Abstract
We have previously reported that bacterial flavohemoglobin (HMP) catalyzes both a rapid reaction of heme-bound O2 with nitric oxide (NO) to form nitrate [HMP-Fe(II)O2 + NO → HMP-Fe(III) + NO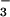 ] and, under anaerobic conditions, a slower reduction of heme-bound NO to an NO− equivalent (followed by the formation of N2O), thereby protecting against nitrosative stress under both aerobic and anaerobic conditions, and rationalizing our finding that NO is rapidly consumed across a wide range of O2 concentrations. It has been alternatively suggested that HMP activity is inhibited at low pO2 because the enzyme is then in the relatively inactive nitrosyl form [koff/kon for NO (0.000008 μM) ≪ koff/kon for O2 (0.012 μM) and KM for O2 = 30–100 μM]. To resolve this discrepancy, we have directly measured heme-ligand turnover and NADH consumption under various O2/NO concentrations. We find that, at biologically relevant O2 concentrations, HMP preferentially binds NO (not O2), which it then reacts with oxygen to form nitrate (in essence NO− + O2 → NO
] and, under anaerobic conditions, a slower reduction of heme-bound NO to an NO− equivalent (followed by the formation of N2O), thereby protecting against nitrosative stress under both aerobic and anaerobic conditions, and rationalizing our finding that NO is rapidly consumed across a wide range of O2 concentrations. It has been alternatively suggested that HMP activity is inhibited at low pO2 because the enzyme is then in the relatively inactive nitrosyl form [koff/kon for NO (0.000008 μM) ≪ koff/kon for O2 (0.012 μM) and KM for O2 = 30–100 μM]. To resolve this discrepancy, we have directly measured heme-ligand turnover and NADH consumption under various O2/NO concentrations. We find that, at biologically relevant O2 concentrations, HMP preferentially binds NO (not O2), which it then reacts with oxygen to form nitrate (in essence NO− + O2 → NO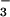 ). During steady-state turnover, the enzyme can be found in the ferric (FeIII) state. The formation of a heme-bound nitroxyl equivalent and its subsequent oxidation is a novel enzymatic function, and one that dominates the oxygenase activity under biologically relevant conditions. These data unify the mechanism of HMP/NO interaction with those recently described for the nematode Ascaris and mammalian hemoglobins, and more generally suggest that the peroxidase (FeIII)-like properties of globins have evolved for handling of NO.
). During steady-state turnover, the enzyme can be found in the ferric (FeIII) state. The formation of a heme-bound nitroxyl equivalent and its subsequent oxidation is a novel enzymatic function, and one that dominates the oxygenase activity under biologically relevant conditions. These data unify the mechanism of HMP/NO interaction with those recently described for the nematode Ascaris and mammalian hemoglobins, and more generally suggest that the peroxidase (FeIII)-like properties of globins have evolved for handling of NO.
The very rapid reaction of oxygenated hemoglobin (oxy Hb) with nitric oxide (NO) has been extensively studied because of its putative importance in modulating NO bioactivity. The heme-bound O2 has partial superoxide character (1–3) and thus reacts with NO to form NO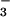 and ferric Hb (refs. 4 and 5; Eq. 1).
and ferric Hb (refs. 4 and 5; Eq. 1).
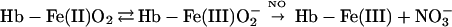 |
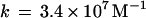 |
1 |
It was originally suggested that this reaction represents the major route of nitrate formation and hence of NO elimination in mammals, but more recent studies have questioned this premise on multiple grounds (6–9) and have shown that NO can escape elimination under physiological conditions by reacting directly with the vacant hemes of partially oxygenated Hb (7). Moreover, further work has provided support for the idea that the NO group bound to the hemes either can transfer oxidatively to a critical cysteine within the proximal heme pocket (to form a bioactive S-nitrosothiol; Eq. 2) or can be eliminated through a nitrate-generating reaction that involves the intermediacy of bound nitroxyl anion (NO−) and that contributes to endogenous methemoglobin formation in mammals (Eq. 3). (The same reaction produces N2O under anaerobic conditions; ref. 10.) Although the concentration of NO and the quaternary structure of Hb (R vs. T) ultimately determine the fate of heme-bound NO in vivo (i.e., Eq. 2 vs. Eq. 3), it is notable that heme iron oxidation effectively supports the redox requirements of both heme-to-thiol NO group transfer and NO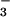 formation.
formation.
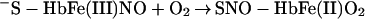 |
2 |
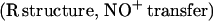 |
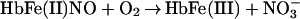 |
3 |
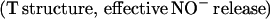 |
The two-domain flavohemoglobins (HMP) are found in certain bacteria and yeasts and protect these organisms against the toxic effects of NO (9, 11–15). The mechanism of NO detoxification has been attributed to a rapid NO oxygenase activity that generates nitrate aerobically (Eq. 4; refs. 12 and 13), and a slower NO reductase activity that operates under strictly anaerobic conditions to produce N2O (Eq. 5; refs. 9, 12, and 15). These reactions are thus directly analogous to those of mammalian hemoglobin, only they are catalytically driven by a flavoreductase domain that utilizes NADH to recycle the oxidized heme (Eqs. 6 and 7; ref. 12).
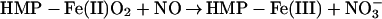 |
4 |
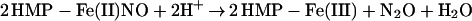 |
5 |
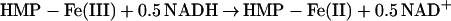 |
6 |
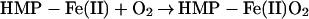 |
7 |
There are caveats, however, with our previous mechanistic studies, which were done at either very high pO2 or complete anaerobiosis (9, 12)—conditions rarely encountered in nature. Gardner et al. (13) have also proposed that HMP has oxygenase activity, but they did not actually determine the identity of the heme ligand during turnover. It has recently been determined that koff/kon for NO (0.000008 μM) ≪ koff/kon for O2 (0.012 μM; ref. 16), indicating that NO should out-compete O2 for binding to HMP at physiological NO/O2 concentrations, and that KM for O2 = 30–100 μM (16, 17). However, whereas these data have been interpreted as evidence for NO inhibition of the oxygenase activity at low O2 (16), we previously reported rapid O2-dependent NO consumption by HMP under these conditions, and rapid turnover of nitrosyl heme upon exposure to O2 (12). Thus, it seems unlikely that the oxygenase function of HMP accounts for protection against NO in biologically relevant situations. Here, we provide a unifying explanation for the “oxygenase/reductase” activities previously ascribed to HMP and reconcile these activities with the recently reported kinetic parameters, by showing that, in fact, HMP is an O2 nitroxylase (or “denitrosylase”). That is, HMP catalyzes what is, in effect, the NO−/O2 reaction.
Materials and Methods
Cell Growth and Enzyme Purification.
A heat-inducible expression system was used for production of HMP (18) as described (12). HMP was purified by anion exchange chromatography on Q Sepharose and MonoQ, and gel filtration on Superdex 200 (12) (Pharmacia).
Spectroscopy.
Assays were performed in 0.1 M phosphate buffer containing 0.1 mM EDTA. Glass syringes without headspace were used to handle solutions. A stopped flow apparatus equipped with a photodiode array detector (Applied Photophysics, Surrey, U.K.) was made anaerobic by flushing both channels with a mixture of 2 μM anaerobic HMP and 0.2 mM NADH. Anaerobiosis was verified by the HMP spectrum. The system was then flushed with anaerobic buffer to remove HMP, leaving the syringes attached. In this configuration, anaerobiosis was maintained for several days. The concentration of NO was manipulated by mixing a saturated NO solution with the desired volume of buffer by using connected glass syringes in a glove box. One syringe was then sealed with a rubber stopper, and connected to the stopped flow apparatus, while flushing ≈0.2 ml from the syringe into the syringe port of the instrument to remove any oxygen introduced during the syringe exchange. Oxygen concentrations were controlled by mixing anaerobic buffer with air-saturated buffer outside the glove box. Two configurations were used to initiate the assay. In the first (used for competition experiments), HMP solutions containing various concentrations of O2 (channel 1) were mixed with anaerobic NADH-containing NO solutions (channel 2). In the second configuration, the preformed, anaerobic ferrous-nitrosyl complex (in the presence of NADH) was mixed with buffer containing different O2 concentrations. Experiments were performed immediately to avoid NADH consumption because of NO reductase activity (12, 15, 16).
Deconvolution of Spectra.
Extinction coefficient spectra of the various liganded forms were generated, and deconvolution of the experimental spectra was done by a least squares regression fitting procedure using mathcad 2000. All spectra were mathematically fitted to 95% confidence.
Results
Ligand Binding During Turnover in the Presence of High O2 Concentrations.
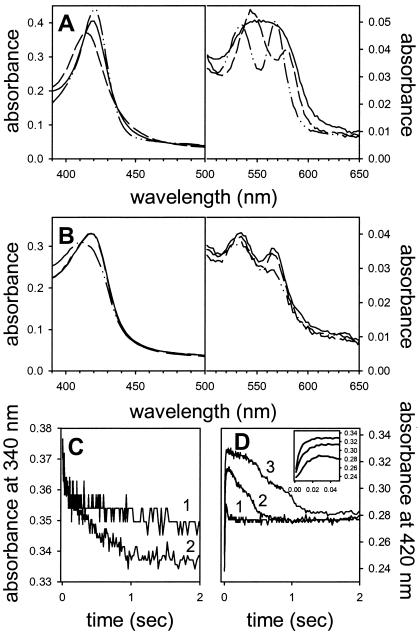
We followed competitive ligand-binding by stopped-flow spectrophotometry in the presence of 125 μM O2 and varying concentrations of NO. The experiments were conducted at 10°C because many reactions would otherwise be completed within the dead time of the instrument (1.3 ms). Steady-state reference spectra of the ligated [Fe(II)NO, Fe(II)O2, Fe(III)NO] forms of HMP are shown in Fig. 1A. The oxy form is characterized by peaks at 415, 543, and 578 nm. The ferric-nitrosyl form has peaks at 419, 533, and 567 nm, and the ferrous-nitrosyl enzyme exhibits a peak at 418 nm and a broad absorbance between 549 and 575 nm. Fig. 1B shows the spectra formed immediately after mixing an aerated (final O2 concentration, 125 μM) HMP-Fe(III) solution with anaerobic NO and NADH. In the presence of 50 or 20 μM NO, the initial spectra show absorbance maxima at 419, 533, and 567 nm, consistent with the formation of ferric nitrosyl HMP (Fe(III)NO). Notably, no oxy HMP is seen. The spectra are broader and less pronounced than those of the pure ferric nitrosyl form, however, suggesting the presence of a small amount of Fe(II)NO. At 10 μM NO, the Soret peak is shifted to 416 nm, and the overall spectrum is consistent with a mixture of species [ferric nitrosyl, oxy and/or ferric HMP (12), and perhaps ferrous nitrosyl]. Fig. 1C shows that NADH substrate is rapidly consumed while the nitrosyl spectra are observed. Thus, the enzyme is evidently consuming NO (see also ref. 12) that is bound to the heme (steady-state). Fig. 1D shows the spectral change at 420 nm (the maximum difference in absorbance between the oxy and nitrosyl forms of HMP, a measure of nitrosyl hemes). NO binding to the hemes is shown to be extremely rapid (Fig. 1D Inset; also see ref. 19), and coincides with very fast initial NADH consumption (Fig. 1C), which we attribute to pre-steady state reduction of the oxidized heme. The concentration of the nitrosyl HMP then decreases coincident with continued NADH consumption and ultimately both flatten out once NO is consumed (Fig. 1 C and D). Taken together, these studies reveal that NO binding to the heme out-competes O2 even at high, supraphysiological ratios of O2 to NO (see more below). Our results are consistent with the previous determination of NO ≫ O2 affinities of HMP reported by Gardner et al. (19); however, whereas these authors had viewed the nitrosyl-ligated HMP complex as enzymatically inactive, our studies clearly show otherwise.
Figure 1.
HMP turnover involves nitrosyl heme. (A) Reference spectra of pure ferrous-nitrosyl [Fe(II)NO; solid line], ferric-nitrosyl [Fe(III)NO; dashed-dotted line], and oxy [Fe(II)O2; dashed line] hemoglobin were generated in the stopped flow apparatus by mixing anaerobic HMP with or without 100 μM NADH plus anaerobic NO or aerobic buffer. Final concentrations were 50 μM NO or 125 μM O2 (B) HMP in aerobic buffer was mixed with a solution of anaerobic NADH and 50 μM (solid line), 20 μM (dashed line), or 10 μM NO (dashed-dotted line), and the spectra were recorded during turnover (16 ms after mixing). The O2 concentration in these experiments was 125 μM. (C) NADH consumption followed at 340 nm (line 1, 10 μM NO; line 2, 20 μM NO), and (D) formation and decomposition of the nitrosyl-heme complex followed at 420 nm in the experiment shown in B. NADH is consumed with a nitrosyl–heme complex present. The Inset in D shows the very rapid formation of the nitrosyl–heme complex. Line 1, 10 μM NO; line 2, 20 μM NO; line 3, 50 μM NO. The traces shown shown in C and D have been averaged from three measurements. Conditions: phosphate buffer (pH 7, 100 mM), 0.1 mM EDTA, 1 cm path length, 10°C.
Reaction of Nitrosyl-HMP with Oxygen.
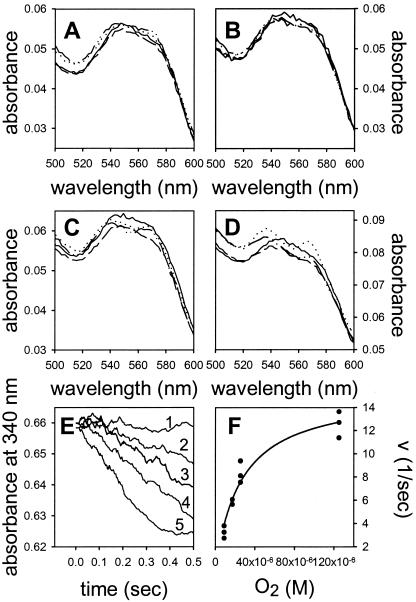
To further verify O2-dependent turnover of the nitrosyl form of HMP, the ferrous nitrosyl complex was preformed by anaerobic incubation of HMP with NADH and NO, and this complex was then mixed in the stopped-flow apparatus with buffer containing various concentrations of O2. [The ferrous nitrosyl complex is comparatively stable under anaerobic conditions, with NO being consumed by reduction to N2O at less than 1% of the aerobic rate of its conversion to nitrate (9, 12, 15, 19).] Fig. 2 shows that the initial spectra (1.3 to 290 ms) are predominantly ferrous nitrosyl at low O2 concentrations (Fig. 2 A and B) and that increasing ferric nitrosyl character develops at higher O2 concentrations (Fig. 2 C and D). Remarkably, even at the highest O2 concentration of 125 μM, features of an oxy spectrum are largely absent. NADH consumption, measured while the spectra in Fig. 2 A–D were recorded, occurs at rates proportional to O2 concentration (Fig. 2E, lines 1–5). The NADH consumption rates followed Michaelis-Menten kinetics, with an apparent KM for oxygen of 25 μM and a kcat of 15 s−1 (NO consumed per heme) at 10°C (Fig. 2E). Thus, we conclude that HMP demonstrates O2-driven (and slower anaerobic) enzymatic turnover of nitrosyl HMP, but does not exhibit dioxygenase activity, which entails binding and activation of O2. Moreover, inasmuch as Fe(II)NO ⇄ Fe(III)NO− (10), and N2O is produced by HMP in the absence of O2 (12, 15), these results [as well as our previous analyses of products (12)] are best rationalized by reaction of O2 with a ferric-NOδ− species to produce nitrate (12). More generally stated, the approach of O2 to the heme-bound NO will decrease the electron density on the iron and increase the nitroxyl anion character of the ligand. The direct binding of NO to FeIII may also explain our previous observation (12) that the enzyme produces some nitrite during turnover (see equations 1 and 2 in ref. 10).
Figure 2.
Nitrosyl-hemoglobin reacts rapidly with oxygen. HMP was incubated anaerobically with NO and NADH to generate the ferrous-nitrosyl heme complex and then mixed with buffer containing different concentrations of oxygen. Oxygen concentrations after mixing were (A) 9 μM, (B) 17 μM, (C) 26 μM, and (D) 125 μM; the final NO concentration was 25 μM. The spectra were recorded 1.3 ms (solid line), 11.5 ms (dashed line), 75 ms (dashed-dotted line), and 290 ms (dotted line) after mixing. Each spectrum is averaged from 2–3 traces. (E) NADH was consumed in an O2-dependent manner while the spectra in A–D were recorded. The absorbance was followed anaerobically (line 1) or in the presence of 9 μM (line 2), 17 μM (line 3), 26 μM (line 4), and 125 μM O2 (line 5). The lines are averaged from 2–3 traces and smoothed by using the moving average technique. (F) NADH consumption as a function of oxygen concentration for the traces shown in E. The line is a least squares fit of the data to the Michaelis-Menten equation. Conditions: phosphate buffer (pH 7, 100 mM), 0.1 mM EDTA, 1 cm path length, 10°C.
Turnover at High NO Concentrations.
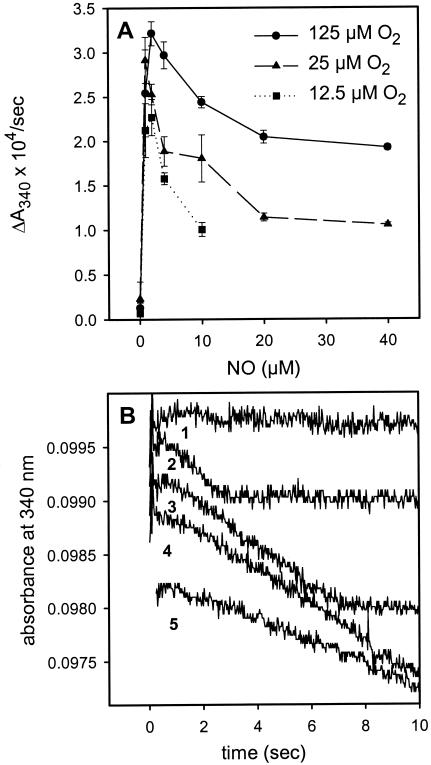
Recent studies have concluded that, at low oxygen concentrations, HMP turnover would occur via NO reduction instead of oxygenation (16, 17), and therefore at a significantly slower rate [NO reductase activity is ≈0.1–1% of the oxygenase activity (9, 15, 16)]. However, our original report showed otherwise (12), and more recent studies by Mills et al. confirm our finding of rapid consumption of high concentrations of NO at low pO2 (17). Moreover, it does not make sense that the enzyme would be inhibited by 1–3 μM NO, as recently suggested (16), because these concentrations are not even toxic to bacteria and HMP protects against far higher concentrations in vivo (11, 12). To further investigate this matter, we measured NADH consumption over a range of NO concentrations. Fig. 3 shows that there is indeed a slight decrease in rate when NO concentrations exceed 2 μM. However, even with NO concentrations of 40 μM and O2 < 10 μM [conditions where the enzyme is in the nitrosyl form (Fig. 3)], rates of NADH consumption remain at about 50% of those measured at very low NO concentrations (Fig. 3A; ref. 17), and the stoichiometry of two NO consumed per NADH oxidized is preserved (Fig. 3B; ref. 12). Our results thus demonstrate that HMP efficiently turns over in the nitrosyl form at low pO2. We conclude that the nitroxyl–oxidation reaction is about 50% of the nitric oxide–oxygenation reaction. That this relative slowing in the denitrosylase mode is functionally insignificant can be understood by appreciating that HMP protects anaerobically by using the far slower NO reductase mechanism (9, 11). Thus, NO does not inhibit the enzyme in any meaningful way.
Figure 3.
Dependence of NADH consumption on NO concentration. (A) HMP (0.2 μM) was incubated in buffer to give the indicated O2 concentrations and mixed with anaerobic buffer containing NADH (100 μM) and NO. (B) Linear NADH consumption at low pO2. The absorbance traces at 12.5 μM O2 from A for no NO (line 1), 1 μM NO (line 2), 2 μM NO (line 3), 5 μM NO (line 4), and 10 μM NO (line 5). Two molecules of NO are consumed per molecule of NADH. Conditions: phosphate buffer (pH 7, 100 mM), 0.1 mM EDTA, 0.2 cm pathlength, 10°C.
Discussion
Here, we establish that HMP catalyzes the reaction of heme-bound NO (in essence NO−) with O2 to produce nitrate. The reaction is analogous to the classical oxy Hb reaction in which O2 is partially reduced (to O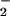 ) at the heme and subsequently reacted with NO to produce nitrate. But NO has a much lower redox potential than O2 (20), and generation of an NO− equivalent is therefore less efficient (7, 10). Nature has evidently overcome this problem by attaching a flavoreductase domain to the hemoglobin and by placing the flavin and heme in unusually close proximity (21). In the absence of O2, the reaction proceeds more slowly, yielding N2O by dimerization and dehydration of HNO (12, 15). Both aerobic and anaerobic reactions proceed via an FeIII (met) hemoglobin intermediate, consistent with reports that HMP is structurally similar to peroxidases (22), and in line with the peroxidase-like function of nematode hemoglobin that consumes NO (23). Further, these data are consistent with our recent studies showing that methemoglobin and nitrate formation in mammals can be rationalized by a nitroxyl-generating reaction mechanism (ref. 10; Eqs. 3 and 5).
) at the heme and subsequently reacted with NO to produce nitrate. But NO has a much lower redox potential than O2 (20), and generation of an NO− equivalent is therefore less efficient (7, 10). Nature has evidently overcome this problem by attaching a flavoreductase domain to the hemoglobin and by placing the flavin and heme in unusually close proximity (21). In the absence of O2, the reaction proceeds more slowly, yielding N2O by dimerization and dehydration of HNO (12, 15). Both aerobic and anaerobic reactions proceed via an FeIII (met) hemoglobin intermediate, consistent with reports that HMP is structurally similar to peroxidases (22), and in line with the peroxidase-like function of nematode hemoglobin that consumes NO (23). Further, these data are consistent with our recent studies showing that methemoglobin and nitrate formation in mammals can be rationalized by a nitroxyl-generating reaction mechanism (ref. 10; Eqs. 3 and 5).
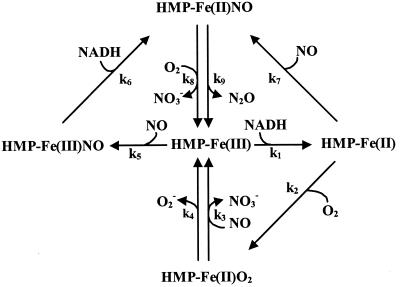
Fig. 4 shows a revised model of HMP turnover that highlights the competitive nature of several reaction pathways. Ferric HMP can react with either NO or NADH to form HMP-Fe(III)NO or HMP-Fe(II) (deoxy), respectively. The affinities for both substrates are high (KMs are low to submicromolar), and one would anticipate that these reactions proceed at significant rates. The former reaction appears to predominate, however, at least in vitro, and, furthermore, NO has much higher affinity for HMP-Fe(II) than O2. Thus, two pathways lead to nitrosyl formation [HMP-Fe(III) + NO and HMP-Fe(II) + NO], whereas only one low-affinity pathway leads to HMP-Fe(II)O2, and accordingly, the binding of NO is favored over O2. The rate-determining step in the “denitrosylase” mechanism appears to be k8/k9, thus explaining the accumulation of HMP-Fe(II)NO at low oxygen concentrations. At high pO2, HMP-Fe(II)NO reacts rapidly with O2, increasing the FeIII character, and HMP-Fe(III) can also rebind NO. Ferric-nitrosyl spectra thus predominate at high O2. By contrast, HMP-Fe(II)O2 spectra are not seen under biologically relevant situations; they are largely an artifact of in vitro conditions (e.g., seen at very low nontoxic (<μM) NO concentrations in the presence of very high nonphysiological (>125 μM) O2 concentrations). Bacteriostatic NO concentrations are in the high micromolar to millimolar range (11, 12, 24), and infected tissues are characterized by very low pO2 (25–27). Pathogenic bacteria are therefore likely to encounter NO at relatively high concentrations and O2 at relatively low concentrations. Our results thus rationalize the protection conferred by HMP against nitrosative stress under anaerobic and microaerobic conditions and more generally give credence to phylogenetic analyses showing that the flavohemoglobin is found in numerous human pathogens that inhabit low pO2 environments.
Figure 4.
Competitive substrate binding in the catalytic cycle of HMP. Ferric HMP can react with either NADH (k1) or NO (k5) to form ferrous HMP or ferric-nitrosyl HMP, respectively. The latter is reduced by NADH to ferrous-nitrosyl HMP (k6). NO (k7) and O2 (k2) compete for ferrous HMP to form the ferrous-nitrosyl or oxy forms, respectively. NO wins out under biologically relevant conditions (see text). The oxy and ferrous-nitrosyl forms complete the cycle by reacting with NO (k3) or O2 (k8), respectively, to generate nitrate and ferric HMP. Because of HMP's higher affinity for NO than O2, and because NO binds both ferric and ferrous heme whereas O2 only binds the ferrous form, the reaction will proceed mainly through the nitrosyl (denitrosylase) pathway (k5 or k1/k7). The slow oxidase (k4) and anaerobic NO reductase (k9) reactions are also depicted, although only the latter is known to have functional significance (9, 11). Reverse reactions and internal electron transfer steps were omitted for clarity. The nitrosyl HMP spectra seen during turnover may be interpreted in terms of a resonance between HMP(FeII)NO ⇄ HMP(Feδ+III)NOδ−, and the position of the relative charge may be dependent on O2. Higher pO2 would increase the FeIII character of the heme through formation of a “dioxynitrosyl” intermediate.
Hemoglobins are of ancient evolutionary origin. Sequence alignments of hemoglobins (28–30) and their identification in Deinococcus (31) and Aquifex (32) [two organisms close to the root of the phylogenetic tree (33)] suggest that they existed ≈2 billion years ago, well before oxygen accumulated in the atmosphere (34). It had been speculated that the ancestral hemoglobin therefore had a redox function (30, 35), and it now seems possible that the nature of this redox reaction was NO transformation and that the function was protection from nitrosative stress. Large amounts of abiotically formed NO were likely present in the early atmosphere and in deep thermal vents (34, 36–38), and “leakage” of NO during denitrification and nitrate respiration may have imposed an additional threat to early life (38, 39). Microbial hemoglobin thus evolved preferential high affinity reactions with NO, which they metabolize to N2O (9, 12, 15). The toxicity of NO is potentiated by O2, and, accordingly, our findings suggest that hemoglobin adapted the NO−/O2 reaction as a means to detoxify the two molecules, while retaining its preference for NO. We recently reported that nematode hemoglobin, which sits at a bridging point in hemoglobin phylogeny, has evolved a different, cysteine-dependent mechanism to achieve NO/O2 detoxification (23). It is important to note, however, that a ferric nitrosyl intermediate is central to both enzymatic mechanisms, and that NO binding to ferric hemes of nematode hemoglobin similarly outcompetes the oxy Hb reaction (NO dioxygenation; Eq. 1). This result appears to have been achieved by substitution of an E7 histidine with glutamine, which dramatically increases the reactivity of NO with FeIII hemes (23), and which is also present in HMP (21, 22). NO also finds a way to preferentially bind hemes of mammalian oxy Hb, from which it transfers oxidatively to key cysteines that preserve its biological activity (7, 8, 10). The picture that emerges is one in which redox reactions and peroxidase (ferric heme)-mediated activities govern the interactions of NO with hemoglobins and help rationalize the molecular basis of its evolution. It is evident from studies with NO that the functional distinction between peroxidases, hemoglobins, and cytochromes is increasingly blurred.
Acknowledgments
We thank Irwin Fridovich for discussions. This work was supported by Grant ES09206 from the National Institutes of Health.
Abbreviations
- HMP
flavohemoglobin
- oxy Hb
oxygenated Hb
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Weiss J J. Nature (London) 1964;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- 2.Wittenberg J B, Wittenberg B A, Peisach J, Blumberg W E. Proc Natl Acad Sci USA. 1970;67:1846–1853. doi: 10.1073/pnas.67.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balagopalakrishna C, Abugo O O, Horsky J, Manoharan P T, Nagababu E, Rifkind J M. Biochemistry. 1998;37:13194–13202. doi: 10.1021/bi980941c. [DOI] [PubMed] [Google Scholar]
- 4.Doyle M P, Hoekstra J W. J Inorg Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 5.Eich R F, Li T, Lemon D D, Doherty D H, Curry S R, Aitken J F, Mathews A J, Johnson K A, Smith R D, Phillips G N, Jr, Olson J S. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 6.Jia L, Bonaventura C, Bonaventura J, Stamler J S. Nature (London) 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 7.Gow A J, Luchsinger B P, Pawloski J R, Singel D J, Stamler J S. Proc Natl Acad Sci USA. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahon T J, Stone A E, Bonaventura J, Singel D J, Stamler J S. J Biol Chem. 2000;275:16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Zeng M, Hausladen A, Heitman J, Stamler J S. Proc Natl Acad Sci USA. 2000;97:4672–4676. doi: 10.1073/pnas.090083597. . (First Published April 11, 2000; 10.1073/pnas.090083597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gow A J, Stamler J S. Nature (London) 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 11.Crawford M J, Goldberg D E. J Biol Chem. 1998;273:12543–12547. doi: 10.1074/jbc.273.20.12543. [DOI] [PubMed] [Google Scholar]
- 12.Hausladen A, Gow A G, Stamler J S. Proc Natl Acad Sci USA. 1998;95:14100–14105. doi: 10.1073/pnas.95.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner P R, Gardner A M, Martin L A, Salzman A L. Proc Natl Acad Sci USA. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Membrillo-Hernandez J, Coopamah M D, Anjum M F, Stevanin T M, Kelly A, Hughes M N, Poole R K. J Biol Chem. 1999;274:748–754. doi: 10.1074/jbc.274.2.748. [DOI] [PubMed] [Google Scholar]
- 15.Kim S O, Orii Y, Lloyd D, Hughes M N, Poole R K. FEBS Lett. 1999;445:389–394. doi: 10.1016/s0014-5793(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 16.Gardner A M, Martin L A, Gardner P R, Dou Y, Olson J S. J Biol Chem. 2000;275:12581–12589. doi: 10.1074/jbc.275.17.12581. [DOI] [PubMed] [Google Scholar]
- 17.Mills C E, Sedelnikova S, Soballe B, Hughes M N, Poole R K. Biochem J. 2001;353:207–213. doi: 10.1042/0264-6021:3530207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Love C A, Lilley P E, Dixon N E. Gene. 1996;176:49–53. doi: 10.1016/0378-1119(96)00208-9. [DOI] [PubMed] [Google Scholar]
- 19.Gardner P R, Gardner A M, Martin L A, Dou Y, Li T, Olson J S, Zhu H, Riggs A F. J Biol Chem. 2000;257:31581–31587. doi: 10.1074/jbc.M004141200. [DOI] [PubMed] [Google Scholar]
- 20.Bartberger M D, Fukuto J M, Houk K N. Proc Natl Acad Sci USA. 2001;98:2194–2198. doi: 10.1073/pnas.041481598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ermler U, Siddiqui R A, Cramm R, Friedrich B. EMBO J. 1995;14:6067–6077. doi: 10.1002/j.1460-2075.1995.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukai M, Mills C E, Poole R K, Yeh S R. J Biol Chem. 2000;276:7272–7277. doi: 10.1074/jbc.M009280200. [DOI] [PubMed] [Google Scholar]
- 23.Minning D M, Gow A J, Bonaventura J, Braun R, Dewhirst M, Goldberg D E, Stamler J S. Nature (London) 1999;401:497–502. doi: 10.1038/46822. [DOI] [PubMed] [Google Scholar]
- 24.De Groote M A, Granger D, Xu Y, Campbell G, Prince R, Fang F C. Proc Natl Acad Sci USA. 1995;92:6399–6403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hays R C, Mandell G L. Proc Soc Exp Biol Med. 1974;147:29–30. doi: 10.3181/00379727-147-38275. [DOI] [PubMed] [Google Scholar]
- 26.Simmen H P, Blaser J. Am J Surg. 1993;166:24–27. doi: 10.1016/s0002-9610(05)80576-8. [DOI] [PubMed] [Google Scholar]
- 27.Anning P B, Sair M, Winlove C P, Evans T W. Am J Respir Crit Care Med. 1999;159:1710–1715. doi: 10.1164/ajrccm.159.6.9801124. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, Riggs A F. Proc Natl Acad Sci USA. 1992;89:5015–5019. doi: 10.1073/pnas.89.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardison R C. Proc Natl Acad Sci USA. 1996;93:5675–5679. doi: 10.1073/pnas.93.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moens L, Vanfleteren J, Van de Peer Y, Peeters K, Kapp O, Czeluzniak J, Goodman M, Blaxter M, Vinogradov S. Mol Biol Evol. 1996;13:324–333. doi: 10.1093/oxfordjournals.molbev.a025592. [DOI] [PubMed] [Google Scholar]
- 31.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, et al. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deckert G, Warren P V, Gaasterland T, Young W G, Lenox A L, Graham D E, Overbeek R, Snead M A, Keller M, Aujay M, et al. Nature (London) 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 33.Pace N R. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 34.Kasting J F. Science. 1993;259:920–925. doi: 10.1126/science.11536547. [DOI] [PubMed] [Google Scholar]
- 35.Hardison R. J Exp Biol. 1998;201:1099–1117. doi: 10.1242/jeb.201.8.1099. [DOI] [PubMed] [Google Scholar]
- 36.Yung Y L, McElroy M B. Science. 1979;203:1002–1004. doi: 10.1126/science.203.4384.1002. [DOI] [PubMed] [Google Scholar]
- 37.Mancinelli R L, McKay C P. Origins Life Evol Biosphere. 1988;18:311–325. doi: 10.1007/BF01808213. [DOI] [PubMed] [Google Scholar]
- 38.Raven J A, Yin Z H. New Phytol. 1998;139:205–219. [Google Scholar]
- 39.Zumft W G. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]