SUMMARY
Objective
Previous studies have shown that Transforming growth factor-β (TGF-β)/TGFβRII-Smad3 signaling is involved in articular cartilage homeostasis. However, the role of TGF-β/ALK5 signaling in articular cartilage homeostasis has not been fully defined. In this study, a combination of in vitro and in vivo approaches was used to elucidate the role of ALK5 signaling in articular cartilage homeostasis and the development of osteoarthritis (OA).
Design
Mice with inducible cartilage-specific deletion of Alk5 were generated to assess the role of ALK5 in OA development. Alterations in cartilage structure were evaluated histologically. The expressions of genes associated with articular cartilage homeostasis and TGF-β signaling were analyzed by qRT-PCR, western blotting and immunohistochemistry. The chondrocyte apoptosis was detected by TUNEL staining and immunohistochemistry. In addition, the molecular mechanism underlying the effects of TGF-β/ALK5 signaling on articular cartilage homeostasis was explored by analyzing the TGF-β/ALK5 signaling-induced expression of proteoglycan 4 (PRG4) using specific inhibitors.
Results
Postnatal cartilage-specific deletion of Alk5 induced an OA-like phenotype with degradation of articular cartilage, synovial hyperplasia, osteophyte formation, subchondral sclerosis, as well as enhanced chondrocyte apoptosis, overproduction of catabolic factors, and decreased expressions of anabolic factors in chondrocytes. In addition, the expressions of PRG4 mRNA and protein were decreased in Alk5 conditional knockout mice. Furthermore, our results showed, for the first time, that TGF-β/ALK5 signaling regulated PRG4 expression partially through the protein kinase A (PKA)-CREB signaling pathway.
Conclusions
TGF-β/ALK5 signaling maintains articular cartilage homeostasis, in part, by upregulating PRG4 expression through the PKA-CREB signaling pathway in articular chondrocytes.
Keywords: Osteoarthritis, Chondrocytes, ALK5, PRG4, CREB
Introduction
Osteoarthritis (OA) is the most common degenerative joint disease, characterized by the progressive fibrillation and degradation of articular cartilage, synovial inflammation and hyperplasia, subchondral bone sclerosis and osteophyte formation1. Chondrocytes are the only cell type in articular cartilage, which are responsible for maintaining the cartilage homeostasis by synthesizing and degrading matrix components in response to environmental cues such as growth factors, cytokines, and biomechanical loading, etc. Failure of chondrocytes to maintain the homeostasis of articular cartilage and/or to repair its damage will lead to articular cartilage degradation2. However, the signaling pathways in chondrocytes involved in homeostasis of articular cartilage remain largely unknown.
Recently, the role of Transforming growth factor-β (TGF-β) signaling in maintaining cartilage homeostasis has received increasing and specific attention. Genetic manipulation of TGF-β pathway components demonstrate that TGF-β/TGFβRII-Smad3 signaling in chondrocytes plays a vital role in articular cartilage homeostasis and OA development3–6. However, the role of TGF-β signaling in articular cartilage homeostasis at the level of ALK5 has not been well clarified. ALK5 expression is decreased in both aging and OA articular cartilage7,8. Overexpression of ALK5 in cho006Edrocytes increases Aggrecan mRNA expression, while knockdown of Alk5 in chondrocytes induces Mmp13 mRNA expression7. Consistently, intraperitoneal injection of ALK5 inhibitor SB-505124 induces proteoglycan loss of articular cartilage in mice9. These results indicate that ALK5 may play an important role in promoting anabolic metabolism and inhibiting catabolic metabolism in chondrocytes. However, in vivo data about the role of ALK5 signaling in articular cartilage homeostasis and OA development are still lacking.
In this study, we utilized mice with inducible deletion of Alk5 specifically in chondrocytes at postnatal stage to elucidate the role of ALK5 signaling in articular cartilage homeostasis and OA development. We revealed that TGF-β/ALK5 signaling was involved in maintaining articular cartilage homeostasis partly by upregulating the expression of PRG4 through protein kinase A (PKA)-CREB signaling.
Materials and methods
Animals
Alk5flox/flox and Col2α1-CreERT2 mice were described previously10,11. Cartilage-specific and inducible Alk5 conditional knockout mice (Alk5flox/flox; Col2α1-CreERT2, hereafter referred to as Alk5Col2ERT2) were created by crossing Alk5flox/flox with Col2a1-CreERT2 mice. The resulting Alk5Col2ERT2 and Cre-negative mice were intraperitoneally injected with tamoxifen (1 mg/10 g body weight, daily for 5 days) at 2 weeks as previously described3,11. Prg4GFPCreERT2/+ mice were kindly provided by professor Matthew L. Warman12. All mice were maintained in a C57BL/6J background. Animal experiments were performed according to protocols approved by the Laboratory Animal Welfare and Ethics Committee of the Third Military Medical University (Chongqing, China).
Histologic assessment
The knee joints isolated from 2-, 3- and 6-month-old mice were fixed in 4% paraformaldehyde overnight, decalcified in 20% formic acid, and embedded in paraffin. Serial sagittal sections were obtained across the medial knee joints and three 5-μm sections were placed on each slide at 50-μm intervals. Each knee yielded about 8–10 slides were stained with Safranin O/Fast Green to assess cartilage destruction by two blinded observers as previously described13. Histologic grading of cartilage degeneration was performed using the Osteoarthritis Research Society International (OARSI) recommended subjective scoring system14. The severity of cartilage destruction was expressed as the summed score (sum of the four highest scores in all slides) and maximal score for the medial femora and medial tibiae separately within each joint. Three sections from each knee were used to measure the thicknesses of articular cartilage (from the articular surface to the chondroosseous junction) by Image J software, and the final measurement is an average of these three sections as previously described15.
Immunohistochemistry
Immunohistochemistry (IHC) was performed with the SP-9000 Histostain-Plus kits (ZSGB-BIO) according to the manufacturer’s instructions. The following antibodies were used: ALK5 (1:100; Santa Cruz Biotechnology), pSmad3 (1:100; Cell Signaling Technology), MMP13 (1:200; Proteintech), ADAMTS5 (1:200; Abcam), AGGRECAN (1:200; Millipore), Cleaved caspase 3 (1:100; Boster), PRG4 (1:500; Abcam), pCREB (1:100; Abcam).
Apoptosis assay
In situ cell death detection kit (Roche) was used to detect apoptotic chondrocytes in articular cartilage according to the manufacturer’s instruction.
Isolation, culture and treatment of femoral head cartilage and primary chondrocytes
Femoral head cartilage and chondrocytes were isolated from 4-week-old mice as previously described16,17. At least three femoral head cartilage were pooled as one sample.
Femoral head cartilage isolated from Alk5Col2ERT2 and Cre-negative mice was cultured in tissue culture method and treated with 1 μM 4-hydroxy tamoxifen (4-OH TM) (Sigma–Aldrich) for 72 h to drive Cre-mediated recombination and delete Alk5, then treated with TGF-β1 (Peprotech) or forskolin (Selleck) for indicated time.
Femoral head cartilage isolated from Prg4GFPCreERT2/+ mice was treated with TGF-β1 with/without other chemical compounds, ALK5 inhibitor SB-505124 (Selleck), PKA inhibitor H89 (Beyotime), CBP-CREB interaction inhibitor (Millipore), for indicated experiments.
Primary chondrocytes were cultured in monolayer cell culture method and used in transfection and phosphorylation experiments.
RNA isolation and quantitative real-time PCR analysis (qRT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen). The expressions of genes were measured with Mx3000P PCR machine (Stratagene) with the cyclophilin A as the internal control. The primers for target genes were listed in Table I. The results were calculated as the relative quantification compared to control group, which was set at 1. Each reaction was run in triplicate and independently repeated three times.
Table I.
Primers and sequences for real-time PCR analysis
| Forward primer | Reverse primer | |
|---|---|---|
| Alk5 | 5′-CATCAGGGTCTGGATCAGGTT-3′ | 5′-GTAACACAATGGTCCTGGCAA-3′ |
| Serpine1 | 5′-TTCAGCCCTTGCTTGCCTC-3′ | 5′-ACACTTTTACTCCGAAGTCGGT-3′ |
| Mmp13 | 5′-CTTCTTCTTGTTGAGCTGGACTC-3′ | 5′-CTGTGGAGGTCACTGTAGACT-3′ |
| Adamts5 | 5′-GGAGCGAGGCCATTTACAAC-3′ | 5′-CGTAGACAAGGTAGCCCACTTT-3′ |
| Aggrecan | 5′-CCTGCTACTTCATCGACCCC-3′ | 5′-AGATGCTGTTGACTCGAACCT-3′ |
| Col2 | 5′-CTGGTGGAGCAGCAAGAGCAA-3′ | 5′-CAGTGGACAGTAGACGGAGGAAAG-3′ |
| Col10 | 5′-ACCCCAAGGACCTAAAGGAA-3′ | 5′-CCCCAGGATACCCTGTTTTT-3′ |
| Cyclophilin A | 5′-CGAGCTCTGAGCACTGGAGA-3′ | 5′-TGGCGTGTAAAGTCACCACC-3′ |
Western blotting
Protein was extracted using ice-cold RIPA lysis buffer containing protease inhibitors (Roche). Equal amount of protein samples were resolved by 12% SDS-PAGE gels and transferred onto a polyvinylidene difluoride membrane (Millipore). Then the protein was probed with the antibody specific to ALK5, pSmad3, MMP13, ADAMTS5, AGGRECAN, pCREB and β-Actin followed by chemiluminescent (Pierce) detection. Intensity values were analyzed with the Image J software and normalized to β-Actin. Each sample was analyzed three times and the mean gray values of immunoblot band were calculated18.
Synovitis assay
Mouse articular joint sections were stained with hematoxylin and eosin (H&E) to detect the thickness of synovium. Histological changes were graded on an arbitrary scale from 0 to 3 (0 = no synovial thickening; 1 = lining of two cell layers; 2 = several extra cell layers; 3 = clear inflammation with cell infiltrate or exudate). Scoring was performed by two blinded observers as previously described19.
Statistical analysis
We used four or eight animals in each group for in vivo studies and three independent biological replicates for in vitro studies. Individual cell experiments were also performed in triplicate as technical replicates. The sample sizes for the groups of interest were enough based on a power of 80% and P < 0.05 using power and sample size calculation online software (http://powerandsamplesize.com/Calculators/) (Supplementary Table 1). The reliability and reproducibility of the histologic assessment and synovitis assay were analyzed by calculating the Intraclass Correlation Coefficient (ICC) for the measurements20. There was a high level of agreement between the two blinded observers (Junlan Huang and Xianxing Wang) (Supplementary Table 2, all ICC > 0.7). All tested variables were tested for normality and homogeneity using Shapiro–Wilk test and Levine’s test, respectively. Quantitative data of histologic grading of cartilage degeneration, MMP13 IHC, ADAMTS5 IHC and TUNEL assay were compared by using the non-parametric Mann–Whitney U test. For the other data, all the tested variables did not violate the assumptions of normality and equal variance. Differences between two groups were evaluated using Student’s unpaired t-test, and comparisons of multiple groups were evaluated using analysis of variance (ANOVA) followed by Tukey’s test. All data were analyzed using GraphPad Prism v.6.01 software and depicted in univariate scatter plots as previously described21. P < 0.05 was considered to be statistically significant.
Result
Postnatal cartilage-specific deletion of Alk5 induces an OA-like phenotype
The physiologic role of ALK5 in articular cartilage homeostasis in vivo has been elusive, as the conventional Alk5 knockout (KO) mice died at the E11.5 stage10 and mice with deletion of Alk5 in chondrocytes died immediately after birth (unpublished data). To determine the specific role of ALK5 signaling in the articular cartilage homeostasis and OA development at the postnatal stage, we generated Alk5Col2ERT2 mice and injected tamoxifen at the age of 2 weeks to specifically inactivate ALK5 signaling in a tamoxifen-inducible and chondrocyte-specific manner (hereafter referred to as Alk5 cKO mice). The protein expressions of ALK5 and pSmad3 were markedly reduced in articular cartilage (both P < 0.001, Fig. 1(A)) and growth plate cartilage (both P < 0.001, Supplementary Figure S1(A)), but not in synovium (P = 0.8934 and P = 0.7566, Supplementary Figure S1(B)) and ligament (P = 0.6401 and P = 0.8519, Supplementary Figure S1(C)) of Alk5 cKO mice, indicating that the Alk5 gene was effectively and specifically deleted in cartilage. We observed that there were no significant differences in body weight, body length, tibia length, femur length and joint morphology, as well as growth plate height (Supplementary Figure S2(A)–1(G)) between Cre-negative and Alk5 cKO mice at 2, 3 and 6 months of age. However, progressive degeneration and lesions of the knee articular cartilage became apparent in Alk5 cKO mice from age 2–6 months. Histologic examination of Alk5 cKO mice at 2 months revealed increased articular cartilage thickness with zonal disruption and loss of proteoglycan content in articular cartilage. Besides, the integrity of articular cartilage was destroyed, with lesions exhibited as either empty lacuna or confined loss of cartilage tissue [Fig. 1(B)]. Furthermore, 3-month-old Alk5 cKO mice showed early OA-like manifestations including loss of proteoglycan content and cartilage tissue and increased number of hypertrophic chondrocytes in articular cartilage [Fig. 1(C)]. The OA-like phenotype became more profound in 6-month-old Alk5 cKO mice, which exhibited as more severe destruction of the articular cartilage associated with greater loss of proteoglycan content and cartilage, osteophyte formation and substantially increased subchondral bone mass [Fig. 1(D) and Supplementary Figure S3(A) (P < 0.001)]. Accordingly, the OARSI scores were significantly increased in 3- and 6-month-old Alk5 cKO mice compared to those in Cre-negative mice (all P < 0.001, Fig. 1(E) and (F)). Taken together, these results demonstrate that postnatal ALK5 signaling plays an essential role in maintaining the integrity and homeostasis of articular cartilage.
Fig. 1. Inducible cartilage-specific Alk5 cKO mice exhibit a progressive OA–like phenotype.

A, Immunohistochemistry (IHC) analysis of ALK5 and pSmad3 protein expressions in articular cartilage of 2 month-old Alk5 cKO mice and Cre-negative control mice. Quantitative data were shown in the right panel (The percentage of positive cells in Cre-negative mice was defined as 1, student’s unpaired t-test, n = 4 mice per group). B, Knee joint samples were dissected from 2-month-old mice, and Safranin O/Fast green staining was performed. Results showed articular cartilage lesions (black arrow), increased number of hypertrophic chondrocytes (yellow arrows) and increased thickness (blue double-ended arrows) in articular cartilage of Alk5 cKO mice. Quantitative data were shown in the right panel (Student’s unpaired t-test, n = 4 mice per group). C, Knee joint samples were dissected from 3-month-old mice, and Safranin O/Fast green staining was performed. Results showed an early features of OA-like phenotype in articular cartilage of 3-month-old Alk5 cKO mice, including tears and clefts in the articular surface (black arrows) and increased number of hypertrophic chondrocytes in articular cartilage (yellow arrows). D, Knee joint samples were dissected from 6-month-old mice, and Safranin O/Fast green staining was performed. Results showed more severe loss of articular cartilage tissue (black arrows), osteophyte formation (red arrow), and subchondral sclerosis (yellow arrows) in 6-month-old Alk5 cKO mice compared with Cre-negative control mice. E and F, OARSI scoring system showed more severe articular cartilage destruction in 3- and 6-month-old Alk5 cKO mice compared with Cre-negative mice (Mann–Whitney U test, n = 8 mice per group). MFC: medial femoral condyle; MTP: medial tibial plateau. Scale bar: 100 μm. Data in were expressed as the mean ± 95% confidence intervals. In A, B, E and F, symbols represent individual mice.
Alk5 cKO mice exhibit increased matrix degradation and chondrocyte apoptosis
Because of the limited amount and difficulty of dissecting mouse knee articular cartilage, we isolate femoral head cartilage, which is much simpler to collect in viable amounts22, to explore the mechanisms underlying the accelerated progression of OA in Alk5 cKO mice. qRT-RCR and western blotting results confirmed that Alk5 gene was effectively deleted in femoral head cartilage after 4-OH TM treatment (all P < 0.001, Fig. 2(A)–(C)). Next, the expressions of genes associated with articular cartilage homeostasis were detected. The mRNA expressions of Aggrecan (P < 0.001) and Col2 (P < 0.001) were decreased in Alk5-deficient femoral head cartilage. Meanwhile, expressions of Mmp13 (P < 0.001) and Adamts5 (P < 0.001) and Col10 (P = 0.01) were significantly increased in Alk5-deficient femoral head cartilage [Fig. 2(D)]. Western blotting and IHC results showed that ACAN (P = 0.001) protein level were significantly decreased while MMP13 (P = 0.012 and P = 0.029, respectively) and ADAMTS5 (P = 0.007 and P = 0.029, respectively) protein levels were significantly increased in Alk5 cKO mice when compared with Cre-negative mice [Fig. 2(E) and (F)].
Fig. 2. Loss of ALK5 signaling alters the expressions of ECM related genes in chondrocytes.
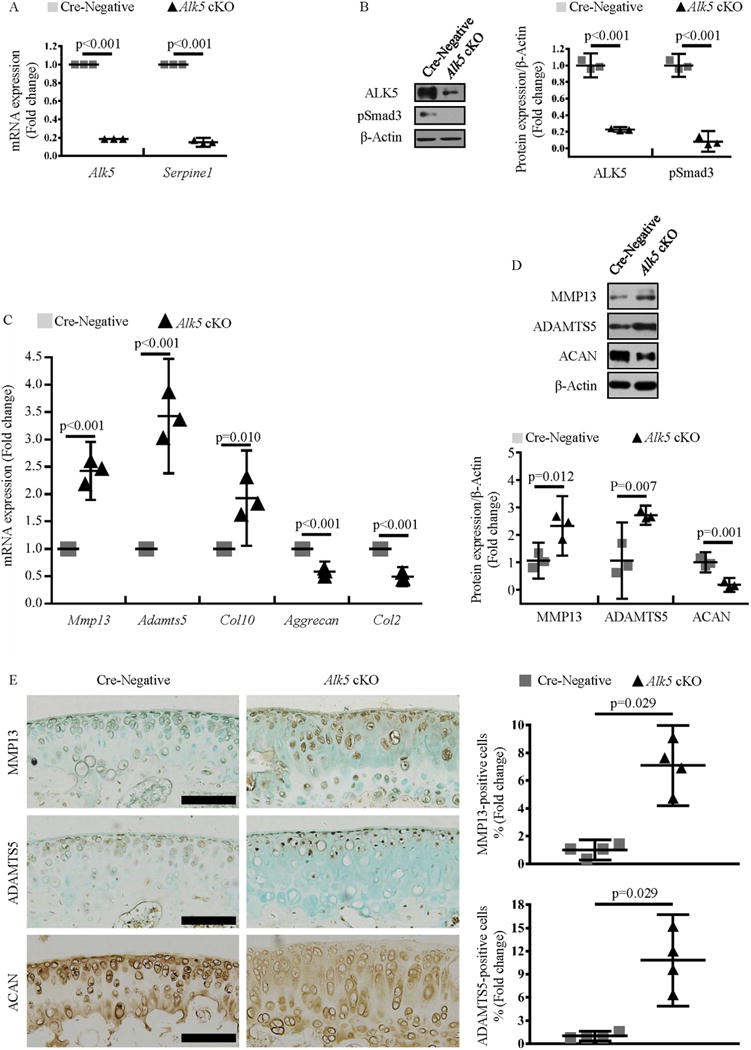
A, Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of Alk5 and Serpine1 mRNA expressions in femoral head cartilage isolated from Alk5Col2ERT2 and Cre-negative control mice after 4-OH TM treatment (Student’s unpaired t-test, n = 4 femoral head cartilage per group). B, Western blotting analysis of ALK5 and pSmad3 protein expressions in femoral head cartilage isolated from Alk5Col2ERT2 and Cre-negative control mice after 4-OH TM treatment. Quantitative data were shown in the right panel (Student’s unpaired t-test, n = 3 femoral head cartilage per group). C, qRT-PCR analysis of Mmp13, Adamts5, Col10, Aggrecan and Col2 mRNA expressions in femoral head cartilage isolated from Alk5Col2ERT2 and Cre-negative mice after 4-OH TM treatment (Student’s unpaired t-test, n = 4 femoral head cartilage per group). D, Western blotting analysis of MMP13, ADAMTS5 and ACAN protein expressions in femoral head cartilage isolated from Alk5Col2ERT2 and Cre-negative control mice after 4-OH TM treatment. Quantitative data were shown in the lower panel (Student’s unpaired t-test, n = 3 femoral head cartilage per group). E, IHC analysis of MMP13, ADAMTS5 and ACAN protein expressions in articular cartilage of 2-month-old Alk5 cKO mice and Cre-negative control mice. Quantitative data were shown in the right panel (The percentage of positive cells in Cre-negative mice was defined as 1, Mann–Whitney U test, n = 4 mice per group). Scale bar: 100 μm. Data were expressed as the mean ± 95% confidence intervals. In A, B, C and D, symbols represent three technical replicates. In E, symbols represent individual mice.
Since it has been reported that chondrocyte apoptosis was strongly related to OA development23,24. We performed TUNEL assay and IHC staining of cleaved caspase 3 to measure apoptosis. TUNEL-positive cells were significantly increased in Alk5 cKO mice, which were mainly located surrounding lesion area (P = 0.029, Fig. 3(A)). Consistently, the number of cleaved caspase 3-positive cells was also markedly increased in Alk5 cKO mice compared with Cre-negative mice (P = 0.003, Fig. 3(B)).
Fig. 3.

Alk5 deficiency increases articular chondrocyte apoptosis. Knee joint samples were dissected from 2-month-old mice, TUNEL assay and cleaved caspase 3 IHC were performed. A, TUNEL staining showed a significant increase in the number of TUNEL positive cells (arrowheads) in articular cartilage of Alk5 cKO mice compared with that in Cre-negative control mice. Quantitative data were shown in the right panel (The percentage of positive cells in Cre-negative mice were defined as 1, Mann–Whitney U test, n = 4 mice per group). B, IHC analysis showed a significant increase in the number of cleaved caspase 3 positive cells in articular cartilage of Alk5 cKO mice compared with that in Cre-negative control mice. Quantitative data were shown in the right panel (The percentage of positive cells in Cre-negative mice was defined as 1, student’s unpaired t-test, n = 4 mice per group). Scale bar: 100 μm. Data were expressed as the mean ± 95% confidence intervals. In A and B, symbols represent individual mice.
Together, these results indicated that Alk5 deficiency directly increased matrix degradation and chondrocyte apoptosis in articular chondrocytes, which may disrupt articular cartilage homeostasis in adults.
Reduced expression of PRG4 in articular cartilage of Alk5 cKO mice
Apart from cartilage destruction, synovial hyperplasia, another key feature associated with OA development that was not observed in Tgfbr2 cKO mice, was also observed in Alk5 cKO mice (P < 0.001, Fig. 4(A)), demonstrating that Alk5 deletion in cartilage leads to a typical OA phenotype. Proteoglycan 4 (PRG4), mainly secreted by synoviocytes and superficial zone chondrocytes, is present in synovial fluid and on the surface of articular cartilage and plays an important role in joint lubrication and synovial homeostasis. Prg4 KO mice develop joint disease with synovial hyperplasia, increased chondrocyte apoptosis, articular cartilage degradation, cellular loss and eventual precocious joint failure25–28. The similar OA phenotypes especially the synovial hyperplasia between Alk5 cKO and Prg4 KO mice indicate that abnormal expression of PRG4 may be involved in the OA phenotype of Alk5 cKO mice. In addition, in vitro study has demonstrated that TGF-β1 induces Prg4 expression in chondrocytes29. Indeed, our IHC results revealed that the PRG4 protein level in articular cartilage, but not in synovium, was reduced in Alk5 cKO mice compared to Cre-negative mice [Fig. 4(B) (P < 0.001) and Supplementary Figure S1(B)]. Meanwhile, the number of superficial zone chondrocytes was decreased in Alk5 cKO mice (P < 0.001, Supplementary Figure S3(B)). Furthermore, Prg4 mRNA expression was decreased in Alk5-deficient femoral head cartilage (P < 0.001, Fig. 4(C)). Similarly, treatment of femoral head cartilage with ALK5 inhibitor SB-505124, significantly reduced the Prg4 mRNA expression in a dose- and time-dependent manner [(P = 0.029, P < 0.001 and P < 0.001, Fig. 4(D)) and (P = 0.016, P < 0.001 and P < 0.001, Fig. 4(E))]. These data suggest a critical role of ALK5 signaling in maintaining cell number and PRG4 expression of superficial zone chondrocytes. The decreased Prg4 expression in chondrocytes partly contributes to the OA phenotype especially synovial hyperplasia observed in Alk5 cKO mice.
Fig. 4. Alk5 cKO mice exhibit enhanced synovial hyperplasia and reduced PRG4 expression.

A, Knee joint samples were dissected from 3-month-old mice, and hematoxylin and eosin staining (H&E) was performed. H&E staining showed increased synovial thickness in Alk5 cKO mice compared with that in Cre-negative mice (student’s unpaired t-test, n = 4 mice per group). B, IHC analysis of PRG4 protein expression in articular cartilage of 2 month-old Alk5 cKO mice and Cre-negative mice. Quantitative data were shown in the right panel (The percentage of positive cells in Cre-negative mice were defined as 1, student’s unpaired t-test, n = 4 mice per group). C, qRT-PCR analysis of Prg4 mRNA expression in femoral head cartilage isolated from Alk5Col2ERT2 and Cre-negative mice after 4-OH TM treatment (Student’s unpaired t-test, n = 4 femoral head per group). D and E, Femoral head cartilage isolated from 1-month-old Cre-negative control mice was treated with increasing concentrations of SB-505124 (0.1, 0.5 and 1 μM) for 24 h (D) or treated with 1 μM SB-505124 for different periods of time (6, 12 and 24 h) (E). qRT-PCR was performed to detect the Prg4 mRNA expression (One-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group). Scale bar: 100 μm. Data were expressed as the mean ± 95% confidence intervals. In A and B, symbols represent individual mice. In C, D and E, symbols represent three technical replicates.
TGF-β1 induces the expression of Prg4 via ALK5 signaling in chondrocytes
Understanding the regulatory role of TGF-β signaling in Prg4 expression may help to elucidate the mechanisms by which TGF-β/ ALK5 signaling participates in the regulation of articular cartilage homeostasis and OA development. We found that TGF-β1 robustly induced the Prg4 mRNA expression in a dose- and time-dependent manner [(P = 0.035, P = 0.002 and P < 0.001, Fig. 5(A)) and (P = 0.001, P < 0.001 and P < 0.001, Fig. 5(B))]. In femoral head cartilage isolated from 1-month-old Prg4GFPCreERT2/+ mice, as described previously, GFPCreERT2 expression mirrors endogenous PRG4 expression12,30. Using this model, we found that TGF-β1 increased the GFP protein levels in femoral head cartilage derived from Prg4GFPCreERT2 mice in a dose- and time-dependent manner [(P = 0.028, P < 0.001 and P < 0.001, Fig. 5(D)) and (all P < 0.001, Fig. 5(E))]. Femoral head cartilage isolated from Alk5Col2ERT2 and Cre-negative mice was treated with 4-OH TM to delete Alk5, followed by treated with TGF-β1 to dissect the role of ALK5 in TGF-β1- induced Prg4 expression. Our data showed that deletion of Alk5 attenuated the TGF-β1-induced mRNA expression of Prg4 (P < 0.001, Fig. 5(G)). Consistently, ALK5 inhibitor SB-505124 decreased TGF-β1-induced Prg4 mRNA and protein expressions (both P < 0.001, Fig. 5(C) and (F)). Furthermore, transfection of constitutively activated ALK5 (CA-ALK5) into primary chondrocytes directly induced the Prg4 expression, while SB-505124 attenuated the CA-ALK5-induced Prg4 expression (P < 0.001, Fig. 5(H)). Taken together, our data demonstrate that TGF-β1 induces Prg4 expression through an ALK5-dependent manner in articular cartilage.
Fig. 5. TGF-β1 induces the expression of Prg4 via ALK5 signaling pathways in chondrocytes.
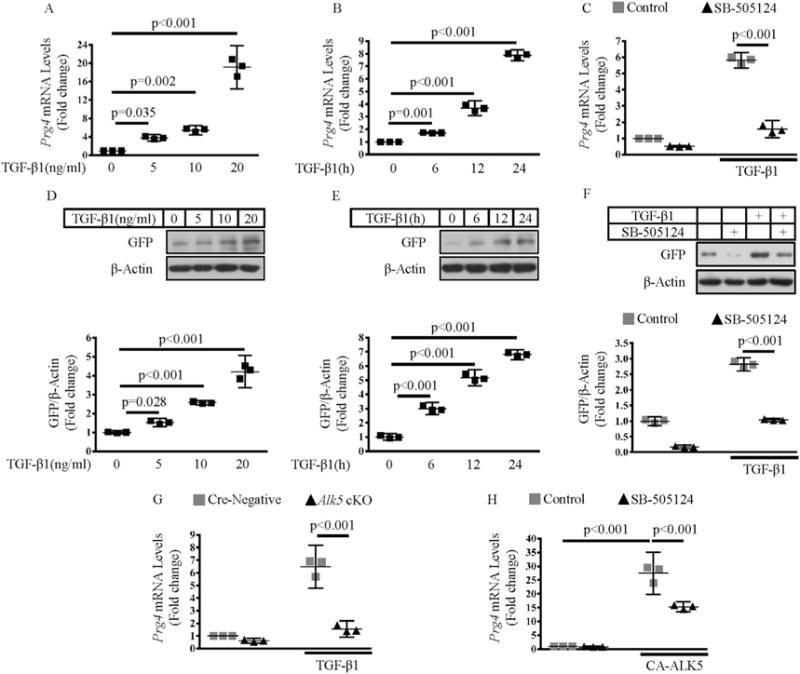
A and B, Femoral head cartilage isolated from Prg4GFPCreERT2/+ mice was treated with increasing concentrations of TGF-β1 (5, 10 and 20 ng/ml) for 24 h (A) or treated with 10 ng/ml TGF-β1 for different periods of time (6, 12 and 24 h) (B). qRT-PCR was performed to detect the Prg4 mRNA expression (One-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group). D and E, Femoral head cartilage isolated from Prg4GFPCreERT2/+ mice were treated with increasing concentrations of TGF-β1 (5, 10 and 20 ng/ml) for 24 h (D) or treated with 10 ng/ml TGF-β1 for different periods of time (6, 12 and 24 h) (E). Western blotting was performed to detect the GFP protein expression. Quantitative data were shown in the lower panel (One-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group). C and F, Femoral head cartilage isolated from Prg4GFPCreERT2/+ mice was pretreated with 1 μM SB-505124 for 30 min, followed by an additional 24 h incubation of 10 ng/ml TGF-β1, 1 μM SB-505124, or a combination of both. qRT-PCR and western blotting were performed to detect Prg4 mRNA expression (C, two-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group) and the GFP protein expression (F, quantitative data were shown in the lower panel, two-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group), respectively. G, Femoral head cartilage isolated from Alk5Col2ERT2 and Cre-negative control mice was treated with 4-OH TM for 72 h, followed by an additional 24 h incubation of 10 ng/ml TGF-β1. qRT-PCR was performed to detect the Prg4 mRNA expression (two-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group). H, Primary chondrocytes isolated from Prg4GFPCreERT2/+ mice were transfected with constitutively activated ALK5 (CA-ALK5) expression plasmid or pcDNA3.1/Myc empty vector. 12 h after transfection, cells were treated with 1 μM SB-505124 for another 12 h qRT-PCR was performed to detect the Prg4 mRNA expression (Two-way ANOVA followed by Tukey’s test, n = 4 mice per group). Data were expressed as the mean ± 95% confidence intervals. In A-H, symbols represent three technical replicates.
TGF-β1/ALK5 regulates Prg4 expression through PKA-CREB pathway
Previous studies demonstrated that the expression of Prg4 could be upregulated by cyclic adenosine monophosphate (cAMP)-dependent PKA/cyclic AMP-responsive element-binding protein (CREB) signaling in epiphyseal chondrocytes30. Meanwhile, TGF-β signaling has been shown to activate PKA in a Smad-dependent manner31,32. To examine whether PKA signaling is crucial for the induction of Prg4 expression by TGF-β signaling in articular cartilage, we examined whether H89, a PKA inhibitor, would affect Prg4 expression induced by TGF-β1. Indeed, H89 treatment significantly attenuated the TGF-β1-induced Prg4 mRNA and GFP protein expressions (both P < 0.001, Fig. 6(A) and (B)). To further confirm the functional significance of TGF-β1 mediated PKA activation, phosphorylation of CREB on Ser133, a key event for the induction of gene transcription by PKA signaling, was tested33. We found that TGF-β1 stimulated the phosphorylation of CREB on Ser133 (P < 0.001), while H89 attenuated TGF-β1-induced phosphorylation of CREB in chondrocytes (P < 0.001, Fig. 6(C)). In contrast, pretreatment with H89 had no effect on the phosphorylation of Smad3 induced by TGF-β1 [Fig. 6(C)], which implied that the activation of PKA was downstream of Smad activated by TGF-β1. Furthermore, we found that phosphorylation of CREB activated by TGF-β1 could be attenuated by SB-505124 (P < 0.001, Fig. 6(D)) or deletion of Alk5 in chondrocytes (P < 0.001, Fig. 6(E)). Similarly, IHC results showed that the pCREB-positive cells in articular cartilage were significantly decreased in Alk5 cKO mice (P < 0.001, Fig. 6(F)). Phosphorylation of CREB recruits CREB binding protein (CBP)/p300 co-activators to activate cAMP response element (CRE)-driven gene transcription33. In addition to CBP/P300, CREB also interacts with another co-activator family, termed cAMP-regulated transcriptional co-activators (CRTCs), which increase CREB activity following their association with CREB34. Previous studies have demonstrated that, besides the phosphorylation of CREB, CREB/CRTCs interaction is also important for the expression of Prg430, and TGF-β signaling could increase the association of CREB and CRTCs in ovary granulosa cells35. Therefore, we examined whether administration of an inhibitor of CBP-CREB interaction would affect the expression of Prg4 induced by TGF-β1. qRT-PCR and western blotting results showed that the inhibitor of CBP-CREB interaction attenuated TGF-β1-induced Prg4 mRNA and GFP protein expressions (both P < 0.001, Fig. 6(G) and (H)). We next examined whether down-regulated expression of Prg4 in Alk5-deficient femoral head cartilage could be rescued by activating PKA/CREB signaling using forskolin, a PKA/CREB agonist36. qRT-PCR results showed that forskolin could partially rescue the reduced expression of Prg4 in Alk5-deficient femoral head cartilage (all P < 0.001, Fig. 6(I)). Taken together, these data suggest that TGF-β/ALK5 signaling induces Prg4 expression by increasing the phosphorylation of CREB and CREB/CRTCs interaction in chondrocytes.
Fig. 6. TGF-β1/ALK5 signaling induces Prg4 expression via the PKA-CREB signaling pathway in chondrocytes.

A, B, G and H. Femoral head cartilage isolated from Prg4GFPCreERT2/+ mice was pretreated with selective inhibitor for 30 min, followed by an additional 24 h incubation with TGF-β1, inhibitor, or a combination of both. qRT-PCR and western blotting were performed to detect the expression of Prg4 mRNA (A and G, two-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group) and the GFP protein expression (B and H, quantitative data were shown in the lower panel, two-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group), respectively. C and D, Primary chondrocytes isolated from Cre-negative mice were pretreated with 10 μM H89 (C) or 1 μM SB-505124 (D) for 30 min, followed by an additional 30 min incubation of 10 ng/ml TGF-β1,10 μM H89/1 μM SB-505124, or a combination of both. Western blotting was performed to detect the pCREB and pSmad3 protein expressions. Quantitative data were shown in the right panel (two-way ANOVA followed by Tukey’s test, n = 4 mice per group). E, Western blotting analysis of pCREB in Cre-negative or Alk5-deficient primary chondrocytes treated with TGF-β1 for 30 min. Quantitative data were shown in the right panel (two-way ANOVA followed by Tukey’s test, n = 4 mice per group). F, IHC analysis of pCREB protein expression in articular cartilage of 2-month-old Alk5 cKO mice and Cre-negative control mice. Quantitative data were shown in the right panel (The percentage of positive cells in Cre-negative mice was defined as 1, student’s unpaired t-test, n = 4 mice per group). I, qRT-PCR analysis of Prg4 mRNA expression in Cre-negative or Alk5-deficient femoral head cartilage treated with increasing concentrations of forskolin (two-way ANOVA followed by Tukey’s test, n = 3 femoral head cartilage per group). J. Schematic illustration of the mechanisms of ALK5 signaling in the maintenance of articular cartilage. Data were expressed as the mean ± 95% confidence intervals. In F, symbols represent individual mice. In A, B, C, D, E, G and H, symbols represent three technical replicates.
Discussion
Previous studies employing transgenic mice overexpressing a dominant-negative form of TGFβRII (dn-TGFβRII) in skeletal tissue and Smad3 KO mice have shown that TGF-β/TGFβRII-Smad3 signaling plays a vital role in articular cartilage homeostasis and OA development4–6. Furthermore, deletion of Tgfbr2 in chondrocytes at the postnatal stage also leads to an OA-like phenotype, which demonstrates that TGF-β/TGFβRII signaling in chondrocytes plays a vital role in postnatal articular cartilage maintenance3. In conventional TGF-β signaling, ALK5 needs to form heteromeric complexes with TGFβRII to transmit TGF-β signals to downstream molecules37, which indicates that ALK5 may play important and similar role in articular cartilage homeostasis as TGFβRII. Recent studies, however, suggest that ALK5 and TGFβRII receptors may have differential role in certain situation. ALK5 and TGFβRII receptors have independent roles in regulating tooth initiation38. Moreover, TGFβRII may be dispensable for the TGF-β signaling, as TGF-β2 is able to elicit signals in the Tgfbr2 deficient cranial neural crest cells through the ALK5/TGFβRIII receptor complex, inducing a Smad-independent TRAF6/TAK1/p38 signaling pathway39. These data imply that the role of ALK5 in the development of OA may be different from that of TGFβRII. Thus, investigating the role of TGF-β signaling in articular cartilage maintenance at the level of ALK5 is essential for the in-depth understanding of the role of TGF-β signaling in articular cartilage homeostasis and OA development.
In order to obtain a higher Cre recombination efficiency and better compare the OA phenotype with Tgfbr2 cKO mice, we selected the same time and dosage of tamoxifen injection as previously described3,11. IHC results showed that the expressions of ALK5 and pSmad3 were markedly reduced in articular cartilage and growth plate cartilage, but not in synovium and ligament of Alk5 cKO mice, indicating Col2a1-CreERT2 mediated recombination was specific in cartilaginous tissues when tamoxifen is injected at 2 weeks, which was consistent with previous studies11,40.
The progressive OA-like phenotype in Alk5 cKO mice found in this study demonstrated that postnatal ALK5 signaling in articular chondrocytes indeed played an important role in maintaining the integrity and homeostasis of articular cartilage. Alk5 cKO mice exhibited decreased cartilage ECM with enhanced expressions of matrix degrading enzymes including MMP13 and ADAMTS5. Mmp13 and Adamts5 are critical downstream target genes involved in the TGF-β/TGFβRII signaling pathway during OA development3. Our data showed that Alk5 deficiency also led to increased expressions of Mmp13 and Adamts5 in articular cartilage, indicating that the expressions of these two catabolic factors may be inhibited by the canonical TGF-β signaling pathway depending on the formation of TGFβRII/ALK5 heteromeric complexes.
PRG4 could provide articular cartilage boundary lubrication and delay the progression of OA through inhibiting the expressions of catabolic factors, chondrocyte apoptosis and synovial cell overgrowth25–27,41. Besides, recent study showed that Prg4-expressing superficial zone chondrocytes served as a stem/progenitor cells population for all deeper layers of mature articular cartilage12, and PRG4 play an important role in maintaining the number of superficial zone chondrocytes and biological functions of superficial zone28. Consistently, Prg4 KO mice develop joint disease with synovial hyperplasia, increased chondrocyte apoptosis and articular cartilage degradation25–28, which were also observed in our Alk5 cKO mice. Most importantly, the expression of PRG4 was reduced in articular cartilage of Alk5 cKO mice and ALK5-deficient femoral head cartilage. All these data demonstrate that PRG4 is involved in the maintenance of articular cartilage homeostasis by TGF-β/ALK5 signaling and the decreased PRG4 expression in chondrocytes partly contributes to the OA phenotype especially synovial hyperplasia observed in Alk5 cKO mice.
Prg4 was already known to be positively regulated by TGF-β in chondrocytes29, and Prg4 mRNA expression was reduced in dn-TGFβRII transgenic mice42. Furthermore, inhibition of Smad3 phosphorylation attenuated TGF-β1 induced PRG4 protein expression43. However, the detailed mechanisms were not well understood. We here found that ALK5 signaling is also critical for mediating the stimulatory effect of TGF-β1 on Prg4 expression. Besides TGF-β signaling, PKA/CREB30 signaling has been found to be essential for the expression of Prg4 in cartilage. PKA, a ubiquitous serine/threonine protein kinase, phosphorylates CREB at the regulatory Ser133 site, as well as recruits CBP/p300 coactivators and CRTCs to activate cAMP response element (CRE)-driven gene transcription33,34. In recent years, PKA/CREB signaling was found to play a vital role in mediating TGF-β-induced responses. TGF-β stimulates PKA/CREB signaling events in both mesangial and MES cells, and inhibition of PKA attenuated TGF-β-induced phosphorylation of CREB31,44. These studies raise the possibility that the activation of PKA signaling may be involved in the TGF-β signaling induced expression of Prg4. In current study, we found that PKA/CREB signaling, as a downstream of ALK5/Smad3 activated by TGF-β, was indispensable for the TGF-β1-induced Prg4 expression, and both increased phosphorylation of CREB and enhanced CREB/CRTCs interaction in chondrocytes were involved in the TGF-β1/ALK5 signaling induced-Prg4 expression. Furthermore, we revealed that forskolin, a PKA agonist36, could rescue the down-regulation of Prg4 in Alk5-deficient femoral head cartilage. It would be interesting to investigate whether activation of PKA-CREB signaling or injection of recombinant PRG4 could alleviate the OA-like phenotype in Alk5 cKO mice.
TGF-β signaling plays a crucial and complex role in OA development45,46. Conventional Smad3 global KO5 and chondrocyte-specific Tgfbr23, Alk5 or Smad3 KO4 mice develop a degenerative joint disease resembling human OA. In contrast, transgenic expression of active TGF-β1 in osteoblastic cells also causes an OA-like phenotype9. These data indicate that distinct TGF-β signaling, mediated by different combination of TGF-β signaling related molecules, has differential effect on different joint tissues. In our current study, we just deleted Alk5 in articular cartilage and proved that ALK5 in chondrocytes basically plays a chondro-protective role in joint tissue homeostasis, furthermore deletion of Alk5 specifically in other joint tissues is expected to dissect the accurate role of ALK5 in cartilage homeostasis. In addition, Alk5 cKO mice showed characteristic cartilage lesions, shown as either empty lacuna or confined loss of cartilage tissue within (inside) articular cartilage that is not commonly observed in other OA models (including Tgfbr2 cKO and Smad3 cKO mice). Interestingly, cartilage-specific deletion of core clock transcription factor Bmal1 (Bmal1 cKO) mice also exhibited this characteristic lesions. More importantly, the phosphorylated Smad2/3 and Serpine1 expressions are also significantly reduced in Bmal1 cKO mice. While, previous reports showed that TGF-β signaling could also upregulate the Bmal1 mRNA expression in human articular cartilage47, whether circadian rhythm is involved in the lesions of Alk5 cKO mice needs to be studied.
In summary, using inducible chondrocyte-specific inactivation approach, we found that Alk5 deficiency in chondrocytes leads to the age-associated progressive OA-like phenotype. Down-regulated PKA-CREB signaling and PRG4 expression may partly contribute to this phenotype. Our data suggest that TGF-β/ALK5 signaling induces the expression of PRG4 by activating the PKA-CREB signaling pathway. These findings strongly suggest that ALK5 signaling plays a chondro-protective role in joint tissue homeostasis at postnatal stage, which will deepen our understanding about the mechanisms of OA and facilitate the development of effective therapeutic agents for OA.
Supplementary Material
Acknowledgments
We thank professor Yeguang Chen (Tsinghua University, China) for providing constructs, professor Yang Chai (University of Southern California, USA) for providing Alk5flox/flox mice, and professor Matthew L. Warman (Boston Children’s Hospital, USA) for providing Prg4GFPCreERT2/+ mice.
Fundings
This work was supported by Special Funds for Major State Basic Research Program of China (973 program) (No. 2014CB942904), International (Regional) Cooperation and Exchange (No. 81220108020), National Natural Science Foundation of China (No. 81472074), and Basic and Advanced Research Project in Chongqing (CSTC2013jcyjC00009).
Footnotes
Author contributions
All authors listed have read and approved all versions of the manuscript.
Conception and design: Quan Wang, Xiaolan Du, Yangli Xie, Lin Chen.
Analysis and interpretation of the data: Quan Wang, Qiaoyan Tan, Wei Xu, Huabing Qi, Junlan Huang, Xianxing Wang Yangli Xie.
Drafting of the article: Quan Wang, Yangli Xie, Lin Chen. Critical revision of the article for important intellectual content: Di Chen, Yangli Xie, Lin Chen.
Final approval of the article: Quan Wang, Xiaolan Du, Yangli Xie, Lin Chen.
Provision of study materials or patients: Quan Wang, Jingyuan Guo, Siru Zhou, Zhenhong Ni, Liang Kuang, Zuqiang Wang, Nan Su, Liang Chen, Xiaolan Du.
Statistical expertise: Qiaoyan Tan, Wei Xu.
Administrative, technical, or logistic support: Bo Chen, Wanling Jiang, Yu Gao, Hangang Chen, Xiaolan Du.
Collection and assembly of data: All authors.
Conflict of interest
The authors declare that they have no competing interests.
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.joca.2017.07.010.
References
- 1.Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthr Lancet. 2015;386:376–87. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Shen J, Jin H, Im HJ, Sandy J, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61–9. doi: 10.1111/j.1749-6632.2011.06258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen J, Li J, Wang B, Jin H, Wang M, Zhang Y, et al. Deletion of the transforming growth factor beta receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheum. 2013;65:3107–19. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CG, Thuillier D, Chin EN, Alliston T. Chondrocyte-intrinsic Smad3 represses Runx2-inducible MMP-13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheum. 2012;64:3278–89. doi: 10.1002/art.34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–52. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–45. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 8.Hui W, Young DA, Rowan AD, Xu X, Cawston TE, Proctor CJ. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann Rheum Dis. 2016;75:449–58. doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–12. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–73. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O’Keefe RJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozhemyakina E, Zhang M, Ionescu A, Ayturk UM, Ono N, Kobayashi A, et al. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015;67:1261–73. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng T, Yi L, Huang J, Luo F, Wen X, Du X, et al. Genetic inhibition of fibroblast growth factor receptor 1 in knee cartilage attenuates the degeneration of articular cartilage in adult mice. Arthritis Rheum. 2012;64:3982–92. doi: 10.1002/art.34645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Jia H, Ma X, Tong W, Doyran B, Sun Z, Wang L, et al. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc Natl Acad Sci U S A. 2016;113:14360–5. doi: 10.1073/pnas.1608938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanton H, Golub SB, Rogerson FM, Last K, Little CB, Fosang AJ. Investigating ADAMTS-mediated aggrecanolysis in mouse cartilage. Nat Protoc. 2011;6:388–404. doi: 10.1038/nprot.2010.179. [DOI] [PubMed] [Google Scholar]
- 17.Jonason JH, Hoak D, O’Keefe RJ. Primary murine growth plate and articular chondrocyte isolation and cell culture. Methods Mol Biol. 2015;1226:11–8. doi: 10.1007/978-1-4939-1619-1_2. [DOI] [PubMed] [Google Scholar]
- 18.Ranstam J. Repeated measurements, bilateral observations and pseudoreplicates, why does it matter? Osteoarthr Cartil. 2012;20:473–5. doi: 10.1016/j.joca.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–64. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 20.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 21.Weissgerber TL, Milic NM, Winham SJ, Garovic VD. Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol. 2015;13:e1002128. doi: 10.1371/journal.pbio.1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudek M, Gossan N, Yang N, Im H-J, Ruckshanthi JPD, Yoshitane H, et al. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J Clin Investig. 2015;126:365–76. doi: 10.1172/JCI82755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoartr Cartil. 2007;15:27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16:26035–54. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Investig. 2005;115:622–31. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coles JM, Zhang L, Blum JJ, Warman ML, Jay GD, Guilak F, et al. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum. 2010;62:1666–74. doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci U S A. 2013;110:5852–7. doi: 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karamchedu NP, Tofte JN, Waller KA, Zhang LX, Patel TK, Jay GD. Superficial zone cellularity is deficient in mice lacking lubricin: a stereoscopic analysis. Arthritis Res Ther. 2015;18:64. doi: 10.1186/s13075-016-0967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niikura T, Reddi AH. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312–21. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa H, Kozhemyakina E, Hung HH, Grodzinsky AJ, Lassar AB. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014;28:127–39. doi: 10.1101/gad.231969.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Zhu Y, Sharma K. Transforming growth factor-beta1 stimulates protein kinase A in mesangial cells. J Biol Chem. 1998;273:8522–7. doi: 10.1074/jbc.273.14.8522. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Duan CJ, Binkley C, Li G, Uhler MD, Logsdon CD, et al. A transforming growth factor beta-induced Smad3/Smad4 complex directly activates protein kinase A. Mol Cell Biol. 2004;24:2169–80. doi: 10.1128/MCB.24.5.2169-2180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrisani OM. CREB-mediated transcriptional control. Crit Rev Eukaryot Gene Expr. 1999;9:19–32. [PubMed] [Google Scholar]
- 34.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–51. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang WL, Lee MT, Wu LS, Chen YJ, Mason J, Ke FC, et al. CREB coactivator CRTC2/TORC2 and its regulator calcineurin crucially mediate follicle-stimulating hormone and transforming growth factor beta1 upregulation of steroidogenesis. J Cell Physiol. 2012;227:2430–40. doi: 10.1002/jcp.22978. [DOI] [PubMed] [Google Scholar]
- 36.Alasbahi RH, Melzig MF. Forskolin and derivatives as tools for studying the role of cAMP. Pharmazie. 2012;67:5–13. [PubMed] [Google Scholar]
- 37.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H, Oka K, Bringas P, Kaartinen V, Chai Y. TGF-beta type I receptor Alk5 regulates tooth initiation and mandible patterning in a type II receptor-independent manner. Dev Biol. 2008;320:19–29. doi: 10.1016/j.ydbio.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwata J, Hacia JG, Suzuki A, Sanchez-Lara PA, Urata M, Chai Y. Modulation of noncanonical TGF-beta signaling prevents cleft palate in Tgfbr2 mutant mice. J Clin Investig. 2012;122:873–85. doi: 10.1172/JCI61498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagao M, Cheong CW, Olsen BR. Col2-Cre and tamoxifen-inducible Col2-CreER target different cell populations in the knee joint. Osteoarthr Cartil. 2016;24:188–91. doi: 10.1016/j.joca.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan MZ, Erez A, Guse K, Dawson B, Bertin T, Chen Y et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5:176ra34. doi: 10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramaswamy G, Sohn P, Eberhardt A, Serra R. Altered responsiveness to TGF-beta results in reduced Papss2 expression and alterations in the biomechanical properties of mouse articular cartilage. Arthritis Res Ther. 2012;14:R49. doi: 10.1186/ar3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DuRaine GD, Chan SM, Reddi AH. Effects of TGF-beta1 on alternative splicing of superficial zone protein in articular cartilage cultures. Osteoarthr Cartil. 2011;19:103–10. doi: 10.1016/j.joca.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Singh LP, Green K, Alexander M, Bassly S, Crook ED. Hexosamines and TGF-beta1 use similar signaling pathways to mediate matrix protein synthesis in mesangial cells. Am J Physiol Ren Physiol. 2004;286:F409–16. doi: 10.1152/ajprenal.00007.2003. [DOI] [PubMed] [Google Scholar]
- 45.Shen J, Li S, Chen D. TGF-beta signaling and the development of osteoarthritis. Bone Res. 2014;2:14002. doi: 10.1038/boneres.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Kraan PM. The changing role of TGFbeta in healthy, ageing and osteoarthritic joints. Nat Rev Rheumatol. 2017;13:155–63. doi: 10.1038/nrrheum.2016.219. [DOI] [PubMed] [Google Scholar]
- 47.Akagi R, Akatsu Y, Fisch KM, Alvarez-Garcia O, Teramura T, Muramatsu Y, et al. Dysregulated circadian rhythm pathway in human osteoarthritis: NR1D1 and BMAL1 suppression alters TGF-beta signaling in chondrocytes. Osteoarthritis Cartilage. 2017;25:943–51. doi: 10.1016/j.joca.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


