Abstract
Histotripsy is a noninvasive, non-thermal ablation technique that uses high-amplitude, focused ultrasound pulses to fractionate tissue via acoustic cavitation. The goal of this study was to show the potential of histotripsy with electronic focal steering to achieve rapid ablation of a tissue volume at a rate matching or exceeding current clinical techniques (~1–2 mL/min). Treatment parameters were established in tissue-mimicking phantoms and applied to ex vivo tissue. 6-μs pulses were delivered by a 250 kHz array. The focus was electrically steered to 1000 locations at 200 Hz PRF (0.12 % duty cycle). MRI and histology of the treated tissue shows a distinct region of necrosis in all samples. Mean lesion volume was 35.6 ± 4.3 mL, generated at 0.9–3.3 mL/min, a speed faster than any current ablation method for a large volume. These results suggest that histotripsy has the potential to achieve noninvasive, rapid, homogenous ablation of a tissue volume.
Keywords: Therapeutic ultrasound, Cavitation, Non-invasive tissue ablation, Electronic focal steering, Histotripsy
Introduction
Surgical resection has long been considered the gold standard for solid tumor intervention (Liang et al. 2014; McWilliams et al. 2010; Scheele et al. 1995). However, minimally invasive local ablation techniques are increasingly indicated as first-option treatments for select cases due to lower cost, shorter associated hospital stays, and lower risk of complications (McWilliams et al. 2010; Jansen et al. 2005; Livraghi et al. 2008; Tombesi et al. 2013). Further motivating this transition, surgery is often feasible for only a small fraction of patients (9–27% of those presenting with hepatocellular carcinoma for example) due to the presence of various complicating factors including compromised organ function, tumor location and morphology, and patient frailty (Curley 2001; Jansen et al. 2005). Despite these advantages, current minimally invasive techniques including radio frequency ablation (RFA), microwave ablation (MWA), laser ablation, and cryo-therapy have limited applicability due to their inability to effectively treat tumors greater than 3 cm in diameter and those with more than 3 nodules (Tateishi et al. 2005; Tombesi et al. 2013).
Clinical alternatives to surgically resecting larger tumors and those with multiple tumor nodules include stereotactic body radiation (SBRT), transcatheter arterial chemoembolization (TACE), and high intensity focused ultrasound (HIFU). SBRT, a technique that uses multiple overlapping radiation beams, can treat moderately sized tumors. However, for tumors >3 cm in diameter, its efficacy diminishes and the risk of damaging healthy tissue increases due to the toxicity associated with radiation (Feigenberg et al. 2015; Timmerman et al. 2006). TACE has produced a significant tumor response in 17–62% of patients with tumors larger than 5 cm, but the rate of complete tumor ablation is very low (0–5% in hepatocellular carcinoma, for example) (Jansen et al. 2005). Thus, TACE is generally used as a palliative or adjuvant therapy. HIFU has been used noninvasively to treat very large tumors (>10 cm)(Wu et al. 2004a; Wu et al. 2004b) but is hampered by generally long treatment times which average approximately 5.5 hours for tumors roughly 8 cm in diameter, presenting a major obstacle to widespread clinical application (Wu et al. 2003; Wu et al. 2004a). Several studies have demonstrated in vivo HIFU ablation rates of ≥ 2 mL/min for targets ≤ 8 mL using optimized electronic focal steering trajectories (Kim et al. 2012; Köhler et al. 2009). However, this technique does not appear scalable to larger tissue targets while maintaining a high ablation rate due to concerns for damaging surrounding tissue structures. For thermal therapies like RFA, MWA, laser therapy, cyrotherapy, and HIFU, perfusion-mediated convection (due to blood flow and commonly referred to as the “heat sink effect”) presents a major challenge for achieving homogeneous coagulative necrosis in highly vascularized tissues like the liver, resulting in incomplete ablation and often requiring repeated treatment (McWilliams et al. 2010; Leslie & Kennedy 2006; McWilliams et al. 2010).
Histotripsy is a noninvasive, non-thermal, ultrasound ablation method that uses short-duration, high-amplitude, focused ultrasound pulses at very low-duty cycle to generate controlled cavitation, which fractionates target tissues into liquid-appearing homogenate. Our previous in vivo studies have shown that histotripsy is not affected by the heat sink effect and can produce homogenous tissue disruption in highly vascular organs, such as the liver and kidney, noninvasively through the ribcage and other overlying tissues (Kim et al. 2014; Vlaisavljevich et al. 2013). Khokhlova et al. (2016) showed that histotripsy is capable of lysing large hemotomas (20 mL) in vitro at a clinically relevant rate of 1.3 mL/min using mechanical focal steering. Vlaisavljevich et al. (2013) demonstrated the ability to generate a 60 mL lesion within 60 minutes in an in vivo porcine model by mechanically steering the therapy focus of a transducer that used the shock scattering mechanism with multi-cycle (≥3 cycles) pulses to generate and maintain a cavitation bubble cloud (Maxwell et al. 2011). Subsequent studies in our lab have shown that the intrinsic threshold mechanism can achieve greater precision and, because it uses 1–1.5 cycle pulses, is expected to generate less tissue heating per pulse (Lin et al. 2014).
Electronic focal steering has been used with therapeutic ultrasound for tissue volume ablation, particularly with ultrasound thermal therapy (Clement, et al. 2000; Clement and Hynynen 2002; McDannold et al. 2010; Pernot et al. 2003). Tavakkoli et al. (1997) and Arefiev et al. (1998) demonstrated that cavitation-induced tissue disruption can be achieved by electronically steering the therapy focus while the transducer and target remain in a fixed position. In these early proof of principle studies where a high treatment rate was not the primary objective, the ablation rate was a fairly modest 0.05 mL/min. In our group, Zhang et al. (2016) demonstrated that histotripsy with electronic focal steering can be combined with mechanical focal steering, resulting in higher efficiency for thrombolysis applications than when mechanical steering is used alone; however, the steering range used for this application was small (± 1 mm).
A major advantage of electronic focal steering is the ability to mitigate the cavitation memory effect more efficiently and practically than by mechanical steering alone. Each histotripsy pulse generates a cloud of cavitation microbubbles, which expand and collapse within <1 ms. Following the violent collapse of a cavitation cloud, remnant cavitation bubbles can persist for seconds as unstabilized nuclei. When the pulse repetition period (PRF) is less than the dissolution time, unstabilized nuclei are preferentially re-excited in a phenomenon known as the cavitation memory effect (Apfel and Holland 1991; Duryea et al. 2014; Holland and Apfel 1990; Roberts et al. 2006; Wang et al. 2009; Wang et al. 2012; Xu et al. 2007). T.-Y. Wang et al. (2012) found that the spatial distribution and extent of the cavitation bubble cloud were highly dependent on PRF. When pulses were separated by a short time interval (high PRF), bubbles tended to nucleate in roughly the same location and form more spatially confined clouds. At sufficiently low PRF, when unstabilized nuclei are allowed to dissolve, each pulse generates a more randomized, more spatially distinct cavitation cloud from the population of nanometer sized nuclei believed to be innate to water-based media (Leighton 2012). As a result, using a lower PRF reduces or eliminates the cavitation memory effect. Wang et al. (2012) showed that the number of pulses required to fractionate 25% of the target volume is reduced by more than an order of magnitude by increasing the time between pulses from 2 ms to >200 ms. Additionally, previous studies have employed an active acoustic technique termed bubble-removal to mitigate the cavitation memory effect. Duryea et al. (2014, 2015a, 2015b) found that applying a low-amplitude ultrasound pulse with many cycles (~1000) following a cavitation event promoted the coalescence (by means of the secondary Bjerknes force) and aggregate translation (primary Bjerknes force) of residual nuclei from the focus.
We hypothesize that histotripsy combined with electronic focal steering using a phased array transducer can achieve rapid, complete necrosis of a large target volume. Histotripsy with electronic focal steering can steer to thousands of overlapping focal locations (which together comprise a large volume) during the off-time between adjacent pulses necessary for residual nuclei to dissolve at a given focus. In this manner, the treatment time for a volume target can be shortened while maintaining high per-pulse fractionation efficiency. In this study, we test this hypothesis by exploring the feasibility of histotripsy with electronic focal steering in an ex vivo tissue model using a 256 element phased array transducer. First, cavitation and the resulting lesion were generated at a single focal site in a tissue-mimicking phantom and monitored using optical imaging to establish acoustic and electronic focal steering parameters (PRF, dose, and focal spacing). Acoustic and steering parameters derived from phantom experiments were then applied to volume ablation experiments using electronic focal steering performed in ex vivo bovine liver samples. Lesion size and the completeness of tissue ablation were assessed with MRI and histology.
Methods
Transducer Characterization
The transducer used for this study was a 250 kHz, 256-element phased array with a 30 cm diameter aperture and a 15 cm focal distance, generating approximately 1.5-cycle, 6-microsecond acoustic pulses. It was fabricated in-house using methods previously described (Kim et al. 2014) and controlled by a field-programmable gate array (FPGA) such that each element was individually addressable. Though the transducer was designed with transcranial brain applications in mind, the methods established herein for treatment planning are generalizable to other transducer configurations and target tissues.
All calibration procedures and ex vivo experiments were performed in a glass tank containing roughly 120 L of filtered tap water degassed to approximately 20% O2 saturation as measured by a dissolved oxygen probe (Orion Start A323, Thermo Scientific, Waltham, MA). The beam profile at the geometric focus was characterized at low pressure (270kPa) using a calibrated needle hydrophone (HNR-0500, Onda Corporation, Sunnyvale, CA) affixed to a motorized 3D positioning system. The full width at half maximum (FWHM) dimensions of the main lobe at the geometric focus were determined to be 3.2 mm in the lateral direction and 6.9 mm along the acoustic axis. At the geometric focus the amplitude of the side lobes in both the lateral and axial directions were measured to be approximately 20% that of the main lobe. Because the pulses are <2 cycles in duration, grating lobes are not observed. The transducer’s electronic focal steering range was characterized similarly by steering the array throughout a regular square grid of positions in the axial plane separated by 5 mm intervals. For each electronic steering focus position on the grid, the 3D positioner moved the needle hydrophone to the current electronic focal steering position where it recorded pressure measurements. The full width half maximum (FWHM) dimensions of the electronic focal steering pressure profile refer to where the peak negative pressure at the steered position ≥ 50% of the peak negative pressure at the geometric focus, which were determined to be 80 mm in the lateral direction and 85 mm along the acoustic axis. Driven at its maximum voltage, the array was capable of generating a bubble cloud in the free field at up to approximately 60 mm off axis in the lateral direction, corresponding to a 22° maximum steering angle for these conditions.
The waveform at the geometric focus was characterized at high-amplitude using a fiber optic probe hydrophone (FOPH) fabricated in-house as described previously (Parsons et al. 2006). At P- levels greater than approximately 10 MPa, the pressure could not be directly measured with the FOPH due to cavitation generated at the fiber tip. For P- ≥ 10 MPa, the transducer’s output was approximated by dividing the array into a number of wedge-shaped subapertures which were driven and measured separately. For each driving voltage used, the array was divided into the minimum number of subapertures necessary to maintain the condition P- ≤ 10 MPa for an individual subaperture. For each measurement, 100 waveforms were collected per subaperture and averaged during post-processing. To approximate the total transducer output, the averaged subaperture waveforms were then summed. A previous study demonstrated reasonable agreement between P- values obtained by subaperture summation and driving all elements simultaneously up to 30 MPa in 1,3 butanediol (Maxwell et al. 2013). At the maximum driving voltage, a P- value of 80 MPa was recorded by linear summation of the subapertures. Fig. 1 shows the summed waveform. It is expected that nonlinear acoustic effects are underestimated in this summed waveform.
Fig. 1.
Acoustic waveform at the transducer’s geometric focus measured by FOPH using subaperture summation. The timescale has been set such that t=0 corresponds to the arrival of the pulse.
Single Lesion Experiments in Red Blood Cell Phantoms
To establish treatment parameters including PRF, the number of pulses to deliver (dose) for a given electronic focal steering site, and the spacing between adjacent electronic focal steering sites in subsequent tissue experiments, single lesions were first generated in tissue-mimicking phantoms. Phantoms were composed of agarose hydrogel and a thin layer of red blood cells (RBCs), allowing cavitation and resulting damage to be directly visualized on a per-pulse basis (Maxwell et al. 2010).
Bovine blood was obtained from a local slaughterhouse (Dunbar Meat Packing Company, Milan, MI) where it was mixed with citrate-phosphate-dextrose solution at 10:1 ratio (C7165, Sigma-Aldrich Corporation, St. Louis, MO). Blood was stored at 4 °C and used for experimentation within 14 days. All procedures involving animal tissue were performed in accordance with guidelines established by the University of Michigan’s Committee on Use and Care of Animals. RBC phantoms were prepared in a manner similar to that previously described (Maxwell et al. 2010) and measured 127 mm x 70 mm x 13 mm as shown in fig. 2b. During experimentation RBC phantoms were submerged and centered in a clear, 33 μm thick polyethylene bag (McMaster-Carr, Elmhurst, IL) containing approximately 2 L of degassed phosphate buffered saline (PBS) to prevent osmotic lysis, thereby maintaining consistent optical contrast. Acoustic attenuation through the polyethylene bag containing PBS was measured using a needle hydrophone and found to be −0.3 dB (3.6%) relative to P- in the free field.
Fig. 2.
(a) Diagram of experimental setup. (b) Photograph of RBC phantom prior to treatment.
The setup for the RBC phantom experiments is shown in fig. 2a. Optical monitoring was performed by positioning a high-speed digital camera (Phantom V210, Vision Research Inc., Wayne, NJ) with a telephoto lens (AF Nikkor, Nikon Corporation, Minato, Tokyo, Japan) facing the broad side of the phantom and centered on the transducer’s geometric focus. Images had resolution of approximately 46 microns/pixel. Continuous-wave (CW) illumination was provided by a film projector (Carousel 4400, Kodak Company, Rochester, NY) positioned on the opposite side of the water tank from the camera. During experimentation the FPGA supplied a trigger signal at 50 ms after the arrival of the histotripsy pulse. The timing of the trigger was set such that the camera would capture an image of the cavitation damage sustained by the phantom after visible cavitation activity had ceased. Commands were sent to the FPGA, camera, and 3D positioner from a PC running a control script written in MATLAB. The script directed the FPGA to fire 200 histotripsy pulses at the geometric focus at a given PRF, during which time the camera would record images. The script then directed the 3D positioner to move the RBC phantom to a new position where the process would repeat with the next PRF to be tested. Focal sites were spaced 15 mm apart in the lateral direction and 19 mm in the axial direction. PRFs tested ranged from 0.1 Hz to 1.2 Hz, each of which was used once per phantom (N = 7). P-was tuned to 50 MPa for all RBC phantom experiments detailed here as this was the estimated minimum in situ pressure used for subsequent experiments in ex vivo tissue.
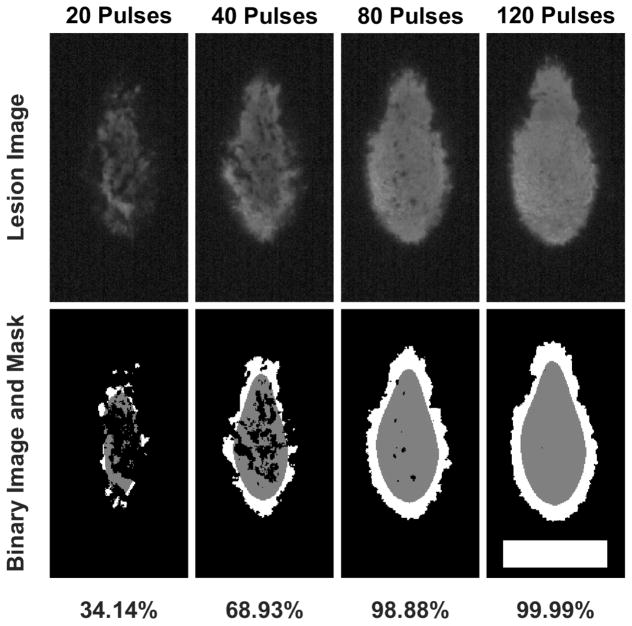
Optical images of fractionation were post-processed using a MATLAB script based on techniques previously described (Lin et al. 2014; Maxwell et al. 2010; Wang et al. 2012). Each image was converted to two separate binary images. The first defined regions of fractionation and the second, through a series of morphological operations, defined a smooth region inset approximately 0.5 mm from the jagged lesion boundary. The extent of fractionation was then defined as the ratio of the number of bright pixels within the smoothed, inset region to the number of total pixels comprising this region. This procedure is detailed in fig. 3.
Fig. 3.
First row: Lesions generated in an RBC phantom by successive pulses. Second row: segmented images. Extent of fractionation is defined as (gray pixels)/(total pixels in enclosed by gray region). Scale bar: 5 mm.
Volume Tissue Ablation Experiments in Ex Vivo Bovine Liver
A total of 5 whole bovine livers were obtained from a local slaughterhouse (Dunbar Meat Packing Company, Milan, MI) and used for experimentation within 8 hours after harvest. Each sample was cut into sections measuring approximately 8 cm x 8 cm x 10 cm. These sectioned samples were then submerged for 5 hours in a vacuum desiccation chamber (model F42025-0000, Bel-Art, SP Industries, Inc., Wayne, NJ) partially filled with degassed PBS. Samples were then secured in exposure fixtures fabricated from polycarbonate film approximately 125 microns thick (McMaster-Carr, Elmhurst, IL) using degassed porcine skin gelatin (50 g: 1 L PBS). Acoustic attenuation through the fixture was measured to be approximately 0.2 dB (5%).
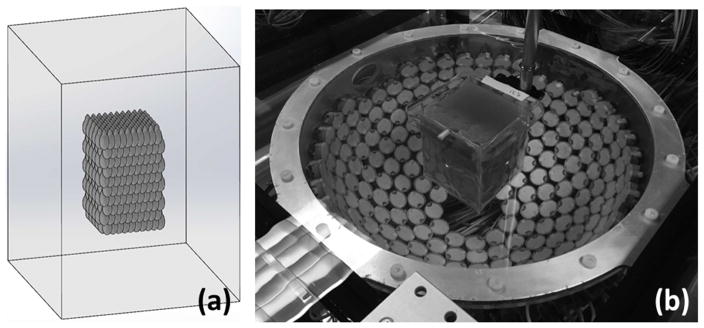
Each sample was secured to a 3D motion system and aligned with the transducer using low-power lasers such that the geometric focus was centered within the sample in the lateral directions and 5.7 cm from the sample’s bottom face along the acoustic axis (see fig. 4a). Results from RBC phantom experiments were used to guide treatment parameters including PRF, dose, and the spacing of electronic focal steering sites for volume tissue ablation experiments in ex vivo bovine liver. Tissue samples were treated by electronically scanning the therapy focus at 200 Hz over 1000 sites (or .2 Hz per focal site). Focal sites were arranged in a modified hexagonal close-packed structure with 2.5 mm center-center spacing in the lateral plane and 4.1 mm plane-plane spacing in the axial direction. It was estimated that the total volume of the lesion would be 34 mL based on a CAD model of the electronic focal steering grid shown in fig. 4a. Three doses were tested, 120 pulses, 250 pulses, and 500 pulses (N=6 for each dose). Total treatment times were 10 min, 21 min, and 42 min, respectively.
Fig. 4.
(a) CAD rendering of liver sample (shown in wireframe outline) and packed treatment foci. (b) photograph of experimental setup.
Tavakkoli et al. (1997) suggested that like thermal HIFU treatment, cavitation-induced tissue disruption using electronic focal steering could be made more efficient by employing a randomized electronic focal steering pattern. Though the rationale for this hypothesis was not explicitly stated, the authors may have been alluding to the treatment rate limitation imposed by the cavitation memory effect (Tavakkoli et al. 1997). Previous experience in our lab supports this idea; randomly ordering the temporal sequence of focal sites in a volume ablation treatment has been found to fractionate tissue more effectively than using a raster scan. Thus, the electronic focal steering scanning sequence, initially generated as a raster pattern, was randomized by applying the MATLAB function randperm and this pattern was then repeated during treatment.
Magnetic resonance imaging (MRI) was used to estimate the treatment volume. Following treatment, samples were stored at 4 degrees C and scanned by MRI within 48 hours. Scans were performed with the sample still in its fixture using a 7-Tesla, small bore, Magnetic Resonance Imaging scanner (Agilent Technologies, Walnut Creek, CA) using a T2*-weighted, gradient-echo sequence. The imaging parameters were TR: 2 s, TE: 10 ms, Matrix: 180 x 180 x 50, FOV: 9 x 9 x 10 cm, Resolution: 0.05 x 0.05 x 0.2 cm, sampling rate: 50 kHz, flip angle: 90 degrees, and slice gap: 0 cm. The resulting series of images represented rectangular volumes encapsulating the lesion within each sample. MR images were reconstructed from the MR acquisition data using the ifft2 function in MATLAB. Images were then normalized and segmented to isolate the region of ablation using a threshold midway between the respective mean pixel intensities of regions of untreated and treated tissue. For each set of MR images, the image-slice located closest to the center of the lesion was used to establish the pixel intensity threshold for segmenting the entire image stack. Several images were segmented by hand using the imfreehand function due to the presence of vasculature or other structures intersecting the lesion boundary with pixel intensity similar to the lesion. To estimate the total volume of the lesion, the sum of the lesion areas from each image was multiplied by the distance separating each imaging plane.
Routine hematoxylin and eosin (H&E) staining was performed for histological evaluation of tissue fractionation extent and to validate the MRI segmentation procedure by assessing the tissue histology and completeness of homogenization throughout the sectioned plane after formalin-fixed paraffin-embedded (FFPE) tissue specimens were processed according to standard protocols. Sectioning planes bisected the lesion along the axis of acoustic propagation. H&E slides were scanned and digitized into .svs format at 400x total optical magnification using a digital whole slide scanner (AT2, Leica Biosystems, Wetzlar, Germany).
A pathologist (JS) examined each digital slide and demarcated the boundary between necrotic and viable tissue using digital pathology software (ImageScope, Leica Biosystems, Wetzlar, Germany). Tissue was deemed completely necrotic if there was confluent cell death (≥95%) with cell loss and only residual cellular debris. Since the treatment was done after ceased blood supply to the tissue, there was no inflammatory response to the necrosis in the tissue. Regions classified as viable were those containing morphologically viable tissue and cells with intact architecture and cytology.
The extent of tissue fractionation within the lesion boundary was assessed for each sample by randomly selecting 32 square regions measuring approximately 250 microns per side within the lesion boundary and counting the number of intact hepatocyte nuclei, intact hepatocytes, and hepatocyte clusters within each region based on visual inspection by a single pathologist (J.S.). Those regions which encompassed large cracks in the tissue or were located within 1 mm of the lesion boundary were excluded and a substitute was selected at a different location. Three control regions were selected within untreated tissue far from the lesion boundary. Intact cell nuclei were defined as those with smooth and complete nuclear membrane and visible chromatin pattern or nucleoli with or without intact cytoplasm or cell membrane. Nuclei with irregular or incomplete nuclear membrane or appear to be smudgy or fragmented were excluded from the count. Hepatocytes were considered intact if they contained an intact nucleus, cytoplasm, and cell membrane. Clusters were defined as two or more adjacent, intact hepatocytes. Previous studies in our lab have demonstrated that the nucleus is the cell structure most resistant to histotripsy damage and that the area-density of healthy-appearing nuclei, which appear prominently in H&E sections, can be used as a proxy for the extent of histotripsy tissue fractionation in ex vivo tissue (Wang et al. 2009; Winterroth et al. 2011). The authors acknowledge that using only one observer (J.S.) to evaluate histological data introduces an element of subjectivity into the analysis and is a limitation of this study.
Results
RBC Phantom Results
A total of 49 single-focus lesions were formed in RBC phantoms at PRFs ranging from 0.1 to 1.2 Hz (N=7 for each PRF). The extent of fractionation within the lesion boundary (defined as the number of bright pixels within a binarized, smoothed, and inset boundary region to the number of total pixels comprising this smoothed region) decreased with increasing PRF for a given therapy dose. Fig. 5 shows representative images for lesions generated with 120 pulses and at various PRFs. It was found that for 0.1 Hz and 0.2 Hz, at approximately 95 and 120 pulses, respectively, fractionation approached saturation (>99%). However, among PRFs tested, those > 0.2 Hz required greater than 200 pulses in order to achieve a comparable level of fractionation. The PRF-dependence observed is believed to result from the cavitation memory effect described previously (Wang et al. 2012). Based on the results from RBC phantom experiments, 0.2 Hz PRF was chosen as the per-point treatment PRF for subsequent ex vivo tissue experiments with electrical focal steering. From fig. 5 it can be seen that the extent of fractionation achieved by 0.2 Hz PRF after 100 pulses is approximately equal to that of 0.4 Hz after 200 pulses. In an effort to minimize potential heating with future in vivo studies in mind, the emphasis was placed on per-pulse fractionation efficiency, rather than gains which may have been possible with respect to time-efficiency. Because previous experiments in our lab have shown that tissue is more resistant to fractionation than RBC phantoms (Miller et al. 2016), 120, 250, and 500 pulses were chosen as the doses to test for ex vivo experiments.
Fig. 5.
Top row: Representative images of lesions in an RBC phantom after sustaining 120 pulses at various PRFs. Bottom row: Percent of material unfractionated within a region inset approximately 0.5 mm from the lesion boundary. Error bars represent ± SD. N=7 for each PRF tested. Scale bar = 5 mm.
The profile of the lesion was analyzed across 7 RBC phantoms by averaging the images from each sample corresponding to 120 pulses delivered at 0.2 Hz to form a composite profile. The mean extent of the 7 lesions was 4.1 ± 0.2 mm in the lateral direction and 8.6 ± 0.7 mm in the axial direction. The profile and mean dimensions were used to establish the spacing of the electronic focal steering grid used for subsequent ex vivo tissue experiments.
Ex Vivo Bovine Hepatic Tissue Experiments
MRI Volume Measurements
To validate histotripsy with electrical focal steering, a total of 18 lesions were generated in ex vivo bovine liver samples using doses of 120, 250, and 500 pulses (N=6 for each dose). MR imaging was used to estimate lesion volume for all samples. Representative images appear in fig. 6. The volume of each individual sample was calculated as the mean of values derived from axial and coronal image stacks. The lesion volumes for each dose group (mean ± SD) were found to be 34.1 ± 1.1 mL for 120 pulses, 32.1 ± 1.5 mL for 250 pulses, and 40.7 ± 3.1 mL for 500 pulses. 1-way ANOVA comparing these data found p-values of 0.02, 6.5x10−4, and 1.2x10−4 for 120 vs. 250, 120 vs. 500, and 250 vs. 500 pulses, respectively. The larger lesion volume observed in the group treated with 500 pulses may be due to the breakdown of supporting tissue structures, allowing the homogenate to behave more like a single-phase fluid and permitting expansion of cavitation activity at the tissue-fluid boundary. Remaining structures, possibly blood vessels or bile ducts, appeared faint but slightly more prominent in MR images of lesions treated with 120 and 250 pulses than those treated with 500 pulses.
Fig. 6.
MR images of ex vivo tissue treated with 120, 250, and 500 pulses. Image slices shown were near the center of the lesion. Lesion segmentation boundary appears in black. Ultrasound propagation direction: bottom to top of images. Scale bar: 1 cm.
Histological Analysis of Fractionation Extent
To assess the extent of fractionation achieved by histotripsy with electric focal steering, samples treated were analyzed microscopically using H&E staining. Digitized H&E slides were examined by a pathologist (JS) and a region of confluent tissue necrosis (≥95% cell death) was demarcated. Thirty-two randomly selected regions within the lesion boundary of each digital slide were examined for the presence of intact nuclei, hepatocytes, and viable hepatocyte clusters.
In all samples, a distinct lesion consisting of tissue necrosis grossly similar to respective MR images was observed. The area within the lesion boundary consisted primarily of necrotic tissue homogenate with sparsely scattered nuclear debris. Rare intact nuclei, hepatocytes, or hepatocyte clusters were present and were found almost exclusively around vessels or near the lesion margins. Occasionally, residual vessels were observed in the lesion as well. The higher resilience of vascular structures to histotripsy damage (Vlaisavljevich et al. 2014) likely provided nearby hepatocytes a degree of protection from cavitation activity. The presence of residual intact nuclei, hepatocytes, and vessels present in the lesion was found to be inversely correlated with dose. Viable hepatocytes in untreated tissue were arranged in cords and were typically polygonal with abundant eosinophilic cytoplasm and round nuclei with prominent nucleoli. Representative images appear in fig. 7.
Fig. 7.
Representative H&E staining of tissue sample treated with 250 pulses. Magnification power is specified in terms of total original optical magnification (objective lens power x eyepiece or camera lens). (a) Low power view of lesion and surrounding tissue. The lesion margin was demarcated by the black line for (a), (c), and (d). The color of each border in magnified views (b–f) corresponds to a highlighted square region in views (a), (c), and (e). (b) 400x view of the center of the lesion showing one healthy-appearing, intact cell nucleus (H) and one pyknotic nucleus (P) surrounded by homogenate. (c), 40x & (d), 400x views of lesion margin showing a sharp demarcation between viable-appearing hepatocytes and necrotic cells. A few residual vessels (V), intact cells, nuclei can be seen in the necrotic tissue near the lesion margin. (e), 40x & (f), 400x views. Residual cell clusters (CC) were occasionally found within the necrotic lesion, often around residual vessels or bile ducts (BD).
The average percent remaining of intact-appearing cells relative to untreated tissue was 1.4 ± 1.1 %, 0.13 ± 0.2%, and 0.05 ± 0.06% for 120, 250, 500 pulses, respectively (see fig. 8). Even though sparse intact-appearing cells were found within the necrotic homogenate in treated area for all samples but one from the 500-pulses group, the pathologist’s (JS’s) analysis suggested that these cells are most likely not viable due to lack of blood supply and would eventually be lysed and cleaned up by inflammatory cells together with the necrotic debris if the treatment were applied in vivo. Since we only looked at a fixed time point post-treatment in an ex vivo organ model, it is impossible to accurately predict the fate of these cells in live animal. However, judging from their appearance and environment in the tissue, it is reasonable to anticipate their death within a short period of time.
Fig. 8.
(a) Area-density of remaining cell clusters within the lesion. (b) Percent of intact cells and cell nuclei remaining within the lesion after treatment relative to untreated tissue. *, **, and *** indicate significance at p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001, respectively.
Peripheral damage and boundary expansion
MR images and histology revealed isolated regions of tissue damage outside of the predicted lesion perimeter. This damage manifests in two patterns highlighted in Fig. 9. In all samples, small irregular elongated areas of tissue damage outside the lesion margin were observed in MR images and histology. Peripheral damage was densest in post-focal tissue immediately adjacent to the distal margin of the lesion and took the form of elongated, streak-like necrotic areas running parallel to the acoustic axis. These features are indicative of a phenomenon known as bubble tunneling (Caskey et al. 2009; Maxwell et al. 2010; Williams and Miller 2003). Features with similar morphology appeared pre-focally in tissue that lay between the transducer and the proximal margin of the lesion but were more sparsely distributed. Streak-like features are similarly observed in RBC phantom experiments in this and previous studies (Kim et al. 2011; Maxwell et al. 2010; Wang et al. 2011) and are believed to correspond to the cavitation and translation of pre-existing, sub-micron gas pockets in the sample. Peripheral damage was also occasionally noted near the lateral margins of the lesion and tended to be less elongated, sometimes roughly spherical. This form of peripheral damage is expected to be less pronounced in an in vivo animal model where the impurities introduced to ex vivo tissue samples during preparation, and which may function as cavitation nuclei, would be absent.
Fig. 9.
MR image (a) and H&E staining (b–f) of sample treated with 500 pulses. Magnification power is specified in terms of total original optical magnification (objective lens power x eyepiece or camera lens). Ultrasound propagation direction is indicated by a white arrow (US) in (a). The color of each border in magnified views (c–f) corresponds to a highlighted square region in views (b), (c), and (e). (a) Border expansion and scalloped edge feature is indicated by a black arrow. Border expansion is also observed on contralateral and proximal margins but is not annotated. Streak-like features indicative of bubble tunneling can be observed in pre and post-focal tissue. (b) Low power view of lesion and surrounding tissue. Lesion margin is delineated by black line. Regions of peripheral tissue necrosis are highlighted in blue. (c) 20x view of pre-focal peripheral tissue necrosis in red square in (b). (d) 400x view of region of necrotic debris in pre-focal tissue. (e) 20x view of post-focal peripheral tissue damage. (f) 400x view of region of necrotic debris in post-focal tissue.
A slight expansion of the lesion perimeter was observed in all directions when >250 pulses were applied. 1-way ANOVA shows no significant difference between the volume of lesions generated with 120 and 250 pulses (p=0.82). However, the analysis did find a significant difference between the volume of lesions generated with 120 and 500 pulses (p=6x10−5) as well as 250 and 500 pulses (p=3x10−5). A possible explanation is that after the complete destruction of tissue components which lend structural support, the necrotic homogenate may behave more like a single-phase fluid and thus permit expansion of cavitation activity at the tissue-fluid boundary, resulting in a slight expansion of the lesion boundary. Further, it was noted in MR images of several samples treated with 500 pulses, that along the lesion boundary there exists a region of intermediate pixel intensity (Fig. 9a). Corresponding regions on H&E slides do not appear noticeably different that other regions of homogenate. The scalloped pattern of the boundary corresponds to the dimensions of individual foci appearing at the intervals prescribed by the electronic focal steering grid.
Discussion
This study demonstrates the feasibility of using histotripsy with electronic focal steering to ablate a volume of ex vivo target tissue rapidly. By electronically steering the focus of a phased array histotripsy transducer, it was possible to excite rapidly cavitation events throughout a large ensemble of overlapping foci such that while remnant nuclei at a given focus were dissolving, the other focal locations in the ensemble could be treated before returning to the given focus. This approach helped to mitigate the rate-limitation imposed by the cavitation memory effect.
The cavitation memory effect imposes both temporal and spatial constraints on the design of an effective electronic focal steering sequence. The temporal constraint can be seen in the single lesion RBC phantom experiments described above. When the local pulse repetition period (PRP) for a given electronic focal steering site is too short, residual cavitation nuclei lack sufficient time to dissolve before the arrival of subsequent pulses. Upon the arrival of subsequent pulses, residual nuclei are preferentially re-excited. Under these conditions in a host medium like tissue or gel that does not readily permit chaotic fluid mixing during and after cavitation bubble-cloud collapse, clouds from one pulse to the next share highly correlated spatial distributions. However, when the local PRP is sufficiently long, residual nuclei have time to dissolve and subsequent clouds nucleate from a more randomized spatial distribution of individual sites within the therapy focus. A previous study in our lab showed that greater randomization of individual bubbles within the cloud resulted in more complete and efficient fractionation of the target (Wang et al. 2012). The spatial constraint of the cavitation memory effect in the context of electronic focal steering arises due to the proximity or overlap of electronic focal steering sites. If a region of the sound field with high enough gain (i.e. a side lobe or the shoulder of the main lobe) is incident on a population of residual nuclei from a previous pulse, they can be re-excited in a manner that compromises the formation of a robust bubble cloud at the intended, current focus. Furthermore, this re-excitement of residual nuclei is expected to prolong the time required for their dissolution. Thus, a minimum separation distance is required for adjacent pulses as well as minimum time-period for which this condition must be maintained. This explains why an electronic focal steering following a raster pattern, for example, would yield low tissue fractionation efficiency.
In this study, a very low local PRF (0.2 Hz) was applied to each focus individually to address the temporal constraint of the cavitation memory effect. Electronic focal steering sites were ordered in a temporally random sequence to reduce the impact of interactions between spatially proximal foci, thereby addressing the spatial constraint of the memory effect. The subject of a separate, ongoing study is designing structured steering sequences to mitigate further the impact of the cavitation memory effect and thereby improve treatment efficiency. These sequences will impose minimum spatial limits on proximal foci such that after a given electronic focal steering location has been pulsed, immediately subsequent pulses will be directed at locations separated from the given location for a certain time-period.
The hemispherical transducer used in this study was designed for transcranial brain applications. For treatment of abdominal organs, such as the liver, pancreas, and kidney, we plan to design and construct an array transducer tailored for these applications. Geometrical constrains will require a smaller aperture and higher f-number which may result in greater focal heating due to nonlinear acoustic propagation. However, nonlinear effects are expected to take place only in regions of high focal gain at or near the focus (Kim et al. 2013; Yuldashev et al. 2013).
In this ex vivo study, heating was not measured. In our previous study, where a higher frequency (750kHz) transducer (f# = 0.8) was used to treat the in vivo porcine liver at 200 Hz PRF through full rib coverage without using aberration correction, a temperature increase of 4.1 ± 1.8 °C in the ribcage was observed (Kim et al. 2014). For the driving electronics used in the current study, additional signal conditioning may retain the high amplitude peak negative phase to ensure cavitation generation while reducing high frequency components which contribute to heating.
The size of the target lesion in this study was limited by the number of electronic focal steering locations that could be stored in the FPGA’s memory (1024) rather than the electronic focal steering range. With a greater number of available electronic focal steering locations, the global PRF and treatment rate could be increased as long as steering range and thermal constraints are not exceeded. Future iterations of the system will include an expanded memory capacity. Used in conjunction with mechanical focal steering, it will be feasible to treat a tissue volume of arbitrary size.
When it is necessary to ablate a region that exceeds the electronic focal steering range, it is possible to combine electrical and mechanical focal steering in a manner similar to that developed by Zhang et al. (2016) to cover the entire volume while maintaining a high ablation rate. This approach will likely prove useful for in vivo settings like abdominal targets which require a higher f-number than that used in the current study, resulting in a diminished maximum steering angle. Additionally, the use of composite or single crystal materials which generate higher surface pressure as well as soft tissue aberration correction techniques currently under investigation may help to offset in vivo pressure losses.
The center frequency of the transducer used for this study was 250 kHz, the lowest our lab has used for a histotripsy study. This frequency was selected to take advantage of the lower attenuation and large focal zone and electronic focal steering range associated with lower frequencies. However, as the center frequency decreases, the probability of cavitating regions outside the targeted focus increases since these regions experience P- on the order of several MPa (Apfel and Holland 1991). Furthermore, the cavitation memory effect is expected to be more pronounced at lower frequency due to the formation of larger, longer-lived bubble clouds which result in longer dissolution times for residual nuclei (Epstein and Plesset 1950; Vlaisavljevich et al. 2015b). Peripheral damage and bubble tunneling may be reduced in future systems by using a transducer with higher center frequency (Vlaisavljevich et al. 2015).
Conclusion
This study demonstrates that histotripsy with electronic focal steering is capable of noninvasively ablating a large tissue volume rapidly and uniformly in an ex vivo bovine hepatic tissue model by mitigating the impact of the cavitation memory effect. This technique is highly repeatable and fairly resilient to overtreatment. Histotripsy with electronic focal steering has the potential to achieve rapid, complete necrosis of a large tissue volume at a rate exceeding any current ablation treatment for large-volume targets.
Acknowledgments
This work was supported by a Research Scholar Grant from the American Cancer Society (RSG-13-101-01-CCE), a grant from National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health under Award Number R01EB008998, and a grant from National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under Award Number R21NS093121. Disclosure notice: Drs. Tim Hall, Charles Cain, and Zhen Xu have financial interests and/or other relationship with HistoSonics Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol. 1991;17:179–185. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- Arefiev A, Prat F, Chapelon JY, Tavakkoli J, Cathignol D. Ultrasound-induced tissue ablation: Studies on isolated, perfused porcine liver. Ultrasound Med Biol. 1998;24:1033–1043. doi: 10.1016/s0301-5629(98)00046-5. [DOI] [PubMed] [Google Scholar]
- Caskey CF, Qin S, Dayton PA, Ferrara KW. Microbubble tunneling in gel phantoms. J Acoust Soc Am. 2009;125:EL183–EL189. doi: 10.1121/1.3097679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement GT, Hynynen K. Micro-receiver guided transcranial beam steering. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:447–453. doi: 10.1109/58.996562. [DOI] [PubMed] [Google Scholar]
- Clement GT, White J, Hynynen K. Investigation of a large-area phased array for focused ultrasound surgery through the skull. Phys Med Biol. 2000;45:1071–1083. doi: 10.1088/0031-9155/45/4/319. [DOI] [PubMed] [Google Scholar]
- Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist. 2001;6:14–23. doi: 10.1634/theoncologist.6-1-14. [DOI] [PubMed] [Google Scholar]
- Duryea AP, Cain CA, Roberts WW, Tamaddoni HA, Hall TL. Removal of Residual Bubble Nuclei Following a Cavitation Event: A Parametric Study. IEEE Trans Ultrason Ferroelectr Freq Control. 2015a;62:1605–1614. doi: 10.1109/TUFFC.2014.006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea AP, Roberts WW, Cain CA, Hall TL. Removal of Residual Cavitation Nuclei to Enhance Histotripsy Fractionation of Soft Tissue. IEEE Trans Ultrason Ferroelectr Freq Control. 2015b;62:2068–2078. doi: 10.1109/tuffc.2015.007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea AP, Tamaddoni HA, Cain CA, Roberts WW, Hall TL. Removal of Residual Nuclei Following a Cavitation Event Using Low-Amplitude Ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:1619–1626. doi: 10.1109/TUFFC.2014.006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein PS, Plesset MS. On the Stability of Gas Bubbles in Liquid-Gas Solutions. J Chem Phys. 1950;18:1505–1509. [Google Scholar]
- Feigenberg SJ, Cohen R, Sharma NK, Husain Z, Chen S, Dawson LA. Principles and Practice of Stereotactic Radiosurgery. 2. New York: Springer-Verlag; 2015. Stereotactic Body Radiation Therapy. [Google Scholar]
- Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- Jansen MC, van Hillegersberg R, Chamuleau RaFM, van Delden OM, Gouma DJ, van Gulik TM. Outcome of regional and local ablative therapies for hepatocellular carcinoma: A collective review. Eur J Surg Oncol. 2005;31:331–347. doi: 10.1016/j.ejso.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Khokhlova TD, Monsky WL, Haider YA, Maxwell AD, Wang YN, Matula TJ. Histotripsy Liquefaction of Large Hematomas. Ultrasound Med Biol. 2016;42:1491–1498. doi: 10.1016/j.ultrasmedbio.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Fifer CG, Gelehrter SK, Owens GE, Berman DR, Vlaisavljevich E, Allen SP, Ladino-Torres MF, Xu Z. Developmental Impact and Lesion Maturation of Histotripsy-Mediated Non-Invasive Tissue Ablation in a Fetal Sheep Model. Ultrasound Med Biol. 2013;39:1047–1055. doi: 10.1016/j.ultrasmedbio.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Kim Y, Maxwell AD, Hall TL, Xu Z, Lin K-W, Cain C. Rapid Prototyping Fabrication of Focused Ultrasound Transducers. IEEE Trans Ultrason. 2014a;61:1559–1574. doi: 10.1109/TUFFC.2014.3070. [DOI] [PubMed] [Google Scholar]
- Kim Y, Vlaisavljevich E, Owens GE, Allen SP, Cain Ca, Xu Z. In vivo transcostal histotripsy therapy without aberration correction. Phys Med Biol. 2014b;59:2553–2568. doi: 10.1088/0031-9155/59/11/2553. [DOI] [PubMed] [Google Scholar]
- Kim Y, Wang T, Xu Z, Cain Ca. Lesion Generation Through Ribs Using Histotripsy Therapy Without Aberration Correction. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:2334–2343. doi: 10.1109/TUFFC.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Keserci B, Partanen A, Rhim H, Lim HK, Park MJ, Köhler MO. Volumetric MR-HIFU ablation of uterine fibroids: Role of treatment cell size in the improvement of energy efficiency. Eur J Radiol Elsevier Ireland Ltd. 2012;81:3652–3659. doi: 10.1016/j.ejrad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Köhler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, Moonen CTW, Ehnholm GJ. Volumetric HIFU ablation guided by multiplane MRI thermometry. AIP Conf Proc. 2009;1113:228–230. [Google Scholar]
- Leighton TG. The Acoustic Bubble. Boston: Academic Press; 2012. [Google Scholar]
- Leslie Ta, Kennedy JE. High-intensity focused ultrasound principles, current uses, and potential for the future. Ultrasound Q. 2006;22:263–272. doi: 10.1097/01.ruq.0000237259.25885.72. [DOI] [PubMed] [Google Scholar]
- Liang P-C, Lai H-S, Shih TT-F, Wu C-H, Huang K-W. The pilot experience upon surgical ablation of large liver tumor by microwave system with tissue permittivity feedback control mechanism. BMC Surg. 2014;14:82. doi: 10.1186/1471-2482-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KW, Kim Y, Maxwell A, Wang TY, Hall T, Xu Z, Fowlkes J, Cain C. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: Microtripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:251–265. doi: 10.1109/TUFFC.2014.6722611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- Maxwell AD, Cain Ca, Hall TL, Fowlkes JB, Xu Z. Probability of Cavitation for Single Ultrasound Pulses Applied to Tissues and Tissue-Mimicking Materials. Ultrasound Med Biol. 2013;39:449–465. doi: 10.1016/j.ultrasmedbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Wang T-Y, Cain Ca, Fowlkes JB, Sapozhnikov Oa, Bailey MR, Xu Z. Cavitation clouds created by shock scattering from bubbles during histotripsy. J Acoust Soc Am. 2011;130:1888. doi: 10.1121/1.3625239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Wang TY, Yuan L, Duryea AP, Xu Z, Cain Ca. A tissue phantom for visualization and measurement of ultrasound-induced cavitation damage. Ultrasound Med Biol. 2010;36:2132–2143. doi: 10.1016/j.ultrasmedbio.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Clement G, Black P, Jolesz F, Hynynen K. Transcranial MRI-guided focused ultrasound surgery of brain tumors: Initial findings in three patients. Neurosurgery. 2010;66:323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams J, Lee E, Yamamoto S, Loh C, Kee S. Image-Guided Tumor Ablation: Emerging Technologies and Future Directions. Semin Intervent Radiol. 2010a;27:302–313. doi: 10.1055/s-0030-1261789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams JP, Yamamoto S, Raman SS, Loh CT, Lee EW, Liu DM, Kee ST. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol Elsevier Inc. 2010b;21:S204–S213. doi: 10.1016/j.jvir.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Miller RM, Zhang X, Maxwell AD, Cain CA, Xu Z. Bubble-Induced Color Doppler Feedback for Histotripsy Tissue Fractionation. IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63:408–419. doi: 10.1109/TUFFC.2016.2525859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JE, Cain Ca, Fowlkes JB. Cost-effective assembly of a basic fiber-optic hydrophone for measurement of high-amplitude therapeutic ultrasound fields. J Acoust Soc Am. 2006;119:1432–1440. doi: 10.1121/1.2166708. [DOI] [PubMed] [Google Scholar]
- Pernot M, Aubry J-F, Tanter M, Thomas J-L, Fink M. High power transcranial beam steering for ultrasonic brain therapy. Phys Med Biol. 2003;48:2577–2589. doi: 10.1109/TMI.2010.2076829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WW, Hall TL, Ives K, Wolf JS, Fowlkes JB, Cain Ca. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–738. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of Colorectal Liver Metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma: An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- Tavakkoli J, Birer A, Arefiev A, Prat F, Chapelon JY, Cathignol D. A piezocomposite shock wave generator with electronic focusing capability: Application for producing cavitation-induced lesions in rabbit liver. Ultrasound Med Biol. 1997;23:107–115. doi: 10.1016/s0301-5629(96)00175-5. [DOI] [PubMed] [Google Scholar]
- Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- Tombesi P, Di Vece F, Sartori S. Resection vs thermal ablation of small hepatocellular carcinoma: What’s the first choice? World J Radiol. 2013;5:1–4. doi: 10.4329/wjr.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Kim Y, Allen S, Owens G, Pelletier S, Cain C, Ives K, Xu Z. Image-Guided Non-Invasive Ultrasound Liver Ablation Using Histotripsy: Feasibility Study in an In Vivo Porcine Model. Ultrasound Med Biol. 2013;39:1398–1409. doi: 10.1016/j.ultrasmedbio.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Kim Y, Owens G, Roberts W, Cain C, Xu Z. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys Med Biol. 2014;59:253–70. doi: 10.1088/0031-9155/59/2/253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Lin K-W, Maxwell A, Warnez MT, Mancia L, Singh R, Putnam AJ, Fowlkes B, Johnsen E, Cain C, Xu Z. Effects of Ultrasound Frequency and Tissue Stiffness on the Histotripsy Intrinsic Threshold for Cavitation. Ultrasound Med Biol. 2015a;41:1651–1667. doi: 10.1016/j.ultrasmedbio.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Lin K-W, Warnez MT, Singh R, Mancia L, Putnam AJ, Johnsen E, Cain C, Xu Z. Effects of tissue stiffness, ultrasound frequency, and pressure on histotripsy-induced cavitation bubble behavior. Phys Med Biol IOP Publishing. 2015b;60:2271–2292. doi: 10.1088/0031-9155/60/6/2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T-Y, Xu Z, Hall TL, Fowlkes JB, Cain Ca. An Efficient Treatment Strategy for Histotripsy by Removing Cavitation Memory. Ultrasound Med Biol. 2012;38:753–766. doi: 10.1016/j.ultrasmedbio.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xu Z, Winterroth F, Hall TL, Fowlkes JB, Rothman ED, Roberts WW, Cain Ca. Quantitative ultrasound backscatter for pulsed cavitational ultrasound therapy-histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:995–1005. doi: 10.1109/tuffc.2009.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Xu Z, Hall TL, Fowlkes JB, Roberts WW, Cain Ca. Active focal zone sharpening for high-precision treatment using histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:305–315. doi: 10.1109/TUFFC.2011.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Miller DL. The use of transparent aqueous gels for observing and recording cavitation activity produced by high intensity focused ultrasound. 2003 IEEE Symp Ultrason. 2003;2:1455–1458. [Google Scholar]
- Winterroth F, Xu Z, Wang TY, Wilkinson JE, Fowlkes JB, Roberts WW, Cain Ca. Examining and analyzing subcellular morphology of renal tissue treated by histotripsy. Ultrasound Med Biol. 2011;37:78–86. doi: 10.1016/j.ultrasmedbio.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Wang Z-B, Chen W-Z, Bai J, Zhu H, Qiao T-Y. Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J Urol. 2003;170:2237–2240. doi: 10.1097/01.ju.0000097123.34790.70. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang Z-B, Chen W-Z, Zhu H, Bai J, Zou J-Z, Li K-Q, Jin C-B, Xie F-L, Su H-B. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol. 2004a;11:1061–1069. doi: 10.1245/ASO.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, Zheng G, Zhou Y, Xu G, Li M, Zhang C, Ye H, Feng R. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview. Ultrason Sonochem. 2004b;11:149–154. doi: 10.1016/j.ultsonch.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Xu Z, Hall TL, Fowlkes JB, Cain Ca. Optical and acoustic monitoring of bubble dynamics at a tissue-fluid interface in ultrasound tissue erosion. AIP Conf Proc. 2007;829:343–347. doi: 10.1121/1.2710079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuldashev PV, Shmeleva SM, Ilyin Sa, Sapozhnikov Oa, Gavrilov LR, Khokhlova Va. The role of acoustic nonlinearity in tissue heating behind a rib cage using a high-intensity focused ultrasound phased array. Phys Med Biol. 2013;58:2537–59. doi: 10.1088/0031-9155/58/8/2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Owens GE, Cain CA, Gurm HS, Macoskey J, Xu Z. Histotripsy Thrombolysis on Retracted Clots. Ultrasound Med Biol. 2016;42:1903–1918. doi: 10.1016/j.ultrasmedbio.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]