ABSTRACT
Monitoring of water and surface sediment in a French eutrophic lake (Lake Aydat) was carried out over a 2-year period in order to determine whether akinetes in sediment could be representative of the most recent bloom and to estimate their germination potential. Sediment analysis revealed two akinete species, Dolichospermum macrosporum and Dolichospermum flos-aquae, present in the same proportions as observed for the pelagic populations. Moreover, similar spatial patterns observed for vegetative cells in the water column and akinete distributions in the sediment suggest that akinetes in the sediment may be representative of the previous bloom. However, the relationship between akinetes in the sediment and vegetative cells in the water column was not linear, and other factors may interfere. For example, our results highlighted horizontal transport of akinetes during the winter. The benthic overwinter phase did not seem to influence the percentages of intact akinetes, which remained stable at approximately 7% and 60% for D. macrosporum and D. flos-aquae, respectively. These percentages may thus be the result of processes that occurred in the water column. The intact overwintering akinetes showed germination rates of up to 90% after 72 h for D. flos-aquae or 144 h for D. macrosporum. The difference in akinete germination rates between these two species demonstrates different ecological strategies, which serve to expand the window for germination in time and space and thus optimize colonization of the water column by nostocalean cyanobacteria.
IMPORTANCE Cyanobacteria have the ability to proliferate and to form blooms. These blooms can then affect the local ecology, health, and economy. The akinete, a resistant cell type that persists in sediment, is an important intermediate phase between previous and future blooms. We monitored the water column and the surface sediment of a French eutrophic lake (Lake Aydat) to investigate the relationship between vegetative cells in the water column and akinetes in the sediment. This study focused on the characterization of spatiotemporal akinete distributions, cellular integrity, and germination potential. Species-specific ecological strategies were highlighted and may partly explain the temporal succession of species in the water column. Akinetes may also be used to understand past nostocalean blooms and to predict future ones.
KEYWORDS: cyanobacterial bloom, akinete, annual life cycle, cellular integrity, germination rate
INTRODUCTION
Over the past decades, the recurrent degradation of freshwater systems due to anthropization has led to a rocketing number of cyanobacterial proliferations (1). Key to the ecological success of cyanobacteria in temperate zones is their ability to overcome unfavorable conditions in the benthic compartment (2). Cyanobacterial orders use different strategies to persist in sediments. For example, Nostocales can produce akinetes, specialized resting cells that are able to survive when environmental conditions are unfavorable for vegetative cells (3). These unfavorable conditions are generally seasonal and occur at the end of the summer or during the autumn, due to factors such as decreases in the photoperiod or light intensity (4) or the temperature (5). Induction of akinete formation can also be triggered by other factors, such as depletion of phosphorus (6) or potassium (7). The high resistance of akinetes means that they are able to survive in sediment for several decades (8, 9). As a result, some studies have used akinetes as indicators of former cyanobacterial blooms (10, 11). However, no clear relationship between the abundance of past vegetative cells in the water column and akinetes present in sediments has yet been demonstrated. Moreover, although the general factors triggering akinete induction are known, more precise details concerning the specific levels remain unknown, i.e., whether the production of akinetes is the same for all species under environmental conditions. In addition, it is not known whether this production of akinetes is stable over time for each species and thus whether akinetes in the sediment can be considered good indicators of past cyanobacterial blooms. In the spring and/or when environmental conditions become favorable again, akinetes germinate into young filaments, called hormogonia, and colonize the water column to initiate the next proliferation (12). Like akinete formation, the germination process is understood in a general way but not sufficiently to estimate the contribution of germinating akinetes to the next cyanobacterial proliferation. Indeed, because the growth requirements for Nostocales species differ, the potential for akinete germination should depend on specific factors, which need to be investigated more thoroughly.
In this context, our first aim was to determine whether akinetes present in surface sediment are representative of the most recent bloom. Our second aim was to investigate the distribution of benthic akinetes and their viability dynamics during the winter. Finally, our last aim was to estimate the specific germination potential of two akinete species in the spring. We carried out fieldwork in the French eutrophic Lake Aydat, monitoring the water column and sediment for bloom-forming and non-bloom-forming species (Dolichospermum macrosporum and Dolichospermum flos-aquae, respectively) over a 2-year period (Fig. 1). We also investigated the global distribution of akinetes in the surface sediment, based on the number of intact akinetes present, and lastly focused on akinete germination in order to estimate the real pool of viable akinetes.
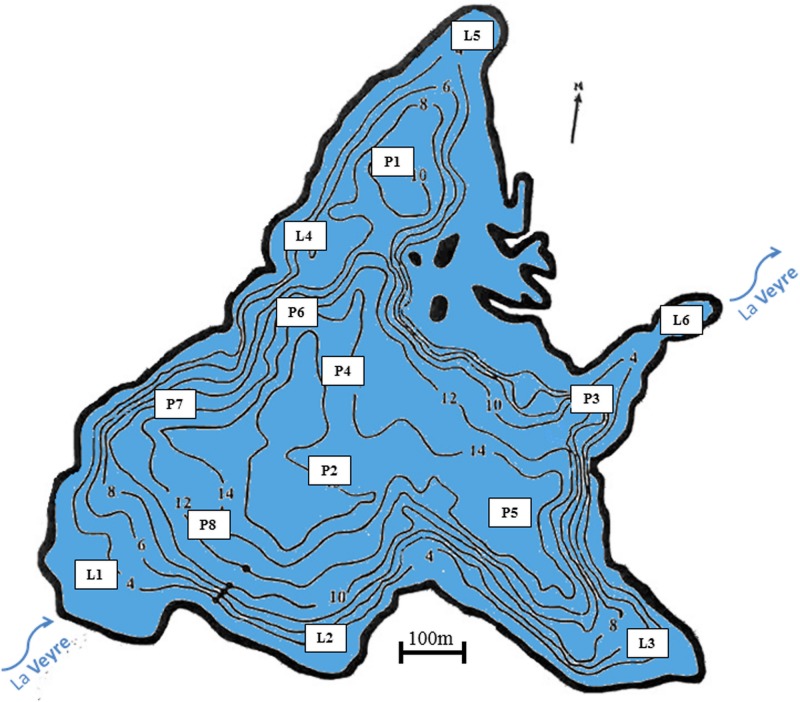
FIG 1.
Bathymetric map of Lake Aydat, showing sampling station locations. The base bathymetric map was created using Surfer (version 7.02).
RESULTS
Vegetative cells and akinete dynamics.
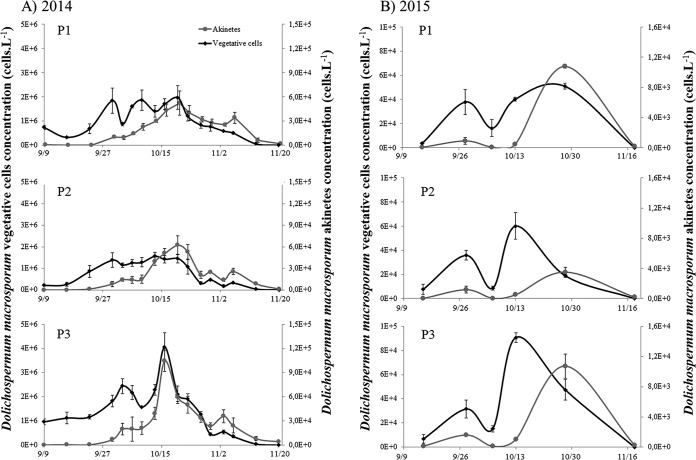
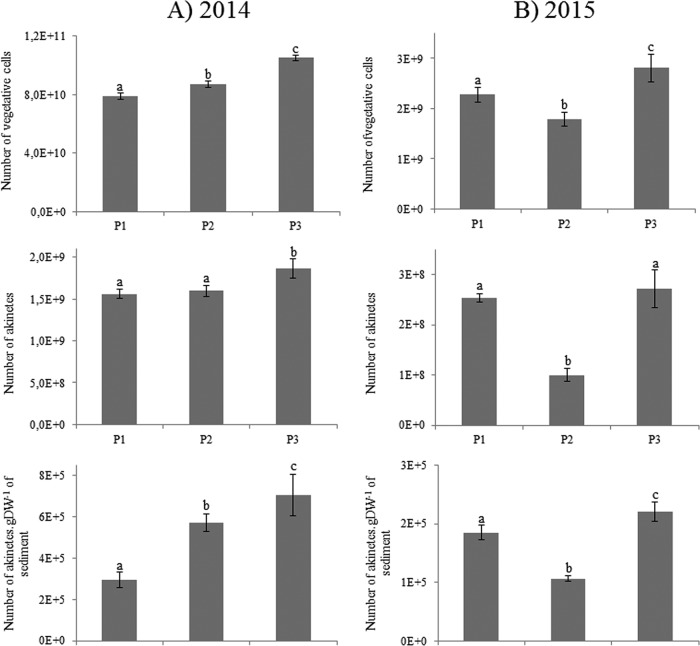
Dolichospermum macrosporum was the dominant cyanobacterial species observed (Fig. 2), whereas D. flos-aquae was occasionally observed in small quantities during the 2-year study period (data not shown). In 2014, at the three locations studied, the abundance of D. macrosporum vegetative cells started to increase at the beginning of September, showed a maximum in mid-October, and then declined progressively until November (Fig. 3A). During this period, the maximum abundances were similar for the P1 and P2 stations, where values reached 1.96 × 106 and 1.56 × 106 vegetative cells · liter−1, respectively, and higher for the P3 station, with a value of 4.06 × 106 vegetative cells · liter−1. The akinete abundance followed the same dynamics, slightly offset in time, with a start at the end of September. The maximum abundance of akinetes at the three points was observed on the same date as the maximum abundance of vegetative cells. Maximum concentrations of akinetes reached 5.19 × 104 and 6.22 × 104 cells · liter−1 for P1 and P2, respectively. Once again, the maximum value was observed at P3, i.e., 10.50 × 104 cells · liter−1 (Fig. 3A). The total numbers of vegetative cells showed significant differences among the stations and appeared significantly smaller for P1 than for P2 and P3 (Fig. 4A). Interestingly, the total numbers of akinetes in the sediment showed the same trend, with P1 values being less than P2 values, which were less than those for P3. The proportions of akinetes versus vegetative cells in the water column were 1.8/100 to 2/100 during the bloom event (Fig. 4). In 2015, the total abundance of vegetative cells was very low and represented only 3% of the 2014 bloom. The kinetics of vegetative cells were also different, with two peaks, on 28 September 2015 and 30 October 2015 (Fig. 3B). The second peak was higher, with maximal densities between 3.51 × 104 and 5.09 × 104 cells · liter−1. In terms of akinete concentrations, the maximal abundances were reached on 30 October 2015 at all stations, with higher values being obtained for P1 (1.08 × 104 cells · liter−1)and P3 (1.07 × 104 cells · liter−1) and a lower value for P2 (3.51 × 103 cells · liter−1). The total numbers of both akinete and vegetative cells followed the same trend, with higher values being observed at P3 than at P2 (Fig. 4B). The proportions of akinetes versus vegetative cells in the water column in 2015 stayed relatively low and were in the same range as observed in 2014, with values of 5.6/100, 9.6/100, and 11.1/100 for P2, P3, and P1, respectively.
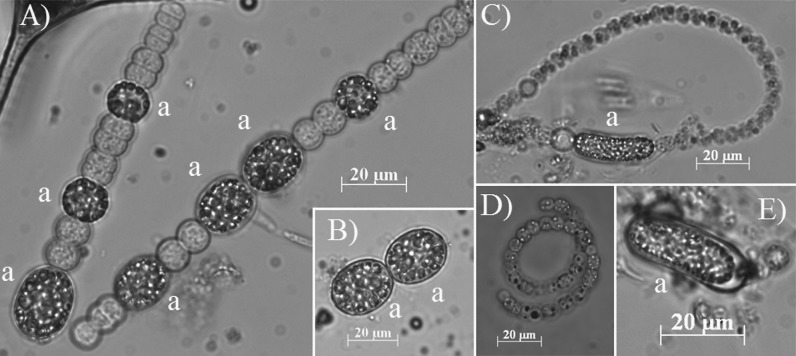
FIG 2.
Photomicrographs, in natural light, of both vegetative cells and akinetes (a) of D. macrosporum (A and B) and D. flos-aquae (C to E).
FIG 3.
Temporal variations in D. macrosporum vegetative cells and akinetes from the 2014 (A) and 2015 (B) blooms for each studied location.
FIG 4.
Estimated numbers of Dolichospermum macrosporum vegetative cells and akinetes in the water column and akinetes in sediment in 2014 (A) and 2015 (B). Lowercase letters represent statistical differences between each analyzed condition.
Spatiotemporal distributions of akinetes in sediment.
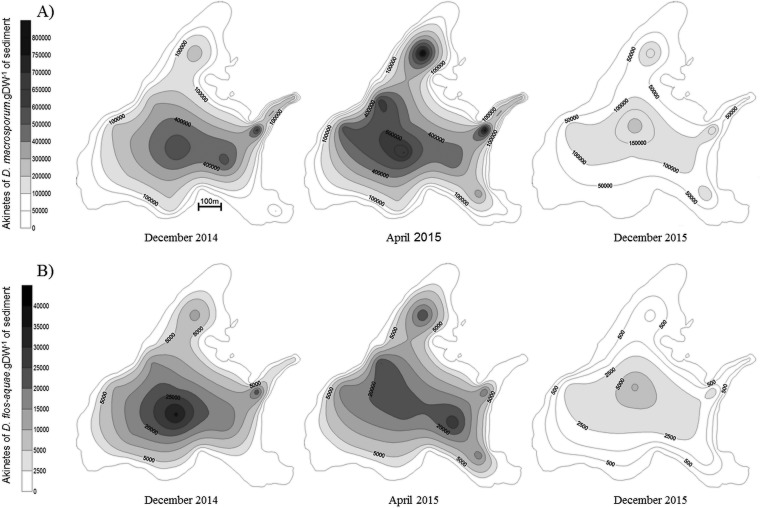
The two species (D. macrosporum and D. flos-aquae) that were present in the water column were also present in sediment (Fig. 5). It was found that akinete abundances in sediment for the three studied dates were markedly different for the two species, with D. macrosporum being more abundant, as was also the case for the water column. Moreover, relative akinete abundances showed the same trends as for the water column; D. macrosporum was more abundant in 2014 than in 2015, with values of 40,000 to 705,000 and 2,600 to 221,000 akinetes · g (dried weight) of sediment, respectively (Fig. 5A). Thus, the overall akinete abundance of D. macrosporum in sediment in 2015 represented only 36% of that in 2014. This trend was even more marked for D. flos-aquae, with an abundance of akinetes present in sediment in 2014 (150 to 36,400 akinetes · g [dried weight] of sediment) 4 times higher than that in 2015 (0 to 4,150 akinetes · g [dried weight] of sediment) (Fig. 5B).
FIG 5.
Spatial distributions of akinetes in Lake Aydat sediment in December 2014, April 2015, and December 2015 for D. macrosporum (A) and D. flos-aquae (B).
The akinete distribution also varied with the season when sampling took place. For both Dolichospermum species, the numbers of benthic akinetes in deep areas were higher in spring than in winter (Student's t test, P ≤ 0.003) (see Table S1 in the supplemental material). In contrast, the numbers of akinetes present at littoral sites were always smaller in spring than in winter (Student's t test, P ≤ 0.03) (Table S1).
Furthermore, akinete concentrations were always significantly higher in deep areas than in littoral areas, for all studied species and every sampling date (Student's t test, P < 0.001) (Fig. 5; also see Table S1). For example, the numbers of D. flos-aquae akinetes in December 2014 were approximatively 4 times higher in deep areas than in littoral ones.
Akinete physiological states in sediment.
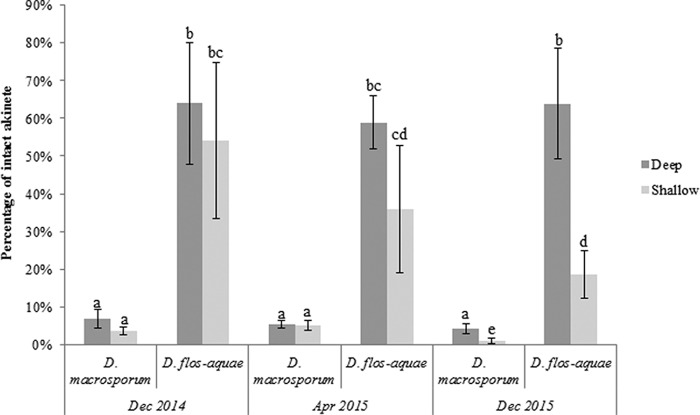
The percentage of intact akinetes in Lake Aydat sediments may depend on the Dolichospermum species (multivariate analysis of variance [MANOVA], P ≤ 1.74 × 10−9) (Fig. 6). Indeed, the percentages of intact akinetes for D. macrosporum were between 1% ± 1% and 6.8% ± 2.4%, whereas the percentages for D. flos-aquae were significantly higher, fluctuating between 18.6% ± 6.3% and 63.9% ± 14.6%. For each species, there were no significant differences in the percentages of intact akinetes based on area and sampling date (Tukey's test, P > 0.05) except for December 2015, when the values for littoral areas were statistically lower than the others.
FIG 6.
Percentages of intact akinetes in Lake Aydat sediment according to the species, the area (pelagic versus littoral zones), and the sampling period. Lowercase letters represent statistical differences between each analyzed condition.
Akinete germination.
Different states of akinetes were observed and considered (Fig. 7), including (i) intact akinetes (Fig. 7A), (ii) germinating akinetes, which appeared to be divided into several cells (Fig. 7B and C), and (iii) young filaments (hormogonia) (Fig. 7D and E), where the number of cells exceeded 8 cells.
FIG 7.
Photomicrographs, in natural light, of D. macrosporum akinete germination, from intact akinete (top left) to hormogonia (young filament) (bottom right).
(i) In situ akinete germination.
The percentages of germinating akinetes were dependent on the species and the sampling location (two-way analysis of variance [ANOVA], P < 0.001). Indeed, the percentages of germinating akinetes in Lake Aydat sediment were 5% ± 3% in deep areas and 30% ± 7.5% in littoral areas for D. macrosporum (Fig. 8). For D. flos-aquae, results were more homogeneous, with 8.9% germinated akinetes in deep zones and 7.5% in littoral areas. D. macrosporum had a significantly higher rate of germinating akinetes in littoral areas than under other conditions (P > 0.00017).
FIG 8.
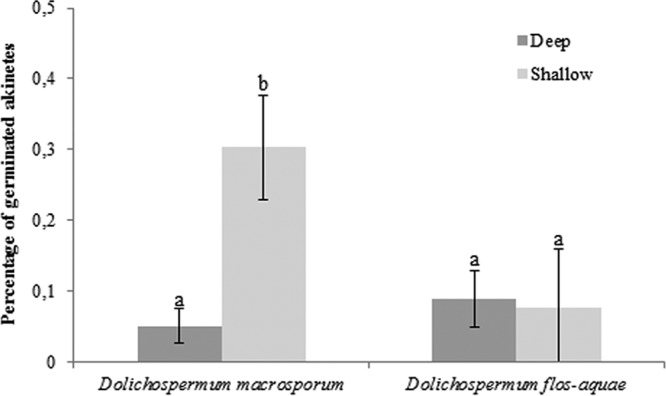
Percentages of germinated akinetes in Lake Aydat sediment in April 2015 according to the species and the lake zone (pelagic versus littoral zones). Lowercase letters represent statistical differences between each analyzed condition.
(ii) Akinete germination experiments.
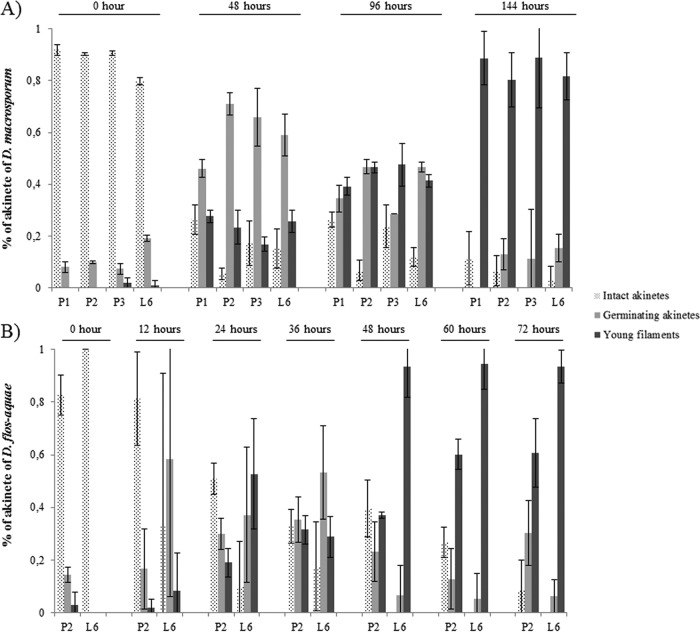
The experimental patterns were similar overall for the two species; at the beginning, the percentages of intact akinetes were high, between 80% and 92% for D. macrosporum (Fig. 9A) and between 82% and 100% for D. flos-aquae (Fig. 9B), and then they decreased to reach values close to 0% at 144 h for D. macrosporum and at 72 h for D. flos-aquae. In contrast, the percentages of filaments formed were almost 0 at the beginning of the experiments and more than 80% at the end of the experiments, except for D. flos-aquae akinetes from P2 (60%). At 48 h, the percentages of young filaments of D. macrosporum varied from 17% to 27%, depending on the sample location, whereas those for D. flos-aquae were 60% to 94%. Temporal differences were also observed for germinating akinetes. A peak was clearly visible at 48 h for D. macrosporum, with values ranging from 46% to 71%, whereas the percentages of germinating akinetes for D. flos-aquae were only 7% to 23% at the same time. The higher percentages of germinating akinetes for D. flos-aquae occurred earlier, between 12 and 36 h, with values of 17% to 58%.
FIG 9.
Percentages of akinete germination for D. macrosporum (A) and D flos-aquae (B).
DISCUSSION
Are akinetes retrieved from the sediment good representatives of past blooms?
Akinete formation occurs in the water column and is triggered by conditions that are unfavorable for the growth of vegetative cells (13–16). Despite the diversity of factors known to potentially drive akinete production (temperature, light intensity, and nutrient concentrations), our results showed stable proportions of planktonic akinetes versus vegetative cells in the water column, with low mean values of 2% to 8.75%, depending on the year. This difference in akinete production between the 2 years is considered to be negligible, compared to the variation in total cyanobacterial biomass between these 2 years. Because stress conditions were extremely different between the bloom-forming year and the non-bloom-forming year (lower temperature, less daylight, and earlier mixing conditions in 2015), we can assume that the abundance of akinetes formed in the water column is proportional to the abundance of vegetative cells, regardless of the environmental conditions. Based on this assumption, the abundance of akinetes formed during a proliferation can facilitate estimation of the total abundance of cyanobacterial cells in a past bloom event. Although akinetes are cells known to be resistant to a wide range of stress factors (e.g., lack of light [17], temperature variations [18], and desiccation [19]) and to be well conserved through time in sediment (8, 9), the question of whether the abundance of akinetes found in the sediment can be used as a good indicator of past proliferation remains (10, 11). At a qualitative level, akinetes in sediment can be used to reveal the Nostocales diversity of the water column. In Lake Aydat, the two cyanobacterial species found in the water column, D. macrosporum and D. flos-aquae, were also observed in the sediment, despite a very low planktonic abundance of D. flos-aquae. At a quantitative level, our results showed identical patterns for the numbers of vegetative cells in the water column and the akinete distributions in the sediment for the 2 years of this study. Therefore, as expected, a bloom-forming year corresponds to a greater abundance of akinetes in the surface sediment. However, we did not obtain similar proportions from year to year for vegetative cells versus benthic akinetes, and thus the past abundance of vegetative cells cannot be exactly calculated by using the number of benthic akinetes present in the sediment. Nevertheless, benthic akinetes can indicate global trends regarding bloom-forming versus non-bloom-forming years. This interannual variability is the result of the balance between the number of akinetes reaching the sediment at the end of the summer season and those leaving the sediment after germination and the inoculation process (12). Therefore, even if the production of akinetes (according to nostocalean biomass) in a water column is quite constant, the loss of akinetes through the sedimentation and inoculation processes may be very different, depending on the year and the biotic and abiotic stresses (such as temperature, light intensity, and parasitism).
The benthic spatial distribution of akinetes and the sampling season must also be taken into account to explain the variability of the benthic akinete and planktonic vegetative cell proportions. Our results highlight a heterogeneous benthic distribution of akinetes, showing great seasonal differences. This benthic distribution may be the result of both planktonic distribution and benthic transport. It is commonly acknowledged that wind can influence planktonic cyanobacterial distribution, creating areas of accumulation (20), and the same applies to akinetes in surface sediments in Spanish lakes (2); the authors showed that sediment close to a dam accumulated 11 times more akinetes than did that in shallower upstream areas, due to the influence of river currents. In Lake Aydat, akinete counts have shown greater concentrations in the deeper parts of the lake, as well as at the lake outlet, in both deep and littoral zones. The lake outlet hot spot in the sediment is probably due to the combination of two phenomena induced by dominant winds and the Veyre river current, i.e., high levels of accumulation of cyanobacteria in the water column and horizontal transport of benthic akinetes from the littoral zone to the deep zone during the winter period. The natural redistribution of fine particles (including cyanobacterial cells) from shallow areas to deep areas has already been observed in other lakes (21, 22). This secondary sedimentation can also be influenced by numerous parameters, such as wind, river flow, sedimentary composition, and lake geomorphology (2, 23, 24). This phenomenon may be amplified by the basaltic lava flow present along the northeastern edge of Lake Aydat (25), where there is no persistent sedimentation. Thus, sampling from the deepest part of the lake at the end of winter seems the best strategy to evaluate the benthic stock of akinetes.
Benthic akinete viability.
Few studies have highlighted that akinetes in sediment can be divided into two categories, intact and nonintact (12, 19). The percentage of intact Cylindrospermopsis raciborskii akinetes in sediments from the pelagic area of Lake Melangsee (Germany) in winter was estimated to be 20% to 30% (12). In this study, intact akinetes from the sediment of Lake Aydat were approximately 7% and 65% for D. macrosporum and D. flos-aquae, respectively. This high level of species-dependent variability may be explained by species-specific sensitivity to abiotic factors in both the water column and the sediment. Indeed, with the same abiotic factors, D. macrosporum akinetes present a significantly lower survival rate than D. flos-aquae akinetes (19). Furthermore, the impact of biotic factors can also be species specific. For example, Bdellovibrio-like bacteria can affect Microcystis aeruginosa but leave Microcystis wesenbergii unaffected, suggesting strict host specificity (26). Viral lysis may also be linked to host specificity. It was shown that an isolated cyanophage (Ma-LMM01) caused lysis of only 1 of the 16 Microcystis aeruginosa genotypes tested (27). In Lake Aydat, D. macrosporum akinetes were specifically affected by the chytrid Rhizosiphon akinetum (28, 29). The high prevalence of species-specific infection of D. macrosporum akinetes observed in the water column in 2014 and 2015 (30) may partly explain why the number of intact akinetes was particularly low for this species.
The viability of akinetes can also be affected during overwintering, for example, through bioturbation by invertebrates, which can reduce the pool of intact akinetes (31, 32). To a lesser extent, parasitism is also a factor in the sediment compartment (33). However, the akinete integrity did not change between December 2014 and April 2015, suggesting a 1-year scale on which the integrity of benthic akinetes was not affected during overwintering. Therefore, we can assume that the proportion of intact akinetes counted in the surface sediment during the winter is a valid reflection of what happened in the water column and during the sedimentation process.
Akinete germination.
Most studies of akinete germination focused on laboratory strains that exhibit rates of germination close to 100% when culture conditions are optimal (34–36). Some studies were also interested in the germination process of akinetes from natural sediment, but apparently the rate of germination was rarely calculated. A germination rate of approximately 70% for Anabaena ucrainica akinetes isolated from Daimon-Ike (Japan) was highlighted previously (37). In our case, the rate of D. flos-aquae akinete germination is of the same order of magnitude. In contrast, this rate was very low for D. macrosporum, due to the presence of a large percentage of akinetes lysed by species-specific parasitism; this seriously affects the size of akinete inocula for the next few years. Considering only the fraction of intact akinetes, we obtained very high rates of germination (up to 90%) for both species. This finding indicates that the pool of intact akinetes corresponds to the pool of viable akinetes. Even if almost all of the intact akinetes are able to germinate in the next few years, this step in the life cycle also seems to follow a species-specific process. D. flos-aquae has a response that is twice as fast as that of D. macrosporum under the same controlled conditions. This finding suggests that akinete germination in the lake is not synchronous for every species, allowing a shift in the time of water colonization. This may indicate different strategies for water column colonization, to avoid competition between D. flos-aquae and D. macrosporum. The most important environmental parameters, independent of the cyanobacterial species, triggering akinete germination are the internal nitrogen quota (3), sediment mixing (38), and increases in temperature (12, 37) and light intensity (39–43). These favorable environmental parameters for germination lead to the definition of more favorable areas for akinete germination. For example, a study proposed the hypothesis that shallow sediments are more important than pelagic sediments for the recruitment of Gloeotrichia echinulata in Lake Erken (Sweden) (38). This idea was confirmed with Anabaena (i.e., Dolichospermum [44]) and with the Aphanizomenon genus in Lake Limmaren (Sweden) (45). The higher rates of germinating akinetes, at least for D. macrosporum, in littoral sediments than in pelagic sediments may confirm the importance of shallow areas in Lake Aydat. However, germinating akinetes of D. macrosporum and D. flos-aquae were also observed in deep sediments (depths of >9 m) below the euphotic zone all year round (data not shown). Thus, akinetes located in deep zones have to be integrated in the potential inoculum. However, because favorable conditions for germination, especially temperature, are not uniform throughout the lake, akinete germination probably does not occur at the same time, as governed by the lake bathymetry. We can infer that akinete germination in lakes occurs first in littoral areas, where light is not a limiting factor and the water temperature increases earlier in the year. The warm water then spreads progressively to the deeper areas, inducing akinete germination. Akinetes located in deeper areas can access light during sediment-mixing phases (3). These processes may extend the germination window in time and space and thus optimize the colonization of the water column.
Conclusions.
This study provided an overview to determine whether akinetes in sediments are representative of previous blooms and to improve the characterization of germination potential. As shown for past microbial diversity (46, 47), sediments represent an archive of past cyanobacterial blooms in the lake water column. Despite all of the parameters involved in the annual dynamics of akinetes (sedimentation and inoculation processes and benthic transport), we conclude that studying benthic akinetes allows determination of past planktonic diversity and illustrates clear trends regarding cyanobacterial abundances from the bloom event in the water column of the previous year. We also showed that the potential inoculum for subsequent years is composed of all intact akinetes whatever their location (i.e., deep versus littoral sediments). Species-specific abilities regarding akinete integrity and germination suggest different ecological strategies, leading to a complex process of water colonization. Further studies need to be undertaken to characterize the long-term persistence of akinetes in sediments, incorporating the notion of akinete integrity.
MATERIALS AND METHODS
Lake description and sampling strategy.
Lake Aydat (45°39′48″N, 002°59′04″E) is located in the French Massif Central region, 838 m above sea level. This dimictic natural dam lake was created by the damming of the river Veyre by a basaltic flow about 7,500 years ago (48). It is a small eutrophic lake with a maximum depth of 15 m, a surface area of 60 ha, and a large catchment area of 30,000 ha. It has recurrent Dolichospermum blooms at the end of summer and the beginning of autumn (28).
Lake Aydat was sampled at least 1 time per week, depending on the cyanobacterial concentration, from September to November during the 2014 and 2015 cyanobacterial blooms. Sampling was carried out on the whole water column, with a phytoplankton net with 20-μm pores (Hydro-Bios, Altenholz, Germany). Samples were collected from 0.5 m above the sediment to the surface water column, corresponding to depths of 9.5, 15, and 10 m for the sampling points P1, P2, and P3, respectively (Fig. 1). Concentrated water for each sample was normalized to a final volume of 2 liters, and 160 ml of these 2 liters was then fixed with 10 ml of Lugol's iodine solution (Sigma-Aldrich, St. Louis, MO, USA). Fixed samples were kept in the dark at 4°C until further analysis.
After each cyanobacterial proliferation (December 2014 and December 2015) and before the cyanobacterial recruitment of 2015 (April), surface sediment sampling was carried out using a sediment corer (UWITEC, Mondsee, Austria) (2, 12, 19). A total of 14 points were sampled, i.e., 8 in the pelagic area (P1, P2, and P3 [which have already been sampled, in the water column] and P4, P5, P6, P7, and P8) and 6 in the littoral area (L1, L2, L3, L4, L5, and L6) (Fig. 1). Points in the pelagic area corresponded to depths of 9 to 15 m, whereas the maximum depth for the littoral sediment was 3 m. Then, supernatant water from each core was discarded, and the mobile surface sediment containing recent akinetes was pipetted and kept at 4°C in the dark until analysis (19). This mobile surface sediment corresponds to the first 0.5 cm of sediment, in accordance with the annual rate of sedimentation in Lake Aydat (49, 50). Therefore, in this study, deep sediment corresponds to surface sediment from pelagic areas and shallow sediment corresponds to surface sediment from littoral areas.
Microscopic counts of cyanobacterial blooms.
Cyanobacterial cell abundance was estimated using 0.25- to 10-ml (depending on the biomass) subsamples from the samples fixed with Lugol's iodine, settled for 24 h in counting chambers. Vegetative cells and akinetes were counted in at least 30 randomly selected optical fields at a magnification of ×200 with an inverted microscope (Axiovert 200 M; Zeiss, Oberkochen, Germany), following the method of Utermöhl (51). Each sample was counted at least three times. Species determination was performed by microscopic examination, based on morphological criteria described in taxonomic keys known from reference books (52–54). The two species were distinguished on the basis of the morphology of their trichomes and the form and size of their akinetes (Fig. 2). D. macrosporum exhibits straight single trichomes and rounded akinetes, while D. flos-aquae has coiled trichomes that form solitary or, more usually, entangled twisted masses, with cylindrical or slightly ellipsoid and often slightly bent akinetes. The recurrence of D. macrosporum and D. flos-aquae in Lake Aydat has been highlighted in numerous studies for decades (19, 28, 29, 55, 56).
Extraction of akinetes from sediment.
Akinetes were extracted from sediments using a density gradient protocol with Ludox, as described previously (19). Briefly, 3 ml of fresh sediment was diluted with 7 ml of distilled water and 4 ml of Ludox TM-50 (Sigma-Aldrich). The solutions were sonicated for 30 s (frequency, 50%; power, 80 W; Sonopuls; Bandelin, Berlin, Germany) and centrifuged at 10,000 × g for 30 min. The first 6 ml was pipetted and kept in the dark at 4°C for further analysis.
Estimation of sediment akinete abundance.
Species determination for akinetes was based on morphological criteria (2, 10–12, 19, 28). This determination was completed by analyzing the morphology of young filaments issued from germinating akinetes (see Fig. S1 in the supplemental material), using taxonomic keys known from reference books (52–54). Akinete abundance was estimated for the 14 studied points, i.e., 8 points in pelagic areas and 6 points in littoral areas. Six replicates per sample were analyzed in this study. Before counting, binding with SYTOX green (Invitrogen, Carlsbad, CA, USA) was carried out by adding 1 μl of 50 mM SYTOX green to 1 ml of the akinete-containing solution and incubating the solution for at least 30 min in the dark (29); 2 ml of this solution was then filtered through an 8-μm mesh (TETP filter; Merck Millipore, Tullagreen, Ireland). Akinetes were counted at a magnification of ×160 with an epifluorescence microscope (Laborlux S; Leica Microsystems GmbH, Wetzlar, Germany), and 40 fields were counted for each filter. Two series of counts were performed for each filter (19), one at 546 nm to distinguish intact akinetes still containing chlorophyll pigments and one at 488 nm to discriminate damaged akinetes with SYTOX green staining.
Akinete germination experiments.
The in situ akinete germination corresponds to the germinating akinetes observed in the surface sediments sampled in April 2015. Extracted akinetes from Lake Aydat sediment (at P1, P2, P3, and L6) were kept under controlled conditions and observed every 48 h for D. macrosporum and every 12 h for D. flos-aquae (Fig. 9). Two experiments were performed in order to follow the germination of the akinetes. The first experiment, over a period of 6 days, was carried out with four different samples (P1, P2, P3, and L6), sampled at 0, 48, 96, and 144 h. The second experiment was shorter (72 h), with more closely spaced sampling (0, 12, 24, 36, 48, 60, and 72 h), and was performed with 2 samples (P2 and L6). In order to collect a sufficient number of akinetes for germination experiments, these resistant cells were concentrated after extraction, with the Ludox protocol described above, from 36 ml of sediment per sample. Then, supernatants containing akinetes were filtered through a 10-μm filter under a pressure of 400 mmHg. The filter was rinsed with distilled water in order to detach the akinetes. The solution obtained was filtered on first 100-μm and then 50-μm nylon filters, in order to remove particles such as larger organic and mineral particles and Chroococcales colonies. Finally, the akinetes were concentrated again during a last filtration with a 10-μm filter, under pressure. Akinetes were collected from the filter using 2.8 ml of BG11 medium (Sigma-Aldrich). The germination experiments were performed in triplicate for each analyzed sample. Each replicate of 800 μl of BG11 medium with akinetes was split into cellular culture wells (48-well plate for cellular tissue; VWR, Radnor, PA, USA) and placed in continuous white light (6 μmol · m−2 · s−1) at 20°C. For each sampling time, 80 μl of homogenized solution of each replicate was analyzed. These solutions were bound with SYTOX green under the same conditions as described above. Then, the 80 μl of solution was filtered through a 47-mm TETP filter and placed on a slide under a cover, and cells were counted by performing transects of the whole sample (magnification of ×200), using an epifluorescence microscope (Zeiss Axiovert 200 M).
Statistical analyses.
The normality of all data and the variance homoscedasticity were evaluated using Shapiro-Wilk and Levene tests, with PAST (version 3.04) and XLSTAT (version 2015.3.01.19199) software. If samples did not follow a normal distribution, then they were normalized with a log10 function (57) and the first two tests were performed again. Estimations of the numbers of D. macrosporum cells present during the whole blooms were performed by calculating the integrals of cellular kinetics (Fig. 3), using OriginPro software (version 8.0725). In this way, the total numbers of cells were estimated for each studied point, for both vegetative cells and akinetes in the water column (Fig. 4). Then, one-way ANOVA, coupled with the post hoc Tukey's test with a 5% confidence interval, was performed in order to determine differences in the numbers of vegetative cells or akinetes, depending on the sampling station (P1, P2, and P3), for the 2 years covered by the survey (Fig. 4). Student's t tests were performed for each studied species in order to highlight differences based on the sampling area and the year of sampling for akinete distributions in sediments (Table S1).
In order to detect the differences in intact akinete distributions in Lake Aydat sediments, MANOVA was carried out with R Commander (version 1.6-1). A post hoc Tukey's test with a 5% confidence interval was also performed (Fig. 4). Two-way ANOVA with a post hoc Tukey's test with a 5% confidence interval was also performed with the PAST software, to highlight the significant differences in in situ akinete germination in April 2015, depending on the species studied and the site location (littoral versus pelagic areas) (Fig. 8). Maps representing the spatial distribution of akinetes in surface sediment were produced using Surfer software (version 7.02), using kriging as the gridding method.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kristell Crenn, Léa Chartrain, and Fanny Perrière for their help during samplings. We also thank Marianne Coulon and Fran Van Wyk de Vries for helping to improve the English text. Finally, we thank the reviewers for improving the manuscript and for their helpful comments.
B.L. was supported by a CIFRE Ph.D. fellowship from the Association Nationale de la Recherche et de la Technologie in collaboration with ATHOS Environnement in the context of Conventions Industrielles de Formation par la Recherche. This work was also funded by l'Agence de l'Eau Loire-Bretagne.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01571-17.
REFERENCES
- 1.Merel S, Walker D, Chicana R, Snyder S, Baurès E, Thomas O. 2013. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ Int 59:303–327. doi: 10.1016/j.envint.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Cirés S, Wörmer L, Agha R, Quesada A. 2013. Overwintering populations of Anabaena, Aphanizomenon and Microcystis as potential inocula for summer blooms. J Plankton Res 35:1254–1266. doi: 10.1093/plankt/fbt081. [DOI] [Google Scholar]
- 3.Hense I, Beckmann A. 2006. Towards a model of cyanobacteria life cycle: effects of growing and resting stages on bloom formation of N2-fixing species. Ecol Model 195:205–218. doi: 10.1016/j.ecolmodel.2005.11.018. [DOI] [Google Scholar]
- 4.Myers JH, Beardall J, Allinson G, Salzman S, Robertson S, Gunthorpe L. 2011. Potential triggers of akinete differentiation in Nodularia spumigena (Cyanobacteriaceae) isolated from Australia. Hydrobiologia 671:165–180. doi: 10.1007/s10750-011-0714-4. [DOI] [Google Scholar]
- 5.Li R, Watanabe M, Watanabe MM. 1997. Akinete formation in planktonic Anabaena spp. (cyanobacteria) by treatment with low temperature. J Phycol 33:576–584. doi: 10.1111/j.0022-3646.1997.00576.x. [DOI] [Google Scholar]
- 6.Rother JA, Fay P. 1977. Sporulation and the development of planktonic blue-green algae in two Salopian meres. Proc R Soc Lond B Biol Sci 196:317–332. doi: 10.1098/rspb.1977.0043. [DOI] [Google Scholar]
- 7.Sukenik A, Kaplan-Levy RN, Viner-Mozzini Y, Quesada A, Hadas O. 2013. Potassium deficiency triggers the development of dormant cells (akinetes) in Aphanizomenon ovalisporum (Nostocales, Cyanoprokaryota). J Phycol 49:580–587. doi: 10.1111/jpy.12069. [DOI] [PubMed] [Google Scholar]
- 8.Wood SA, Jentzsch K, Rueckert A, Hamilton DP, Cary SC. 2009. Hindcasting cyanobacterial communities in Lake Okaro with germination experiments and genetic analyses. FEMS Microbiol Ecol 67:252–260. doi: 10.1111/j.1574-6941.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 9.Livingstone D, Jaworski GHM. 1980. The viability of akinetes of blue-green algae recovered from the sediments of Rostherne Mere. Br Phycol J 15:357–364. doi: 10.1080/00071618000650361. [DOI] [Google Scholar]
- 10.Eilers JM, Kann J, Cornett J, Moser K, St Amand A. 2004. Paleolimnological evidence of change in a shallow, hypereutrophic lake: Upper Klamath Lake, Oregon, USA. Hydrobiologia 520:7–18. doi: 10.1023/B:HYDR.0000027718.95901.ae. [DOI] [Google Scholar]
- 11.Van Geel B, Mur LR, Ralska-Jasiewiczowa M, Goslar T. 1994. Fossil akinetes of Aphanizomenon and Anabaena as indicators for medieval phosphate-eutrophication of Lake Gosciaz (Central Poland). Rev Palaeobot Palynol 83:97–105. doi: 10.1016/0034-6667(94)90061-2. [DOI] [Google Scholar]
- 12.Rücker J, Tingwey EI, Wiedner C, Anu CM, Nixdorf B. 2009. Impact of the inoculum size on the population of Nostocales cyanobacteria in a temperate lake. J Plankton Res 31:1151–1159. doi: 10.1093/plankt/fbp067. [DOI] [Google Scholar]
- 13.Baker PD. 1999. Role of akinetes in the development of cyanobacterial populations in the lower Murray River, Australia. Mar Freshw Res 50:265–279. doi: 10.1071/MF98090. [DOI] [Google Scholar]
- 14.Cirés S, Wörmer L, Wiedner C, Quesada A. 2013. Temperature-dependent dispersal strategies of Aphanizomenon ovalisporum (Nostocales, Cyanobacteria): implications for the annual life cycle. Microb Ecol 65:12–21. doi: 10.1007/s00248-012-0109-8. [DOI] [PubMed] [Google Scholar]
- 15.Everson S, Fabbro L, Kinnear S, Wright P. 2011. Extreme differences in akinete, heterocyte and cylindrospermopsin concentrations with depth in a successive bloom involving Aphanizomenon ovalisporum (Forti) and Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju. Harmful Algae 10:265–276. doi: 10.1016/j.hal.2010.10.006. [DOI] [Google Scholar]
- 16.Mehnert G, Rucker J, Wiedner C. 2014. Population dynamics and akinete formation of an invasive and a native cyanobacterium in temperate lakes. J Plankton Res 36:378–387. doi: 10.1093/plankt/fbt122. [DOI] [Google Scholar]
- 17.Sutherland JM, Herdman M, Stewart WDP. 1979. Akinetes of the cyanobacterium Nostoc PCC 7524: macromolecular composition, structure and control of differentiation. J Gen Microbiol 115:273–287. doi: 10.1099/00221287-115-2-273. [DOI] [Google Scholar]
- 18.Hori K, Okamoto J, Tanji Y, Unno H. 2003. Formation, sedimentation and germination properties of Anabaena akinetes. Biochem Eng J 14:67–73. doi: 10.1016/S1369-703X(02)00136-5. [DOI] [Google Scholar]
- 19.Legrand B, Lamarque A, Sabart M, Latour D. 2016. Characterization of akinetes from cyanobacterial strains and lake sediment: a study of their resistance and toxic potential. Harmful Algae 59:42–50. doi: 10.1016/j.hal.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Kanoshina I, Lips U, Leppänen J. 2003. The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae 2:29–41. [Google Scholar]
- 21.Evans RD. 1994. Empirical evidence of the importance of sediment resuspension in lakes. Hydrobiologia 284:5–12. doi: 10.1007/BF00005727. [DOI] [Google Scholar]
- 22.Verspagen JMH, Snelder EOFM, Visser PM, Jöhnk KD, Ibelings BW, Mur LR, Huisman J. 2005. Benthic-pelagic coupling in the population dynamics of the harmful cyanobacterium Microcystis. Freshw Biol 50:854–867. doi: 10.1111/j.1365-2427.2005.01368.x. [DOI] [Google Scholar]
- 23.Pourriot R, Meybeck M. 1995. Limnologie générale. Elsevier Masson, Paris, France. [Google Scholar]
- 24.Kravchuk ES, Ivanova EA, Gladyshev MI. 2011. Spatial distribution of resting stages (akinetes) of the cyanobacteria Anabaena flos-aquae in sediments and its influence on pelagic populations. Mar Freshw Res 62:450–461. doi: 10.1071/MF10256. [DOI] [Google Scholar]
- 25.Gallois L, Glangeaud P. 1910. Les régions volcaniques du Puy-de-Dôme. Ann Geogr 19:169–173. doi: 10.3406/geo.1910.7892. [DOI] [Google Scholar]
- 26.Gerphagnon M, Macarthur DJ, Latour D, Gachon CMM, Van Ogtrop F, Gleason FH, Sime-Ngando T. 2015. Microbial players involved in the decline of filamentous and colonial cyanobacterial blooms with a focus on fungal parasitism. Environ Microbiol 17:2573–2587. doi: 10.1111/1462-2920.12860. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, Takashima Y, Tomaru Y, Shirai Y, Takao Y, Hiroishi S, Nagasaki K. 2006. Isolation and characterization of a cyanophage infecting the toxic cyanobacterium Microcystis aeruginosa. Appl Environ Microbiol 72:1239–1247. doi: 10.1128/AEM.72.2.1239-1247.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerphagnon M, Latour D, Colombet J, Sime-Ngando T. 2013. Fungal parasitism: life cycle, dynamics and impact on cyanobacterial blooms. PLoS One 8:e60894. doi: 10.1371/journal.pone.0060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerphagnon M, Latour D, Colombet J, Sime-Ngando T. 2013. A double staining method using SYTOX green and calcofluor white for studying fungal parasites of phytoplankton. Appl Environ Microbiol 79:3943–3951. doi: 10.1128/AEM.00696-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerphagnon M, Colombet J, Latour D, Sime-Ngando T. 2017. Spatial and temporal changes of parasitic chytrids of cyanobacteria. Sci Rep 7:6056. doi: 10.1038/s41598-017-06273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearns CM, Hairston NG, Kesler DH. 1996. Particle transport by benthic invertebrates: its role in egg bank dynamics. Hydrobiologia 332:63–70. doi: 10.1007/BF00020780. [DOI] [Google Scholar]
- 32.Karlson AML, Nascimento FJA, Suikkanen S, Elmgren R. 2012. Benthic fauna affects recruitment from sediments of the harmful cyanobacterium Nodularia spumigena. Harmful Algae 20:126–131. doi: 10.1016/j.hal.2012.09.001. [DOI] [Google Scholar]
- 33.Hargreaves KR, Anderson NJ, Clokie MRJ. 2013. Recovery of viable cyanophages from the sediments of a eutrophic lake at decadal timescales. FEMS Microbiol Ecol 83:450–456. doi: 10.1111/1574-6941.12005. [DOI] [PubMed] [Google Scholar]
- 34.Rai AK, Pandey GP. 1981. Influence of environmental stress on the germination of Anabaena vaginicola akinetes. Ann Bot 48:361–370. doi: 10.1093/oxfordjournals.aob.a086134. [DOI] [Google Scholar]
- 35.Sili C, Ena A, Materassi R, Vincenzini M. 1994. Germination of desiccated aged akinetes of alkaliphilic cyanobacteria. Arch Microbiol 162:20–25. doi: 10.1007/BF00264368. [DOI] [Google Scholar]
- 36.Van Dok W, Hart BT. 1997. Akinete germination in Anabaena circinalis (cyanophta). J Phycol 33:12–17. doi: 10.1111/j.0022-3646.1997.00012.x. [DOI] [Google Scholar]
- 37.Tsujimura S, Okubo T. 2003. Development of Anabaena blooms in a small reservoir with dense sediment akinete population, with special reference to temperature and irradiance. J Plankton Res 25:1059–1067. doi: 10.1093/plankt/25.9.1059. [DOI] [Google Scholar]
- 38.Karlsson-Elfgren I, Rengefors K, Gustafsson S. 2004. Factors regulating recruitment from the sediment to the water column in the bloom-forming cyanobacterium Gloeotrichia echinulata. Freshw Biol 49:265–273. doi: 10.1111/j.1365-2427.2004.01182.x. [DOI] [Google Scholar]
- 39.Yamamoto Y. 1976. Effect of some physical and chemical factors on the germination of akinetes of Anabaena cylindrica. J Gen Appl Microbiol 22:311–323. doi: 10.2323/jgam.22.311. [DOI] [Google Scholar]
- 40.Huber AL. 1985. Factors affecting the germination of akinetes of Nodularia spumigena (Cyanobacteriaceae). Appl Environ Microbiol 49:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fay P. 1988. Viability of akinetes of the planktonic cyanobacterium Anabaena circinalis. Proc R Soc Lond B Biol Sci 234:283–301. doi: 10.1098/rspb.1988.0049. [DOI] [Google Scholar]
- 42.Barbiero RP, Kann J. 1994. The importance of benthic recruitment to the population development of Aphanizomenon flos-aquae and internal loading in a shallow lake. J Plankton Res 16:1581–1588. doi: 10.1093/plankt/16.11.1581. [DOI] [Google Scholar]
- 43.Baker PD, Bellifemine D. 2000. Environmental influences on akinete germination of Anabaena circinalis and implications for management of cyanobacterial blooms. Hydrobiologia 427:65–73. doi: 10.1023/A:1003988426561. [DOI] [Google Scholar]
- 44.Komárek J. 2015. Review of the cyanobacterial genera implying planktic species after recent taxonomic revisions according to polyphasic methods: state as of 2014. Hydrobiologia 764:259–270. [Google Scholar]
- 45.Karlsson-Elfgren I, Brunberg AK. 2004. The importance of shallow sediments in the recruitment of Anabaena and Aphanizomenon (Cyanophyceae). J Phycol 40:831–836. doi: 10.1111/j.1529-8817.2004.04070.x. [DOI] [Google Scholar]
- 46.Domaizon I, Savichtcheva O, Debroas D, Arnaud F, Villar C, Pignol C, Perga ME. 2013. DNA from lake sediments reveals the long-term dynamics and diversity of Synechococcus assemblages. Biogeosciences 10:3817–3838. doi: 10.5194/bg-10-3817-2013. [DOI] [Google Scholar]
- 47.Capo E, Debroas D, Arnaud F, Domaizon I. 2015. Is planktonic diversity well recorded in sedimentary DNA? Toward the reconstruction of past protistan diversity. Microb Ecol 70:865–875. doi: 10.1007/s00248-015-0627-2. [DOI] [PubMed] [Google Scholar]
- 48.Sabart M, Crenn K, Perrière F, Abila A, Leremboure M, Colombet J, Jousse C, Latour D. 2015. Co-occurrence of microcystin and anatoxin-a in the freshwater lake Aydat (France): analytical and molecular approaches during a three-year survey. Harmful Algae 48:12–20. doi: 10.1016/j.hal.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Lavrieux M, Disnar JR, Chapron E, Breheret JG, Jeremy J, Miras Y, Reyss JL, Andrieu-Ponel V, Arnaud F. 2013. 6,700-year sedimentary record of climatic and anthropic signals in Lake Aydat (French Massif Central). Holocene 23:1317–1328. doi: 10.1177/0959683613484616. [DOI] [Google Scholar]
- 50.Sarazin G, Michard G, Al Gharib I, Bernat M. 1992. Sedimentation rate and early diagenesis of particulate organic nitrogen and carbon in Lake Aydat (Puy-de-Dôme, France). Chem Geol 98:307–316. doi: 10.1016/0009-2541(92)90191-7. [DOI] [Google Scholar]
- 51.Utermöhl H. 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int Ver Theor Angew Limnol 9:1–38. [Google Scholar]
- 52.Bourrelly P. 1966. Les algues d'eau douce: les algues vertes. Editions N; Boubee & Cie, Paris, France. [Google Scholar]
- 53.Geitler L. 1932. Cyanophyceae. Akademische Verlagsgesellschaft, Leipzig, Germany. [Google Scholar]
- 54.Komárek J. 2013. Süsswasserflora von Mitteleuropa, bd. 19/3: cyanoprokaryota, 3 teil. Heterocytous genera. Springer Spektrum, Berlin, Germany. [Google Scholar]
- 55.Amblard C. 1988. Seasonal succession and strategies of phytoplankton development in two lakes of different trophic states. J Plankton Res 10:1189–1208. doi: 10.1093/plankt/10.6.1189. [DOI] [Google Scholar]
- 56.Rasconi S, Niquil N, Sime-Ngando T. 2012. Phytoplankton chytridiomycosis: community structure and infectivity of fungal parasites in aquatic ecosystems. Environ Microbiol 14:2151–2170. doi: 10.1111/j.1462-2920.2011.02690.x. [DOI] [PubMed] [Google Scholar]
- 57.Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biol Rev 81:259–291. doi: 10.1017/S1464793106007007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.