Abstract
It has been debated for several years as to whether the antidiabetic drug pioglitazone increases the risk for bladder cancer. A series of recent large population studies yielded conflicting results. To investigate why the observational studies yielded conflicting results, we conducted stratified analyses to analyze the potential confounders behind these discordant outcomes. A total of 2,764,731 participants from observational (OB) studies and 9,999 from randomized control trials (RCTs) were identified for these analyses. The stratified analysis revealed that the study type, adjustment for age/sex, treatment duration, cumulative dose, agents used in a control group, mean period of follow-up and study population region might contribute to the discordant outcomes. In terms of population regions, pioglitazone increased the risk for bladder cancer could be found in European population, and patients who undergo treatment with pioglitazone for longer durations (>12 months) or are administrated a larger cumulative dose (>28,000 mg) might require more attention, and the long-term effects (≥3.6 years) of pioglitazone needs be monitored more carefully.
Introduction
Thiazolidinediones (TZDs), agonists of the peroxisome-proliferator-activated receptor (PPAR) γ, reduce blood glucose levels primarily by increasing insulin sensitivity in peripheral tissues, without causing hypoglycemia1,2. These agents have been widely used in patients with diabetes mellitus (DM). However, troglitazone was discontinued because it caused hepatotoxicity3, and the safety of rosiglitazone was disputed due its effect on the cardiovascular system4,5. In contrast, pioglitazone has been shown to prevent the progression to diabetes and major cardiovascular events6,7 as well as nonalcoholic steatohepatitis6, thereby indicating a broader prospect for its clinical applications. However, globally, safety concerns pertaining to the potential effect of pioglitazone in increasing bladder cancer risk have been raised and debated for many years8–23. A series of large clinical studies24–26, such as the Kaiser Permanente Northern California (KPNC) study, United Kingdom Clinical Practice Research Datalink (UKCPRD) research, and Four European Countries Datasets (FECD) research, obtained discordant results, which led to more debate regarding this issue. Although this observation is still debated, the U.S. Food and Drug Administration (FDA) did warn about this risk on December 12, 2016 (http://www.fda.gov/Drugs/DrugSafety/ucm519616.htm).
We conducted stratified analyses to investigate why the observational studies yield conflicting results and to analyze potential confounders that caused inconsistent results in the previous studies, as well as to determine optimal future study designs. In addition, as pioglitazone use may be related to other malignancies as well24,27, risk for other cancers types were also evaluated in the study.
Methods
Literature Search
Embase, PubMed, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov were searched from inception through Jan 5, 2017 without language restriction. Two independent reviewers (Y.W. and R.Z.) searched and selected studies separately. Disagreements were resolved by discussion between the reviewers, and if necessary, consultation with other authors included in this study. Our search strategy included the following terms pertinent to pioglitazone: peroxisome proliferator activated receptor agonist/activator, PPAR, thiazolidinediones, TZDs, pioglitazone, Actos; and terms pertinent to cancer: cancer, tumor, carcinoma, neoplasm, malignancy (eAppendix 1 in the supplement). References of relevant studies were manually screened for eligible sources of data.
Inclusion and Exclusion Criteria
All human studies that evaluated patients with DM, reported pioglitazone therapy, and provided cancer outcomes were included. Observational (OB) studies and randomized controlled trials (RCTs) that provided either relative risk estimates such as risk ratios (RR), hazard ratios (HR) or odds ratios (OR) and 95% confidence intervals (CI) for cancers or raw data were eligible. Studies with the greatest number of patients and the latest publications were selected when overlapping subjects were included in more than one study. Trials reporting serious adverse events or adverse events related to cancer following pioglitazone exposure were also included.
We excluded duplicate reports and abstracts from meeting proceedings. Studies were also excluded if they were animal research, reviews, comments or replies.
Data Extraction
Three reviewers (Y.W., H.Q. and R.Z.) independently extracted data from the primary texts and supplementary appendixes of all trials. Disagreements among the three reviewers were resolved by discussion, and if necessary, consultation with the other two reviewers (Y.Z. and H.Z.). The following data were collected for OB studies: authors, year of publication, age and sex, sample size, number of cancer events in both groups, RR, HR, OR with 95% CI, study type, adjustment factors, dose/duration response gradient, types of medications used in exposed and control groups, mean period of follow-up, target disease, and population region. For RCTs, data included trial registry number, number of study sites, study phase, number of cancer events in both groups, types of medications used in interventional and control groups, duration of follow-up, target disease, and population region.
For relative risk estimates of each study, we selected the most adjusted value (that is, the multivariable association measure with the highest number of covariates, to reduce the biases as much as possible) and corresponding 95% CI, in addition to raw events data. Unadjusted estimates were selected if the outcome was not adjusted for any variable.
Quality Assessment
Three reviewers (Y.W., R.Z. and H.Q.) independently assessed the quality of all studies. The Newcastle-Ottawa quality assessment scale28 was used to assess the risk of bias of cohort studies and case-control studies. The highest-quality score was 9 (maximum), and studies with scores ≥7 were considered as having a low risk of bias, scores of 4–6 as having a moderate risk of bias, and scores <4 as having a high risk of bias. The item “was follow-up long enough for outcomes to occur” for cohort studies was removed owing to the adequate duration of follow-up is uncertain and was analyzed as a result of this study. The Cochrane Collaboration’s tool29 was used to assess the risk of bias of RCTs. The judgmental items were “random sequence generation”, “allocation concealment”, “blinding of participants and personnel”, “blinded assessment of bladder cancer events”, “incomplete outcome data”, and “selective reporting”.
Definitions
The primary outcomes were included to examine the association between pioglitazone use and bladder cancer risk and whether this association varied based on the study design (i.e., study type, adjusted factors, intervention measures, comparator agents, follow-up duration, and study population)30. The secondary outcome was defined as the relationship between pioglitazone use and other cancer risks.
Statistical Analysis
As the incidence rates of cancers involved in our study are relatively rare (<5%) in overall and subgroup analyses, the distinctions among the RR, HR, and OR can be ignored31. We pooled relative risk estimates and reported pooled OR with corresponding 95% CI using random effects models according to the methodology proposed by DerSimonian and Laird32, with weights calculated by the inverse variance method in cases of heterogeneity. Otherwise, fixed-effects models were used. Subgroup analyses were conducted to explore whether the association between pioglitazone use and bladder cancer risk were varied by study design.
The MOOSE guidelines33 for meta-analysis were followed, and PRISMA criteria34 were performed for reporting our meta-analysis. Publication bias was evaluated visually by funnel plots and quantified by the Egger’s test and the Begg’s test35,36. Heterogeneity across trials was assessed by theI 2 statistic, with values greater than 50% indicating significant statistical heterogeneity37. If significant statistical heterogeneity was detected, a sensitivity analysis was conducted using the “leave one out” approach38 to identify the source of heterogeneity. This “leave one out” approach was also used for sensitivity analysis to detect the influence of a single study on the overall bladder cancer risk. In addition, sensitivity analyses were also conducted by study quality analysis, which was restricted to the highest-quality studies with Newcastle-Ottawa scale scores of 8–9, and to test the robustness of overall bladder cancer risk and subgroup analysis.
Results with 2-sided P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using Stata Statistical Software: version 12.0 (STATA Corp, College Station, TX).
Results
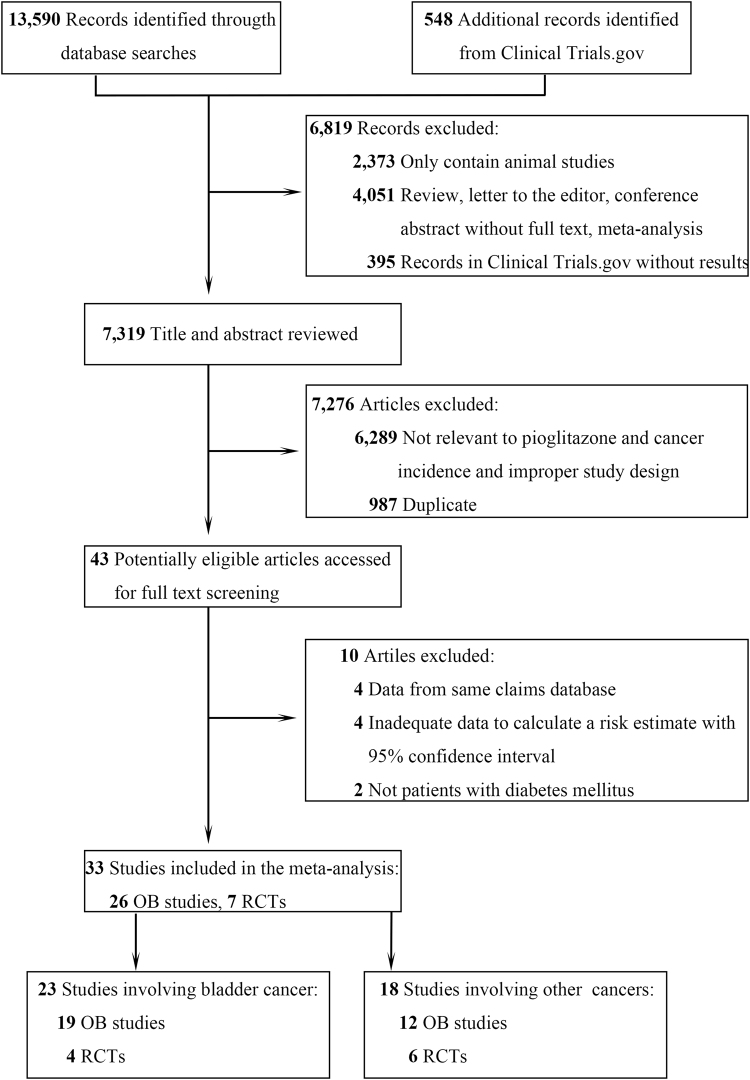
Figure 1 outlines the procedure used for the literature search. For analysis of the primary outcomes (see Definitions in Methods), 19 OB studies9,12–22,24–27,39–41 and 4 RCTs (including clinical trials NCT00494312 and NCT00736099)10,42 published between 1986 and 2016 were identified. From the OB studies, 2,764,731 participants were included. Of these participants, 343,176 (12.4%) were exposed to pioglitazone, and 13,264 (0.5%) developed bladder cancer. The mean period of follow-up ranged from 2.1 to 7.9 years. In the RCTs, 9,999 participants were included. Of these participants, 4,515 (45.2%) were exposed to pioglitazone, and 24 (0.2%) developed bladder cancer. The mean period of follow-up ranged from 72 weeks to 48 months (eTable 1 and eReferences in the Supplement). For analysis of the secondary outcome (see Definitions in Methods), 12 OB studies9,13,17,24,27,39,43–48 and 6 RCTs (including clinical trials NCT00736099, NCT00676338, NCT00879970, and NCT00637273)10,42 were identified, and 17 other site-specific cancers were assessed.
Figure 1.
Flowchart of the Literature Search and Study Selection Abbreviations: OB, observational studies. RCTs, randomized controlled trials.
Part 1: Analysis with observational studies
Pioglitazone and overall bladder cancer risk at the global level
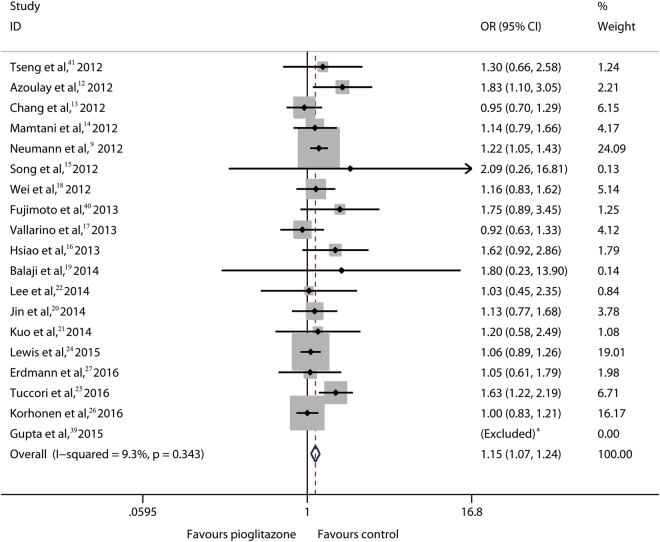
First, we conducted a pooled analysis to determine whether pioglitazone use was associated with the bladder cancer risk at the global level. To date, a majority of the studies related to this topic are OB studies. Only 4 RCTs were identified; thus, at first the OB studies were analyzed. The results showed that the risk for bladder cancer risk increased by 15% (OR, 1.15; 95% CI, 1.07–1.24; P < 0.001) (Fig. 2 ), indicating that pioglitazone use was associated with overall bladder cancer risk based on the existing evidence.
Figure 2.
Bladder Cancer Risks Related to Pioglitazone Use Versus Control for Patients with DM in Global from OB Studies Abbreviations: DM, diabetes mellitus. aGiven that both the exposed and control groups did not report bladder cancer incidence, the OR was not estimable.
Study type and pioglitazone related bladder cancer risk at the global level
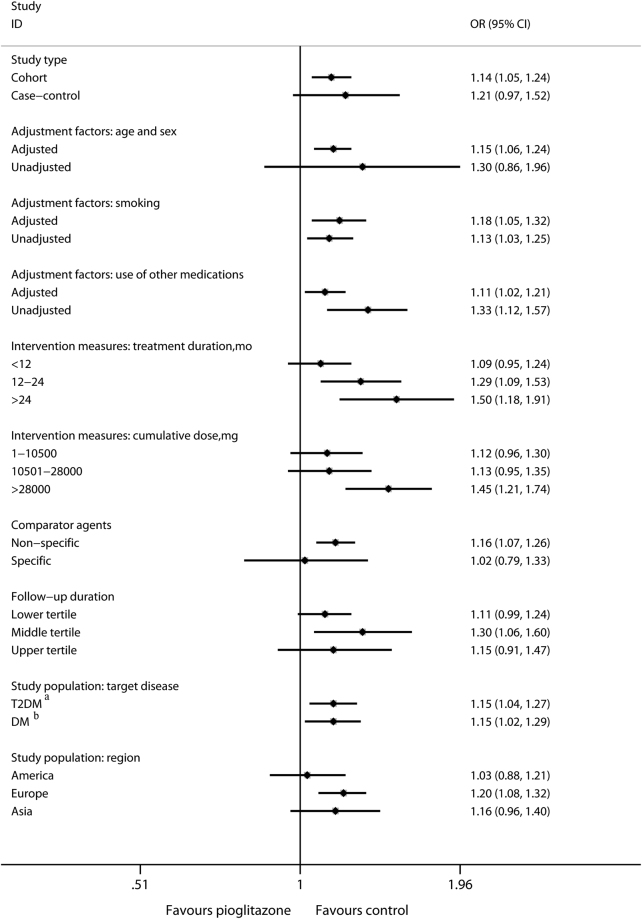
Next, to explore the reason behind the inconsistent outcomes observed in previous individual studies, the potential confounders for study outcome, which are generally derived from study design (study type, adjustment factors, intervention measures, comparator agents, follow-up duration, and study population)30, were analyzed. In terms of the study type, cohort studies showed a positive result with regard to the relationship between pioglitazone and bladder cancer risk (OR, 1.14; 95% CI, 1.05–1.24; P = 0.001), whereas case control studies showed negative results (OR, 1.21; 95% CI, 0.97–1.52; P = 0.10) (Fig. 3 ), suggesting that study type might be a confounder for the global outcomes.
Figure 3.
Subgroup Analyses of Bladder Cancer Risk Related to Pioglitazone Use Versus Control for Patients with DM in Global from OB Studie. Abbreviations: T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes mellitus. aWith T1DM excluded. bWith T1DM included.
Adjustment factors and pioglitazone related bladder cancer risk at the global level
With regard to adjustment factors, the main cancer risk factors that are commonly adjusted in medication related cancer risk studies include age/sex, smoking, unhealthy diet and lack of physical activity, and the use of other medications49. Although the risk factor “unhealthy diet and lack of physical activity” could not be analyzed (refer to the limitations list in the Discussion section for the reasons), the remaining 3 factors were evaluated. The adjustment for both smoking and use of other medications did not influence the outcomes (smoking: adjusted, OR, 1.18; 95% CI, 1.05–1.32; P = 0.006 vs. non-adjusted, OR, 1.13; 95% CI, 1.03–1.25; P = 0.01; use of other medications: adjusted, OR, 1.11; 95% CI, 1.02–1.21; P = 0.02 vs. non-adjusted, OR, 1.33; 95% CI, 1.12–1.57; P = 0.001), whereas adjustment for age/sex significantly affected the results (adjusted: OR, 1.15; 95% CI, 1.06–1.24; P = 0.001 vs. non-adjusted: OR, 1.30; 95% CI, 0.86–1.96; P = 0.22) (Fig. 3 ). These results indicated that adjustment for age/sex might also be a confounder that affected previous outcomes, and the results of the studies adjusted for age/sex seem more credible.
Intervention measures and pioglitazone related bladder cancer risk at the global level
With regard to intervention measures, administration routes as well as the treatment duration and cumulative dose were commonly considered50–52. However, pioglitazone is only orally administrated, only the duration and cumulative dose were assessed in this study. Of the included OB trials, in 13, the duration or cumulative dose-response relation was evaluated, and in 8, the unified categories were used. These 8 trials were analyzed. Increased risks of bladder cancer were identified in longer duration and larger cumulative dose subgroups (duration: 12–24 months, OR, 1.29; 95% CI, 1.09–1.53; P = 0.003; >24 months, OR, 1.50; 95% CI, 1.18–1.91; P = 0.001; cumulative dose: >28,000 mg, OR, 1.45; 95% CI, 1.21–1.74; P < 0.001), while the shorter duration and smaller cumulative dose subgroups did not increased risks of bladder cancer (duration: <12 months, OR, 1.09; 95% CI, 0.95–1.24; P = 0.21; cumulative dose: 1–10,500 mg, OR, 1.12; 95% CI, 0.96–1.30; P = 0.17; 10,501–28,000 mg, OR, 1.13; 95% CI, 0.95–1.35; P = 0.16) (Fig. 3 ). These results indicated that the treatment duration and cumulative dose might also be confounders, and that long durations and large cumulative doses of pioglitazone use should be carefully monitored.
Comparator agents and pioglitazone-related bladder cancer risk at the global level
The medication used in the control group can also be a potential confounder53. Of the included OB trials, non-specific agents were used in the control groups of 17 studies, and specific agents (insulin and rosiglitazone) were reported in 2. A positive relationship was identified in the non-specific comparator subgroup (OR, 1.16; 95% CI, 1.07–1.26; P < 0.001), whereas the result was negative in the specific comparator subgroup (OR, 1.02; 95% CI, 0.79–1.33; P = 0.86) (Fig. 3 ), indicating that comparator agents might influence the outcomes. Moreover, insulin and rosiglitazone have been reported to potentially influence bladder cancer risks54,55, this likely leads to the incorrect estimation of the association between pioglitazone use and bladder cancer risk. Thus, the results of studies that used non-specific agents as the control group might be more reasonable.
Follow-up duration and pioglitazone related bladder cancer risk at the global level
The follow-up duration mainly refers to the mean period of follow-up, as the maximum period cannot describe the maturity of the study data and the quality of follow-up56. This factor was analyzed using the tertiles method (the lower and upper cut-off points were 3.6 and 4.8 years, respectively) in our study. The middle tertile exhibited a positive association between pioglitazone and bladder cancer risk (OR, 1.30; 95% CI, 1.06–1.60; P = 0.01), whereas the lower (OR, 1.11; 95% CI, 0.99–1.24; P = 0.08) and upper tertiles (OR, 1.15; 95% CI, 0.91–1.47; P = 0.25) did not show any association (Fig. 3 ), indicating that the follow-up duration might influence the outcomes. However, the pooled result of the upper tertile was inexplicable at the global level.
Study population and pioglitazone related bladder cancer risk at the global level
The study population commonly includes the target disease and the population region57. In terms of target disease, the relationship between pioglitazone use and bladder cancer risk was positive both in the T2DM (with T1DM excluded) and DM (with T1DM included) subgroups (T2DM: OR, 1.15; 95% CI, 1.04–1.27; P = 0.006 vs. DM: OR, 1.15; 95% CI, 1.02–1.29; P = 0.02) (Fig. 3 ). However, for the population region, the European region exhibited an increased risk of bladder cancer after pioglitazone exposure (OR, 1.20; 95% CI, 1.08–1.32; P < 0.001), whereas the results in both American (OR, 1.03; 95% CI, 0.88–1.21; P = 0.68) and Asian (OR, 1.16; 95% CI, 0.96–1.40; P = 0.12) regions were negative (Fig. 3 ), indicating that the population region might be a confounder at the global level.
Study design and pioglitazone related bladder cancer risk in different population regions
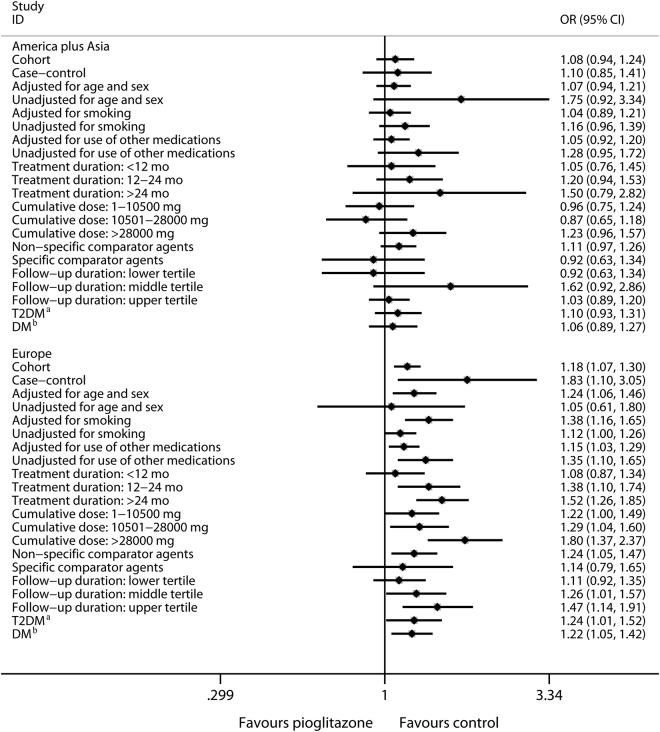
The population region is a confounder that influences outcomes objectively58–60. Therefore, we re-analyzed the potential confounders in different population regions. For American and Asian regions, the results were negative (P > 0.05), further confirming the negative pooled results (Fig. 4, Table 1 ). In the European region, the results were positive (P < 0.05) except for the subgroups unadjusted for age/sex (OR, 1.05; 95% CI, 0.61–1.80; P = 0.86), moking (OR, 1.12; 95% CI, 1.00–1.26; P = 0.052), the shortest treatment duration (OR, 1.08; 95% CI, 0.87–1.34; P = 0.49) and follow-up period (OR, 1.11; 95% CI, 0.92–1.35; P = 0.28), and specific agent (rosiglitazone) used in the control group (OR, 1.14; 95% CI, 0.79–1.65; P = 0.49). As mentioned above, the results of studies adjusted for age/sex and smoking, with longer treatment durations, and non-specific agents used in the control group were considered more credible; therefore, the positive results achieved in these subgroups in both Europe and globally further confirmed the positive pooled European and global results (Fig. 4, Table 1). Interestingly, the subgroup results of the longest follow-up were positive in the European populations (OR, 1.47; 95% CI, 1.14–1.91; P = 0.003) but negative in the American and Asian populations (OR, 1.03; 95% CI, 0.89–1.20; P = 0.68). This finding might explain the inexplicable negative result of the longest follow-up subgroup at global level (Fig. 4, Table 1). Moreover, when the bladder cancer risk related to pioglitazone was considered based on different population regions, the study type no longer influenced the outcomes (Fig. 4, Table 1). Taken together, these results indicated that a positive relationship between pioglitazone and bladder cancer risk may be present in the European population. Thus, it is important to pay more attention to European patients, especially those in whom the treatment duration is long; the long-term effects of pioglitazone need to be monitored closely in these cases. In addition, future related studies should adjust for age/sex and smoking, and adopt non-specific agents in the control group.
Figure 4.
Subgroup Analyses of Bladder Cancer Risk Related to Pioglitazone Use Versus Control for Patients with DM in Europe, and America plus Asia, Respectively from OB Studies. aWith T1DM excluded. bWith T1DM included.
Table 1.
Bladder Cancer Risk Related to Pioglitazone Use in Patients with DM Compared Globally and Regionally from OB Studies.
| Global | Sub-regional | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomea | OR (95% CI) | P-Value | American plus Asian Regions | European Region | |||||
| Outcomea | OR (95% CI) | P-Value | Outcomea | OR (95% CI) | P-Value | ||||
| Study type | |||||||||
| Cohort | + | 1.14 (1.05, 1.24) | 0.001 | — | 1.08 (0.94, 1.24) | 0.26 | + | 1.18 (1.07, 1.30) | 0.001 |
| Case-control | — | 1.21 (0.97, 1.52) | 0.10 | — | 1.10 (0.85, 1.41) | 0.47 | + | 1.83 (1.10, 3.05) | 0.02 |
| Adjustment factors | |||||||||
| Age and sex | |||||||||
| Adjusted | + | 1.15 (1.06, 1.24) | 0.001 | — | 1.07 (0.94, 1.21) | 0.30 | + | 1.24 (1.06, 1.46) | 0.009 |
| Unadjusted | — | 1.30 (0.86, 1.96) | 0.22 | — | 1.75 (0.92, 3.34) | 0.09 | — | 1.05 (0.61, 1.80) | 0.86 |
| Smoking | |||||||||
| Adjusted | + | 1.18 (1.05, 1.32) | 0.006 | — | 1.04 (0.89, 1.21) | 0.64 | + | 1.38 (1.16, 1.65) | < 0.001 |
| Unadjusted | + | 1.13 (1.03, 1.25) | 0.01 | — | 1.16 (0.96, 1.39) | 0.13 | — | 1.12 (1.00, 1.26) | 0.052 |
| Use of other medications | |||||||||
| Adjusted | + | 1.11 (1.02, 1.21) | 0.02 | — | 1.05 (0.92, 1.20) | 0.45 | + | 1.15 (1.03, 1.29) | 0.01 |
| Unadjusted | + | 1.33 (1.12, 1.57) | 0.001 | — | 1.28 (0.95, 1.72) | 0.11 | + | 1.35 (1.10, 1.65) | 0.004 |
| Intervention measures | |||||||||
| Treatment duration, mo | |||||||||
| < 12 | — | 1.09 (0.95, 1.24) | 0.21 | — | 1.05 (0.76, 1.45) | 0.78 | — | 1.08 (0.87, 1.34) | 0.49 |
| 12–24 | + | 1.29 (1.09, 1.53) | 0.003 | — | 1.20 (0.94, 1.53) | 0.14 | + | 1.38 (1.10, 1.74) | 0.006 |
| > 24 | + | 1.50 (1.18, 1.91) | 0.001 | — | 1.50 (0.79, 2.82) | 0.21 | + | 1.52 (1.26, 1.85) | < 0.001 |
| Cumulative dose, mg | |||||||||
| 1–10500 | — | 1.12 (0.96, 1.30) | 0.17 | — | 0.96 (0.75, 1.24) | 0.78 | + | 1.22 (1.00, 1.49) | 0.048 |
| 10501–28000 | — | 1.13 (0.95, 1.35) | 0.16 | — | 0.87 (0.65, 1.18) | 0.37 | + | 1.29 (1.04, 1.60) | 0.02 |
| > 28000 | + | 1.45 (1.21, 1.74) | < 0.001 | — | 1.23 (0.96, 1.57) | 0.10 | + | 1.80 (1.37, 2.37) | < 0.001 |
| Comparator agents | |||||||||
| Agents use in control group | |||||||||
| Specific | + | 1.16 (1.07, 1.26) | < 0.001 | — | 1.11 (0.97, 1.26) | 0.12 | + | 1.24 (1.05, 1.47) | 0.01 |
| Non-specific | — | 1.02 (0.79, 1.33) | 0.86 | — | 0.92 (0.63, 1.34) | 0.66 | — | 1.14 (0.79, 1.65) | 0.49 |
| Follow-up Duration | |||||||||
| Mean period of follow-up, y | |||||||||
| Lower tertile, < 3.6 | — | 1.11 (0.99, 1.24) | 0.08 | — | 0.92 (0.63, 1.34) | 0.66 | — | 1.11 (0.92, 1.35) | 0.28 |
| Middle tertile, 3.6–4.8 | + | 1.30 (1.06, 1.60) | 0.013 | — | 1.62 (0.92, 2.86) | 0.10 | + | 1.26 (1.01, 1.57) | 0.04 |
| Upper tertile, ≥ 4.8 | — | 1.15 (0.91, 1.47) | 0.25 | — | 1.03 (0.89, 1.20) | 0.68 | + | 1.47 (1.14, 1.91) | 0.003 |
| Study population | |||||||||
| Target disease | |||||||||
| T2DMb | + | 1.15 (1.04, 1.27) | 0.006 | — | 1.10 (0.93, 1.31) | 0.24 | + | 1.24 (1.05, 1.47) | 0.01 |
| DMc | + | 1.15 (1.02, 1.29) | 0.02 | — | 1.06 (0.89, 1.27) | 0.48 | + | 1.14 (0.79, 1.65) | 0.49 |
| Study population region | |||||||||
| America | — | 1.03 (0.88, 1.21) | 0.68 | NA | NA | NA | NA | NA | NA |
| Europe | +1 | 1.20 (1.08, 1.32) | < 0.001 | NA | NA | NA | NA | NA | NA |
| Asia | — | 1.16 (0.96, 1.40) | 0.12 | NA | NA | NA | NA | NA | NA |
Abbreviations: DM, diabetes mellitus; OB, observational studies; T2DM, type 2 diabetes; T1DM, type 1 diabetes; NA, not available.
aThe outcome includes statistical increased risk of bladder cancer ( + ) and non-association (-) related to pioglitazone use.
bWith T1DM excluded.
cWith T1DM included.
Pioglitazone and other cancer risks
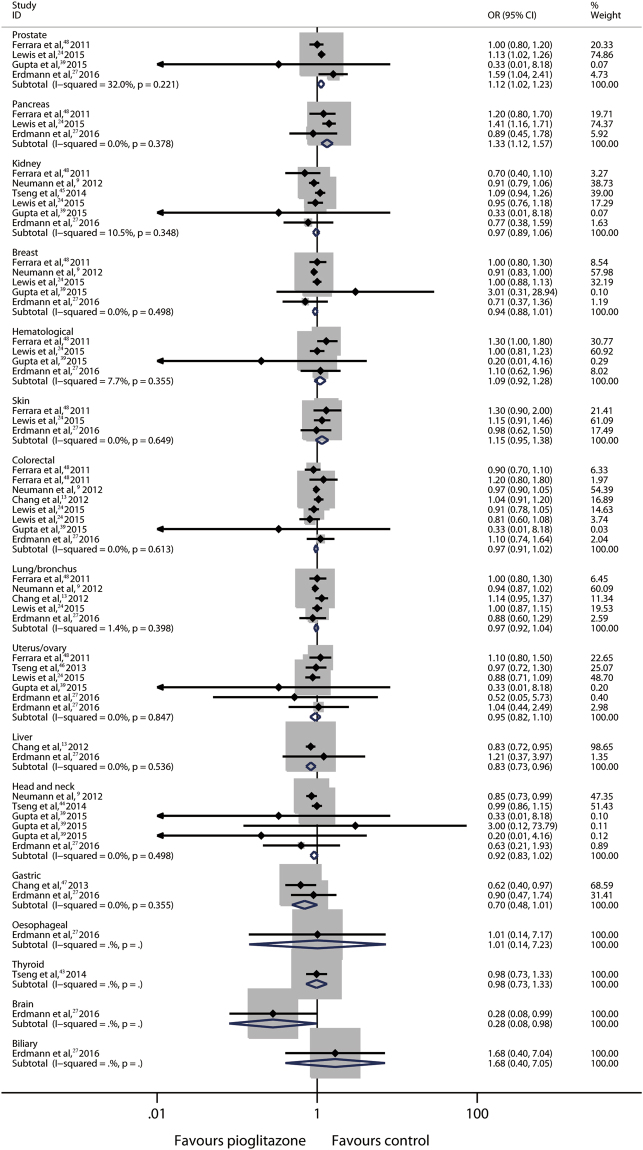
In addition to bladder cancer, pioglitazone exposure has also been reported to be associated with prostate and pancreatic cancer risks24,27. To examine the relationship between pioglitazone and the other cancers types, 16 site-specific cancers assessed in 12 related OB studies were analyzed. However, one-third of the results of the initial analysis yielded significant heterogeneities (I 2 > 50%, esophagus, thyroid, brain and biliary cancers were only reported by one study each, and it was not possible to test heterogeneity, eFigure 1 in the Supplement). Therefore, sensitivity analyses were conducted using the “leave one out” approach38, and one study (Vallarino et al.17) was identified as a common contributor for these heterogeneities (I 2 decreased to ≤32% with Vallarino et al.17 excluded) (Fig. 5 ). The re-summary results showed that pioglitazone use was associated with increased risks of prostate and pancreatic cancer (prostate: OR, 1.12; 95% CI, 1.02–1.23; P = 0.02; pancreatic: OR, 1.33; 95% CI, 1.12–1.57; P = 0.001), and decreased risks of liver and brain cancer (liver: OR, 0.83; 95% CI, 0.73–0.96; P = 0.01; brain: OR, 0.28; 95% CI, 0.08–0.98; P = 0.047, with only one report) (Fig. 5 ). These results indicated that pioglitazone use might also be related to other cancer risks.
Figure 5.
Other Cancer Risks in Patients with DM Receiving Pioglitazone Versus Control from OB Studiesa. aWith Vallarino et al.17 excluded.
Part 2: Reanalysis using OB studies and RCTs
Heretofore, only 4 RCTs related to pioglitazone and bladder cancer risk and a positive pooled result of RCTs have been reported61. Therefore, we combined all the OB studies with the 4 RCTs and reanalyzed the results. Given that the 4 RCTs were not involved in the adjustment factors and intervention measures analyses, we included comparator agents, follow-up duration, and study population in the subgroup analyses. As for other cancers, 17 site-specific cancers were analyzed. All the re-analytical results with OB studies plus RCTs were similar to those obtained from the OB studies (eFigure 2–5 in the Supplement).
Sensitivity Analysis
For overall bladder cancer risk, sensitivity analyses were performed both using “study quality” analysis (eTable 2 in the supplement) and using the “leave one out” approach, and none of the results showed significant changes. For the subgroup analyses of bladder cancer risk, “study quality” sensitivity analysis was conducted, and the results remained similar. With regard to risks for other cancer types, a sensitivity analysis was conducted using the “leave one out” approach, the results of which are presented in the “Pioglitazone and other cancer risks” section of the Results.
Publication Bias Analysis
No significant bias was detected by Egger’s test (P = 0.22) or Begg’s test (P = 0.57 [continuity corrected]) (eFigure 6, 7 in the supplement).
Discussion
Global safety concerns about the association of pioglitazone use with bladder cancer risk have been present and debated since the PROspective pioglitAzone Clinical Trial in macroVascular Events (PROactive) study was conducted, more than a decade ago10. Recently, the discordant results obtained from several large-population studies further increased the uncertainty24–26. Here, we identified certain regional differences that might be extremely important factors that contribute to the inconsistent outcomes of the previous studies.
Our analysis suggested that pioglitazone use was associated with bladder cancer risk at the global level (15% increase), which was consistent with the results of most of the previous meta-analyses (17% to 23% increase)051,52,61–64. In addition, the negative results were achieved from the subgroups unadjusted for age/sex, short treatment durations and small cumulative doses, and the specific agents (insulin and rosiglitazone) used as comparators during the stratified analysis with potential confounders. These also strengthen the positive relationship, as adjusted results are more credible, and diabetic patients require long-term treatment65, and hence insulin and rosiglitazone may influence bladder cancer risk54,55.
Most importantly, our results indicated for the first time that the association of pioglitazone use and bladder cancer risk exhibits a significant regional variation. While the pooled and stratified results were negative in the American and Asian regions, results from studies conducted in the European region showed a striking positivity from the pooled and most of the stratified analyses. Although the subgroups unadjusted for age/sex and smoking, short treatment duration and follow-up period, and rosiglitazone used in the control group showed negative results, based on the reasons mentioned above, the results further confirmed a positive relationship in European populations. This regional difference might explain the discordant results of several previous studies, such as the KPNC and UKCPRD24,25. Similarly, the risk factors for stroke, disabling sequelae from bacterial meningitis, and obesity were also reported to have regional differences58–60. Interestingly, when the relationship was considered in terms of different population regions, the study type no longer influenced the outcomes, and the inexplicable results of follow-up duration analysis at the global level could be interpreted reasonably. Based on these findings, we suggest that the bladder cancer risk related to pioglitazone should be considered according to the different population regions, and the European population require more attention. Notably, although we identified the regional differences across continents, the possibility of variation among different countries and regions may exist. Whether the regional differences that we observed in this study are associated with race and ethnicity remain unclear, as only the KPNC study24 described the racial composition of its study population. Further studies are required to clarify these issues.
Moreover, when performing future studies, the authors need to be aware of the long-term effects of prolonged pioglitazone use and should adjust for age/sex and smoking and adopt non-specific agents in the control group. Although our results indicated that the study design did not influence the outcomes of the American and Asian populations, it still had an effect on the European population. In the European population, positive results were achieved with long treatment durations (>12 months) and follow-up periods (≥3.6 years); therefore, we should pay more attention to this population. In addition, adjustment for age/sex and smoking, and the specific comparator agent (rosiglitazone) influenced the outcomes; therefore, these factors should also be noticed in future related studies.
Our results indicated that pioglitazone use might also be related to increased risk for other cancer types, such as prostate and pancreatic cancer, which is consistent with the observations of the KPNC study24, and decreased risks for liver and brain cancer. Additionally, one study recently suggested that pioglitazone might influence cancer progression66. However, studies on these other cancers are very limited and more studies are needed to answer several key questions in this field: Does pioglitazone influence the risk and/or progression of other cancers? Does the association between pioglitazone use and risk for other cancer types show regional differences? If so, what is the mechanism behind the role of pioglitazone in tumorigenesis?
Our study has important implications in future clinical practice and trials in that (1) more attention regarding bladder cancer risk related to pioglitazone should be given to European, especially those with long treatment durations and a large cumulative dose; (2) studies should adjust for age/sex and smoking, and adopt non-specific agents in the control group; and (3) the risks of other site-specific cancers, such as prostate, pancreatic, liver and brain cancer, need to be addressed.
These are several limitations to our study. First, among the main cancer risk factors, the factor “unhealthy diet and lack of physical activity” was not analyzed because it was reported by only one study12 in the form of alcohol intake. Second, some factors that were adjusted for in previous individual trials were not analyzed for the following reasons. Only one18 and two trials22,24 have adjusted for body mass index (BMI) and income, respectively; therefore, the data are inadequate for meaningful analyses. The comorbidities selected for adjustment different from trial to trial, and the diabetes duration and date of cohort entry were defined inconsistently among the trials9,12–22,24–27,39–41; thus, these 3 factors were also unsuitable for analysis. Only 3 trials adjusted for hemoglobin A1c concentration (HbA1c)12,24,25. Although the analyzed results were accordant with our conclusions, more data might be required for further analysis. The results obtained from analysis at the global level showed that adjustment for HbA1c affected the outcome (adjusted: OR, 1.40; 95% CI, 0.98–2.02; P = 0.07 vs. non-adjusted: OR, 1.12; 95% CI, 1.03–1.23; P = 0.01, efigure 8 in the supplement). However, only 3 adjusted trials were included, of which 212 , 25 were conducted in Europe and 124 was conducted in America; thus, this regional difference might contribute to the negative pooled results of the adjusted trials at the global level. When the association was analyzed by region, the results of both the HbA1c-adjusted and unadjusted groups were positive in the European regions (adjusted: OR, 1.68; 95% CI, 1.30–2.16; P < 0.001 vs. non-adjusted: OR, 1.13; 95% CI, 1.01–1.25; P = 0.03) but negative in the American and Asian regions (adjusted: OR, 1.06; 95% CI, 0.89–1.26; P = 0.51 vs. non-adjusted: OR, 1.11; 95% CI, 0.94–1.31; P = 0.23) (efigure 9 in the supplement), which further confirmed our conclusions. Third, the variation of diagnostic criteria for bladder cancer and other cancers among these studies could not be assessed in our study. Finally, most of the included studies were observational in design and patient-level data for each of the studies could not be obtained due to authorization limit. However, most of these studies have adjusted for major potential confounders, such as age/sex, smoking, and comorbidities, for bias control.
Conclusions
In summary, the risk of bladder cancer with pioglitazone use might needs to be considered in the European population, and patients with a longer treatment duration (>12 months) or a larger cumulative dose (>28,000 mg) might should be followed up more carefully. The long-term effects (≥3.6 years) of pioglitazone might also need to be noted. In addition, future related studies should adjust for age/sex and smoking and adopt non-specific agents in the control group. Moreover, pioglitazone use may relate to risks for other cancer types.
Access to research materials
Information about how the data can be accessed is available from the corresponding author.
Electronic supplementary material
Acknowledgements
Dr HT. Zheng had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Na Wu (Department of Epidemiology, College of Preventive Medicine, Third Military Medical University, Chongqing, China), for additional statistical support, which was provided without compensation. This study was supported by grants from National Key R&D Program of China (No. 2016YFC1101100), National Natural Science Foundation of China (No. 81471039, No. 81270893, No. 81228023, No. 81401601, and No. 81402202) and the Natural Science Foundation Project of Chongqing (CSTC2014jcyjjq10006, CSTC2012jjB10023, and CSTC2016jcyjA0518). Role of the Sponsors: The finding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author Contributions
Study concept and design: H.Q., Y.Z., Y.W., H.Z. Acquisition, analysis, or interpretation of data: H.Q., Y.Z., Y.W., H.Z. Drafting of the manuscript: H.Q., Y.Z. Critical revision of the manuscript for important intellectual content: X.R., Z.L., H.Z. Statistical analysis: Y.W., H.Q., R.Z. Obtained funding: Y.Z., H.Z. Administrative, technical, or material support: G.Y., H.Z. Study supervision: H.Z.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Hua Qu, Yi Zheng and Yuren Wang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16074-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet. 1999;354:141–148. doi: 10.1016/S0140-6736(98)10364-1. [DOI] [PubMed] [Google Scholar]
- 2.Campbell IW. The Clinical Significance of PPAR Gamma Agonism. Curr Mol Med. 2005;5:349–363. doi: 10.2174/1566524053766068. [DOI] [PubMed] [Google Scholar]
- 3.Bjornsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–38. doi: 10.1016/j.dld.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Mitka M. Panel recommends easing restrictions on rosiglitazone despite concerns about cardiovascular safety. JAMA. 2013;310:246–247. doi: 10.1001/jama.2013.8141. [DOI] [PubMed] [Google Scholar]
- 5.Woodcock J, Sharfstein JM. & Hamburg, M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med. 2010;363:1489–1491. doi: 10.1056/NEJMp1010788. [DOI] [PubMed] [Google Scholar]
- 6.Cusi K, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 7.Kernan WN, et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis JD, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann A, et al. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953–1962. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dormandy JA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 11.Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011;54:2009–2015. doi: 10.1007/s00125-011-2171-z. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay L, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ. 2012;344:e3645. doi: 10.1136/bmj.e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang CH, et al. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology. 2012;55:1462–1472. doi: 10.1002/hep.25509. [DOI] [PubMed] [Google Scholar]
- 14.Mamtani R, et al. Association between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort study. J Natl Cancer Inst. 2012;104:1411–1421. doi: 10.1093/jnci/djs328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song SO, et al. The risk of bladder cancer in korean diabetic subjects treated with pioglitazone. Diabetes Metab J. 2012;36:371–378. doi: 10.4093/dmj.2012.36.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao FY, Hsieh PH, Huang WF, Tsai YW, Gau CS. Risk of bladder cancer in diabetic patients treated with rosiglitazone or pioglitazone: a nested case-control study. Drug Saf. 2013;36:643–649. doi: 10.1007/s40264-013-0080-4. [DOI] [PubMed] [Google Scholar]
- 17.Vallarino C, et al. Comparing pioglitazone to insulin with respect to cancer, cardiovascular and bone fracture endpoints, using propensity score weights. Clin Drug Investig. 2013;33:621–631. doi: 10.1007/s40261-013-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2012;75:254–259. doi: 10.1111/j.1365-2125.2012.04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balaji V, Seshiah V, Ashtalakshmi G, Ramanan SG, Janarthinakani M. A retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427. doi: 10.4103/2230-8210.131223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin SM SS, Jung CH. Risk of bladder cancer among patients with diabetes treated with a 15 mg pioglitazone dose in Korea: a multi-center retrospective cohort study. J Korean Med Sci. 2014;29:5. doi: 10.3346/jkms.2014.29.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo H W TM, Ho SC, Yang CY. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:4. doi: 10.1016/j.kjms.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Lee MY, Hsiao PJ, Yang YH, Lin KD, Shin SJ. The association of pioglitazone and urinary tract disease in type 2 diabetic Taiwanese: bladder cancer and chronic kidney disease. PLoS One. 2014;9:e85479. doi: 10.1371/journal.pone.0085479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin D, et al. Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia. 2015;58:493–504. doi: 10.1007/s00125-014-3456-9. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JD, et al. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA. 2015;314:265–277. doi: 10.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 25.Tuccori M, et al. Pioglitazone use and risk of bladder cancer: population based cohort study. BMJ. 2016;352:i1541. doi: 10.1136/bmj.i1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korhonen, P. et al. Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries. Bmj354 (2016). [DOI] [PMC free article] [PubMed]
- 27.Erdmann E, Harding S, Lam H, Perez A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes Metab. 2016;18:266–273. doi: 10.1111/dom.12608. [DOI] [PubMed] [Google Scholar]
- 28.Wells, G.A. et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 29.Higgins JP, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Report No. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, et al. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ. 2016;352:i610. doi: 10.1136/bmj.i610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenhouse JB, L. S. The handbook of research synthesis and meta-analysis. 2nd edn, 423 (Russell Sage Foundation, 2009).
- 39.Gupta S, et al. Pioglitazone and the risk of bladder cancer: An Indian retrospective cohort study. Indian J Endocrinol Metab. 2015;19:639–643. doi: 10.4103/2230-8210.163187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujimoto K, et al. Possible link of pioglitazone with bladder cancer in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2013;99:e21–23. doi: 10.1016/j.diabres.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Tseng CH. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012;35:278–280. doi: 10.2337/dc11-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nissen SE, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 43.Tseng CH. Pioglitazone and thyroid cancer risk in Taiwanese patients with type 2 diabetes 2. J Diabetes. 2014;6:448–450. doi: 10.1111/1753-0407.12149. [DOI] [PubMed] [Google Scholar]
- 44.Tseng CH. Pioglitazone and oral cancer risk in patients with type 2 diabetes. Oral Oncol. 2014;50:98–103. doi: 10.1016/j.oraloncology.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Tseng CH. Pioglitazone does not affect the risk of kidney cancer in patients with type 2 diabetes. Metabolism. 2014;63:1049–1055. doi: 10.1016/j.metabol.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Tseng CH. Pioglitazone does not affect the risk of ovarian cancer: analysis of a nationwide reimbursement database in Taiwan. Gynecol Oncol. 2013;131:135–139. doi: 10.1016/j.ygyno.2013.07.087. [DOI] [PubMed] [Google Scholar]
- 47.Chang SS, Hu HY. Association of thiazolidinediones with gastric cancer in type 2 diabetes mellitus: a population-based case-control study. BMC Cancer. 2013;13:420. doi: 10.1186/1471-2407-13-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrara A, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care. 2011;34:923–929. doi: 10.2337/dc10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Cancerhttp://www.who.int/mediacentre/factsheets/fs297/en/ (2015).
- 50.Seal BS, Anderson S, Shermock KM. Factors Associated with Adherence Rates for Oral and Intravenous Anticancer Therapy in Commercially Insured Patients with Metastatic ColonCancer. J Manag Care Spec Pharm. 2016;22:227–235. doi: 10.18553/jmcp.2016.22.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Z, Shen Z, Lu Y, Zhong S, Xu C. Increased risk of bladder cancer with pioglitazone therapy in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2012;98:159–163. doi: 10.1016/j.diabres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Bosetti C, et al. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist. 2013;18:148–156. doi: 10.1634/theoncologist.2012-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 54.Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer. 2013;132:2186–2191. doi: 10.1002/ijc.27878. [DOI] [PubMed] [Google Scholar]
- 55.Han E, et al. Rosiglitazone Use and the Risk of Bladder Cancer in Patients With Type 2 Diabetes. Medicine (Baltimore) 2016;95:e2786. doi: 10.1097/MD.0000000000002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shuster JJ. Median follow-up in clinical trials. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 1991;9:191–192. doi: 10.1200/JCO.1991.9.1.191. [DOI] [PubMed] [Google Scholar]
- 57.Rohrig B, du Prel JB, Blettner M. Study design in medical research: part 2 of a series on the evaluation of scientific publications. Dtsch Arztebl Int. 2009;106:184–189. doi: 10.3238/arztebl.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Donnell MJ, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 59.Edmond K, et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–328. doi: 10.1016/S1473-3099(10)70048-7. [DOI] [PubMed] [Google Scholar]
- 60.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner RM, et al. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78:258–273. doi: 10.1111/bcp.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferwana M, et al. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med. 2013;30:1026–1032. doi: 10.1111/dme.12144. [DOI] [PubMed] [Google Scholar]
- 63.He S, et al. Pioglitazone prescription increases risk of bladder cancer in patients with type 2 diabetes: an updated meta-analysis. Tumour Biol. 2014;35:2095–2102. doi: 10.1007/s13277-013-1278-x. [DOI] [PubMed] [Google Scholar]
- 64.Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ. 2012;184:E675–683. doi: 10.1503/cmaj.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker JJ, Johnson JA, Wild SH. Diabetes treatments and cancer risk: the importance of considering aspects of drug exposure. Lancet Diabetes Endocrinol. 2013;1:132–139. doi: 10.1016/S2213-8587(13)70028-7. [DOI] [PubMed] [Google Scholar]
- 66.Darash-Yahana M, et al. Breast cancer tumorigenicity is dependent on high expression levels of NAF-1 and the lability of its Fe-S clusters. Proc Natl Acad Sci USA. 2016;113:10890–10895. doi: 10.1073/pnas.1612736113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.