Abstract
Sarcopenia impairs survival in patients with hepatocellular carcinoma (HCC). This study aimed to clarify the factors that contribute to decreased skeletal muscle volume in patients with HCC. The third lumbar vertebra skeletal muscle index (L3 SMI) in 351 consecutive patients with HCC was calculated to identify sarcopenia. Sarcopenia was defined as an L3 SMI value ≤ 29.0 cm2/m2 for women and ≤ 36.0 cm2/m2 for men. The factors affecting L3 SMI were analyzed by multiple linear regression analysis and tree-based models. Of the 351 HCC patients, 33 were diagnosed as having sarcopenia and showed poor prognosis compared with non-sarcopenia patients (p = 0.007). However, this significant difference disappeared after the adjustments for age, sex, Child–Pugh score, maximum tumor size, tumor number, and the degree of portal vein invasion by propensity score matching analysis. Multiple linear regression analysis showed that age (p = 0.015) and sex (p < 0.0001) were significantly correlated with a decrease in L3 SMI. Tree-based models revealed that sex (female) is the most significant factor that affects L3 SMI. In male patients, L3 SMI was decreased by aging, increased Child–Pugh score (≥56 years), and enlarged tumor size (<56 years). Maintaining liver functional reserve and early diagnosis and therapy for HCC are vital to prevent skeletal muscle depletion and improve the prognosis of patients with HCC.
Keywords: hepatocellular carcinoma, skeletal muscle depletion, sarcopenia, prognostic factor
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. Typically, patients with HCC have a poor clinical course as the prognosis is strongly affected by liver functional reserve and clinical cancer stage [1,2]. The recurrence rate of HCC is extremely high, which is also associated with the poor prognosis [3]. To identify patients with a high mortality risk and to choose the most adequate treatment, a precise prediction of the prognosis of patients with HCC is essential. Thus, several prognostic staging systems, such as Barcelona Clinic Liver Cancer (BCLC) [4], Cancer of the Liver Italian Program (CLIP) [1], and Japan Integrated Staging (JIS) [2], most of which take both clinical cancer stage and liver functional reserve into consideration, have been developed.
Recently, skeletal muscle depletion or sarcopenia, initially defined as the loss of skeletal muscle mass that occurs with aging [5], has garnered attention as a new and promising prognostic factor for various malignancies, including HCC [6,7,8,9,10]. Skeletal muscle volume depletion assessed by computed tomography (CT) predicts poor prognosis of all cancer stages [11], and for sorafenib-treated patients with HCC [12]. Sarcopenia and rapid skeletal muscle depletion are also involved in worse survival in patients with liver cirrhosis [13,14]. These findings strongly suggest that sarcopenia is a significant factor that predicts the prognosis of patients with HCC and liver cirrhosis.
Several pathological conditions, including advanced organ failure, inflammatory disease, malignancy, endocrine disease, sedentary lifestyle, and malnutrition are associated with skeletal muscle depletion [5,15]. In liver disease, the following points can be considered as the main mechanisms of sarcopenia: protein energy malnutrition; imbalance of protein synthesis and breakdown; increased expression of myostatin, a cytokine that strongly suppresses skeletal muscle growth; and increased production of reactive oxygen species and inflammatory cytokines [16]. Therefore, sarcopenia could be a result of various pathological conditions, such as poor liver functional reserve or advanced cancer stages, which in turn affects survival of patients with HCC. However, the precise factors that enhance the progression of sarcopenia and worsen the prognosis of HCC patients have not been evaluated.
The purpose of this study is to identify the factors that contribute to skeletal muscle depletion in HCC patients, especially focusing on liver functional reserve and cancer progression. Based on the results of this study, we will also discuss how to prevent skeletal muscle depletion and improve prognosis in patients with HCC.
2. Materials and Methods
2.1. Patients, Treatment, and Follow-Up Strategy
We evaluated 351 consecutive HCC patients in our hospital between May 2006 and December 2015. HCC nodules were detected using imaging modalities, including dynamic CT, dynamic magnetic resonance imaging (MRI), and abdominal arteriography. HCC was diagnosed based on a typical hypervascular tumor stain on angiography and typical dynamic study findings of enhanced staining in the early phase and attenuation in the delayed phase. The treatment plan in each case was according to the Clinical Practice Guidelines for HCC issued by the Japan Society of Hepatology (JSH) [17]. Patients were thereafter followed on an outpatient basis and had dynamic CT, MRI, or ultrasound every three months after the initial treatment. Recurrent HCC was diagnosed when the typical findings of HCC were observed, and the treatment was still based on the aforementioned guidelines for HCC. Overall survival was defined as the interval from the date of the initial treatment to the date of death or December 2015 for surviving patients. All study participants provided verbal informed consent, which was considered sufficient because this study followed an observational research design that did not require new human biological specimens, and instead relied only on preexisting materials. The study design—including this consent procedure—was approved by the ethics committee of the Gifu University School of Medicine on 7 June 2017 (ethic approval code: 29–26).
2.2. Image Analysis of Skeletal Muscle Volume and Definition of Sarcopenia
Skeletal muscle volume was measured using a CT image that had been taken solely for the purpose of diagnosing HCC prior to the initial treatment. A transverse CT image at the third lumbar vertebra (L3) in the inferior direction was assessed. The muscles in the L3 region—including psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis—were analyzed using SYNAPSE VINCENT software (version 3.0, Fujifilm Medical, Tokyo, Japan), which enables specific tissue demarcation using Hounsfield unit (HU) thresholds. The muscles were quantified within a range of −29 to +150 HU [18], and tissue boundaries were manually corrected as needed. The cross-sectional areas of the muscle (cm2) at the L3 level computed from each image were normalized by the square of the height (m2) to obtain the L3 skeletal muscle index (L3 SMI, cm2/m2), which was used as an indicator of skeletal muscle volume in previous reports [11,12,16]. Sarcopenia was defined as an L3 SMI value ≤29.0 cm2/m2 for women and ≤36.0 cm2/m2 for men, which is according to a previous study [11].
2.3. Statistical Analysis
Overall survival was estimated using the Kaplan–Meier method, and differences between curves were evaluated using the log-rank test. To exclude the effect of possible confounding factors between sarcopenia and non-sarcopenia groups, we performed rigorous adjustments for the following six factors using a propensity score matching analysis: age, sex, Child–Pugh score (CPS), tumor size, tumor number, and the degree of portal vein invasion, which were considered as prognostic factors for HCC patients by previous studies [1,2,4], and showed significant differences between the two groups. The propensity score matching analysis was performed based on the following algorithm: 1:1 optimal match with calipers of width 0.2 of the standard deviation of the logit of the propensity score and no replacement [19]. To identify which of the six factors contributed to decreased L3 SMI, we conducted a multiple linear regression analysis. A tree-based model analysis, which uses the binary recursive partitioning process of the population, was also performed; thus, L3 SMI is similar in patients within each group but different between groups [20,21]. Statistical significance was defined as p < 0.05. All statistical analyses were performed using R version 3.3.1 (The R Project for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
3. Results
3.1. Baseline Characteristics and Laboratory Data of Patients
The baseline characteristics and laboratory data of the 351 patients (242 male and 109 female; average age, 70.4 years) are shown in Table 1. The average L3 SMI for all enrolled patients was 43.7 cm2/m2, and 33 patients were classified into the sarcopenia group. The average tumor size was 4.2 cm, and 188 patients received curative treatment.
Table 1.
Baseline demographic and clinical characteristics.
| Variables | Total (n = 351) |
|---|---|
| Sex (male/female) | 242/109 |
| Age (years) | 70.4 ± 10.3 |
| Etiology (HBV/HCV/HBV + HCV/others) | 43/204/3/101 |
| BMI (kg/m2) | 23.1 ± 3.4 |
| L3 SMI (cm2/m2) | 43.7 ± 8.6 |
| Sarcopenia (yes/no) | 33/318 |
| Child–Pugh score (5/6/7/8/9/10/11) | 179/84/52/20/9/6/1 |
| ALB (g/dL) | 3.6 ± 0.6 |
| ALT (IU/L) | 46.9 ± 44.3 |
| T-Bil (mg/dL) | 1.2 ± 1.0 |
| PLT (×104/μL) | 13.1 ± 7.8 |
| PT (%) | 85.3 ± 16.7 |
| FPG (mg/dL) | 110.6 ± 34.2 |
| HbA1c (%) | 6.0 ± 1.1 |
| AFP (ng/dL) | 11,557 ± 73,374 |
| PIVKA-II (mAU/mL) | 21,056 ± 125,773 |
| Tumor size (cm) | 4.2 ± 3.7 |
| Tumor number (1/≥2) | 193/158 |
| Vp (0/1/2/3/4) | 289/15/15/15/17 |
| Stage (I/II/III/IV) | 79/126/100/46 |
| Curability of initial treatment (yes/no) | 188/163 |
| Oral administration of BCAA (yes/no) | 153/198 |
| Co-existing diseases (yes/no) | |
| Renal disease | 22/329 |
| Heart disease | 45/306 |
| Respiratory disease | 16/335 |
| Neurologic disease | 22/329 |
| Malignant disease (except HCC) | 27/324 |
Values are presented as average ± standard deviation. HBV, hepatitis B virus; HCV, hepatitis C virus; BMI, body mass index; L3 SMI, third lumbar vertebra skeletal muscle index; ALT, alanine aminotransferase; T-Bil, total bilirubin; PLT, platelet count; PT, prothrombin time; FPG, fasting plasma glucose; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonists-II; Vp, the degree of portal vein invasion; BCAA, branched-chain amino acids; HCC, hepatocellular carcinoma.
3.2. Comparison of Overall Survival in Sarcopenia and Non-Sarcopenia Groups before and after Adjustments for Possible Confounding Factors
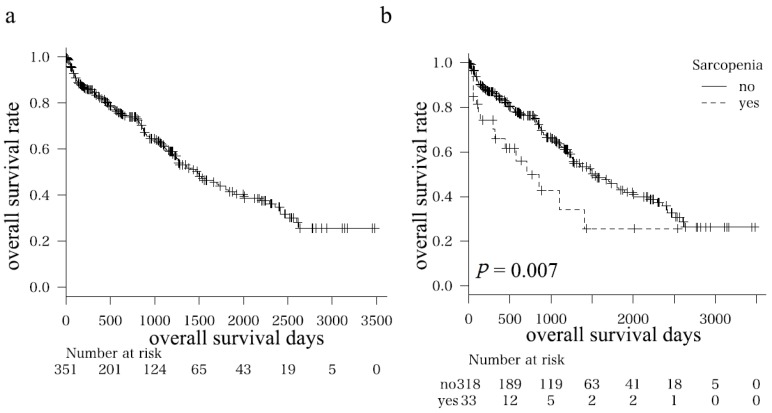
The one-, three-, and five-year overall survival rates of all enrolled patients were 83.0, 61.8, and 42.2%, respectively (Figure 1a). Table 2 shows the clinical characteristics and laboratory data of the sarcopenia (n = 33) and non-sarcopenia (n = 318) groups. Significant differences in sex (male/female = 30/3 vs. 212/106, p = 0.003), body mass index (BMI; kg/m2, 20.8 vs. 23.3, p < 0.0001), the value of L3 SMI (cm2/m2; 30.8 vs. 45.1, p < 0.0001), CPS (5/6/7/8/9/10/11 = 15/7/5/2/0/3/1 vs. 164/77/47/18/9/3/0, p = 0.039), total bilirubin (mg/dL; 1.6 vs. 1.2, p = 0.045), maximum tumor size (cm; 5.6 vs. 4.0, p = 0.020), the degree of portal vein invasion (Vp 0/1/2/3/4 = 24/1/2/2/4 vs. 265/14/13/13/13, p = 0.040), curability of initial treatment (yes/no, 12/21 vs. 175/142, p = 0.039), and prevalence rate of neurologic disease (yes/no, 6/27 vs. 16/302, p = 0.011) were found. Sarcopenia patients died significantly earlier than non-sarcopenia patients (p = 0.007, Figure 1b).
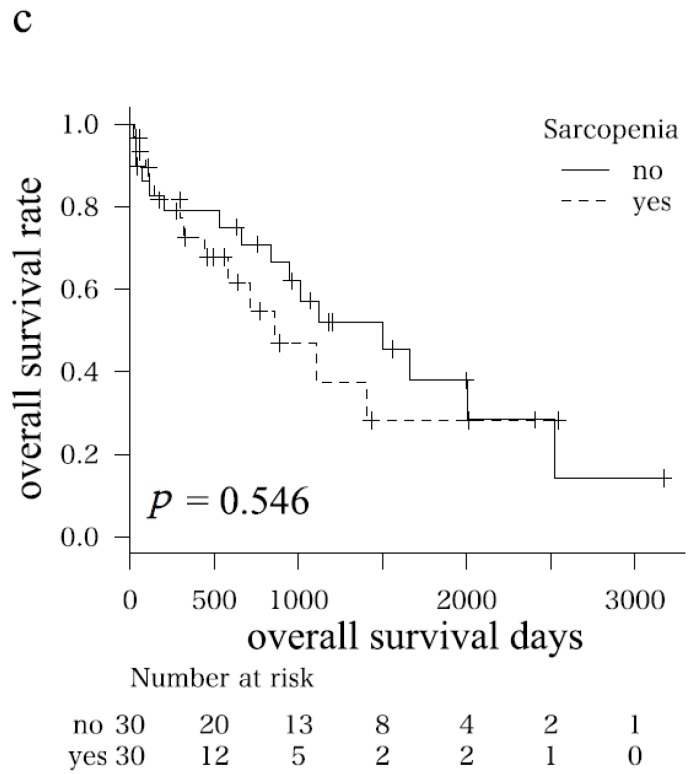
Figure 1.
Kaplan–Meier curves for overall survival time in (a) all patients; (b) subgroups (i.e., sarcopenia and non-sarcopenia groups); and (c) subgroups after adjustments for possible confounding factors (age, sex, Child–Pugh score, maximum tumor size, tumor number, and the degree of portal vein invasion) using propensity score matching analysis.
Table 2.
Baseline demographic and clinical characteristics of sarcopenia and non-sarcopenia groups.
| Variables | Sarcopenia (n = 33) | Non-Sarcopenia (n = 318) | p Value |
|---|---|---|---|
| Sex (male/female) | 30/3 | 212/106 | 0.003 |
| Age (years) | 72.6 ± 1.8 | 70.2 ± 0.6 | 0.197 |
| Etiology (HBV/HCV/HBV + HCV/other) | 3/22/0/8 | 40/182/3/93 | 0.868 |
| BMI (kg/m2) | 20.8 ± 0.6 | 23.3 ± 0.2 | <0.0001 |
| L3 SMI (cm2/m2) | 30.8 ± 1.3 | 45.1 ± 0.4 | <0.0001 |
| Child–Pugh score (5/6/7/8/9/10/11) | 15/7/5/2/0/3/1 | 164/77/47/18/9/3/0 | 0.039 |
| ALB (g/dL) | 3.5 ± 0.1 | 3.6 ± 0.03 | 0.315 |
| ALT (IU/L) | 52.2 ± 7.7 | 46.4 ± 2.5 | 0.805 |
| T-Bil (mg/dL) | 1.6 ± 0.2 | 1.2 ± 0.06 | 0.045 |
| PLT (×104/μL) | 14.5 ± 1.4 | 13.0 ± 0.4 | 0.276 |
| PT (%) | 89.1 ± 2.9 | 84.9 ± 0.9 | 0.176 |
| FPG (mg/dL) | 113.3 ± 6.0 | 110.3 ± 2.0 | 0.958 |
| HbA1c (%) | 6.2 ± 0.2 | 6.0 ± 0.07 | 0.356 |
| AFP (ng/dL) | 4133 ± 12983 | 12,319 ± 4158 | 0.549 |
| PIVKA-II (mAU/mL) | 35,910 ± 21,910 | 19,475 ± 7149 | 0.476 |
| Tumor size (cm) | 5.6 ± 0.6 | 4.0 ± 0.2 | 0.020 |
| Tumor number (1/≥2) | 20/13 | 173/145 | 0.505 |
| Vp (0/1/2/3/4) | 24/1/2/2/4 | 265/14/13/13/13 | 0.040 |
| Stage (I/II/III/IV) | 8/7/12/6 | 71/117/88/42 | 0.303 |
| Curability of initial treatment (yes/no) | 12/21 | 176/142 | 0.039 |
| Oral administration of BCAA (yes/no) | 17/16 | 136/182 | 0.361 |
| Co-existing diseases (yes/no) | |||
| Renal disease | 2/31 | 20/298 | 1.000 |
| Heart disease | 5/28 | 40/278 | 0.593 |
| Respiratory disease | 0/33 | 16/302 | 0.381 |
| Neurologic disease | 6/27 | 16/302 | 0.011 |
| Malignant disease (except HCC) | 0/33 | 27/291 | 0.093 |
Values are presented as average ± standard deviation. HBV, hepatitis B virus; HCV, hepatitis C virus; BMI, body mass index; L3 SMI, third lumbar vertebra skeletal muscle index; ALT, alanine aminotransferase; T-Bil, total bilirubin; PLT, platelet count; PT, prothrombin time; FPG, fasting plasma glucose; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonists-II; Vp, the degree of portal vein invasion; BCAA, branched-chain amino acids; HCC, hepatocellular carcinoma.
To clarify the effects of sarcopenia on the prognosis of HCC patients, a propensity score matching analysis was performed after adjusting for liver functional reserve and tumor-related factors. Thirty patients from both sarcopenia and non-sarcopenia groups were chosen (Table 3). No significant differences in all variables except BMI (20.7 vs. 23.2, p = 0.002) and L3 SMI (30.5 vs. 46.8, p < 0.0001) were found; the adjustments were performed properly. Interestingly, the significant difference in the overall survival between sarcopenia and non-sarcopenia patients observed in the initial analysis (Figure 1b) disappeared after the adjustments for possible confounding factors (p = 0.546, Figure 1c).
Table 3.
Baseline demographic and clinical characteristics of sarcopenia and non-sarcopenia groups after adjustments for possible confounding factors using propensity score matching analysis.
| Variables | Sarcopenia (n = 30) | Non-Sarcopenia (n = 30) | p Value |
|---|---|---|---|
| Sex (male/female) | 27/3 | 28/2 | 1.000 |
| Age (years) | 71.8 ± 9.7 | 73.0 ± 10.7 | 0.642 |
| Etiology (HBV/HCV/HBV + HCV/other) | 3/21/0/6 | 3/20/1/6 | 0.918 |
| BMI (kg/m2) | 20.7 ± 3.0 | 23.2 ± 2.8 | 0.002 |
| L3 SMI (cm2/m2) | 30.5 ± 6.4 | 46.8 ± 7.4 | <0.0001 |
| Child–Pugh score (5/6/7/8/9/10/11) | 14/7/5/2/0/1/1 | 14/9/6/0/1/0/0 | 0.660 |
| ALB (g/dL) | 3.6 ± 0.7 | 3.6 ± 0.6 | 0.984 |
| ALT (IU/L) | 48.8 ± 57.7 | 41.8 ± 22.7 | 0.543 |
| T-Bil (mg/dL) | 1.4 ± 1.0 | 1.0 ± 0.5 | 0.106 |
| PLT (×104/μL) | 14.5 ± 13.0 | 13.4 ± 4.9 | 0.656 |
| PT (%) | 89.8 ± 14.4 | 87.7 ± 15.5 | 0.589 |
| FPG (mg/dL) | 114.8 ± 40.2 | 113.6 ± 47.9 | 0.918 |
| HbA1c (%) | 6.3 ± 1.6 | 5.8 ± 1.2 | 0.226 |
| AFP (ng/dL) | 3531 ± 12,997 | 1480 ± 4268 | 0.423 |
| PIVKA-II (mAU/mL) | 22,979 ± 89,165 | 10,893 ± 46,387 | 0.518 |
| Tumor size (cm) | 5.0 ± 3.9 | 4.4 ± 3.8 | 0.534 |
| Tumor number (1/≥2) | 18/12 | 18/12 | 1.000 |
| Vp (0/1/2/3/4) | 24/1/2/1/2 | 22/3/1/2/2 | 0.852 |
| Stage (I/II/III/IV) | 8/7/10/5 | 7/11/5/7 | 0.427 |
| Curability of initial treatment (yes/no) | 12/18 | 17/13 | 0.301 |
Values are presented as average ± standard deviation. HBV, hepatitis B virus; HCV, hepatitis C virus; BMI, body mass index; L3 SMI, third lumbar vertebra skeletal muscle index; ALT, alanine aminotransferase; T-Bil, total bilirubin; PLT, platelet count; PT, prothrombin time; FPG, fasting plasma glucose; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonists-II; Vp, the degree of portal vein invasion.
3.3. Significant Factors that Affect L3 SMI Based on Multiple Linear Regression Analysis and Tree-Based Models
Of the six possible confounding factors (age, sex, CPS, tumor size, tumor number, and the degree of portal vein invasion), age (p = 0.015) and sex (p < 0.0001) were significantly correlated with the value of L3 SMI based on multiple linear regression analysis (Table 4). The following regression equation with an intercept of 46.94 (p < 0.0001) was obtained from Equation (1) and (2):
| L3 SMI (cm2/m2) = 46.94 − 0.10 × [Age] + 5.20 (for men) | (1) |
| L3 SMI (cm2/m2) = 46.94 − 0.10 × [Age] (for women) | (2) |
Table 4.
Significant factors affecting L3 SMI by multiple linear regression analysis.
| Variables | Std. Coefficient | Std. Error | t Value | p Value |
|---|---|---|---|---|
| Intercept | 46.94 | 3.06 | 15.33 | <0.0001 |
| Age | −0.10 | 0.04 | −2.44 | 0.015 |
| Sex (vs. man) | 5.20 | 0.95 | 5.46 | <0.0001 |
Std. coefficient, standard coefficient; Std. error, standard error.
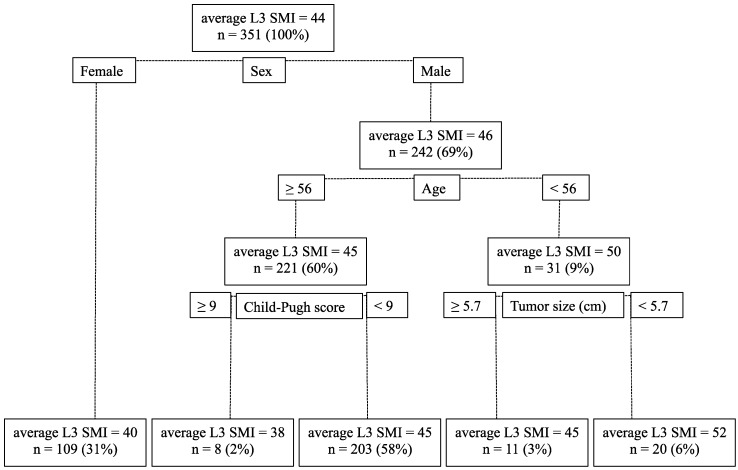
Furthermore, according to the tree-based models, the most significant factor contributing to the value of L3 SMI was sex; the average L3 SMI in men was 46 cm2/m2 and that in women was 40 cm2/m2. In men, the most significant factor for decreased L3 SMI was aging; the average L3 SMI in men ≥ 56 and < 56 years old was 45 and 50 cm2/m2, respectively. In men ≥ 56 years old, a higher CPS was involved in the loss of skeletal muscle mass; the average L3 SMI with CPS ≥ 9 and < 9 was 38 and 45 cm2/m2, respectively. In men < 56 years old, an enlarged tumor size was involved in the loss of skeletal muscle mass; the average L3 SMI with tumor size ≥ 5.7 and < 5.7 cm was 45 and 52 cm2/m2, respectively. However, these factors observed in men did not affect the L3 SMI in women. The decision tree for this analysis is shown in Figure 2.
Figure 2.
The decision tree for the tree-based models. The differences in L3 SMI between each group become larger with the six factors (age, sex, Child–Pugh score, maximum tumor size, tumor number, and the degree of portal vein invasion).
4. Discussion
The results of this study showed that sarcopenia impairs survival in patients with HCC. These findings are consistent with those of previous studies [6,7,8,9,10,11,12]. Thus, skeletal muscle volume measurement using CT, which is commonly used in the clinical setting for HCC, is useful to predict the prognosis of patients with this malignancy.
Here, patients in the sarcopenia group had poorer liver functional reserve, larger tumor size, and more severe portal vein invasion, which significantly contributed to the lower curability of the initial treatment for HCC. Moreover, men, older patients, and those with poorer liver functional reserve and larger tumor size had a lower skeletal muscle volume. Several studies revealed that liver functional reserve and clinical cancer stage are significant prognostic factors for HCC and that, similar to the results in our study, the progression of underlying liver cirrhosis and HCC, in addition to aging, are critically involved in the development of sarcopenia and subsequent poor prognosis of these patients [1,2,4]. The results of the propensity score matching analysis showed that the significant differences in overall survival between the sarcopenia and non-sarcopenia groups disappeared after adjustments for the probable confounding factors associated with the patients’ characteristics and tumor factors. This finding may suggest that the sarcopenia group had clinical characteristics—such as old age, poor liver functional reserve, and progressed cancer stage—that affect survival.
Several clinical studies have revealed that sarcopenia might be an independent prognostic factor for HCC [11,12]. In addition, HCC patients with sarcopenia more often have complications with chemotherapy toxicity and this might make the prognosis of the patients more serious [22,23]. On the other hand, skeletal muscle volume in HCC patients was determined, at least in part, by sex, age, CPS, and tumor burden in this study. Based on the findings, it could be said that, at least in men, sarcopenia is a prognostic factor possibly affected by already-known prognostic factors, including liver functional reserve and clinical cancer stage [1,2,4]. Most of the existing prognostic staging systems, such as CLIP and JIS [1,2,4], take liver functional reserve and clinical cancer stage into consideration in predicting prognosis for HCC as accurately as possible. Thus, these findings strongly suggest that maintaining liver functional reserve and early detection and therapy for HCC, which can increase the curability of initial treatment, are effective strategies to improve the prognosis. In addition, reducing the amount of tumor burden and maintaining liver functional reserve, which have been reported repeatedly to improve prognosis for HCC [1,2,4], could be effective measures to prevent skeletal muscle depletion; however, future prospective study is required to evaluate this hypothesis further.
Other possible strategies to prevent skeletal muscle depletion or to increase skeletal muscle mass include nutritional and exercise therapies, both of which have been shown to improve outcomes in patients with liver cirrhosis [24,25]. Oral supplementation with branched chain amino acids is one of the most promising methods [26,27,28]. Exercise therapy might also be promising in preventing skeletal muscle depletion [29]. Moreover, poor dietary or sedentary lifestyle is considered one of the main causes of sarcopenia [15]; thus, appropriate assessment and modification of lifestyle could be useful to prevent decreased skeletal muscle volume and subsequently to improve the prognosis of patients with HCC and liver cirrhosis. Furthermore, pathological conditions—except liver diseases—that can lead to sarcopenia must also be considered. The aging of HCC patient population is advancing and elderly patients are easily complicated with several diseases which cause sarcopenia. In the present study, the prevalence of patients with neurologic disease, which decreases activities of daily living levels, was significantly high in the sarcopenia group. Therefore, in order to prevent sarcopenia, such patients might especially be recommended to start rehabilitation as early as possible.
In the present study, aging was critically involved in the complication with sarcopenia in men. One of the reasons of this phenomenon might be that decreasing of testosterone caused by aging because this sex hormone is known to promote the growth of skeletal muscle [30]. On the other hand, aging, progression of CPS, and enlargement of tumor size did not have effects on the loss of skeletal muscle volume in women. Because women usually store abundance of fat and generate their energy more preferentially from fat stores than from skeletal muscle stores [31], women might be more resistant to sarcopenia compared to men. In addition to the present study, several clinical trials have defined the optimal cutoff values of skeletal muscle volume which are different between men and women [7,11,32]. These findings may suggest that setting the different cutoff values on every sex is reasonable to evaluate sarcopenia.
This study has some limitations. First, we used our own cutoff values for L3 SMI (i.e., 29.0 cm2/m2 for women and 36.0 cm2/m2 for men), which are not similar to those of the JSH (i.e., 38.0 cm2/m2 for women and 42.0 cm2/m2 for men) [16], to determine sarcopenia because the latter could not stratify the risk of mortality for HCC patients. We also did not use the cutoff values reported by the previous reports [7,32], both of which are widely accepted as appropriate values in western countries, because they are not applicable to Japanese HCC patients whose BMIs are small and differ considerably from Western populations. Second, because of the retrospective design of our study, muscle strength such as grip strength and walking speed, which is usually regarded as a diagnostic criterion for sarcopenia [15,16], was not assessed. Future prospective studies that examine the most optimal cutoff values for L3 SMI to diagnose sarcopenia and whether sarcopenia itself worsens the prognosis of patients with HCC should be performed.
5. Conclusions
We demonstrated that L3 SMI, an indicator of skeletal muscle volume, was significantly decreased in female HCC patients. In male patients, L3 SMI was significantly affected by aging, liver functional reserve (≥56 years), and tumor size (<56 years). These findings strongly suggest that more emphasis should be put on maintaining liver functional reserve and reducing tumor burden, both of which are well-reported prognostic factors for HCC [1,2,4], especially in patients with sarcopenia. In conclusion, in addition to the measure for sarcopenia, maintaining liver functional reserve and early detection and curative therapy for HCC are effective ways to improve the prognosis of chronic liver disease patients, especially those with HCC.
Acknowledgments
No funding sources support the preparation of this manuscript.
Author Contributions
Kenji Imai designed the study, analyzed the data, and wrote the manuscript. Koji Takai supervised the treatment for the participants. Satoshi Watanabe, Tatsunori Hanai, Atsushi Suetsugu, and Makoto Shiraki contributed to select the participants and collect the data. Masahito Shimizu mainly reviewed and amended the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: The cancer of the liver italian program (clip) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M., Chung H., Osaki Y. Prognostic staging system for hepatocellular carcinoma (clip score): Its value and limitations, and a proposal for a new staging system, the japan integrated staging score (jis score) J. Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 3.Poon R.T. Prevention of recurrence after resection of hepatocellular carcinoma: A daunting challenge. Hepatology. 2011;54:757–759. doi: 10.1002/hep.24569. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: The bclc staging classification. Semin. Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997;127:990S–991S. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara N., Nakagawa H., Kudo Y., Tateishi R., Taguri M., Watadani T., Nakagomi R., Kondo M., Nakatsuka T., Minami T., et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., Baracos V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 8.Sabel M.S., Lee J., Cai S., Englesbe M.J., Holcombe S., Wang S. Sarcopenia as a prognostic factor among patients with stage iii melanoma. Ann. Surg. Oncol. 2011;18:3579–3585. doi: 10.1245/s10434-011-1976-9. [DOI] [PubMed] [Google Scholar]
- 9.Tan B.H., Birdsell L.A., Martin L., Baracos V.E., Fearon K.C. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:6973–6979. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 10.Van Vledder M.G., Levolger S., Ayez N., Verhoef C., Tran T.C., Ijzermans J.N. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br. J. Surg. 2012;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 11.Iritani S., Imai K., Takai K., Hanai T., Ideta T., Miyazaki T., Suetsugu A., Shiraki M., Shimizu M., Moriwaki H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 2015;50:323–332. doi: 10.1007/s00535-014-0964-9. [DOI] [PubMed] [Google Scholar]
- 12.Imai K., Takai K., Hanai T., Ideta T., Miyazaki T., Kochi T., Suetsugu A., Shiraki M., Shimizu M. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int. J. Mol. Sci. 2015;16:9612–9624. doi: 10.3390/ijms16059612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanai T., Shiraki M., Nishimura K., Ohnishi S., Imai K., Suetsugu A., Takai K., Shimizu M., Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Hanai T., Shiraki M., Ohnishi S., Miyazaki T., Ideta T., Kochi T., Imai K., Suetsugu A., Takai K., Moriwaki H., et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 2016;46:743–751. doi: 10.1111/hepr.12616. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa H., Shiraki M., Hiramatsu A., Moriya K., Hino K., Nishiguchi S. Japan society of hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016;46:951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 17.Clinical practice guidelines for hepatocellular carcinoma—The Japan society of hepatology 2009 update. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2010;40:2–144. doi: 10.1111/j.1872-034X.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 18.Mitsiopoulos N., Baumgartner R.N., Heymsfield S.B., Lyons W., Gallagher D., Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 19.Austin P.C. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: A systematic review and suggestions for improvement. J. Thorac. Cardiovasc. Surg. 2007;134:1128–1135. doi: 10.1016/j.jtcvs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Pociot F., Karlsen A.E., Pedersen C.B., Aalund M., Nerup J., European Consortium for I.G.S. Novel analytical methods applied to type 1 diabetes genome-scan data. Am. J. Hum. Genet. 2004;74:647–660. doi: 10.1086/383095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee M., Muenz D.G., Chang J.T., Papaleontiou M., Haymart M.R. Tree-based model for thyroid cancer prognostication. J. Clin. Endocrinol. Metab. 2014;99:3737–3745. doi: 10.1210/jc.2014-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mir O., Coriat R., Blanchet B., Durand J.P., Boudou-Rouquette P., Michels J., Ropert S., Vidal M., Pol S., Chaussade S., et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE. 2012;7:e37563. doi: 10.1371/journal.pone.0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prado C.M., Antoun S., Sawyer M.B., Baracos V.E. Two faces of drug therapy in cancer: Drug-related lean tissue loss and its adverse consequences to survival and toxicity. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:250–254. doi: 10.1097/MCO.0b013e3283455d45. [DOI] [PubMed] [Google Scholar]
- 24.Jones J.C., Coombes J.S., Macdonald G.A. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transplant. 2012;18:146–151. doi: 10.1002/lt.22472. [DOI] [PubMed] [Google Scholar]
- 25.Plauth M., Cabre E., Riggio O., Assis-Camilo M., Pirlich M., Kondrup J., Ferenci P., Holm E., Vom Dahl S., Muller M.J., et al. Espen guidelines on enteral nutrition: Liver disease. Clin. Nutr. 2006;25:285–294. doi: 10.1016/j.clnu.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Marchesini G., Bianchi G., Merli M., Amodio P., Panella C., Loguercio C., Rossi Fanelli F., Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/S0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 27.Moriwaki H., Shiraki M., Fukushima H., Shimizu M., Iwasa J., Naiki T., Nagaki M. Long-term outcome of branched-chain amino acid treatment in patients with liver cirrhosis. Hepatol. Res. 2008;38:S102–S106. doi: 10.1111/j.1872-034X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 28.Muto Y., Sato S., Watanabe A., Moriwaki H., Suzuki K., Kato A., Kato M., Nakamura T., Higuchi K., Nishiguchi S., et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin. Gastroenterol. Hepatol. 2005;3:705–713. doi: 10.1016/S1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 29.Vincent H.K., Raiser S.N., Vincent K.R. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res. Rev. 2012;11:361–373. doi: 10.1016/j.arr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossmann M., Hoermann R., Gani L., Chan I., Cheung A., Gow P.J., Li A., Zajac J.D., Angus P. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin. Endocrinol. 2012;77:323–328. doi: 10.1111/j.1365-2265.2012.04347.x. [DOI] [PubMed] [Google Scholar]
- 31.Riggio O., Angeloni S., Ciuffa L., Nicolini G., Attili A.F., Albanese C., Merli M. Malnutrition is not related to alterations in energy balance in patients with stable liver cirrhosis. Clin. Nutr. 2003;22:553–559. doi: 10.1016/S0261-5614(03)00058-X. [DOI] [PubMed] [Google Scholar]
- 32.Montano-Loza A.J., Meza-Junco J., Prado C.M., Lieffers J.R., Baracos V.E., Bain V.G., Sawyer M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012;10:166–173, 173.e1. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]