ABSTRACT
Intestinal bacteria employ microbial metabolites from the microbiota and chemical signaling during cell-to-cell communication to regulate several cellular functions. Pathogenic bacteria are extremely efficient in orchestrating their response to these signals through complex signaling transduction systems. Precise coordination and interpretation of these multiple chemical cues is important within the gastrointestinal (GI) tract. Enteric foodborne pathogens, such as enterohemorrhagic Escherichia coli (EHEC) and Salmonella enterica serovar Typhimurium, or the surrogate murine infection model for EHEC, Citrobacter rodentium, are all examples of microorganisms that modulate the expression of their virulence repertoire in response to signals from the microbiota or the host, such as autoinducer-3 (AI-3), epinephrine (Epi), and norepinephrine (NE). The QseBC and QseEF two-component systems, shared by these pathogens, are involved in sensing these signals. We review how these signaling systems sense and relay these signals to drive bacterial gene expression; specifically, to modulate virulence. We also review how bacteria chat via chemical signals integrated with metabolite recognition and utilization to promote successful associations among enteric pathogens, the microbiota, and the host.
KEYWORDS: chemical signaling, Enterobacteriaceae, Escherichia, Salmonella, intestinal metabolites
COMMENSALS AND PATHOGENS IN THE GUT
The large and diverse bacterial community in the human gut also has important functions in the physiology of the intestine. The intestinal mucosa forms a physical barrier that keeps the microbiota on the luminal side. The mucus layer is composed of mucin, glycoproteins, trefoil peptides, and surfactant phospholipids (Fig. 1). Altogether, they constitute a nutrient-rich mucus layer, which has an important role as a protective barrier against microorganisms (1). The biogeography of the gastrointestinal (GI) tract is diverse in its composition and density and its distinct chemical and physical features (2). The density and composition of bacterial communities change according to their location in the gut. Throughout the GI tract, there is significant variation in the physicochemical conditions and substrate availability that impact bacterial growth, differentially promoting or hampering the colonization of certain niches by various species. In the proximal colon, the high concentration of sugar substrates in the mucus allows the expansion of the saccharolytic members of the microbiota (Table 1). Inversely, the lower availability of sugar substrates in the distal colon triggers proteolysis, which decreases the bacterial growth rate and the diversity of the microbiota (1, 3). The resident microbiota, together with all chemical and physical features of the intestine, contribute to shape the metabolic landscape within the gut, producing a multitude of characterized and as-yet-unknown intestinal metabolites. Moreover, the microbial composition of the human GI tract varies with age, diet, host genetics, and external insults like antibiotic treatments (4, 5).
FIG 1.
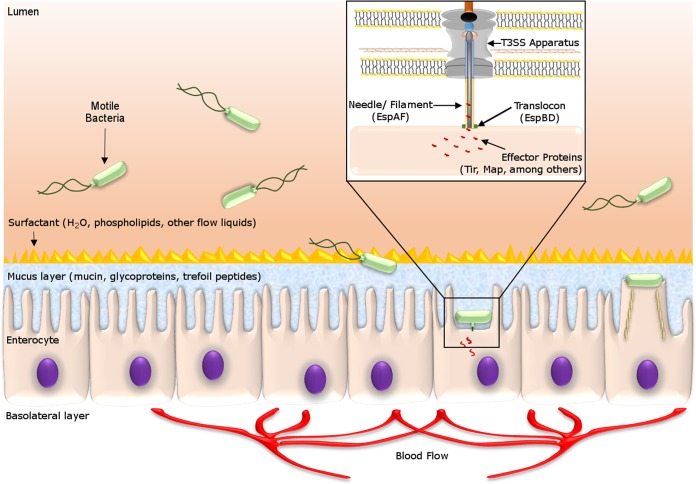
The epithelial layers and compounds in the human small intestine comprise a rich environment for bacterial species and also display an important mucosal barrier that separates enterocytes from the luminal environment. After passing the mucosal layer and finding the enterocytes, EHEC expresses a T3SS, which forms a structure similar to a needle that allows the bacteria to inject secreted proteins into host cells. These proteins orchestrate changes in the actin and myosin filaments that promote the formation of the pedestal that characterizes the A/E lesion (14, 39).
TABLE 1.
The different physicochemical conditions and substrate availabilities for bacterial growth along the gut to favor the colonization of beneficial bacteria
| Section of colon | Characteristica |
|---|---|
| Proximal | High concn of sugars and substrates |
| Saccharolysis | |
| pH of approximately 5.0 to 6.0 | |
| High bacterial growth and diverse microbiota | |
| Distal | Low availability of substrate |
| Proteolysis | |
| Neutral pH | |
| Low bacterial growth and microbiota diversity |
The intestinal environment hosts a diverse microbial community that modifies and shapes the composition of the metabolites and chemical signals within the gut. Pathogenic bacteria employ multiple systems to sense and respond to these cues to interact with the microbiota and the host. The ratio between bacteria and host cells in the human body is still unresolved; previous studies have estimated a 10:1 ratio (6–8), although recently it was calculated to be closer to a 1:1 ratio (9). However, this does not change the important role of microbiota-host interactions (10) and may reflect the microbiota's fluctuation in this association. The intestinal microbiota is thought to be an essential contributor to human health. However, despite the protection offered by different commensal bacteria, pathogens are capable of invading and exploiting this balanced system. These pathogens compete for nutrients and highjack general metabolites from the host or the resident microbiota, employing them as signals (6, 7).
The balance between commensal and potentially pathogenic bacteria is a central element of human health. The microbiota benefits the host by enabling fermentation of nondigestible diet components, such as complex sugars and lipid molecules. This metabolic breakdown leads to vitamin K production, absorption of important ions, and changes in basic cell functions, such as controlling the proliferation and differentiation of epithelial cells. The microbiota also impacts the functioning of the immune system (1, 4, 5).
Enterohemorrhagic Escherichia coli (EHEC) and Salmonella enterica serovar Typhimurium are two major foodborne pathogens that cause gastroenteritis outbreaks worldwide (11–14). EHEC is an important human pathogen that colonizes the large intestine but is a commensal in other hosts, such as cattle, its main reservoir (15). Intestinal disease caused by EHEC cannot be replicated in mice with the same clinical aspects as observed in human infections. Thus, an important surrogate murine infection model is Citrobacter rodentium, a natural murine pathogen that shares many virulence traits with EHEC (16). S. Typhimurium also causes gastroenteritis, but unlike EHEC, it may progress to systemic infection (13, 17). Recent studies investigating the relationship between these pathogens and covering the resident microbiota have started to elucidate how these pathogens outmaneuver the host defenses.
FROM FOOD SOURCES TO MICROBIAL METABOLISM
The composition of the intestinal microbiota is impacted by diet, lifestyle, and host genetics. Diet influences nutrient availability in the gut and changes the composition of the intestinal microbiota. Pathogenic bacteria generally compete directly against commensals for nutrients and colonization sites within the intestine (18, 19).
Fermenter bacteria present in the proximal colon, such as members of the Bacteroidetes, Firmicutes, and Actinobacteria phyla, break down more complex dietary carbohydrates. These organisms produce short-chain fatty acids (SCFAs), particularly acetate, propionate, and butyrate. These metabolites are important energy sources that aid host cell differentiation and nutrient absorption by the colonic epithelium (3, 18, 20).
Mice fed with acetylated starch have increased bacterial acetate levels in their feces, leading the protection against an initial EHEC colonization (21). Moreover, EHEC-infected mice coinfected with Bifidobacterium longum, which has a subset of carbohydrate transporters, can produce enough acetate via bacterial sugar metabolism to promote host defenses against enteric pathogens (21). Details are discussed further in Metabolites Influencing EHEC Pathogenesis below.
THE CHALLENGE OF GI TRACT COLONIZATION
Enteric bacteria face many challenges in colonizing the GI tract. These organisms must survive the host immune defenses and tolerate the presence of toxic chemicals, including hydrochloric acid and bile salts. In addition to host immune defenses, specific cues, dependent on the intestinal niche location and infection timing, play a role in gene expression that allows bacteria to thrive during this competition. Bacterial acid resistance systems allow them to survive several hours at low pHs, ranging between 2.0 and 6.0, during the passage through the human stomach (22). High tolerance to bile is another critical characteristic for GI bacteria, as the bile in the small intestine is detrimental to bacterial survival (23). Bacteria employ multiple systems to be able to survive, including efflux pump systems that secrete toxic compounds; in parallel, these organisms may also trigger changes in bacterial membrane permeability to avoid the excess of some ions. Another stress that bacteria in the GI tract must resist is cationic antimicrobial peptides found in the gut. Gram-negative bacteria may chemically change their lipid A chains, inducing distinct lipopolysaccharide layer modifications to fortify the bacterial defenses as a shield to resist antimicrobial peptides (24). Surviving differences in oxygen levels is also a key ability for bacteria that inhabit the GI tract. The GI tract features variable oxygen levels, from a relatively anaerobic lumen to a more oxygenated area adjacent to the mucosal surface that is generated by diffusion from the capillary network of the microvilli. The ability of enteric bacteria to switch from aerobiosis to anaerobiosis or microaerobiosis is advantageous (22, 23). Bacteria employ multiple strategies to colonize the inhospitable GI tract. By sensing different metabolites, bacteria successfully colonize the gut; unfortunately, among them, pathogenic bacteria also have metabolite-sensing systems to compete against the microbiota.
BACTERIAL METABOLITE SENSING
Bacteria need to adapt their metabolism quickly to survive hostile environments. Metabolite-sensing mechanisms are found both in commensal and pathogenic bacteria. Commensal E. coli colonizes the human GI tract within a few hours after birth, initiating a mutually beneficial relationship (14). Pathogenic E. coli employs complex virulence mechanisms that are activated by chemical signaling and nutrients, both essential for intestinal colonization and evasion of host defenses (19, 25).
EHEC and S. Typhimurium employ cell-to-cell communication to regulate their virulence programs and cause gastroenteritis (12–14). Similarly, Citrobacter rodentium uses these cell-cell communication systems to trigger intestinal colitis in mice (16). These enteric pathogens have in common an intricate signaling system employing cues from both the host and the gut microbiota. The chemical and nutrient signaling in these bacteria is mediated by two-component systems (TCS), such as QseBC and QseEF, that sense these differences and initiate a complex signaling cascade that drives the virulence program in these pathogens (Fig. 2) (15, 19). The bacterial enteric community is a complex system and suffers constant exposure to threats, such as potential pathogens.
FIG 2.
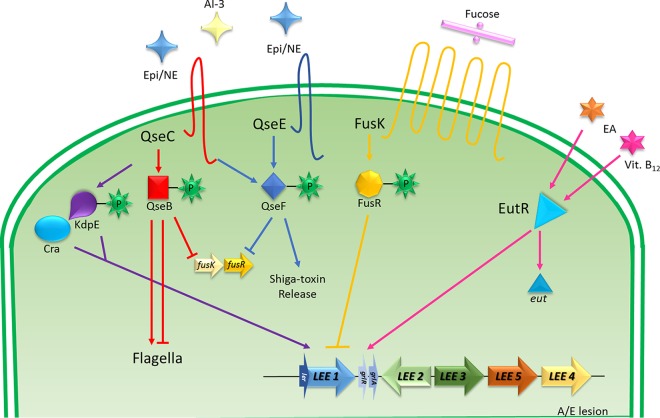
Chemical signaling by Epi/NE, AI-3, fucose, and ethanolamine (EA) in EHEC interacts with transmembrane sensor kinase receptors and activates a TCS via response regulators, which may activate or repress the signal transduction. The QseC sensor kinase has a central role in the activation of the QseB response regulator by Epi, NE, or AI-3; QseB regulates the expression of flagella and represses the expression of fusK/-R. QseC can also phosphorylate KdpE, which together with Cra, activates the pathway responsible for LEE activation. QseC also may activate the response regulator QseF, which stimulates the production of Shiga toxin, but it is able to repress the expression of fusK/-R. The cognate sensor FusK is activated by fucose and phosphorylates FusR to inhibit LEE expression (28, 97, 104, 128). The regulation of the eut operon is also implicated: eut encodes the EutR transcription factor, which recognizes the presence of ethanolamine (EA) and vitamin B12 to modulate virulence gene expression. EutR can bind in the LEE in the absence of EA or B12, but transcription occurs only in the presence of both. In the presence of EA and B12, eut expression promotes EA metabolism, providing an advantage in the competition for nutrients, and also promotes host colonization (129).
Enterics maintain a successful homeostatic community, but the resident microbiota may be manipulated by bacterial pathogens, such as EHEC. EHEC pathogenesis is characterized by a low infectious dose, and therefore, only a few CFU are enough to colonize the host. EHEC scavenge metabolites from different microorganisms, such as Bacteriodes thetaiotaomicron, a highly prevalent saccharolytic bacterium in the intestine. B. thetaiotaomicron can break down plant starches and other complex carbohydrates to simpler sugar molecules that can be utilized by other members of the microbiota (26, 27). For example, fucose released from the mucus by B. thetaiotaomicron can be used by pathogens like EHEC to trigger their infectious processes (28).
BACTERIAL PATHOGENESIS CONTROLLED BY INTESTINAL CHEMICAL SENSING
E. coli O157:H7 is the most common and serious EHEC serotype that causes human illness. Its lethality is caused by the presence of the potent Shiga toxin, carried on a lambdoid prophage. This toxin can cause hemolytic uremic syndrome (HUS), a life-threatening condition (14, 29, 30). EHEC harbors a pathogenicity island named the locus of enterocyte effacement (LEE), which encodes a microscopic “needle,” a type III secretion system (T3SS), that injects many different effector proteins into the host cell (Fig. 1) (19, 28, 31–35). In addition to the T3SS, the LEE also contains genes that encode an adhesin (intimin) (36) and its own receptor, the translocated intimin receptor (Tir) (37, 38), which is translocated into the epithelial cell (33–35). Together, these effectors lead to extensive host cytoskeletal rearrangements and intestinal infection (14, 39).
Animal models of EHEC infection are limited, and they do not mirror all of the disease facets, such as the low infectious dose that is enough to trigger the diarrheagenic disease (12). Different EHEC infection models have been tested in very distinct animals, such as ferrets, greyhound dogs, monkeys, Caenorhabditis elegans, rats, baboons, and chickens (40–46), but these models reproduce only a few aspects of the disease. The models most used to mimic EHEC pathogenesis are pigs, rabbits, and mice (47). Briefly, piglets are used in EHEC infections. However, these infections do not result in attaching and effacing (A/E) lesions; gnotobiotic piglets show a watery but not bloody diarrhea (48, 49), while conventional animals, such as suckling piglets, exhibit more severe neurological diseases rather than renal toxicity effects from Shiga toxin (50). Rabbits are also employed in different models, such as 3-day-old infant (51), suckling, up to 10-week-old young (52–54), or adult (55) rabbits. Rabbits are susceptible to oral administration of EHEC (52), but they do not develop HUS, since rabbits lack the renal receptor globotriasylceramide (Gb3) for Shiga toxin (56). The 3-day-old infant New Zealand White rabbit infection model is one of the most reproducible animal models for EHEC, with the pathogen reaching consistent levels of colonization in the large intestine, forming A/E lesions, causing diarrhea, and persisting in the intestine, causing a delay in the animals' growth (51, 57, 58). Conversely, mice have Gb3 receptors, and Shiga toxin causes acute renal damage and death in mice (59, 60). However, mice are naturally resistant to colonization by EHEC, and thus, murine models need a depleted microbiota to allow EHEC colonization (47). Many different mouse models are used to address this issue (60), such as pretreatment with antibiotics (61, 62), germfree conditions (63–65), or diet-induced changes (66), all of which modify the microbiota environment to promote intestinal disease. Despite recent advances in the field, EHEC animal models do not reproduce the full clinical illness seen during human infection (47). Altogether, C. rodentium infection in mice appears to be the only natural murine model to study some aspects that EHEC and C. rodentium infection have in common (47). C. rodentium also harbors the LEE pathogenicity island and forms A/E lesions on the murine gut (67–69). Most of the EHEC virulence genes have been described previously by studying the function of the C. rodentium orthologs during murine infection (67–69).
Salmonella is also an important foodborne pathogen. The Salmonella enterica serovar Typhimurium murine infection models have been extensively applied to study Salmonella gastroenteritis (13). The gastroenteritis caused by S. Typhimurium requires two steps that are mediated by two distinct T3SSs (70) encoded by two Salmonella pathogenicity islands (SPIs), SPI1 and SPI2. The SPI1-encoded T3SS is active during the initial contact with host epithelial cells and translocates bacterial proteins across the host membrane, and the SPI2-encoded T3SS is expressed within the phagosome and translocates effectors across the vacuolar membrane. The SPI1 system is required for invasion of nonphagocytic cells, induction of intestinal inflammatory responses, intestinal colonization, and diarrhea (71, 72). The SPI2-encoded T3SS has an important role in bacterial survival in macrophages and the establishment of systemic disease (73). The S. Typhimurium murine infection model has been extensively employed in the study of many virulence traits (71–73).
Intestinal chemical sensing plays an important role in S. Typhimurium colonization of the gut in murine models pretreated with antibiotics (74). The changes in the composition of the microbiota promote pathogen expansion mediated by the oxidation of carbohydrates (74). Probiotic bacteria present in the intestinal resident microbiota are key elements to this complex bacterial ecosystem. Members of the Bacteroidetes and Firmicutes phyla are prevalent anaerobic bacteria in healthy individuals. Both metabolize nondigestible proteins and complex carbohydrates, such as gluten and fiber or mucus saccharides, respectively (75). In the absence of other sources, bacteria rely on fermentation of these carbohydrates and proteins to obtain the carbon and nitrogen that are essential to the biosynthesis of bacterial proteins, carbohydrates, lipids, and nucleotides. Although reactive oxygen and nitrogen species are generated by the inflammatory host response during inflammation induced by S. Typhimurium, they oxidize luminal compounds (trimethylamine and thiosulfate), forming exogenous electron acceptors (trimethylamine N-oxide and tetrathionate) that can be used by S. Typhimurium (76). The presence of these acceptors enables facultative anaerobic bacteria, such as S. Typhimurium, to utilize microbiota-derived products to generate energy, balance redox reactions, and acquire carbon for the biosynthesis of other products essential to bacteria during infection (75).
The large majority of human pathogens require iron as a nutrient to support growth and colonization. Iron is essential to all living cells, although a tight concentration control is essential to avoid its toxicity, due to the redox potential; invading bacteria, such as S. Typhimurium, have evolved many systems to ensure the uptake of iron that is available intracellularly in the host (77). This important host-pathogen interface is linked to pathogen proliferation, virulence, and persistence (78, 79). S. Typhimurium's ability to grow in the host regardless of the iron level is mediated by Feo proteins, which take up unbound ferrous iron (Fe2+) (77, 80). The proliferation of invasive bacterial pathogens is limited by the bioavailability of iron. The innate immune system is capable of limiting iron availability in the presence of invading microbes, also known as nutritional immunity; however, pathogens possess mechanisms to circumvent this and cause disease (79). During inflammation, iron becomes even more limited, and therefore, Salmonella relies on siderophores to bind to ferric iron (Fe3+), sequestering Fe3+ from host proteins. Enterobactin is the most common siderophore among the Enterobacteriaceae. Salmochelin is another siderophore present in Salmonella, a C-glucosylated derivative of enterobactin (81), but different than enterobactin, it cannot bind to the antimicrobial lipocalin-2, which prevents bacterial iron acquisition; thus, salmochelin confers an important advantage to S. Typhimurium versus microbiota members during intestinal colonization (82). However, the probiotic E. coli strain Nissle is also able to evade iron sequestration by lipocalin-2. S. Typhimurium and E. coli Nissle compete for iron sources during inflammation (83). In this case, iron uptake and evasion of lipocalin-2 are beneficial to the host, since the competition favors colonization by the probiotic E. coli Nissle during S. Typhimurium inflammation (83). The nutrient-limiting condition and different bacterial survival strategies are very important players to understand for insight into how pathogens interact with indigenous microbiota and the surrounding metabolites and, specifically, their strategies to explore distinct niches.
METABOLITES INFLUENCING EHEC PATHOGENESIS
Various metabolites and compounds may influence the course of EHEC infection. Recent experiments with EHEC-infected mice suggest that food choice has a significant impact on Shiga toxin expression (66). EHEC-infected mice fed a low-fiber diet exhibited higher weight loss and a lower Shiga toxin-dependent survival rate. These data suggest that a high-fiber diet renders these animals more resilient in the face of Shiga toxin exposure. A high-fiber diet led to a reduction in the commensal E. coli population compared to those of other species, which benefits EHEC colonization during gut competition. However, the findings for different levels of dietary fiber are contradictory. Regarding EHEC colonization and Shiga toxin production, this murine model mostly reflects Shiga toxin effects and not the GI phase of infection, because EHEC does not promote A/E lesion formation in the mouse intestine (66). Distinct metabolites are very important to pathogens, while these organisms must scavenge and exploit all signals to quickly adapt themselves during colonization.
The SCFAs have an important role during EHEC pathogenesis, because they impact EHEC gene regulation. Butyrate works as a signal in EHEC by enhancing the expression of the T3SS and flagellar genes. Butyrate activates the flagellar master regulator, flhDC, through the leucine-responsive regulatory protein (Lrp), a regulator of virulence genes. Moreover, butyrate, acetate, and propionate also activate downstream genes independently of flhDC activation (84, 85).
Other metabolites repress signals and limit EHEC colonization in hostile environments (86). Biotin, present at high levels in the small intestine, reduces the presence of EHEC in this GI compartment. Conversely, the lower levels of biotin found in the large intestine favor EHEC colonization (86). A novel system was recently described to sense d-serine levels, acting to repress the injection of effector proteins into the host cell via the T3SS. This system functions in a concentration-dependent manner via the DlsT/YhaJ d-serine sensor system. d-Serine can either act as a carbon source or modulate a set of stress-dependent genes in E. coli strains of different pathotypes and, consequently, modulate the gene expression of unique virulence factors (87).
Other bacterial interactions may be very important during EHEC pathogenesis. Recently, the probiotic bacterium Bifidobacterium longum was reported to change the EHEC outcome in the gut through the production of acetate, preventing translocation of the Shiga toxin through the intestinal epithelium and ultimately avoiding HUS. This protection is attributed to a higher acetate concentration accompanied by increased consumption of carbohydrates by this probiotic B. longum gut member. In vitro, acetate protects the permeability of epithelial cell monolayers, which directly affects EHEC infection, but the complete mechanism is still not fully elucidated (88). The composition of the intestinal microbiota and different metabolites seem to directly affect the GI trespassers (21), which may be an explanation for the differential outcomes of GI tract infections in distinct individuals.
CHEMICAL SIGNALING IN BACTERIA
Bacteria employ different mechanisms to explore the GI tract and sense distinct cues. This chemical signaling among bacteria regulates different aspects of their transcriptomes and is usually referred to as quorum sensing (QS). QS was first described in the environmental Vibrio fischeri, a Gram-negative marine bacterium. When V. fischeri bacteria are at high cell density in a squid or fish light organ, the bacterial QS system activates their bioluminescence genes within the light organ. This is achieved through diffusible chemicals called autoinducers (AIs). AIs act as hormonelike molecules (89).
THE AI-3–EPINEPHRINE/NOREPINEPHRINE INTERKINGDOM SIGNALING SYSTEM
Among these QS signaling systems, the bacterial AI-3 molecule has been shown to be present in different bacterial species (90) and to have a key role in interkingdom signaling. AI-3 is produced by the human GI microbiota and cross signals with the host hormones epinephrine (Epi) and norepinephrine (NE) (25). The AI-3–Epi/NE system has been implicated in controlling the pathogenesis of EHEC (Fig. 2) (25, 91). This system has also been shown to promote virulence gene expression in S. Typhimurium (57, 92, 93) and Citrobacter rodentium (94), among other species (95). Bacteria sense these interkingdom chemical signals through histidine sensor kinases anchored in the bacterial inner membrane. The QseC and QseE receptors are two kinases that have been described to sense these cues (28, 96, 97). QseC and QseE phosphorylate their cognate response regulators (RRs) in the cytoplasm, as well as noncognate RRs, to modulate the expression of the virulence genes in EHEC (Fig. 2) (62, 67).
The stress hormones Epi and NE are derived from tyrosine-containing catechol and amine groups (98). These catecholamines are neurotransmitters of the central and peripheral nervous system that are responsible for regulating many body functions, including the fight-or-flight response, cognitive activity, and mood, as well as functions of the liver, lung, and adipose tissue and the cardiovascular system, and they also affect intestinal peristaltic movements (99, 100). Mammalian cells recognize these neurotransmitters through adrenergic receptors, a subset of the family of G protein-coupled receptors (GPCRs). In contrast, bacterial cells do not encode GPCR receptors in their genomes but have the membrane-bound histidine sensor kinase receptors that are able to sense these hormones (96, 101).
THE QseBC AND QseEF TCSs
The QseBC two-component system (TCS) is found in Proteobacteria, including animal and plant pathogens (57, 102, 103). The QseC histidine sensor kinase increases its autophosphorylation in the presence of AI-3–Epi/NE molecules and relays this information to its cognate RR QseB and two noncognate RRs, QseF and KdpE (Fig. 2) (96, 104). The QseBC TCS is involved in chemical signaling that regulates flagella and motility in EHEC (11). QseBC regulates the expression of flhDC, which encode the master flagellar regulator. QseB directly activates flhDC expression and binds directly to the low- and high-affinity binding sites of the flhDC promoter. QseBC initiates flagellar class 1 gene expression, dependent on the presence of flhDC FliA (sigma 28) (101).
TCSs enable bacteria to sense their specific signals but may also trigger their response to different cues; therefore, TCSs can cross-communicate with other TCSs, depending on the specificity of the signal sensor kinases that together form a broader and more complex regulatory system (105). QseC activates its cognate RR QseB, and it also phosphorylates the RRs KdpE and QseF (Fig. 2) (67). The KdpDE system is regulated by the potassium concentration, while the RR KdpE is phosphorylated by the histidine kinase KdpD (106). QseC phosphorylates both KdpE and QseF, activating LEE and Shiga toxin expression (104).
The QseE sensor kinase senses Epi/NE, sulfate, and phosphate (62). QseE only phosphorylates its cognate RR, QseF (97, 107). QseF affects the expression of Shiga toxin (67) and the small RNA GlmY (108, 109), which works in concert with GlmZ to posttranscriptionally regulate the expression of LEE and EspFu (an effector necessary for A/E lesion formation) in EHEC (108, 109).
EHEC and C. rodentium share the QseBC and QseEF systems that sense Epi and NE and which regulate their virulence genes similarly (94). Both adrenergic hormones activate virulence in C. rodentium via the QseC and QseE sensors. qseC, qseE, and qseEC mutants are attenuated during murine infections (94). QseC and QseE also sense Epi and NE in the mouse intestine, as shown in infection studies with dopamine-hydroxylase knockout (Dbh−/−) mice, which are unable to produce Epi and NE (94).
QseC and QseE also regulate the virulence expression and pathogenesis of S. Typhimurium in swine and mice (57, 92, 93). QseC regulates the expression of flagellum and motility genes and sifA (93). SifA is necessary for S. Typhimurium's survival inside macrophages (110). A ΔqseC mutant has decreased colonization in swine (92) and is attenuated for systemic infection in mice (93). The qseE mutant has reduced invasion of epithelial cells and reduced intramacrophage survival. Additionally, the qseEC double mutant is slightly attenuated only in epithelial cell invasion, which mirrors the phenotype of the ΔqseC mutant, and it has an intermediate intramacrophage replication defect in comparison to the intramacrophage replication of the qseE single mutant. The QseC sensor kinase participates actively in S. Typhimurium's pathogenesis via the expression of virulence genes and phenotypically during intramacrophage replication as observed in vivo and in vitro infections.
The expression of the SPI1 T3SS effector genes, such as sipA and sopB, which occurs during the invasion of epithelial cells, is activated by epinephrine via QseE. The expression of these genes is also decreased in the ΔqseEC mutant, albeit to a lesser extent than for those described above, congruent with its attenuated-invasion phenotype. The expression of SPI2 T3SS genes, such as sifA, is important during intramacrophage replication, which is also decreased in the qseE and the qseEC mutants. During systemic murine infections, the qseE mutant is highly attenuated, while the double mutant has an intermediate phenotype (111). The transcriptional expression levels of both the SPI1 and SPI2 genes during mouse infections show the importance of the QseEF TCS system during S. Typhimurium pathogenesis.
In transcriptomic analyses of the QseC regulon, QseC regulates the expression of the virulence stress-related periplasmic protein (VisP). VisP binds to peptidoglycan and interacts with the LpxO-lipid A-modifying enzyme, inhibiting lipid A hydroxylation. VisP promotes LpxO inhibition and, thus, reduction of lipid A hydroxylation, which is important for intravacuolar survival upon stresses like acidification and exposure to hydrogen peroxide and heavy metals like cadmium chloride (112). More studies are necessary for full elucidation of the role of VisP during systemic infection. Nevertheless, both VisP and LpxO also have independent functions during S. Typhimurium colitis. VisP integrates chemical signaling, stress responses, and lipopolysaccharide (LPS) modifications during S. Typhimurium pathogenesis (112). The QseC and QseE adrenergic sensors modulate several aspects of EHEC, S. Typhimurium, and C. rodentium pathogenesis. Some arms of these signaling systems converge among these pathogens, while others diverge.
SUGAR REGULATION OF VIRULENCE
Sugars are the primary carbon sources for bacterial cells, and they also play an important role in bacterial pathogenesis (28, 113). Both pathogenic and commensal bacteria compete for carbon sources like glucuronate, mannose, fucose, and ribose to colonize and proliferate in the gut. EHEC uses breakdown products from commensal bacteria, such as mucin-derived sugars, to gain a competitive advantage during intestinal colonization (114). Sugar availability influences the microbiota composition and the expansion of bacterial pathogens (113). The expression of the LEE in EHEC is inhibited during glycolytic metabolism (115). Conversely, EHEC growth within a gluconeogenic environment activates the expression of the LEE genes (115). This sugar-dependent regulation is achieved through two transcription factors: KdpE and Cra (115). The glucose concentration in the environment triggers KdpE phosphorylation by the KdpD histidine sensor kinase. Cra, also known as FruR, uses sugar fluctuations to activate or inhibit the expression of its target genes (115, 116). In addition to regulating the expression of the LEE, Cra and KdpE also regulate catabolic and osmotic stress (115, 116). These regulatory mechanisms provide fine tuning that allows the bacterial cells to adapt themselves to environmental fluctuations related to different concentrations of the carbon sources available and change their basic metabolism, as well as their virulence expression.
Fucose is an abundant carbohydrate in the gut. EHEC employs the FusKR system to exploit fucose as a signal and compete against commensal E. coli for intestinal colonization. Bacteroides thetaiotaomicron, an abundant member of the microbiota, is able to grow in mucin and produces multiple fucosidases that harvest fucose from the mucus, increasing fucose availability in the intestinal lumen. Fucose is sensed by EHEC via the histidine sensor kinase FusK to modulate LEE gene expression and sugar metabolism (28, 117). Hence, EHEC exploits B. thetaiotaomicron to promote the expression of its virulence repertoire (28, 118).
The sugar chemical signaling in EHEC is interconnected with the adrenergic sensing system. QseC and QseE sense Epi and activate QseB and QseF, both of which repress the expression of fusKR. Another level of interaction occurs through the QseC-phosphorylated RR KdpE, which directly interacts with Cra during gluconeogenesis to promote virulence gene expression (Fig. 2). To prevent superfluous energy expenditure, chemical signaling guides EHEC through the biogeography of the GI tract (28). The regulation of virulence may exploit available signals and metabolites; many adaptive conditions are employed to promote pathogens against their competitors.
THE ROLE OF OTHER METABOLITES IN BACTERIAL PATHOGENESIS
Ethanolamine (EA) is an abundant source of nitrogen and carbon in the intestine, and it also serves as a signal for pathogenic bacteria, such as EHEC and S. Typhimurium, to activate virulence gene expression (119, 120). The resident microbiota does not metabolize EA efficiently; however, EHEC and S. Typhimurium both utilize EA as an important nitrogen source to promote their expansion in the gut and as a signal to control virulence gene expression. Several EHEC virulence genes are induced by EA via the expression of the EutR factor, an EA receptor. Genes induced by EA via EutR include genes encoding fimbrial adhesins, flagella, Shiga toxin, and the LEE (95, 121).
APPROACHES TO INTERFERE WITH BACTERIAL PATHOGENESIS
Recent studies on the chemistry of the microbiome are helping to elucidate how bacteria can scavenge compounds for their own benefit (6, 7, 18). Bacteria employ chemical signaling to communicate among themselves upon the availability of favorable or detrimental compounds. Different aspects of this signaling have been shown in the bacterial cell-to-cell communication field (25, 122–124). The advent of novel technologies will help to obtain more detailed information on the intricate microbiota-pathogen-host relationships to directly identify single molecules or more complex compounds present during in vivo infections. The first requirement is to identify and functionally study the effects of nutrient and metabolite changes in specific sites (95).
The approach of designing new compounds based on the regulation of virulence was employed to describe the N-phenyl-4-(3-phenylthioureido)benzenesulfonamide molecule, named LED209. This compound came from a large library of 150,000 small organic molecules screened to identify antagonists of AI-3, using an LEE1::LacZ expression reporter in EHEC. It was described as preventing the activation of transcriptional factors of EHEC by blocking the expression of LEE virulence genes that are mediated by the activation of AI-3 (57). After extensive structure-function studies, LED209 was shown to be a prodrug highly selective for the QseC sensor kinase, impairing QseC's function by preventing the activation of virulence genes both in vitro and during murine infection. LED209 does not interfere with bacterial growth, potentially reducing selective pressure toward drug resistance (102). This strategy may minimize the development of antibiotic resistance, a high concern for the development of new antimicrobials.
Elucidating the function of intestinal metabolites and bacterial chemical signaling systems may promote the discovery of new antivirulence targets; such targets could include uncharacterized membrane proteins and TCSs related to the signaling cascades, without any disruption of basic metabolic pathways. Additional studies are necessary to understand these complex sensing networks in different isolates, since they may have different regulation or functions in pathogens other than those previously studied (25, 94, 125–127). A better knowledge of all intestinal metabolites and the microbial mechanisms for sensing them will contribute to novel therapeutics.
ACKNOWLEDGMENTS
Funding was provided by CNPq grant 441884/2014-8 and FAPESP grant 2014/06779-2 to C.G.M.
REFERENCES
- 1.Guarner F, Malagelada JR. 2003. Gut flora in health and disease. Lancet 361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 2.Simon GL, Gorbach SL. 1986. The human intestinal microflora. Dig Dis Sci 31:147S–162S. doi: 10.1007/BF01295996. [DOI] [PubMed] [Google Scholar]
- 3.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel JC, Galan JE. 2006. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J Cell Biol 175:453–463. doi: 10.1083/jcb.200605144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marteau P, Pochart P, Doré J, Béra-Maillet C, Bernalier A, Corthier G. 2001. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl Environ Microbiol 67:4939–4942. doi: 10.1128/AEM.67.10.4939-4942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper LV, Gordon JI. 2001. Commensal host-bacterial relationships in the gut. Science 292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 8.Savage DC. 1977. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 9.Sender R, Fuchs S, Milo R. 2016. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Sender R, Fuchs S, Milo R. 2016. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperandio V, Torres AG, Kaper JB. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 12.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haraga A, Ohlson MB, Miller SI. 2008. Salmonellae interplay with host cells. Nat Rev Microbiol 6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 14.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 15.Hughes DT, Sperandio V. 2008. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. 2014. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol 12:612–623. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- 17.Almeida F, Seribelli AA, da Silva P, Medeiros MI, Dos Prazeres Rodrigues D, Moreira CG, Allard MW, Falcao JP. 2017. Multilocus sequence typing of Salmonella Typhimurium reveals the presence of the highly invasive ST313 in Brazil. Infect Genet Evol 51:41–44. doi: 10.1016/j.meegid.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. 2012. Host-gut microbiota metabolic interactions. Science 336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Doria JD, Sperandio V. 2013. Nutrient and chemical sensing by intestinal pathogens. Microbes Infect 15:759–764. doi: 10.1016/j.micinf.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macfarlane S, Macfarlane GT. 2003. Regulation of short-chain fatty acid production. Proc Nutr Soc 62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Smith MP, Chapin KC, Baik HS, Bennett GN, Foster JW. 1996. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol 62:3094–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thanassi DG, Cheng LW, Nikaido H. 1997. Active efflux of bile salts by Escherichia coli. J Bacteriol 179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Needham BD, Trent MS. 2013. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol 11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. 2003. Bacteria-host communication: the language of hormones. Proc Natl Acad Sci U S A 100:8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Gordon JI. 2003. Honor thy symbionts. Proc Natl Acad Sci U S A 100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonnenburg ED, Sonnenburg JL, Manchester JK, Hansen EE, Chiang HC, Gordon JI. 2006. A hybrid two-component system protein of a prominent human gut symbiont couples glycan sensing in vivo to carbohydrate metabolism. Proc Natl Acad Sci U S A 103:8834–8839. doi: 10.1073/pnas.0603249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature 492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. 2013. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karmali MA, Petric M, Lim C, Fleming PC, Steele BT. 1983. Escherichia coli cytotoxin, haemolytic-uraemic syndrome, and haemorrhagic colitis. Lancet ii:1299–1300. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A 92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croxen MA, Finlay BB. 2010. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol 8:26–38. [DOI] [PubMed] [Google Scholar]
- 33.Elliott SJ, Wainwright LA, McDaniel TK, Jarvis KG, Deng YK, Lai LC, McNamara BP, Donnenberg MS, Kaper JB. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol 28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 34.Elliott SJ, Yu J, Kaper JB. 1999. The cloned locus of enterocyte effacement from enterohemorrhagic Escherichia coli O157:H7 is unable to confer the attaching and effacing phenotype upon E. coli K-12. Infect Immun 67:4260–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellies JL, Elliott SJ, Sperandio V, Donnenberg MS, Kaper JB. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol Microbiol 33:296–306. doi: 10.1046/j.1365-2958.1999.01473.x. [DOI] [PubMed] [Google Scholar]
- 36.Jerse AE, Yu J, Tall BD, Kaper JB. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A 87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenny B, Abe A, Stein M, Finlay BB. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect Immun 65:2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511–520. doi: 10.1016/S0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 39.Hueck CJ. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62:379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woods JB, Schmitt CK, Darnell SC, Meysick KC, O'Brien AD. 2002. Ferrets as a model system for renal disease secondary to intestinal infection with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. J Infect Dis 185:550–554. doi: 10.1086/338633. [DOI] [PubMed] [Google Scholar]
- 41.Raife T, Friedman KD, Fenwick B. 2004. Lepirudin prevents lethal effects of Shiga toxin in a canine model. Thromb Haemost 92:387–393. doi: 10.1160/TH03-12-0759. [DOI] [PubMed] [Google Scholar]
- 42.Zotta E, Lago N, Ochoa F, Repetto HA, Ibarra C. 2008. Development of an experimental hemolytic uremic syndrome in rats. Pediatr Nephrol 23:559–567. doi: 10.1007/s00467-007-0727-4. [DOI] [PubMed] [Google Scholar]
- 43.Siegler RL, Obrig TG, Pysher TJ, Tesh VL, Denkers ND, Taylor FB. 2003. Response to Shiga toxin 1 and 2 in a baboon model of hemolytic uremic syndrome. Pediatr Nephrol 18:92–96. doi: 10.1007/s00467-002-1035-7. [DOI] [PubMed] [Google Scholar]
- 44.Chou TC, Chiu HC, Kuo CJ, Wu CM, Syu WJ, Chiu WT, Chen CS. 2013. Enterohaemorrhagic Escherichia coli O157:H7 Shiga-like toxin 1 is required for full pathogenicity and activation of the p38 mitogen-activated protein kinase pathway in Caenorhabditis elegans. Cell Microbiol 15:82–97. doi: 10.1111/cmi.12030. [DOI] [PubMed] [Google Scholar]
- 45.Kang G, Pulimood AB, Koshi R, Hull A, Acheson D, Rajan P, Keusch GT, Mathan VI, Mathan MM. 2001. A monkey model for enterohemorrhagic Escherichia coli infection. J Infect Dis 184:206–210. doi: 10.1086/322011. [DOI] [PubMed] [Google Scholar]
- 46.Beery JT, Doyle MP, Schoeni JL. 1985. Colonization of chicken cecae by Escherichia coli associated with hemorrhagic colitis. Appl Environ Microbiol 49:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritchie JM. 2014. Animal models of enterohemorrhagic Escherichia coli infection. Microbiol Spectr 2:EHEC-0022-2013. doi: 10.1128/microbiolspec.EHEC-0022-2013. [DOI] [PubMed] [Google Scholar]
- 48.Francis DH, Collins JE, Duimstra JR. 1986. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun 51:953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzipori S, Wachsmuth IK, Chapman C, Birden R, Brittingham J, Jackson C, Hogg J. 1986. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis 154:712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- 50.Dean-Nystrom EA, Pohlenz JF, Moon HW, O'Brien AD. 2000. Escherichia coli O157:H7 causes more-severe systemic disease in suckling piglets than in colostrum-deprived neonatal piglets. Infect Immun 68:2356–2358. doi: 10.1128/IAI.68.4.2356-2358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritchie JM, Thorpe CM, Rogers AB, Waldor MK. 2003. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect Immun 71:7129–7139. doi: 10.1128/IAI.71.12.7129-7139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farmer JJ III, Potter ME, Riley LW, Barrett TJ, Blake PA, Bopp CA, Cohen ML, Kaufmann A, Morris GK, Remis RS, Thomason BM, Wells JG. 1983. Animal models to study Escherichia coli O157:H7 isolated from patients with haemorrhagic colitis. Lancet i:702–703. [DOI] [PubMed] [Google Scholar]
- 53.Potter ME, Kaufmann AF, Thomason BM, Blake PA, Farmer JJ III. 1985. Diarrhea due to Escherichia coli O157:H7 in the infant rabbit. J Infect Dis 152:1341–1343. doi: 10.1093/infdis/152.6.1341. [DOI] [PubMed] [Google Scholar]
- 54.Pai CH, Kelly JK, Meyers GL. 1986. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun 51:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De SN, Chatterje DN. 1953. An experimental study of the mechanism of action of Vibrio cholerae on the intestinal mucous membrane. J Pathol Bacteriol 66:559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- 56.Zoja C, Corna D, Farina C, Sacchi G, Lingwood C, Doyle MP, Padhye VV, Abbate M, Remuzzi G. 1992. Verotoxin glycolipid receptors determine the localization of microangiopathic process in rabbits given verotoxin-1. J Lab Clin Med 120:229–238. [PubMed] [Google Scholar]
- 57.Rasko DA, Moreira CG, Li DR, Reading NC, Ritchie JM, Waldor MK, Williams N, Taussig R, Wei S, Roth M, Hughes DT, Huntley JF, Fina MW, Falck JR, Sperandio V. 2008. Targeting QseC signaling and virulence for antibiotic development. Science 321:1078–1080. doi: 10.1126/science.1160354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature 492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wadolkowski EA, Sung LM, Burris JA, Samuel JE, O'Brien AD. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun 58:3959–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohawk KL, O'Brien AD. 2011. Mouse models of Escherichia coli O157:H7 infection and Shiga toxin injection. J Biomed Biotechnol 2011:258185. doi: 10.1155/2011/258185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wadolkowski EA, Burris JA, O'Brien AD. 1990. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun 58:2438–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calderon Toledo C, Rogers TJ, Svensson M, Tati R, Fischer H, Svanborg C, Karpman D. 2008. Shiga toxin-mediated disease in MyD88-deficient mice infected with Escherichia coli O157:H7. Am J Pathol 173:1428–1439. doi: 10.2353/ajpath.2008.071218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eaton KA, Friedman DI, Francis GJ, Tyler JS, Young VB, Haeger J, Abu-Ali G, Whittam TS. 2008. Pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ-free mice. Infect Immun 76:3054–3063. doi: 10.1128/IAI.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tyler JS, Beeri K, Reynolds JL, Alteri CJ, Skinner KG, Friedman JH, Eaton KA, Friedman DI. 2013. Prophage induction is enhanced and required for renal disease and lethality in an EHEC mouse model. PLoS Pathog 9:e1003236. doi: 10.1371/journal.ppat.1003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taguchi H, Takahashi M, Yamaguchi H, Osaki T, Komatsu A, Fujioka Y, Kamiya S. 2002. Experimental infection of germ-free mice with hyper-toxigenic enterohaemorrhagic Escherichia coli O157:H7, strain 6. J Med Microbiol 51:336–343. doi: 10.1099/0022-1317-51-4-336. [DOI] [PubMed] [Google Scholar]
- 66.Zumbrun SD, Melton-Celsa AR, Smith MA, Gilbreath JJ, Merrell DS, O'Brien AD. 2013. Dietary choice affects Shiga toxin-producing Escherichia coli (STEC) O157:H7 colonization and disease. Proc Natl Acad Sci U S A 110:E2126–E2133. doi: 10.1073/pnas.1222014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vazquez A, Barba J, Ibarra JA, O'Donnell P, Metalnikov P, Ashman K, Lee S, Goode D, Pawson T, Finlay BB. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci U S A 101:3597–3602. doi: 10.1073/pnas.0400326101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gruenheid S, Sekirov I, Thomas NA, Deng W, O'Donnell P, Goode D, Li Y, Frey EA, Brown NF, Metalnikov P, Pawson T, Ashman K, Finlay BB. 2004. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol 51:1233–1249. doi: 10.1046/j.1365-2958.2003.03911.x. [DOI] [PubMed] [Google Scholar]
- 69.Hemrajani C, Marches O, Wiles S, Girard F, Dennis A, Dziva F, Best A, Phillips AD, Berger CN, Mousnier A, Crepin VF, Kruidenier L, Woodward MJ, Stevens MP, La Ragione RM, MacDonald TT, Frankel G. 2008. Role of NleH, a type III secreted effector from attaching and effacing pathogens, in colonization of the bovine, ovine, and murine gut. Infect Immun 76:4804–4813. doi: 10.1128/IAI.00742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hansen-Wester I, Hensel M. 2001. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect 3:549–559. doi: 10.1016/S1286-4579(01)01411-3. [DOI] [PubMed] [Google Scholar]
- 71.Galan JE, Curtiss R III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A 86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galan JE, Curtiss R III. 1990. Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun 58:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shea JE, Hensel M, Gleeson C, Holden DW. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci U S A 93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faber F, Tran L, Byndloss MX, Lopez CA, Velazquez EM, Kerrinnes T, Nuccio SP, Wangdi T, Fiehn O, Tsolis RM, Baumler AJ. 2016. Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature 534:697–699. doi: 10.1038/nature18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faber F, Baumler AJ. 2014. The impact of intestinal inflammation on the nutritional environment of the gut microbiota. Immunol Lett 162:48–53. doi: 10.1016/j.imlet.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Baumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cassat JE, Skaar EP. 2013. Iron in infection and immunity. Cell Host Microbe 13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nairz M, Schroll A, Sonnweber T, Weiss G. 2010. The struggle for iron—a metal at the host-pathogen interface. Cell Microbiol 12:1691–1702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 79.Parrow NL, Fleming RE, Minnick MF. 2013. Sequestration and scavenging of iron in infection. Infect Immun 81:3503–3514. doi: 10.1128/IAI.00602-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrews SC, Robinson AK, Rodriguez-Quinones F. 2003. Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 81.Hantke K, Nicholson G, Rabsch W, Winkelmann G. 2003. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A 100:3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. 2009. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M. 2013. Probiotic bacteria reduce salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe 14:26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakanishi N, Tashiro K, Kuhara S, Hayashi T, Sugimoto N, Tobe T. 2009. Regulation of virulence by butyrate sensing in enterohaemorrhagic Escherichia coli. Microbiology 155:521–530. doi: 10.1099/mic.0.023499-0. [DOI] [PubMed] [Google Scholar]
- 85.Tobe T, Nakanishi N, Sugimoto N. 2011. Activation of motility by sensing short-chain fatty acids via two steps in a flagellar gene regulatory cascade in enterohemorrhagic Escherichia coli. Infect Immun 79:1016–1024. doi: 10.1128/IAI.00927-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang B, Feng L, Wang F, Wang L. 2015. Enterohemorrhagic Escherichia coli senses low biotin status in the large intestine for colonization and infection. Nat Commun 6:6592. doi: 10.1038/ncomms7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Connolly JP, Gabrielsen M, Goldstone RJ, Grinter R, Wang D, Cogdell RJ, Walker D, Smith DG, Roe AJ. 2016. A highly conserved bacterial d-serine uptake system links host metabolism and virulence. PLoS Pathog 12:e1005359. doi: 10.1371/journal.ppat.1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukuda S, Toh H, Taylor TD, Ohno H, Hattori M. 2012. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 3:449–454. doi: 10.4161/gmic.21214. [DOI] [PubMed] [Google Scholar]
- 89.Engebrecht J, Silverman M. 1984. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A 81:4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walters M, Sircili MP, Sperandio V. 2006. AI-3 synthesis is not dependent on luxS in Escherichia coli. J Bacteriol 188:5668–5681. doi: 10.1128/JB.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sperandio V, Mellies JL, Nguyen W, Shin S, Kaper JB. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc Natl Acad Sci U S A 96:15196–15201. doi: 10.1073/pnas.96.26.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bearson BL, Bearson SM. 2008. The role of the QseC quorum-sensing sensor kinase in colonization and norepinephrine-enhanced motility of Salmonella enterica serovar Typhimurium. Microb Pathog 44:271–278. doi: 10.1016/j.micpath.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Moreira CG, Weinshenker D, Sperandio V. 2010. QseC mediates Salmonella enterica serovar Typhimurium virulence in vitro and in vivo. Infect Immun 78:914–926. doi: 10.1128/IAI.01038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreira CG, Russell R, Mishra AA, Narayanan S, Ritchie JM, Waldor MK, Curtis MM, Winter SE, Weinshenker D, Sperandio V. 2016. Bacterial adrenergic sensors regulate virulence of enteric pathogens in the gut. mBio 7:e00826-16. doi: 10.1128/mBio.00826-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kendall MM, Sperandio V. 2016. What a dinner party! Mechanisms and functions of interkingdom signaling in host-pathogen associations. mBio 7:e01748-15. doi: 10.1128/mBio.01748-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. 2006. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A 103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reading NC, Rasko DA, Torres AG, Sperandio V. 2009. The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc Natl Acad Sci U S A 106:5889–5894. doi: 10.1073/pnas.0811409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Furness JB. 2000. Types of neurons in the enteric nervous system. J Auton Nerv Syst 81:87–96. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 99.Eisenhofer G, Kopin IJ, Goldstein DS. 2004. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 100.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. 2012. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol 303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 101.Clarke MB, Sperandio V. 2005. Transcriptional regulation of flhDC by QseBC and sigma (FliA) in enterohaemorrhagic Escherichia coli. Mol Microbiol 57:1734–1749. doi: 10.1111/j.1365-2958.2005.04792.x. [DOI] [PubMed] [Google Scholar]
- 102.Curtis MM, Russell R, Moreira CG, Adebesin AM, Wang C, Williams NS, Taussig R, Stewart D, Zimmern P, Lu B, Prasad RN, Zhu C, Rasko DA, Huntley JF, Falck JR, Sperandio V. 2014. QseC inhibitors as an antivirulence approach for Gram-negative pathogens. mBio 5:e02165-14. doi: 10.1128/mBio.02165-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parker CT, Russell R, Njoroge JW, Jimenez AG, Taussig R, Sperandio V. 2017. Genetic and mechanistic analyses of the periplasmic domain of the enterohemorrhagic Escherichia coli QseC histidine sensor kinase. J Bacteriol 199:e00861-16. doi: 10.1128/JB.00861-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hughes DT, Clarke MB, Yamamoto K, Rasko DA, Sperandio V. 2009. The QseC adrenergic signaling cascade in enterohemorrhagic E. coli (EHEC). PLoS Pathog 5:e1000553. doi: 10.1371/journal.ppat.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mellies JL, Barron AM, Carmona AM. 2007. Enteropathogenic and enterohemorrhagic Escherichia coli virulence gene regulation. Infect Immun 75:4199–4210. doi: 10.1128/IAI.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heermann R, Jung K. 2010. The complexity of the ‘simple’ two-component system KdpD/KdpE in Escherichia coli. FEMS Microbiol Lett 304:97–106. doi: 10.1111/j.1574-6968.2010.01906.x. [DOI] [PubMed] [Google Scholar]
- 107.Yamamoto K, Hirao K, Oshima T, Aiba H, Utsumi R, Ishihama A. 2005. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J Biol Chem 280:1448–1456. doi: 10.1074/jbc.M410104200. [DOI] [PubMed] [Google Scholar]
- 108.Gopel Y, Luttmann D, Heroven AK, Reichenbach B, Dersch P, Gorke B. 2011. Common and divergent features in transcriptional control of the homologous small RNAs GlmY and GlmZ in Enterobacteriaceae. Nucleic Acids Res 39:1294–1309. doi: 10.1093/nar/gkq986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gruber CC, Sperandio V. 2015. Global analysis of posttranscriptional regulation by GlmY and GlmZ in enterohemorrhagic Escherichia coli O157:H7. Infect Immun 83:1286–1295. doi: 10.1128/IAI.02918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beuzon CR, Meresse S, Unsworth KE, Ruiz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J 19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moreira CG, Sperandio V. 2012. Interplay between the QseC and QseE bacterial adrenergic sensor kinases in Salmonella enterica serovar Typhimurium pathogenesis. Infect Immun 80:4344–4353. doi: 10.1128/IAI.00803-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moreira CG, Herrera CM, Needham BD, Parker CT, Libby SJ, Fang FC, Trent MS, Sperandio V. 2013. Virulence and stress-related periplasmic protein (VisP) in bacterial/host associations. Proc Natl Acad Sci U S A 110:1470–1475. doi: 10.1073/pnas.1215416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, Sonnenburg JL. 2013. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502:96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Connolly JP, Finlay BB, Roe AJ. 2015. From ingestion to colonization: the influence of the host environment on regulation of the LEE encoded type III secretion system in enterohaemorrhagic Escherichia coli. Front Microbiol 6:568. doi: 10.3389/fmicb.2015.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Njoroge JW, Nguyen Y, Curtis MM, Moreira CG, Sperandio V. 2012. Virulence meets metabolism: Cra and KdpE gene regulation in enterohemorrhagic Escherichia coli. mBio 3:e00280-12. doi: 10.1128/mBio.00280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Njoroge JW, Gruber C, Sperandio V. 2013. The interacting Cra and KdpE regulators are involved in the expression of multiple virulence factors in enterohemorrhagic Escherichia coli. J Bacteriol 195:2499–2508. doi: 10.1128/JB.02252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carlson-Banning KM, Sperandio V. 2016. Catabolite and oxygen regulation of enterohemorrhagic Escherichia coli virulence. mBio 7:e01852-16. doi: 10.1128/mBio.01852-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Curtis MM, Hu Z, Klimko C, Narayanan S, Deberardinis R, Sperandio V. 2014. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe 16:759–769. doi: 10.1016/j.chom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. 2011. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A 108:17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kendall MM, Gruber CC, Parker CT, Sperandio V. 2012. Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic Escherichia coli O157:H7. mBio 3:e00050-12. doi: 10.1128/mBio.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anderson CJ, Clark DE, Adli M, Kendall MM. 2015. Ethanolamine signaling promotes Salmonella niche recognition and adaptation during infection. PLoS Pathog 11:e1005278. doi: 10.1371/journal.ppat.1005278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Connell JL, Kim J, Shear JB, Bard AJ, Whiteley M. 2014. Real-time monitoring of quorum sensing in 3D-printed bacterial aggregates using scanning electrochemical microscopy. Proc Natl Acad Sci U S A 111:18255–18260. doi: 10.1073/pnas.1421211111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang M, Schaefer AL, Dandekar AA, Greenberg EP. 2015. Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. Proc Natl Acad Sci U S A 112:2187–2191. doi: 10.1073/pnas.1500704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim MK, Ingremeau F, Zhao A, Bassler BL, Stone HA. 2016. Local and global consequences of flow on bacterial quorum sensing. Nat Microbiol 1:15005. doi: 10.1038/nmicrobiol.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moreira CG, Palmer K, Whiteley M, Sircili MP, Trabulsi LR, Castro AF, Sperandio V. 2006. Bundle-forming pili and EspA are involved in biofilm formation by enteropathogenic Escherichia coli. J Bacteriol 188:3952–3961. doi: 10.1128/JB.00177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Juarez-Rodriguez MD, Torres-Escobar A, Demuth DR. 2013. ygiW and qseBC are co-expressed in Aggregatibacter actinomycetemcomitans and regulate biofilm growth. Microbiology 159:989–1001. doi: 10.1099/mic.0.066183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. 2011. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol Microbiol 80:1516–1529. doi: 10.1111/j.1365-2958.2011.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Njoroge J, Sperandio V. 2012. Enterohemorrhagic Escherichia coli virulence regulation by two bacterial adrenergic kinases, QseC and QseE. Infect Immun 80:688–703. doi: 10.1128/IAI.05921-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luzader DH, Clark DE, Gonyar LA, Kendall MM. 2013. EutR is a direct regulator of genes that contribute to metabolism and virulence in enterohemorrhagic Escherichia coli O157:H7. J Bacteriol 195:4947–4953. doi: 10.1128/JB.00937-13. [DOI] [PMC free article] [PubMed] [Google Scholar]