Abstract
Modulation of posttranslational modifications (PTMs), such as protein acetylation, is considered a novel therapeutic strategy to combat the development and progression of cardiovascular diseases. Protein hyperacetylation is associated with the development of numerous cardiovascular diseases, including atherosclerosis, hypertension, cardiac hypertrophy, and heart failure. In addition, decreased expression and activity of the deacetylases Sirt1, Sirt3, and Sirt6 have been linked to the development and progression of cardiac dysfunction. Several phytochemicals exert cardioprotective effects by regulating protein acetylation levels. These effects are mainly exerted via activation of Sirt1 and Sirt3 and inhibition of acetyltransferases. Numerous studies support a cardioprotective role for sirtuin activators (e.g., resveratrol), as well as other emerging modulators of protein acetylation, including curcumin, honokiol, oroxilyn A, quercetin, epigallocatechin-3-gallate, bakuchiol, tyrosol, and berberine. Studies also point to a cardioprotective role for various nonaromatic molecules, such as docosahexaenoic acid, alpha-lipoic acid, sulforaphane, and caffeic acid ethanolamide. Here, we review the vast evidence from the bench to the clinical setting for the potential cardioprotective roles of various phytochemicals in the modulation of sirtuin-mediated deacetylation.
1. Introduction
Cardiovascular diseases (CVDs) have remained the leading cause of death worldwide for the past two decades. In 2012, coronary artery disease alone was responsible for 7.4 million deaths, and CVDs accounted for 17.3 million deaths worldwide [1]. Based on current estimates, the number of CVD-related deaths is expected to increase to 23.6 million by 2030 [1]. Coronary heart disease, hypertension, peripheral vascular disease, myocardial infarctions, strokes, and heart failure are the most prevalent CVDs [2]. Their aetiology is diverse and includes various risk factors, such as aging, obesity, smoking, and diabetes [1]. Abundant natural biologically active substances, also known as functional molecules, from diverse sources can potentially decrease risk factors for CVDs, thereby significantly reducing the incidence of CVDs [3–7].
The search for new functional molecules to prevent CVDs faces a major challenge due to the growing list of pathogenic mechanisms attributed to a multitude of interrelated pathways. Research on the role of posttranslational modifications (PTMs) in the development and progression of CVDs has spiked dramatically, with modulation of PTMs currently considered a potential therapeutic strategy [8–10].
PTMs are important regulators of the synthesis, subcellular localization, and enzymatic activity of proteins. PTMs also modulate signal transduction pathways and cellular metabolism (reviewed in [11]). PTMs respond rapidly to both internal and external (environmental) stimulation, allowing for efficient signal transmission and amplification. Among PTMs, most research thus far has focused on protein phosphorylation, although protein acetylation has emerged as a key regulatory mechanism and an attractive therapeutic target in the field of chronic diseases ([8–10], reviewed in [12]). Although acetylation was first described in the early 1960s, detailed studies of its biological role did not take place until the late 1990s. The first description of protein acetylation focused on histones as targets of histone acetyltransferases, which transfer acetyl groups from acetyl coenzyme A to specific lysine residues on histones, exposing DNA to transcription [13]. Conversely, histone deacetylases (HDACs) remove acetyl groups from acetylated lysine amino acids on histones, allowing chromatin condensation and therefore silencing of gene transcription [13]. Later research described the deacetylation of histone and nonhistone proteins, orchestrated by a subgroup of deacetylases known as sirtuins [14]. A classification system of HDACs was later developed based on their molecular targets and mechanism of action. Briefly, there are four classes of HDACs in mammals, all of which utilize zinc as a cofactor, except for sirtuins (class III), which utilize nicotinamide dinucleotide (NAD+) as a substrate in enzymatic activity [14, 15]. As NAD+ levels vary according to nutrient availability, the activity of sirtuins is strongly associated with the metabolic status of the cell, with their activity increasing under conditions of calorie restriction and exercise [15–17].
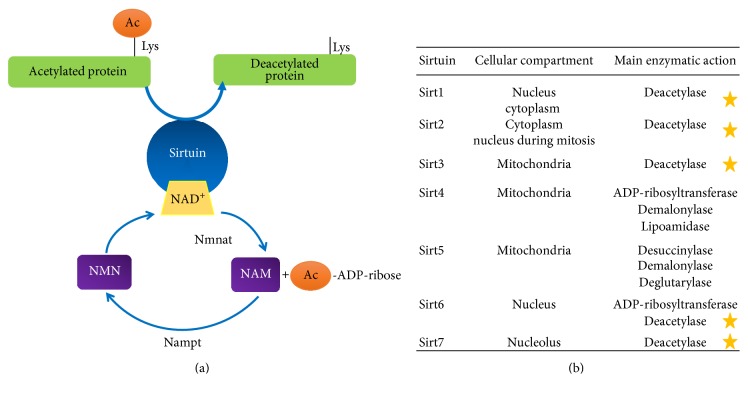
Sirtuins are widely distributed in prokaryotic and eukaryotic species, with seven isoforms characterized in humans [18, 19]. They share a common catalytic domain but differ with regard to their N- and C-terminal sequences, which determine their susceptibility to regulation by sirtuin-activating compounds [20] and PTMs (reviewed in [21]). The molecular targets and subcellular localization of different sirtuins vary, with some sirtuins present in more than one organelle. Although all sirtuins are classified as deacetylases, Sirt4 and Sirt5 have weak deacetylase activity and function as demalonylases and deacylases [22, 23] (Figure 1).
Figure 1.
(a) NAD+-dependent deacetylation reaction performed by sirtuins. NAD+ is synthesized from its precursor NMN and degraded into NAM + acetyl-ADP-ribose once sirtuins utilize it for their activation [10–12]. Activated sirtuins interact with their target protein and transfer the acetyl group from target lysine residues to ADP-ribose. (b) Sirtuins1–7, their subcellular localization, and the enzymatic activity they perform; yellow stars indicate deacetylase activity [13, 14, 17, 18]. NAD: nicotinamide dinucleotide; NMN: nicotinamide mononucleotide; Nmnat: nicotinamide mononucleotide adenylyltransferase; Nampt: nicotinamide phosphoribosyltransferase.
2. Protein Acetylation and CVDs
Research on the impact of protein acetylation in CVDs has focused mainly on Sirt1, Sirt3, and Sirt6, with little information regarding the function of the remaining sirtuins. Sirt1 and Sirt3 are involved in the regulation of important cellular mechanisms (e.g., apoptosis and cell survival), as well as the regulation of reactive oxygen species levels, hypertrophic and fibrotic responses, and mitochondrial biogenesis and function (Figure 2). Recent evidence suggests that impairments in the sirtuin family are associated with the development and progression of CVDs, as discussed below. Sirt1 modulates early embryogenesis, and homozygous sirt1−/− mice die shortly after birth [43]. As shown in deletion studies of crossbred mice, sirt1−/+ mice develop dilated cardiomyopathy [44] and exhibit increased cardiac injury induced by ischemia/reperfusion (I/R) [45]. These deleterious effects are associated with inhibition of Sirt1-mediated activation of forkhead box protein O1 (FOXO1) and O3 (FOXO3), which are responsible for the transcription of antioxidant enzymes, such as superoxide dismutase and catalases [44–46]. Sirt1 deficiency results in activation of proliferative and proinflammatory pathways involving tumour necrosis factor-α and nuclear factor-κB (NF-κB), leading to cardiac hypertrophy, fibrosis, and heart failure [24, 45] (Figure 2). With aging, the deleterious effects of Sirt1 deficiency become more prominent [47, 48]. Sirt1 stimulation and overexpression appears to provide protection against age-related cardiac diseases [48–50]. In addition, low to moderate overexpression of Sirt1 (2.5–7.5 times) mitigates cardiac hypertrophy induced by aging [48], provides protection against oxidative stress-induced cardiotoxicity [45], and improves endothelial function [26, 51]. Sirt1 stimulation and overexpression also has a range of other beneficial effects (Table 1). Sirt1 expression increases (12-fold) in hearts of dogs with experimental heart failure induced by rapid pacing [55]. Transgenic mice with cardiac-specific overexpression (20-fold) of Sirt1 exhibit mitochondrial dysfunction and dilated cardiomyopathy [64]. Thus, it appears that moderate stimulation of Sirt1 is beneficial for cardiac function, whereas excessive stimulation has deleterious effects on the heart.
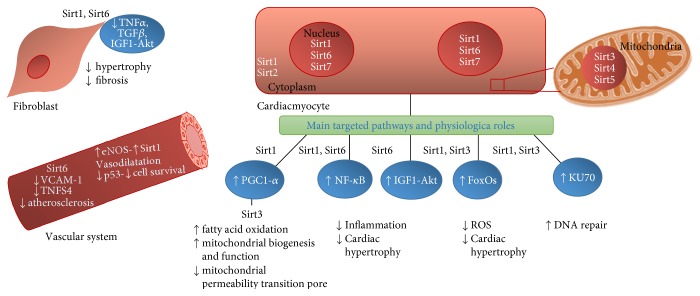
Figure 2.
Targeted pathways by sirtuins in cardiac fibroblasts, cardiac myocytes, and in the vascular system. Sirt1 and Sirt6 prevent fibrosis and fibroblast hypertrophy by repressing growth factors such as TGF-β and IGF1, as well as inflammatory cytokines like TNF-α [24, 25]. At the vascular level, Sirt1 activation induces vasodilatation and promotes cell survival via deacetylation of eNOS and p53. The activity of eNOS and p53 increases in a Sirt1-dependent manner [26], whereas Sirt6 inhibits VCAM and TNFS protecting against atherosclerosis [27]. Sirt1 in the cardiac myocyte promotes mitochondrial biogenesis and function mainly through the activation of PGC1-α and Sirt3 [28], which activates mitochondrial dehydrogenases, enzymes from the electron transport chain, and the synthase and represses cyclophilin D, protecting the cell from the opening of the mitochondrial permeability transition pore [29–38]. Nuclear sirtuins 1 and 6 prevent cardiac hypertrophy and inflammation through the inactivation of the NF-κB pathway [24, 25], as well as IGF-Akt by Sirtuin 6 [25]. Sirtuins 1 and 3 are also regulators of oxidative stress through the regulation of FoxOs, and both promote DNA repair through the activation of Ku70 [39–42].
Table 1.
Cardioprotective effect and mechanism of action of resveratrol in preclinical studies.
| Target HDAC or HAT | Molecular pathway | Experimental model | Cardiovascular effect | Reference |
|---|---|---|---|---|
| ↑ Sirt1 | ↑ PGC-1α ↑ Bcl2 ↓ Bax, caspase 3 ↑ SOD, SDH, Cyt-c oxidase |
TAC induced myocardial infraction In vivo Hypoxia induced dysfunction In vitro |
↑ LVEF ↓ fibrosis ↓ apoptosis |
[52] |
| ↑ Sirt3 | ↓ TGF-β/Smad3 | TAC induced heart failure In vivo |
↓ fibrosis ↓ collagen deposition ↓ cardiac hypertrophy Prevented decrease in cardiac FS Preserved diastolic function |
[53] |
| ↑ Sirt1 | ↑ SOD | Chronic heart failure model In vivo Ang II or antimycin A induced oxidative stress In vitro |
↑ FS ↑ LVEF ↑ survival ↓ apoptosis |
[54] |
| ↑ Sirt3 | ↑ SOD | Dox-induced mitochondrial dysfunction In vivo In vitro |
↓ oxidative stress ↑ ATP mitochondrial production |
[32] |
| ↑ Sirt1 | ↓ p38MAPK ↓ caspase 3 ↓ Bax ↑ Bcl-2 ↑ SOD1 |
Dox-induced heart failure In vivo |
↑ FS ↓ apoptosis ↓ oxidative stress |
[55] |
| ↑ Sirt1 | ↑ AMPK | Dox-induced cardiotoxicity In vitro |
↑ survival | [56] |
| ↑ Sirt3 | ↓ p53 ↓ Bax, Cyt-c |
Dox-induced cardiotoxicity In vivo |
↓ apoptosis Attenuated loss of diastolic and systolic function. |
[57] |
| ↑ Sirt1 | ↓ USP7 ↓ p300 ↓ Bax, caspase 3 ↓ p53 |
Dox-induced cardiotoxicity in young and aged hearts In vivo |
↑ FS ↑ EF ↓ LVEDS ↓ apoptosis |
[58] |
| ↑ Sirt1 | ↑ PI3K-Akt ↓ TNF-α ↓ FAS/FADD/caspase 8 ↓ caspase 3 ↑ FoxO3 |
Exercise during aging In vivo |
↑ FS ↓ fibrosis ↓ apoptosis |
[41] |
| ↑ Sirt1 | ↓ ac-FoxO1 ↓ Bim, Bax ↓ p53 |
Aging In vivo |
↑ FS ↑ LVEF ↓ fibrosis ↓ apoptosis |
[40] |
| ↑ Sirt1 | ↑ SOD ↑ GSH |
High glucose-induced mitochondrial oxidative stress. In vitro |
↓ oxidative stress | [59] |
| ↑ Sirt1 | ↓ p53 ↑ SDF-1 |
NE-induced hypertrophy In vitro Hypertension model In vivo |
↓ hypertrophy ↑ bioavailable NO ↓ apoptosis |
[60] |
| In T1DM: ↑ Sirt1, Sirt2, Sirt3, and Sirt5. In T2DM: ↑ Sirt1 and Sirt2 ↓ Sirt3, which was initially elevated |
↓ B-MHC ↓ Akt |
T1DM-induced cardiomyopathy In vivo T2DM-induced cardiomyopathy In vivo |
In T1DM rats: ↓ cardiac atrophy In T2DM rats: ↓ cardiac hypertrophy |
[61] |
| ↑ Sirt1, Sirt3, Sirt4, and Sirt7 | ↓ caspase 3 | H2O2-induced apoptosis In vitro |
↓ apoptosis | [62] |
| Most effects abolished when using sirtinol | ↑ SOD1, SOD3, GPx1, catalase. ↓ NOX2, NOX4 ↑ GTP cyclohidrolase 1 and biopterin |
In vivo
Apo-lipoprotein E Knockout mice |
↓Oxidative stress Reversed eNOS uncoupling |
[63] |
AMPK: adenosine monophosphate-activated kinase; Ang II: angiotensin II; B-MHC: myosin heavy chain B; Cyt-c: cytochrome c; Dox: doxorubicin; FS: fractional shortening; Gpx1: glutathione peroxidase 1; GSH: glutathione; LVEF: left ventricular ejection fraction; NE: norepinephrine; NOX: NAD(P)H oxidase; PGC1-α: peroxisome proliferator activator of transcription (PPARy) co-activator 1α; TAC: transverse aortic constriction; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; SDF-1: stroma cell derived factor 1; SHD: succinate dehydrogenase; SOD: superoxide dismutase. USP7: ubiquitin-specific-processing protease 7.
Sirt3 regulates mitochondrial protein acetylation, and severe hyperacetylation of mitochondrial proteins occurs in sirt3−/− knockout mice [29]. Such hyperacetylation has direct implications for ATP-dependent cellular processes, as hyperacetylation of enzymes involved in the Krebs cycle and electron transport chain translates into severe depletion of ATP (as much as 50%) [30], as well as compromised cardiac myocyte function. As demonstrated in previous studies, sirt3−/− mice develop cardiac hypertrophy, fibrosis, and mitochondrial dysfunction in an age-dependent manner [31]. In addition, sirt3−/− mice are more sensitive to damage induced by I/R injury [32–35] and microvascular dysfunction [34]. In contrast, overexpression of Sirt3 in mice hearts provides protection against cardiac hypertrophy and fibrosis [36, 37]. It also provides protection against oxidative stress-induced damage and apoptosis in the myocardium [38]. These protective effects are associated with activation of the antioxidant defence response, mediated by FoxO3a, which preserves mitochondrial energy production via the activation of mitochondrial dehydrogenases, thereby preventing mitochondrial permeability transition pore (mPTP) opening [38]. mPTP opening is followed by increases in Ca2+ overload, in addition to depletion of ATP and mitochondrial swelling, which eventually cause necrosis and apoptosis in cardiac myocytes [65]. In animal models of metabolic syndrome and ventricular dysfunction, mitochondria are prone to mPTP opening as compared with controls, concomitant with decreased Sirt3 expression and a hyperacetylated mitochondrial profile [66]. In biopsies of patients with heart failure, Sirt3 expression was lower in obese patients than in nonobese patients. Interestingly, acetylation profiles of patients with end-stage heart failure are correlated with body mass index and cardiac remodelling [66].
As shown in similar studies, the level of protein acetylation is closely associated with the metabolic status of the cell, and it varies with nutritional status [33]. Moreover, persistent hyperacetylation in the heart, as occurs in Sirt3 knockout mice, results in increased sensitivity to hemodynamic stress [67]. In addition, an increase in the NADH : NAD ratio inhibits Sirt3, resulting in mitochondrial hyperacetylation [67]. Restoring Sirt3 activity via normalization of the NADH/NAD ratio reverses protein hyperacetylation in complex I-deficient hearts, as well as in hearts with cardiac remodelling. Thousands of mitochondrial acetylation sites have been identified in acetylome analyses of sirt3−/− mice [68] and human failing hearts [8]. Among these, hyperacetylation of the malate-aspartate shuttle and regulators of mPTP opening are linked to the development of cardiac dysfunction [68]. Significant mitochondrial lysine hyperacetylation occurs in humans with end-stage heart failure, as shown by a myocardial acetylproteomic study [8].
Of note, homozygous sirt3−/− mice do not express any specific phenotype at birth, with cardiac development and function appearing normal under physiological conditions. Nevertheless, when exposed to I/R injury or agonist-induced cardiac hypertrophy, sirt3−/− mice exhibit severe mitochondrial hyperacetylation, in addition to decreased mitochondrial and myocardial function and lower survival rates [30]. This highlights the role of Sirt3 in the maladaptation observed during stressful cardiac conditions [35] and suggests that an NAD precursor, as well as sirtuin-activating compounds (STACs), could be used as cardiac therapy.
Sirt6 is a negative regulator of the insulin-like growth factor-1-protein kinase B pathway, which is implicated in the development of heart failure. Although sirt6−/− mice develop cardiac hypertrophy and heart failure, transgenic mice overexpressing this sirtuin are protected against both events. Likewise, Sirt6 expression levels are reduced in patients with failing hearts and in those with atherosclerosis [25, 69]. Sirt6 expression levels are also decreased in murine models [25]. Notably, sirt6−/− deficiency is associated with overexpression of proinflammatory cytokines, such as tumour necrosis factor superfamily member 4 and vascular cell adhesion molecule 1. These findings suggest that maintaining Sirt6 expression might be a novel therapeutic strategy against both cardiac and vascular dysfunction. Figure 2 summarizes the main reported targets and physiological roles of sirtuins in CVD prevention.
3. Regulation by Phenolic Compounds and Synthetic Molecules of Protein Acetylation in CVDs
The potential roles of several molecules as sirtuin activators have been studied due to their cardioprotective effects, which have been described both in vitro and in vivo (Figure 3). Most of these molecules are phenolic compounds, such as resveratrol (trans-3,5,4′-trihydroxystilbene) [17], but some synthetic molecules have been developed and successfully tested [20]. As phenolic compounds are hydrophobic, they can permeate the cell membrane to perform biological functions. The rate at which they enter the cell depends on both the size of the molecule and hydrophobicity of the attached functional groups [27, 70].
Figure 3.
Phytochemicals with beneficial effects in CVDs through modulation of protein acetylation.
The mechanism by which phenolic molecules promote the activity of sirtuins, specifically that of Sirt1, may involve allosteric activation and direct binding to a negatively charged amino acid from the N terminus, [54]. The binding of STACs lowers the Km of the substrate and thus enhances enzymatic activity. Although sirtuins share a common catalytic domain, they differ in their N- and C-terminal sequences. Thus, the mechanism by which phenolic molecules promote the activity of Sirt1 cannot be generalized to other sirtuin isoforms. As shown in previous research, activators of other sirtuins, such as Sirt3, directly interact with the protein, but the specific mechanism remains unclear [37]. Targeting adenosine monophosphate protein kinases (AMPKs) upregulates both Sirt1 activity and that of the peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1-α), a transcriptional coactivator, thereby indirectly increasing nuclear and mitochondrial sirtuin activity [28]. Other approaches for Sirt activation involve NAD+ precursors, such as nicotinamide riboside or nicotinamide mononucleotide [71], and augmentation of NAD+ availability via inhibition of glycohydrolases CD38 and CD157, which convert NAD+ to nicotinamide mononucleotide [72]. Detailed descriptions of the mechanism underlying the actions of STACs and the role of sirtuins in CVDs can be found elsewhere [73, 74]. The following sections provide a comprehensive review of natural STACs that regulate protein acetylation in CVDs.
3.1. Resveratrol
Resveratrol is a polyphenol found in grapes and red wine. It is one of the best studied phytochemicals, and it is known to provide protection against CVDs. It was first described as a Sirt1 activator by Howitz et al., who demonstrated that resveratrol was capable of reducing the Km of both the acetylated target of Sirt1 and that of NAD+ (35- and 5-fold, resp.) [17]. The mechanism underlying the activity of resveratrol was later questioned, with some proposing that it was dependent on the fluorophore utilized to label the evaluated peptide [75]. However, later research confirmed that resveratrol was an allosteric activator of Sirt1 [54].
Resveratrol has been evaluated in different models of cardiac disease, including chronic conditions such as heart failure and atherosclerosis, or damage associated with acute events, such as I/R (Table 1). In vitro, resveratrol decreases oxidative stress, inhibits hypertrophy, promotes cell survival, and inhibits apoptosis [38, 52, 56, 59, 60, 62, 76]. In vivo, supplementation with resveratrol decreases hypertrophy and fibrosis [52]. It also preserves cardiac function in models of heart failure induced by norepinephrine [60], doxorubicin [56–58], steptozotocin [61], and angiotensin II [53]. Furthermore, resveratrol prevents cardiac dysfunction in models of acute myocardial infarction induced by left coronary flow occlusion [76]. These cardioprotective effects are dependent on the activation of Sirt1 [52, 56, 59, 60, 62, 76] and Sirt3 [38, 53], as both gene silencing and specific sirtuin antagonists block the beneficial response. Moreover, in models of atherosclerosis, such as the apolipoprotein E (apoE−/−) mouse, resveratrol reverses endothelial nitric oxide synthase (eNOS) uncoupling and reduces oxidative stress. As resveratrol also enhances the activity of FoxO, the underlying mechanism possibly involves FoxO via NAD-dependent deacetylation, which contributes to cellular stress resistance [39]. Although sirtuin expression has not been studied in this model, treatment with sirtinol, a sirtuin inhibitor, abolished the beneficial effects of resveratrol supplementation [63].
The decrease in the activity of sirtuins with age is well known. Therefore, several models have studied the effects of supplementation with resveratrol on cardiac disease in a senescence setting. In senescence-accelerated mice, supplementation with resveratrol resulted in the recovery of Sirt1 activity [40]. Resveratrol supplementation also provided protection against hypertrophy and apoptosis, as well as preservation of left ventricular function, as compared with hearts from unsupplemented aged mice [40]. The addition of resveratrol to an exercise regime in aged rats potentiated the increase in Sirt1 activity achieved by exercise alone, and this translated into decreased fibrosis, apoptosis, and improved fractional shortening [41]. The molecular pathways involved in these in vitro and in vivo studies are summarized in Table 1.
3.2. Curcumin
Curcumin (diferuloylmethane), a polyphenol derived from the turmeric plant, is the second most well-studied phenolic compound for the treatment of CVDs. It modulates cardiac acetylation, mainly via the stimulation of Sirt1 [42, 77–79] and inhibition of histone acetyltransferase p-300 (p-300-HAT) [80–84].
The potential of curcumin-induced activation of Sirt1 as a mechanism to improve vascular function was studied in human THP-1-machrophage-derived foam cells [77]. Curcumin activated Sirt1 and decreased cellular cholesterol levels, preventing the formation of atherosclerotic plaques [77]. The authors attributed their findings to Sirt1-dependent activation of the ATP binding transporter cassette 1, which increased cholesterol efflux [77]. Another study showed that curcumin improved vascular function by Sirt1-dependent activation of eNOS [79]. By deacetylating eNOS, Sirt1 stimulated endothelium-dependent NO synthesis and protected endothelial cells against premature senescence induced by oxidative stress [79].
Curcumin-induced inhibition of p300-HAT is associated with decreased acetylation, which provides protection against cardiac injury. In murine models of myocardial infraction, curcumin-induced inhibition of p300-HAT resulted in decreased infract sizes, in addition to the prevention of cardiac hypertrophy and fibrosis and preservation of ventricular function [42, 80, 81]. The beneficial effects of curcumin have been attributed to is downregulation of transcription factors, such as NF-κB, GATA binding protein 4, and transforming growth factor β1, that are normally activated in the presence of myocardial damage (Table 2). In models of both chronic and acute myocardial damage, curcumin-induced inhibition of p-300-HAT result in decreased apoptosis in response to deacetylation of p53, as well as inhibition of proapoptotic Bax and caspase 3 [42, 80]. These cardioprotective effects were attributed to Sirt1 stimulation [77, 79]. Based on the current literature, decreased cardiac acetylation, either by activation of Sirt1 or inhibition of p-300-HAT, appears to induce similar responses, such as inhibition of proinflammatory and profibrotic transcription factors, as well as activation of antioxidant enzymes, that ultimately preserve cardiac function (Table 2) [81–83].
Table 2.
Cardioprotective effect and mechanism of action of curcumin in preclinical studies.
| Target HDAC or HAT | Molecular pathway | Experimental model | Cardiovascular effect | Reference |
|---|---|---|---|---|
| ↑ Sirt1 | ↓ TGF-β, Col III, Col I | TAC induced myocardial infraction In vivo Ang II-Induced hypertrophy In vitro |
↓ infract area ↓ fibrosis ↓ hypertrophy |
[77] |
| ↑ Sirt1 | ↑ SOD ↑ Bcl2, ↓ Bax |
Isolated ischemia-reperfusion model Ex vivo TAC induced myocardial infraction In vivo Simulated ischemia-reperfusion model In vitro |
Improved post-ischemic cardiac function ↓ myocardial infract size ↓ apoptotic index ↓ oxidative stress Preserved serum CK activity ↓ LDH serum levels |
[42] |
| ↑ Sirt1 | ↑ eNOS ↓ p21 |
H2O2-induced endothelial premature senescence In vitro |
↓ premature senescence ↓ oxidative stress ↓ apoptosis Preverved NO synthesis |
[79] |
| ↑ Sirt1 | ↑ AMPKα ↑ LXR-α ↑ ATP binding cassette transporter 1 |
Atherogenic model In vitro |
Antiatherogenic ↓ cellular cholesterol ↑ cholesterol efflux from THP-1 |
[78] |
| ↓ p300-HAT | ↓ acetylation of histones 3 and 4 | LPS-induced cardiac hypertrophy In vivo |
↓ cardiac hypertrophy | [83] |
| ↓ p300-HAT | ↓ TGF-β/Smad2 | High glucose-induced cardiac hypertrophy In vitro Streptozotocin-induced cardiac dysfunction In vivo |
↓ cardiac hypertrophy ↓ extracellular matrix production ↑ diastolic function |
[81] |
| ↓ p300-HAT | ↓ GATA4 ↓ NF-κB ↓ acetylation of histones 3 and 4 |
TAC induced myocardial Infraction In vivo PE-induced hypertrophy In vitro |
↓ LV wall thickness Preserved systolic function ↓ hypertrophy |
[77] |
| ↓ p300-HAT | ↓ Ac-p53 ↓ ANF, β-MHC ↓ Bax, Cyt c, caspase 3, and PARP |
TAC induced myocardial Infraction In vivo Ang II-Induced hypertrophy In vitro |
↓ hypertrophy ↓ apoptosis |
[80] |
| ↓ p300-HAT | ↓ GATA4 ↓ p53 |
Hypoxia-induced hypertrophy model In vitro |
Stabilized mitochondrial membrane potential Restored lactate, acetyl-coA pyruvate, and glucose levels |
[82] |
AMPK: adenosine monophosphate-activated kinase; ANF: atrial natriuretic factor; Ang II: angiotensin II; B-MHC: myosin heavy chain B; CK: creatine kinase; Cyt-c: cytochrome c; EF: ejection fraction; eNOS: endothelial nitrix oxide synthase; LDH: lactate dehydrogenase; LXR-α: liver X receptor α; LV: left ventricular; NE: norepinephrine; PAI-I: plasminogen activator inhibitor 1; PARP: poly(ADP-ribose) polymerase; PGC1-α: peroxisome proliferator activator of transcription (PPARy) coactivator 1α; PE: phenylephrine TAC: transverse aortic constriction; SOD: superoxide dismutase.
3.3. Honokiol
Honokiol, a biphenolic compound obtained from the bark of the magnolia tree, was recently evaluated in a murine model of cardiac hypertrophy and fibrosis [37]. Remarkably, the authors demonstrated that honokiol was not only capable of preventing agonist-induced heart failure but it also reversed preexisting fibrosis and ventricular failure. The cardioprotective effects of honokiol were associated with a dose-dependent increase in Sirt3 activity. Regarding the mechanism of action, the authors showed that honokiol entered mitochondria and directly interacted with Sirt3, although the precise binding site for activation remains unclear.
3.4. Oroxylin A
Oroxylin A (OA) is derived from the root of Scutellaria baicalensis. Based on its chemical structure, with hydroxyl groups at C-5 and C-7 and a methoxy group at C-6, it is classified as a flavone [85]. As demonstrated in pharmacokinetic studies involving animal models, OA is highly bioavailable after oral infection, which increases its potential as a bioactive compound [85]. Previous studies reported that OA functioned as a Sirt3 activator in human breast cancer cells [86] and as an acute Sirt3 activator in an in vitro model of cardiac myocyte insulin resistance [87, 88]. Via the activation of Sirt3, OA prevented loss of contractile function in response to insulin overstimulation, as evidenced by preserved peak shortening [88]. OA also appeared to reduce angiotensin-induced hypertrophy and cell death in cardiac myoblasts, pointing to a potential cardioprotective effect. In addition, OA decreased mitochondrial hyperacetylation and energetic debacle in a dose-dependent manner [89]. Based on the current evidence, OA appears to increase Sirt3 activity in cardiac cells, although no precise mechanism of action has been described thus far.
3.5. Other Emerging Regulators of Protein Acetylation in CVDs
Information on regulators of protein acetylation other than the aforementioned is scarce but encouraging. Details on phytochemicals capable of modulating cardiac acetylation via the activation of sirtuins in models of CVDs are presented in Figure 3. They include quercetin, epigallocatechin-3-gallate, bakuchiol, tyrosol, and berberine [81–86]. Nutraceuticals that function as activators of Sirt1 include docosahexaenoic acid, alpha-lipoic acid, sulforaphane, and caffeic acid ethanolamide, although the mechanisms by which they activate sirtuins remain to be elucidated [26, 89–97]. Most of these functional molecules share common molecular targets and exert their actions, for example, by stimulation of AMPK-α, eNOS, PGC1-α, and superoxide dismutase or inhibition of NF-κB and proapoptotic molecules (e.g., Bax and caspase 3). Table 3 summarizes the findings of experimental models and the results obtained for each reported phytochemical, specifying the molecular pathways and targets involved.
Table 3.
Other emerging cardioprotective phytochemicals regulating protein acetylation.
| Phytochemical | Target HDAC or HAT | Molecular pathway | Model | Cardiovascular effect | Reference |
|---|---|---|---|---|---|
| Honokiol | ↑ Sirt3 | ↓ collagen, B-MHC, and ANF | TAC induced heart failure model In vivo PE and Ang II-induced cardiac hypertrophy In vitro |
Blocks cardiac hypertrophic response Ameliorates preexisting hypertrophy ↓ oxidative stress |
[37] |
| Oroxylin A | ↑ Sirt3 | ↑ aldehyde dehydrogenase | Insulin-induced cardiac dysfunction In vitro |
Preserved cardiac myocyte contractility | [87] |
| Epigallocatechin-3-gallate | ↑ Sirt1 | ↑ AMPK-α ↑ eNOS |
High-fat diet-induced hypercholesterolemia In vivo |
↓ serum cholesterol ↓ oxidative stress Improved morphology of myocardial tissue |
[90] |
| ↓ Ac-FoxO1 ↓ Nrf2 |
High-glucose-induced-autophagy In vitro |
↓ ROS ↓ autophagy |
[89] | ||
| Quercetin | ↑ Sirt1 | ↑ AMPK-α ↑ eNOS ↓ NOX2 ↓ NOX4 ↓ NF-κB |
OxLDL-induced endothelial oxidative stress In vitro |
Preserved mitochondrial function ↓ inflammation |
[91] |
| Berberine | ↑ Sirt1 | ↑ SOD ↑ Bcl-2 ↓ Bax, caspase 3 |
Ischemia/reperfusion-induced myocardial Infraction In vivo Simulated ischemia/reperfusion model In vitro |
↓ infract size ↓ oxidative stress ↓ apoptosis ↓ LDH Maintained LVEF and LVFS Inhibited increase in IL-6 and TNF-α |
[92] |
| Bakuchiol | ↑ Sirt1 | GC-1α ↑ Bcl2 ↓ Bax, caspase 3 ↑ SOD, SDH, Cyt-c oxidase |
Ischemia reperfusion-induced myocardial infraction Ex vivo Simulated ischemia/reperfusion model In vitro Rat cardiac myocytes |
↓ apoptosis ↓ oxidative stress Maintained mitochondrial bioenergetics |
[93] |
| n-Tyrosol | ↑ Sirt1 | ↑ Akt ↑ eNOS ↑ Foxo3a |
TAC induced myocardial infraction In vivo |
↓ infract size ↓ apoptosis ↓ fibrosis ↑ LVIDd ↑ EF ↑ FS |
[94] |
| α-Lipoic acid | ↑ Sirt1 | ↓ PARP-2 | TAC-induced cardiac hypertrophy In vivo Ang II-induced hypertrophy In vitro |
↓ cardiac hypertrophy | [95] |
| Docosahexaenoic acid | ↑ Sirt1 | ↑ eNOS |
In vitro
Ex vivo |
↑ NO synthesis ↑ bioavailable NO |
[26] |
| Sulforaphane | ↑ Sirt1 | ↑ Nrf2, NQo1, HO-1 ↓ PAI-I, TNF-α, CTFG, TGF-β Preserved LKB1/AMPK/PGC-1α |
T2DM-induced cardiomyopathy In vivo |
↓ cardiac remodeling ↓ cardiac dysfunction ↓ cardiac lipid accumulation ↓ oxidative stress ↓ inflammation ↓ fibrosis |
[96] |
| Caffeic acid ethanolamide | ↑ Sirt1 ↑ Sirt3 |
↑ SOD, HIF1-α | Isoproterenol-induced cardiac dysfunction In vivo In vitro |
Restored oxygen consumption rates Preserved ATP levels ↓ cardiac remodeling ↓ oxidative stress Preserved mitochondrial function |
[97] |
AMPK: adenosine monophosphate-activated kinase; ANF: atrial natriuretic factor; Ang II: angiotensin II; B-MHC: myosin heavy chain B; CTFG: connective tissue growth factor; Cyt-c: cytochrome c; Dox: doxorubicin; EF: ejection fraction; eNOS: endothelial nitrix oxide synthase; FS: fractional shortening; HIF1-α: hypoxia inducible factor 1-α; HO-1: heme oxygenase; LDH: lactate dehydrogenase; LKB1; liver kinase B 1; LVID internal diameter in diastole; left ventricular, LVEF: left ventricular ejection fraction; NE: norepinephrine; NQo1: NAD(P)H quinone dehydrogenase 1; PAI-I: plasminogen activator inhibitor 1; PARP-2: poly(ADP-ribose) polymerase 2; PGC1-α: peroxisome proliferator activator of transcription (PPARy) coactivator 1α; PE: phenylephrine TAC: transverse aortic constriction; T2DM: type 2 diabetes mellitus; SHD: succinate dehydrogenase; SOD: superoxide dismutase.
4. Clinical Evidence for Cardioprotective Properties of Natural Modulators of Protein Acetylation
Experimental data supports cardioprotective properties of natural modulators of protein acetylation, but little is known about their effects in a clinical setting. To date, resveratrol is the only phytochemical that has been tested as a sirtuin activator in humans. As discussed previously, resveratrol mimics calorie restriction effects in vitro and in vivo. A recent study of obese patients demonstrated that supplementation with resveratrol for 30 days significantly increased Sirt1 expression via activation of AMPK and that it improved muscle mitochondrial respiration by increasing fatty acid oxidation [98]. This translated into decreased hepatic lipid accumulation and reduced inflammation [98]. The study did not measure variations in cardiac function. Nevertheless, it is well known that obesity is an independent risk factor for CVDs [1]. In this context, the observed protection against inflammation and lipid accumulation might decrease the risk of vascular and cardiac pathologies in obese patients.
In contrast to the synergetic effects of resveratrol and physical activity observed in murine models, supplementation with resveratrol blunted the cardioprotective effects achieved by 8 weeks of physical exercise in men over 60. This study detected no changes in Sirt1 expression in either group [99]. As the roles of both physical activity and resveratrol as activators of Sirt1 have been demonstrated in experimental models of senescence, it appears that the human response to both stimuli is different with regard to the activation of the AMPK/Sirt1/PGC1-α axis, as discussed previously [100].
The role of resveratrol as a Sirt1 activator was evaluated in postmenopausal woman of with a normal weight and glucose tolerance [101]. In this group, resveratrol supplementation was associated with no major improvements in metabolic parameters or modification of Sirt1 expression [101]. Although sirtuin deficiency is uncommon in healthy individuals, it has been reported in obese patients and patients with metabolic syndrome [98, 102]. Additionally, sirtuin activity was decreased in a study of heart failure patients [8]. In common with vitamin supplementation in the absence of any vitamin deficit [103], supplementation with sirtuin activators is not expected to have any benefit when basal levels of Sirt1 expression and activity are normal. The aforementioned might explain why supplementation with resveratrol has a positive effect under conditions of obesity [98] but no effects under conditions of normal weight and glucose tolerance [101].
5. Bioavailability of Cardioprotective Phytochemicals
Although experimental models have revealed promising cardioprotective effects of phytochemicals, their bioactivity in the clinical setting remains to be explored. After oral ingestion of phenolic compounds, two factors mainly determine their biological activity: absorption and metabolic stability. Absorption in the small intestine varies according to the hydrophobicity of the compounds and affinity of membrane transporters, and only aglycones can be efficiently absorbed [27, 104–106]. Most polyphenols must be hydrolysed by intestinal enzymes or microflora in order to permeate the intestinal epithelium [105, 106]. Once they are absorbed and reach the liver, their stability depends on their sensitivity to metabolism by phase II enzymes.
Only a few pharmacokinetic studies of phenolic compounds have been reported in humans. A study of resveratrol absorption and metabolism in healthy subjects showed that after oral ingestion of resveratrol, around 92% of the administered dose was excreted in urine and faeces [27]. In an experimental study, the final amount of resveratrol absorbed in liver fractions was extremely low due to rapid formation of conjugates of resveratrol, mainly by sulfation and glucuronidation [104].
Some studies have explored the potential impact of functional groups on the bioavailability of polyphenols. Methylated polyphenols easily permeated the intestinal epithelium, without previous conjugation, in contrast to unmethylated compounds [104]. In the presence of hepatic phase II enzymes, methylated polyphenols were more stable than their unmethylated counterparts. These properties should encourage more in-depth studies of the potential biological effects of naturally methylated polyphenols, considering the benefits of their pharmacokinetic profiles.
Recently, the role of nanocarriers as a novel strategy to increase the bioavailability of polyphenols has been studied (reviewed in [107]). Besides increasing absorption and protecting polyphenols from enzymatic degradation, nanocarriers can be configured to release material in a controlled and prolonged manner, maintaining bioactivity for longer periods. Two studies that examined the potential of nanocurcumin as a sirtuin activator reported significant improvements in its bioactivity [82, 84]. Although neither study directly compared the biological effects of curcumin versus those of nanocurcumin, the biological effects of nanocurcumin were observed at lower concentration than that of free curcumin [42, 80, 81] (Table 2).
A number of studies have examined other nanoencapsulated polyphenols, although they did not evaluate their activity as modulators of acetylation. Recent evidence indicates that cardiac muscle and vessels are targets for nanotechnology-based therapies that could reach the myocardium through dysfunctional permeable endothelium [108]. In a murine model of heart failure, passive cardiac accumulation of high concentrations of nanocarriers occurred after a single application [108]. Compared to normal heart tissues, the accumulation of nanovectors was more than 10 times higher in the heart failure murine model [108]. This approach using nanocarriers, represents a potential avenue for functional molecules, which may be translated into innovative treatments to improve patient CVD outcomes.
Recent studies revealed that the microbiome can significantly modify the extent to which phenolic compounds are metabolized [109, 110]. It is well known that intestinal and colonic microorganisms vary according to a patient's physiological status [111]. Thus, preliminary pharmacokinetic studies should ideally be performed in systems simulating both healthy and unhealthy gastrointestinal tracts.
6. Potential Toxicity of Phytochemicals
Although numerous health benefits are associated with the consumption of phytochemicals, caution is needed when selecting an exploratory dose because of their hormetic behaviour. Plants synthesize phytochemicals and activate adaptive molecular pathways to protect themselves against cellular stress. Although exogenous administration of phytochemicals to organisms can have protective effects at specific doses, they can also have prooxidant and cytotoxic effects at relatively high concentrations [112].
Regarding cardioprotection and the toxicity of phytochemicals, a recent study compared heart function in rats after 21 days of supplementation with increasing doses of resveratrol [113]. The authors reported that 2.5 mg/kg/d and 25 mg/kg/d protected ex vivo against I/R induced injury but that higher doses had adverse effects on cardiac function [113]. Interestingly, in this study, both rats and rabbits showed greater tolerance to a synthetic resveratrol formulation, Longevinex, which contains small amounts of quercetin and ferulic acid, than to resveratrol alone. This finding might be explained by increased flavonoid stability and metabolic competition when administered in combination than when administered singly, with the combination therapy potentially decreasing, as well as having a prooxidant effect. Clinical trials reported no adverse effects of resveratrol doses ranging from 0.4 mg/kg/d to 5 g/d [114, 115]. The apparent higher tolerance observed in humans than in animal models might be explained by lower resveratrol bioavailability and metabolic competition with other nutrients present in a patient's diet, in addition to differences in the gastrointestinal tracts of humans and animals.
To our knowledge, there are no reports on the pharmacokinetic profiles of the remaining reviewed molecules in humans. However, as shown by in vitro and in vivo studies, most phytochemicals exhibit a similar bimodal dose-response curve. Supplementation with epigallocatechin-3-gallate at 30 and 60 mg/kg abolished anxiety in mice [116, 117]. Remarkably, increasing the dose to 100 mg/kg induced 100% mortality in less than 24 h [116, 117]. Epigallocatechin-3-gallate is the most abundant catechin in green tea. Although moderate consumption of this beverage has been associated with health benefits, more than 1 L per day increased the risk of cancer in humans [118], although this finding was attributed to the temperature of the beverage and not only to its bioactive substances [118, 119]. Although many studies in the literature support the potential cardioprotective effects of the phytochemicals reviewed in the present work, in-depth studies of their bioavailability and pharmacokinetic profiles are missing. Further research is needed to address this issue.
7. Conclusion
Protein hyperacetylation is associated with the development of several CVDs, including atherosclerosis, hypertension, cardiac hypertrophy, and heart failure. The underlying mechanisms include activation of proinflammatory cytokines and proapoptotic molecules and inhibition of mitochondrial biogenesis and function, in addition to downregulation of enzymes involved in antioxidant defence. Decreased expression and activity of the deacetylases Sirt1, Sirt3, and Sirt6 are associated with the development and progression of the aforementioned pathologies.
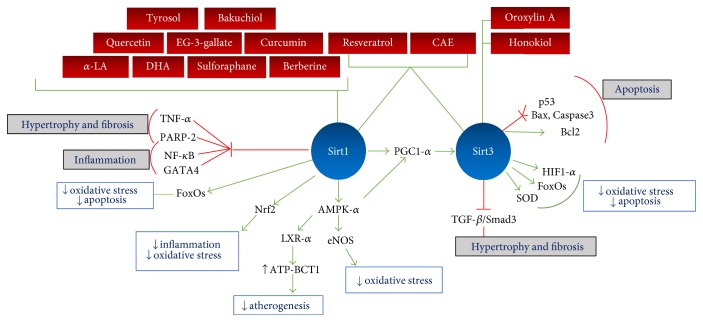
The potential cardioprotective roles of several phytochemicals as regulators of sirtuin-mediated protein deacetylation have been studied. Preclinical evidence suggests that by activating Sirt1 and/or Sirt3, some bioactive phytochemicals can protect the cardiovascular system from the negative consequences of hyperacetylation (Figure 4). In the clinical setting, only resveratrol has been validated as a Sirt1 activator in obese patients, with conflicting results found in other clinical trials performed with men over 60 and postmenopausal women. As healthy subjects show no benefit from supplementation, it appears that sirtuin activators should be evaluated only in specific patient groups, such as obese subjects or those with metabolic syndrome or heart failure, with a previous reported deficiency. Pharmacokinetic studies in humans are required to determine the optimum dose selection. The low bioavailability of phytochemicals limits their biological effects. Various strategies, including nanodelivery systems, aimed at overcoming this problem are currently under way. Initial results of these studies appear promising.
Figure 4.
Cardioprotective effects of sirtuin activators and the molecular pathways involved. Red boxes state the phytochemicals regulating the activity of Sirt1, Sirt3, or both, as indicated by the green connecting lines. Green lines indicate activation of the indicated targets, whereas red lines indicate inhibition. Gray boxes indicate inhibition of cellular responses and white boxes indicate stimulation of them. CAE: caffeic acid ethanolamide; a-LA: alpha-lipoic acid; DHA docosahexaenoic acid.
Acknowledgments
This work was partially supported by Xignus Research Foundation as well endowed Chair in Cardiology/Grupo de Enfoque Medicina Cardiovascular Tec de Monterrey, CONACYT Grant CB-256577, Fronteras de la Ciencias 0682 (Gerardo García-Rivas), and Red Temática Farmoquímicos del CONACYT. Niria Treviño-Saldaña was supported by a graduate scholarship program from CONACYT. The authors thank Dr. Aurora Valdez for her methodological assessment during the organization of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mozaffarian D., Benjamin E. J., Go A. S., et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Mendis S., Puska P., Norrving B., editors. World Health Organization. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: 2011. Obtained from http://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/ [Google Scholar]
- 3.Egert S. A., Bosy-Westphal J. Seiberl. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. British Journal of Nutrition. 2009;7(102):1065–1074. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 4.Faridi V., Njike S., Dutta A., Ali D., Katz D. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. The American Journal of Clinical Nutrition. 2008;1(88):58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 5.García-Rivas G., Youker K. A., Orrego C., et al. Standardized extract from black bean coat (Phaseolus vulgaris L.) prevents adverse cardiac remodeling in a murine model of non-ischemic cardiomyopathy. RSC Advances. 2015;5:90858–90865. doi: 10.1039/c5ra07715j. [DOI] [Google Scholar]
- 6.Rodríguez-Sánchez D., Silva-Platas C., Rojo R. P., et al. Activity-guided identification of acetogenins as novel lipophilic antioxidants present in avocado pulp (Persea americana) Journal of Chromatography B. 2013;30:942–943. doi: 10.1016/j.jchromb.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Sanchez D. G., Flores-García M., Silva-Platas C., et al. Isolation and chemical identification of lipid derivatives from avocado (Persea americana) pulp with antiplatelet and antithrombotic activities. Food & Function. 2001;6(1):193–203. doi: 10.1039/c4fo00610k. [DOI] [PubMed] [Google Scholar]
- 8.Horton J. L., Martin O. J., Lai L., et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;2, article e84897 doi: 10.1172/jci.insight.84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadtochiy S. M., Redman E., Rahman I., Brookes P. S. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovascular Research. 2011;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bong-Hyun A., Hyun-Seok K., Shiwei S., et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh C. T., Graneau-Tsodikova S., Gatto G. J. Protein posttranslational modifications: the chemistry of proteome diversifications. Angewandte Chemie (International Ed. in English) 2005;45:7342–7372. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- 12.Verdin E., Ott M. 50 years of protein acetylation: from gene regulation to epigenetics; metabolism and beyond. Nature Reviews Molecular Cell Biology. 2014;16(4):258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 13.Allfrey V. G., Faulkner R., Mirsky A. E. Acetylation and methylation of histones and their possible role in the regulation of Rna synthesis. Proceedings of the National Academy of Sciences. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su-ju L., Kaeberlein M., Andalis A. A., et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 15.Brachmann C. B., Sherman J. M., Devine S. E., Cameron E. E., Pillus L., Boeke J. D. The SIR2 gene family; conserved from bacteria to humans; functions in silencing; cell cycle progression; and chromosome stability. Genes Development. 1995;23:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- 16.Michan S., Sinclai R. D. Sirtuins in mammals: insights into their biological function. Biochemical Journal. 2007;404(1):1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howitz K. T., Bitterman K. J., Cohen H., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 18.Frye R. A. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochemical and Biophysical Research Communications. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 19.Frye R. A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochemical and Biophysical Research Communications. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 20.Smith J. J., Kenney R. D., Gagne D. J., et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Systems Biology. 2009;10:p. 31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flick F., Lūscher B. Regulation of sirtuin function by posttranslational modifications. Frontiers in Pharmacology. 2012;3:p. 29. doi: 10.3389/fphar.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haigis M. C., Mostoslavsky R., Haigis K., et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 23.Du J., Zhou Y., Su X., et al. Sirt5 is an NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2016;334(6057):806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X., Lui Q., Wang M., et al. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS One. 2011;6(11, article e27081) doi: 10.1371/journal.pone.0027081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu S., Yin M., Koroleva M., et al. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. Aging. 2016;8(5):1064–1078. doi: 10.18632/aging.100975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung S. B., Kwon S. K., Kwon M., et al. Docosahexaenoic acid improves vascular function via up-regulation of SIRT1 expression in endothelial cells. Biochemical and Biophysical Research Communications. 2013;437:114–119. doi: 10.1016/j.bbrc.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Walle T., Hsieh F., DeLegge H. M., Oatis J. E., Walle U. K. High absorption but very low bioavailability or oral resveratrol in humans. Drug Metabolism and Disposition. 2004;23(12):1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 28.Higashida K., Kim S. H., Jung S. R., Asaka M., Holloszy J. O., Dong---Ho H. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS Biology. 2014;12(1):p. 10. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombard D. B., Alt F. W., Cheng H. L., et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Molecular and Celullar Biology. 2007;24:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn B. H., Kim H. S., Song S., et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proceedings of the National Academy of Sciences. 2008;38:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hafner A. V., Dai J., Gomes A. P., et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging. 2010;2(12):914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter G. A., William R. U., Brookes P. S., Nadtochy S. M. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. American Journal of Physiology Heart and Circulatory Physiology. 2014;306:H1602–H1609. doi: 10.1152/ajpheart.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L., Vaitheesvaran B., Hartil K., et al. N6-acetylation differences suggest acetylation coordinates organ-specific fuel switching. Journal of Proteome Research. 2011;10(9):4134–4149. doi: 10.1021/pr200313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He X., Zeng H., Chen J. X. Ablation of SIRT3 causes coronary microvascular dysfunction and impairs cardiac recovery post myocardial ischemia. International Journal of Cardiology. 2016;215:349–357. doi: 10.1016/j.ijcard.2016.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parodi-Rullán R. M., Chapa-Dubocq X., Rullán P. J., Jang S., Javadov S. High sensitivity of SIRT3 deficient hearts to ischemia-reperfusion is associated with mitochondrial abnormalities. Frontiers in Pharmacology. 2017;16(8):p. 275. doi: 10.3389/fphar.2017.00275. 14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundersan N. R., Gupta M., Kim G., Rajamohan S. B., Isbatan A., Gupta M. P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. Journal of Clinical Investigation. 2009;119(9):2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillai V. B., Samant S., Sundersan N. R., et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial SIRT3. Nature Communications. 2015;6:p. 6656. doi: 10.1038/ncomms7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung K. G., Cole L. K., Xiang B., et al. Sirtuin-3 (SIRT3) protein attenuates doxorubicin-induced oxidative stress and improves mitochondrial respiration in H9c2 cardiomyocytes. The Journal of Biological Chemistry. 2015;290:10981–10993. doi: 10.1074/jbc.M114.607960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi Y., Furukawa-Hibi Y., Chen C., et al. SIRT1 is critical regulator of FoxO-mediated transcription in response to oxidative stress. International Journal of Molecular Medicine. 2005;16(2):237–243. doi: 10.3892/ijmm.16.2.237. [DOI] [PubMed] [Google Scholar]
- 40.Sin T. K., Yu A. P., Yung B. Y., et al. Modulating effect of SIRT1 activation induced by resveratrol on FoxO1-associated apoptotic signalling in senescent heart. The Journal of Physiology. 2014;592(12):2535–2548. doi: 10.1113/jphysiol.2014.271387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin C. H., Lin C. C., Ting W. J., et al. Resveratrol enhanced FoxO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. AGE. 2014;36:p. 9705. doi: 10.1007/s11357-014-9705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y., Duan W., Lin Y., et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radical Biology & Medicine. 2013;65:667–679. doi: 10.1016/j.freeradbiomed.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 43.McBurney M. W., Yang X., Jardine K., et al. The mammalian SIR2α protein has a role in embryogenesis and gametogenesis. Molecular and Cellular Biology. 2003;23(1):38–54. doi: 10.1128/mcb.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Planavila A., Dominguez E., Navarro M., et al. Dilated cardiomyopathy and mitochondrial dysfunction in Sirt1-deficient mice: a role for Sirt1-Mef2 in adult heart. Journal Molecular and Cellular Biology. 2012;53(4):521–531. doi: 10.1016/j.yjmcc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Hsu C. P., Zhai P., Yamamoto T., et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122(21):2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan X.-H., Liu X.-H., Hong X., et al. CD38 deficiency protects the heart from ischemia/reperfusion injury through activating SIRT1/FOXOs-mediated antioxidative stress pathway. Oxidative Medicine and Cellular Longevity. 2016;2016:14. doi: 10.1155/2016/7410257.7410257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfluger P. T., Herranz D., Velasco-Miguel S., Serrano M., Tschop M. H. Sirt1 protects against high-fat diet-induced metabolic damage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alcendor R. R., Gao S., Zhai P., et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circulation Research. 2007;100(10):1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 49.Ruan Y., Dong C., Patel J., et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cellular Physiology and Biochemistry. 2015;35:1116–1124. doi: 10.1159/000373937. [DOI] [PubMed] [Google Scholar]
- 50.Prola A., Da Silva J. P., Guilbert A., et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death & Differentiation. 2017;24:343–356. doi: 10.1038/cdd.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson A. M., Martin K. A., Rzucidlo E. M. Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLos One. 2014;9(1, article e85495) doi: 10.1371/journal.pone.0085495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanno M., Kuno A., Yano T., et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. The Journal of Biological Chemistry. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tongshuai C., Jingyuan L., Junni L., et al. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGFB/Smad3 pathway. The American Journal of Physiology. 2015;308:H424–H434. doi: 10.1152/ajpheart.00454.2014. [DOI] [PubMed] [Google Scholar]
- 54.Hubbard B. P. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alcendor R. R., Kirshenbaum L. A., Imai S., Vatner S. F., Sadoshima J. Silent information regulator 2α a longevity factor and class III histone deacetylase; is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circulation Research. 2004;95(10):971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- 56.Lou Y., Wang Z., Xu Y., et al. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. International Journal of Molecular Medicine. 2015;36:873–880. doi: 10.3892/ijmm.2015.2291. [DOI] [PubMed] [Google Scholar]
- 57.Zhang C., Feng Y., Qu S., et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovascular Research. 2011;90:538–545. doi: 10.1093/cvr/cvr022. [DOI] [PubMed] [Google Scholar]
- 58.Sin T. K., Tam B. T., Yung B. Y., et al. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. The Journal of Physiology. 2015;593(8):1887–1899. doi: 10.1113/JP270101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ungvari Z., Labinskyy N., Mukhopadhyay P., et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. The American Journal of Physiology. 2009;2009(297):H1876–H1881. doi: 10.1002/jcb.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thandapilly S. J., Louis X. L., Yang T., et al. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes; by activating NO-AMPK pathway. European Journal of Pharmacology. 2011;668(1-2):217–224. doi: 10.1016/j.ejphar.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 61.Bagul P. K., Dinda A. K., Banerjee S. K. Effect of resveratrol on sirtuins expression and cardiac complications in diabetes. Biochemical and Biophysical Research Communications. 2015;468:221–227. doi: 10.1016/j.bbrc.2015.10.126. [DOI] [PubMed] [Google Scholar]
- 62.Yu W., Fu Y. C., Zhou X. H., et al. Effects of resveratrol on H2O2-induced apoptosis and expression of SIRTs in H9c2 cells. Journal of Cellular Biochemistry. 2009;107:741–747. doi: 10.1002/jcb.22169. [DOI] [PubMed] [Google Scholar]
- 63.Xia N., Daiber A., Habermeier A., et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. Journal of Pharmacology and Experimental Therapeutics. 2010;335(1):149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 64.Kawashima T., Inuzuka Y., Okuda J., et al. Constitutive SIRT1 overexpression impairs mitochondria and reduces cardiac function in mice. Journal of Molecular and Cellular Cardiology. 2011;51(6):1026–1036. doi: 10.1016/j.yjmcc.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 65.García-Rivas G. J., Torre-Amione G. Abnormal mitochondrial function during ischemia reperfusion provides targets for pharmacological therapy. Methodist DeBakey Cardiovascular Journal. 2009;5(3):2–7. doi: 10.14797/mdcj-5-3-2. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Rivas G., Morales J. A., Vega-Sevilla L., Silva-Platas C., García N. Regulation of mitochondrial permeability transition by Sirt3-catalyzed cyclophilin D deacetylation and its relevance for ventricular dysfunction in metabolic syndrome. Mitochondr Physiol Network. 2013;18(08) [Google Scholar]
- 67.Karamanlidis G., Lee C. F., Garcia-Menendez L., et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metabolism. 2013;18:239–25045d. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee C. F., Chavez J. D., Garcia-Menendez L., et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134(12):883–894. doi: 10.1161/circulationaha.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sundersan N. R., Vasudevan P., Zhong L., et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nature Medicine. 2012;18:1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen X., Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metabolism & Disposition. 2006;34:1786–1792. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 71.Canto C., Houtkooper R. H., Pirinen E., et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabolism. 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Camacho-Pereira J., Tarragó M. G., Chini C. C., et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metabolism. 2016;23(6):1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonkowski M. S., Sinclair D. A. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nature Reviews Molecular Cell Biology. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsushima S., Sadoshima J. The role of sirtuins in cardiac disease. The American Journal of Physiology-Heart and Circulatory Physiology. 2015;309(9):H1375–H1389. doi: 10.1152/ajpheart.00053.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pacholec M., Bleasdalle J. E., Chrunyk B., et al. SRT1720; SRT2183; SRT1460; and resveratrol are not direct activators of SIRT1. The Journal of Biological Chemistry. 2009;285:8340–8835. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong W., Tatsuo S., Shou-Dong W., Qian Z., Jian-Feng H., Jue W. Resveratrol upregulates cardiac SDF-1 in mice with acute myocardial infarction through the deacetylation of cardiac p53. PLoS One. 2015;10(6, article e0128978) doi: 10.1371/journal.pone.0128978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao J., Sheng C., Zhang X., Guo M., Ji X. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Design; Development and Therapy. 2016;10:1267–1277. doi: 10.2147/DDDT.S104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin X.-l., Liu M.-H., Hu H.-J., et al. Curcumin enhanced cholesterol efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRa signaling in THP-1 macrophage-derived foam cells. DNA and Cell Biology. 2015;34:561–572. doi: 10.1089/dna.2015.2866. [DOI] [PubMed] [Google Scholar]
- 79.Sun Y., Hu X., Hu G., Xu C., Jiang H. Curcumin attenuates hydrogen peroxide-induced premature senescence via the activation of SIRT1 in human umbilical vein endothelial cells. Biological and Pharmaceutical Bulletin. 2015;38:1134–1141. doi: 10.1248/bpb.b15-00012. [DOI] [PubMed] [Google Scholar]
- 80.Morimoto T., Sunagawa Y., Kawamura T., et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. Journal of Clinical Investigation. 2008;118:868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bugyei-Twim A., Advani A., Advani S. L., et al. Highglucose induces Smad activation via the transcriptional coregulator p300 and contributes to cardiac fibrosis and hypertrophy. Cardiovascular Diabetology. 2014;13:p. 89. doi: 10.1186/1475-2840-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ray A., Rana S., Banerjee D., et al. Improved bioavailability of targeted curcumin delivery efficiently regressed cardiac hypertrophy by modulating apoptotic load within cardiac microenvironment. Toxicology and Applied Pharmacology. 2016;290:54–65. doi: 10.1016/j.taap.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 83.Chowdhury R., Nimmanapalli R., Graham T., Reddy G. Curcumin attenuation of lipopolysaccharide induced cardiac hypertrophy in rodents. ISRN Inflammation. 2013;2013:8. doi: 10.1155/2013/539305.539305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nehra S., Bhardwaj V., Ganju L., Saraswat D. Nanocurcumin prevents hypoxia induced stress in primary human ventricular cardiomyocytes by maintaining mitochondrial homeostasis. PLoS One. 2015;10(9, article e0139121) doi: 10.1371/journal.pone.0139121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li T., Feng Z., Yao M., Liao Q., Zhongxiang Z., Zheng L. Comparative pharmacokinetic and tissue distribution study of baicalin; baicalein; wogonoside; wogonin and oroxylin-A after oral administration of component compatibility of SHT and total flavonoids fractions of Radix scutellariae to rats. Analytical Methods. 2014;6:5799–5807. doi: 10.1039/c4ay00701h. [DOI] [Google Scholar]
- 86.Wei L., Zhou Y., Dai Q., et al. Oroxylin A induces dissociation of hexokinase II from the mitochondria and inhibits glycolysis by SIRT3-mediated deacetylation of cyclophilin D in breast carcinoma. Cell Death & Disease. 2013;4, article e601 doi: 10.1038/cddis.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu N., Ren J., Zhang Y. Mitochondrial aldehyde dehydrogenase obliterates insulin resistance-induced cardiac dysfunction through deacetylation of PGC-1α. Oncotarget. 2016;7:76398–76414. doi: 10.18632/oncotarget.11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Treviño-Saldaña N., García-Rivas J. G. Cardioprotective Effect of Oroxylin A as as Modulator of Mitochondrial Protein Acetylation in a Model of Hypertrophy and Cell Injury. New Orleans, LA, USA: ISHR conference meeting; 2017. [Google Scholar]
- 89.Liu J., Tang Y., Feng Z., et al. Acetylated FoxO1 mediates high-glucose induced autophagy in H9c2 cardiomyoblasts: regulation by a polyphenol -(−)-epigallocatechin-3-gallate. Metabolism Clinical and Experimental. 2014;63:1314–1323. doi: 10.1016/j.metabol.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 90.Zhong W., Huan X. D., Cao Q., Yang J. Cardioprotective effect of epigallocatechin-3-gallate against myocardial infarction in hypercholesterolemic rats. Experimental and therapeutic Medicine. 2015;9:405–410. doi: 10.3892/etm.2014.2135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Chan H., Chu P. M., Tsai K. L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Molecular Nutrition & Food Research. 2015;59:1905–1917. doi: 10.1002/mnfr.201500144. [DOI] [PubMed] [Google Scholar]
- 92.Yu L., Li Q., Yu B., et al. Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: role of silent information regulator 1. Oxidative Medicine and Cellular Longevity. 2016;2016:16. doi: 10.1155/2016/1689602.1689602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng J., Yang Y., Zhou Y., et al. Bakuchiol attenuates myocardial ischemia reperfusion injury by maintaining mitochondrial function: the role of silent information regulator 1. Apoptosis. 2016;21:532–545. doi: 10.1007/s10495-016-1225-6. [DOI] [PubMed] [Google Scholar]
- 94.Samson M. S., Mahesh T., Suresh V. P., Debayon P., Nilanjana M. Akt/FOXO3a/SIRT1 mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: switching gears towards survival and longevity. Journal of Agricultural and Food Chemistry. 2008;56(20):9692–9698. doi: 10.1021/jf802050h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang L., Zou J., Chai E., Qi Y., Zhang Y. Alpha-lipoic acid attenuates cardiac hypertrophy via downregulation of PARP-2 and subsequent activation of SIRT-1. European Journal of Pharmacology. 2014;744:203–210. doi: 10.1016/j.ejphar.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z., Wang S., Zhou S., et al. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. Journal of Molecular and Cellular Cardiology. 2014;77:42–52. doi: 10.1016/j.yjmcc.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 97.Lee S. Y., Ku H. C., Kuo Y. H., et al. Caffeic acid ethanolamide prevents cardiac dysfunction through sirtuin dependent cardiac bioenergetics preservation. Journal of Biomedical Science. 2015;22:p. 80. doi: 10.1186/s12929-015-0188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Timmers S., Konings E., Bilet L., et al. Calorie restriction-like effects of 30 days of resveratrol (resVidaTM) supplementation on energy metabolism and metabolic profile in obese humans. Cell Metabolism. 2011;5:p. 14. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gliemann L., Schmidt J. F., Olesen J., et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. The Journal of Physiology. 2013;591(20):5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skrobuk P., von Kraemer S., Semenova M. M., Zitting A., Koistinen H. A. Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells. Diabetologia. 2012;11:3051–3060. doi: 10.1007/s00125-012-2691-1. [DOI] [PubMed] [Google Scholar]
- 101.Yoshino J., Conte C., Fontana L., et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metabolism. 2012;16(5):p. 658. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirschey M. D., Shimazu T., Jing E., et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Molecular Cell. 2011;44(2):177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.CRN. B vitamins and Cardiovascular Disease. 4th. Washington, DC, USA: The Benefits of Nutritional Supplements; 2012. https://www.crnusa.org/sites/default/files/pdfs-benefits/14CRN-BenefitsBook-Bcvd.pdf. [Google Scholar]
- 104.Wen X., Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metabolism and Disposition. 2006;(34):1786–1792. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 105.Manach C., Scalbert S., Morand C., Remesy C., Jiménez L. Polyphenols: food sources and bioavailability. The American Journal of Clinical Nutrition. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 106.Surangi H., Rupasinghe V. Flavonoid bioavailability and attempts for bioavailability enhacement. Nutrients. 2013;5:3367–3387. doi: 10.3390/nu5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guerrero-Beltrán C. E., Bernal-Ramírez J., Lozano O., et al. Silica nanoparticles induce cardiotoxicity interfering with energetic status and Ca2+ handling in adult rat cardiomyocytes. American Journal of Physiology. Heart and Circulatory Physiology. 2017;312(4):p. H645. doi: 10.1152/ajpheart.00564.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruiz-Esparza G. U., Segura-Ibarra V., Cordero-Reyes A. M., et al. A specifically designed nanoconstruct associates, internalizes, traffics in cardiovascular cells, and accumulates in failing myocardium: a new strategy for heart failure diagnostics and therapeutics. European Journal of Heart Failure. 2016;18(2):169–178. doi: 10.1002/ejhf.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guinane C. M., Cotter P. D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therapeutic Advances in Gastroenterology. 2013;6:295–308. doi: 10.1177/1756283x13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ozdal T., Sela D. A., Xiao J., Boyacioglu D., Chen F., Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8:p. 78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sekirov I., Russel C. L., Antunes C. M., Finlay B. Gut microbiota in health and disease. Physiological Reviews. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 112.Bouayed J., Bohn T. Exogenous antioxidants: double-edged swords in cellular redox state. Oxidative Medicine and Cellular Longevity. 2010;3(4):228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Juhasz B., Mukherjee S., Dipak K., Das D. K. Hormetic response of resveratrol against cardioprotection. Experimental & Clinical Cardiology. 2010;15(4):e134–e138. [PMC free article] [PubMed] [Google Scholar]
- 114.Boocock D. J., Faust G. E., Patel K. R., et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential chemopreventive agent. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(6):1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 115.Anton S. D., Embry C., Marsiske M., et al. Safety and metabolic outcomes of resveratrol supplementation in older adults: results of a twelve-week, placebo-controlled pilot study. Experimental Gerentology. 2014;57:181–187. doi: 10.1016/j.exger.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vignes M., Maurice T., Lante F., Nedjar M., Thethi K., Guiramand J. Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG) Brain Research. 2006;1110:102–115. doi: 10.1016/j.brainres.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 117.Almeida L., Vaz-da-Silva M., Falcão A., Soares E. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Molecular Nutrition & Food Research. 2009;53:S7–S1. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 118.Kinjo Y., Cui Y., Akiba S., Watanabe S. Mortality risks of oesophageal cancer associated with hot tea, alcohol, tobacco and diet in Japan. Journal of Epidemiology. 1998;8:235–243. doi: 10.2188/jea.8.235. [DOI] [PubMed] [Google Scholar]
- 119.Islami F., Boffetta P., Ren J. Pedoeim L. High-temperature beverages and foods and esophageal cancer risk – a systematic review. International Journal of Cancer. 2009;125:491–524. doi: 10.1002/ijc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]