Abstract
Objective
To determine whether there is a threshold of detectable HIV RNA under 1,000 copies/mL after antiretroviral therapy initiation associated with 10-year all-cause mortality.
Design
This study included nearly 8,000 patients from a US-based multicenter clinical cohort who started antiretroviral therapy between 1 January 1998 and 31 December 2013. Viral load was assessed six months after initiation of therapy. Patients were followed from six months after therapy initiation (between 1 July 1998 and 30 June 2014) until death, and data were administratively censored after 10 years or on 31 December 2014.
Methods
We used nonparametric multiple imputation to account for left-censored viral load measurements, Cox proportional hazards models to estimate all-cause mortality hazard ratios, Nelson-Aalen cumulative hazard estimates to construct risk curves, and inverse probability of exposure weights to standardize estimated hazard ratios and risk curves to the total study population.
Results
Plots of standardized hazard ratio estimates and 95% confidence intervals indicated there was no demonstrable viral load threshold between 30 and 500 copies/mL associated with a marked increase in 10-year mortality. The standardized 10-year risk of mortality among patients with viral loads between 400 and 999 copies/mL six months after starting treatment was comparable to the risk of mortality among patients with viral loads between 1,000 and 4 million copies/mL (20% vs. 23%).
Conclusion
Incomplete suppression of plasma HIV RNA six months after starting therapy is associated with substantial 10-year all-cause mortality risk, highlighting the importance of rapid viral load suppression after therapy initiation.
Keywords: antiretroviral therapy, viral suppression, mortality, multiple imputation, left censoring, clinical cohort
INTRODUCTION
The goal of antiretroviral therapy (ART) is to restore and maintain the health of people infected with human immunodeficiency virus (HIV) through suppression of HIV replication. In clinical practice, a concentration of HIV ribonucleic acid (RNA) below the detection limits of available assays is considered evidence of viral suppression. Despite advances in ART[1], not all HIV patients on treatment are able to achieve and maintain suppressed viral loads.[2]
Low, detectable HIV RNA under 1,000 copies/mL has been studied as a potential risk factor for drug resistance, virologic failure, cancer, and mortality.[3–14] However, the clinical significance of detectable viral loads in this range remains unclear, despite studies suggesting that patients with HIV or acquired immune deficiency syndrome (AIDS) who have low, detectable viral loads are at higher risk of experiencing adverse health outcomes compared to patients with undetectable viral loads.[3–5, 7, 8, 10, 12–14]
Uncertainty about the effects of low, detectable viral load is due in part to variability in its definition. Clinical and epidemiologic studies typically set the upper bound of low viral load between 200 and 1,000 copies/mL, while the lower bound tends to be fixed at the detection limit of the viral load assay used in the study.[3–17] Defining a range of low, detectable viral load is further complicated when viral load measurements are obtained from multiple assays with different limits of detection, and when a considerable proportion of those measurements fall below varying detection limits.
As access to effective ART scales up and the sensitivity of viral load assays improves over time, the number of HIV patients with low, detectable levels of HIV RNA observed in clinical practice will grow. Therefore, it is important to understand the implications of low, detectable viral load. The objective of this study is to determine whether a clinically significant threshold of detectable viral load under 1,000 copies/mL can be defined based on the relationship between a single viral load measurement collected six months after ART initiation and 10-year mortality.
METHODS
Study population
We used data from the Center for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS), a multicenter clinical cohort that currently includes over 30,000 HIV patients in the United States. CNICS maintains a clinical data repository from electronic medical record systems to support HIV research.[18] The CNICS cohort includes patients aged 18 years and older who initiated primary care in or after 1995 at eight CFAR sites: Case Western Reserve University; Fenway Community Health Center of Harvard University; Johns Hopkins University; University of Alabama at Birmingham; University of California, San Diego; University of California, San Francisco; University of North Carolina at Chapel Hill; and University of Washington. CNICS is a dynamic cohort, with approximately 1,400 new patients enrolling and 10% of patients leaving care annually.[18]
All participants provided written informed consent to be included in the CNICS cohort, or contributed data with a waiver of written informed consent where approved by local institutional review boards. Upon entry into CNICS, demographic and historical information, including prior diagnoses and antiretroviral treatment, was collected. After enrollment, patient data were prospectively captured at clinic visits and included prescribed medications, laboratory test results, and conditions diagnosed by providers. CNICS participants were typically seen in clinical care every three to four months, though frequency of follow-up is patient specific.
A total of 27,865 patients entered the CNICS cohort between 1 January 1998 and 31 December 2013. Patients who initiated monotherapy or dual therapy prior to or with no history of starting combination ART (defined as three or more ART drugs prescribed concurrently) (n=3,499), initiated ART prior to entering CNICS (n=6,405), initiated ART after 31 December 2013 (n=282), had no history of initiating ART (n=4,067), died within six months of starting ART (n=130), or did not have at least one viral load measurement six months (−30/+90 days) after ART initiation (n=3,431) were excluded from our study. Patients with missing race/ethnicity information (n=93), no recorded CD4 count six months (−30/+90 days) after ART initiation (n=322), no pre-ART viral load measurement recorded between 60 days prior to CNICS entry and ART initiation (n=284), or pre-ART viral load measurements that suggested unrecorded prior exposure to treatment (<1,000 copies/mL) (n=1,408) were also excluded. A total of 7,944 patients were included in the final study sample.
Mortality ascertainment
The outcome of interest was time to death from any cause. All CNICS sites regularly query the National Death Index and state death certificate records to confirm recorded dates of deaths and capture unrecorded deaths among CNICS patients no longer in care. We used all-cause mortality as our endpoint because cause of death data were unavailable for approximately 35% of deaths recorded in CNICS.
Because vital records are maintained on all patients enrolled in CNICS, including those not currently retained in care, we included deaths among patients no longer being seen in a CNICS clinic in the analyses. We ended the study period on 31 December 2014 to account for reporting delays, which can result in underestimated mortality, and to allow sufficient time for CNICS sites to verify vital records.
Viral load assessment
The exposure of interest was HIV RNA six months (−30/+90 days) after ART initiation. For patients who had more than one viral load during the 120-day window, we used the measurement that was collected closest to six months after the date of ART initiation. Viral loads measurements were determined by quantitative amplification assays and expressed as the number of HIV copies per milliliter of blood plasma (copies/mL). Viral load assays used in this study varied over time and by CNICS site; the lower limits of detection for the most commonly used assays were 20, 30, 40, 48, 50, 75, and 400 copies/mL. Viral load measurements six months after ART initiation ranged from 6 to over 4 million copies/mL.
Statistical analysis
The majority of viral load observations included in our analyses were reported to be below specified limits of detection. We assumed that these viral load measurements were left censored at random conditional on observed covariates, and used a nonparametric multiple imputation approach with a left-censoring score model to account for missing data. For each viral load observation, we used logistic regression to estimate the conditional probability of left censoring given age, sex, race/ethnicity, male-to-male sexual contact, injection drug use, smoking status, at-risk alcohol use, pre-ART viral load, year of ART initiation, ART regimen, CD4 count, clinical AIDS status, status of other clinical conditions (chronic hepatitis B, chronic hepatitis C, past diagnosis of any cancer excluding nonmelanoma skin cancer), statin use, study site, and death.[19] Restricted quadratic splines were used to model age and CD4 count, with knots at the 5th, 35th, 65th, and 95th percentiles.[20] We stratified the study cohort into five groups based on quintiles of the predicted probability of being left censored.
Next, we computed nonparametric maximum likelihood estimates of the distribution function of viral load using the Turnbull estimator[21, 22], stratified by quintiles of the left-censoring score, and used these estimates to impute left-censored viral load observations. We imputed all viral load observations that were too low to be quantified using assays with detection limits of 20, 30, 40, 48, 50, 75, or 400 copies/mL, and imputed values were bounded between zero and the assay detection limit. One hundred imputed datasets were generated for analysis.
The start of follow-up for each patient was six months after the date of ART initiation. Patients were followed until death, and data were administratively censored after 10 years or on 31 December 2014. Hazard ratios for 10-year all-cause mortality from six months post-ART initiation were estimated using the following Cox proportional hazards model[23]:
where xk,1 is 1 if viral load is between 20 and <k and 0 otherwise, xk,2 is 1 if viral load is between k and 999 copies/mL and 0 otherwise, and x3 is 1 if viral load is above 999 copies/mL and 0 otherwise. The reference category was viral load <20 copies/mL. We estimated hazard ratios for each of the three viral load categories, for possible threshold values of k between 30 and 500 copies/mL. Efron’s approximation was used to handle tied event times.[24]
We used inverse probability of exposure weights[24, 25] to control for differences at baseline among patients across the four viral load categories (<20, 20 to < k, k to 999, and >999 copies/mL) and standardize estimates to the total study population. Sex, race/ethnicity, male-to-male sexual contact, and injection drug use were assessed at entry into the CNICS cohort. Pre-ART viral load, year of ART initiation, and ART regimen were assessed at ART initiation. Age, smoking status, at-risk alcohol use, CD4 count, clinical AIDS status, status of other clinical conditions (chronic hepatitis B, chronic hepatitis C, past diagnosis of any cancer excluding nonmelanoma skin cancer), statin use, and study site were assessed at study baseline. Restricted quadratic splines were used to model age and CD4 count. Using a multinomial logistic regression model for each k, we estimated the conditional probability of being in each viral load category, and calculated stabilized weights. The weights had a mean of 1.0 across all k for all imputations, with an overall minimum of 0.13 and overall maximum of 15.
We generated combined point estimates of hazard ratios for 10-year all-cause mortality by averaging across the 100 log hazard ratio estimates from the imputed datasets. We calculated robust standard errors for standardized hazard ratios, and used Rubin’s variance estimator[26] to combine variance within and between imputations. These overall hazard ratio and variance estimates were used to construct 95% Wald confidence intervals. The proportional hazards assumption was evaluated by examining plots of the log cumulative hazard by time and testing the product term of viral load and time; no notable violations of this assumption were identified. We computed mortality risk over time using the Nelson-Aalen cumulative hazard function[27, 28], and constructed risk curves[29] averaged across all imputations and stratified by viral load category.
Additionally, for comparison, we replaced left-censored observations with half of the detection limit, and calculated hazard ratios for 10-year all-cause mortality and generated risk curves. In this alternative analysis, the weights used to standardize hazard ratio estimates to the total study population had a mean of 0.96 (range: 0.16, 19) across all k, after truncating at the 99.97 percentile.[24] We used SAS version 9.4 (SAS Institute Inc., Cary, NC) for analyses, and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) for figures.
RESULTS
We identified 7,944 CNICS patients (49,118 person-years) who met our study inclusion criteria (Table 1). Of the patients included in the study, the median age at baseline (six months after ART initiation) was 40 (interquartile range [IQR]: 32, 46) years, 83% were male, 45% were white/Caucasian, 37% were black/African American, 62% identified as men who have sex with men, and 12% reported having ever injected drugs. The median pre-ART viral load was 74,827 (IQR: 22,394, 237,780) copies/mL, median year of ART initiation was 2007 (IQR: 2003, 2010), and median CD4 count was 349 (IQR: 193, 532) cells/mm3. At baseline, 29% of study patients had been diagnosed with AIDS, 47% were prescribed a non-nucleoside reverse transcriptase inhibitor-based regimen, and 40% had been prescribed a protease inhibitor-based regimen. Patients were followed for a median of 6.2 (IQR: 3.5, 10) years, and 862 deaths from any cause were recorded during the study period.
Table 1.
Demographic, clinical, and behavioral characteristics of 7,944 CNICS patients six months after ART initiation, between 1 July 1998 and 30 June 2014, averaged over 100 imputations.
| Characteristic | Total n=7,944 |
<20 cpm n=4,545 |
20–999 cpm n=2,184 |
>999 cpm n=1,215 |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Age, years | 40 (32, 46) | 40 (32, 47) | 40 (33, 47) | 39 (32, 45) |
| Malea | 6,566 (82.7) | 3,790 (83.4) | 1,833 (83.9) | 943 (77.6) |
| Race/ethnicitya | ||||
| White, non-Hispanic | 3,581 (45.1) | 2,163 (47.6) | 971 (44.5) | 447 (36.8) |
| Black, non-Hispanic | 2,908 (36.6) | 1,482 (32.6) | 835 (38.2) | 591 (48.6) |
| Other, non-Hispanic | 401 (5.1) | 254 (5.6) | 101 (4.6) | 46 (3.8) |
| Hispanic | 1,054 (13.3) | 646 (14.2) | 277 (12.7) | 131 (10.8) |
| MSM, evera | 4,917 (61.9) | 2,946 (64.8) | 1,331 (60.9) | 640 (52.7) |
| IDU, evera | 979 (12.3) | 477 (10.5) | 277 (12.7) | 225 (18.5) |
| Smoking, ever | 2,605 (32.8) | 1,451 (31.9) | 708 (32.4) | 466 (36.7) |
| At-risk alcohol use, ever | 1,197 (15.1) | 654 (14.4) | 317 (14.5) | 226 (18.6) |
| Pre-ART viral load, cpmb | 74,827 (22,394; 237,780) | 56,048 (17,593; 170,000) | 111,880 (36,295; 372,848) | 93,928 (30,900; 294,966) |
| Year of ART initiationb | 2007 (2003, 2010) | 2008 (2004, 2010) | 2006 (2002, 2009) | 2004 (2001, 2008) |
| ART regimenb | ||||
| NNRTI-based | 3,747 (47.2) | 2,463 (54.2) | 852 (39.0) | 432 (35.6) |
| PI-based | 3,188 (40.1) | 1,519 (33.4) | 1,048 (48.0) | 621 (51.1) |
| INSTI-based | 431 (5.4) | 313 (6.9) | 87 (4.0) | 31 (2.6) |
| Other | 578 (7.3) | 229 (5.5) | 198 (9.1) | 131 (10.8) |
| CD4 count, cells/mm3 | 349 (193, 532) | 405 (245, 574) | 321 (184, 496) | 191 (71, 355) |
| Clinical AIDS diagnosis | 2,313 (29.1) | 1,101 (24.2) | 740 (33.9) | 472 (38.9) |
| Chronic hepatitis B | 209 (2.6) | 91 (2.0) | 66 (3.0) | 52 (4.3) |
| Chronic hepatitis C | 650 (8.2) | 327 (7.2) | 177 (8.1) | 146 (12.0) |
| Past cancer diagnosis | 429 (5.4) | 240 (5.3) | 121 (5.5) | 68 (5.6) |
| Statin use, ever | 250 (3.2) | 167 (3.7) | 59 (2.7) | 24 (2.0) |
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; cpm, copies per milliliter; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor
Assessed at entry into CNICS cohort.
Assessed at ART initiation.
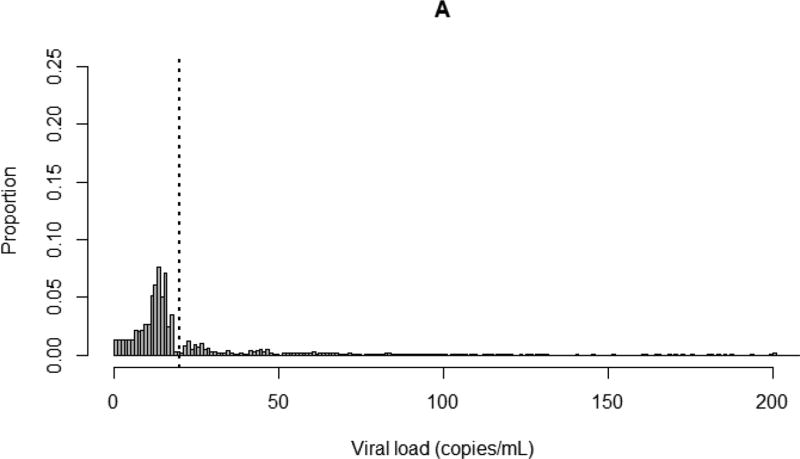
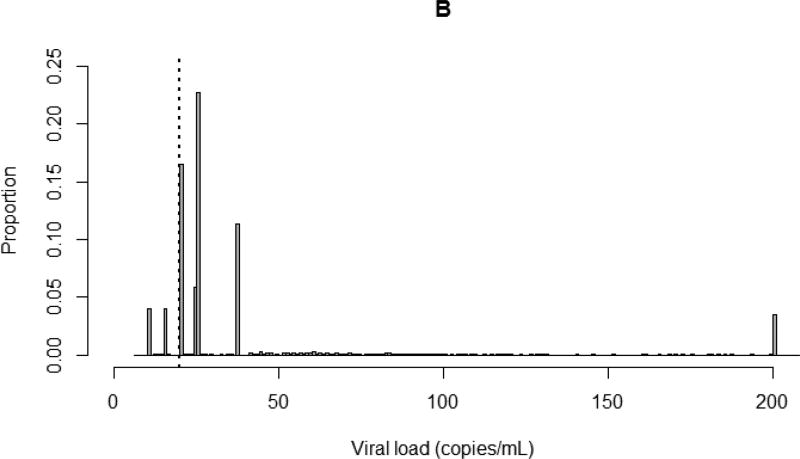
Of the patients included in this study, 68% had viral loads six months after ART initiation that were left censored at assay detection limits (Appendix 1). After imputation, an average of 57% of all viral load measurements (84% of left-censored observations) fell below 20 copies/mL. Fifteen percent of study patients had viral loads of at least 1,000 copies/mL six months after ART initiation. Plots of the distribution of viral load comparing nonparametric multiple imputation to simple substitution (replacing left-censored observations with half of the detection limit) are shown in Figure 1.
Figure 1.
Distribution of viral loads up to 200 copies/mL for 7,944 CNICS patients six months after ART initiation. Dotted line indicates 20 copies/mL. A. After nonparametric multiple imputation of left-censored viral load observations, averaged over 100 imputations; B. After substitution of left-censored viral load observations with half of assay detection limits.
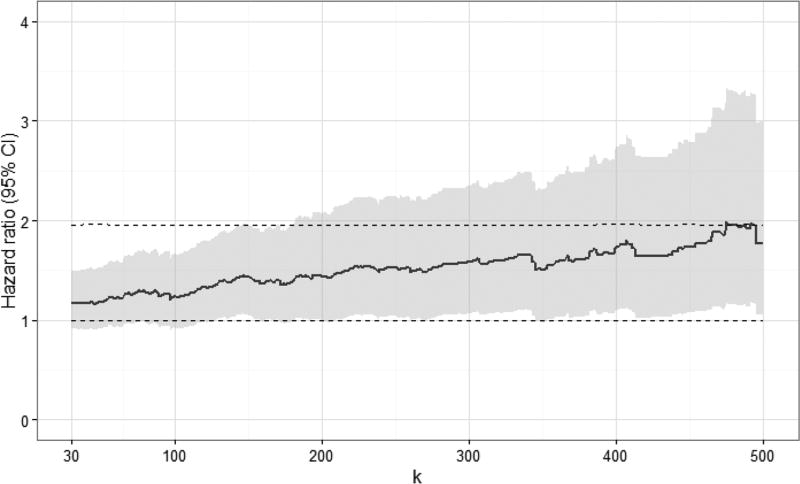
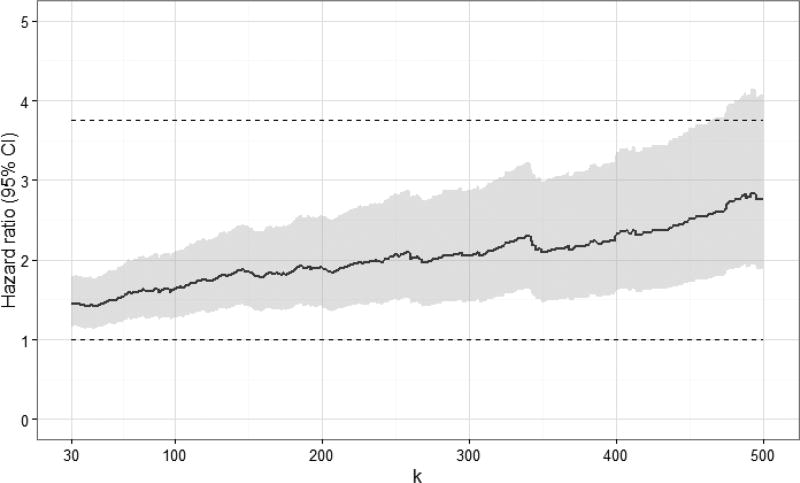
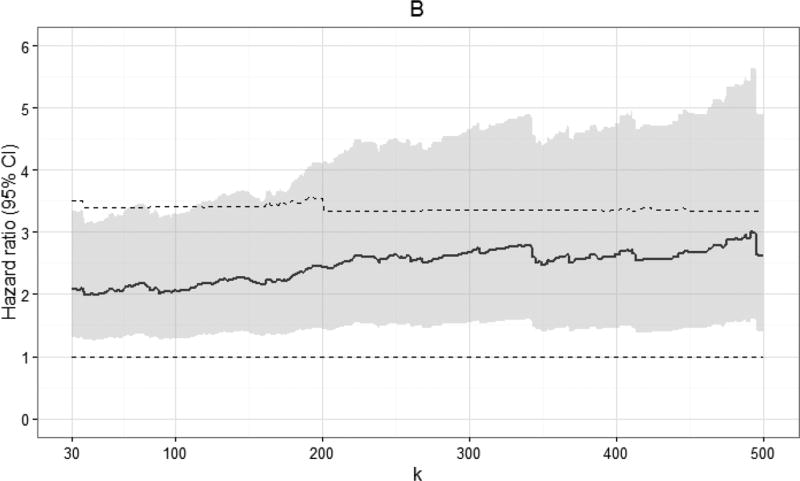
Standardized hazard ratio estimates at specific values of k are shown in Table 2 (see Appendix 2a for crude hazard ratio estimates). Viral load measurements were categorized as <20 copies/mL (reference group), 20 to < k copies/mL, k to 999 copies/mL, and >999 copies/mL. In aggregate, viral load measurements of 20 to 999 copies/mL were not associated with increased 10-year all-cause mortality, when compared to viral loads under 20 copies/mL (standardized hazard ratio [HR]: 1.18, 95% confidence interval [CI]: 0.93, 1.50). When comparing k to 999 copies/mL to <20 copies/mL, we observed an increase in the standardized 10-year hazard ratio for mortality at values of k discernable at 130 copies/mL (standardized HR: 1.39, 95% CI: 1.02, 1.88). As expected, viral loads >999 copies/mL were strongly associated with increased mortality (standardized HR: 1.96, 95% CI: 1.56, 2.46). Plots of standardized hazard ratio estimates and 95% confidence intervals by k indicated there was no demonstrable viral load threshold between 30 and 500 copies/mL associated with a marked increase in 10-year mortality (Figure 2; see Appendix 2b for plots of crude hazard ratios).
Table 2.
Standardized hazard ratio estimates for 10-year all-cause mortality for 7,944 CNICS patients, by selected viral load threshold values of k, combined from 100 imputations.
| Standardizeda hazard ratio (95%
confidence interval) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
k, cpm |
No.b of deaths |
No.b of Patients |
<20 cpmc |
No.b of deaths |
No.b of patients |
20 to
<k cpm |
No.b of deaths |
No.b of patients |
k to 999 cpm |
No. of deaths |
No. of patients |
>999 cpm |
|
|
|
|
|
|
||||||||
| 20 | 310 | 4545 | 1 | — | — | — | 230 | 2184 | 1.18 (0.93, 1.50) | 322 | 1215 | 1.96 (1.56, 2.46)d |
| 30 | 54 | 535 | 1.17 (0.75, 1.84) | 176 | 1649 | 1.17 (0.91, 1.50) | ||||||
| 40 | 75 | 708 | 1.19 (0.81, 1.73) | 155 | 1476 | 1.18 (0.91, 1.52) | ||||||
| 50 | 96 | 934 | 1.17 (0.83, 1.66) | 134 | 1215 | 1.18 (0.92, 1.53) | ||||||
| 75 | 121 | 1273 | 1.10 (0.79, 1.47) | 109 | 911 | 1.29 (0.98, 1.70) | ||||||
| 100 | 142 | 1454 | 1.12 (0.85, 1.50) | 88 | 730 | 1.25 (0.93, 1.68) | ||||||
| 130 | 152 | 1592 | 1.09 (0.83, 1.44) | 78 | 592 | 1.39 (1.02, 1.88) | ||||||
| 200 | 174 | 1776 | 1.11 (0.85, 1.44) | 56 | 409 | 1.44 (1.00, 2.07) | ||||||
| 300 | 189 | 1912 | 1.11 (0.86, 1.43) | 41 | 272 | 1.58 (1.07, 2.35) | ||||||
| 400 | 197 | 1991 | 1.12 (0.87, 1.45) | 33 | 193 | 1.74 (1.10, 2.74) | ||||||
| 500 | 201 | 2041 | 1.11 (0.86, 1.43) | 29 | 143 | 1.77 (1.05, 2.99) | ||||||
Abbreviation: cpm, copies/mL.
Standardized estimates controlled for age, sex, race/ethnicity, male-to-male sexual contact, injection drug use, smoking, at-risk alcohol use, pre-ART viral load, year of ART initiation, ART regimen, CD4 count, clinical AIDS status, status of other clinical conditions (chronic hepatitis B, chronic hepatitis C, past diagnosis of any cancer excluding nonmelanoma skin cancer), statin use, and study site.
Averaged over 100 imputations, rounded to nearest integer.
Viral load <20 copies/mL was reference category across k.
Hazard ratio for viral loads >999 copies/mL was unchanged across k.
Figure 2.
Standardized hazard ratios and 95% confidence intervals for 10-year all-cause mortality for 7,944 CNICS patients, by viral load threshold k, for viral loads between k and 999 copies/mL, combined from 100 imputations. Standardized estimates controlled for age, sex, race/ethnicity, male-to-male sexual contact, injection drug use, smoking, at-risk alcohol use, pre-ART viral load, year of ART initiation, ART regimen, CD4 count, clinical AIDS status, status of other clinical conditions (chronic hepatitis B, chronic hepatitis C, past diagnosis of any cancer excluding nonmelanoma skin cancer), statin use, and study site. Upper dashed line indicates hazard ratio for 10-year all-cause mortality for viral loads >999 copies/mL; lower dashed line indicates hazard ratio of 1.
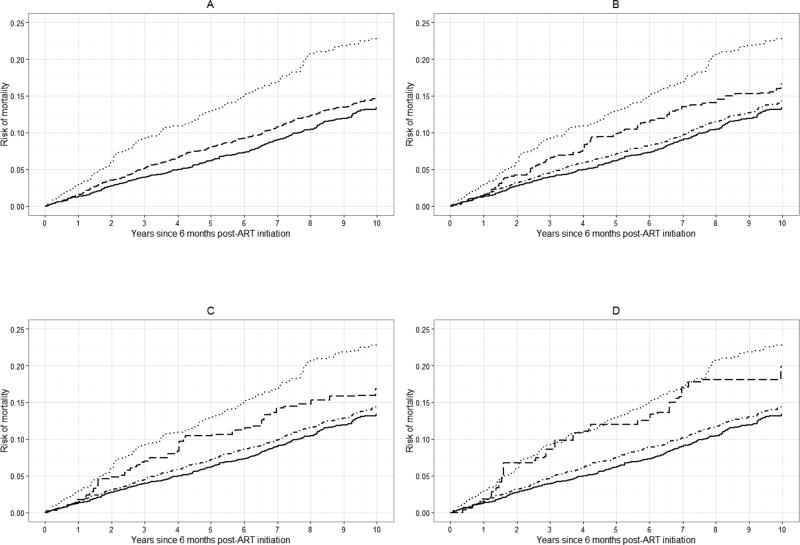
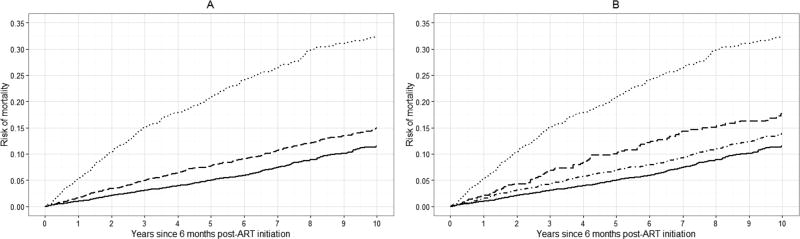
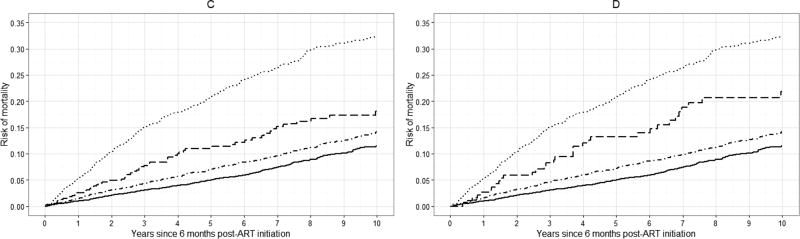
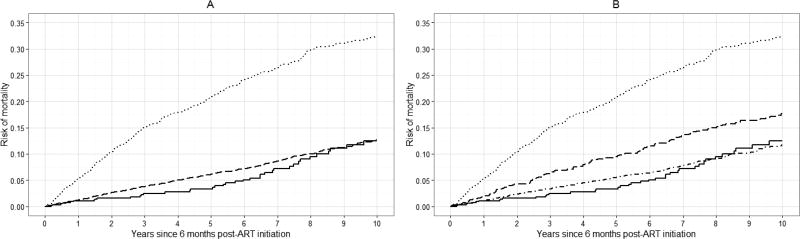
Standardized risk curves for all-cause mortality at specific values of k are shown in Figure 3 (see Appendix 2c for crude risk curves). Again, we observed an increase in the standardized risk of 10-year mortality with increasing viral load at baseline. The average standardized risk of 10-year mortality was approximately 14% among patients with viral loads between 20 and 400 copies, which was similar to the risk among patients with viral loads <20 copies/mL (13%). There was a 20% standardized risk of death among patients with viral loads between 400 and 999 copies/mL, comparable to the risk among patients with viral loads >999 copies/mL (23%).
Figure 3.
Standardized risk curves for all-cause mortality for 7,944 CNICS patients, for selected viral load threshold values of k, stratified by viral load category, averaged over 100 imputations. Standardized estimates controlled for age, sex, race/ethnicity, male-to-male sexual contact, injection drug use, smoking, at-risk alcohol use, pre-ART viral load, year of ART initiation, ART regimen, CD4 count, clinical AIDS status, status of other clinical conditions (chronic hepatitis B, chronic hepatitis C, past diagnosis of any cancer excluding nonmelanoma skin cancer), statin use, and study site. Solid line represents viral loads <20 copies/mL, dot-dashed line represents viral loads between 20 and k copies/mL, dashed line represents viral loads between k and 999 copies/mL, dotted line represents viral loads >999 copies/mL. A. k = 20 copies/mL; B. k = 130 copies/mL; C. k = 200 copies/mL; D. k = 400 copies/mL.
Hazard ratio estimates for 10-year all-cause mortality and risk curves, generated after replacing left-censored viral load observations with half of the detection limit, are shown in Appendix 3a–e. With this simple substitution approach, the majority (58%) of viral load observations were between 20 and 50 copies/mL, while a comparatively low proportion (8%) of viral loads fell below 20 copies/mL. Patterns in hazard ratio estimates were similar to what we observed with estimates calculated using multiply-imputed viral loads, though the magnitude of hazard ratio estimates was higher across all values of k using the substitution method of handling left-censored viral load observations. We also observed similar trends in the risk curves for mortality, though there was a notably less steep trajectory in the standardized risk of mortality over time among patients with viral loads <20 copies/mL based on data generated from simple substitution.
DISCUSSION
The objective of this study was to determine whether there was a threshold of HIV viral load under 1,000 copies/mL early after the start of therapy associated with increased mortality, while systematically accounting for undetectable viral load results. We did not identify a clear low-level viral load threshold between 30 and 500 copies/mL that corresponded with a marked increase in 10-year all-cause mortality. Rather, we observed a gradual increase in standardized hazard ratio estimates with increasing viral load, discernable at 130 copies/mL. The average standardized 10-year mortality risk among patients with viral loads between 400 and 999 copies/mL at baseline approached the standardized risk of mortality among patients with viral loads between 1,000 and 4 million copies/mL (20% vs. 23%).
The US Department of Health and Human Services and the AIDS Clinical Trials Group currently define virologic failure as one confirmed viral load measurement over 200 copies/mL.[30] Here, using a single measurement after six months of therapy, we observed a 44% increase in the hazard of death among patients with viral loads between 200 and 999, compared to those with viral loads under 20 copies/mL (standardized HR: 1.44, 95% CI: 1.00, 2.07). The average standardized 10-year mortality risk among patients with low-level viral loads between 200 and 999 copies/mL at baseline was 17%, which was higher than the average standardized risk of mortality among patients with viral loads under 20 copies/mL (14%).
In this study, exposure status was based on one viral load measurement collected approximately six months after ART initiation. Because a single detectable viral load measurement could represent either a transient increase in viral load or sustained low-level HIV RNA concentrations in the detectable range, which are likely disparate risk factors, using time-varying measures of viral load to assess exposure is warranted for future analysis. That said, we observed a clear pattern of increasing 10-year mortality risk with increasing viral load, based on one viral load measurement under 1,000 copies/mL after six months of therapy. We also observed that a single viral load measurement at or above 1,000 copies/mL six months after ART initiation was strongly associated with 10-year mortality. This suggests that a single viral load measurement collected six months after initiating ART remains highly informative regarding the risk of death over 10 years.
By using data from CNICS, the largest clinical cohort of HIV patients in US, we expect our study findings to be generalizable to treated adult HIV patients engaged in clinical care at academic medical centers in the US. Because patients who died within six months of initiating ART were excluded from the study population, we expect that the results of this study are applicable to patients who start treatment early enough in the disease course to be effective. There may be unmeasured confounding that impacts our findings, and we note that a viral load measurement collected shortly after starting therapy may be a proxy for unmeasured variables, such as socioeconomic status. We assumed that variables included in the analyses were measured without error, which is unlikely for self-reported behaviors such as tobacco, alcohol, and illicit drug use; however, we do not expect measurement error of confounders to be differential by exposure or outcome.
We did not account for adherence, switching, or cessation of ART regimen in the analyses. For each treatment-naïve patient, we considered the first recorded date of concurrent prescription of three or more ART drugs as an indicator of starting a combination ART regimen, and ignored changes in treatment. Approximately 85% of patients included in our study had viral loads under 1,000 copies six months after ART initiation, and we assumed that patients not taking their medication as prescribed were likely assigned to the highest viral load category (>999 copies/mL). We did not evaluate values of k above 500 copies/mL due to the relatively small number of deaths among patients with viral loads between 500 and 999 copies/mL six months after starting therapy. We also note that, because we had limited follow-up for patients whose viral loads were assessed with ultrasensitive assays, it would be prudent to reevaluate our estimates after additional person-time has accumulated in the CNICS cohort.
Nearly 70% of viral load observations included in our analyses were left censored. Simple substitution methods (e.g., replacement with a constant value such as the assay detection limit, half the detection limit, the detection limit divided by the square root of two, or zero) are often used to account for left-censored exposure data but can result in substantial bias, particularly when the proportion of censoring is high.[31, 32] Another approach is to impute left-censored exposure data by maximum likelihood[32, 33], but this is problematic when the data do not closely follow a known parametric distribution.[34] Here, we used nonparametric multiple imputation to account for left-censored viral loads. This allowed us to effectively compare undetectable viral load observations collected over time using assays with different detection limits, without having to rely on distributional assumptions. As shown in Figure 1, simple substitution resulted in the majority of viral loads being amassed at specific values determined by assay detection limits, while multiple imputation produced a more biologically plausible depiction of the underlying distribution of viral load.
Using simple substitution resulted in far fewer left-censored viral loads that were categorized as under 20 copies/mL (8% vs. 57% with multiple imputation). Standardized hazard ratio estimates indicated an increased hazard of death for patients at all low-level viral loads between 20 and 999 copies/mL at baseline, due to a markedly lower risk of death among patients with viral loads under 20 copies/mL compared to patients in the same viral load category based on multiply-imputed data (4% vs. 13%). Almost 60% of patients with viral loads under 20 copies/mL based on data generated by simple substitution received care from the same study site and initiated ART during a narrow time period, whereas patients in the same viral load category based on multiply-imputed data represented all study sites and started ART during all years included in the study period. Using simple substitution resulted in violations of positivity, and fitting the weight model to data generated after simple substitution yielded extreme values, which we did not observe when fitting the same weight model to data generated from multiple imputation. Due to these factors, the hazard ratio and risk estimates we calculated using our nonparametric multiple imputation approach were attenuated but likely less biased than estimates calculated using simple substitution data.
Detectable viral loads under 1,000 copies/mL may indicate ongoing low-level HIV replication due to inadequate response to treatment, drug resistance, drug interactions, or incomplete adherence to therapy or care. Occurrences of low, detectable viral load, whatever the underlying cause, will be more commonly observed in HIV patients as access to antiretroviral therapy increases and assay sensitivity improves over time. While we observed an increased hazard of death with low-level viral loads, discernable at 130 copies/mL, this association was largely driven by the elevated mortality risk experienced by patients with viral loads between 400 and 999 copies/mL. Patients with viral loads in this higher range, which suggested partial response to treatment, faced a similar long-term risk of mortality as patients with high viral loads that indicated overt treatment failure. Low-level viral loads between 400 and 999 copies/mL shortly after starting ART appear to place patients at a significantly higher 10-year risk of death than patients with viral loads under 20 copies/mL, and occurrences of viral loads in this range may need to be treated similarly as viral loads that exceed 1,000 copies/mL. Given the importance of rapidly achieving virologic suppression after initiating treatment, further investigation of the causes of unsuppressed viral loads between 400 and 999 copies/mL is warranted.
Acknowledgments
We thank the patients, investigators, and staff at the study sites of the Center for AIDS Research Network of Integrated Clinical Systems.
Sources of funding:
This project was supported by grants T32 AI007001, R01 AI100654, R24 AI067039, and P30 AI50410 from the National Institutes of Health. Other support was provided by grants P30 AI094189 and U01 DA036935.
Appendix 1
Number (%) of 7,944 CNICS patients with viral load measurements observed or left censored at lower limits of detection for assays most commonly used during study period, by year of start of follow up.
| Limit of detection
(copies/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Start of follow-up |
All patients |
20 | 30 | 40 | 48 | 50 | 75 | 400 |
| Total | 7,944 | 212 (3.1) | 299 (4.4) | 1,095 (16.0) | 365 (5.3) | 1,626 (23.7) | 820 (12.0) | 238 (3.5) |
| 1998 | 85 | 0 | 0 | 0 | 0 | 32 (37.7) | 0 | 15 (17.7) |
| 1999 | 268 | 1 (0.4) | 0 | 1 (0.4) | 0 | 117 (43.7) | 0 | 44 (16.4) |
| 2000 | 376 | 0 | 0 | 1 (0.3) | 0 | 174 (46.3) | 3 (0.8) | 39 (10.4) |
| 2001 | 445 | 1 (0.2) | 0 | 0 | 0 | 191 (42.9) | 5 (1.1) | 47 (10.6) |
| 2002 | 384 | 0 | 1 (0.3) | 1 (0.3) | 1 (0.3) | 168 (43.8) | 26 (6.8) | 21 (5.5) |
| 2003 | 438 | 0 | 23 (5.3) | 1 (0.2) | 0 | 129 (29.5) | 95 (21.7) | 21 (4.8) |
| 2004 | 493 | 0 | 47 (9.5) | 1 (0.2) | 0 | 146 (29.6) | 87 (17.7) | 13 (2.6) |
| 2005 | 451 | 0 | 46 (10.2) | 2 (0.4) | 0 | 122 (27.1) | 112 (24.8) | 16 (3.6) |
| 2006 | 521 | 0 | 51 (9.8) | 0 | 0 | 186 (35.7) | 99 (19.0) | 15 (2.9) |
| 2007 | 547 | 0 | 50 (9.1) | 14 (2.6) | 3 (0.6) | 199 (36.4) | 113 (20.7) | 14 (2.6) |
| 2008 | 624 | 0 | 64 (10.3) | 66 (10.6) | 72 (11.5) | 157 (25.2) | 94 (15.1) | 7 (1.1) |
| 2009 | 720 | 0 | 37 (5.1) | 187 (26.0) | 146 (20.3) | 77 (10.7) | 70 (9.7) | 4 (0.6) |
| 2010 | 662 | 0 | 0 | 275 (41.5) | 121 (18.3) | 48 (7.3) | 48 (7.3) | 5 (0.8) |
| 2011 | 665 | 61 (9.2) | 0 | 288 (43.3) | 66 (9.2) | 34 (5.1) | 50 (7.5) | 0 |
| 2012 | 577 | 123 (21.3) | 2 (0.4) | 213 (36.9) | 26 (4.5) | 8 (1.4) | 47 (8.2) | 1 (0.2) |
| 2013 | 495 | 91 (18.4) | 0 | 200 (40.4) | 16 (3.2) | 6 (1.2) | 37 (7.5) | 0 |
| 2014 | 193 | 41 (21.2) | 0 | 63 (32.6) | 14 (7.3) | 0 | 19 (9.8) | 0 |
Appendix 2a
Crude hazard ratio estimates for 10-year all-cause mortality for 7,944 CNICS patients, by selected viral load threshold values of k, combined from 100 imputations.
| Crude hazard ratio
(95% confidence interval) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
k, cpm |
No.a of deaths |
No.a of patients |
<20 cpmb |
No.a of deaths |
No.a of patients |
20 to
<k cpm |
No.a of deaths |
No.a of patients |
k to
999 cpm |
No. of deaths |
No. of patients |
>999 cpm |
|
|
|
|
|
|
||||||||
| 20 | 310 | 4545 | 1 | — | — | — | 230 | 2184 | 1.43 (1.15, 1.78) | 322 | 1251 | 3.75 (3.18, 4.43)c |
| 30 | 54 | 535 | 1.37 (0.89, 2.11) | 176 | 1649 | 1.45 (1.17, 1.80) | ||||||
| 40 | 75 | 708 | 1.44 (1.00, 2.06) | 155 | 1476 | 1.43 (1.14, 1.78) | ||||||
| 50 | 96 | 934 | 1.41 (1.01, 1.95) | 134 | 1215 | 1.45 (1.16, 1.80) | ||||||
| 75 | 121 | 1273 | 1.30 (0.97, 1.73) | 109 | 911 | 1.61 (1.28, 2.04) | ||||||
| 100 | 142 | 1454 | 1.32 (1.01, 1.73) | 88 | 730 | 1.64 (1.28, 2.11) | ||||||
| 130 | 152 | 1592 | 1.30 (1.00, 1.68) | 78 | 592 | 1.80 (1.39, 2.33) | ||||||
| 200 | 174 | 1776 | 1.32 (1.04, 1.69) | 56 | 409 | 1.90 (1.42, 2.55) | ||||||
| 300 | 189 | 1912 | 1.34 (1.06, 1.70) | 41 | 272 | 2.06 (1.47, 2.88) | ||||||
| 400 | 197 | 1991 | 1.34 (1.07, 1.69) | 33 | 193 | 2.33 (1.62, 3.35) | ||||||
| 500 | 201 | 2041 | 1.34 (1.06, 1.68) | 29 | 143 | 2.77 (1.88, 4.07) | ||||||
Abbreviation: cpm, copies/mL.
Averaged over 100 imputations, rounded to nearest integer.
Viral load <20 copies/mL was reference category across k.
Hazard ratio for viral loads >999 copies/mL was unchanged across k.
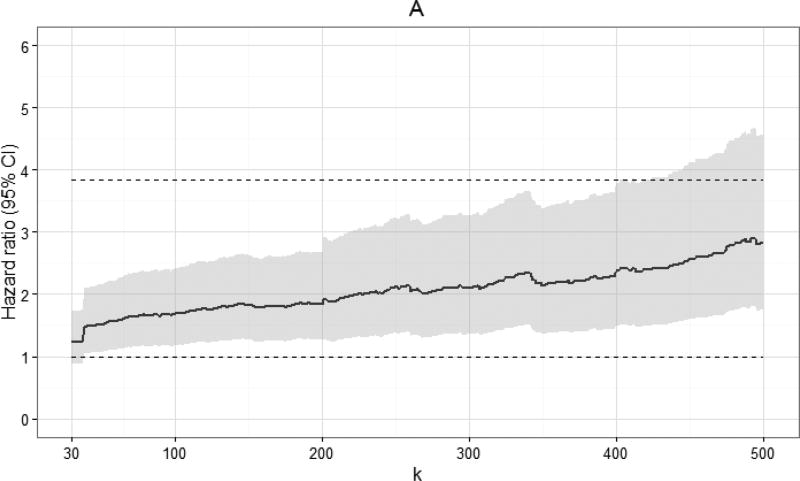
Appendix 2b
Crude hazard ratios and 95% confidence intervals for 10-year all-cause mortality for 7,944 CNICS patients, by viral load threshold k, for viral loads between k and 999 copies/mL, combined from 100 imputations. Upper dashed line indicates hazard ratio for 10-year all-cause mortality for viral loads >999 copies/mL; lower dashed line indicates hazard ratio of 1.
Appendix 2c
Crude risk curves for all-cause mortality for 7,944 CNICS patients, for selected viral load threshold values of k, stratified by viral load category, averaged over 100 imputations. Solid line represents viral loads <20 copies/mL, dot-dashed line represents viral loads between 20 and k copies/mL, dashed line represents viral loads between k and 999 copies/mL, dotted line represents viral loads >999 copies/mL. A. k = 20 copies/mL; B. k = 130 copies/mL; C. k = 200 copies/mL; D. k = 400 copies/mL.
Appendix 3a
Crude hazard ratio estimates for 10-year all-cause mortality for 7,944 CNICS patients, by selected viral load threshold values of k, with left-censored viral load observations substituted with half of assay detection limit.
| Crude hazard ratio
(95% confidence interval) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
k, cpm |
No. of deaths |
No. of patients |
<20 cpma |
No. of deaths |
No. of patients |
20 to
<k cpm |
No. of deaths |
No. of patients |
k to
999 cpm |
No. of deaths |
No. of patients |
>999 cpm |
|
|
|
|
|
|
||||||||
| 20 | 39 | 674 | 1 | — | — | — | 501 | 6055 | 1.19 (0.86, 1.65) | 322 | 1251 | 3.83 (2.75, 5.34) |
| 30 | 276 | 3623 | 1.15 (0.82, 1.61) | 225 | 2432 | 1.24 (0.88, 1.74) | 3.83 (2.75, 5.34) | |||||
| 40 | 336 | 4552 | 1.08 (0.78, 1.51) | 165 | 1503 | 1.48 (1.05, 2.10) | 3.83 (2.75, 5.35) | |||||
| 50 | 339 | 4640 | 1.08 (0.77, 1.50) | 162 | 1415 | 1.52 (1.07, 2.15) | 3.83 (2.75, 5.35) | |||||
| 75 | 356 | 4919 | 1.07 (0.77, 1.48) | 145 | 1136 | 1.66 (1.16, 2.36) | 3.84 (2.75, 5.35) | |||||
| 100 | 375 | 5091 | 1.08 (0.78, 1.50) | 126 | 964 | 1.69 (1.18, 2.42) | 3.83 (2.75, 5.35) | |||||
| 130 | 384 | 5221 | 1.08 (0.78, 1.50) | 117 | 834 | 1.79 (1.25, 2.58) | 3.84 (2.75, 5.35) | |||||
| 200 | 404 | 5395 | 1.09 (0.79, 1.52) | 97 | 660 | 1.84 (1.27, 2.67) | 3.84 (2.75, 5.35) | |||||
| 300 | 461 | 5787 | 1.14 (0.83, 1.59) | 40 | 268 | 2.10 (1.35, 3.27) | 3.83 (2.75, 5.34) | |||||
| 400 | 468 | 5862 | 1.15 (0.83, 1.59) | 33 | 193 | 2.37 (1.49, 3.78) | 3.83 (2.75, 5.34) | |||||
| 500 | 472 | 5912 | 1.15 (0.83, 1.59) | 29 | 143 | 2.83 (1.75, 4.57) | 3.83 (2.75, 5.34) | |||||
Abbreviation: cpm, copies/mL.
Viral load <20 copies/mL was reference category across k.
Appendix 3b
Standardized hazard ratio estimates for 10-year all-cause mortality for 7,944 CNICS patients, by selected viral load threshold values of k, with left-censored viral load observations substituted with half of assay detection limit.
| Standardizeda hazard ratio
(95% confidence interval) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
k, cpm |
No. of deaths |
No. of patients |
<20 cpmb |
No. of deaths |
No. of patients |
20 to
<k cpm |
No. of deaths |
No. of patients |
k to
999 cpm |
No. of deaths |
No. of patients |
>999 cpm |
|
|
|
|
|
|
||||||||
| 20 | 39 | 674 | 1 | — | — | — | 501 | 6055 | 1.84 (1.19, 2.82) | 322 | 1251 | 3.34 (2.13, 5.24) |
| 30 | 276 | 3623 | 1.72 (1.07, 2.76) | 225 | 2432 | 2.09 (1.30, 3.35) | 3.51 (2.17, 5.66) | |||||
| 40 | 336 | 4552 | 1.80 (1.16, 2.78) | 165 | 1503 | 2.00 (1.27, 3.15) | 3.39 (2.16, 5.32) | |||||
| 50 | 339 | 4640 | 1.81 (1.17, 2.79) | 162 | 1415 | 2.01 (1.27, 3.18) | 3.40 (2.17, 5.32) | |||||
| 75 | 356 | 4919 | 1.76 (1.14, 2.72) | 145 | 1136 | 2.17 (1.36, 3.46) | 3.40 (2.17, 5.34) | |||||
| 100 | 375 | 5091 | 1.80 (1.17, 2.78) | 126 | 964 | 2.08 (1.30, 3.33) | 3.41 (2.18, 5.35) | |||||
| 130 | 384 | 5221 | 1.78 (1.15, 2.74) | 117 | 834 | 2.22 (1.38, 3.58) | 3.41 (2.17, 5.34) | |||||
| 200 | 404 | 5395 | 1.86 (1.18, 2.92) | 97 | 660 | 2.44 (1.45, 4.10) | 3.54 (2.22, 5.66) | |||||
| 300 | 461 | 5787 | 1.76 (1.15, 2.71) | 40 | 268 | 2.68 (1.54, 4.65) | 3.35 (2.14, 5.25) | |||||
| 400 | 468 | 5862 | 1.79 (1.16, 2.75) | 33 | 193 | 2.63 (1.46, 4.74) | 3.35 (2.14, 5.26) | |||||
| 500 | 472 | 5912 | 1.77 (1.15, 2.73) | 29 | 143 | 2.63 (1.41, 4.90) | 3.35 (2.14, 5.25) | |||||
Abbreviation: cpm, copies/mL.
Standardized estimates controlled for age, sex, race/ethnicity, male-to-male sexual contact, injection drug use, smoking, at-risk alcohol use, pre-ART viral load, year of ART initiation, ART regimen, CD4 count, clinical AIDS status, status of other clinical conditions (chronic hepatitis B, chronic hepatitis C, past diagnosis of any cancer excluding nonmelanoma skin cancer), statin use, and study site.
Viral load <20 copies/mL was reference category across k.
Appendix 3c
Crude and standardized hazard ratios and 95% confidence intervals for 10-year all-cause mortality for 7,944 CNICS patients, by viral load threshold k, for viral loads between k and 999 copies/mL, with left-censored viral load observations substituted with half of assay detection limit. Standardized estimates controlled for age, sex, race/ethnicity, male-to-male sexual contact, injection drug use, smoking, at-risk alcohol use, pre-ART viral load, year of ART initiation, ART regimen, CD4 count, clinical AIDS status, status of other clinical conditions (chronic hepatitis B, chronic hepatitis C, past diagnosis of any cancer excluding nonmelanoma skin cancer), statin use, and study site. Upper dashed line indicates hazard ratio for 10-year all-cause mortality for viral loads >999 copies/mL; lower dashed line indicates hazard ratio of 1. A. Crude; B. Standardized.
Appendix 3d
Crude risk curves for all-cause mortality for 7,944 CNICS patients, for selected viral load threshold values of k, stratified by viral load category, with left-censored viral load observations substituted with half of assay detection limit. Solid line represents viral loads <20 copies/mL, dot-dashed line represents viral loads between 20 and k copies/mL, dashed line represents viral loads between k and 999 copies/mL, dotted line represents viral loads >999 copies/mL. A. k = 20 copies/mL; B. k = 130 copies/mL; C. k = 200 copies/mL; D. k = 400 copies/mL.
Appendix 3e
Standardized risk curves for all-cause mortality for 7,944 CNICS patients, for selected viral load threshold values of k, stratified by viral load category, with left-censored viral load observations substituted with half of assay detection limit. Standardized estimates controlled for age, sex, race/ethnicity, male-to-male sexual contact, injection drug use, smoking, at-risk alcohol use, pre-ART viral load, year of ART initiation, ART regimen, CD4 count, clinical AIDS status, status of other clinical conditions (chronic hepatitis B, chronic hepatitis C, past diagnosis of any cancer excluding nonmelanoma skin cancer), statin use, and study site. Solid line represents viral loads <20 copies/mL, dot-dashed line represents viral loads between 20 and k copies/mL, dashed line represents viral loads between k and 999 copies/mL, dotted line represents viral loads >999 copies/mL. A. k = 20 copies/mL; B. k = 130 copies/mL; C. k = 200 copies/mL; D. k = 400 copies/mL.
Footnotes
Disclaimers: None.
Author contributions:
Study concept: JSL, SRC, CJA, JJE. Study design: JSL, SRC, JJE, CJA, DBR, DPD, WCM. Data acquisition: JJE, RDM, MK, CM, KM, EG. Data analysis: JSL, SRC. Data interpretation: JSL, SRC, JJE. Drafting of manuscript: JSL, SRC, JJE. Revision of manuscript: All authors. Final approval of manuscript: All authors.
References
- 1.Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. The New England journal of medicine. 2008;359(4):339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 2.Deeks SG. Determinants of virological response to antiretroviral therapy: implications for long-term strategies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;30(Suppl 2):S177–184. doi: 10.1086/313855. [DOI] [PubMed] [Google Scholar]
- 3.Achenbach CJ, Buchanan AL, Cole SR, Hou L, Mugavero MJ, Crane HM, et al. HIV viremia and incidence of non-Hodgkin lymphoma in patients successfully treated with antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(11):1599–1606. doi: 10.1093/cid/ciu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boillat-Blanco N, Darling KE, Schoni-Affolter F, Vuichard D, Rougemont M, Fulchini R, et al. Virological outcome and management of persistent low-level viraemia in HIV-1-infected patients: 11 years of the Swiss HIV Cohort Study. Antiviral therapy. 2014 doi: 10.3851/IMP2815. [DOI] [PubMed] [Google Scholar]
- 5.Charuratananon S, Sungkanuparph S. Rate of and predicting factors for virologic failure in HIV-infected patients with persistent low-level viremia under antiretroviral therapy. Journal of the International Association of Providers of AIDS Care. 2015;14(1):12–16. doi: 10.1177/2325957414527168. [DOI] [PubMed] [Google Scholar]
- 6.Cohen C. Low-level viremia in HIV-1 infection: consequences and implications for switching to a new regimen. HIV clinical trials. 2009;10(2):116–124. doi: 10.1310/hct1002-116. [DOI] [PubMed] [Google Scholar]
- 7.The Antiretroviral Therapy Cohort Collaboration. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS (London, England) 2015;29(3):373–383. doi: 10.1097/QAD.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 8.Delaugerre C, Gallien S, Flandre P, Mathez D, Amarsy R, Ferret S, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PloS one. 2012;7(5):e36673. doi: 10.1371/journal.pone.0036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastburn A, Scherzer R, Zolopa AR, Benson C, Tracy R, Do T, et al. Association of low level viremia with inflammation and mortality in HIV-infected adults. PloS one. 2011;6(11):e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(10):1489–1496. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 11.Silva J, Pereira K, Rijo J, Alberto T, Cabanas J, Gomes P, et al. A retrospective observational study of low-level viraemia and its immunological and virological significance: which outcome to expect. Journal of the International AIDS Society. 2014;17(4 Suppl 3):19668. doi: 10.7448/IAS.17.4.19668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sungkanuparph S, Groger RK, Overton ET, Fraser VJ, Powderly WG. Persistent low-level viraemia and virological failure in HIV-1-infected patients treated with highly active antiretroviral therapy. HIV medicine. 2006;7(7):437–441. doi: 10.1111/j.1468-1293.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 13.Taiwo B, Gallien S, Aga E, Ribaudo H, Haubrich R, Kuritzkes DR, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. The Journal of infectious diseases. 2011;204(4):515–520. doi: 10.1093/infdis/jir353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenhende MA, Perrier A, Bonnet F, Lazaro E, Cazanave C, Reigadas S, et al. Risk of virological failure in HIV-1-infected patients experiencing low-level viraemia under active antiretroviral therapy (ANRS C03 cohort study) Antiviral therapy. 2015 doi: 10.3851/IMP2949. [DOI] [PubMed] [Google Scholar]
- 15.Charpentier C, Landman R, Laouenan C, Joly V, Hamet G, Damond F, et al. Persistent low-level HIV-1 RNA between 20 and 50 copies/mL in antiretroviral-treated patients: associated factors and virological outcome. The Journal of antimicrobial chemotherapy. 2012;67(9):2231–2235. doi: 10.1093/jac/dks191. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CY, Luo YZ, Wu PY, Liu WC, Yang SP, Zhang JY, et al. Antiretroviral therapy (ART) management of Low-Level Viremia in Taiwan (ALLEVIATE) Journal of the International AIDS Society. 2014;17(4 Suppl 3):19785. doi: 10.7448/IAS.17.4.19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do T, Duncan J, Butcher A, Liegler T. Comparative frequencies of HIV low-level viremia between real-time viral load assays at clinically relevant thresholds. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2011;52(Suppl 1):S83–89. doi: 10.1016/j.jcv.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. International journal of epidemiology. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moons KG, Donders RA, Stijnen T, Harrell FE., Jr Using the outcome for imputation of missing predictor values was preferred. Journal of clinical epidemiology. 2006;59(10):1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology (Cambridge, Mass) 2011;22(6):874–875. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peto R. Experimental Survival Curves for Interval-Censored Data. Journal of the Royal Statistical Society Series C (Applied Statistics) 1973;22(1):86–91. [Google Scholar]
- 22.Turnbull BW. The Empirical Distribution Function with Arbitrarily Grouped, Censored and Truncated Data. Journal of the Royal Statistical Society, Series B. 1976;38(3):290–295. [Google Scholar]
- 23.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 24.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchanan AL, Hudgens MG, Cole SR, Lau B, Adimora AA. Worth the weight: using inverse probability weighted Cox models in AIDS research. AIDS research and human retroviruses. 2014;30(12):1170–1177. doi: 10.1089/aid.2014.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 27.Nelson W. Theory and applications of hazard plotting for censored failure data. Technometrics. 1972;14(4):945–966. [Google Scholar]
- 28.Aalen O. Nonparametric inference for a family of counting processes. The Annals of Statistics. 1978;6(4):701–726. [Google Scholar]
- 29.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Computer methods and programs in biomedicine. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Department of Health and Human Services, editor. Panel of Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2013. p. C5. [Google Scholar]
- 31.Helsel D. Much ado about next to nothing: incorporating nondetects in science. The Annals of occupational hygiene. 2010;54(3):257–262. doi: 10.1093/annhyg/mep092. [DOI] [PubMed] [Google Scholar]
- 32.Jin Y, Hein MJ, Deddens JA, Hines CJ. Analysis of lognormally distributed exposure data with repeated measures and values below the limit of detection using SAS. The Annals of occupational hygiene. 2011;55(1):97–112. doi: 10.1093/annhyg/meq061. [DOI] [PubMed] [Google Scholar]
- 33.Cole SR, Chu H, Nie L, Schisterman EF. Estimating the odds ratio when exposure has a limit of detection. International journal of epidemiology. 2009;38(6):1674–1680. doi: 10.1093/ije/dyp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillespie BW, Chen Q, Reichert H, Franzblau A, Hedgeman E, Lepkowski J, et al. Estimating population distributions when some data are below a limit of detection by using a reverse Kaplan-Meier estimator. Epidemiology (Cambridge, Mass) 2010;21(Suppl 4):S64–70. doi: 10.1097/EDE.0b013e3181ce9f08. [DOI] [PubMed] [Google Scholar]