Abstract
NADPH oxidases are a family of enzymes capable of transferring electrons from NADPH to molecular oxygen. A major function of NADPH oxidases is the activation of molecular oxygen into reactive oxygen species. Increased activity of NADPH oxidases has been implicated in various pathologies, including cardiovascular disease, neurological dysfunction, and cancer. Thus, NADPH oxidases have been identified as a viable target for the development of novel therapeutics exhibiting inhibitory effects on NADPH oxidases. Here, we describe the development of new assays for measuring the activity of NADPH oxidases enabling the high-throughput screening for NADPH oxidase inhibitors.
Keywords: NADPH oxidase, reactive oxygen species, fluorescent probes, high-throughput screening, HPLC
Introduction
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Nox1-5, Duox1-2) are a family of enzymes capable of transferring electrons from the reduced form of NADPH to molecular oxygen (Fig. 1) (1–4). One-electron reduction of molecular oxygen leads to the formation of the superoxide radical anion (O2•−), which undergoes dismutation to hydrogen peroxide (H2O2).
Figure 1.

The enzymatic function of NADPH oxidases.
O2•− and H2O2 may initiate a cascade of various reactive oxygen and nitrogen species (Fig. 2), capable of irreversible modification of cellular biomolecules (e.g., DNA, proteins, lipids) (5). Thus, NADPH oxidases have been proposed as a promising target for drug development in a range of pathologies, including cardiovascular diseases, neurodegeneration, and cancer (6–12). Development of new inhibitors of NADPH oxidases has been an active area of research over the last decade, but with only limited success, as reviewed elsewhere (13–19). New and preferably isoform-specific inhibitors are still needed both for basic research and potential therapeutic applications (13–15, 19–24).
Figure 2.
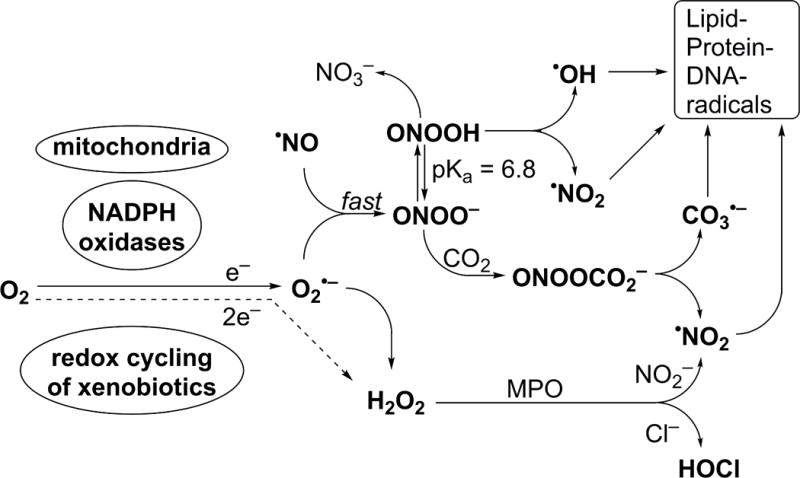
Cascade of reactive oxygen and nitrogen species initiated by one- or two-electron reduction of molecular oxygen, leading to oxidation of biomolecules.
One of the major limitations of the research on NADPH oxidases and in the development of new inhibitors was the lack of the rigorous and reliable assays and probes for use in cell-free systems and intact cells (25). Despite many artifacts, lucigenin and luminol were the most frequently used probes for measuring Nox activity. However, the situation recently changed, due to a better understanding of the chemistry of the probes, including the reaction mechanism, stoichiometry and kinetics, and the development of new probes and assays (26–29). These rigorous approaches have also enabled the establishment of the protocol for high-throughput screening (HTS) assays for faster development of new inhibitors of NADPH oxidases. In this review, we discuss the major limitations of the chemiluminescent probes for O2•− and H2O2, and describe in detail the recent developments in the rigorous, high-throughput assays of NADPH oxidases.
Spectrophotometric probes for O2•−
The spectrophotometric probes for O2•− are mostly based on the reducing potential of O2•−, and include ferricytochrome c (cyt c3+) and nitroblue tetrazolium (NBT) (30–33). Monitoring of the superoxide dismutase (SOD)-inhibitable reduction of cyt c3+ is widely used to rigorously determine the superoxide flux in cell-free assays, but may be not optimal in systems capable of significant reduction of cyt c3+ via superoxide-independent pathways (e.g., diaphorase-like activity, reduction by thiols, etc.) (34–37). Nitroblue tetrazolium has been used to detect O2•− both in cell-free and cellular systems (32, 38–40). Upon reduction, the blue, water-insoluble formazan product is formed via two one-electron reduction processes, with the NBT radical cation as an intermediate. Similar to cyt c3+, NBT is a substrate for diaphorases, indicating the lack of specificity of the probe for O2•− (41, 42). The confounding aspect of the use of NBT for O2•− detection is the ability of the NBT radical cation to transfer an electron to molecular oxygen, leading to redox cycling and generation of O2•− by the probe (43, 44).
Chemiluminescent probes for O2•−
Chemiluminescent and bioluminescent probes have been widely used in biological systems, thanks to high sensitivity and the possibility for real-time monitoring of cellular events. Several chemiluminescent probes for superoxide have been developed, but two of them, lucigenin and luminol-based probes, gained the most popularity.
Lucigenin is a dication, which upon one-electron reduction forms a lucigenin radical cation, with a subsequent reaction with superoxide leading to chemiluminescence. However, lucigenin is a substrate for diaphorase activity, and the one-electron reduction product, the lucigenin radical cation, has been demonstrated to rapidly reduce oxygen, leading to redox cycling and generation of superoxide and bringing into question the applicability of lucigenin for O2•− detection (45–49). Recently, it was demonstrated that lucigenin produces a chemiluminescence signal even in tissues from transgenic animals lacking the NADPH oxidase enzymes, and cytochrome P450 enzymes were shown to be responsible for lucigenin-derived chemiluminescence (50, 51). Clearly, the data obtained with lucigenin as a probe used to measure NADPH oxidase activity require reevaluation.
The other chemiluminescent probe widely used to monitor NADPH oxidase-derived O2•– is luminol and its analog, L-012 (52–55). In the presence of superoxide, luminol undergoes oxidative transformation, involving the formation of luminol radical, which upon further reaction with O2•− leads to a luminescence signal (56). A similar mechanism was proposed for the L-012 probe. Similar to lucigenin, the major limitation of luminol and L-012 probes is the capability of the probe-derived radical to reduce oxygen to superoxide and the formation of the luminol radical in superoxide-independent pathways (45, 57). It was demonstrated that peroxidases in the presence of H2O2 oxidize L-012 to produce a chemiluminescence signal, inhibitable by superoxide dismutase (57). This suggests that luminol and L-012, while useful to monitor oxidative burst in neutrophils, should be used with caution, as they may report the peroxidatic activity (e.g., myeloperoxidase [MPO]) in addition to NADPH oxidase-derived superoxide (58). This should be always kept in mind because NADPH-oxidase-enriched membrane preparations from neutrophils typically contain large amounts of MPO (58).
Fluorescent probes for O2•–
Hydroethidine (HE, also known as dihydroethidium, Fig. 3) remains the most widely used probe for the detection of O2•−. The initial work on the application of the HE probe involved measurement of superoxide from activated neutrophils (59). Although it was initially assumed that ethidium (E+) is the product of HE reaction with O2•−, it has been demonstrated that the actual product is 2-hydroxyethidium (2-OH-E+) (60). Importantly, this product is not formed by other biologically relevant oxidants, indicating its value as a specific marker for O2•− (61, 62). The mechanism of transformation of HE into 2-OH-E+ by superoxide involves one-electron oxidation of HE into the HE radical cation (HE•+), which upon reaction with O2•− leads to formation of 2-OH-E+ (63, 64). In contrast to the luminol-derived radical, to the best of our knowledge, HE•+ does not react with oxygen; thus, no artifactual generation of superoxide was reported for this probe. The major limitation of the probe is that, though it forms an O2•− -specific product, the probe by itself is not selective for O2•−. Both one- and two-electron oxidants can react with the probe, forming several additional products, including E+ and diethidium (E+-E+, Fig. 4) (62, 65). Detection of E+-E+ may be applied to monitor the peroxidatic activity in the investigated systems. Recently, an additional hypochlorous acid (HOCl)-specific product from HE was reported and proposed for use as a specific marker of MPO activity in vitro and in vivo (66, 67). The chemical reactivity of HE toward various cellular oxidizing, nitrating, and chlorinating species, while enabling simultaneous monitoring of different species, requires chromatographic techniques for selective detection and quantification of 2-OH-E+, when used for measurements of NADPH oxidase activity (62, 68–71). High-performance liquid chromatography (HPLC)-based analyses of HE and its oxidation products is important, as probe availability is one of the factors controlling the yield of the oxidation products, similar to other reactive oxygen species probes. Because conversion of HE into 2-OH-E+ is a multistep process involving HE•+, the steady-state concentration of this short-lived intermediate will also affect the yield of 2-OH-E+. Thus, the presence of one-electron oxidants capable of oxidation of HE to HE•+ may increase the yield of 2-OH-E+ at a steady flux of superoxide, as recently demonstrated (61). Here, the HPLC-based profiling of the oxidation products enables proper interpretation of the data, as one-electron oxidants will also produce dimeric products (e.g., E+-E+). Thus, in case of the increased production of 2-OH-E+ without an increased level of E+-E+, one can conclude the increased production of O2•−. When the levels of both 2-OH-E+ and E+-E+ are increased, the formation of one-electron oxidants can be concluded, but the data may not allow for the assessment of superoxide production.
Figure 3.
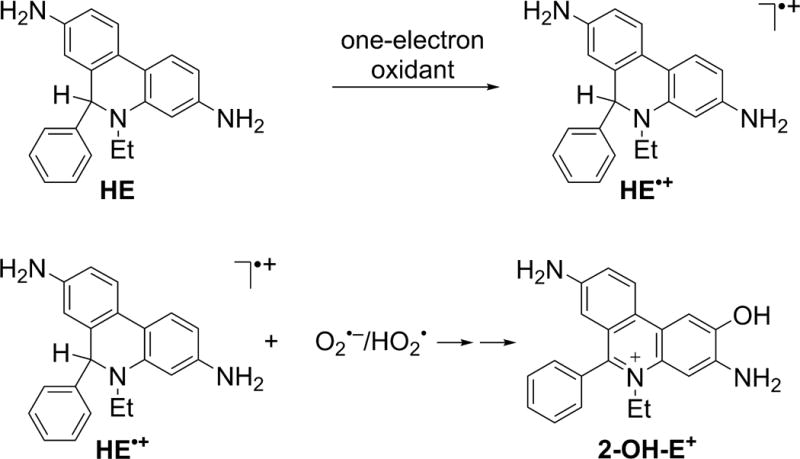
Involvement of HE•+ intermediate in the conversion of HE to 2-OH-E+.
Figure 4.
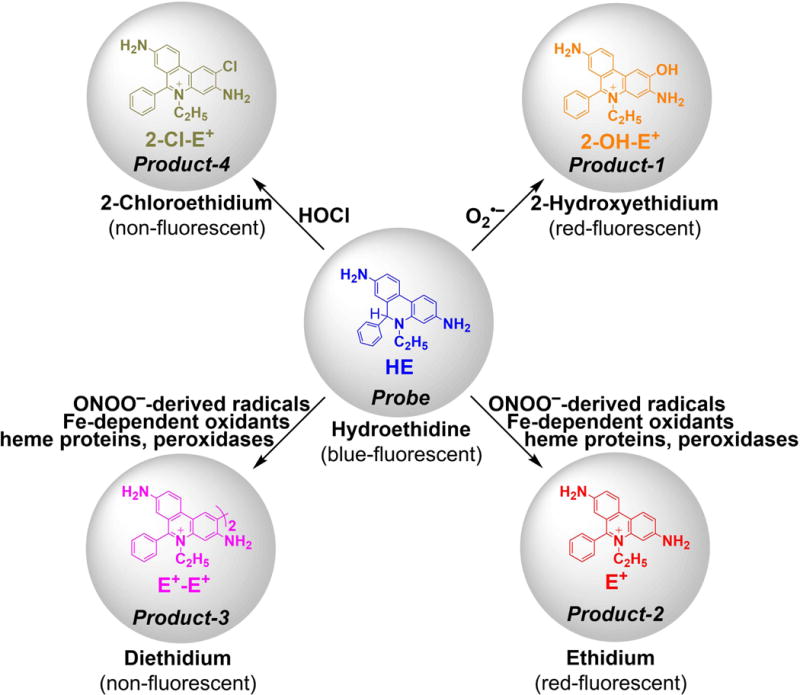
Dependence of the products of HE oxidation on the identity of the oxidant.
To minimize nonspecific oxidation of HE by intracellular components and to selectively detect extracellular superoxide (derived from NADPH oxidase), a cell-membrane-impermeable analog of HE was designed, synthesized, and chemically characterized (72). Upon two-electron reduction of propidium iodide, a dye typically used to stain necrotic cells, hydropropidine (HPr+, Fig. 5) is formed, which is structurally similar to HE but bears quaternary ammonium cationic moiety, which significantly suppresses its cellular uptake. In fact, upon incubation with RAW 264.7 macrophages, more than 99% of the probe was detected in the extracellular space, while a significant portion of the HE probe was taken up by cells (Fig. 5).
Figure 5.

Hydropropidine as a cell membrane-impermeable probe for superoxide. (A) Chemical structures of HPr+ and the superoxide-specific oxidation product, 2-OH-Pr2+. (B) Comparison of the cell-medium distribution of HE and HPr+ probes upon incubation with RAW 264.7 cells. (Reprinted from Free Radic. Biol. Med., vol. 54, Michalski, R., Zielonka, J., Hardy, M., Joseph, J., & Kalyanaraman, B., Hydropropidine: a novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide, 135–147. Copyright 2013, with permission from Elsevier.) (72)
Analysis of the chemical reactivity of HPr+ toward biologically relevant oxidants indicates that it behaves very similarly to HE, and in the presence of O2•− forms a specific hydroxylated product, 2-hydroxypropidium (2-OH-Pr2+). It was demonstrated that HPr+ selectively detects extracellular superoxide produced by RAW 264.7 macrophages activated to produce O2•− by treatment with the phorbol 12-myristate 13-acetate ester (PMA) or menadione (72). Similar to 2-OH-E+, 2-OH-Pr2+ can bind to DNA leading to a more than 10-fold increase in the fluorescence yield. Thus, plate-reader-based assays using HPr+ should include DNA for improved sensitivity. Although DNA intercalators may affect 2-OH-Pr2+ binding, leading to a decrease in the fluorescence signal, this limitation can be overcome by increasing the concentration of DNA.
EPR spin trapping of superoxide radical anion
Electron paramagnetic resonance (EPR) is a spectroscopic technique that selectively detects species carrying unpaired electron(s) and, thus, is regarded as the most rigorous method to detect free radicals. While most biologically relevant free radicals are short-lived and do not accumulate sufficiently to meet the detection limit of the EPR technique, the use of spin traps enables detection of spin adducts, which are characterized by a significantly longer lifetime that leads to their accumulation during incubation (73–75). Compared to most luminescent probes, EPR spin trapping is advantageous in that the product is formed in a single step, limiting the possibility of interference by other components during the detection process. The most widely used spin traps for O2•− are cyclic nitrones, including DMPO (5,5-dimethyl-1-pyrroline-N-oxide), BMPO (5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide), DEPMPO (5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide), and DIPPMPO (5-(diisopropoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide), which upon reaction with superoxide form the corresponding spin adducts (Fig. 6) with characteristic EPR spectrum. Although early spin trapping studies of neutrophil-generated oxidants employed a DMPO spin trap, the short lifetime of the superoxide spin adduct and its conversion into the hydroxyl radical adduct were confusing factors in the analyses of the identities of the radicals trapped (76–78). A DEPMPO spin trap was used to detect O2•− produced by NADPH oxidase in intact HL60 cells differentiated into neutrophil-like cells (dHL60) and stimulated with PMA (Fig. 7) (79). The EPR signal was inhibited by SOD but not by catalase, indicating that superoxide was responsible for the adduct formed, consistent with the spectral features observed, which are characteristic of a superoxide spin adduct.
Figure 6.
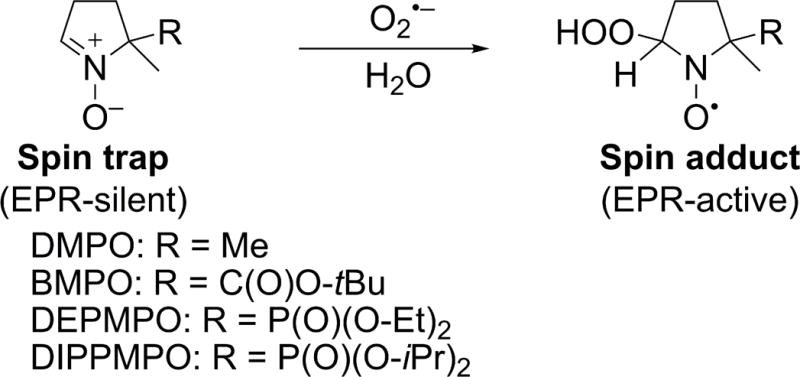
Spin trapping of O2•− by selected cyclic nitrones.
Figure 7.
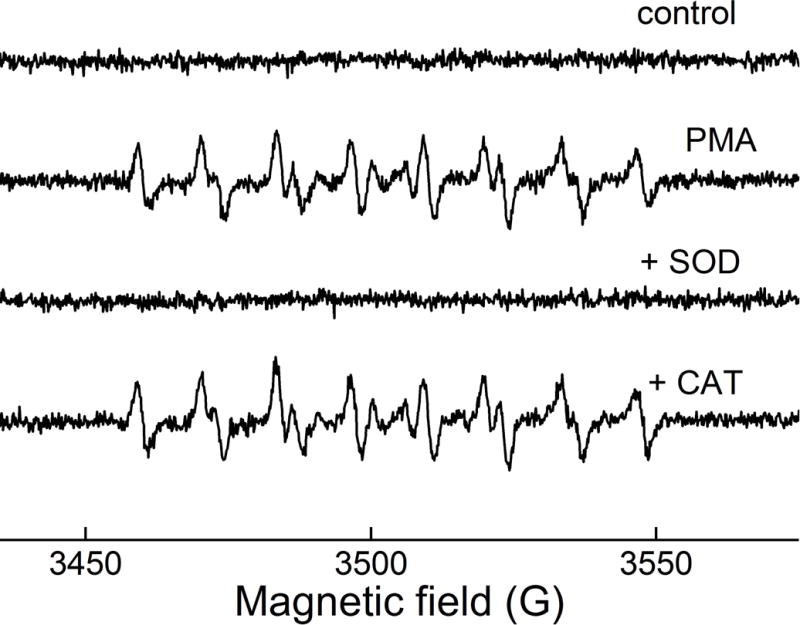
EPR spin trapping of superoxide generated by NADPH oxidase. DEPMPO spin trap was incubated with dHL60 cells in the absence (control) or presence of PMA. Where indicated, SOD or catalase was also present. (This research was originally published in Journal of Biological Chemistry. Zielonka, J., Cheng, G., Zielonka, M., Ganesh, T., Sun, A., Joseph, J., Michalski, R., O’Brien, W. J., Lambeth, J. D., & Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014; 289: 16176–16189. © the American Society for Biochemistry and Molecular Biology.) (79)
To prevent degradation of the superoxide spin adduct within cells and extend its lifetime, the DIPPMPO spin trap has been recently covalently linked to cyclodextrin (CD) (80, 81). The CD-conjugated DIPPMPO was demonstrated to detect O2•− generated by macrophages stimulated with PMA. In the same work, the EPR spin trapping was also extended to undetached cells, opening the possibility of studying superoxide generation by NADPH oxidases present in adherent cells in their natural (surface-attached) state (74, 80).
Although EPR spin trapping is a highly valuable tool in the characterization of the radical species produced by NADPH oxidase enzymes, its use in the high-throughput screening assays is currently limited to confirmatory assays, due to significantly lower throughput as compared to luminescence-based assays.
Amplex Red-based assays for H2O2
10-Acetyl-3,7-dihydroxyphenoxazine (Amplex Red) is a fluorogenic probe that, upon one-electron oxidation, undergoes conversion to red-fluorescent resorufin (Fig. 8) (82). Although Amplex Red does not react directly with H2O2, in the presence of horseradish peroxidase (HRP), it can quantitatively detect H2O2 with a high sensitivity. Because the oxidation requires HRP, the assay is limited to cell-free assays or measurement of extracellular H2O2. Although Amplex Red-based assays may be useful in monitoring NADPH oxidase activity, their application may be limited in cell-free systems, because NADPH and reduced glutathione (GSH) interfere with the assay, complicating interpretation of the results (83). Both NAD(P)H and GSH are substrates for HRP used in the assay (84, 85). In addition, quantification of H2O2 by Amplex Red may be affected by exposure of samples to visible light, or the presence of peroxynitrite (ONOO–) (86–89). Thus, the results of real-time monitoring of H2O2 formation should be validated by end-point measurements, and catalase should be always used to confirm the identity of the oxidant.
Figure 8.
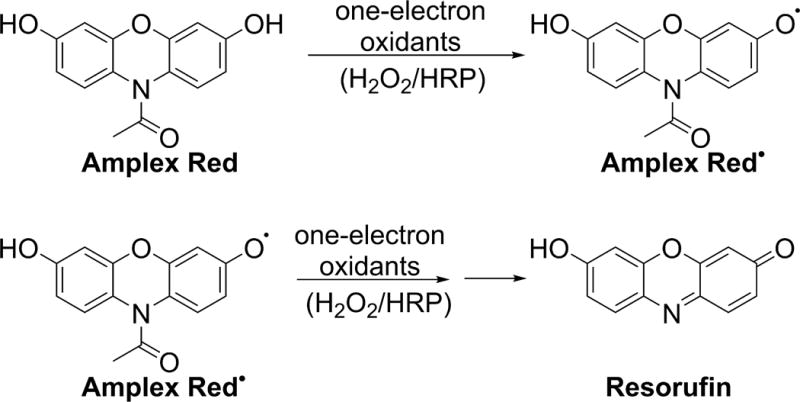
Involvement of Amplex Red-derived radical in the oxidative conversion of Amplex Red into resorufin.
Boronate-based assays for H2O2
Over the last decade, a new generation of probes for H2O2 have been developed based on oxidative transformation of aromatic boronic acids and esters into phenolic products (Fig. 9) (90, 91). A wide spectrum of probes has been reported with detection modalities ranging from spectrophotometry through fluorimetry to bioluminescence and PET imaging. Boronates can be oxidized not only by H2O2 but also by other biologically relevant oxidants, including ONOO–, HOCl, and selected protein hydroperoxides (Fig. 9) (91–97). It was shown that in the presence of ONOO– or excess of HOCl, additional, oxidant-specific products are formed (92, 94). Thus, by profiling the products formed, in addition to the use of specific inhibitors and scavengers, the identity of the oxidant(s) can be determined (98–100). In specific instances, where the involvement of other oxidants can be excluded, boronate-based probes can be used to selectively monitor the activity of NADPH oxidases (24, 79, 91, 101). Several boronate-based fluorogenic probes have been applied to monitor the activity of NADPH oxidases, including coumarin boronic acid (CBA), which upon reaction with H2O2 undergoes oxidation to 7-hydroxycoumarin (COH, also known as umbelliferone) (79, 102, 103). The major limitation of boronic probes is their relatively low reactivity toward H2O2, reducing the possibility of quantitative analyses of H2O2 but, on the other hand, allowing the bioorthogonal mode of detection (90).
Figure 9.

Oxidation of aromatic boronic acids into phenolic products.
Simultaneous detection of O2•− and H2O2
Progress in HPLC column technology over the last decade enables a significant reduction in the time needed to perform chromatographic analyses, from 30–60 min to 60 s or less per sample. For example, the HPLC-based analysis of HE and its oxidation products initially took 60–75 min per sample, was later shortened to 10 min, and most recently has been shortened to less than 90 s per sample (69, 79, 95). With such rapid assays, multiple probes and their products can be separated and detected simultaneously, providing an opportunity for real-time, simultaneous monitoring of NADPH-oxidase-derived O2•− and H2O2. While HE conversion to 2-OH-E+ is used to monitor O2•− formation, the extent of oxidation of the CBA probe to COH reflects the production of H2O2 (Fig. 10) (24, 79).
Figure 10.
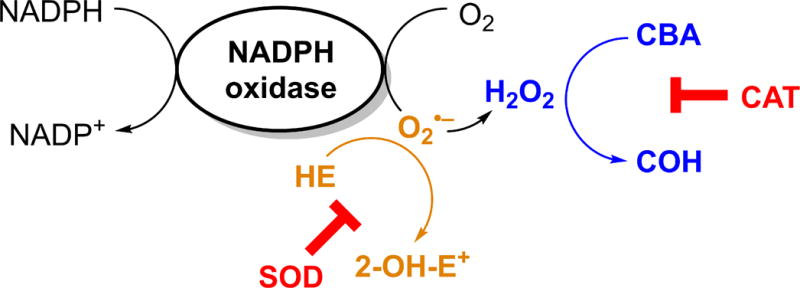
Principles of simultaneous detection of NADPH oxidase-derived O2•− and H2O2 by a mixture of HE and CBA probes.
Using both probes, simultaneous monitoring of O2•− and H2O2 is possible, both in a cell-free xanthine/xanthine oxidase system and in cellular models of different isoforms of NADPH oxidases (Fig. 11) (79). Simultaneous monitoring of O2•− and H2O2 generated by different isoforms of NADPH oxidases enables direct comparison of the identity of the species released from the enzyme. In the case of NADPH oxidase-2 (Nox2) and NADPH oxidase-5 (Nox5), the products of both O2•− and H2O2 were detected, whereas in case of NADPH oxidase-4 (Nox4), only the H2O2 product level was increased when compared with the cell line without an overexpression of Nox4. This is consistent with previous reports and indicates that O2•− undergoes rapid dismutation to H2O2 within the active center of the enzyme (104–106).
Figure 11.
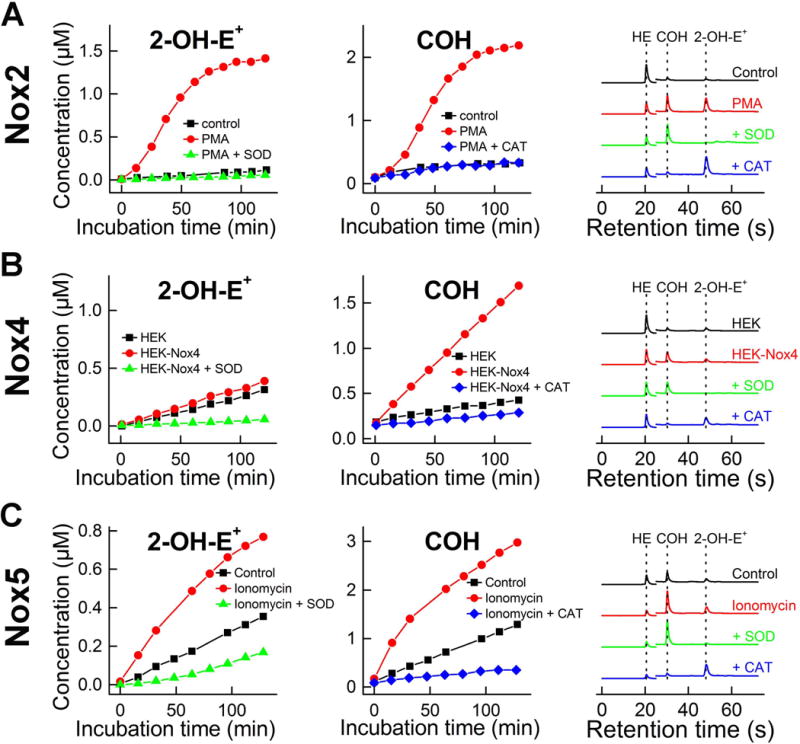
Simultaneous monitoring of superoxide and H2O2 generated by different isoforms of NADPH oxidase. Cells were stimulated, where necessary, as indicated and incubated with HE and CBA probes. During the incubation the media were probed repeatedly at different time points, and analyzed by rapid HPLC for the formation of 2-OH-E+ and COH. (A) Nox2 model: dHL60 cells stimulated with PMA; (B) Nox4 model: HEK 293 cells with stably overexpressed Nox4; (C) Nox5 model: HEK 293 cells with stably overexpressed Nox5 and stimulated with ionomycin. (This research was originally published in Journal of Biological Chemistry. Zielonka, J., Cheng, G., Zielonka, M., Ganesh, T., Sun, A., Joseph, J., Michalski, R., O’Brien, W. J., Lambeth, J. D., & Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014; 289: 16176–16189. © the American Society for Biochemistry and Molecular Biology.) (79)
Monitoring of oxygen and NADPH consumption
In addition to monitoring the formation of O2•− and H2O2, the activity of NADPH oxidase can be measured by monitoring the rate of oxygen consumption. When the mitochondrial respiration does not change and/or is relatively small, the rates of oxygen consumption may reflect the activity of NADPH oxidase, which provides the basis for probe-free assays. Due to the recent developments in monitoring oxygen consumption by cells in a multi-well plate format, these measurements can be conveniently carried out in 24-well and 96-well plates, providing a medium-throughput capability. Using the Seahorse XF96 extracellular flux analyzer, oxygen consumption rates (OCR) can be monitored in real time and up to four different compounds can be injected during the analysis, providing the opportunity to interrogate the effects of different compounds on basal and mitochondrial respiration and on the activity of NADPH oxidase (Fig. 12). Using dHL60 neutrophil-like cells, it was demonstrated that activation of NADPH oxidase by treatment with PMA leads to several-fold increase in oxygen consumption rates (Fig. 12A), which was impeded by commercially available inhibitors of NADPH oxidase activity (e.g., diphenyleneiodonium (DPI, Fig. 12C), but not by inhibitors of mitochondrial respiration, rotenone + antimycin A (Fig. 12B). Analysis of the effect of the commercial, nonspecific inhibitor of NADPH oxidases and other flavoproteins, DPI indicates that while the compound can inhibit both the basal respiration (mitochondrial function) and response to PMA (the activity of NADPH oxidase, Fig. 12C), the concentration dependence of these two effects is different, with relatively selective inhibition of NADPH oxidase activity at submicromolar DPI concentrations (Fig. 12D) (24).
Figure 12.
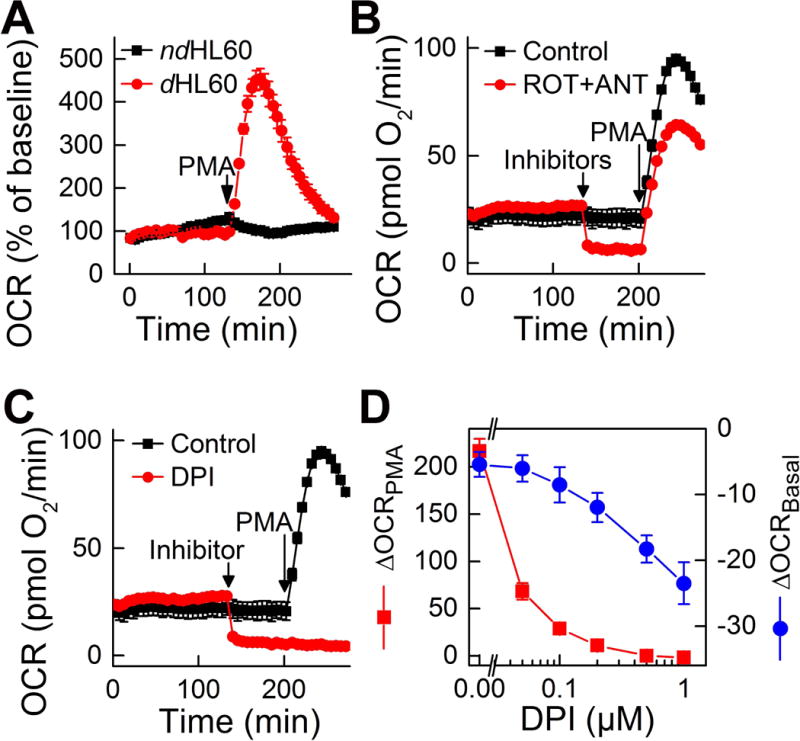
Measurement of NADPH oxidase activity by monitoring the rates of OCR. (A) Comparison of nondifferentiated and differentiated HL60 cells in their response to PMA stimulation. (B) Effect of mitochondrial inhibitors (1 μM rotenone, ROT, and 1 μM antimycin A, ANT) on basal and PMA-stimulated oxygen consumption by dHL60 cells. (C,D) Effect of diphenyleneiodonium (DPI, 10 μM) on basal (ΔOCRBasal) and PMA-stimulated (ΔOCRPMA) oxygen consumption by dHL60 cells. (This research was originally published in Journal of Biological Chemistry. Zielonka, J., Cheng, G., Zielonka, M., Ganesh, T., Sun, A., Joseph, J., Michalski, R., O’Brien, W. J., Lambeth, J. D., & Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014; 289:16176–16189, and Zielonka, J., Zielonka, M., VerPlank, L., Cheng, G., Hardy, M., Ouari, O., Ayhan, M. M., Podsiadly, R., Sikora, A., Lambeth, J. D., & Kalyanaraman, B. Mitigation of NADPH Oxidase 2 Activity as a Strategy to Inhibit Peroxynitrite Formation. J. Biol. Chem. 2016; 291:7029–7044. © the American Society for Biochemistry and Molecular Biology) (24, 79)
Due to the constant production of NADPH in cells and multiple pathways of its consumption, the monitoring of its consumption rates for measurement of the activity of NADPH oxidases in intact cells is impractical. However, in cell-free assays, when NADPH is added as a bolus, monitoring the rates of consumption of NADPH provides another probe-free mode of measurement of the activity of NADPH oxidases. Spectrophotometric (λ = 340 nm) monitoring of NADPH consumption was recently applied in the confirmatory assays for inhibitors of NADPH oxidases (22).
Development of the workflow for HTS of inhibitors of NADPH oxidase activity in intact cells
With the recent developments discussed above, it is now possible to establish rapid, yet rigorous assays for NADPH oxidases, that could be used in the HTS campaign, focused on the discovery of new inhibitors. Although several reports on HTS campaigns have been reported, with possible isoform-specific inhibitors of NADPH oxidases identified, in many cases the subsequent studies using more-rigorous assays revealed the lack of inhibition and interference of the positive hits with the assays used (22, 23, 107). Based on the progress that has been made in understanding the chemistry of HE and its analogs as the probes for O2•−, and of boronates as the probes for H2O2, a new workflow to screen the potential inhibitors of NADPH oxidases in intact cells was proposed (Fig. 13). The workflow and choice of probes were optimized for the Nox2 isoform, which produces significant fluxes of O2•− and H2O2 in extracellular space. For other Nox isoforms, this workflow may need to be modified, depending on the identity (O2•− and H2O2) and location (intra- vs. extracellular space) of the species to be detected. For the primary assays of O2•− and H2O2, HPr+ and CBA-based assays were used, respectively. It was shown that stimulation of dHL60 cells with PMA leads to oxidation of the HPr+ and CBA probes, accompanied by an increase in the fluorescence intensity (Fig. 14). While the HPr+-derived signal was inhibitable by SOD but not by CAT (Fig. 14A), the CBA-derived fluorescence signal was inhibited by CAT but not by SOD (Fig. 14B). The fluorescence intensity was decreased in case of both probes when commercially available inhibitors, DPI and VAS2870, were applied. All these data confirm that the HPr+ and CBA probes measure the activity of NADPH oxidase, by reporting O2•− and H2O2 production, respectively. These assays were performed in 384-well plates, demonstrating the HTS compatibility.
Figure 13.
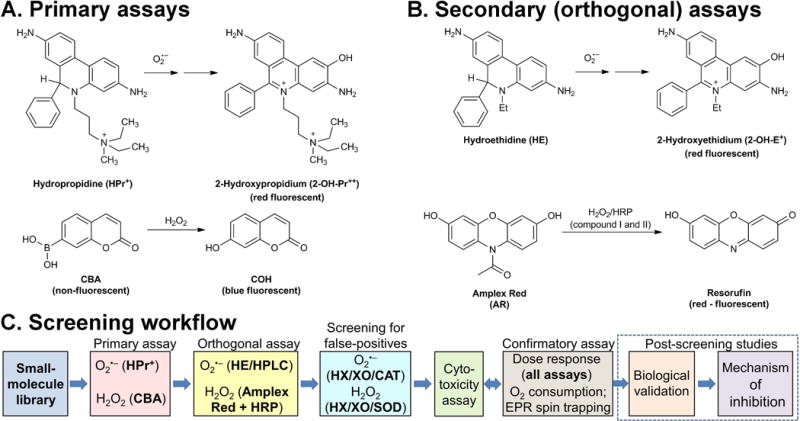
Probe chemistry and assay design. (A) Probes and products formed in primary assays. (B) Probes and products formed in orthogonal assays. (C) The workflow scheme for screening of Nox inhibitors. (This research was originally published in Journal of Biological Chemistry. Zielonka, J., Cheng, G., Zielonka, M., Ganesh, T., Sun, A., Joseph, J., Michalski, R., O’Brien, W. J., Lambeth, J. D., & Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014; 289: 16176–16189. © the American Society for Biochemistry and Molecular Biology.) (79)
Figure 14.
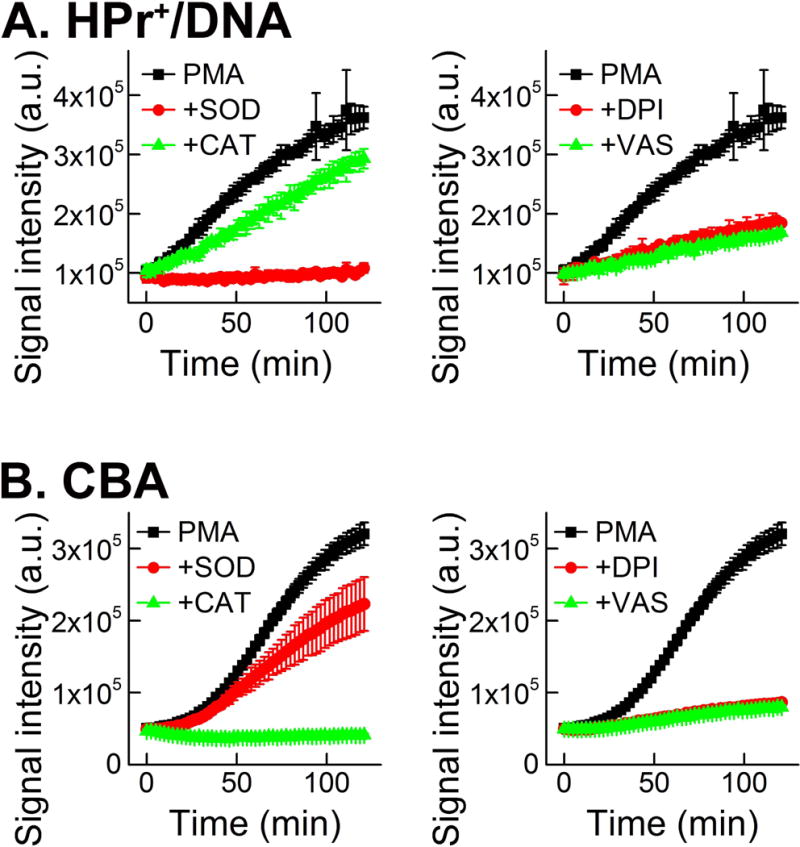
Monitoring NADPH oxidase-2 activity in dHL60 cells using primary assays. (A) Effect of SOD, catalase, DPI and VAS2870 on the time-dependent increase in fluorescence intensity due to oxidation of HPr+ probe, induced by PMA. (B) Same as in (A), but CBA probe was used. (This research was originally published in Journal of Biological Chemistry. Zielonka, J., Cheng, G., Zielonka, M., Ganesh, T., Sun, A., Joseph, J., Michalski, R., O’Brien, W. J., Lambeth, J. D., & Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014; 289: 16176–16189. © the American Society for Biochemistry and Molecular Biology.) (79)
For the secondary assays, the HE probe coupled with rapid HPLC-based detection of 2-OH-E+ was proposed for monitoring NADPH oxidase-derived O2•−. For the detection of H2O2, the Amplex Red-based assay was used. As shown in Figure 15, the stimulation of the dHL60 cells with PMA led to the appearance of the HPLC peak of 2-OH-E+ and time-dependent increase in Amplex Red-derived fluorescence. The HPLC peak of 2-OH-E+ was inhibited by SOD but not by CAT (Fig. 15A), while the Amplex Red-derived fluorescence signal was inhibited by CAT but not by SOD (Fig. 15B). Again, both secondary assays described could be carried out in a 384-well plate format, demonstrating their applicability in the HTS campaign. Using these four primary and secondary assays for O2•− and H2O2, a small, focused library of potential inhibitors of NADPH oxidase-2 was screened, resulting in identification of several positive hits, which decrease the rates of probe oxidation by more than 50% when used at 10 μM concentration (79). Interestingly, the same hits were identified in all four assays. One of the positive hits identified, called compound 43 (Fig. 16A), was further tested in confirmatory assays, as described below. At a 10 μM concentration, this compound did not interfere with the assays (when tested using hypoxanthine and xanthine oxidase as a source of O2•− and H2O2) nor did it induce cell death (as measured by monitoring cellular ATP levels) (79).
Figure 15.
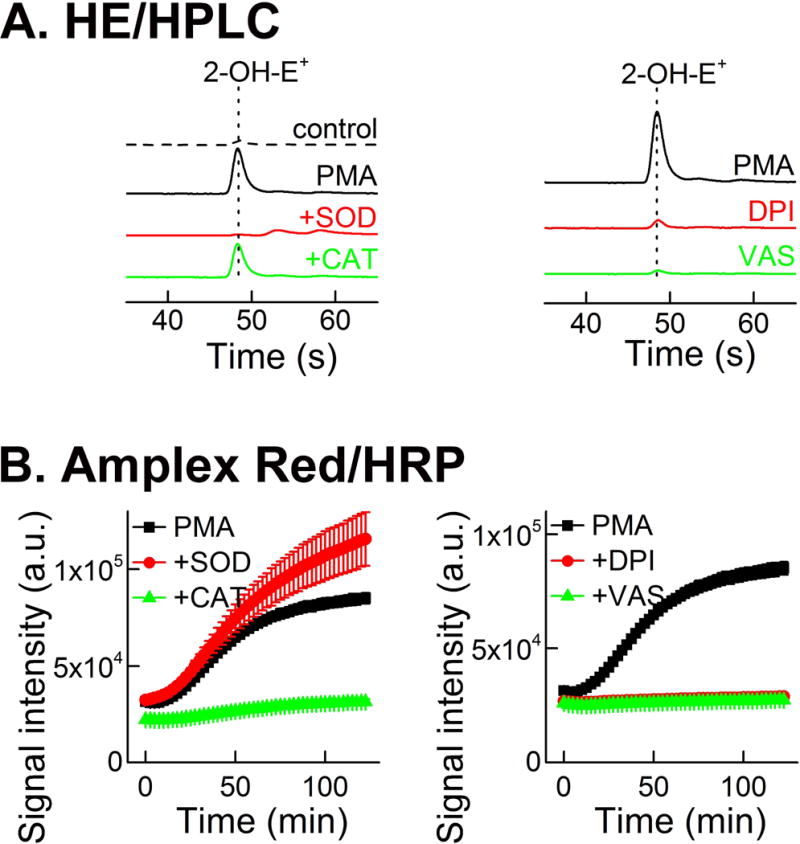
Monitoring NADPH oxidase-2 activity in dHL60 cells using secondary assays. (A) Effect of SOD, catalase, DPI, and VAS2870 on the yield of 2-OH-E+ formed from HE probe by dHL60 cells stimulated with PMA, as measured by rapid HPLC analyses. (B) Effect of SOD, CAT, DPI, and VAS2870 on the time-dependent increase in fluorescence intensity due to oxidation of Amplex Red probe, induced by PMA. (This research was originally published in Journal of Biological Chemistry. Zielonka, J., Cheng, G., Zielonka, M., Ganesh, T., Sun, A., Joseph, J., Michalski, R., O’Brien, W. J., Lambeth, J. D., & Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014; 289: 16176–16189. © the American Society for Biochemistry and Molecular Biology.) (79)
Figure 16.
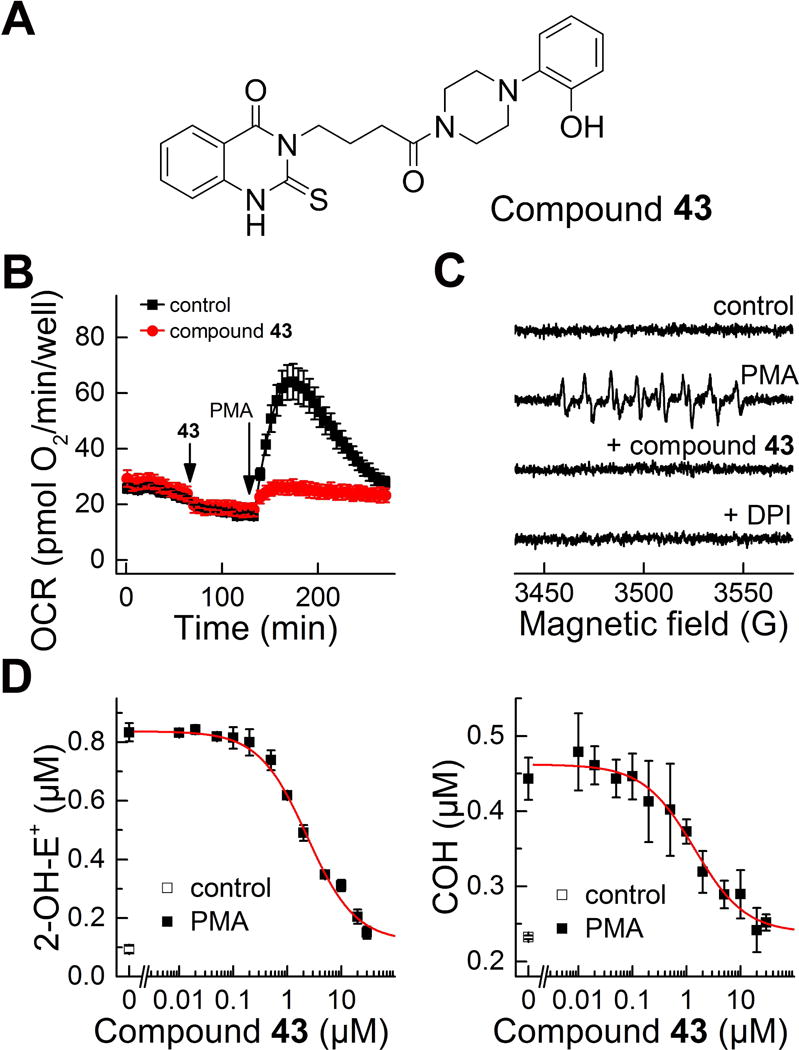
Confirmatory assays used for further characterization of the positive hits from HTS campaign. (A) Structure of the identified hit (compound 43 from ref. (79)). (B-C) Effect of the identified hit on the PMA-stimulated oxygen consumption rates (B) and formation of DEPMPO superoxide spin adduct (C). (D) Concentration dependence of the compound 43 on PMA-stimulated probe oxidation by dHL60 cells in the HPLC-based assays for simultaneous monitoring of O2•− and H2O2. (This research was originally published in Journal of Biological Chemistry. Zielonka, J., Cheng, G., Zielonka, M., Ganesh, T., Sun, A., Joseph, J., Michalski, R., O’Brien, W. J., Lambeth, J. D., & Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014; 289: 16176–16189. © the American Society for Biochemistry and Molecular Biology.) (79)
Multi-well plate-based oximetry on the Seahorse XF96 extracellular flux analyzer was used for probe-free monitoring of the effects of compound 43 on NADPH oxidase-dependent oxygen consumption. As shown in Figure 16B, in contrast to DPI (Fig. 12) compound 43 did not significantly affect the basal (mitochondrial) respiration, but it prevented the PMA-induced burst in oxygen consumption, indicating selective inhibition of NADPH oxidase activity. Furthermore, treatment of dHL60 cells with compound 43, similar to treatment with DPI, prevented the PMA-stimulated formation of the superoxide spin adduct to DEPMPO, as tested using the EPR spin trapping technique (Fig. 16C). Finally, the dose dependence of the effects of compound 43 on PMA-stimulated activity of NADPH oxidase in dHL60 cells was studied using rapid HPLC-based simultaneous detection of O2•− and H2O2. Both the formation of 2-OH-E+ (product of the reaction between HE and O2•−) and of COH (product of the reaction between CBA and H2O2) were inhibited with similar IC50 value (2.3 μM) (Fig. 16D).
The experiments described above established a set of assays suitable for potential use in an HTS campaign for discovery of new inhibitors of NADPH oxidases. In the follow-up study, the proposed workflow was used to screen a library of more than 2,000 bioactive compounds at the Broad Institute (24). All three plate-reader-based assays (using HPr+, CBA, and Amplex Red probes) were compatible with HTS and showed good plate-to- plate reproducibility. The correlation of the results from the assays performed led to identification of 49 (2.4%) compounds showing inhibitory effects in all three assays (Fig. 17). The selected positive hits identified were further tested in confirmatory assays and showed inhibitory activity on NADPH oxidase activity in other cellular models of NADPH oxidase, RAW 264.7 macrophages, stimulated with PMA. Furthermore, the compounds capable of inhibiting NADPH oxidase activity were demonstrated to be able to mitigate peroxynitrite formation by activated macrophages, as measured using a novel LC-MS (liquid chromatography-mass spectrometry)-based, peroxynitrite-specific assay (24).
Figure 17.

Results of screening of a library of bioactive compounds (~ 2,000) using three probes: HPr+ in the presence of DNA as a probe for O2•−, and CBA or Amplex Red in the presence of HRP as probes for H2O2. (A) Correlation of the results of the three assays for Nox2 activity. (B) Results of screening as a percentage of positive hits in one, two or all three assays. (This research was originally published in Journal of Biological Chemistry. Zielonka, J., Zielonka, M., VerPlank, L., Cheng, G., Hardy, M., Ouari, O., Ayhan, M. M., Podsiadly, R., Sikora, A., Lambeth, J. D., & Kalyanaraman, B. Mitigation of NADPH Oxidase 2 Activity as a Strategy to Inhibit Peroxynitrite Formation. J. Biol. Chem. 2016; 291:7029–7044. © the American Society for Biochemistry and Molecular Biology) (24)
Whether the identified positive hits directly bind to NADPH oxidase subunits, or affect the upstream events leading to NADPH oxidase activation, remains to be established by the follow-up studies (Fig. 13C, post-screening studies) including cell-free assays of NADPH oxidase activity and analysis of the effects of the identified inhibitors on the phosphorylation status and assembly of NADPH oxidase subunits. Interestingly, some of the identified positive hits included promazines, a class of compounds identified as NADPH oxidase-2 inhibitors in an independent study, which showed the inhibitory activity also in cell-free assays (23). This opens up the possibility of repurposing compounds already in use in the clinic for treatment of pathologies associated with increased activity of the members of the family of NADPH oxidases.
Conclusions and perspectives
Although many probes widely used to measure NADPH oxidase activity suffer from serious limitations and their use needs to be discontinued, currently several reliable assays for O2•− and H2O2 can be adapted to monitor the activity of NADPH oxidases in a high-throughput manner. Using the appropriate models of isoforms of NADPH oxidase, these assays can be used in HTS campaigns using large chemical libraries, with the aim of discovering new, isoform-specific inhibitors of NADPH oxidases. Further medicinal, chemistry-based work on positive hits may improve their potency while minimizing toxicity, providing a large therapeutic window for potential clinical trials.
The other, complementary approach includes the screening of smaller libraries of FDA-approved drugs and agents, which could lead to rapid translation of the positive hits “from bench to bedside” via a drug repurposing strategy. In fact, the promazine-based inhibitors identified independently by different groups using the HTS assays were demonstrated to inhibit superoxide levels in mouse brains in vivo, demonstrating the high potential of such a strategy (23).
Acknowledgments
Funding: This work was supported by a grant from NIH (R01 AA022986) to B.K. and from the French National Research Agency (ANR-16-CE07-0023-01) to O.O. and M.H. A.S. and R.M. were supported by a grant from Polish National Science Centre, No. 2015/18/E/ST4/00235.
Abbreviations
- 2-OH-E+
2-hydroxyethidium
- 2-OH-Pr2+
2-hydroxypropidium
- BMPO
5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide
- CAT
catalase
- CBA
coumarin-7-boronic acod
- CD
cyclodextrin
- COH
7-hydroxycoumarin
- cyt c3+
ferricytochrome c
- DEPMPO
5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide
- DIPPMPO
5-(diisopropoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- DPI
diphenyleneiodonium
- E+
ethidium
- E+-E+
diethidium
- EPR
electron paramagnetic resonance
- GSH
glutathione
- HE
hydroethidine (or dihydroethidium)
- HE•+
HE radical cation
- HOCl
hypochlorous acid
- HPLC
high-performance liquid chromatography
- HPr+
hydropropidine
- HRP
horseradish peroxidase
- HTS
high-throughput screening
- L-012
8-amino-5-chloro-2,3-dihydro-7-phenyl-pyrido[3,4-d]pyridazine
- MPO
myeloperoxidase
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
- NBT
nitroblue tetrazolium
- Nox2
NADPH oxidase-2
- Nox4
NADPH oxidase-4
- Nox5
NADPH oxidase-5
- OCR
oxygen consumption rate
- PMA
phorbol 12-myristate 13-acetate
- SOD
superoxide dismutase
- VAS2870
1,3-benzoxazol-2-yl-3-benzyl-3H-[1,2,3]triazolo[4,5-d]pyrimidin-7-yl sulfide
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest.
References
- 1.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 3.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. Oxford University Press; USA: 2015. [Google Scholar]
- 6.Bonner MY, Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci. 2012;69:2435–2442. doi: 10.1007/s00018-012-1017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today. 2006;11:524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Hecker L, Cheng J, Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci. 2012;69:2365–2371. doi: 10.1007/s00018-012-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streeter J, Thiel W, Brieger K, Miller FJ., Jr Opportunity Nox: The future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc Ther. 2013;31:125–137. doi: 10.1111/j.1755-5922.2011.00310.x. [DOI] [PubMed] [Google Scholar]
- 10.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 11.Krause KH, Lambeth D, Kronke M. NOX enzymes as drug targets. Cell Mol Life Sci. 2012;69:2279–2282. doi: 10.1007/s00018-012-1006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayernia Z, Jaquet V, Krause KH. New insights on NOX enzymes in the central nervous system. Antioxid Redox Signal. 2014;20:2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altenhofer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxid Redox Signal. 2015;23:406–427. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borbely G, Szabadkai I, Horvath Z, Marko P, Varga Z, Breza N, Baska F, Vantus T, Huszar M, Geiszt M, Hunyady L, Buday L, Orfi L, Keri G. Small-molecule inhibitors of NADPH oxidase 4. J Med Chem. 2010;53:6758–6762. doi: 10.1021/jm1004368. [DOI] [PubMed] [Google Scholar]
- 15.Cifuentes-Pagano E, Meijles DN, Pagano PJ. The quest for selective Nox inhibitors and therapeutics: Challenges, triumphs and pitfalls. Antioxid Redox Signal. 2014;20:2741–2754. doi: 10.1089/ars.2013.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 18.Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cifuentes-Pagano E, Csanyi G, Pagano PJ. NADPH oxidase inhibitors: A decade of discovery from Nox2ds to HTS. Cell Mol Life Sci. 2012;69:2315–2325. doi: 10.1007/s00018-012-1009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem. 2010;53:7715–7730. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 21.Cifuentes-Pagano E, Saha J, Csanyi G, Ghouleh IA, Sahoo S, Rodriguez A, Wipf P, Pagano PJ, Skoda EM. Bridged tetrahydroisoquinolines as selective NADPH oxidase 2 (Nox2) inhibitors. Medchemcomm. 2013;4:1085–1092. doi: 10.1039/C3MD00061C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano K, Chen WS, Chueng AL, Dunne AA, Seredenina T, Filippova A, Ramachandran S, Bridges A, Chaudry L, Pettman G, Allan C, Duncan S, Lee KC, Lim J, Ma MT, Ong AB, Ye NY, Nasir S, Mulyanidewi S, Aw CC, Oon PP, Liao S, Li D, Johns DG, Miller ND, Davies CH, Browne ER, Matsuoka Y, Chen DW, Jaquet V, Rutter AR. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid Redox Signal. 2015;23:358–374. doi: 10.1089/ars.2014.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seredenina T, Chiriano G, Filippova A, Nayernia Z, Mahiout Z, Fioraso-Cartier L, Plastre O, Scapozza L, Krause KH, Jaquet V. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radic Biol Med. 2015;86:239–249. doi: 10.1016/j.freeradbiomed.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Zielonka J, Zielonka M, VerPlank L, Cheng G, Hardy M, Ouari O, Ayhan MM, Podsiadly R, Sikora A, Lambeth JD, Kalyanaraman B. Mitigation of NADPH oxidase 2 activity as a strategy to inhibit peroxynitrite formation. J Biol Chem. 2016;291:7029–7044. doi: 10.1074/jbc.M115.702787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maghzal GJ, Krause KH, Stocker R, Jaquet V. Detection of reactive oxygen species derived from the family of NOX NADPH oxidases. Free Radic Biol Med. 2012;53:1903–1918. doi: 10.1016/j.freeradbiomed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Kalyanaraman B, Hardy M, Podsiadly R, Cheng G, Zielonka J. Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling. Arch Biochem Biophys. 2017;617:38–47. doi: 10.1016/j.abb.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debowska K, Debski D, Hardy M, Jakubowska M, Kalyanaraman B, Marcinek A, Michalski R, Michalowski B, Ouari O, Sikora A, Smulik R, Zielonka J. Toward selective detection of reactive oxygen and nitrogen species with the use of fluorogenic probes–limitations, progress, and perspectives. Pharmacol Rep. 2015;67:756–764. doi: 10.1016/j.pharep.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalyanaraman B, Hardy M, Zielonka J. A critical review of methodologies to detect reactive oxygen and nitrogen species stimulated by NADPH oxidase enzymes: Implications in pesticide toxicity. Curr Pharmacol Rep. 2016;2:193–201. doi: 10.1007/s40495-016-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Koppenol WH, van Buuren KJ, Butler J, Braams R. The kinetics of the reduction of cytochrome c by the superoxide anion radical. Biochim Biophys Acta. 1976;449:157–168. doi: 10.1016/0005-2728(76)90130-4. [DOI] [PubMed] [Google Scholar]
- 31.Bielski BHJ, Shiue GG, Bajuk S. Reduction of nitro blue tetrazolium by CO2- and O2- radicals. J Phys Chem. 1980;84:830–833. [Google Scholar]
- 32.Nauseef WM. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim Biophys Acta. 2014;1840:757–767. doi: 10.1016/j.bbagen.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler J, Koppenol WH, Margoliash E. Kinetics and mechanism of the reduction of ferricytochrome c by the superoxide anion. J Biol Chem. 1982;257:10747–10750. [PubMed] [Google Scholar]
- 34.Nisimoto Y, Otsuka-Murakami H, Iwata S. NADPH-cytochrome c reductase from human neutrophil membranes: Purification, characterization and localization. Biochem J. 1994;297(Pt 3):585–593. doi: 10.1042/bj2970585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nisimoto Y, Otsuka-Murakami H, Iwata S, Isogai Y, Iizuka T. Characterization of superoxide dismutase-insensitive cytochrome c reductase activity in HL-60 cytosol as NADPH-cytochrome P450 reductase. Arch Biochem Biophys. 1993;302:315–321. doi: 10.1006/abbi.1993.1217. [DOI] [PubMed] [Google Scholar]
- 36.Ginsburgh CL, Everse J. Studies on the reduction of cytochrome c by thiols. Bioorg Chem. 1978;7:481–492. [Google Scholar]
- 37.Hu TM, Ho SC. Kinetics of redox interaction between cytochrome c and thiols. J Med Sci. 2011;31:109–115. [Google Scholar]
- 38.Choi HS, Kim JW, Cha YN, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem. 2006;27:31–44. doi: 10.1080/15321810500403722. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 40.Keshari RS, Verma A, Barthwal MK, Dikshit M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J Cell Biochem. 2013;114:532–540. doi: 10.1002/jcb.24391. [DOI] [PubMed] [Google Scholar]
- 41.Thayer WS. Superoxide-dependent and superoxide-independent pathways for reduction of nitroblue tetrazolium in isolated rat cardiac myocytes. Arch Biochem Biophys. 1990;276:139–145. doi: 10.1016/0003-9861(90)90020-y. [DOI] [PubMed] [Google Scholar]
- 42.Schor NA, Stedman RB, Epstein N, Schally G. Rat splenic D-T diaphorase and NAD(P)H-nitroblue tetrazolium reductase. Their use to assess the action of polycyclic hydrocarbons in the lymphatic system. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41:83–93. doi: 10.1007/BF02890273. [DOI] [PubMed] [Google Scholar]
- 43.Auclair C, Torres M, Hakim J. Superoxide anion involvement in nbt reduction catalyzed by NADPH-cytochrome P-450 reductase: A pitfall. FEBS Lett. 1978;89:26–28. doi: 10.1016/0014-5793(78)80514-6. [DOI] [PubMed] [Google Scholar]
- 44.Picker SD, Fridovich I. On the mechanism of production of superoxide radical by reaction mixtures containing NADH, phenazine methosulfate, and nitroblue tetrazolium. Arch Biochem Biophys. 1984;228:155–158. doi: 10.1016/0003-9861(84)90056-0. [DOI] [PubMed] [Google Scholar]
- 45.Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O2.- Free Radic Biol Med. 1993;15:447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 46.Liochev SI, Fridovich I. Lucigenin (bis-n-methylacridinium) as a mediator of superoxide anion production. Arch Biochem Biophys. 1997;337:115–120. doi: 10.1006/abbi.1997.9766. [DOI] [PubMed] [Google Scholar]
- 47.Vasquez-Vivar J, Hogg N, Pritchard KA, Jr, Martasek P, Kalyanaraman B. Superoxide anion formation from lucigenin: An electron spin resonance spin-trapping study. FEBS Lett. 1997;403:127–130. doi: 10.1016/s0014-5793(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 48.Vasquez-Vivar J, Martasek P, Hogg N, Karoui H, Masters BS, Pritchard KA, Jr, Kalyanaraman B. Electron spin resonance spin-trapping detection of superoxide generated by neuronal nitric oxide synthase. Methods Enzymol. 1999;301:169–177. doi: 10.1016/s0076-6879(99)01080-0. [DOI] [PubMed] [Google Scholar]
- 49.Wardman P, Burkitt MJ, Patel KB, Lawrence A, Jones CM, Everett SA, Vojnovic B. Pitfalls in the use of common luminescent probes for oxidative and nitrosative stress. J Fluoresc. 2002;12:65–68. [Google Scholar]
- 50.Rezende F, Prior KK, Lowe O, Wittig I, Strecker V, Moll F, Helfinger V, Schnutgen F, Kurrle N, Wempe F, Walter M, Zukunft S, Luck B, Fleming I, Weissmann N, Brandes RP, Schroder K. Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radic Biol Med. 2017;102:57–66. doi: 10.1016/j.freeradbiomed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Rezende F, Lowe O, Helfinger V, Prior KK, Walter M, Zukunft S, Fleming I, Weissmann N, Brandes RP, Schroder K. Unchanged NADPH oxidase activity in Nox1-Nox2-Nox4 triple knockout mice: What do NADPH-stimulated chemiluminescence assays really detect? Antioxid Redox Signal. 2016;24:392–399. doi: 10.1089/ars.2015.6314. [DOI] [PubMed] [Google Scholar]
- 52.Nishinaka Y, Aramaki Y, Yoshida H, Masuya H, Sugawara T, Ichimori Y. A new sensitive chemiluminescence probe, L-012, for measuring the production of superoxide anion by cells. Biochem Biophys Res Commun. 1993;193:554–559. doi: 10.1006/bbrc.1993.1659. [DOI] [PubMed] [Google Scholar]
- 53.Imada I, Sato EF, Miyamoto M, Ichimori Y, Minamiyama Y, Konaka R, Inoue M. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal Biochem. 1999;271:53–58. doi: 10.1006/abio.1999.4107. [DOI] [PubMed] [Google Scholar]
- 54.Sohn HY, Gloe T, Keller M, Schoenafinger K, Pohl U. Sensitive superoxide detection in vascular cells by the new chemiluminescence dye L-012. J Vasc Res. 1999;36:456–464. doi: 10.1159/000025688. [DOI] [PubMed] [Google Scholar]
- 55.Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, Kleschyov AL, Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36:101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Merenyi G, Lind J, Eriksen TE. Luminol chemiluminescence: Chemistry, excitation, emitter. J Biolumin Chemilumin. 1990;5:53–56. doi: 10.1002/bio.1170050111. [DOI] [PubMed] [Google Scholar]
- 57.Zielonka J, Lambeth JD, Kalyanaraman B. On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: A reevaluation. Free Radic Biol Med. 2013;65:1310–1314. doi: 10.1016/j.freeradbiomed.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Ganesh T, Diebold BA, Zhu Y, McCoy JW, Smith SM, Sun A, Lambeth JD. Thioxo-dihydroquinazolin-one compounds as novel inhibitors of myeloperoxidase. ACS Med Chem Lett. 2015;6:1047–1052. doi: 10.1021/acsmedchemlett.5b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J Leukoc Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- 60.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Michalski R, Michalowski B, Sikora A, Zielonka J, Kalyanaraman B. On the use of fluorescence lifetime imaging and dihydroethidium to detect superoxide in intact animals and ex vivo tissues: A reassessment. Free Radic Biol Med. 2014;67:278–284. doi: 10.1016/j.freeradbiomed.2013.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zielonka J, Zhao H, Xu Y, Kalyanaraman B. Mechanistic similarities between oxidation of hydroethidine by fremy’s salt and superoxide: Stopped-flow optical and EPR studies. Free Radic Biol Med. 2005;39:853–863. doi: 10.1016/j.freeradbiomed.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Zielonka J, Sarna T, Roberts JE, Wishart JF, Kalyanaraman B. Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch Biochem Biophys. 2006;456:39–47. doi: 10.1016/j.abb.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mitohydroethidine in mitochondria: Identification of homo- and heterodimers. Free Radic Biol Med. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maghzal GJ, Cergol KM, Shengule SR, Suarna C, Newington D, Kettle AJ, Payne RJ, Stocker R. Assessment of myeloperoxidase activity by the conversion of hydroethidine to 2-chloroethidium. J Biol Chem. 2014;289:5580–5595. doi: 10.1074/jbc.M113.539486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Talib J, Maghzal GJ, Cheng D, Stocker R. Detailed protocol to assess in vivo and ex vivo myeloperoxidase activity in mouse models of vascular inflammation and disease using hydroethidine. Free Radic Biol Med. 2016;97:124–135. doi: 10.1016/j.freeradbiomed.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Kalyanaraman B, Dranka BP, Hardy M, Michalski R, Zielonka J. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes–the ultimate approach for intra- and extracellular superoxide detection. Biochim Biophys Acta. 2014;1840:739–744. doi: 10.1016/j.bbagen.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zielonka J, Hardy M, Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: Ramifications in superoxide measurements. Free Radic Biol Med. 2009;46:329–338. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: A unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 71.Fernandes DC, Gonçalves RC, Laurindo FRM. Measurement of superoxide production and NADPH oxidase activity by HPLC analysis of dihydroethidium oxidation. In: Touyz RM, Schiffrin EL, editors. Hypertension: Methods and protocols. Springer New York; New York, NY: 2017. pp. 233–249. [DOI] [PubMed] [Google Scholar]
- 72.Michalski R, Zielonka J, Hardy M, Joseph J, Kalyanaraman B. Hydropropidine: A novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radic Biol Med. 2013;54:135–147. doi: 10.1016/j.freeradbiomed.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ouari O, Hardy M, Karoui H, Tordo P. Recent developments and applications of the coupled EPR/spin trapping technique (EPR/st) Electron Paramagnetic Resonance. 2011;22:1–40. [Google Scholar]
- 74.Abbas K, Babic N, Peyrot F. Use of spin traps to detect superoxide production in living cells by electron paramagnetic resonance (EPR) spectroscopy. Methods. 2016;109:31–43. doi: 10.1016/j.ymeth.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Davies MJ. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods. 2016;109:21–30. doi: 10.1016/j.ymeth.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 76.Britigan BE, Rosen GM, Chai Y, Cohen MS. Do human neutrophils make hydroxyl radical? Determination of free radicals generated by human neutrophils activated with a soluble or particulate stimulus using electron paramagnetic resonance spectrometry. J Biol Chem. 1986;261:4426–4431. [PubMed] [Google Scholar]
- 77.Britigan BE, Rosen GM, Thompson BY, Chai Y, Cohen MS. Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986;261:17026–17032. [PubMed] [Google Scholar]
- 78.Pou S, Cohen MS, Britigan BE, Rosen GM. Spin-trapping and human neutrophils. Limits of detection of hydroxyl radical. J Biol Chem. 1989;264:12299–12302. [PubMed] [Google Scholar]
- 79.Zielonka J, Cheng G, Zielonka M, Ganesh T, Sun A, Joseph J, Michalski R, O’Brien WJ, Lambeth JD, Kalyanaraman B. High-throughput assays for superoxide and hydrogen peroxide: Design of a screening workflow to identify inhibitors of NADPH oxidases. J Biol Chem. 2014;289:16176–16189. doi: 10.1074/jbc.M114.548693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abbas K, Hardy M, Poulhes F, Karoui H, Tordo P, Ouari O, Peyrot F. Medium-throughput ESR detection of superoxide production in undetached adherent cells using cyclic nitrone spin traps. Free Radic Res. 2015;49:1122–1128. doi: 10.3109/10715762.2015.1045504. [DOI] [PubMed] [Google Scholar]
- 81.Hardy M, Bardelang D, Karoui H, Rockenbauer A, Finet JP, Jicsinszky L, Rosas R, Ouari O, Tordo P. Improving the trapping of superoxide radical with a beta-cyclodextrin- 5-diethoxyphosphoryl-5-methyl-1-pyrroline-n-oxide (DEPMPO) conjugate. Chemistry (Easton) 2009;15:11114–11118. doi: 10.1002/chem.200901342. [DOI] [PubMed] [Google Scholar]
- 82.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: Applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 83.Votyakova TV, Reynolds IJ. Detection of hydrogen peroxide with Amplex Red: Interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys. 2004;431:138–144. doi: 10.1016/j.abb.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 84.Kashem MA, Dunford HB. Kinetics of the oxidation of reduced nicotinamide adenine dinucleotide by horseradish peroxidase compounds I and II. Biochem Cell Biol. 1986;64:323–327. doi: 10.1139/o86-045. [DOI] [PubMed] [Google Scholar]
- 85.Harman LS, Carver DK, Schreiber J, Mason RP. One- and two-electron oxidation of reduced glutathione by peroxidases. J Biol Chem. 1986;261:1642–1648. [PubMed] [Google Scholar]
- 86.Zhao B, Summers FA, Mason RP. Photooxidation of Amplex Red to resorufin: Implications of exposing the Amplex Red assay to light. Free Radic Biol Med. 2012;53:1080–1087. doi: 10.1016/j.freeradbiomed.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Debski D, Smulik R, Zielonka J, Michalowski B, Jakubowska M, Debowska K, Adamus J, Marcinek A, Kalyanaraman B, Sikora A. Mechanism of oxidative conversion of Amplex(R) Red to resorufin: Pulse radiolysis and enzymatic studies. Free Radic Biol Med. 2016;95:323–332. doi: 10.1016/j.freeradbiomed.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Summers FA, Zhao B, Ganini D, Mason RP. Photooxidation of Amplex Red to resorufin: Implications of exposing the Amplex Red assay to light. Methods Enzymol. 2013;526:1–17. doi: 10.1016/B978-0-12-405883-5.00001-6. [DOI] [PubMed] [Google Scholar]
- 89.Zhao B, Ranguelova K, Jiang J, Mason RP. Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex Red. Free Radic Biol Med. 2011;51:153–159. doi: 10.1016/j.freeradbiomed.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lippert AR, Van de Bittner GC, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc Chem Res. 2011;44:793–804. doi: 10.1021/ar200126t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem Res Toxicol. 2012;25:1793–1799. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: Mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic Biol Med. 2009;47:1401–1407. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: Direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sikora A, Zielonka J, Lopez M, Dybala-Defratyka A, Joseph J, Marcinek A, Kalyanaraman B. Reaction between peroxynitrite and boronates: EPR spin-trapping, HPLC analyses, and quantum mechanical study of the free radical pathway. Chem Res Toxicol. 2011;24:687–697. doi: 10.1021/tx100439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: High-throughput real-time analyses. J Biol Chem. 2012;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Michalski R, Zielonka J, Gapys E, Marcinek A, Joseph J, Kalyanaraman B. Real-time measurements of amino acid and protein hydroperoxides using coumarin boronic acid. J Biol Chem. 2014;289:22536–22553. doi: 10.1074/jbc.M114.553727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: Direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zielonka J, Sikora A, Adamus J, Kalyanaraman B. Detection and differentiation between peroxynitrite and hydroperoxides using mitochondria-targeted arylboronic acid. Methods Mol Biol. 2015;1264:171–181. doi: 10.1007/978-1-4939-2257-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zielonka J, Podsiadly R, Zielonka M, Hardy M, Kalyanaraman B. On the use of peroxy-caged luciferin (PCL-1) probe for bioluminescent detection of inflammatory oxidants in vitro and in vivo – identification of reaction intermediates and oxidant-specific minor products. Free Radic Biol Med. 2016;99:32–42. doi: 10.1016/j.freeradbiomed.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smulik R, Debski D, Zielonka J, Michalowski B, Adamus J, Marcinek A, Kalyanaraman B, Sikora A. Nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH. Biological implications. J Biol Chem. 2014;289:35570–35581. doi: 10.1074/jbc.M114.597740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Woolley JF, Naughton R, Stanicka J, Gough DR, Bhatt L, Dickinson BC, Chang CJ, Cotter TG. H2O2 production downstream of FLT3 is mediated by p22phox in the endoplasmic reticulum and is required for STAT5 signalling. PLoS One. 2012;7:e34050. doi: 10.1371/journal.pone.0034050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dickinson BC, Peltier J, Stone D, Schaffer DV, Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol. 2011;7:106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brewer TF, Garcia FJ, Onak CS, Carroll KS, Chang CJ. Chemical approaches to discovery and study of sources and targets of hydrogen peroxide redox signaling through NADPH oxidase proteins. Annu Rev Biochem. 2015;84:765–790. doi: 10.1146/annurev-biochem-060614-034018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD. Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. Nox4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]


