Abstract
Members of the B cell lymphoma-2 (Bcl-2) family are pivotal arbiters of mitochondrially mediated apoptosis, a process of fundamental importance during tissue development, homeostasis, and disease. At the structural and mechanistic level, the mammalian members of the Bcl-2 family are increasingly well understood, with their interplay ultimately deciding the fate of a cell. Dysregulation of Bcl-2-mediated apoptosis underlies a plethora of diseases, and numerous viruses have acquired homologs of Bcl-2 to subvert host cell apoptosis and autophagy to prevent premature death of an infected cell. Here we review the structural biology, interactions, and mechanisms of action of virus-encoded Bcl-2 proteins, and how they impact on host-virus interactions to ultimately enable successful establishment and propagation of viral infections.
Keywords: Bcl-2, apoptosis, autophagy, structural biology, poxvirus, herpesvirus, asfarvirus, iridovirus, adenovirus, host-pathogen interactions
1. Introduction
From the observation of specific changes in cell morphology upon cellular suicide, and ending in engulfment of the cell by phagocytes, Kerr et al. in 1972 concluded that there must be a genetically programmed form of cell death responsible for cell deletion, that they called apoptosis [1]. From these early origins, it is now recognised that apoptosis is one of a spectrum of programmed cell death (PCD) pathways that includes not only apoptosis but also autophagy, necroptosis and more specialised forms of PCD such as pyroptosis, ferroptosis, anoikis, entosis, pathanatos, netosis, and cornification [2]. The genetic and molecular basis of these different pathways are still being determined.
Correct apoptosis regulation is key to homeostasis, and regulates the clearance of cells that are no longer required in development, and are damaged, dangerous, or infected [3,4]. Apoptosis is an important regulator of the immune response, and pathogens have acquired genes that both prevent the cell from initiating apoptosis during their replicative stage, and initiating apoptosis to release their progeny. Using molecular mimicry of host proteins, pathogens have evolved mechanisms to overcome host cell defences. Such host-pathogen interactions are poorly understood, but several of these pathways are regulated by the activity of proteins of the B-cell lymphoma-2 (Bcl-2) family, a group of about 20 proteins, and numerous studies have been performed to further understand and characterise its mechanism [5,6,7,8,9]. Ultimately, the caspase cascade is activated [10], enabling disassembly of the apoptotic cell followed by its phagocyte-mediated engulfment and elimination via lysosomes [11].
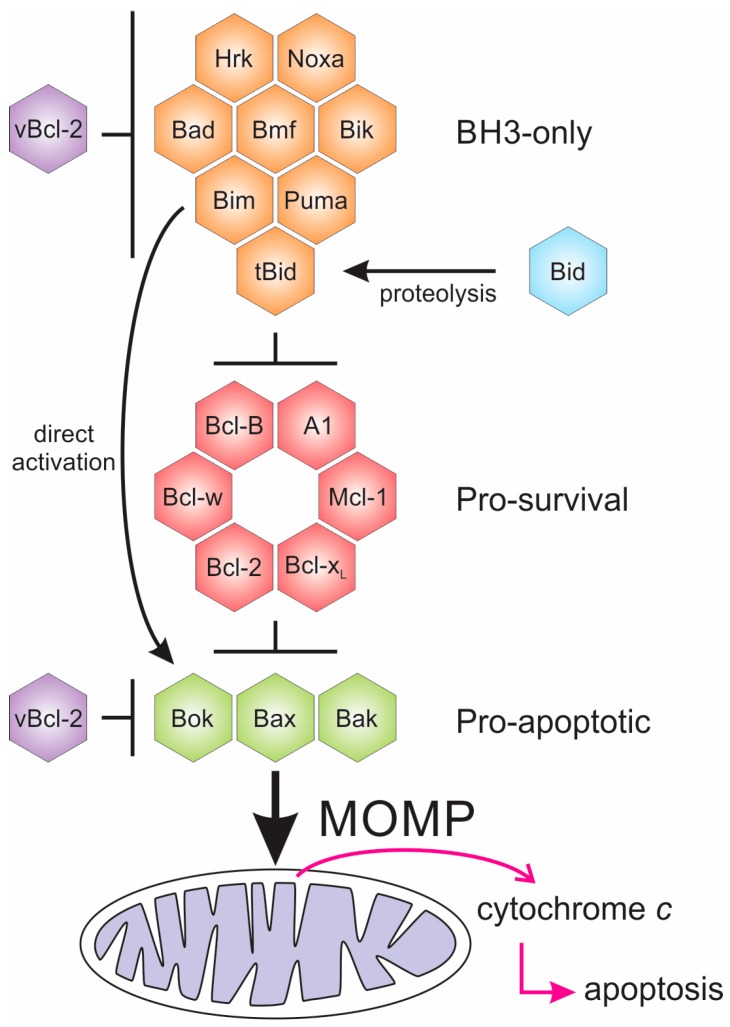
Bcl-2 proteins arose early in metazoan evolution [12,13], and are characterised by the existence of short conserved sequence regions, the Bcl-2 homology (BH) motifs [14] (Figure 1). Two phylogenetically [15] and structurally [8] separate groups of proteins constitute the Bcl-2 family, one group consists of intrinsically disordered proteins (IDP) [7,16], while the other group have a globular α-helical fold structure known as the “Bcl-2 fold” [8]. The former group are IDPs and are exclusively pro-apoptotic, while the latter folded group are either pro-survival or pro-apoptotic. Both Bcl-2 family groups bear BH motifs. The pro-apoptotic BH3-only proteins bear only the BH3 motif (Figure 1), and in mammals includes the proteins Bim, Bad, Bmf, Hrk, Puma, Bik, and Noxa. The BH3-only proteins acquire secondary structure upon binding to their folded Bcl-2 targets, neutralising or activating this second group of the Bcl-2 family. The Bcl-2 fold generates a hydrophobic groove that accommodates a BH3 motif. The Bcl-2 family in mammals includes the pro-survival members Bcl-2, Bcl-xL, Mcl-1, Bcl-w, Bcl-B, and A1/Bfl-1 [17] while the pro-apoptotic group includes Bax and Bak, and a member Bok that as yet, has a less-well defined role [18]. The pro-apoptotic group all bear the BH3-motif, whereas the pro-survival proteins do not always bear this motif [8]. Bid bears only a BH3-motif, but has a folded α-helical structure [19,20,21], and is activated by caspase cleavage in the α1-α2 loop to form truncated Bid (tBid), the C-terminal fragment that bears the BH3-motif. It is not clear how the caspase cleaved Bid (cBid) decomposes to form N-Bid, the N-terminal fragment, and tBid, the C-terminal fragments, as cBid is stable [19]. Though tBid retains some secondary structure [22], like the other BH3-only proteins, tBid is intrinsically disordered [22,23].
Figure 1.
Pathways for Bcl-2 protein action. In mammals, a tripartite mechanism regulated by the Bcl-2 family controls the integrity of the mitochondrial outer membrane (MOM). Activation of Bax and Bak leads to MOM permeabilisation (MOMP), and escape of factors such as cytochrome c from the mitochondrial intermembrane space to initiate the caspase cascade that is the defining step in apoptosis. BH3-only proteins either activate Bax and/or Bak either by removal of their inhibition by pro-survival proteins, or by direct interaction. The BH3-only protein Bid is activated by proteolytic cleavage that releases its BH3-motif for interaction. Viral Bcl-2 (vBcl-2) orthologues can act on the BH3-only proteins, or directly block the action of Bax and Bak to prevent apoptosis initiation. Activation steps are shown as arrows and inhibition as bars.
Bcl-2 proteins are not only pivotal in higher organisms such as mammals, worms and flies, but have been identified in evolutionary ancient species such as sponges [24,25] and hydra [26]. Key molecular and mechanistic features appear to be well preserved, with the identification of pro-survival Bcl-2 and pro-apoptotic Bak in sponges [27], along with representatives of pro-survival Bcl-2, as well as BH3-only proteins and Bak like proteins in hydra [28]. The key role of the membrane is preserved, and interactions between the pro-survival Bcl-2 and Bax-like proteins are conserved in hydra, with the Bcl-2 orthologue HyBcl-2 co-localising with HyBax on the periphery of mitochondria [29,30]. The Bcl-2 family probably forms part of a primitive immune response for cnidarians, and Bax has been shown to be upregulated in response to disease in the coral Acropora hyacinthus [31]. The demosponge Geodia cydonium Bcl-2 orthologue, BHP2, is the most ancient Bcl-2 protein to be described at a molecular level to date [32]. Structurally, the Bcl-2 fold and the BH3 motif-in-groove interaction is conserved, as shown for the sponge Bcl-2 BHP2 (Figure 2), although subtle differences allow BHP2 to discriminate between most human pro-apoptotic Bcl-2 proteins to be selective for its sponge counterparts [32].
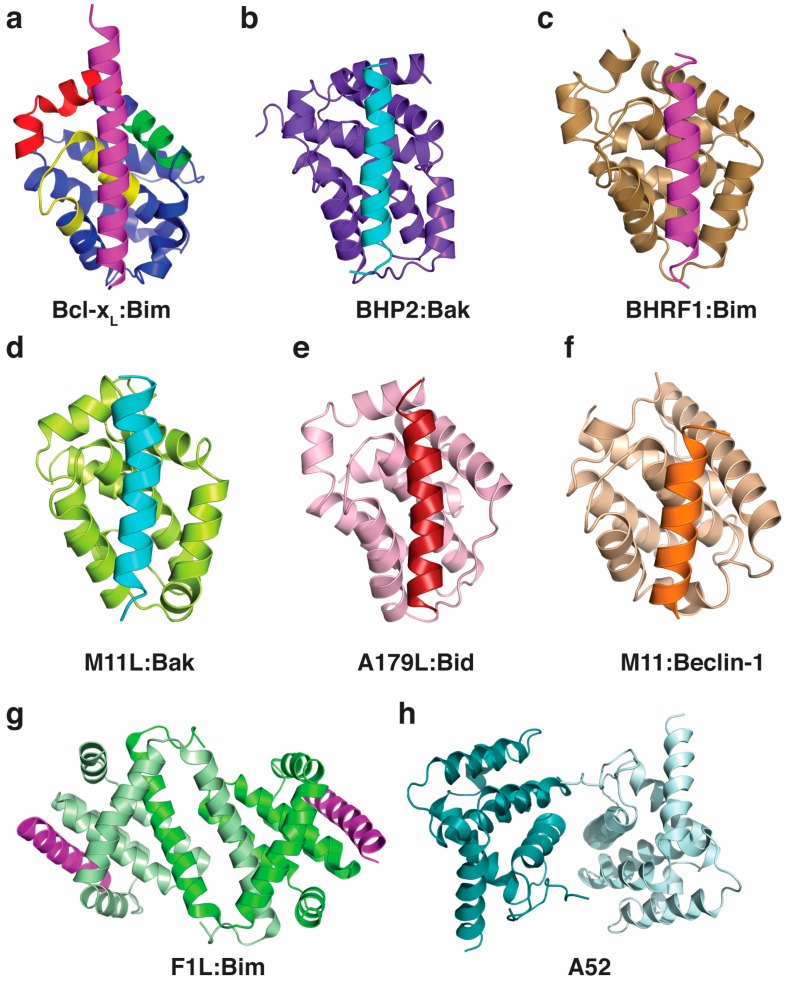
Figure 2.
Structures of Bcl-2 family members. (A) Human Bcl-xL:Bim complex [33] (PDB ID 1PQ1); (B) G. cydonium BHP2:LB-Bak-2 complex [32] (PDB ID 5TWA); (C) EBV BHRF1:Bim complex [34] (PDB ID 2WH6); (D) Myxomavirus M11L:Bak complex [35] (PDB ID 2JBY); (E) African swine fever virus A179L:Bid complex [36] (PDB ID 5UA4); (F) Murine γ-herpesvirus 68 M11:Beclin-1 complex [37] (PDB ID 3BL2); (G) Vaccinia virus F1L:Bim complex [38] (PDB ID 4D2M); (H) Vaccinia virus A52 [39] (PDB ID 2VVW).
It is now emerging that the apoptotic machinery is closely associated with other cellular regulatory pathways such as autophagy, the unfolded protein response, and endoplasmic reticulum (ER) stress signalling [40]. Viral subversion of the cellular Unfolded Protein Response (UPR) is a mechanism that is increasingly recognised as being fundamental for host immunity [41]. The UPR is a multimodal response to perturbed ER function (“ER stress”) that results from unfolded proteins accumulating in the ER faster than they are able to be folded, leading to shut down of translation, an increase in the rate of protein folding, activation of degradation pathways of the ubiquitin-proteasome or autophagy, and ultimately apoptosis if the stress is unrelieved. Thus, ER stress, autophagy, and apoptosis are all tightly linked and regulated by viruses.
The gatekeepers of mitochondrial integrity are the pro-apoptotic proteins Bax and Bak that have overlapping roles [42]. Bax and Bak are necessary for instigation of apoptosis; however, the details of their mode of action are still disputed. In mammals, after apoptotic stimuli, cytosolic Bax migrates to the mitochondrial outer membrane (MOM) to generate pores in the mitochondrial outer membrane that allows the escape of apoptogenic factors that have activated the caspase cascade (Figure 1). The apoptotic programme is not conserved in all aspects; for example, apoptotis in Caenorhabditis elegans differs from that in mammals. In C. elegans, there is a single folded Bcl-2 protein (CED-9) present in this organism that is associated with mitochondria [43], and it interacts with the caspase activator CED-4 to inhibit apoptosis. The CED-9:CED-4 interaction is antagonised by the BH3-only protein, Egl-1, to release CED-4 and activate the caspase CED-3 and the caspase cascade.
2. An Expanding Family of Viral Bcl-2 Orthologues has been Discovered
The importance of apoptosis and the Bcl-2 proteins in immune cell regulation and innate immunity responses has created an evolutionary pressure for viruses to acquire the genes for the pro-survival Bcl-2 proteins [44]. There are a multitude of large DNA viruses that mimic pro-survival Bcl-2 (vBcl-2) proteins, hijacking the intrinsic apoptotic pathway for their benefit; these are summarised in Table 1.
Table 1.
Pro-survival Bcl-2 proteins encoded by viruses.
| Virus-Encoded Pro-Survival Bcl-2 | Reference |
|---|---|
| γ-herpesviruses 68 M11 | [45] |
| Adenovirus E1B19K | [46] |
| Epstein-Barr virus BHRF1 | [34,47] |
| Epstein-Barr virus BALF1 | [34,47] |
| Kaposi’s sarcoma-associated herpesvirus Ks-Bcl-2 | [48,49] |
| Turkey herpesvirus vnr-13 | [50] |
| African swine fever virus A179L | [36,51] |
| Grouper iridovirus GIV66 | [52,53] |
| Myxoma virus M11L | [35,54] |
| Vaccinia virus F1L | [55,56,57] |
| Variola virus F1L | [58] |
| Ectromelia virus EMV025 | [59] |
| Sheeppox virus SPPV14 | [60] |
| Deerpox virus DPV022 | [61,62] |
| Fowlpox virus FPV029 | [63,64] |
| Canarypox CNP058 | [65] |
| Lumpy skin disease virus LD17 | [60] |
| Orfvirus ORFV125 | [66] |
3. Membrane Interactions
The accumulation and oligomerisation of Bax and Bak at the intracellular membrane is the key event and the point of no return in apoptosis, and it is the least well understood at a molecular level [67]. vBcl-2 orthologues also play a role here, with many localising to intracellular membranes such as the mitochondrial membrane, ER and nuclear envelope in the host cell. The presence of a putative hydrophobic transmembrane (TM) region for many of the Bcl-2 family indicates the importance of this interaction, though the exact molecular mechanisms remain ill-defined, Bax and Bak accumulate on the mitochondrial surface and ultimately lead to its disruption and leakage. Viral control over membrane disruption is thus crucial to maintaining the host cell viability for replication [68].
The folded Bcl-2 proteins are partitioned between the cytosol and intracellular membranes and trafficking between the two environments occurs. Differences in cytosol-membrane partitioning are dependent on their rate of translocation, which is in turn dependent on their TM regions [69]. Bak and Bcl-2 are predominantly membrane-associated [70,71], while others, including Bax and Bcl-xL are predominantly cytosolic but become membrane integrated after an apoptotic stimulus [72,73]. Trafficking of Bax and Bak between the membrane and cytosol is a process dependent on Bcl-xL and Bcl-2 [69,74,75,76]. In addition to the requirement for the TM region the interaction between the pro-survival and proteins requires an exposed BH3-motif on Bax and Bak, a process that necessarily requires a conformational change from their solution conformation where the key residues of the BH3-motif are buried [77]. This suggests an interaction of the BH3 motif of Bax or Bak in the groove of the pro-survival protein, although this was one of the first interactions observed in the Bcl-2 family [78] there remains a deficit of structural data on this interaction.
BH3-only proteins are also associated with intracellular membranes (see [7] for a discussion), some such as Bik bear hydrophobic C-terminal regions suggestive of membrane interacting proteins. The interaction with BH3-only proteins releases the TM region from the BH3-binding groove in pro-survival proteins [79,80], potentially releasing it for membrane binding. Full biological activity of pro-survival Bcl-2 is not observed if the C-terminal region is truncated from these molecules even though binding to their BH3-targets is improved [79]. The same behaviour has been observed for viral Bcl-2 orthologues, where C-terminal deletions reduce the pro-survival activity (See Opgenorth et al. 1992 [81] for M11L truncation). Similarly, deletion of the C-terminal TM region of Bax impairs its membrane localisation and biological activity [82]. A combination of biophysical, biochemical and genetic studies have shown that there is multiple redundancy in the interactions [17,83,84]. Several models have been put forward for BH3-only protein PCD activation but most BH3-only proteins are able to activate Bax or Bak [85]. Thus, the membrane interaction is critical to the pro-survival or pro-apoptotic activity of the Bcl-2 family and further complicates an already complex multilevel-redundant regulation mechanism for mammalian apoptosis.
Many vBcl-2 orthologues, including the first viral Bcl-2 orthologue found, adenovirus E1B 19K, though without an obvious hydrophobic C-terminal region, are closely associated with intracellular membranes, the ER and nuclear envelope [86] and the association with membranes is required for its function [87]. Frog virus 3 Bcl-2 orthologue 97R localises to the ER and deletion of the C-terminal 29 residues inactivates the protein [88]. The African swine fever virus Bcl-2 orthologue A179L, is closely associated with viral factories, and though it lacks an obvious TM region, it is associated with the ER and mitochondrial membranes. However, the mutant G85A A179L loses its ability to keep cells alive, but also associates with ER membranes [89], probably indicating that a competent BH3-binding is required for ER association, as this mutant destroys binding to the BH3-only proteins [36]. EBV BHRF1 is associated with membranes [47]. Combined, these features attest to the importance of membrane association or integration to the activity of the folded Bcl-2 proteins, including those encoded by viruses.
Though many questions remain about the exact nature of the molecular assemblies of Bax and Bak that disrupt the MOM, a more consistent mechanism is now emerging where Bax and Bak undergo a series of conformational changes to form high-order aggregates to create the membrane disrupting pores [90]. However, in solution Bax is a monomeric and relatively rigid protein with little evidence of conformational mobility [77,91], a finding consistent with fluorescence cross correlation studies showing that Bax associates with mitochondria prior to oligomerisation [92] into ring-like pores [93,94]. In a defined system consisting of only cBid, Bax and Bcl-xL, Bleicken et al. showed that the interactions between these apoptotic regulators is spatially regulated. When embedded in the membrane, Bax is proposed to form a positive feed-back loop recruiting Bax, and Bcl-xL inhibits this process by preventing Bax oligomer growth and translocating membrane Bax to the cytosol [92]. Accumulating evidence suggests that the TM regions are intermolecular interaction sites. Andreau-Fernandez et al. showed that the TM region of Bax interacts with the TM regions of Bcl-2 and Bcl-xL [95]. Earlier structural studies on Bcl-w where a near-full-length sequence was well behaved [80], showed that like Bax [77] the C-terminal tail lies in the BH3-binding groove. Furthermore, the presence of the TM region in Bcl-xL [79,96] and Bcl-w [80] reduces their affinity for BH3-motifs. In the case of Bcl-xL is dimeric when the TM region is present [92] and monomeric in its absence, as shown by structural studies [97]. Nuclear Magnetic Resonance (NMR) investigation of the Bcl-xL:membrane interaction showed that it has an α-helical C-terminal tail that anchors the folded globular Bcl-2 domain head to the membrane [98]. BH3-ligand displacement of the C-terminal residues of the α9 residues from the groove of Bcl-w renders them unstructured in solution [80], and a likely mechanism to drive Bcl-w to the membrane [79]. Biochemical studies showed that like Bcl-xL, Bak has a transmembrane C-terminal anchor [99]. Combined, these studies indicate that the C-terminal region of the Bcl-2 fold are not by-standers, and play an important role in not only membrane targeting and anchoring, but also the protein-protein interactions of these molecules. The observation that the viral pro-survival proteins mimic these membrane localisation and activities suggests the importance of modulating PCD in infected cells.
Other models for the membrane oligomerisation include an initial dimerisation of Bax or Bak [100] prior to their oligomerisation at the membrane surface by unfolding an interaction. In this model, it is proposed that membrane rupture is caused by disordered clustering of Bak or Bax dimers. A “hit and run” mechanism has been suggested, where an initial weak interaction induces subsequent conformational changes in Bax or Bak. A second site has been proposed for binding BH3-only proteins on Bax, though it is a low affinity interaction that initiates apoptosis [101]. Structural investigation of detergent treated Bax in the presence of BH3-motifs gave a symmetrical Bax dimer with the BH3 bound in the groove; this was proposed to be the active from of Bax that further oligomerises to form pores [102]. Structures of Bim and Bid BH3-motifs in the groove of domain swapped dimer have been determined [103], similar domain-swapped dimers have been observed for Bcl-xL, that also retain the ability to bind BH3 motifs [104]. A caveat on these studies is that they were performed with C-terminally truncated Bax and may not reflect membrane interactions in their entirety [92]. Further studies will be required to elucidate the exact mechanisms.
4. Viral Bcl-2-mediated Subversion of Programmed Cell Death
Considering the importance of Bcl-2 proteins in regulating apoptosis, as well as autophagy in higher organisms [11,84], it is unsurprising that numerous viruses have acquired sequence, functional and structural homologs of Bcl-2 to subvert host apoptosis as well as autophagy signalling for their own ends. Prevention of premature host cell death during the initial stages has been shown to be critical for successful infection of EBV [68] and demonstrates the pivotal role that disarming of host apoptotic defences plays in preventing clearance of virus to enable successful infection and propagation. However, whilst prevention of premature host cell apoptosis is highly desirable, ultimately, viruses also rely on host cell apoptosis at a later stage to aid viral dissemination, for example an avian reovirus triggers apoptosis to enable optimal release and dissemination of viral progeny [105].
The earliest identified virus encoded Bcl-2 homologs were E1B 19K from adenovirus and BHRF1 from EBV which both display substantial sequence identity (18% and 16% identical to human Bcl-2 respectively) to mammalian Bcl-2 and contain the hallmark BH1 and BH2 motifs. Functional studies of E1B 19K determined that it is a potent inhibitor of apoptosis that is induced by stimuli including Fas ligand, TNFα, and adenoviral infection. Mechanistically, E1B 19K engages Bax [106], Bak [107] and Bik [108], and is functionally interchangeable with Bcl-2 during adenovirus infection and transformation [46].
One mechanism for modulating apoptosis is to manipulate the BH3-only and Bax family through interaction in the binding groove (Figure 2). This interaction has now been extensively studied and binding affinities measured (Table 2), but the implications of binding specific BH3-motif bearing proteins is not always clear. For example, Bim is a universal Bcl-2 binder and binds all mammalian pro-survival proteins with relatively high affinity, yet is not bound by all vBcl-2 proteins (for example the variola virus F1L). Some viral Bcl-2 proteins are capable of binding nearly all pro-apoptotic proteins (e.g., A179L, FPV039), while others have a much more specific ligand range (Table 2). The specificities of the viral Bcl-2 proteins for their BH3-targets have been determined (Table 2), and in general, these interactions are of lower affinity than the pro-survival Bcl-2, probably reflecting a balancing act by the virus, as they need to block apoptosis during their replicative stage; however apoptosis is necessary for the escape of viral progeny on maturation. The cell type specificity of the virus also probably plays a role in deciding which host Bcl-2 proteins are inhibited, and this is an area for further investigation.
Table 2.
Affinities (in nM) of different pro-survival Bcl-2 proteins for peptides spanning the BH3 motif of endogenous pro-apoptotic Bcl-2 family members or Beclin-1 (measurements taken from: [34,35,36,57,58,60,62,64,110,137,138,139,140,141,142]).
| Poxviral Bcl-2 | ||||||
| Pro-death | SPPV14 | M11L | MVA_F1L | VAR_F1L | DPV022 | FPV039 |
| Bad | >2000 | >1000 | NB | NB | NB | 653 |
| Bid | 341 | 100 | NB | 3200 | NB | 2 |
| Bik | >2000 | >1000 | NB | NB | NB | 30 |
| Bim | 26 | 5 | 250 | NB | 340 | 10 |
| Bmf | 67 | 100 | NB | NB | NB | 16 |
| Hrk | 63 | >1000 | NB | NB | NB | 24 |
| Noxa | >2000 | >1000 | NB | NB | NB | 28 |
| Puma | 65 | >1000 | NB | NB | NB | 24 |
| Bak | 46 | 50 | 4300 | 2640 | 6930 | 76 |
| Bax | 32 | 75 | 1850 | 960 | 4040 | 76 |
| Beclin-1 | n/a | n/a | n/a | n/a | NB | n/a |
| Asfarviral Bcl-2 | Herpesviral Bcl-2 | |||||
| A179L | BHRF1 | Ks-Bcl-2 | M11 | N1L | ||
| Bad | 258 | >2000 | >1000 | NB | >1000 | |
| Bid | 26 | 109 | 112 | 232 | 152 | |
| Bik | 190 | >2000 | >1000 | NB | n/a | |
| Bim | 6 | 18 | 29 | 131 | 72 | |
| Bmf | 254 | >2000 | >1000 | 300 | n/a | |
| Hrk | 1487 | >1000 | >1000 | 719 | n/a | |
| Noxa | 1575 | >2000 | >1000 | 132 | n/a | |
| Puma | 31 | 70 | 69 | 370 | n/a | |
| Bak | 29 | 150 | <50 | 76.3 | 71 | |
| Bax | 26 | 1,400 | 980 | 690 | n/a | |
| Beclin-1 | n/a | n/a | 40.2 | n/a | ||
| Human Bcl-2 | Sponge Bcl-2 | |||||
| Bcl-2 | Bcl-w | Bcl-xL | Mcl-1 | A1 | BHP2 | |
| Bad | 16 | 30 | 5.3 | >100,000 | 15,000 | NB |
| Bid | 6800 | 40 | 82 | 2100 | 1 | NB |
| Bik | 850 | 12 | 43 | 1700 | 58 | NB |
| Bim | 2.6 | 4.3 | 4.6 | 2.4 | 1 | NB |
| Bmf | 3 | 9.8 | 9.7 | 1100 | 180 | NB |
| Hrk | 320 | 49 | 3.7 | 370 | 46 | 3760 |
| Noxa | >100,000 | >100,000 | >100,000 | 24 | 20 | NB |
| Puma | 3.3 | 5.1 | 6.3 | 5 | 1 | NB |
| Bak | >1000 | 500 | 50 | 10 | 3 | 66 |
| Bax | 100 | 58 | 130 | 12 | n/a | NB |
| Beclin-1 | n/a | n/a | 2300 | n/a | n/a | n/a |
MVA = modified vaccinia virus Ankara, VAR = variole virus, n/a = not available, NB = no binding.
5. Herpesviridae-Encoded Bcl-2 Homologs
Many members of the herpesviridae encode Bcl-2 like proteins. Epstein-Barr virus (or human herpesvirus 4) is a large DNA virus belonging to the γ-herpesviridae and harbours two Bcl-2 homologs, BHRF1 and BALF1. BHRF1 was shown to be an enhancer of cell survival [47]. Biochemical and structural studies revealed that BHRF1 adopts a Bcl-2 fold [34,109] and is bound to the BH3-only proteins Bim, Bid, and Puma, as well as Bak and Bax [34,110] (Table 2). Mechanistically, BHRF1 was shown to rely on the sequestration of Bim [111] and Bak [34] to inhibit apoptosis, and to confer chemoresistance in a Burkitt lymphoma mouse model, similar to Bcl-2 [34]. BHRF1 was also shown to be constitutively overexpressed in a sub-set of EBV transformed B-cells, thus rendering them resistant to apoptosis [112]. The function of a second EBV-encoded Bcl-2 homolog, BALF1, remains controversial. Initial data suggested that BALF1 acts as a pro-survival Bcl-2 protein [113], however a second report showed that BALF1 is pro-apoptotic and inhibits the other EBV-encoded pro-survival protein BHRF1 [114]. Subsequently, others reported that both BHRF1 and BALF1 are required for successful EBV-induced B-cell transformation [68]. The identification of BHRF1 in transformed B-cells sparked interest in developing antagonists against BHRF1 for targeted cancer therapy, and the feasibility of such an approach was recently demonstrated via the use of an engineered protein that bound BHRF1 with picomolar affinity [115]. No small molecule antagonists for BHRF1 have been reported yet; however their development is underway [116].
Kaposi’s sarcoma herpesvirus (KSHV or human herpesvirus 8) is also a large DNA virus and a member of the γ-herpesviridae. KSHV encodes a Bcl-2 homolog, Ks-Bcl-2 [117] that adopts a Bcl-2 fold [49] and is able to bind Bim, Bid, Bik, Bmf, Hrk, Noxa, and Puma [110] (Table 2). Conflicting data exist for binding of Bax and Bak, with one report indicating that neither bind Ks-Bcl-2 [48], whereas a subsequent study revealed a high affinity interaction for Bak (50 nM) and moderate affinity for Bax (980 nM) [109]. During viral infection, Ks-Bcl-2 appears to play a pivotal role in completion of the lytic cycle, as a Ks-Bcl-2 deletion virus of KSHV does not complete the lytic replication cycle. Interestingly, the Ks-Bcl-2-related ORF16 from rhesus rhadinovirus is able to functionally replace Ks-Bcl-2 during the lytic cycle, in contrast to other endogenous mammalian Bcl-2 proteins, such as Bcl-xL or other herpesvirus-encoded vBcl-2 proteins including M11 and vMIA [118]. Another rhadinovirus, herpesvirus saimiri, also encodes a Bcl-2 homolog named ORF16 [119], which was shown to be anti-apoptotic and bound Bak and Bax in pull-down assays.
Murine γ-herpesvirus 68 encodes M11 [120] and was identified as an inhibitor of Fas and TNF induced apoptosis [45,121], but biochemical studies demonstrated binding to Bim, Bid, Bmf, Noxa, Puma, and Hrk, as well as Bak and Bax via the canonical ligand binding groove of its Bcl-2 fold [37]. Thus, M11 can inhibit the major mitochondrial pathways to apoptosis, however functional studies [37,122] indicate that the mitochondrial pathway may not be the primary target for M11 (see below).
Cytomegalovirus (CMV or human herpesvirus 5) is a large DNA virus belonging to the β-herpesviridae. CMV encodes proteins that directly target host pro-apoptotic proteins Bax and Bak, but appear to be neither sequence nor structural homologs of Bcl-2. Human CMV encodes vMIA, which has been shown to inhibit Bax [123] and Bak oligomerisation [124,125]. Interestingly, the interaction of vMIA with Bax does not involve the canonical Bcl-2 ligand binding groove. Unexpectedly, vMIA bound to an alternative binding site distinct from the canonical BH3 binding groove in Bax, which was mapped using NMR to define an interaction site comprising primarily of the loops connecting α1-α2, α3-α4, and α5-α6. Furthermore, the vMIA-Bax interaction was of high affinity with a Kd of 22 nM [126]. Whilst human CMV vMIA appears to be able to neutralise both Bax and Bak, in mouse CMV Bax and Bak are neutralised by two proteins with single specificity [127]. MCMV-encoded m38.5 has been shown to be mitochondrially localised, and to inhibit Bax activation [128,129], whereas Bak inhibition is achieved via m41.1 [130,131].
Amongst the α-herpesviruses, a virus-encoded homolog of the endogenous turkey pro-survival Bcl-2 protein NR13 [132], Bcl-B [133], Boo, Diva, or NRH [134] in mammals, has a Bcl-2 fold [135,136] and has been termed vnr-13 [50]. Though little is known about vnr-13, it was shown to localise to the outer mitochondrial membrane, and inhibit apoptosis after serum deprivation [50].
6. Poxviridae-Encoded Bcl-2 Homologs
The poxviridae encompass a number of families that encode for Bcl-2 proteins. Vaccinia virus is a large DNA virus, the prototypical member of the orthopoxviruses, and encodes F1L, a potent inhibitor of intrinsic apoptosis [55,56] that displays no recognisable sequence identity to Bcl-2. Structural studies revealed that F1L adopts an unusual Bcl-2 fold featuring a domain-swapped dimer configuration, in marked contrast to mammalian pro-survival Bcl-2 proteins, which are all monomeric [57,143]. Furthermore, F1L only bound a highly restricted subset of pro-apoptotic Bcl-2 including Bim [56,144] and Bak [145,146] (Table 2), and was shown to inhibit Bak activation by functionally replacing Mcl-1 during infection [147]. Although F1L is also able to inhibit Bax-mediated apoptosis [144], this activity is likely via an indirect mechanism as F1L does not engage Bax in a cellular context. Like other Bcl-2 family proteins F1L is localised to mitochondrial membranes through its C-terminal residues, and this region is necessary for full pro-survival activity [148]. Mechanistically, the interaction of F1L with Bim was identified as the primary mechanism underlying F1L-mediated inhibition of apoptosis in the context of a live viral infection [143]. Interestingly, the F1L homolog in variola virus, the causative agent of smallpox and another member of the orthopoxviridae, appears to utilise a different mechanism for apoptosis inhibition, despite adopting a near identical structure and sequence [58]. Unlike its vaccinia virus counterpart, variola virus F1L only binds Bid, Bak, and Bax, and not Bim (Table 2), and only inhibits Bax-mediated apoptosis [58]. A homolog of vaccinia virus F1L found in another orthopoxvirus, Ectromelia virus EMV025, was also shown to be anti-apoptotic by inhibiting Bax and Bak activation by directly engaging Bim and Bak [59].
Myxomavirus is a member of the leporipoxviridae and encodes for M11L, another potent inhibitor of intrinsic apoptosis lacking detectable sequence similarity with Bcl-2 [149]. M11L is able to engage several host pro-apoptotic Bcl-2 proteins including Bak [150], Bax, Bim, and Bid [35] (Table 2). Structural studies showed that M11L adopts a compact, monomeric Bcl-2 fold [35,151] where the canonical ligand binding groove is used to engage pro-apoptotic Bcl-2 proteins [35]. Interestingly, functional studies revealed that M11L primarily acts by sequestering Bak and Bax [35], in contrast to vaccinia virus F1L which acts primarily via Bim sequestration [143].
Orf virus is a parapoxvirus and encodes a readily identifiable Bcl-2 homolog, ORFV125, that potently inhibits intrinsic apoptosis [66]. Functional studies revealed that ORFV125 interacts with several BH3-only proteins including Bim, Puma, Hrk, Bik, and Noxa as well as active Bax but not Bak [152].
Among the avipoxviridae, both fowlpoxvirus FPV039 and canarypoxvirus CNP058 have been shown to suppress apoptosis. FPV039 inhibits apoptosis [153] after overexpression of all BH3-only proteins [153], and was shown to adopt a Bcl-2 fold and engage all major host pro-apoptotic Bcl-2 proteins [64] (Table 2). Interestingly, the closely related canarypoxvirus CNP058 also inhibits apoptosis in transfected cells, but engaged a different subset of pro-apoptotic Bcl-2 proteins, largely with weaker affinities than FPV039 [64].
Other Bcl-2 proteins-encoded by poxviruses include sheeppoxvirus SPPV14 and deerpoxvirus DPV022. SPPV14 displayed a broader spectrum of pro-apoptotic Bcl-2 interactions by binding Bim, Bid, Bmf, Hrk, Puma, as well as Bax and Bak [60] (Table 2). In contrast, DPV022 only engaged Bim, Bax and Bak [62] (Table 2). Intriguingly, DPV022 also adopted a domain-swapped Bcl-2 fold similar to those observed for vaccinia and variola virus F1L [57,58,143], suggesting that this particular topology for Bcl-2 proteins may be more widely found in nature.
7. Asfarviridae and iridoviridae-Encoded Bcl-2 Homologs
African swine fever virus is a large double stranded DNA virus, the only member of the asfarviridae, and encodes A179L [154]. A179L adopts a Bcl-2 fold [36], and displays extreme promiscuity by binding all host pro-apoptotic Bcl-2 proteins [36,155]. A179L localised to mitochondria [89] and potently inhibits apoptosis in cell culture assays [51]. Amongst the iridoviridae, grouper iridovirus was shown to encode GIV66, which inhibited apoptosis in a grouper kidney cell culture model [156]. However, the structural and functional basis of GIV66-mediated apoptosis inhibition has not been established.
8. Other Functional Roles of Viral Bcl-2 Homologs
Although the vast majority of virus-encoded Bcl-2 proteins primarily interfere with host cell intrinsic apoptosis signalling by targeting endogenous pro-apoptotic host Bcl-2 proteins, a number of studies have revealed that vBcl-2 proteins also harbour other activities. Several vBcl-2 proteins have been shown to inhibit autophagy. Murine γ-herpesvirus 68-encoded M11 utilises the canonical Bcl-2 ligand binding groove to bind the BH3 motif of Beclin-1, a key autophagy regulator [37,122]. Another member of the herpesviridae, KSHV, also targets Beclin-1 using Ks-Bcl-2 [157]. However, this ability to engage Beclin-1 is not limited to the herpesviridae, with African swine fever A179L displaying autophagy inhibitory activity in addition to anti-apoptotic activity [89]. Adenoviral E1B 19K was also shown to bind Beclin-1, and thus inhibit autophagy [158]. However, to date, no autophagy inhibitor has been identified amongst the poxviridae.
Another virus-encoded Bcl-2 protein with multiple functionalities is the vaccinia virus F1L. In addition to the anti-apoptotic activity mediated by sequestering Bim, F1L was also shown to inhibit inflammasome activation [159] via an unusual unstructured N-terminal extension prior to the Bcl-2 fold [38]. In addition to the ability to mediate inflammasome activation, the N-terminus of F1L was also proposed to act as a caspase-9 inhibitor [160,161], however, a subsequent study suggested that the F1L N-terminus is not involved in apoptosis inhibition [38].
F1L is not the only vaccinia virus-encoded Bcl-2 protein with dual functionality. N1L was shown to adopt a Bcl-2 fold (albeit lacking a TM anchoring region) and inhibit both intrinsic apoptosis by targeting several pro-apoptotic Bcl-2 proteins as well as modulating NF-κB signalling. Interestingly, N1L also adopts a Bcl-2 fold with dimeric topology; however, dimerisation is not achieved via a domain swap as seen in F1L and DPV022, but rather via a novel interface centering on the α1 and α6 helices [138,162]. Furthermore, the ability to manipulate both apoptosis and NF-κB signalling is mediated via two discrete sites on N1L [163].
In addition to N1L, several other NF-κB inhibitors that adopt Bcl-2 folds have now been identified in vaccinia virus. These include B14 [39] andA52 [39], as well as A46 [164,165], A49 [166] and K7 [167]. Whilst all four proteins inhibit NF-κB, they are distinguished by substantial differences in mechanism, cellular activity, and structure. Similar to N1L, B14 and A52 form dimers utilising an interface involving α1 and α6 helices, with small but significant differences in the orientation of monomeric chains with each other within the dimers amongst the three proteins [39]. Furthermore, A46 also forms dimers, however, this involves an interface formed by α4 and α6 helices of the C-terminal Bcl-2 like domain. Intriguingly, A46 harbours an additional N-terminal domain that mediates tetramerisation of A46, thus adding an additional layer of quaternary structure-based regulation [168]. In contrast, A49 and K7 are monomeric in solution. Unlike N1L, B14, A52 and K7 do not bind pro-apoptotic Bcl-2 proteins. However, K7 harbours dual functionality that is similar to N1L, and in addition to inhibition of NF-κB, it also binds to the human DEAD-box RNA helicase DDX3 to inhibit induction of the IFN-β promoter [169,170].
9. Concluding Remarks
Virus-encoded Bcl-2 proteins have demonstrated the remarkable adaptability of the Bcl-2 fold, and its ability to modulate signalling that involves several cell death-associated pathways via multiple mechanisms. Whilst mammalian pro-survival Bcl-2 proteins display several distinct rules of engagement for their interactions with pro-apoptotic Bcl-2, the picture is not as clear amongst the virus-encoded homologs of Bcl-2. In mammals, key interactions between pro-survival and pro-apoptotic Bcl-2 are typically characterised by high affinities, with some such as Bcl-xL:Bim interactions straying into picomolar affinities. The caveat with the binding studies is that they have been performed with C-terminally truncated molecules, and probably overestimate true affinities [79,80]. Furthermore, all mammalian pro-survival Bcl-2 proteins target Bim, the sole universal pro-apoptotic BH3-only protein [135] and either Noxa or Bad, but not both [139]. In contrast, key interactions between virus-encoded pro-survival proteins tend to display weaker affinities, typically with dissociation constants (Kd) in the nanomolar range, although the dimeric poxvirus-encoded pro-survival proteins F1L [57,58] and DPV022 [62] only display low nanomolar or micromolar Kd values. It remains to be determined if these markedly weaker affinities are related to the different oligomeric state of the virus-encoded proteins. Furthermore, the Bad/Noxa dyad does not apply to virus-encoded pro-survival Bcl-2, with fowlpoxvirus FPV039 [64] and ASFV A179L [36] binding both Noxa and Bad. Indeed, both of these proteins bind all major pro-apoptotic Bcl-2 proteins, another feature not previously observed amongst their mammalian counterparts. Lastly, Bim is not a universal target amongst virus-encoded Bcl-2, with variola virus F1L showing no affinity for Bim, and instead displaying weak binding to Bid [58] whilst being a potent inhibitor of Bax-mediated apoptosis in cellular assays. Overall the virus-encoded Bcl-2 pro-survival proteins display weaker affinities for host pro-apoptotic proteins. This could suggest that only small perturbations of the overall balance between pro-survival and pro-apoptotic proteins in a host cell are sufficient to impede apoptosis progression; however, the functional relevance of these lower affinities remains to be clarified.
When considering mechanisms and the long-standing debate on the precise mechanism of action of cellular pro-survival Bcl-2, current models and mechanisms have not been entirely resolved [84]. BH3-only proteins antagonise the pro-survival Bcl-2 family that in turn keep Bax and Bak in check, but they can also activate Bax and Bak (Figure 1). Direct binding to Bax and Bak has been observed though the affinities are generally low, for example, Bim binds full length Bax with a Kd of 3.1 μM compared with Kd in low nanomolar ranges for the pro-survival proteins [171]. While the exact details of the membrane pore generated by Bak and Bax remain under investigation, it is clear that the interactions at the membrane are crucial to the apoptotic response in mammals, and viruses also appear to exploit this. vMIA [172] and other viral Bcl-2 proteins translocate to the MOM, and a possible role for them would be to inhibit pore formation by either preventing pore growth through sequestration or retrotranslocation of the components into the cytosol as in the case of Bcl-xL [74,92]. It is becoming apparent for the best understood virus-encoded Bcl-2 proteins that, in contrast to mammalian apoptosis, multiple mechanisms of action exist, though many of these need to be clarified with quantitative structure and binding studies that are complemented by live viral infection models. Whereas myxomavirus M11L was shown to act by sequestering Bax and Bak [35], vaccinia virus F1L was shown to only require neutralisation of Bim in a viral infection setting [143]. For EBV BHRF1, a combination of neutralisation of Bim [111] and Bak [34] was required.
When considering the role of membranes in Bcl-2 activity, and the observation that it is Bak and Bax accumulation at the membrane that is critical for intrinsic apoptosis to proceed, the association of virus-encoded Bcl-2 proteins with membranes is not unexpected. Nearly all apoptosis inhibitory vBcl-2 proteins harbour transmembrane anchoring regions to direct their subcellular localisation, chiefly to the MOM. Interestingly, different vBcl-2 proteins appear to inhibit different stages of Bax activation and translocation to the outer mitochondrial membrane [173]. E1B 19K and BHRF1, are examined for their ability to block Bax activation at different steps and thereby reveal the timing of mitochondrial changes during apoptosis. BHRF1 inhibited Bax activation but not upstream of apoptotic signalling events, whereas E1B19K permitted the initial stages of Bax activation to proceed, but prevented the subsequent oligomerisation of Bax. Furthermore, CMV-encoded m38.5 and vMIA appear to block Bax downstream of translocation to mitochondria, when Bax has already undergone structural changes [125].
These data suggest that no universal mechanism exists that enables virus-encoded Bcl-2 to subvert premature host cell apoptosis, and that the precise mechanism reflects the unique circumstances under which viral infection takes place. In particular, the mechanism of action may be heavily influenced by the initial site of contact and tissue type. A pertinent example is CMV-encoded m38.5, with an m38.5 deletion virus showing no overt signs of impaired replication in visceral organs, whereas in salivary glands a 10–100 fold difference was observed [128]. This suggests that particular tissues may be more prone to infection; however, this aspect and the observation that expression patterns of pro-apoptotic Bcl-2 proteins vary amongst tissues has not been adequately addressed for the vast majority of viruses. Interestingly, viruses can manipulate the host cell apoptosis program on many levels, with evidence emerging that viruses can manipulate the caspase cascade [174] and the endogenous levels of the host Bcl-2 family members [175]. These findings add an additional layer of complexity to the quest of identifying the precise molecular mechanism of action of vBcl-2 proteins, and ultimately suggest that more sophisticated approaches may be required to answer these questions.
Acknowledgments
This work was supported by the National Health and Medical Research Council Australia (Project Grant APP1007918 to Marc Kvansakul) and the Australian Research Council (Fellowship FT130101349 to Marc Kvansakul).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V.L., El-Deiry W.S., Fulda S., et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasser A., Cory S., Adams J.M. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardwick J.M., Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youle R.J., Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 6.Kvansakul M., Hinds M.G. The Bcl-2 family: Structures, interactions and targets for drug discovery. Apoptosis. 2015;20:136–150. doi: 10.1007/s10495-014-1051-7. [DOI] [PubMed] [Google Scholar]
- 7.Kvansakul M., Hinds M.G. The structural biology of BH3-only proteins. Methods Enzymol. 2014;544:49–74. doi: 10.1016/B978-0-12-417158-9.00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Kvansakul M., Hinds M.G. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis. 2013;4:e909. doi: 10.1038/cddis.2013.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delbridge A.R., Grabow S., Strasser A., Vaux D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 10.Fuentes-Prior P., Salvesen G.S. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem. J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marino G., Niso-Santano M., Baehrecke E.H., Kroemer G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanave C., Santamaria M., Saccone C. Comparative genomics: The evolutionary history of the Bcl-2 family. Gene. 2004;333:71–79. doi: 10.1016/j.gene.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Zmasek C.M., Godzik A. Evolution of the animal apoptosis network. Cold Spring Harb. Perspect. Biol. 2013;5:a008649. doi: 10.1101/cshperspect.a008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aouacheria A., Rech de Laval V., Combet C., Hardwick J.M. Evolution of Bcl-2 homology motifs: Homology versus homoplasy. Trends Cell Biol. 2013;23:103–111. doi: 10.1016/j.tcb.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aouacheria A., Brunet F., Gouy M. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators. Mol. Biol. Evol. 2005;22:2395–2416. doi: 10.1093/molbev/msi234. [DOI] [PubMed] [Google Scholar]
- 16.Rautureau G.J., Day C.L., Hinds M.G. Intrinsically disordered proteins in Bcl-2 regulated apoptosis. Int. J. Mol. Sci. 2010;11:1808–1824. doi: 10.3390/ijms11041808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamas-Din A., Kale J., Leber B., Andrews D.W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke F., Voss A., Kerr J.B., O’Reilly L.A., Tai L., Echeverry N., Bouillet P., Strasser A., Kaufmann T. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ. 2012;19:915–925. doi: 10.1038/cdd.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou J.J., Li H., Salvesen G.S., Yuan J., Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/S0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 20.McDonnell J.M., Fushman D., Milliman C.L., Korsmeyer S.J., Cowburn D. Solution structure of the proapoptotic molecule BID: A structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/S0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 21.Billen L.P., Shamas-Din A., Andrews D.W. Bid: A Bax-like BH3 protein. Oncogene. 2008;27:S93–S104. doi: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Tjandra N. Structural insights of tBid, the caspase-8-activated Bid, and its BH3 domain. J. Biol. Chem. 2013;288:35840–35851. doi: 10.1074/jbc.M113.503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Y., Bobkov A.A., Plesniak L.A., Marassi F.M. Mapping the interaction of pro-apoptotic tBID with pro-survival BCL-XL. Biochemistry. 2009;48:8704–8711. doi: 10.1021/bi901171n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiens M., Diehl-Seifert B., Muller W.E. Sponge Bcl-2 homologous protein (BHP2-GC) confers distinct stress resistance to human HEK-293 cells. Cell Death Differ. 2001;8:887–898. doi: 10.1038/sj.cdd.4400906. [DOI] [PubMed] [Google Scholar]
- 25.Wiens M., Miller W.E.G. Cell death in Porifera: Molecular players in the game of apoptotic cell death in living fossils. Can. J. Zool. 2006;84:307–321. doi: 10.1139/z05-165. [DOI] [Google Scholar]
- 26.David C.N., Schmidt N., Schade M., Pauly B., Alexandrova O., Bottger A. Hydra and the evolution of apoptosis. Integr. Comp. Biol. 2005;45:631–638. doi: 10.1093/icb/45.4.631. [DOI] [PubMed] [Google Scholar]
- 27.Wiens M., Belikov S.I., Kaluzhnaya O.V., Schroder H.C., Hamer B., Perovic-Ottstadt S., Borejko A., Luthringer B., Muller I.M., Muller W.E. Axial (apical-basal) expression of pro-apoptotic and pro-survival genes in the lake baikal demosponge Lubomirskia baicalensis. DNA Cell Biol. 2006;25:152–164. doi: 10.1089/dna.2006.25.152. [DOI] [PubMed] [Google Scholar]
- 28.Lasi M., Pauly B., Schmidt N., Cikala M., Stiening B., Kasbauer T., Zenner G., Popp T., Wagner A., Knapp R.T., et al. The molecular cell death machinery in the simple cnidarian Hydra includes an expanded caspase family and pro- and anti-apoptotic Bcl-2 proteins. Cell Res. 2010;20:812–825. doi: 10.1038/cr.2010.66. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Taubenberger A., Vos M.J., Bottger A., Lasi M., Lai F.P., Fischer M., Rottner K. Monomeric red fluorescent protein variants used for imaging studies in different species. Eur. J. Cell Biol. 2006;85:1119–1129. doi: 10.1016/j.ejcb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Bottger A., Alexandrova O. Programmed cell death in Hydra. Semin. Cancer Biol. 2007;17:134–146. doi: 10.1016/j.semcancer.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth T.D., Knack B., Ukani L., Seneca F., Weiss Y., Leggat W. In situ hybridisation detects pro-apoptotic gene expression of a Bcl-2 family member in white syndrome-affected coral. Dis. Aquat. Organ. 2015;117:155–163. doi: 10.3354/dao02882. [DOI] [PubMed] [Google Scholar]
- 32.Caria S., Hinds M.G., Kvansakul M. Structural insight into an evolutionarily ancient programmed cell death regulator - the crystal structure of marine sponge BHP2 bound to LB-Bak-2. Cell Death Dis. 2017;8:e2543. doi: 10.1038/cddis.2016.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X., Dai S., Zhu Y., Marrack P., Kappler J.W. The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/S1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- 34.Kvansakul M., Wei A.H., Fletcher J.I., Willis S.N., Chen L., Roberts A.W., Huang D.C., Colman P.M. Structural basis for apoptosis inhibition by Epstein-Barr virus BHRF1. PLoS Pathog. 2010;6:e1001236. doi: 10.1371/journal.ppat.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kvansakul M., van Delft M.F., Lee E.F., Gulbis J.M., Fairlie W.D., Huang D.C., Colman P.M. A structural viral mimic of prosurvival Bcl-2: A pivotal role for sequestering proapoptotic Bax and Bak. Mol. Cell. 2007;25:933–942. doi: 10.1016/j.molcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Banjara S., Caria S., Dixon L.K., Hinds M.G., Kvansakul M. Structural Insight into African Swine Fever Virus A179L-Mediated Inhibition of Apoptosis. J. Virol. 2017;91 doi: 10.1128/JVI.02228-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ku B., Woo J.S., Liang C., Lee K.H., Hong H.S., E X., Kim K.S., Jung J.U., Oh B.H. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog. 2008;4:e25. doi: 10.1371/journal.ppat.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caria S., Marshall B., Burton R.L., Campbell S., Pantaki-Eimany D., Hawkins C.J., Barry M., Kvansakul M. The N Terminus of the Vaccinia Virus Protein F1L Is an Intrinsically Unstructured Region That Is Not Involved in Apoptosis Regulation. J. Biol. Chem. 2016;291:14600–14608. doi: 10.1074/jbc.M116.726851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham S.C., Bahar M.W., Cooray S., Chen R.A., Whalen D.M., Abrescia N.G., Alderton D., Owens R.J., Stuart D.I., Smith G.L., et al. Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-κB rather than apoptosis. PLoS Pathog. 2008;4:e1000128. doi: 10.1371/journal.ppat.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pihan P., Carreras-Sureda A., Hetz C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017;24:1478–1487. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssens S., Pulendran B., Lambrecht B.N. Emerging functions of the unfolded protein response in immunity. Nat. Immunol. 2014;15:910–919. doi: 10.1038/ni.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsten T., Ross A.J., King A., Zong W.X., Rathmell J.C., Shiels H.A., Ulrich E., Waymire K.G., Mahar P., Frauwirth K., et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/S1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen F., Hersh B.M., Conradt B., Zhou Z., Riemer D., Gruenbaum Y., Horvitz H.R. Translocation of C. elegans CED-4 to nuclear membranes during programmed cell death. Science. 2000;287:1485–1489. doi: 10.1126/science.287.5457.1485. [DOI] [PubMed] [Google Scholar]
- 44.Neumann S., El Maadidi S., Faletti L., Haun F., Labib S., Schejtman A., Maurer U., Borner C. How do viruses control mitochondria-mediated apoptosis? Virus Res. 2015;209:45–55. doi: 10.1016/j.virusres.2015.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G.H., Garvey T.L., Cohen J.I. The murine gammaherpesvirus-68 M11 protein inhibits Fas- and TNF-induced apoptosis. J. Gen. Virol. 1999;80:2737–2740. doi: 10.1099/0022-1317-80-10-2737. [DOI] [PubMed] [Google Scholar]
- 46.Chiou S.K., Tseng C.C., Rao L., White E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J. Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henderson S., Huen D., Rowe M., Dawson C., Johnson G., Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc. Natl. Acad. Sci. USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng E.H., Nicholas J., Bellows D.S., Hayward G.S., Guo H.G., Reitz M.S., Hardwick J.M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Q., Petros A.M., Virgin H.W., Fesik S.W., Olejniczak E.T. Solution structure of a Bcl-2 homolog from Kaposi sarcoma virus. Proc. Natl. Acad. Sci. USA. 2002;99:3428–3433. doi: 10.1073/pnas.062525799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aouacheria A., Banyai M., Rigal D., Schmidt C.J., Gillet G. Characterization of vNR-13, the first alphaherpesvirus gene of the bcl-2 family. Virology. 2003;316:256–266. doi: 10.1016/j.virol.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Brun A., Rivas C., Esteban M., Escribano J.M., Alonso C. African swine fever virus gene A179L, a viral homologue of bcl-2, protects cells from programmed cell death. Virology. 1996;225:227–230. doi: 10.1006/viro.1996.0592. [DOI] [PubMed] [Google Scholar]
- 52.Lu L., Zhou S.Y., Chen C., Weng S.P., Chan S.M., He J.G. Complete genome sequence analysis of an iridovirus isolated from the orange-spotted grouper, Epinephelus coioides. Virology. 2005;339:81–100. doi: 10.1016/j.virol.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Tsai C.T., Ting J.W., Wu M.H., Wu M.F., Guo I.C., Chang C.Y. Complete genome sequence of the grouper iridovirus and comparison of genomic organization with those of other iridoviruses. J. Virol. 2005;79:2010–2023. doi: 10.1128/JVI.79.4.2010-2023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham K.A., Opgenorth A., Upton C., McFadden G. Myxoma virus M11L ORF encodes a protein for which cell surface localization is critical in manifestation of viral virulence. Virology. 1992;191:112–124. doi: 10.1016/0042-6822(92)90172-L. [DOI] [PubMed] [Google Scholar]
- 55.Wasilenko S.T., Stewart T.L., Meyers A.F., Barry M. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. USA. 2003;100:14345–14350. doi: 10.1073/pnas.2235583100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer S.F., Ludwig H., Holzapfel J., Kvansakul M., Chen L., Huang D.C., Sutter G., Knese M., Hacker G. Modified vaccinia virus Ankara protein F1L is a novel BH3-domain-binding protein and acts together with the early viral protein E3L to block virus-associated apoptosis. Cell Death Differ. 2006;13:109–118. doi: 10.1038/sj.cdd.4401718. [DOI] [PubMed] [Google Scholar]
- 57.Kvansakul M., Yang H., Fairlie W.D., Czabotar P.E., Fischer S.F., Perugini M.A., Huang D.C., Colman P.M. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008;15:1564–1571. doi: 10.1038/cdd.2008.83. [DOI] [PubMed] [Google Scholar]
- 58.Marshall B., Puthalakath H., Caria S., Chugh S., Doerflinger M., Colman P.M., Kvansakul M. Variola virus F1L is a Bcl-2-like protein that unlike its vaccinia virus counterpart inhibits apoptosis independent of Bim. Cell Death Dis. 2015;6:e1680. doi: 10.1038/cddis.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta N., Taylor J., Quilty D., Barry M. Ectromelia virus encodes an anti-apoptotic protein that regulates cell death. Virology. 2015;475:74–87. doi: 10.1016/j.virol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 60.Okamoto T., Campbell S., Mehta N., Thibault J., Colman P.M., Barry M., Huang D.C., Kvansakul M. Sheeppox virus SPPV14 encodes a Bcl-2-like cell death inhibitor that counters a distinct set of mammalian proapoptotic proteins. J. Virol. 2012;86:11501–11511. doi: 10.1128/JVI.01115-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banadyga L., Lam S.C., Okamoto T., Kvansakul M., Huang D.C., Barry M. Deerpox virus encodes an inhibitor of apoptosis that regulates Bak and Bax. J. Virol. 2011;85:1922–1934. doi: 10.1128/JVI.01959-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burton D.R., Caria S., Marshall B., Barry M., Kvansakul M. Structural basis of Deerpox virus-mediated inhibition of apoptosis. Acta Crystallogr. D Biol. Crystallogr. 2015;71:1593–1603. doi: 10.1107/S1399004715009402. [DOI] [PubMed] [Google Scholar]
- 63.Banadyga L., Gerig J., Stewart T., Barry M. Fowlpox virus encodes a Bcl-2 homologue that protects cells from apoptotic death through interaction with the proapoptotic protein Bak. J. Virol. 2007;81:11032–11045. doi: 10.1128/JVI.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anasir M.I., Caria S., Skinner M.A., Kvansakul M. Structural basis of apoptosis inhibition by the fowlpox virus protein FPV039. J. Biol. Chem. 2017;292:9010–9021. doi: 10.1074/jbc.M116.768879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tulman E.R., Afonso C.L., Lu Z., Zsak L., Kutish G.F., Rock D.L. The genome of canarypox virus. J. Virol. 2004;78:353–366. doi: 10.1128/JVI.78.1.353-366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westphal D., Ledgerwood E.C., Hibma M.H., Fleming S.B., Whelan E.M., Mercer A.A. A novel Bcl-2-like inhibitor of apoptosis is encoded by the parapoxvirus ORF virus. J. Virol. 2007;81:7178–7188. doi: 10.1128/JVI.00404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uren R.T., Iyer S., Kluck R.M. Pore formation by dimeric Bak and Bax: An unusual pore? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Altmann M., Hammerschmidt W. Epstein-Barr virus provides a new paradigm: A requirement for the immediate inhibition of apoptosis. PLoS Biol. 2005;3:e404. doi: 10.1371/journal.pbio.0030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Todt F., Cakir Z., Reichenbach F., Emschermann F., Lauterwasser J., Kaiser A., Ichim G., Tait S.W., Frank S., Langer H.F., et al. Differential retrotranslocation of mitochondrial Bax and Bak. EMBO J. 2015;34:67–80. doi: 10.15252/embj.201488806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zong W.X., Li C., Hatzivassiliou G., Lindsten T., Yu Q.C., Yuan J., Thompson C.B. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akao Y., Otsuki Y., Kataoka S., Ito Y., Tsujimoto Y. Multiple subcellular localization of Bcl-2: Detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res. 1994;54:2468–2471. [PubMed] [Google Scholar]
- 72.Wolter K.G., Hsu Y.T., Smith C.L., Nechushtan A., Xi X.G., Youle R.J. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsu Y.T., Wolter K.G., Youle R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edlich F., Banerjee S., Suzuki M., Cleland M.M., Arnoult D., Wang C., Neutzner A., Tjandra N., Youle R.J. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Todt F., Cakir Z., Reichenbach F., Youle R.J., Edlich F. The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria. Cell Death Differ. 2013;20:333–342. doi: 10.1038/cdd.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schellenberg B., Wang P., Keeble J.A., Rodriguez-Enriquez R., Walker S., Owens T.W., Foster F., Tanianis-Hughes J., Brennan K., Streuli C.H., et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol. Cell. 2013;49:959–971. doi: 10.1016/j.molcel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki M., Youle R.J., Tjandra N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/S0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 78.Oltvai Z.N., Milliman C.L., Korsmeyer S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-O. [DOI] [PubMed] [Google Scholar]
- 79.Wilson-Annan J., O’Reilly L.A., Crawford S.A., Hausmann G., Beaumont J.G., Parma L.P., Chen L., Lackmann M., Lithgow T., Hinds M.G., et al. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J. Cell Biol. 2003;162:877–887. doi: 10.1083/jcb.200302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hinds M.G., Lackmann M., Skea G.L., Harrison P.J., Huang D.C., Day C.L. The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. EMBO J. 2003;22:1497–1507. doi: 10.1093/emboj/cdg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Opgenorth A., Graham K., Nation N., Strayer D., McFadden G. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L and myxoma growth factor. J. Virol. 1992;66:4720–4731. doi: 10.1128/jvi.66.8.4720-4731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nechushtan A., Smith C.L., Hsu Y.T., Youle R.J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tait S.W., Green D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Czabotar P.E., Lessene G., Strasser A., Adams J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 85.Hockings C., Anwari K., Ninnis R.L., Brouwer J., O’Hely M., Evangelista M., Hinds M.G., Czabotar P.E., Lee E.F., Fairlie W.D., et al. Bid chimeras indicate that most BH3-only proteins can directly activate Bak and Bax, and show no preference for Bak versus Bax. Cell Death Dis. 2015;6:e1735. doi: 10.1038/cddis.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.White E., Blose S.H., Stillman B.W. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol. Cell Biol. 1984;4:2865–2875. doi: 10.1128/MCB.4.12.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao L., Modha D., White E. The E1B 19K protein associates with lamins in vivo and its proper localization is required for inhibition of apoptosis. Oncogene. 1997;15:1587–1597. doi: 10.1038/sj.onc.1201323. [DOI] [PubMed] [Google Scholar]
- 88.Ring B.A., Ferreira Lacerda A., Drummond D.J., Wangen C., Eaton H.E., Brunetti C.R. Frog virus 3 open reading frame 97R localizes to the endoplasmic reticulum and induces nuclear invaginations. J. Virol. 2013;87:9199–9207. doi: 10.1128/JVI.00637-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hernaez B., Cabezas M., Munoz-Moreno R., Galindo I., Cuesta-Geijo M.A., Alonso C. A179L, a new viral Bcl2 homolog targeting Beclin 1 autophagy related protein. Curr. Mol. Med. 2013;13:305–316. doi: 10.2174/156652413804810736. [DOI] [PubMed] [Google Scholar]
- 90.Cosentino K., Garcia-Saez A.J. Bax and Bak Pores: Are We Closing the Circle? Trends Cell Biol. 2017;27:266–275. doi: 10.1016/j.tcb.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barnes C.A., Mishra P., Baber J.L., Strub M.P., Tjandra N. Conformational Heterogeneity in the Activation Mechanism of Bax. Structure. 2017;25:1310–1316.e1313. doi: 10.1016/j.str.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bleicken S., Hantusch A., Das K.K., Frickey T., Garcia-Saez A.J. Quantitative interactome of a membrane Bcl-2 network identifies a hierarchy of complexes for apoptosis regulation. Nat. Commun. 2017;8:73. doi: 10.1038/s41467-017-00086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grosse L., Wurm C.A., Bruser C., Neumann D., Jans D.C., Jakobs S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016;35:402–413. doi: 10.15252/embj.201592789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salvador-Gallego R., Mund M., Cosentino K., Schneider J., Unsay J., Schraermeyer U., Engelhardt J., Ries J., Garcia-Saez A.J. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 2016;35:389–401. doi: 10.15252/embj.201593384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andreu-Fernandez V., Sancho M., Genoves A., Lucendo E., Todt F., Lauterwasser J., Funk K., Jahreis G., Perez-Paya E., Mingarro I., et al. Bax transmembrane domain interacts with prosurvival Bcl-2 proteins in biological membranes. Proc. Natl. Acad. Sci. USA. 2017;114:310–315. doi: 10.1073/pnas.1612322114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao Y., Fujimoto L.M., Hirshman N., Bobkov A.A., Antignani A., Youle R.J., Marassi F.M. Conformation of BCL-XL upon Membrane Integration. J. Mol. Biol. 2015;427:2262–2270. doi: 10.1016/j.jmb.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muchmore S.W., Sattler M., Liang H., Meadows R.P., Harlan J.E., Yoon H.S., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 98.Yao Y., Nisan D., Fujimoto L.M., Antignani A., Barnes A., Tjandra N., Youle R.J., Marassi F.M. Characterization of the membrane-inserted C-terminus of cytoprotective BCL-XL. Protein Expr. Purif. 2016;122:56–63. doi: 10.1016/j.pep.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iyer S., Bell F., Westphal D., Anwari K., Gulbis J., Smith B.J., Dewson G., Kluck R.M. Bak apoptotic pores involve a flexible C-terminal region and juxtaposition of the C-terminal transmembrane domains. Cell Death Differ. 2015;22:1665–1675. doi: 10.1038/cdd.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dewson G., Kratina T., Czabotar P., Day C.L., Adams J.M., Kluck R.M. Bak activation for apoptosis involves oligomerization of dimers via their α6 helices. Mol. Cell. 2009;36:696–703. doi: 10.1016/j.molcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Gavathiotis E., Suzuki M., Davis M.L., Pitter K., Bird G.H., Katz S.G., Tu H.C., Kim H., Cheng E.H., Tjandra N., et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Czabotar P.E., Westphal D., Dewson G., Ma S., Hockings C., Fairlie W.D., Lee E.F., Yao S., Robin A.Y., Smith B.J., et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 103.Robin A.Y., Krishna Kumar K., Westphal D., Wardak A.Z., Thompson G.V., Dewson G., Colman P.M., Czabotar P.E. Crystal structure of Bax bound to the BH3 peptide of Bim identifies important contacts for interaction. Cell Death Dis. 2015;6:e1809. doi: 10.1038/cddis.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Neill J.W., Manion M.K., Maguire B., Hockenbery D.M. BCL-XL dimerization by three-dimensional domain swapping. J. Mol. Biol. 2006;356:367–381. doi: 10.1016/j.jmb.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez-Grille J., Busch L.K., Martinez-Costas J., Benavente J. Avian reovirus-triggered apoptosis enhances both virus spread and the processing of the viral nonstructural muNS protein. Virology. 2014;462–463:49–59. doi: 10.1016/j.virol.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han J., Sabbatini P., Perez D., Rao L., Modha D., White E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 107.Farrow S.N., White J.H., Martinou I., Raven T., Pun K.T., Grinham C.J., Martinou J.C., Brown R. Cloning of a Bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 108.Han J., Wallen H.D., Nunez G., White E. E1B 19,000-molecular-weight protein interacts with and inhibits CED-4-dependent, FLICE-mediated apoptosis. Mol. Cell Biol. 1998;18:6052–6062. doi: 10.1128/MCB.18.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang Q., Petros A.M., Virgin H.W., Fesik S.W., Olejniczak E.T. Solution structure of the BHRF1 protein from Epstein-Barr virus, a homolog of human Bcl-2. J. Mol. Biol. 2003;332:1123–1130. doi: 10.1016/j.jmb.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 110.Flanagan A.M., Letai A. BH3 domains define selective inhibitory interactions with BHRF-1 and KSHV BCL-2. Cell Death Differ. 2008;15:580–588. doi: 10.1038/sj.cdd.4402292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desbien A.L., Kappler J.W., Marrack P. The Epstein-Barr virus Bcl-2 homolog, BHRF1, blocks apoptosis by binding to a limited amount of Bim. Proc. Natl. Acad. Sci. USA. 2009;106:5663–5668. doi: 10.1073/pnas.0901036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelly G.L., Long H.M., Stylianou J., Thomas W.A., Leese A., Bell A.I., Bornkamm G.W., Mautner J., Rickinson A.B., Rowe M. An Epstein-Barr virus anti-apoptotic protein constitutively expressed in transformed cells and implicated in burkitt lymphomagenesis: The Wp/BHRF1 link. PLoS Pathog. 2009;5:e1000341. doi: 10.1371/journal.ppat.1000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marshall W.L., Yim C., Gustafson E., Graf T., Sage D.R., Hanify K., Williams L., Fingeroth J., Finberg R.W. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J. Virol. 1999;73:5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bellows D.S., Howell M., Pearson C., Hazlewood S.A., Hardwick J.M. Epstein-Barr virus BALF1 is a BCL-2-like antagonist of the herpesvirus antiapoptotic BCL-2 proteins. J. Virol. 2002;76:2469–2479. doi: 10.1128/jvi.76.5.2469-2479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Procko E., Berguig G.Y., Shen B.W., Song Y., Frayo S., Convertine A.J., Margineantu D., Booth G., Correia B.E., Cheng Y., et al. A computationally designed inhibitor of an Epstein-Barr viral Bcl-2 protein induces apoptosis in infected cells. Cell. 2014;157:1644–1656. doi: 10.1016/j.cell.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caria S., Chugh S., Nhu D., Lessene G., Kvansakul M. Crystallization and preliminary X-ray characterization of Epstein-Barr virus BHRF1 in complex with a benzoylurea peptidomimetic. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012;68:1521–1524. doi: 10.1107/S1744309112043333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sarid R., Sato T., Bohenzky R.A., Russo J.J., Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nat. Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 118.Gallo A., Lampe M., Gunther T., Brune W. The Viral Bcl-2 Homologs of Kaposi’s Sarcoma-Associated Herpesvirus and Rhesus Rhadinovirus Share an Essential Role for Viral Replication. J. Virol. 2017;91 doi: 10.1128/JVI.01875-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nava V.E., Cheng E.H., Veliuona M., Zou S., Clem R.J., Mayer M.L., Hardwick J.M. Herpesvirus saimiri encodes a functional homolog of the human Bcl-2 oncogene. J. Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Virgin H.W., 4th, Latreille P., Wamsley P., Hallsworth K., Weck K.E., Dal Canto A.J., Speck S.H. Complete sequence and genomic analysis of murine γherpesvirus 68. J. Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roy D.J., Ebrahimi B.C., Dutia B.M., Nash A.A., Stewart J.P. Murine γherpesvirus M11 gene product inhibits apoptosis and is expressed during virus persistence. Arch. Virol. 2000;145:2411–2420. doi: 10.1007/s007050070030. [DOI] [PubMed] [Google Scholar]
- 122.Sinha S., Colbert C.L., Becker N., Wei Y., Levine B. Molecular basis of the regulation of Beclin 1-dependent autophagy by the γ-herpesvirus 68 Bcl-2 homolog M11. Autophagy. 2008;4:989–997. doi: 10.4161/auto.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goldmacher V.S., Bartle L.M., Skaletskaya A., Dionne C.A., Kedersha N.L., Vater C.A., Han J.W., Lutz R.J., Watanabe S., Cahir McFarland E.D., et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karbowski M., Norris K.L., Cleland M.M., Jeong S.Y., Youle R.J. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 125.Norris K.L., Youle R.J. Cytomegalovirus proteins vMIA and m38.5 link mitochondrial morphogenesis to Bcl-2 family proteins. J. Virol. 2008;82:6232–6243. doi: 10.1128/JVI.02710-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma J., Edlich F., Bermejo G.A., Norris K.L., Youle R.J., Tjandra N. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc. Natl. Acad. Sci. USA. 2012;109:20901–20906. doi: 10.1073/pnas.1217094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cam M., Handke W., Picard-Maureau M., Brune W. Cytomegaloviruses inhibit Bak- and Bax-mediated apoptosis with two separate viral proteins. Cell Death Differ. 2010;17:655–665. doi: 10.1038/cdd.2009.147. [DOI] [PubMed] [Google Scholar]
- 128.Manzur M., Fleming P., Huang D.C., Degli-Esposti M.A., Andoniou C.E. Virally mediated inhibition of Bax in leukocytes promotes dissemination of murine cytomegalovirus. Cell Death Differ. 2009;16:312–320. doi: 10.1038/cdd.2008.152. [DOI] [PubMed] [Google Scholar]
- 129.Arnoult D., Skaletskaya A., Estaquier J., Dufour C., Goldmacher V.S. The murine cytomegalovirus cell death suppressor m38.5 binds Bax and blocks Bax-mediated mitochondrial outer membrane permeabilization. Apoptosis. 2008;13:1100–1110. doi: 10.1007/s10495-008-0245-2. [DOI] [PubMed] [Google Scholar]
- 130.Fleming P., Kvansakul M., Voigt V., Kile B.T., Kluck R.M., Huang D.C., Degli-Esposti M.A., Andoniou C.E. MCMV-mediated inhibition of the pro-apoptotic Bak protein is required for optimal in vivo replication. PLoS Pathog. 2013;9:e1003192. doi: 10.1371/journal.ppat.1003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Handke W., Luig C., Popovic B., Krmpotic A., Jonjic S., Brune W. Viral inhibition of BAK promotes murine cytomegalovirus dissemination to salivary glands. J. Virol. 2013;87:3592–3596. doi: 10.1128/JVI.02657-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee R.M., Gillet G., Burnside J., Thomas S.J., Neiman P. Role of Nr13 in regulation of programmed cell death in the bursa of Fabricius. Genes Dev. 1999;13:718–728. doi: 10.1101/gad.13.6.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ke N., Godzik A., Reed J.C. Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J. Biol. Chem. 2001;276:12481–12484. doi: 10.1074/jbc.C000871200. [DOI] [PubMed] [Google Scholar]
- 134.Aouacheria A., Arnaud E., Venet S., Lalle P., Gouy M., Rigal D., Gillet G. Nrh, a human homologue of Nr-13 associates with Bcl-Xs and is an inhibitor of apoptosis. Oncogene. 2001;20:5846–5855. doi: 10.1038/sj.onc.1204740. [DOI] [PubMed] [Google Scholar]
- 135.Rautureau G.J., Yabal M., Yang H., Huang D.C., Kvansakul M., Hinds M.G. The restricted binding repertoire of Bcl-B leaves Bim as the universal BH3-only prosurvival Bcl-2 protein antagonist. Cell Death Dis. 2012;3:e443. doi: 10.1038/cddis.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rautureau G.J., Day C.L., Hinds M.G. The structure of Boo/Diva reveals a divergent Bcl-2 protein. Proteins. 2010;78:2181–2186. doi: 10.1002/prot.22728. [DOI] [PubMed] [Google Scholar]
- 137.Ku B., Woo J.S., Liang C., Lee K.H., Jung J.U., Oh B.H. An insight into the mechanistic role of Beclin 1 and its inhibition by prosurvival Bcl-2 family proteins. Autophagy. 2008;4:519–520. doi: 10.4161/auto.5846. [DOI] [PubMed] [Google Scholar]
- 138.Aoyagi M., Zhai D., Jin C., Aleshin A.E., Stec B., Reed J.C., Liddington R.C. Vaccinia virus N1L protein resembles a B cell lymphoma-2 (Bcl-2) family protein. Protein Sci. 2007;16:118–124. doi: 10.1110/ps.062454707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen L., Willis S.N., Wei A., Smith B.J., Fletcher J.I., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 140.Smits C., Czabotar P.E., Hinds M.G., Day C.L. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure. 2008;16:818–829. doi: 10.1016/j.str.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 141.Willis S.N., Chen L., Dewson G., Wei A., Naik E., Fletcher J.I., Adams J.M., Huang D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fletcher J.I., Meusburger S., Hawkins C.J., Riglar D.T., Lee E.F., Fairlie W.D., Huang D.C., Adams J.M. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc. Natl. Acad. Sci. USA. 2008;105:18081–18087. doi: 10.1073/pnas.0808691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Campbell S., Thibault J., Mehta N., Colman P.M., Barry M., Kvansakul M. Structural insight into BH3 domain binding of vaccinia virus antiapoptotic F1L. J. Virol. 2014;88:8667–8677. doi: 10.1128/JVI.01092-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taylor J.M., Quilty D., Banadyga L., Barry M. The vaccinia virus protein F1L interacts with Bim and inhibits activation of the pro-apoptotic protein Bax. J. Biol. Chem. 2006;281:39728–39739. doi: 10.1074/jbc.M607465200. [DOI] [PubMed] [Google Scholar]
- 145.Postigo A., Cross J.R., Downward J., Way M. Interaction of F1L with the BH3 domain of Bak is responsible for inhibiting vaccinia-induced apoptosis. Cell Death Differ. 2006;13:1651–1662. doi: 10.1038/sj.cdd.4401853. [DOI] [PubMed] [Google Scholar]
- 146.Wasilenko S.T., Banadyga L., Bond D., Barry M. The vaccinia virus F1L protein interacts with the proapoptotic protein Bak and inhibits Bak activation. J. Virol. 2005;79:14031–14043. doi: 10.1128/JVI.79.22.14031-14043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Campbell S., Hazes B., Kvansakul M., Colman P., Barry M. Vaccinia virus F1L interacts with Bak using highly divergent Bcl-2 homology domains and replaces the function of Mcl-1. J. Biol. Chem. 2010;285:4695–4708. doi: 10.1074/jbc.M109.053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stewart T.L., Wasilenko S.T., Barry M. Vaccinia virus F1L protein is a tail-anchored protein that functions at the mitochondria to inhibit apoptosis. J. Virol. 2005;79:1084–1098. doi: 10.1128/JVI.79.2.1084-1098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Everett H., Barry M., Lee S.F., Sun X., Graham K., Stone J., Bleackley R.C., McFadden G. M11L: A novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med. 2000;191:1487–1498. doi: 10.1084/jem.191.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang G., Barrett J.W., Nazarian S.H., Everett H., Gao X., Bleackley C., Colwill K., Moran M.F., McFadden G. Myxoma virus M11L prevents apoptosis through constitutive interaction with Bak. J. Virol. 2004;78:7097–7111. doi: 10.1128/JVI.78.13.7097-7111.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Douglas A.E., Corbett K.D., Berger J.M., McFadden G., Handel T.M. Structure of M11L: A myxoma virus structural homolog of the apoptosis inhibitor, Bcl-2. Protein Sci. 2007;16:695–703. doi: 10.1110/ps.062720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Westphal D., Ledgerwood E.C., Tyndall J.D., Hibma M.H., Ueda N., Fleming S.B., Mercer A.A. The orf virus inhibitor of apoptosis functions in a Bcl-2-like manner, binding and neutralizing a set of BH3-only proteins and active Bax. Apoptosis. 2009;14:1317–1330. doi: 10.1007/s10495-009-0403-1. [DOI] [PubMed] [Google Scholar]
- 153.Banadyga L., Veugelers K., Campbell S., Barry M. The fowlpox virus BCL-2 homologue, FPV039, interacts with activated Bax and a discrete subset of BH3-only proteins to inhibit apoptosis. J. Virol. 2009;83:7085–7098. doi: 10.1128/JVI.00437-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Neilan J.G., Lu Z., Afonso C.L., Kutish G.F., Sussman M.D., Rock D.L. An African swine fever virus gene with similarity to the proto-oncogene Bcl-2 and the Epstein-Barr virus gene BHRF1. J. Virol. 1993;67:4391–4394. doi: 10.1128/jvi.67.7.4391-4394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Galindo I., Hernaez B., Diaz-Gil G., Escribano J.M., Alonso C. A179L, a viral Bcl-2 homologue, targets the core Bcl-2 apoptotic machinery and its upstream BH3 activators with selective binding restrictions for Bid and Noxa. Virology. 2008;375:561–572. doi: 10.1016/j.virol.2008.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lin P.W., Huang Y.J., John J.A., Chang Y.N., Yuan C.H., Chen W.Y., Yeh C.H., Shen S.T., Lin F.P., Tsui W.H., et al. Iridovirus Bcl-2 protein inhibits apoptosis in the early stage of viral infection. Apoptosis. 2008;13:165–176. doi: 10.1007/s10495-007-0152-y. [DOI] [PubMed] [Google Scholar]
- 157.Pattingre S., Tassa A., Qu X., Garuti R., Liang X.H., Mizushima N., Packer M., Schneider M.D., Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 158.Piya S., White E.J., Klein S.R., Jiang H., McDonnell T.J., Gomez-Manzano C., Fueyo J. The E1B19K oncoprotein complexes with Beclin 1 to regulate autophagy in adenovirus-infected cells. PLoS ONE. 2011;6:e29467. doi: 10.1371/journal.pone.0029467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gerlic M., Faustin B., Postigo A., Yu E.C., Proell M., Gombosuren N., Krajewska M., Flynn R., Croft M., Way M., et al. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proc. Natl. Acad. Sci. USA. 2013;110:7808–7813. doi: 10.1073/pnas.1215995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu E., Zhai D., Jin C., Gerlic M., Reed J.C., Liddington R. Structural determinants of caspase-9 inhibition by the vaccinia virus protein, F1L. J. Biol. Chem. 2011;286:30748–30758. doi: 10.1074/jbc.M111.280149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhai D., Yu E., Jin C., Welsh K., Shiau C.W., Chen L., Salvesen G.S., Liddington R., Reed J.C. Vaccinia virus protein F1L is a caspase-9 inhibitor. J. Biol. Chem. 2010;285:5569–5580. doi: 10.1074/jbc.M109.078113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cooray S., Bahar M.W., Abrescia N.G., McVey C.E., Bartlett N.W., Chen R.A., Stuart D.I., Grimes J.M., Smith G.L. Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein. J. Gen. Virol. 2007;88:1656–1666. doi: 10.1099/vir.0.82772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.De Motes C.M., Cooray S., Ren H., Almeida G.M.F., McGourty K., Bahar M.W., Stuart D.I., Grimes J.M., Graham S.C., Smith G.L. Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence. PLoS Pathog. 2011;7:e1002430. doi: 10.1371/journal.ppat.1002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fedosyuk S., Grishkovskaya I., de Almeida Ribeiro E., Jr., Skern T. Characterization and structure of the vaccinia virus NF-κB antagonist A46. J. Biol. Chem. 2014;289:3749–3762. doi: 10.1074/jbc.M113.512756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim Y., Lee H., Heo L., Seok C., Choe J. Structure of vaccinia virus A46, an inhibitor of TLR4 signaling pathway, shows the conformation of VIPER motif. Protein Sci. 2014;23:906–914. doi: 10.1002/pro.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neidel S., Maluquer de Motes C., Mansur D.S., Strnadova P., Smith G.L., Graham S.C. Vaccinia virus protein A49 is an unexpected member of the B-cell Lymphoma (Bcl)-2 protein family. J. Biol. Chem. 2015;290:5991–6002. doi: 10.1074/jbc.M114.624650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kalverda A.P., Thompson G.S., Vogel A., Schroder M., Bowie A.G., Khan A.R., Homans S.W. Poxvirus K7 protein adopts a Bcl-2 fold: Biochemical mapping of its interactions with human DEAD box RNA helicase DDX3. J. Mol. Biol. 2009;385:843–853. doi: 10.1016/j.jmb.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 168.Fedosyuk S., Bezerra G.A., Radakovics K., Smith T.K., Sammito M., Bobik N., Round A., Ten Eyck L.F., Djinovic-Carugo K., Uson I., et al. Vaccinia Virus Immunomodulator A46: A Lipid and Protein-Binding Scaffold for Sequestering Host TIR-Domain Proteins. PLoS Pathog. 2016;12:e1006079. doi: 10.1371/journal.ppat.1006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oda S., Schroder M., Khan A.R. Structural basis for targeting of human RNA helicase DDX3 by poxvirus protein K7. Structure. 2009;17:1528–1537. doi: 10.1016/j.str.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 170.Schroder M., Baran M., Bowie A.G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 2008;27:2147–2157. doi: 10.1038/emboj.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Merino D., Giam M., Hughes P.D., Siggs O.M., Heger K., O’Reilly L.A., Adams J.M., Strasser A., Lee E.F., Fairlie W.D., et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J. Cell Biol. 2009;186:355–362. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Poncet D., Larochette N., Pauleau A.L., Boya P., Jalil A.A., Cartron P.F., Vallette F., Schnebelen C., Bartle L.M., Skaletskaya A., et al. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 2004;279:22605–22614. doi: 10.1074/jbc.M308408200. [DOI] [PubMed] [Google Scholar]
- 173.Cross J.R., Postigo A., Blight K., Downward J. Viral pro-survival proteins block separate stages in Bax activation but changes in mitochondrial ultrastructure still occur. Cell Death Differ. 2008;15:997–1008. doi: 10.1038/cdd.2008.14. [DOI] [PubMed] [Google Scholar]
- 174.Connolly P.F., Fearnhead H.O. Viral hijacking of host caspases: An emerging category of pathogen-host interactions. Cell Death Differ. 2017;24:1401–1410. doi: 10.1038/cdd.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Collins-McMillen D., Kim J.H., Nogalski M.T., Stevenson E.V., Chan G.C., Caskey J.R., Cieply S.J., Yurochko A.D. Human Cytomegalovirus Promotes Survival of Infected Monocytes via a Distinct Temporal Regulation of Cellular Bcl-2 Family Proteins. J. Virol. 2015;90:2356–2371. doi: 10.1128/JVI.01994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]