Abstract
Purpose
Intervertebral disc degeneration is a major cause of back pain. Novel therapies for prevention or reversal of disc degeneration are needed. It is desirable for potential therapies to target both inflammation and matrix degeneration.
Materials and Methods
The combined regenerative potential of link protein N-terminal peptide (LN) and fullerol on annulus fibrosus (AF) cells was evaluated in a 3D culture model.
Results
Interleukin-1α (IL-1α)-induced AF cell degeneration was counteracted by fullerol, LN, and fullerol + LN, with the latter having the greatest effect on matrix production as evaluated by real-time polymerase chain reaction and glycosaminoglycan assay. IL-1α-induced increases in pro-inflammatory mediators (interleukin-6 and cyclooxygenase-2) and matrix metalloproteinases (MMP-1, -2, -9, and -13) were also counteracted by fullerol and LN.
Conclusion
Our data demonstrate that LN and fullerol individually, and in combination, promote matrix production and have anti-inflammatory and anti-catabolic effects on AF cells.
Keywords: Back pain, fullerol, inflammation, intervertebral disc degeneration, link N peptide
Introduction
Low back pain is a major health problem in the United States and worldwide with tremendous socioeconomic impact (1). An estimated 80% of the population will experience back pain at some point during their lifetime (2). With an aging baby boomer population, it is a problem that is expected to increase in prevalence.
Symptomatic intervertebral disc (IVD) degeneration is a major cause of low back pain and contributes to the pathophysiology of other important etiologies including spinal arthritis, myelopathy, and radiculopathy (3,4). Current mainstays of treatment for low back pain are divided into nonoperative (anti-inflammatory medication, physical therapy, and pain management) and operative modalities (spinal fusion, discectomy, and decompression). However, both forms of treatment leave room for improvement, in that neither actively targets or reverses the underlying disease process: IVD degeneration (5).
Novel biologic therapies utilizing bone morphogenetic proteins (BMP-2 and -7), growth and differentiation factor 5, and platelet-rich plasma (PRP) to stimulate disc matrix production are currently being investigated but have so far been limited by short half-life, high cost, and safety concerns (6–10). Two other novel agents, fullerol and link protein N-terminal peptide (LN), have been shown to be potentially beneficial in the treatment of IVD degeneration.
Owing to its unique cage nanostructure, fullerol (a polyhydroxylated, water-soluble, and biocompatible fullerene derivative) is several hundred times more powerful than conventional antioxidants and demonstrates excellent cell membrane penetration (11,12). In vitro and in vivo studies have documented the efficacy of fullerol in preventing IVD degeneration by scavenging reactive oxygen species (ROS), which are thought to play a major role in disc degeneration (11,12).
Link protein is a glycoprotein that stabilizes the interaction between aggrecan and hyaluronan, two major matrix structural components (13). LN is the resulting N-terminal 16-amino peptide (DHLSDNYTLDHDRAIH) following in vivo proteolytic cleavage of link protein at His16-Ile17 residues (14). Studies have shown that LN stimulates the synthesis of disc matrix by increasing disc cell production of aggrecan and collagen II through a complex Smad/BMP signaling cascade (15,16). LN has also been shown to potentially restore disc structure and function (17–20).
As both inflammation and disc cell degeneration play important roles in the progression of IVD degeneration, it is desirable for potential therapies to address both anti-inflammation and matrix regeneration. In the present study, we sought to investigate the combined protective antioxidant/anti-inflammatory properties of fullerol with the regenerative properties of LN in an in vitro rabbit annulus fibrosus (AF) cell model. We found that fullerol and LN together promoted disc matrix production and decreased inflammation by modulating the anabolic and catabolic metabolism within disc cells. This study may provide insight into a successful integrated strategy for treating disc degeneration and back pain.
Materials and methods
Isolation of AF cells from the IVD
Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Virginia. Inner AF cells were isolated and pooled from three male New Zealand White rabbits (8–10 weeks old, Charles River, MA) as reported previously (21). After euthanasia, the inner AF from lumbar discs (L3–L5) was harvested under sterile conditions. To avoid contamination, the inner AF layer next to the nucleus pulposus was discarded. The remaining AF were cut into small pieces and digested with 0.025% collagenase (Serva, Heidelberg, Germany) for 2–4 h at 37 °C with gentle shaking. After centrifugation, cells were cultured in complete Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) medium containing 15% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA) and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Cells at passages 2–5 were used for later experiments. Culture media were changed every 3 days.
Monolayer culture
Cells were plated in a 6-well plate with 1 × 105 cells and treated under different conditions. For the control condition, cells were treated with DMEM/F12. Cells were treated with or without 10 ng/mL Interleukin-1α (IL-1α, R&D, Minneapolis, MN) plus fullerol (1 μM, MER Corporation, Tucson, AZ) based on our prior study (12) or LN (10ng/mL, Anaspect, Fremont, CA) for 7 days. LN at concentrations 10 and 100 ng/mL demonstrated similar effects on collagen II mRNA expression, thus we utilized 10 ng/mL for later experiments. Culture medium was changed every other day.
3D culture
For 3D culture, AF cells were cultured in a pellet culture system as described previously (21). Cells (2 × 105) were centrifuged at 500 g for 5 min in a 15-mL tube and cultured overnight. Cells were treated with DMEM/F12 containing 1% FBS and 37.5 mg/mL ascorbic acid and 1 × insulin, transferrin, and selenous acid premix (Life Technologies) with the same conditions as described in the monolayer culture. TGF-β3 (10 ng/mL, Peprotech, Rocky Hill, NJ) was used as a positive control for zymography assay. Media were changed every other day. Pellets were collected at days 7 and 14 for RNA isolation and glycosaminoglycan (GAG) assay.
Gelatin and collagen zymography
Zymography was performed as previously described with media harvested on day 7 after 3D culture (16). Briefly, gelatinolytic zymography was performed on 10% polyacrylamide resolving gels containing 1 mg/mL gelatin to determine matrix metalloproteinases (MMPs), MMP-2 and MMP-9, activities. To evaluate the activity of MMP-1 and MMP-13, equal amounts of culture media were separated on a 10% gel with 10 mg/mL of collagen I. Gels were scanned with a Chemidoc Imaging system (Bio-Rad, Hercules, CA) and analyzed with ImageJ software (NIH, Bethesda, MD).
RNA isolation and real-time reverse transcription polymerase chain reaction
Total RNA was isolated with Trizol reagent (Life Technologies) and reverse transcribed to complementary DNA (cDNA) by an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Reverse transcription of 0.5 μg RNA was performed with iScript Reverse Transcription Supermix (Bio-Rad) and the cDNA was diluted for either 5-(target genes) or 50-fold (reference gene) with nuclease-free water. The quantitative polymerase chain reaction (PCR) was performed using SYBR green and gene-specific primer sets (Table 1) on an iQ 5 (Bio-Rad). The mRNA expression of target genes was normalized to 18s. Fold changes in gene expression were presented as 2−(averageΔΔCt). The ΔCt of each stimulated sample was related to the respective ΔCt of each control sample.
Table 1.
Primer sequence for real-time PCR.
| Name | Length | Tm | Sequences | Gene ID |
|---|---|---|---|---|
| mmp13 forward | 175 | 57.6 | 5′-CCTTCTGGTCTTCTGGCTCAC-3′ | NM_001082037.1 |
| mmp13 reverse | 5′-AGTGCTCCTGGGTCCTTGG-3′ | |||
| mmp1 forward | 199 | 55.2 | 5′-TGGAGTGCCTGATGTG-3′ | NM_001171139.1 |
| mmp1 reverse | 5′-CTTGACCCTTGGAGACTTTGG-3′ | |||
| collagen-II forward | 60 | 55 | 5′-ACAGCAGGTTCACCTATAC −3′ | XM_002723439.3 |
| collagen-II reverse | 5′-CCCACTTACCGGTGTT −3′ | |||
| aggrecan forward | 176 | 60 | 5′-AGGATGGCTTCCACCAGTGC −3′ | XM_008251723.2 |
| aggrecan reverse | 5′-TGCGTAAAAGACCTCACCCTCC-3′ | |||
| interleukin-6 forward | 148 | 53.1 | 5′-CGGCGGTGAATAATGAGAC −3′ | NM_001082064.2 |
| interleukin-6 reverse | 5′-ATCAGGTGTTGGATGTTCTTC −3′ | |||
| cox-2 forward | 106 | 55 | 5′-AACTCCCAATCCGCATGCTA −3′ | NP_007552.1 |
| cox-2 reverse | 5′-GCTTGATTTAAGCGTCCGGG −3′ | |||
| 18s forward | 167 | 55 | 5′-ATCAGATACCGTCGTAGTTC −3′ | NR_033238.1 |
| 18s reverse | 5″-TTCCGTCAATTCCTTTAAG-3″ |
Sulfated GAG assay
Cells were cultured for 7 days and digested with papain extraction buffer at 60 °C overnight (22). Amino sugars were determined using a dimethylmethylene blue (Crescent Chemicals, Islandia, NY) colorimetric assay with chondroitin sulfate C as a standard. DNA content was analyzed using the Hoechst dye with a calf thymus DNA as a standard. The GAG content was normalized to DNA content.
Statistical analyses
Experimental data were presented as mean ± standard error of the mean. Statistical differences were calculated using one-way analysis of variance with Bonferroni post hoc test. Data comparing two groups were analyzed with a Student’s t-test (Prism 6, GraphPad, La Jolla, CA). A p value less than 0.05 was considered statistically significant. All experiments were repeated three separate times, and each time with three technical repeats.
Results
IL-1α-induced AF cell degeneration in a dose-dependent manner
To optimize IL-1α-induced AF cell degeneration, we first treated rabbit AF cells with different concentrations of IL-1α in a monolayer culture. After 3 days in culture, collagen II mRNA decreased significantly at 1 ng/mL, and aggrecan mRNA decreased at 10 ng/mL (Figure 1). Although 50 ng/mL of IL-1α resulted in the lowest collagen II and aggrecan mRNA expression, we observed floating cells in the culture media. Therefore, we decided to use 10 ng/mL IL-1α for later experiments.
Figure 1.
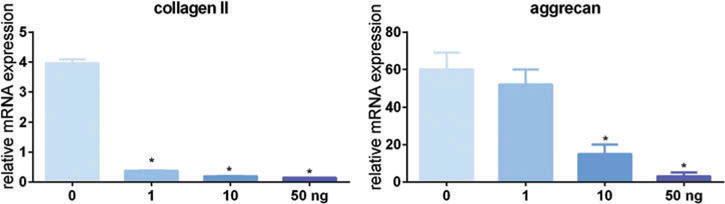
IL-1α-induced AF cell degeneration in a dose-dependent manner. Rabbit AF cells were cultured in a monolayer for 3 days. Cells were treated with or without IL-1α to induce AF cell degeneration. Cells were harvested for RNA isolation and the gene expression of collagen II and aggrecan was measured with real-time reverse transcription PCR. The target gene was normalized to 18s. *p < 0.05 versus control.
Fullerol and LN protect AF cells from IL-1α-induced matrix degradation
When rabbit AF cells were cultured in a cell culture plate for 7 days, either fullerol or LN significantly induced both collagen II and aggrecan mRNA expression (Figure 2). The addition of IL-1α significantly reduced both collagen II and aggrecan mRNA expression but fullerol and LN reversed this effect. In cells treated with IL-1α, fullerol, and LN, the collagen II level increased about 15-fold as compared with control, and over 20-fold as compared with IL-1α treatment (p < 0.05). Treatment with LN increased the aggrecan mRNA level, and combination therapy with LN and fullerol demonstrated the greatest increase as compared with IL-1α treatment.
Figure 2.
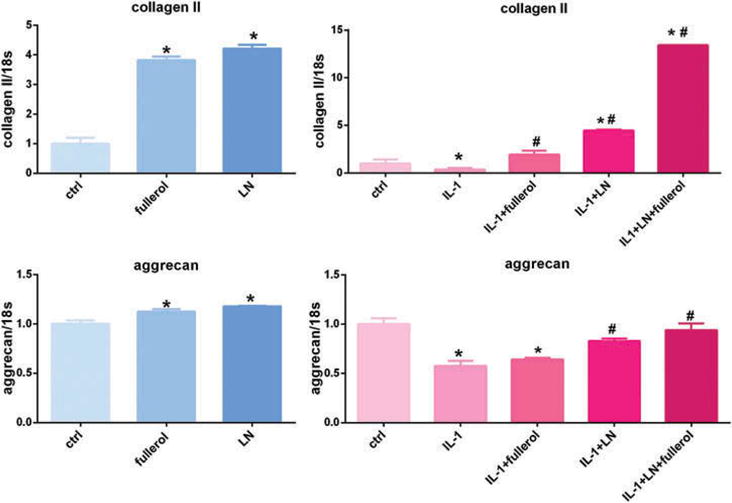
Link N and fullerol increased both collagen II and aggrecan expression in monolayer culture. Rabbit AF cells were cultured in a monolayer for 7 days. Cells were treated with or without IL-1α with addition of LN, fullerol, or LN + fullerol for 7 days in monolayer culture. The gene expression of collagen II and aggrecan was measured with real-time reverse transcription PCR. *p < 0.05 versus control; #p < 0.05 versus IL-1α-treated group.
Since cells may undergo phenotypic changes in a monolayer culture, we performed the same experiment in a 3D pellet culture system for 7 and 14 days. Fullerol and LN significantly increased both collagen II and aggrecan mRNA expression as compared with control in a 3D culture at day 7 (Figure 3). Both collagen II and aggrecan expression were significantly reduced when AF cells were treated with IL-1α, and this was rescued by fullerol and LN co-treatment. Collagen II levels were similar to control and aggrecan expression was higher than baseline levels when cells were treated with both fullerol and LN with IL-1α stimulation. Fullerol and LN individually rescued matrix production but this was not as effective as the combination of both fullerol and LN. Similar results were observed when the pellets were harvested on day 14 (Figure 4) but to a slightly lesser extent.
Figure 3.
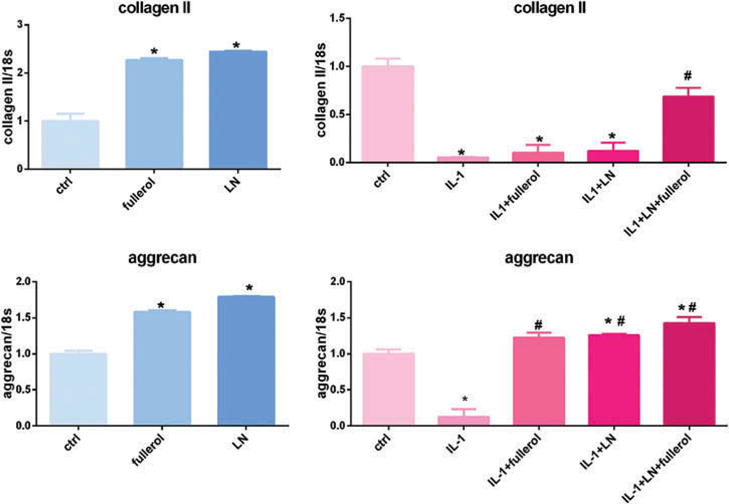
Link N and fullerol increased both collagen II and aggrecan expression in a 3D culture model. Cells were treated with or without IL-1α with addition of LN, fullerol, or LN + fullerol for 7 days. The cell pellets were harvested for RNA isolation and the gene expression of collagen II and aggrecan was measured with real-time reverse transcription PCR. *p < 0.05 versus control; #p < 0.05 versus IL-1α-treated group.
Figure 4.
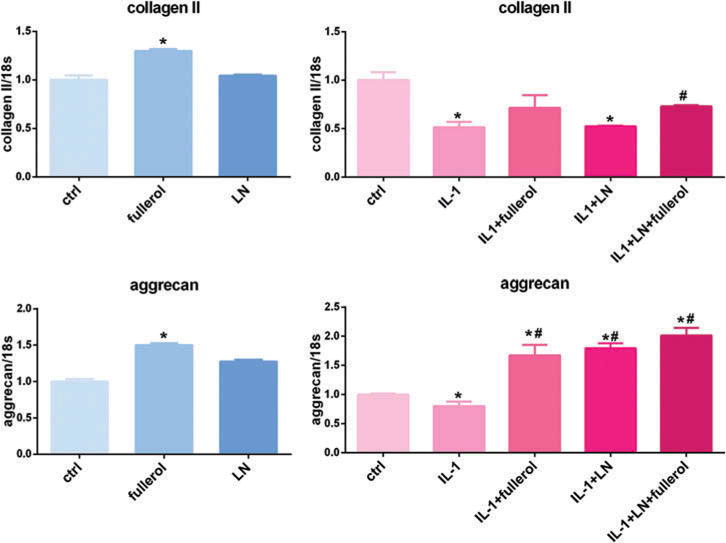
Link N and fullerol increased collagen II and aggrecan expression in 3D culture for 14 days. Cells were treated with or without IL-1α with addition of LN, fullerol, or LN + fullerol. The cell pellets were harvested for RNA isolation and the gene expression of collagen II and aggrecan was measured with real-time reverse transcriptionPCR. *p < 0.05 versus control; #p < 0.05 versus IL-1α-treated group.
Fullerol and LN promote proteoglycan synthesis in AF cells
GAGs are linear polysaccharides and one of the most important extracellular matrix components of the IVD (23,24). Cellular GAG content was significantly increased in AF cells treated with LN but not in those treated with fullerol as compared with controls after 7 days in 3D cultures. GAG content was significantly decreased by IL-1α; however, this effect was counteracted by fullerol, LN, and fullerol + LN. Fullerol + LN and LN alone induced the highest levels of GAG production when stimulated with IL-1α, and this level is significantly higher as compared with untreated cells (Figure 5).
Figure 5.
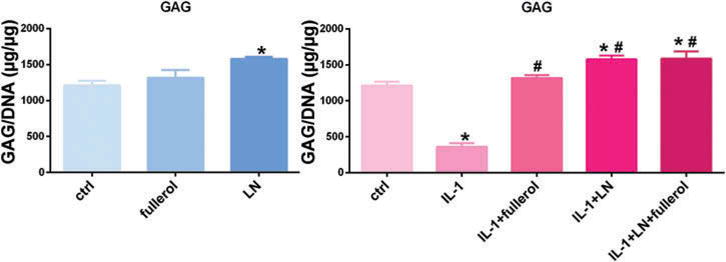
Biochemistry assay shows Link N and fullerol combined increased GAG expression. Rabbit AF cells were cultured in pellet culture for 7 days. Cells were treated with or without IL-1α with addition of LN, fullerol, or LN + fullerol. *p < 0.05 versus control; #p < 0.05 versus IL-1α-treated group.
Fullerol and LN inhibit IL-1α-induced upregulation of IL-6 and COX-2 in AF cells
In normal 3D culture conditions without the addition of IL-1α, the mRNA expression of interleukin-6 (IL-6) was unchanged by either LN or fullerol, while COX-2 mRNA expression was increased by fullerol. IL-1α induced a significant increase in IL-6 and cyclooxygen-ase-2 (COX-2) mRNA expression, which was decreased by fullerol, LN, and fullerol + LN (Figure 6). IL-6 mRNA demonstrated the greatest decrease in IL-1α-induced expression with fullerol + LN. However, in the case of COX-2, fullerol alone, rather than fullerol + LN, resulted in the greatest decrease in COX-2 mRNA expression.
Figure 6.
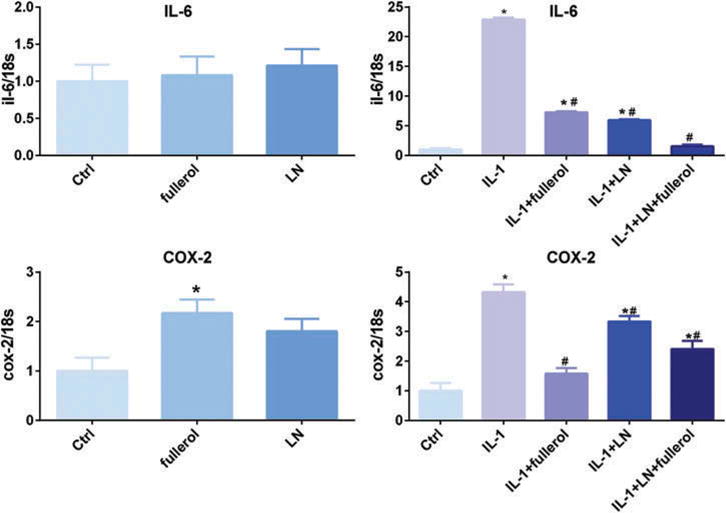
Link N and fullerol decreased mRNA expression levels of IL-6 and COX-2. Cells were treated with or without IL-1α with addition of LN, fullerol, or LN + fullerol in a pellet culture for 7 days. The cell pellets were harvested for RNA isolation and the gene expression of IL-6 and COX-2 was measured with real-time reverse transcriptionPCR. *p < 0.05 versus control; #p < 0.05 versus IL-1α-treated group.
Fullerol and LN inhibit IL-1α activation of MMPs
In 3D culture for 7 days, IL-1α was found to increase the expression of matrix degradation enzymes MMP-1 and -13 while fullerol and LN each were found to have minimal effect on their expression as compared with control (Figure 7A). In the presence of IL-1α-induced inflammation, co-treatment with fullerol or LN each had a protective effect, decreasing MMP-1 and -13 mRNA expression as compared with cells treated with IL-1α alone. Further, co-treatment with fullerol and LN resulted in a significantly greater decrease in MMP-1 and -13 mRNA expression, comparable to the baseline, as compared with either agent individually. These results were confirmed with collagen zymography (Figure 7B). Also, fullerol and LN decreased the activity of MMP-2 and -9 as detected by gelatin zymogrphy. As expected, TGF-β3 decreased the activity of MMP-2 and -13.
Figure 7.
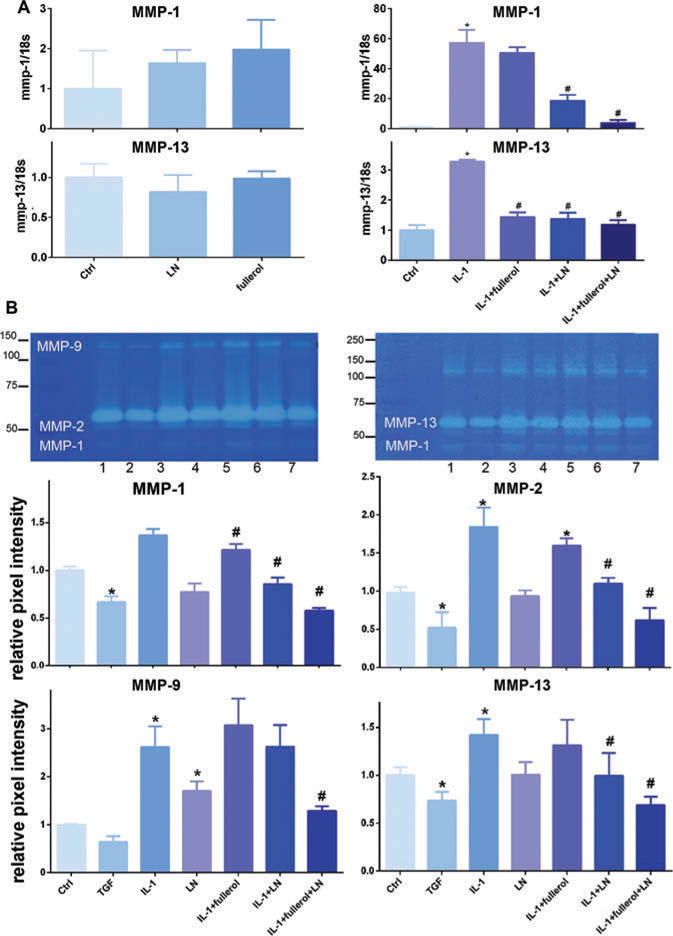
Link N and fullerol increased anabolic metabolites through decreasing MMP expression and activity. A, the gene expression of MMP-1 and -3 were measured with real-time reverse transcriptionPCR. B, zymographic images. The culture media were subjected to gelatin and collagen zymography. Bar graphs show intensity of gel bands. *p < 0.05 versus control; #p < 0.05 versus IL-1α-treated group. TGF-β3 was used as a positive control.
Discussion
During IVD degeneration, desiccation and acidification of the cellular environment lead to cell death and dysfunction. Additionally, collagen fiber disruption in the AF and mirco-trauma to the endplates allows for vessel and nerve ingrowth into the IVD (5). Although the exact pathogenesis of IVD degeneration is not well understood, it is thought to be an active process that is initiated by noxious stimuli (repetitive mechanical stress, osmotic pressure, and reduced glucose) and propagated by oxidative stress and cytokines resulting in a mismatch between anabolic and catabolic metabolisms (5,12,25). Thus, anti-inflammatory and antioxidative biological therapies to regenerate the cellular matrix have attracted a great deal of attention. Although prior studies suggested that fullerol or LN can combat disc degeneration via anti-inflammatory/antioxidative effects and matrix regeneration, respectively, their individual effects seemed insufficient to alter the clinical course of disease. The combination of LN and fullerol may impact both inflammation and matrix degeneration simultaneously to counteract the two core pathological changes involved in IVD degeneration.
By stimulating disc anabolism, biologic therapies seek to push the balance in favor of disc regeneration. Experimental biologic therapies utilizing BMP-2 and -7, growth and differentiation factor 5, and PRP to stimulate disc matrix production are currently being investigated but have so far been limited by high cost and safety concerns (6–10). Prior studies have shown that LN and fullerol are individually effective at combating the processes that contribute to IVD degeneration by increasing cell matrix production and decreasing ROS and cytokine-induced inflammation (11–15). LN has been shown to upregulate the expression of aggrecan and collagen II and inhibit the expression of catabolic regulators IL-1β and MMP-1 (16). The mechanism of action of LN is not yet fully understood but involves LN binding BMP-RII on chondrocytes and initiating a complex Smad/BMP signaling cascade that promotes production of the IVD matrix (15,20). Fullerol prevents the catabolic effect of inflammation by scavenging ROS, effectively interrupting the positive feedback loop between ROS and Tumor necrosis factor-α (TNF-α)-induced inflammation in IVD cells (11,12). In the present study, we confirmed that fullerol and LN markedly increase the mRNA expression of collagen II and aggrecan in 3D cultures as compared with control. In an inflammatory 10 ng/mL IL-1α condition, fullerol and LN together increased the expression of collagen II and aggrecan mRNA significantly (Figure 3). Both fullerol and LN individually promote AF matrix regeneration with greater increases in aggrecan expression as compared with increases in collagen II expression, as confirmed using GAG assay (Figure 5). In disc degeneration, biochemical alteration of the extracellular matrix occurs early, with aggrecan being particularly susceptible to proteolytic damage and loss (26,27). The protective effects of fullerol on aggrecan expression might be involved in its ROS scavenging activity. Although fullerol and LN promoted cellular matrix production in a monolayer culture, the increase in collagen II was even more pronounced than in 3D culture. This suggests that cell phenotype might be influenced by monolayer culture.
Oxidative stress due to an imbalance between free radical burden and cellular scavenging mechanisms is implicated in the degenerative diseases of skeletal tissue by triggering cellular senescence and apoptosis, and promoting inflammation, fibrosis, and pain nociception (28). During IVD degeneration, elevated pro-inflammatory cytokines result in increased intracellular ROS levels through mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, creating a positive feedback loop for inflammation (29). In disc tissue, oxidative stress due to nitric oxide has been shown to contribute to matrix destruction, promote apoptosis, and suppress proteoglycan synthesis (30). Here we showed that the mRNA level of IL-6 and COX-2 was significantly induced by IL-1α in rabbit AF cells, which was suppressed by fullerol alone or fullerol combined with LN treatment. Surprisingly, LN alone also decreased the expression of IL-6 and COX-2 by IL-1α (Figure 6). This could be due to the enhanced anabolic metabolism of AF cells induced by LN that provides resistance to inflammatory stimuli, although elucidating a detailed mechanism requires further investigation.
A cohort of pro-inflammatory cytokines including IL-1, IL-6, and TNF-α is upregulated in the degenerated IVD. IVD cells produce IL-1 and TNF-α, which induce cartilage catabolism at molecular and morphological levels by increasing production of degradative enzyme MMPs, nitric oxide, and inflammatory mediators such as prostaglandin E2 while decreasing proteoglycan content (5,25,31). Further, cells from the acidic degenerated IVD environment have been found to express more MMP-13 and aggrecanase in response to IL-1 than those from nondegenerate IVDs (25). Herein, we show that IL-1α increased the expression and activity of MMP-1 and MMP-13 significantly, which was counteracted by the combination of fullerol and LN treatment (Figure 7). Similarly, fullerol and LN combined inhibit the activity of MMP-2 and -9 induced by IL-1α. This suggests that both LN and fullerol decrease catabolic metabolism, which may contribute to matrix regeneration.
There are several limitations to the current study. First, this was an in vitro study performed using rabbit AF cells, which may not perfectly represent the in vivo condition. We used a pellet culture system that most closely recreates the in vivo 3D environment. We pooled AF cells from three rabbits for the experiments, which may compromise the biological significance. Second, we used IL-1α in our experiments to stimulate IVD degenerative changes in AF cells. However, IVD degeneration is a complex process, involving mechanical stress, oxidation, and a combination of inflammatory cytokines, of which IL-1α plays a crucial, albeit incomplete role. Other studies have used an acute annulus-puncture model to create disc degeneration in rabbits but this method is also limited since multiple factors, rather than a single injury, are involved in the induction of disc degeneration in humans (32,33). Future studies investigating the combined effects of LN and fullerol on the whole IVD using an in vivo model are needed. Further, in order for LN and fullerol to be of therapeutic potential, the question of drug delivery must be addressed. In this in vitro model, we incorporated LN and fullerol directly into the cell media but in order to be of therapeutic value, we must find a way to safely deliver LN and fullerol into the degenerated IVD without causing further damage to the disc.
Conclusion
IVD is a major cause of chronic back pain and disability that results in considerable social and medical costs. The development of IVD degeneration is a complex process known to be associated with proteolytic degradation, oxidative stress, and inflammation. So far there is no disease-modifying remedy for disc degeneration. In the present study, we seek to identify a better biological treatment strategy with a combination of antioxidative and matrix-producing agents, fullerol and LN, on rabbit AF cell regeneration. Our results demonstrate that fullerol and LN reverse IL-1α-induced disc degeneration by modulating anabolic and catabolic metabolism toward regeneration and through anti-inflammatory pathways (Figure 8). These results suggest that fullerol and LN combined may demonstrate a beneficial effect on disc regeneration and shed light on their potential clinical application as therapeutic agents for the treatment of IVD degeneration.
Figure 8.
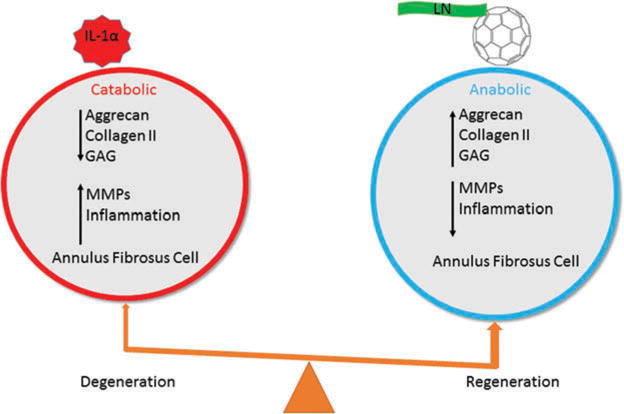
Possible mechanisms under which fullerol and LN act on rabbit AF cells.
Acknowledgments
Funding
We appreciate the funding support in part from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21AR057512 and RO1AR064792), and North American Spine Society.
Footnotes
Color versions of one or more of the figures in this article can be found online at www.tandfonline.com/icts.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: National Health and Nutrition Examination Survey 2009–2010. Arthritis Care Res (Hoboken) 2016. 2016 Oct 8; doi: 10.1002/acr.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Casterl LD, Kalsbeek WD, Carey TS. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–116. doi: 10.22203/ecm.v030a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976) 1995;20:1878–1883. doi: 10.1097/00007632-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Freemont AJ, Watkins A, Le Maitre C, Jeziorska M, Hoyland JA. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002;196:374–379. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- 6.Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc–in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976) 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 7.Gruber HE, Norton HJ, Hanley EN. Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2000;25:2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc. Spine (Phila Pa 1976) 2007;32:1174–1180. doi: 10.1097/01.brs.0000263369.95182.19. [DOI] [PubMed] [Google Scholar]
- 9.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An HS. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976) 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Chee A, Thonar EJ, An HS. Intervertebral disk repair by protein, gene, or cell injection: a framework for rehabilitation-focused biologics in the spine. Pm&R. 2011;3:S88–S94. doi: 10.1016/j.pmrj.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Jin L, Shen FH, Balian G, Li X. Fullerol nanoparticles suppress inflammatory response and adipogenesis of vertebral bone marrow stromal cells: a potential novel treatment for intervertebral disc degeneration. Spine J. 2013;13:1571–1580. doi: 10.1016/j.spinee.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Jin L, Yao L, Shen FH, Shimer AL, Li X. Antioxidative nanofullerol prevents intervertebral disk degeneration. Int J Nanomedicine. 2014;9:2419–2430. doi: 10.2147/IJN.S60853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mwale F, Demers CN, Petit A, Roughley PJ, Poole AR, Steffen T, Aebi M, Antoniou J. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J Cell Biochem. 2003;88:1202–1213. doi: 10.1002/jcb.10479. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, McKenna LA, Dean M. An N-terminal peptide from link protein stimulates synthesis of cartilage proteoglycans. Biochem Soc Trans. 1997;25:427S. doi: 10.1042/bst025427s. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Weitzmann MN, Sangadala S, Hutton WC, Yoon ST. Link protein N-terminal peptide binds to bone morphogenetic protein (BMP) type II receptor and drives matrix protein expression in rabbit intervertebral disc cells. J Biol Chem. 2013;288:28243–28253. doi: 10.1074/jbc.M113.451948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Hutton WC, Yoon ST. ISSLS Prize winner: effect of link protein peptide on human intervertebral disc cells. Spine (Phila Pa 1976) 2013;38:1501–1507. doi: 10.1097/BRS.0b013e31828976c1. [DOI] [PubMed] [Google Scholar]
- 17.Mwale F, Wang HT, Roughley P, Antoniou J, Haglund L. Link N and mesenchymal stem cells can induce regeneration of the early degenerate intervertebral disc. Tissue Eng Part A. 2014;20:2942–2949. doi: 10.1089/ten.tea.2013.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mwale F, Demers CN, Petit A, Roughley P, Poole R, Steffen T, Aebi M, Antoniou J. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J Cell Biochem. 2003;88:1202–1213. doi: 10.1002/jcb.10479. [DOI] [PubMed] [Google Scholar]
- 19.Mwale F, Masuda K, Pichika R, Epure LM, Yoshikawa T, Hemmad A, Roughley PJ, Antoniou J. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R120. doi: 10.1186/ar3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbott RD, Purmessur D, Monsey RD, Brigstock DR, Laudier DM, Iatridis JC. Degenerative grade affects the responses of human nucleus pulposus cells to link-N, CTGF, and TGFβ3. J Spinal Disord Tech. 2013 May;26(3):E86–94. doi: 10.1097/BSD.0b013e31826e0ca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin L, Liu Q, Scott P, Zhang D, Shen F, Balian G, Li X. Annulus fibrosus cell characteristics are a potential source of intervertebral disc pathogenesis. Plos One. 2014;9:e96519. doi: 10.1371/journal.pone.0096519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L, Ding M, Saadoon O, Vess E, Fernandez A, Zhao P, Jin L, Li X. A novel culture platform for fast proliferation of human annulus fibrosus cells. Cell Tissue Res. 2017;367:339–350. doi: 10.1007/s00441-016-2497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iatridis JC, MacLean JJ, O’Brien M, Stokes IA. Measurements of proteoglycan and water content distribution in human lumbar intervertebral. Discs Spine (Phila Pa 1976) 2007;32:1493–1497. doi: 10.1097/BRS.0b013e318067dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oegema TR. Biochemistry of the intervertebral disc. Clin Sports Med. 1993;12:419–439. [PubMed] [Google Scholar]
- 25.Hughes SPF, Freemont AJ, Hukins D, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. Bone Joint J. 2012;94-B:1298–1304. doi: 10.1302/0301-620X.94B10.28986. [DOI] [PubMed] [Google Scholar]
- 26.Mwale F, Ciobanu I, Giannitsios D, Roughley P, Steffen T, Antoniou J. Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine (Phila Pa 1976) 2011;36:E131–E138. doi: 10.1097/BRS.0b013e3181d52b9e. [DOI] [PubMed] [Google Scholar]
- 27.Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12:20141191. doi: 10.1098/rsif.2015.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziskoven C, Jäger M, Kircher J, Patzer T, Bloch W, Brixius K, Krauspe R. Physiology and pathophysiology of nitrosative and oxidative stress in osteoarthritic joint destruction. Can J Physiol Pharmacol. 2011;89:455–466. doi: 10.1139/y11-055. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Fujita N, Hosogane N, Watanabe K, Ishii K, Toyama Y, Takubo K, Horiuchi K, Miyamoto T, Nakamura M, Matsumoto M. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther. 2015;17:316. doi: 10.1186/s13075-015-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu GZ, Ishihara H, Osada R, Kimura T, Tsuji H. Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine (Phila Pa 1976) 2001;26:134–141. doi: 10.1097/00007632-200101150-00005. [DOI] [PubMed] [Google Scholar]
- 31.Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976) 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Kong MH, Do DH, Miyazaki M, Wei F, Yoon SH, Wang JC. Rabbit model for in vivo study of intervertebral disc degeneration and regeneration. J Korean Neurosurg Soc. 2008;44:327–333. doi: 10.3340/jkns.2008.44.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin L, Ding M, Oklopcic A, Aghdasi B, Xiao L, Li Z, Jevtovic-Todorovic V, Li X. Nanoparticle fullerol alleviates radiculopathy via NLRP3 inflammasome and neuropeptides. Nanomedicine. 2017 Apr 9; doi: 10.1016/j.nano.2017.03.015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


