Abstract
Mice carrying one inactivated Fhit allele (Fhit +/− mice) are highly susceptible to tumor induction by N-nitrosomethylbenzylamine, with 100% of Fhit +/− mice exhibiting tumors of the forestomach/squamocolumnar junction vs. 25% of Fhit +/+ controls. In the current study a single N-nitrosomethylbenzylamine dose was administered to Fhit +/+, +/−, and −/− mice to compare carcinogen susceptibility in +/- and −/− Fhit-deficient mice. At 29 weeks after treatment, 7.7% of wild-type mice had tumors. Of the Fhit −/− mice 89.5% exhibited tumors (average 3.3 tumors/mouse) of the forestomach and squamocolumnar junction; half of the −/− mice had medium (2 mm diameter) to large (>2 mm) tumors. Of the Fhit +/− mice 78% exhibited tumors (average 2.4 tumors/mouse) and 22% showed medium to large tumors. Untreated Fhit-deficient mice have been observed for up to 2 years for spontaneous tumors. Fhit +/− mice (average age 21 mo) exhibit an average of 0.94 tumors of different types; Fhit −/− mice (average age 16 mo) also showed an array of tumors (average 0.76 tumor/mouse). The similar spontaneous and induced tumor spectra observed in mice with one or both Fhit alleles inactivated suggests that Fhit may be a one-hit tumor suppressor gene in some tissues.
Deletion at chromosome region 3p14.2 is among the earliest events observed in many cancers and is observed in very early preneoplastic lesions of lung, cervix, and breast (1–5). The ≈2-Mb fragile FHIT gene is the likely target of these disease-associated deletions, which result in loss of portions of one or both FHIT alleles, with concomitant loss of at least half of the normal complement of Fhit protein. Whether loss of one FHIT allele predisposes to further genetic changes is not known, but clonal expansion of the 3p14.2-deleted cells occurs. In the majority of frankly malignant lesions of most cancer types, one or both FHIT alleles are damaged with resultant reduction or loss of the proapoptatic Fhit protein (refs. 6 and 7 for review), loss that has been correlated with worse outcome in some tumor types (8–11).
The FHIT gene, in both mouse and human (12–15), encompasses a constitutive chromosomal fragile site, which is susceptible to DNA gaps and breaks on exposure to carcinogens, a susceptibility that explains the frequent alterations to the gene in preneoplastic and neoplastic lesions. Because the FHIT locus is so susceptible to damage and is inactivated early in many cancers and its loss may have prognostic significance in a large fraction of cancers, Fhit interacting proteins, substrates, and effectors are attractive targets for inclusion in cancer prevention and therapy strategies (16, 17). To establish models for exploring the role of Fhit in cancer induction, prevention, and therapy, we have developed mouse strains carrying one or two inactivated Fhit alleles (18) and have studied the development of spontaneous and N-nitrosomethylbenzylamine (NMBA)-induced tumors in adult mice with intact or inactivated Fhit alleles. In a previous study, we had observed that 100% of Fhit +/− mice (heterozygous for an inactivated Fhit gene) developed tumors of the gastrointestinal tract after eight doses of NMBA, whereas only 25% of wild-type mice showed tumors (18). Also, 60% of the NMBA-induced Fhit +/− mice developed sebaceous tumors, whereas none of the NMBA-treated wild-type mice did; these sebaceous tumors were larger and apparently more frequent in the NMBA-treated Fhit +/− mice than in untreated littermates. Thus, the NMBA-induced phenotype was similar to the Muir-Torre syndrome (MTS) phenotype observed in a subset of hereditary nonpolyposis colon cancer cases exhibiting MSH2 mutation (19). We concluded that Fhit is a gatekeeper tumor suppressor for the NMBA-induced MTS-like phenotype.
In the current study, we have observed aged cohorts of mice with two, one, or no intact Fhit alleles for development of disease and have compared susceptibility of all three genotypes to a single dose of the gastric carcinogen, NMBA. More than 50% of +/− and −/− mice developed spontaneous tumors in a broad spectrum of tissues, compared with 8% in mice with two intact Fhit alleles. More than 75% of +/− and −/− mice developed multiple gastric tumors by 29 weeks after the single carcinogen dose, compared with 8% of wild-type mice. For both spontaneous and induced tumors, the frequency of tumor development was not significantly different in the Fhit-deficient mice of +/− and −/− genotypes, suggesting that loss of one Fhit allele had nearly the same effect on tumor development as loss of both Fhit alleles; that is, as previously demonstrated for the p27Kip1 protein (20), Fhit protein may be haploid insufficient for tumor suppression in some mouse tissues.
Materials and Methods
Phenotyping of Untreated Mice.
(C57BL/6J × 129/SvJ) F1, F2, F3, and F4 mice (B6129 F1-F4), of which 12 were Fhit +/+, 23 Fhit +/−, and 24 Fhit −/− (5–27 months of age), were produced in the Kimmel Cancer Center animal facility and observed weekly for up to 2 years. When animals appeared unwell, either because of visible lesions, dull coat, hunched appearance, weight loss, or advanced age, animals were killed and autopsied; tissues of most organs, as well as apparently diseased tissues, were removed, fixed in buffered formalin, and examined histologically by two pathologists after hematoxylin/eosin staining. Lesions were photographed and sections were taken for immunohistochemical analyses.
Carcinogenicity Study.
Thirteen Fhit +/+, 23 Fhit +/−, and 38 Fhit −/− mice (7–10 months of age) were given an intragastric dose of NMBA (Ash Stevens, Detroit), 2 mg/kg body weight. All of the mice were killed 29 weeks after the NMBA dose and were examined for end-point tumor incidence. At autopsy, whole esophagi and stomachs were removed and opened longitudinally. Other tissues with apparent tumors also were examined. The number of animals bearing tumors in the esophagus, the forestomach, the squamocolumnar junction with the glandular stomach, and other tissues were scored. The tissues were fixed in buffered formalin and examined histologically, after hematoxylin/eosin staining, for the presence of hyperkeratosis, parakeratosis, dysplasia, papillomas, adenomas, and carcinomas.
Statistical Analysis.
Tumor incidence differences were analyzed by two-tailed Fisher's exact test (Biostat, www.matforsk.no/ola/fisher.htm).
Immunohistochemistry.
Tissue sections were mounted on silane-coated slides, dewaxed, rehydrated, and pretreated with citrate buffer (pH 5.5) for CD3 or Target Retrieval Solution (Dako) for CD79a antigen for 5 min in a pressure cooker.
For B cell antigen receptor complex, CD79a, detection, a goat polyclonal antiserum (Santa Cruz Biotechnology) was used at a dilution of 1:20 for 1 h, after blocking with rabbit serum (Zymed) for 10 min. CD79a staining was detected with biotinylated rabbit anti-goat (Zymed) for 20 min, streptavidin-conjugated peroxidase (Zymed) for 20 min, and 3,3′-diaminobenzidine tetrahydrochloride (Sigma) for 10 min. For T cell CD3 complex detection, an anti-CD3 rabbit polyclonal antiserum (Dako) was used at 1:100 for 1 h after blocking with goat serum (BioGenex Laboratories, San Ramon, CA) for 10 min. Biotinylated goat anti-rabbit (BioGenex) was applied for 20 min and detected with streptavidin-conjugated peroxidase (Zymed) for 20 min and 3,3′-diaminobenzidine tetrahydrochloride (Sigma) for 10 min. For detection of B cell IgG, a Histostain-Plus Kit (Zymed) was used; detection also was based on a biotin-streptavidin-peroxidase system. As a control, PBS was substituted for primary antibody.
For Fhit staining, endogenous peroxidase was inhibited with 3% hydrogen peroxide, and nonspecific binding sites were blocked with normal goat serum. Slides were incubated with the primary rabbit antibody, anti-glutathione S-transferase murine Fhit (1:4,000 dilution, overnight), followed by incubation with biotinylated goat anti-rabbit antibody. Negative controls were not incubated with the primary antibody. Slides were then incubated with streptavidin horseradish peroxidase (Dako, 1:1,000 dilution). Fhit protein was localized by a final incubation with 3,3′-diaminobenzidine tetrahydrochloride (Sigma). Slides were counterstained with hematoxylin, dehydrated, and coverslipped.
Results and Discussion
Genotypes and Phenotypes.
A total of 59 B6129 F1–F4 hybrid mice were placed on a time study and observed, untreated, for up to 2 years. Twelve mice had Fhit +/+ genotype, 23 +/−, and 24 −/−. All mice developed normally, were fertile, and were born at the expected ratios. During the time study six +/− mice (26%) and seven −/− mice (29%) were found dead for unknown reasons. The average age of the dead animals was 19.5 mo (+/−) and 18.2 mo (−/−); none of the +/+ littermates died spontaneously in the same period (P = 0.05 +/+ vs. Fhit/−). Because several Fhit-deficient mice showed apparent large abscesses, we considered the possibility that infections could have caused the ≈30% mortality and have tabulated the infections observed in all of the mice autopsied. Two of 12 (16%) wild-type mice showed abscesses in the liver by the end of the experiment. Of the Fhit +/− mice, 17% showed evidence of infection in different tissues and of the −/− mice, 29% showed evidence of infection. The increased mortality and infection in −/− mice and the observed lymphoid hyperplasia (see below) suggested that Fhit-deficient mice might exhibit some form of immune deficiency. In preliminary experiments, we have characterized the percentage of peripheral blood lymphocytes, monocytes, and granulocytes in the three mouse strains. In wild-type mice, granulocytes represented 15% of the total, whereas in +/− and −/− mice granulocytes represented 2–6% in three experiments.
The sebaceous tumors observed in the first NMBA experiment (18) were large and frequent (60% of mice) in Fhit +/− mice of the F1 generation. Sebaceous tumors have never been observed in wild-type mice with or without NMBA treatment. In untreated Fhit +/− and −/− mice of generations F1–F4, 20% of each genotype showed spontaneous sebaceous tumors, probably an underestimation because autopsies did not include the entire skin of the mice. Thus, sebaceous tumors, in humans a hallmark of MTS (19), occur spontaneously in Fhit-deficient mice and are increased in size and frequency in mice-treated with eight doses of NMBA, considered a low-dose treatment. In a second eight-dose NMBA experiment (unpublished results) 27% of Fhit +/− (F2 generation) mice showed sebaceous tumors, with one mouse exhibiting four such tumors and another showing a large sebaceous tumor (7 × 4 mm). These NMBA experiments show that the Fhit-deficient mice are susceptible to an MTS-like phenotype, expression of which is enhanced by genetic damage to other genes. As noted previously (18), this result suggests that the action of the carcinogen, NMBA, mimics the MSH2 deficiency of human MTS and that Fhit acts as a gatekeeper in the wild-type mice.
In the current cohorts of single-dose NMBA-treated mice (see below), fewer and smaller sebaceous tumors were observed (≈10 and 13% of −/− and +/− mice, respectively; these tumors were not examined histologically, nor were the mice thoroughly examined for presence of skin lesions), confirming the conclusion that the eight-dose NMBA treatment induced both gastric and sebaceous tumors in Fhit-deficient mice. These mice probably exhibited fewer sebaceous tumors than the aged, untreated mice because they were killed at less than 1 year of age.
NMBA-Induced Gastric Tumors.
A preliminary experiment (unpublished results) to compare NMBA sensitivity of Fhit +/− and Fhit −/− mice was designed with an eight-dose NMBA protocol, as previously reported for Fhit +/− mice (18). All of the Fhit +/− (15/15) and −/− (8/8) mice developed tumors compared with only 25% of +/+ controls. There was no observable difference between Fhit +/− and −/− mice in number and types of tumors occurring in the esophagus, forestomach, and squamocolumnar junction with the glandular stomach (unpublished results). Thus, we designed a second experiment in which mice of all three genotypes, 13 Fhit +/+, 23 +/−, and 38 −/−, were treated with a single intragastric dose of NMBA and were killed 29 weeks later. Results of this experiment are shown in Table 1 and Fig. 1. The fraction of tumor bearing mice was ≈8% for Fhit +/+ mice, 78% for +/−, and 89% for −/− mice. The number of tumor-bearing animals among Fhit-deficient mice was ≈10-fold higher than in wild-type littermates and the incidence of gastric tumors per mouse in Fhit-deficient mice was 12- to 16-fold higher than in wild-type mice; the trend toward more tumors in Fhit −/− relative to Fhit +/− mice was not statistically significant at this NMBA dose and time of observation. Carcinogen-induced lesions observed in the forestomach of the three mouse genotypes were mainly hyperplasias and papillomas with squamous cell carcinomas occurring in a few Fhit-deficient mice (Fig. 2). Fifty three percent of Fhit −/− and 61% of Fhit +/− mice showed multiple tumors ≥ 2 mm. The frequency of small tumors (<2 mm) was similar in +/− and −/− mice; large tumors (>2 mm) were observed in 24% of −/− mice and 17% of +/− mice. These results demonstrated that there is little difference in NMBA susceptibility in Fhit-deficient mice, whether they are missing one or both Fhit alleles. Although unexpected, this result is consistent with observations of similar frequencies of spontaneous and induced sebaceous tumors in the two genotypes and similar tumor frequency observed in the preliminary eight-dose NMBA treatment of the two deficient genotype strains.
Table 1.
Incidence of gastric tumors induced by NMBA in wild-type and Fhit-deficient mice
| Genotype | Total tumors | Tumor-bearing mice | Tumors/mouse |
|---|---|---|---|
| +/+ | 3 | 1/13 (7.7%) | 0.2 |
| +/− | 55 | 18/23 (78.3%) | 2.4 |
| −/− | 125 | 34/38 (89.5%) | 3.3 |
A total of 74 B6129F1–F4 mice (7–10 months of age) were given an intragastric dose of NMBA, 2 mg/kg body weight. All of the mice were killed 29 weeks after the NMBA dose. Tumor incidence was analyzed by two-tailed Fisher's exact test: +/− vs. +/+, P = 6.4 × 10−5; −/− vs. +/+, P = 1.3 × 10−7; +/− vs. −/−, P = 0.3.
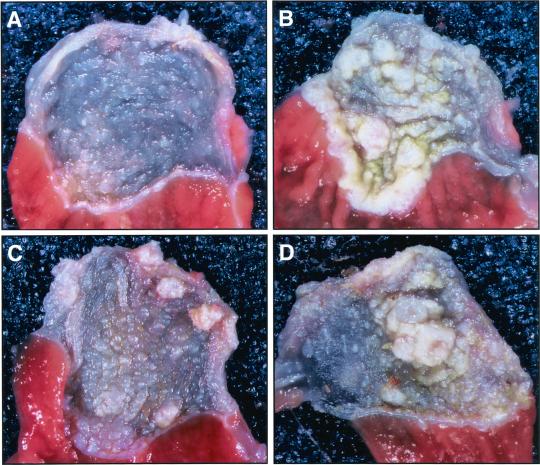
Figure 1.
Gross anatomy of murine forestomach after NMBA treatment. Typical aspects of NMBA-induced pathology in forestomachs of Fhit +/+ mouse 54 (A), Fhit +/− mouse 59 (B), Fhit −/− mouse 15 (C), and Fhit −/− mouse 17 (D) are shown. (Magnification: ×5.)
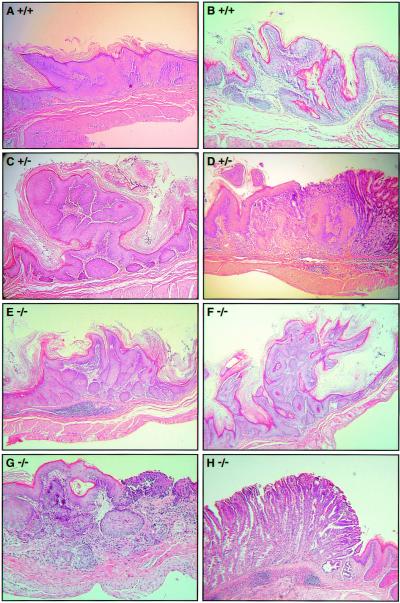
Figure 2.
NMBA-induced histopathology of murine forestomach. Examples of the carcinogen-induced lesions observed in the three strains of mice: hyperplasia in Fhit +/+ mouse 43 (A), early papilloma in Fhit +/+ mouse 53 (B), papilloma in Fhit +/− mouse 55 (C), squamous cell carcinoma in Fhit +/− mouse 62 (D), focal hyperplastic lesion in Fhit −/− mouse 17 (E), papilloma in Fhit −/− mouse 20 (F), squamous cell carcinoma in Fhit −/− mouse 8 (G), and foveolar hyperplasia of the glandular stomach in Fhit −/− mouse 28 (H). (Magnification: ×200.)
In the previous study of NMBA-induced tumors in Fhit +/− mice (18), we examined a number of the larger tumors for Fhit expression and observed that several of the tumors showed loss of Fhit protein by immunohistochemical analysis. We concluded that the second Fhit allele was probably inactivated in these tumors, although we did not observe genomic alterations. In the current study we also have examined a number of the tumors by immunohistochemistry (Fig. 3) and have observed that Fhit protein is still expressed in some of the tumors of Fhit +/− mice; in Fig. 3D, most of the cells of the early papilloma exhibit weak Fhit staining, whereas in Fig. 3E many of the cells of the squamous cell carcinoma from +/− mouse 62 express a low level of Fhit (compare with Fig. 3B showing Fhit negative cells of a basal cell hyperplasia of −/− mouse 16).
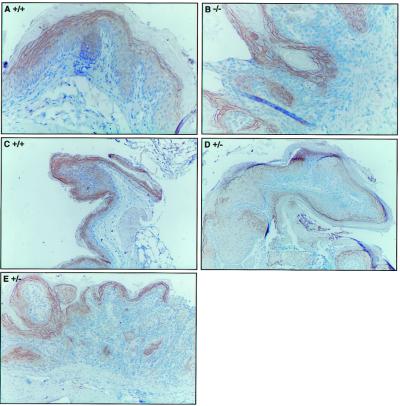
Figure 3.
Immunohistochemical detection of Fhit in NMBA-treated forestomach tissue. Forestomach sections were analyzed for expression of Fhit protein by immunohistochemistry using the rabbit anti- glutathione S-transferase-Fhit antiserum. (A) Normal epithelium in +/+ mouse 53; (B) basal cell hyperplasia in −/− mouse 16; (C) early papilloma in +/+ mouse 53; (D) early papilloma in +/− mouse 55; and (E) squamous cell carcinoma in +/− mouse 62. Note that the staining procedure results in nonspecific staining (B) of the highly keratinized outer layer. (Magnifications: A and B, ×200; C–E, ×100.)
In summary, the results of the carcinogen studies strongly suggested that loss of one or both Fhit alleles causes similar susceptibility to tumors of the murine upper gastrointestinal tract and suggested that Fhit, like p27Kip1 (20), could be a haploinsufficient tumor suppressor in some tissues.
Spontaneous Tumor Phenotypes.
Mice were killed and autopsied between 5 and 27 months of age. Tissues from 13 different organs (see Table 2) were examined for gross pathology and histology, although not all organs were examined in all mice. Twelve mice were Fhit +/+ (average age 22.4 ± 2.3 mo), 17 were +/− (21.5 ± 3.5 mo), and 17 were −/− (16.3 ± 5.9 mo). The number of tumor-bearing animals among Fhit-deficient mice, +/− and −/−, was 6-fold higher than in wild-type mice, and the incidence of tumors per mouse in Fhit-deficient mice was at least 10-fold higher than in wild-type littermates (see Table 2). The difference in tumor incidence between Fhit +/− and −/− mice was not statistically significant. Fhit-deficient mice displayed a wide spectrum of spontaneous tumors (Fig. 4). Wild-type mice spontaneously developed two types of tumors: lung tumors, both adenocarcinomas and carcinoid (Fig. 4D), and lymphoma. We found approximately the same incidence of lung tumors in Fhit-deficient mice (Fig. 4E, for example); and have not tabulated them in our comparison of the three genotypes. A total of 8.3% (1/12) of wild-type mice were diagnosed with lymphoma; a slightly increased incidence of lymphoma, mainly polymorphous B cell lymphomas (for example see Fig. 4H), was found in 20.6% of Fhit-deficient mice (7/34). Several aged mice of all three genotypes showed hyperplasia of lymphoid tissues and 15% (5/34) of Fhit-deficient mice showed atypical hyperplasia, a preneoplastic condition. Both Fhit-deficient genotypes also showed more frequent perivascular atypical B cell infiltrates in internal organs, mainly lungs and kidneys. In total, 68% (23/34) of Fhit-deficient mice displayed some type of lymphoid lesion (hyperplasia, lymphoid infiltrates, or lymphoma) compared with 33% (4/12) of +/+ littermates (P < 0.05). A number of other tumors were found only in Fhit-deficient mice (Table 3). The most frequently occurring were sebaceous tumors (20%, Fig. 4F), liver hemangiomas (12%, Fig. 4A), fundic polyps of the stomach (9%, Fig. 4C), and colonic submucosal stromal and neurogenic tumors (6%). Six other types of tumors (18%) occurred only once, three in Fhit +/− and three in −/− mice (Table 3, Fig. 4 B–G). The data in Tables 2 and 3 confirm that for spontaneous tumors of various tissues there is little difference in tumor susceptibility in the Fhit +/− and −/− mouse strains.
Table 2.
Incidence of spontaneous tumors in wild-type and Fhit-deficient mice
| Genotype | Total tumors | Tumor-bearing mice | Tumors/mouse |
|---|---|---|---|
| +/+ | 1 | 1/12 (8.3%) | 0.08 |
| +/− | 16 | 9/17 (52.9%) | 0.94 |
| −/− | 13 | 9/17 (52.9%) | 0.76 |
Autopsies were performed after CO2 asphyxiation. Tissues from 13 different organs were fixed in 10% phosphate-buffered formalin: skin, pancreas, spleen, kidneys, thymus, lungs, heart, stomach, salivary glands, small intestine, colon-rectum, lymph nodes, and liver. Tissues were examined histologically after hematoxylin and eosin staining for the presence of hyperplasia, dysplasia, papillomas, adenomas, and carcinomas. Tumor incidence was analysed by two-tailed Fisher's exact test: −/+ vs. +/+, P = 0.02; −/− vs. +/+, P = 0.02.
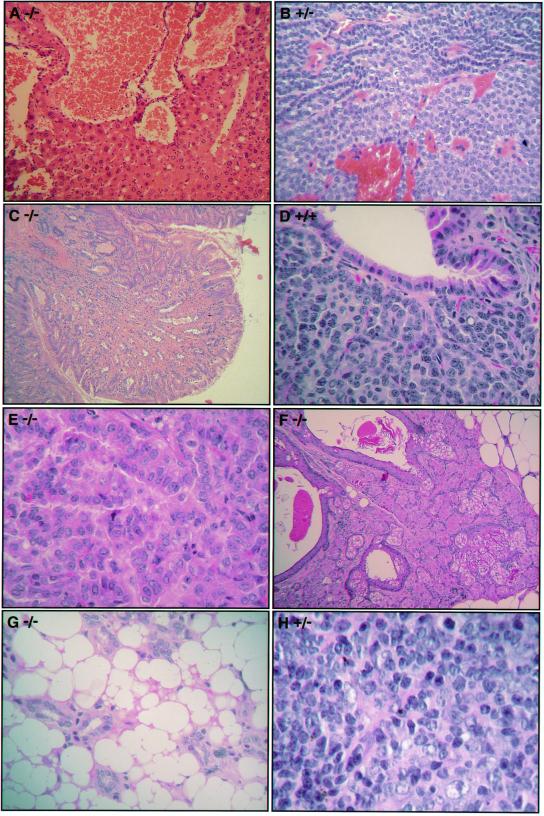
Figure 4.
Histopathology of spontaneous tumors. Examples of the spontaneous lesions observed in aging mice of all three genotypes. Mouse 6F2 (−/−) liver hemangioma (A), mouse 1047 (+/−) parathyroid adenoma (B), mouse 3027 (−/−) fundic polyp (C), mouse 6173–4 (+/+) lung carcinoid (D), mouse 3103 (−/−) lung adenocarcinoma (E), mouse 3103 (−/−) sebaceous tumor (F), mouse 789 (−/−) angiolipoma (G), and mouse 977 (+/−) large B cell lymphoma (H). (Magnifications: A–C and F, ×100; D, ×200; E, G, and H, ×400.)
Table 3.
Spontaneous tumor spectrum in Fhit-deficient mice
| Lymphomas | Sebaceous tumors | Liver tumors | Gastric polyps | Colonic submucosal tumors |
|---|---|---|---|---|
| +/+ | +/− | +/− Hemangioma | +/− | +/− Neuroma |
| +/− | +/− | +/− Hemangioma | −/− | +/− Lipoma |
| +/− | +/− | +/− Adenoma | −/− | |
| +/− | +/− | −/− Hemangioma | ||
| −/− | −/− | |||
| −/− | −/− | |||
| −/− | −/− | |||
| −/− | ||||
| Thymoma | Uterine leiomyoma | Subcutaneous | ||
| −/− | +/− | angiolipoma | ||
| −/− | ||||
| Warthin's tumor | Parathyroid adenoma | Benign stromal tumor | ||
| +/− | +/− | −/− |
The spontaneous tumor spectrum observed in Fhit-deficient mice does not obviously coincide with that of other tumor suppressor knockout strains, although there is apparent partial overlap with the tumor spectrum of Vhl conditional knockouts (21), which showed hemangiomas of the liver, and a carcinogen-induced tumor phenotype partially overlapping with Msh2 knockout mice (22). This finding suggests that the Fhit signal pathway(s) is not entirely within the pathway of other known tumor suppressor proteins.
As for p27Kip1 protein (20), a 50% reduction in Fhit protein, a condition that could frequently occur because of the fragility of the Fhit locus in mouse and human, must provide a selective advantage for clonal growth in a diversity of tissues, which includes sebaceous glands, liver, and hematopoietic tissues in mice. With exposure to carcinogens that act on multiple organs, a wide variety of cancers of epithelial origin probably would be observed. It is likely that Fhit-deficient mice will thus prove useful in understanding the role of FHIT in cancer and will provide model systems for the study of carcinogenicity of specific compounds and the study of development, prevention, and treatment of common cancers, as recently demonstrated by prevention of the NMBA-induced upper gastric cancers in Fhit +/− mice by treatment with viral vectors carrying the FHIT gene (16, 17).
Finally, the observation that FHIT is haploinsufficient for tumor suppression may explain a number of early observations showing that some tumors retain an intact copy of FHIT and express reduced levels of Fhit protein.
Acknowledgments
We thank Almeta Mathis for manuscript preparation, Dr. Rossano Cesari for technical assistance, and the Kimmel Cancer Center transgenic facility for generation of Fhit knockout mice. This study was supported by Program Project Grants CA77738 and CA21124 and Cancer Center Support Grant CA56036 from the U.S. Public Health Science
Abbreviations
- NMBA
N-nitrosomethylbenzylamine
- MTS
Muir-Torre syndrome
References
- 1.Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, Ghirelli C, Tornielli S, Sard L, Huebner K, Pierotti M A, et al. Cancer Res. 1998;58:5032–5037. [PubMed] [Google Scholar]
- 2.Ahmadian M, Wistuba I I, Fong K M, Behrens C, Kodagoda D R, Saboorian M H, Shay J, Tomlinson G E, Blum J, Minna J A, Gazdar A F. Cancer Res. 1997;57:3664–3668. [PubMed] [Google Scholar]
- 3.Wistuba I I, Lam S, Behrens C, Virmani A K, Fong K M, LeRiche J, Samet J M, Srivastava S, Minna J D, Gazdar A F. J Natl Cancer Inst. 1997;89:1366–1373. doi: 10.1093/jnci/89.18.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birrer M J, Hendricks D, Farley J, Sundborg M J, Bonome T, Walts M J, Geradts J. Cancer Res. 1999;59:5270–5274. [PubMed] [Google Scholar]
- 5.Connolly D C, Greenspan D L, Wu R, Ren X, Dunn R L, Shah K V, Jones R W, Bosch F X, Munoz N, Cho K R. Clin Cancer Res. 2000;6:3505–3510. [PubMed] [Google Scholar]
- 6.Huebner K, Garrison P N, Barnes L D, Croce C M. Annu Rev Genet. 1998;32:7–31. doi: 10.1146/annurev.genet.32.1.7. [DOI] [PubMed] [Google Scholar]
- 7.Croce C M, Sozzi G, Huebner K. J Clin Oncol. 1998;17:1618–1624. doi: 10.1200/JCO.1999.17.5.1618. [DOI] [PubMed] [Google Scholar]
- 8.Capuzzi D, Santoro E, Hauck W W, Kovatich A, Rosato F, Baffa R, Huebner K, McCue P A. Cancer. 1999;88:24–34. [PubMed] [Google Scholar]
- 9.Tomizawa Y, Nakajima T, Kohno T, Saito R, Yamaguchi N, Yokota J. Cancer Res. 1998;58:5478–5483. [PubMed] [Google Scholar]
- 10.Lee J I, Soria J-C, Hassan K, Liu D, Tang X, El-Naggar A, Hong W K, Mao L. Cancer Res. 2001;61:837–841. [PubMed] [Google Scholar]
- 11.Ingvarsson S, Sigbjornsdottir B I, Huiping C, Jonasson J G, Agnarsson B A. Cancer Detect Prev. 2001;25:318–324. [PubMed] [Google Scholar]
- 12.Ohta M, Inoue H, Cotticelli M G, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 13.Zimonjic D B, Druck T, Ohta M, Kastury K, Popescu N C, Huebner K. Cancer Res. 1997;57:1166–1170. [PubMed] [Google Scholar]
- 14.Glover T W, Hoge A, Miller D E, Ascara-Wilke J E, Adam A, Dagenais S L, Wilke C M, Dierick H A, Beer D G. Cancer Res. 1998;58:3409–3414. [PubMed] [Google Scholar]
- 15.Pekarsky Y, Druck T, Cotticelli M G, Ohta M, Shou J, Mendrola J, Montgomery J C, Buchberg A M, Manenti G, Fong L Y Y, et al. Cancer Res. 1998;58:3401–3408. [PubMed] [Google Scholar]
- 16.Dumon K R, Ishii H, Fong L Y Y, Zanesi N, Fidanza V, Mancini R, Vecchione A, Baffa R, Trapasso F, During M J, et al. Proc Natl Acad Sci USA. 2001;98:3346–3351. doi: 10.1073/pnas.061020098. . (First Published February 27, 2001; 10.1073/pnas.061020098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii H, Dumon K, Vecchione A, Trapasso F, Mimori K, Alder H, Mori M, Sozzi G, Baffa R, Huebner K, Croce C M. Cancer Res. 2001;61:1578–1589. [PubMed] [Google Scholar]
- 18.Fong L Y Y, Fidanza V, Zanesi N, Lock L, Siracusa L, Mancini R, Sprashvili Z, Ottey M, Martin S E, Druck T, et al. Proc Natl Acad Sci USA. 2000;97:4742–4747. doi: 10.1073/pnas.080063497. . (First Published April 11, 2000; 10.1073/pnas.080063497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse R, Rutten A, Lamberti C, Hosseiny-Malayeri H R, Wang Y, Ruelfs C, Jungck M, Mathiak M, Ruzicka T, Hartschuh W, et al. Am J Hum Genet. 1998;63:63–70. doi: 10.1086/301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fero M L, Randel E, Gurley K E, Roberts J M, Kemp C J. Nature (London) 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haase V H, Glickman J N, Socolovsky M, Jaenisch R. Proc Natl Acad Sci USA. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reitmair A H, Redston M, Cai J C, Chuang T C Y, Bjerknes M, Cheng M, Hay K, Gallinger S, Bopat B, Mak T W. Cancer Res. 1996;56:3842–3849. [PubMed] [Google Scholar]