Abstract
A systematic review was conducted to evaluate the status and intake of iron, vitamin A, iodine, folate and zinc in women of reproductive age (WRA) (≥15–49 years) and pregnant women (PW) in Ethiopia, Kenya, Nigeria and South Africa. National and subnational data published between 2005 and 2015 were searched via Medline, Scopus and national public health websites. Per micronutrient, relevant data were pooled into an average prevalence of deficiency, weighted by sample size (WAVG). Inadequate intakes were estimated from mean (SD) intakes. This review included 65 surveys and studies from Ethiopia (21), Kenya (11), Nigeria (21) and South Africa (12). In WRA, WAVG prevalence of anaemia ranged from 18–51%, iron deficiency 9–18%, and iron deficiency anaemia at 10%. In PW, the prevalence was higher, and ranged from 32–62%, 19–61%, and 9–47%, respectively. In WRA, prevalence of vitamin A, iodine, zinc and folate deficiencies ranged from 4–22%, 22–55%, 34% and 46%, while in PW these ranged from 21–48%, 87%, 46–76% and 3–12% respectively. Inadequate intakes of these micronutrients are high and corresponded with the prevalence figures. Our findings indicate that nationally representative data are needed to guide the development of nutrition interventions and public health programs, such as dietary diversification, micronutrient fortification and supplementation.
Keywords: iron, anaemia, vitamin A, folate, zinc, iodine, deficiency, intake, women, Africa
1. Introduction
Micronutrient deficiencies in women of reproductive age (WRA) are known to impair health, pregnancy outcomes and growth as well as the development of their offspring [1,2]. Women are vulnerable to micronutrient deficiencies due to inadequate dietary intake, lack of availability of food, inequitable distribution of food within the same household, lack of knowledge about the importance of dietary diversity and frequent occurrence of infectious diseases [3]. In several developing countries, societal norms and gender-based discrimination require women to put their family members before their own health and nutritional needs, and consequently increase the risk of micronutrient deficiency even further in this population group [3,4]. Thus, WRA in low- and middle-income countries often enter pregnancy malnourished, and the additional demands of pregnancy may further exacerbate micronutrient deficiencies in these pregnant women (PW) [1,2].
The most common micronutrient deficiencies in women are iron, vitamin A, iodine, folate and zinc [5]. It is well known that iron deficiency has adverse effects on productivity and cognition in the general population and is the leading cause of anaemia during pregnancy, contributing to 20% of all maternal and perinatal mortality and low birth weight [6,7]. Vitamin A deficiency (VAD) can cause impaired vision (e.g., night blindness) and immune function [8], and may result in preterm birth and infant mortality [9]. Iodine deficiency impairs mental functioning, with an estimated intellectual loss of 7.4 to 15 Intelligence Quotient (IQ) points in infants born to mothers with a poor iodine status [10,11]. Folate deficiency at the time of conception can cause neural tube defects in infants [12]. Zinc deficiency has been suggested as a risk factor with adverse long-term effects on growth, immunity, and metabolic status of surviving offspring [13].
Globally anaemia and VAD prevalence in women are decreasing; however, regions of Africa (and Southeast Asia) still have alarming rates of anaemia in WRA, including PW, and the prevalence of VAD is also reported to be high in PW [14,15]. Precise data on iodine, folate and zinc status in African women are lacking; nevertheless, the prevalence of corresponding micronutrient deficiencies based on inadequate intakes is estimated to be high [16]. The high nutritional burden in women has been recognized by the UN Sustainable Development Goals with a target to address the nutritional needs of adolescent girls, as well as pregnant and lactating women, by 2030 [17]. To develop public health strategies and monitor programmes to reach these goals, data on micronutrient status and intake in WRA and PW are essential. For African countries, these data are largely absent and mostly outdated. Therefore, this study aims to perform a systematic review to evaluate recent data on micronutrient status and dietary intake of iron, vitamin A, iodine, folate and zinc in WRA (≥15–49 years) and PW in four of the seven largest and rapidly growing countries in Africa [18,19], including Ethiopia, Kenya, Nigeria and South Africa.
2. Methods
2.1. Search Strategy
A systematic approach was followed to select all studies with data on iron, vitamin A, iodine, folate and zinc status and intake in Ethiopia, Kenya, Nigeria and South Africa. A literature search was conducted on Medline, Scopus, World Health Organisation (WHO) and The United Nations Children’s Fund (Vitamin and Mineral Nutrition Information System) databases. A combination of the following terms was used to search abstracts and titles:
(Anaemia OR iron OR vitamin A OR iodine OR folate OR zinc) AND (deficiency OR intake OR status OR prevalence) AND (Ethiopia OR Kenya OR Nigeria OR South Africa) AND (women OR adult OR adolescent OR pregnant).
Full-text articles were obtained and reviewed to identify those that met the selection criteria below. The reference lists of all articles of interest were checked for additional studies. Websites of public health organizations were searched and local experts were contacted to get access to additional studies and surveys.
2.2. Inclusion Criteria
After the initial search, data from different study types including national surveys, population-based observational (cross-sectional or longitudinal) studies, or baseline or control group data from intervention studies were screened to determine eligibility based on the following inclusion criteria:
- National and subnational surveys or studies reporting data on either anaemia or iron or vitamin A or iodine or folate or zinc status in apparently healthy PW and/or WRA (aged ≥15–49 years) in each country as assessed by the following biomarkers:
- Iron:
- Anaemia: For WRA Hb < 120 g/L; for PW Hb < 110 g/L. Roughly 50% of anaemia cases are caused by iron deficiency (18), therefore, anaemia prevalence was also included;
- Iron Deficiency (ID): Serum ferritin < 15 μg/L, regardless of correction for inflammation;
- Iron Deficiency Anaemia (IDA): Combination of anaemia and ID [20]
- VAD: serum retinol < 0.7 nmol/L or (20 μg/dL) [21] for WRA and PW;
- Folate deficiency: Serum folate < 3 ng/mL (<6.8 nmol/L) severe deficiency and 3–5.9 ng/mL (6.8–13.4 nmol/L) possible deficiency [22];
- Zinc deficiency: Serum zinc < 10.7 umol/L (70 μg/dL) for WRA and 8.6 umol/L (56 μg/dL) for PW [23];
Dietary intake data of iron, vitamin A, zinc, or folate measured at individual level and for iodine, consumption of iodized salt at household level in each country.
Studies and surveys conducted and published 2005–2015.
2.3. Data Extraction
2.3.1. Status Data
For micronutrient deficiencies, we extracted the reported prevalence of anaemia, ID, IDA, VAD, iodine, folate and zinc deficiency when meeting the inclusion criteria. For the biochemical markers of micronutrient status, we extracted the means and when reported, standard deviations (SD) from each data source and separately reported clinical and subclinical deficiencies per micronutrient. For example, the prevalence of clinical signs of VAD (i.e., night blindness, Bitot’s spots, corneal xerosis, xeropthalmia) [21] and iodine deficiency (i.e., goitre) [25] were reported separately from their subclinical signs (e.g., serum retinol and UIE).
2.3.2. Intake Data
Information on daily dietary intake of iron, vitamin A, folate and zinc in WRA and PW was included as reported. For iodine, little data were available and, therefore, data on household consumption of iodized salt was used. When data was reported as median (and ranges), it was converted to mean by taking an average of median and the interquartile ranges (IQR). For converting the ranges to standard deviation (SD), the difference between high and low IQR was divided by 1.35. When variation was expressed in standard errors of mean (SEM) or confidence intervals, these were converted to SDs.
2.3.3. Data Analysis
When, within a country, more than one study and/or survey was included in the status data of the same micronutrient, the results of these were pooled for each micronutrient and biomarker separately into a weighted mean, which was the weighted average (WAVG) of the sample size of these studies and/or surveys. Therefore, the WAVG is based on all available subnational data and national data. Per country and per micronutrient, the WAVG for PW and WRA were calculated separately. All data were handled and analysed using an Excel spreadsheet (2016).
2.3.4. Calculating Inadequacy of Micronutrient Intakes
The Estimated Average Requirement (EAR) cut-point method provides a way to estimate the prevalence of inadequate nutrient intake in a population. The proportion of subjects with intake below the EAR was used to estimate the prevalence of inadequate intake of micronutrients in the population. As per Institute of Medicine (IOM) guidelines [26], a conversion factor of 1.6 was used for iron, 1.5 for vitamin A, 1.4 for folate and 1.2 for zinc for calculating EAR from Recommended Daily Allowance (RDA) set by WHO/FAO (Food and Agriculture Organization) [27]. For iron intake in pregnancy, the EAR for pregnancy set by IOM was used [26]. When values for vitamin A intake were reported as beta-carotene, they were converted to retinol activity equivalents (RAE) by dividing with 12 [26]. The bioavailability of 12% was used for dietary iron and the “lowest bioavailability” was used for dietary zinc. For each survey/study, the prevalence of inadequate intake for iron, vitamin A, folate and zinc were estimated by comparing the reported mean and SD to the corresponding EAR, assuming a normal distribution of the data [28]. For iodine, percentage of households consuming inadequately iodized salt (<15 ppm) or non-iodized was included as reported.
3. Results
3.1. Data Availability
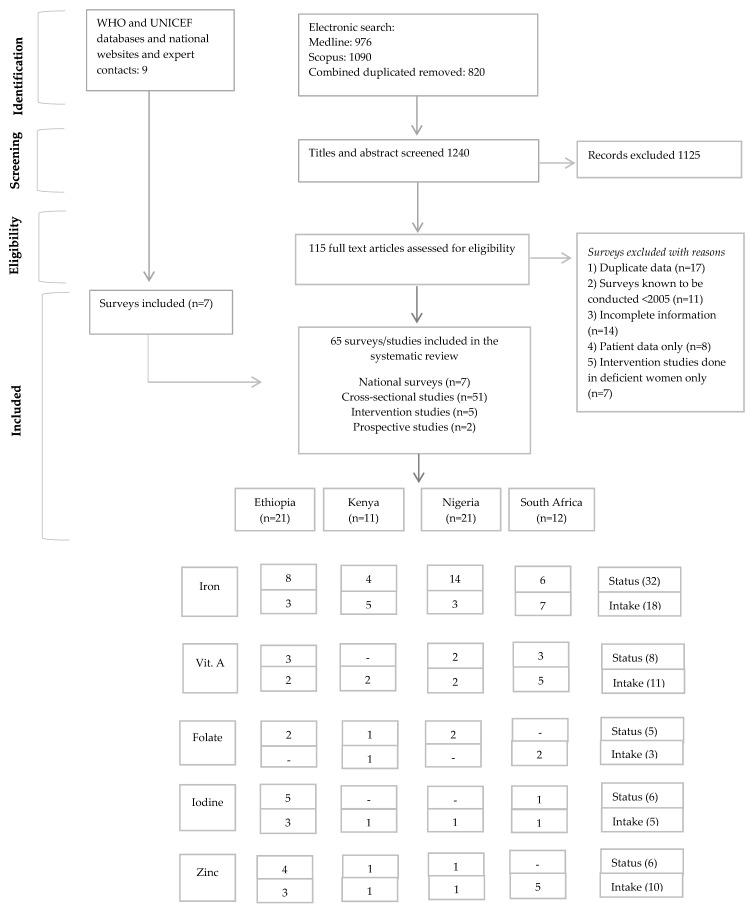
A total of 115 studies and nine surveys were identified from the literature search, whereas only 65 datasets met the inclusion criteria (Figure 1). Out of these, 21 were included for Ethiopia, 11 for Kenya, 21 for Nigeria and 12 for South Africa. The data from Ethiopia are predominantly from rural areas, whereas the data from Kenya, Nigeria and South Africa represent an equal number of rural and urban studies (Table 1).
Figure 1.
PRISMA flow diagram [36] of the identification of literature for inclusion in this systematic review.
Table 1.
Characteristics of the studies and surveys included from Ethiopia, Kenya, Nigeria and South Africa.
| Reference | Year of Survey | Study Location | State/Province | Age | n | Study Design | Data Included | |
|---|---|---|---|---|---|---|---|---|
| Ethiopia | ||||||||
| National data | ||||||||
| Ethiopia Public Health Institute, 2013 [29] | 2011 | Nationwide | National | 15–49 years | 7908 | National Food consumption survey | Iron, vitamin A, zinc intake | |
| Ethiopian Public Health Institute, 2015 [30] | 2015 | Nationwide | National | - | 1741 | National (micronutrient) survey | Iron, vitamin A, zinc status | |
| DHS, 2011 [31] | 2011 | Nationwide | National | 15–49 years | 15782 (WRA) + 1173 (PW) | Demographic and Health Survey (DHS) | Iron status | |
| Subnational data | ||||||||
| Abuye, 2008 [37] | 2008 * | 5 states, cluster sampling method was applied to select the study population | Amhara, Tigray, Oromiya, SNNP & Benishangul-Gumuz | - | 6960 | Cross-sectional study | Iodine status | |
| Amare 2012 [38] | 2005 | Gondar city (urban setting; two-stage probability sampling method was used for selecting the study population) | Amhara | >18 years | 356 | Cross-sectional study | Iron and vitamin A intake | |
| Abriha, 2014 [39] | 2014 | Mekelle town (urban setting) | Amhara | 16–40 years pregnant (all trimesters) | 619 | Cross-sectional study | Iron status | |
| Bogale, 2009 [40] | 2007 | Sidama zone (rural area) | Sidama | 28 years | 99 | Cross-sectional study | Iodine status and household consumption of iodized salt | |
| Ersino, 2013 [41] | 2009 | Tulu Health Center (rural area) | Sidama | 27.7 ± 5.6 years pregnant † | 172 | Cross-sectional study | Iodine status and household consumption of iodized salt | |
| Gebremedhin, 2011 [42] | 2011 | Sidama zone (rural area with primarily subsistent farming) | Sidama | 15–≥35 years pregnant (all trimesters) | 700 | Cross-sectional study | Zinc status | |
| Gebremedhin, 2014 [43] | 2011 | Sidama (rural area with primarily subsistent farming) | Sidama | 15–49 years pregnant (all trimesters) | 750 | Cross-sectional study | Iron status | |
| Gebreselassie, 2013 [44] | 2011 | Kebeles of Sidama zone (rural area) | Sidama | ~29 years pregnant (all trimesters) | 700 | Cross-sectional study | Vitamin A status | |
| Gebreegziabher, 2013 [45] | 2009 | Rural communities of Sidama zone, | Sidama | 30.8 ± 7.8 years | 202 | Cross-sectional study | Iodine status | |
| Gibson, 2008 [46] | 2008 * | Sidama (rural area with primarily subsistent farming) | Sidama | 28 years pregnant † | 99 | Cross-sectional study | Iron, vitamin A, folate status iron and zinc intake | |
| Haidar, 2009 [47] | 2005 | Tigray; Affar; Amhara; Oromiya; Benishangul-Gumuz; Southern Nations, SNNP; Harari regions; Addis Ababa and Dire Dawa city administrations (80% of the population was from rural setting) | 9 regions | 15–49 years | 970 | Cross-sectional study | Iron status | |
| Haider, 2010 [48] | 2005 | Tigray; Affar; Amhara; Oromiya; Benishangul-Gumuz; Southern Nations, SNNP; Harari regions; Addis Ababa and Dire Dawa city administrations (80% of the population was from rural setting) | 9 regions | 15–49 years | 970 | Cross-sectional study | Folate status | |
| Hambidge, 2006 [49] | 2006 * | Alamura (rural area with resource poor setting) | Rift Valley | 27.8 ± 4.7 years pregnant (3rd trimester) | 17 | Cross-sectional study | Zinc intake | |
| Joray, 2015 [50] | 2010 | Sidama (rural area) | Sidama | 18–50 years | 35 | Baseline data of an intervention trial | Zinc status | |
| Kedir, 2013 [51] | 2010 | Haramaya district (primarily rural area) | Oromia | 25 ± 5 years | 1678 | Cross-sectional study | Iron status | |
| Kedir, 2014 [52] | 2012 | Haramaya district (10 administrative rural unit rural) | Oromia | 27 ± 5.9 years pregnant (all trimesters) | 435 | Cross-sectional study | Iodine status | |
| Mulu, 2011 [53] | 2005 | University of Gondar Hospital | Northwest | 16–45 years | 25 | Cross-sectional study | Vitamin A status | |
| Stoecker, 2009 [54] | 2009 * | Sidama region (rural area with primarily subsistent farming) | Sidama | 27.7 ± 4.7 years pregnant (2nd & 3rd trimester) | 99 | Cross-sectional study | Iron status | |
| Kenya | ||||||||
| National data | ||||||||
| DHS, 2015 [32] | 2014 | Nationwide | National | 15–49 years | 34139 (HH) | Demographic and Health Survey (DHS) | Household consumption of iodized salt | |
| Subnational data | ||||||||
| Adongo, 2013 [55] | 2013 * | Kalacha Location, Marsabit County (pastoral area with livestock) | Eastern | 15–49 years | 224 | Cross-sectional survey | Iron status and intake | |
| Gitau, 2008 [56] | 2008 * | Makongeni, Thika District | Central | - | 100 | Cross-sectional study | Iron status | |
| Kamau-Mbuthia, 2007 [57] | 2004–2005 ** | Provincial General Hospital, Nakuru, (urban area) | Rift valley | ~25 years pregnant † | 716 | Cross-sectional study | Iron and zinc intake | |
| Mwangi, 2014 [58] | 2011–2012 | Nyanza Province (rural area) | Nyanza | 15–45 years pregnant (2nd trimester) | 470 | Baseline of intervention trial | Iron status | |
| Mitheko, 2015 [59] | 2015 * | Naivasha | Nakuru | pregnant † | 172 | Cross-sectional study | Zinc status | |
| Ouma, 2006 [60] | 2003–2005 ** | 3 hospitals in Nyanza province (primarily rural area) | Nyanza | <20 years pregnant (2nd & 3rd trimester) | 488 | Baseline of intervention trial | Iron status | |
| Othoo, 2014 [61] | 2014 * | Ndhiwa Maternal and Child Health clinic | Nyanza | pregnant † | 162 | Cross-sectional study | Iron and vitamin A intake | |
| Shipala, 2012 [62] | 2008 | 2 health facilities in Bungoma | Western | <19 years pregnant † | 384 | Cross-sectional study | Iron and folate intake | |
| Van Eijk, 2008 [63] | 2003–2005 ** | 3 hospital in Nyanza province | Nyanza | <20 years pregnant † | 458 | Baseline of intervention trial | Folate status | |
| Waswa, 2011 [64] | 2011 * | Moi University, Eldoret Town (urban area) | Nyanza | 20–25 years | 260 | Cross-sectional survey | Iron and vitamin A status | |
| Nigeria | ||||||||
| National data | ||||||||
| DHS, 2008 [33] | 2008 | Nationwide | National | 15–49 years | 32079 (HH) | Demographic and Health Survey | Household consumption of iodized salt | |
| Subnational data | ||||||||
| De Moura, 2015 [65] | 2011 | 31 local government area in Akwa-Ibom (primarily rural area) | Akwa-Ibom | 18–49 years | 622 | Cross-sectional survey | Iron, vitamin A and zinc intake | |
| Dim, 2007 [66] | 2005 | University of Nigeria teaching hospital, Enugu city (urban) | Enugu | 21 ± 7 years pregnant (all trimesters) | 530 | Cross-sectional study | Iron status | |
| Dim, 2014 [67] | 2012 | University of Nigeria teaching hospital, Enugu city (urban) | Enugu | 16–45 years pregnant (all trimesters) | 200 | Cross-sectional study | Iron status | |
| Ezugwu, 2013 [68] | 2009–2010 | University of science and technology teaching hospital, Enugu city (urban) | Enugu | 25 ± 5 years pregnant (all trimesters) | 1306 | Cross-sectional study | Iron status | |
| Idowu, 2005 [69] | 2005 * | Ogun | Abeokuta | >15 years pregnant (all trimesters) | 477 | Cross-sectional study | Iron status | |
| Kagu, 2007 [70] | 2005–2006 | Federal Medical Center, Nguru (rural area with subsistence farming and fishing) | Yobe | 13–48 years pregnant (all trimesters) | 1040 | Prospective study | Iron status | |
| Miri-Dashe, 2014 [71] | 2014 * | Plateau State Specialist Hospital, Jos (urban area) | Plateau | 18–65 years pregnant (all trimesters) | 125 (WRA) + 134 (PW) | Cross-sectional study | Iron status | |
| Nwizu, 2011 [72] | 2011 * | Aminu Kano Teaching hospital, Kano (urban area) | Kano | >15 years pregnant (all trimesters) | 300 | Cross-sectional study | Iron status | |
| Okwu & Ukoha 2008 [73] | 2008* | Owerri (majority urban and some rural areas were included) | Imo | >18 years pregnant † | 1387 | Cross-sectional study | Iron status | |
| Olatunbosun, 2014 [74] | 2012 | University of Uyo Teaching Hospital, Uyo City (urban) | Akwa Ibom | 17–45 years pregnant (2nd & 3rd trimester) | 400 | Cross-sectional study | Iron status | |
| Otemuyiwa, 2012 [75] | 2012 * | Adekunle Ajasin University, Akungba-Akoko & Obafemi Awolowo University (rural), Ile-Ife (urban) | Osun | 15–35 years | 203 | Cross-sectional study | Iron intake | |
| Obasi, 2013 [76] | 2013 * | Federal teaching hospital Abakaliki & st Vincent Hospital Ndubia (rural) | Ebonyi | 15–40 years pregnant (2nd trimester) | 295 | Cross-sectional study | Iron status | |
| Ofojekwu, 2013 [77] | 2013 * | Federal School of Medical Laboratory Science and the School of Nursing and Midwifery in Jos City (urban) | Plateau | 19–30 years | 46 | Cross-sectional study | Iron status | |
| Olubukola, 2011 [78] | 2008 | University college hospital & Adeoyo maternity hospital in Ibadan (urban) | Oyo | 27 years pregnant (all trimesters) | 2702 | Cross-sectional Study | Iron status | |
| Shu & Ogbodo 2005 [79] | 2005 * | University of Nigeria teaching hospital, Enugu City (urban) | Enugu | 18–42 years pregnant (all trimesters) | 74 | Cross-sectional study | Iron status | |
| Ugwuja, 2009 [80] | 2007–2008 | Federal medical Centre, Abakaliki (rural) | Ebonyi | 15–40 years pregnant (2nd trimester) | 349 | Cross-sectional study | Iron status | |
| Ugwuja, 2010 [81] | 2007–2008 | Fedearl medical centre, Abakaliki (rural, with mixed population) | Ebonyi | 15–40 years pregnant (2nd trimester) | 349 | Cross-sectional study | Zinc status | |
| VanderJagt, 2011 [82] | 2011 * | Jos teaching university hospital, Jos City (urban) | Plateau | 28 ± 6.1 years pregnant (all trimesters) | 143 | Cross-sectional study | Folate status | |
| VanderJagt, 2009 [83] | 2009 * | Jos teaching university hospital, Jos city (urban) | Plateau | 27.4 ± 5.4 years pregnant (3rd trimester) | 98 | Cross-sectional study | Folate status | |
| Williams, 2008 [84] | 2006 | University of Calabar Teaching Hospital, Calabar (urban) | Cross River State | pregnant (all trimesters) | 101 | Cross-sectional study | Vitamin A intake and status | |
| South Africa | ||||||||
| National data | ||||||||
| NFCS, 2007 [34] | 2005 | Nationwide | National | 16–35 years | ~2400 | National survey | Iron, vitamin A and iodine status and household consumption of iodized salts | |
| Shisana, 2014 [35] | 2012 | Nationwide | National | 16–35 years | ~1300 | National health and nutrition survey | Iron and vitamin A status | |
| Subnational data | ||||||||
| Dolman, 2013 [85] | 2005/12 | North West (rural and urban) | North West | ~47 years | 1068 | Prospective Urban and Rural Epidemiological (PURE) study | Iron, vitamin A, folate and zinc intake | |
| Faber, 2005 [86] | 2005* | Ndunakazi (rural) | KwaZulu-Natal | 29.7 ± 7.6 years | 118 | Cross-sectional survey | Iron and vitamin A status | |
| Gichohi-Wainaina, 2015 [87] | 2005 | North West (rural and urban) | North West | 32–86 years | 678 | PURE cohort study (baseline data) | Iron status | |
| Hattingh, 2008 [88] | 2008 * | Mangaung (urban) | Bloemfontein | 25–34 years | 279 | Cross-sectional survey | Iron, vitamin A and zinc intake | |
| Kolahdooz, 2013 [89] | 2013 * | Empangeni (rural) | KwaZulu-Natal | 19–50 years | 40 | Cross-sectional study | Iron, vitamin A and zinc intake | |
| Lawrie, 2008 [90] | 2008 * | Workers at Helen Joseph and Coronation hospital, Gauteng (urban) | Gauteng | >18 years | 631 | Cross-sectional study | Iron status | |
| Mostert, 2005 [91] | 2005 * | Dikgale primary health care clinic, Limpopo (rural, resource poor setting) | Limpopo | 13–40 years pregnant † | 46 | Cross-sectional study | Iron, vitamin A and zinc intake | |
| Oldewage-Theron, 2011 [92] | 2011 * | Informal settlement in Vaal region (a peri-urban area, resource poor setting) | Gauteng | - | 426 | Cross-sectional study | Iron, vitamin A, folate and zinc intake | |
| Oldewage-Theron, 2014 [93] | 2008–2009 | Qwa-Qwa (rural households) | Free State | 39.8 ± 13.5 years | 83 | Single system case study (baseline data) | Iron Status and intake | |
| Pisa, 2012 [94] | 2005 | North West (rural and urban) | North West | >35 years | 1264 | Prospective Urban and Rural (PURE) Epidemiological study (baseline data) | Iron intake | |
* Year of survey not available, hence year of publication was assumed to be the year of survey; CG-control group; HH-households; WRA-women of reproductive age; PW-pregnant women; ** data collection was done in 2002–2005, but were included due to scarce data in Kenya; † trimester not mentioned in the study; for pregnant women, unless data was reported for only one trimester, an average prevalence/intake of the two or three trimesters was included.
Of the 65 data sources, seven were national and 58 were subnational data (51 cross-sectional studies, five intervention studies and two prospective cohorts). For national data, three surveys were from Ethiopia [29,30,31], one from Kenya (iodine data only) [32], and Nigeria (iodine data only) [33] each, and two were from South Africa [34,35]. The majority of the data was on iron status and intake and fewer data was found on vitamin A, iodine, folate and zinc. For 13 studies the sample size was <100; for the remaining studies and surveys it ranged from 100 to 32,079.
3.2. Prevalence of Micronutrient Deficiencies in WRA
3.2.1. Status
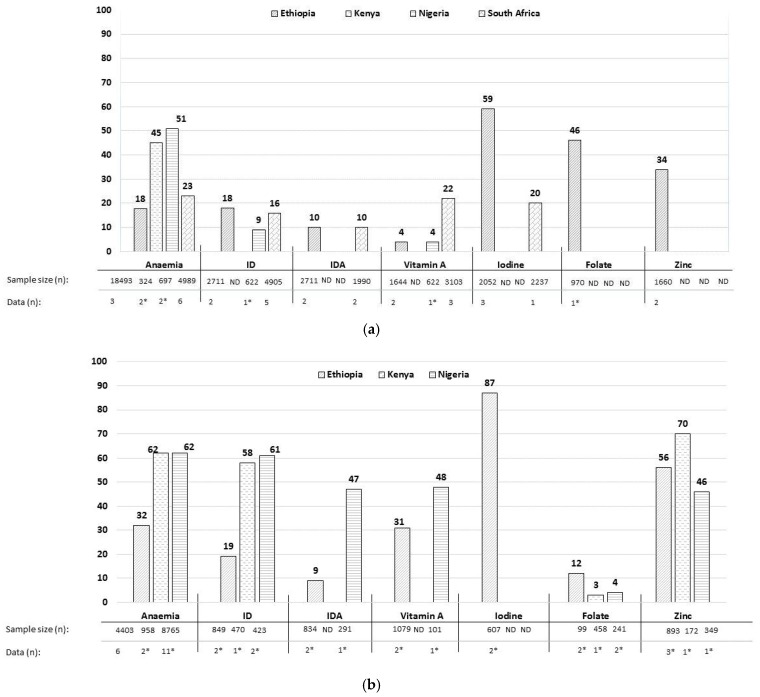
The most complete data in WRA were found for Ethiopia followed by South Africa, Nigeria and Kenya. Anaemia rates ranged from 18 to 51% (WAVG) in the four countries (Figure 2a). The prevalence of ID ranged from 9% to 18% in Ethiopia, Nigeria and South Africa, and that of IDA was 10% in Ethiopia and South Africa each (Figure 2a). The prevalence of VAD ranged from 4% to 22% (WAVG) in Ethiopia, Nigeria and South Africa (Figure 2a), with no status data for Kenya. Iodine deficiency rates ranged from 59% in Ethiopia to 20% in South Africa (Figure 2a), and no iodine status data were found for Kenya and Nigeria. The prevalence of folate and zinc deficiency was only reported in Ethiopia and was 46% and 34%, respectively (Figure 2a).
Figure 2.
(a) Prevalence of micronutrient deficiencies (%) in women of reproductive age in Ethiopia, Kenya, Nigeria and South Africa. The prevalence in percentage (%) are reported as weighted average (WAVG), i.e., an average prevalence that was weighted for the sample size of the studies (subnational data) and national surveys (national data); data (n) show number of studies and surveys included; * only subnational data; ID: iron deficiency; IDA: iron deficiency anaemia; ND: no data. (b) Prevalence of micronutrient deficiencies (%), in pregnant women in Ethiopia, Kenya and Nigeria. The prevalence in percentage (%) are reported as weighted average (WAVG), i.e., an average prevalence that was weighted for the sample size of the studies (subnational data) and national surveys (national data); data (n) show number of studies and surveys included; * only subnational data; ID: iron deficiency; IDA: iron deficiency anaemia; ND: no data.
3.2.2. Intake Data
Mean dietary iron intake ranged from 3.8 to 97.8 mg/d (Table 2) and 34–100% of the WRA in Kenya, Nigeria and South Africa had inadequate intakes. Iron intake in Ethiopia was reported to be high (47–97.8 mg/d) and only 8–12% of the WRA had inadequate intake. Mean dietary vitamin A intake ranged from 71 to 2477 μg/d, and 3–100% had inadequate intake (Table 2). Mean dietary folate intake was reported only for South Africa and ranged from 82 to 334 μg/d and 47–98% had inadequate intake (Table 2). Mean dietary zinc intake ranged from 3.8–16.2 mg/d and 23–96% had inadequate intake (Table 2) in Ethiopia, Nigeria and South Africa. The percentage of households not consuming adequately iodized salt (>15 ppm) ranged from 23% to 98% in Ethiopia, Nigeria and South Africa (Table 2). Information on intake of adequately iodized salt was not available for Kenya and reported the intake of non-iodized salt (1% of the households) only.
Table 2.
Intake of micronutrients and iodized salt intake in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa.
| Country | Reference | Age Group | n | Dietary Information | Iron | Vitamin A | Folate | Zinc | Iodine | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/d | Inadequate Intake (%) | μg RE/d | Inadequate Intake (%) | μg/d | Inadequate Intake (%) | mg/d | Inadequate Intake (%) | Households not Consuming Adequately Iodized Salt (%) | |||||
| Women of reproductive age | |||||||||||||
| Ethiopia | Amare, 2012 [38] | >18 years | 255 | 24 h dietary recall | 97.8 (54.6) | 8% | 216(25) | 100% | |||||
| Bogale, 2009 [40] | 28 years | 99 | Plasma mass spectrometer for iodine content of salt | 98% | |||||||||
| Ethiopian Public Health Institute, 2015 [30] | households | 3221 | Titration method | 74% | |||||||||
| NFC Survey, 2013 ^ [29] | 15–49 years | 7908 | 24 h dietary recall | 47.2 (33.8) | 12% | 71 (129) † | 99% | 7.6 (5.7) | 53% | ||||
| Kenya | Adongo, 2013 [55] | 15–49 years | 224 | 24 h dietary recall | 11.8 (5.2) | 94% | |||||||
| DHS, 2014 [32] | households | 34139 | Rapid test kit for iodine content of salt | 1% * | |||||||||
| Waswa, 2011 [64] | 20–25 years | 260 | FFQ | 12.6 (6.6) | 86% | 368 (287) | 53% | ||||||
| Nigeria | De Moura, 2015 ^ [65] | >18 years | 579 | 24 h dietary recall | 11.2 (4) | 98% | 2477 (961) | 3% | 11.2 (4) | 23% | |||
| DHS, 2008 [33] | households | 32079 | - | 48% | |||||||||
| Otemuyiwa, 2013 [75] | 15–35 years | 203 | FFQ | 13.5 (4.5) | 92% | ||||||||
| South Africa | Dolman, 2013 | 47 years | 1068 | FFQ | 11.5 (5.2) | 94% | 657 (495) | 28% | 334(162) | 47% | 8.6 (3.8) | 46% | |
| Kolahdooz, 2013 [89] | 19–50 years | 40 | 24 h dietary recall | 24 (10) | 45% | 216 (336) † | 66% | 8.3 (3.6) | 49% | ||||
| Hattingh, 2008 ^ [88] | 25–34 years | 279 | FFQ | 26.7 (15.9) | 34% | 2221(1472) | 11% | 16.2(9.3) | 20% | ||||
| NFCS, 2007 [34] | 16–35 years | 2237 | Iodometric titration method for iodine content of salt | 23% | |||||||||
| Pisa, 2012 [94] | >35 years | 1264 | QFFQ | 12.5 (9.1) | 79% | ||||||||
| Oldewage-Theron, 2014 [93] | 19–75 years | 84 | 24 h recall | 6.4 | |||||||||
| Oldweage-Theron, 2011 [92] | na | 426 | 24 h recall | 3.8 (2) | 100% | 176 (617) | 61% | 82 (103) | 98% | 3.8 (2.4) | 96% | ||
| Pregnant women | |||||||||||||
| Ethiopia | Ersino, 2013 [41] | 27.7 ± 5.6 years | 172 | Plasma mass spectrometer for iodine content of salt | >90% | ||||||||
| Gibson, 2008 [46] | 28 years | 99 | 1 day weigh record | 28.3 | 5.4 | ||||||||
| Hambidge, 2006 [49] | 27.8 ± 4.7 years | 17 | 24 h weigh record | 6 (3.2) | 97% | ||||||||
| Kenya | Kamau-Mbuthia, 2007 [57] | ~26 years | 716 | 24 h dietary recall | 16.1 | 9.4 | |||||||
| Shipala, 2012 [62] | <19 years | 384 | FFQ | 26.8 | 364 | ||||||||
| Othoo, 2014 [61] | 21–25 years | 162 | Semi-structured Questionnaire | 18.5 | 436 | ||||||||
| Nigera | Williams, 2008 [84] | - | 101 | 24 h dietary recall | 2645 (189) | 3% | |||||||
| South Africa | Mostert, 2005 [91] | 13–40 years | 46 | 24 h dietary recall | 9.6 (4.3) | 99% | 574 (428) | 48% | 8.1 (4.3) | 84% | |||
Intake data presented as mean (SD); ^ Data were reported as median and IQR, but converted to mean; * households consuming iodized salts; † vitamin A reported RAE (retinol activity equivalent).FFQ-Food Frequency Questionnaire; QFFQ- Quantitative Food Frequency Questionnaire.
3.3. Prevalence of Micronutrient Deficiencies in PW
3.3.1. Status
The most complete data in PW were found for Ethiopia, followed by Nigeria and Kenya. No status data for PW from South Africa met the inclusion criteria. The prevalence of anaemia was higher in PW than WRA and ranged from 32% to 62%; 19–61% had ID (Figure 2b). IDA rates were 9% in Ethiopia and 47% in Nigeria (Figure 2b). None of the national surveys reported VAD (based on serum retinol) and the prevalence is therefore based on subnational data only. VAD prevalence was higher in PW than WRA and ranged from 31% to 48% in Ethiopia, and Nigeria (Figure 2b). Iodine deficiency was only reported in Ethiopia, at 87% (Figure 2b). Limited data from five subnational studies were found on folate status, with no national surveys reporting it. Folate deficiency was 12% in Ethiopia, 3% in Kenya and 4% in Nigeria (Figure 2b). Zinc deficiency was at 56% for Ethiopia, 70% for Kenya and 46% for Nigeria (Figure 2b).
3.3.2. Intake Data
Mean dietary iron intakes ranged from 9.6 to 28.3 mg/d and up to 99% of the PW had inadequate intakes (Table 2). Mean vitamin A intakes ranged from 436 to 2645 μg/d and 3–48% had inadequate intakes (Table 2) in Kenya, Nigeria and South Africa. Mean dietary folate intake was reported only in Kenya at 364 μg/d (Table 2). Mean dietary zinc intake ranged from 5.4 to 9.4 mg/d and 84 to 99% had inadequate intakes in Ethiopia, Kenya and South Africa (Table 2). More than 90% of households with PW consumed inadequately iodized salt in Ethiopia; no specific data on iodized salt consumption in households with PW were found in the other three countries (Table 2).
3.4. Biomarker Data in WRA and PW
Mean haemoglobin concentration ranged from 92 to 147 g/L and mean serum ferritin concentration from 9 to 85 μg/L (Supplementary Figure S1a,b) in PW and WRA. The prevalence of night blindness was reported in PW at 1.1–1.5% (Supplementary Table S1) and serum retinol ranged from 0.84 to 1.49 μmol/L (Supplementary Figure S2a,b) in WRA and PW. UIE ranged from 17 to 188 μg/L (Supplementary Figure S3) and goitre prevalence was reported in Ethiopia only and ranged from 16% to 85% in PW and WRA (Supplementary Table S2). Mean serum folate ranged from 12.6 to 31.3 ng/mL (Supplementary Figure S4) and mean serum zinc concentration ranged from 6.6 to 12.5 μmol/L in PW and WRA (Supplementary Figure S5).
4. Discussion
4.1. Main Findings and Their Significance
This systematic review, based on national and subnational data published from 2005 to 2015, provides an overview of the prevalence of micronutrient deficiencies in WRA and PW in the four African countries. Anaemia rates ranged from 18% to 51% in WRA in the four countries, ID ranged from 9% to 16% and IDA was at 10%. The prevalence of VAD deficiency in WRA was highest in South Africa at 22%, while that of iodine deficiency was highest in Ethiopia (59%) (Figure 2a). Data on folate (46%) and zinc (34%) deficiencies were only available for Ethiopian WRA (Figure 2a). As expected, the prevalence of anaemia in PW was high, ranging from 32% to 62%, while that of ID and IDA ranged from 19% to 61% and 9–47%, respectively (Figure 2b). Based on limited data, the prevalence of folate deficiency was low in PW (<12%), while zinc deficiency and VAD were high (>30%) (Figure 2b). Iodine deficiency in PW was reported at 87% in Ethiopia only. Notably, no micronutrient status data in PW from South Africa were found. Data on inadequate intake of iron, vitamin A, folate, zinc and household consumption of iodized salt largely corresponded with the prevalence figures for micronutrient deficiencies.
4.2. Strengths and Limitations of This Study
This systematic review is the first to provide an overview of both status and dietary intakes of iron, vitamin A, iodine, folate and zinc in WRA and PW in Ethiopia, Kenya, Nigeria and South Africa. A strength of this review is the use of national and subnational data, which were pooled to determine the prevalence of deficiency in WRA and PW. The restriction of the studies to the years 2005–2015 increases the possibility of data being reflective of the current situation, thereby increasing the validity of this study. This review may help guide public health practitioners and policy makers in advocating for public health strategies to prevent micronutrient deficiencies in WRA and PW, especially where nationally representative data are lacking.
However, there are a few limitations to this systematic review. Most limitations are mentioned in a similar publication on children (0–19 years) from the four countries [95] and will not be discussed again. Some limitations specifically related to this review are noted here. Firstly, prevalence estimates from subnational data can either over- or underestimate the national prevalence of micronutrient deficiencies, as large regional differences in micronutrient status may exist within countries. This is especially the case for data on PW and for countries such as Kenya and Nigeria, where subnational data provided substantial weight to the overall figures on micronutrient deficiencies due to a lack of national data. Nevertheless, this bias has been minimised by removing the data that included deficient populations only (Figure 1). Secondly, for folate, iodine, zinc and vitamin A (PW) very limited data were found, and therefore the WAVG for these micronutrients may not represent the national situation. Lastly, intakes from supplements, such as for iron and folate from common supplementation programs in PW, were not considered by most intake data studies. Therefore, figures for inadequate intakes are crude approximations and should be interpreted as such.
4.3. Findings on Micronutrient Status and Intake in WRA
The prevalence of anaemia, 18–51% in WRA, is a moderate to severe public health problem as per the WHO criteria [20]. Stevens et al. estimated (using data from 1995–2011) that Central and West Africa had the highest anaemia prevalence at about 50% in WRA [15], which is in line with our findings. However, a recent systematic review from 21 African countries (Ethiopia is the only country in common with our review) reported an overall anaemia prevalence of 23% (range 12.5–36%) based on 2003–2010 DHS data in WRA (including PW) [96]. The overall lower anaemia prevalence in this review could be due to the lower anaemia rates in these countries or the type of data (national vs. subnational data from high endemic regions) included. About half of all anaemia is estimated to be attributable to iron deficiency, depending on the geographic and disease environment. Much of the other half is caused by infectious diseases and deficiencies of other micronutrients [20]. Our data on low iron intakes correspond with the figures for anaemia and iron deficiency. However, for Ethiopia iron intake was relatively high at 47.2–97.8 mg/d [29,38], whereas the prevalence of ID in Ethiopia was comparable to other countries (Figure 2a). This high intake is most likely attributed to contaminant iron and consumption of teff [97], which is rich in iron but has poor bioavailability.
The prevalence of VAD is particularly high in South Africa at 22% vs. 4% in Ethiopia and Nigeria. Similar findings of higher VAD prevalence are also reported in 0–19-year-old South African children compared to the other three countries [95], implying that VAD is a problem of public health significance in South Africa. Vitamin A intakes had large variation, from very low intakes of 71 ug/d in Ethiopia to high intakes of 2477 ug/d in Nigeria; these intakes could be influenced by seasonal variation, mandatory vitamin A fortification policies or differences in intake of vitamin-A-rich organ meat [98,99].
Data on the prevalence of iodine deficiency in WRA were only found for Ethiopia (59%) and South Africa (20%) and were partly consistent with households not consuming adequately iodized salt (23–98%) in these countries. Although household use of adequately iodized salt is used as proxy for iodine intake, certain processed foods containing iodized salt could significantly contribute to iodine intake [100]; this may explain some of the discrepancies between iodine status in WRA and household use of adequate iodized salt.
A decrease in folate deficiencies after the introduction of folic acid fortification of staple food has been reported in population surveys by many countries, including South Africa [101]. However, our review indicates that there are limited data available to confirm these reductions. Ethiopia is yet to implement mandatory fortification of staples with micronutrients including folic acid, which could explain the high folate deficiency rate of 46% in WRA in Ethiopia.
Data on the prevalence of zinc deficiency were found for Ethiopia only (34%). Estimates based on the bioavailability of zinc, physiological requirements and predicted zinc absorption suggest that inadequate zinc intakes occur in over 25% of the population in Southeast Asia and Africa [102]. Thus, the prevalence of zinc deficiency could be substantial (>20%) in the included African countries with no data currently available.
4.4. Findings on Micronutrients Status and Intake in PW
For PW, we did not find any status data from South Africa that met the inclusion criteria (as most data were from before 2005), thus the findings are largely applicable to the other three countries. The prevalence of anaemia in PW of 32–61% is a moderate to severe public health problem as per the WHO criteria [20]. These findings are in line with a recent review that estimated anaemia prevalence in PW > 50% in Central and West Africa [15]. Africa also has the highest prevalence of IDA in pregnant women [15]; this resonates with the findings on IDA at 47% in Nigeria. However, it was only 9% in Ethiopia. It should be noted that the prevalence of ID and IDA in the current review may be underestimated because of uncertainty about the correction of ferritin levels for inflammation and/or infection [103]. Because of increased needs during pregnancy, the recommendation for iron intake increases by 50% compared to that in WRA, and therefore pregnant women are more vulnerable to inadequate iron intake, which is also indicated in our study. However, it is unclear from several intake studies whether iron intake from supplements (as are often prescribed for PW) was considered.
Next to young children, PW are at the highest risk for VAD due to their increased demands for vitamin A and the potential health consequences associated with its deficiency during this life stage. The data on VAD rates (31–48%) based on serum retinol in PW in this review are based on three subnational studies and are higher than those reported by the WHO for the African region (14% (9.7–19%; 95%CI)) [104]. However, when comparing clinical data on night blindness as an indicator for VAD (see Table S1), the data show a lower prevalence (1.1 to 1.5%) compared to WHO reports for Africa (9.8%) [104].
Except for Ethiopia, we found no iodine status data in PW. This is of concern as PW are particularly vulnerable to iodine deficiency due to their increased needs. Assessments of median UIE in 6–12-year-old children could be used to estimate the iodine status of the general adult population; these, however, cannot be extrapolated to PW [24]. Therefore, assessments of iodine status in PW are highly recommended.
The prevalence of folate deficiency in Kenya, Nigeria and Ethiopia was at 3%, 4% and 12%, respectively. Nevertheless, the included studies are small and not nationally representative, with the exception of one study from Ethiopia [48]. Dietary data on folate intake is limited; however, the intake of folic acid supplements during pregnancy could explain the lower level of folate deficiency in these countries . Nevertheless, our data on prevalence of folate deficiency are in contrast to the high prevalence of neural tube defects in sub-Saharan Africa, with figures ranging from 0.16 to 7 per 1000 live births [105]. Therefore, more data are needed to conclude whether folate status is sufficient in both PW and WRA in African countries.
Similar to folate, the data on zinc are limited and mostly based on subnational data, with deficiency reported at 46% to 70% in PW in Ethiopia, Kenya and Nigeria. Zinc deficiency is common in developing countries and low maternal circulating zinc concentrations have been associated with pregnancy complications [13]. As diet is the main factor that determines zinc status [106], inadequate intake of zinc due to a primarily plant-based diet is by far the most likely cause of zinc deficiency in these populations. It is widely acknowledged that many PW do not meet the recommended intakes of zinc [107] and our data also show that 84% to 97% of PW have inadequate intake.
4.5. Implications for Policies and Programmes
It is critical that WRA enter pregnancy with the best possible macro- and micronutrient status and receive adequate nutrition during pregnancy for their health and for the wellbeing of their offspring. The adverse consequences of a poor maternal diet are even more severe in adolescent pregnancy; however, data on adolescents are largely lacking. To facilitate the nutritional health and wellbeing of PW and WRA in developing countries, population-level sustainable and globally standardized dietary monitoring is essential. In the absence of national dietary surveys, conducting regular smaller surveys in different regions in the country can also provide important information and insights to policy makers and governments.
As the majority of the population in these countries relies on staple foods, the fortification of staples (e.g., flour, oils, salt) has been implemented by various African countries; nevertheless, progress is not consistent across countries. South Africa and Nigeria have been practising mandatory fortification of flours for more than a decade, Kenya has recently started such an initiative, and Ethiopia is in the planning phase. Moreover, it is important to note that with legislation on mandatory food fortification, the quality of fortified foods is not automatically guaranteed as compliance to legislation is found to be low [108]. In addition to food-based programmes, daily or intermittent iron supplementation, alone or together with other micronutrients, should be strengthened, especially for PW to improve their intake. Currently, coverage and compliance to supplementation programmes throughout pregnancy is far from optimum [32] despite clear recommendations by the WHO and national bodies [109,110]. Moreover, the coverage of supplementation programs has been worse in women of poor socioeconomic status and with unplanned pregnancies (especially in adolescents).
Therefore, the fortification of other commonly consumed foods that are widely available and affordable, such as condiments and cooking aids (including seasonings and bouillon cubes) has the potential to improve the nutritional status of women and their families and has the advantage of reaching women prior to pregnancy [111,112]. Recent studies have shown that bouillon cubes fortified with vitamin A or iodine can make a large contribution to the recommended daily intake of these micronutrients in poor population groups and can help decrease the prevalence of micronutrient deficiencies [100,113]. Next to this, dietary diversification and improved access to foods that have high micronutrient bioavailability, including animal products, are also important strategies.
5. Conclusions
In conclusion, the available data indicate that the prevalence of anaemia and iron deficiencies in PW and WRA in Ethiopia, Kenya, Nigeria and South Africa are a public health concern based on the WHO criteria. Limited data indicate that vitamin A, iodine, zinc and folate nutrition may also be suboptimal in both WRA and PW in these countries. Underlying these deficiencies are inadequate dietary intakes, as indicated by the available studies. Therefore, nationally representative data on micronutrient status and intake of especially vitamin A, iodine, zinc and folate in PW and WRA are urgently needed to further guide the development of public health programmes and monitor the impact of these programmes on a regular basis. These programs should focus on improving micronutrient intake through stimulating dietary diversity and the fortification of commonly consumed and affordable food products. In addition, supplementation programmes need to be scaled up for pregnant women.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/9/10/1096/s1, Figure S1: Mean (SD) haemoglobin (g/L) and serum ferritin (µg/L) concentration in women of reproductive years (a) and pregnant women (b) in four African countries., Figure S2: Mean (SD) Serum retinol (µmol/L) concentration in women of reproductive age group (a) and pregnant women (b) in four African countries., Figure S3: Median Urinary Iodine Excretion (µg/L) in women of reproductive age and pregnant women in Ethiopia and South Africa., Figure S4: Mean (SD) Serum folate (ng/L) concentration in women of reproductive age (WRA) and pregnant women in Ethiopia and Nigeria, Figure S5: Mean (SD) Serum zinc (µmol/L) concentration in women of reproductive age (WRA) and pregnant women in Ethiopia, Kenya and Nigeria., Table S1: Clinical signs of VAD, night blindness in pregnant women., Table S2: Goiter prevalence in women of reproductive age and pregnant women in Ethiopia.
Author Contributions
Rajwinder Harika and Ans Eilander initiated the research design. Rajwinder Harika, Mieke Faber, Folake Samuel, Judith Kimiywe, Afework Mulugeta and Ans Eilander contributed to data collection. Rajwinder Harika and Ans Eilander did the data analysis and interpretation. All authors contributed to the writing of the manuscript.
Conflicts of Interest
Rajwinder Harika and Ans Eilander are employees of Unilever. Unilever sells food products globally, including in African countries.
References
- 1.Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de O.M., Ezzati M., Grantham-McGregor S., Katz J., Martorell R., et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 2.Grieger J.A., Clifton V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients. 2014;7:153–178. doi: 10.3390/nu7010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darnton-Hill I. Global burden and significance of multiple micronutrient deficiencies in pregnancy. Nestle Nutr. Inst. Workshop Ser. 2012;70:49–60. doi: 10.1159/000337421. [DOI] [PubMed] [Google Scholar]
- 4.Darnton-Hill I., Webb P., Harvey P.W., Hunt J.M., Dalmiya N., Chopra M., Ball M.J., Bloem M.W., de Benoist B. Micronutrient deficiencies and gender: Social and economic costs. Am. J. Clin. Nutr. 2005;81:1198s–1205s. doi: 10.1093/ajcn/81.5.1198. [DOI] [PubMed] [Google Scholar]
- 5.Muthayya S., Rah J.H., Sugimoto J.D., Roos F.F., Kraemer K., Black R.E. The global hidden hunger indices and maps: An advocacy tool for action. PLoS ONE. 2013;8:e67860. doi: 10.1371/journal.pone.0067860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozuki N., Lee A.C., Katz J. Moderate to severe, but not mild, maternal anemia is associated with increased risk of small-for-gestational-age outcomes. J. Nutr. 2012;142:358–362. doi: 10.3945/jn.111.149237. [DOI] [PubMed] [Google Scholar]
- 7.Steer P.J. Maternal hemoglobin concentration and birth weight. Am. J. Clin. Nutr. 2000;71(Suppl. 5):1285s–1287s. doi: 10.1093/ajcn/71.5.1285s. [DOI] [PubMed] [Google Scholar]
- 8.Bates C.J. Vitamin A. Lancet. 1995;345:31–35. doi: 10.1016/S0140-6736(95)91157-X. [DOI] [PubMed] [Google Scholar]
- 9.Tielsch J.M., Rahmathullah L., Katz J., Thulasiraj R.D., Coles C., Sheeladevi S., Prakash K. Maternal night blindness during pregnancy is associated with low birthweight, morbidity, and poor growth in South India. J. Nutr. 2008;138:787–792. doi: 10.1093/jn/138.4.787. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann M.B. Iodine deficiency. Endocr. Rev. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann M.B. The Importance of Adequate Iodine during Pregnancy and Infancy. World Rev. Nutr. Diet. 2016;115:118–124. doi: 10.1159/000442078. [DOI] [PubMed] [Google Scholar]
- 12.Hibbard B.M., Hibbard E.D., Jeffcoate T.N. Folic acid and reproduction. Acta Obstet. Gynecol. Scand. 1965;44:375–400. doi: 10.3109/00016346509155874. [DOI] [PubMed] [Google Scholar]
- 13.Brown K.H., Rivera J.A., Bhutta Z., Gibson R.S., King J.C., Lönnerdal B., Ruel M.T., Sandtröm B., Wasantwisut E., Hotz C., et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004;25(Suppl. 2):S99–S203. [PubMed] [Google Scholar]
- 14.Stevens G.A., Bennett J.E., Hennocq Q., Lu Y., De-Regil L.M., Rogers L., Danaei G., Li G.Q., White R.A., Flaxman S.R., et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health. 2015;3:e528–e536. doi: 10.1016/S2214-109X(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 15.Stevens G.A., Bennett J.E., Hennocq Q., Lu Y., De-Regil L.M., Rogers L., Danaei G., Li G., White R.A., Flaxman S.R., et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health. 2013;1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lartey A. Maternal and child nutrition in Sub-Saharan Africa: Challenges and interventions. Proc. Nutr. Soc. 2008;67:105–108. doi: 10.1017/S0029665108006083. [DOI] [PubMed] [Google Scholar]
- 17.International Food Policy Research Institute . Global Nutrition Report 2016: From Promise to Impact: Ending Malnutrition by 2030. International Food Policy Research Institute; Washington, DC, USA: 2016. [Google Scholar]
- 18.United Nations Department of Economic and Social Affairs, List of African Countries by Population. [(accessed on 6 March 2016)];2015 Available online: http://statisticstimes.com/population/african-countries-by-population.php.
- 19.Frayne B., Crush J., McLachlan M. Urbanization, nutrition and development in Southern African cities. Food Secur. 2014;6:101–112. doi: 10.1007/s12571-013-0325-1. [DOI] [Google Scholar]
- 20.World Health Organisation Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. [(accessed on 21 April 2016)];2001 WHO/NHD/01.3. Available online: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf.
- 21.World Health Organisation . Indicators for Assessing Vitamin A Deficiency and Thier Application in Monitoring and Evaluating Intervention Programmes. World Health Organisation; Geneva, Switzerland: 1996. [Google Scholar]
- 22.World Health Organisation . Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. Vitamin and Mineral Nutrition Information System. World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- 23.De Benoist B., Darnton-Hill I., Davidsson L., Fontaine O., Hotz C. Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG Interagency Meeting on Zinc Status Indicators. Food Nutr. Bull. 2007;28(Suppl. 3):S480–S484. doi: 10.1177/15648265070283S306. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organisation (WHO) Vitamin and Mineral Nutrition Information System. World Health Organisation; Geneva, Switzerland: 2013. [(accessed on 6 March 2016)]. Urinary iodine concentrations for determining iodine status deficiency in populations. Available online: http://www.who.int/nutrition/vmnis/indicators/urinaryiodine. [Google Scholar]
- 25.World Health Organisation (WHO) Vitamin and Mineral Nutrition Information System. World Health Organisation; Geneva, Switzerland: 2014. [(accessed on 6 March 2016)]. Goitre as a determinant of the prevalence and severity of iodine deficiency disorders in populations. Available online: http://apps.who.int/iris/bitstream/10665/133706/1/WHO_NMH_NHD_EPG_14.5_eng.pdf. [Google Scholar]
- 26.Institute of Medicine . Dietary Reference Intakes: Applications in Dietary Assessment. National Academy Press; Washington, DC, USA: 2000. [Google Scholar]
- 27.Food and Agriculture Organisation & World Health Organisation . Human Vitamin and Mineral Requirements. Food and Agriculture Organisation & World Health Organisation; Rome, Italy: 2001. [Google Scholar]
- 28.Hartung J.E.B., Klosener K.H. Statistik Lehr-Und Handbuch der Angewandten Statistik. 6th ed. Oldenbourg; Munich, Germany: 1987. [Google Scholar]
- 29.Ethiopian Public Health Institute . Ethiopia National Food Consumption Survey. Ethiopian Public Health Institute; Addis Ababa, Ethiopia: 2013. [Google Scholar]
- 30.Ethiopian Public Health Institute . Ethiopian National Micronutrient Survey 2014/2015: Preliminary Report. Ethiopian Public Health Institute; Addis Ababa, Ethiopia: 2016. [Google Scholar]
- 31.Central Statistical Agency. ICF International . Ethiopia Demographic and Health Survey 2011. Central Statistical Agency; Addis Ababa, Ethiopia: ICF International; Calverton, MD, USA: 2012. [Google Scholar]
- 32.Kenya National Bureau of Statistics . Kenya Demographic and Health Survey 2014. Kenya National Bureau of Statistics; Nairobi, Kenya: 2015. [Google Scholar]
- 33.National Population Commission (NPC) [Nigeria] and ICF Macro . Nigeria Demographic and Health Survey 2008. National Populaton Commission and ICF Macro; Abuja, Nigeria: 2009. [Google Scholar]
- 34.Department of Health and Nutrition National Food Consumption Survey—Fortification Baseline (NFCS-FB): South Africa, 2005. [(accessed on 28 May 2016)];2007 Available online: http://www.sajcn.co.za/index.php/SAJCN/article/view/286/281.
- 35.Shisana O., Labadarios D., Rehle T., Simbayi L., Zuma K., Dhansay A., Reddy P., Parker W., Hoosain E., Naidoo P., et al. South African National Health and Nutrition Examination Survey (SANHANES-1) HRSC Press; Cape Town, South Africa: 2014. [Google Scholar]
- 36.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Abuye C., Berhane Y., Ersumo T. The role of changing diet and altitude on goitre prevalence in five regional states in Ethiopia. East Afr. J. Public Health. 2008;5:163–168. doi: 10.4314/eajph.v5i3.38997. [DOI] [PubMed] [Google Scholar]
- 38.Amare B., Moges B., Moges F., Fantahun B., Admassu M., Mulu A., Kassu A. Nutritional status and dietary intake of urban residents in Gondar, Northwest Ethiopia. BMC Public Health. 2012;12:752. doi: 10.1186/1471-2458-12-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abriha A., Yesuf M.E., Wassie M.M. Prevalence and associated factors of anemia among pregnant women of Mekelle town: A cross sectional study. BMC Res. Notes. 2014;7:888. doi: 10.1186/1756-0500-7-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogale A., Abebe Y., Stoecker B.J., Abuye C., Ketema K., Hambidge K.M. Iodine status and cognitive function of women and their five year-old children in rural Sidama, southern Ethiopia. East Afr. J. Public Health. 2009;6:296–299. [PubMed] [Google Scholar]
- 41.Ersino G., Tadele H., Bogale A., Abuye C., Stoecker B.J. Clinical assessment of goiter and low urinary iodine concentration depict presence of severe iodine deficiency in pregnant Ethiopian women: A cross-sectional study in rural Sidama, southern Ethiopia. Ethiop. Med. J. 2013;51:133–141. [PubMed] [Google Scholar]
- 42.Gebremedhin S., Enquselassie F., Umeta M. Prevalence of prenatal zinc deficiency and its association with socio-demographic, dietary and health care related factors in rural Sidama, Southern Ethiopia: A cross-sectional study. BMC Public Health. 2011;11:898. doi: 10.1186/1471-2458-11-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gebremedhin S., Enquselassie F., Umeta M. Prevalence and correlates of maternal anemia in rural Sidama, Southern Ethiopia. Afr. J. Reprod. Health. 2014;18:44–53. [PubMed] [Google Scholar]
- 44.Gebreselassie S.G., Gase F.E., Deressa M.U. Prevalence and correlates of prenatal vitamin A deficiency in rural Sidama, Southern Ethiopia. J. Health Popul. Nutr. 2013;31:185–194. doi: 10.3329/jhpn.v31i2.16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gebreegziabher T.T., Gase F.E., Deressa M.U. Lack of dietary sources of iodine and the prevalence of iodine deficiency in rural women from Sidama zone, southern Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2013;13:8401–8414. [Google Scholar]
- 46.Gibson R.S., Abebe Y., Stabler S., Allen R.H., Westcott J.E., Stoecker B.J., Krebs N.F., Hambidge K.M. Zinc, gravida, infection, and iron, but not vitamin B-12 or folate status, predict hemoglobin during pregnancy in Southern Ethiopia. J. Nutr. 2008;138:581–586. doi: 10.1093/jn/138.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haidar J.A., Pobocik R.S. Iron deficiency anemia is not a rare problem among women of reproductive ages in Ethiopia: A community based cross sectional study. BMC Blood Disord. 2009;9:7. doi: 10.1186/1471-2326-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haidar J., Melaku U., Pobocik R.S. Folate defciency in women of reproductive age in nine administrative regions of Ethiopia: An emerging public health problem. S. Afr. J. Clin. Nutr. 2010;23:132–137. doi: 10.1080/16070658.2010.11734327. [DOI] [Google Scholar]
- 49.Hambidge K.M., Abebe Y., Gibson R.S., Westcott J.E., Miller L.V., Lei S., Stoecker B.J., Arbide I., Teshome A., Bailey K.B., et al. Zinc absorption during late pregnancy in rural southern Ethiopia. Am. J. Clin. Nutr. 2006;84:1102–1106. doi: 10.1093/ajcn/84.5.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joray M.L., Yu T.W., Ho E., Clarke S.L., Stanga Z., Gebreegziabher T., Hambidge K.M., Stoecker B.J. Zinc supplementation reduced DNA breaks in Ethiopian women. Nutr. Res. 2015;35:49–55. doi: 10.1016/j.nutres.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kedir H., Berhane Y., Worku A. Khat chewing and restrictive dietary behaviors are associated with anemia among pregnant women in high prevalence rural communities in eastern Ethiopia. PLoS ONE. 2013;8:e78601. doi: 10.1371/journal.pone.0078601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kedir H., Berhane Y., Worku A. Subclinical Iodine Deficiency among Pregnant Women in Haramaya District, Eastern Ethiopia: A Community-Based Study. J. Nutr. Metab. 2014;8:e78926. doi: 10.1155/2014/878926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulu A., Kassu A., Huruy K., Tegene B., Yitayaw G., Nakamori M., Van Nhien N., Bekele A., Wondimhun Y., Yamamoto S., et al. Vitamin A deficiency during pregnancy of HIV infected and non-infected women in tropical settings of Northwest Ethiopia. BMC Public Health. 2011;11:569. doi: 10.1186/1471-2458-11-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoecker B.J., Abebe Y., Hubbs-Tait L., Kennedy T.S., Gibson R.S., Arbide I., Teshome A., Westcott J., Krebs N.F., Hambidge K.M. Zinc status and cognitive function of pregnant women in Southern Ethiopia. Eur. J. Clin. Nutr. 2009;63:916–918. doi: 10.1038/ejcn.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adongo A.O., Shell-Duncan B., Prisca T.J. Effect of settlement on nutrition and health status of pastoral Gabra women of reproductive age in Kalacha Location, Marsabit County, Kenya. Public Health Nutr. 2013;16:1622–1630. doi: 10.1017/S136898001200496X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gitau G., Kimiywe J.J.W. Gender and Inequality in Developing Countries. Arise Publishers; New Delhi, India: 2008. Food Intake and Iron Status of Lactating and Non lactating mothers in makongeni, Thika, Kenya. [Google Scholar]
- 57.Kamau-Mbuthia E., Elmadfa I. Diet quality of pregnant women attending an antenatal clinic in Nakuru, Kenya. Ann. Nutr. Metab. 2007;51:324–330. doi: 10.1159/000107674. [DOI] [PubMed] [Google Scholar]
- 58.Mwangi M.N., Maskey S., Andang'o P.E.A., Shinali N.K., Roth J.M., Trijsburg L., Mwangi A.M., Zuilhof H., Lagen B., Savelkoul H.F.J., et al. Diagnostic utility of zinc protoporphyrin to detect iron deficiency in Kenyan pregnant women. BMC Med. 2014;12:229. doi: 10.1186/s12916-014-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitheko A., Kimiywe J., Njeru P. Prevalence of Maternal Serum Zinc Deficiency and Its Associated Factors among Pregnant Women in Naivasha, Kenya. Eur. J. Nutr. Food Saf. 2015;5:354–355. doi: 10.9734/EJNFS/2015/20850. [DOI] [Google Scholar]
- 60.Ouma P., Parise M.E., Hamel M.J., Ter Kuile F.O., Otieno K., Ayisi J.G., Kager P.A., Steketee R.W., Slutsker L., van Eijk A.M. A randomized controlled trial of folate supplementation when treating malaria in pregnancy with sulfadoxine-pyrimethamine. PLoS Clin. Trials. 2006;1:e28. doi: 10.1371/journal.pctr.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Othoo D.A., Waudo J.N., Kuria E.N. Dietary assessment of vitamin and iron among pregnant women at Ndhiwa sub distract hospital-Kenya. Afr. J. Food Agric. Nutr. Dev. 2014;14:2114–2128. [Google Scholar]
- 62.Shipala E.K., Wafula S.W., Ettyang G.A., Were E.O. Nutrient intake among pregnant teenage girls attending ante-natal clinics in two health facilities in bungoma south district, western Kenya. East Afr. Med. J. 2012;89:94–99. [PubMed] [Google Scholar]
- 63.Van Eijk A.M., Ouma P.O., Williamson J., Ter Kuile F.O., Parise M., Otieno K., Hamel M.J., Ayisi J.G., Kariuki S., Kager P.A., et al. Plasma folate level and high-dose folate supplementation predict sulfadoxine-pyrimethamine treatment failure in pregnant women in western Kenya who have uncomplicated malaria. J. Infect. Dis. 2008;198:1550–1553. doi: 10.1086/592715. [DOI] [PubMed] [Google Scholar]
- 64.Waswa J. Influence of percieved body image on nutrient intake and nutritional health of female students of Moi University. East Afr. J. Public Health. 2011;8:123–131. [PubMed] [Google Scholar]
- 65.De Moura F.F., Moursi M., Lubowa A., Ha B., Boy E., Oguntona B., Sanusi R.A., Maziya-Dixon B. Cassava intake and Vitamin A status among women and preschool children in Akwa-Ibom, Nigeria. PLoS ONE. 2015;10:e0129436. doi: 10.1371/journal.pone.0129436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dim C.C., Onah H.E. The prevalence of anemia among pregnant women at booking in Enugu, South Eastern Nigeria. Medscape Gen. Med. 2007;9:11. [PMC free article] [PubMed] [Google Scholar]
- 67.Dim C.C., Ugwa E.O., Anyaehie U.B., Obioha K.C. A comparison of capillary and venous blood haematocrits of pregnant women in Nigeria: The impact on diagnosis and prevalence of anaemia in pregnancy. BioMed Res. Int. 2014;2014:467056. doi: 10.1155/2014/467056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ezugwu E.C., Mbah B.O., Chigbu C.O., Onah H.E. Anaemia in pregnancy: A public health problem in Enugu, southeast Nigeria. J. Obstet. Gynaecol. 2013;33:451–454. doi: 10.3109/01443615.2013.771158. [DOI] [PubMed] [Google Scholar]
- 69.Idowu O.A., Mafiana C.F., Dapo S. Anaemia in pregnancy: A survey of pregnant women in Abeokuta, Nigeria. Afr. Health Sci. 2005;5:295–299. doi: 10.5555/afhs.2005.5.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kagu M.B., Kawuwa M.B., Gadzama G.B. Anaemia in pregnancy: A cross-sectional study of pregnant women in a Sahelian tertiary hospital in Northeastern Nigeria. J. Obstet. Gynaecol. 2007;27:676–679. doi: 10.1080/01443610701612144. [DOI] [PubMed] [Google Scholar]
- 71.Miri-Dashe T., Osawe S., Tokdung M., Daniel N., Choji R.P., Mamman I., Deme K., Damulak D., Abimiku A. Comprehensive reference ranges for hematology and clinical chemistry laboratory parameters derived from normal Nigerian adults. PLoS ONE. 2014;9:e93919. doi: 10.1371/journal.pone.0093919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nwizu E.N., Iliyasu Z., Ibrahim S.A., Galadanci H.S. Socio-demographic and maternal factors in anaemia in pregnancy at booking in Kano, northern Nigeria. Afr. J. Reprod. Health. 2011;15:33–41. [PubMed] [Google Scholar]
- 73.Okwu G.N., Ukoha A.I. Studies on the predisposing factors of iron deficiency anaemia among pregnant women in Nigerian community. Pak. J. Nutr. 2008;7:151–156. doi: 10.3923/pjn.2008.151.156. [DOI] [Google Scholar]
- 74.Olatunbosun O.A., Abasiattai A.M., Bassey E.A., James R.S., Ibanga G., Morgan A. Prevalence of anaemia among pregnant women at booking in the university of Uyo teaching hospital, Uyo, Nigeria. BioMed Res. Int. 2014 doi: 10.1155/2014/849080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Otemuyiwa I.O., Adewusi S.R.A. Food choice and meal consumption pattern among undergraduate students in two universities in southwestern Nigeria. Nutr. Health. 2012;1:233–245. doi: 10.1177/0260106013510994. [DOI] [PubMed] [Google Scholar]
- 76.Obasi I.O., Nwachukwu N. Comparative iron related anaemia at pregnancy in Ebonyi state, South-east Nigeria. J. Med. Sci. 2013;13:425–431. doi: 10.3923/jms.2013.425.431. [DOI] [Google Scholar]
- 77.Ofojekwu M.J.N., Nnanna O.U., Okolie C.E., Odewumi L.A., Isiguzoro I.O.U., Lugos M.S.D. Hemoglobin and serum iron concentrations in menstruating nulliparous women in Jos, Nigeria. Lab. Med. 2013;44:121–124. doi: 10.1309/LMM7A0F0QBXEYSSI. [DOI] [Google Scholar]
- 78.Olubukola A., Odunayo A., Adesina O. Anemia in pregnancy at two levels of health care in Ibadan, south west Nigeria. Ann. Afr. Med. 2011;10:272–277. doi: 10.4103/1596-3519.87042. [DOI] [PubMed] [Google Scholar]
- 79.Shu E.N., Ogbodo S.O. Role of ascorbic acid in the prevention of iron-deficiency anaemia in pregnancy. Biomed. Res. 2005;16:40–44. [Google Scholar]
- 80.Ugwuja E.I., Akubugwo E.I., Ibiam A.U., Onyechi U. Impact of maternal iron deficiency and anaemia on pregnancy and its outcomes in Nigerian population. Int. J. Nutr. Wellness. 2009;10:1–11. [Google Scholar]
- 81.Ugwuja E.I., Akubugwo E.I., Ibiam U.A., Obidoa O. Impact of maternal copper and zinc status on pregnancy outcomes in a population of pregnant Nigerians. Pak. J. Nutr. 2010;9:678–682. doi: 10.3923/pjn.2010.678.682. [DOI] [Google Scholar]
- 82.Vanderjagt D.J., Ujah I.A., Ikeh E.I., Bryant J., Pam V., Hilgart A., Crossey M.J., Glew R.H. Assesment of the vitamin B12 status of pregnant women in Nigeria using plasma holotranscobalamin. Int. Sch. Res. Not. Obstet. Gynecol. 2011;2011:365894. doi: 10.5402/2011/365894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vanderjagt D.J., Ujah I.A., Patel A., Kellywood J., Crossey M.J., Allen R.H., Stabler S.P., Obande O.S., Glew R.H. Subclinical vitamin B12 deficiency in pregnant women attending an antenatal clinic in Nigeria. J. Obstet. Gynaecol. 2009;29:288–295. doi: 10.1080/01443610902812709. [DOI] [PubMed] [Google Scholar]
- 84.Williams I.O., Eka O.U., Essien E.U. Vitamin A status of pregnant women in Calabar metropolis, Nigeria. Pak. J. Biol. Sci. 2008;11:1702–1707. doi: 10.3923/pjbs.2008.1702.1707. [DOI] [PubMed] [Google Scholar]
- 85.Dolman R.C., Wentzel-Viljoen E., Jerling J.C., Feskens E.J., Kruger A., Pieters M. The use of predefined diet quality scores in the context of CVD risk during urbanization in the South African Prospective Urban and Rural Epidemiological (PURE) study. Public Health Nutr. 2014;17:1706–1716. doi: 10.1017/S1368980013002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faber M., Swanevelder S., Benade A.J. Is there an association between the nutritional status of the mother and that of her 2-year-old to 5-year-old child? Int. J. Food Sci. Nutr. 2005;56:237–244. doi: 10.1080/09637480500145913. [DOI] [PubMed] [Google Scholar]
- 87.Gichohi-Wainaina W.N., Melse-Boonstra A., Swinkels D.W., Zimmermann M.B., Feskens E.J., Towers G.W. Common variants and haplotypes in the TF, TNF-α, and TMPRSS6 genes are associated with iron status in a female black South African population. J. Nutr. 2015;145:945–953. doi: 10.3945/jn.114.209148. [DOI] [PubMed] [Google Scholar]
- 88.Hattingh Z., Walsh C.M., Bester C.J., Oguntibeju O.O. An analysis of dietary micronutrient intakes in two age groups of black South African women. West Indian Med. J. 2008;57:431–437. [PubMed] [Google Scholar]
- 89.Kolahdooz F., Spearing K., Sharma S. Dietary adequacies among South African adults in rural KwaZulu-Natal. PLoS ONE. 2013;8:e67184. doi: 10.1371/journal.pone.0067184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawrie D., Coetzee L.M., Glencross D.K. Iron deficiency anaemia in healthy South African women despite iron fortification. S. Afr. Med. J. 2008;98:606–607. [PubMed] [Google Scholar]
- 91.Mostert D., Steyn N.P., Temple N.J., Olwagen R. Dietary intake of pregnant women and their infants in a poor black South African community. Curationis. 2005;28:12–19. doi: 10.4102/curationis.v28i4.1002. [DOI] [PubMed] [Google Scholar]
- 92.Oldewage-Theron W., Kruger R. Dietary diversity and adequacy of women caregivers in a peri-urban informal settlement in South Africa. Nutrition. 2011;27:420–427. doi: 10.1016/j.nut.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 93.Oldewage-Theron W.H., Egal A.A., Grobler C. Is overweight and obesity associated with iron status in low-income men and women? A case study from Qwa-Qwa, South Africa. Integr. Food Nutr. Metab. 2014;1:107–113. [Google Scholar]
- 94.Pisa P.T., Behanan R., Vorster H.H., Kruger A. Social drift of cardiovascular disease risk factors in Africans from the North West Province of South Africa: The PURE study. Cardiovasc. J. Afr. 2012;23:371–378. doi: 10.5830/CVJA-2012-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harika R., et al. Are Low Intakes and Deficiencies in Iron, Vitamin A, Zinc, and Iodine of Public Health Concern in Ethiopian, Kenyan, Nigerian, and South African Children and Adolescents. Food Nutr. Bull. 2017;38:405–428. doi: 10.1177/0379572117715818. [DOI] [PubMed] [Google Scholar]
- 96.Haverkate M., Smits J., Meijerink H., van der Ven A. Socioeconomic determinants of haemoglobin levels of african women are less important in areas with more health facilities: A multilevel analysis. J. Epidemiol. Community Health. 2014;68:116–122. doi: 10.1136/jech-2012-202336. [DOI] [PubMed] [Google Scholar]
- 97.Abebe Y., Bogale A., Hambidge K.M., Stoecker B.J., Arbide I., Teshome A., Krebs N.F., Westcott J.E., Bailey K.B., Gibson R.S. Inadequate intakes of dietary zinc among pregnant women from subsistence households in Sidama, Southern Ethiopia. Public Health Nutr. 2008;11:379–386. doi: 10.1017/S1368980007000389. [DOI] [PubMed] [Google Scholar]
- 98.Nel J., van Stuijvenberg M.E., Schoeman S.E., Dhansay M.A., Lombard C.J., du Plessis L.M. Liver intake in 24-59-month-old children from an impoverished South African community provides enough vitamin A to meet requirements. Public Health Nutr. 2014;17:2798–2805. doi: 10.1017/S1368980013003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faber M., Laubscher R. Seasonal availability and dietary intake of beta-carotene-rich vegetables and fruit of 2-year-old to 5-year-old children in a rural South African setting growing these crops at household level. Int. J. Food Sci. Nutr. 2008;59:46–60. doi: 10.1080/09637480701664852. [DOI] [PubMed] [Google Scholar]
- 100.Abizari A.R., Dold S., Kupka R., Zimmermann M.B. More than two-thirds of dietary iodine in children in northern Ghana is obtained from bouillon cubes containing iodized salt. Public Health Nutr. 2017;20:1107–1113. doi: 10.1017/S1368980016003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Modjadji S.E.P., Alberts M., Mamabolo R.L. Folate and iron status of South African non-pregnant rural women of childbearing, age, before and after fortification of foods. S. Afr. J. Clin. Nutr. 2007;20:89–93. doi: 10.1080/16070658.2007.11734132. [DOI] [Google Scholar]
- 102.Wessells K.R., Brown K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE. 2012;7:e50568. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thurnham D.I., McCabe L.D., Haldar S., Wieringa F.T., Northrop-Clewes C.A., McCabe G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010;92:546–555. doi: 10.3945/ajcn.2010.29284. [DOI] [PubMed] [Google Scholar]
- 104.World Health Organisation (WHO) WHO Global Database on Vitamin A Deficiency. World Health Organisation; Geneva, Switzerland: 2009. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. [Google Scholar]
- 105.Rabiu T.B., Tiamiyu L.O., Awoyinka B.S. Awareness of spina bifida and periconceptional use of folic acid among pregnant women in a developing economy. Child Nerv. Syst. 2012;28:2115–2119. doi: 10.1007/s00381-012-1879-5. [DOI] [PubMed] [Google Scholar]
- 106.Brown K.H., Wuehler S.E., Peerson J.M. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food Nutr. Bull. 2001;22:113–125. doi: 10.1177/156482650102200201. [DOI] [Google Scholar]
- 107.Caulfield L.E., Black R.E. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors 2004. World Health Organization; Geneva, Switzerland: 2004. Zinc deficiency; pp. 257–279. [Google Scholar]
- 108.Ogunmoyela O.A., Adekoyeni O., Aminu F., Umunna L.O. A Critical Evaluation of Survey Results of Vitamin A and Fe Levels in the Mandatory Fortified Food Vehicles and Some Selected Processed Foods in Nigeria. Niger. Food J. 2013;31:52–62. doi: 10.1016/S0189-7241(15)30077-1. [DOI] [Google Scholar]
- 109.World Health Organisation . Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women. World Health Organisation; Geneva, Switzerland: 2012. [PubMed] [Google Scholar]
- 110.Ministry of Health, Republic of Kenya . National Iron and Folic Acid Supplementation Communication Strategy. Government of Kenya; Nairobi, Kenya: 2013. [Google Scholar]
- 111.Bhutta Z.A., Das J.K., Rizvi A., Gaffey M.F., Walker N., Horton S., Webb P., Lartey A., Black R.E. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet. 2013;382:452–477. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 112.Hess S.Y., Brown K.H., Sablah M., Engle-Stone R., Aaron G.J., Baker S.K. Results of Fortification Rapid Assessment Tool (FRAT) surveys in sub-Saharan Africa and suggestions for future modifications of the survey instrument. Food Nutr. Bull. 2013;34:21–38. doi: 10.1177/156482651303400104. [DOI] [PubMed] [Google Scholar]
- 113.Engle-Stone R., Nankap M., Ndjebayi A.O., Brown K.H. Simulations based on representative 24-h recall data predict region-specific differences in adequacy of vitamin A intake among Cameroonian women and young children following large-scale fortification of vegetable oil and other potential food vehicles. J. Nutr. 2014;144:1826–1834. doi: 10.3945/jn.114.195354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.