Abstract
Background
Bronchoscopic procedures are common in the clinical setting, with estimates indicating 500,000 are undertaken per year in the USA alone. These procedures are generally regarded as safe. Unfortunately, a risk of cross-contamination between patients, with possible subsequent infection, is associated with the re-usable technology typically used in these procedures.
Objective
Our objective was to conduct an early cost-effectiveness analysis (CEA) of single-use flexible video bronchoscope technology compared with the current reusable technology in a US hospital intensive care setting.
Methods
We conducted a CEA to determine an incremental cost-effectiveness ratio (ICER), and constructed a decision analytic model based on the best available evidence from a literature search and a Delphi panel. We also conducted several one- and two-way sensitivity analyses and a probabilistic sensitivity analysis to illuminate the uncertainty associated with the estimates.
Results
The literature search showed ample evidence of risk, albeit little of it was quantifiable. Estimates from the Delphi method found approximately a 3% risk of cross-contamination and approximately a 21% risk of subsequent infection. Pneumonia was estimated as the most likely manifestation of infection. The CEA showed a saving of $US118 per procedure and elimination of 0.7% of the risk of infection with the single-use technology. Relevant sensitivity analyses generally validated this result.
Conclusion
This study suggests that implementation of the single-use technology in the intensive care unit is cost effective in most scenarios. However, this result should be interpreted with caution because of the lack of certain knowledge on this particular topic.
Electronic supplementary material
The online version of this article (doi:10.1007/s41669-017-0012-9) contains supplementary material, which is available to authorized users.
Keywords: Probabilistic Sensitivity Analysis, Delphi Method, Subsequent Infection, Health Economic Evaluation, Bacterial Spore
Key Points for Decision Makers
| Risks of cross-contamination and post-endoscopic infection from bronchoscopic procedures is under-researched. |
| A single-use flexible video bronchoscope would eliminate any given risk of cross-contamination. |
| Early assessment of the cost effectiveness of single-use bronchoscopes indicates potential hospital savings and patient benefits from infections avoided. |
Introduction
Although definitive assessment of cost effectiveness may require long-term evidence from randomized trials, it is important to begin to estimate likely cost effectiveness early in the life cycle of new technologies [1]. Such estimates can help prioritize internal development plans, indicate which parameters need further research and inform early adopters of the technology [1–3]. Single-use flexible video bronchoscopes is one such new technology in an area with limited evidence.
Bronchoscopes give healthcare professionals both visualization of and access to the affected tissue to investigate symptoms, confirm diagnoses or treat a patient. For instance, the instrument allows for visual orientation into an individual lobe or segment bronchi or allows for bronchoalveolar lavage. Estimates indicate 500,000 bronchoscopic procedures take place per year in the USA alone [4]. Bronchoscopic procedures are common in clinical settings because they are generally recognized as safe [5]. Complications associated with flexible bronchoscopy are usually minor and relate to procedure or sedation [5]. Common complications include bleeding, pneumothorax and infection; however, other risks, such as sore throat, heart attack and fever, also exist [6–8].
A single-use flexible video bronchoscope would eliminate any given risk of cross-contamination. The decision as to whether or not a hospital should buy and implement the new and possibly better technology requires economical, ethical and clinical considerations. This study provides a health economic perspective on the issue by conducting a cost-effectiveness analysis (CEA) of single-use flexible video bronchoscopes and comparing this with reusable flexible video bronchoscopes when applied in a typical intensive care unit (ICU). ICUs are characterized by patients with generally low immune responses, and they often rely on mechanical ventilation, bypassing normal immune responses in the upper airways. This patient group is therefore particularly prone to infection. However, the risk of cross-contamination and infection is not well investigated. Given the limited clinical evidence, this study should be interpreted as an early assessment of the likely cost effectiveness of single-use flexible video bronchoscopes.
Reprocessing of Reusable Flexible Bronchoscopes
When a procedure is completed, the standard reusable flexible bronchoscope needs to be reprocessed prior to reuse. All parts of the reusable technology are reused. Flexible bronchoscopes initially receive manual cleaning (removing organic debris and microorganisms) at the site before being moved to a designated reprocessing work area for leak testing and possible automated cleaning. Depending on the device material, it undergoes either disinfection (the elimination of all microorganisms other than a small number of bacterial spores) or sterilization (the complete destruction of all forms of microbiological life) [9]. When this reprocessing has been performed according to the approved labelling from the manufacturer, the flexible bronchoscopes are stored in an appropriate storage cabinet. Each flexible bronchoscope requires a specific reprocessing regime. This results in a range of different instructions on product labels, such as the varying use of detergents [10]. Some healthcare facilities also use automated endoscope reprocessors (AERs) to implement a mechanical disinfection method. Since the bronchoscopes are in contact with mucous membranes and have a moderate degree of infection risk if contaminated at the time of use, they are categorized as semi-critical devices [11] and should therefore optimally be sterilized. However, the device materials do not always permit this reprocessing method. If the reusable bronchoscope is heat-labile, low-temperature reprocessing should be applied, such as high-level disinfection (HLD) [10]. HLD procedures vary [9] but commonly involve the elimination of certain microorganisms to an acceptable extent. The US FDA maintains an updated list of approved sterilants and high-level disinfectants for this purpose [12].
Risks of Cross-Contamination and Post-Endoscopic Infection
The risk of adverse events due to inappropriate cleaning, disinfection or rinsing, or lack of leak testing and drying as a cause of cross-contamination is well described in the literature [13–15]. Some of these failures are associated with human error and some suggest additional or improved training as part of the solution [16]. Yet some of these cases involving inadequate cleaning might be due to difficult conditions for personnel [17].
Biofilm formation is of special concern in reprocessing [18, 19]. A biofilm can be defined as a microbially derived sessile community characterized by cells that are irreversibly attached to a substratum, interface or each other and that are embedded in a matrix of extracellular polymeric substances that produce and exhibit an altered phenotype with respect to growth rate and gene transcription [20]. The structure and physiological attributes of biofilms make microorganisms in biofilms, in contrast to a normal planktonic state, very resistant to antimicrobial agents, whether antibiotics, disinfectants or germicides [20].
Outbreaks of post-endoscopic infection and cross-contamination related to biofilm development inside endoscope channels and AERs have been reported in the literature [15, 21]. Biofilms can be removed from artificial surfaces by physical methods, e.g. thorough brushing of bronchoscope channels combined with chemical treatment [9]. Unfortunately, because of the composition and nature of the flexible bronchoscope construction, it is difficult to consistently brush and clean properly [21–23]. This is particularly so when the bronchoscope is damaged [19, 24]. It is difficult to know whether the flexible bronchoscope is damaged [18, 21, 22, 24], and therefore a potential risk exists for bacterial colonization of cracks, grooves and pits.
The resistance of microorganisms in biofilms to decontamination is an issue of concern not only for the bronchoscopes but also for the AERs. If a contaminated reusable bronchoscope is introduced to an AER, it could allow the formation of biofilm in the AER itself. This AER could then contaminate the next, originally sterile, reusable bronchoscope being reprocessed, thereby acting as a source of contamination [25]. In addition, bacterial spores are not necessarily eliminated by exposure to disinfectants [9]. A few disinfectants, termed chemical sterilants, are able to kill bacterial spores after prolonged exposure (3–12 h) [9].
Although much effort is being put into reprocessing reusable bronchoscopes, reports are continually being published showing problems with cross-contamination despite strict adherence to reprocessing labelling instructions [15, 18]. Complications during these procedures are a reality in which clinicians work and to which patients are compelled to submit. Nonetheless, the risk of cross-contamination of a pathogen from one patient to another as a result of inadequate bronchoscope reprocessing might be preventable. However, research in this context is still sparse, and no direct quantified risk has been identified in the literature. In 2015 alone, the FDA published new guidelines for reprocessing, issued safety communications on bronchoscopes and AERs, required manufacturers to conduct post-marketing surveillance studies and sent warning letters to at least three major manufactures [26–28]. It is in the interests of patients, clinicians and suppliers in this industry to further investigate the reality. Until then, we are dealing with uncertainty.
In an effort to minimize this risk of cross-contamination, a range of alternative pathways could be taken, such as the use of single-use protective sheets, thorough and repetitive education of healthcare personnel or further development of reusable bronchoscope materials and AERs [29]. Still, these methods of dealing with risk will only minimize, not eliminate, the uncertainty.
Methods
Health Economic Evaluation
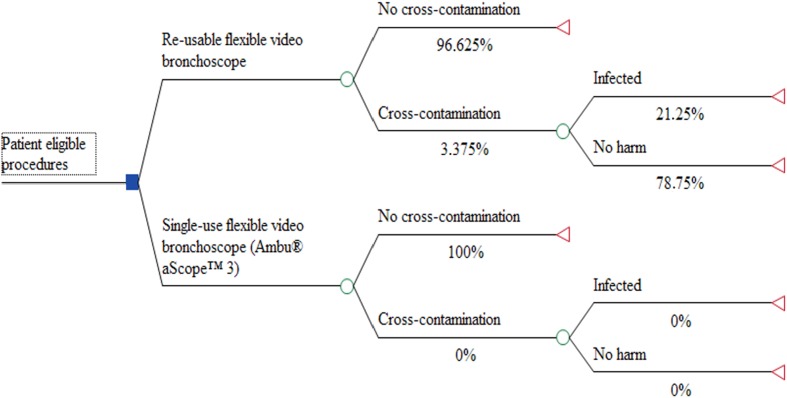
We constructed a decision analytic model on the basis of the best available evidence to estimate the short-term costs and benefits of single-use flexible video bronchoscopes compared with reusable flexible video bronchoscopes [30, 31] (Fig. 1). The setting was a US hospital ICU. The time horizon was short term (within 1 year). Costs were estimated in $US, year 2015–2016 values. The model was drawn up in TreeAgePro 2014 with the Healthcare Module addition.
Fig. 1.
Decision analytic model for cost effectiveness of single-use flexible video bronchoscopes
Risk
To inform the model with data on the effect, we conducted a literature search using a mix of methods, primarily a PICO (population, intervention, comparator, outcome) search of PubMed, the Cochrane Library, and Embase using the medical subject heading (MeSH) terms ‘bronchoscopy’, ‘risk’, ‘cross-contamination’, ‘reusable’, ‘single-use’, ‘disposable’, ‘infection’, ‘prevention’ and ‘reprocessing’ (period: 1980–2015). Typical manifestations of infection in this context are also worthy of attention in this process. Only studies from Europe, the USA and Canada were included. We screened and evaluated the articles and extracted the relevant data. Many reports in the literature indicate a non-quantifiable risk of cross-contamination and subsequent infection due to bronchoscopy [15, 32, 33]. A broad consensus exists that a risk is indeed generally present and that cases are under-reported [5, 6, 15, 17, 24, 34, 35]. To inform the decision model with sufficient data, we needed to find or estimate the risk of cross-contamination and infection. Both the effect of using reusable flexible video bronchoscopes and the effect of using single-use flexible video bronchoscope were needed for quantitative analysis. We therefore obtained expert consensus using the Delphi method to structure the communication and deliver the circumstances described in detail below. The literature findings support or validate the estimation of the risk of cross-contamination and infection for the estimates provided from a panel of experts. The uncertainties associated with the estimates of the effects given by the Delphi panel are also reflected in the applied probability distributions.
We used the findings from the literature review to identify international experts and researchers from different continents who were relevant for inclusion in the Delphi panel. The Delphi method is a method of structured communication allowing a group of individuals to deal with a complex problem [36, 37]. We used the sub-version ‘conventional Delphi’ [36, 37]. First, a questionnaire was sent to the identified group. Questionnaires were completed and returned, and we summarized the results. A new questionnaire (see the Electronic Supplementary Material [ESM]) based on the results of the first round was then sent back to the respondent group (for further details see the ESM). This allowed the respondents to re-evaluate their original answers. This technique combines polling and conference procedures in a way that facilitates unrestricted professional estimation [38]. The level of expertise was rated by the authors on the basis of either the frequency of appearance in the literature, the frequency of citation or via conversation with the expert in question. Of 14 contacts, eight completed the process. All eight experts completed both questionnaires. Expert identities were anonymised; however, all were experienced clinicians and researchers within the field and together they represented both Europe and North America. We calculated the standard error of the sample mean based on the results from this method, and used this as input in the approximation of the relevant statistical distribution for probabilistic sensitivity analysis (PSA).
Cost
The cost per procedure using a reusable flexible video bronchoscope was estimated based on literature findings [39–43] at $US221, year 2015 values. This is the mean cost per use over the lifespan of the reusable technology.
Given existing variance in the literature estimates, special attention was paid to this parameter in the two-way sensitivity analysis. We based our calculations for single-use flexible video bronchoscopes on the Ambu® aScope™ 3. The cost of using a single-use flexible video bronchoscope (Ambu® aScope™ 3) per procedure, including the monitor (Ambu® aView™), was estimated at $US305. This estimate is based on the price of one single-use flexible video bronchoscope (for US hospitals, the purchase price would be approximately $US300) and the recommended number of monitors needed based on the average number of procedures from the identified cost analyses in the literature. As these recommendations are based on estimates, and this technology is likely to increase waste handling, some uncertainty is connected with this parameter. We are dealing with a group of patients who already have various conditions (those for which they were admitted to the ICU setting), and so a cross-contaminated and subsequently infected patient would have additional pneumonia, not pneumonia alone. We were therefore interested in finding the marginal cost in a setting similar to our case setting. We considered ventilator-associated pneumonia (VAP), assuming it would be the clinically most appropriate substitute for our infection manifestation, as it also constitutes a problem supplementary to the original condition for which the patient was admitted. The cost per VAP case was found in the literature in a systematic review of US clinical settings [44] that identified the average marginal cost of typical infections. The average marginal cost of VAP was identified as $US28,383 per case.
Sensitivity Analyses
We conducted several analyses to test the robustness of the base-case results and to provide adequate insight for the decision maker, applying both deterministic sensitivity analyses and PSA. We conducted one-way (univariate) sensitivity analysis for all parameters in the model to explore the impact on the incremental cost-effectiveness ratio (ICER) of changing the value of the parameter while keeping all other parameter values unchanged and conducted two-way sensitivity analyses for different price levels for the new technology. PSA was performed to estimate the decision uncertainty by using the specified distributions in a second-order Monte Carlo simulation with 1000 samples of mean ICER. Results from the PSA are presented in an ICER scatterplot to illustrate the likelihood of savings and associated reduction in risk of an adverse event (such as nosocomial infection with pneumonia).
Results
Results from the Delphi exercise showed reasonably similar values during both rounds of questionnaires. When asked to estimate the typical condition an infected patient in this context would have, all answered “pneumonia” in both rounds. See Table 1 for the final results from the Delphi exercise as well as the other parameter values used.
Table 1.
All parameter values used in the model and their respective standard errors, distributions and sources
| Parameter | Base-case value (SE) | Distribution | Source |
|---|---|---|---|
| Effects | |||
| Reusable flexible video bronchoscope risk of cross-contamination | 3.375% (0.4199) | Beta | Delphi panel |
| Reusable flexible video bronchoscope risk of subsequent infection | 21.25% (2.7951) | Beta | Delphi panel |
| Single-use flexible video bronchoscope risk of cross-contamination | 0% (0) | NA | NA |
| Single-use flexible video bronchoscope risk of subsequent infection | 0% (0) | NA | NA |
| Costs | |||
| Reusable flexible video bronchoscope cost per procedure | $US221 (44) | Gamma | [35–39] |
| Single-use flexible video bronchoscope cost per procedure | $US305 (15) | Gamma | Producer (Ambu A/S) |
| Cost per case of VAP | $US28.383 (4257) | Gamma | [40] |
NA not available, SE standard error, VAP ventilator-associated pneumonia
The CEA base-case results indicate that the single-use flexible video bronchoscope technology is the preferred technology, as this option is less costly and more effective in regard to cross-contamination and subsequent infection. Using the current technology is estimated to have an average cost of $US424 and to hold a 0.7% risk of infection. The newer technology has an average cost per use of $US305 and a 0% risk of infection. Results show a possible saving of $US118.56 per procedure and the elimination of a 0.7% risk of infection if the single-use option is adopted instead of the current technology. Table 2 presents an overview of the different one-way sensitivity analyses performed.
Table 2.
Base-case result and one-way sensitivity analyses
| Scenario | Δ cost ($) | Δ effect (avoided risk of infection) | ICER (cost per avoided infection) |
|---|---|---|---|
| Base-case (see Table 1) | –119 | 0.0072 | –16,554 |
| Below various one-way sensitivity analyses | |||
| When cost of use of the reusable technology is $100 | 0.68 | 0.0072 | 68 |
| When cost of use of the reusable technology is $200 | –99 | 0.0072 | –13,795 |
| When cost of use of the reusable technology is $300 | –199 | 0.0072 | –27,684 |
| When cost of use of the reusable technology is $400 | –299 | 0.0072 | –41,573 |
| When cost of use of the reusable technology is $500 | –399.32 | 0.0072 | –55,462 |
| When cross-contamination for reusable technology is set to 0% | 220 | 1.0000 | 220 |
| When cross-contamination for reusable technology is set to 2.5% | –65 | 0.0053 | –12,296 |
| When cross-contamination for reusable technology is set to 5% | –216 | 0.0106 | –20,340 |
| When cross-contamination for reusable technology is set to 7.5% | –366.03 | 0.0159 | –23,021 |
| When cross-contamination for reusable technology is set to 10% | –516 | 0.0212 | –24,361 |
| When infection rate for reusable technology is set to 10% | –11 | 0.0034 | –3307 |
| When infection rate for reusable technology is set to 15% | –59 | 0.0051 | –11,665 |
| When infection rate for reusable technology is set to 20% | –107.74 | 0.0068 | –15,845 |
| When infection rate for reusable technology is set to 25% | –156 | 0.0085 | –18,352 |
| When infection rate for reusable technology is set to 30% | –204 | 0.0102 | –20,024 |
| When infection rate for reusable technology is set to 35% | –252 | 0.0119 | –21,218 |
| When infection rate for reusable technology is set to 40% | –300.75 | 0.0136 | –22,114 |
ICER incremental cost-effectiveness ratio
Figure 2 uses two-way sensitivity analysis graphs to illustrate that the higher the internal cost of reprocessing, quality assurance and repairs associated with the reusable technology, the more advantageous it is to use the single-use technology (as indicated in red).
Fig. 2.
Two-way sensitivity analyses. Red area indicates net savings from single-use technology compared with reusable bronchoscopes. Blue area indicates that re-usable technology is cheaper. Potential health benefits from avoided infections are not included in the two-way sensitivity analyses
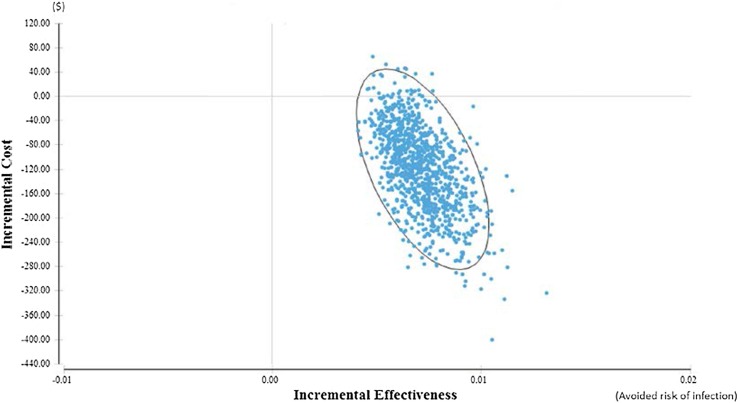
A scatterplot from the PSA is illustrated in Fig. 3. The majority of calculated ICERs are in the south-eastern quadrant, indicating a likelihood of net savings for the hospital of over 97%. A 95% confidence ellipse has been applied to the scatterplot.
Fig. 3.
Scatterplot from probabilistic sensitivity analyses using 1000 s-order samples
Discussion
This is the first study to utilize a CEA with the aim of indicating whether implementation of a single-use flexible video bronchoscope (Ambu® aScope™ 3) is cost effective when solely looking at cross-contamination and possible subsequent infections with bronchoscopes in a typical ICU setting compared with current best practice involving reusable flexible video bronchoscopes.
Based on limited evidence, the model suggests that implementation of the single-use technology in the ICU is cost saving and associated with increased patient safety. PSA and other sensitivity analyses generally confirmed this. However, the results should be interpreted with caution because definite knowledge is lacking on this particular topic.
Despite this lack, it can be argued that it is still necessary to undertake health economic evaluations very early in the adoption process for new health technologies to direct attention to possible improvements [3]. In constructing the decision model, the decision makers have an opportunity to see a simplified model of the real world. The results provide an intuitive and visual approach to the decision at hand. The gaps in the evidence have been highlighted in the literature review, and the Delphi method has been used to accommodate this issue. As with any model, this analysis has its limitations, which in this case involve data availability and our assumptions.
The risks of cross-contamination and post-endoscopic infection were estimated by a panel of experts using the Delphi method. The method is characterized by its considerable uncertainty, and it was only possible to obtain the full participation of eight of the 14 international (anonymous) experts we contacted. We have no reason to question the validity of the panel estimates. The advantages of making the expert panel anonymous is that it allows for open answers about what is sometimes a sensitive topic. Possible disadvantages include the lack of transparency. Other possible limitations of this health economic evaluation concern the study perspective, the model structure, the time horizon and other complications and costs. When thinking outside of the healthcare sector perspective, there are likely to be more outcomes that have not been included. An example of this would be ICU patients’ delayed recovery due to cross-contamination and subsequent infection, which would delay their return to the labour market. The benefit of implementing the new safer technology is thereby underestimated from a societal perspective. To make the results as precise as possible, a clear delineation of the study perspective was chosen, as it deals with a very complex reality. Looking at the workflow in an ICU setting, availability because of repairs or downtime in relation to reprocessing could be of concern. Therefore, implementing a single-use technology could mean the constant availability of flexible video bronchoscopes. This is important when dealing with one or perhaps multiple emergency situations. This scenario was not included in the analysis but could mean the benefits of implementing the new single-use technology are underestimated. The lack of availability can also result in personnel waiting time and thereby added costs per procedure.
Environmental factors, such as increased waste disposal and handling with disposable single-use flexible video bronchoscopes, could influence the result through an overestimation of the benefits. Ethical considerations are also a subject for discussion. Some would argue that patient safety should always come first and that safer technology should always be implemented regardless of the associated cost. This could also mean that certain healthcare providers might not offer certain treatments. Others would argue that resources are scarce and prioritization should be based on specific evaluations to secure the best safety for the money.
Finally, our study only applies to an ICU setting with immunocompromised patients, and the probabilities of risk have been estimated based on this setting. Therefore, the choice of strategy might differ in case settings other than ICUs.
Remarkably few cases of cross-contamination have been reported in the literature, and more research, preferably prospective, is needed to increase the level of evidence on this topic.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Christoffer Lilja Terjesen performed the modelling, including data collection and writing of the manuscript. Lars Ehlers and Julia Kovaleva contributed to the conception of the study, the overall design, and critical revision. All authors approved the manuscript.
Compliance with Ethical Standards
Funding
No funding was received for this study.
Conflict of interest
No conflicts of interests exist for Christoffer Lilja Terjesen, Julia Kovaleva or Lars Ehlers.
Data availability statement
All data input and details on modelling supporting this study are provided in the manuscript and the supplementary information accompanying this paper. Readers should be able to replicate the model in TreeAge, Microsoft® Excel or other preferred software.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s41669-017-0012-9) contains supplementary material, which is available to authorized users.
References
- 1.Sculpher M, Drummond M, Buxton M. The iterative use of economic evaluation as part of the process of health technology assessment. J Health Serv Res Policy. 1997;2(1):26–30. doi: 10.1177/135581969700200107. [DOI] [PubMed] [Google Scholar]
- 2.Dong H, Buxton M. Early assessment of the likely cost-effectiveness of a new technology: a Markov model with probabilistic sensitivity analysis of computer-assisted total knee replacement. Int J Technol Assess Health Care. 2006;22(2):191–202. doi: 10.1017/S0266462306051014. [DOI] [PubMed] [Google Scholar]
- 3.Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making? Health Econ. 2006;15:677–687. doi: 10.1002/hec.1093. [DOI] [PubMed] [Google Scholar]
- 4.Muscarella LF. Dear Los Angeles Times: the risk of disease transmission during gastrointestinal endoscopy. Gastroenterology Nurs. 2004;27:271–278. doi: 10.1097/00001610-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Wang K, Mehta AC, Turner JF, editors. Flexible bronchoscopy. 3. Chichester: Wiley-Blackwell; 2011. [Google Scholar]
- 6.Kupeli E, Karnac D, Mehta AC. Murray and Nadel’s textbook of respiratory medicine: flexible bronchoscopy. 5. Philadelphia: Elsevier-Saunders; 2010. pp. 485–505. [Google Scholar]
- 7.Kraft M. Goldman-Cecil medicine: approach to the patient with respiratory disease. 24. Philadelphia: Elsevier-Saunders; 2011. pp. 512–516. [Google Scholar]
- 8.Reynolds HY. Goldman-Cecil medicine: respiratory structure and function: mechanisms and testing. 24. Philadelphia: Elsevier-Saunders; 2011. pp. 523–527. [Google Scholar]
- 9.Rutala WA, Weber DJ, The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Centers for Disease Control and Prevention; 2008. http://www.cdc.gov/hicpac/Disinfection_Sterilization/2_approach.html. Accessed 12 July 2016.
- 10.Center for Biologics Evaluation and Research. Reprocessing medical devices in health care settings: validation methods and labeling. Guidance for industry and Food and Drug Administration staff. US Food and Drug Administration. Silver Spring, MD: US FDA; 2015. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM253010.pdf. Accessed 12 July 2016.
- 11.US Food and Drug Administration. Medical devices. What are reusable medical devices? Silver Spring, MD: US FDA; 2015. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ReprocessingofReusableMedicalDevices/ucm454619.htm. Accessed 12 July 2016.
- 12.US Food and Drug Administration. Medical Devices. FDA-cleared sterilants and high level disinfectants with general claims for processing reusable medical and dental devices: March 2015. Silver Spring, MD: US FDA; 2015. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofReusableMedicalDevices/ucm437347.htm. Accessed 12 July 2016.
- 13.Silva CV, Magalhães VD, Pereira CR, Kawagoe JY, Ikura C, Ganc AJ. Pseudo-outbreak of Pseudomonas aeruginosa and Serratia marcescens related to bronchoscopes. Infect Control Hosp Epidemiol. 2003;24:195–197. doi: 10.1086/502195. [DOI] [PubMed] [Google Scholar]
- 14.Bou R, Aguilar A, Perpinan J, Ramos P, Peris M, Lorente L, et al. Nosocomial outbreak of Pseudomonas aeruginosa infections related to a flexible bronchoscope. J Hosp Infect. 2006;64:129–135. doi: 10.1016/j.jhin.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Kovaleva J, Peters FTM, van der Mei HC, Degener JE. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013;26:231–254. doi: 10.1128/CMR.00085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristensen MS, Møller J. Airway management behaviour, experience and knowledge among Danish anaesthesiologists: room for improvement. Acta Anaesthesiol Scand. 2001;45:1181–1185. doi: 10.1034/j.1399-6576.2001.450921.x. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan A. Epidemiology and prevention of infections related to endoscopy. Curr Infect Dis Rep. 2003;5:467–472. doi: 10.1007/s11908-003-0088-5. [DOI] [PubMed] [Google Scholar]
- 18.Pajkos A, Vickery K, Cossart Y. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J Hosp Infect. 2004;58:224–229. doi: 10.1016/j.jhin.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Buss AJ, Been MH, Borgers RP, Stokroos I, Melchers WJG, Peters FTM, et al. Endoscope disinfection and its pitfalls: requirement for retrograde surveillance cultures. Endoscopy. 2008;40:327–332. doi: 10.1055/s-2007-995477. [DOI] [PubMed] [Google Scholar]
- 20.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vickery K, Pajkos A, Cossart Y. Removal of biofilm from endoscopes: evaluation of detergent efficiency. Am J Infect Control. 2004;32:170–176. doi: 10.1016/j.ajic.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Herve R, Keevil CW. Current limitations about the cleaning of luminal endoscopes. J Hosp Infect. 2012;83:22–29. doi: 10.1016/j.jhin.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Marion-Ferey K, Pasmore M, Stoodley P, Wilson S, Husson GP, Costerton JW. Biofilm removal from silicone tubing: an assessment of the efficacy of dialysis machine decontamination procedures using an in vitro model. J Hosp Infect. 2003;53:64–71. doi: 10.1053/jhin.2002.1320. [DOI] [PubMed] [Google Scholar]
- 24.Kovaleva J, Meessen NEL, Peters FTM, Been MH, Arends JP, Borgers RP, et al. Is bacteriologic surveillance in endoscope reprocessing stringent enough? Endoscopy. 2009;41:913–916. doi: 10.1055/s-0029-1215086. [DOI] [PubMed] [Google Scholar]
- 25.Schelenz S, French G. An outbreak of multidrug-resistant Pseudomonas aeruginosa infection associated with contamination of bronchoscopes and an endoscope washer-disinfector. J Hosp Infect. 2000;46:23–30. doi: 10.1053/jhin.2000.0800. [DOI] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. Medical devices. Reprocessing of reusable medical devices: information for manufacturers. Silver Spring, MD: US FDA; 2015. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofReusableMedicalDevices/. Accessed 12 July 2016.
- 27.US Food and Drug Administration. Medical devices. FDA recommends health care facilities transition from custom ultrasonics endoscope washer/disinfectors to alternate reprocessing methods: FDA Safety Communication. Silver Spring, MD: US FDA; 2015. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm472462.htm. Accessed 12 July 2016.
- 28.US Food and Drug Administration. Medical devices. Infections associated with reprocessed flexible bronchoscopes: FDA safety communication. Silver Spring, MD: US FDA; 2015. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm462949.htm. Accessed 12 July 2016.
- 29.Centers for Disease Control and Prevention. Health Alert Network. Immediate need for healthcare facilities to review procedures for cleaning, disinfecting, and sterilizing reusable medical devices. Atlanta, GA: CDC; 2015. http://emergency.cdc.gov/han/han00382.asp. Accessed 12 July 2016.
- 30.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015. [Google Scholar]
- 31.Briggs A, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2008. [Google Scholar]
- 32.Kirschke DL, Jones TF, Craig AS, Chu PS, Mayernick GG, Patel JA, et al. Pseudomonas aeruginosa and Serratia marcescens contamination associated with a manufacturing defect in bronchoscopes. N Engl J Med. 2003;348:214–220. doi: 10.1056/NEJMoa021791. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan A, Wolfenden LL, Song X, Mackie K, Hartsell TL, Jones HD, et al. An outbreak of Pseudomonas aeruginosa infections associated with flexible bronchoscopes. N Engl J Med. 2003;348:221–227. doi: 10.1056/NEJMoa021808. [DOI] [PubMed] [Google Scholar]
- 34.Mughal MM, Minai OA, Culver DA, Mehta AC. Reprocessing the bronchoscope: the challenges. Semin Respir Crit Care Med. 2004;25:443–449. doi: 10.1055/s-2004-832717. [DOI] [PubMed] [Google Scholar]
- 35.Mehta AC, Prakash UBS, Garland R, Haponik EF, Moses L, Schaffner W, et al. American College of Chest Physicians and American Association for Bronchology Consensus Statement. Chest. 2005;128:1742–1755. doi: 10.1378/chest.128.3.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linstone HA, Turoff M, editors. The Delphi method: techniques and applications. 1. Boston: Addison-Wesley Publishing; 1975. [Google Scholar]
- 37.Yousuf MI. The Delphi technique. Essays Educ. 2007;20:1–10. [Google Scholar]
- 38.Bryman A, Bell E. Business research methods. 3. Oxford: Oxford University Press; 2011. [Google Scholar]
- 39.Aïssou M, Coroir M, Debes C, Camus T, Hadri N, Gutton C, et al. Cost analysis comparing single-use (Ambu® aScope™) and conventional reusable fiberoptic flexible scopes for difficult tracheal intubation. Ann Fr Anesth Reanim. 2013;32:291–295. doi: 10.1016/j.annfar.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Liu SS, Brodsky JB, Macario A. Cost identification analysis of anesthesia fiberscope use for tracheal intubation. J Anesth Clin Res. 2012;3:3–6. [Google Scholar]
- 41.Gupta D, Wang H. Cost-effectiveness analysis of flexible optical scopes for tracheal intubation: a descriptive comparative study of reusable and single-use scopes. J Clin Anesth. 2011;23:632–635. doi: 10.1016/j.jclinane.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Tvede MF, Kristensen MS, Nyhus-Andreasen M. A cost analysis of reusable and disposable flexible optical scopes for intubation. Acta Anaesthesiol Scand. 2012;56:577–584. doi: 10.1111/j.1399-6576.2012.02653.x. [DOI] [PubMed] [Google Scholar]
- 43.McCahon RA, Whynes DK. Cost comparison of re-usable and single-use fibrescopes in a large English teaching hospital. Anaesthesia. 2015;70:699–706. doi: 10.1111/anae.13011. [DOI] [PubMed] [Google Scholar]
- 44.Anderson DJ, Kirkland KB, Kaye KS, Thacker PA, II, Kanafani ZA, Auten G, et al. Underresourced hospital infection control and prevention programs: penny wise, pound foolish? Infect Control Hosp Epidemiol. 2007;28:767–773. doi: 10.1086/518518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data input and details on modelling supporting this study are provided in the manuscript and the supplementary information accompanying this paper. Readers should be able to replicate the model in TreeAge, Microsoft® Excel or other preferred software.