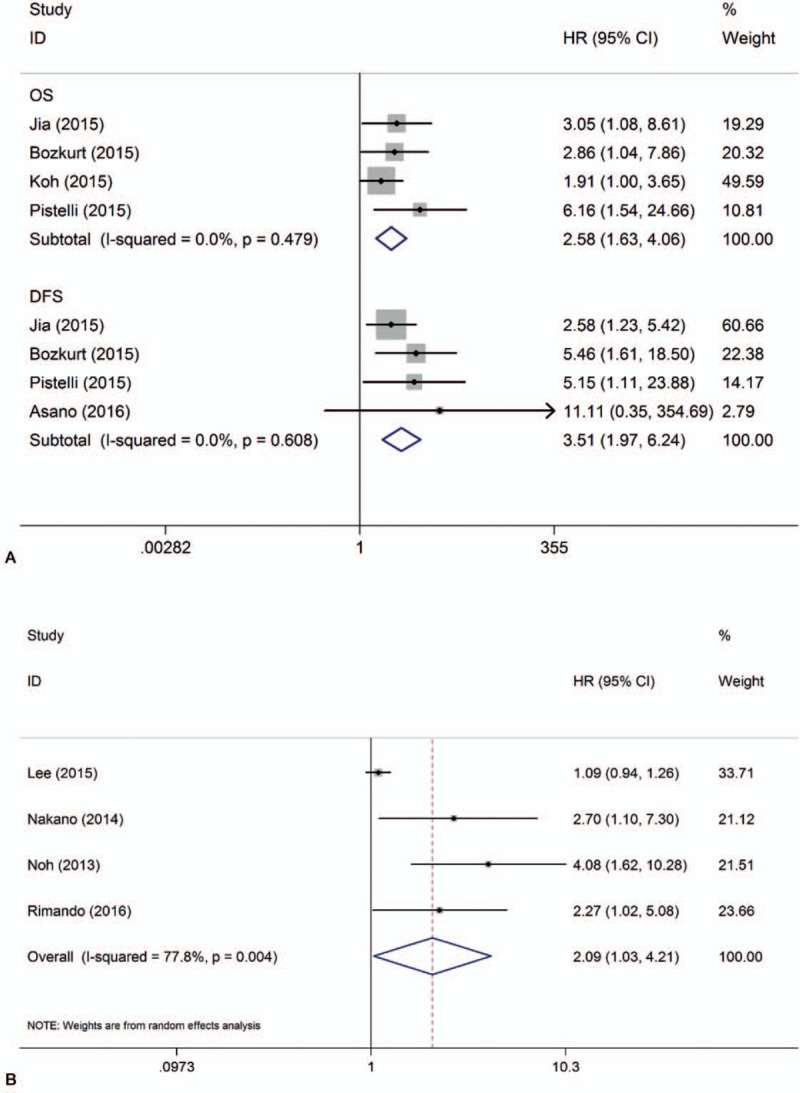
Forrest plots of survival: (A) HR for survival in TNBC; (B) HR for cancer-specific survival.
3.5. NLR and CSS
Four studies involving 4186 patients estimated the association between NLR and CSS. Patients with higher NLR had poorer CSS (HR = 2.09, 95% CI = 1.04–4.21) than those with lower NLR (Fig. 3), with a significant heterogeneity (I2 = 78%). Similarly, stability of the pooled result was influenced by the study by Lee et al[16] in sensitivity analysis (Supplementary Figure 3). As there were too few studies in this pooled analysis, we did not perform subgroup analysis and publication bias analysis.
3.6. Publication bias
Begg and Egger test (Fig. 4, supplementary Figure 4) revealed obvious publication bias (OS: P>|t| = .034; DFS: P >|t|< .001). To determine the origin of this bias, the trim-and-fill method was applied. For DFS, there were 4 potential publications, and 2 hypothesized publications for OS. Recalculation of HR indicated that high NLR might be a risk factor for both OS (HR = 1.72, 95% CI = 1.25–2.36) and DFS (HR = 1.49, 95% CI = 1.14–1.94) even though there existed heterogeneity.
Figure 4.
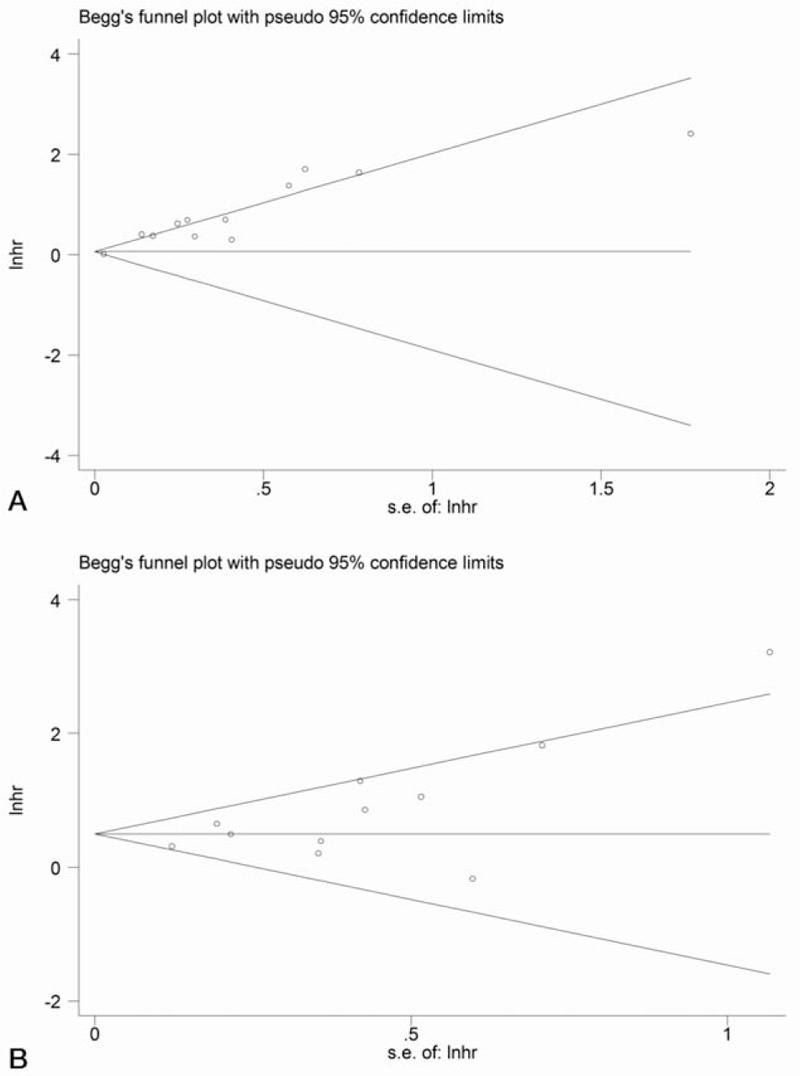
Begg funnel plot: (A) estimated publication bias in pooled analysis of disease-free survival; (B) estimated publication bias in pooled analysis of overall survival.
4. Discussion
Although the mechanisms of the association between tumor and inflammation have not been fully understood, it is sure that tumor and inflammation interacts with each other.[37,38] On the one hand, regional hypoxia and reactive oxygen species (ROS) is considered to be a potential factor to drive inflammatory response in tumor.[39–42] On the other hand, cancer-related inflammation plays a necessary role in the prognosis of cancer.[38,43–46] According to that, several targets related to inflammation including cyclooxygenase, chemokine (C-C motif) ligand 2, CXC chemokine receptor 4 (CXCR4), nuclear factor kappa B, and so on have been established to apply in the treatment of cancer.[37,43,47–50] Besides, biomarkers associated with systemic inflammatory response such as neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and C-reactive protein were developed to predict the prognosis of cancer.[51] Particularly in recent years, an increasing evidence demonstrated that pretreatment NLR could be a predictor of prognosis in variates of cancers.[36,52–54] It could be mainly explained by TME and different functions of neutrophils and lymphocytes.
TME is defined as a complex tissue composed not only of tumor cells but also of stromal cells, inflammatory cells, vasculature, and extracellular matrices (ECMs).[55] TME plays a critical role in regulating tumor immunity that is the basis of immunotherapy and further affects progression of cancer. Neutrophils in TME are divided into 2 types: N1 and N2. The former owns anti-tumor effect, while the later has immunosuppressive effect. Recruited neutrophils produce protein or chemokines such as CXCR, cytokines, vascular endothelial growth factor, or matrix metalloproteinase 9 to promote proliferation, invasion, and angiogenesis of tumor and more intratumoral neutrophil infiltration is associated with poorer prognosis in varieties of carcinoma.[56,57] In contrast, lymphocytes are known to have the function of immunosurveillance and anti-tumor effects.[58–60] Besides, pooled analysis indicates that a decreased number of tumor-infiltrating lymphocytes in breast cancer tissue predicts low pathology complete response rate to chemotherapy as well as shorter DFS and OS.[61,62] Neutrophil-to-lymphocyte ratio might indirectly reflect immunosuppressive versus anti-tumor effect in cancer patients. Therefore, patients with an elevated NLR might be inclined to show a poorer outcome.
In this study, a system review with a meta-analysis of previous publications aimed to study correlations between pretreatment NLR and survival was performed. The result demonstrated that high NLR correlated with poor OS (HR = 1.87, 95% CI = 1.41–2.48), DFS (HR = 1.72, 95% CI = 1.30–2.27), and CSS (HR = 2.09, 95% CI = 1.04–4.21). We got stable results even though we excluded Suppan and Koh's study, which we identified as the potential origin of heterogeneity in our pooled analysis. What is more, we found that the correlation was stronger in TNBC with a higher HR (OS: HR = 2.58, 95% CI = 1.63–4.06; DFS: HR = 3.51, 95% CI = 1.97–6.24).
Although there has been 1 meta-analysis examining the role of NLR in predicting survival of breast cancer patients so far, our study possessed the following several advantages: First, comparing with previous one that included only 8 studies, we included the newest publications from 2015 to 2016 in our analysis, which to date, contained the most study (18 publications) and patients number (5523 for DFS, 7002 for OS) to ensure the reliability of the conclusion. In particular, we involved 11 studies from Asian population. Second, we had a more strict inclusion criterion. For instance, we depleted studies came from the same center to avoid duplicated data in pooled analysis, while the previous meta-analysis used 2 studies by Azab et al[25,34] and Forget et al.[24,32] In addition, we had chosen HR and relative risk (RR) (only 1 study) as the parameters of correlation in the synthesized analysis. By contrast, another meta-analysis included Cihan et al[12] that used OR to measure the association. Lastly, this is the first article to examine HR for DFS and OS in TNBC patients, and we also studied the influence of high NLR on CSS.
However, the following limitations must be considered when interpreting the findings in our study. First significant heterogeneity was observed in each pooled analysis. Aiming at this, we tried our best to find out the source of heterogeneity by subgroup and sensitivity analysis. What is more, those heterogeneous studies hardly altered pooled results. Second, there was publication bias in DFS and OS pooled analysis. However, trim-and-filled method demonstrated that the publication bias did not affect stability of the result. Third, all involved studies used different cut-off values that might affect extrapolation and operability of the results in predicting the survival of patients. Finally, there were only 4 studies in pooled analysis for CSS, and subgroup analysis was not performed.
5. Conclusion
Our meta-analysis suggested that high NLR was associated with poor prognosis in breast cancer patients. As an easily available clinical index, NLR could serve as a predictor of patients’ survival to assist with treatment decisions. Thus, more high quality of perspective studies is necessary to be conducted to validate its role in breast cancer.
Supplementary Material
Footnotes
Abbreviations: CSS = cancer-specific survival, DFS = disease-free survival, HR = hazard ratio, NLR = neutrophil to lymphocyte ratio, OR = odds ratio, OS = overall survival, RFS = recurrence-free survival, RR = relative risk, TNBC = triple-negative breast cancer.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol 2015;1:505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [4].Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Allen MD, Jones LJ. The role of inflammation in progression of breast cancer: friend or foe? (Review). Int J Oncol 2015;47:797–805. [DOI] [PubMed] [Google Scholar]
- [6].Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015;12:584–96. [DOI] [PubMed] [Google Scholar]
- [7].Jiang Y, Xu H, Jiang H, et al. Pretreatment neutrophil-lymphocyte count ratio may associate with gastric cancer presence. Cancer Biomark 2016;16:523–8. [DOI] [PubMed] [Google Scholar]
- [8].Minardi D, Scartozzi M, Montesi L, et al. Neutrophil-to-lymphocyte ratio may be associated with the outcome in patients with prostate cancer. SpringerPlus 2015;4:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 2015;113:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015;236:297–304. [DOI] [PubMed] [Google Scholar]
- [11].Chen J, Hong D, Zhai Y, et al. Meta-analysis of associations between neutrophil-to-lymphocyte ratio and prognosis of gastric cancer. World J Surg Oncol 2015;13:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cihan YB, Arslan A, Cetindag MF, et al. Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev 2014;15:4225–31. [DOI] [PubMed] [Google Scholar]
- [13].Ulas A, Avci N, Kos T, et al. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? J BUON 2015;20:714–22. [PubMed] [Google Scholar]
- [14].Chen J, Deng Q, Pan Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. FEBS Open Bio 2015;5:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suppan C, Bjelic-Radisic V, La Garde M, et al. Neutrophil/Lymphocyte ratio has no predictive or prognostic value in breast cancer patients undergoing preoperative systemic therapy. BMC Cancer 2015;15:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee SK, Choi MY, Bae SY, et al. Immediate postoperative inflammation is an important prognostic factor in breast cancer. Oncology 2015;88:337–44. [DOI] [PubMed] [Google Scholar]
- [17].Koh YW, Lee HJ, Ahn JH, et al. Prognostic significance of the ratio of absolute neutrophil to lymphocyte counts for breast cancer patients with ER/PR-positivity and HER2-negativity in neoadjuvant setting. Tumour Biol 2014;35:9823–30. [DOI] [PubMed] [Google Scholar]
- [18].Zhang P, Zong Y, Liu M, et al. Prediction of outcome in breast cancer patients using test parameters from complete blood count. Mol Clin Oncol 2016;4:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim YY, Park HK, Lee KH, et al. Prognostically distinctive subgroup in pathologic N3 breast cancer. J Breast Cancer 2016;19:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jia W, Wu J, Jia H, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One 2015;10:e0143061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bozkurt O, Karaca H, Berk V, et al. Predicting the role of the pretreatment neutrophil to lymphocyte ratio in the survival of early triple-negative breast cancer patients. J BUON 2015;20:1432–9. [PubMed] [Google Scholar]
- [22].Nakano K, Hosoda M, Yamamoto M, et al. Prognostic significance of pre-treatment neutrophil: lymphocyte ratio in Japanese patients with breast cancer. Anticancer Res 2014;34:3819–24. [PubMed] [Google Scholar]
- [23].Dirican A, Kucukzeybek BB, Alacacioglu A, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol 2015;20:70–81. [DOI] [PubMed] [Google Scholar]
- [24].Forget P, Bentin C, Machiels JP, et al. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 2014;113(Suppl 1):i82–7. [DOI] [PubMed] [Google Scholar]
- [25].Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol (Northwood, London, England) 2013;30:432. [DOI] [PubMed] [Google Scholar]
- [26].Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer 2013;16:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yao M, Liu Y, Jin H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. OncoTargets Ther 2014;7:1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pistelli M, De Lisa M, Ballatore Z, et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer 2015;15:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Asano Y, Kashiwagi S, Onoda N, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol 2016;23:1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rimando J, Campbell J, Kim JH, et al. The pretreatment neutrophil/lymphocyte ratio is associated with all-cause mortality in black and white patients with non-metastatic breast cancer. Front Oncol 2016;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hong J, Mao Y, Chen X, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol 2016;37:4135–42. [DOI] [PubMed] [Google Scholar]
- [32].Forget P, Machiels JP, Coulie PG, et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol 2013;20(Suppl 3):S650–60. [DOI] [PubMed] [Google Scholar]
- [33].Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer 2016;16:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 2012;19:217–24. [DOI] [PubMed] [Google Scholar]
- [35].Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol 2014;32:2142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013;33(Suppl 1):S79–84. [DOI] [PubMed] [Google Scholar]
- [38].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [39].Wu Y, Antony S, Meitzler JL, et al. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett 2014;345:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tafani M, Sansone L, Limana F, et al. The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid Med Cell Longev 2016;2016:3907147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett 2017;387:95–105. [DOI] [PubMed] [Google Scholar]
- [42].Blaser H, Dostert C, Mak TW, et al. TNF and ROS crosstalk in inflammation. Trends Cell Biol 2016;26:249–61. [DOI] [PubMed] [Google Scholar]
- [43].Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Matsumoto H, Koo SL, Dent R, et al. Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol 2015;68:506–10. [DOI] [PubMed] [Google Scholar]
- [45].Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sharon Y, Raz Y, Cohen N, et al. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res 2015;75:963–73. [DOI] [PubMed] [Google Scholar]
- [47].Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 2012;22:33–40. [DOI] [PubMed] [Google Scholar]
- [48].Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- [49].Zelenay S, van der Veen AG, Bottcher JP, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 2015;162:1257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu Q, Tan Q, Zheng Y, et al. Blockade of Fas signaling in breast cancer cells suppresses tumor growth and metastasis via disruption of Fas signaling-initiated cancer-related inflammation. J Biol Chem 2014;289:11522–35. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [51].Wulaningsih W, Holmberg L, Garmo H, et al. Prediagnostic serum inflammatory markers in relation to breast cancer risk, severity at diagnosis and survival in breast cancer patients. Carcinogenesis 2015;36:1121–8. [DOI] [PubMed] [Google Scholar]
- [52].Cheng H, Long F, Jaiswar M, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep 2015;5:11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Na N, Yao J, Cheng C, et al. Meta-analysis of the efficacy of the pretreatment neutrophil-to-lymphocyte ratio as a predictor of prognosis in renal carcinoma patients receiving tyrosine kinase inhibitors. Oncotarget 2016;7:44039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Luo Y, She DL, Xiong H, et al. Pretreatment neutrophil to lymphocyte ratio as a prognostic predictor of urologic tumors: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett 2016;370:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol 2016;37:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shen MX, Hu PP, Donskov F, et al. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One 2014;9:e98259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].West NR, Milne K, Truong PT, et al. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 2011;13:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949–55. [DOI] [PubMed] [Google Scholar]
- [60].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [61].Wang K, Xu J, Zhang T, et al. Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: a meta-analysis. Oncotarget 2016;7:44288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014;25:1536–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


