Abstract
Rationale
Advancing marijuana prevention and intervention efforts is important given decreasing perception of harm among adolescents and increasing marijuana legalization.
Objectives
This study evaluates how a monitored abstinence protocol may contribute to emotional functioning and changes in marijuana problems that can enhance successful outcomes for non-treatment seeking adolescent marijuana users.
Methods
Adolescent marijuana users (n=26) and demographically matched controls (n=30) completed 28-days of monitored abstinence confirmed by bi-weekly urine toxicology. Participants were given measures of emotional functioning, marijuana use symptoms, and reward sensitivity during monitored abstinence.
Results
All participants (N=56) completed the protocol, and 69% of marijuana users (n=18 of 26) were confirmed abstinent for 28-days, with all users showing decreasing marijuana use. Reductions in subsyndromal depression, positive marijuana use expectancies, and poor sleep quality were observed by the end of the monitored abstinence period (n=26, ps<.05). Marijuana users also reported more attentional impulsivity and less responsiveness to reward stimuli during the second week of abstinence compared to controls. Later age of onset of regular marijuana use and more cumulative lifetime use was associated with a greater degree of emotional change and increased recognition of the negative effects of marijuana use.
Conclusions
Monitored abstinence programs may be beneficial in reducing marijuana use, subsyndromal emotional distress symptoms, and changing beliefs about marijuana use. Future prevention and intervention efforts may consider targeting reward sensitivity and impulsivity, in addition to marijuana use, expectancies, and emotional functioning.
Introduction
Adolescent marijuana use is linked to poorer neural health and psychological distress symptoms (Filbey et al. 2015; Jacobus et al. 2015; Moitra et al. 2016). Neural and mental health vulnerabilities (e.g., negative affect) and use-related problems (marijuana-related consequences, poor self-efficacy, marijuana expectancies, craving and withdrawal) play a role in maintenance of problematic marijuana use patterns after initiation of use, barriers and failed attempts to quit or cut back on use, and consequently, increasing prevalence rates of marijuana use disorders (Budney et al. 2007; Hasin et al. 2015; Tims et al. 2002; Womack et al. 2016; Zvolensky et al. 2017). Focusing efforts on better understanding cannabis-related processes and barriers that may promote use and influence behavioral interventions for adolescent marijuana users is a critical public health concern (Hogue et al. 2014; Zvolensky et al. 2017).
Contingency Management (CM) is an evidence-based treatment for reducing marijuana use (Budney et al. 2007; Cooper et al. 2015; Davis et al. 2015; Dennis et al. 2004). Biochemical verification (e.g., urine toxicology) is typically an important aspect of abstinence-based CM. Vouchers are given as positive reinforcement for negative drug screening (Budney et al. 2006; Copeland et al. 2016; Kaminer et al. 2014; Schuster et al. 2016; Stanger et al. 2009). Limited work pointedly explores how a monitored abstinence protocol with adolescents simultaneously influences trajectories of subsyndromal mental health symptoms (e.g., depression and anxiety), sleep disturbance, marijuana use expectancies and consequences, and reward sensitivity in non-treatment seeking marijuana users compared to matched controls (Angarita et al. 2016; Boden et al. 2013; Brackenbury et al. 2016; Gates et al. 2016; Hayaki et al. 2010; Moitra et al. 2016; Moitra et al. 2015). Barriers to treatment success (e.g., CM) may be associated with cannabis-related problems (e.g., withdrawal and craving) and processes that can influence emotional processing (e.g., negative affect), cognitive attributions and self efficacy (e.g., marijuana effect expectancies), and risk taking behaviors (e.g., continued use and functional consequences) (Budney et al. 2001; Cornelius et al. 2008; Fox et al. 2011; Khurana et al. 2015; Stanger et al. 2013; Tims et al. 2002; Zvolensky et al. 2017).
We recently examined neural health changes and neural recovery in adolescent marijuana users pre- and post monitored abstinence and found alterations in cortical thickness that continue to persist after 28-days of monitored abstinence, and associations between cortical thickness and lifetime marijuana use and age of marijuana use onset. Findings also suggest resolution of cerebral blood flow differences (Jacobus et al. 2012; Jacobus et al. 2014). Secondary aims of the larger neuroimaging study included characterization of stress and reward-related addiction cycle symptoms (Koob and Volkow 2010) in the sample. Gaining a better understanding of how physiological symptoms (craving, withdrawal), mental health symptoms, and cannabis-related factors and barriers may be affected by common behavioral interventions targeting marijuana use (e.g., CM) may help uncover potential treatment interfering factors for adolescent marijuana users that have clinical implications in preventing or treating problematic use (Brown et al. 2013; Zvolensky et al. 2017).
Therefore, this study aimed to evaluate 1) the influence of 28-days of monitored abstinence on changes in subsyndromal emotional functioning, sleep difficulties, marijuana withdrawal, marijuana craving, marijuana expectancies, and marijuana-related problems, and 2) characterize reward sensitivity and attention impulsivity measured after cessation of marijuana use in a sample of adolescent (ages 15–18, average age 17) marijuana users. Associations between age of marijuana use onset and lifetime marijuana use was also explored. The sample included n=26 marijuana users and n=30 demographically matched controls on age, gender, ethnicity, and family history of substance use disorder, who completed bi-weekly urine toxicology for 4 weeks (9 total toxicology screens) and repeated administration of self-report instruments assessing emotional functioning and marijuana use symptoms over the 28-day protocol. We hypothesized that following completion of monitored abstinence, marijuana users would report less depression and anxiety symptoms, sleep-related problems, and marijuana-related problems and symptoms (consequences, expectancies, craving, withdrawal) by day 28 of the protocol compared to baseline; and minimal group differences would be observed at follow-up. Notably, the marijuana users recruited for the study were not treatment-seekers or experiencing severe levels of mental health distress, despite regular use of marijuana.
Methods
Participants
Adolescents (N=56) were recruited from local San Diego schools and included 26 marijuana users (MJ; lifetime marijuana episodes (use days) ≥ 200, past month marijuana use episodes range 1–28, past three-month average marijuana use days range 7–30) and 30 control teens (CON; lifetime marijuana episodes ≤ 7, no past month marijuana use, past three-month average marijuana use days per month range 0–1) with minimal substance use histories (see Table 1). A district-approved research flyer that described a paid research opportunity at the University of California, San Diego was distributed throughout San Diego high schools. Teens and demographically matched controls were screened for substance use and exclusionary criteria.
Table 1.
Demographic Characteristics at Day 0 unless otherwise noted.
| CON (n=30) M (SD) [range] or % |
MJ (n=26) M (SD) [range] or % |
|
|---|---|---|
| Age, in years | 17.4 (0.8) | 17.7 (0.7) |
| % Male | 73% | 73% |
| % White | 73% | 81% |
| Grade point average | 3.7 (0.6) | 3.4 (0.8) |
| Annual household income | 155K (79) | 202K (188) |
| WASI Vocabulary T score | 58.2 (7.7) | 54.8 (8.9) |
| % Family history negative for substance use disorder | 47% | 35% |
| Lifetime marijuana use days* | 0.9 [0.0–7.0] | 408.2 [200.0–740.0] |
| Past month marijuana use days, Day 0* | 0.1 [0.0–1.0] | 18.1 [1.0–28.0] |
| Past month marijuana use days, Day 28* | 0.0 [0.0–0.0] | 0.7 [0.0–4.0] |
| Average marijuana use days per montha, Day 0* | 0.1 [0.0–1.0] | 22.1 [7.0–30.0] |
| Average marijuana hits per day in past month, Day 0 | NA | 10 [2.0–25.0] |
| THCCOOH/creatinine, Day 0*,b | 0.0 [0.0–0.0] | 1.2 [0.0–9.5] |
| THCCOOH/creatinine, Day 28 | 0.0 [0.0–0.0] | 0.3 [0.0–3.8] |
| Average cigarette use per month | 0.0 (0.0) | 11.1 (29.6) |
| Lifetime alcohol use days* | 6.1 (14.4) | 110.7 (88.3) |
| Past month alcohol use days, Day 0* | 0.2 [0.0–4.0] | 3.3 [0.0–11.0] |
| Past month alcohol use days, Day 28* | 0.1 [0.0–3.0] | 0.9 [0.0–5.0] |
| Lifetime other drug use episodes* | 0.0 (0.0 | 6.2 (8.3) |
| Past month other drug use days, Day 0* | 0.0 [0.0–0.0] | 0.5 (0.0–3.0] |
| Past month other drug use days, Day 28 | 0.0 [0.0–0.0] | 0.2 [0.0–2.0] |
| Days since marijuana use, Day 0* | 327.6 [4–1102]c | 4.5 [1.0–18.0] |
| Days since marijuana use, Day 28* | 354.7 [32–1127]c | 27.5 [3.0–43.0] |
| Days since alcohol use, Day 0* | 191.1 [16–1103]d | 30.2 [3.0–369.0] |
| Days since alcohol use, Day 28* | 201.7 [2.0–1131.0]d | 44.7 [2.0–398.0] |
| Age of onset, regular marijuana use*,e | NA | 15.3 (0.9) |
| Age of onset, regular alcohol usec | NA | 15.5 (1.5) |
Notes:
p<.05;
CON=control teens, MJ= marijuana teen users; WASI=Wechsler Abbreviated Scale of Intelligence; THCCOOH= 11-nor-9-carboxy-Δ9-tetrahydrocannabinol;
Average over three months prior to baseline;
THCCOOH/creatinine ratios in ng/mg;
n=7;
n=14;
>1 time/week for 52 weeks.
Ninety-six percent of participants in the MJ group met current Diagnostic and Statistical Manual for Mental Disorder-Fourth Edition (DSM-IV) cannabis abuse or dependence criteria, while 15% met current alcohol abuse or dependence criteria. Only one individual in the CON group met current abuse criteria for alcohol use, and none of the individuals in the CON group met cannabis abuse/dependence criteria. Comprehensive screening interviews were administered to adolescents and parents/guardians; adolescents provided assent for their own participation and guardians were required to provide consent in accordance with the University of California, San Diego Human Research Protections Program. Exclusionary criteria included history of a DSM-IV Axis I disorder other than alcohol or cannabis use disorder, use of psychoactive medications, learning disability or mental retardation, neurological condition (e.g., migraine), or traumatic brain injury with loss of consciousness >2 min; prenatal alcohol or drug exposure; premature birth; left handedness; and non-fluency in English. Participants completed all appointments at the University of California, Department of Psychiatry and asked to refrain from all intoxicants during participation (28 days). Self-report measures were administered during the toxicology appointments (see Table 2).
Table 2.
Study procedures and self-report measures administered
| Day of Week | 0 | 1 | 4 | 7 | 10 | 14 | 17 | 21 | 27 | 28 |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline & Toxicology (Tox) Visit # | Baseline | Tox 1 | Tox 2 | Tox 3 | Tox 4 | Tox 5 | Tox 6 | Tox 7 | Tox 8 | Tox 9 |
| Beck Depression Inventory-II | ● | ● | ● | ● | ● | |||||
| State Trait Anxiety Inventory | ● | ● | ● | ● | ● | |||||
| Pittsburgh Sleep Quality Index | ● | ● | ● | ● | ● | |||||
| Sleep Habit Questionnaire | ● | ● | ||||||||
| MJ Craving Questionnaire | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| MJ Withdrawal Discomfort Scale | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| MJ Effect Expectancy Questionnaire | ● | ● | ||||||||
| MJ Problem Scale | ● | ● | ||||||||
| BIS/BAS | ● | |||||||||
| Barratt Impulsiveness Scale-11 | ● |
Abstinence not required at Day 0 (no toxicology testing), days since last MJ use ranged from 1–18 for marijuana users; days since alcohol use ranged from 3–369 for marijuana users
Participants (CON and MJ) were compensated $10 for each successful urine toxicology screen (9 toxicology appointments over 28 days, see Table 2). CON did not test positive for urine marijuana metabolites at baseline or over the course of the study. Participants were not required to be abstinent at the Day 0 (baseline) appointment, and days since last use of marijuana (for MJ group) ranged from 1–18 at Day 0; 80% of MJ reported use within 1–5 days of the Day 0 appointment and 73% tested positive for marijuana metabolites in urine (15ng 11-nor-9-carboxy tetrahydrocannabinol (THCCOOH)/mL cut-off concentration). Starting at the first toxicology appointment, THCCOOH to creatinine concentration ratios were examined in relation to published data on these ratios determined in marijuana users during sustained monitored abstinence (Smith et al. 2009) for confirmation of abstinence over the course of 4 weeks. New cannabis use was determined by dividing each THCCOOH normalized to creatinine concentration by the previously collected THCCOOH normalized to creatinine concentration (urine 2/urine 1) and comparing this ratio to the 95% CI ratio for the time interval between the collections. For example, the 95% limit for the U2/U1 ratio was 1.59 when the collection interval was ≤ 24 h and 0.91, 0.51, 0.24, and 0.14 for collections ranging from 1–4 days, respectively. A successful urine toxicology screen was determined by determining the time difference between the urine specimens, selecting the correct metabolite ratio for this time frame, and comparing the obtained U2/U1 ratio for the participant to the 95% limit for the specific time difference (Smith et al. 2009). Breath alcohol with the Alco-Sensor IV Breathalyzer (Intoximeters 2005) was also evaluated for all participants at each urine toxicology screen appointment and sobriety from alcohol was confirmed for all participants (less than 0.02 g/100 mL). Fifty-six individuals (n=26 MJ users) finished the 28-day protocol (60 enrolled); 8 of n=26 users reported ≤4 days of cannabis use during the monitored abstinence period; however, biweekly toxicology screening showed a trend of decreasing THCCOOH/creatinine ratios among all users that completed. Loss to follow-up was relatively small and within the acceptable range for clinical trials (<15%) (Fewtrell et al. 2008; Kristman et al. 2004); the four individuals that did not complete the protocol (13%) were marijuana users that continued to use during monitored abstinence and failed to complete the final appointments. Those four individuals were not included in the final sample (n=26) or any statistical analysis presented in this manuscript.
Measures
Substance Use and Mental Health Assessment
The Customary Drinking and Drug Use Record assessed quantity and frequency of lifetime marijuana, alcohol, cigarette, and other drug use and age of marijuana use onset (Brown et al. 1998). The Timeline Followback quantified self-reported substance use (e.g., marijuana, alcohol) at each visit during the 28-day monitored abstinence protocol (Sobell and Sobell 1992).
Marijuana symptoms, expectancies, and consequences questionnaires were administered throughout the protocol (see Table 2). The Marijuana Craving Questionnaire (MCQ) is a 10-item self-report questionnaire (total scale values range from 10 (no craving)-70 (high craving)) that evaluates intention and desire to smoke marijuana, anticipated pleasure, and anticipated relief from negative affect and withdrawal (Budney et al. 2001). The Marijuana Withdrawal Discomfort Scale (MWDS) is a 30-item self-report form on which participants rate the severity of withdrawal symptoms (none (0) to severe (3)) over the past 24-hours (Budney et al. 2004); these symptoms change with marijuana use but include experiences related to mood and sleep that CON may also experience. Total MWDS scores range from 0–90. The Marijuana Problem Scale (MPS) assesses 19 functional problems (no problem (0) to serious problem (2)) associated with marijuana use (Budney et al. 2001) and total scores range from 0–38. The Marijuana Effect Expectancy Questionnaire (MEEQ) provides a measure of appraisal on six subscales (cognitive/behavioral impairment (total score range 5–50), relaxation/tension (total score range 5–40), social/sexual facilitation (total score range 5–45), perceptual/cognitive enhancement (total score range 5–40), global negative effects (total score range 5–45), and craving/physical effects (total score range 5–30); this 48-item instrument asks participants to identify a value between 1 (disagree strongly) and 5 (agree strongly) for each item to identify if a participant expects marijuana-related effects to occur in one or more of these domains (Schafer and Brown 1991). High scores reflect a high level of expectancy on the corresponding subscale.
Emotional Functioning, Reward Sensitivity, and Demographics
The Beck Depression Inventory Second Edition (BDI-II) and Spielberger State Trait Anxiety Inventory (STAI) assessed depressive symptoms and state anxiety (Beck et al. 1996; Spielberger et al. 1970). State Trait Anxiety scores were converted to gender-normed T-scores for high-school age boys and girls (Spielberger et al. 1970). The Family History Assessment Module (Rice et al. 1995) evaluated family history of psychiatric and substance use disorders. The Pittsburgh Sleep Quality Index (PSQI) (Buysse et al. 1989) is a brief self-report measure administered to capture sleep quality via a global summary score. The PSQI contains 18 items and yields seven subscales (range better (0) – worse (3)) that measure sleep onset latency, efficiency, duration, disturbance, days of dysfunction, overall quality (range 0 (better)-21(worse); poor sleep quality threshold >5), and sleep medication usage. The Behavioral Inhibition System and Behavioral Approach System scales consist of 24 items (BIS/BAS) (Carver and White 1994) that measure avoidance (BIS) and approach (BAS) sensitivities reflective of reward sensitivity personality traits. Four response options range from very true (1) to very false for me (4); BAS subscales include reward responsiveness, fun seeking, and drive. The Barratt Impulsiveness Scale (BIS-11) (Patton et al. 1995) is a 30-item self-report measure administered to assess impulsivity; items are on a 4-point scale and range from rarely (1) to almost/always (4). Barratt subscales examined include cognitive impulsivity (i.e., attention difficulties), motor impulsivity (i.e., acting without thinking), and non-planning impulsivity (i.e., poor planning).
The Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary subtest was included as an estimate of premorbid intellectual functioning (Wechsler 1999). Parental income and grade point average were collected during a comprehensive clinical interview at baseline.
Data Analysis
Demographic comparisons and substance use
Analysis of variance (ANOVA) and Chi-square tests evaluated differences between groups on demographic variables and to identify appropriate covariates for subsequent analysis.
Primary analyses
Repeated-measures analysis of variance (ANOVA) examined the main effect of group, time, and Group x Time interactions on dependent variables of emotional distress (BDI-II, Cronbach’s alpha range .70–.82; STAI, Cronbach’s alpha range .51–.75), sleep quality (PSQI, Cronbach’s alpha range .64–.74), marijuana withdrawal (MWDS, Cronbach’s Alpha range .72–.88), and marijuana craving (MCQ, Cronbach’s alpha range .92–.93) over time in both groups, despite anticipated changes in the MJ group only. When Mauchly’s test suggested violations of sphericity, Greenhouse-Geisser corrections were used to determine statistical significance. Changes on marijuana expectancy symptoms (MEEQ, Cronbach’s alpha range .77–.87) and marijuana problems (MPS, Cronbach’s alpha range .77–.83) were examined in the MJ group only. One-way ANOVA examined between-group differences on measures of reward sensitivity (BIS/BAS, Cronbach’s alpha=.85) (time point 9) and attention impulsivity (BIS-11, Cronbach’s alpha=.81) (time point 6) (see Table 2).
Secondary exploratory analyses: bivariate correlations
We focused on four secondary a priori analyses for measures in which we observed a change over time. These correlations focused on two key variables 1) cumulative marijuana use (lifetime use), and 2) age of marijuana use onset. These variables show robust associations with neurodevelopmental and mental health functioning outcomes in the research literature (Volkow et al. 2016) and with neural health in this sample in particular (Jacobus et al. 2012; Jacobus et al. 2014). Therefore, the study addressed three key questions: is age of MJ use onset or cumulative MJ use associated with 1) self-reported changes in depression, anxiety, or sleep quality over monitored abstinence, 2) changes in MJ use expectancies, withdrawal, and craving over monitored abstinence, or 3) reward sensitivity and attentional impulsivity. We also examined if change in MJ use expectancies was related to change in emotional distress over monitored abstinence, given the increasing attention to how beliefs about marijuana use may distinctly influence treatment outcomes and use patterns (Brackenbury et al. 2016).
Results
Demographics and substance use
Groups did not differ on any demographic variable other than substance use, as anticipated (e.g. lifetime marijuana use, alcohol use, other drug use, ps<.05, see Table 1). Lifetime and past 28-day alcohol use (measured at Day 28 of the protocol) and lifetime other drug use episodes at Day 0 were identified as covariates. All significant findings were re-examined controlling for these variables; however, the significant associations reported below remained unchanged.
Emotional Functioning
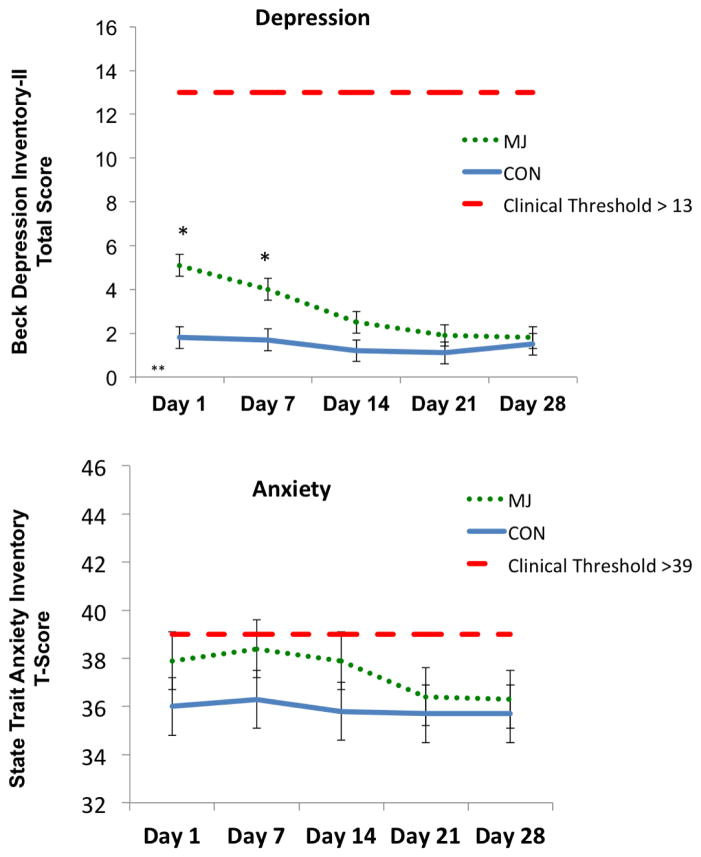
The main effect of time F(2,106)=7.7, p<.01, partial η2=.13, and main effect of group F(1,51)=5.2, p=.02, partial η2=.09, predicted self-reported depression scores. The Group by Time Interaction was also significant, F(2,106)=3.8, p=.02, partial η2=.07. Follow-up analysis revealed significant decrease in depression scores for MJ (Day 1>Day 14, 21, 28) but not CON (p>.05), and between group differences (MJ>CON) on Day 1 and Day 7. Between-group differences were no longer present after Day 14 (ps<.05, see Figure 1). The average percent reduction in scores relative to baseline for MJ was 36.6% compared to 9.5% for controls. The suggested minimal clinically significant difference cutoff is 17% (Button et al. 2015). No significant differences (between-group, within-subject, or Group by Time interactions) were identified for self-reported anxiety (ps>.05)
Figure 1.
Self-reported emotional functioning scores. *p<.05, (MJ>CON); **p<.05; (MJ Day 1>MJ Day 14, 21, 28). Cohen’s d=.35 (percent reduction in BDI-II scores at Day 28 relative to Day 1, MJ>CON)
Sleep
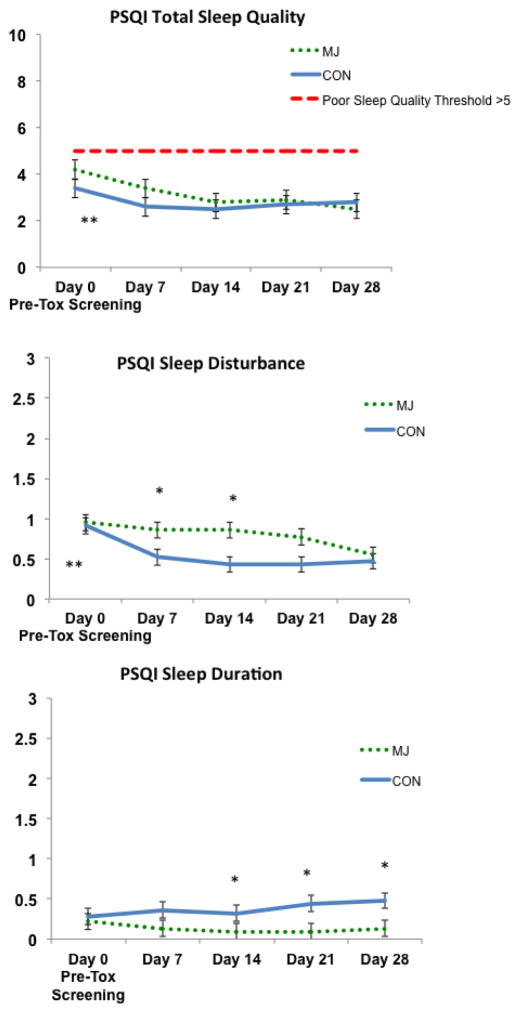
A main effect of time was observed on total sleep quality, F(3,141)=4.8, p<.01, partial η2=.10.. The main of group (F(1,44)=.192, p=.60) and Group by Time interaction (F(3,141)=.40, p=.76) was not significant. Follow-up analysis reveals this main effect is largely driven by within-group change in the MJ group (Day 0 > Day 14, 21, 28) (ps<.05); a significant within-subject change was not observed for CON (ps>.05).
A main effect of time, F(4, 41)=5.0, p<.01, partial η2=.33, and main effect of group (trend) F(1,44)=3.9, p=.05, partial η2=.08, was observed for sleep disturbance. The Group by Time interaction was not significant, F(4,41)=2.0, p=.10. These findings appear driven by within-subject change in the MJ group (Day 0>Day28) and between-group differences (MJ>CON) at Day 7 and Day 14 that resolve by Day 21; a significant within-subject change was not observed for CON (ps>.05).
A main effect of group was found for sleep duration F(1,47)=5.6, p=.02, partial η2=.12, driven by differences at Day 14, 21, and 28. In general, CON report sleeping more hours per night compared to MJ. The main effect of time, F(4,44)=.48, p=.75 and Group by Time interaction F(4,44)=.55, p=.70 were not significant for sleep duration. No significant between-subjects, within-subjects, or Group by Time interactions were observed for the subscales onset latency, efficiency, days of dysfunction, and sleep medication usage (ps>.05) (see Figure 2).
Figure 2.
Self-reported sleep quality; *p<.05, main effect of group (CON>MJ); **p<.05, within-subject effect (Sleep Quality: MJ Day 0> MJ Day 14, 21, 28) (Sleep Disturbance: MJ Day 0 > MJ Day 28). Cohen’s d=.39 (change in PSQI Total Sleep Quality index from Day 0 to Day 28 MJ>CON)
Marijuana Use Expectancies and Consequences
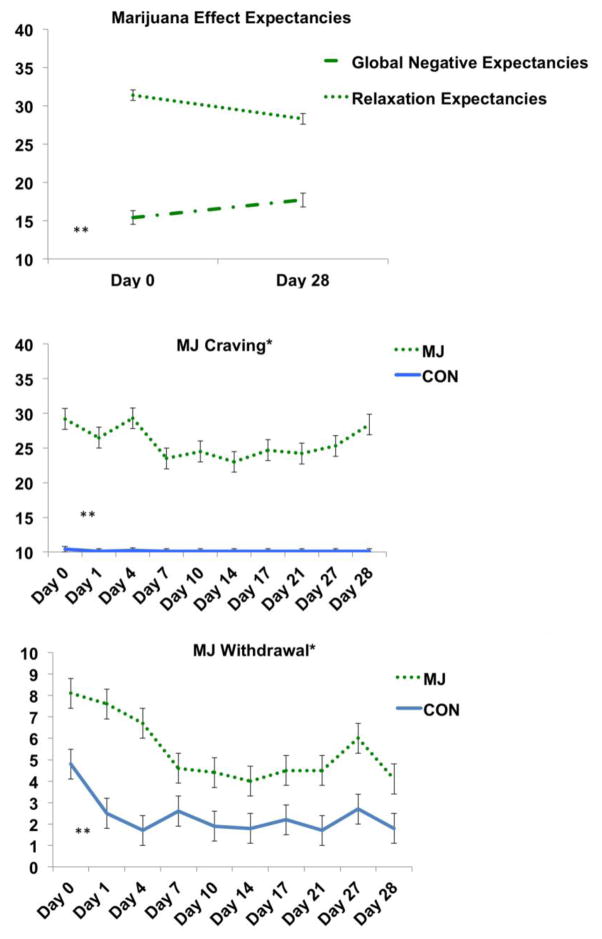
In the user group only, we found a within-subjects effect for the global negative effects (F(1,25)=12.6, p<.01, partial η2=.34) and relaxation/tension subscales (F(1,25)=14.0, p<.01, partial η2=.36) of the MEEQ. MJ decreased their expectations of relaxation and increased their expectation of the global negative effects of marijuana from Day 0 to Day 28 (see Figure 3). We did not see significant changes on the subscales of cognitive/behavioral impairment, social/sexual facilitation, perceptual/cognitive enhancement, or craving/physical effects (ps>.05). We observed a trend in decreased marijuana related problems from Day 0 to Day 27 on the MPS (p=.06).
Figure 3.
Marijuana expectancies scores, and craving and withdrawal scales. *p<.05, main effect of group (MJ>CON, all time points); **p<.05, within-subject effect for MJ group (Expectancies: MJ Day 0>MJ Day 28) (Craving: MJ Day 0 > MJ Days 7–21) (Withdrawal: MJ Day 0 > MJ Day 7–21, 28).
As expected, the Group by Time interaction significantly predicted marijuana cravings scores, F(4, 201)= 2.7, p=.03, η2=.05, MJ>CON. The MJ group reported higher craving scores at all time points (ps<.01); within the MJ group, we found a significant difference between Day 0 craving and follow-up appointments (Day 0 > Days 7–21, ps<.05) that was not observed in the CON group (ps>.05).
Similarly, the main effect of group predicted MJ withdrawal symptoms (F(1,49)= 12.06, p<.01, partial η2=.20), although the Group by Time interaction was not significant (F(3,94)=1.69, p=.15), the within-subject effect (F(3,194)=7.0, p<.01, partial η2=.12) was explored in the MJ group and differences (ps<.05) were observed (Day 0 > Day 7–21, 28). We did not observe a significant within-subject effect in the CON group (ps>.05) (see Figure 3).
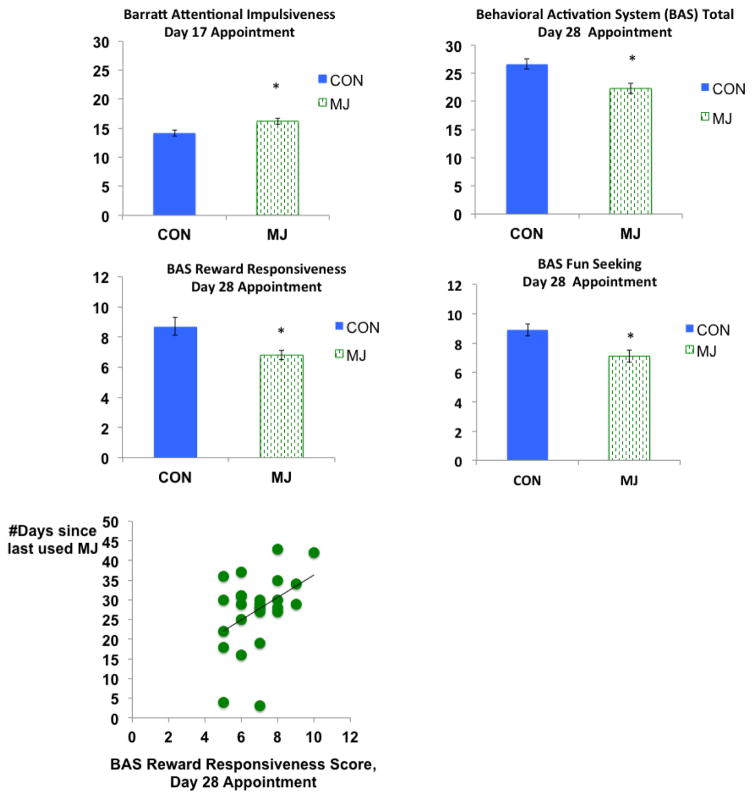
Reward Motivation
Between-group differences (MJ<CON) were observed on the Behavioral Approach System (BAS) Total score F(1,54)=8.0, p<.01, partial η2=.13; BAS Fun Seeking score F(1,55)=10.6, p<.01, partial η2=.16; and BAS Reward Responsiveness score F(1,54)=6.0, p=.01, partial η2=.10, on Day 28. These relationships remained significant after controlling for depression at Day 1 and 28-day follow-up (ps<.05), as depression was not found to be correlated with BIS/BAS scores. We found significant correlations between days since last use of marijuana and BAS reward responsiveness (r=.42, p=.03) as more days since last use was linked to higher scores on the reward responsiveness subscale; this relationship remained after removing two individuals who may have used more recently during the monitored abstinence period (r=.44, p=.03). We did not see significant between-group differences on the BAS drive subscale, F(1,55)=1.48, p=.23 or BIS scale, F(1,55)=.72, p=.40.
Between-group differences (MJ>CON) were identified on the Barratt Impulsiveness Scale Attention subscale F(1,50)=7.76, p<.01, partial η2=.14; attentional impulsivity was measured on Day 17. We did not observe significant between-group differences on the motor impulsivity subscale, F(1,50)=1.3, p=.26 or non-planning impulsivity subscale F(1,50)=.32, p=.58.
Bivariate Correlations
In the MJ group, we observed a significant positive correlation between lifetime marijuana use episodes and change in self-reported anxiety by Day 28 (r=.39, p=.04), as more lifetime use at Day 0 was linked to a larger decrease in anxiety over the 28 days; lifetime marijuana use was not associated with baseline anxiety (p=.13). The same directional relationship for change in anxiety was observed for change in depression (r=.37), although it did not reach statistical significance (p=.06). A negative correlation was observed for age of MJ initiation and change in global negative expectancies (r=−.42, p=.03); later age of MJ use onset was linked to increased recognition of the global negative effects of marijuana by Day 28. Later age of MJ initiation was associated with a greater degree of change in perceived sleep quality by Day 28 (r=.42, p=.03). We did not find a relationship between change in emotional functioning and changes in expectancies (rs<.18, ps>.35).
Discussion
The current findings expand the literature in several ways including: 1) MJ demonstrated decreased self-reported subsyndromal depression symptoms by week three of monitored abstinence, and greater changes in depression and anxiety symptoms were observed in those reporting more lifetime marijuana use at baseline; 2) group differences in perceptions of sleep quality and sleep disturbance resolved by Day 28, although MJ continued to report less sleep than controls; 3) MJ reported increased expectation of global negative effects and less expectation that marijuana helps reduce tension and anxiety after completing 28-days of abstinence; and 4) MJ reported less incentive sensitivity and more attentional impulsivity compared to controls, measured after self-reported subsyndromal emotional symptoms substantially decreased (~ day 14 of the protocol). Findings also support the extant literature identifying withdrawal and craving symptoms following cessation of use (Cohen-Zion et al. 2009; Crowley et al. 1998; Duffy and Milin 1996; Milin et al. 2008; Vandrey et al. 2005). Craving and withdrawal symptoms were highest during the first week of the protocol and decreases were observed within the first two weeks of abstinence. We speculate slight increases on these scales toward the end of the protocol are related to anticipation of re-initiation of marijuana use. Follow-up interviews were not conducted past 28-days; therefore, plans for reinitiation of use is speculative, but is consistent with research showing poor longer-term efficacy (>1month) for abstinence-based CM protocols with adolescents (Schuster et al. 2016).
It remains unclear as to whether depression or substance use presents first, if these conditions simultaneously emerge, or both are associated with extraneous clinical factors (Feingold et al. 2017; Gilder and Ehlers 2012; Womack et al. 2016). Our preliminary data suggest that marijuana users experiencing subsyndromal depression symptoms at the start of a monitored abstinence period may experience a reduction in those symptoms. The average percent reduction in BDI-II scores (~37%) relative to baseline for the MJ group was above the minimal clinically significant difference cutoff (>17%) (Button et al. 2015), which suggests clinically important improvements were observed in some individuals.
Sleep difficulties often correspond with depression symptoms (Gates et al. 2016; Maple et al. 2016; Ogeil et al. 2015). Participants in our study reported fairly minimal sleep difficulties (PSQI Total scores <5, clinical cut-off for sleep disturbance); however, perceived sleep quality and perceived disturbance no longer differed compared to controls by day 28 of the protocol. This change is notable as perception of general sleep quality is associated with enhanced likelihood for quit success compared to reported sleep duration and efficiency (Babson et al. 2013a; Babson et al. 2013b; Vandrey et al. 2011). Abstinence may influence perception of sleep quality, despite continued differences in quantity of sleep reported after several weeks of abstinence.
Significant changes on the expectancies subscales relaxation and tension reduction and global negative impairment were encouraging, as marijuana users reported less expectation that marijuana helps reduce tension and anxiety and increased perception of global negative consequences by the end of the protocol; those who initiated regular use at a later age reported greater change in these expectancy scales. Expectancies and coping motives may mediate substance use severity in high-risk youth (Fanale et al. 2017; Kristjansson et al. 2012; Vangsness et al. 2005). We also observed differences in reward sensitivity and impulsivity during later time points (following initial decreases in depression and anxiety) that were linked to recency of marijuana use. As expected, the marijuana users scored higher on the Barratt Impulsiveness Scale attention domain, as impulsivity traits are likely to impact vulnerability to substance misuse (attention domain; e.g., trouble with concentration) (Cservenka et al. 2012; Day et al. 2013; Dougherty et al. 2013; Gruber et al. 2012). Conversely, the controls showed higher scores on approach system (BAS) subscales (e.g., reward responsiveness, fun seeking), suggesting controls may be more responsive to reward and reward cues compared to the marijuana users in this investigation; although the degree to which high BAS score predict problematic substance use outcomes may be moderated by high-order cognitive functioning abilities (e.g, inhibitory control), and the BAS/BIS imbalance (Kim-Spoon et al. 2016; Prince van Leeuwen et al. 2011).
Our BAS findings are similar to work conducted by Wright and colleagues (Wright et al. 2016), in which they also found decreased behavioral approach scores in marijuana users ages 18–25. The authors suggest depressive symptoms may underlie decreased BAS scores (vs. impulsivity traits) (McFarland et al. 2006), although depression symptoms in the users was not statistically different from controls at the time of measurement in our protocol (~week 3, day 17). BAS scales are suggested to represent many difference facets of impulsivity, (e.g., sensation seeking, sensitivity to reward) (Dawe and Loxton 2004; Ross et al. 2009). While increased BAS sensitivity is linked to substance use in adults and adolescents (Johnson et al. 2003); research suggests that higher BAS (combined with low BIS) scores may be linked to lifetime experimentation versus repeated problematic use (Prince van Leeuwen et al. 2011). Our findings of low BAS scores in our users and lack of group differences on BIS scores may underlie neurocognitive vulnerabilities, marijuana-related changes in dopaminergic pathways, and/or a premature neurodevelopmental changes in reward sensitivity for substance users moving beyond experimentation (Chung et al. 2015; Takahashi et al. 2007; Urosevic et al. 2012; Wahlstrom et al. 2010).
Limitations include the small sample size and limited Type I error control, reliance on self-report measures, and lack of follow-up beyond the 28-day monitored abstinence period. Marijuana users also reported alcohol use (Subbaraman and Kerr 2015); therefore changes may be influenced by alcohol use patterns. Further, the sample size is small and multiple comparison corrections were not stringent, therefore replication is important. We cannot rule out regression to the mean as a possible explanation for changes observed without randomization and multiple measurements of each construct assessed. Each impulsivity instrument was administered once, and therefore, we could not examine change over time for this construct. Our non-clinical sample is also predominately male, Caucasian, and from higher-income households; therefore, generalizability may be limited. Studies using randomized controlled trial designs will allow inferences to be made about the efficacy of CM protocols.
We observed notable changes in depression symptoms, sleep quality, and self-reported marijuana use expectancies following participation in a 28-day monitored abstinence protocol. Many individuals use marijuana to cope with various degrees of depression, anxiety, and sleep problems and therefore the potential medical application of marijuana for treatment of mental health symptoms continues to be explored despite mixed findings (Babson et al. 2017; Feingold et al. 2017; Haj-Dahmane and Shen 2014), however this study supports the extant adolescent research literature that consistently shows marijuana use during neurodevelopment likely has a deleterious impact on neural health and emotional functioning (Lisdahl et al. 2013; Volkow et al. 2014). Future work in our laboratory will continue to explore treatment approaches that target substance misuse and substance-related processes and treatment barriers in the context of neurodevelopmental vulnerabilities and the neurobiology of addiction.
Figure 4.
Self-reported reward responsiveness and impulsivity traits, *p<.05, main effect of group (Barratt Attentional Impulsiveness: MJ>CON) (BAS subscales: CON>MJ)
Acknowledgments
This study was supported by National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism Grants R01 AA013419, U01 AA021692, U01 DA041089, P20 DA024194, K23 AA025399, and NCATS KL2 TR001444
References
- Angarita GA, Emadi N, Hodges S, Morgan PT. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addiction science & clinical practice. 2016;11:9. doi: 10.1186/s13722-016-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babson KA, Boden MT, Bonn-Miller MO. The impact of perceived sleep quality and sleep efficiency/duration on cannabis use during a self-guided quit attempt. Addictive behaviors. 2013a;38:2707–13. doi: 10.1016/j.addbeh.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Babson KA, Boden MT, Harris AH, Stickle TR, Bonn-Miller MO. Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. Journal of substance abuse treatment. 2013b;44:438–43. doi: 10.1016/j.jsat.2012.08.224. [DOI] [PubMed] [Google Scholar]
- Babson KA, Sottile J, Morabito D. Cannabis, Cannabinoids, and Sleep: a Review of the Literature. Curr Psychiatry Rep. 2017;19:23. doi: 10.1007/s11920-017-0775-9. [DOI] [PubMed] [Google Scholar]
- Beck AT, RAS, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Boden MT, McKay JR, Long WR, Bonn-Miller MO. The effects of cannabis use expectancies on self-initiated cannabis cessation. Addiction. 2013;108:1649–57. doi: 10.1111/add.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury LM, Ladd BO, Anderson KG. Marijuana use/cessation expectancies and marijuana use in college students. The American journal of drug and alcohol abuse. 2016;42:25–31. doi: 10.3109/00952990.2015.1105242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PC, Budney AJ, Thostenson JD, Stanger C. Initiation of abstinence in adolescents treated for marijuana use disorders. Journal of substance abuse treatment. 2013;44:384–90. doi: 10.1016/j.jsat.2012.08.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of studies on alcohol. 1998;59:427–38. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Archives of general psychiatry. 2001;58:917–24. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of consulting and clinical psychology. 2006;74:307–16. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addiction science & clinical practice. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, Welton NJ, Ades AE, Lewis G. Minimal clinically important difference on the Beck Depression Inventory--II according to the patient’s perspective. Psychological medicine. 2015;45:3269–79. doi: 10.1017/S0033291715001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994:67. [Google Scholar]
- Chung T, Paulsen DJ, Geier CF, Luna B, Clark DB. Regional brain activation supporting cognitive control in the context of reward is associated with treated adolescents’ marijuana problem severity at follow-up: A preliminary study. Developmental cognitive neuroscience. 2015;16:93–100. doi: 10.1016/j.dcn.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Zion M, Drummond SP, Padula CB, Winward J, Kanady J, Medina KL, Tapert SF. Sleep architecture in adolescent marijuana and alcohol users during acute and extended abstinence. Addictive behaviors. 2009;34:976–9. doi: 10.1016/j.addbeh.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K, Chatters R, Kaltenthaler E, Wong R. Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report. Health Technol Assess. 2015;19:1–130. doi: 10.3310/hta19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Gates P, Pokorski I. A Narrative Review of Psychological Cannabis Use Treatments With And Without Pharmaceutical Adjunct. Current pharmaceutical design. 2016 doi: 10.2174/1381612822666160831094811. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addictive behaviors. 2008;33:1500–5. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug and alcohol dependence. 1998;50:27–37. doi: 10.1016/s0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug and alcohol dependence. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ML, Powers MB, Handelsman P, Medina JL, Zvolensky M, Smits JA. Behavioral therapies for treatment-seeking cannabis users: a meta-analysis of randomized controlled trials. Evaluation & the health professions. 2015;38:94–114. doi: 10.1177/0163278714529970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neuroscience and biobehavioral reviews. 2004;28:343–51. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Day AM, Metrik J, Spillane NS, Kahler CW. Working memory and impulsivity predict marijuana-related problems among frequent users. Drug and alcohol dependence. 2013;131:171–4. doi: 10.1016/j.drugalcdep.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk R. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials. Journal of substance abuse treatment. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, Shannon EE, Acheson A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology. 2013;226:307–19. doi: 10.1007/s00213-012-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy A, Milin R. Case study: withdrawal syndrome in adolescent chronic cannabis users. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1618–21. doi: 10.1097/00004583-199612000-00013. [DOI] [PubMed] [Google Scholar]
- Fanale CM, Maarhuis P, Wright BR, Caffrey K. The effect of attitudinal barriers to mental health treatment on cannabis use and mediation through coping motives. Addictive behaviors. 2017;69:35–41. doi: 10.1016/j.addbeh.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Feingold D, Rehm J, Lev-Ran S. Cannabis use and the course and outcome of major depressive disorder: A population based longitudinal study. Psychiatry research. 2017;251:225–234. doi: 10.1016/j.psychres.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, Koletzko B, Lucas A. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Archives of disease in childhood. 2008;93:458–61. doi: 10.1136/adc.2007.127316. [DOI] [PubMed] [Google Scholar]
- Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Developmental cognitive neuroscience. 2015;16:16–22. doi: 10.1016/j.dcn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CL, Towe SL, Stephens RS, Walker DD, Roffman RA. Motives for cannabis use in high-risk adolescent users. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2011;25:492–500. doi: 10.1037/a0024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates P, Albertella L, Copeland J. Cannabis withdrawal and sleep: A systematic review of human studies. Substance abuse. 2016;37:255–69. doi: 10.1080/08897077.2015.1023484. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Ehlers CL. Depression symptoms associated with cannabis dependence in an adolescent American Indian community sample. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2012;21:536–43. doi: 10.1111/j.1521-0391.2012.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2012;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. Chronic stress impairs alpha1-adrenoceptor-induced endocannabinoid-dependent synaptic plasticity in the dorsal raphe nucleus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:14560–70. doi: 10.1523/JNEUROSCI.1310-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA psychiatry. 2015;72:1235–42. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Hagerty CE, Herman DS, de Dios MA, Anderson BJ, Stein MD. Expectancies and marijuana use frequency and severity among young females. Addictive behaviors. 2010;35:995–1000. doi: 10.1016/j.addbeh.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue A, Henderson CE, Ozechowski TJ, Robbins MS. Evidence base on outpatient behavioral treatments for adolescent substance use: updates and recommendations 2007–2013. Journal of clinical child and adolescent psychology: the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2014;43:695–720. doi: 10.1080/15374416.2014.915550. [DOI] [PubMed] [Google Scholar]
- Intoximeters. Alco-Sensor IV with Memory Operators Manual. St. Louis, MO: 2005. [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology. 2012;222:675–84. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Castro N, Brumback T, Meruelo AD, Tapert SF. Neuropsychological Performance in Adolescent Marijuana Users With Co-Occurring Alcohol Use: A Three-Year Longitudinal Study. Neuropsychology. 2015 doi: 10.1037/neu0000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. Journal of studies on alcohol and drugs. 2014;75:729–43. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Turner RJ, Iwata N. BIS/BAS levels and psychiatric disorder. Journal of psychopathology and behavioral assessment. 2003:25. [Google Scholar]
- Kaminer Y, Burleson JA, Burke R, Litt MD. The efficacy of contingency management for adolescent cannabis use disorder: a controlled study. Substance abuse. 2014;35:391–8. doi: 10.1080/08897077.2014.933724. [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Experimentation versus progression in adolescent drug use: A test of an emerging neurobehavioral imbalance model. Development and psychopathology. 2015;27:901–13. doi: 10.1017/S0954579414000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Holmes C, Lee J, Chiu P, King-Casas B. Behavioral and neural inhibitory control moderates the effects of reward sensitivity on adolescent substance use. Neuropsychologia. 2016;91:318–326. doi: 10.1016/j.neuropsychologia.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson SD, Agrawal A, Lynskey MT, Chassin LA. Marijuana expectancies and relationships with adolescent and adult marijuana use. Drug and alcohol dependence. 2012;126:102–10. doi: 10.1016/j.drugalcdep.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristman V, Manno M, Cote P. Loss to follow-up in cohort studies: how much is too much? Eur J Epidemiol. 2004;19:751–60. doi: 10.1023/b:ejep.0000036568.02655.f8. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple KE, McDaniel KA, Shollenbarger SG, Lisdahl KM. Dose-dependent cannabis use, depressive symptoms, and FAAH genotype predict sleep quality in emerging adults: a pilot study. The American journal of drug and alcohol abuse. 2016;42:431–40. doi: 10.3109/00952990.2016.1141913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland BR, Shankman SA, Tenke CE, Bruder GE, Klein DN. Behavioral activation system deficits predict the six-month course of depression. Journal of affective disorders. 2006;91:229–34. doi: 10.1016/j.jad.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Milin R, Manion I, Dare G, Walker S. Prospective assessment of cannabis withdrawal in adolescents with cannabis dependence: a pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:174–8. doi: 10.1097/chi.0b013e31815cdd73. [DOI] [PubMed] [Google Scholar]
- Moitra E, Anderson BJ, Stein MD. Reductions in Cannabis Use Are Associated with Mood Improvement in Female Emerging Adults. Depression and anxiety. 2016;33:332–8. doi: 10.1002/da.22460. [DOI] [PubMed] [Google Scholar]
- Moitra E, Christopher PP, Anderson BJ, Stein MD. Coping-motivated marijuana use correlates with DSM-5 cannabis use disorder and psychological distress among emerging adults. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2015;29:627–32. doi: 10.1037/adb0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogeil RP, Phillips JG, Rajaratnam SM, Broadbear JH. Risky drug use and effects on sleep quality and daytime sleepiness. Hum Psychopharmacol. 2015;30:356–63. doi: 10.1002/hup.2483. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Prince van Leeuwen A, Creemers HE, Verhulst FC, Ormel J, Huizink AC. Are adolescents gambling with cannabis use? A longitudinal study of impulsivity measures and adolescent substance use: the TRAILS study. Journal of studies on alcohol and drugs. 2011;72:70–8. doi: 10.15288/jsad.2011.72.70. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism, clinical and experimental research. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Ross SR, Benning SD, Patrick CJ, Thompson A, Thurston A. Factors of the psychopathic personality inventory: criterion-related validity and relationship to the BIS/BAS and five-factor models of personality. Assessment. 2009;16:71–87. doi: 10.1177/1073191108322207. [DOI] [PubMed] [Google Scholar]
- Schafer J, Brown SA. Marijuana and cocaine effect expectancies and drug use patterns. Journal of consulting and clinical psychology. 1991;59:558–65. doi: 10.1037//0022-006x.59.4.558. [DOI] [PubMed] [Google Scholar]
- Schuster RM, Hanly A, Gilman J, Budney A, Vandrey R, Evins AE. A contingency management method for 30-days abstinence in non-treatment seeking young adult cannabis users. Drug and alcohol dependence. 2016;167:199–206. doi: 10.1016/j.drugalcdep.2016.08.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. Journal of analytical toxicology. 2009;33:185–9. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. A technique for assessing self-reported alcohol consumption. Humana Press; New York, NY: 1992. Timeline follow back. [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto, CA, USA: 1970. [Google Scholar]
- Stanger C, Budney AJ, Bickel WK. A developmental perspective on neuroeconomic mechanisms of contingency management. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2013;27:403–15. doi: 10.1037/a0028748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug and alcohol dependence. 2009;105:240–7. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaraman MS, Kerr WC. Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcoholism, clinical and experimental research. 2015;39:872–9. doi: 10.1111/acer.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Yamagata S, Kijima N, Shigemasu K, Ono Y, Ando J. Continuity and change in behavioral inhibition and activation systems: A longitudinal behavioral genetic study. Personality and Individual Differences. 2007;43:1616–1625. [Google Scholar]
- Tims FM, Dennis ML, Hamilton N, BJB, Diamond G, Funk R, Brantley LB. Characteristics and problems of 600 adolescent cannabis abusers in outpatient treatment. Addiction. 2002;97(Suppl 1):46–57. doi: 10.1046/j.1360-0443.97.s01.7.x. [DOI] [PubMed] [Google Scholar]
- Urosevic S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental psychology. 2012;48:1488–500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug and alcohol dependence. 2005;78:205–10. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug and alcohol dependence. 2011;117:38–44. doi: 10.1016/j.drugalcdep.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangsness L, Bry BH, LaBouvie EW. Impulsivity, negative expectancies, and marijuana use: a test of the acquired preparedness model. Addictive behaviors. 2005;30:1071–6. doi: 10.1016/j.addbeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;371:879. doi: 10.1056/NEJMc1407928. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, Bloomfield MA, Curran HV, Baler R. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA psychiatry. 2016;73:292–7. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain and cognition. 2010;72:146–59. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Womack SR, Shaw DS, Weaver CM, Forbes EE. Bidirectional Associations Between Cannabis Use and Depressive Symptoms From Adolescence Through Early Adulthood Among At-Risk Young Men. Journal of studies on alcohol and drugs. 2016;77:287–97. doi: 10.15288/jsad.2016.77.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NE, Scerpella D, Lisdahl KM. Marijuana Use Is Associated with Behavioral Approach and Depressive Symptoms in Adolescents and Emerging Adults. PloS one. 2016;11:e0166005. doi: 10.1371/journal.pone.0166005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Paulus DJ, Garey L, Manning K, Hogan JBD, Buckner JD, Rogers AH, Kathryn McHugh R. Perceived barriers for cannabis cessation: Relations to cannabis use problems, withdrawal symptoms, and self-efficacy for quitting. Addictive behaviors. 2017;76:45–51. doi: 10.1016/j.addbeh.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]