Abstract
Recent genetic, molecular and postmortem studies suggest impaired D2R trafficking in patients with schizophrenia (SZ). Imaging and preclinical studies have shown agonist-induced D2R internalization can be imaged with positron emission tomography (PET) using dopamine-D2 receptor (D2R) radiotracers combined with psychostimulant challenge. This is feasible if radiotracer binding is measured when post-challenge dopamine (DA) levels have returned to baseline, following the initial competition phase between DA and radiotracer for binding to D2R. Here we used “late”-phase imaging post-challenge to test the hypothesis that impaired D2R internalization in SZ leads to blunted late phase displacement, or a faster return to baseline, in patients compared to healthy controls (HC). We imaged 10 patients with SZ and 9 HC with PET and [11C]raclopride at baseline and twice (3–5hr and 6–10hr) following 0.5 mg/kg dextro-amphetamine. We measured binding potential relative to non-displaceable compartment (BPND) and derived percent reduction from baseline (ΔBPND) for each post-amphetamine scan. To test the hypothesis that time course of return of striatal BPND to baseline differed between SZ and HC, we implemented a linear model with ΔBPND as dependent variable, time post-amphetamine as repeated measure, and time post-amphetamine and diagnostic group as fixed effects. Neither diagnostic group nor interaction of diagnostic group-by-time post-amphetamine significantly affected striatal ΔBPND (F=1.38, p=0.26; F=0.51, p=0.61). These results show similar pattern of return of BPND to baseline as a function of time in patients with SZ and HC, suggesting striatal D2R internalization as measured by our imaging paradigm is normal in patients with SZ.
Introduction
Multiple imaging studies using positron emission tomography (PET) and single-photon emission computed tomography (SPECT) have provided evidence of increased dopamine (DA) release in the striatum of patients with schizophrenia (SZ) using dopamine-D2 receptor (D2R) radiotracers combined with an amphetamine challenge 1–5. This paradigm measures a change in D2R radiotracer binding after amphetamine, or “displacement” of the radiotracer, due to competition between DA and the radiotracer for binding to the D2 receptors. Competition is measureable when imaging is performed within a certain timeframe after amphetamine administration, capturing the peak levels of psychostimulant-induced increased DA levels around the synapse. However, D2R radiotracers’ uptake measured at later timepoints, when DA levels, as measured with microdialysis, had returned to pre-challenge levels, were observed to remain well below baseline in rodents 6, 7, cats 8, monkeys 9, 10 and humans 4, 11, an observation referred to as “late phase” displacement.
Purely competitive binding models do not account for this “late phase” displacement. Laruelle and others proposed that the late phase decrease in binding potential might reflect agonist-induced internalization of D2R, with the resultant lowered affinity of radiotracer for the internalized receptors 12–14. Support for this mechanism as an explanation of the prolonged binding potential decrease came from subsequent in vitro 15–17 and in vivo 6, 18 studies. Tests of a wide range of D2R radiotracers found on average about a 50% lower affinity for internalized D2R compared to cell surface bound receptors 15, 19, 20, a change sufficient to explain the “late phase” decrease in binding potential after amphetamine challenge 19, 21. Consistent with this idea, a PET study in a mouse model deficient in D2R internalization failed to detect the “late phase” effect 6, providing further support that the prolonged effect is due to D2R internalization. Thus, based on this converging evidence from preclinical studies, “late phase” imaging of D2R radiotracers after a psychostimulant challenge can be used to probe the magnitude and timeline of D2R internalization in the human brain.
In parallel, recent studies have suggested that intrinsic factors related to D2R trafficking may be impaired in schizophrenia 22–25. Animal models of schizophrenia susceptibility genes such as dysbindin (DTNBP1) 22, 23, 26 and DISC1 27 have suggested impaired D2R trafficking. This impairment can lead to prolonged stimulation by the agonist and explain some of the behavioral findings showing that DA agonist exposure produces psychosis at higher rates in SZ compared to healthy controls (HC) 1.
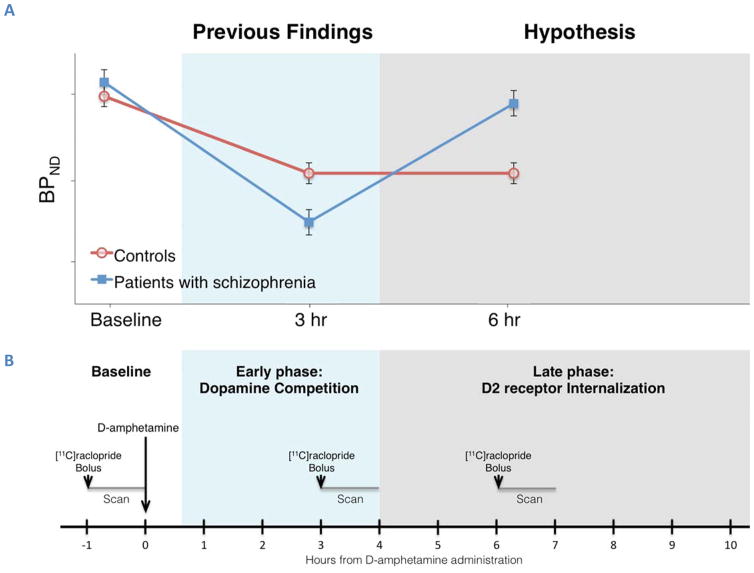
Here we used “late phase” imaging post-challenge to test the hypothesis that D2R internalization is impaired in schizophrenia. We used PET imaging with [11C]raclopride in groups of patients with SZ and matched HC over an extended interval of time following amphetamine. We expected that, if D2R internalization is impaired in SZ, patients will show faster recovery to baseline binding potential, or smaller magnitude of displacement, compared to HC in the later time points after amphetamine (Figure 1A).
Figure 1. Hypothesis and PET study design for imaging the effect of D2R internalization on the reduction of D2R radiotracer binding by psychostimulant challenge.
Upper panel (A): Hypothesis: If D2R internalization is impaired in SZ, this group will show blunted late phase (>4 hr) displacement compared to HC. Lower panel (B): Timeline and study design for PET imaging with [11C]raclopride. Participants were scanned three times: at baseline conditions and at 3, 5–7 or 8–10 hours after oral administration of D-amphetamine 0.5 mg/kg (long arrow). The D2R radiotracer [11C]raclopride was administered as a bolus over 30 sec (short arrow) and emission data was collected for 60 min (gray lines).
Methods
Study population
This study was approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI) and Columbia University Medical Center (CUMC). All participants provided written informed consent. Consent for patients with SZ additionally required (1) presence of a study advocate and (2) determination of capacity by a clinician unrelated to the study using the MacArthur Competence Assessment Tool for Clinical Research 28. The inclusion criteria for patients with SZ were: age 18–55 years; DSM-IV criteria for schizophrenia, schizophreniform or schizoaffective disorder; negative urine toxicology; stable as an outpatient and free from antipsychotic medication for at least three weeks; and agreeable to begin treatment with antipsychotic medication immediately following participation in this study. Patients with schizophrenia were excluded for current (last 6 months) diagnosis of substance abuse, or substance dependence (excluding nicotine), and any current use of amphetamines, opiates, cocaine, sedative-hypnotics, or cannabis, even if these did not meet criteria for abuse or dependence.
Healthy control participants had no current or past DSM-IV Axis I diagnosis, as well as no history of, or current, substance use (except for nicotine use in some), and no first-degree family history of schizophrenia.
All participants were free of significant medical and neurological illnesses, did not use psychotropic medications or substances of abuse (confirmed with urine drug toxicology), had no clinically significant brain abnormalities on a T1-weighted MRI scan, and were not pregnant or nursing. Groups were matched for age, sex, ethnicity, tobacco use, and parental socioeconomic status. Participants were recruited through advertisements, word of mouth, and referrals from clinicians and other researchers.
Assessments
A clinical psychologist (N.O.) used the Diagnostic Interview for Genetic Studies (DIGS) 29 to confirm the diagnosis of schizophrenia or schizoaffective disorder in patients and to confirm the absence of psychiatric comorbidity in patients and healthy controls.
All participants completed additional clinical, neuropsychological and cognitive assessments in the week prior to their PET scans, including the Hollingshead interview for socio-economic status (SES) for participants and their parents 30, the Psychotomimetic States Inventory (PSI) 31, and Positive and Negative Syndrome Scale (PANSS) 32. The PSI and the positive symptom subscale of the PANSS were repeated twice each on the second PET scan day, 3–5 and 7 hours after amphetamine administration. All clinical and neurocognitive assessments were administered by trained raters with pre-established intra- and inter-rater reliability.
PET data acquisition
Participants with schizophrenia were admitted to the inpatient unit of NYSPI the day before the first PET scan and stayed at least until the day after amphetamine administration, i.e. a minimum of 3 nights. Each participant underwent three 60-min PET scans with [11C]raclopride: a baseline PET scan on one day (first), and then two post-amphetamine PET scans the following day, with at least 3 hours between radiotracer injections (Figure 1). Since the exact timing of return to baseline in each group could not be predicted, we spread the scans at different intervals after amphetamine, with an early (second) and a late (third) scan for each of the subjects separated by a few hours, and matched the groups on this variable. As a result, the second and third 60-min scans were acquired 3–5 and 6–10 hours, respectively, after oral (PO) administration of dextro-amphetamine (0.5 mg/kg). At the start of each scan, participants received a bolus injection of [11C]raclopride over 30 seconds. Data were acquired in list mode on a Biograph mCT PET-CT scanner (Siemens/CTI, Knoxville TN), binned into a sequence of frames of increasing duration and reconstructed by filtered back projection using manufacturer-provided software (for detailed methods see Supplement).
PET data analysis
PET data were motion corrected and registered to the individual’s T1-weighted MRI scan using SPM2 software. Regions of interest (ROIs) were drawn on each subject’s MRI and transferred to the coregistered PET data. ROIs included the whole striatum (STR) (a priori) and its subregions (exploratory): the associative striatum (AST), including pre-commissural dorsal caudate, post-commissural caudate and pre-commissural dorsal putamen, the limbic striatum, which comprises the ventral striatum (VST), and the sensorimotor striatum (SMST), which comprises the post-commissural putamen 33. The cerebellum was included as a reference region.
Time activity curves were formed as the mean activity in each ROI in each frame. Data were analyzed using the simplified reference tissue model (SRTM) 34 to determine the binding potential relative to the non-displaceable compartment (BPND).
The primary outcome measure was the relative reduction in BPND for [11C]raclopride (ΔBPND), reflecting amphetamine-induced radiotracer displacement, calculated according to:
MRI data acquisition
MRI studies were performed using a 3.0T GE MR750 system (GE Medical Systems, Waukesha WI, USA) and a 32-channel head coil (Nova Medical, Wilmington MA, USA). High-resolution T1-weighted images were acquired and used for PET coregistration and ROI delineation (see supplemental methods for details of MRI acquisition and analysis).
Statistical analyses
Clinical and demographic measures were compared between groups using t-tests and Fisher’s exact tests. To test the hypothesis that the time course of return of BPND to baseline following amphetamine differed between SZ and HC, we implemented a linear model with striatal ΔBPND as dependent variable, time post-amphetamine as repeated measure, and time post-amphetamine and diagnostic group as fixed effects. Main effects of diagnostic group, time post-amphetamine, and the interaction of diagnostic group-by-time post-amphetamine were tested at the α = 0.05 significance level. Tests were applied to the whole STR. On an exploratory basis, the striatal subregions AST, VST, and SMST were also tested separately.
Results
Schizophrenia and healthy control groups did not significantly differ by sex or age (Table 1). Average participant and parental SES scores were lower in SZ than HC.
Table 1.
Demographics and PET Parameters
| Healthy Control N=9 |
Schizophrenia N=10 |
p1 | |
|---|---|---|---|
| Demographics | |||
| Sex | M=6, F=3 | M=7, F=3 | 1.00 |
| Age 2 | 31.9 (7.5) | 34.3 (11.7) | 0.60 |
| Ethnicity 2 | 4AA, 2C, 1As, 2His | 6AA, 2C, 1As, 1His | |
| SES of participant 2, 3 | 45.1 (15.2) | 20.9 (8.1) | <0.01 |
| Parental SES 2 | 47.9 (6.1) | 36.3 (12.9) | 0.04 |
| Tobacco Users (average use) | 2 (0.3 packs/day) | 4 (0.9 packs/day) | |
| Antipsychotic medication status | - | 5 drug-naïve, 5 drug-free 4 | |
| PANSS Total score 2 | 33.4 (3.3) | 67.6 (25.3) | 0.002 |
| PANSS Positive score 2 | 7.3 (0.5) | 17 (7.3) | 0.002 |
| PANSS Negative score 2 | 9.8 (3.6) | 16.6 (6.1) | 0.01 |
| PANSS General score 2 | 16.4 (0.74) | 34.0 (14.1) | 0.003 |
| PET Parameters | |||
| Hours Post-amphetamine 5 | 3.87 (1.09), 8.07 (1.80) | 3.92 (1.09), 7.89 (2.04) | 0.91, 0.84 |
| Amphetamine level (ng/mL) 5 | 67.0 (19.9), 64.1 (18.7) | 69.2 (12.2), 63.0 (14.5) | 0.79, 0.89 |
| Radiotracer Mass (μg) 6 | 1.9 (0.5), 2.5 (0.5), 2.0(0.9), 1.5(0.5) | 2.3 (2.1), 3.2 (1.8), 2.5 (1.4), 3.8 (2.3) | 0.53, 0.37, 0.33, 0.06 |
| Radiotracer Dose (mCi) 6 | 10.2 (2.9), 11.3 (1.4), 11.1 (2.9), 10.8 (1.1) | 8.4 (2.8), 11.1 (3.3), 11.3 (2.5), 9.1 (4.0) | 0.20, 0.90, 0.92, 0.36 |
Notes:
p-values for 2-tailed t test for age, SES, PANSS total and subscale scores, and PET parameters; Fisher exact test for sex.
mean (SD)
Socio-economic status (SES) as measured by the Hollingshead Interview
Antipsychotic medication status was considered “drug-naïve” if lifetime exposure <6 weeks and none in past 3 weeks, and “drug-free” if none in past 3 weeks.
mean (SD) for scan2, scan3 (i.e. first and second post-amphetamine scans, respectively). See Supplementary Tables 1 and 2 for details.
mean (SD) for scans at baseline (i.e. baseline), 3 hours (h), 5–7h, and 8–10h post-amphetamine. See Supplementary Tables 1 and 2 for details.
Average injected radiotracer dose and mass did not significantly differ between baseline and post-amphetamine scans or between groups (Table 1). Time post-amphetamine and average plasma amphetamine levels at scan times did not differ between groups. (See Supplementary Tables 1 and 2 for demographic and PET results stratified by subgroups of participants with scans at 3 and 6 hours, 5 and 10 hours, and 3 and 10 hours post-amphetamine).
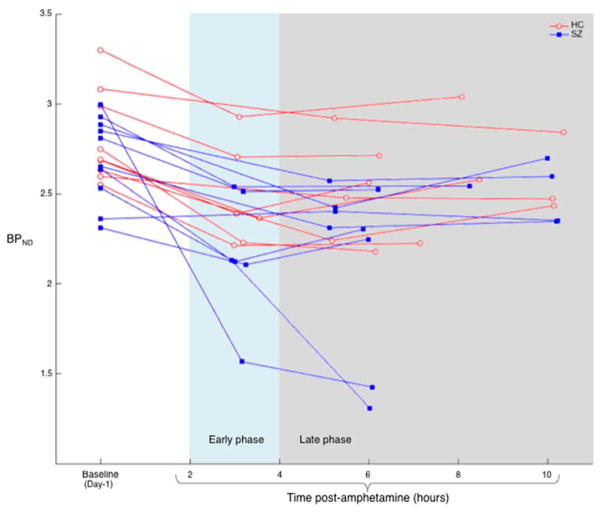
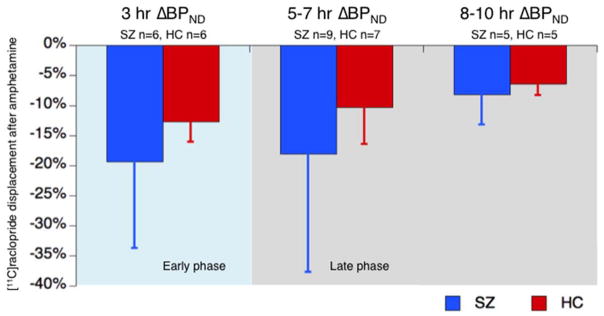
Measured values of striatal BPND at baseline, early and late post-amphetamine time points for each participant are shown in Figure 2. Table 2 shows group averages of BPND for the whole striatum and its subregions, at baseline and early and late time points. ΔBPND’s are also calculated. Group mean striatal ΔBPND’s for each time range are plotted in Figure 3. The linear model for each ROI found no significant effects of diagnostic group (STR: F = 1.38, p = 0.26; AST: F = 0.93, p = 0.35; VST: F = 4.14, p = 0.07; SMST: F = 1.26, p = 0.27) or interaction of diagnostic group by time post-amphetamine (STR: F = 0.51, p = 0.61; AST: F = 0.52, p = 0.60; VST: F = 0.09, p = 0.92; SMST: F = 0.78, p = 0.48). There was a significant effect of time post-amphetamine on ΔBPND in STR and SMST (STR: F = 3.58, p = 0.05; AST: F = 1.55, p = 0.24, VST: F = 2.70, p = 0.12; SMST: F = 9.33, p = 0.002). To assess for effects of motion and differences in motion between groups on the outcome measure, we examined the parameters generated by SPM software during the realignment procedure an indirect indication of motion. We did note that the SPM realignment tool applied slightly, but significantly larger corrections to the SCZ group than the HC group (e.g., 6.6 ± 5.6 mm average translation across frames in SCZ, 3.3 ± 3.4 mm in HC, p = 0.01). When average translation was added to the statistical model as a covariate, the main results were unchanged qualitatively (no significant effect of group or group by time interaction in any brain region). Thus, while we cannot completely rule out the possibility that different amounts of motion between groups masked a true group difference in the rate of return to baseline, the available data do not indicate that motion affected the test of our hypothesis.
Figure 2. Individual striatal binding potentials of [11C]raclopride at baseline and two post-amphetamine time points.
Whole striatum [11C]raclopride binding potential (BPND) plotted at baseline and two post-amphetamine time points for 10 participants with schizophrenia (SZ, blue filled squares) and 9 healthy controls (HC, red open circles). Baseline scans were acquired 1 day before (Day-1) amphetamine administration and post-amphetamine scans. Linear model showed that time post-amphetamine significantly affected ΔBPND (F = 3.58, p = 0.05), but there were no significant effects of diagnostic group (F = 1.38, p = 0.26) or diagnostic group by time post-amphetamine interaction (F = 0.51, p = 0.61) on ΔBPND, suggesting that the groups did not differ in rate of D2R return to baseline.
Table 2.
Binding potentials of [11C]raclopride at baseline and post-amphetamine at early and late time points
| Region | Healthy Controls (N=9) | Schizophrenia (N=10) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline BPND (scan1) |
Post-amph BPND (scan2) |
Post-amph BPND (scan3) |
ΔBPND (scans1to2) |
ΔBPND (scans1to3) |
Baseline BPND (scan1) |
Post-amph BPND (scan2) |
Post-amph BPND (scan3) |
ΔBPND (scans1to2) |
ΔBPND (scans1to3) |
|
| Striatum | 2.81 ± 0.26 | 2.50 ± 0.29 | 2.56 ± 0.28 | −0.11 ± 0.04 | −0.09 ± 0.05 | 2.7 ± 0.24 | 2.27 ± 0.30 | 2.23 ± 0.48 | −0.15 ± 0.13 | −0.17 ± 0.19 |
| AST | 2.68 ± 0.23 | 2.44 ± 0.29 | 2.48 ± 0.25 | −0.09 ± 0.05 | −0.07 ± 0.05 | 2.58 ± 0.27 | 2.24 ± 0.30 | 2.19 ± 0.47 | −0.13 ± 0.13 | −0.14 ± 0.20 |
| SMST | 3.27 ± 0.40 | 2.74 ± 0.35 | 2.89 ± 0.42 | −0.16 ± 0.06 | −0.12 ± 0.06 | 3.11 ± 0.26 | 2.49 ± 0.37 | 2.48 ± 0.57 | −0.20 ± 0.13 | −0.20 ± 0.19 |
| VST | 2.47 ± 0.22 | 2.23 ± 0.29 | 2.23 ± 0.26 | −0.10 ± 0.08 | −0.10 ± 0.07 | 2.39 ± 0.16 | 1.96 ± 0.28 | 1.91 ± 0.40 | −0.18 ± 0.12 | −0.20 ± 0.17 |
Notes:
BPND and ΔBPND values given for mean ± SD. P-values based on 2 sample t test of HC v. SZ were not statistically significant (<0.05) for any measures.
Scan1 is baseline; Scan2 is either 3 or 5–7 hours post-amphetamine (post-amph); Scan3 is either 5–7 or 8–10 hours post-amph. See Supplementary Tables 1 and 2 for additional details.
Associative Striatum (AST), Sensorimotor Striatum (SMST), Ventral Striatum (VST)
Figure 3. Average striatal [11C]raclopride displacement (ΔBPND) after amphetamine.
Average percent change from baseline of [11C]raclopride binding potential (ΔBPND) in whole striatum in three post-amphetamine time ranges for participants with schizophrenia (SZ, blue) and healthy controls (HC, red). Error bars represent standard deviations from mean.
Furthermore, post-hoc analysis controlling for nicotine use did not significantly affect the results.
Discussion
Dopamine dysfunction in schizophrenia is thought to involve a presynaptic dysregulation and an additional D2R-specific dysfunction as evidenced by the supersensitivity to D2R stimulation even in the presence of overall low presynaptic dopamine release 35. This supersensitivity of D2R to DA may involve any one of different cellular mechanisms or even combinations of those. Here we tested for one potential mechanism that could explain the increased sensitivity of D2R to agonist-induced stimulation, which is impaired internalization. Testing for impaired internalization in vivo in patients with schizophrenia is important because, if confirmed, it would allow the field to better target the impaired mechanism itself, opening the door to “new age” therapies that go beyond D2 blockade and address more specifically the precise cellular processes that may be altered. This may circumvent the type of side effects associated with a more global, or less targeted approach, of D2 antagonism. In other words, a better mechanistic understanding would lead to better treatment and management for patients.
We previously demonstrated that the late phase decrease in binding potential of [11C]raclopride after amphetamine is reflective of D2R internalization 6, 10, 15, 19. We used this paradigm to examine differences in magnitude of D2R internalization in the striatum of patients with schizophrenia and healthy controls. We found that both groups showed similar decreases in BPND during late phase imaging after amphetamine, suggesting that D2R trafficking in schizophrenia is not impaired within this extended time frame. Our negative findings suggest that aspects of D2R signaling other than internalization may be altered in schizophrenia 36. The alleviation of positive symptoms of schizophrenia specifically after therapeutic blockade of D2R rather than other dopaminergic receptors suggests that, in addition to excess presynaptic release, homeostatic regulation of D2R signaling is likely to be compromised. Abnormal regulation of intracellular signaling molecules such as Akt and GSK3 downstream from D2R internalization may be at play, or abnormal distribution of D2R across the cellular components within the striatal synapse may also play a role 25, 37–40.
The data for the early displacement in this study is consistent with previous work. The groups differed, at least numerically, showing a larger displacement in patients compared to controls. Patients with schizophrenia also showed larger variability, consistent with previous studies. In particular, two patients showed extreme values of roughly 50% displacement at 3 hours and at 5 hours (see Figure 2). Extreme values have also been observed previously in most imaging cohorts examining either amphetamine induced dopamine release 41 or even alpha-methyl-para-tyrosine induced depletion paradigm 42. This consistency in patterns with prior work provides indirect validation to the results we showed here, and some assurance that our conclusions are supported by the data despite the small sample and the limitations we describe below.
Limitations
Our imaging paradigm was limited in terms of the optimal time window as we had to restrict imaging to 2 days and 3 scans per subject to minimize experimental burden on the subjects, in addition to avoiding nighttime scanning for feasibility issues, such as availability of radiochemistry and access to scanning facilities, as well as personnel. It is possible that the divergence between groups may have occurred later than the latest point we obtained at 10 hours, such as 24 hours. Imaging 24 hours after amphetamine may have provided additional confidence in our conclusions of no group differences. However, even though data in nonhuman primates 9 showed measureable displacement at 24 hours, the data in humans 11 failed to detect a significant displacement in healthy volunteers at 24 hours, which means that testing patients for no displacement compared to controls at 24 hours would not have yielded a measureable difference between groups. Nevertheless, a later time point than what we obtained may have been beneficial, especially in light of the fact that oral amphetamine may have had delayed effect on dopamine compared to intravenous (IV) amphetamine. The plasma concentration of amphetamine peaks approximately 3 to 4 hours after PO administration 43–45, while plasma levels measured 20 minutes after IV administration are already declining (20 min concentration ≈ 80% of 10 minute concentration) 46. A delay in peak amphetamine levels may lead to delayed dopamine release and remaining elevations of dopamine around the synapse that could explain persistent displacement at the later time-point. However, the data from the Toronto group 11 showed no displacement at 24 hours in healthy research participants using PO amphetamine. Overall, we think it is unlikely that our conclusion would have differed with a later imaging time-point since the graphs of the change over time do not suggest any trend in this direction.
Second, it is possible that a larger cohort may have led to increased confidence that we did not fail to detect a small effect. Mitigating against this possibility however, is the fact that the observed sample mean of late phase displacement (compared to baseline) was numerically greater in SCZ than HC.
It is also possible that our group of patients with schizophrenia failed to include, or was heterogeneous in, the subset of these patients who may have D2R internalization impairments. For example, if internalization is only impaired in those individuals with decreased dysbindin expression, genetic profiles of this sample may be useful in identifying a subset of individuals with the ‘Bray haplotype’ – as this is the only genetic marker in the dysbindin gene that has so far been associated with decreased dysbindin expression 47. Nevertheless our results suggest that impaired internalization is not an overall characteristic of SZ in general, and should be tested for in subsets with a specific genetic profile in future studies.
Better methods with higher temporal and cellular resolution may be needed to provide more definitive information in this regard. For example, examining trafficking-related parameters in dopaminergic neurons from cell lines obtained from patients with extreme levels of amphetamine-induced displacements compared to controls may be a more productive approach. This might be accomplished through a combination of in vivo imaging with induced pluripotent stem cell (IPSC) research in the same subjects in order to compare the cellular phenotype with the observed imaging results, and derive a mechanistic understanding of the PET signal. Such an approach combines the advantages of insights from in vivo imaging with the cellular resolution of in vitro examinations.
Supplementary Material
Acknowledgments
Funding for this study was provided by grant R21 MH099509 from the National Institute of Mental Health (NIMH) to Dr. Abi-Dargham. Dr. Weinstein was supported by a T32 grant from NIMH (MH018870). Dr. van de Giessen was supported by a Rubicon grant from the Netherlands Organization for Scientific Research (825.12.009). The authors would also like to acknowledge the contributions of Dr. Mette Skinbjerg in early stages of project development, Rassil Ghazzoui in participant recruitment, Seth Baker in data collection, Nadia-Tina Dandan in data entry, and Dr. Cliff Cassidy in early stages of data interpretation.
Footnotes
Supplementary Information is available at Molecular Psychiatry’s website.
Conflict of Interest
The authors have no competing financial interests in relation to the work described.
References
- 1.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. The American journal of psychiatry. 1998;155(6):761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 3.Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biological psychiatry. 2009;65(12):1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biological psychiatry. 1999;46(1):56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 6.Skinbjerg M, Liow JS, Seneca N, Hong J, Lu S, Thorsell A, et al. D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: a PET study in a receptor internalization-deficient mouse model. NeuroImage. 2010;50(4):1402–1407. doi: 10.1016/j.neuroimage.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houston GC, Hume SP, Hirani E, Goggi JL, Grasby PM. Temporal characterisation of amphetamine-induced dopamine release assessed with [11C]raclopride in anaesthetised rodents. Synapse. 2004;51(3):206–212. doi: 10.1002/syn.10296. [DOI] [PubMed] [Google Scholar]
- 8.Ginovart N, Wilson AA, Houle S, Kapur S. Amphetamine pretreatment induces a change in both D2-Receptor density and apparent affinity: a [11C]raclopride positron emission tomography study in cats. Biological psychiatry. 2004;55(12):1188–1194. doi: 10.1016/j.biopsych.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Narendran R, Slifstein M, Hwang DR, Hwang Y, Scher E, Reeder S, et al. Amphetamine-induced dopamine release: duration of action as assessed with the D2/3 receptor agonist radiotracer (−)-N-[(11)C]propyl-norapomorphine ([11C]NPA) in an anesthetized nonhuman primate. Synapse. 2007;61(2):106–109. doi: 10.1002/syn.20346. [DOI] [PubMed] [Google Scholar]
- 10.Narendran R, Hwang DR, Slifstein M, Hwang Y, Huang Y, Ekelund J, et al. Measurement of the proportion of D2 receptors configured in state of high affinity for agonists in vivo: a positron emission tomography study using [11C]N-propyl-norapomorphine and [11C]raclopride in baboons. J Pharmacol Exp Ther. 2005;315(1):80–90. doi: 10.1124/jpet.105.090068. [DOI] [PubMed] [Google Scholar]
- 11.Cardenas L, Houle S, Kapur S, Busto UE. Oral D-amphetamine causes prolonged displacement of [11C]raclopride as measured by PET. Synapse. 2004;51(1):27–31. doi: 10.1002/syn.10282. [DOI] [PubMed] [Google Scholar]
- 12.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20(3):423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain research Brain research reviews. 2000;31(2–3):371–384. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 14.Ginovart N. Imaging the dopamine system with in vivo [11C]raclopride displacement studies: understanding the true mechanism. Mol Imaging Biol. 2005;7(1):45–52. doi: 10.1007/s11307-005-0932-0. [DOI] [PubMed] [Google Scholar]
- 15.Guo N, Guo W, Kralikova M, Jiang M, Schieren I, Narendran R, et al. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(3):806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quelch DR, Withey SL, Nutt DJ, Tyacke RJ, Parker CA. The influence of different cellular environments on PET radioligand binding: an application to D2/3-dopamine receptor imaging. Neuropharmacology. 2014;85:305–313. doi: 10.1016/j.neuropharm.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chugani DC, Ackermann RF, Phelps ME. In vivo [3H]spiperone binding: evidence for accumulation in corpus striatum by agonist-mediated receptor internalization. J Cereb Blood Flow Metab. 1988;8(3):291–303. doi: 10.1038/jcbfm.1988.64. [DOI] [PubMed] [Google Scholar]
- 18.Skinbjerg M, Ariano MA, Thorsell A, Heilig M, Halldin C, Innis RB, et al. Arrestin3 mediates D(2) dopamine receptor internalization. Synapse. 2009;63(7):621–624. doi: 10.1002/syn.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laruelle M, Guo N, Guo W, Jiang M, Schieren I, Abi-Dargham A, et al. Impact of dopamine D2 receptor internalization on binding parameters of D2 PET radiotracers. NeuroImage. 2008;41:T36–T36. [Google Scholar]
- 20.Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, et al. In vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (−)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse. 2004;52(3):188–208. doi: 10.1002/syn.20013. [DOI] [PubMed] [Google Scholar]
- 21.Hwang DR, Narendran R, Laruelle M. Positron-labeled dopamine agonists for probing the high affinity states of dopamine subtype 2 receptors. Bioconjug Chem. 2005;16(1):27–31. doi: 10.1021/bc049834n. [DOI] [PubMed] [Google Scholar]
- 22.Papaleo F, Weinberger DR. Dysbindin and Schizophrenia: it’s dopamine and glutamate all over again. Biological psychiatry. 2011;69(1):2–4. doi: 10.1016/j.biopsych.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN, et al. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Molecular psychiatry. 2012;17(1):85–98. doi: 10.1038/mp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schubert KO, Focking M, Prehn JH, Cotter DR. Hypothesis review: are clathrin-mediated endocytosis and clathrin-dependent membrane and protein trafficking core pathophysiological processes in schizophrenia and bipolar disorder? Molecular psychiatry. 2012;17(7):669–681. doi: 10.1038/mp.2011.123. [DOI] [PubMed] [Google Scholar]
- 25.Beaulieu JM. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. Journal of psychiatry & neuroscience : JPN. 2012;37(1):7–16. doi: 10.1503/jpn.110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, et al. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(46):19593–19598. doi: 10.1073/pnas.0904289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su P, Li S, Chen S, Lipina TV, Wang M, Lai TK, et al. A dopamine D2 receptor-DISC1 protein complex may contribute to antipsychotic-like effects. Neuron. 2014;84(6):1302–1316. doi: 10.1016/j.neuron.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Appelbaum PS, Grisso T The MacArthur Treatment Competence Study. I: Mental illness and competence to consent to treatment. Law Hum Behav. 1995;19(2):105–126. doi: 10.1007/BF01499321. [DOI] [PubMed] [Google Scholar]
- 29.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- 30.Hollingshead AB. Working paper published by the author. New Haven, Connecticut: 1975. Four factor index of social status. [Google Scholar]
- 31.Mason OJ, Morgan CJ, Stefanovic A, Curran HV. The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophrenia research. 2008;103(1–3):138–142. doi: 10.1016/j.schres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 33.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. JCerebBlood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JL, Urban N, Slifstein M, Xu X, Kegeles LS, Girgis RR, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Molecular psychiatry. 2013;18(8):909–915. doi: 10.1038/mp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(45):18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan HY, Chen AG, Chen Q, Browne LB, Verchinski B, Kolachana B, et al. Epistatic interactions of AKT1 on human medial temporal lobe biology and pharmacogenetic implications. Molecular psychiatry. 2012;17(10):1007–1016. doi: 10.1038/mp.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71(2):278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eichel K, Jullie D, von Zastrow M. beta-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation. Nature cell biology. 2016;18(3):303–310. doi: 10.1038/ncb3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irannejad R, Kotowski SJ, von Zastrow M. Investigating signaling consequences of GPCR trafficking in the endocytic pathway. Methods Enzymol. 2014;535:403–418. doi: 10.1016/B978-0-12-397925-4.00023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 42.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith CT, Dang LC, Cowan RL, Kessler RM, Zald DH. Variability in paralimbic dopamine signaling correlates with subjective responses to d-amphetamine. Neuropharmacololy. 2016;108:394–402. doi: 10.1016/j.neuropharm.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith CT, Weafer J, Cowan RL, Kessler RM, Palmer AA, de Wit H, et al. Individual differences in timing of peak positive subjective responses to d-amphetamine: Relationship to pharmacokinetics and physiology. J Psychopharmacol. 2016;30(4):330–343. doi: 10.1177/0269881116631650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angrist B, Corwin J, Bartlik B, Cooper T. Early pharmacokinetics and clinical effects of oral D-amphetamine in normal subjects. Biological psychiatry. 1987;22(11):1357–1368. doi: 10.1016/0006-3223(87)90070-9. [DOI] [PubMed] [Google Scholar]
- 46.Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64(5):350–362. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bray NJ, Preece A, Williams NM, Moskvina V, Buckland PR, Owen MJ, et al. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Human molecular genetics. 2005;14(14):1947–1954. doi: 10.1093/hmg/ddi199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.