Abstract
Combination cancer treatment has emerged as a critical approach to achieve remarkable anticancer effect. In this study, we prepared a theranostic nanoformulation that allows for photoacoustic imaging as well as combination gene and photothermal therapy. Gold nanorods (GNR) were coated with dipicolyl amine (DPA), which forms stable complexes with Zn2+ cations. The resulting nanoparticles, Zn(II)/DPA-GNR, recognize phosphate-containing molecules, including siRNA, because of the specific interaction between Zn(II) and the phosphates. We chose anti-polo-like kinase 1 siRNA (siPLK) as our example for gene silencing. The strong complexation between Zn(II)/DPA-GNR and siPLK provided high stability to the nano-complexes, which efficiently delivered siRNA into the targeted cancer cells in vitro and in vivo. The particle served as a theranostic agent because the GNRs of nano-complexes permitted effective photothermal therapy as well as photoacoustic imaging upon laser irradiation. This gene/photothermal combination therapy using siPLK/Zn(II)DPA-GNRs exhibited significant antitumor activity in a PC-3 tumor mouse model. The concept described in this work may be extended to the development of efficient delivery strategies for other polynucleotides as well as advanced anticancer therapy.
Keywords: dipicolylamine, metal-organic complexes, siRNA, gold nanorod, photothermal therapy, photoacoustic imaging, combination therapy, theranostics.
Introduction
RNA interference (RNAi) has been widely used for cancer therapy because of its naturally occurring unique sequence-specific post-transcriptional gene silencing effect. Recently significant progresses have been made in this research field.1-3 However, due to the anionic charge of siRNA phosphodiester backbone and its large molecular weight, the delivery of siRNA based therapeutics is restricted by a relatively poor pharmacokinetics and low cellular internalization following systemic administration 4, 5. Despite promising pre-clinical studies, there are currently no clinically successful siRNA formulations for tumor treatment. Although numerous tumor therapeutic siRNA delivery carriers have been developed based on cationic polymers, cationic lipids, or amino acids that efficiently bind anionic RNAi, such as small interfering RNA (siRNA) and microRNA (miRNA) 6-9, these strong cationic carriers cause destabilization of cell membranes and severe cytotoxicity to normal tissues 10-14. This non-specific cell cytotoxicity has proven to be a major barrier in the clinical application of RNAi delivery systems. To overcome these limitations, more study is required to develop efficient siRNA delivery nanoplatforms without using strong cationic derivatives.
Recently, we developed a hyaluronic acid (HA)-based nanoparticle system for delivering siRNA that uses Zn(II)-dipicolylamine (Zn-DPA) receptors for high siRNA binding affinity 15,16. The Zn-DPA receptors are metal-organic complexes that have high affinity for phosphate-containing molecules such as siRNA through specific interactions between the coordinated zinc ions of the DPA and the anionic phosphate moieties 17-19. Various Zn-DPA analogues have been developed as useful tools for the sensing of biologically important phosphate derivatives 20-22. However, the application of Zn-DPA analogues for siRNA delivery has not been fully explored. Furthermore, these Zn-DPA analogue-based nanoparticle systems are significantly less cationic in character than conventional siRNA delivery carriers and therefore, have tremendous potential for the development of non-toxic and efficient therapeutic siRNA carriers.
As a nanocarrier for siRNA delivery, multi-functional gold nanorod particles are attractive hybrid materials composed of an inorganic metallic gold core surrounded by an organic or biomolecular layer. These nanomaterials are promising candidates in drug delivery owing to their tunable core size, monodispersity, low toxicity, unique dimensions and tunable surface functionalities as well as the ability for photoacoustic tomography (PAT) 23-26. Especially because of their large surface-to-volume ratio, GNRs are ideal nanocarriers which can provide sufficient surface area for coupling Zn-DPA receptors to complex with siRNA. Furthermore, application of thermotherapy to destroy specific cells is a well-known strategy for the treatment of cancer since it is a noninvasive method and a relatively painless treatment for patients 27-29. Due to the GNRs' strong plasmonic resonance peak, GNRs efficiently convert light energy into surface-localized heat generated as a consequence of electron phonon collisions, known as photothermal conversion, when the near-infrared light matches the plasmon resonance wavelength 30, 31. Heat transfer from the surface of GNRs to the surrounding cellular environment is highly localized, decaying exponentially within a few nanometers and therefore is thought to minimize adverse effects on normal cells 32.
Thus, these properties would reduce damages to healthy cells during photothermal therapy of the tumor region and provide synergistic effect associated with combination therapy of siRNA agents and photothermal therapy, which will lead to enhanced anti-tumor efficacy. Although single-arm treatment is still widely used for many different cancers, this conventional method remains less effective than combination therapy 33. For the successful cancer treatment, combination therapy is an emerging potential method that combines two or more different therapeutic drugs or treatments by working in a synergistic or additive manner 34. Moreover, this combination therapy requires a lower therapeutic dosage of each individual drug or treatment and could prevent the toxic effects on normal cells while simultaneously producing cytotoxic effects on cancer cells as combination therapy targets different pathways to enhance cytotoxicity to cancer cells. In nanomedicine, the exploitation of multifunctional nanoparticles for effective delivery of combined therapeutic agents or treatments in order to improve cancer therapy has arisen as one of the promising strategies 35, 36.
Polo-like kinase 1 (PLK1) is a member of serine/threonine protein kinases and the most extensively studied member of polo-like kinases. PLK1 has been reported to play a critical role in regulating multiple processes including mitosis, meiosis, spindle assembly, centrosome maturation, anaphase promoting complex (APC), and cytokinesis 37, 38. It has been found that the concentration and activity of PLK1 is important for the processes of cell cycle 39, 40. In addition, PLK1 has also been regarded as a proto-oncogene that is overexpressed in several different cancers, including prostate cancer, breast cancer, and non-small-cell lung cancer, being often correlated with poor patient prognosis while its expression level in normal tissues is often very low or undetectable 41-44. Preclinical studies indicate that pharmacological or genetic inhibition of PLK1 triggers mitotic arrest and enhances cell death in cancer cells. Notably, high expression level of PLK1 in tumors is correlated with low survival rate of patients 42, 45. Recently, numerous inhibitors of PLK1 have been developed to treat several types of cancers 46-50. Thus, PLK1 inhibition is a promising therapeutic approach for cancer therapy.
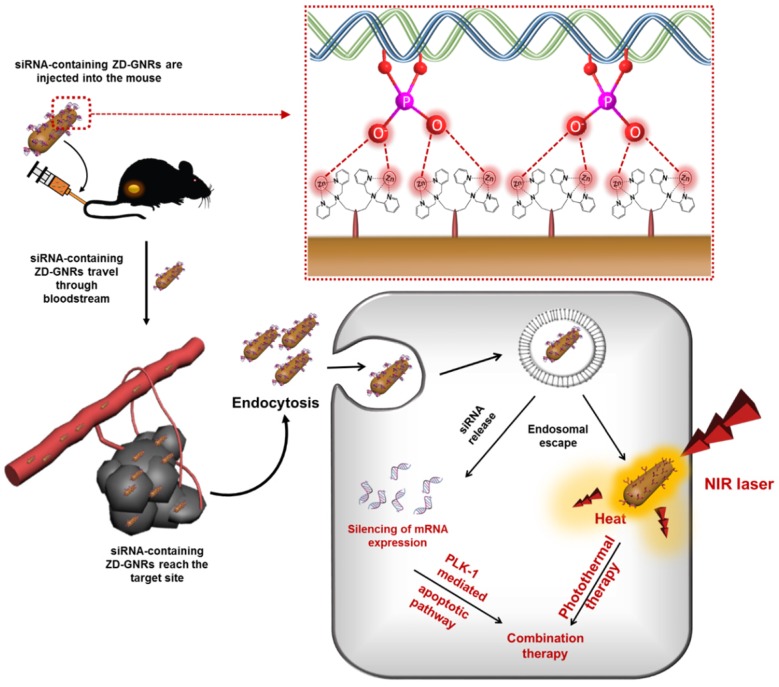
Herein, we developed a novel nanoformulation by conjugating Zn-DPA (ZD) onto gold nanorods (GNRs) and, subsequently, loading the particle with siRNA. The resulting particles facilitate the release of entrapped siRNA within tumor cells and cause a prominent gene silencing effect. Moreover, due to the unique photoacoustic (PA) property of GNRs, ZD-GNR serves as a theranostic agent for simultaneous PA imaging and therapy of cancer by generating heat upon laser irradiation. Figure 1 illustrates the process of developing siRNA complexed Zn-DPA conjugated GNRs (siRNA/ZD-GNRs) and working principle of combination therapy of both gene-silencing of siRNA and photothermal effect upon laser irradiation.
Figure 1.
Schematic illustration of specific interaction between the Zn(II)-dipicolylamine (Zn-DPA) and phosphate groups of siRNA and combined anti-PLK1 gene therapy/photothermal therapy upon laser irradiation after the accumulation of siPLK/ZD-GNRs at the target tumor tissues.
Results and Discussion
Fabrication and characterization of siRNA/ZD-GNRs
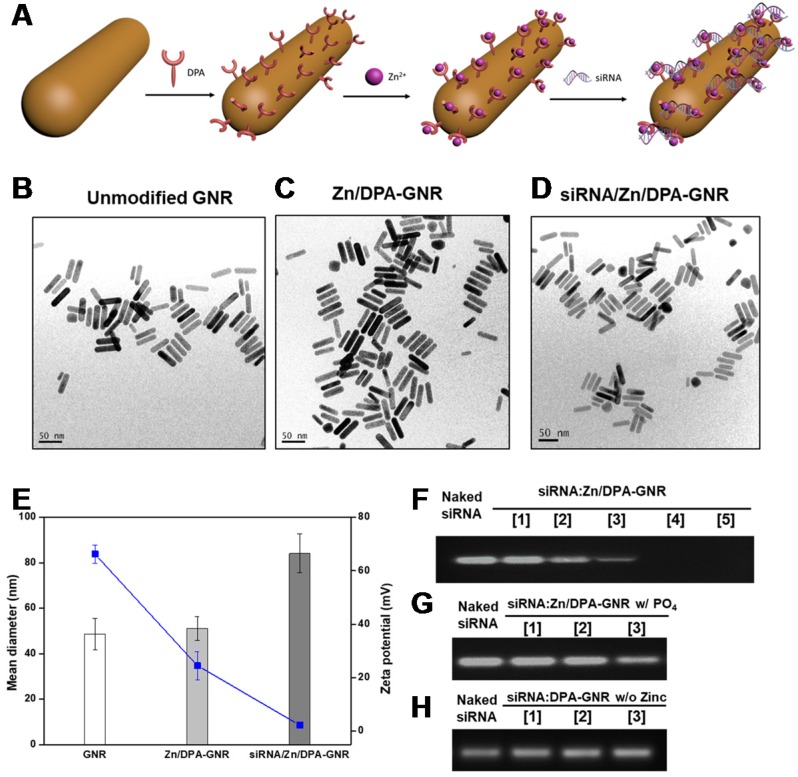
Figure 1 illustrates the process of developing siRNA complexed Zn-DPA conjugated GNRs (siRNA/ZD-GNRs) and working principle of combination therapy of both gene-silencing of siRNA and photothermal effect upon laser irradiation. For the fabrication of siRNA/ZD-GNRs, we coupled lipoic acid-functionalized bis-DPA molecules onto the surface of gold nanorods (GNRs). First, lipoic acid-modified bis-DPA was synthesized and confirmed by 1H-NMR and LCMS (Figure S2 and Figure S3 in the Supporting Information). Second, the DPA-GNRs were synthesized through a ligand exchange reaction that allows replacing hexadecyltrimethylammonium bromide (CTAB) with lipoic acid-modified bis-DPA by forming covalent Au-S bonds between the CTAB-stabilized GNRs and lipoic acid-modified bis-DPA 51. Excess lipoic acid-modified bis-DPA was removed using repeated centrifugation and dispersed in distilled water.
To find the ideal molar ratio of [DPA]/[GNRs] and obtain uniform and stable bis-DPA functionalized GNRs, we investigated the conversion of bis-DPA (%) from various molar ratios of [DPA]/[GNRs]. As can be seen in Table S1 in the Supporting Information, we found that low molar feed ratios of [DPA] to [GNRs], such as 10:1 (DPA-GNR1), 50:1 (DPA-GNR2), or 100:1 (DPA-GNR3), resulted in particle aggregation, despite high incorporation of bis-DPA. At the molar feed ratio of 500:1 (DPA-GNR4), we obtained uniform, non-aggregated bis-DPA functionalized GNRs, as demonstrated by transmission electron microscopy (TEM) (Figure 2c). Additionally, the high density of DPA attached to the GNR surface is expected to have a high affinity for siRNA by specific interactions between coordinated zinc ions of DPA and anionic phosphates of the RNA. Dynamic light scattering (DLS) was employed to characterize the particle size and zeta potential of siRNA/ZD-GNRs and the intermediates. After DPA-GRNs were complexed with zinc ion, the mean diameter of DPA-GNRs slightly increased from 48.6 ± 6.8 to 51.13 ± 5.2 nm and the surface charge decreased but remained positive from 66.2 ± 3.4 to 24.5 ± 5.2 mV due to the complexation of zinc ions with the bis-DPA molecules (Figure 1 and 2b).
Figure 2.
Characterization of siRNA/ZD-GNRs. (a) Schematic illustration of assembly of siRNA/ZD-GNRs. (b-d) Representative TEM images of GNRs (b), ZD-GNRs (c), and siRNA/ZD-GNRs (d). (e) Size distribution and zeta-potential of GNRs, ZD-GNRs and siRNA/ZD-GNRs (n = 3). (f-h) Electrophoretic retardation analysis of siRNA binding with ZD-GNRs: (f) (Lanes 1-5: complexes at siRNA/ZD-GNR molar ratios of 600, 300, 200, 100, and 50; (g) After addition of phosphate ions (Lanes 1-3: complexes at molar ratio of 200, 100, and 50); and (h) siRNA binding with DPA-GNRs without zinc ions (Lanes 1-3: complexes at molar ratios of 200, 100 and 50).
The formation of siRNA/ZD-GNR complexes (siRNA/ZD-GNR) was investigated by the agarose gel retardation assay by using a siRNA (siLuc) that targets firefly luciferase (fluc) gene as a model siRNA. When the molar ratio of siRNA/ZD-GNR reached 100, the ZD-GNRs completely bound siRNA to form nano-complexes (Figure 2f). After formulation of siLuc/ZD-GNRs, the complexes formed spherical structures with an average hydrodynamic diameter of 84.1 ± 8.6 nm (Figure 2d, 2e). As seen from Figure 2e, the zeta potential of the siLuc/ZD-GNR complexes decreased to 2.3 ± 1.0 mV due to the complexation of negatively-charged phosphate molecules of siRNA onto the ZD-GNRs. To confirm that the siLuc/ZD-GNR complexes were based on coordination between the zinc ions with DPAs and phosphate molecules of siRNA, the complexation between DPA-GNR and siRNA without zinc ions as well as decomplexation of siLuc/ZD-GNR after the addition of sodium phosphate were investigated in a retardation test (Figure 2g, 2h). As shown in Figure 2h, without zinc ions, ZD-GNRs did not complex with siRNA at any concentration. Furthermore, the compact coordination of siRNA with Zn/DPA ligands was disrupted after the addition of phosphate ions whereas other salts (e.g. NaCl, MgCl2) did not affect the siLuc/ZD-GNR complexes (Figure 2g and Figure S4 in the Supporting Information). These results demonstrated the strong and selective binding of phosphate molecules of siRNA with Zn/DPA on the surface of GNRs.
Cellular uptake and intracellular distribution of siRNA/ZD-GNRs
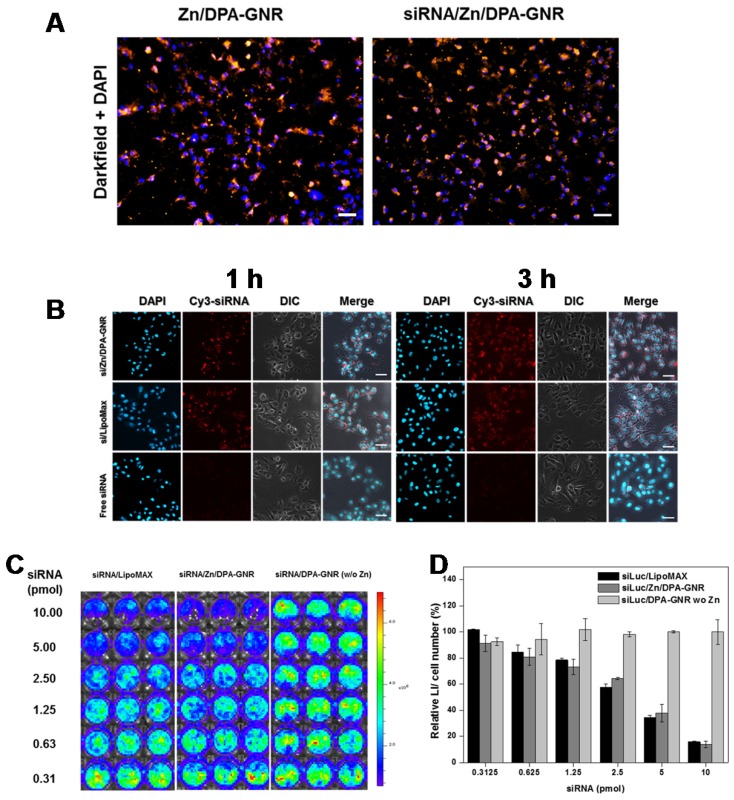
Cellular uptake of siRNA/ZD-GNRs was examined in 143B cancer cells by dark-field microscopy. As shown in Figure 3a, 143B cells treated with both ZD-GNRs and siRNA/ZD-GNRs for 3 h displayed a strong golden color, suggesting that GNR-based nano-complexes had been taken up by the cells and were located within the cytosol. In order to provide additional evidence of intracellular distribution, Cy3-siRNA/ZD-GNRs, Cy3-siRNA/ LipoMax and free Cy3-siRNA were incubated with 143B cancer cells (Figure 3b and Figure S5 in the Supporting Information). The intracellular fluorescence signals of siRNA/ZD-GNRs in 143B cancer cells were monitored by confocal laser scanning microscopy (CLSM). After 1 h incubation, 143B cells with Cy3-siRNA/ZD-GNRs exhibited a strong and bright red fluorescence in the cytosol. This indicated the uptake of the nanoparticles into the cells and their location at the endo/lysosomal compartments in the cytosol. After 3 h incubation of 143B cells with Cy3-siRNA/ZD-GNRs, the red fluorescence was broadly found and evenly distributed within the cytosol, which can be ascribed to the released siRNA from internalized siRNA/ZD-GNRs. Interestingly, it was found that intracellular distribution using Cy3-siRNA/LipoMax showed a similar uptake pattern compared to siRNA/ZD-GNRs. In contrast, 143B cells treated with free Cy3-siRNA exhibited a negligible red fluorescence within the cytosol due to the difficulty of cell permeation of large, negatively charged siRNA molecules. The mechanism regarding endosomal escape of siRNA/ZD-GNRs may be due to proton sponge effect 23, 52. Because the zinc ion or pyridine molecule of DPA or ZD-DPA would complex with chloride, protons or anions such as pyrophosphate, ADP and ATP, respectively, inducing the entering of water molecules to the lysosome. This process results in swelling and rupture of lysosome, with siRNA/ZD-GNRs released into the cytosol. However, the detailed mechanism remains elusive. Collectively, these results imply that siRNA/ZD-GNRs can be localized within endo/lysosomes and effectively internalized within cells after escaping from endo/lysosomes and eventually siRNA can be released from the complexes, such as the mechanism of Lipomax, for siRNA delivery42-44.
Figure 3.
(a) Dark-field light scattering and DAPI images of ZD-GNRs and siRNA/ZD-GNRs after incubation with 143B cells for 3 h. (b) CLSM images of 143B cells treated with Cy3-labeled siRNA/ZD-GNRs for 1 and 3 h. (c-d) Suppression of fLuc gene expression of 143B-fLuc cells after treatment with siLuc/LipoMax, siLuc/ZD-GNRs or siLuc/DPA-GNRs without zinc ions. Scale bar: 20 μm.
In Vitro Gene Silencing Effect of siRNA/ZD-GNRs
An ideal siRNA delivery nanoparticle must traffic its siRNA payload successfully from endo/lysosomes into the cytosol where the siRNA must exhibit gene silencing effects. The degree of gene silencing by siLuc/ZD-GNRs was examined in 143B-fluc cells using bioluminescence imaging (BLI). Luciferase activity was measured in cells treated with free siLuc, siLuc/LipoMax, siLuc/DPA-GNRs without zinc ions, negative control siRNA (siNC) with ZD-GNRs (siNC/ZD-GNRs), and siNC/LipoMax. As expected from a cellular uptake study in Figure 2b, free siLuc did not display any distinct fluc gene silencing effect at any concentration (Figure S6 in the Supporting Information). Moreover, siLuc/DPA-GNRs without zinc ions, siNC/ZD-GNRs or siNC/LipoMax also did not achieve obvious gene silencing effect in the target cells (Figure 3c, d and Figure S6 in the Supporting Information). In particular, a remarkably high gene silencing efficacy in cells treated with siLuc/ZD-GNRs was found in a dose-dependent manner (Figure 3c,d). As the concentration of siRNA increased, the expression of fluc from the 143B cells was reduced to 84.55 ± 5.32%, 57.60 ± 2.52%, and 15.77 ± 0.55% when 0.625, 2.5, and 10 pmol siLuc/ZD-GNRs were added, respectively. These observations demonstrate that ZD-GNRs, as an siRNA delivery carrier, can bind to the siRNA molecules by forming compact complexes through specific interactions between coordinated zinc ions of DPA and anionic phosphates of the siRNA, allow its cellular uptake, promote endo/lysosome escape, and finally release siRNA molecules into the cytosol, where unique sequence-specific gene silencing effect occurs.
Photothermal Properties of the ZD-GNRs
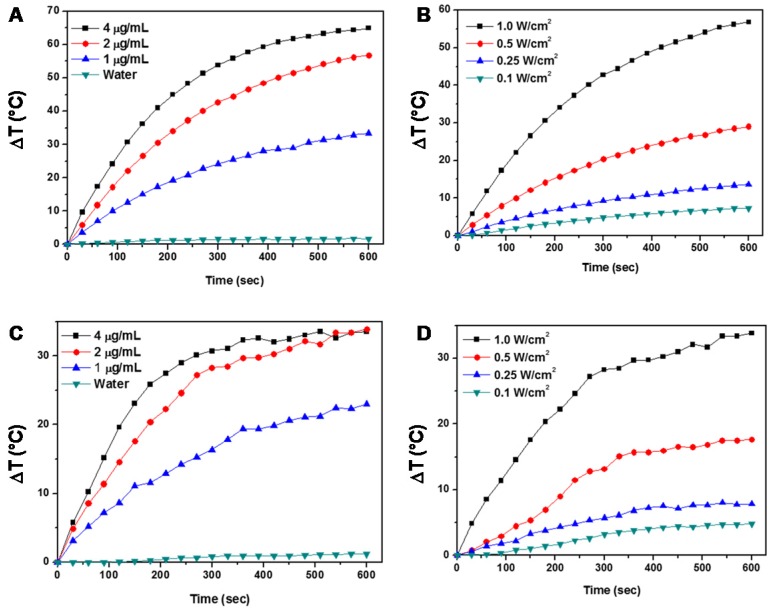
We next investigated the photothermal effect of the ZD-GNRs. Because the gold nanorods have peak absorbance around 800 nm, 808 nm laser source is suitable for photothermal experiments45. Different concentrations of ZD-GNRs were evaluated at fixed laser power (1.0 W/cm2) for 10 min. The temperature of the ZD-GNR solution increased in a dose-dependent manner (Figure 4a). The ZD-GNR solution (4 μg/mL) showed rapid temperature increase to about 65 °C within 10 min. As shown in Figure 3b, we also observed temperature change of ZD-GNRs at a fixed concentration (2 μg/mL) and irradiated with different laser powers, which showed laser-power-dependent temperature increase. More importantly, to confirm the photothermal properties of ZD-GNRs after being taken up by cells, 143B cells were treated with ZG-GNRs for 5 h and the medium was washed twice and replaced with fresh medium. The temperature change of GD-GNRs in cell culture medium was monitored upon laser irradiation. The cell culture without particles showed almost no heating effect. However, the Zn-GNR treatment (2 μg/mL) followed by 1.0 W/cm2 laser treatment raised the medium temperature by about 30 °C within 10 min (Figure 3c), which is high enough for cell killing.46 Taken together, the results above confirmed that the siRNA/ZD-GNRs can effectively deliver siRNA into cells, silence target genes, and have photothermal effect by gold nanorods upon laser irradiation.
Figure 4.
Photothermal studies of ZD-GNRs. (a-b) Temperature changes of ZD-GNRs at different concentrations irradiated with an 808 nm laser at power density of 1.0 W/cm2 (a) and with different laser powers at a fixed concentration (2 μg/mL) (b). (c-d) Temperature variation of 143B cells treated with ZD-GNRs in culture medium at different concentrations irradiated with an 808 nm laser at power density of 1.0 W/cm2 (c) and with different laser powers at a fixed particle concentration (2 μg/mL) (d).
In Vitro Combination Treatment with Gene and Photothermal-therapy
To demonstrate the potency of siRNA/ZD-GNRs for combination therapy of cancer, we chose polo-like kinase 1 (PLK1), a serine/threonine protein kinase which plays a critical role in mitosis as a therapeutic target.34-36 The RT-PCR analysis showed that PLK1 mRNA was abundant in three types of prostate carcinoma cell lines including PC-3, DU145, and LNCaP, but was negligible in normal MCF10A cells (Figure S7 in the Supporting Information). After confirming the successful cell uptake of ZD-GNRs and siPLK/ZD-GNRs by using ICP-OES analysis (Figure S8 in the Supporting Information), we first investigated the cytotoxicity of siPLK/ZD-GNRs on these prostate carcinoma cell lines.
The siRNA-free ZD-GNRs showed negligible cell death on all three cell lines for concentration up to 50 μg/mL and 48 h treatment (Figure S9 in the Supporting Information). Moreover, there is no obvious damage to the cells treated with negative control siRNA loaded GNR formula (siNC/ZD-GNRs) for siRNA concentration up to 20 pmol (Figure S10 in the Supporting Information). As shown in Figure S11 in the Supporting Information, the silencing PLK1 by siPLK/ZD-GNR nanocomplex decreased the viability of cancer cells in a dose-dependent manner.
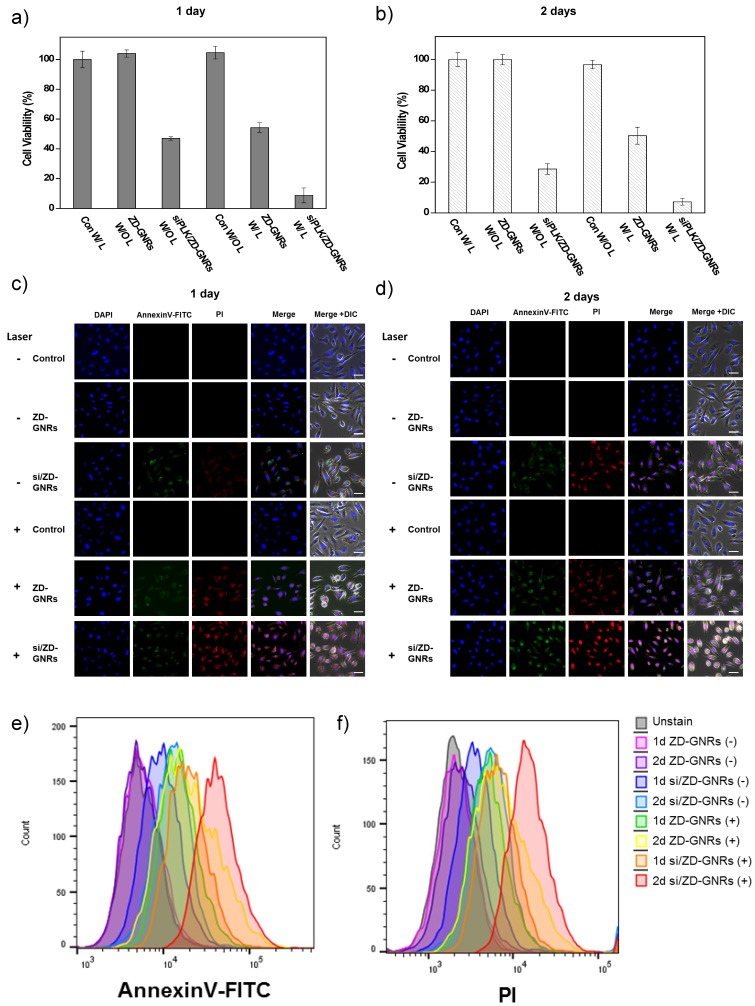
Next, combined gene and photothermal therapy of siPLK/ZD-GNRs was conducted. The prostate cancer cells were incubated with siPLK/ZD-GNRs, ZD-GNRs, or PBS for 24 h and 48 h. After being washed and placed in fresh cell culture medium, the cells with and without 808 nm laser irradiation were examined by a cell counting kit-8 (CCK-8) assay. As shown in Figure 5a and b, siPLK/ZD-GNRs with laser irradiation produced significantly higher cytotoxic effect against PC-3 cells than ZD-GNRs only with laser or siPLK/ZD-GNRs without laser. Similar results were also seen for DU145 and LNCaP cells (Figure S12a, b and S13a,b in the Supporting Information). The cytotoxicity of siPLK/ZD-GNRs was further evaluated using Annexin V-FITC and PI staining (Figure 5c, d), which distinguishes non-apoptotic cells (no fluorescence), early apoptotic cells (green fluorescence), necrotic cells (red fluorescence) and late apoptotic and necrotic cells (green and red fluorescence)47. In the case of siPLK/ZD-GNRs only without laser, the cells showed prominent green fluorescence due to early apoptosis induced by PLK1 depletion at day 1 and weak red fluorescence because of the presence of a small number of late apoptotic and necrotic cells at day 2. When PC-3 cells were incubated with siPLK/ZD-GNRs followed by laser irradiation, both green and red fluorescence appeared, showing the efficacy of combination therapy of PLK1 depletion and PTT induced apoptosis and necrosis in cancer cells. Notably, even though treatment with ZD-GNRs and laser irradiation also induced apoptosis and necrosis, siPLK/ZD-GNRs with laser led to the highest extent of apoptosis and necrosis. Similar results were also found from the FACS analysis (Figure 5e,f). The combination therapy was also highly effective for DU145 and LNCaP cells (Figure S12c-f and S13c-f in the Supporting Information). Moreover, mRNA level of PLK1 was also evaluated using reverse transcription polymerase chain reaction (RT-PCR) (Figure S14 in the Supporting Information), manifesting the effectiveness of gene silencing by siPLK/ZD-GNRs.
Figure 5.
In vitro combinational gene/photothermal therapy of PC-3 cells with siPLK/ZD-GNRs. (a-b) In vitro cytotoxicity of saline, ZD-GNRs, and siPLK/ZD-GNRs with and without 808 nm laser irradiation at power density of 0.5 W/cm2 against prostate cancer cells after incubation for 1 day (a) and 2 days (b). (c-d) Confocal microscopy images of PC-3 cells treated with PI and Annexin V (AV) after treatment after incubation for 1 day (c) and 2 days (d) (Blue fluorescence is associated with DAPI; the green fluorescence is expressed by Annexin V-FITC, and the red fluorescence is released from PI). (e-f) Scale bar: 20 μm. Flow cytometry data of PC-3 cells treat with PI and Annexin V (AV) after treatment after incubation for 1 day (e) and 2 days (f).
In Vivo Photoacoustic Imaging of siPLK/ZD-GNRs
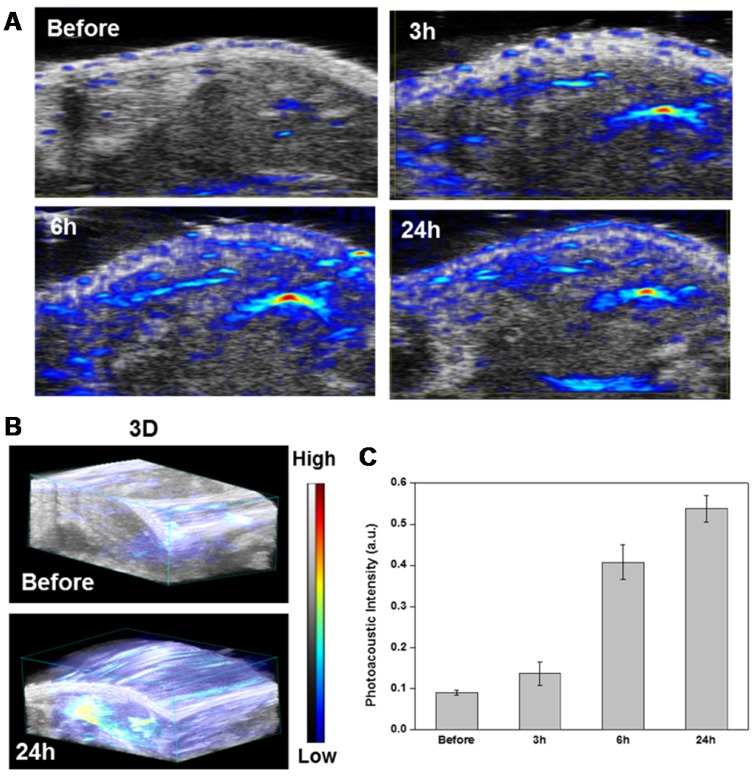
Photoacoustic imaging (PAI) is recognized as a promising imaging tool with high contrast and spatial resolution to investigate distribution and accumulation of metallic particles such as gold in target tumor tissue48. Before performing the gene and photothermal combination therapy, PAI of siPLK/ZD-GNRs was monitored in PC-3 tumor-bearing mice. When the tumor volume reached ~100 mm3, the mice were treated by an intravenous injection of the siPLK/ZD-GNRs (100 µL in saline, 0.1 mg/mL). As shown in Figure 6, after treatment with siPLK/ZD-GNRs, the PA signal in the tumor region gradually increased with time and the PA intensity at 24 h was 6.0 times higher than that before injection. Additionally, the 3D-PAI images showed remarkably strong intensity in the tumor region at 24 h post-injection, indicating excellent tumor accumulation of siPLK/ZD-GNRs, which was also confirmed by biodistribution of gold content (Figure S15 in the Supporting Information).
Figure 6.
In vivo photoacoustic imaging of siPLK/ZD-GNRs. (a-b) 2D images (a) and 3D images (b) of the PC-3 tumor region at different time points after intravenous injection of siPLK/ZD-GNRs. (c) histogram of PA intensity profile of the tumor as a function of time.
In Vivo Combined Gene and Photothermal Therapy
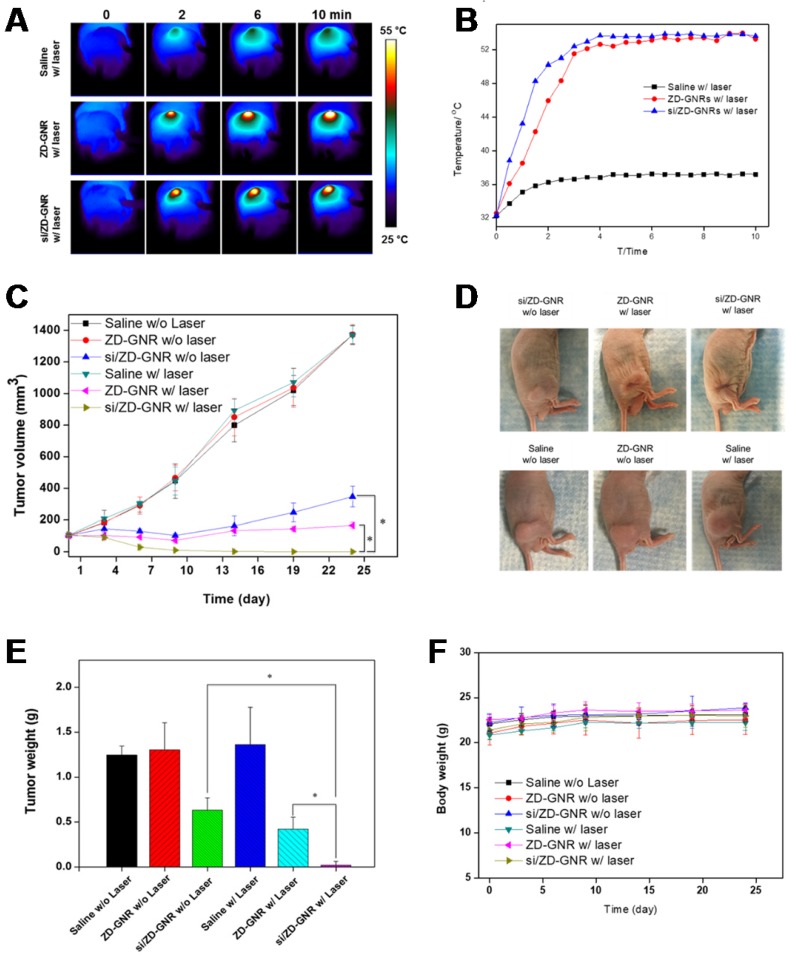
Before the in vivo therapeutic experiment, we first performed the serum stability of the siPLK/ZD-GNRs because serum nuclease attack in bloodstream is one of major challenges for systemic delivery of siRNA.53 When serum stability was examined in the 40% fetal bovine serum (FBS) solution at 37 °C, the free siRNA was broken down within 12 h incubation, whereas siPLK/ZD-GNRs were stable to serum solution even after 24 h incubation (Figure S16 in the Supporting Information). These results indicate that siPLK/ZD-GNRs are stable from nuclease attack in the bloodstream when performing in vivo combination treatment because of their strong complexation. Based on the excellent in vitro therapeutic behavior and in vivo tumor accumulation of siPLK/ZD-GNRs, this nanoplatform was investigated for anti-tumor efficacy on PC-3 tumor-bearing mice. Free ZD-GNRs and the siPLK/ZD-GNRs were administered intravenously when the tumor volume reached ~100 mm3. At 24 h post-injection, the tumors were irradiated with 808 nm laser at a 0.5 W/cm2 laser power for 10 min and the temperature increase was monitored using an infrared thermal camera for in vivo thermal imaging. As shown in Figure 6a,b, no obvious temperature change was observed in the saline group. In contrast, both ZD-GNRs and siPLK/ZD-GNRs treated mice displayed an increase of ~21 ºC in the tumor region, which is high enough to ablate the tumor tissue49. Figure 6c shows the changes in PC-3 tumor volumes in nude male mice after intravenous injection of saline with or without laser irradiation, the ZD-GNRs with or without laser irradiation, and the siPLK/ZD-GNRs with or without laser irradiation. The control groups (saline with or without laser irradiation, ZD-GNRs without laser irradiation) did not show any substantial delay of tumor growth. The treatments with siPLK/ZD-GNRs without laser irradiation or ZD-GNRs with laser irradiation were effective in delaying tumor growth but not tumor regression. The siPLK/ZD-GNRs with laser irradiation is the most efficacious in tumor reduction compared to all the other study groups (Figure 6c,d). Moreover, it was found that the expression of PLK1 was significantly down-regulated in the siPLK/ZD-GNRs with laser irradiation group, indicating that the combination treatment induced the apoptosis of target tumor tissues (Figure S17 in the Supporting Information). At the end of the in vivo experiment, the PC-3 tumor tissues were dissected from tumor-bearing mice and weighed, which was consistent with the tumor's volume measured by a caliper (Figure 6e). We also investigated the body weight for the possible in vivo systemic toxicity of siPLK/ZD-GNRs with or without laser irradiation, and no significant body weight change was found in any of the treatment group. Altogether, the improved antitumor efficacy of siPLK/ZD-GNRs with laser irradiation can be explained by the synergy of combined systemic gene silencing induced apoptosis and locally focused photothermal therapy.
Conclusions
We successfully developed siRNA-complexed Zn(II)-DPA conjugated gold nanorods (siRNA/ZD-GNRs) through an artificial siRNA receptor, Zn(II)-DPA, which can strongly bind siRNA molecules for combined gene and photothermal tumor treatment. The introduction of gold nanorods with Zn(II)-DPA as a siRNA delivery carrier provided not only sufficient complexation with siRNA in the form of stable nanoparticles, which show strong gene-silencing activity, but also photothermal property of GNRs upon laser irradiation. Moreover, the application of siPLK/ZD-GNRs for gene/photothermal combination therapy demonstrates synergistic antitumor efficacy, which is significantly better than individual gene therapy or PTT alone. Additionally, due to the photoacoustic character of GNRs, siRNA/ZD-GNRs have great potential as a theranostic agent for simultaneous PA imaging and therapy of cancers. The approach suggested in this work may provide a valuable general strategy for efficient combination treatment of cancer.
Materials and Methods
The synthetic schemes and experimental details are described in the Supporting Information section. All animal experiments were conducted in accordance with a protocol approved by the CC/NIH ACUC committee.
Materials
CTAB-stabilized GNRs were purchased from Nanopartz Inc. (Loveland, CO). Cells were purchased from the American Type Culture Collection (Rockville, MD). Other chemicals and biological materials were obtained from Sigma-Aldrich (St. Louis, MO) or Invitrogen (Carlsbad, CA). All the siRNAs were synthesized by Thermo Fisher Scientific (Waltham, MA). The sequences of siRNA firefly luciferase (siLuc) are 5′-GCACUCUGAUUGACAAAUACGAUUU-3′ (sense) and 5′-AAAUCGUAUUUGUCAAUCAGAGUGC-3′ (antisense), the sequences of siRNA negative control (siNC) are 5'-AAUUCUCCGAACGUGUCACGU-3′ (sense) and 5′-ACGUGACACGUUCGGAGAAUU-3′ (antisense), and the sequence of siRNA targeting Plk1 mRNA (siPLK) are 5′-UGAAGAAGAUCACCCUCCUUAdTdT-3′ (sense) and antisense strand, 5′-UAAGGAGGGUGAUCUfUCUfUCfAdTdT-3′ (antisense).
Synthesis of DPA-GNRs
To prepare gold nanorods modified with dipycolylamine (DPA-GNRs), DPA-GNRs was synthesized by a ligand exchange reaction between CTAB-stabilized GNRs and lipoic acid-modified DPA. The lipoic acid-modified DPA in ethanol (2 mL) was slowly added to the 10 mL of CTAB-stabilized GNRs (2 nM) colloid, and the solution was kept at room temperature for 12 h. The DPA modified GNR (DPA-GNR) solution was centrifuged 3 times at 6000 rpm to remove unreacted lipoic acid-modified DPA species from 6 mL of ethanl/distilled water (1:5, v/v) and dispersed in 2 mL of distilled water. To determine the conversion of DPA and composition ratio, the unreacted lipoic acid-modified DPA in the supernatant was collected and the concentration was determined by measuring the UV absorbance intensity at 350 nm.
Formulation of siRNA/ZD-GNRs
siRNA/ZD-GNRs were prepared by a metal-organic complex technique. DPA-GNRs at 2 mg/mL were dispersed in ultrapure water (UPW) for 30 min and then 5 mL of 10 mM zinc nitrate hexahydrate was mixed with 5 mL of DPA-GNRs (2 mg/mL in UPW). The resulting mixture was incubated at room temperature for 30 min. After centrifugation (5000 rpm, 3x) to remove excess zinc, ZD-GNR were dispersed in UPW. At the molar ratio of 100 the ZD-GNR dispersion (2 μL, 0.1 μM) was mixed with 1 μL of siRNA (20 μM) (siRNA against firefly luciferase (siLuc) or siRNA against PLK1 (siPLK)), and incubated at room temperature for 30 min. The siRNA/ZD-GNRs were then kept at 4 ºC for in vitro or in vivo applications.
Agarose Gel Electrophoresis
siRNA/ZD-GNRs were evaluated by agarose gel retardation assay. The gels were prepared with 2% agarose in Tris-acetate-EDTA buffer containing 0.5 μg/mL ethidium bromide and 2 mM of zinc ion. Twenty microliters of complexes containing solution with 1 μg siRNA was electrophoresed with Tris-acetate-EDTA (TAE) running buffer. Gel electrophoresis was carried out at 100 V for 30 min and the gel was imaged using a LAS-3000 gel documentation system (Fujifilm Life Science, Japan). To assess the decomplexation of siRNA, siRNA/ZD-GNRs were incubated with 0.1 M sodium phosphate, sodium chloride or magnesium chloride for 30 min and the released siRNA fraction was imaged.
Inductively Coupled Plasma Emission Spectrometry (ICP-OES)
Quantification of siRNA/ZD-GNRs taken up in cells was performed using ICP-OES. Prostate cancer cells seeded onto a 6-well plate were incubated for 24 h and then treated with ZD-GNRs (3 µg/mL) or siRNA/ZD-GNRs (3 µg/mL) for another 24 h. Cells were then harvested and digested by 200 µL of aqua regia. 50 µL of ZD-GNRs were also digested in 100 µL of aqua regia. The digested samples were diluted with ultrapure water to 5 mL and the gold concentration was measured by ICP-OES (Agilent 700 Series, Santa Clara, CA).
Gene Silencing Effects of siRNA/ZD-GNRs in Cells
143B cells stably expressing firefly luciferase (fluc) reporter gene were maintained in MEM medium with 10% FBS at 37 ºC in a humidified 5% CO2 atmosphere. To confirm gene silencing effect of siRNA/ZD-GNRs, 143B cells were seeded in 96-well plates and treated with siLuc/ZD-GNRs (8 μL; 10 pmol siRNA) dispersed in 92 μL of cell culture medium. Parallel experiments for positive control (siLuc/LipoMAX) and negative control (siNC/ZD-GNRs or siNC/LipoMAX) were also carried out. After 24 h incubation, the fLuc level was quantified using a bioluminescence imaging system (Xenogen IVIS-100).
Darkfield Light Scattering Imaging
Darkfield images were taken with a Zeiss Axioskop 2 plus microscope equipped with a black-white CCD camera. All images were taken at 60X magnification under the same lighting conditions using an Olympus IX-81 inverted epifluorescence microscope.
In Vitro Confocal Microscopy Imaging
143B cells were seeded onto coverslip-bottom culture chambers (LabTEK) in 200 μL of MEM supplemented with 10% FBS and 1% antibiotics (penicillin 100 U/mM, streptomycin 0.1 mg/mL). After incubation for 24 h (37 ºC, 5% CO2), the medium was carefully aspirated and replaced with 1 mL of medium containing siRNA/ZD-GNRs. The cells were incubated for 1 and 6 h and then washed three times with PBS. The cells were fixed with 4% w/v formaldehyde for 5 min. Cells were then washed with PBS and mounted with antifade mounting medium with DAPI (Vectashield, Burlingame, CA). Confocal microscopy was performed with an Olympus Fluoview FV10i (Olympus, Tokyo, Japan).
Measurement of Photothermal Effect of ZD-GNRs
ZD-GNRs were diluted into different concentrations with distilled water. Aliquots of samples were exposed to an 808 nm NIR laser (Laserglow Technologies, Toronto, Ontario, Canada) for 10 min at different power densities. A laser energy meter (Coherent Portland, OR) was used to measure and calibrate the laser densities. The temperature increase of the solutions was recorded with an infrared thermal imaging system (FLIR® Systems, Oregon, USA).
Measurement of In Vitro Photothermal Effect after Cell Uptake of ZD-GNRs
143B cells (3 x 105 cells/well) were seeded onto 6-well flat-bottomed plates in 2 mL of MEM cell culture medium supplemented with 10% FBS and 1% antibiotics and incubated for 24 h. The medium was aspirated and incubated with various concentrations of ZD-GNRs for 24 h at 37 ºC. The cells were washed twice with PBS to remove the remaining ZD-GNRs, and the cells were harvested and resuspended in MEM medium. The samples were exposed to an 808 nm NIR laser for 10 min at different power densities. The temperature changes of the cell culture medium were recorded with an IR thermal camera.
In Vitro Photothermal Effect of the siPLK/ZD-GNRs Irradiated with an NIR Laser
0.2 mL of siPLK/ZD-GNRs in a 1 mL tube was irradiated with an 808 nm laser at different power densities for 10 min. The spot size of the laser was adjusted to cover the entire solution surface. The thermal images and temperature increase of the aqueous solutions were recorded by using an infrared thermal camera. The water control was also treated under the same conditions.
Prostate Carcinoma Cell Culture
Three human prostate cancer cell lines, including PC-3, LNCaP and DU145 were used in this study. The PC-3 cells were cultured in F12 (Life Technologies, Carlsbad), LNCaP cells were cultured in RPMI-1640 (Life Technologies, Carlsbad), and DU145 cells were cultured in MEM (Life Technologies, Carlsbad) supplemented with 10% FBS and 1% penicillin-streptomycin (Gibco, Grand Island, NY) in a humidified incubator at 37 ºC with 5% CO2.
In Vitro Combined Gene and Photothermal-therapy
Three cancer cells (PC-3, DU145, and LNCaP) were seeded at a density of 5 x 103 cells/well in 96-well well plate. After 24 h, the cells were washed twice with PBS and incubated with various concentrations of siPLK/ZD-GNRs, ZD-GNRs for 24 h or 48 h at 37 ºC. The cells were then washed with PBS, and 100 μL of fresh medium was added. The cells were irradiated with or without an 808 nm laser at a power density of 0.5 W/cm2 for 5 min, respectively. After incubation for another 2 h, the viability of cancer cells was examined using the standard CCK-8 assay.
RT-PCR Analysis
The total RNA content was prepared from siPLK/ZD-GNRs treated PC-3, DU145, and LNCaP cell lysates after TRIzol extraction, and then RNA quantity was quantified using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA). To synthesize cDNA, 1 µg of RNA was used by Verso cDNA synthesis Kit (Thermo Fisher Scientific, Waltham, MA). The sequences of the forward and reverse primers of PLK1 were 5′-CTGCCTGACCATTCCACCAA and 5′- GTGCCGTCACGCTCTATGTA, respectively. The sequences of the forward and reverse primers of β-actin were 5′-ACAGAGCCTCGCCTTTGC and 5′-AGAGGCGTACAGGGATAGCA, respectively. The PCR reaction (94°C for 30 s, 60°C for 30 s, 72°C for 30 s) was run for 30 cycles after an initial single cycle of 94°C for 2 min to activate the Taq polymerase. For final elongation, single step was performed at 72°C for 15 min. After amplification, PCR products were analyzed by gel electrophoresis in 1.8% agarose gels and visualized by GelRedTM (Biotium, Hayward, CA) staining.
Serum Stability of siRNA/ZD-GNRs
FBS was mixed with 20 µL of complexes containing solution with 1 μg siRNA (free siRNA or siRNA/ZD-GNRs) at the final concentration of 40% and then incubated at 37 °C during the indicated period. The samples were then analyzed by agarose gel (2%) under TBE (Tris-borate-EDTA) running buffer. Gel electrophoresis was carried out at 100 V for 30 min and the gel was subsequently imaged using a LAS-3000 gel documentation system (Fujifilm Life Science, Japan).
In Vivo Photoacoustic Imaging
The PC-3 xenograft tumor was established by inoculating 10 million cells into the right flank of athymic C3H/HeN nude mice (20-25 g, Harlan, Indianapolis, IN). When tumors reached 100 mm3 in volume, the mice (n = 3) were treated by an intravenous injection of the siPLK/ZD-GNRs (100 µL in saline, 0.1 mg/mL). The PA image of the whole tumor region was scanned by using the VisualSonic Vevo 2100 LAZR system (40 MHz, 256-element linear array transducer) at 0, 3, 6, and 24 h postinjection.
In Vivo Biodistribution by ICP-OES
To study the biodistribution of the particles in various organs, when PC-3 tumors reached a size of 100 mm3 in volume, 250 μL suspension of siPLK/ZD-GNRs was intravenously injected into PC-3 tumor mice. The mice were sacrificed at 3, 6, 12, 24, and 48 h post-injection and organs including tumor, heart, liver, spleen, lung and kidneys were dissected and weighted. Each group had three mice as replicates. All the samples were stored at -80 °C before ICP-OES measurement.
In Vivo Combined Gene and Photothermal-therapy
When tumors reached a size of 100-150 mm3 in volume, saline (250 µL), saline with laser irradiation (250 µL), ZD-GNRs (250 µL), ZD-GNRs with laser irradiation (250 µL), siPLK/ZD-GNRs (250 µL; siRNA 1.5 nmol), and siPLK/ZD-GNRs with laser irradiation (250 µL; siRNA 1.5 nmol) were injected intravenously at days 0, 3, and 6 (4 mice/group). At 24 h after the injection of the third dose, the PC-3 tumors were exposed to an 808 nm laser (0.5 W/cm2) for 10 min. The body weights of mice were recorded, and tumor volumes were calculated as a x b2/2, where a and b are the largest and smallest diameters, respectively.
In Vivo Gene Expression Evaluation in Tumor Tissue by RT-PCR
Nude mice bearing PC-3 tumors (100-150 mm3) were intravenously injected with saline (250 µL), saline with laser irradiation (250 µL), ZD-GNRs (250 µL), ZD-GNRs with laser irradiation (250 µL), siPLK/ZD-GNRs (250 µL; siRNA 1.5 nmol), or siPLK/ZD-GNRs with laser irradiation (250 µL; siRNA 1.5 nmol) at days 0, 3 and 6. At 24 h post-injection of the third dose, the PC-3 tumors were exposed to an 808 nm laser (0.5 W/cm2) for 10 min. After the in vivo experiment, the collected tumor tissue was homogenized by PreCellys (Bertin Technologies, Montigny-Le-Bretonneux, France) in 500 μL of TRIzol, and then centrifuged at 12,000g at 4 °C for 15 min, and then RNA was extracted from lysates and purified. To synthesize cDNA, 1 µg of RNA was used by Verso cDNA synthesis Kit (Thermo Fisher Scientific, Waltham, MA). The sequences of the forward and reverse primers of PLK1 were 5′-CTGCCTGACCATTCCACCAA and 5′-GTGCCGTCACGCTCTATGTA, respectively. The sequences of the forward and reverse primers of β-actin were 5′-ACAGAGCCTCGCCTTTGC and 5′-AGAGGCGTACAGGGATAGCA, respectively. The PCR reaction (94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s) was run for 30 cycles after an initial single cycle of 94 °C for 2 min to activate the Taq polymerase. For final elongation, single step was performed at 72 °C for 15 min. After amplification, PCR products were analyzed by gel electrophoresis in 1.8% agarose gels and visualized by GelRedTM (Biotium, Hayward, CA) staining.
Statistical Analysis
The statistical significance of differences between experimental and control groups was determined with Sigma Plot using t-test (p < 0.05 was considered statistically significant).
Supplementary Material
Supplementary figures.
Figure 7.
Combinational gene/photothermal therapy of siPLK/ZD-GNRs on PC-3 tumor-bearing mice. (a-b) Thermographic images (a) and temperature changes of the tumor area (b) of the mice treated with saline, ZD-GNRs, and siPLK/ZD-GNRs upon 10 min laser exposure. (c) Changes in tumor volumes after intravenous injection of the samples with or without laser exposure. (d) Representative images of PC-3 tumor-bearing mice at day 23 after treatment. (e) Weights of excised tumors (n = 4/group). (f) Body weight of tumor-bearing mice after treatment. (*P < 0.05).
Acknowledgments
This work was supported in part, by the Intramural Research Program (IRP) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH) and the Global Innovative Research Center (GiRC) Program (2012K1A1A2A01055811) of the National Research Foundation of Korea.
References
- 1.Moazed D. Small RNAs in transcriptional gene silencing and genome defence. Nature. 2009;457:413–420. doi: 10.1038/nature07756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. Lancet Oncol. 2012;13:e249–258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 3.de Fougerolles A, Vornlocher H-P, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takakura Y, Nishikawa M, Yamashita F, Hashida M. Influence of physicochemical properties on pharmacokinetics of non-viral vectors for gene delivery. J Drug Targeting. 2002;10:99–104. doi: 10.1080/10611860290016694. [DOI] [PubMed] [Google Scholar]
- 6.Howard KA, Rahbek UL, Liu X. et al. RNA interference in vitro and in vivo using a chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Semple SC, Akinc A, Chen J. et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 8.Suh MS, Shim G, Lee HY. et al. Anionic amino acid-derived cationic lipid for siRNA delivery. J Control Release. 2009;140:268–276. doi: 10.1016/j.jconrel.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Lin Q, Chen J, Zhang Z, Zheng G. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine. 2014;9:105–120. doi: 10.2217/nnm.13.192. [DOI] [PubMed] [Google Scholar]
- 10.Choi YJ, Kang SJ, Kim YJ, Lim Y-b, Chung HW. Comparative studies on the genotoxicity and cytotoxicity of polymeric gene carriers polyethylenimine (PEI) and polyamidoamine (PAMAM) dendrimer in Jurkat T-cells. Drug Chem Toxicol. 2010;33:357–366. doi: 10.3109/01480540903493507. [DOI] [PubMed] [Google Scholar]
- 11.Hunter AC. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv Drug Del Rev. 2006;58:1523–1531. doi: 10.1016/j.addr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly (ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Kuo JhS, Jan Ms, Chiu HW. Mechanism of cell death induced by cationic dendrimers in RAW 264.7 murine macrophage-like cells. J Pharm Pharmacol. 2005;57:489–495. doi: 10.1211/0022357055803. [DOI] [PubMed] [Google Scholar]
- 14.Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–4171. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Choi KY, Bhirde A. et al. Sticky nanoparticles: a platform for siRNA delivery by a bis(zinc(II) dipicolylamine)-functionalized, self-assembled nanoconjugate. Angew Chem Int Ed Engl. 2012;51:445–449. doi: 10.1002/anie.201105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi KY, Silvestre OF, Huang X. et al. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano. 2014;8:4559–4570. doi: 10.1021/nn500085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee H-W, Choi SJ, Yoo SH. et al. A bifunctional molecule as an artificial flavin mononucleotide cyclase and a chemosensor for selective fluorescent detection of flavins. J Am Chem Soc. 2009;131:10107–10112. doi: 10.1021/ja9018012. [DOI] [PubMed] [Google Scholar]
- 18.Kwon TH, Kim HJ, Hong JI. Phosphorescent thymidine triphosphate sensor based on a donor-acceptor ensemble system using intermolecular energy transfer. Chemistry. 2008;14:9613–9619. doi: 10.1002/chem.200801260. [DOI] [PubMed] [Google Scholar]
- 19.Rhee HW, Lee SH, Shin IS. et al. Detection of kinase activity using versatile fluorescence quencher probes. Angew Chem Int Ed Engl. 2010;122:5039–5043. doi: 10.1002/anie.201000879. [DOI] [PubMed] [Google Scholar]
- 20.Hwang L, Ayaz-Guner S, Gregorich ZR. et al. Specific enrichment of phosphoproteins using functionalized multivalent nanoparticles. J Am Chem Soc. 2015;137:2432–2435. doi: 10.1021/ja511833y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plaunt AJ, Harmatys KM, Wolter WR, Suckow MA, Smith BD. Library synthesis, screening, and discovery of modified Zinc(II)-bis(dipicolylamine) probe for enhanced molecular imaging of cell death. Bioconjug Chem. 2014;25:724–737. doi: 10.1021/bc500003x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuen KKY, Jolliffe KA. Bis[zinc(II)dipicolylamino]-functionalised peptides as high affinity receptors for pyrophosphate ions in water. Chem Commun (Camb) 2013;49:4824–4826. doi: 10.1039/c3cc40937f. [DOI] [PubMed] [Google Scholar]
- 23.Bahadur K, Thapa B, Bhattarai N. Gold nanoparticle-based gene delivery: promises and challenges. Nanotechnol Rev. 2014;3:269–280. [Google Scholar]
- 24.Webb JA, Bardhan R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale. 2014;6:2502–2530. doi: 10.1039/c3nr05112a. [DOI] [PubMed] [Google Scholar]
- 25.Huang P, Lin J, Li W. et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew Chem Int Ed Engl. 2013;52:13958–13964. doi: 10.1002/anie.201308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song J, Yang X, Jacobson O. et al. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano. 2015;9:9199–9209. doi: 10.1021/acsnano.5b03804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang P, Lin J, Wang S. et al. Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials. 2013;34:4643–4654. doi: 10.1016/j.biomaterials.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Huang P, Nie L. et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv Mater. 2013;25:3055–3061. doi: 10.1002/adma.201204623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang C, Diao S, Wang C. et al. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv Mater. 2014;26:5646–5652. doi: 10.1002/adma.201401825. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Takashima M, Yuba E, Harada A, Kono K. PEGylated PAMAM dendrimer-doxorubicin conjugate-hybridized gold nanorod for combined photothermal-chemotherapy. Biomaterials. 2014;35:6576–6584. doi: 10.1016/j.biomaterials.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Yang H-W, Liu H-L, Li M-L. et al. Magnetic gold-nanorod/PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials. 2013;34:5651–5660. doi: 10.1016/j.biomaterials.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Wang J, Chen C. Gold nanorods based platforms for light-mediated theranostics. Theranostics. 2013;3:223–238. doi: 10.7150/thno.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia J, Zhu F, Ma X, Cao ZW, Li YX, Chen YZ. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov. 2009;8:111–128. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 34.Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30:679–692. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 35.Woodcock J, Griffin JP, Behrman RE. Development of novel combination therapies. New Engl J Med. 2011;364:985–987. doi: 10.1056/NEJMp1101548. [DOI] [PubMed] [Google Scholar]
- 36.Batist G, Gelmon KA, Chi KN. et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15:692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 37.Barr FA, Silljé HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–441. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 38.Kasahara K, Goto H, Izawa I. et al. PI 3-kinase-dependent phosphorylation of Plk1-Ser99 promotes association with 14-3-3γ and is required for metaphase-anaphase transition. Nat Commun. 2013;4:1882. doi: 10.1038/ncomms2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petronczki M, Lénárt P, Peters J-M. Polo on the rise—from mitotic entry to cytokinesis with Plk1. Dev Cell. 2008;14:646–659. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Martin BT, Strebhardt K. Polo-like kinase 1: target and regulator of transcriptional control. Cell Cycle. 2006;5:2881–2885. doi: 10.4161/cc.5.24.3538. [DOI] [PubMed] [Google Scholar]
- 41.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nature reviews cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 42.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 43.Simizu S, Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat Cell Biol. 2000;2:852–854. doi: 10.1038/35041102. [DOI] [PubMed] [Google Scholar]
- 44.Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794–2797. [PubMed] [Google Scholar]
- 45.Dietzmann K, Kirches E, Von Bossanyi P, Jachau K, Mawrin C. Increased human polo-like kinase-1 expression in gliomas. J Neurooncol. 2001;53:1–11. doi: 10.1023/a:1011808200978. [DOI] [PubMed] [Google Scholar]
- 46.Peters U, Cherian J, Kim JH, Kwok BH, Kapoor TM. Probing cell-division phenotype space and Polo-like kinase function using small molecules. Nat Chem Biol. 2006;2:618–626. doi: 10.1038/nchembio826. [DOI] [PubMed] [Google Scholar]
- 47.Ellis PM, Chu QS, Leighl N. et al. A phase I open-label dose-escalation study of intravenous BI 2536 together with pemetrexed in previously treated patients with non-small-cell lung cancer. Clin Lung Cancer. 2013;14:19–27. doi: 10.1016/j.cllc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Frost A, Mross K, Steinbild S. et al. Phase I study of the Plk1 inhibitor BI 2536 administered intravenously on three consecutive days in advanced solid tumours. Current Oncol. 2012;19:28–35. doi: 10.3747/co.19.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olmos D, Barker D, Sharma R. et al. Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17:3420–3430. doi: 10.1158/1078-0432.CCR-10-2946. [DOI] [PubMed] [Google Scholar]
- 50.Ma WW, Messersmith WA, Dy GK. et al. Phase I study of Rigosertib, an inhibitor of the phosphatidylinositol 3-kinase and Polo-like kinase 1 pathways, combined with gemcitabine in patients with solid tumors and pancreatic cancer. Clin Cancer Res. 2012;18:2048–2055. doi: 10.1158/1078-0432.CCR-11-2813. [DOI] [PubMed] [Google Scholar]
- 51.Song J, Pu L, Zhou J, Duan B, Duan H. Biodegradable theranostic plasmonic vesicles of amphiphilic gold nanorods. ACS Nano. 2013;7:9947–9960. doi: 10.1021/nn403846v. [DOI] [PubMed] [Google Scholar]
- 52.Ding Y, Jiang Z, Saha K. et al. Gold nanoparticles for nucleic acid delivery. Mol Ther. 2014;22:1075–1083. doi: 10.1038/mt.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.