Abstract
Polarized radial arrays of cytoplasmic microtubules (MTs) with minus ends clustered at the cell center define the organization of the cytoplasm through interaction with microtubule motors bound to membrane organelles or chromosomes. It is generally assumed that the radial organization results from nucleation of MTs at the centrosome. However, radial MT array can also be attained through self-organization that requires the activity of a minus-end-directed MT motor, cytoplasmic dynein. In this study we examine the role of cytoplasmic dynein in the self-organization of a radial MT array in cytoplasmic fragments of fish melanophores lacking the centrosome. After activation of dynein motors bound to membrane-bound organelles, pigment granules, the fragments rapidly form polarized radial arrays of MTs and position pigment aggregates at their centers. We show that rearrangement of MTs in the cytoplasm is achieved through dynein-dependent MT nucleation. The radial pattern is generated by continuous disassembly and reassembly of MTs and concurrent minus-end-directed transport of pigment granules bearing the nucleation sites.
In animal cells, cytoplasmic MTs are commonly organized into polarized radial arrays (1). Minus ends of MTs are clustered, whereas plus ends are free and display the behavior known as dynamic instability (2), alternating phases of growth and shortening. Therefore radial organization allows MTs to efficiently explore the intracellular space. In interphase, a radial array of MTs directs membrane trafficking and determines steady-state positions of organelles (3) through interaction with organelle-bound microtubule motors of the kinesin family [translocation mostly to MT plus ends or to the cell periphery (4)] or cytoplasmic dynein [translocation to the minus ends or to the center (5)]. During mitosis, the focal points of MT asters serve as the poles of the mitotic spindle. Interaction of MTs extending from the poles with the kinetochores of chromosomes, with each other, and with the cell cortex provides the structural basis for formation and positioning of the mitotic spindle (6, 7). Therefore polarized radial MT arrays play a key role in the organization of cytoplasm in interphase and during mitosis.
Focusing of the minus ends into a radial array is generally assumed to be the result of MT outgrowth from the centrosome, which provides stable nucleation templates (6). Polarized MT assemblies can also form through a self-organization mechanism, which requires the activity of MT motors (8). Information about the role of MT motors in the organization of focused MT arrays was provided to a large extent by the studies of the formation of MT asters and mitotic spindle poles in mitotic cell extracts (9–13). In the extracts, clustering of the MT minus ends was performed by a minus-end-directed motor, cytoplasmic dynein, which formed a large multisubunit complex with its activator dynactin and a high-molecular-weight protein, NuMa (9, 10, 13). To explain the dynein-dependent self-organization of MTs into focused arrays, a model was presented based on the inherent capacity of MT motors to recognize the intrinsic polarity of MTs (9, 14). The model required organization of dynein molecules into multivalent complexes, which were capable of interacting with more than one MT, and assumed that dynein molecules remained attached to MTs when they reached the minus end. The assembly of asters was explained by the unidirectional transport of MTs with plus ends leading, which resulted in the clustering of the minus ends (9, 14).
In support of the MT transport model, multimolecular assemblies of MT motors generated radial MT arrays in purified systems in vitro (15, 16). Polarity of MTs in the formed asters was indeed determined by the polarity of a MT motor (15, 16). Furthermore, in mitotic cell extracts short MT seeds were moved by cytoplasmic dynein to the mitotic spindle poles (11). Therefore, the results of in vitro studies indicate that MT motors are capable of arranging MTs into focused arrays by producing force for the unidirectional MT movement. However, such MT self-organization as a result of MT transport requires vigorous movement of MTs in the cytoplasm and therefore is apparently inconsistent with the role of MTs as immobile “rails” that direct the transport of membrane organelles and chromosomes.
In this study, we have attempted to elucidate the role of cytoplasmic dynein in the self-organization of a polarized radial MT array in vivo by examining the formation of MT asters in cytoplasmic fragments of melanophores. In melanophores thousands of membrane-bounded organelles, pigment granules, are rapidly transported to the cell center to form a tight aggregate or redisperse uniformly throughout the cytoplasm (17, 18). The granules move by means of the minus-end-directed MT motor cytoplasmic dynein (aggregation; ref. 19) or a plus-end-directed kinesin-related motor (dispersion; refs. 20 and 21). Microsurgically produced cytoplasmic fragments of melanophores lacking the centrosome rapidly form a polarized radial array after stimulation of minus-end-directed movement with adrenaline and position the pigment aggregate to a focal point of converging MTs (22, 23). Formation of the focused array depends on the activity of cytoplasmic dynein bound to the pigment granules (23). Therefore, melanophore fragments provide an excellent model system for the study of the role of cytoplasmic dynein in the establishment of radial MT organization.
Materials and Methods
Cell Culture.
Cultures of black tetra melanophores were prepared as described in (24). Aggregation of pigment granules was induced with 10−5 adrenaline and pigment dispersion with 5 mM caffeine.
Preparation of Cy3-Tubulin.
Porcine brain tubulin depleted of microtubule-associated proteins was labeled with Cy3-reactive dye (Amersham Pharmacia) as described (25) and used at a needle concentration of 10–15 mg/ml. For fluorescence speckle microscopy (26) needle concentration of tubulin was reduced to 0.3–0.5 mg/ml.
Production and Purificaton of Dynein Inhibitors.
Monoclonal antibody 74.1 against an intermediate chain of cytoplasmic dynein (ref. 27; a gift from Kevin Pfister, University of Virginia School of Medicine) was purified from ascitic fluid by chromatography on protein A–Agarose (Sigma). The recombinant 50-kDa subunit of dynactin complex, dynamitin (28), was expressed in bacteria (the expression vector was provided by Richard Vallee, University of Massachusetts Medical School) and purified by ammonium sulfate precipitation and Mono Q chromatography (29). Dynein antibody and dynamitin were used at needle concentrations of 3.3 and 15 mg/ml, respectively.
Microsurgery and Microinjection.
To prepare fragments, melanophore processes were dissected with microneedles with a 0.1-μm tip diameter. Microinjection was performed as described (24). After microinjection with fluorescently tagged tubulin subunits, cells were incubated for at least 1 h at 30°C to allow incorporation of labeled tubulin into microtubules.
Imaging and Data Analysis.
Cells injected with Cy3-tubulin were treated with the oxygen-depleting agent Oxyrase (Oxyrase Company, Mansfield, OH) to reduce photodamage and photobleaching (25). Dishes with Oxyrase-treated cells were covered with a layer of mineral oil (Squibb) to retard gas exchange. Injected cells were observed on a Nikon Diaphot 300 inverted microscope equipped with a Plan ×100 1.25 NA objective. Images were collected with a slow-scan back-illuminated cooled charge-coupled device camera (CH350; Roper Scientific, Trenton, NJ) driven by metamorph imaging software (Universal Imaging, Media, PA). The values for the rates of growth and shortening of MTs were calculated as displacements of MT ends within 3-s time intervals traced with a mouse-driven cursor with scion image software. The proportion of MT polymer in cytoplasmic fragments of melanophores was determined by a fluorescence ratiometric procedure as described (30, 31).
Results
Microsurgically produced cytoplasmic fragments of melanophores rapidly (≈10 min) form a radial MT array and aggregate pigment granules at the center after stimulation with adrenaline, which activates dynein motors located on the pigment granules (ref. 23; Fig. 1a). Conceivably, the radial arrangement of MTs in the fragments could be achieved by the transport of MTs, by remodeling of a MT array through concurrent disassembly and reassembly, or through a synergistic activity of the two mechanisms.
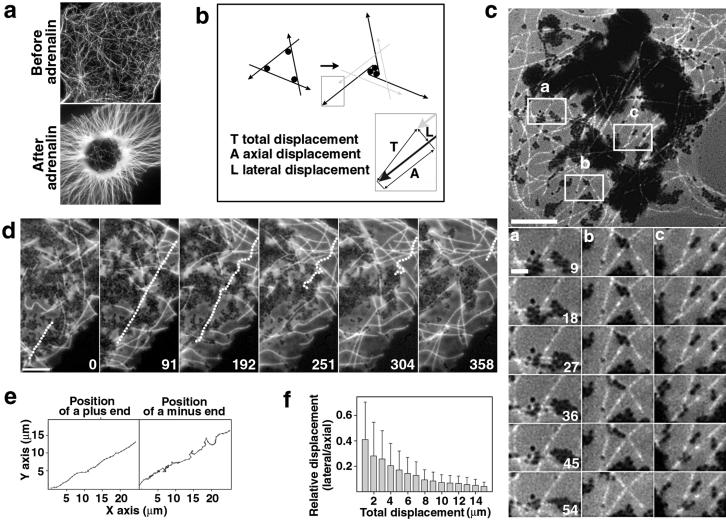
Figure 1.
Organization of a polarized radial array does not involve MT transport. (a) Live fluorescence images of MTs in a fragment before and after stimulation with adrenaline. (b) A model proposed to describe the organization of a radial array through the transport of MTs (black thick lines; arrows indicate plus ends) by multivalent minus-end-directed MT motors (black circles). Gray lines indicate the initial position of MTs. The diagram at the bottom illustrates the direction of movement of a MT end during the formation of a radial array through a transport mechanism. The attainment of a radial organization depends on the axial and lateral displacement of MT ends. (c) Behavior of fluorescent speckles produced by injection of melanophores with fluorescently tagged tubulin subunits at a low (0.5 mg/ml) concentration. (Upper) MTs with speckles at low magnification. (Lower) Time sequences of speckles on MTs in the regions indicated in the upper panel. Numbers indicate the time in seconds for all three time sequences [scale bars, 10 μm (Upper) and 2 μm (Lower)]. (d and e) Fishtailing of a MT in a fragment stimulated with adrenaline. (d) Time sequence. Contours of a fishtailing MT are shown as a dotted line. The plus end is at the top and the minus end is at the bottom. The time in seconds is indicated at the lower right corner of each panel (scale bar, 5 μm). (e) Life histories of a plus end (Left) and a minus end (Right) of a MT shown in d. (f) Processivity of the lateral displacement of the minus ends of fishtailing MTs. The ratio of lateral to axial displacement of a MT minus end (shift at right angles to a MT axis vs. shift along the axis) was plotted against the total displacement (total distance covered by an end) at increasing time intervals (for the definition of total, axial, and lateral displacement see also bottom part of b).
Vectorial transport of MTs by dynein motors located on pigment granules could result in the clustering of minus ends similar to the focusing of MTs in mitotic cell extracts (9, 10, 13). This mechanism (9, 14) requires significant movement of cytoplasmic MTs (Fig. 1b). To test for MT transport, we followed the behavior of fluorescently tagged MTs during self-organization by high-resolution digital fluorescence microscopy (24). MTs were tagged with a fluorescent dye by injection of intact melanophores with labeled tubulin subunits (24). In the fragments stimulated with adrenaline, the ends of MTs continuously moved, displaying behavior consistent with MT gliding along their tracks. To determine whether the movement of MT ends was indeed due to such transport, we injected melanophores with labeled tubulin subunits at a low (<1 mg/ml) needle concentration. Stochastic association of labeled tubulin subunits with growing MT ends produced speckled variations of fluorescence intensity along MT (26), which served as internal reference marks. After stimulation with adrenaline, fluorescence speckles remained stationary on most MTs (Fig. 1c). Axial displacement was extremely rare (seven MTs in nine examined fragments), and shifts were small (<0.5 μm). Infrequently, the MTs that contacted local pigment aggregates displayed vigorous movement—fishtailing—which appeared to result from the application of external force by MT motors bound to pigment granules (Fig. 1d; the fishtailing MT is shown as a dotted line). Although the fraction of MTs that exhibited such behavior was small (<10%), we sought to determine whether the fishtailing movement might contribute to the organization of a radial MT array.
Clustering of minus ends through movement in the cytoplasm requires progressive lateral displacement of MTs (Fig. 1a). Therefore, to examine the contribution of fishtailing to the establishment of the radial organization, we attempted to determine whether the ends of a given MT moved continuously away from the initial axis. Locations of plus (growing) and minus (shortening) ends were determined at increasing time intervals after stimulation with adrenaline, and the data were plotted as life histories of individual MT ends (Fig. 1e). Analysis of the plots indicated that plus ends advanced essentially along the initial MT axis (Fig. 1e Left). Minus ends displayed both lateral and axial movement, but the lateral shifts appeared to be nonprocessive (Fig. 1e Right). Therefore these data strongly argued against the involvement of MT transport in the establishment of radial organization. To completely rule out the transport mechanism we performed formal analysis of the processivity of lateral displacement for the minus ends. We calculated the values for axial displacement (shift along a MT axis), lateral displacement (shift at right angles to the axis), and total displacement (total distance covered by a minus end), as shown in Fig. 1b (Lower). Averaging of the data for 52 minus ends of fishtailing MTs in eight fragments clearly showed that the ratio of lateral and axial displacements declined when the total displacement increased (Fig. 1f). Therefore lateral shifts in the positions of the minus ends were indeed not processive. Taken together, these data clearly indicate that the movement of MTs does not contribute to the organization of a radial array.
Limited nonprocessive movement of MTs invokes a mechanism that depends on concurrent disassembly and reassembly at the ends. We found that in the fragments stimulated with adrenaline, MTs continuously grew from the plus ends and shortened from the minus ends. Furthermore, in contrast to the fragments with established radial array, where most of the minus ends were stabilized at the pigment aggregate (23), during aggregation the fraction of stable minus ends was insignificant (not shown). Therefore the dynamic properties of MTs were consistent with the organization of a radial array through a disassembly–reassembly mechanism. However, the generation of a radial pattern by this mechanism would require nucleation at local sites that eventually accumulate in the center of a fragment. Our previous work (32) documented continuous outgrowth of MTs from the aggregates of pigment granules. Therefore we hypothesized that pigment aggregates could serve as primary sites for MT nucleation in the absence of the centrosome.
The standard test for nucleation involves recovery of MTs after disruption with cold or nocodazole. In intact cells, regrowth of MTs occurs predominantly from the centrosome. To test the nucleating capacity of pigment aggregates, we labeled MTs with fluorescent tubulin subunits. Fragments were treated with adrenaline for 15 min to allow for pigment aggregation and the formation of a radial array and then chilled on ice for 30 min. Incubation in the cold completely depolymerized MTs, but aggregates of pigment granules remained essentially intact (Fig. 2a). Sequences of fluorescence images acquired after the fragments were transferred to room temperature indicated that MTs initially appeared inside or in close proximity to the pigment aggregates (Fig. 2a). Growth through the cytoplasm to the periphery of a fragment gradually reestablished the radial organization (Fig. 2a). We concluded that the pigment aggregates in the fragments had a capacity for MT nucleation similar to that of the centrosomes in intact cells.
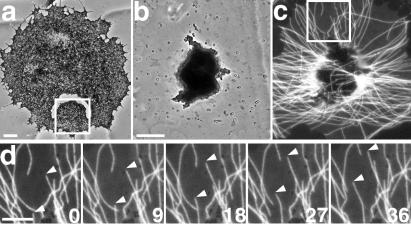
Figure 2.
Pigment aggregates are capable of MT nucleation. (a) Live images of MTs during recovery after cold treatment. Numbers in the lower right corner of each panel indicate the time in minutes and seconds after transfer of a chilled fragment to room temperature (scale bar, 10 μm). (b) MTs in a fragment before and after pigment aggregation. (Upper) Live images of MTs (scale bar, 5 μm). (Lower) Change in MT length after aggregation. (c) The ratio of MT densities before and after stimulation with adrenaline in control (left bar) or pigment-free (right bar) fragments. MT densities were determined by measuring the total MT length in the same fragments before and 20 min after stimulation with adrenaline.
MT nucleating activity could be an inherent property of pigment granules or might be attained because of the stimulation with adrenaline. To discriminate between these possibilities we have compared the amount of MT polymer in the same fragments before and after pigment aggregation. Nucleation of MTs on a MT organizing center, such as the centrosome, allows polymerization at a lower minimal concentration of free tubulin subunits (“critical concentration”) and therefore increases the amount of MT polymer in the cytoplasm (31). Thus, if the granules acquired nucleating capacity in response to aggregating stimuli, the level of MT polymer should be significantly higher in the aggregated compared with the dispersed state. To quantify polymer levels we took advantage of the fact that in small (10–15 μm) fragments we could trace every MT and measure total MT length in the same fragment before and after pigment aggregation. We found that in dispersed state the MT polymer level defined by the total MT length was low, as expected in the case of self-nucleation of MTs in the cytoplasm (31). In contrast, in the aggregated state the total MT length was about 2-fold higher (Fig. 2b). The increase in the polymer level clearly depended on pigment granules, because in pigment-free fragments produced by microdissection of cells with aggregated pigment granules, MT polymer level did not increase in response to treatment with adrenaline (Fig. 2c). These results strongly suggested that nucleation of MTs on pigment granules was provoked by the treatment with adrenaline.
The most noticeable effect of adrenaline is the activation of dynein motors essential for the organization of MTs. Although the primary role of dynein motors appeared to be transport of pigment granules (23), they could also be involved in MT nucleation. To test for the role of cytoplasmic dynein in MT nucleation, we injected fragments with established radial arrays with dynein inhibitors, a monoclonal antibody to dynein intermediate chain (74.1; ref. 27), or a recombinant (29) 50-kDa subunit of the dynein activator dynactin (dynamitin; ref. 28). The two inhibitors, at needle concentrations of 3.3 and 15 mg/ml, respectively, completely inhibited aggregation of pigment granules in intact melanophores (not shown). Injection of the fragments with dynein inhibitors resulted in suppression of MT outgrowth from the pigment aggregates, immediate release of MT minus ends, gradual randomization of the MT network (Fig. 3b), and rapid decrease in MT density (Fig. 3c). The level of MT polymer, estimated with a single cell ratiometric fluorescence assay (30, 31), decreased by about 40% (Fig. 3d) within 20 min after injection of the dynein inhibitors, returning to the level characteristic for the dispersed state. The rates of growth (8.6 ± 1.1 μm/min, mean ± SD) and shortening (7.6 ± 1.6 μm/min, mean ± SD) in the fragments injected with dynein inhibitors were similar to the rates of growth and shortening in control noninjected fragments (7.7 ± 0.5 and 5.1 ± 0.6, mean ± SD, respectively). Therefore the effects of dynein inhibitors were consistent with inhibition of MT nucleation and uncapping of MT minus ends associated with pigment aggregates. Taken together, these results indicated that cytoplasmic dynein was directly or indirectly involved in MT nucleation and capping of the MT minus ends.
Figure 3.
Dynein inhibitors disorganize radial MT arrays in the fragments. (a) Immunoblot of the extract of black tetra melanophores with antibody against the intermediate chain of cytoplasmic dynein (antibody 74.1). (b) Live images of MTs after injection of antibody 74.1 (3.3 mg/ml needle). Numbers in the lower right corner indicate the time in minutes after injection (scale bar, 5 μm). (c) Kinetics of the decrease in the level of MT polymer after injection of 74.1 antibody. The level of MT polymer at each time point was calculated as the difference between mean fluorescence intensities in a region containing and a region lacking MT. (d) Effect of injecting dynein inhibitors on the level of MT polymer. Error bars indicate the 99% confidence interval. MT polymer level was determined by single-cell fluorescence ratiometric assay. Pigment-free fragments were dissected from cells with aggregated pigment granules. Needle concentrations of nonimmune mouse IgG, dynein antibody 74.1 (14), and recombinant subunit of dynactin (p50, dynamitin) were 3.5, 3.3, and 15 mg/ml respectively.
The dynein-dependent nucleation of MTs on pigment granules in the fragments could reflect a mechanism that complements nucleation at the centrosome in intact melanophores. However, in cells the steady-state concentration of free tubulin subunits should be lower than in the fragments, because centrosomes presumably have a higher nucleating capacity than pigment aggregates and therefore induce the formation of a larger amount of MTs (31). To determine whether pigment granules were capable of MT nucleation at the low concentration of free tubulin subunits typical of intact melanophores, we tested for the formation of a pigment aggregate on the noncentrosomal side of a wound, which isolated a portion of the cytoplasm from the influence of the centrosomal radial array. Melanophores were injected with fluorescent tubulin subunits, and a wound was produced with a sharp glass microneedle (Fig. 4a). Remarkably, adrenaline resulted in the formation of a local pigment aggregate on the noncentrosomal side of a wound (Fig. 4b). Digital fluorescence microscopy demonstrated that MTs continuously emerged from a noncentrosomal pigment aggregate and grew toward the preexisting cell margin or toward the wound (Fig. 4d). Thus, the behavior of MTs surrounding a local pigment aggregate that formed in cells was very similar to the behavior of MTs in the fragments. These results strongly suggest that the presence of the centrosome does not preclude nucleation on pigment aggregates. Therefore MT nucleation on pigment granules, which accumulated in the centrosomal region in response to the treatment with adrenaline, contributes to the organization of the radial MT array in intact melanophores.
Figure 4.
Outgrowth of microtubules from a local pigment aggregate formed on the noncentrosomal side of a wound in a whole melanophore. (a) Phase-contrast image of a melanophore with dispersed pigment with a U-shaped wound produced with a glass microneedle. (b) Magnified phase-contrast image of a region outlined in a 10 min after induction of aggregation with adrenaline. The pigment aggregate has been formed on the noncentrosomal side of the wound. (c and d) Live fluorescence images of microtubules that surround the pigment aggregate shown in b. (c) Distribution of microtubules around the pigment aggregate. (d) Time sequence of microtubules at a high magnification in the boxed region shown in c. MTs continuously emerged from the pigment aggregate and grew to the wound or to the cell margin by the addition of subunits to the plus ends (indicated by arrowheads). Numbers indicate the time in seconds [scale bars, 20 μm (a), 10 μm (b and c), and 5 μm (d)].
Discussion
Self-Organization of a Radial Microtubule Array in Melanophore Fragments.
Our results shed light on the role of the activity of cytoplasmic dynein and MT dynamics in the organization of a polarized radial MT array in the absence of the centrosome in vivo. The formation of a radial array is achieved through concurrent disassembly and reassembly of otherwise immobile MTs. Growth of MTs is initiated on noncentrosomal sites that provide nucleation templates but, unlike the centrosome, do not anchor the minus ends tightly. Frequent release and depolymerization from minus ends allows for rapid reorganization of a MT array. The spatial distribution of MTs at any given moment is determined by the location of noncentrosomal nucleation sites. However, the nucleation sites themselves never remain stationary, but are rapidly transported to MT minus ends. Nucleation and frequent release of MTs superimposed on minus-end-directed transport of nucleation sites eventually results in the formation of a radial array.
We hypothesize that the following sequence of events leads to the organization of random MTs into a radial array in melanophore fragments (Fig. 5). At an early stage of the self-organization, rapid transport of pigment granules to the MT minus ends induces the formation of local pigment aggregates (Fig. 5b). MT nucleation on local aggregates and growth in all directions produce microasters (Fig. 5c). Transport of pigment granules along MTs extending between the local pigment aggregates results in the fusion of microasters and the formation of a single radial array with the pigment aggregate at the center (Fig. 5d). In our model, cytoplasmic dynein plays a dual role in the self-organization mechanism by participating in MT nucleation and by supporting minus-end-directed transport of nucleation sites.
Figure 5.

A model for the formation of a radial MT array in melanophore fragments. (a) In the dispersed state, pigment granules and microtubules are distributed randomly in a fragment. (b) After stimulation with adrenaline, transport of pigment granules to the MT minus ends results in the formation of local pigment aggregates. (c) Nucleation of MTs on the pigment aggregates produces microasters. (d) Fusion of microasters results in the formation of a single radial array of microtubules with the pigment aggregate at the center.
Assembly of MT Arrays by Cytoplasmic Dynein.
Organization of MTs into interphase radial arrays (33, 34), mitotic spindle poles (13, 28), or bundles in axonal shafts (35) requires the activity of cytoplasmic dynein. Given that cytoplasmic dynein is a force-producing enzyme, it seems reasonable to assume that it functions in MT organization by providing the driving force for unidirectional transport of MTs. It is not clear, however, if the force produced by dynein motors attached to membrane organelles or anchored in the cytoplasm is sufficient to overcome the drag force for the MT movement. Although some live observations document movement of individual MTs (25, 36), others indicate that MTs generally remain stationary in the cytoplasm (37, 38). In our experiments MTs never displayed persistent lateral or axial displacement characteristic of the transport mechanism. Radial organization was attained through concurrent disassembly and reassembly of MTs.
Our results indicate that mechanical force produced by cytoplasmic dynein is used in the transport of MT nucleation and capping sites to the MT minus ends. Remarkably, the same mechanism seems to operate in intact cells, with radial organization already established through the MT nucleation at the centrosome. In melanophores, accumulation at the cell center of the pigment granules bearing noncentrosomal nucleating sites apparently increases the density of MTs in centrosomal radial arrays and therefore enhances the efficiency of pigment aggregation. Surprisingly, the assembly of the centrosome itself also seems to depend on the dynein-dependent transport of large particles containing the integral centrosomal components pericentrin (39), PCM1 (40), and γ-tubulin (41), a protein directly involved in the organization of MT nucleating templates. Therefore transport of components of microtubule-organizing centers by cytoplasmic dynein seems to reflect a general mechanism for the construction of radial MT arrays.
Our results indicate that cytoplasmic dynein may play an even more critical role in MT nucleation than merely transporting the nucleation sites. It also seems to account for the MT nucleation and minus-end capping activity displayed by pigment granules, which are critical for the attainment of radial MT organization but separate from the production of the mechanical force. Frequent release of MTs from the pigment granules indeed suggests that the molecular mechanism of dynein-dependent minus-end capping is fundamentally different from the capping of the minus ends at the centrosome, which apparently involves tight attachment to the γ-tubulin ring complex. Therefore, in addition to participation in the minus-end-directed transport of membrane organelles or chromosomes, cytoplasmic dynein may play an important role in the control of MT assembly by capping of MT minus ends or MT nucleation.
Possible Mechanisms of Dynein Involvement in MT Nucleation.
We envision three possible mechanisms of MT nucleation on noncentrosomal sites that require cytoplasmic dynein. It may promote nucleation indirectly, by binding protein complexes that serve as templates for the MT nucleation. A possible candidate for the nucleation template located on the pigment granules is the γ-tubulin ring complex responsible for MT nucleation at the centrosome. Binding of the γ-tubulin ring complex to the pigment granules may be mediated by pericentrin, which is known to interact with a light intermediate chain (LIC1) of cytoplasmic dynein (42, 43). We feel, however, that mediation by pericentrin is an unlikely possibility because immunostaining of melanophores with γ-tubulin antibody did not detect γ-tubulin on the pigment granules (23). Alternatively, cytoplasmic dynein may be involved in the transport of a different, as yet unidentified factor that induces MT capping or nucleation. However, we favor a third possibility, that the dynein/dynactin complex may be directly involved in MT nucleation. Indeed, in melanophores aggregation stimuli dramatically enhance the ability of dynein/dynactin to bind MTs (43). Furthermore, partially purified cytoplasmic dynein has been observed to stimulate MT assembly in vitro (J. R. McIntosh, personal communication). Each dynein/dynactin complex possesses three MT-binding sites located on the dynein heavy chains (44) and on the p150Glued subunit of the dynactin (45). Several dynein/dynactin complexes closely spaced on the surface of a pigment granule could provide a sufficient number of binding sites for the 12 tubulin dimers that form a nucleus for the MT growth (46). Although the details of the molecular mechanism remain to be determined, we believe that dynein-dependent nucleation of MTs may provide a mechanism that cooperates with the centrosome in the organization of a radial MT array.
Acknowledgments
The authors are grateful to K. K. Pfister for providing 74.1 antibody and R. B. Vallee for a gift of p50 cDNA. We thank Timothy Mitchison and anonymous reviewers for helpful comments and A. S. Kashina, S. M. King, and A. E. Cowan for critical reading of the manuscript. This work was supported by National Institues of Health Grant GM62290-01 and National Science Foundation Grant MCB 9996320 (to V.R.) and Russian Basic Science Foundation Grant 99-04-49436 (to I.V.).
Abbreviation
- MTs
microtubules
References
- 1.Kellog D R, Moritz M, Alberts B. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- 2.Mitchison T J, Kirschner M A. Nature (London) 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 3.Lane J, Allan V. Biochim Biophys Acta. 1998;1376:27–55. doi: 10.1016/s0304-4157(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein L S, Philp A V. Annu Rev Cell Dev Biol. 1999;15:141–183. doi: 10.1146/annurev.cellbio.15.1.141. [DOI] [PubMed] [Google Scholar]
- 5.Holzbaur E, Vallee R B. Annu Rev Cell Biol. 1994;10:331–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- 6.Wateres J C, Salmon E D. Curr Opin Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- 7.Compton D A. Annu Rev Biochem. 2000;69:95–114. doi: 10.1146/annurev.biochem.69.1.95. [DOI] [PubMed] [Google Scholar]
- 8.Sharp D J, Rogers G C, Scholey J M. Biochim Biophys Acta. 2000;1496:128–141. doi: 10.1016/s0167-4889(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 9.Verde F, Berrez J M, Antony C, Karsenti E. J Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merdes A, Ramyar K, Vechio J D, Clevelend D W. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- 11.Heald R, Tournebize R, Habermann A, Karsenti E, Hyman A. Nature (London) 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 12.Gaglio T, Saredi A, Bingham J B, Hashbani M J, Gill S R, Schroer T A, Compton D A. J Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaglio T, Dionne M A, Compton D A. J Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman A A, Karsenti E. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]
- 15.Urritia R, McNiven M, Albanesi J P, Murphy D B, Kachar B. Proc Natl Acad Sci USA. 1991;88:6701–6705. doi: 10.1073/pnas.88.15.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedelec F J, Surrey T, Maggs A C, Leibler S. Nature (London) 1997;389:305–308. doi: 10.1038/38532. [DOI] [PubMed] [Google Scholar]
- 17.Haimo L H, Thaler C D. BioEssays. 1994;16:727–732. [Google Scholar]
- 18.Tuma M C, Gelfand V I. Pigm Cell Res. 2000;12:283–294. doi: 10.1111/j.1600-0749.1999.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson H, Wallin M. Cell Motil Cytoskeleton. 1997;38:397–410. doi: 10.1002/(SICI)1097-0169(1997)38:4<397::AID-CM9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Rodioinov V I, Gyoeva F K, Gelfand V I. Proc Natl Acad Sci USA. 1991;88:4956–4960. doi: 10.1073/pnas.88.11.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuma C, Zill A, LeBot N, Vernos I, Gelfand V I. J Cell Biol. 1998;143:1547–1558. doi: 10.1083/jcb.143.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNiven M A, Wang M, Porter K R. Cell. 1984;37:753–765. doi: 10.1016/0092-8674(84)90411-2. [DOI] [PubMed] [Google Scholar]
- 23.Rodionov V I, Borisy G G. Nature (London) 1997;386:170–173. doi: 10.1038/386170a0. [DOI] [PubMed] [Google Scholar]
- 24.Rodionov V I, Lim S-S, Gelfand V I, Borisy G G. J Cell Biol. 1994;126:1455–1464. doi: 10.1083/jcb.126.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keating T J, Peloquin J G, Rodionov V I, Moncilovich D, Borisy G G. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterman-Storer C M, Salmon E D. Biophys J. 1998;75:2059–2069. doi: 10.1016/S0006-3495(98)77648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillmann J F, III, Pfister K. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Echeverri C J, Paschal B M, Vaughan K T, Valle R B. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittmann T, Hyman A. Methods Cell Biol. 1999;61:137–143. doi: 10.1016/s0091-679x(08)61978-0. [DOI] [PubMed] [Google Scholar]
- 30.Zhai Y, Borisy G G. J Cell Sci. 1994;107:881–890. doi: 10.1242/jcs.107.4.881. [DOI] [PubMed] [Google Scholar]
- 31.Rodionov V I, Nadezhdina E S, Borisy G G. Proc Natl Acad Sci USA. 1999;96:115–120. doi: 10.1073/pnas.96.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodionov V I, Borisy G G. Science. 1997;275:215–218. doi: 10.1126/science.275.5297.215. [DOI] [PubMed] [Google Scholar]
- 33.Purohit A, Tynan S H, Vallee R B, Doxsey S J. J Cell Biol. 1999;147:481–491. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintine N J, Gill S R, Eckley D M, Crego C L, Compton D A, Schroer T A. J Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad F J, Echeverri C J, Vallee R B, Baas P J. J Cell Biol. 1998;140:246–256. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dent E W, Callaway J L, Szebenyi G, Baas P, Kalil K. J Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waterman-Storer C, Salmon E D. J Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang S, Svitkina T M, Borisy G G, Popov S V. Nat Cell Biol. 1999;1:399–403. doi: 10.1038/15629. [DOI] [PubMed] [Google Scholar]
- 39.Young A, Dictenberg J, Purohit A, Tuft R, Doxsey S. Mol Biol Cell. 2000;11:2047–2056. doi: 10.1091/mbc.11.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubo A, Saski H, Yaba-Kubo A, Tsukita S, Shina N. J Cell Biol. 1999;147:969–979. doi: 10.1083/jcb.147.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Wong M L, Alberts B, Mitchison T. Nature (London) 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- 42.Tynan S H, Purohit A, Doxsey S J, Vallee R B. J Biol Chem. 2000;275:32763–32768. doi: 10.1074/jbc.M001536200. [DOI] [PubMed] [Google Scholar]
- 43.Waterman-Storer C M, Karki S, Holzbaur E L F. Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gee M, Heuser J E, Vallee R B. Nature (London) 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- 45.Reese E L, Haimo L T. J Cell Biol. 2000;151:155–165. doi: 10.1083/jcb.151.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Carlier M F, Didry D, Pantalony D. Biophys J. 1997;73:418–427. doi: 10.1016/S0006-3495(97)78081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]